Page 1

Bio-Scale™ Mini
UNOsphere SUPrA™
Cartridges, 1 and 5 ml
Instruction Manual
Catalog #
732-4200
732-4201
732-4202
For technical support, contact your local Bio-Rad office, or in the
U.S., call 1-800-4BIORAD (1-800-424-6723).
Page 2

Page 3

Table of Contents
Section 1...Introduction. .......................................1
Section 2...Connection to Bio-Rad’s Low-
..............Pressure Chromatography
..............Instruments ........................................5
Section 3 ..Connection to Other Liquid
..............Chromatography Systems................10
..............3.1 BioLogic DuoFlow™
Systems.................................11
3.2 HPLC Systems ......................11
3.3 FPLC Systems .......................12
Section 4 ..Getting Started.................................13
4.1 Screening Buffers and
Conditions for UNOsphere
SUPrA....................................14
4.2 Scaling Up the Separation .....16
Section 5 ..Care of the Cartridge........................19
5.1 Cleaning.................................19
Page 4

5.2 Sanitation...................................
5.3 Autoclaving ............................21
5.4 Storage..................................21
Section 6 ..Technical Assistance ........................22
Section 7 ..Ordering Information.........................23
Section 8 ..References .......................................25
Page 5

Page 6

Section 1
Introduction
Bio-Scale™ Mini cartridges have a patent-pending,
double-wall design that provides extra durability and
allows easy, reliable runs with aqueous buffers most
commonly used for protein purification. The
polypropylene Luer fittings and internal sealing
surfaces assure leak-free operation at pressures up
to 45 psi. The cartridges are convenient, disposable,
and supplied ready for use. Cartridges are available
for a variety of chromatographic techniques including
desalting, ion exchange, and affinity chromatography.
See Ordering Information (Section 7) for a listing of
the complete Bio-Scale Mini cartridge product line.
1
Page 7

UNOsphere SUPrA™ medium is a chromatographic
support based on recombinant protein A. The
media are designed for process-scale purification of
monoclonal antibodies. The protein A ligand is
produced in E. coli without the use of material from
animal origin. The UNOsphere base bead is a
macroporous polymeric bead that is designed for
robust and scaleable applications. See Tables 1 and
2 on pages 4–5 for the technical description of the
product.
UNOsphere SUPrA media are built on the proven
UNOsphere base bead, which insures an easy
scale-up path for process applications. The
outstanding flow pressure performance of
UNOsphere chromatography media allow its use in
process applications without concern for exceeding
the pressure limits of the media or chromatography
system. The flow characteristics of UNOsphere
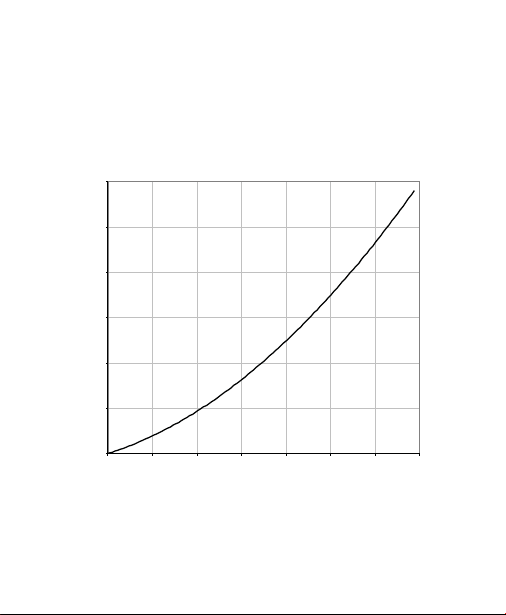
SUPrA packed in a large column format are shown
in Figure 1.
2
Page 8
UNOsphere SUPrA affinity chromatography media
come with full regulatory support and are backed by
the support of Bio-Rad’s global application and
development team.
Fig. 1. Flow performance of UNOsphere SUPrA media in
Bio-Rad's EasyPack™ column (20 x 20 cm) packed to 13.1%
axial compression.
0.0
0.5
1.0
1.5
2.0
2.5
3.0
0 100 200 300 400 500 600 700
3
Pressure (Bar)
Linear Flow (cm/hr)
Page 9

Table 1. Bio-Scale Mini UNOsphere SUPrA
Cartridge Specifications.
Sizes 1 and 5 ml bed volumes
Dimensions 1 ml: 40 x 5.6 mm, length x inner
diameter
5 ml: 40 x 12.6 mm, lenghth x inner
inner diameter
Maximum pressure tolerance 45 psi
Recommended flow rates 1 ml: 1–2 ml/min (240–480 cm/hr)
5 ml: 5–10 ml/min (140–480 cm/hr)
Maximum flow rate 1 ml: 3 ml/min (730 cm/hr)
5 ml: 15 ml/min (722 cm/hr)
Fittings Female Luer fitting inlet and
male Luer fitting outlet
Column material Polypropylene
Frit material Polyethylene (HDPE)
Shipping condition 20% ethanol
Storage recommendation 20% ethanol
Autoclavability Not autoclavable
4
Page 10

Table 2. Technical Description of UNOsphere
SUPrA Affinity Chromatography Media.
Composition Highly crosslinked polyacrylamide polymer
Particle size range 53–61 µm
Ligand Recombinant protein A
Coupling chemistry Epoxy
Dynamic binding capacity* 150 cm/hr 30 ± 3 mg/ml
300 cm/hr 25 ± 2 mg/ml
450 cm/hr 20 ± 2 mg/ml
Chemical stability** 10 mM hydrochloric acid
6 M guanidine hydrochloride
0.1 M arginine (pH 2.8)
0.1 M citrate (pH 2.8)
0.1 M glycine (pH 2.8)
Working pH range 3–11
Cleaning-in-place (CIP) 6 M guanidine hydrochloride
10 mM hydrochloric acid
0.1 M sodium hydroxide
1 M acetic acid/20% ethanol
Recommended mobile phase 100–600 cm/hr
velocity range
Temperature stability 4–40°C
Delivery conditions 50% slurry in 20% ethanol
Storage conditions 4–8°C
* Minimum 20 mg/ml at 300 cm/hr; 10% breakthrough capacity determined
with 1.0 mg/ml polyclonal human IgG in 1.1 x 10 cm column.
** No significant change in chromatographic performance after 24-hour storage
at room temperature.
5
Page 11

Section 2
Connecting to Bio-Rad's
Low-Pressure
Chromatography
Instruments
The Bio-Scale™ Mini cartridges are ideal for use with
Bio-Rad's BioLogic™ LP system, Econo gradient
pump, and Model EP-1 Econo pump, and all lowpressure chromatography instruments. Bio-Scale
Mini cartridges can be conveniently connected
directly to the system using the Luer lock fittings on
the cartridge.
1. Install 1.6 mm ID tubing in the pumphead.
Adjust platen pressure screw (on pumphead).
Using a screwdriver or coin, turn the screw
counterclockwise as far as it will go, then turn
clockwise three full turns. Assemble with fittings
and lock rings as shown in Figure 1.
6
Page 12

(Use orange lock rings and medium size barb
fittings with 1.6 mm tubing.)
Fig. 1. Biologic LP setup.
7
Platen pressure
screw
See detail
2 3
1
9
456
7 8
0
C .
Alarm
Lock ring
Tubing
Luer fitting
Page 13

2. To maximize gradient accuracy and apply
samples efficiently, install 1.6 mm ID tubing from
the pump to the MV-6 sample inject valve (if
available). If using the MV-6 sample inject valve,
turn the knob counterclockwise as far as it will
go so it will now correspond to the printed
diagram on the valve. (See Figure 2.)
Fig. 2. Connecting to a MV-6 valve.
8
SAMPLE LOOP
INJECT
PORT
MV-6
BIOLOGIC LP
SYSTEM OR ECONO
GRADIENT PUMP
TO
INJECT
VALVE
FILL
TO
COLUMN
WASTE
BIOLOGIC LP
SYSTEM OR ECONO
GRADIENT PUMP
SAMPLE LOOP
INJECT
PORT
FILL
MV-6
INJECT
TO
VALVE
"INJECT" POSITION"FILL" POSITION
TO
COLUMN
WASTE
Page 14

9
Fig. 3. Column and fittings.
Inlet fitting
Outlet fitting
Page 15

3. Connect the inlet of the cartridge to the male
Luer fitting on the MV-6 sample inject valve. (See
Figure 2.) If not using the MV-6 sample inject
valve, connect a barb to male Luer fitting on the
1.6 mm ID tubing, then connect to the top of the
female Luer on the Bio-Scale mini cartridge. For
optimum performance, a cartridge should be
mounted vertically with the arrow on the
cartridge pointing downward.
4. Connect the cartridge outlet to the 1.6 mm ID
tubing leading to the BioLogic LP optics module
or Econo UV monitor. It is recommended to use
the shortest length (approximately 10 cm) of
1.6 mm ID tubing. Connect a barb to female
Luer fitting to the 1.6 mm ID tubing, then
connect to the bottom of the male Luer on the
Bio-Scale Mini cartridge.
10
Page 16

Section 3
Connecting to Other Liquid
Chromatography Systems
The Bio-Scale™ Mini cartridges can be connected
to any liquid chromatography system, provided that
the maximum pressure limit (3 bar, 45 psi, or
300 KPa) of the cartridges is not exceeded. It is
recommended that the system pressure limit be set
according to the cartridge pressure limit. Pressures
in excess of 3.4 bar are usually caused by
restrictions in tubing or detector cells downstream
from the cartridge. Bio-Rad offers two fittings kits for
easy connection of a Bio-Scale Mini cartridge to a
BioLogic DuoFlow™, HPLC- or FPLC-type system.
11
Page 17

3.1 BioLogic DuoFlow Systems
The Bio-Scale Mini cartridge to BioLogic system
fittings kit (catalog #732-0113) includes ¼–28
female to male Luer and ¼–28 female to female
Luer to connect one Bio-scale Mini cartridge to the
BioLogic DuoFlow system.
3.2 HPLC Systems
The Luer to 10–32 adaptor fittings kit (catalog
#732-0112), provides fittings necessary to connect
the cartridge to nut and ferrule type fittings found
on most HPLC systems. Alternatively, the cartridge
can be connected to HPLC systems via a low dead
volume
1/16 in union with a new piece of stainless
steel tubing attached to the union. Simply slip a
short length of the 0.8 mm ID tubing over
1/16 in OD
stainless steel tubing to a distance of 1 cm.
12
Page 18

3.3 FPLC Systems
The Luer to M6 adaptor fittings kit (catalog
#732-0111), provides fittings necessary to connect
the cartridge to the M6 fittings found on FPLC or
related systems.
13
Page 19

Section 4
Getting Started
4.1 Screening Buffers and Conditions for
UNOsphere SUPrA™
Buffers
A1: 0.02 M sodium phosphate, 0.02 M sodium citrate,
pH 7.5.
A2: 0.02 M boric acid, 0.02 M sodium phosphate,
0.02 M sodium citrate, 1.0 M sodium sulfate, pH 9.0.
B: 0.02 M sodium citrate, 0.1 M sodium chloride,
pH 2.5.
Conditions
Equilibrate column: with 10 CV buffer A1 or A2 (see
note below).
Inject: MAb sample either as is or diluted 1:10 into
buffer A1 or A2 (see below).
14
Page 20

Wash: buffer A1 or A2 until effluent absorbance
returns to baseline.
Elute: in a 10 CV linear gradient to 100% B, or
Desired % buffer B.
Strip: with 5 CV buffer B.
Use buffer A1 for binding human and guinea pig
IgG. Use buffer A2 for all others. The recommended
column equilibration interval is excessive under
most conditions, but should be used as a default
until specific equilibration requirements are established
for your particular system. In addition, be aware of
solubility limitations of antibodies that require high
salt concentrations (A2 buffer) for binding.
Characterize product solubility thoroughly under
loading conditions. If the antibody fails to remain
fully soluble for the longest possible duration from
equilibration to completion of sample load when
adjusted to load conditions, then load by using
online dilution technique.
15
Page 21

Initial selectivity screening should be conducted with
a linear gradient. Knowledge of a monoclonal's
subclass may suggest a particular range of conditions,
but variation from one monoclonal to another is
sufficient to risk incomplete or no elution.
The choice of citrate for the low pH buffer is
predicated on the broad pH range achievable with
phosphate/citrate systems. If a higher pH range is
required, add boric acid to the binding buffer.
(Note: if CHT™ ceramic hydroxyapatite is used as
the subsequent polishing column, once scouting is
finished replace citrate with glycine or other
nonchelating salt for further process development.
Citrate buffers are incompatible with CHT.)
4.2 Scaling Up the Separation
For quick scale up, two or three cartridges of the
same type can be connected in series.
Backpressure will increase with cartridges in series,
so care should be taken to maintain pressures
≤45 psi.
16
Page 22

Bio-Scale™ Mini cartridges are available in 1 and 5 ml
cartridge format. The UNOsphere SUPrA media are
also available in larger amounts from 25 ml bottles
to bulk quantities.
Section 5
Care of the Cartridge
5.1 Cleaning
During operation it is recommended that the column
bed is periodically cleaned to remove bound
substances that can adversely impact the separation
performance of the column. The accumulated
substances fall into two general categories: a)
difficult to remove precipitated or denatured substances,
and b) substances that are hydrophobically bound
to the column bed. To ensure that all bound substances
are released and washed out of the column, the
following cleaning-in-place (CIP) protocols are
recommended.
17
Page 23

CIP Protocols
The following protocols are suggested to remove
precipitated or denatured substances from the bed.
Wash the bed with 2–5 column volumes in reverse
flow with one of the following solutions:
• 6 M guanidine hydrochloride
• 10 mM hydrochloric acid
• 0.1 M sodium hydroxide
• 1 M acetic acid/20% ethanol
Followed by a reverse flow wash with at least
5 column volumes of binding buffer, neutral pH (7–8).
To remove any hydrophobically bound substances
from the bed, wash the column with 2–5 column
volumes in reverse flow of a nonionic
surfactant/detergent, followed by a reverse-flow wash
with at least 5 column volumes of neutral pH binding
buffer.
18
Page 24

Suggested contact time per cycle is 15 min at room
temperature.
5.2 Sanitization
To reduce the potential for microbial contamination
of the cartridge, the column can be periodically
washed with a solution consisting of 0.1 M sodium
hydroxide. Allow to stand for 1 hour, then wash with
buffer until a neutral pH is reached.
5.3 Storage
To store UNOsphere SUPrA™ for long periods,
equilibrate the media with a 20% ethanol/water
solution and store at 4ºC.
5.4 Autoclaving
Bio-Scale™ Mini cartridges are not autoclavable.
19
Page 25

Section 6
Technical Assistance
For additional information and technical assistance,
contact your local Bio-Rad representative as listed
on the back cover of our catalog, or in the U.S., call
technical support at 1-800-4BIORAD.
20
Page 26

Section 7
Ordering Information
Bio-Scale™ Mini Cartridges*
Description 5 x 1 ml 1 x 5 ml 5 x 5 ml 1 x 1 ml
UNOsphere™Q Support 732-4100 731-4102 731-4104
UNOsphere S Support 732-4110 731-4112 731-4114
UNOsphere Rapid S 732-4400 732-4401 732-4402
UNOsphere SUPrA 732-4201 732-4202 — 732-4200
Macro-Prep®High Q Support 732-4120 732-4122 732-4124
Macro-Prep High S Support 732-4130 732-4132 732-4134
Macro-Prep DEAE Support 732-4140 732-4142 732-4144
Bio-Gel®P-6 Support — 732-4502 732-4504
Affi-Prep®Protein A Support 732-4600 732-4602 —
Profinity™IMAC Support 732-4610 732-4612 732-4614
Affi-Gel®DEAE Blue Support — 732-4632 732-4634
Affi-Gel Blue Support — 732-4642 732-4644
* For the most up-to-date list of cartridge offerings, please visit
us online at www.bio-rad.com/cartridges/.
• Larger package sizes of media are available for process-scale
chromatography. Inquire with your local Bio-Rad representative.
21
Page 27

Fittings Kits
22
Catalog # Description
732-0111 Luer to M6 Adaptor Fittings Kit, includes Luer to
732-0112 Luer to 10–32 Adaptor Fittings Kit, includes Luer
732-0113 Luer to BioLogic™ System Fittings Kit, includes
M6 fitting to connect to an FPLC system
to polypropylene/PTFE 10–32 fittings to connect
1 cartridge to an HPLC system
¼–28 female to male Luer and ¼–28 female to
female Luer to connect one cartridge to the
BioLogic DuoFlow™ system
Page 28

Section 8
References
1. Harris ELV and Angal S, Protein Purification
Methods: A Practical Approach, IRL Press,
Oxford (1989)
2. Scopes RK, Protein Purification: Principles and
Practice (Second Edition), Springer-Verlag, New
York (1987)
3. Snyder LR and Kirkland JJ, Introduction to
Modern Liquid Chromatography (Second
Edition), Wiley, New York (1979)
4. Gagnon P, Avoiding Instrument-associated
Aberrations in Purification Scale-up and
Scale-down, BioPharm 10, 42–45 (1997)
5. Gagnon P, Purification Tools for Monoclonal
Antibodies Validated Bio Systems (1996)
23
Page 29

FPLC is a trademark of GE Healthcare Group Companies.
Luer-Lok is trademark of Becton, Dickinson and Co. Triton
is a trademark of Union Carbide Corp. Upchurch is a trademark
of Upchurch Scientific.
24
Page 30

Page 31

Page 32

Bio-Rad
Laboratories, Inc.
Life Science
Group
Bulletin 0000 Rev A US/EG
Web site www.bio-rad.com USA 800 4BIORAD
Australia 61 02 9914 2800 Austria 01 877 89 01 Belgium 09 385 55 11
Brazil 55 21 3237 9400 Canada 905 364 3435 China 86 21 6426 0808
Czech Republic 420 241 430 532 Denmark 44 52 10 00
Finland 09 804 22 00 France 01 47 95 69 65 Germany 089 318 84 0
Greece 30 210 777 4396 Hong Kong 852 2789 3300
Hungary 36 1 455 8800 India 91 124 4029300 Israel 03 963 6050
Italy 39 02 216091 Japan 03 6361 7000 Korea 82 2 3473 4460
Mexico 52 555 488 7670 The Netherlands 0318 540666
New Zealand 0508 805 500 Norway 23 38 41 30 Poland 48 22 331 99 99
Portugal 351 21 472 7700 Russia 7 495 721 14 04
Singapore 65 6415 3188 South Africa 27 861 246 723
Spain 34 91 590 5200
Taiwan 886 2 2578 7189 United Kingdom 020 8328 2000
Sweden 08 555 12700 Switzerland 061 717 95 55
00-0000 0000 Sig 0308
10014997 Rev B
 Loading...
Loading...