Fujifilm SonoSite S-2 Service manual

i

Manufacturer
FUJIFILM SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021-3904
USA
Telephone: 1-888-482-9449 or 1-425-951-1200
Fax: 1-425-951-1201
EC Authorized Representative
FUJIFILM SonoSite B.V.
Joop Geesinkweg 140
1114 AB Amsterdam, The Netherlands
Australia Sponsor
FUJIFILM SonoSite Australasia Pty Ltd
114 Old Pittwater Rd
Brookvale, New South Wales 2100, Australia
Caution:
SonoSite SII, SonoHD2, SonoMB, SonoSite and the SONOSITE logo are registered and unregistered trademarks of FUJIFILM SonoSite, Inc. in
various jurisdictions.
DICOM is the registered trademark of the National Electrical Manufacturers Association for its standards publications relating to digital
communications of medical information.
Non-SonoSite product names may be trademarks or registered trademarks of their respective owners.
FUJIFILM is a registered and unregistered trademark of FUJIFILM Corporation in various jurisdictions.
The FUJIFILM SonoSite ultrasound system(s) referenced in this document may be covered by one or more of the following U.S. patents: US
8,861,822; US 8,858,436; US 8,834,372; US 8,805,047; US 8,439,840; US 8,398,408; US 8,355,554; US 8,216,146; US 8,213,467; US
8,147,408; US 8,137,278; US 8,088,071; US 8,066,642; US 8,052,606; US 7,819,807; US 7,804,970; US 7,740,586; US 7,686,766; US
7,604,596; US 7,591,786; US 7,588,541; US 7,534,211; US 7,449,640; US 7,169,108; US 6,962,566; US 6,648,826; US 6,575,908; US
6,569,101; US 6,471,651; US 6,416,475; US 6,383,139; US 6,364,839; US 6,203,498; US 6,135,961; US 5,893,363; US 5,817,024; US
5,782,769; US 5,722,412; AU: 730822; AU: 727381; CA: 2,372,152; CA: 2,371,711; CN 103237499B; CN 101231457B; CN 98108973.9; CN
98106133.8; CN 97113678.5; DE 69831698.3; DE 69830539.6; DE 69730563.5; DE 602004027882.3; DE 602004023816.3; DE 60034670.6;
DE 60029777.2; EP 1589878; EP 1552792; EP 1180971; EP 0875203; EP 0815793; EP 1180970; EP 0881492; ES 2229318; ES 159878; ES
1552792; ES 0881492; FR 158978; FR 1552792; FR 1180970; FR 0881492; FR 0875203; FR 0815793; GB 158978; GB 1552792; GB 1180971;
GB 1180970; GB 0881492; GB 0875203;GB 0815793; IT 1589878; IT 1552792; IT 0881492; IT 0815793; JP 5782428; JP 4696150; KR 532359;
KR 528102; NO 326814; NO 326202 and pending.
Federal (United States) law restricts this device to sale by or on the order of a physician.
P22358-02 10/2018
Copyright © 2018 FUJIFILM SonoSite, Inc.
All rights reserved.
ii

Introduction ........................................................................................1
Audience ..............................................................................................1
Contact Information..............................................................................1
Conventions .........................................................................................2
Labeling symbols..................................................................................2
.............................................................................................................7
System Specifications ..........................................................................7
System Dimensions..............................................................................7
Display Dimensions..............................................................................7
Environmental limits .............................................................................7
Operating (system, battery, and transducer)........................................7
Shipping and storage (system and transducer)....................................7
Shipping and storage (battery)............................................................. 7
Electrical specifications ........................................................................7
Battery specifications ...........................................................................8
Imaging Modes.....................................................................................8
Image and Clip Storage........................................................................8
Compatible accessories and peripherals .............................................8
Safety...................................................................................................11
Electrical safety ....................................................................................11
Electrical safety classification...............................................................13
Equipment safety..................................................................................13
Battery safety .......................................................................................14
Clinical safety .......................................................................................15
Hazardous materials ............................................................................15
Electromagnetic compatibility...............................................................16
Electrostatic discharge .........................................................................16
Separation distance..............................................................................17
Guidance and manufacturer’s declaration............................................18
Immunity testing requirements .............................................................21
Standards.............................................................................................21
Electromechanical Safety Standards ...................................................21
EMC Standards Classification..............................................................21
Acoustic standards...............................................................................21
Biocompatibility standards....................................................................21
Airborne Equipment Standards ............................................................22
DICOM Standard..................................................................................22
HIPAA Standard...................................................................................22
System Overview................................................................................23
About the System.................................................................................23
Theory of Operation .............................................................................23
Description of Operating Modes...........................................................25
Additional System Feature Performances............................................26
Front End Overview..............................................................................27
Back End Overview..............................................................................28
Control Subsystem...............................................................................29
Power Supply and Control....................................................................30
Battery Pack (VBAT) ............................................................................30
1

Battery Charger....................................................................................31
DICOM .................................................................................................31
Troubleshooting.................................................................................33
System and Subsystem Diagnosis....................................................... 33
System Repair...................................................................................... 34
Test Equipment .................................................................................... 34
Failure (Assert) Codes .........................................................................34
Verifying a System Assert Code ..........................................................34
DICOM .................................................................................................35
User Interface....................................................................................... 36
Battery..................................................................................................36
Replacement Procedures .................................................................. 37
Required Tools.....................................................................................37
User Interface Removal .......................................................................37
Required Parts .....................................................................................37
User Interface Removal .......................................................................37
User Interface Parts Transfer............................................................... 40
Install User Interface ............................................................................42
Base Assembly Disassembly for Repair and/or Replacement............. 43
Required Tools.....................................................................................44
Power Button PCA Replacement .........................................................44
Required Parts .....................................................................................44
Power Button PCA Removal ................................................................ 44
Power Button PCA Replacement .........................................................44
Back Enclosure Replacement .............................................................. 45
Back Enclosure Removal ..................................................................... 45
Back Enclosure Replacement .............................................................. 45
Internal Mini-Dock Replacement ..........................................................46
Required Parts .....................................................................................46
Internal Mini-Dock Removal ................................................................. 46
Internal Mini-Dock Replacement ..........................................................47
DTC/STC Replacement .......................................................................47
Required Parts .....................................................................................47
DTC Removal.......................................................................................48
DTC Replacement................................................................................49
Dual Fan Cable Assembly Replacement .............................................50
Required Parts .....................................................................................50
Dual Fan Cable Assembly Removal ....................................................50
Dual Fan Cable Assembly Replacement .............................................50
Power Supply PCBA Removal .............................................................51
Required Parts .....................................................................................51
Power Supply PCBA Removal .............................................................51
Power Supply PCBA Replacement ......................................................51
SD Card Replacement ......................................................................... 52
Main PCBA Replacement ....................................................................52
Required Parts .....................................................................................52
Main PCBA Removal ...........................................................................53
Main PCBA Replacement ....................................................................53
2

Mid-Frame Replacement......................................................................55
Required Parts .....................................................................................55
Mid-Frame Replacement......................................................................55
Maintenance........................................................................................57
Periodic Maintenance...........................................................................57
Performance Testing..........................................................................59
Overview ..............................................................................................59
Recommend Test Equipment...............................................................59
Setting Up Performance Tests .............................................................59
Basic Operational Tests .......................................................................60
2D Performance Tests .........................................................................60
2D Performance / Image Quality ..........................................................60
Axial Measurement Accuracy...............................................................61
Lateral Measurement Accuracy............................................................61
Penetration...........................................................................................62
Additional Performance Tests ..............................................................62
Color Doppler (Color) ...........................................................................62
Color Power Doppler (CPD) .................................................................63
M Mode Imaging...................................................................................63
Tissue Harmonic Imaging.....................................................................63
Image Quality Verification Test/Livescan .............................................63
Printer...................................................................................................64
Battery Charging ..................................................................................64
Video Output ........................................................................................64
Replacement Parts List......................................................................65
Base Assembly Components ...............................................................65
Additional Base Assembly Components ..............................................67
User Interface Components .................................................................68
Parts Unique to STC Systems..............................................................69
Enclosure Parts....................................................................................70
Transducer Nest Frame Assembly.......................................................71
Ordering Replacement Parts................................................................72
Service Event Reporting....................................................................73
Service Event Report Form.................................................................. 74
Service Event Report Instructions ........................................................75
Returning Products to FUJIFILM SonoSite ..........................................76
Shipping Instructions............................................................................76
3

4

Chapter 1: Introduction
Before servicing the SonoSite SII ultrasound system, please read this manual.
The ultrasound system has multiple configurations and feature sets. All are described in this service manual
but not every option may apply to your system. System features depend on your system configuration,
transducer, and exam type.
Refer to the SII Ultrasound System User Guide for additional information regarding safety, system controls,
operation, capabilities, and specifications.
Audience
The intended audience of this manual is properly trained field and in-house service personnel.
Contact Information
Questions and comments are encouraged. FUJIFILM SonoSite is interested in your feedback regarding the
service manual. If you encounter difficulty with the system, use the information in this manual to help correct
the problem. If the problem is not covered here, contact FUJIFILM SonoSite Technical Support as follows:
Technical Support (USA, Canada) 1-877-657-8118
Technical Support fax: 1-425-951-6700
Technical Support e-mail: ffss-service@fujifilm.com
FUJIFILM SonoSite website: www.sonosite.com
International Technical Support: Contact your local representative or call (USA)
+425-951-1330
European Service Center United Kingdom
Tel: 0044 01462341151
e-mail: uk-service@fujifilm.com
France
Tel: 0033 01/82880702
e-mail: ERAF-service@fujifilm.com
Germany
Tel: 0049 069/80884030
e-mail: ERAF-service@fujifilm.com
Spain
Tel: 0034 911238451
e-mail: ERAF-service@fujifilm.com
Italy
Tel 0039 02 94753655
e-mail: ERAF-service@fujifilm.com
Asia/Pacific Tel: (+65) 63805581
e-mail: FFSS-APACME-service@fujifilm.com
Chapter 1: Introduction 1

Conventions
LOT
REF
These conventions are used in this service manual:
•A WARNING describes precautions necessary to prevent injury or loss of life.
•A Caution describes precautions necessary to protect the products.
• Numbered steps must be performed in a specific order.
• Bulleted lists present information in list format but do not imply a sequence.
Labeling symbols
The following symbols are used on the products, packaging, and containers.
Table 1: Labeling Symbols
Symbol Definition
Alternating Current (AC)
Class 1 device indicating manufacturer’s declaration of conformance with
Annex VII of 93/42/EEC
Class 1 device requiring verification by the Notified Body of sterilization or
measurement features, or to a Class IIa, IIb, or III device requiring
verification or auditing by the Notified Body to applicable Annex(es) of
93/42/EEC
Attention, see the user guide
Follow instructions for use.
Device complies with relevant Australian regulations for electronic devices.
Batch code, date code, or lot code type of control number
Biological risk
Authorized representative in the European Community
Canadian Standards Association. The “C” and “US” indicators next to this
mark signify that the product has been evaluated to the applicable CSA
and ANSI/UL Standards, for use in Canada and the US, respectively.
Catalog number
2 Chapter 1: Introduction
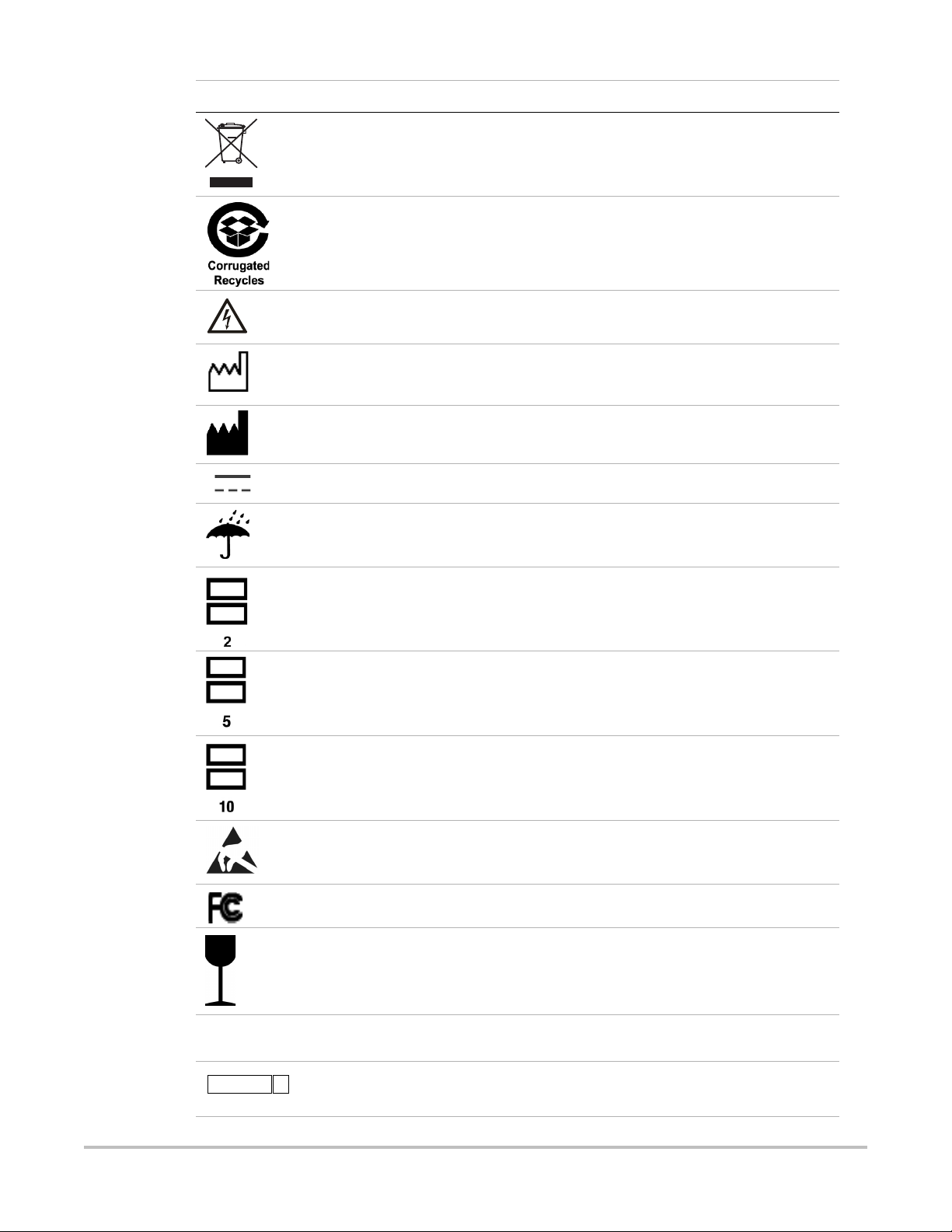
Table 1: Labeling Symbols (Continued)
STERILE R
Symbol Definition
Collect separately from other household waste (see European Commission
Directive 93/86/EEC). Refer to local regulations for disposal.
Corrugated recycle
Dangerous voltage
Date of manufacture
Manufacturer
Direct Current (DC)
Do not get wet.
Do not stack over 2 high.
Do not stack over 5 high.
Do not stack over 10 high.
Electrostatic sensitive devices
Device complies with relevant FCC regulations for electronic devices.
Fragile
GEL Gel
Sterilized using irradiation
Chapter 1: Introduction 3

Table 1: Labeling Symbols (Continued)
STERILE EO
SN
Symbol Definition
Sterilized using ethylene oxide
Hot
Indoor use only.
Non-ionizing radiation
Paper recycle
Serial number type of control number
Temperature limitation
Atmospheric pressure limitation
Humidity limitation
Submersible. Protected against the effects of temporary immersion.
Water-Tight Equipment. Protected against the effects of extended
immersion.
Handle transducer with care.
To avoid tipping, do not move the system using the handle on the front of
the SonoSite SII ultrasound system.
When moving the system, push the stand using the tray assembly.
Type BF patient applied part
(B = body, F = floating applied part)
Defibrillator proof type CF patient applied part
A mandatory action that the user shall read the accompanying
documentation for more information.
4 Chapter 1: Introduction

Table 1: Labeling Symbols (Continued)
Symbol Definition
Pollution Control Logo. (Applies to all parts/products listed in the China
RoHS disclosure table. May not appear on the exterior of some
parts/products because of space limitations.)
China Compulsory Certificate mark (“CCC Mark”). A compulsory safety
mark for compliance to Chinese national standards for many products sold
in the People’s Republic of China.
Maximum weight load
WARNING:
Connect Only
Accessories and
Peripherals
Recommended
by FUJIFILM
SonoSite
WARNING: Connect Only
Accessories and Peripherals
Recommended by FUJIFILM SonoSite
Chapter 1: Introduction 5

6 Chapter 1: Introduction

Chapter 2:
Specifications
System Specifications
This chapter contains information regarding system specifications and accessory compatibility. The
information applies to the ultrasound system, transducers, accessories, and peripherals.
System Dimensions
Height: 17.6 in. (44.7 cm)
Max height with stand: 59.5 in. (151 cm)
Min height with stand: 49 in. (124.5 cm)
Width: 11.3 in. (28.7 cm)
Depth: 4.8 in. (12.2 cm)
Weight:12.5 lbs. (5.7 kg)
Weight with stand 57.5 lbs. (26.1 kg)
Display Dimensions
Length: 9.7 in. (24.6 cm)
Height: 7.3 in. (18.5 cm)
Diagonal: 12.1 in. (30.7 cm)
Environmental limits
Note: The temperature, pressure, and humidity limits apply only to the ultrasound system and
transducers.
Operating (system, battery, and transducer)
10–40°C (50–104°F), 15–95% R.H.
700 to 1060hPa (0.7 to 1.05 ATM)
Shipping and storage (system and transducer)
-20–60°C (-4–140°F), 15–95% R.H.
500 to 1060hPa (0.5 to 1.05 ATM)
Shipping and storage (battery)
-20–60°C (-4–140°F), 15–95% R.H. (For storage longer than 30 days, store at or below room temperature.)
500 to 1060hPa (0.5 to 1.05 ATM)
Electrical specifications
Power Supply Input: 100-240 VAC, 50/60 Hz, 2.0 A Max @ 100 VAC.
Power Supply Output 1: 15 VDC, 5.0A Max (system)
Power Supply Output 2: 12 VDC, 2.3A Max (battery)
Combined output not exceeding 75W.
Chapter 2: 7

Battery specifications
The battery is comprised of six lithium-ion cells plus electronics, a temperature sensor, and battery contacts.
Run time is up to two hours, depending on imaging mode and display brightness.
Imaging Modes
2D (256 gray shades)
Color power Doppler (CPD) (256 colors)
Color Doppler (Color) (256 colors)
M Mode
Tissue Harmonic Imaging (THI)
Image and Clip Storage
The number of images and clips you can save varies with imaging mode and file format.
Compatible accessories and peripherals
FUJIFILM SonoSite has tested the SonoSite SII ultrasound system with the following accessories and
peripherals and has demonstrated compliance to the requirements of IEC60601-1-2:2007.
You may use these FUJIFILM SonoSite accessories and third-party peripherals with the SonoSite SII
ultrasound system.
WARNING:
WARNING:
Accessories and peripherals compatible with SonoSite SII ultrasound system
Description Part Number Maximum Cable Length
C11x transducer P07678 6.0 ft/1.8 m
C35x transducer P21981 6.0 ft/1.8 m
rC60xi transducer P21070 5.5 ft/1.7 m
rC60xi transducer armored P21636 5.5 ft/1.7 m
rP19x transducer P21015 6.0 ft/1.8 m
rP19x transducer armored P21556 6.0 ft/1.8 m
HFL38xi transducer P20311 5.5 ft/1.7 m
HFL38xi transducer armored P20377 5.5 ft/1.7 m
HFL50x transducer P07693 5.5 ft/1.7 m
Use of the accessories with medical systems other than the SonoSite SII
ultrasound system may result in increased emissions or decreased
immunity of the medical system.
Use of accessories other than those specified may result in increased
emissions or decreased immunity of the ultrasound system.
HSL25x P20679 7.5 ft/2.3 m
ICTx transducer P07690 5.5 ft/1.7 m
L25x transducer P07691 7.5 ft/2.3 m
8 Chapter 2:

Accessories and peripherals compatible with SonoSite SII ultrasound system (Continued)
L25x transducer armored P22950 7.5 ft/2.3 m
L38xi transducer P12742 5.5 ft/1.7 m
L38xi transducer armored P19626 5.5 ft/1.7 m
L52x transducer (Vet) V00033 7.5 ft/2.3 m
L52x transducer armored (Vet) V20962 7.5 ft/2.3 m
P10x transducer P07696 6.0 ft/ 1.8m
P11x transducer P16665 6.5 ft/2.0 m
Bar code scanner P14166 4.8 ft/1.5 m
Kit, PowerPack P13559 —
Battery for PowerPack P13123 —
Black & white printer P13745 —
Hybrid black & white printer P20006
Black & white printer power
— 3.3 ft/1 m
cable
Black & white printer USB
— 10.8 ft/3.3 m
cable
Dual USB Footswitch P14689 9.8 ft/3.0 m
SII Stand P21432 —
Storage bin kit, Universal/Edge
P22048 —
stands
Power cord (system) P00848 (USA) 10 ft/3 m
Power Supply/Battery Charger P09823 6.8 ft/ 2 m
PowerPark P12822 —
USB wireless adapter P17725 —
EU compliant wireless adapter P22625
SII Battery Pack P20664 —
Wireless mounting kit P17724
Chapter 2: 9

10 Chapter 2:

Chapter 3: Safety
This chapter contains electrical and clinical safety information required by regulatory agencies. The
information applies to the ultrasound system, transducers, accessories, and peripherals.
Electrical safety
This system meets EN60601-1, Class I/internally-powered equipment requirements and Type BF and Type
CF isolated patient-applied parts safety requirements.
This system complies with the applicable medical equipment requirements published in the Canadian
Standards Association (CSA), European Norm Harmonized Standards, and Underwriters Laboratories (UL)
safety standards. See “Standards” on page 21.
For maximum safety observe the following warnings and cautions.
WARNING:
WARNING:
WARNING:
To avoid the risk of injury, do not operate the system in the presence of
flammable gasses or anesthetics. Explosion can result.
To avoid the risk of electrical shock or injury, do not open the system
enclosures. All internal adjustments and replacements, except battery
replacement, must be made by a qualified technician.
To avoid the risk of electrical shock:
• This equipment must be connected only to a supply mains with protective
earth.
• Use only properly grounded equipment. Shock hazards exist if the power
supply is not properly grounded. Grounding reliability can be achieved only
when equipment is connected to a receptacle marked “Hospital Only” or
“Hospital Grade” or equivalent. The grounding wire must not be removed or
defeated.
• When using the system in an environment where the integrity of the protective
earth conductor arrangement is in doubt, operate the system on battery
power only and disconnect the power supply.
• Do not let the bar code scanner touch the patient.
• Do not touch any of the following:
• The power supply and the patient at the same time
• The ungrounded signal input/output connectors on the back of the
ultrasound system
• The system battery contacts (inside the battery compartment)
• The system transducer connector when the transducer is disconnected
Chapter 3: Safety 11

• Do not connect the system power supply or docking system to a multiple
portable socket outlet (MPSO) or extension cord.
• Before using the transducer, inspect the transducer face, housing, and cable.
Do not use the transducer if the transducer or cable is damaged.
• Always disconnect the power supply from the system before cleaning the
system.
• Do not use any transducer that has been immersed beyond the specified
cleaning or disinfection level. See Chapter 7, “Maintenance”
• Use only accessories and peripherals recommended by FUJIFILM SonoSite,
including the power supply. Connection of accessories and peripherals not
recommended by FUJIFILM SonoSite could result in electrical shock. Contact
FUJIFILM SonoSite or your local representative for a list of accessories and
peripherals available from or recommended by FUJIFILM SonoSite.
WARNING:
WARNING:
WARNING:
Caution:
To avoid the risk of electrical shock and fire hazard:
• Inspect the power supply, AC power cords, cables, and plugs on a regular
basis. Ensure that they are not damaged.
• The power cord set that connects the power supply of the ultrasound system
or the stand to mains power must only be used with the power supply or
docking system, and cannot be used to connect other devices to mains
power.
To prevent injury to the operator/bystander, the transducer must be removed
from patient contact before the application of a high-voltage defibrillation pulse.
To avoid possible electrical shock or electromagnetic interference, verify proper
operation and compliance with relevant safety standards for all equipment
before clinical use. Connecting additional equipment to the ultrasound system
constitutes configuring a medical system. FUJIFILM SonoSite recommends
verifying that the system, all combinations of equipment, and accessories
connected to the ultrasound system comply with JACHO installation
requirements and/or safety standards such as AAMI-ES1, NFPA 99 OR IEC
Standard 60601-1-1 and electromagnetic compatibility standard IEC 60601-1-2
(Electromagnetic compatibility), and are certified according to IEC Standard
60950 (Information Technology Equipment (ITE)).
Do not use the system if an error message appears on the LCD display. Note
the error code and call FUJIFILM SonoSite Technical Support for further
assistance.
Caution:
To avoid increasing the system and transducer connector temperature, do not
block the airflow to the ventilation holes on the side of the system.
12 Chapter 3: Safety

Electrical safety classification
Class I equipment The ultrasound system is classified as Class I equipment
when powered from the external power supply or mounted
on the stand because the external power supply is a Class
1 protectively earthed power supply.
The stand has no protective earth. Ground bond testing is
not applicable to the ultrasound system or the stand.
Note:AC powered peripherals that may be used with the system
are Class I and are individually protectively earthed. Ground bond
testing may be conducted on each AC powered peripheral.
Internally powered
equipment
Type BF applied parts Ultrasound transducers
Type CF applied parts ECG module/ECG leads
IPX-7 (watertight
equipment)
Non AP/APG Ultrasound system power supply, docking system, and
Ultrasound system not connected to the power supply
(battery only)
Ultrasound transducers
peripherals. Equipment is not suitable for use in the
presence of flammable anaesthetics.
Equipment safety
To protect your ultrasound system, transducers, and accessories, follow these precautions.
Caution:
Caution:
Caution:
Excessive bending or twisting of cables can cause a failure or intermittent
operation.
Improper cleaning or disinfecting of any part of the system can cause
permanent damage. For cleaning and disinfecting instructions, see Chapter 7,
“Maintenance”
Do not submerge the transducer connector in solution. The cable is not
liquid-tight beyond the transducer connector/cable interface.
Caution:
Caution:
Caution:
Chapter 3: Safety 13
Do not use solvents such as thinner or benzene, or abrasive cleaners on any
part of the system.
Remove the battery from the system if the system is not likely to be used for a
month or more.
Do not spill liquid on the system.

Battery safety
To prevent the battery from bursting, igniting, or emitting fumes and causing personal injury or equipment
damage, observe the following precautions.
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
The battery has a safety device. Do not disassemble or alter the battery.
Charge the batteries only when the ambient temperature is between 0° and
40°C (32° and 104°F).
Do not short-circuit the battery by directly connecting the positive and negative
terminals with metal objects.
Do not touch battery contacts.
Do not heat the battery or discard it in a fire.
Do not expose the battery to temperatures over 60°C (140°F). Keep it away
from fire and other heat sources.
Do not charge the battery near a heat source, such as a fire or heater.
Do not leave the battery in direct sunlight.
Do not pierce the battery with a sharp object, hit it, or step on it.
Do not use a damaged battery.
Do not solder a battery.
The polarity of the battery terminals isfixed and cannot be switched or
reversed. Do not force the battery into the system.
Do not connect the battery to an electrical power outlet.
Do not continue recharging the battery if it does not recharge after two
successive six hour charging cycles.
WARNING:
WARNING:
WARNING:
Caution:
Do not ship a damaged battery without instructions from FUJIFILM SonoSite
Technical Support. (See “Contact Information” on page 1.)
If the battery leaks or emits an odor, remove it from all possible flammable
sources.
Periodically check to make sure that the battery charges fully. If the battery fails
to charge fully, replace it.
To avoid the battery becoming damaged and causing equipment damage,
observe the following precautions:
• Do not immerse the battery in water or allow it to get wet.
• Do not put the battery into a microwave oven or pressurized container.
• If the battery emits an odor or heat, is deformed or discolored, or in any way
appears abnormal during use, recharging or storage, immediately remove it
and stop using it. If you have any questions about the battery, consult
FUJIFILM SonoSite or your local representative.
• Store the battery between -20°C (-4°F) and 60°C (140°F).
• Use only FUJIFILM SonoSite batteries.
• Do not use or charge the battery with non-FUJIFILM SonoSite equipment.
Only charge the battery with the system.
14 Chapter 3: Safety

Clinical safety
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
WARNING:
Non-medical (commercial) grade peripheral monitors have not been verified or
validated by FUJIFILM SonoSite as being suitable for diagnosis.
To avoid the risk of a burn hazard, do not use the transducer with high
frequency surgical equipment. Such a hazard may occur in the event of a
defect in the high frequency surgical neutral electrode connection.
Do not use the system if it exhibits erratic or inconsistent behavior.
Discontinuities in the scanning sequence are indicative of a hardware failure
that must be corrected before use.
Some transducer sheaths contain natural rubber latex and talc, which can
cause allergic reactions in some individuals. Refer to 21 CFR 801.437, User
labeling for devices that contain natural rubber.
Perform ultrasound procedures prudently. Use the ALARA (as low as
reasonably achievable) principle and follow the prudent use information
concerning MI and TI.
FUJIFILM SonoSite does not currently recommend a specific brand of acoustic
standoff. If an acoustic standoff is used, it must have a minimum attenuation
of .3dB/cm/MHz.
Some FUJIFILM SonoSite transducers are approved for intraoperative
applications if a market-cleared sheath is used.
To avoid injury and reduce risk of infection to the patient, observe the following:
• Follow Universal Precautions when inserting and maintaining a medical
device for interventional and intraoperative procedures.
• Appropriate training in interventional and intraoperative procedures as
dictated by current relevant medical practices as well as in proper operation
of the ultrasound system and transducer is required. During vascular access,
the potential exists for serious complications including without limitation the
following: pneumothorax, arterial puncture, guidewire misplacement, and
risks normally associated with local or general anesthesia, surgery, and
post-operative recovery.
WARNING:
To avoid device damage or patient injury, do not use the P10x or rP19x needle
guide bracket on patients with pacemakers or medical electronic implants. The
needle guide bracket for the P10x and rP19x transducers contains a magnet
that is used to ensure the bracket is correctly oriented on the transducer. The
magnetic field in direct proximity to the pacemaker or medical electronic
implant may have an adverse effect.
Hazardous materials
WARNING:
Chapter 3: Safety 15
Products and accessories may contain hazardous materials. Ensure that
products and accessories are disposed of in an environmentally responsible
manner and meet federal and local regulations for disposing hazardous
materials.

Electromagnetic compatibility
The ultrasound system has been tested and found to comply with the electromagnetic compatibility (EMC)
limits for medical devices to IEC 60601-1-2:2001. These limits are designed to provide reasonable
protection against harmful interference in a typical medical installation.
WARNING:
Caution:
The Sonosite SII ultrasound system should not be used adjacent to or stacked
with other equipment. If such use occurs, verify that the SonoSite SII
ultrasound system operates normally in that configuration.
Medical electrical equipment requires special precautions regarding EMC and
must be installed and operated according to these instructions. Portable and
mobile RF communications equipment can affect the ultrasound system.
Electromagnetic interference (EMI) from other equipment or interference
sources could result in performance disruption of the ultrasound system.
Evidence of disruption may include image degradation or distortion, erratic
readings, equipment ceasing to operate, or other incorrect functioning. If this
occurs, survey the site to determine the source of disruption, and take the
following actions to eliminate the source(s).
• Turn equipment in the vicinity off and on to isolate disruptive equipment.
• Relocate or re-orient interfering equipment.
• Increase distance between interfering equipment and your ultrasound
system.
• Manage use of frequencies close to ultrasound system frequencies.
• Remove devices that are highly susceptible to EMI.
• Lower power from internal sources within facility control (such as paging
systems).
• Label devices susceptible to EMI.
• Educate clinical staff to recognize potential EMI-related problems.
• Eliminate or reduce EMI with technical solutions (such as shielding).
• Restrict use of personal communicators (cell phones, computers) in areas
with devices susceptible to EMI.
• Share relevant EMI information with others, particularly when evaluating new
equipment purchases which may generate EMI.
• Purchase medical devices that comply with IEC 60601-1-2 EMC Standards.
Caution:
To avoid the risk of increased electromagnetic emissions or decreased
immunity, use only accessories and peripherals recommended by FUJIFILM
SonoSite. Connection of accessories and peripherals not recommended by
FUJIFILM SonoSite to the ultrasound system may result in malfunction of the
ultrasound system or other medical electrical devices in the area. Contact
FUJIFILM SonoSite or your local representative for a list of accessories and
peripherals available from or recommended by FUJIFILM SonoSite. See the
FUJIFILM SonoSite accessories user guide.
Electrostatic discharge
Caution:
16 Chapter 3: Safety
Electrostatic discharge (ESD), or static shock, is a naturally occurring
phenomenon. ESD is common in conditions of low humidity, which can be
caused by heating or air conditioning. ESD is a discharge of the electrical
energy from a charged body to a lesser or non-charged body. The degree of
discharge can be significant enough to cause damage to a transducer or an
ultrasound system. The following precautions can help reduce ESD: anti-static
spray on carpets, anti-static spray on linoleum, and anti-static mats.

Separation distance
PPP
Recommended separation distances between portable and mobile RF communications equipment
and the SonoSite SII ultrasound system
The SonoSite SII ultrasound system is intended for use in an electromagnetic environment
in which radiated radio frequency (RF) disturbances are controlled. The customer or the
user of the SonoSite SII ultrasound system can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and the SonoSite SII ultrasound system as recommended below,
according to the maximum output power of the communications equipment.
Rated
maximum
output power
of transmitter
Watts
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
11.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended
separation distance (d) in meters (m) can be estimated using the equation applicable to
the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note: At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reflection from structures, objects, and people.
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d=1.2
80 MHz to 800 MHz
d=1.2
800 MHz to 2.5 GHz
d=2.3
Chapter 3: Safety 17

Guidance and manufacturer’s declaration
WARNING:
The SonoSite SII wireless adapter contains an IEEE 802.11 transmitter that utilizes the ISM frequency band
from 2.412 to 2.4835 GHz and implements two methods of transmission:
• IEEE 802.11b with Complementary Code Keying (CCK), Differential Quaternary Phase Shift Keying
(DQPSK), and Differential Binary Phase Shift Keying (DBPSK) at 16 dB
• IEEE 802.11g with Orthogonal Frequency Division Multiplexing (OFDM) at 13 dBm
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions
The SonoSite SII ultrasound system is intended for use in the electromagnetic environment
specified below. The customer or the user of the SonoSite SII ultrasound system should
assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment
RF emissions
ClSPR 11
Other equipment, even equipment that complies with CISPR emission
requirements, can interfere with the SonoSite SII ultrasound system.
Group 1 The SonoSite SII ultrasound system uses RF
energy only for its internal function. Therefore, its
RF emissions are very low and are not likely to
cause any interference in nearby electronic
equipment.
RF emissions
ClSPR 11
Harmonic emissions
IEC 61000-3-2
Voltage
fluctuations/flicker
emissions
IEC 61000-3-3
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The SonoSite SII ultrasound system is intended for use in the electromagnetic environment
specified below. The customer or the user of the SonoSite SII ultrasound system should
assure that it is used in such an environment.
Immunity
Test
Electrostatic
Discharge
(ESD)
IEC 61000-4-2
IEC 60601 Test
Level
±6.0KV contact
±8.0KV air
Class A The SonoSite SII ultrasound system is suitable for
use in all establishments other than domestic and
those directly connected to the public low-voltage
Class A
Complies
power supply network which supplies buildings
used for domestic purposes.±
Compliance
Level
±6.0KV contact
±8.0KV air
Electromagnetic
Environment
Floors should be wood,
concrete or ceramic tile. If
floors are covered with
synthetic material, the
relative humidity should be
at least 30%.
18 Chapter 3: Safety

Guidance and Manufacturer’s Declaration - Electromagnetic Immunity (Continued)
P
The SonoSite SII ultrasound system is intended for use in the electromagnetic environment
specified below. The customer or the user of the SonoSite SII ultrasound system should
assure that it is used in such an environment.
Immunity
Test
Electrical fast
Transient burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips,
short
interruptions
and voltage
variations on
power supply
input lines
IEC
61000-4-11
IEC 60601 Test
Level
±2KV for power
supply lines
±1KV for
input/output lines
±1KV line(s) to
line(s)
±2KV line(s) to earth
>5% U
T
(>95% dip in UT) for
0.5 cycle
40% U
T
(60% dip in UT) for 5
cycles
70% U
T
(30% dip in UT) for
25 cycles
>5% U
T
(>95% dip in UT) for
5s
Compliance
Level
±2KV for power
supply lines
±1KV for
input/output lines
±1KV line(s) to
line(s)
±2KV line(s) to
earth
>5% U
T
(>95% dip in UT)
for 0.5 cycle
40% U
T
(60% dip in UT)
for 5 cycles
70% U
T
(30% dip in UT)
for 25 cycles
>5% U
T
(>95% dip in UT)
for 5s
Electromagnetic
Environment
Mains power quality should
be that of a typical
commercial or hospital
environment.
Mains power quality should
be that of a typical
commercial or hospital
environment.
Mains power quality should
be that of a typical
commercial or hospital
environment. If the user of
the SonoSite SII ultrasound
system requires continued
operation during power
mains interruptions, it is
recommended that the
SonoSite SII ultrasound
system be powered from an
uninterruptible power supply
or a battery.
Power
Frequency
Magnetic Field
IEC 61000-4-8
Conducted RF
IEC 61000-4-6
3 A/m 3 A/m Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
3Vrms
150 kHz to 80 MHz
3 Vrms Portable and mobile RF
communications equipment
should be used no closer to
any part of the SonoSite SII
ultrasound system including
cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.
Recommended Separation
Distance
d = 1.2
Chapter 3: Safety 19

Guidance and Manufacturer’s Declaration - Electromagnetic Immunity (Continued)
P
P
The SonoSite SII ultrasound system is intended for use in the electromagnetic environment
specified below. The customer or the user of the SonoSite SII ultrasound system should
assure that it is used in such an environment.
Immunity
Test
Radiated RF
IEC 61000-4-3
Radiated RF
IEC 61000-4-3
(continued)
IEC 60601 Test
Level
3Vim
80 MHz to 2.5 GHz
Compliance
Level
3 V/m
Electromagnetic
Environment
d = 1.2
80 MHz to 800 MHz
d = 2.3
800 MHz to 2,5 GHz
Where P is the maximum
output power rating of the
transmitter in watts (W)
according to the transmitter
manufacturer and d is the
recommended separation
distance in meters (m).
Field strengths from fixed
RF transmitters, as
determined by an
electromagnetic Site
a
survey
, should be less than
the compliance level in each
b
frequency range
.
Interference may occur in
the vicinity of equipment
marked with the following
symbol:
Note:UT is the AC mains voltage prior to application of the test level.
At 80 MHz and 800 MHz, the higher frequency range applies.
These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a. Field strengths from fixed transmitters such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which the FUJIFILM SonoSite ultrasound
system is used exceeds the applicable RF compliance level above, the FUJIFILM SonoSite ultrasound system
should be observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as re-orienting or relocating the FUJIFILM SonoSite ultrasound system.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
FCC Caution: Changes or modifications not expressly approved by the party responsible for compliance
could void the user’s authority to operate the equipment.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
• This device may not cause harmful interference.
• This device must accept any interference received, including interference that may cause undesired
operation.
20 Chapter 3: Safety
 Loading...
Loading...