Page 1
Chelex®100
and Chelex 20
Chelating Ion
Exchange Resin
Instruction Manual
Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547
LIT200 Rev B
Page 2

Introduction
Chelex chelating ion exchange resin has unusually
high preference for copper, iron, and other heavy metals over monovalent cations such as sodium and
potassium. Its selectivity for divalent over monovalent
ions is approximately 5,000 to 1, and it has a very
strong attraction for transition metals, even in highly
concentrated salt solution.
Technical Description
Chelating resin is available as Analytical Grade
Chelex 100 resin, Biotechnology Grade Chelex 100
resin, and Technical Grade Chelex 20 resin. The
Analytical Grade Chelex 100 resin has been exhaustively sized, purified, and converted to make it suitable for accurate, reproducible analytical techniques.
Biotechnology Grade Chelex 100 resin is analytical
grade resin which is certified to contain less than 100
micro-organisms per gram of resin. Technical Grade
Chelex 20 resin is coarse mesh resin useful for large
1
Page 3
scale clean-up, for example metals from waste waters,
CH2COOH CH2COOH CH2COO- CH2COO-
Ø–CH2–NH+Ø–CH2NH+Ø–CH2–NH
+
Ø–CH2–N
CH2COOH
CH2COO- CH2COO- CH2COO-
pH 2.21 pH 3.99 pH 7.41 pH 12.30
where analytical purity is not a major concern.
Chelex 100 resin and Chelex 20 resin are styrene
divinylbenzene copolymers containing paired iminodiacetate ions which act as chelating groups in binding polyvalent metal ions. Chelex chelating resin is
classed with the weakly acidic cation exchange resins
by virtue of its carboxylic acid groups, but it differs
from ordinary exchangers because of its high selectivity for metal ions and its much higher bond strength.
Chelex chelating resin is efficiently regenerated
in dilute acid and operates in basic, neutral, and
weakly acidic solutions of pH 4 or higher. At very
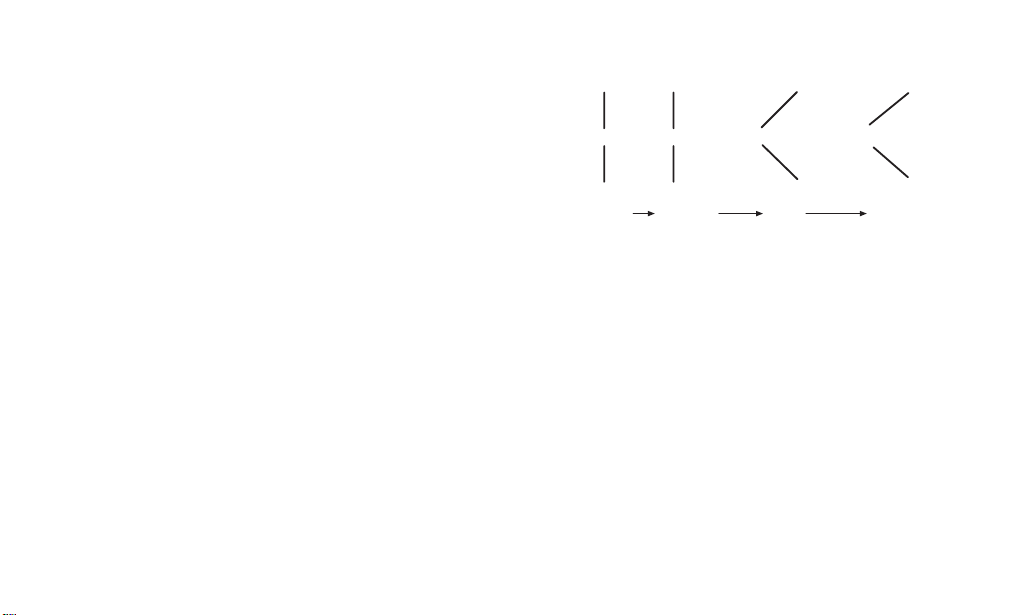
low pH, the resin acts as an anion exchanger. Figure 1
shows the zwitterionic forms of the Chelex resin as a
function of pH.
Fig. 1. Change in structure of Chelex resin with
increasing pH.
Selectivity for Heavy Metal Ions
The selectivity of Chelex resin for metal cations
corresponds to that of iminodiacetic acid. A list of
selectivity factors for several divalent cations is given
in Table 1. The selectivity factor is a quantitative measure of the affinity that Chelex resin displays for a particular cation compared to its affinity for a reference
cation, in this case Zn
+2
.
2 3
Page 4

Table 1. Selectivity for Divalent Cations
Hg
Cu
UO
Ni
Pb
Zn
Co
Cd
+2
1060 Fe
+2
126 Mn
+2
+2
+2
+2
+2
+2
5.70 Ba
4.40 Ca
3.88 Sr
1.00 Mg
0.615 Na
0.390
+2
0.130
+2
0.024
+2
0.016
+2
0.013
+2
0.013
+2
0.009
+1
0.0000001
Actual selectivity values for any particular system
depend on the pH, ionic strength, and the presence of
other complex-forming species. Thus Hg
+2
appears
high in the selectivity series in the presence of nitrate
ions, but low in the series in the presence of chloride
ions, with which it forms a complex. The approximate
order of selectivity for cations in nitrate or chloride
solutions is:
+2
Cu
>>Pb+2>Fe+3>Al+3>Cr+3>Ni+2>Zn+2>Ag
>Co+2>Cd+2>Fe+2>Mn+2>Ba+2>Ca+2>>>Na
4 5
+
+
A selectivity series for cations in an acetate buffer
system at pH 5 is:
+2
>Cu+2>>Fe+2>Ni+2>Pb+2>Mn+2>>
Pd
+2
Ca
= Mg+2>>> Na
+
The selectivity for various cations in aqueous
solutions at pH 4 is:
+2
>Cu+2>Pb+2>>>Ni+2>Zn+2>Cd+2>Co+2>Fe+2>
Hg
+2
Mn
>Ca+2>>>Na
+
The selectivity at pH 9 in the presence of 1.5 M
(NH
Co
+2
is:
4)2SO4
>Ni+2>Cd+2>Cu+2>Zn+2>Ca+2>>>Na
+
Page 5

Instructions for Use
Chelex resins may be used with either a batch
method or a column method.
Batch Method
The batch method is the addition of resin directly
into the sample followed by stirring.
1. Weigh out about 5 grams of resin for every
100 ml of sample. For larger scale applications or
when a more exact amount of resin is needed, use
the capacity guidelines given below to calculate
the resin volume for the specific sample metal
concentration.
2. Add resin to the sample and stir or shake (gently)
for 1 hour.
3. Filter or decant the sample from the resin.
Column Method
The column method involves pouring a column
with the Chelex resin and passing the sample through
6 7
to achieve the separation. Although large mesh material (50-100 mesh) allows rapid flow rates and the
ability to process large volumes of solution, resolution
may be sacrificed. On the other hand, small mesh
material (200-400 mesh) can achieve very high resolution and analytical results, but will require longer process time due to the slow flow rate.
1. Calculate the amount of resin required based on
the expected metal concentration. If the metal
concentration is unknown, begin with 5 grams of
resin for 100 ml of sample, and then optimize the
volumes after obtaining the results.
2. Prepare a buffer with a pH and ionic concentration
that will allow the metal to be ion-exchanged easily
onto the column. Use the information from Table 1,
and the selectivity comparisons of different pH
solutions mentioned above, to optimize the buffer.
For unknown solutions, use deionized water.
3. Slurry the resin in the buffer, and pour the column. Allow several bed volumes to pass through
the column to insure a well packed bed.
Page 6

4. Slowly add the sample to the column, taking care
not to disturb the resin bed.
5. Initiate flow. Discard initial buffer from the void
volume.
6. If a metal free solution is the goal, collect effluent.
If the concentrated metals are of interest, allow all
of the sample to pass through the column, then
elute the metals off the resin with a solution containing a counterion of higher selectivity than the
bound metal.
Ion Exchange
The quantity of cations exchanged is a function of
pH. Exchange is very low below pH 2, increases
sharply from pH 2 to 4, and reaches a maximum above
pH 4. Any metal removed from solution is replaced by
an equivalent amount of the ions originally on the
resin. Usually an alkali metal form is best. The resin is
supplied in the sodium form, but may be used in the
potassium or ammonium form as well. These weakly
held ions allow other ions to be readily adsorbed. The
8
capacity of the sodium form of the resin is 0.4 meq/ml
(defined as Cu(NH
3)4
+2
uptake).
Elution, Regeneration, and Conversion
Metals can be removed with Chelex chelating
resin using either the batch or the column technique,
though the column technique is generally more efficient. The most effective agents to elute the metals
from the resin are acids. Concentrated salt solutions
are often useful for selective elution, but are generally
inefficient in removing strongly absorbed metals.
Regeneration of the resin to a salt form is a twostep process. The resin is first converted to the hydrogen form using acid, then converted to the desired
ionic form using the hydroxide of the cation desired.
The regeneration to the sodium form of resin loaded
with copper would proceed as follows:
Resin-Cu + 2 HCl Resin-H + CuCl
Resin-H + NaOH Resin-Na + H2O
The following sequence should be used: 2 bed
volumes in 1 N HCl, 5 bed volumes water rinse, 2 bed
9
2
Page 7

= average MW
∑ MW
n
volumes 1 N NaOH, 5 bed volumes water rinse. In
some cases, especially with strongly complexed metals such as iron, complete regeneration can be accomplished only by using the two-step regeneration
procedure. Single-step conversions are adequate when
going from weakly held to strongly held ions. Thus
the calcium form is prepared from the sodium form
using 2 bed volumes of 2 N calcium chloride.
Chelex 100 resin undergoes volume changes
when its ionic form is altered or the external medium
is changed. The resin swells 100% in going from the
hydrogen to a monovalent salt form. Therefore, normal precautions, such as wrapping columns with tape,
should be taken to protect against glass breakage.
Using the resin in the calcium form is a method that
has been used to prevent shrinkage upon elution. The
resin volume in water of different ionic forms is:
+
Na
1.00; H+0.45; Cu+20.60; Fe+20.45; Zn+20.55;
+2
Ca
0.53; K+1.06; Li+0.98; Ag+0.70; Cr+30.53.
The resin volume of the sodium form in various
solvents is (the resin exhibits appreciable capacity in
organic solvents):
water 1.00, acetone 0.47, methanol 0.70, ethanol
0.45, isopropanol 0.48, ethyl acetate 0.96.
Calculating Capacity
A step-by-step method is used for determining the
approximate amount of resin needed to remove heavy
metal ions from aqueous systems.
1. Determine total volume of solution to be treated
to remove heavy metals.
Example: 10 liters
2. Calculate the average molecular weight of metals
to be exchanged.
Example: Using Cu
227.9/3=76 grams/mol
+2
, Cd+2, Cr+3;
10
11
Page 8

Example: = 6.58 ml
2.63 meq
0.4 meq/ml
3. Calculate the total weight of the metals. Usually,
Example: = 2.63 meq
100 mg
76 g/mol
2 eq/mol
metals are measured in parts per million (ppm). In
aqueous solutions, ppm can be assumed to be
mg/liter.
Cd
Cr
+2
= 3 ppm
+2
= 5 ppm
+3
= 2 ppm
Example: Cu
10 ppm or 10 mg/liter
10 mg/liter x 10 liters = 100 mg
4. Convert the weight determined in step 3 to equivalence. Equivalence = weight in grams/equivalent
weight, where equivalent weight = molecular
weight/average valence.
5. The wet capacity of Chelex resin is 0.40 meq/ml.
Knowing this, the volume of resin needed can be
calculated.
6. Convert the volume in step 5 to weight. The density of Chelex resin is 0.65 g/ml.
Example: 6.58 ml x 0.65 g/ml = 4.3 grams
pH Stability
Chelex resin is stable over the entire pH range and
functionally active from pH 2-14.
Flow Rates
If a tightly held cation is to be isolated from a solu-
tion of weakly held cations, a flow rate in excess of 20
cm/min can often be used. Separations between similar
species and efficient regeneration and conversion
require lower flow rates, usually less than 4 cm/min.
12
13
Page 9

Buffering
Chelex resin in the hydrogen form has a pH of
2–3. The pH in the sodium form is about 11 and may
be slowly lowered by extended water washing.
However, a more satisfactory procedure for adjusting
the pH is to use a buffer. Thus, a sodium form at
pH 6.3 can be prepared by rinsing with 4 bed volumes
of 0.5 M sodium acetate buffer, followed by 5 bed
volumes of water.
Storage
Chelex resin is stable for at least 2 years when
stored sealed in the original container at 22 °C. It should
be stored in a salt form such as sodium or ammonium. If
left in the hydrogen form for more than a few hours, the
resin has a tendency to lose chelating capacity. Should
such a loss occur, the resin can be regenerated by heating it at 60 °C in 30-50% alkali for 24 hours. Free iminodiacetic acid produced upon long standing (detected by
its odor) may be extracted by methanol or by heating to
14
80 °C in 3 N ammonium hydroxide for 2 hours. The
resin is autoclavable in the sodium form.
Applications of Chelex Resins
Chelex 100 resin has found many uses. These
include analysis of trace metals in natural waters,
reagents, biochemical, and physiological fluids;
removal of trace metals from reagents, biochemicals,
physiological fluids, culture media, soils, and enzyme
systems; recovery of metals from process streams; and
chromatography of closely related metals.
Trace Metal Removal
Chelex resin offers a rapid and thorough method
for removing trace metal contaminants that could have
an effect on biological fluids or biological systems
under study. A unique ion exchange resin that is more
selective for multivalent metals than the standard
cation exchange resins, Chelex resin will scavenge
multivalent metal ion contaminants without altering
the concentration of nonmetallic ions. In most cases,
15
Page 10

neither column nor batch treatment with Chelex 100
resin has any effect on protein concentration or
enzyme activity. Where low protein recovery is a
problem, the protein can be dialyzed against a buffer
containing Chelex 100 resin. Table 2 gives examples
of trace metal removal with Chelex 100 resin.
Glyphosate Concentration with Chelex
100 Resin
Chelex 100 resin may be used to concentrate
glyphosate [N-(phosphonomethyl) glycine] when the
resin is in the iron form. When environmental water or
an aqueous extract of crops such as soybeans, grapes,
cabbage, or alfalfa is applied to Chelex 100 resin
(100-200 mesh, iron form), glyphosate complexes
with the iron form Chelex 100 resin. The glyphosate
may subsequently be eluted with 6 M HCl and applied
®
to AG
1-X8 resin (200-400 mesh, chloride form) for
anion exchange cleanup. The glyphosate along with
AMPA (aminomethyl phosphonic acid), its breakdown product, may then be quantified with the
®
Aminex
glyphosate analysis column.
16
Metal Analysis with Chelex Resin
Many methods of metal analysis, such as neutron
activation and atomic absorption, depend on the prior
separation and concentration of the metals from such
samples as air, soil, industrial waste waters, and biological extracts. Trace metals can be concentrated by
adsorption to Chelex chelating resin. The use of
Chelex resin to preconcentrate samples for analysis
has been extensively reviewed.
nanogram levels of Cd, Co, Cu, Fe, Mn, Ni, Pb, and
Zn can be achieved by using Chelex 100 resin 200400 mesh, with graphite furnace atomic adsorption
spectrometry.
2
Chelex 100 resin is also effective in
concentrating traces of Cd, Co, Cu, Mn, Ni, Pb, and
Zn from various food digests. Average recoveries
were 98.1% determined with standards.
1
Determination of sub-
17
3
Page 11

Table 2. Sample Applications with
Chelex 100 Resin
Application Reference
Preparation of metal-free apo- O’Keefe, E. T., Hill, R. L. and
enzyme Bell, J. E., Biochem., 19, 4954
Removal of calcium from Chiesi, M. and Inesi, G.,
sarcoplasmic reticulum vesicles Biochem.19, 2912 (1980).
Removal of extraneously bound Barker, R., et al., Biochem.
metal ions from enzymes prior J., 177, 289 (1979).
to NMR and ESR studies
Batch removal of calcium from Raymond, F A. and
whole blood Weinshilboum, R. M., Clin.
Removal of metals from ATP Sontheimer, G. M., et al.,
Removal of metals from buffer, Knapp, G., et al., J. Anal.
brine, and biological solutions
Removal of metals from S100b Baudier, J., et al.,
and melittin Biochem., 26, 2886 (1987).
Purification of dinucleotides Reinhardt, C. G. and Krugh,
(1980).
Chim. Acta, 58, 185 (1975).
Biochem., 26, 2701 (1987).
Atomic Spectrometry, 2, 611
(1987); Laue, T. M., et al.,
Biochem., 28, 4762 (1989).
T. R., Biochem., 17, 4845
(1978).
Application Reference
Removal of metals from cell Bosron, W. F., Kennedy
suspension F. S. and Vallee, B. L.,
Batch removal of metals from Agarwal, M., Bennett,
urine R. B., Stump, I. G. and D’Avria,
Removal of metals from enzyme Dunn, M. F., Pattison,
solutions S. E., Storm, M. C. and Quiel,
Removal of metals from guinea Amiraian, K., McKinney,
pig complement J. A. and Duchna, L.,
Removal of aldehydes and Ray, W. J. and
peroxides from polyethylene Pavathingal, J. M., Anal.
glycol Biochem., 146, 307 (1985).
Removal of interfering Brandt, S. J., Dougherty,
components from myo-inositol R. W., Lapetina, E . G. and
Calcium removal from protein Putnam-Evans, C. L.,
kinases Harmon, A. C. and Cormier,
Removal of divalent cations Devlin, C. C. and Grisham,
from NMR stock solutions C. M., Biochem., 29, 6192
Biochem., 14, 2275 (1975).
J. M., Anal. Chem., 47, 924
(1975).
E., Biochem., 19, 718 (1980).
Immunology, 26, 1135 (1974).
Niedel, J. E., Proc. Nat. Acad.
Sci., 82, 3277 (1985).
M. J., Biochem., 29, 2488 (1990).
(1990). Chuknyisky, P. P.,
Rifkind, J. M., Tarien, E., Beal,
R. B. and Eichhorn, G. L.,
Biochem., 29, 5987 (1990).
18
19
Page 12

Application Reference
Removal of paramagnetic Kochoyan, M., Leroy,
impurities from oligonucleotides J. L. and Guéron, M.,
for NMR analysis Biochem., 29, 4799 (1990).
Trace metal removal from buffer Pai, S.-C., Chen, T.-C.,
solutions and seawater Wong, G. T. F. and Hung, C.-
Iron removal from bacteria Trost, J. T. and Blankenship,
Trace metal ion removal from Yong, G., Leone, C. and
buffer solutions Strothkamp, K. G., Biochem.,
Removal of multivalent cation Swanson, J. E. and Feigenson,
contaminants G. W., Biochem., 29, 8291
Schroeder, S. A., Roongta, V.,
Fu, J. M., Jones, C. R. and
Gorenstein, D. G., Biochem., 28,
8292 (1989).
C., Anal. Chem., 62, 774 (1990).
R. E., Biochem., 28, 9898 (1989).
29, 9684 (1990). Severns, J. C.
and McMillin, D. R., Biochem.,
29, 8592 (1990) Shang, Z., Liao,
Y.-D., Wu, F. Y.-H. and Wu, C.W., Biochem., 28, 9790 (1989).
Chung, H. K. and Ingle, J. D.,
Anal. Chem., 62, 2547 (1990).
Grimshaw, C. E., Shahbaz, M.
and Putney, C. G., Biochem.,
29, 9936 (1990).
(1990).
Application Reference
Purification of NMR reagents Brito, R. M. M., Rudolph, F.B.
Calcium removal from Vorherr, T., James, P.,
calmodulin Krebs, J., Enyedi, A.,
Purification of zinc isotopes Gökmen, I. G., Aras,
from human blood and excrement N. K., Gordon, G. E., Wastney,
Calcium removal from buffer Kinoshita, C.M., Ying,
solutions S.-C., Hugli, T.E., Siegel, J. N.,
Removal of zinc from buffer Jefferson, J. R., Hunt, J. B.
solutions and
DNA extraction for PCR Walsh, P. S., Metzger,
and Rosevear, P. R., Biochem.,
30, 1461 (1991). Ray, W. J.,
Burgner, J. W. and Post, C. B.,
Biochem., 29, 2770 (1990).
McCormick, D. J., Penniston,
J. T. and Carafoli, E., Biochem.,
29, 355 (1990).
M. E. and Henkin, R. I., Anal.
Chem., 61, 2757 (1989).
Potempa, L. A., Jiang, H.,
Houghten, R. A. and Gewurz,
H., Biochem., 28, 9840 (1989).
Thielens, N. M., Dorsselaer, A.
V., Gagnon, J. and Arlaud, G. J.,
Biochem., 29, 3570 (1990).
Ginsburg, A., Biochem., 29,
6687 (1990).
D. A. and Higuchi, R.,
BioTechniques, 10, 506 (1991).
20
21
Page 13

Table 3. Other Examples of Metal
Preconcentration and Separation on Chelex
100 Resin
Metal Sample Reference
As (V), Industrial Chandra, M., et al., Reactive
As (III). solutions Polymers, 8, 85 (1988).
Cu, Cd, Mn, River Liu, Y. and Ingle, Jr., J. D.,
Zn, Pb water Anal. Chem., 61, 525 (1989).
Cd, Cu, Pb, Environmental Figura, P. and McDuffie, B.,
Zn samples Anal. Chem., 52, 1433 (1980).
Uranium Natural Pakains, P., Anal. Chim. Acta,
waters 120, 289 (1980).
Cd, Co, Cr, Natural Kingston, H. M. and
Cu, Fe, Mn, waters R. R., Anal. Chem., 55, 1160
Mo, Ni, Sc, (1983); Greenberg, R. R. and
Sn,Th, U, V, Kingston, H. M., J. Radioanal.
Zn Chem., 71, 147 (1982).
Cd, Co, Cu, Seawater Kingston, H. M., Barnes, I. L.,
Fe, Mn, Ni, Brady, T. J. and Rains, T. C., Pb,
Zn Anal. Chem., 50, 2064 (1978).
Cd, Zn, Pb, Seawater Sturgeon, R. E., Berman, S. S.,
Fe, Mn, Cu, Desaulniers, J. A. H.,
Ni, Co, Cr Mykytiuk, A. P, McLaren, J. W.
and Russell, D. S., Anal. Chem.,
52, 1585 (1980).
Metal Sample Reference
Fe, Cd, Zn, Seawater Mykytiuk, A. P., Russell, D. S.
Cu, Ni, Pb, U, and Sturgeon, R. E., Anal.
Co Chem., 52, 1281 (1980).
Cd, Pb, Ni, Seawater Rasmussen, L., Anal. Chim.
Cu, Zn Acta, 125, 117 (1961).
Cd, Ce, Co, Seawater Kingston, H. M. and
Cu, Fe, Mn, Greenberg, R. R., Environ.
Mo, Ni, Pb, Inter., 10, 153 (1984).
Sc, Sn, Th,
U, Zn
Fe, Mn, Cu, Seawater Paulson, A. J., Anal. Chem.,
Ni, Cd, Pb, Zn 58, 183 (1986).
Fe, Zn, Mn Biological Pella, P. A., Kingston, H. M.
V Biological Fassett, J. D. and Kingston,
V Seawater Dupont, V., Auger, Y., Jeandel,
materials and Sieber, J . R., Anal. Chem.,
55, 1193 (1983).
materials H. M., Anal. Chem., 57, 2474
(1985).
C. and Wartel, M., Anal. Chem.,
63, 520 (1991).
22
23
Page 14

Ordering Information
Catalog Pkg.
Number Product Description Size
142-2822 Analytical Grade Chelex
142-2832 Analytical Grade Chelex
142-2825 Analytical Grade Chelex
142-2842 Analytical Grade Chelex
143-2832 Biotechnology Grade Chelex
745-7001 Technical Grade Chelex
100 Resin, 50-100 mesh,
sodium form 500 g
100 Resin, 100-200 mesh,
sodium form 500 g
100 Resin, 100-200 mesh,
iron form 100 g
100 Resin, 200-400 mesh,
sodium form 500 g
100 Resin, 100-200 mesh,
sodium form 100 g
20 Resin, 20-50 mesh, sodium form 10 kg
24
 Loading...
Loading...