GE Healthcare 259cx, 256cx, 250cx User Manual

GE Healthcare
Corometrics™ 250cx Series Monitor
Operator’s Manual
Corometrics 250cx Series Monitor
English
2036946-001 C (paper)
© 2007 General Electric Company.
All Rights Reserved.

GE Healthcare
Corometrics™ 250cx Series Monitor
Operator’s Manual
Corometrics 250cx Series Monitor
English
2036946-001 C (paper)
© 2007 General Electric Company.
All Rights Reserved.

GUARANTEE
All equipment sold by GE Medical Systems Information Technologies, is fully guaranteed as to materials and workmanship for a period of 1 year. GE Medical Systems Information Technologies reserves the right to perform guarantee service operations in its own factory, at an authorized repair station, or in the customer’s installation.
Our obligation under this guarantee is limited to repairing, or, at our option, replacing any defective parts of our equipment, except fuses or batteries, without charge, if such defects occur in normal service.
Claims for damage in shipment should be filed promptly with the transportation company. All correspondence covering the instrument should specify the model and serial numbers.
GE MEDICAL SYSTEMS Information Technologies
A GE Healthcare Company
GE Medical Systems Information Technologies will make available on request such circuit diagrams, component diagrams, component parts lists, descriptions, calibration instructions, or other information which will assist the users or appropriately qualified technical personnel to repair those parts of the equipment which are classified by GE Medical Systems Information Technologies as repairable. Refer to the 250/250cx Series Service Manual for further information.
NOTE: In addition to software version 4.50, the information in this manual also applies to previous software revisions of Corometrics 250cx Series Monitor. There are no user-apparent differences among these software versions. Due to continuing product innovation, specifications in this manual are subject to change without notice.
NOTE: For technical documentation purposes, the abbreviation GE is used for the legal entity name, GE Medical Systems Information Technologies
Ohmeda Oximetry and other trademarks (OxyTip+®, PIr™, TruSat™, TruSignal™, TruTrak+®, SuperSTAT™) are the property of GE Medical Systems Information Technologies, a division of General Electric Corporation. All other product and company names are the property of their respective owners.
MASIMO SET® is a trademark of Masimo Corporation. Possession or purchase of this device does not convey any express or implied license to use the device with replacement parts which would, alone, or in combination with this device, fall within the scope of one or more of the patents relating to the device.
NELLCOR®, OxiMax®, C-LOCK® and SatSeconds™ are trademarks of Nellcor Puritan Bennett. TAT-5000™, Exergen® and TemporalScanner™ are trademarks of Exergen Corporation.
CAUTION: In the United States of America, Federal Law restricts this device to sale by or on the order of a physician.
Corometrics and Marquette are registered trademarks of GE Medical Systems Information Technologies. GE is a registered trademark of General Electric Company. All other product and brand names are trademarks or registered trademarks of their respective companies. ©2005, 2006, 2007 GE Medical Systems Information Technologies. All rights reserved. No part of this manual may be reproduced without the permission of GE Medical Systems Information Technologies.
T-2 |
Corometrics 250cx Series Monitor |
Revision C |
|
2036946-001 |
16-Sept-2007 |

CE Marking Information
CE Marking Information
0086
Compliance
A GE brand Corometrics 250cx Series Monitor bears CE mark CE-0086 indicating its conformity with the provisions of the Council Directive 93/ 42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive.
The device is manufactured in India; the CE mark is applied under the authority of Notified Body BSI (0086).
The country of manufacture and appropriate Notified Body can be found on the equipment labeling.
The product complies with the requirements of standard EN 60601-1-2 “Electromagnetic Compatibility—Medical Electrical Equipment” and standard EN 60601-1 “General Requirements for Safety.”
Components of the Certified Systems
The IEC electromagnetic compatibility (EN) standards require individual equipment (components and accessories) to be configured as a system for evaluation. For systems that include a number of different equipments that perform a number of functions, one of each type of equipment shall be included in the evaluation.
The equipment listed below is representative of all possible combinations. For individual equipment certification, refer to the appropriate declarations of conformity.
Component Description:
250cx Series Maternal/Fetal Monitor
Model 146 Fetal Acoustic Stimulator
Intrauterine Pressure Transducer
TOCO Transducer
FECG Cable/Legplate
Ultrasound Transducers (x2)
Blood Pressure Hose and Cuff
MSpO2 Interconnect Cable and Sensor
MECG Cable
FECG/MECG Adapter Cable
Remote Event Marker
RS-232C Interconnect Cables (x3)
Central Nurses Station Interconnect Cable
Model 2116B Keyboard and Interconnect Cable
Model 1563AAO Telemetry Cable
Exergen TemporalScannerTM TAT-5000 Assembly 2036641-001
Revision C |
250cx Series Maternal/Fetal Monitor |
CE-1 |
|
2036946-001 |
|

CE Marking Information
Exceptions
The Monitor System EMC: Immunity Performance
None
Be aware that adding accessories or components, or modifying the medical device or system may degrade the EMI performance. Consult with qualified personnel regarding changes to the system configuration.
CE-2 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

1
2
Contents
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
General Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Responsibility of the Manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Responsibility of the User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Definitions of Terminology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Monitor Contraindications, Warnings, and Precautions . . . . . . . . . . . . . . . . . . . . 1-5
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
Equipment Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-10 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
About the Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Illustrations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Fetal Monitoring Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Surveillance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Maternal Monitoring Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Blood Pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 Pulse Oximetry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4 Heart/Pulse Rate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Series Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
The 250cx Series Monitor Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4 System Parameters (256cx and 259cx) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4 Fetal Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5 Maternal Parameters (259cx only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6 Adding Fetal Movement Detection and/or Spectra Alerts . . . . . . . . . . . . . . . . . . . 2-6
Revision C |
250cx Series Maternal/Fetal Monitor |
i |
|
2036946-001 |
|

3
4
Controls, Indicators, and Connectors . . . . . . . . . . . . . . . 3-1
Front Panel Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Front Panel Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Display Example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Primary Labor Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
FHR Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
UA Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Additional Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
Maternal NIBP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
MHR/P Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
MSpO2 Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-12
Waveform Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-12
Time and Waveform Message Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-12
Battery-Backed RAM Status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Softkeys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Mode Title Softkeys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Waveform Softkeys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Dedicated Softkey Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-14
Rear Panel Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-16
Setup Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Loading Strip Chart Recorder Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Interruption of Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Self-Test Routine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Setup Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Using the Trim Knob Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
General Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Play Song . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Song Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Temp Done Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Brightness . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Paper Speed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Date . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
MSpO2 Print Interval . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
FSpO2 Print Interval . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
FSpO2 Trace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
ii |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

5
6
Preparing the Monitor for Patient Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Fetal Heart Rate Monitoring . . . . . . . . . . . . . . . . . . . . . . . 5-1
Ultrasound (External Method) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
US/US2 Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Alert . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Alarm Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
FECG (Internal Method) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Artifact Elimination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
FECG Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Audio Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Alarm Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Fetal Heart Rate Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-6 |
FHR Threshold Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-6 |
FHR High Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-7 |
Sample Clinical Exceptions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-8 |
Active Signal Quality Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-8 |
Resolved Signal Quality Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-8
100% Signal Loss . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Silencing an Audio Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Single Fetal Heart Rate Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Dual Fetal Heart Rate Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Heartbeat Coincidence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11 Fetal Heart Rate Offset . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11 Activating the Fetal Heart Rate Offset Feature . . . . . . . . . . . . . . . . . . . . . . 5-11
De-Activating the Fetal Heart Rate Offset Feature . . . . . . . . . . . . . . . . . . . |
5-12 |
Uterine Activity Monitoring . . . . . . . . . . . . . . . . . . . . . . . 6-1
Tocotransducer (External Method) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Establishing a Baseline . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Initial Referencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Accounting for Belt Tension . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4 More About Referencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4 Out of Range Condition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4 Manually Setting the Baseline at the Default Value . . . . . . . . . . . . . . . . . . . . 6-4 Manually Overriding the Baseline Default Value . . . . . . . . . . . . . . . . . . . . . . 6-4
Revision C |
250cx Series Maternal/Fetal Monitor |
iii |
|
2036946-001 |
|

7
8
Automatic Baseline “Zeroing” . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Internal Method - Intrauterine Pressure (IUP) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
Why You Must Zero the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
Maternal Heart/Pulse Rate Monitoring . . . . . . . . . . . . . . 7-1
MHR/P Source . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
MHR/P Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Source . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
HR/PR Trace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Alarm Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
MECG Lead . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
MECG Pacer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-6
Maternal ECG Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7
Theory and Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7
Pacemaker Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7
MECG Waveform . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
Maternal Non-Invasive Blood Pressure Monitoring . . . . 8-1
Blood Pressure Safety Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-4
NIBP Determination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-5
SuperSTAT NIBP Determination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-5
Accelerated Determination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-6
Systolic Search . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-6
NIBP Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-7
Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-7
Target . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-7
NIBP Done Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
Alarm Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
NIBP Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
Patient Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
Blood Pressure Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-9
Hydrostatic Effect . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-10
Manual Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-10
Automatic Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-10
Taking a Manual Reading Between Auto Determinations . . . . . . . . . . . . . . 8-11
iv |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

9
10
Venous Return in Auto Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-11 Adjusting the Interval Time Between Automatic Determinations . . . . . . . . . 8-11 NIBP Interval Button Shortcut . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-12
Terminating a Determination in Progress . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-13 |
Smart BP Feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-13
Enabling/Disabling Smart BP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-13
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-13
Maternal Pulse Oximetry Monitoring . . . . . . . . . . . . . . . . 9-1
MSpO2 Technology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
Which Module is Installed? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3 Theory of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-4 Ohmeda TruSignal™ Oximetry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-4 TruSignal™ Enhanced SpO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-4 Signal processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-4 Masimo SET® . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-4
Signal Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-4 |
Nellcor OxiMax® . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Automatic Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-6
SatSeconds™ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-6 |
SatSeconds “Safety Net” . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-8 |
Using SatSeconds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-8 |
MSpO2 Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-9
Response Time (Nellcor 506 Technology Only) . . . . . . . . . . . . . . . . . . . . . . . . . . 9-9
Response Time (Nellcor NELL-3 Technology Only) . . . . . . . . . . . . . . . . . . . . . . . 9-9
Sensitivity (Masimo Technology Only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-9
Averaging Time (Masimo Technology Only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Print Interval . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
%O2 Trace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Alarm Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
MSpO2 Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
MSpO2 Pulse Beat Audio . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-11
The MSpO2 Waveform . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-11
Module and Probe Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-11
Modules and Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
No Implied License . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
Alarm Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
Master Alarm Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
Revision C |
250cx Series Maternal/Fetal Monitor |
v |
|
2036946-001 |
|

Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
Alarm Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-4
Alarm Silence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-4
Alarm Setting Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-5
Maternal Alarm Occurring During Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-5
Alarm Behavior . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-5
Fetal Heart Rate Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
FHR Patient Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
Active Patient Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
Resolved Patient Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
FHR Signal Quality Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
Active Signal Quality Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
Resolved Signal Quality Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-7 |
Silencing an FHR Audio Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-7 |
Maternal Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Maternal Patient Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Active Patient Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Resolved Patient Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Signal Quality Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Active Signal Quality Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-8
Resolved Signal Quality Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. . 10-8 |
Silencing a Maternal Audio Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 10-8 |
Alarms Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-9 |
11 Recorder Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
11-1 |
Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Off Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
On Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Maternal-Only Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
What is the Maternal-Only Mode? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Printing Style . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Changing Recorder Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Functionality with a QS System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
Paper Versus Electronic Strip Charts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5 Fetal Heart Rate Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-6
Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-6
Multiple Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-6
SpO2 Scale . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Standard Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
Blood Pressure Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
Maternal Pulse Oximetry Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-9
vi |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

12
13
14
Annotations from a Central Information System . . . . . . . . . . . . . . . . . . . . . . . . . 11-9 Multiple Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-9
Summary of Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-10
Adjustable Recorder Font Size . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-13
Chart Style Vital Signs Printing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-14
Enabling/Disabling Chart-Style Printing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-14 Examples of Printing Styles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-15 Chart-Style Printing Examples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-15 Real-Time Printing Example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-15 Chart-Style 7-Minute Exception for NIBP . . . . . . . . . . . . . . . . . . . . . . . . . . 11-15
Strip Chart Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-16 Paper-Low, Paper-Out, and Paper-LoadING Error Conditions . . . . . . . . . . . . . 11-18
Maternal Vital Signs History . . . . . . . . . . . . . . . . . . . . . . |
12-1 |
What is the Maternal Vital Signs History Screen? . . . . . . . . . . . . . . . . . . . . . . . |
. 12-3 |
Using the Maternal Vital Signs History Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . |
12-4 |
Displaying the Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 12-4 |
Selecting the HX Interval . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 12-4 |
Printing the Maternal Vital Signs History Screen . . . . . . . . . . . . . . . . . . . . . . . . |
. 12-5 |
Printing the Entire Vital Signs History . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 12-5 |
Printing a Page of the Vital Signs History . . . . . . . . . . . . . . . . . . . . . . . . . |
. 12-5 |
Stopping the Printing of Maternal Vital Signs History . . . . . . . . . . . . . . . . . . 12-5
Heartbeat Coincidence . . . . . . . . . . . . . . . . . . . . . . . . . . 13-1
Heartbeat Coincidence Theory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-3
Using the Heartbeat Coincidence Feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-3
Enabling/Disabling Heartbeat Coincidence Detection . . . . . . . . . . . . . . . . . . . . . 13-3
Display Indicator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-3
Strip Chart Annotation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-5
Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-1
Waveform Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
Selecting the Waveform . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
Waveform Speed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
ECG Size . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
MECG Lead Select . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
MECG Pacer Label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
Moving Gap . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-4
Freezing Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-4
Revision C |
250cx Series Maternal/Fetal Monitor |
vii |
|
2036946-001 |
|

15
16
17
Printing a Waveform Snapshot . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-5 |
Recorder On . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-5 |
Recorder in Maternal-Only Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-6 |
Recorder Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-6 |
Stopping a Print Command . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-6 |
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
15-1 |
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-3
Monitor Exterior . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-3
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-4
Tocotransducer and Ultrasound Transducer . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-4
Leg Plates and MECG Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-4
Maternal NIBP Cuffs and Hoses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-5
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-5
Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-5
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-5
SpO2 Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-6
Maternal SpO2 Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-6
NIBP Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-6
Disposal of Product Waste . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-7
Patient Applied Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-7
Packaging Material . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-7
Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-7
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
16-1 |
General Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-3
Ultrasound Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-4
FECG Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-5
External Uterine Activity Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-5
Internal UA Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-6
MECG Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-7
Blood Pressure Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-7
Maternal Pulse Oximetry Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16-8
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . |
17-1 |
General Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17-3
Operating Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17-4
viii |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

18
A
B
Strip Chart Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17-11
Supplies & Accessories . . . . . . . . . . . . . . . . . . . . . . . . . 18-1
General Add-Ons Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-3
Paper Supplies Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-3
Ultrasound Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-3
FECG Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-4
Tocotransducer Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-4
IUPC Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-4
MECG Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-5
NIBP Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-5
MSpO2 Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-6
Peripheral Device Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18-6
Factory Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .A-1
Table of Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-3
Fetal Movement Detection . . . . . . . . . . . . . . . . . . . . . . . . .B-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Availability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-3
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-3
Using Fetal Movement Detection While Monitoring . . . . . . . . . . . . . . . . . . . . . . . . B-3
Enabling/Disabling Fetal Movement Detection . . . . . . . . . . . . . . . . . . . . . . . . . . .B-3 Display Indicator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-4 Strip Chart Annotation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-4 Using the FM Remote Marker to Complement the Patient Record . . . . . . . . . . . .B-4
C Spectra Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-1
Important Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-3
Revision C |
250cx Series Maternal/Fetal Monitor |
ix |
|
2036946-001 |
|

Using the Spectra Alert Option . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-4 |
Enabling/Disabling Spectra Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-4 |
Methodology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-4 |
Alert Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-6
Active Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-6
Silencing Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-6
Resolved Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-7
Alert Suspension Feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-7
Enabling/Disabling the Alert Suspension Feature . . . . . . . . . . . . . . . . . . . . .C-7
Suspending Audio Alerts (and the Nurse Call Interface) . . . . . . . . . . . . . . . .C-7
Restoring Audio Alerts (and the Nurse Call Interface) . . . . . . . . . . . . . . . . . .C-7
Alert Parameters Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-9
Resetting Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-12
False Pattern Recognition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-12
Mode Switching . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-12
Trend Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-13
Uterine Contraction Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-13
Enabling/Disabling UC Frequency Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-14
UC Frequency in UA Display Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-14
UC Frequency Histogram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-15
Enabling/Disabling UC Chime . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-15
Nurse Call Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-16
Alert Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-17
D Frequently Asked
Questions D-1
FAQs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .D-3
x |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

1 Safety
Revision C |
250cx Series Maternal/Fetal Monitor |
1-1 |
|
2036946-001 |
|

Safety:
For your notes
1-2 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

Safety: General Information
General Information
General Use
If the monitor is cold to the touch or below ambient temperature, allow it to stabilize before use.
To ensure patient safety, use only parts and accessories manufactured or recommended by GE Medical Systems Information Technologies. Parts and accessories used shall meet the requirements of EN60601-1-1.
Disposable devices are intended for single use only. They should not be reused.
Test all functions periodically and whenever the integrity of the monitor is in doubt.
Refer to the “Maternal/Fetal Monitoring, Clinical Applications Manual” for information concerning the limitations of internal and external fetal heart rate monitoring techniques.
Responsibility of the Manufacturer
GE Medical Systems Information Technologies is responsible for the effects on safety, reliability, and performance if:
assembly operations, extensions, readjustments, modifications, or repairs are carried out by persons authorized by GE Medical Systems Information Technologies;
the electrical installation of the relevant room complies with the requirements of appropriate regulations; and
the monitor is used in accordance with the instructions of use.
Responsibility of the User
This device is intended for use by clinical professionals who are expected to know the medical procedures, practices, and terminology required to monitor obstetrical patients. This manual documents all possible parameters available in the 250cx Series monitor. It is the responsibility of each hospital to ensure that the Labor and Delivery staff is trained in all aspects of the selected model.
The 250cx Series monitor is only one clinical indicator of fetal status during labor. The monitor is designed to assist the perinatal staff in assessing the status of a patient. The monitor does not replace observation and evaluation of the mother and fetus at regular intervals by a qualified care provider, who will make diagnoses and decide on treatments or interventions. Visual assessment of the monitor display and strip chart must be combined with knowledge of patient history and risk factors to properly care for the mother and fetus.
Revision C |
250cx Series Maternal/Fetal Monitor |
1-3 |
|
2036946-001 |
|

Safety: Definitions of Terminology
Definitions of Terminology
Six types of special notices are used throughout this manual. They are: Danger, Warning, Caution, Contraindication, Important, and Note. The warnings and cautions in this Safety section relate to the equipment in general and apply to all aspects of the monitor. Be sure to read the other chapters because there are additional warnings and cautions which relate to specific features of the monitor.
When grouped, warnings and cautions are listed alphabetically and do not imply any order of importance.
|
Definitions of Terminology |
|
|
|
|
Danger |
|
A DANGER notice indicates an imminently |
|
|
hazardous situation which, if not avoided, will result |
|
|
in death or serious injury. |
|
|
|
Warning |
|
A WARNING indicates a potentially hazardous |
|
|
situation which, if not avoided, could result in death |
|
|
or serious injury. |
|
|
|
Caution |
|
A CAUTION indicates a potentially hazardous |
|
|
situation which, if not avoided, may result in minor |
|
|
or moderate injury. Cautions are also used to avoid |
|
|
damage to equipment. |
|
|
|
Contraindication |
|
A CONTRAINDICATION describes any special |
|
|
symptom or circumstance that renders the use of a |
|
|
remedy or the carrying out of a procedure |
|
|
inadvisable, usually because of a risk. |
|
|
|
Important |
|
An IMPORTANT notice indicates an emphasized |
|
|
note. It is something you should be particularly |
|
|
aware of; something not readily apparent. |
|
|
|
Note |
|
A NOTE indicates a particular point of information; |
|
|
something on which to focus your attention. |
|
|
|
1-4 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

Safety: Monitor Contraindications, Warnings, and Precautions
Monitor Contraindications, Warnings, and
Precautions
Warnings
WARNINGS
ACCIDENTAL SPILLS—In the event that fluids are accidentally spilled onto the monitor, remove the monitor from operation and inspect for damage.
APPLICATION—This monitor is not designed for direct cardiac connection.
CONDUCTIVE CONNECTIONS—Avoid making any conductive connections to applied parts (patient connection) which are likely to degrade safety.
CONDUCTIVE PARTS—Ensure that the conductive parts of the lead electrodes and associated connectors do not contact other conductive parts including earth.
CONNECTIONS—The correct way to connect a patient to the monitor is to plug the electrode leads into the patient cable which in turn connects to the monitor. The monitor is connected to the wall socket by the power cord. Do not plug the electrode leads into the power cord, a wall socket, or an extension cord.
DEFIBRILLATION—During defibrillation, all personnel must avoid contact with the patient and monitor to avoid a dangerous shock hazard. In addition, proper placement of the paddles in relation to the electrodes is required to minimize harm to the patient.
DEFIBRILLATION PROTECTION—When used with the GE Medical Systems Information Technologies-recommended accessories, the monitor is protected against the effects of defibrillator discharge. If monitoring is disrupted by the defibrillation, the monitor will recover.
ELECTRICAL SHOCK—To reduce the risk of electrical shock, do not remove monitor cover. Refer servicing to qualified personnel.
ELECTROMAGNETIC INTERFERENCE—Be aware that strong electromagnetic fields may interfere with monitor operation. Interference prevents the clear reception of signals by the monitor. If the hospital is close to a strong transmitter such as TV, AM or FM radio, police or fire stations, a HAM radio operator, an airport, or cellular phone, their signals could be picked up as monitor signals. If you feel interference is affecting the monitor, contact your Service Representative to check the monitor in your environment. Refer to “Electromagnetic Interference” on page 1-9 for additional information.
Revision C |
250cx Series Maternal/Fetal Monitor |
1-5 |
|
2036946-001 |
|

Safety: Monitor Contraindications, Warnings, and Precautions
WARNINGS
ELECTROSURGERY—The monitor is not designed for use with high-frequency surgical devices. In addition, measurements may be affected in the presence of strong electromagnetic sources such as electrosurgery equipment.
EQUIPMENT USE—The use of this equipment is restricted to one patient at a time.
EXPLOSION HAZARD—Do not use this equipment in the presence of flammable anesthetics or inside an oxygen tent.
GROUNDING—Do not defeat the three-wire grounding feature of the power cord by means of adaptors, plug modifications, or other methods. A dangerous shock hazard to both patient and operator may result.
INOPERABLE MECG—The MECG trace is not visible during a MECG LEADS OFF condition or an overload (saturation) of the frontend amplifier during differential input voltage of more than ± 300mV.
INSTRUCTIONS—For continued and safe use of this equipment, it is necessary to follow all listed instructions. However, the instructions provided in this manual in no way supersede established medical procedures concerning patient care. The monitor does not replace observation and evaluation of the patient, at regular intervals, by a qualified care provider who will make diagnoses and decide on treatments and interventions.
INTERFACING OTHER EQUIPMENT—Monitoring equipment must be interfaced with other types of medical equipment by qualified biomedical engineering personnel. Be certain to consult manufacturers’ specifications to maintain safe operation.
LEAKAGE CURRENT TEST—The interconnection of auxiliary equipment with this device may increase the total leakage current. When interfacing with other equipment, a test for leakage current must be performed by qualified biomedical engineering personnel before using with patients. Serious injury or death could result if the leakage current exceeds applicable standards. The use of accessory equipment not complying with the equivalent safety requirements of this equipment may lead to a reduced level of safety of the resulting system. Consideration relating to the choice shall include: use of the accessory in the patient vicinity; and evidence that the safety certification of the accessory has been performed in accordance with the appropriate EN60601.1 and/or EN60601.1.1 harmonized national standard.
LINE ISOLATION MONITOR TRANSIENTS—Line isolation monitor transients may resemble actual cardiac waveforms, and thus cause incorrect heart rate determinations and alarm activation (or inhibition).
1-6 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

Safety: Monitor Contraindications, Warnings, and Precautions
WARNINGS
MRI USE—Do not use the electrodes during MRI scanning; conducted current could potentially cause burns.
PATIENT CABLES AND LEADWIRES—Do not use patient cables and electrode leads that permit direct connection to electrical sources. Use only “safety” cables and leadwires. Use of non-safety patient cables and lead wires creates risk of inappropriate electrical connection which may cause patient shock or death.
PACEMAKER PATIENTS—Rate meters may continue to count the pacemaker rate during occurrences of cardiac arrest or some arrhythmias. Do not rely entirely upon rate meter alarms. Keep pacemaker patients under close surveillance. Refer to Chapter 16, “Troubleshooting” for disclosure of the pacemaker pulse rejection capability of the 250cx Series Monitor.
RF INTERFACE—Known RF sources, such as cell phones, radio or TV stations, and two-way radios, may cause unexpected or adverse operation of this device.
SIMULTANEOUS DEVICES—Do not simultaneously connect more than one device that uses electrodes to detect ECG and/or respiration to the same patient. Use of more than one device in this manner may cause improper operation of one or more of the devices.
STRANGULATION—Make sure all patient cables, leadwires, and tubing are positioned away from the patient’s head to minimize the risk of accidental strangulation.
WATER BIRTHS—Do not use the monitor to directly monitor patients during water births, in whirlpool or submersion water baths, during showers, or in any other situation where the mother is immersed in water. Doing so may result in electrical shock hazard.
EXTERNAL VGA CONNECTIONS—Connect only to GE recommended display. ONLY remove cover plate if external display is used.
TELEMETRY CONNECTIONS—Connect only to GE recommended telemetry systems. Contact your GE service representative for more information.
Revision C |
250cx Series Maternal/Fetal Monitor |
1-7 |
|
2036946-001 |
|

Safety: Monitor Contraindications, Warnings, and Precautions
WARNINGS
COLOR DISPLAY—Certain colors may have limited visibility at a distance. Color-blind individuals may experience this more often.
EXERGEN® TAT-5000™ —Cable assembly 2036641-001 cannot be field serviced. Do NOT attempt any repairs to this assembly. This assembly must be returned to the factory for any repairs. This assembly, as shipped, is important to patient safety.
DISPOSAL—This product consists of devices that may contain mercury, which must be recycled or disposed of in accordance with local, state, or country laws. (Within this system, the backlight lamps in the monitor display contain mercury.)
Cautions
CAUTIONS
STATIC ELECTRICITY—This assembly is extremely static sensitive and should be handled using electrostatic discharge precautions.
ANNUAL SERVICING—For continued safety and performance of the monitor, it is recommended that the calibration, accuracy, and electrical safety of the monitor be verified on an annual basis by a GE Medical Systems Information Technologies Service Representative.
DAILY TESTING—It is essential that the monitor and accessories be inspected every day. It is recommended practice to initiate the monitor’s self-test feature at the beginning of each monitoring session; follow the instructions in Chapter 4, “Setup Procedures”.
ENVIRONMENT—The performance of the monitor has not been tested in certain areas, such as x-ray and imaging suites. The monitor is not recommended for use in these environments.
EQUIPMENT CONFIGURATION—The equipment or system should not be used adjacent to, or stacked with, other equipment. If adjacent or stacked use is necessary, the equipment or system should be tested to verify normal operation in the configuration in which it is being used.
PERFORMANCE—Report all problems experienced with the monitor. If the monitor is not working properly, contact your Service Representative for service. The monitor should not be used if it is not working properly.
1-8 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

Safety: Monitor Contraindications, Warnings, and Precautions
Electromagnetic Interference
This device has been tested and found to comply with the Medical Electrical Equipment-General Requirements for Safety-Collateral Standard: Electromagnetic Compatibility, EN60601-1-2:2001, Medical Device Directive 93/42/EEC. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation.
However, because of the proliferation of radio-frequency transmitting equipment and other sources of electrical noise in the health-care and home environments (e.g. cellular phones, mobile two-way radios, electrical appliances), it is possible that high levels of such interference due to proximity or strength of a source, may result in disruption of performance of this device.
Refer to the Electromagnetic Immunity information in this product’s service manual for EN 60601-1-2 (2001) compliance information and safety information for this product.
This equipment generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with these instructions, may cause harmful interference with other devices in the vicinity. Disruption or interference may be evidenced by erratic readings, cessation of operation, or incorrect functioning. If this occurs, the use site should be surveyed to determine the source of this disruption, and actions should be taken to eliminate the source.
The user is encouraged to try to correct the interference by one or more of the following measures:
Turn equipment in the vicinity off and on to isolate the offending equipment.
Reorient or relocate the other receiving device.
Increase the separation between the interfering equipment and this equipment.
If assistance is required, contact your GE Medical Systems Service Representative.
Revision C |
250cx Series Maternal/Fetal Monitor |
1-9 |
|
2036946-001 |
|
Safety: Equipment Symbols
Equipment Symbols
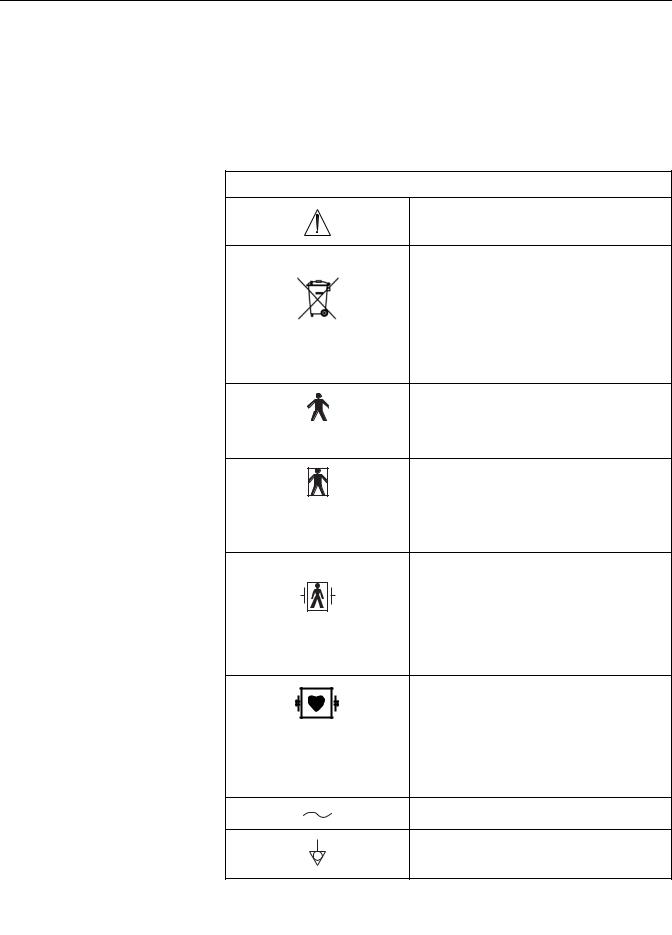
The following is a list of symbols used on products manufactured by GE Medical
Systems Information Technologies. Some symbols may not appear on your unit.
Equipment Symbols
ATTENTION: Consult accompanying documents.
WASTE OF ELECTRICAL AND ELECTRONIC EQUIPMENT (WEEE): This symbol indicates that the waste of electrical and electronic equipment must not be disposed as unsorted municipal waste and must be collected separately. Please contact an authorized representative of the manufacturer for information concerning the decommissioning of your equipment.
TYPE B EQUIPMENT: Type B equipment is suitable for intentional external and internal application to the patient, excluding direct cardiac application.
TYPE BF EQUIPMENT: Type BF equipment is suitable for intentional external and internal application to the patient, excluding direct cardiac application. Type BF equipment has an F-type applied part.
DEFIBRILLATOR-PROOF TYPE BF EQUIPMENT: Type BF equipment is suitable for intentional external and internal application to the patient, excluding direct cardiac application. Type BF equipment is type B equipment with an F-type isolated (floating) part. The paddles indicate the equipment is defibrillator proof.
TYPE CF EQUIPMENT: Type CF equipment is suitable for intentional external and internal application to the patient including direct cardiac application. Type CF equipment is F-type applied part that provides a higher degree of protection against electric shock than that provided by Type BF applied parts.
ALTERNATING CURRENT (AC).
EQUIPOTENTIALITY.
1-10 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

Safety: Equipment Symbols
|
Equipment Symbols |
|
|
|
|
O |
|
POWER OFF: disconnection from the mains. |
|
|
|
I |
|
POWER ON: connection to the mains. |
|
|
|
|
|
VGA connection. |
|
|
|
Revision C |
250cx Series Maternal/Fetal Monitor |
1-11 |
|
2036946-001 |
|

Safety: Equipment Symbols
1-12 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|

2 Introduction
Revision C |
250cx Series Maternal/Fetal Monitor |
2-1 |
|
2036946-001 |
|

Introduction:
For your notes
2-2 |
250cx Series Maternal/Fetal Monitor |
Revision C |
|
2036946-001 |
|
 Loading...
Loading...