Page 1

NH3-selective electrodes
Manual
8.109.8031EN
Page 2

Page 3

Metrohm AG
CH-9100 Herisau
Switzerland
Phone +41 71 353 85 85
Fax +41 71 353 89 01
info@metrohm.com
www.metrohm.com
NH3-selective electrodes
8.109.8031EN
Manual
11.2011 ebe
Page 4

Teachware
Metrohm AG
CH-9100 Herisau
teachware@metrohm.com
This documentation is protected by copyright. All rights reserved.
Although all the information given in this documentation has been
checked with great care, errors cannot be entirely excluded. Should you
notice any mistakes please send us your comments using the address
given above.
Documentation in additional languages can be found on
http://documents.metrohm.com.
Page 5

■■■■■■■■■■■■■■■■■■■■■■
Table of contents
1 Introduction 1
2 Mode of operation 2
3 Selecting the electrode 4
4 Assembling the electrode 5
5 Storing the electrode 8
6 Performing measurements 9
6.1 Measured quantities ............................................................. 9
6.2 General notes ........................................................................ 9
6.3 Performing direct measurements ..................................... 10
6.4 Performing standard addition ........................................... 11
Table of contents
6.5 Performing measurements with very low concentra-
tions ..................................................................................... 12
6.6 Influence of dissolved particles on the measurement ..... 13
7 Troubleshooting 14
7.1 Problems and their solutions ............................................. 14
8 Technical specifications 16
9 Accessories 17
9.1 Scope of delivery ................................................................ 17
9.2 Optional accessories ........................................................... 18
Index 19
NH3-selective electrodes
■■■■■■■■
III
Page 6

Page 7

■■■■■■■■■■■■■■■■■■■■■■
1 Introduction
The NH3-selective gas membrane electrodes are combined electrodes, i.e.
they contain one measuring electrode and one reference electrode.
They enable the rapid, simple, inexpensive and precise determination of
dissolved ammonia (NH3) in aqueous systems, e.g. in natural water, sewage, boiler feed water, beer, etc.
In addition, the NH3-selective gas membrane electrodes can detect the following substances:
■ Ammonium ions (NH
■ Organic nitrogen (N) after a Kjeldahl digestion
Discolored or turbid samples do not compromise the measurement, which
means that, generally speaking, no pre-distillation is necessary.
The membrane of the NH3-selective electrode is gas-permeable and
water-repellent, i.e. water cannot dampen the membrane and cannot permeate the pores. In the case of samples that contain surface-active substances, or in the case of nonaqueous systems, fluid makes its way into
the membrane. This leads to difficulties, e.g. in the case of sewage samples (detergents) or with nylon or dye samples (nonaqueous systems). An
ammonia determination of such samples should therefore be carried out
by suspending the electrode above the solution instead of immersing it.
+
) after their conversion into ammonia
4
1 Introduction
NH3-selective electrodes
■■■■■■■■
1
Page 8
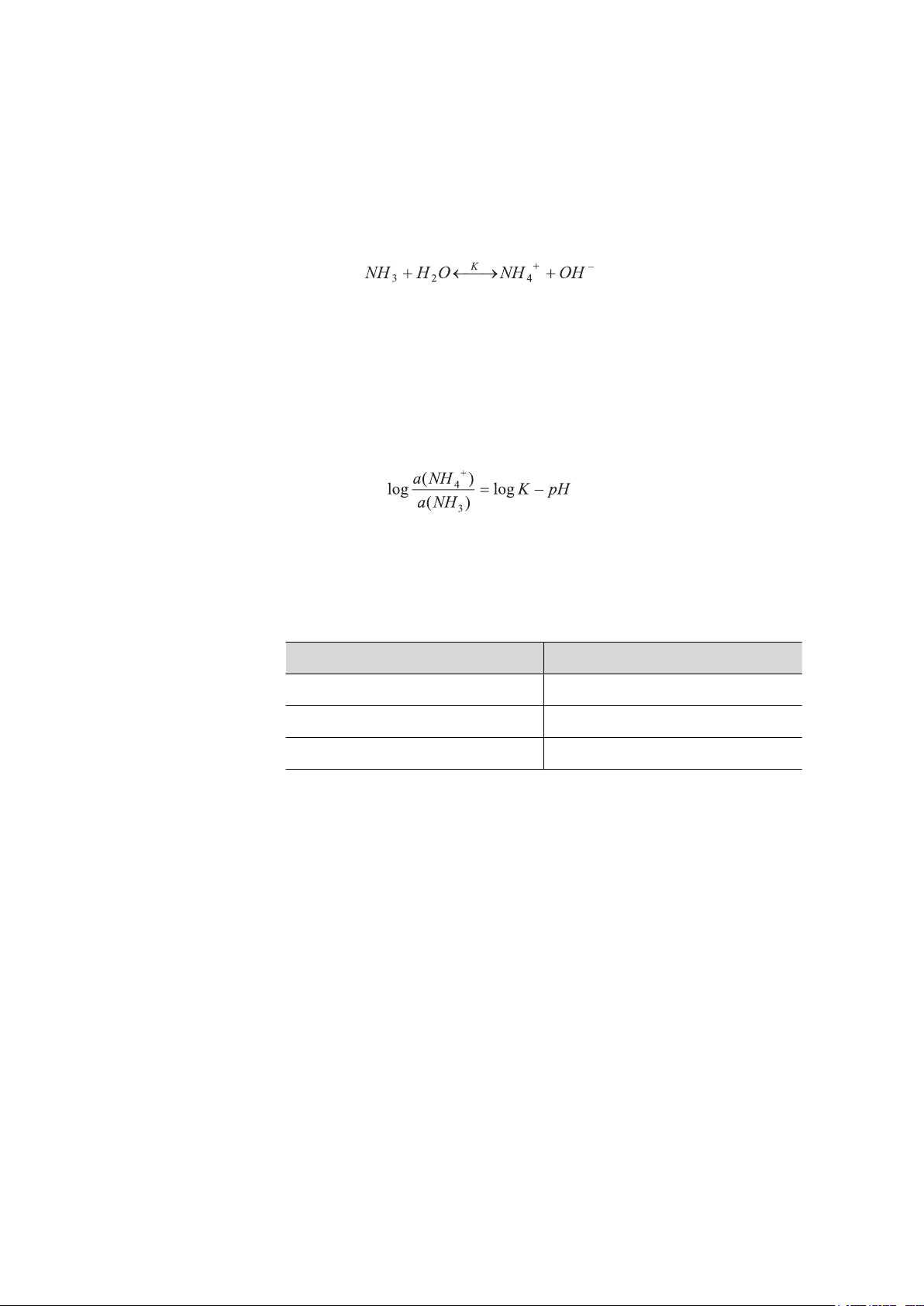
2 Mode of operation
Ammonia (NH3) reacts in water with the formation of ammonium ions
+
(NH
):
4
The equilibrium of this reaction, i.e. the relative proportion of ammonia
and ammonium ions, is determined by the pH value of the solution. In
acidic solutions, ammonia reacts almost completely to ammonium ions. At
a pH value of 9.2, the ratio of ammonia to ammonium ions is approximately 1 : 1. If the pH value of the solution is known, then the ratio of
ammonia to ammonium ions can also be calculated directly. The following
applies:
■■■■■■■■■■■■■■■■■■■■■■
(1)
(2)
The value of the equilibrium constant K is also dependent on the temperature.
Table 1
Temperature dependency of the ammonia – ammonium ions
equilibrium
Temperature in °C – log(K)
5 4.13
25 4.78
35 5.08
The addition of an excessive amount of a strong base, e.g. concentrated
caustic soda (NaOH), causes the ammonium ions to be transformed into
ammonia in their entirety. Determination of the ammonium concentration
is thus indirectly possible by means of the determination of ammonia. In
the case of the NH3-selective electrode, a gas-permeable, hydrophobic
membrane separates the measuring solution from the measuring electrolyte. Ammonia now diffuses through the membrane for as long as the
partial pressure on the two sides is identical in size. The partial pressure of
ammonia is always proportional to its concentration. The measuring electrolyte is comprised of a sufficiently concentrated ammonium chloride
solution, which means that the ammonium concentration can be assumed
to be constant.
■■■■■■■■
2
The potential U of the measuring electrode is proportional to the hydroxide concentration of the measuring electrolyte:
NH3-selective electrodes
Page 9

■■■■■■■■■■■■■■■■■■■■■■
2 Mode of operation
(3)
UN is the Nernst potential (slope) of the electrode. Because of the fact that
the hydroxide concentration is proportional to the ammonia concentration
(equation 4), the electrode obeys the Nernst equation for the ammonia
concentration as well:
(4)
(5)
Using equation 5, either the ammonia concentration or the ammonium
concentration can be determined from the measured data. U‘0 is determined essentially by means of the internal reference electrode. This
responds to the existing chloride concentration contained in the non-refillable reference electrolyte (gel).
NH3-selective electrodes
■■■■■■■■
3
Page 10

3 Selecting the electrode
In the following you will find a list of criteria that is intended to make it
easier for you to select the correct electrode:
6.0506.100, 6.1255.000 6.0506.150, 6.1255.050
5 · 10–6…10–2 mol/L 10–4…1 mol/L
■■■■■■■■■■■■■■■■■■■■■■
Clean sample (e.g. drinking water,
boiler feed water or mineral
water)
Long-term measurement, moni-
More rapid response time close to
the detection limit
Lower detection limit Better signal stability with higher
Complete membrane modules for
simple replacement
Membrane module individually
tested and certified
Sewage sample
toring
More rapid regeneration time
after high concentrations
concentration
Less expensive replacement of
contaminated membranes (e.g.
oleaginous sewage)
Replacement membranes without
certificate
■■■■■■■■
4
NH3-selective electrodes
Page 11

■■■■■■■■■■■■■■■■■■■■■■
4 Assembling the electrode
The way that you assemble your electrode depends on the type of the
electrode.
■ The electrode 6.0506.100 is supplied with complete membrane mod-
ules.
■ The electrode 6.0506.150 is supplied with a membrane module with
separate membranes. The membrane must first be mounted on the
membrane module. It can be replaced as needed.
Mounting the membrane on the membrane module
Electrode 6.0506.150
Unscrew the cover from the membrane module.
1
4 Assembling the electrode
Make ready the package with the membranes.
2
Each membrane is stored between two white paper strips. This prevents the membranes from sticking together.
Use the tweezers provided to take out one membrane, holding it on
3
its narrow side.
NH3-selective electrodes
■■■■■■■■
5
Page 12

■■■■■■■■■■■■■■■■■■■■■■
Place the membrane on the screw thread of the membrane module
4
and use your thumb to hold it in place.
Place the membrane carefully lengthwise over the opening of the
5
screw thread.
NOTE
The part of the membrane which lies above the opening of the
screw thread may not come into contact with fingers or other
objects under any circumstances. The water-repellent effect of the
membrane would otherwise be reduced as a result.
Pull the membrane slightly at its sides and press it firmly against the
6
screw thread.
The membrane must lie taut and wrinkle-free above the opening.
Screw the cover on the membrane module over the taut membrane.
7
■■■■■■■■
6
NH3-selective electrodes
Page 13

■■■■■■■■■■■■■■■■■■■■■■
4 Assembling the electrode
Check once again to ensure that the visible part of the membrane
8
lies taut and wrinkle-free above the opening.
Preparing the electrode for use
CAUTION
The membrane of the membrane module may not come into contact
with fingers or with other objects under any circumstances. The waterrepellent effect of the membrane would otherwise be reduced as a
result.
Electrode 6.0506.100 and 6.0506.150
Fill the membrane module with 2 mL of the measuring electrolyte
1
(6.2316.030).
Remove the measuring electrode from the electrode carrier and rinse
2
with distilled water.
Introduce the measuring electrode into the membrane module and
3
screw both parts together.
Prior to initial use, shake the assembled NH3-selective electrode sev-
4
eral times as you would a fever thermometer in order to remove any
air bubbles from the membrane.
Before making the first measurement, condition the electrode for at
5
least 10 minutes in distilled water.
NH3-selective electrodes
■■■■■■■■
7
Page 14

5 Storing the electrode
The type of storage depends on the storage duration. Observe the following notes:
Storage between measurements
■ Store electrode in distilled water.
Storage duration between 1 and 5 days
■ Store the electrode in measuring electrolyte (6.2316.030).
Storage duration of more than 5 days
Unscrew membrane module.
1
Rinse the membrane module thoroughly with distilled water inside
2
and outside and place it in dry storage.
Store pH glass electrode in c(KCl) = sat. (6.2308.000).
3
■■■■■■■■■■■■■■■■■■■■■■
■■■■■■■■
8
NH3-selective electrodes
Page 15

■■■■■■■■■■■■■■■■■■■■■■
6 Performing measurements
6.1 Measured quantities
Ammonia concentrations are usually specified in one of the following
units:
■ mol/L
■ ppm (NH
■ ppm (N)
)
3
6 Performing measurements
Table 2
Conc. in mol/L N content in ppm NH3 content in ppm
6.2 General notes
■ Standard and sample solutions must always be measured at the same
stirring speed and at the same temperature. A difference in temperature of 1 °C results in a deviation of approx. 2%.
■ If possible, use narrow, high measuring vessels (minimum ratio of sur-
face to volume).
■ Immediately prior to the measurement, a sodium hydroxide solution
must be added to each measuring solution. The solutions should have
a pH value of 11...14 after this addition and the concentration of all
dissolved particles should not exceed 1 mol/L.
■ Alkali samples must be measured immediately. The ammonia loss in a
stirred alkali sample of 100 mL is approx. 50% within six hours.
For purposes of storage, the samples must be acidulated with hydrochloric acid to approx. pH 6 (approx. 0.5 mL c(HCl) = 1 mol/L per liter
of sample) and stored in well-sealed vessels.
Do not add the sodium hydroxide solution until just before the measurement.
Conversion factors for various concentration specifications
–4
10
10
10
10
–3
–2
–1
1.4 1.7
14 17
140 170
1400 1700
1 14000 17000
NH3-selective electrodes
■■■■■■■■
9
Page 16

6.3 Performing direct measurements
■ The electrode must be conditioned in distilled water for at least 10
minutes between the measurements. If measurements are being performed at very high concentrations, then the electrode should be conditioned for 30 minutes.
■ If a standard or sample solution is measured repeatedly, then it must
be stored in a sealed or covered vessel between the measurements.
This ensures that ammonia will not escape.
■ Ammonia dissipates very rapidly out of solution when ammonia con-
centrations are > 1 mol/L. These kinds of samples must therefore be
diluted.
■ You will find many additional useful notes on working with the NH
selective electrode in Application Bulletin 133.
6.3 Performing direct measurements
Direct measurement is a simple and rapid method for testing numerous
samples across a wide range of concentrations.
Observe the following notes when performing direct measurements:
■■■■■■■■■■■■■■■■■■■■■■
-
3
■ Select the concentrations of the standard solutions (e.g. NH
Cl solu-
4
tions) in such a way that the ammonia concentration of the sample
solution to be anticipated falls in the middle of the calibration range.
■ Make sure that the temperatures of all standard and sample solutions
are identical.
■ The diffusion of ammonia through the membrane is slowed down con-
siderably in the presence of ammonia concentrations < 6 · 10–5 mol/L.
The response time of the electrode is prolonged accordingly. A special
measurement technique is required for this concentration range (see
Chapter 6.5, page 12).
■ Ammonia dissipates very rapidly out of solution when ammonia con-
centrations are > 1 mol/L. These kinds of samples must therefore be
diluted (see Chapter 6.6, page 13).
■■■■■■■■
10
NH3-selective electrodes
Page 17

■■■■■■■■■■■■■■■■■■■■■■
6.4 Performing standard addition
Standard addition is a considerably more convenient method for the measurement of ion concentrations with an ion-selective electrode (ISE). If you
need to measure only a few samples, then this method is very simple,
because you do not need to incorporate a calibration curve. Furthermore,
all matrix effects are eliminated, because the electrode is calibrated in the
sample solution.
Observe the following notes when performing standard additions:
■ Add defined volumes of a standard solution of the measurement ion to
the sample in a series of steps.
The concentration in the sample solution is calculated on the basis of
the resulting change in voltage and the initial voltage prior to the addition of the standard solution. Modern ion meters, such as the 781 pH/
Ion Meter or the 867 pH Module, can perform a standard addition
automatically when a corresponding dosing device is attached to the
instrument.
■ In order to guarantee a reliable evaluation of the standard addition,
care must be taken to ensure that the buret volume and the concentration of the standard solution are adjusted to the respective measuring
conditions. The individual addition volumes must be selected in such a
way that the potential difference is at least 15 mV after each additive
step. At least four volume additions should be carried out.
6 Performing measurements
Table 3
Recommended concentrations of the standard solution for the
standard addition
Buret volume in mL c
Standard solution
: c
Sample
5 40 : 1
10 20 : 1
20 10 : 1
50 5 : 1
Example of a standard addition
NOTE
This sample is valid for standard additions with any number of ionselective electrodes.
Sample concentration
5 mg/L
NH3-selective electrodes
■■■■■■■■
11
Page 18

6.5 Performing measurements with very low concentrations
Buret volume 10 mL
Sample size 10 mL
ISA/TISAB 10 mL
Total volume 20 mL
■■■■■■■■■■■■■■■■■■■■■■
Factor c
Standard solution/cSample
20
This results in a sample concentration in the measuring solution of 2.5
mg/L. The optimum concentration of the standard solution is thus 2.5
mg/L · 20 = 50 mg/L. Please note that this is merely to be considered a
guideline for standard additions. Even if you deviate from this recommendation, precise measurements will still be possible.
6.5 Performing measurements with very low concentrations
NOTE
The electrode 6.0506.100 or the membrane module 6.1255.000 must
be used for measurements with very low concentrations.
The response time of the electrode is relatively long with low ammonia
concentrations. The response time decreases with increasing concentration. You can improve the response time of the electrode and the precision of the result for ammonia concentrations < 6 · 10–5 mol/L with the
following measures:
■■■■■■■■
12
■ Condition the NH
-selective electrode prior to the measurement in an
3
ammonia-free pH 4 buffer solution.
■ Dilute the measuring electrolyte 1 : 9 with distilled water.
■ Work with sealed measuring vessel.
■ Use as large a sample volume as possible so that the ratio of surface to
volume is as low as possible. This will minimize the absorption of
ammonia from the air.
Even when these measures are applied, the response time of the electrode
may still be as long as ten minutes, not only for the samples but also
when conditioning in pH 4 buffer.
NH3-selective electrodes
Page 19

■■■■■■■■■■■■■■■■■■■■■■
6 Performing measurements
6.6 Influence of dissolved particles on the measurement
Water vapor is a potential interference factor. It can enter through the
membrane and change the concentration of ammonium chloride in the
measuring electrolyte. This leads to a potential drift. The introduction of
water should not be a problem, however, if the following conditions are
met:
■ The total concentration of the dissolved particles is approx. 0.1 mol/L
(osmotic pressure).
■ The electrode and the sample solution are maintained at the same
temperature.
The addition of the sodium hydroxide solution to samples with small ionic
strength results automatically in the correct concentration of particles in
solution. Samples with a total ion concentration > 1 mol/L should be
diluted prior to the measurement, although the ammonia concentration
should not be too close to the detection limit.
Samples with high total ion concentration (i.e. with high osmotic pressure)
and with low ammonia concentration can be measured after the osmotic
pressure of the measuring electrolyte has been increased. This is increased
through the addition of sodium nitrate (2.125 g NaNO3 per 50 mL measuring electrolyte).
NH3-selective electrodes
■■■■■■■■
13
Page 20

7.1 Problems and their solutions
7 Troubleshooting
7.1 Problems and their solutions
Problem Cause Remedy
■■■■■■■■■■■■■■■■■■■■■■
The electrode slope
is too large (> 62
mV).
The electrode slope
is too small.
The electrode was insufficiently prepared or conditioned for too short a time
in distilled water.
The membrane has a tear. Replace the membrane or the membrane
The calibration solutions
are contaminated or they
have been used too long.
Either too little sodium
hydroxide solution was
added, or none at all.
The pH glass electrode
(measuring electrode) is
possibly defective.
The measuring electrode
was in dry storage.
Refill the electrode and condition for at least
30 minutes in distilled water.
module.
Use fresh calibration solutions.
Add 1 mL sodium hydroxide solution with
c(NaOH) = 10 mol/L per 100 mL measuring
solution.
For the test, calibrate the pH glass electrode
separately in pH buffer solution or perform an
extensive electrode test with 780/781 pH/Ion
Meter, 867 pH Module or a Titrando. Replace
the measuring electrode if required.
Soak the measuring electrode for 6 to 12
hours in pH 7 buffer solution.
The measuring
range is exceeded.
■■■■■■■■
14
The measuring device is
possibly defective.
The electrode is not connected correctly.
The calibration is no longer
accurate.
There is no measuring electrolyte in the membrane
module.
The pH glass electrode
(measuring electrode) is
possibly defective.
Check the measuring device.
Check the electrode connection.
Recalibrate the electrode.
Unscrew the membrane module and refill it
with 2 mL measuring electrolyte.
For the test, calibrate the pH glass electrode
separately in pH buffer solution or perform an
extensive electrode test with 780/781 pH/Ion
NH3-selective electrodes
Page 21

■■■■■■■■■■■■■■■■■■■■■■
Problem Cause Remedy
Meter, 867 pH Module or a Titrando. Replace
the measuring electrode if required.
7 Troubleshooting
The measuring signal is noisy, the
measured values are
unstable.
A potential drift
occurs.
There is not enough measuring electrolyte in the
membrane module.
The pH glass electrode
(measuring electrode) is
possibly defective.
The membrane is contaminated (e.g. with oleaginous
sample).
The membrane is defective
(punctured, discolored,
dampened); measuring
electrolyte runs out.
The wrong measuring electrolyte is in the membrane
module.
The membrane is severely
contaminated: not only
gases, but also sample
passes through the membrane.
Unscrew the membrane module, empty it and
refill it with 2 mL measuring electrolyte.
For the test, calibrate the pH glass electrode
separately in pH buffer solution or perform an
extensive electrode test with 780/781 pH/Ion
Meter, 867 pH Module or a Titrando. Replace
the measuring electrode if required.
Replace the membrane or the membrane
module.
Check the membrane module for tears,
replace the membrane or the membrane module if necessary.
Fill the membrane module only with the measuring electrolyte supplied.
Replace the membrane or the membrane
module.
NH3-selective electrodes
The total ion concentration
of the sample is > 1 mol/L.
Ammonia escapes too
quickly.
Temperature fluctuations
occur.
The measuring electrode
was in dry storage.
Dilute the sample.
Use only closed measuring vessels.
Observe uniform tempering of the sample,
equipping the measuring vessel with a thermostat if necessary.
Soak the measuring electrode for 6 to 12
hours in pH 7 buffer solution.
■■■■■■■■
15
Page 22

8 Technical specifications
Measuring range
6.0506.100 5 · 10–6…10–2 mol/L (NH3)
0.1…170 ppm (NH3)
6.0506.150 10–4…1 mol/L (NH3)
10…17000 ppm (NH3)
■■■■■■■■■■■■■■■■■■■■■■
Temperature
range
pH range (measuring electrode)
Minimum immersion depth
Shaft length 133 mm
Shaft diameter 12 mm
Shaft material PEEK
Module material
6.0506.100 POM black
6.0506.150 POM black/white
Electrode plug-in
head
0…50 °C
pH 0…14
2 mm
Metrohm plug-in head G
■■■■■■■■
16
NH3-selective electrodes
Page 23

■■■■■■■■■■■■■■■■■■■■■■
9 Accessories
NOTE
Subject to change without notice.
9.1 Scope of delivery
6.0506.100 NH3-selective electrode
9 Accessories
Qty.
Order no. Description
1 – Measuring electrode with Metrohm plug-in
head G and certificate
3 – Membrane modules with certificates
1 6.2316.030 Measuring electrolyte (50 mL)
1 8.109.8031ML Manual for NH3-selective electrodes, Ger-
man/English
6.0506.150 NH3-selective electrode
Qty.
Order no. Description
1 – Measuring electrode with Metrohm plug-in
head G and certificate
1 – Package with 20 membranes each
1 – Membrane module
1 6.2316.030 Measuring electrolyte (50 mL)
1 – Tweezers
1 – Pasteur pipette
1 8.109.8031ML Manual for NH3-selective electrodes, Ger-
man/English
NH3-selective electrodes
■■■■■■■■
17
Page 24

9.2 Optional accessories
9.2 Optional accessories
6.0506.100 NH3-selective electrode
Order no. Description
6.1255.000 Replacement module kit for NH3-selective electrode
6.0506.100
Comprised of: 3 replacement membrane modules with certificates, 1 measuring
electrolyte (50 mL).
6.0506.150 NH3-selective electrode
■■■■■■■■■■■■■■■■■■■■■■
Order no.
6.1255.050 Replacement module kit for NH3-selective electrode
Comprised of: 1 replacement membrane module, 1 package of 20 membranes,
1 tweezers.
8.109.8032ML Leaflet for membrane module (mounting instructions)
Description
6.0506.150
■■■■■■■■
18
NH3-selective electrodes
Page 25

■■■■■■■■■■■■■■■■■■■■■■
Index
Index
A
Accessories ............................... 17
Alkali sample .............................. 9
Application Bulletin 133 ........... 10
C
Concentration
Converting ........................... 9
High ................................... 13
Low .................................... 12
Conversion factor
Concentration ...................... 9
D
Difficulties
Nonaqueous system ............. 1
Surface-active substance ....... 1
Direct measurement ................. 10
E
Electrode
Conditioning .................. 7, 10
Putting into operation .......... 7
Response time .................... 12
Screwing together ................ 7
Selecting .............................. 4
Storage ................................ 8
Equilibrium constant K ................ 2
Equilibrium reaction
NH3 – NH
Temperature dependency ..... 2
Error search .............................. 14
M
Measuring unit ........................... 9
Membrane
Mounting ............................. 5
Membrane module
Equipping ............................. 5
Method
Direct measurement ........... 10
Standard addition ............... 11
N
Number of samples
Large .................................. 10
Small .................................. 11
R
Response time
Electrode ............................ 12
+
.......................... 2
4
Improvement ...................... 12
S
Sample
Storage ................................ 9
Scope of delivery ...................... 17
Selection criteria
Electrode .............................. 4
Standard addition ..................... 11
Concentration .................... 11
Example ............................. 11
Storage duration
Electrode .............................. 8
T
Technical specifications ............ 16
Temperature dependency
Equilibrium reaction .............. 2
Temperature difference
Measurement ....................... 9
Troubleshooting ....................... 14
W
Water vapor
Interference factor .............. 13
NH3-selective electrodes
■■■■■■■■
19
 Loading...
Loading...