Erbe Vio 200 S User manual

VIO 200 S
User Manual
04.07
V 1.2.x
ERBE


VIO 200 S
User Manual

Dr. Jürgen Forster
Technical Editor
Tel.: (+ 49) 70 71 75 52 46
E-mail: jforster@erbe-med.de
I shall be very pleased if this User Manual can make your work easier and help you to use all the functions of your unit safely.
It has been produced with great care by me in collaboration with development engineers and quality personnel. The use of
publishing software and digital photography provided the documentation team with layout flexibility. Our object was to
achieve a clear combination of text and photographs. The translation of the text into your language is subjected to a strict
quality control. The User Manual is digitally printed only on delivery of your machine. All information is up to date. The
ERBE documentation team would like to improve its products for your benefit, and I would therefore be very pleased to receive suggestions, criticism and questions as well as positive comments.
EN ISO 9001 EN ISO 13485
User Manual Art. No. 80104-951
All rights to this User Manual, in particular rights of duplication, dissemination and translation, are reserved. No part of this
User Manual may be reproduced in any form (by photocopying, microfilming or other methods) or processed, duplicated or
disseminated by the use of electronic systems without the written consent of ERBE Elektromedizin GmbH.
The information contained in this User Manual can be changed or expanded without prior notice and without obligation on
the part of ERBE Elektromedizin GmbH.
Printed by ERBE Elektromedizin
Printed in Germany
Copyright © ERBE Elektromedizin GmbH, Tübingen 2007

Table of Contents
Chapter Title Page
Table of Contents
1 Safety Instructions ......................................................................... 9
Intended use .............................................................................................................9
Combination with other equipment .........................................................................9
Safety notations........................................................................................................9
Meaning of the note ................................................................................................. 9
Who must read this User Manual?.........................................................................10
Compliance with safety information......................................................................10
Structure of safety instructions .............................................................................. 10
Operating errors by persons without training ........................................................11
Risks due to the environment................................................................................. 11
Electric shock......................................................................................................... 12
Fire / explosion ...................................................................................................... 13
Burns ......................................................................................................................15
Risks due to incorrect use of the neutral electrode ................................................19
Defective unit......................................................................................................... 20
Interference caused by the unit ..............................................................................20
Damage to the unit and accessories ....................................................................... 22
Notes ...................................................................................................................... 23
Art. No. 80104-951
04 / 2007
2 Safety Features............................................................................. 25
NESSY...................................................................................................................25
How do I receive information about the safety status of the neutral electrode?.... 26
Automatic monitoring of equipment output error..................................................29
Automatic monitoring of the ON time................................................................... 29
Protection from operating errors............................................................................ 30
3 Description of the Controls......................................................... 31
Controls on the front panel .................................................................................... 31
Controls on the back ..............................................................................................33
4 Working with the Electrosurgical Unit: a Tutorial .................... 35
Re this chapter........................................................................................................35
Setting options for the unit..................................................................................... 36
Factory unit settings............................................................................................... 37
Operation of the unit using Focus, Selection and Plus/Minus buttons ..................38
Connect unit and switch on....................................................................................42
Select/switch program............................................................................................ 43
Connect footswitches, instruments and neutral electrodes ....................................45
Using the unit (cutting/coagulation) ......................................................................49
Amend and save program settings ......................................................................... 52
Create new program............................................................................................... 61
5 / 120

Table of Contents
Rename program ....................................................................................................62
Delete program .......................................................................................................64
Amend setup settings..............................................................................................65
Amend service settings...........................................................................................66
Procedure with errors shown on unit display .........................................................67
5 Description of socket hardware..................................................69
Purchasing further receptacles................................................................................69
Sockets for different modes and instrument connectors.........................................69
Monopolar socket ...................................................................................................69
Bipolar socket.........................................................................................................71
Socket for patient plate...........................................................................................72
6 Monopolar Modes.........................................................................73
AUTO CUT ............................................................................................................73
ENDO CUT Q ........................................................................................................75
ENDO CUT I..........................................................................................................76
SOFT COAG ..........................................................................................................77
FORCED COAG....................................................................................................79
7 Bipolar Modes............................................................................... 83
BIPOLAR SOFT COAG........................................................................................83
8 APC socket (only available with the APC module).................... 87
APC socket .............................................................................................................87
9 APC Modes and Argon-Assisted Modes
(only available with APC module) ............................................... 89
FORCED APC........................................................................................................89
Argon-assisted AUTO CUT Mode ........................................................................91
Argon-assisted SOFT COAG mode ......................................................................93
Argon-assisted FORCED COAG Mode ...............................................................95
10 Installation..................................................................................... 99
Ambient conditions ................................................................................................99
Electrical installation............................................................................................100
Install electrosurgical unit on overhead support...................................................102
Installing the unit on an ERBE equipment cart....................................................103
11 Cleaning and Disinfection ......................................................... 105
Safety Instructions................................................................................................105
Wipe disinfection..................................................................................................106
Instructions for cleaning and disinfection ............................................................106
Art. No. 80104-951
04 / 2007
6 / 120
12 Status Messages, Error Messages ........................................... 107
Status messages ....................................................................................................107
Operating error messages .....................................................................................108
System error messages .........................................................................................109
13 General Technical Data..............................................................111

Table of Contents
14 Information on electromagnetic compatibility (EMC) ............. 113
Guidelines for avoiding, recognising and rectifying unwanted
electromagnetic effects on other equipment or systems, which
are the result of operating the VIO system. ......................................................... 113
15 Maintenance, Customer Service, Warranty, Disposal ............ 119
Maintenance.........................................................................................................119
Customer service..................................................................................................119
Warranty............................................................................................................... 119
Disposal................................................................................................................ 120
Art. No. 80104-951
04 / 2007
7 / 120

Table of Contents
Art. No. 80104-951
04 / 2007
8 / 120
CHAPTER 1
Safety Instructions
1 • Safety Instructions
Intended use
The VIO 200 S is an electrosurgical unit for cutting and coagulation. Thanks to its
performance features it offers universal applications.
Combination with other equipment
You can combine this unit with matching ERBE equipment: e.g. APC 2, EIP 2. You
will then have a well-conceived, coordinated system.
Safety notations
Art. No. 80104-951
DANGER
indicates an imminently hazardous situation which, if not avoided,
will result in death or serious injury.
WARNING
indicates a potentially hazardous situation which, if not avoided,
could result in death or serious injury.
04 / 2007
indicates a potentially hazardous situation which, if not avoided,
may result in minor or moderate injury.
CAUTION
CAUTION
used without the safety alert symbol indicates a potentially hazardous situation which, if not avoided, may result in property damage.
Meaning of the note
"Note:"
Refers a) to manufacturer's information that relates directly or indirectly to the safety of people or protection of property. The information does not relate directly to a
risk or dangerous situation.
Refers b) to manufacturer's information that is important or useful for operating or
servicing the unit.
9 / 120

1 • Safety Instructions
Who must read this User Manual?
Knowledge of the User Manual is absolutely essential for correct operation of the
unit.
Therefore everyone who is concerned with
• preparing,
• adjusting,
• operating,
• disassembling, as well as
• cleaning and disinfecting
the unit must read the User Manual.
Please pay particular attention to the safety instructions in each chapter.
Compliance with safety information
Working with medical equipment is associated with certain risks to patients, medical personnel and the environment. Risks cannot be entirely eliminated by design
measures alone.
Safety does not depend soley on the equipment. Safety depends to a large extent on
the training of medical personnel and correct operation of the equipment.
The safety instructions in this chapter must be read, understood and applied by everyone who is working with the equipment.
Structure of safety instructions
The safety instructions are structured according to the following risks:
• Operating errors by persons without training
• Risks due to the environment
• Electric shock
• Fire / explosion
• Burns
• Risks due to incorrect use of the neutral electrode
• Defective unit
• Interference caused by the unit
• Damage to the unit and accessories
•Notes
Art. No. 80104-951
04 / 2007
10 / 120

1 • Safety Instructions
Operating errors by persons without training
WARNING
Operating errors by persons without training
Persons without training can operate the unit incorrectly.
Risk of injury or death for patients and medical staff! Risk of damage to property.
¨ The equipment may only be used by persons who have been
trained on how to use it properly according to this User Manual.
¨ Training may only be carried out by persons who are suitable on
the basis of their knowledge and practical experience.
¨ In the event of uncertainties or if you have any questions, please
contact ERBE Elektromedizin. You will find the addresses in
the address list at the end of this User Manual.
Risks due to the environment
Art. No. 80104-951
CAUTION
Interference with the unit by portable and mobile HF communication devices (e.g. mobile phones, WLAN equipment)
Electromagnetic waves emitted by portable and mobile HF communication devices can effect the unit.
The unit may fail or not perform properly.
¨ Please see the table "Recommended separation distances be-
tween portable and mobile HF communications equipment and
the equipment" at the end of this User Manual.
04 / 2007
Unsuitable temperature or level of humidity during operation
If you operate the equipment at an unsuitable temperature or level
of humidity, it may sustain damage, fail, or not perform properly.
¨ Operate the equipment at a suitable temperature and level of hu-
midity. You will find the tolerances for temperature and humidity in the Technical Data.
¨ If other ambient conditions have to be observed for operation of
the equipment, you will also find them in the Technical Data.
CAUTION
CAUTION
Unsuitable temperature or humidity in transit or storage
If you transport or store the equipment at an unsuitable temperature
or level of humidity, it may sustain damage and fail.
¨ Transport and store the equipment at a suitable temperature and
level of humidity. You will find the tolerances for temperature
and humidity in the Technical Data.
¨ If other ambient conditions have to be observed for transport
and storage of the equipment, you will also find them in the
Technical Data.
11 / 120

1 • Safety Instructions
CAUTION
Insufficient acclimatization time, unsuitable temperature during acclimatization
If the device was stored or transported below or above a certain
temperature, it will take a certain time and temperature to acclimatize.
If do not observe the rules, the device can sustain damage and fail.
¨ Acclimatize the device according to the rules in the Technical
Data.
CAUTION
Overheating of the device due to poor ventilation
If ventilation is poor, the device can overheat, sustain damage, and
fail.
¨ Install the device in such a way that there is an unobstructed cir-
culation of air around the housing. Installation in confined wall
recesses is prohibited.
CAUTION
Penetration of liquid into the device
The housing is not absolutely watertight. If liquid penetrates, the
device can sustain damage and fail.
¨ Make sure no liquid can penetrate the device.
¨ Do not place vessels containing liquids on top of the device.
Electric shock
WARNING
Defective grounded power outlet, inferior-quality power cord,
incorrect line voltage, multiple power outlets, extension cords
Risk of electric shock and other injuries to the patient and medical
personnel! Risk of damage to property.
¨ Connect the unit / the equipment cart to a properly installed
grounded power outlet.
¨ Only use the ERBE power cord or an equivalent power cord for
this purpose. The power cord must bear the applicable national
test symbol.
¨ Check the power cord for damage. You must not use a damaged
power cord.
¨ The supply voltage must match the voltage specified on the
unit's rating plate.
¨ Do not use multiple power outlets.
¨ Do not use extension cords.
Art. No. 80104-951
04 / 2007
12 / 120
1 • Safety Instructions
WARNING
Incorrect line fuse, defective device
Risk of electric shock to the patient and medical personnel! Risk of
damage to property.
¨ Blown line fuses may only be replaced by a competent techni-
cian. Only replacement fuses that have the same rating as the
one specified on the unit’s rating plate may be used.
¨ When a fuse has been changed, the function of the unit must be
verified. If the unit does not function properly or there are any
concerns, please contact ERBE.
WARNING
Connection of unit / equipment cart and power supply during
cleaning and disinfection
Risk of electric shock to the medical personnel!
¨ Switch off the device. Unplug the power cord of the device/
equipment cart.
Art. No. 80104-951
Fire / explosion
In electrosurgery electric sparks and arcs occur at the instrument. Flammable gases,
vapours, and liquids can be set alight or caused to explode.
DANGER
Flammable anesthetics
Risk of explosion to the patient and medical personnel! Risk of
damage to property.
04 / 2007
¨ Do not use flammable anesthetics when an operation is being
performed on the head or thorax.
¨ If use is unavoidable, you must extract the anesthetics before
performing electrosurgery.
WARNING
Flammable gas mixture in TUR (Transurethral Resection) and
TCR (Transcervical Endometrial Resection)
Hydrogen and oxygen can ascend into the roof of the bladder, the
upper part of the prostate, and the upper part of the uterus. If you
resect into this gas mixture, it could combust.
Risk of combustion to the patient!
¨ Allow the gas mixture to escape through the resectoscope
sheath.
¨ Do not resect into the gas mixture.
DANGER
Flammable endogenous gases in the gastrointestinal tract
Risk of explosion to the patient!
¨ Extract the gases before performing electrosurgery or irrigate
with CO
.
2
13 / 120

1 • Safety Instructions
DANGER
Combustion-supporting gases, e.g. oxygen, nitrous oxide
The gases can accumulate in materials like cotton wool or gauze.
The materials become highly flammable.
Risk of fire to the patient and medical personnel! Risk of damage to
property.
¨ Do not use combustion-supporting gases when an operation is
being performed on the head or thorax.
¨ If use is unavoidable, you must extract the combustion-support-
ing gases before performing electrosurgery.
¨ Remove any jeopardized (e.g. cotton wool or gauze) materials
before performing electrosurgery.
¨ Check the oxygen-carrying tubes and connections for leaks.
¨ Check the endotracheal tubes and their cuffs for leaks.
¨ Before using argon plasma coagulation (APC) in the tracheo-
bronchial system it is absolutely essential that you observe the
specific safety information and instructions in the User Manual
for the argon plasma unit!
WARNING
Active or hot instruments in contact with combustible materials
Materials like gauze, swabs, and cloths can catch fire.
Risk of fire to the patient and medical personnel! Risk of damage to
property.
¨ Do not bring active or hot instruments into contact with com-
bustible materials.
¨ Put instruments down in a safe place: sterile, dry, non-conduc-
tive, and easy to see. Instruments that have been put down must
not come into contact with the patient, medical personnel, or
combustible materials.
WARNING
Flammable detergents and disinfectants, flammable solvents
in adhesives used on the patient and on the device / equipment cart
Risk of fire and explosion to the patient and medical personnel!
Risk of damage to property.
¨ Use products that are not flammable.
If the use of flammable products is unavoidable, proceed as follows:
¨ Allow the products to evaporate completely before switching on
the device.
¨ Check whether flammable liquids have accumulated under the
patient, in body recesses such as the navel, or in body cavities
such as the vagina. Remove any liquids before performing electrosurgery.
Art. No. 80104-951
04 / 2007
14 / 120
1 • Safety Instructions
WARNING
Ignition of anesthetics, skin cleansers, and disinfectants in
potentially explosive atmospheres
If you place the device in a potentially explosive atmosphere, anesthetics, skin cleansers, and disinfectants can ignite.
Risk of fire and explosion to the patient and medical personnel!
Risk of damage to property.
¨ Do not place the device in potentially explosive atmospheres.
Burns
WARNING
Damaged device, damaged accessories, modified device, and
modified accessories
Risk of burns and injury to the patient and medical personnel! Risk
of damage to property.
¨ Check the device and accessories for damage every time before
using them (e.g. footswitch, cords of instruments and the neutral
electrode, equipment cart).
¨ You must not use damaged equipment or damaged accessories.
Exchange defective accessories.
¨ If the equipment or equipment cart is damaged, please contact
our customer service.
¨ For your safety and that of the patient: Never attempt to perform
repairs or make modifications yourself. Any modification will
invalidate liability on the part of ERBE Elektromedizin GmbH.
Art. No. 80104-951
04 / 2007
WARNING
HF leakage current flows through metal parts
The patient must not have contact with electrically conductive objects. That includes metal parts of the operating table, for example.
HF current can be discharged through points of contact accidentally (HF leakage current).
Risk of burns to the patient!
¨ Position the patient on dry, antistatic drapes.
¨ If the drapes can become wet during the operation due to sweat,
blood, irrigation liquid, urine, etc., lay a waterproof sheet over
the drapes.
WARNING
HF leakage current flows through monitoring electrodes
HF current can be discharged through points of contact between the
skin and monitoring electrodes accidentally (HF leakage current).
Risk of burns to the patient!
¨ Position monitoring electrodes as far away as possible from the
surgical field (area where electrosurgical instruments are used).
¨ Do not use needle electrodes for monitoring during electrosur-
gery.
15 / 120
1 • Safety Instructions
¨ Where possible, use monitoring electrodes that contain devices
to limit high-frequency current.
WARNING
HF leakage current flows through skin-to-skin points of contact
HF current can be discharged through skin-to-skin points of contact
accidentally (HF leakage current).
Risk of burns to the patient!
¨ Prevent skin-to-skin points of contact. For example, lay dry
gauze between the patient's arms and body.
WARNING
Unintentional activation of the instrument
Risk of burns to the patient and medical personnel!
¨ Put instruments down in a safe place: sterile, dry, non-conduc-
tive, and easy to see. Instruments that have been put down must
not come into contact with the patient, medical personnel, or
combustible materials.
¨ Instruments that have been put down must not come into contact
with the patient, not even indirectly. An instrument can come
into contact with the patient indirectly through electrically conductive objects or wet drapes, for example.
CAUTION
Hot instruments
Even non-active instruments that are still hot can burn the patient or
medical personnel.
¨ Put instruments down in a safe place: sterile, dry, non-conduc-
tive, and easy to see. Instruments that have been put down must
not come into contact with the patient, medical personnel, or
combustible materials.
¨ Instruments that have been put down must not come into contact
with the patient, not even indirectly. An instrument can come
into contact with the patient indirectly through electrically conductive objects or wet drapes, for example.
WARNING
Unintentional activation of the instrument during an endoscopic application
If the instrument is activated and remains activated during an endoscopic application, the patient can suffer burns when the instrument
is removed.
All points that come into contact with the active part of the instrument are at risk. The cause of unintentional activation can be a fault
in the footswitch or device or operator error, for example.
You will recognize unintentional activation from the continuous activation signal.
Risk of burns to the patient!
Art. No. 80104-951
04 / 2007
16 / 120
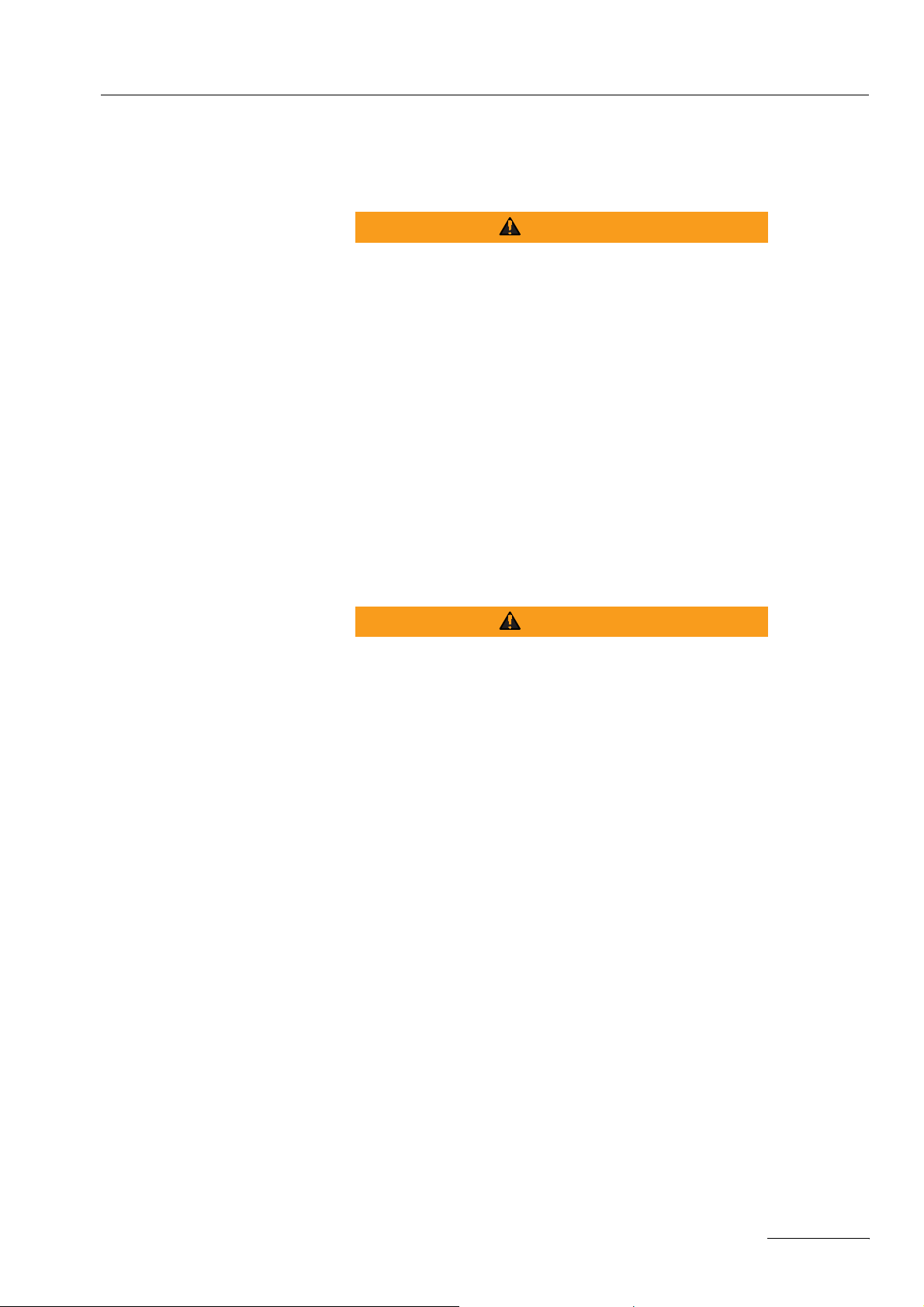
1 • Safety Instructions
¨ Turn off the power switch on the electrosurgical unit immedi-
ately. Only then should the instrument be removed from the patient’s body.
WARNING
Capacitive coupling between the cords of two instruments
When one instrument is activated, current can be transferred to the
cord of another instrument (capacitive coupling).
The patient can suffer burns if the non-active but still live instrument has direct or indirect contact with the patient.
Risk of burns to the patient!
¨ Lay the cords of instruments in such a way that they are as far
apart as possible.
¨ Put instruments down in a safe place: sterile, dry, non-conduc-
tive, and easy to see.
¨ Instruments that have been put down must not come into contact
with the patient, medical personnel, or combustible materials.
¨ Instruments that have been put down must not come into contact
with the patient, not even indirectly. An instrument can come
into contact with the patient indirectly through electrically conductive objects or wet drapes, for example.
Art. No. 80104-951
WARNING
Power setting too high, ON time too long, effects too high
The higher the power setting the longer the ON time of the unit and
the higher the effect the higher the risk of accidental tissue damage.
Risk of accidental tissue damage to the patient!
04 / 2007
¨ Set power as low as possible relative to the required surgical ef-
fect.
¨ Activate the unit for as short a time as possible relative to the re-
quired surgical effect.
¨ The temperature at the neutral electrode site increases during
long and continuous activations; therefore, ensure that the cooling phases between activations are sufficient.
¨ Set effect as low as possible relative to the required surgical ef-
fect.
¨ If you are unable to achieve a surgical effect with a power set-
ting / ON time / effect level that is sufficient judging from experience, this can be due to a problem with the electrosurgical unit
or accessories:
¨ Check the instrument for soiling with insulating tissue rem-
nants.
¨ Check the neutral electrode to make sure it is secure.
¨ Check the connectors on all cords to make sure they are secure.
17 / 120
1 • Safety Instructions
WARNING
Activation of the unit with no knowledge of active settings
If the user does not understand the active settings of the unit, he can
cause the patient accidental tissue damage.
¨ Check the active settings on the display of the unit, after:
switching on the unit, connecting up an instrument, and changing the program.
WARNING
The user was not informed of a change in maximum ON time
Risk of accidental tissue damage to the patient!
¨ All users must be informed of any change in maximum ON time
at an early stage. That is, before the user works with the modified maximum ON time for the first time.
¨ The temperature at the neutral electrode site increases during
long and continuous activations; therefore, ensure that the cooling phases between activations are sufficient.
WARNING
Tissue structures / vessels with a cross-section that is small
or becoming smaller
If monopolar HF current flows through parts of the body with a relatively small cross-section, there is a risk of unintentional coagulation for the patient!
¨ If possible, use the bipolar coagulation technique.
WARNING
Activation signal not audible
You do not hear the signal when the electrosurgical unit is activated.
Risk of burns to the patient and medical personnel!
¨ Adjust the activation signal so that it is clearly audible.
WARNING
Undesirable contact between the active instrument and metal
objects in the patient's body
Contact with metal hemostats, etc.
Risk of burns to the patient!
¨ Do not touch metal objects (e.g. implants) in the patient's body
with the active instrument.
Art. No. 80104-951
04 / 2007
18 / 120
CAUTION
A hand-held metal instrument is touched with the active
instrument (electrode)
Risk of hand burns!
¨ Such practice is not recommended. The risk of burns can not be
ruled out.
1 • Safety Instructions
Risks due to incorrect use of the neutral electrode
WARNING
Positioning the neutral electrode above the heart
Risk of ventricular fibrillation and cardiac arrest for the patient!
¨ Do not position the neutral electrode over the heart or in the re-
gion of the heart.
WARNING
Incorrect application of the neutral electrode
Risk of burns to the patient!
¨ Apply the entire contact surface of the neutral electrode to a
muscular part of the body with good blood circulation.
¨ Apply the neutral electrode as close as possible to the surgical
site.
¨ Insert the contact tab of the neutral electrode completely into the
connecting clamp. The contact tab must not touch the patient's
skin. (For reusable cord with disposable pads only.)
¨ Align the symmetry line of the neutral electrode towards the op-
erating field. The current should flow from the active electrode
(instrument) to the symmetry line of the neutral electrode (Fig.
1-1).
¨ Check the neutral electrode regularly, for good contact.
¨ Check the neutral electrode especially when the patient has been
repositioned and after surgical steps where the device was activated frequently and for a long time.
Art. No. 80104-951
04 / 2007
Fig. 1-1
WARNING
Short circuit in the connecting cord or in the clip of a dual surface neutral electrode
With the NESSY setting "NE: either way" setup and a short circuit
in the connecting cord or in the clip of a dual surface neutral electrode the device can no longer monitor the contact with the patient's
skin or the application direction of the contact surface. You will not
receive a warning if the electrodes becomes detached from the skin.
You will not receive a warning if the application direction of the
neutral electrode is incorrect.
Risk of burns to the patient!
19 / 120
1 • Safety Instructions
¨ To rule out the possibility of a short circuit in the connecting
cord and the clip before use, see Chapter 2 of this Manual "Safety Features" for NESSY.
Note: ERBE recommends the use of split return electrodes in combination with the
NESSY setting set to “NE: dynamic” or “NE: dual surface”. With this combination
the optimal use of the safety monitoring functions are given (see chapter 2 “NESSY
Safety Features). If the unit is activated in a monopolar mode using a cable with a
short, the unit will give an audible warning signal and will display a "B-B" error
message on the screen.
Defective unit
WARNING
Undesirable rise in output level due to failure of electrosurgical unit
Risk of accidental tissue damage to the patient!
¨ Observe the error message: "Activation has been interrupted". If
the display shows this message a second time, please inform
Technical Service.
WARNING
Routine safety testing not being done
Risk of injury or death for patients and medical staff! Risk of damage to property.
¨ Have the device checked for safety at least once a year.
¨ You must not use a device that is not safe.
WARNING
Failure of display elements
If display elements fail, you can no longer operate the device safely.
Risk of injury or death for patients and medical staff!
¨ You must not use the unit.
Interference caused by the unit
WARNING
Interference with cardiac pacemakers, internal defibrillators,
or other active implants
Activation of the electrosurgical unit may affect the performance of
active implants or damage them.
Risk of injury or death for patients!
¨ In the case of patients having active implants, consult the man-
ufacturer of the implant or the competent department of your
hospital prior to performing surgery.
¨ Do not position the neutral electrode near cardiac pacemakers,
internal defibrillators, or other active implants.
Art. No. 80104-951
04 / 2007
20 / 120
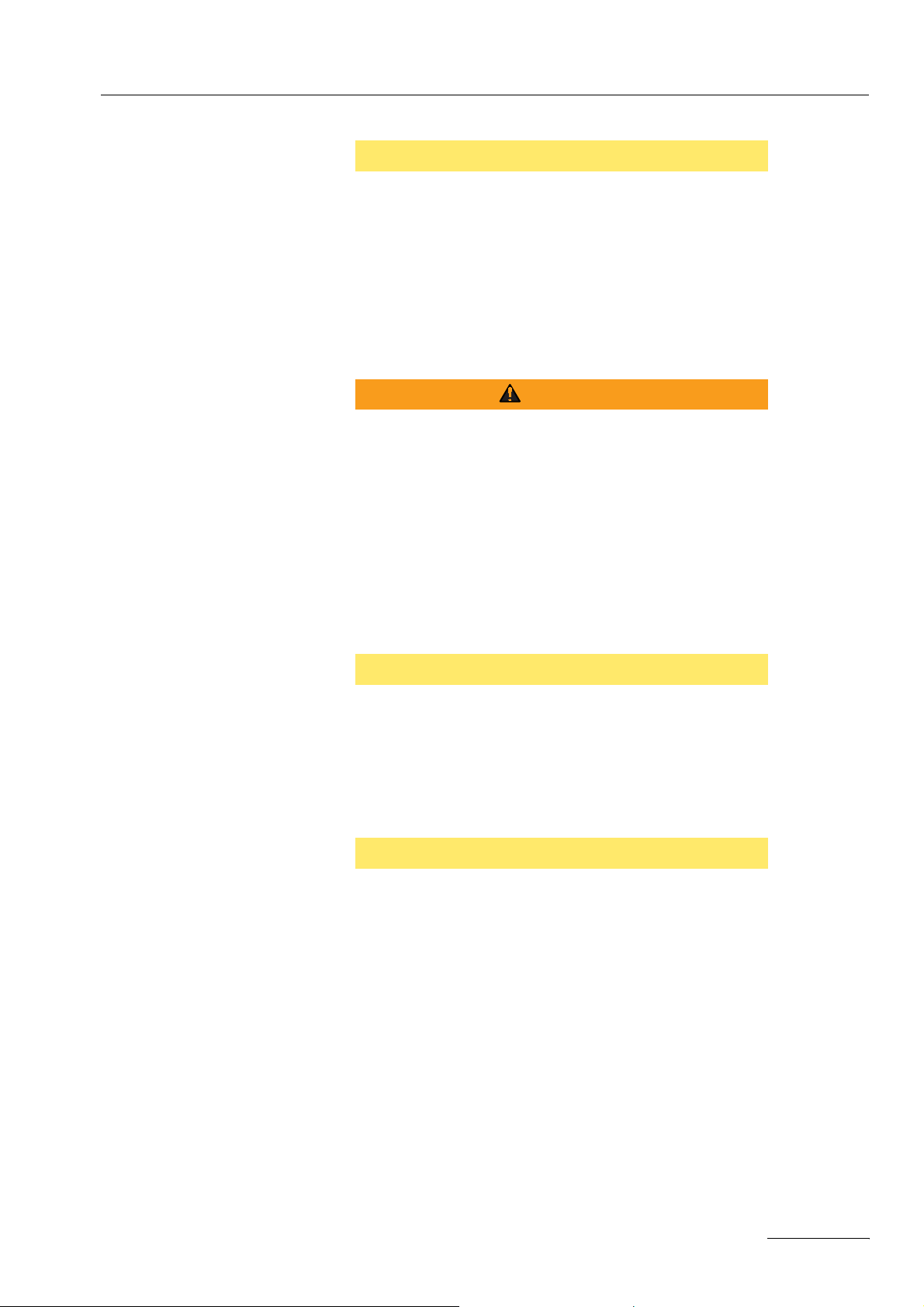
1 • Safety Instructions
CAUTION
Interference with electronic equipment due to the electrosurgical unit
The activated electrosurgical unit can affect the performance of
electronic equipment by causing interference.
¨ Position the electrosurgical unit, the cords of the instruments,
and the cord of the neutral electrode as far away as possible from
electronic equipment.
¨ Position the cords as far away as possible from the cords of elec-
tronic equipment.
WARNING
Low-frequency currents stimulate nerves and muscles (Neuromuscular Stimulation)
Low-frequency currents arise either due to low-frequency power
sources or partial rectification of the HF current. During cutting
procedures, forced coagulation and spray coagulation, the unavoidable electric arcs between an active electrode and the tissue have the
effect that a portion of the high-frequency alternating current is rectified. Spasms or muscle contractions can occur.
Risk of injury to the patient.
¨ Set effect as low as possible relative to the required surgical ef-
fect.
Art. No. 80104-951
CAUTION
Use of non-approved internal cables by Technical Service
04 / 2007
This can result in the increased emission of electromagnetic waves
or reduce the immunity of the device.
The unit may fail or not perform properly.
¨ Technical Service may only use the internal cables that are list-
ed in the service manual for the device.
CAUTION
Stacked devices
If you stack the device next to other equipment or with other equipment, the devices can affect each other.
The unit may fail or not perform properly.
¨ The device may only be stacked next to or with VIO series units.
¨ If it is necessary to operate the device near other equipment or
stacked together with other equipment, check whether the devices are affecting each other: Are the devices behaving unusually? Do errors occur?
21 / 120
1 • Safety Instructions
Damage to the unit and accessories
CAUTION
Electric load on instrument too high
The instrument can be damaged.
¨ The ERBE instructions for use of the instruments indicate the
maximum electrical capacity of the instrument. Check that the
instrument is suitable for the required mode, the required power
limitation, and the required effect. This can be done with the
help of the performance diagrams for each mode.
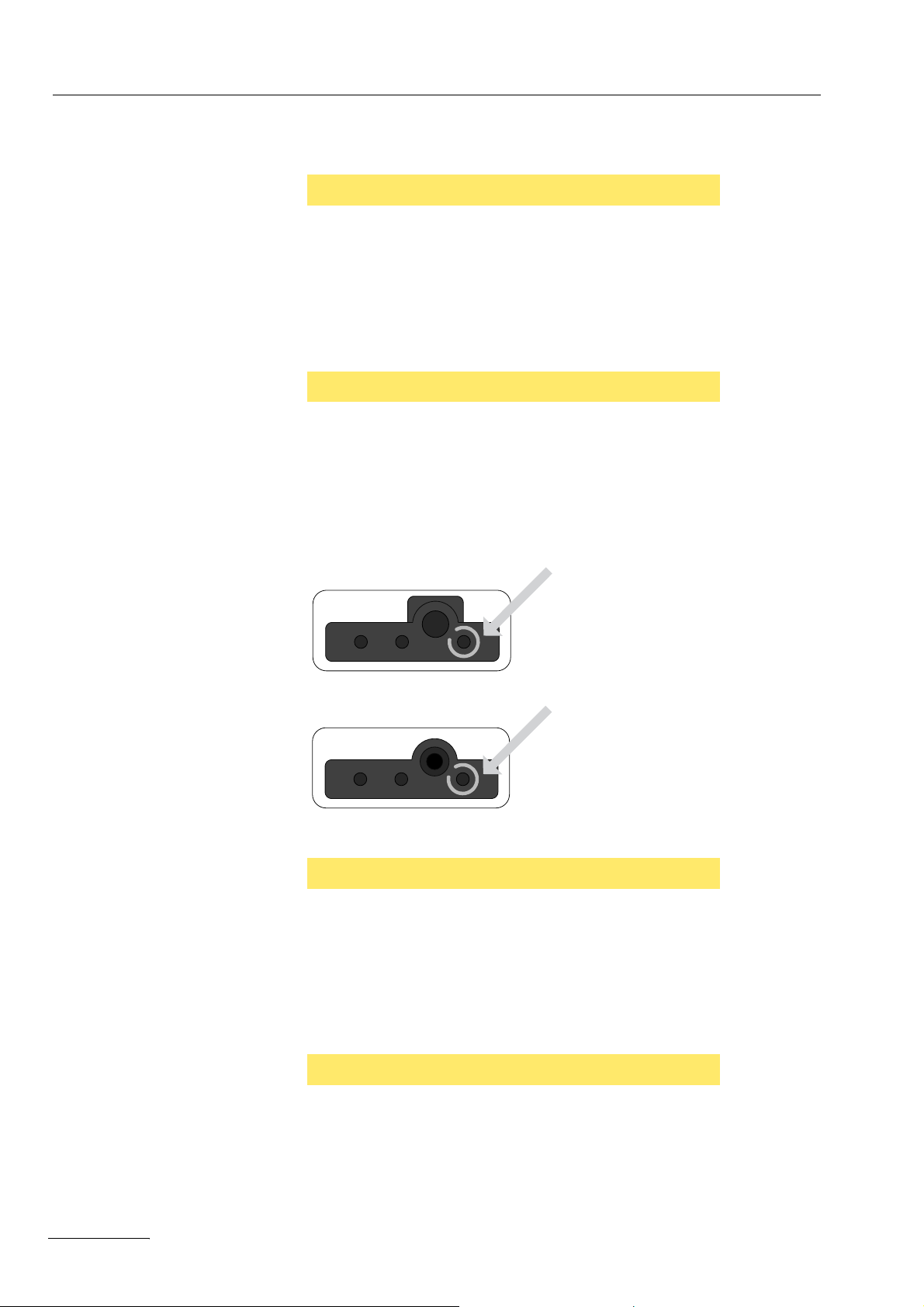
CAUTION
Mix-up of receptacles on monopolar receptacle modules
20140-622, 20140-623
If the receptacles are mixed up, the unit will be damaged.
¨ If you use a connecting cord with a monopolar 4 mm dia. con-
nector, you may only plug the connector into the receptacle with
the blue ring. The correct receptacle is marked with an arrow on
the illustration.
Fig. 1-2
CAUTION
Very long activation cycles without cooling phases
The electrosurgical unit is designed and tested for a relative ON
time of 25 % (conforming to IEC 60601-2-2). If you perform very
long activation cycles without appropriate cooling phases, the unit
can be damaged.
¨ Keep to the 25 % relative ON time (see also Technical Data, Op-
erating Mode). If you operate the unit for a lengthy period.
CAUTION
Alcohol-based spray disinfectant for fast disinfection
With membrane keyboards and paint surfaces there is the risk of
cracks. Propanol and ethanol will erode surfaces.
¨ Do not use these substances.
Art. No. 80104-951
04 / 2007
22 / 120

1 • Safety Instructions
CAUTION
Alternate use of disinfectant solutions based on different
active ingredients
A color reaction may occur with plastics.
¨ Do not use these substances alternately.
Notes
Art. No. 80104-951
Grounding
Note: If necessary, the equipment can be connected to the external grounding system of the room with the grounding pin on the back of the unit and/or Cart using a
connecting cable designed for this purpose. Affects of low frequency leakage currents due to a defective grounding system within the room may be eliminated
through external grounding.
Use of a defibrillator
Note: The equipment conforms to the requirements of Type CF and is protected
against the effects of a defibrillator discharge.
Membrane keyboards
Note: If alcohol-based disinfectants are used on units with membrane keyboards,
this remove the anti-glare finish. However, the user surfaces remain fully functional.
04 / 2007
23 / 120

1 • Safety Instructions
Art. No. 80104-951
04 / 2007
24 / 120
CHAPTER 2
Safety Features
2 • Safety Features
NESSY
What is NESSY?
The NESSY settings
1 Dual surface NE / "NE: Dynamic"
Art. No. 80104-951
04 / 2007
setting
2 Dual surface NE / "NE: Dual sur-
face" setting
The unit is equipped with a Neutral Electrode Safety System (NESSY), which monitors the neutral electrode, warns of critical situations, and thus prevents burns. How
effective the monitoring is depends on whether you choose a single surface or dual
surface neutral electrode and on the NESSY setting.
On delivery, the unit is set to Neutral electrode: Dynamic. To utilize this setting, you
require a dual surface neutral electrode.
In the unit's service programs, a technician can carry out various NESSY settings
according to your requirements. The following table shows you what effects the settings will have on the safety of monitoring.
• You will see the safety level in the first column. 1 = highest safety level.
• In the second column you can see the combination of neutral electrode (NE) /
setting in the service programs.
• In columns 3 - 6 you can see what safety level NESSY offers with various combinations.
Unit - NE connection
z z z z
z z z
Skin - NE
contact
NE application direction
Higher safety for patients
with low skin resistance
3 Dual surface NE / "NE: Either
way" setting
4 Single surface NE / "NE: Either
way" setting
4 Single surface NE / "NE: Single
surface" setting
Short circuit in the connecting
cord or in the clip of a dual sur-
face neutral electrode with the
NESSY setting "NE: Either way"
setup
z Partial,
observe warning
z
z
With the NESSY setting NE: Either way setup and a short circuit in the connecting
cord or in the clip of a dual surface neutral electrode the device can no longer monitor the contact with the patient's skin or the application direction of the contact surface. You will not receive a warning if the electrodes becomes detached from the
skin. You will not receive a warning if the application direction of the neutral electrode is incorrect.
Partial,
observe warning
25 / 120

2 • Safety Features
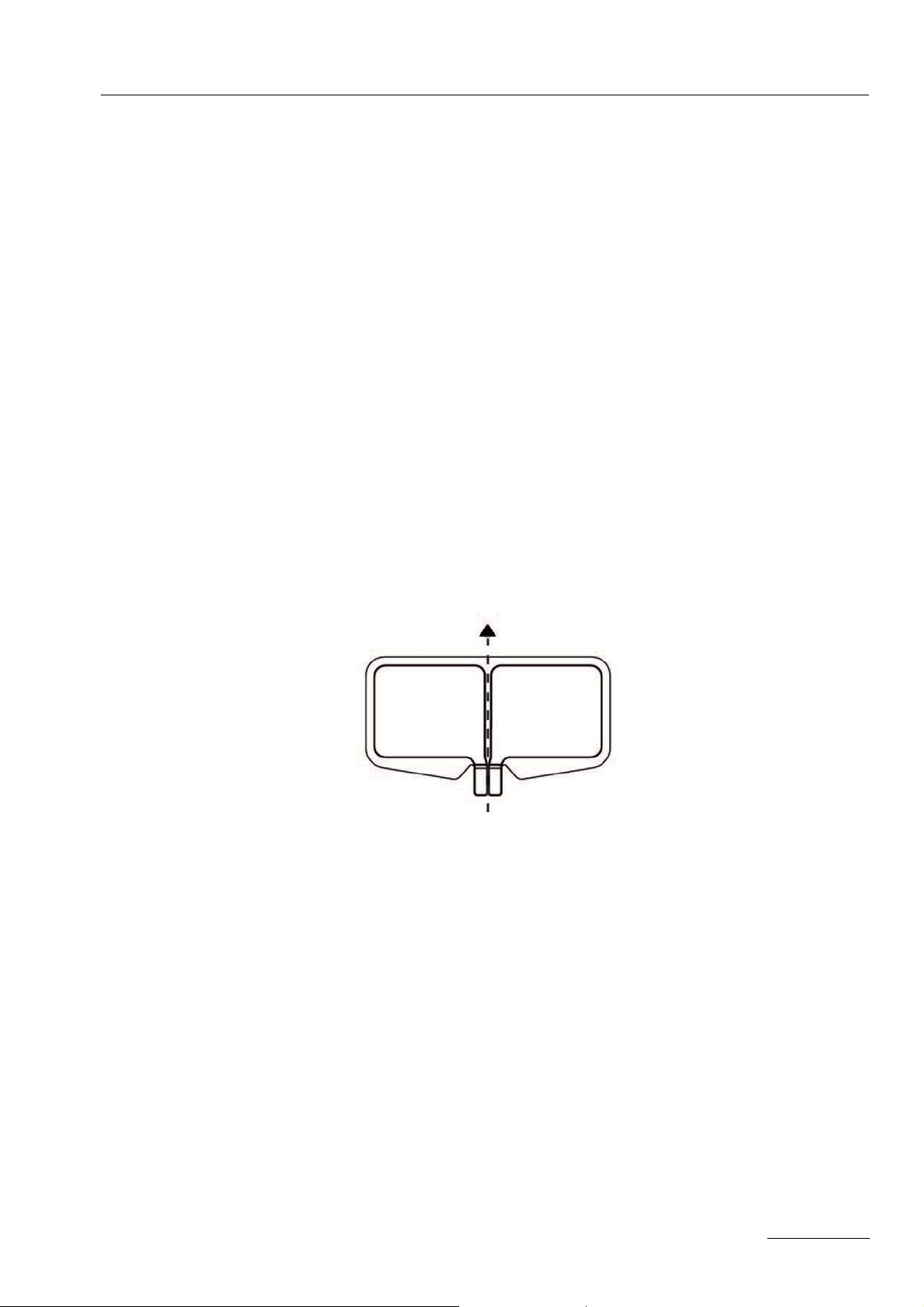
Observe the indicator lights
A check of the connecting cable can be performed before use as follows:
• Switch on the Unit. Set the NESSY setting to "NE: either way". Connect the
cable to the return electrode receptacle.
• If the connecting cord or the clip of a reusable cable do not have shorts the display of the dual surface (1) and the display of the single surface (2) will light up
red. If the displays lights up green, a short of the cable is detected by the unit.
Fig. 2-1
The displays of neutral electrodes (1) and (2) light up red.
How do I receive information about the safety status of the neutral
electrode?
No electrode connected
Single surface electrode con-
nected. "Neutral electrode:
Single surface" setup
Fig. 2-2
The neutral electrode socket is equipped with indicator lights, which represent a
dual surface electrode (1) and a single surface electrode (2) respectively. Call up the
NESSY window using the Focus button. Here you can check which setting is active
in the unit's service programs.
• Neutral electrode: Dynamic
• Neutral electrode: Dual surface
• Neutral electrode: Either way
• Neutral electrode: Single surface
If the unit is set for a dual surface / dynamic electrode and you connect a single surface electrode, the dual surface indicator light will illuminate red. If the unit is set
for a single surface electrode and you connect a dual surface electrode, the single
indicator light will illuminate red. In both cases you can only activate monopolar
mode if you connect the correct electrode.
If you switch on the unit without having connected an electrode, the indicator lights
will illuminate red. It is not possible to activate monopolar mode.
If you connect a single surface electrode, the unit only monitors the connection between unit and electrode. If this is faultless, the electrode symbol illuminates green
(safety status Green). Monopolar mode can be activated.
Art. No. 80104-951
04 / 2007
26 / 120
If the connection to the unit is interrupted, or if the electrode contact tab is not fully
inserted into the connection clamp, the electrode symbol illuminates red (safety status Red). Monopolar mode cannot be activated. If you attempt activation, an audible
warning signal is emitted. If a single surface electrode is connected, the contact between the electrode and the patient's skin is not monitored! You will not receive a
warning if the electrode becomes detached from the skin and there is a danger of
burns.
2 • Safety Features
Dual surface neutral electrode
connected. "Neutral electrode:
Dual surface" or "Neutral elec-
trode: Either way" setup
To optimally utilize the unit's monitoring functions, ERBE recommends connecting
a dual surface electrode, and in particular the ERBE NESSY Omega electrode.
Apart from many other advantages, this electrode virtually eliminates any possibility of excessive heating of the tissue and skin at the edges of the electrode.
Contact between skin and electrode
If you connect a dual surface electrode, the unit not only monitors the connection
between unit and electrode, but also the contact between skin and electrode. If everything is OK, the electrode symbol illuminates green (safety status Green). Monopolar mode can be activated.
If the connection with the unit is interrupted, or if the contact tab is not fully inserted
into the connection clamp, or if the contact with the skin is so bad that there is a danger of burns, the electrode symbol illuminates red (safety status Red). Monopolar
mode cannot be activated. If you attempt activation, an audible warning signal is
emitted.
Application direction of the contact surface relative to the conduction direction
When dual surface electrodes are used, NESSY also monitors the direction of application of the contact surface relative to the conduction direction. The high-frequency current is not, as a rule, distributed evenly over the contact surface of the neutral
electrode. The current flows to the proximal corners or edges. There it can be larger
than at the distal corners or edges. For this reason, when applying the neutral electrode, ensure that the neutral electrode's line of symmetry points toward the operating field.
Art. No. 80104-951
04 / 2007
Fig. 2-3
NESSY compares the currents that flow through the two surfaces of the neutral electrode. If the currents differ slightly from each other, an indicator window appears on
the display. Monopolar mode can still be activated, but you should correct the position of the neutral electrode as soon as possible.
If the currents differ too greatly from each other, the dual surface electrode symbol
on the VIO illuminates red. Monopolar mode cannot be activated. If you attempt activation, an audible warning signal is emitted. A warning message appears on the
display: When applying the neutral electrode, ensure that the line of symmetry
points toward the operating field.
27 / 120
2 • Safety Features
Checking function of the NESSY
window when a dual surface
electrode is connected with
"Neutral electrode: Dual sur-
face" or "Neutral electrode:
Either way" setup
If you press the Focus button at the neutral electrode socket, you will move to the
NESSY window.
In the NESSY window the measured contact resistance between the skin and the
electrode is displayed as a numerical value and a white bar.
Fig. 2-4
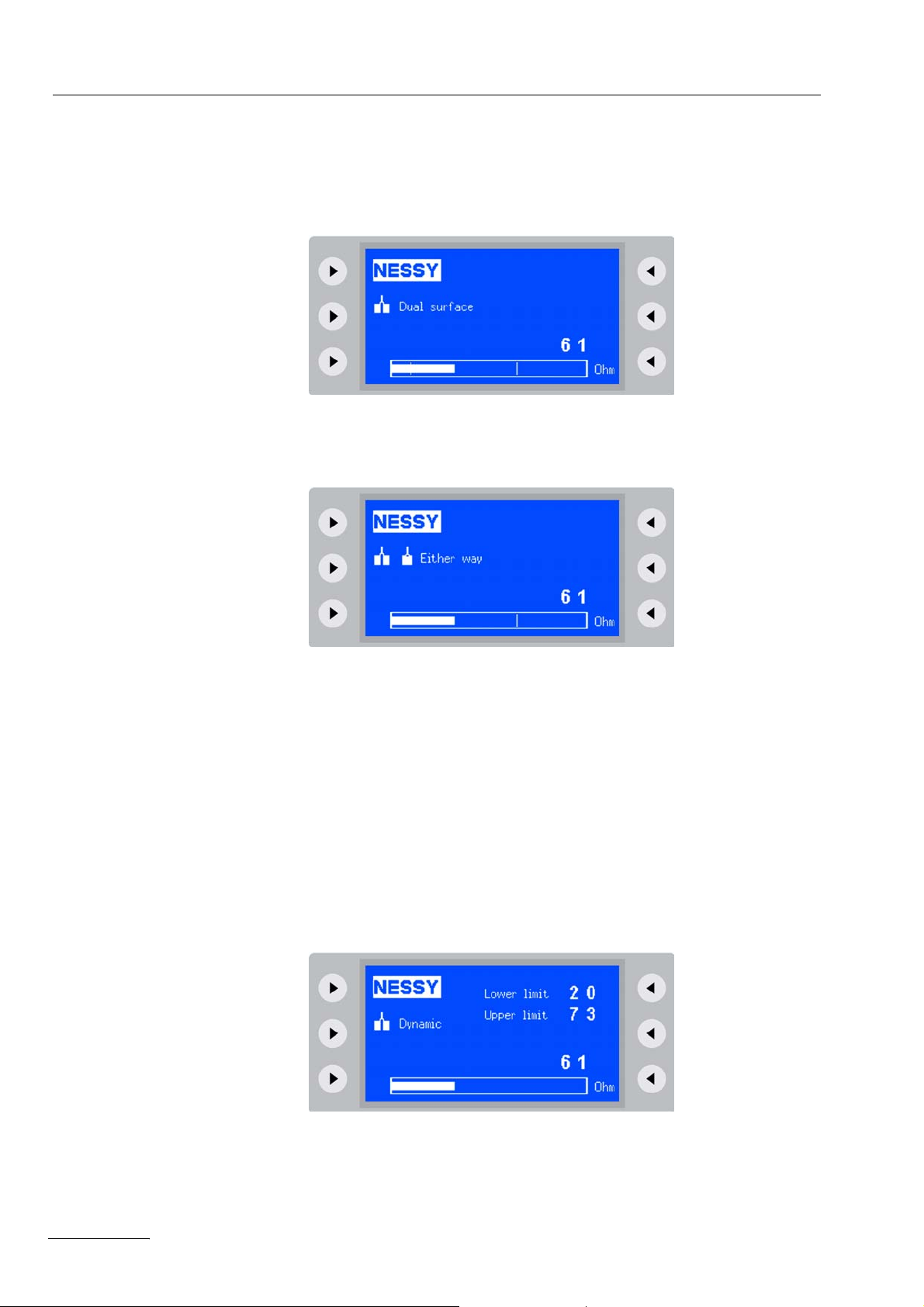
With the setup setting Neutral electrode: Dual surface the permissible resistance
range of 20 to 120 ohms is shown by two vertical lines.
Dual surface neutral electrode
connected. "Neutral electrode:
Dynamic" setup
Checking function of the NESSY
window when a dual surface
electrode is connected with
"Neutral electrode: Dynamic"
setup
Fig. 2-5
With the setup setting Neutral electrode: Either way the maximum permissible resistance of 120 ohms is shown by a vertical line. No lower limit value can be specified with this setting.
The monopolar sockets of the unit and the APC socket can only be activated when
the resistor value is within the permissible range.
The Neutral electrode: Dynamic setup offers extra safety for patients with low skin
resistance, for example, patients with little subcutaneous fatty tissue, children and
infants. Even with these patients, critical detachment of the neutral electrode from
the skin is detected in good time.
If you press the Focus button at the neutral electrode socket, you will move to the
NESSY window.
Art. No. 80104-951
04 / 2007
28 / 120
Fig. 2-6
In the Nessy window the measured contact resistance between the skin and the electrode is displayed as a numerical value and a white bar, and the permissible resistance range by two numerical values.

2 • Safety Features
The lower limit value is 20 ohms. The upper limit value is not fixed at 120 ohms but
depends on the lowest measured contact resistance between the skin and neutral
electrode (measurement value). The upper limit value is reduced in relation to the
measurement value to the extent that critical detachment of the neutral electrode
from the skin is detected in good time.
The monopolar sockets of the unit and the APC socket can only be activated when
the resistor value is within the permissible range.
Art. No. 80104-951
The NESSY window as a visual
aid to applying a dual surface
The NESSY window when con-
necting a single surface
04 / 2007
electrode
electrode
When you apply a dual surface electrode to the patient's skin, first change to the
NESSY window. With the aid of its displays, you can recognize how good the skin
contact is. Ideally the contact resistance should be between 20 and 120 ohms.
To check a single surface electrode it is sufficient to observe the indicator lights.
When a single surface electrode is connected, the NESSY window does not give any
visual assistance. The contact between electrode and skin cannot be measured when
a single surface electrode is used.
Automatic monitoring of equipment output error
The unit is equipped with an automatic monitoring system for the HF output parameters. This system monitors any divergence between the actual value and the setpoint of the HF output parameters selected and emits warning signals or switches
off the HF generator if the divergence is so great that the required quality of the respective effect (CUT or COAG) is no longer guaranteed. For the operating surgeon
the display of any equipment output error allows him to immediately see, in the
event of divergence or absence of the required effect, whether this defect has been
caused by the unit. With the unit, any divergence of the HF output parameters from
the HF output parameters actually selected can only be caused by loads with an excessively low resistance, e.g. too large coagulation electrodes, short circuit between
active electrode and patient plate or by a defect in the unit.
Automatic monitoring of the ON time
With proper use, a high-frequency generator is only briefly activated to carry out a
cut or coagulation using a fingerswitch, pedal or AUTO START. This generally
only takes a few seconds. A defect in the unit, in the accessories or in usage may
cause the high-frequency generator to be switched on unintentionally. To prevent
major damage being caused by accidental activation of a high-frequency generator
the unit is equipped with a monitor which automatically monitors the ON time of
the high-frequency generator.
Custom adaptation of maximum
ON time
When a predetermined maximum ON time is exceeded, the monitor emits a visual
and acoustic signal and automatically switches off the HF generator. However, the
HF generator can be switched back on at any time, resulting in renewed monitoring
of the ON time. This prevents major damage being caused by the accidental activation of an HF generator for indefinitely long periods.
In view of the risk of thermal tissue damage due to the accidental switch-on, a HF
generator which has been switched on accidentally should be switched off again automatically, as far as possible immediately. As the unit cannot automatically distinguish between the intentional and accidental switch-on of a HF generator, the automatic switch-off of a HF generator should not take place too quickly as this would
hinder the operating surgeon with cutting or coagulation. Setting of the ON time can
only be carried out by a technician in the service programs.
29 / 120

2 • Safety Features
WARNING
The user was not informed of a change in maximum ON time
Risk of accidental tissue damage to the patient!
¨ All users must be informed of any change in maximum ON time
at an early stage. That is, before the user works with the modified maximum ON time for the first time.
¨ The temperature at the neutral electrode site increases during
long and continuous activations; therefore, ensure that the cooling phases between activations are sufficient.
Protection from operating errors
To prevent operating errors the front panel and the menus are designed so as to automatically monitor and signal illogical or incomplete settings.
All receptacles of the applied part are arranged in the receptacle strip next to the
front panel. These receptacles are designed so that only connectors of the proper accessories can be inserted (provided that only the accessories supplied or recommended by the manufacturer of the unit are used).
You can connect 2 or 3 instruments simultaneously to the unit according to the number of sockets available. However, for reasons of safety these can be activated only
alternately. Only one socket conducts HF voltage at any time.
Whenever the power switch is switched on, an automatic test program is run inside
the unit, designed to detect and signal the following defects in the operator controls
of the unit and the connected accessories:
• If a button on the front panel has short-circuited due to an error or was pressed
when the power switch was switched on, this error will be indicated acoustically and by an error number and message after switch-on of the power switch.
• If a button on the electrode handle has short-circuited due to an error or has
been bypassed at low resistance (e.g. due to moisture in the electrode handle) or
was pressed while the power switch was switched on, this error will be indicated acoustically and by an error number and message after switch-on of the
power switch.
• If a contact of the footswitch has short-circuited due to an error, or a pedal is
jammed or a pedal was pressed while the power switch was switched on, this
error will be indicated acoustically and by an error number and message.
Art. No. 80104-951
04 / 2007
30 / 120
 Loading...
Loading...