Page 1
CONTENTSCONTENTS
CONTENTS
CONTENTSCONTENTS
GENERAL SAFETY GUIDELINES ..................................... 1
I. DESCRIPTION..................................................................... 3
I. 1. Front panel (Fig. I. 1) ...................................................... 3
I. 2. Rear panel (Fig. I. 2) ......................................................... 3
II. STARTING-UP / INSTALLATION ................................ 3
II. 1. Use in direct oxygen therapy ......................................... 3
III. CLEANING - MAINTENANCE .................................... 4
III. 1. Cleaning .......................................................................... 4
III. 2. Everyday disinfection ................................................... 4
GENERAL SAFETY GUIDELINES
Only persons who have read and understood this entire
manual should be allowed to operate the Mark 5 Plus.
USE OF OXYGEN
• Oxygen is not a flammable gas, but it accelerates the
combustion of materials. To avoid all risks of fire, the Mark
5 Plus should be kept away from all flames, incandescent
sources and sources of heat (cigarettes), as well as any
combustible products such as oil, grease, solvents, aerosols, etc.
• Do not use in an explosive atmosphere.
• Avoid letting oxygen accumulate on an upholstered seat or
other fabrics. In the event the concentrator is operating while
not supplying oxygen to a patient, position it so that the gas
flow is diluted in the ambient air.
• Place the device in a ventilated area free from smoke and
atmospheric pollution (rear filter unobstructed).
USE AND MAINTENANCE OF THE DEVICE
• Use the power cord provided, and check that the electrical
characteristics of the power socket used match those indicated
on the manufacturer’s plate on the rear panel of the machine.
• We recommend avoiding the use of extension cords or
adapters, as they are potential sources of sparks and fire.
• The MARK 5 Plus must only be used for oxygen therapy
and only on medical prescription. The indicated daily
duration and flow must be followed, otherwise it may present
a risk to the health of the patient.
• Do not use in a specifically magnetic environment (MRI, etc.).
III. 3. Maintenance ................................................................... 4
IV. USEFUL INFORMATION .............................................. 4
IV. 1. Accessories and spare parts ........................................ 4
IV. 2. Materials in direct or indirect contact with the patient 4
IV. 3. Operating principles ..................................................... 4
IV. 4. Alarms - Safety devices ................................................ 5
IV. 5. Mark 5 Plus function .................................................... 5
IV. 6. Technical characteristics .............................................. 5
IV. 7. Standards ......................................................................... 6
IV. 8. Symbols - Abbreviations .............................................. 6
IV. 9. Method for disposing of waste ................................... 6
IV. 10. Method for disposing of device ................................ 6
IV. 12. Troubleshooting .......................................................... 7
•The Mark 5 Plus has an audible alarm to warn the user of
problems. In order that the alarm may be heard, the maximum
distance that the user can move away from it must be
determined to suit the surrounding noise level.
Conformity with IEC601-1 (§ 6.8.2 b):
"The manufacturer, assembler, installer or distributor are not
considered to be responsible themselves for the consequences
on the safety, reliability and characteristics of a device unless:
- The assembly, fitting, extensions, adjustments, modifications
or repairs have been performed by persons authorized by the
party in question,
- The electrical installation of the corresponding premises
complies with IEC /NEC requirements.
- The device is used in accordance with the instructions for use."
If the replacement parts used for the periodic servicing by an
approved technician do not comply with the manufacturer’s
specifications, the manufacturer is not responsible in the
event of an accident.
•Do not open the device while in operation: risk of electrical
shock.
This device complies with the requirements of the FDA
Quality System Regulation and the 93/42/EEC European
directive but its operation may be affected by other devices
being used near by, such as diathermy and high frequency
electro-surgical equipment, defibrillators, short wave
therapy equipment, mobile telephones, CB and other
portable devices, microwave ovens, induction plates or
even remote control toys or any other electromagnetic
interferences which exceed the levels specified by the EN
60601-1-2 standard.
11
1
11
5/005/00
5/00
5/005/00
code 2010-1024CE code 2010-1024CE
code 2010-1024CE
code 2010-1024CE code 2010-1024CE
Page 2
I.1I.1
I.1
I.1I.1
44
4
44
1
2
/
1
1
1
/
2
2
2
/
6
1
2
5
1
3
/
33
3
33
cc
c
cc
bb
b
bb
22
2
22
aa
a
aa
2
2
5
/
1
4
3
1
/
2
4
AVAILABLE ACCESSORIES
IF PRESCRIBED BY A PHYSICIAN
11
1
11
66
6
66
55
5
55
Humidifier: P / N 9012-8774
Cannula: P / N 9012-8780
Extension Tubing
25ft. (7.6m): P / N 9012-8781
Tubing Adapter P / N 9012-8783
The above items are available from
Nidek Medical Products, Inc.
I.2I.2
I.2
I.2I.2
99
9
99
1010
10
1010
77
7
77
1111
11
1111
88
8
88
code 2010-1024CEcode 2010-1024CE
code 2010-1024CE
code 2010-1024CEcode 2010-1024CE
22
2
22
Page 3
I. DESCRIPTION
II. STARTING UP / INSTALLATION
The Mark 5 Plus is an oxygen concentrator designed to
satisfy oxygen therapy prescriptions at home or in the clinic.
It provides a continuous flow of oxygen enriched Product by
separating the oxygen and nitrogen contained in ambient air.
It can be used either to administer oxygen with nasal cannulas
or another probe or mask type of device.
The Mark 5 Plus is easy to use.
The single flow adjustment knob allows:
• the device to be easily adjusted to the prescribed flow rate,
• the equipment supplier or medical staff to limit flows to a
specific range of flow rates with an internal locking device.
It has a power failure alarm and an operating fault alarm.
Note: the performances described pertain to the use of the
Mark 5 Plus with the accessories recommended by Nidek
Medical Products, Inc.
Attention : Les performances peuvent être dégradées au delà
de 4000 mètres. Dans ce cas veuillez consulter votre technicien
pour un réglage précis.
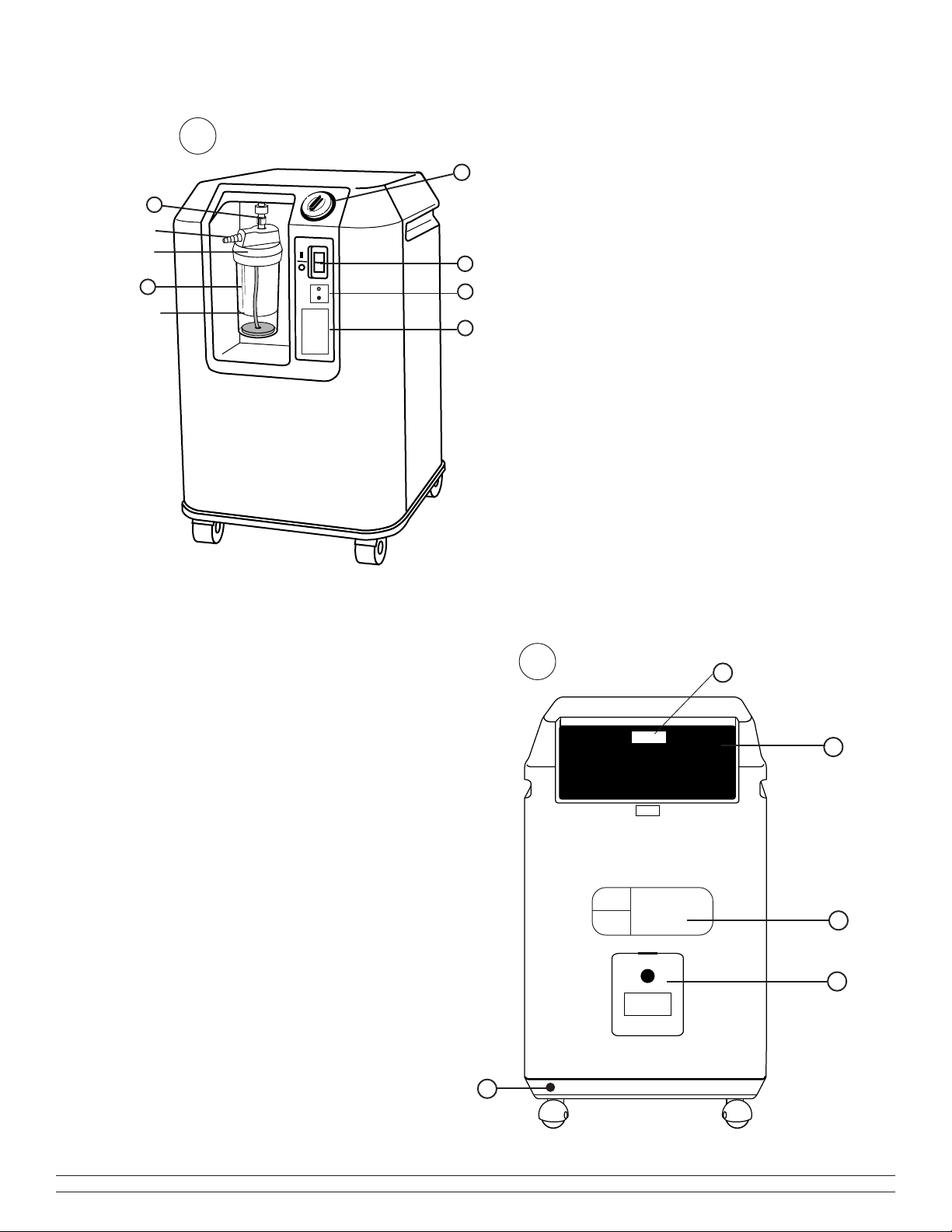
I. 1. Front panel (Fig.
I. 1I. 1
I. 1)
I. 1I. 1
1 Start/stop (on/off) Rocker Switch
2 Humidifier (space reserved)
a) Flask
b) Lid
c) Outlet connector
3 Oxygen enriched air outlet
4 Flow adjustment knob (l/min.)
5 Safety instructions
6 Oxygen monitor (when installed).
II. 1. Use in direct oxygen therapy
a - Ensure that the switch (1) is in the 0/(OFF) position.
b - Turn the flow adjustment knob (4) to the prescribed value.
This knob may have already been locked in the medically
prescribed position. In this case, do not force it. Only the
technician or medical personnel are authorized to release it.
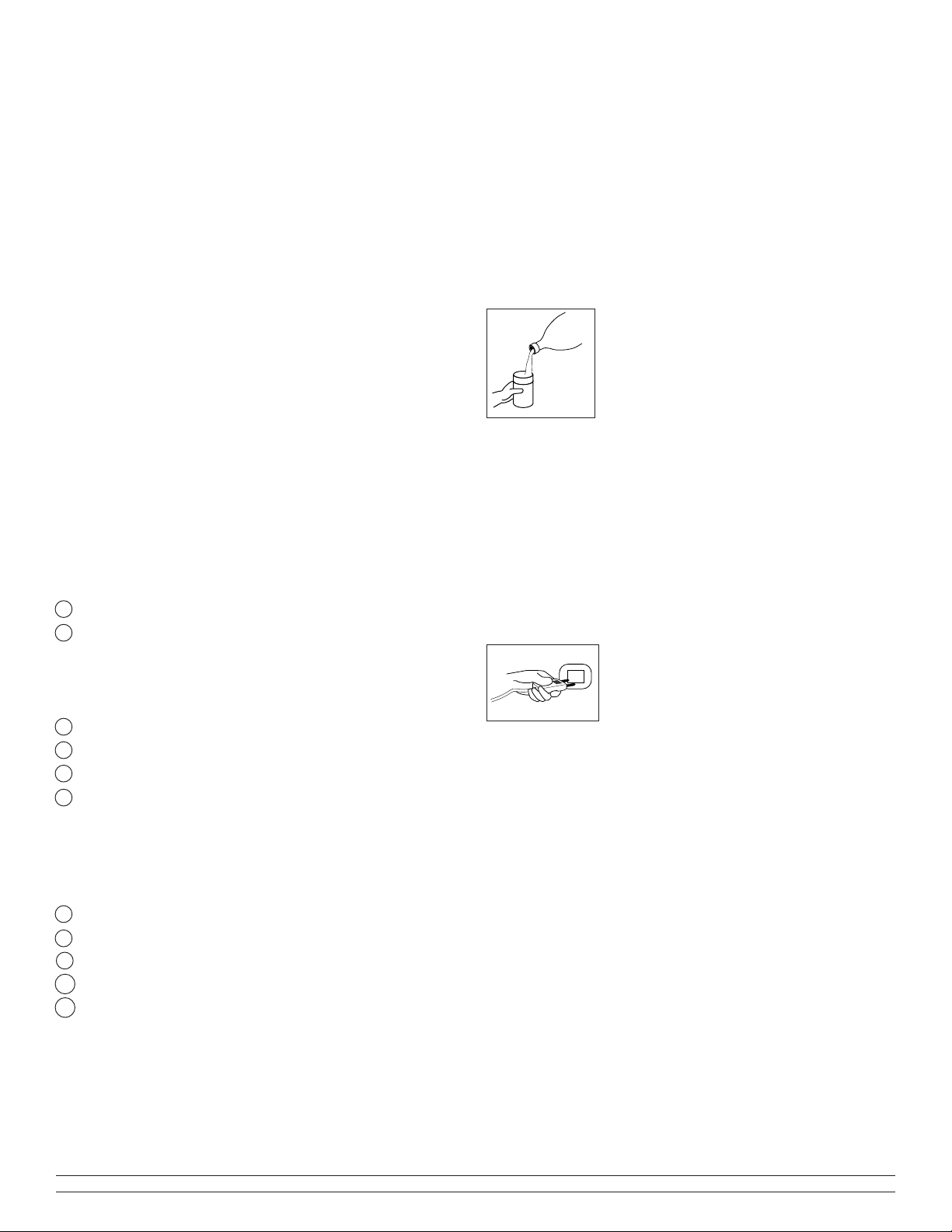
c - If used with a humidifier:
Unscrew the flask and fill it with water up to the line (see the
humidifier instructions). Then screw the
humidifier flask onto its lid until there are
no leaks from it.
d - Connect the oxygen tube to the
humidifier outlet nozzle or to the
concentrator outlet if a humidifier has not
been prescribed. The tube between the cannula and the Mark
5 Plus should be limited to 60 feet (20 meters) long, in order
to ensure that the oxygen flow rate remains within
specification values.
e - Ensure that all of the parts are connected correctly so as to
avoid leaks.
f - Plug the power cable into a power outlet of correct voltage
and frequency as defined on the manufacturer's label (8).
g - Press the switch to the start position I/
ON (LED lit). Units without OCSI will sound
an alarm for a few seconds. Units with OCSI
will suppress the green LED until oxygen
concentration exceeds the set point.
h - Check that the oxygen flows out of the administration device
(nasal cannulas or other) by placing the orifice(s) on the
surface of a glass of water. The flow should disturb the surface
of the water.
I I
I. 2. Rear panel (Fig.
I
I I
. 2. 2
. 2)
. 2. 2
7 Dust filter
8 Manufacturer’s label
9 Electrical power cord
10 Hour meter (located under the dust filter)
11 Internal filter access panel (service provider)
33
3
33
i - Adjust the nasal cannulas or mask to suit your face.
Remark: the required oxygen concentration is normally obtained
within five minutes after the unit is switched on.
At the end of the treatment, press the Rocker Switch (1) to
place it in the O/(OFF) position to stop the device. The
oxygen enriched air flow continues for approximately 1
minute after the device is stopped.
For the equipment supplier or medical staff:
The flow adjustment knob may be locked to limit it to a given
range of values. (See procedure in the maintenance manual).
code 2010-1024CE code 2010-1024CE
code 2010-1024CE
code 2010-1024CE code 2010-1024CE
Page 4

III. CLEANING - MAINTENANCE
III. 1. Cleaning
Only the outside of the Mark 5 Plus is to be cleaned, with a
soft, dry cloth or, if necessary, a damp sponge, then
thoroughly dried with wipes and an alcohol based solution.
Acetone, solvents or any other inflammable products
must not be used.
Do not use abrasive powders.
The entire oxygen administration circuit (oxygen therapy nasal
cannulas, etc.) must be changed.
III. 3. Maintenance
No special maintenance needs to be carried out by the
patient. Your equipment supplier performs periodic
maintenance operations to assure continued reliable service
from the Mark 5 Plus.
IV. USEFUL INFORMATION
IV. 1. Accessories and spare parts
The removable dust filter (7) must be cleaned in soapy water
weekly or after approximately 100 hours of use. More frequent
cleaning is recommended in dusty
1010
10
1010
77
7
77
enviroments.
Fit a dry filter.
1111
11
1111
10 Hour meter
11 Ventilation grill
7 Removable dust filter
III. 2. Everyday disinfection
Due to the presence of the bacterial filter inside the device,
everyday disinfection only concerns the external oxygen
therapy accessories: humidifier, probes, nasal cannulas (refer
to the respective instructions for use).
The use of alcohol based solutions means that the device
must be switched off.
a - The following minimum guidelines must be observed:
• Humidifier : (If prescribed by a physician)
Daily:
- Empty the water from the humidifier.
- Rinse the humidifier flask under running water.
- Fill humidifier up to the mark with distilled water.
Regularly:
- Disinfect the humidifier parts by immersing them in a
disinfectant solution (in general, we recommend using water
containing a small amount of chlorine bleach).
- Rinse and dry.
- Check that the humidifier lid seal is in good condition.
The accessories used with the Mark 5 Plus must:
- be oxygen compatible,
- be biocompatible,
- comply with the general requirements of the FDA or the
93/42/EEC European Directive as appropriate.
The connectors, tubes, nasal cannulas, probes or masks must
be designed for oxygen therapy.
The accessories with a Nidek Medical part number reference,
or included in the set of accessories supplied with the device,
comply with these requirements.
Contact your dealer to obtain these accessories.
Remarks:
• The use of certain administration accessories which are not
specified for use with this concentrator may reduce its
performances and void the manufacturer’s responsibility
(ISO 8359).
IV. 2. Materials in direct or indirect contact with the
patient
Concentrator casing ............................................................ABS
Mains cable .......................................................................... PVC
Dust filter ..................................................................... Polyester
ON/OFF switch ............................................................... Nylon
Casters ........................................................................ Elastomer
Flow adjustment knob ............................ABS/Polycarbonate
Gas outlet ............................................................................. Steel
Printed labels..................................................... Polypropylene
Pipe/ Tubing ............................. Aluminium, PVC or silicone
Humidifier ............................................................. Polypropylene
Support..................................... Chromed brass/Polypropylene
Filter ..................................................................... Polypropylene
Tuyau d'alimentation de NEBAL ........Silicone
IV. 3. Operating principle
• Oxygen tubing and nasal cannula:
Follow the manufacturer’s instructions.
b - For each new patient:
The humidifier must be sterilized if possible or changed.
The Mark 5 Plus must be cleaned and disinfected as per the
above instructions. The bacterial filter inside the device should
be changed. The dust filter may be changed as well.
code 2010-1024CEcode 2010-1024CE
code 2010-1024CE
code 2010-1024CEcode 2010-1024CE
The compressor sends filtered ambient air to a rotary distribution valve, which allows compressed air to pass to the column
in production. The columns contain a molecular sieve, whose
function is to adsorb the nitrogen and thus allow oxygen to
pass.
The oxygen enriched product is then directed to a pressure
reducing valve through the flow control valve to the oxygen
outlet fitting.
44
4
44
Page 5

During this time, the column which is being "regenerated" is
connected to the ambient air and flow of oxygen enriched
product is passed through it (from the column "in production"). In this way, when one column is in production, the
other is in a nitrogen desorption or "regeneration" phase. The
oxygen enriched product finally passes through a bacterial
filter located prior to the oxygen outlet fitting.
IV. 4. Alarms - Safety devices
IV. 4. 1. Alarms
• No voltage detection:
In the event of a loss of power, a continuous audible alarm is
activated (and the green light is extinguished if equipped
with oxygen monitor).
• Process fault:
In the case of a process fault, a visible and audible (after 15
minutes) alarm is activated (continuous red light or lighted alarm
and audible alarm, see p. 7).
IV. 4. 2. Safety devices
A red light indicates a concentration below the preset level.
When the light is red for more than 15 minutes (±2 minutes),
a continuous audible alarm is activated. Call the equipment
supplier to service the machine.
Note: when the Mark 5 Plus is started, the Oxygen Monitor
operates as follows:
1) in addition to the normal Mark 5 Plus test, LED indicator
lights are suppressed until oxygen concentration reaches
normal levels.
2) the light remains lit for a few minutes (5-10 minutes at
maximum) until the concentration of the gas supplied reaches
and exceeds the preset level.
3) The red light is off and the green light is lit after this period,
showing that the concentrator is operating satisfactorily.
IV. 5. 2. Maintenance of the Oxygen Monitor:
- No special maintenance is required,
- The equipment supplier checks that the oxygen monitor is
still operating correctly when the routine checks are performed
on the Mark 5 Plus.
The alarm set-point is factory set and there is no means to
adjust the settings. Models operating at 50 Hz are set at 83%;
60 Hz models are set at 85%.
• Compressor motor:
Thermal safety is ensured by a thermal switch situated in the
stator winding (145 ± 5 oC).
• "Ambient air” valve:
In the case of a negative pressure in the molecular sieve
columns, this valve allows ambient air to enter.
• Electrical protection of the Mark 5 Plus :
A 5A circuit breaker is incorporated into the START/STOP
switch of all 230V models. A 10 A circuit breaker is optionally
available with 115V models.
• Safety valve:
This is fitted on the compressor outlet and is calibrated to 42
psig (2.8 bar.).
• Class II devices with insulated casings (IEC 60601
standard).
IV. 5. Oxygen Monitor function (Optional)
IV. 6. Technical characteristics
Dimensions: L x W x H: 15 x 15 x 66 in. (381 x 381 x
660mm)
Castor diameter: 1.5 in. (38 mm).
Tilt angle (transport with humidifier fitted): 70
o
.
Weight: 55-60lbs. (25-28kg.) (varies with model)
Noise level < 46 dBA to 52 dBA(to ISO 8359)
Flow values:
0.125 / 0.25/ 0.5 / 1/ 1.5/ 2/ 2.5/ 3/ 3.5/ 4/ 4.5/ 5 l/min.
(some models may have other values)
Accuracy of flow supplied:
In compliance with the ISO 8359 standard, the flow supplied
is equal to the flow set on the flow selector, accurate to within
± 10 % or 200 ml/min., whichever is the larger of the two.
Average oxygen content:
• at 2 l/min. 93%.
• at 4 l/min. 91%.
• at 5 l/min. 90%.
(values at 21 oC and at one atmosphere pressure ).
IV. 5. 1. Operating principle (oxygen concentration indication
module)
The Oxygen Monitor (6) is an electronic module capable of
checking the effective oxygen concentration supplied by the
Mark 5 Plus concentrator.
The Oxygen Monitor detects any drop in the concentration
below a pre-set level and activates an audible and visual alarm.
A green light indicates that the concentration is above the
pre-set oxygen concentration level.
55
5
55
Max. recommended flow: 5 l/min.
The variation of the maximum recommended flow does not
exceed ± 10 % of the indicated value when a back pressure
of 1 psig(7kPa) is applied to the output of the device. The
maximum outlet pressure is 9 psig(62 kPa).
Electrical power supply:
115 V Units 230 V Units
Frequency: 60Hz 50/60Hz
Average Power : 420 - 440 VA 400-420 VA
ProtectionClass: ClassIIb Class IIb
Mains Protection: 10A(optional) 5A
code 2010-1024CE code 2010-1024CE
code 2010-1024CE
code 2010-1024CE code 2010-1024CE
Page 6

Filters:
At the rear of the device: a dust filter.
At the compressor input: a filter cartridge (technician only).
Before the oxygen outlet: a bacterial filter < 0.3 µm. (technician
only)
: Fragile - handle with care.
: Oxygen concentration warning light.
Air circulation:
A blower cools the compressor compartment.
Environmental limit conditions:
The performances of the device (especially the oxygen concentration) are quoted at 70oF (21oC) and one atmosphere.
They may change with temperature and altitude. For further
information, please consult the maintenance manual.
- The device must only be stored, transported and used in the
vertical position.
- Ambient temperature of between 10 oC and 40 oC (operation).
- Storage temperature range from 0 oC to 50 oC.
- Relative humidity of between 30 % and 75 % (operation and
storage).
- IPXX: No particular protection against penetration by liquids
or solids (complies with the EN 60601-1 standard: spilling of
a glass of water).
IV. 7. Standards
ISO 8359: Oxygen concentrators for medical use.
EN 60601: Electrical Safety-Medical Devices.
IV. 9. Method for disposing of waste
All waste from the Mark 5 Plus (patient circuit, filter, etc.)
must be disposed of using the appropriate methods.
IV. 10. Method for disposing of the device
In order to preserve the environment, the concentrator must
only be disposed of using the appropriate methods.
Furthermore, as part of the marking (directive 93/42 /
EEC), the serial number of the device disposed of must be
sent to the Nidek Medical technical service department if the
unit has the mark.
Mark 5 Plus Serial No. ________________________
Date first used: ______________________________
____________________________________________
IV. 8. Symbols - Abbreviations
I : ON
O : Off (power switched off).
: Type B device
: Class II device
: Do not smoke.
0413 : Complies with the 93/42/EEC directive certified
by the approved organization no 0413.
: Do not expose to open flames.
: Do not grease.
: Technical information.
: Consult the accompanying documents.
: Keep in the vertical position.
Maintained by: ______________________________
____________________________________________
Your distributor: _____________________________
Address : ____________________________________
____________________________________________
____________________________________________
Telephone : _________________________________
The manufacturer’s instructions for the preventive
maintenance of the devices defined in the maintenance
manual and any updates to it must be followed.
The work must be carried out by suitably trained
technicians.
Only use original spare parts.
Upon request, the supplier can provide circuit diagrams,
spare parts lists, technical details or any other information
of use to qualified technical personnel for parts of the
device which are designated as being the manufacturer’s
responsibility by the manufacturer as repairable.
code 2010-1024CEcode 2010-1024CE
code 2010-1024CE
code 2010-1024CEcode 2010-1024CE
66
6
66
Page 7

IV. 12. Fault Conditions and Corrections.
ObservationsObservations
Observations
ObservationsObservations
The I-O (ON/OFF)button is in the
ON position. The green indicator
does not light up and the device
does not operate.
The continuous alarm sounds.
Red light of Oxygen monitor
remains lighted.
The alarm test does not work.
The compressor operates and the
I-O (ON/OFF) button is in the ON
position but the green indicator
does not light up.
The I-O (ON/OFF) button is lit and
the compressor is operating but
there is no flow. The audible alarm
sounds (continuously).
The I-O (ON/OFF) button is lit, the
compressor is operating, there is a
flow but the audible alarm sounds
continuously.
Probable causesProbable causes
Probable causes
Probable causesProbable causes
Power cable not plugged
in correctly.
Power failure.
Oxygen concentration is too low.
Internal electrical fault.
Faulty indicator.
Pneumatic connection broken or
other pressure problem.
Internal electrical fault.
Pneumatic circuit fault.
SolutionsSolutions
Solutions
SolutionsSolutions
Check the cable connection.
Check the fuses or circuit
breaker on the power panel.
Contact your equipment supplier.
Contact your equipment supplier.
Contact your equipment supplier.
Stop the device by pressing the
I-O (ON/OFF) button and contact
your equipment supplier.
Stop the device and contact
your equipment supplier.
The compressor stops in midcycle, then starts again after a few
minutes.
The oxygen enriched air flow is
interrupted at the nasal cannula
outlet.
The flow at the nasal cannula
outlet is irregular.
Nidek Medical Products, Inc.
3949 Valley East Industrial Drive
Birmingham, Alabama 35217 U.S.A.
Tel: 205-856-7200 Fax: 205-856-0533
Compressor thermal safety
device has been activated.
Fan not working.
Dirty Filters.
Tube disconnected or humidifier
not tight.
Cannula Tubing kinked.
Pneumatic circuit problem.
Stop the device and wait for it to
cool down.Check that the patient
circuit is not obstructed. Clean
cabinet filter.Start up again.
Reset the circuit breaker (4) if
necessary by pressing the I-O
(ON/OFF) switch. If the device
does not start, contact your
equipment supplier.
Check that tubing connections
are secure and that the tubing is
not kinked.
Contact your equipment supplier
EU Representative
mdi Europa GmbH
Wittekamp 30
D-30163 Hanover
Germany
Tel: +49-511-39-08 95 30
Fax: +49-511-39-08 95 39
info@mdieuropa.com
www.mdieuropa.com
.
77
7
77
5/005/00
5/00
5/005/00
code 2010-1024CE code 2010-1024CE
code 2010-1024CE
code 2010-1024CE code 2010-1024CE
 Loading...
Loading...