
UPDATE
Propaq CS® Directions For Use
Update for Software Version 3.3X
Propaq Encore® Reference Guide
Update for Software Version 2.3X
Keep this update in the back pocket of your manual.

Copyright © 2001 by Welch Allyn Protocol, Inc.
Protocol
Protocol, Inc. Welch Allyn Protocol, Inc. is protected under various patents and patents pending. Masimo® and
SET
NELLCOR®, DURASENSOR®, C-LOCK® and OXISENSOR® are registered trademarks of Mallinckrodt, Inc.
Copyright Notice:
All rights are reserved. The software is protected by United States of America copyright laws and international
treaty provisions applicable all over the world. Under such laws, you are licensed to use the copy of the
, Propaq®, Propaq® CS, Acuity®, and Flexible Monitoring® are registered trademarks of Welch Allyn
®
are registered trademarks and Signal Extraction Technology™ is a trademark of Masimo Corporation.
®
Software in this Product is Copyright© 2001 by Welch Allyn Protocol, Inc., or its vendors.
Welch Allyn® is a registered trademark of Welch Allyn, Inc.
software incorporated with this instrument as intended in the operation of the product in which it is embedded,
but the software may not be copied, decompiled, reverse-engineered, disassembled or otherwise reduced to
human-perceivable form. This is not a sale of the software or any copy of the software; all right, title and
ownership of the software remains with Welch Allyn Protocol or its vendors. Welch Allyn Protocol will make
available specifications necessary for interoperability of this software on request; however, users should be
aware that use of Welch Allyn Protocol hardware and software with devices or software not sold by Welch Allyn
Protocol or its authorized dealers and affiliates may lead to erroneous results and consequent danger in patient
care, and may also void Welch Allyn Protocol's warranty.
Disclaimers:
Welch Allyn Protocol, Inc. cautions the reader of this manual:
• This manual may be wholly or partially subject to change without notice.
• All rights are reserved. No one is permitted to reproduce or duplicate, in any form, the whole or part of this
manual without permission from Welch Allyn Protocol, Inc.
• Welch Allyn Protocol, Inc. will not be responsible for any injury to the user or other person(s) that may result
from accidents during operation of the Propaq monitor.
• Welch Allyn Protocol, Inc. assumes no responsibility for usage not in accordance with this manual that
results in illegal or improper use of the Propaq monitor.
• No implied license: Possession or purchase of this device does not convey any express or implied license to
use the device with unauthorized sensors or cables which would, alone, or in combination with this device,
fall within the scope of one or more of the patents relating to this device.
For information concerning this document or any Welch Allyn Protocol product, contact:
Welch Allyn Protocol, Inc.
Customer Service
8500 SW Creekside Place
Beaverton, Oregon 97008-7107 USA
Within USA, toll free:
Phone: (800) 289-2500
Phone Technical Services: (800) 289-2501
WorldWide:
Phone: (503) 526-8500
Fax: (503) 526-4200
Fax Technical Services: (503) 526-4910
Internet:
http://www.protocol.com
Email Technical Services: solutions@protocol.com
Email Marketing Dept.: marketing@protocol.com
Protocol Medical Systems, Ltd.: NW Europe
Derby Service Centre, St. Georges House, Vernon
Gate
Derby DE1 1UQ, United Kingdom
Phone: 44 1332 206208 Fax: 44 1332 206209
Email: uk@protocol.com
Welch Allyn U.K. Ltd.
Cublington Road, Aston Abbotts
Buckinhamshire HP22 4ND, England
Phone: 44-1296-682140 Fax: 44-1296-682104
0123
Welch Allyn Italia Srl
Via Napo Torriani, 28, 20124 Milano, Italy
Phone: 39-02-6699-291 Fax: 39-02-6671-3599
Welch Allyn GmbH Germany
Postfach 31, Zollerstrasse 2-4
72471 Jungingen, Germany
Phone: 49-7477-92-710 Fax: 49-7477-92-7190
Welch Allyn France
814 Rue Charles de Gaulle
77100 Mareuil les Meaux, France
Phone: 01-6009-3366 Fax: 01-6009-6797
Welch Allyn – South Asia and Pacific
P.O. Box 39-293 Howick, Auckland, New Zealand
Phone: 64-9-532-9524 Fax: 64-9-532-9526
Welch Allyn Hong Kong
Room 601, Kornhill Metro Tower
1 Kornhill Road, Quarry Bay, H.K.
Phone: 852-2886-8980 Fax: 852-2886-8360
Welch Allyn Protocol, Inc. – Latin America
MD Intl., 7324 SW 48th Street, Miami, FL 33155 USA
Phone: (305) 669-9003 Fax: (305) 669-8951
Update Part No: 810-1285-01
Rev. A 09/01
Printed in USA

Contents
English . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
About this Update. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
General Warnings and Cautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
SpO2 Monitoring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
NIBP Trends and SEARCH Message. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
Update Propaq CS DFU 3.3X/Propaq Encore Reference Guide 2.3X 3
English
About this Update
This Update describes the changes in the operation of the Propaq® CS
monitor (software version 3.3X) and Propaq® Encore monitor (software
version 2.3X). These changes are primarily associated with the Pulse
Oximetry (SpO2) options that are available for use with the Propaq CS and
Propaq Encore monitors:
• Masimo® Pulse Oximetry option (motion tolerant).
• Nellcor® Pulse Oximetry option (motion tolerant).
• Nellcor® Pulse Oximetry option (without motion tolerance).
For all other operating information for these monitors, refer to these
documentation kits:
• 810-1102-XX Propaq CS 3.XX Directions For Use Kit
• 810-0867-XX Propaq Encore 2.XX Reference Guide Kit
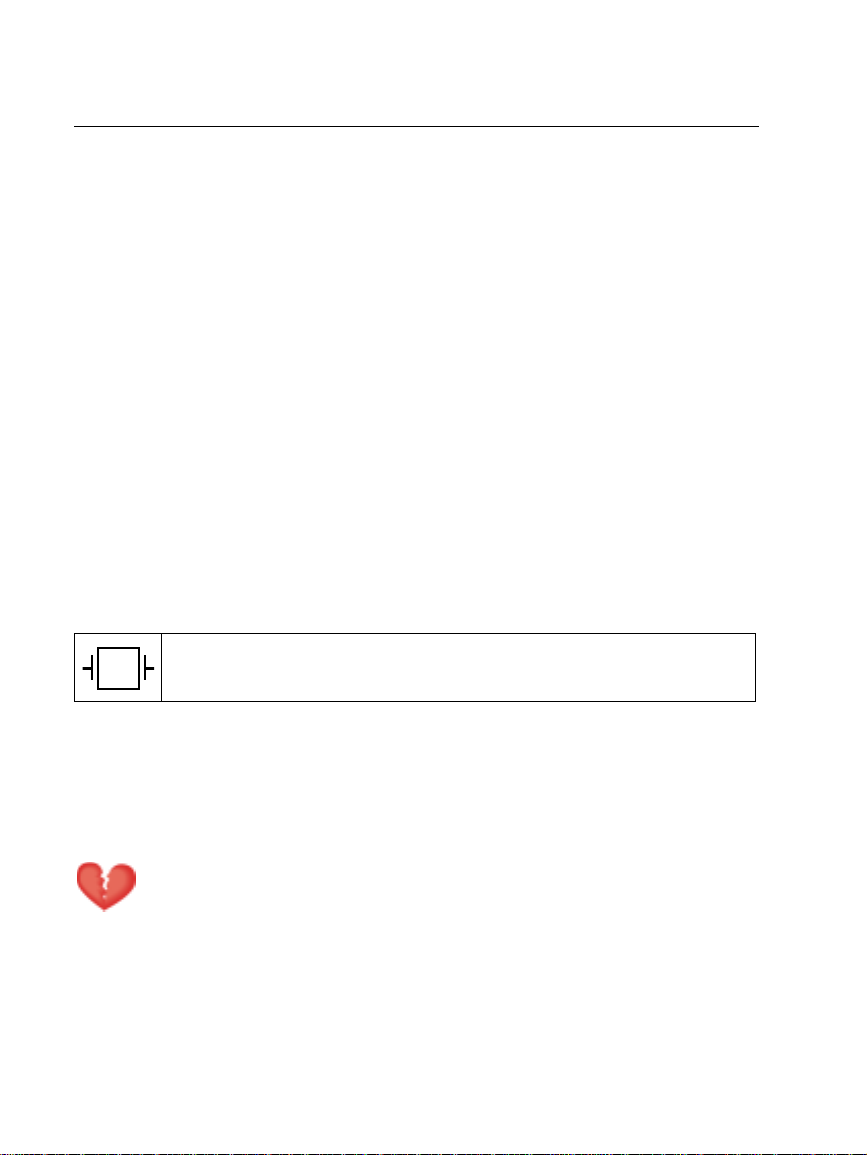
Symbols
Protected during defibrillation
General Warnings and Cautions
Familiarize yourself with all warnings and cautions before using the Propaq
monitor.
Warning
Do not operate this product in the presence of flammable anesthetics or other flammable
substance in combination with air, oxygen-enriched environments, or nitrous oxide; explosion can
result.
The pulse oximetry channel should NOT be used as an apnea monitor.
4 Welch Allyn

English (Cont.)
This monitor is to be operated by qualified personnel only. The operator of this monitor should read
this entire manual, the monitor
Directions For Use
This monitor should only be repaired by qualified service personnel. The operator should not
attempt to open the monitor case or perform any maintenance on the monitor except for
procedures explicitly described in this manual that can be performed by operators such as
inspection and cleaning.
When using a power adapter with this monitor, be sure to connect the power adapter to a threewire, grounded, hospital-grade receptacle. Do not under any circumstances attempt to remove the
grounding conductor from the power plug of the power adapter. Do not plug the power adapter into
an extension cord. If there is any doubt about the integrity of the protective earth ground of the
receptacle for the power adapter, do not plug in the power adapter; operate the monitor only on
battery power. Contact your biomedical engineering department for assistance in identifying the
proper power receptacle and making appropriate power connections.
Place the Propaq monitor and accessories in locations where they cannot harm the patient if they
fall from their shelf or mount. Lift the monitor only by its handle; do not lift it by any attached cables.
Safe interconnection between the Propaq monitor and other devices must comply with applicable
medical systems safety standards such as IEC 60601-1-1. Within certain governmental
jurisdictions, all interconnected accessory equipment must be labeled by an approved testing
laboratory. After interconnection with accessory equipment, risk (leakage) current and grounding
requirements must be maintained.
As with all medical equipment, carefully route the patient cabling to reduce the possibility of patient
entanglement or strangulation.
before operating the monitor.
Reference Guide
or
Directions For Use
, and all accessory
SpO2 Monitoring
Warning
Oxygen saturation measurements using pulse oximetry are highly dependent on proper placement
of the sensor and patient conditions. Patient conditions such as shivering and smoke inhalation
may result in erroneous oxygen saturation readings. If pulse oximetry measurements are suspect,
verify the reading using another clinically accepted measurement method, such as arterial blood
gas measurements on a co-oximeter.
Tissue damage can be caused by incorrect application or use of a sensor (e.g., wrapping the
sensor too tightly, applying supplemental tape, failing to periodically inspect the sensor site,
leaving a sensor on too long in one place). Refer to the Directions for Use provided with each
sensor for specific instructions on application and use, and for description, warnings, cautions, and
specifications.
Sensors exposed to ambient light while not applied to a patient can exhibit semi-normal saturation
readings. Be sure the sensor is securely placed on the patient and check its application often to
ensure accurate readings.
Inaccurate measurements may be caused by venous pulsations.
Update Propaq CS DFU 3.3X/Propaq Encore Reference Guide 2.3X 5
 Loading...
Loading...