Page 1
©2014 Welch Allyn, Inc.
MM 721357 Ver. C
Standard laryngoscope handles
Directions for use
1
Intended
About this
use
The
laryngoscope blades which are used to examine
placement of a tracheal
document
This directions for use apply to Welch Allyn reusable standard laryngoscope handles REF:
60200, 60300, 60400, 60305. Welch Allyn
used
MacIntosh
Warnings and
WARNING: Welch Allyn reusable standard laryngoscope handles must be reprocessed
after each use.
WARNING: The reprocessing procedure and the equipment and materials described
must be followed and conducted by persons trained and familiar
reprocessing.
WARNING: Consult cleaning and disinfecting agent manufacturer
proper preparation and use.
WARNING: Repeated
inspection procedures to assure damage has not occurred to the handle.
WARNING: High level disinfection and/or sterilization are not achieved by these
methods.
WARNING: Discard any component that shows evidence of damage or
deterioration.
WARNING: Do not modify this equipment. Any modification of this
lead to patient injury. Any modification of this equipment voids the
WARNING: Personnel shall
appropriate personal protective equipment when handling
equipment.
WARNING:
CAUTION:
CAUTION: Do not immerse/soak handle, damage to handle may occur.
CAUTION: If the device will be unused for several months or longer, remove
batteries prior to storing the device.
standard laryngoscope handle is an accessory used w i t h c o m p at i b l e r i g i d st a n d a r d
and visualize
tube.
reusable standard
with
Welch Allyn standard laryngoscope blades MacIntosh
REF:
6924X, and Miller REF: 6804X and REF 68470.
a patient’s airway
laryngoscope handles may be
REF:
and
aid
6904X, English
cautions
with
medical device
reprocessing may
follow their facility policies
Laryngoscope equipment is not suitable for use in intense
Failure
to follow these instructions may cause damage to this han d l e .
degrade
elements of the handle.
and
procedures
potentially
instructions
product warranty.
and
contaminated
magnetic
for their
Follow
equipment may
wear
the
fields
Page 2
2
Directions for use
Welch Allyn standard laryngoscope handles
Reprocessing
instructions
These reprocessing instructions refer to procedures for cleaning and intermediate level
disinfection.
between each use using
• Cleaning
Standard
laryngoscope handles must be reprocessed prior to first use and
the
and intermediate level
following method as outlined in this
disinfection
Welch Allyn has validated the above instruction as being capable of preparin g these
laryngoscope handles for re-use. The user must ensure that the reprocessing as actually
performed by the user's personnel, with the user's equipment and materials, achieves the
desired result. This may require validation and routine monitoring of the user's actual proce ss
NOTE: The main handle and bottom cap components of handles marked “AUTOCLAVE” are
compatible with the autoclave methods identified which are provided for facilities who wish to
autoclave after cleaning and intermediate level disinfection.
Cleaning and intermediate level disinfection instructions
Point of use: 1.
Preparation
for
decontamination:
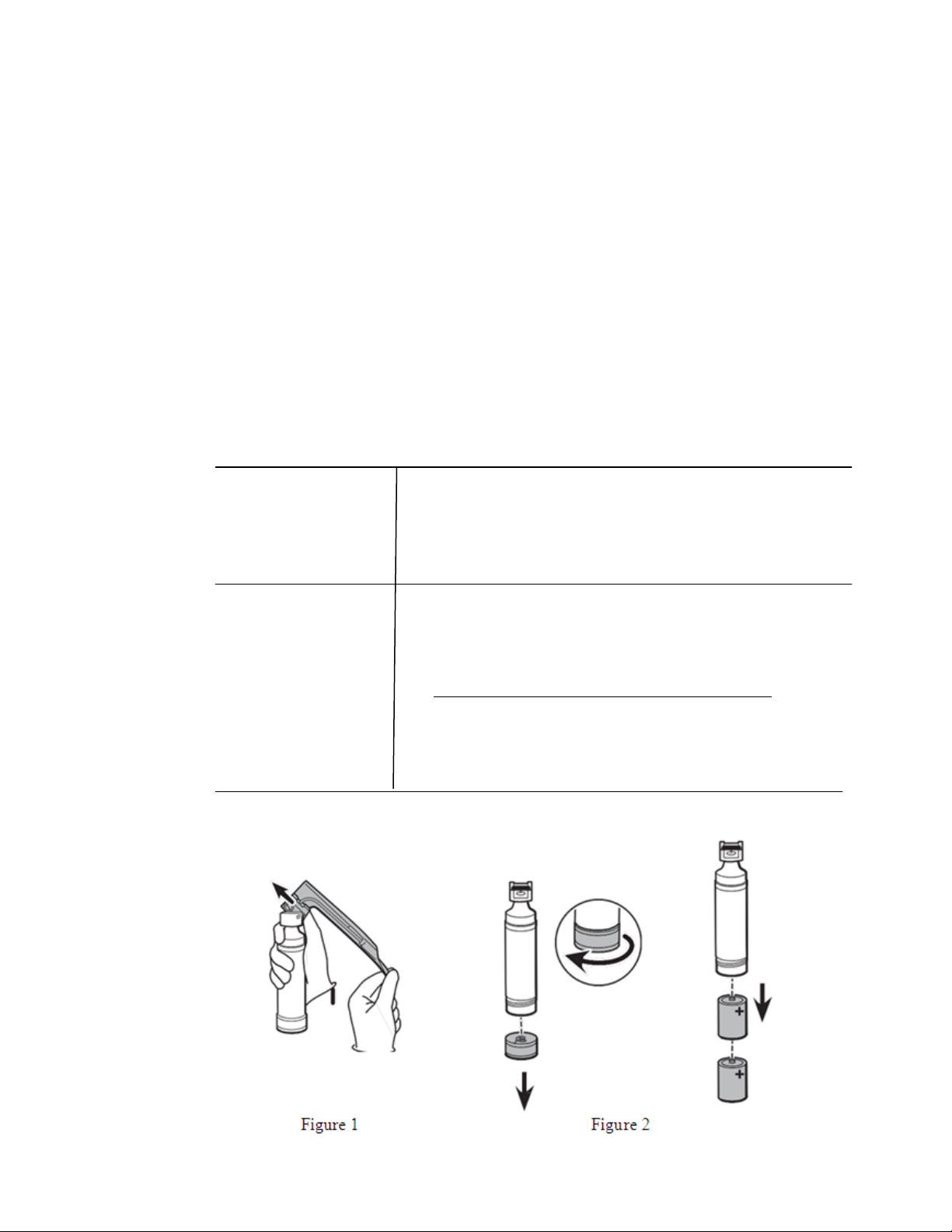
Separate
blade assembly from handle and place handle
into suitable containment for subsequent reprocessing
per figure 1. Do not place handle with sharp devi ce s.
2. Prevent the handle from drying (i.e. wrap/cover in moist
germicidal
wipe).
1. Select an appropriate quaternary ammonium
isopropanol based germicidal cleaner labeled suitable
for use on healthcare equipment and capable
intermediate level disinfection. Reference EPAregistered disinfectants:
http://www.epa.gov/oppad001/chemregindex.htm
Outside of the U.S., please consult applicable
regulatory body for equivalent quaternary ammonium
isopropanol germicidal cleaner.
2. Remove batteries per figure 3.
document:
Page 3

Directions for use
Cleaning
and
intermediate level
disinfection:
Drying:
Maintenance,
Inspection and Testing
Storage: Store handle per facility practice to allow device to remain
End of
reprocessing
Welch Allyn standard laryngoscope handles
3
1. Follow the ge r m i c i da l w i p e manufacturer’s instructions
to clean all exposed surfaces of the handle and end
cap.
2. If necessary, brush with a dry, soft-bristled brush and
re-wipe to loosen/remove excessive visible soil.
3. After all visible soil is removed, re-wipe to wet all
surfaces and allow adequate contact time for
disinfection as directed by the germicidal wipe
manufacturer.
CAUTION: Only use quaternary ammonium
isopropanol based germicidal wipes.
Allow components to air dry.
1. Inspect
each
component
area (per f
igure
3)
for
damage
or
deterioration.
WARNING: Discard any component that
shows
evidence of damage or deterioration.
Contact Welch Allyn for component replacement.
2. Reassemble batteries into handle per figure 4 with
new or batteries in known good condition.
3 . Attach handle to a clean and disinfected test blade
assembly in known working condition. Verify
•
Blade assembly engages and locks onto handle.
•
Blade assembly deploys into its locked position on
handle
•
Light output is satisfactory.
If the la mp fails to
replace the
AND lamp illuminates.
light or output is low
batteries.
that:
, check or
clean, dry, and ready for service.
instructions for intermediate level disinfection.
Page 4

4
Directions for use
Autoclave
Welch Allyn standard laryngoscope handles
instructions
NOTE: The main handle and bottom cap components of handles marked “AUTOCLAVE” are
compatible with the autoclave methods identified which are provided for facilities who wish to
autoclave after cleaning and intermediate level disinfection.
Disassembly: Remove batteries per figure 2 and set aside.
After battery removal, select ONE of the following autoclave methods below
main handle and bottom cap (only):
Gravity autoclave: Follow equipment manufacturer and
set-up and operation of autoclave e quipment. Gravity autoclave settings are as
follows:
•
Temperature: 132 C (270 F)
•
Exposure time: 3 minutes (unwrapped)
•
Minimum dry time: 1
minute
Pre-vacuum autoclave: Follow equipment manufacturer and
set-up and operation of autoclave e quipment.
follows:
•
Temperature: 132 C (270 F)
•
Exposure time: 3 minutes (unwrapped)
•
Minimum dry time: 1
minute
Pre-vacuum
each
Maintenance,
Inspection and Testing
1. Inspect
deterioration.
component
WARNING: Discard any component that
evidence of damage or deterioration.
Contact Welch Allyn for component replacement.
2. Reassemble b a t t e r i e s i n t o h andle per figure 4.
3. Attach handle to a clean and disinfected test blade
assembly in known working condition. Verify
•
Blade assembly engages and locks onto handle.
•
Blade assembly deploys into its locked position on
handle AND lamp
•
Light output is satisfactory.
If the la mp fails to
replace the
illuminates.
batteries
light or output is low
Storage: Store handle per facility practice to allow device to remain
clean, dry, and ready for service.
facility
procedures in the
facility
procedures in the
autoclave settings are as
area per f
igure 3 for
damage
shows
, check or
for
that:
the
or
Page 5

Directions for use
Welch Allyn standard laryngoscope handles
5
Maintenance
Replace
the
batteries
1. Unscrew bottom cap of handle per figure 5 and remove batteries.
2. Alkaline batteries are supplied with your handle for maximum performance and
3. Insert batteries and reinstall bottom cap per figure 5.
4. Reprocess repaired assembly as appropriate per these instructions
instructions
are recommended as replacements; however
used.
•
Large handle, REF 60200 uses two “D” size
•
Medium handle, REF 60300 uses two “C” size
•
Penlight handle, REF 60400 uses two “AA” size
•
Stubby handle, REF 60305 uses two “AA” size
carbon-zinc
batteries may also be
Figure 5
Page 6

6
Directions for use
Specifications
Electrical:
For information about electromagnetic compatibility (EMC) see Welch Allyn website:
http://www.welchallyn.com
Operating:
32
(0 C)
Approvals:
Con fo rm s t o A S TM F 965 and
Warranty:
One year
Service Information:
For
Welch Allyn
Storage/Transport:
104 F
(40 C)
F
-4 F
(-20 C)
120 F
(49 C)
ISO-7376-1, IEC/EN
The
CE
mark on this product indicates that it has been tested to and
with the provisions noted within the
Complies with EMC Framework of Australia
Technical
Support or to obtain information about any Welch Allyn product,
Technical
Support: w
ww.welchallyn.com/support.
93/42/EEC
Welch Allyn standard laryngoscope handles
60601-1,
Medical Device
IEC/EN
60601-1-2
conforms
Directive.
contact
Welch Allyn, Inc.
4341 State Street Road
Skaneateles Falls, NY 13153-0220 USA
www.welchallyn.com
Authorized European Repre sentative Address:
Regulatory Affairs Representative
Welch Allyn, Limited
Navan Business Park
Dublin Road
Navan, County Meath, Republic
of Ireland
 Loading...
Loading...