GE Healthcare Lunar enCORE Specification

GEHealthcare
LunarenCORE
SafetyandSpecificationManual
Rev 3 - Part number: LU43618EN 5/2009
GEMedicalSystemsLUNAR Contact Numbers
Headquarters
GE Medical Systems Lunar 3030 OhmedaDr. Madison, WI53718
USA
+1 (800) 437-1171
China
No. 19 ChangjiangRoad Wuxi, Jiangsu, 214028 P.R.C.
+86-510-85225888
+86-510-85226688 (fax)
www.gehealthcare.com
DPXSeries YZB/USA2099 SFDA(I)20023301115
ProdigySeries YZB/USA0509SFDA(I)20043301375
iDXA YZB/USA1104-2007 SFDA(I)20073302084
GE Medical Systems ITGmbH Munzinger Strasse 3-5 D-79111 Freiburg, Germany +49 212 2802 652
+49 0761 45 43 233 (fax)
France
11 Avenue Morane Saulnier
78 457 VELIZY
+33-1-34-49-5365 +33-1-34-49-5406 (fax)
Germany
BeethovenStr. 239 D-42655 Solingen Germany +49-212-2802-0 +49-212-2802-390 (fax)
Asia/Pacific
4-7-127 Asahigaoka Hino-shi, Tokyo191-8503 Japan +81-42-585-5111 +81-42-585-3077 (fax)
GEMedicalSystemsLUNARrecommendsviewingtheinstructionsfor navigatingtheLunar iDXA,PRODIGY™,PRODIGY™Advance, PRODIGY™Primo,PRODIGY™Pro,DPX™NT/Pro/MD+/Duo/Bravo™SafetyInformationandTechnicalSpecificationsbeforeproceedingthroughtheonlineguidefor thefirsttime.

TableofContents
Introduction |
3 |
Search |
3 |
LicenseandWarrantyInformation |
4 |
GeneralProductInformation |
5 |
TrainingInformation |
5 |
Cautionsfor DEXADeterminations |
6 |
Precautionsfor StandardOperatingProcedures |
6 |
Patents |
7 |
StandardOperatingProcedures |
7 |
Scanner TableAssembly |
7 |
SystemSafety |
8 |
Operator Safety |
8 |
PatientSafety |
9 |
MechanicalSafety |
14 |
ExternalSymbols |
14 |
InternalSymbols |
15 |
Labels |
15 |
EmergencyStopButtonandFailsafeCircuit |
19 |
Registration |
20 |
Facilities |
20 |
ElectricalSafety |
20 |
Scatter Radiation |
23 |
SystemMaintenance |
30 |
ArchiveImageFiles |
30 |
TestEmergencyStopButton |
31 |
PreventiveMaintenance |
31 |
DisposeofMaterials |
32 |
SpaceRequirements |
32 |
ComponentSpecifications |
34 |
FunctionalSpecifications |
35 |
EnvironmentalSpecifications |
36 |
Power Specifications |
37 |
X-RayGenerator Specifications |
38 |
GEMEDICALSYSTEMSX-RayTubeHeadAssembly |
44 |
CompatibleComponents |
51 |
FDACertifiedComponents |
52 |
Index |
55 |
- 2 of57-

Introduction
Thismanualcontainssafetyandmaintenanceinformation,andtechnicalspecifications,for your bonedensitometer.
ThismanualshouldbeusedwiththeLunar enCORETMOnlineHelpyoureceivedwithyour system.
Theinformationinthismanualissubjecttochangewithoutnotice.Youmayuseor copythesoftwaredescribedinthismanual onlyinaccordancewiththetermsofyour softwarelicense,productwarranty,or servicecontractagreements.
Nopartofthispublicationmaybereproducedfor anypurposewhatsoever,storedinaretrievalsystem,or transmittedinany formor byanymeans,mechanical,photocopying,recordingor otherwise,withouttheexpresswrittenpermissionofGEMedical SystemsLunar.
Anyreproduction,photocopyingandrecordinginwholeor partisprohibited.Anyinformationcontainedhereinshallnotbedisclosedtoanycompanyviewedasacompetitor toGEMedicalSystemsLunar.
GEMedicalSystemsLunar makesnowarrantyofanykindwithregardtothismaterial,andshallnotbeheldliablefor errorscontainedhereinor for incidentalor consequentialdamagesinconnectionwiththefurnishingsor useofthismanual.
TheinformationcontainedinthemanualisconfidentialandproprietarytoGEMedicalSystemsLunar.Thisinformationisprovided onlytoauthorizedrepresentativesofGEMedicalSystemsLunar'scustomerssolelyfor thepurposeoffacilitatingtheuseofGEMedicalSystemsLunar'sproducts.Noinformationcontainedhereinmaybedisclosedtoanyunauthorizedpersonfor anypurpose whatsoever withoutprior writtenconsentofGEMedicalSystemsLunar.
ReadtheUser andtheSafetyandSpecificationmanualsthoroughlybeforeusingthesystemor attemptingtoserviceanycomponents.Unauthorizedservicemayvoidsystemwarrantiesor servicecontracts.ConsulttheGEMedicalSystemsLunar Customer ServiceDepartmentbeforeattemptinganyservice:800-437-1171 (U.S.A).
Lunar isaregisteredtrademarkofGEMedicalSystemsLunar.Allother productandbrandnamesareregisteredtrademarksor trademarksoftheir respectivecompanies.
Copyright©1999,2000,2001,2002,2003,2004,2005,2006,2007,2008,2009
GEMedicalSystemsLunar,Madison,Wisconsin.Allrightsreserved.
Search
Youcansearchfor topicsandcontentwithintheonlinehelp.
1. ClicktheSearchtabintheonlinehelpwindow.
2. Typethecontentfor whichyouaresearching.
- 3 of57-

3.ClickList Topics.
4.Clickanydisplayed topicnametodisplaythedesiredtopic.
License and Warranty Information
Pleasecarefullyreadthefollowingtermsandconditionsbeforeinstallingor operatingtheGEMedicalSystemsLunar Software ("Software").Byinstallingor usingtheSoftwareinyour GEMedicalSystemsLunar product,Youindicateyour acceptanceofthese termsandconditions.IfYoudonotagreewiththetermsandconditions,donotinstallor operatetheSoftwareandreturnittoGE MedicalSystemsLunar.
TheSoftwarehasbeenprovidedtoYoufor useonaspecificGEMedicalSystemsLunar product.TheSoftwareisprovidedunder thetermsofthisAgreementandislicensedtoYou,notsold.Your rightstousetheSoftwarearesubjecttothetermsandconditions containedwithinthisLicenseAgreementandGEMedicalSystemsLunar reservesanyrightsnotexpresslygrantedtoYou.This Licenseisnon-exclusiveandanon-transferablelicensetousetheGEMedicalSystemsLunar Software.Re-distributionofSoftware or anydocumentationprovidedtoyoubyGEMedicalSystemsLunar isstrictlyprohibited.
Thisproductincludessomesoftwarecomponentsthatarelicensedunder theGNUGeneralPublicLicense(GPL).Sourcecodefor GPLcomponentsisavailableuponrequest.
ThetermsandconditionsofthisLicenseAgreementandLimitedSoftwareWarrantyareasfollows:
1. LICENSE. This License allows You to:
(a)usetheSoftwareonaproductinaccordancewiththeaccompanyingdocumentation.To"use"theSoftwaremeansthatthe Softwareiseither loadedinthetemporarymemoryofacomputer or installedonanypermanentmemoryor mediaofacomputer (e.g.,harddisk,CD-ROM,opticaldisk,zipdisk,andthelike);
(b)makeone(1)copy,inmachine-readableform,oftheSoftwareasprovidedtoYousolelyfor thepurposesofbackup;provided
thatsuchcopyincludesthereproductionofanycopyrightnoticeor other proprietarynoticeappearinginor onsuchSoftware.
2. LICENSE RESTRICTIONS.
(a)YOUMAYNOT,EXCEPTASEXPRESSLYPROVIDEDFORINTHISLICENSE:(i)DECOMPILE,DISASSEMBLE,ORREVERSEENGINEERTHE SOFTWARE(excepttotheextentapplicablelawsspecificallyprohibitsuchrestriction);(ii)COPY,MODIFY,ADAPT,TRANSFER,TRANSLATE,RENT,LEASE,GRANTASECURITYINTERESTIN,ORLOANTHESOFTWAREORANYPORTIONTHEREOF;(iii)CREATEDERIVATIVE WORKSBASEDUPONTHESOFTWAREORANYPORTIONTHEREOF;OR(iv)REMOVEANYCOPYRIGHTORPROPRIETARYNOTICESOR LABELSINORONTHESOFTWARE.
(b)YouunderstandthatGEMedicalSystemsLunar mayupdateor revisetheSoftware,andinsodoingincur noobligationtofur-
nishsuchupdatestoYouunder thisLicense.GEMedicalSystemsLunar hasnoobligationtoimprove,updateor supporttheSoftwareinthefuture.
(c) Intheeventtheinstrumentor productdesignatedfor theSoftwareissoldor otherwisetransferredtoathirdparty,thatpartyis notauthorizedtousetheSoftwareunlesstheyfirstpaytoGEMedicalSystemsLunar theapplicablelicensefeeandagreetothe termsandconditionsofaSoftwareLicenseAgreement.Upontransfer oftheSoftwareor anycopythereof,theLicensegranted hereunder shallterminateimmediately.
3. TERM AND TERMINATION.
ThisLicenseiseffectiveuntilterminated.ThisLicensewillterminateimmediatelywithoutnoticefromGEMedicalSystemsLunar or judicialresolutionifYoufailtocomplywithanyprovisionoftheLicense.UponanyterminationofthisLicense,Youagreetoreturn or destroytheSoftware,allaccompanyingwrittenmaterialsandallcopiesthereofinanyform.Section5 willsurviveanytermination.
4. EXPORT LAW.
Youagreethatneither theSoftwarenor anydirectproductthereofisbeingor willbeshipped,transferredor re-exported,directly or indirectlyintoanycountryprohibitedunder UnitedStateslawor regulationspromulgatedthereunder.
5. WARRANTY.
GEMedicalSystemsLunar warrantsthat,tothebestofour knowledge,thesoftwareprovidedwiththisLicensewillperformas describedintheproduct'soperator'smanualandthetechnicalspecificationfor thisSoftware.Thislimitedwarrantyiscontingent uponproper useoftheSoftwareanddoesnotcover anySoftwarewhichhasbeenmodified,subjectedtomaliciouslogic,unusual physicalor electricalstress,or usedoncomputer equipmentnotspecifiedbyGEMedicalSystemsLunar.
GEMedicalSystemsLunar doesnotwarrantthatthefunctionscontainedinthisSoftwarewillmeetyour requirements,or thatthe operationoftheSoftwarewillbeuninterruptedor errorfree.StatementsmadeaboutthisSoftwaredonotconstitutewarranties
- 4 of57-

andshallnotberelieduponbyYouindecidingwhether topurchasetheGEMedicalSystemsLunar productor usetheSoftware.IN NOEVENTSHALLGEMEDICALSYSTEMSLUNARBELIABLETOYOUFORANYDAMAGESARISINGOUTOFTHEUSEORINABILITYTOUSE SUCHSOFTWARE.
THESOLEANDEXCLUSIVEREMEDYINTHEEVENTOFDEFECTISEXPRESSLYLIMITEDTOTHEREPLACEMENTOFTHESOFTWAREPROVIDED.IFFAILUREOFTHESOFTWAREHASRESULTEDFROMACCIDENTORABUSE,GEMEDICALSYSTEMSLUNARSHALLHAVENO RESPONSIBILITYTOREPLACETHESOFTWARE.
GEMedicalSystemsLunar willconsider thiswarrantytobevoidifYoufailtocomplywiththetermsintheSoftwareLicenseAgreement.
6. TITLE.
Title,ownershiprights,andintellectualpropertyrightsintheSoftwareshallremainwithGEMedicalSystemsLunar.ThisSoftwareis protectedbythecopyrightlawsandtreaties.
7. MISCELLANEOUS.
ThisAgreementrepresentsthecompleteagreementconcerningthisLicenseandmaybeamendedonlybyawritingexecutedby bothparties.TheLicenseisgovernedbythelawsoftheStateofWisconsin,U.S.A.withoutregardtoitsconflictoflawsprinciples.If anyprovisionofthisAgreementisheldbyacourtofcompetentjurisdictiontobeunenforceable,thatprovisionshallbeenforcedto themaximumextentpermissibleand/or reformedonlytotheextentnecessarytomakeitenforceable,andtheremainingprovisionsofthisAgreementwillnotbeaffectedor impairedinanyway.Ifanylegalactionor proceedingisbroughtfor theenforcementofthisAgreement,or becauseofanyallegeddispute,breach,defaultor misrepresentationinconnectionwithanyofthe provisionsofthisAgreement,thesuccessfulor prevailingpartyshallbeentitledtorecover reasonableattorneys'feesandother costsincurredinsuchactionor proceeding,inadditiontoanyother relieftowhichsuchpartymaybeentitled.
General Product Information
Thebonedensitometer isdesignedtoestimatethebonemineraldensityandbodycomposition(leanandfattissuemass)of patientswhenmedicallyindicatedbytheir physicians.Themanualsprovideinstructionsfor operatingthesoftwareandscan table,systeminformation,andmaintenanceinformation.
Variables Affecting Scan Results
Scanresultscanbeaffectedbyoperator techniqueandpatientvariability:
1.Operator techniquereferstopatientpositioningandscananalysis.Tominimizetechniquevariables,1)establishconsistentpositioningandscananalysisroutinesbyusinganatomicallandmarkswhenpositioningpatients,and2)during analysis,manipulaterawscandataonlywhenabsolutelynecessary.
2.Patientvariabilityreferstochangesinthepatient'smedicalhistory,metabolism,anddiet.Italsoreferstodiagnosticproceduresthatinvolveradionuclideuptakeandmedicaltreatment,andthepresenceofexternalradiation(particularlythe useofother radiation-generatingdevicesinthevicinityofthesystem).Tominimizepatientvariability,1)thoroughlyfamil- iarizeyourselfwiththepatient'shistory,and2)installthescanner inanenvironmenteffectivelyshieldedfromother sourcesofexternalradiation.
CAUTION: United States Federal Law restricts this device to the sale, distribution, and use by or on the order of a physician (USA only).
Training Information
GEMedicalSystemsLunar or authorizedGEMedicalSystemsLunar distributorsprovideindividual,hands-ontrainingaspartof theinstallationprocedurefor your system.(GEMedicalSystemsLunar distributorsprovidetrainingfor systemsinstalledoutside theUnitedStates.)AnApplicationsSpecialistprovidesinformationonsoftwareandhardwareoperations,andreviewsthewarningsandcautionsinthemanuals.
IMPORTANT: Only trained technologists should operate the system. New technologists should receive training prior to unsupervised operation of the system. Additional training sessions are available on request for a nominal fee. For more information, contact the GE Medical Systems Lunar Customer Service Department at 800-334-5831, or your local GE representative.
- 5 of57-

Cautions for DEXA Determinations
YoushouldbeawareofthefollowingfactorswhichmayaffecttheclinicalaccuracyofDEXAspineestimates:markeddistortionsof skeletalarchitecture-e.g.,osteophytes,degenerativediscdisease,spinalarthritis,spondylolisthesis,kyphoscoliosis,andvertebral fractures-andsignificantcalciumdepositsintheaortacanfalselyelevatespinebonemineralvalues.Regionsthatcontainthese dystrophiccalcificationscanbeexcludedfromthescananalysisinsomecases.Thescanner canbeusedtomonitor changesin bonemineralover timeinpatientswiththesedisorders,butcautionmustbetakenininterpretation.UseDEXAestimatesasanaid toother methodsintheevaluationofpatientbonemineralstatusintheclinicalsetting.
Inaddition,spineestimateswillbedifficulttointerpretfor patientswithorthopedicmetaldevicesandprevioussurgicalinterventions,suchasbonegrafts.Radiographiccontrastmaterialandradiopharmaceuticalsusedfor myelograms,bariumenemas, andother diagnostictestspreventaccurateestimates.Bariumclearsthebodywithinafewdays,buttheoil-baseddyesusedin myelogramsseveralyearsagomayremainwithinthebodyfor years.Athree-daywaitingperiodissufficienttimefor bariumand mostradiopharmaceuticalstobecompletelydischargedfromthebody.
Femur estimateswillbedifficulttointerpretfor patientswithorthopedicmetaldevicesandprevioussurgicalinterventions.The mostcommoncomplicatingfactorsfor femur estimatesareprostheticdevicesandsurgicalimplantsintheregionofthebone scan.Resultsmaybeadverselyaffectedifthepatienthasdifficultywiththedesired25°inwardrotationofthelegor withmaintainingthispositionwithoutmovement.
TotalBodyestimatesrequireconsistentpatientpositioningfor accurateresultsandwillbedifficulttointerpretfor patientswith orthopedicmetaldevicesandprevioussurgicalinterventions.Theoperator shouldpayparticular attentiontothelocationofthe patient'sarms,keepingthepositioningthesamefor eachscan.Resultsmaybeaffectedifthepatientmovesduringthescan.
Precautions for Standard Operating Procedures
1.Donotattempttooperatethescanner withoutfirstreadingthismanual.
2.Donotremovetheassemblypanelsor attemptanyrepairswithoutprior instructionsfromauthorizedGEMedicalSystemsLunar personnel.
3.PerformtheQualityAssuranceprocedureeachmorning.Ifanytestfails,checkthepositionofthecalibrationblockand reruntheQAprocedure.Ifatestfailsagain,contactGEMedicalSystemsLunar Support.Also,callGEMedicalSystems Lunar ifmorethantwofailuresoccur inaone-weekperiod.Iftheroomtemperaturechangesmorethe5°Cduringthe day,thenperformanother DailyQA.
4.Ifthepatientisor mightbepregnant,alwayscontactthepatient'sphysicianbeforeperformingascan.
5.Remainintheroomwiththepatientwhileascanisinprogress. Assurethepatientdoesnotmoveduringthemeasurement. Minimizetheamountoftimethepatientliesflatonthescantable.
6.Restrictaccesstotheroomtoauthorizedpersonnel.
7.Donotattempttoserviceanyofthesystem'selectricalcomponentswhilethescantableisturnedON.Highvoltageis usedtoproducex-rays.
8.Radiationsafetyinformationislocatedwithinthismanualyoureceivedwithyour system. Reviewthisinformationbefore operation.
9.To stop the scanner in an emergency,presstheemergencystopbuttononthescanarm.DO NOT usetheemergency stopbuttontoroutinelyabortascan.
10.Removeanyfluidswhicharespilledonpador anysurfaceoftableimmediately.
11.Allsurfacesshouldbecleanedtomeetsite'sguidelinesfor handlingbloodandbodyfluids.Padmaterialmaybedamagedbycertainchemicals Useappropriatehospitalgradedisinfectantfollowedbymilddetergent.
12.Donotgeneratex-raysthroughtheuseofremoteapplications.
-6 of57-
13.Protectthecomputer againstmaliciouslogicandunauthorizednetworkaccess. Onlyallowauthorizeduser access. Pre- ventvirusattacksthroughtheuseoffirewalls,anti-virussoftwareandsoftwarepatchupdates.Contactyour localGErepresentativefor moreinformation.
14.DPXDuo: Extendthestepthefulldistancetoprovidemaximumsurfaceareafor thepatienttogetonandoffthetable withoutriskofinjury.
15.DPXDuo: Donotplaceanexcessiveloadonfootrest(stirrup),drawers,or legextension.
16.DPXDuo: Donotsitonlegextensiontable.
Patents
Thisproductiscoveredbytheclaimsofoneor moreofthefollowingpatents:
U.S.patents#5,040,546,#5,306,306,#5,480,439,#5,533,084,#6,038,281,#6,081,582,#U520050249331A1, #U520050247882A1,#U520050247880A1
Standard Operating Procedures
1.Quality Assurance:Everymorning,beforeyoustartpatientmeasurements,completethedailyQualityAssuranceprocedure.Refer tochapter 2 oftheenCOREOperator'sManual.Makesureyousaveyour printedresultsfor futurereference.
2.Measure Patients:Iftimeallows,enter thePrimary,Secondary,andAdditionaldatafor thepatientsyouexpecttomeasureduringtheday.Refer tochapter 3 oftheenCOREOperator'sManualtomeasureapatient.
3.Analyze Results:Analyzeandprintresultsimmediatelyafter eachpatientmeasurementiftimeallows.Otherwise, analyzeallofthepatientfilesafter thelastpatienthasbeenmeasured.Refer tochapter 4 oftheenCOREOperator'sManualtoanalyzeresults.
4.Archive image files:Archiveyour imagefilesbeforeyouleavefor theday.Intheunlikelyeventofacomputer malfunction,itisveryimportantthatyouhavearchivedfilesofallofyour patientmeasurementstorebuildyour database. Refer toArchiveimagefilesonpage30 for archiveprocedures.
5.Shut down computer:Attheendoftheday,selectExitfromtheMainscreen,selectShutDownfromtheClosewindow, andclickOKtoclosetheprogram.
Note:Donotturnoffthescanner attheendofthedayfor stationarysystems.
Scanner Table Assembly
Note: Do not attempttoservicethescanner tableassembly.PleasecallGEMEDICALSYSTEMSLunar Supportor your GEMEDICAL SYSTEMSLunar distributor.
Scanner table
Thescanner tableisusedtosupportthepatientduringameasurementor generalexamination(DPXDuo).Inaddition,thex-ray sourceassemblyandother electronicsarecontainedinsidethescanner table.
Scanner arm
Thelaser light,emittedfromanapertureonthescanner arm,helpsyoulocatethemeasurementstartposition.Positioning switchesletyoumovethescanner armuntilthelaser lightislocatedatthecorrectstartposition.Thestartpositionisdifferentfor eachmeasurementtype.
TheDPXDuoandDPXBravoscanner armhasareleaseandlockingmechanismallowingtheupper armtoswivelwhenthe scanner isidle. Thescanner armmustbeinthelockedpositionover thescanner tabletoperformameasurement.
- 7 of57-

Display panel
Thefollowingdescribestheindicatorslocatedonthescanner armdisplaypanel:
Indicator |
Status (on) |
Green(power) |
Power issuppliedtothescanner table. |
Yellow(x-ray) |
X-raytubeassemblyissupplyingx-rays. |
Yellow(shutter) |
Shutter isopen. |
Amber (laser) |
Laser ison. |
Emergency stop button
Pushtheredemergencystopbuttontostopthescanner armandimmediatelyshutdownx-raysinanemergency.Donotusethe emergencystopbuttontoroutinelystopthescanner duringnormaloperation.
Positioning switches
Thepositioningswitchesmovethescanner armanddetector tothemeasurementstartposition(thelaser lightindicatesthepositionofthedetector).TheBack/Frontswitchmovesthedetector acrossthewidthofthescanner table.TheLeft/Rightswitchmoves thescanner armdownthelengthofthescanner table.
Swing arm position sensing switches (DPX Duo, DPX Bravo)
Theswingarmpositionsensingswitchesdetectthelockingstatusoftheswingarmandtheswingarmlatch. Theswingarmlatch mustbelockedandtheswingarmmustbeinthelockedpositionover thescantablebeforeameasurementcanbeperformed. Releaseoftheswingarmlatchduringameasurementwillabortthescanandthemeasurementdatawillbelost.
iDXA Start Scan button
Thestartscanbuttoninitiatesthepatientmeasurement. Thestartscanbuttonislocatedonthedisplaypanelnear thepositioning switches.
System Safety
Obeythesesafetyguidelinesatalltimes:
●Readthemanualbeforeyouoperatethescanner.
●Thetechnologistoperatingthescanner mustremainintheroomwiththepatientduringthemeasurement.
●Donotattempttoservicethescanner.PleasecallGEMEDICALSYSTEMSLunar Supportor your GEMEDICALSYSTEMS Lunar Distributor.
●Whenthescanner isnotinuse,makesuretheShutter Open,X-ray,andLaser lightsareoff.
●Donotputexcessivepressureonthescanner arm.
●Usethescanner tablefor patientmeasurementsandexaminations(DPXDuo)only:donotsit,standor lieonthetablefor other purposes.
●Donotletliquidstouchthecomputer or scanner tablemechanicsandelectronics.
Operator Safety
Personnel monitors
Personnelmonitorsarenotnecessarytooperatethescanner.
Itisnotlikelythatyoucanreceivemorethan25%ofthemaximumpermissiblex-raydosefromthescanner.However,somefacil- itieschoosetousepersonnelmonitors.Refer toyour city,countyor stateHealthDepartmentor RadiationSafetyOfficer for your facility'spolicy.
Filmbadgesandthermalluminescentdosimeter (TLD)badgesareobtainedfromasupplier accreditedbytheNationalVoluntary LaboratoryAccreditationProgramfor personneldosimetryprocessing.
Thefollowingisasamplesituationfor aclinicmeasuringanAP spineandDualFemur on5 subjectsper daywithanexposurerate of0.18mR/hr atadistanceof2 metersestimatedfromtheiDXAisodosecurves.
- 8 of57-
SampleCalculationfor EstimatedExposureper Year fromScatter withiDXADensitometer
ScanType |
Mode |
AverageScans/Day |
ScanTime/Day |
Equivalent2.5 mA |
ScanTime/day |
||||
|
|
|
(sec/day) |
(sec/day) |
AP Spine |
Standard |
5 |
260 |
260 |
DualFemur |
Standard |
5 |
535 |
535 |
2.5 mAScanTimeper Day(sec) |
|
|
|
795 |
2.5 mAScanTimeper Day(hours) |
|
|
0.221 |
|
2.5 mAScanTimeper Week(hours) |
|
|
1.11 |
|
2.5 mAScanTimeper Year (hours) |
|
|
57.5 |
|
2.5 mAExposurefromIsodosePlots(mR/hr) |
|
|
0.18 |
|
TotalExposurefor 1 Year (mR) |
|
|
|
10.3 |
TotalAbsorbedDosefor 1 Year (mRad)0.92 Rad/R |
|
|
9.5 |
|
X-ray and shutter graphics
Duringameasurementor QualityAssuranceprocedure,x-rayandshutter graphicsareshownonthecomputer monitor.The graphicsaregreentoindicatex-raysareoffandtheshutter isclosed,andyellowtoindicatex-raysareonandtheshutter isopen.
X-rays offand shutter closed (green):
X-rays on and shutter open (yellow):
X-ray shutter
Whenpower tothescanner isinterruptedduringameasurementor QualityAssuranceprocedure,theshutter closesandthex- raytubestopsgeneratingx-radiation.
X-ray power supply
Thex-raytubeassemblyuseshighvoltagetogeneratex-rays. DO NOT touchinternalcomponents. DO NOT attempttoservice internalcomponents.
Patient Safety
Pinch points
TheWarninglabelidentifiesthelocationofpossiblepinchpoints.
Whenthescanner armisinmotion,makesurepossiblepinchpointareasareclear atalltimes.Patientlimbsmustremaininside theboundariesofthetabletop.Apinchpointispossiblebetweenthescanner armandtable.
- 9 of57-

Laser Safety
DO NOT STARE INTO THE LASER BEAMduringpatientpositioningandQualityAssuranceprocedures.Thelabelthatfollowsis locatedonthescanner armandshowsthelocationofthelaser aperture.
Radiation Safety
X-ray exposure: Thesystemmakesradiationwhenelectricvoltageissuppliedto,andcurrentflowsthrough,thex-raytube. Duringameasurement,theshutter openstoletabeamofradiationpassthroughthescanner tableandpatient. ThenominalradiationfieldattheiDXAscanner tabletopis18.4 mmx3.3 mm,attheProdigy tabletopis19.5 mmx3.4 mmandattheDPXseries tabletopitis2 mm. Leadoxideshieldingsurroundsthex-raytubeinsertinsidethetubehousingassemblyandreducesradiation levelsaroundthescanner table.
Skin entrance dose: AVictoreenmodel530 PrecisionElectrometer/Dosemeter withaModel660-5 IonChamber wasusedto measuretheX-rayentrancedose. Refer tothe"CurrentandTypicalDoseTables" for irradiationtimesandskinentrancedoses.
Measurement modes
Patientthicknessdeterminestheappropriatemeasurementmode.Theprogramselectstheappropriatemodebasedonthe patient'sheightandweight.
LunarenCORESystems
|
iDXA, PRODIGY, PRODIGY Advance |
DPX Series |
Mode |
Patient thickness |
Patient thickness |
Thick |
>25 cm |
>25 cm |
Standard |
13-25 cm |
15-25 cm |
Thin |
<13 cm |
<15 cm |
Current andtypicaldose information forLunariDXAmodes
|
|
|
|
|
Estimated |
|
|
|
Typical Meas- |
|
Skin |
|
|
|
urement Area |
Irradiation |
Entrance |
|
ModeA |
Current |
L x W cm x cm |
times |
Dose |
Site |
(mA) B |
C,D |
(sec)C,D,E |
(μGy)F,G |
|
AP Spine |
Thick |
2.500 |
19.0 x18.0 |
109 |
329 |
AP Spine |
Standard |
2.500 |
19.0 x18.0 |
52 |
146 |
AP Spine |
Thin |
0.625 |
19.0 x18.0 |
52 |
37 |
AP Spine |
QuickView |
2.500 |
19.0 x18.0 |
23 |
47 |
Femur |
Thick |
2.500 |
20.5 x17.0 |
112 |
329 |
Femur |
Standard |
2.500 |
20.5 x17.0 |
54 |
146 |
Femur |
Thin |
0.625 |
20.5 x17.0 |
54 |
37 |
Femur |
QuickView |
2.500 |
20.5 x17.0 |
24 |
47 |
DualFemur |
Thick |
2.500 |
2 x20.5 x17.0 |
224 |
329 |
DualFemur |
Standard |
2.500 |
2 x20.5 x17.0 |
107 |
146 |
DualFemur |
Thin |
0.625 |
2 x20.5 x17.0 |
107 |
37 |
DualFemur |
QuickView |
2.500 |
2 x20.5 x17.0 |
48 |
47 |
APVAH |
Thick |
2.500 |
42.7 x18.0 |
117 |
146 |
APVAH |
Standard |
2.500 |
42.7 x18.0 |
117 |
146 |
APVAH |
Thin |
0.625 |
42.7 x18.0 |
117 |
37 |
- 10 of57-

Forearm |
Standard |
0.188 |
14.2 x10.0 |
24 |
10 |
Hand |
Standard |
0.188 |
25.3 x18.0 |
69 |
10 |
TotalBody |
Thick |
0.188 |
196.8 x66 |
796 |
6 |
TotalBody |
Standard |
0.188 |
196.8 x66 |
436 |
3 |
TotalBody |
Thin |
0.188 |
196.8 x66 |
436 |
3 |
LVAH |
Standard |
2.500 |
42.7 x20.0 |
271 |
329 |
LVAH |
Thin |
0.625 |
60.0 x20.0 |
381 |
82 |
LateralSpine |
Standard |
2.500 |
19.0 x18.0 |
104 |
329 |
Orthopedic |
|
|
|
|
|
Femur |
Thick |
2.500 |
23.7 x15.0 |
109 |
329 |
Orthopedic |
|
|
|
|
|
Femur |
Standard |
2.500 |
23.7 x15.0 |
53 |
146 |
Orthopedic |
|
|
|
|
|
Femur |
Thin |
0.625 |
23.7 x15.0 |
53 |
37 |
SmallAnimal |
Standard |
0.188 |
75.8 x25.0 |
264 |
10 |
AAllmodesare100kV,±1kV.
BTubecurrentis±1%atthemaximumcurrent.
CImagingtimemeasuredfromshutter opentoshutter close,90%to100%ofindicatedvalue.
DSizesofmeasurementareasandirradiationtimeswillbelessthanthoselistedaboveifyouusetheSmartScanfeature.
EMeasurementlengthsandtimesaredependentonpatientheightandproductversion.
FDosemeasurementsareconstrainedbyDailyQAlimits.
GIrradiationtimesanddosevaluesdonotconsider a“sweepretry” featurewhichcandoublethedosefor asingletransverse
sweepwithinanentirescan. Ifaretryoccursaslightincreaseinirradiationtimeandskinentrancedosewouldbeexpected. The retryfeaturereducesneedtorescanentirepatient.
H Theactivationofthespinegeometryapplicationpermitsamaximumscanlengthupto69.5 cm.
Current andtypicaldose information forLunar PRODIGY,PRODIGY Advance,PRODIGY Pro modes
|
|
|
|
|
Estimated |
|
|
|
Typical Meas- |
|
Skin |
|
|
|
urement Area |
Irradiation |
Entrance |
|
Mode1 |
Current |
L x W cm x cm |
times |
Dose |
Site |
(mA) 2 |
4,5 |
(sec)3,4,5 |
(μGy)6,7 |
|
AP Spine |
Thick |
3.000 |
15.1 x12.1 |
56 |
83 |
AP Spine |
Standard |
3.000 |
15.1 x12.1 |
28 |
37 |
AP Spine |
Thin |
0.750 |
15.1 x12.1 |
28 |
9 |
AP Spine |
QuickView |
3.000 |
15.1 x12.1 |
14 |
12 |
Femur |
Precise |
3.000 |
15.1 x12.1 |
56 |
83 |
Femur |
Thick |
3.000 |
15.1 x12.1 |
56 |
83 |
Femur |
Standard |
3.000 |
15.1 x12.1 |
28 |
37 |
Femur |
Thin |
0.750 |
15.1 x12.1 |
28 |
9 |
Femur |
QuickView |
3.000 |
15.1 x12.1 |
14 |
12 |
DualFemur |
Thick/Precise |
3.000 |
2 x15.1 x12.1 |
112 |
83 |
DualFemur |
Standard |
3.000 |
2 x15.1 x12.1 |
55 |
37 |
DualFemur |
Thin |
0.750 |
2 x15.1 x12.1 |
55 |
9 |
DualFemur |
QuickView |
3.000 |
2 x15.1 x12.1 |
28 |
12 |
- 11 of57-
Forearm |
Standard |
0.150 |
13.4 x10.0 |
21 |
2 |
Hand |
Standard |
0.150 |
23.5 x18.0 |
61 |
2 |
TotalBody |
Thick |
0.150 |
151.5 x60 |
532 |
0.8 |
TotalBody |
Standard |
0.150 |
151.5 x60 |
295 |
0.4 |
TotalBody |
Thin |
0.150 |
151.5 x60 |
295 |
0.4 |
LateralBMD |
Standard |
3.000 |
15.1 x12 |
56 |
83 |
LVA |
Standard |
3.000 |
38.7 x15.0 |
175 |
83 |
APVA |
Thick |
3.000 |
38.7 x15 |
85 |
37 |
APVA |
Standard |
3.000 |
38.7 x15 |
85 |
37 |
APVA |
Thin |
0.750 |
38.7 x15 |
85 |
9 |
OrthopedicFemur |
Thick |
3.000 |
20.2 x15 |
91 |
83 |
OrthopedicFemur |
Standard |
3.000 |
20.2 x15 |
44 |
37 |
OrthopedicFemur |
Thin |
0.750 |
20.2 x15 |
44 |
9 |
SmallAnimal |
Standard |
0.15 |
75.7 x25.0 |
261 |
1.8 |
Current andtypicaldose information forLunar PRODIGY Primo modes |
|
|
|||
|
|
|
|
|
Estimated |
|
|
|
Typical Meas- |
|
Skin |
|
|
|
urement Area |
Irradiation |
Entrance |
|
Mode1 |
Current |
L x W cm x cm |
times |
Dose |
Site |
(mA) 2 |
4,5 |
(sec)3,4,5 |
(μGy)6,7 |
|
AP Spine |
Thick |
1.500 |
15.1 x12.1 |
96 |
74 |
AP Spine |
Standard |
1.500 |
15.1 x12.1 |
56 |
42 |
AP Spine |
Thin |
0.375 |
15.1 x12.1 |
56 |
10 |
Femur |
Thick |
1.500 |
15.1 x12.1 |
96 |
74 |
Femur |
Standard |
1.500 |
15.1 x12.1 |
56 |
42 |
Femur |
Thin |
0.375 |
15.1 x12.1 |
56 |
10 |
DualFemur |
Thick |
1.500 |
2 x15.1 x12.1 |
193 |
74 |
DualFemur |
Standard |
1.500 |
2 x15.1 x12.1 |
112 |
42 |
DualFemur |
Thin |
0.375 |
2 x15.1 x12.1 |
112 |
10 |
Forearm |
Standard |
0.150 |
13.4 x10.0 |
21 |
2 |
TotalBody |
Thick |
0.150 |
151.5 x60 |
532 |
0.8 |
TotalBody |
Standard |
0.150 |
151.5 x60 |
295 |
0.4 |
TotalBody |
Thin |
0.150 |
151.5 x60 |
295 |
0.4 |
LateralBMD |
Standard |
3.000 |
15.1 x12 |
56 |
83 |
LVA |
Standard |
3.000 |
38.7 x15.0 |
175 |
83 |
APVA |
Thick |
3.000 |
38.7 x15 |
85 |
37 |
|
|
|
|
|
|
- 12 of57-

APVA |
Standard |
3.000 |
38.7 x15 |
85 |
37 |
APVA |
Thin |
0.750 |
38.7 x15 |
85 |
9 |
Orthopedic |
|
|
|
|
|
Femur |
Thick |
3.000 |
20.2 x15 |
91 |
83 |
Orthopedic |
|
|
|
|
|
Femur |
Standard |
3.000 |
20.2 x15 |
44 |
37 |
Orthopedic |
|
|
|
|
|
Femur |
Thin |
0.750 |
20.2 x15 |
44 |
9 |
Current andtypicaldose information forLunar DPX-PRO/NT/Duo/Bravo modes
|
|
|
|
|
Estimated |
|
|
|
Typical Meas- |
|
Skin |
|
|
|
urement Area |
Irradiation |
Entrance |
|
Mode1 |
Current |
L x W cm x cm |
times |
Dose |
Site |
(mA) 2 |
4,5 |
(sec)3,4,5 |
(μGy)6,7 |
|
AP Spine |
Thick |
1.500 |
15.1 x12.1 |
215 |
41 |
AP Spine |
Standard |
1.500 |
15.1 x12.1 |
108 |
20 |
AP Spine |
Thin |
0.375 |
15.1 x12.1 |
215 |
5 |
|
|
NotAvail- |
|
|
|
AP Spine |
QuickView |
able |
|
|
|
Femur |
Precise |
1.500 |
14.0 x12.0 |
221 |
41 |
Femur |
Thick |
1.500 |
14.0 x12.0 |
221 |
41 |
Femur |
Standard |
1.500 |
14.0 x12.0 |
132 |
20 |
Femur |
Thin |
0.375 |
14.0 x12.0 |
221 |
5 |
|
|
NotAvail- |
|
|
|
Femur |
QuickView |
able |
|
|
|
DualFemur |
Thick/Precise |
1.500 |
2 x14.0 x12.0 |
443 |
41 |
DualFemur |
Standard |
1.500 |
2 x14.0 x12.0 |
264 |
20 |
DualFemur |
Thin |
0.375 |
2 x14.0 x12.0 |
443 |
5 |
|
|
NotAvail- |
|
|
|
DualFemur |
QuickView |
able |
|
|
|
Forearm |
Standard |
0.050 |
11.5 x10.0 |
286 |
3 |
|
|
NotAvail- |
|
|
|
Hand |
Standard |
able |
|
|
|
TotalBody |
Thick |
0.100 |
151.5 x60 |
1337 |
0.3 |
TotalBody |
Standard |
0.100 |
151.5 x60 |
670 |
0.2 |
TotalBody |
Thin |
0.100 |
151.5 x60 |
900 |
0.2 |
LateralBMD |
Standard |
1.500 |
12.0 x12.0 |
189 |
41 |
|
|
NotAvail- |
|
|
|
LVA |
Standard |
able |
|
|
|
|
|
NotAvail- |
|
|
|
APVA |
Thick |
able |
|
|
|
|
|
NotAvail- |
|
|
|
APVA |
Standard |
able |
|
|
|
|
|
- 13 of57- |
|
|
|

|
|
NotAvail- |
|
|
|
APVA |
Thin |
able |
|
|
|
OrthopedicFemur |
Thick |
1.500 |
20.1 x15.0 |
385 |
41 |
OrthopedicFemur |
Standard |
1.500 |
20.1 x15.0 |
223 |
20 |
OrthopedicFemur |
Thin |
0.375 |
20.1 x15.0 |
385 |
5 |
|
|
NotAvail- |
|
|
|
SmallAnimal |
Standard |
able |
|
|
|
Current andtypicaldose information for DPX-MD+ modes. Note,Standardmode isreplacedwith Standard-MDmode.
|
|
|
|
|
Estimated |
|
|
|
Typical Meas- |
|
Skin |
|
|
|
urement Area |
Irradiation |
Entrance |
|
Mode1 |
Current |
L x W cm x cm |
times |
Dose6 |
Site |
(mA) 2 |
4,5 |
(sec)3,4,5 |
(μGy) |
|
AP Spine |
Standard-MD |
0.750 |
15.0 x12.0 |
212 |
20 |
Femur |
Standard-MD |
0.750 |
15.0 x12.0 |
236 |
20 |
OrthopedicFemur |
Standard-MD |
0.750 |
15.0 x12.0 |
336 |
20 |
1Allmodesare76kV,±1kV.
2Tubecurrentis±1%atthemaximumcurrent.
3Imagingtimemeasuredfromshutter opentoshutter close,90%to100%ofindicatedvalue.
4SizesofmeasurementareasandirradiationtimeswillbelessthanthoselistedaboveifyouusetheSmartScanfeature.
5Measurementlengthsandtimesaredependentonpatientheightandproductversion.
6DosemeasurementsareconstrainedbyDailyQAlimits. For example,themaximumspine(standardmode)rangeis30 to85μGy
for Prodigydensitometersand8 to28μGyfor DPXseriesdensitometers.
7 Irradiationtimesanddosevaluesdonotconsider a“sweepretry” featurewhichcandoublethedosefor asingletransverse sweepwithinanentirescan. Ifaretryoccursaslightincreaseinirradiationtimeandskinentrancedosewouldbeexpected. On Lunar ProdigyscannersDF+12000 andabove,allProdigyAdvance,andDPX+NTscannersrunningversion8 softwareand newer,asweepmayberetriedonetimeduringacquisition. Amaximumoftwosweepscanberetriedper scan. Theretryfeature reducesneedtorescanentirepatient.
Mechanical Safety
Thescanner armmovesdowntheentirelengthofthescanner table. Makesurethepatientdoesnotinterferewiththemovement ofthescanner armtopreventpossibleinjury. Inaddition,makesurethattherearenoobjectsbehindthescanner tablethatmight obstructmovementofthescanner arm.
WeightappliedtotheLunar iDXAmustnotexceed204kg(450 pounds).WeightappliedtotheLunar DPX-Pro/NT/MD+ scantable bed mustnotexceed136kg(300 pounds). WeightappliedtotheLunar PRODIGY,PRODIGYAdvance,PRODIGYPrimo, DPXDuo/Bravoscantablebedor footstep(DPXDuo)mustnotexceed159kg(350 pounds).
External Symbols
 Attention:showstheOperator'sManualcontainsimportantsafetyinformationsuchasthelocationofpinch
Attention:showstheOperator'sManualcontainsimportantsafetyinformationsuchasthelocationofpinch
points.
- 14 of57-

 Emergency StopButton:showsthelocationoftheemergencystopbutton.
Emergency StopButton:showsthelocationoftheemergencystopbutton.
 LaserOn:showsthelocationoftheLaser Onindicator.
LaserOn:showsthelocationoftheLaser Onindicator.
 ShutterOpen:showsthelocationoftheShutter Openindicator.
ShutterOpen:showsthelocationoftheShutter Openindicator.
 X-ray On:showsthelocationoftheX-rayOnindicator.
X-ray On:showsthelocationoftheX-rayOnindicator.
Type B Equipment: showsthatthescanner hasTypeBprotectionagainstelectricalshock.
PowerOn:showsthelocationofthePower Onindicator andtheswitchpositionfor Power On.
 PowerOff:showstheswitchpositionfor Power Off.
PowerOff:showstheswitchpositionfor Power Off.
Internal Symbols
 Protective Earth:showsthelocationofaProtectiveEarthterminal.
Protective Earth:showsthelocationofaProtectiveEarthterminal.
 FunctionalEarth:showsthelocationofaFunctionalEarthterminal.
FunctionalEarth:showsthelocationofaFunctionalEarthterminal.
Labels
Laser Caution and Ionizing Radiation Label: Showsthatthescanner usesaClassII laser. The labelincludesthe requiredsymbolsand precaution(Laser Radiation:Donotstareinto beam. ClassII Laser Product).
- 15 of57-

Tube Head Assembly "Lunar iDXA" Label:
Thislabelgivestube headassemblyandxraysourcecharacteristicsinformation.It islocatedonthetube headassemblyandthe footpanelofthe scanner. Thelabel appearancemayvary fromtheonedisplayed here. TheLunar iDXA serieslabelcoversappropriateTubeHead Assemblyfor Lunar iDXA scanners.
Tube Head Assembly “DPX Series” Label: This labelgivestubehead assemblyandx-ray sourcecharacteristics information.Itislocated onthetubehead assemblyandthefoot panelofthescanner. TubeHeadAssembly labelcoversDPX-NT, DPX-MD+,DPXBravo, andDPXDuo.
- 16 of57-
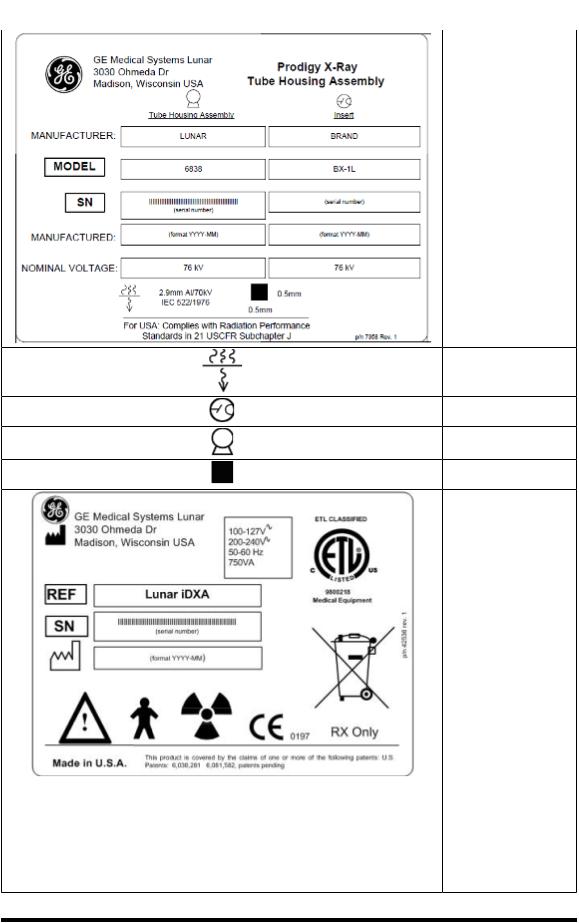
Tube Head Assembly “Prodigy Series” Label:
Thislabelgivestube headassemblyandxraysourcecharacteristicsinformation.It islocatedonthetube headassemblyandthe footpanelofthe scanner. Thelabel appearancemayvary fromtheonedisplayed here. TheProdigyseries labelcoversappropriate TubeHeadAssemblyfor ProdigyandProdigy Advancescanners.
Inherent Filtration:Sym- bolfromEN60417-1, 5381
Tube Insert:Symbol fromEN60417-1,5337
X-ray Source:Symbol fromEN60417-1,5338
Focal Point:Symbol fromEN60417-1,5327
System Label: Thislabel givessysteminput power requirementsand complianceinformation. Itislocatedonthefoot panelofscanners.The Attentionsymbolindicatesneedtoread accompanyingdocuments. Personsymbol referstoTypeBapplied partfor degreeofelectric shockprotectionper EN60601-1. TheFan symboldenotesionizing radiationisgenerated. TheCEmarkshows compliancewiththeMedicalDeviceDirective 93/42/EEC.TheETLmark showscompliancetoUL 60601-1 andCAN/CSA C22.2 No.601 The WasteReceptaclemark indicatesthatthewaste ofelectricalandelectronicequipmentmust notbedisposedas
- 17 of57-

unsortedmunicipal wasteandmustbecollectedseparately.Please contactanauthorized representativeofthe manufacturer for informationconcerningthe decommissioningof your equipment.
High Voltage Power Supply:Thislabelgives highvoltagepower supply(x-raygenerator) information.Itislocated onthehighvoltage power supplyandfoot panelofthescanner. Prodigy/DPXseriesHigh VoltagePower Supply labelcoversallthelatest Lunar productssince theyusethesameHVPS partnumber.
X-ray Controller:This labelshowsx-raycon- troller compliance.Itis locatednear thex-ray controller andonthe footpanelofthe scanner. TheLunar iDXA X-rayController Assemblylabelcoversall theLunar iDXAproducts. Prodigy/DPXseriesXrayController Assembly labelcoversallthelatest Lunar products.Labels showmodel/serial number for thatspecific product.
Collimator Assembly: Thislabelgivescollimator assemblyinformation.Itislocatedon thecollimator andfoot panelofthescanner. TheLunar iDXACollimator Assemblylabel coversallLunar iDXA products.Prodigy/DPX seriesCollimator Assemblylabelcovers latestLunar products. Labelsshowmodel/se-
- 18 of57-
 Loading...
Loading...