GE Healthcare B30 User Manual

GE Healthcare
B30 Patient Monitor
User’s Reference Manual
B30 Patient Monitor
English
2039820-001 C (Paper)
2044678-001 C (CD)
© 2009 General Electric Company.
All Rights Reserved.

B30 patient Monitor User's Reference Manual
Related to software license L-DICU08 Monitoring functions
0459
Conformity according to the Council Directive 93/42/EEC concerning Medical Devices.
All specifications are subject to change without notice.
Document no. 2039820-001 15th July, 2009
GE Medical Systems Information Technologies, Inc. |
GE Healthcare |
|
8200 West Tower Avenue |
3F Building 1, GE Technology Park |
|
Milwaukee, WI USA |
1 Huatuo Road |
|
Zip: 53223 |
Shanghai PRC 201203 |
|
Tel: 1 414 355 5000 (outside US) |
Tel: +86 |
21 3877 7888 |
800 558 5102 (US only) |
Fax: +86 |
21 3877 7451 |
Fax: 1 414 355 3790 |
|
|
www.gehealthcare.com |
|
|
Copyright © 2009 General Electric Company. All rights reserved.
Intended purpose (Indications for use)
The B30 patient monitor is intended for multiparameter patient monitoring. The B30 monitor is indicated for continuous monitoring of hemodynamic parameters (including arrhythmia and ST segment analysis) and respiratory status and creation of limit alarms. The B30 monitor is intended for all hospital patients and all hospital departments including intra-hospital transport but excluding harsh physical environment like MRI.
The Patient side module E-PSM(P)W and accessories are indicated for monitoring of hemodynamic parameters of all hospital patients. The hemodynamic parameters of the module comprise ECG (including ST-Segment and arrhythmia), impedance respiration, oscillometric NIBP (sys/dia/mean), temperature, SpO2 (including monitoring during conditions of clinical patient motion), and invasive blood pressure. Impedance respiration measurement is indicated for patients ages three years and up. The NIBP measurement is indicated for patients who weight 5kg (11 lb) or up.The E-PSM(P)W is intended for all hospital departments including intra-hospital transport but excluding harsh physical environment like MRI.
The extension module N-FCREC (option N-FCREC or N-FC) is indicated for monitoring of CO2 and respiration rate of all hospital patients. CO2 measurements are indicated for patients who weight over 5 kg (11 lb).
The B30 monitor and N-F(C)(REC) Extension Module and E-PSM(P)W Patient Side Module are indicated for use by qualified medical personnel only.
Classifications
In accordance with IEC 60601-1
Class I and internally powered equipment – the type of protection against electric shock.
Type BF or CF equipment. The degree of protection against electric shock is indicated by a symbol on each parameter module.
Equipment not suitable for use in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide.
Continuous operation according to the mode of operation. Portable Monitor.
In accordance with IEC 60529
IPX1 - degree of protection against harmful ingress of water.
In accordance with EU Medical Device Directive
The B30 patient monitor is classified as IIb.
In accordance with CISPR 11:
Group 1, Class B:
•Group 1 contains all ISM (Industrial, scientific and medical) equipment in which there is intentionally generated and/or used conductively coupled radio-frequency energy which is necessary for the internal functioning of the equipment itself.
•Class B equipment is suitable for use in domestic establishments and in establishments directly connected to a low voltage power supply network which supplies buildings used for domestic purposes.
Responsibility of the manufacturer
GE Medical Systems Information Technologies, Inc. is responsible for the effects on safety, reliability and performance of the equipment only if:
•assembly, extensions, readjustments, modifications, servicing and repairs are carried out by personnel authorized by GE.
•the electrical installation of the monitor room complies with appropriate requirements.
•the equipment is used in accordance with the "User's Guide."

Product availability
Some of the products mentioned in this manual may not be available in all countries. Please, consult your local representative for the availability.
Trademarks
Dash, Datex, Ohmeda, S/5, D-fend, D-fend+, Mini D-fend, OxyTip+, ComWheel, ComBar, EarSat, FingerSat, FlexSat are trademarks of GE Healthcare. All other product and company names are property of their respective owners.
End User License Agreement
THIS DOCUMENT IS A LEGAL AGREEMENT BETWEEN YOU, THE "LICENSEE," AND GE HEALTHCARE (“GE”). IF YOU DO NOT AGREE TO ALL THE TERMS OF THIS AGREEMENT, PROMPTLY RETURN THE ENTIRE PACKAGE, INCLUDING ALL ACCESSORIES, IN THEIR ORIGINAL PACKAGE, WITH YOUR SALES RECEIPT TO GE FOR A FULL REFUND.
1.Grant of License. GE grants to Licensee a nonexclusive, nontransferable, restricted license, without right to sublicense, to use the copy of the incorporated software/firmware("Software"), and manuals and documentation related to the Software in connection with Licensee's use of the product for their labeled purpose and only when the instrument is used with authorized accessories and sensors, in accordance with this End User License Agreement ("Software License"). GE reserves all rights not expressly granted to Licensee.
2.Ownership of Software/Firmware. Title to, ownership of, and all rights and interests in, any software and/or firmware and the documentation, and all copies thereof, remain at all times vested in GE or its partners, and they do not pass to Licensee.
3.Assignment. The rights and obligations of the Licensee under this Software License are personal. Accordingly, neither this Software License nor any of such rights and obligations are assignable or transferable by merger or by operation of law or otherwise without the prior written consent of GE. You may not rent, lease, sell, or otherwise dispose of the software/ firmware or the products on a temporary basis. GE may assign this Software License and/or any rights of Licensor hereunder, to any affiliate, or to any purchaser of substantially all of the assets used by GE in the performance of this Software License.
4.Limitation of liability. Other than the attached limited warranty, the Software is being licensed to Licensee "as is," without warranty of any kind, express or implied, including without limitation the warranties of merchantability, fitness for a particular purpose, functionality, use or performance of the Software and compatibility with particular computer systems, computer peripherals or other software packages, title or non-infringement. Some jurisdictions do not allow the disclaimer of implied warranties, so the above disclaimer may not apply to Licensee, in which case the duration of any such implied warranties is limited to the longer of (i) minimum required by law or (ii) thirty (30) days from the date the Software is received by Licensee.
In no case, including without limitation any breach of a fundamental term or a fundamental breach of this Software license, shall GE be liable for any damages, including but not limited to indirect, exemplary, special, consequential or incidental damages of any kind (including without limitation lost profits), even if GE has been advised of the possibility of such damages. These provisions hereof shall apply to the full extent permitted by law.
5. Copy Restrictions. The software/firmware and the accompanying written materials are copyrighted. Unauthorized copying of the software, including software that has been modified, merged, or included with other software, or other written materials is expressly forbidden. You

may be held legally responsible for any copyright infringement that is caused or incurred by your failure to abide by the terms of this license.
6. Use Restriction. As the Licensee, you may physically transfer the products from one location to another provided that the software/firmware is not copied. You may not electronically transfer the software/firmware from the products to any other device. You may not disclose, publish, translate, release or distribute copies of the software/firmware to others. You may not modify, adapt, translate, reverse engineer, decompile, disassemble, or create derivative works based on the software/firmware, unless and to the extent specifically permitted by local law. Your license to the software is not valid for use with any unauthorized data acquisition device. When information of the internal structure of the Software is necessary in order to obtain interoperability of the Software with other software programs, Licensee shall immediately contact GE.
The Software contains proprietary and confidential information of GE and its suppliers and is considered by GE and its suppliers to constitute valuable trade secrets. Licensee will hold the Software in confidence and shall protect the Software with at least the same degree of care with which Licensee protects its own similar confidential information but in no event less than a reasonable standard of care. Licensee agrees that its officers and employees shall protect the confidentiality of the Software and all confidential and non-public information relating thereto and shall not disclose such information to any third party. This obligation of confidentiality shall survive the termination of the Software License.
Licensee agrees to comply with all applicable export and re-export restrictions and regulations imposed by the government of the United States or of the country to which the Software is shipped to Licensee. Licensee shall not commit any act or omission, which will result in a breach of any such export requirements. Licensee shall defend, indemnify and hold GE and all GE's suppliers harmless from any claims arising out of Licensee's violation of such export control laws.
Upon termination by GE or its suppliers of this Software License, Licensee shall (as advised by GE) immediately destroy the Software and all copies thereof or return the same to GE and within two (2) business days thereafter certify to GE in writing that in accordance with instructions from GE or its suppliers, all copies of the Software have been either destroyed or returned to GE, whether same is in tangible or intangible form and Licensee shall further certify that all use thereof is and shall remain terminated.
7.No waiver. The failure of GE to enforce any provision of this Software License shall not be considered a waiver of any subsequent breach of that provision or as a waiver of any other provision hereof.
8.Amendments. This Software License may be modified only by a written instrument expressly agreed to by the parties hereto.
Warranty
This Product is sold by GE Healthcare (“GE”) under the warranty set forth in the following paragraphs. Such warranty is extended only with respect to the purchase of this Product directly from GE or GE’s Authorized Dealers as new merchandise and is extended to the Buyer thereof, other than for the purpose of resale.
For a period of twelve (12) months from the date of original delivery to Buyer, this Product, other than expandable parts, is warranted against functional defects in materials and workmanship and to conform to the description of the Product contained in this manual and accompanying labels and/or inserts, provided that the same is properly operated under the conditions of normal use, that regular periodic maintenance and service is performed and that the replacements and repairs are made in accordance with the instructions provided, using genuine parts and performed by a trained person. The foregoing warranty shall not apply if the Product has been repaired by anyone other than GE or otherwise than in accordance with written instructions provided by GE, or altered by anyone other than GE, or if the Product has been subject to abuse, misuse, negligence, or accident.
GE’s sole and exclusive obligation and Buyer’s sole and exclusive remedy under the above warranty is limited to repairing or replacing, free of charge, at GE’s option, a Product, which is telephonically reported to the nearest GE office or GE’s Authorized Dealers office and which, if so advised by GE, is thereafter returned with a statement of observed deficiency, not later than seven (7) days after the expiration date of the applicable warranty, to the GE office or GE’s Authorized Dealers office during normal business hours, transportation charges prepaid, and which, upon GE’s examination, is found not to conform to the above warranty. GE shall not be otherwise liable for any damages including but not limited to incidental damages, consequential damages, or special damages.
There are no express or implied warranties, which extend beyond the warranty hereinabove set forth. GE makes no warranty of merchantability or fitness for particular purpose with respect to the product or parts thereof.

Table of contents
Table of contents
About this manual
1Safety precautions
2System description
3Monitoring basic
4Alarms
5Monitor setup
6Trends
7Patient data management
8Printing and recording
9Cleaning and care
10Troubleshooting
11ECG
12Pulse oximetry
13Temperature
14Invasive blood pressure
15Impedance respiration
16Non-invasive blood pressure
17Airway gas (CO2)
Index
i

Table of contents
ii

Table of contents
Table of contents
About this manual |
1 |
Intended audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . About-1 Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . About-1 Illustrations and names . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . About-1 Conventions used in this manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . About-2 Related documentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . About-2 Installation and service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . About-3
i

Table of contents
ii

About this manual
About this manual
Intended audience
This manual is intended for clinical professionals. Clinical professionals are expected to have a working knowledge of medical procedures, practices, and terminology, as required for monitoring critically ill patients.
Overview
This User’s Reference Manual describes the functions offered by the B30 patient monitor running the software license L-DICU08. As the monitor setup may vary, some menus, displays and functions described may not be available in the monitor you are using.
This manual is an integral part of the product and describes its intended use. Keep it always close to the equipment. Observance of the manual is a prerequisite for proper product performance and correct operation and ensures patient and operator safety.
NOTE: Before using your monitor, please read the “User’s Guide” or this manual thoroughly. This User’s Reference Manual gives you more specific information about the clinical and technical aspects. Pay special attention to WARNING and CAUTION statements.
The new user of the monitor should begin with sections “Safety precautions” “System description” and “Monitoring basic.” These sections describe the system and the basic operation of the monitor.
The measurement sections describe the measurement technique, setup and how to adjust displays and menus for patient monitoring and special views.
Section “Monitor setup” gives instructions about setting up the system and making changes in the default settings. Section “Cleaning and care” describes cleaning and daily maintenance procedures.
Illustrations and names
All illustrations in this manual are only examples, and may not necessarily reflect your system settings or data displayed in your system. If a particular selection is not available in your system, the selection is shown grayed in the menu.
All names used in examples and illustrations are fictitious.
1

B30 Patient Monitor
Conventions used in this manual
To help you find and interpret information easily, the manual uses consistent text formats:
Hard keys |
Names of the hard keys on the Command board, side panel and |
|
modules are written in the following way: Others. |
Menu items |
Software terms that identify window parts or menu items are written |
|
in bold italic: Lab Data. |
Menu access |
Menu access is described from top to bottom. For example, the |
|
selection of the Monitor Setup hard key, the Screen Setup menu |
|
item and the Waveform Fields menu item would be shown as |
|
Monitor Setup - Screen Setup - Waveform Fields. |
File names etc. |
File names, file paths and text to be entered are written in the |
|
following way: comm.exe. |
Messages |
Messages (alarm messages, informative messages) displayed on the |
|
screen are written inside single quotes: ‘Please wait.’ |
References |
When referring to different sections in this manual or to other |
|
manuals, manual names and section names are enclosed in double |
|
quotes: See section "Cleaning and care." Please refer to "Technical |
|
Reference Manual: Installation." |
WARNING |
This is a WARNING. |
CAUTION |
This is a CAUTION. |
NOTE |
This is a NOTE. |
The following symbols are also used to distinguish procedures:
ECG |
Press the menu key described. |
|
Turn the ComWheel.
Push the ComWheel.
Related documentation
Software options and default settings are described in the “Default Configuration Worksheet” delivered with each monitor.
Available accessories are described in the “Supplies and Accessories” catalog delivered with each monitor.
For more information about the iCentral, see the “iCentral User’s Reference Manual”.
2

About this manual
Installation and service
A separate “Technical Reference Manual” describes installation, interfacing, connectors, service, maintenance and reparation procedures of the monitor.
Medical electrical equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in the “Technical Reference Manual” by qualified personnel.
Service and repairs are allowed for authorized service personnel only.
3

B30 Patient Monitor
4

Table of contents
Table of contents
1 Safety precautions |
1-1 |
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-1 Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-2 ESD precautionary procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3 ESD precautionary procedure training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3 Points to note . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3 Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
i

Table of contents
ii

Safety precautions
1 Safety precautions
The following list contains all the general warnings and cautions you should know before starting to use the system. Warnings and cautions specific to parts of the system can be found in the relevant section.
Warnings
WARNING A WARNING indicates a situation in which the user or the patient may be in danger of injury or death.
•To avoid explosion hazard, do not use the monitor in presence of flammable anesthetics.
•Connect only one patient to the monitor at a time.
•Do not use the monitor without manufacturer approved mounting attached.
•Use only hospital-grade grounded power outlets and power cord.
•To avoid the risk of electric shock, this equipment must only be connected to a supply mains with protective earth.
•Do not use an additional multiple socket outlet, extension cord or adapter of any kind.
•Use only an intact power cord.
•Do not user the power cord for any other product or purpose.
•Do not apply mechanical tension to the power cord, otherwise it may be damaged.
•Some equipment malfunctions may not generate a monitor alarm. Always keep the patient under close surveillance.
•Never install the monitor so that it is above the patient.
•Do not use the monitor in high electromagnetic fields (for example, during MRI).
•Do not connect any external devices to the system other than those specified.
•Do not touch the patient, table, instruments, modules or the monitor during defibrillation.
•Pins of connectors identified with the ESD warning symbol should not be touched. Connections should not be made to these connectors unless ESD precautionary procedures are used. For details, see ”ESD precautionary procedures” page 1-2.
•Use only approved accessories, including mounts and batteries, and defibrillator-proof cables and invasive pressure transducers. For a list of approved supplies and accessories, see the "Supplies and Accessories" catalog delivered with the monitor. Other cables, transducers, batteries and accessories may cause a safety hazard, damage the equipment or system, result in increased emissions or decreased immunity of the equipment or system or interfere with the measurement. Protection against cardiac
defibrillator discharge is due in part to the accessories for pulse oximetry (SpO2), temperature (T) and invasive pressure (P) measurement.
•Single-use accessories are not designed to be re-used. Re-use may cause a risk of contamination and affect the measurement accuracy.
•The monitor or its components should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the monitor and its components should be observed to verify normal operation in configuration in which it will be used.
1-1

B30 Patient Monitor
•When detaching modules, be careful not to drop them. Always support with one hand while pulling out with the other.
•If you accidentally drop the monitor or modules, have them checked by authorized service personnel prior to clinical use.
•If the integrity of the external protective earth conductor arrangement is in doubt, use the monitor with battery operation.
•Vibrations during intrahospital transport may disturb SpO2, ECG, impedance respiration, InvBP and NIBP measurements.
•If the unit fails to respond as described, do not use the monitor until tested and repaired by authorized service personnel.
•The system is intended for use by qualified medical personnel only.
•When using the electrosurgery unit, ensure proper contact of the ESU return electrode to the patient to avoid burns at monitor measurement sites.
•Make sure that the leadwire set clips or snaps do not touch any electrically conductive material including earth.
Cautions
CAUTION |
A CAUTION indicates a situation in which the unit or the devices connected to it |
|
may be damaged. |
|
• Before connecting the power cord to a power supply, check that the local voltage and |
|
frequency correspond with the rating stated on the device plate. |
|
• Leave space for air circulation to prevent the monitor from overheating. |
|
• Do not store or use the monitor outside the temperature and humidity ranges specified in |
|
“Performance” in section “System description” of this manual. |
|
• Refresh the batteries completely every six months. |
ESD precautionary procedures
•To avoid electrostatic charges to build up, it is recommended to store, maintain and use the equipment at a relative humidity of 30% or greater. Floors should be covered by ESD dissipative carpets or similar. Non-synthetic clothing should be used when working with the component.
•To prevent applying a possible electrostatic discharge to the ESD sensitive parts of the equipment, one should touch the metallic frame of the component or a large metal object located close to the equipment. When working with the equipment and specifically when the ESD sensitive parts of the equipment may be touched, a grounded wrist strap intended for use with ESD sensitive equipment should be worn. Refer documentation provided with the wrist straps for details of proper use.
ESD precautionary procedure training
•It is recommended that all potential users receive an explanation of the ESD warning symbol and training in ESD precautionary procedures.
•The minimum content of an ESD precautionary procedure training should include an introduction to the physics of electrostatic charge, the voltage levels that can occur in normal practice and the damage that can be done to electronic components if they are touched by an operator who is electrostatically charged. Further, an explanation should be given of methods to prevent build-up of electrostatic charge and how and why to
1-2

Safety precautions
discharge one’s body to earth or to the frame of the equipment or bond oneself by means of a wrist strap to the equipment or the earth prior to making a connection.
Points to note
•Medical electrical equipment needs special precautions regarding electromagnetic compatibility, EMC, and needs to be installed and put into service according to the EMC information provided in the "Technical Reference Manual" by qualified personnel.
•Portable and mobile RF communications equipment can affect the medical electrical equipment.
•The equipment is suitable for use in the presence of electrosurgery. Please notice the possible limitations in the parameter sections and in “Performance” on page 2-30.
•Service and repairs are allowed for authorized service personnel only.
Disposal
•Dispose of the whole device, parts of it, its packing material and manuals in accordance with local environmental and waste disposal regulations.
Product Compliance
The B30 Patient Monitor is classified in the following categories for compliance:
•This equipment is suitable for connection to public mains as defined in CISPR 11.
•This Monitor conforms to general safety standard for medical devices to IEC 60601-1.
•This Monitor conforms to EMC safety standard to IEC 60601-1-2.
•This Monitor conforms to usability safety standard for medical devices to IEC 60601-1-6.
•Software is developed in accordance with IEC 60601-1-4.
•The application of risk management analysis to medical device conforms to ISO 14971.
•The SpO2 Parameter conforms to ISO 9919.
•The TEMP parameter conforms to EN 12470-4.
•The CO2 parameter conforms to ISO 21647.
•This Monitor conforms to particular safety standard for multifunction patient monitoring equipment to IEC 60601-2-49 with the exception of Subclause 51.103; 51.103.1 and 51.102.4.
NOTE:
−This Monitor conforms to particular safety standard for multifunction patient monitoring equipment to IEC 60601-2-49 with the exception of Subclause 51.103: Both non-latched and latched alarms are selectable for technical alarms.
−If the Latching Alarms selection is active, both technical alarm and physiological alarm messages stay on the screen even if the initial alarm condition goes away.
WARNING The latching alarms function is convenient for the user to analyze and track the old state of the patient. This is a advanced function, which protected by the password in the monitor. This function should be operated by a qualified medical personnel only. The latched technical alarms do not affect safety and effectiveness of monitor.
1-3

B30 Patient Monitor
NOTE:
−This Monitor conforms to particular safety standard for multifunction patient monitoring equipment to IEC 60601-2-49 with the exception of Subclause 51.103.1: A new technical alarm only triggers a visual indication, not the audible alarm.
−During silencing, all new alarms for the same reason and all alarms for a different reason are indicated only visually.
WARNING In clinical conditions, caregivers are more likely to silence alarms when they consider these alarms quietly cacophony and may distract and overwhelm the clinicians. Appropriate visual textual message indicated in B30 still work to direct the caregiver's attention to the unexpected situation when alarms are silenced. However, the alarm silence function should be carefully used, the clinical practitioner should check the monitor’s screen frequently during silencing.
NOTE:
−This Monitor conforms to particular safety standard for multifunction patient monitoring equipment to IEC 60601-2-49 with the exception of Subclause 51.102.4: New PHYSIOLOGICAL ALARM(S) beginning after the activation of SILENCE/ RESET resume audio-visual ALARM manifestations
−Refer to the exception of Subclause 51.103.1 for the same performance and warning.
•The invasive blood pressure parameter conforms to the IEC 60601-2-34 with the exception of Sub-clause 51.300 and 51.207.4
NOTE:
−The invasive blood pressure parameter conforms to the IEC 60601-2-34 with the exception of Sub-clause 51.300: Both non-latched and latched alarms are selectable for technical alarms.
−Refer IEC 60601-2-49 item for monitor’s performance and warnings.
NOTE:
−The invasive blood pressure parameter conforms to the IEC 60601-2-34 with the exception of Sub-clause 51.207.4: A new alarm only triggers a visual indication, not the audible alarm.
−Refer IEC 60601-2-49 item for monitor’s performance and warnings.
•The ECG parameter conforms to IEC 60601-2-27 with the exception of Sub-clause 50.102.8 a); 51.103.2; 51.103.3.4; 51.104.1 and 51.104
NOTE:
−The ECG parameter conforms to IEC 60601-2-27 with the exception of Sub-clause 50.102.8 a): Frequency response: The output signal amplitudes are out of range at Method A 40HZ and Method B
−The ECG high-frequency response limit for monitoring filter and ST filter with 50 Hz power supply frequency is 30 Hz, but not 40 Hz according the 60601-2-27.
WARNING In clinical conditions, the high-frequency response limit of 30 Hz for monitoring filter and ST filter with 50 Hz power supply frequency is adequately and safe for these lethal arrhythmia identification which are available in B30. The diagnostic filter is recommended for diagnostic purposes.
1-4

Safety precautions
NOTE:
−The ECG parameter conforms to IEC 60601-2-27 with the exception of Sub-clause 51.103.2: SILENCE/RESET of PHYSIOLOGICAL ALARMS/Sub-clause 51.104.1: Auditory manifestation of TECHNICAL ALARMS: A new alarm only triggers a visual indication, not the audible alarm.
−Refer IEC 60601-2-49 item for monitor’s performance and warnings.
NOTE:
−The ECG parameter conforms to IEC 60601-2-27 with the exception of Sub-clause 51.103.3.4: New PHYSIOLOGICAL ALARM(s) beginning after activation of SILENCE/ RESET resumed the auditory and visual ALARM manifestations
−Refer IEC 60601-2-49 items for monitor’s performance and warnings.
NOTE:
−The ECG parameter conforms to IEC 60601-2-27 with the exception of Sub-clause 51.104: Both non-latched and latched alarms are selectable for technical alarms.
−Refer IEC 60601-2-49 items for monitor’s performance and warnings.
•The NIBP parameter conforms to IEC 60601-2-30, EN 1060-1, EN 1060-3 with the exception of Sub-clause 22.4.1, 51.103 of IEC 60601-2-30 and Sub-clause 5 of EN 1060-1.
NOTE:
−The NIBP parameter conforms to IEC 60601-2-30 with the exception of Subclause 22.4.1: In single fault condition, the functioning independently of the normal pressure control system does not prevent immediately.
WARNING Prolonged use and frequent blood pressure determinations can lead to venous pooling and congestion. Devices that exert pressure on tissue have been associated with purpura, skin avulsion, compartmental syndrome, ischemia, and/or neuropathy. To minimize these potential problems, especially when monitoring at frequent intervals or over extended periods of time, make sure the cuff is applied appropriately and examine the cuff site and the limb distal to the cuff regularly for signs of impeded blood flow.
NOTE:
−The NIBP parameter conforms to IEC 60601-2-30 with the exception of Sub-clause 51.103: Both non-latched and latched alarms are selectable for technical alarms.
−Refer IEC 60601-2-49 item for monitor’s performance and warnings.
NOTE:
−The NIBP parameter conforms to EN1060-1 with the exception of Sub-clause 5: Abbreviations; “Mean” used instead “M” or “MAP”.
−The word “Mean” is used in monitor instead of “M” or “MAP” as the value for the NIBP pressure mean average.
WARNING The clinical practitioner can understand “Mean” is the abbreviation of NIBP pressure mean average. The B30 monitor is intended for use by qualified medical personnel only.
•The alarm systems of the Monitor conform to IEC 60601-1-8 with the exception of Subclause 6.3.3.1a
NOTE:
1-5

B30 Patient Monitor
−The alarm systems of the Monitor conform to IEC 60601-1-8 with the exception of Sub-clause 6.3.3.1a: An ALARM SYSTEM provided with auditory ALARM SIGNALS shall have at least one set of ALARM SIGNALS.
−The characteristics of auditory alarm signals are different from IEC 60601-1-8 requirement. This is only ms quantitative difference that can’t be defected by human audition, according to the usability study, it has no negative effect in clinical use.
Comformity according to the Council Directive
93/42/EEC concerning Medical Devices.
0459
1-6

Table of contents
Table of contents
2 System description |
2-1 |
Principles of functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1 System introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1 Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-2 Optional components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 Rear panel connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3 Module overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4 Patient Side Module E-PSMW . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4 Patient Side Module E-PSMPW . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5 Extension Module N-FC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6 Extension Module N-FCREC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7 Extension Module N-FREC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7 General module description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8 Inserting and removing a module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8 Keyboards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10 Command Board keys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10 Side panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-11 Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12 Battery indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13 Replacing the batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14 Conditioning a battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15 Symbols and abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15 Equipment safety symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15 Other symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16 Abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19 Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30 Power supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30 Battery operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30 Environmental conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30 Alarm behavior . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30 Defibrillator & IABP synchronization connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30 Hemodynamic modules E-PSM, E-PSMP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-31
Modules with CO2 measurement, N-FC and N-FCREC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-34 Modules with recorder, N-FREC and N-FCREC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-35
i

Table of contents
ii

System description
2 System description
Principles of functions
The B30 monitor is a modular multiparameter patient monitor. The monitor is especially designed for monitoring in PACU, ED, Wards, Step down units, ICU, CCU and OR in regions where anesthesia gas monitoring is not required. It can also be used during transportation within the hospital.
The modular design makes the system flexible and easy to upgrade.
System introduction
The B30 monitor system may consist of the elements shown below.
NOTE: Your system may not include all these components. Consult your local representative for the available components.
1
2
3
Figure 2-1 B30 patient monitor system
|
(1) |
B30 with module(s) |
|
(2) |
Printer (network printer only) |
|
(3) |
Other monitors in the network. NOTE: You cannot view other monitors on the B30 with L- |
|
|
DICU08 software. |
|
NOTE: The allowed cables, batteries, transducers and accessories for the monitor are |
|
|
listed in the “Supplies and Accessories” catalog delivered with the monitor. |
|
WARNING |
If you accidentally drop the monitor, modules or frames, have them |
|
|
checked by authorized service personnel prior to clinical use. |
|
WARNING |
Connect only one patient to the monitor at a time. |
|
WARNING |
Do not use the monitor without manufacturer approved mounting |
|
|
attached. |
|
2-1
B30 Patient Monitor
WARNING |
Before starting to use the system, ensure that the whole combination |
|
complies with the international standard IEC 60601-1-1 and with the |
|
requirements of the local authorities. Do not connect any external devices |
|
to the system other than those specified. |
CAUTION |
The monitor display is fragile. Ensure that it is not placed near a heat source or |
|
exposed to mechanical shocks, pressure, moisture or direct sunlight. |
Components
The main components of the B30 are the monitor frame and the interchangeable modules.
1 2 3
4
5
12
6
11
 7 10 9 8
7 10 9 8
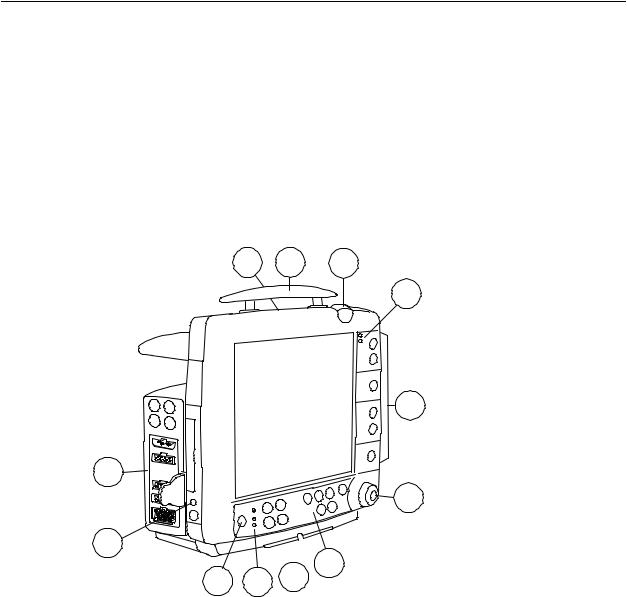
Figure 2-2 B30 monitor front panel
(1)Battery compartment
(2)Transportation handle
(3)Alarm light
(4)Alarm LED indicators
(5)Side panel keys
(6)The ComWheel
(7)Command Board keys
(8)Guide rail for GCX mounting
(9)Mains power and battery LEDs
(10)ON/standby key
(11)Defibrillator & IABP synchronization connector (marked with X5)
(12)Measurement modules, see page 2-4
You can use one E-PSM(P)W and/or one N-Fx module in the monitor at a time.
2-2
System description
Optional components
Optional components are:
•
•
Patient Side Modules E-PSMW and E-PSMPW Extension Modules N-FREC, N-FCREC and N-FC
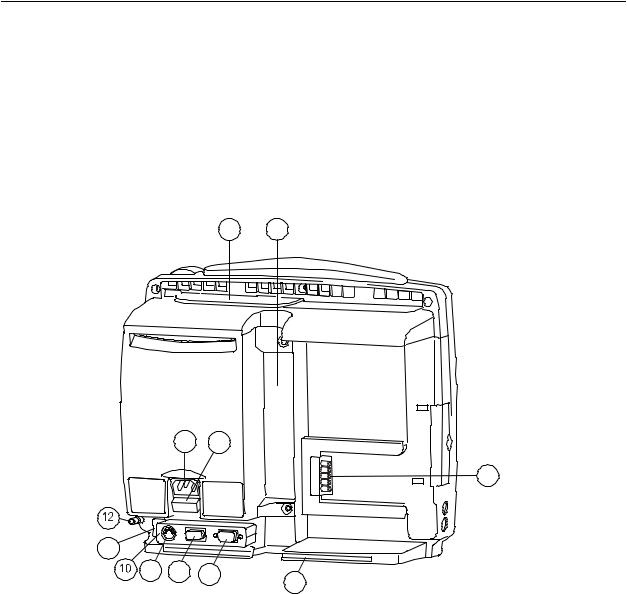
Rear panel connections
1 2
5 6
3
11
9 |
8 |
7 |
4 |
|
|
|
Figure 2-3 Rear panel connections
(1)Battery compartment
(2)Slot for infusion pole mounting
(3)Module connector (marked with X4)
(4)Guide rail for GCX mounting
(5)Receptacle for power cord
(6)Fuse holder
(7)Serial port (marked with X9)
(8)Network ID connector (marked with X8)
(9)Connector for future use (marked with X7)
(10)Accessory: Multi I/O adapter (with connectors 7 - 9 above)
(11)Network connector
(12)Equipotential connector
2-3

B30 Patient Monitor
Module overview
Different modules measure different parameters. See below for module descriptions and features.
Patient Side Module E-PSMW
1
2
3  6
6
4
5
Figure 2-4 Module E-PSMW
(1)Module keys
(2)NIBP connector
(3)Temperature connector: 2-channel measurement
(4)SpO2 connector
(5)ECG (3/5 lead) and impedance respiration connector
(6)Tab for removing the module
2-4
 Loading...
Loading...