Page 1

Nidek Medical Products, Inc®
Mark 5 Nuvo® Lite Oxygen Concentrator
Service Manual
Nidek Medical Products, Inc. 3949 Valley East Industrial Drive
Birmingham, Alabama 35217 USA
Telephone: (205) 856-7200 • 24-Hour Fax: (205) 856-0533
Nidek Medical is a trademark of Nidek Medical Products, Inc.
Mark 5 Nuvo® Lite is a registered trademark of Nidek Medical Products, Inc
2010-8405 Rev E Jan 2011 1 of 48
Page 2

Table of Contents
General Safety
Instructions
Section 1.0
Introduction
1.1
1.2
1.3
Section 2.0
Operational Check and Concentration Test
2.1
Section 3.0
Patient Instructions
3.1
Section 4.0
Home Service Provider Maintenance
4.1
4.2
Section 5.0
Service
5.1
5.2
Production and Use of Oxygen
Use and Maintenance of the Device
Standards and Regulations
2
4
4
5
2.2
2.3
2.4
2.5
3.2
Home Service Provider Responsibility
Important Notice and Symbol Explanations
Functional Specifications
Description of Operation
Operation Check
Alarm System
2.3.1 Power Failure Alarm test
Oxygen Concentration Test and Specification
No Oxygen Flow Alarm test Procedure
General Instructions
Routine Maintenance by the Patient
3.2.1 Cleaning the Cabinet Air Filter
3.2.2 Checking the Alarm System Battery
Routine Maintenance
4.1.1 Cabinet Air Filter
4.1.2 Final Product Filter Replacement
4.1.3 Compressor Inlet Air Filter Replacement
4.1.4 Recording Maintenance
6
7
7
8
8
9
9
9
10
10
11
11
11
11
11
12
12
12
-
2 of 48 Jan 2011 2010-8405 Rev E
Cleaning Unit
4.2.1 Preparing for New Patient Use
Components
Cabinet Removal
5.2.1 Removing Cabinet Back
5.2.2 Caster Replacement
13
13
13
13
13
13
Page 3

5.3
Compressor
5.4
5.5
5.6
5.7
5.8
5.9
5.10
5.11
5.12
5.13
5.14
5.15
6.1
6.2
6.3
6.4
6.5
5.3.1 Compressor Replacement
5.3.2 Capacitor Replacement
Process Control Valve
Sieve Bed Replacement
5.5.1 Sieve Bed Removal
5.5.2 Sieve Bed Installation
Cabinet Fan Replacement
Circuit Board Replacement
5.7.1 Circuit Board Removal
5.7.2 Circuit Board Installation
Product Regulator Check and Setting
5.8.1 Product Regulator Cleaning or Rebuilding
Pressure Switch Replacement
Circuit Breaker Replacement
5.10.1 Circuit Breaker Removal
5.10.2 Circuit Breaker Installation
I/0 (ON/OFF) Power Switch Replacement
5.11.1 I/0 (ON/OFF) Power Switch Removal
5.11.2 I/0 (ON/OFF) Power Switch Installation
Buzzer Replacement
Hour Meter Replacement
Flow Valve Replacement
5.14.1 Flow Valve Removal
5.14.2 Flow Valve Installation
Power Cord Replacement
14
15
15
16
16
16
16
17
17
17
18
18
19
19
19
19
19
20
20
20
20
20
20
20
20
21
Section 6.0
Troubleshooting
Air Pressure Test (P1)
6.1.1 High Air Pressure (P1)
6.1.2 Low Air Pressure (P1)
Product Pressure Test (P2)
6.2.1 Low Product Pressure
6.2.2 High Product Pressure
General Troubleshooting
Troubleshooting Chart
Tool Kit and Pressure Test Gauge
21
23
23
24
24
24
25
26
29
2010-8405 Rev E Jan 2011 3 of 48
Page 4

Appendices
Exploded
Drawings
A-1: OCSI Pneumatic Diagram 30
A-2: Standard Pneumatic Diagram 31
A-2-A: No-Flow Alarm Pneumatic Diagram 32
A-3: 115 Volt Electrical Schematic 33
A-4: 230 Volt Electrical Schematic 34
A-5: Electrical Schematic for No Flow Board 35
A-6: Electrical & Compressor Assembly 36
A-6-A: Electrical & Compressor Assembly Parts 37
A-6-B: Compressor Mounting Bracket Parts 38
A-7: Front Cabinet Standard Assembly 39
A-7-A: Front Cabinet Standard Assembly Parts 40
A-8: Front Cabinet OCSI Assembly 41
A-8-A: Front Cabinet OCSI Assembly Parts 42
A-9: Back Cabinet Assembly 43
A-9-A: Back Cabinet Assembly Parts 44
A-10: Module Assembly 45
A-10-A: Module Assembly Parts 46
A-11: Maintenance Log 48
General Safety Instructions
Production and use of oxygen
Oxygen is not a flammable gas, but accelerates the combustion of materials. To prevent fire risks, the
MARK 5 NUVO Lite should be kept away from flames, incandescent sources or sources of heat
(including cigarettes) and combustible products such as oil, grease, solvents, aerosols, etc.
Do not use in an explosive atmosphere.
Prevent oxygen from accumulating on upholstered seats or any other fabric. If the concentrator
operates without being administered to a patient, locate it so that the flow of product gas produced is
dissipated into the air.
Locate the equipment in a free space (filter to the rear and below) which is well ventilated and free of
fumes or atmospheric pollution.
Use and Maintenance of the Device
Use the electric cable provided and check that the voltage of the mains socket used complies with the
electrical characteristics of the appliance indicated on the manufacturers plate on the rear of the
device.
4 of 48 Jan 2011 2010-8405 Rev E
Page 5

Do not use an extension cord or multiple sockets which can create sparks and therefore pose a fire
risk.
Use of the MARK 5 NUVO Lite must be restricted solely to oxygen therapy on medical prescription
in compliance with the daily rate and duration.
Use in other circumstances may represent a hazard to patient health.
Do not use in a specifically magnetic environment (MRI, etc.).
The MARK 5 NUVO Lite has an audible alarm intended to warn the user of any problems. The user
must determine the maximum distance away from the Nuvo based on the on the sound levels in the
environment, to ensure that the alarm is always audible.
Standards & Regulations
In compliance with UL60601-1 [EN60601-1] (para 6.82.b):
“The manufacturer, assembler, installer or importer are not considered to be responsible for
consequences or the safety, reliability and characteristics of a device unless,
● the assembly, extensions, adjustments modifications or repairs have been performed by
persons authorized by the manufacturer,
● the electrical installation of the corresponding premises complies with appropriate regulations
and codes,
● the device is used in accordance with the instructions for its use.
If the replacement parts used for periodic servicing by an approved technician do not comply with the
manufacturer’s specifications, the manufacturer is absolved of all liability in the event of an incident.
Do not open the equipment when it is powered on: risk of electrocution.
This device complies with the requirements of the FDA Quality System Regulation and EU Directive
93/42/EEC and 2007/47/EC, but its operation may be affected by use in the surrounding area of
appliances such as diathermy, high frequency electro-surgical instruments, defibrillators, short wave
treatment appliances, cell-phones, CB devices and other portables, microwave ovens, induction hot
plates or remote control toys, and more generally, by electromagnetic interference exceeding the
levels specified in standard IEC(EN) 60601-1-2:2001.
As a regulated medical device, both manufacturers and service providers have certain responsibilities
regarding complaints.
FDA defines a complaint as any written, electronic or oral communication that alleges
deficiencies related to the identity, quality, durability, reliability, safety, effectiveness or
performance of a device that has been released for distribution.
2010-8405 Rev E Jan 2011 5 of 48
Page 6

Service providers have a responsibility to evaluate any complaints received from their direct
customers. ( Ref. CFR 820.198 ). Nidek Medical does not have direct links to your customers.
Your evaluation should include the following:
● Determine if the complaint warrants action by Nidek Medical,
● If NO, resolve the complaint with your customer,
● If YES, contact Nidek Medical customer service,
● Work with Nidek Medical to resolve all complaints.
1.0 Introduction
1.1 Home Service Provider Responsibility
All Home Service Providers of the Nidek Medical MARK 5 NUVO Lite Oxygen Concentrator must
assume responsibilities for handling, operational check-out, patient instruction, and maintenance.
These responsibilities are outlined below and throughout this manual.
WARNING
MARK 5 NUVO Lite units must not be used for or with any life-supporting or life sustaining
applications. Patients unable to communicate discomfort while using this device may require
additional monitoring. Advise patients to immediately notify their Home Service Provider(s) and/or
physician(s) in case of an alarm or any discomfort.
As a Home Service Provider, you must do all of the following:
● Inspect the condition of each MARK 5 NUVO Lite unit immediately upon delivery to your
business location. Note any sign of damage, external or internal, on the delivery receipt,
and report it directly to both the freight company and Nidek Medical Products, Inc.
immediately.
● Check the operation of each MARK 5 NUVO Lite before delivery to a patient. Always
operate the unit for a reasonable length of time and check that the oxygen concentration
level is within specifications as referred to in Section 2.4. Test the power failure alarm as
described in Section 2.3 of this manual.
● Deliver MARK 5 NUVO Lite units only to patients authorized by a physician’s
prescription. The MARK 5 NUVO Lite must not be used as a life-supporting or life
sustaining device. A backup supply of oxygen must be available.
● Instruct patients and patient caregivers how to use the MARK 5 NUVO Lite in
conjunction with the Users Manual.
● Instruct patients and patient caregivers to notify their physicians and/or Home Service
Providers if they experience any signs of discomfort.
● Instruct each patient and patient caregivers how to perform routine maintenance of the
6 of 48 Jan 2011 2010-8405 Rev E
Page 7
cabinet air filter and how to check the alarm system. (Refer to Section3.2.)
Describes a hazard or unsafe practice that can result in
Describes a hazard or unsafe practice that can result in
size or
Be available to service each patient at any time. Maintain the MARK 5 NUVO Lite in
accordance with Section 4.0.
Repair components and replace parts only as outlined in this manual. Use only Nidek Medical parts for
replacement in MARK 5 NUVO Lite Oxygen Concentrators.
● Refer to the MARK 5 NUVO Lite Product Warranty if parts replacement is required within
the warranty period.
1.2 Important Notice and Symbol Explanations
As you read the manual, pay special attention to the WARNING, CAUTION, and NOTE messages.
They identify safety guidelines or other important information as follows:
WARNING:
CAUTION:
NOTE:
The following harmonized symbols (pictograms), used for non-English language countries, will be
located in the User’s Guide of the MARK 5 NUVO Lite unit:
Read the accompanying documents; particularly the User's Guide.
Store, ship and use the device in an upright condition.
No smoking within five feet of this device, oxygen-carrying tubing, or
accessories.
Indicates an alarm signal for low oxygen concentration or other problem.
Do not use any oil or grease on or near the device
severe bodily injury or death.
minor bodily injury or property damage.
Provides information important enough to empha
repeat.
1.3 Functional Specifications
Dimensions: 35.6 cm long, 22.9 cm wide, 58.5 cm high
[14 in. long, 9 in. wide, 23 in. tall]
Weight: 13 kg [30 Ib] depending on sound attenuation package
Electrical 115 VAC, 60 Hz, +/- 10% <330 watts(avg)
Requirements: 230 VAC, 50 Hz, +/- 10% <300 watts(avg)
230 VAC, 60 Hz, +/- 10% <330 watts(avg) (280 W for 3 l/min unit)
Capacity: Max. 5 liters per minute ( 3 l/min for 925/60K )
Accuracy: Flow Valve ±10% indicated flow rate +/- 200 ml whichever is greater as
2010-8405 Rev E Jan 2011 7 of 48
Page 8

per ISO 8359 Standard
Concentration: 2 liters per minute at >90%
5 liters per minute at 90% (+ 6.5 / - 3%)
(Based on 21°C [70°F] at sea level)
Response Time: Acceptable concentration is normally achieved in about 90 seconds;
allow 5 minutes to attain full concentration.
Positioning: Operate the unit in an upright position, maintaining at
least six inches of open space on all sides for ventilation.
2.0 Operational Check and Concentration Test
2.1 Description of Operation
Air enters the MARK 5 NUVO Lite Oxygen Concentrator through an external cabinet air filter. This
filtered air enters the compressor via a suction tube and fine filter, which quiets the suction sounds
made by the compressor. Pressurized air then exits the compressor and passes through a heat
exchanger into a pair of 3-way solenoid valves. The heat exchanger reduces the temperature of the
compressed air. Next, the solenoid valve directs the air into one of two sieve beds that contain
molecular sieve. The special characteristic property of molecular sieve is that it physically attracts
(adsorbs) nitrogen when air passes through this material, thus enabling the production of high purity
oxygen.
There are two sieve beds or adsorbent columns; while one produces high purity oxygen, the other is
purged of the nitrogen it adsorbed (collected) while it was producing oxygen. Each column produces
oxygen for approximately five seconds and delivers it to the product storage volume tank integrated
into the sieve module. Oxygen exits the product storage tank through a pressure regulator, flow
control valve, and final product filter. The flow control valve, controls the flow rate of oxygen delivered
to the patient. The MARK 5 NUVO Lite unit delivers up to 95% oxygen concentration at flow rates
from 0.125 to 5 l/min. The remaining constituents of the product gas stream are nitrogen and argon,
both of which are part of the air we breathe, are inert and are completely safe.
2.2 Operational Check
Nidek Medical runs each device through a burn in period and tests every MARK 5 NUVO Lite
Oxygen Concentrator thoroughly after manufacture before releasing for shipment. As the home
service provider, it is your responsibility to perform the following test to ensure that no damage
occurred in shipping or handling.
1. Open and inspect all concentrator cartons upon receipt. Unpack each unit and remove it from
its carton. Inspect the unit itself for damage. If the exterior of the carton is damaged, or the
unit itself is damaged, note it on the freight bill signed by the driver.
2. Plug in the power cord of the unit, and set the I/0 (ON/OFF) switch to the I (ON) position.
Check to see that the following occurs:
● The compressor runs, listen for the sound.
● Exhausted cooling air flows out of the bottom rear of the unit.
8 of 48 Jan 2011 2010-8405 Rev E
Page 9

● OPTIONAL for Units Equipped with Oxygen Concentration Status Indicator (OCSI): The
OCSI green light remains off until the oxygen concentration reaches 85% ± 3% (82% ± 2%
for 50 Hz units) (approximately two minutes).
● OPTIONAL for Units Equipped with No Oxygen Flow Alarm Board: The No Oxygen Flow
board test should be preformed as in Section 2.5 of this manual.
● After performing the above steps, remove the power cord from the wall outlet. Actuate the I/0
(ON/OFF) switch to the I (ON) position and note that the audible alarm sounds intermittently.
(See Section 2.3). If the unit does not initially sound off, plug the unit in and allow the unit to
run approximately 10 minutes to charge the capacitor and repeat the test. Move the switch to
the 0 (OFF) position.
3. Turn the flow valve adjustment knob clockwise until it stops (wide open). The flow valve
should indicate 5 liters/min. and the output of the unit should be 5 liters/min. If not, refer to
Section 5.8 to adjust the product regulator.
4 Perform an oxygen concentration test, as described in Section 2.4.
2.3 Alarm System
The MARK 5 NUVO©Lite Oxygen Concentrator is equipped with a capacitor powered alarm
system, which sounds an intermittent alarm when a power failure occurs and a continuous alarm when
one or more cycle variables are not within specification. It sounds an alarm if the high or low pressure
indicators are activated or if the optional OCSI detects lower than predetermined levels of oxygen
concentration. The alarm remains on until you correct the alarm condition or you set the I/0 (ON/OFF)
switch to the 0 (OFF) position. Refer to Section 6.0 for a list of probable alarm causes.
2.3.1 Power Failure Alarm Test
To test the power failure alarm, perform the following actions:
Unplug the power cord from the wall outlet, and set the I/0 (ON/OFF) switch to the I (ON) position.
If the unit has been stored for a prolonged period, Allow unit to run for approximately 10 minutes to
charge the capacitor and re-test the unit.
This should immediately activate the intermittent audible alarm. If it does not, refer to the
troubleshooting chart in Section 6.0 of this manual.
2.4 Oxygen Concentration Test and Specification
To ensure that the output of oxygen from the device is within specification, you must perform an
oxygen concentration test. Test the unit upon delivery to a patient and at periodic intervals. Home
Service Providers, based on their expertise and documentation, may establish and implement their
own plans for checking oxygen concentration. Consult Nidek Medical’s Service and Maintenance Log
(A-11) for the recommended intervals for testing.
1. If an oxygen humidifier bottle is used, remove it from the oxygen outlet.
2010-8405 Rev E Jan 2011 9 of 48
Page 10
2. Connect a calibrated oxygen concentration analyzer to the oxygen outlet.
3. Set the I/0 (ON/OFF) power switch to the I (ON) position. (It takes approximately five minutes
for the oxygen concentration to stabilize.) Take oxygen concentration readings over a period of
several minutes to reduce any cyclic variations
4. Verify that the product flow rate delivered by the unit matches the patient’s prescription and
does not exceed the capacity of the unit.
5. Disconnect the oxygen analyzer, and reconnect the humidifier bottle (if used) and any other
equipment / accessories that may be required.
6. Adjust the flow valve knob to the prescribed flow rate.
Nidek Medical MARK 5 NUVO Lite Concentration Specifications
Liter Flow Specification
2 l/min greater than 90%
5 l/min 90% + 6.5 / - 3%
NOTE:
Do not measure oxygen concentration output after the product stream passes through a humidifier
bottle. Erroneous readings will result and your oxygen concentration measuring device might be
damaged.
2.5 No Oxygen Flow Alarm Test Procedure
A No Oxygen Flow Alarm board is available as an option with the Nuvo Lite
Oxygen Concentrator. To test the function of “No Flow” alarm, follow the instructions below:
1. Turn Concentrator on and allow the unit to reach normal operating purity.
2. Adjust the Oxygen Flow to desired flow rate.
3. Block the oxygen flow at the patient outlet
4. A continuous audible alarm should sound as long as the Oxygen flow is blocked
3.0 Patient Instructions
3.1 General Instructions
It is important that patients thoroughly understand how to operate the Nidek Medical MARK 5 NUVO
Lite unit. This enables proper treatment as prescribed by a qualified, licensed physician. You must
explain that the purpose of this therapy is to alleviate symptoms. If patients experience any discomfort
or the unit alarms, they must notify their Home Service Provider and/or physician immediately. You,
as the Home Service Provider, are responsible to see that each patient receives the User's Guide.
Explain each step in the operation of the unit to the patient in reference to that guide.
10 of 48 Jan 2011 2010-8405 Rev E
Page 11

3.2 Routine Maintenance by the Patient
n the grille in the back of the unit. Be
To ensure accurate output and efficient operation of the unit, the patient must perform two simple
routine maintenance tasks:
• Clean the cabinet air filter
• Check the alarm system
3.2.1 Cleaning the Cabinet Air Filter
NOTE: The patient must clean this filter weekly, as described below. The filter may require
daily cleaning if the MARK 5 NUVO Lite unit operates in a harsh environment such as a house
heated by wood, kerosene, or oil, or one with excessive cigarette smoke.
1
Remove the dirty cabinet air filter from the back of the MARK 5 NUVO
Lite unit.
2 Wash the dirty filter in warm water with household detergent, and rinse.
3 Use a soft absorbent towel to remove excess water.
4 Reinstall the clean cabinet air filter o
careful that the filter edges are under the tabs.
3.2.2 Checking the Power Alarm System
See Procedure described in Paragraph 2.3.1
4.0 Home Service Provider Maintenance
4.1 Routine Maintenance
The MARK 5 NUVO Lite unit has three filters that require inspection and scheduled maintenance or
replacement.
To ensure that the output of oxygen from the unit is within specification, you must perform an oxygen
concentration test. Test the unit upon delivery to a patient and at periodic intervals. Home Service
Providers, based on their expertise and documentation, should establish and implement their own
practices for checking oxygen concentration. The interval established may be longer or shorter than
90 days, which is the default time period recommended for providers who do not choose to establish
their own method.
Nidek Medical does not require preventive maintenance on the concentrator. You do not need to
perform any maintenance as long as the MARK 5 NUVO Lite unit remains within specifications at
the prescribed flow rate. (Refer to Section 2.4)
4.1.1 Cabinet Air Filter
The external cabinet air filter is located on the back of the unit, You can easily remove it by hand.
Instruct the patient to clean this filter weekly. (Refer to Section 3.2.1.)
2010-8405 Rev E Jan 2011 11 of 48
Page 12

NOTE:
The filter may require more frequent cleaning if the MARK 5 NUVO Lite unit operates in a harsh
environment such as a house heated by wood, kerosene, or oil, or one with excessive cooking,
cigarette smoke or atmospheric dust.
4.1.2 Final Product Filter Replacement
The final product filter does not require periodic replacement; it needs to be replaced only if it restricts
oxygen flow. It is suggested that it be replaced whenever the sieve module is repaired or replaced
and after the compressor is rebuilt.
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back to locate the final product filter.
NOTE: Observe the position of the filter before removal.
3. Separate the silicone tubing from both sides of the filter.
4. Install the new filter with the inlet side in the same position as before. Push the tubing together
so that it overlaps the barbs of the final product filter connections.
5. Record information about the final product filter replacement in Appendix 11 of this manual and
online at www.nidekmedical.com under the ‘Maintenance Log’ tab.
6. Reinstall the cabinet back.
4.1.3 Inlet Air Filter Replacement
The inlet air filter requires inspection at each patient visit. The filter should be replaced every 2 years,
or more often depending on environment.
1. Set the unit I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet air filter to locate the inlet air filter.
3. Remove filter from the unit, and replace with a new filter.
4. Record information about the filter replacement in Appendix 11 of this manual and online at
www.nidekmedical.com under the ‘Maintenance Log’ tab.
5. Reinstall the cabinet air filter.
NOTE: The filter may require more frequent cleaning if the MARK 5 NUVO Lite unit operates in a
harsh environment such as a house heated by wood, kerosene, or oil, or one with excessive cooking,
cigarette smoke or atmospheric dust.
4.1.4 Recording Maintenance
As the Home Service Provider, it is suggested that you record all routine maintenance and repairs
performed on the MARK 5 NUVO Lite unit, including hours and dates of service in Appendix 11 of
this manual and online at www.nidekmedical.com under the ‘Maintenance Log’ tab.
12 of 48 Jan 2011 2010-8405 Rev E
Page 13

4.2 Cleaning Unit
Periodically, use a damp cloth to wipe down the exterior case of the MARK 5 NUVO Lite. If you use
medical disinfectants, be sure to follow manufacture’s instructions.
4.2.1 Preparing for New Patient Use
When you remove the MARK 5 NUVO Lite from a patient’s home, always dispose of the used nasal
cannula and humidifier bottle. Inspect the humidifier tube and clean or replace as needed.
Replace the cabinet air filter between each patient’s use or clean with warm soapy water if it is in good
condition. Clean this filter at least once per week or more frequently if operated in a dusty
environment.
Retest the MARK 5 NUVO Lite before you return it to your inventory.
5.0 Service
5.1 Components
The design of the Nidek Medical MARK 5 NUVO Lite Oxygen Concentrator allows for easy access
and removal of most components. This allows you to perform scheduled maintenance, repair, and
replacement of parts with minimal time and effort. The inlet air filter is conveniently located behind the
cabinet air filter on the cabinet back.
CAUTION: For your safety, be sure to set the I/0 (ON/OFF) switch to the 0
(OFF) position and unplug the power cord before you service the
MARK 5 NUVO Lite Oxygen Concentrator.
NOTE: Record all scheduled maintenance on the Maintenance Log found in Appendix 11. (Refer to
Section 4.1.4.)
5.2 Cabinet Removal
5.2.1 Removing Cabinet Back
Lay the device on its front to access the cabinet back, remove four screws, two at the top near the
handle and two near the bottom of the cabinet.
5.2.2 Caster Replacement
The casters are a push in type that does not require a fastener. Lay the device on its back to access
the casters from the bottom. Pull them straight out away from the bottom.
2010-8405 Rev E Jan 2011 13 of 48
Page 14
5.3 Compressor
The compressor is the pump within the oxygen concentrator that supplies air to the separation process
performed by the sieve beds. The pressure generated by the compressor forces oxygen to flow out of
the top of the sieve columns.
The compressor is the likely cause of two potential specific problems:
a. An insufficient amount of air is supplied to the process, and
b. An excessive sound level.
● Air Supply
Compressor output refers to how much compressed air the compressor can produce. This depends
upon the model of the compressor, length of stroke, piston diameter, speed of rotation and condition of
seals. The cup seals form the seal between the piston and the cylinder wall. As the cup seals wear,
the output begins to gradually decrease.
This reduction in compressor output results in less air, and thus less oxygen, entering the sieve beds.
Therefore, the production of oxygen decreases.
Because this drop in oxygen production occurs over a long period of time, preventive maintenance on
the compressor is not required.
You can continue a patient’s therapy on the MARK 5 NUVO Lite unit as long as the oxygen
concentration level at the prescribed liter flow rate is within Nidek Medical’s specification limits. Refer
to Section 2.4.
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
____ ___
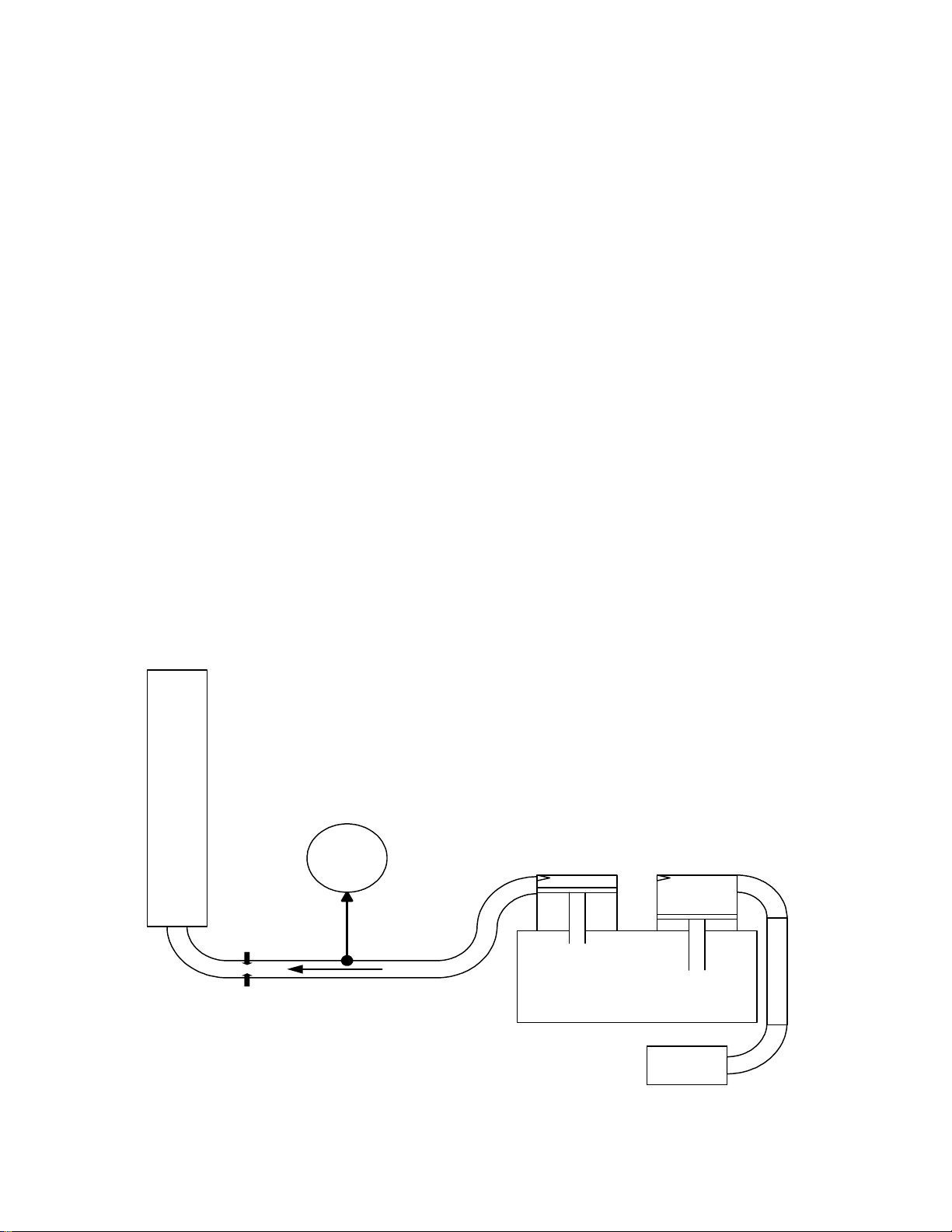
Air Flow
Meter
0 to 100 l/min
Pressu re
1 bar
Pressure
Gauge
Flow
Compressor
Restrictor
Air
Filter
14 of 48 Jan 2011 2010-8405 Rev E
Page 15

● Sound Level
The sound level is largely determined by the condition of the compressor’s bearings.
There are four bearings located within the compressor that allow the inner components of the
compressor to rotate. If the bearings wear to the point that they become loose and noisy, the
compressor becomes noticeably loud and needs servicing. The life of a compressor is determined
primarily by its operating temperature. It is extremely important that the inlet cooling air filters are
cleaned and replaced as required.
5.3.1 Compressor Replacement
Remove Compressor Assembly
To remove the compressor assembly for exchange, follow the steps listed below:
1. Set the unit’s I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back.
3. Disconnect the suction tube and the discharge tube.
4. Disconnect the two compressor power cable leads and the two leads to the capacitor.
5. Disconnect the two springs from the compressor support plate. Slide the compressor
assembly from the cabinet.
6. Remove compressor springs from the compressor with the two screws.
7. Remove heat exchanger from compressor.
8. Remove the heat exchanger fitting from the compressor.
Compressor Assembly Installation
To install a new compressor, follow the steps listed below:
1. Perform the compressor removal procedure in reverse order.
2. Leak test all connections.
5.3.2 Capacitor Replacement
The capacitor helps the compressor to start and run more efficiently. If the compressor cannot start,
the capacitor may be defective and require replacement. The capacitor should be replaced at each
compressor service / module replacement. To replace the capacitor, take the following steps:
1. Set the unit’s I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug
the power cord.
2. Remove the cabinet back.
3. Disconnect the two leads to the capacitor and clip the tie wrap securing the capacitor.
4. To install the new capacitor, connect the leads and replace the capacitor and install a new tie
wrap.
5. Clip excess after securing capacitor.
2010-8405 Rev E Jan 2011 15 of 48
Page 16

5.4 Process Control Valve
The MARK 5 NUVO Lite uses a solenoid powered poppet valve assembly to control the air
separation process. There is a feed port that connects to the compressor and an exhaust port that
discharges through an integral exhaust muffler. There are three possible valve states as follows:
1. Air feed connected to sieve bed A and exhaust connected to sieve bed B.
2. Air feed connected to sieve bed B and exhaust connected to sieve bed A.
3. Both ports open; this is a very short time period during which air
4. pressure builds in the sieve beds.
The control valve of the MARK 5 NUVO Lite requires no scheduled maintenance. If a valve does not
function as required, it is best to replace the complete sieve module as it is probable that one or both
of the beds has been damaged.
5.5 Sieve Bed Replacement
CAUTION:
Do not expose molecular sieve (contents of bed) to air for an extended period of time. Prolonged
exposure of molecular sieve to the moisture in room air results in contamination and permanent
damage to the sieve material. Keep all openings to the sieve beds sealed during periods of storage.
NOTE: It is recommended to replace the sieve beds and control valve as a complete assembly.
5.5.1 Sieve Bed Removal
1. Set the unit’s I/0 (ON/OFF) switch to the 0 (OFF) position and unplug the power cord.
2. Remove the cabinet back.
3. Disconnect the compressor discharge 5/16” tube from the top of the solenoid valve and the
product tube from the regulator.
4. Unplug the solenoid valve electrical leads at the solenoids.
5. Lift the module up and out of the cradle.
5.5.2 Sieve Bed Installation
To install the sieve beds, follow the sieve bed removal procedure in reverse order. It is very important
to tighten ensure that the tubes are fully inserted into the fittings to eliminate leaks.
To check for leaks, take the following steps:
1. Plug in the unit.
2. Set the unit’s I/0 (ON/OFF) switch to I (ON) for three minutes with the flow meter closed to
pressurize the system.
3. Apply soapy water around the tubing connections at the valve; check for leaks.
Caution: There is an electrical shock hazard with the Power ON. Be careful that no water
contacts any of the electrical connections.
16 of 48 Jan 2011 2010-8405 Rev E
Page 17

NOTE: Even small leaks can affect concentrator performance and can cause contamination of the
sieve. Careful leak testing is important.
5.6 Cabinet Fan Replacement
The cabinet fan for the MARK 5 NUVO Lite is located adjacent to the compressor. Refer to the
troubleshooting chart in Section 6.0 of this manual for instances where replacement of the fan may be
required.
To replace the cabinet fan in the Mark5 Nuvo® unit, take the following steps:
1. Set the unit’s I/0 (ON/OFF) switch to the 0 (OFF) position and unplug the power cord.
2. Remove the back cabinet.
3. Disconnect the fan leads.
4. Lift out the fan.
5. Install isolation foam around new fan.
6. Insert into cabinet and connect the fan leads.
7. Reinstall the back cabinet.
5.7 Circuit Board Replacement
There are two printed circuit boards within the MARK 5 NUVO Lite There is a printed circuit board
that controls the alarm system functions and also a printed circuit board that controls the timing logic
for the solenoid valves.
Consult the troubleshooting chart in Section 6.0 to determine which and when to replace the one of
the printed circuit boards.
CAUTION:
The Printed Circuit Boards (PCB) contain components that are sensitive to electrostatic discharge
(ESD) that can damage the board if not handled properly. As when handling any ESD sensitive PCB,
observe standard ESD safety procedures. These procedures include the following:
• Handle the PCB by the edges only.
• Work on a grounded ESD mat.
• Wear a grounded wrist strap.
• Store PCB in anti-static bags only.
5.7.1 Alarm Circuit Board Removal (Note that the Circuit Board on the OCSI unit is
different to the Circuit Board on the standard unit.)
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back.
3. Disconnect the 7-pin connector from the upper circuit board.
4. Disconnect tubing from each end of the black sensor tube on the OCSI unit, noting their
position and orientation.
2010-8405 Rev E Jan 2011 17 of 48
Page 18

5. Non OCSI units: Cut tie-wrap and remove pressure sensor line.
6. Remove the screws that attach the board to the front cabinet.
7. Remove the circuit board.
NOTE: Handle the new circuit board only by the edges to prevent electrostatic damage to the
unit.
For Reinstallation reverse the above procedure.
5.7.2 Timing Circuit Board Removal
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back.
3. Disconnect the 6 and the 4 pin connectors as well as the 6 spade connectors from the Timing
Board. Brown 220V (White 110V) wires towards the module side and Blue 220V (Black 110V)
wires toward the compressor side.
4. Remove the mounting screws.
5. Remove the circuit board.
NOTE: Handle the new circuit board only by the edges to prevent electrostatic damage to the
unit.
For Reinstallation reverse the above procedure.
5.7.3 MARK 5 NUVO Lite units produced after 1 June 2010 have a PCB that includes the functions
of both the Alarm Circuit board and the Timing Board that were used in earlier units.
The new board includes two LED's that are visible inside the unit. They have been added
to assist the service technician in determining that the solenoid valves have power to them and
are cycling. Also, the new board flashes the green LED during the startup phase to indicate that
the device has power. The flashing green LED is continuous when oxygen concentration reaches
the specified level.
5.8 Product Regulator Check and Setting
The product regulator enables you to set the maximum flow of oxygen output by the MARK 5 NUVO
Lite unit. To check for proper adjustment of the product regulator, take the following steps:
1. Set the I/0 (ON/OFF) switch to the I (ON) position.
2. Allow the unit to run for a few minutes.
3. Connect a pressure gauge directly to the patient outlet.
4. The pressure should read 7.1 psig (49 kPa) ± 10%.
5. If it requires adjustment, remove the cabinet back and lift out the module
enough to access the regulator.
6. Adjust the regulator as necessary. Turn the knob clockwise to increase the output pressure.
(Requires a 3/32 hex wrench)
7. Reinsert the module assembly and reinstall the cabinet back.
18 of 48 Jan 2011 2010-8405 Rev E
Page 19

5.8.1 Product Regulator Cleaning or Rebuilding
Clean or rebuild the product regulator if the regulator cannot be adjusted.
I. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord,
2. Remove the back cabinet.
3. Remove the regulator from the tee on top of the solenoid valve assembly.
4. Adjust the product regulator fully counterclockwise to unload the spring. This makes
disassembly and reassembly easier.
5. Remove the diaphragm. (Clean or replace it.)
6. Use a hex-head screwdriver to unscrew the diaphragm stem guide located in the center of the
regulator body to gain access to the seat,
7. Remove the seat. Be careful not to lose the spring located behind the seat.
8. Replace the seat or clean by blowing clean air on and around it.
9. With the spring behind the seat, screw the diaphragm stem guide back into the body of the
regulator. (Do not over tighten.)
10. Install a clean or replacement diaphragm.
11. Put the large spring and slip ring into the bonnet, and screw the bonnet onto the regulator
body.
12. Reinstall the regulator.
13. Reset the product regulator as described in Section 5.8.1.
5.9 Pressure Monitoring Board Replacement (Standard Unit, Non OCSI only)
The high and low pressure alarms are activated by a pressure transducer located on the alarm circuit
board adjacent to the mains switch.
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the back cabinet.
3. Disconnect the 7 pin connector from the circuit board.
4. Disconnect tubing from pressure sensor by cutting tie-wrap.
5. Remove the circuit board and replace with a new one.
6. Test the alarm system, as described in Section 2.3.Reinstall the back cabinet.
5.10 Circuit Breaker Replacement
5.10.1 Circuit Breaker Removal
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back.
3. Disconnect the circuit breaker leads.
4. Unscrew the circuit breaker while you apply pressure to the circuit breaker retaining ring.
5.10.2 Circuit Breaker Installation
Follow the removal procedure for the circuit breaker in reverse order to install the new circuit breaker.
2010-8405 Rev E Jan 2011 19 of 48
Page 20

5.11 I/0 (ON/OFF) Power Switch Replacement
5.11.1 I/0 (ON/OFF) Power Switch Removal
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position and unplug the power cord.
2. Remove both the back cabinet.
3. Disconnect the I/0 (ON/OFF) switch leads from the back of the switch being careful to note the
position of each.
4. Push on the back of the power switch, while holding in its four retaining tabs, and remove the
switch through the front of the control panel.
5.11.2 I/0 (ON/OFF) Power Switch Installation
Follow the removal procedure for the I/0 (ON/OFF) power switch in reverse order to install a new
power switch.
5.12 Buzzer Replacement
The buzzer is a fixed component on the circuit board and is not individually replaceable.
5.13 Hour Meter Replacement
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back.
3. Disconnect the hour meter leads.
4. Remove the hour meter from the front cabinet.
5. Install the new hour meter into its mounting location. Make sure that the hour meter is mounted
right side up.
6. Reconnect the hour meter leads.
7. Reinstall the cabinet back.
5.14 Flow Valve Replacement
5.14.1 Flow Valve Removal
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet back.
3. Remove the two hoses from the back of the flow valve.
4. Remove the knob from the flow valve, and the two Philips screws below the knob.
5. Remove the flow valve.
5.14.2 Flow Valve Installation
To install a new flow valve, follow the flow valve removal procedure in reverse order. Then perform a
leak test on the connections.
20 of 48 Jan 2011 2010-8405 Rev E
Page 21

5.15 Power Cord Replacement
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position and unplug the power cord.
2. Remove the cabinet back. Clip the power cord retaining tie wrap
3. Slide the power cord strain relief reinforcement upwards to remove it from the mounting
location at the bottom of the front cabinet.
4. Disconnect the power cord leads from the terminal quick connects.
5. Connect the leads on the new power cord at the terminal quick connects.
6. Reinstall the power cord strain relief into the base of the unit.
7. Reconnect the cabinet back and install a new power cord retaining tie wrap.
6.0 Troubleshooting
6.1 Air Pressure Test (P1)
Testing the operating pressure is a useful diagnostic tool when a concentrator has low purity
and requires servicing. Units functioning normally do not require operating tests.
Use the following procedure to test the operating pressure of the unit.
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet rear.
3. Remove the air supply tubing going to the control valve and install the test
port tee fitting. Figure 6.1.1 & 6.1.2 shows the normal operating configurations for each type
of unit. Figure 6.1.3 shows the installation of the test ports.
4. Connect the pressure test gauge to the test port.
5. Plug in the power cord, and set the I/0 (ON/OFF) power switch to the ON position. Set the
flow meter to 5 l/min, and allow the unit to run at least five minutes.
6. Observe the maximum and minimum readings on the pressure test gauge.
7. The maximum reading should not exceed 34 psig (235 kPa). The minimum reading
should not be less than 10 psig (70 kPa).
NOTE When you turn the unit on, it will take several minutes to reach normal operating
pressures.
2010-8405 Rev E Jan 2011 21 of 48
Page 22

Figure 6.1.1 normal operating configuration (OCSI unit).
Figure 6.1.2 normal operating configuration (standard unit).
22 of 48 Jan 2011 2010-8405 Rev E
Page 23

Figure 6.1.3 configuration with test ports
6.1.1 High Operating Air Pressure (P1)
Higher than normal operating pressure may indicate any of the following:
► A restrictive exhaust muffler, which does not allow the waste (purge) gas to exit the system
freely.
► Contaminated sieve beds. Change the sieve beds.
6.1.2 Low Operating Air Pressure (P1)
Lower than normal operating pressure may indicate any of the following:
► A restriction in the suction resonator or inlet air filter, this limits the amount of room air
available to the compressor. Disconnect the suction tube at the compressor, and allow the
unit to operate without the suction resonator to see if normal operating pressure returns.
► An improperly operating control valve. Confirm that the control valve does not have a leak.
► A leak in the unit, which allows system pressure to escape. Leak test the unit.
► A compressor with reduced output.
2010-8405 Rev E Jan 2011 23 of 48
Page 24

Ensure that the concentration level at the desired liter flow is within specifications listed in section 2.4.
If it is below specifications, replace or repair the compressor.
6.2 Product Pressure Test (P2)
Testing the product pressure is a useful diagnostic tool when a concentrator has low purity and
requires servicing. Units functioning normally do not require operating tests.
Use the following procedure to test the product pressure of the unit.
1. Set the I/0 (ON/OFF) switch to the 0 (OFF) position, and unplug the power cord.
2. Remove the cabinet rear. Remove the cap from the regulator test port and install the test port
tee. Figure 6.1.1 &
3. Remove the cap from the regulator test port and install the test port tee. Figure 6.1.1 & 6.1.2
shows the normal operating configurations for each type of unit. Figure 6.1.3 shows the
installation of the test ports.
4. Connect the pressure test gauge to the P2 test port.
5. Plug in the power cord, and set the I/0 (ON/OFF) power switch to the ON position. Set the flow
meter to 5 l/min, and allow the unit to run at least five minutes.
6. Observe the maximum and minimum readings on the pressure test gauge.
The maximum reading should not exceed 32 psig (220kPa). The minimum reading should not
be less than 9 psig (62 kPa).
6.2.1 Low Product Pressure (P2)
Lower than normal operating pressure may indicate any of the following:
► An inlet air filter that limits the amount of room air available to the compressor. Disconnect
the suction tube at the compressor, and allow the unit to operate without the suction
resonator to see if normal operating pressure returns.
► An improperly operating control valve. Confirm that the control valve does not have a leak.
► A leak in the unit, which allows system pressure to escape. Leak test the unit.
► A compressor with reduced output. Ensure that the concentration level at the desired liter
flow is within specifications listed in Section 2.4. If it is below specification, replace or repair
the compressor.
6.2.2 High Product Pressure (P2)
Higher than normal operating pressure may indicate any of the following:
► A restrictive exhaust muffler, which does not allow the waste (purge) gas to exit the system
freely.
► Check exhaust muffler for any restrictions
► Contaminated sieve beds. Change the sieve beds.
24 of 48 Jan 2011 2010-8405 Rev E
Page 25

6.3 General Troubleshooting
Before reviewing the troubleshooting chart, the following steps may be useful to isolate any
malfunctions:
1. Turn the concentrator on. If unit does not turn on, refer to troubleshooting chart.
2. Make sure all filters are clean.
3. Connect test pressure gauge to the outlet fitting of the unit. The pressure
should read 7.1 psig (49 kPa) ± 10%.
4. Connect test pressure gauge to the P1 test port on the module. The pressure should be
cycling between approximately 10 and 34 psig (70 and 235 kPa).
5. Make sure the unit is cycling properly by observing the pressure gauge cycle between a high
and a low pressure. If the unit is not cycling properly, refer to troubleshooting chart.
6. Make sure that the unit is leak free by testing all tubing connections and fittings with leak
testing solution. Protect circuit board from solution and start leak test at the heat exchanger,
following the air flow through the unit to the oxygen outlet. Repair all leaks by tightening
connections and fittings.
7. Set the concentrator at 5 l/min and connect pressure test gauge to P2 at the top of the sieve
beds. Determine pressure parameters by observing high and low pressure points on the
gauge. It should cycle between approximately 9 psig and 32 psig (62 to 220 kPa). If
pressures are high or low, refer to the troubleshooting chart.
8. Review troubleshooting chart to isolate and repair any other malfunctions.
The following diagnostic flow chart will help to isolate potential problems.
2010-8405 Rev E Jan 2011 25 of 48
Page 26

Low Oxygen
Concentration
Verify Oxygen
Flow Rate
Measure Air
Pressure
Low Pressure High Pressure
Replace Air
Inlet Filter
Replace
Check for
Leaks
Muffler
Foams
Check the
Compressor
Normal Air
Pressure
Measure the
Oxygen Pressure
Low Pressure High Pressure
Check for
Leaks
Check the
Control
Valve
Normal Pressure
Check for leaks at the:
- regulator outlet
- product tubing
- oxygen outlet
Check
Control
Valve
Replace
Muffler
Foams
Replace
Sieve
Module
26 of 48 Jan 2011 2010-8405 Rev E
Page 27

Problem
Probable Cause
Solution
Compressor runs with
intermittent high pressure
alarm and low oxygen
concentration.
Compressor relief valve
releases (popping sound).
Defective sieve beds.
Restriction in exhaust muffler.
Defective valve.
Defective control valve.
Contaminated sieve beds.
Replace sieve beds.
Replace or clean muffler.
Replace sieve module.
Replace sieve module.
Replace sieve module.
Defective relief valve.
Constant alarm with I/0
(ON/OFF) switch in ON
position. Circuit breaker
repeatedly trips.
Alarm does not sound.
Flow fluctuates. Improperly set or faulty
Defective circuit breaker.
Defective capacitor.
Defective compressor.
Defective circuit board.
Faulty electrical connection.
Faulty electrical connection.
Defective I/0 (ON/OFF) switch.
Defective buzzer.
Defective pressure sensor.
product regulator.
Leak.
Worn compressor.
Defective flow valve.
Kinked tubing
Replace sieve module.
Replace relief valve.
Replace circuit breaker.
Replace capacitor.
Replace compressor.
Replace circuit board.
Repair electrical connection.
Repair electrical connection.
Replace I/0 (ON/OFF) switch.
Replace board.
Replace and test control board.
Check regulator setting/clean,
repair, or replace regulator.
Leak test.
Replace compressor
Replace flow valve.
Check tubing that connects the
top of the sieve beds.
2010-8405 Rev E Jan 2011 27 of 48
Page 28

Problem
Probable Cause
Solution
Cabinet fan does not turn. Defective cabinet fan.
Replace cabinet fan.
Defective electrical
connections.
Limited or Iow flow. Restriction in humidifier or
tubing.
Product regulator set too low.
Leak.
Weak compressor.
Air flow obstruction.
Low concentration. Compressor inlet filter is dirty
or partially blocked.
System leak
Faulty compressor
Check electrical connections.
Replace humidifier or tubing.
Adjust regulator setting.
Leak test and repair leak.
Check system pressure, and
rebuild or exchange compressor.
Check Filter, suction resonator,
and suction tube for obstruction.
Replace inlet filter.
Leak test and repair leak.
Check system pressure, and
rebuild or replace compressor.
Unit temperature too high,
Contaminated sieve beds.
Defective control valve.
Restriction in exhaust muffler,
Restriction in suction
resonator.
Blocked air intake or defective
cabinet filter.
Check that P1 and P2 pressures
are within range.
Replace sieve module.
Repair or replace sieve module.
Replace or clean exhaust muffler.
Check suction resonator and
suction tube for obstruction and
remove.
28 of 48 Jan 2011 2010-8405 Rev E
Page 29

6.5 Tool Kit, Oxygen Purity testing and Pressure Test Gauge
The tools needed for you to properly service the MARK 5 NUVO Lite unit are listed below:
► Requires no special tools; generally available tools including common pliers, channel lock,
wire cutters, needle-nose pliers, slotted-head screwdriver, long Phillips head screwdriver, 8inch adjustable wrench, 7/16-inch socket, 7/16-inch combination wrench, 5/8-inch
combination wrench and 3/8-inch combination wrench.
► For checking the Oxygen purity at the Concentrator.
Nidek Medical recommends Part # 6500-4220
► An accurate pressure test gauge to take both P-1 and P-2 pressure readings on the MARK
5 NUVO Lite unit should be kept available at all times. This gauge kit allows connections
to the pressure test locations.
Kit for testing P1 & P2 pressures with gauge supplied # 6500-0051
Kit for testing P1 & P2 pressures w/o gauge supplied # 6500-0052
Kit tools for Nuvo Lite # 6500-0053
Appendices
Drawings
A-1: OCSI Pneumatic Diagram 30
A-2: Standard Pneumatic Diagram 31
A-2-A: No-Flow Alarm Pneumatic Diagram 32
A-3: 115 Volt Electrical Schematic 33
A-4: 230 Volt Electrical Schematic 34
A-5: Electrical Schematic for No Flow Board 35
A-6: Electrical & Compressor Assembly 36
A-7: Front Cabinet Standard Assembly 39
A-8: Front Cabinet OCSI Assembly 41
A-9: Back Cabinet Assembly 43
A-10: Module Assembly 45
2010-8405 Rev E Jan 2011 29 of 48
Page 30

A-1
Flow Schematic OCSI Option
30 of 48 Jan 2011 2010-8405 Rev E
Page 31

A-2
Flow Schematic Standard Non-OCSI Option
2010-8405 Rev E Jan 2011 31 of 48
Page 32

A-2-A
Flow Schematic No-Flow Option
32 of 48 Jan 2011 2010-8405 Rev E
Page 33

115 Volt Electrical
Schematic
R = Red
W = White
K = Black
BL = Blue
BR = Brown
GR = Green
YL = Yellow
PU = Purple
A-3
115 Volt Electrical Schematic
2010-8405 Rev E Jan 2011 33 of 48
Page 34

230 Volt Electrical
Schematic
R = Red
W = White
K = Black
BL = Blue
BR = Brown
GR = Green
YL = Yellow
PU = Purple
A-4
230 Volt Electrical Schematic
34 of 48 Jan 2011 2010-8405 Rev E
Page 35

230 Volt
No Flow Option
Electrical Schematic.
R = Red
W = White
K = Black
BL = Blue
BR = Brown
GR = Green
YL = Yellow
PU = Purple
NOTE:
230 Volt Version
is Shown
For 115 Volt Version:
A-5
Electrical Schematic – No Flow Alarm Option
1. SUBSTITUTE BK
FOR WHEREVER BL
IS USED
2. SUBSTITUE W FOR
WHEREVER BR IS
USED
3. SUBSTITUTE DPST
SWITCH IN PLACE OF
TPST SWITCH
4. USE TWO BK
VOLTAGE SELECT
JUMPERS INSTEAD
OF ONE BR
2010-8405 Rev E Jan 2011 35 of 48
Page 36

A-6
ELECTRICAL & COMPRESSOR ASSEMBLY
36 of 48 Jan 2011 2010-8405 Rev E
Page 37

400-1513 1 SHUNT, VOLTAGE SELECTION 1 EA
8400-5018 2 HOURMETER AC 100-230 V 50/60 1 EA
9250-1170 2 HOURMETER,UNV W/O CLIP 1 EA
8400-1012 3 SCREW, 8-32 x 5/8"LG.HEX HEAD 2 EA
8400-1010 4 BRACKET,COMPRESSOR 1 EA
8400-1049 5 BRACKET, HEAT EXCHANGER 1 EA
8400-1052 6 FITTING,COMPRESSOR 1 EA
8400-1053 6 FITTING,COMPRESSOR 1 EA
8400-1016 7 SPRING, EXTENSION 2 EA
8400-1094 8 EXCHANGER,HEAT 1 EA
8400-1095 8 EXCHANGER,HEAT 1 EA
8400-1161 9 TUBING,5/16ODx3/16IDx12.5"LG. 1 EA
8400-1162 9 TUBING, 5/16ODx3/16IDx9.75"LG. 1 EA
8400-1163 10 FITTING HX 1 EA
8400-1030 12 GROMMET,.75"IDX1.0"HOLE 2 EA
8400-1013 13 RETAINER, SPRING 1 EA
9250-1062 14 TIE-WRAP, 14" LONG 2 EA
8400-1510 15 WIRING, HARNESS 230 VOLT 1 EA
8400-1500 15 WIRING,HARNESS 115 VOLT 1 EA
8400-1512 15 WIRING,HARNESS UNIVERSAL VOLT 1 EA
9250-1330 16 CORD, POWER, EUROPLUG 1 EA
9250-1311 16* CORD,POWER, TYPE "K" PLUG 1 EA
8400-1018 17 BREAKER,CIRCUIT 10 AMP 115 V 1 EA
8400-1019 17 BREAKER, CIRCUIT 5 AMP 230 V 1 EA
8400-1008 18 SWITCH,POWER UNIVERSAL 1 EA
9250-1012 18 SWITCH,POWER 110 VOLT 1 EA
9250-1023 19 FAN,115V LOW NOISE 1 EA
9250-1024 19 FAN, 230V LOW NOISE 1 EA
8400-1042 20 CAPACITOR,20μF P2 1 EA
8400-2450 21 COMPR. 2450 115V/60HZ 5LPM 1 EA
8400-2460 21 COMPR. 2450 230V/50HZ 5LPM 1 EA
8400-2466 21 COMPR. 2450 230V/60HZ 3LPM 1 EA
8400-0010 22 BAFFLE, COMPRESSOR COOLING 1 EA
8400-1014 23 WASHER SPLIT RETAINING 2 EA
8400-1011 24 LIMITER COMPRESSOR 1 EA
8400-1009 25 CAP, VINYL 3-16" 2 EA
8400-1017 26 GROMMET, SPRING EYE 4 EA
8400-1041 27 FAN ISOLATOR 2 EA
8400-8450 99 KIT, COMP. PLATE ASSY 1 EA
ELECTRICAL AND COMPRESSOR ASSEMBLY PARTS
A-6-A
2010-8405 Rev E Jan 2011 37 of 48
Page 38

8400
-
1017
6
GROMMET, SPRING EYE
4
EA
Part Reference
Call-Out
Qty
Unit of
Number Number Part Description Required Measure
8400-1010 1 BRACKET,COMPRESSOR 1 EA
8400-1013 2 RETAINER, SPRING 1 EA
8400-1016 3 SPRING, EXTENSION 2 EA
8400-1030 4 GROMMET,.75"IDX1.0"HOLE 2 EA
8400-1009 5 CAP, VINYL 3-16" 2 EA
COMPRESSOR MOUNTING BRACKET PARTS
A -6-B
38 of 48 Jan 2011 2010-8405 Rev E
Page 39

A-7
A-7
CABINET, FRONT ASSEMBLY (STD)
2010-8405 Rev E Jan 2011 39 of 48
Page 40

Part Reference
Call-Out
Qty
Unit of
Number Number Part Description Required Measure
8400-0002 1 CABINET,FRONT 1 EA
9251-1330 4 VALVE, FCV 1/8-5.0 LPM 1 EA
8400-1331 5 KNOB,FLOW 0.125TO 5.0 FLOW 1 EA
9251-1332 6 RING,LOCK-OUT 1 EA
9251-1335 7 CLIP,D STYLE 1 EA
8400-1020 8 FITTING, OXYGEN OUTLET 1 EA
8400-1024 9 WASHER, 3/8 STAR O2 OUTLETLITE 1 EA
8400-1022 10 NUT 3/8-24 O2 OUTLET LITE 1 EA
7631-1053 11 FILTER, FINALPRODUCT 1 EA
6956-9674 12 VALVE, CHECK 1/4 HOSE MPC A975 1 EA
8400-1102 13 FOAM, ANTI VIBRATION 1 EA
7854-6051 14 HOSE 5/32 X11/32 X3 LG SILIC 1 EA
6491-1006 15 ADAPTER 1/4 ODTX3/16HOSE 1 EA
7854-6052 16 HOSE 5/32 X11/32 X2 LG SILIC 2 EA
7854-6055 17 HOSE 5/32 X11/32 X 7"LG SIL 1 EA
9250-1041 18 HOSE 5/32 X11/32 X5 LONG SIL 1 EA
8400-1337 19 HOSE 1/8" ID X1/4" OD 3" LG 1 EA
8400-1204 20 BOARD,TIMING OMS 9V 5LPM 1 EA
9250-1045 21 SCREW,PLASTITE#4X3/8" PAN.HD 8 EA
9251-1336 23 SCREW #6 X32 X.5"LG. FLUSH 2 EA
A-7-A
PARTS
CABINET, FRONT ASSEMBLY (STD)
40 of 48 Jan 2011 2010-8405 Rev E
Page 41

A-8
CABINET, FRONT ASSEMBLY OCSI
2010-8405 Rev E Jan 2011 41 of 48
Page 42

Part Reference
Call-Out
Qty
Unit of
Number Number Part Description Required Measure
8400-0002 1 CABINET,FRONT 1 EA
9251-1330 4 VALVE, FCV 1/8-5.0 LPM 1 EA
8400-1331 5 KNOB,FLOW 0.125 TO 5.0 FLOW 1 EA
9251-1332 6 RING,LOCK-OUT 1 EA
9251-1335 7 CLIP,D STYLE 1 EA
8400-1020 8 FITTING, OXYGEN OUTLET 1 EA
8400-1024 9 WASHER,3/8 STAR O2 OUTLET LITE 1 EA
8400-1022 10 NUT3/8-24O2 OUTLET LITE 1 EA
7631-1053 11 FILTER, FINALPRODUCT 1 EA
6956-9674 12 VALVE, CHECK 1/4 HOSE MPC A975 1 EA
8400-1102 13 FOAM, ANTI VIBRATION 1 EA
7854-6051 14 HOSE 5/32 X11/32 X3 LG SILIC 1 EA
6491-1006 15 ADAPTER 1/4 ODTX 3/16 HOSE 1 EA
7854-6052 16 HOSE 5/32 X11/32 X2 LG SILIC 2 EA
7854-6055 17 HOSE 5/32 X11/32 X 7"LG SIL 1 EA
9250-1041 18 HOSE 5/32 X11/32 X5 LONG SIL 1 EA
8400-1337 19 HOSE 1/8" ID X1/4" OD 3" LG 1 EA
8400-1204 20 BOARD,TIMING OMS 9V 5LPM 1 EA
9250-1045 21 SCREW,PLASTITE#4X3/8" PAN.HD 8 EA
9251-1336 23 SCREW #6 X32X.5"LG. FLUSH 2 EA
A-8-A
PARTS
FRONT CABINET ASSEMBLY OCSI MODEL
42 of 48 Jan 2011 2010-8405 Rev E
Page 43

A-9
CABINET, BACK ASSEMBLY
2010-8405 Rev E Jan 2011 43 of 48
Page 44

Part Reference
Call-Out
Qty
Unit of
Number Number Part Description Required Measure
8400-0001 1 CABINET,BACK 1 EA
8400-0022 2 RETAINER,CORD 1 EA
8400-1025 3 FILTER,CABINETINLET 1 EA
8400-1028 4 HOSE, COMPRESSOR INLET. 1 EA
8400-1059 6 ADAPTER, FILTER 1 EA
8400-0029 7 RETAINER,CORD BUCKLE 1 EA
8400-0023 8 RETAINER,CORD RIVET 3 EA
8400-1180 9 FILTER AIR INLET. 1 EA
6814-9228 10 ELBOW, 1/2 DOUBLE BARB, NYLON 1 EA
5190-2233 11 HOLDER TIE WRAP 1 EA
9030-6008 12 TIE-WRAP 4.5" OVERALL 1 EA
8400-0013 13 SCREW, #14x 1-5"LG PLASTITE 4 EA
8400-1185 14 RESONATOR, INLETAIR 1 EA
9250-1030 15 GOMMET,RUBBER 1 EA
9250-1062 16 TIE-WRAP, 14" LONG 1 EA
8400-0032 17 PLUG BUNGEE HOLE 3/16" 1 EA
POWER CORD
A-9-A
PARTS
CABINET BACK ASSEMBLY MODEL
44 of 48 Jan 2011 2010-8405 Rev E
Page 45

A-10
MODULE NEW ASSEMBLY 5LPM
2010-8405 Rev E Jan 2011 45 of 48
Page 46

Part Reference
Call-Out
Qty
Unit of
Number Number Part Description Required Measure
8400-1060 1 REGULATOR, 2-PORT 1 EA
8400-1165 2 FITTING VALVE 5/16"X3/8 1 EA
8400-1200 3 VALVE ASSY 1 EA
8400-1236 28 TEE,ADAPTER NYLON 1 EA
8400-1253 29 O-RING, REGULATORTEE -204 1 EA
8644-9401 30 PLUG, 1/4~ ODTPUSH IN 1 EA
A-10-A
MODULE ASSEMBLY REPLACEMENT PARTS
46 of 48 Jan 2011 2010-8405 Rev E
Page 47

Nidek Medical Oxygen Concentrator Service and Maintenance Log
Model Number ________________
Initial Inspection
1. Upon receipt, check the unit for shipping
damage. Notify shipping company if damaged.
2. Verify that cabinet air filter and the inlet air
filter are in place.
3. Plug the unit into an electrical outlet, turn the
unit 'ON,’ and check the audible/visual alarms.
4. Set the flow meter/flow control at the
maximum
recommended flow rate and allow the unit to run
for 15 minutes.
5. Using a calibrated oxygen analyzer, verify
concentration is greater than 87 percent.
Routine Service Check
Perform routine servicing as shown in the chart
below. Record the activities performed in the log
provided on the following page.
1. Record the elapsed usage time in hours.
2. Check oxygen concentration with a calibrated
oxygen analyzer.
3. Verify audible alarm and indicator light
functions
between patients and every two years.
4. Inspect filters and replace as necessary.
Serial Number ___________________
Between-Patient Maintenance
1. Remove oxygen tubing, cannula, and humidifier
bottle and discard.
2. Wash or replace the humidifier tubing if used.
3. Wash or replace the cabinet air filter.
4. Clean the concentrator cabinet.
5. Check oxygen concentration and flow. If the unit
performs within specification, the final product
filter does not need to be replaced between patients.
Patient/Caregiver Maintenance
1. Inspect the Oxygen tubing, cannula, and
humidifier bottle - clean as needed according to
manufacturer’s instructions.
2. Wash the cabinet air filter weekly with a mild
detergent solution. Dry before reinstalling onto the
device.
The routine service intervals shown below depend
on the conditions in which the devices are used.
They reflect the minimum recommendation when
operated in a clean environment. As conditions can
vary widely, the homecare provider or patient
caregiver is responsible to determine:
- the character of the environment in which the
concentrator is to operate.
- a maintenance schedule with intervals based on
the environment in which the unit is
operating/functioning.
Standard Servicing Intervals are shown below. Intervals used by the homecare service provider
and/or patient caregiver should be more frequent when conditions of usage dictate.
Nidek Medical Oxygen Concentrator Routine Service Intervals
Check Percent
Oxygen
Concentration
OCSI Models:
Every 15,000 hours
or 3 years.
Std Models: Every
5,000 hours or 1
year.
Cabinet Air Filter Inlet Air Filter Final Product Filter Capacitor
Wash the filter
each week in a
mild detergent
solution.
Dry before
reinstalling.
Inspect at each
patient visit.
Replace every 2
years, or more often
depending on
environment.
Replace at each
compressor
service / module
replacement.
Replace at each
compressor
service / module
replacement.
Page 48

Please maintain a log of all
maintenance activities performed on
this unit.
Serial
Number______________Model_____________
Date Hours % O2
Inspection Prior to Putting Into Service
In-Service Checks
Alarms
Check
Additional Information (Work Done, Filter Changes,
Comments, etc)
Medical device regulations require users and service personnel to notify manufacturers of any incidents
that, if repeated, could cause injury to any person.
email: info@nidekmedical.com
Please update maintenance log information upon each service at www.nidekmedical.com under
the 'Maintenance Log' tab.
 Loading...
Loading...