Page 1
Dräger Medical, Inc.
Incubator 8000 IC / SC / NC
Technical Service Bulletin # 19
Re: Retrofitting of warning labels
Update: May 31, 2000
Reference Doc: Complaint # 980046
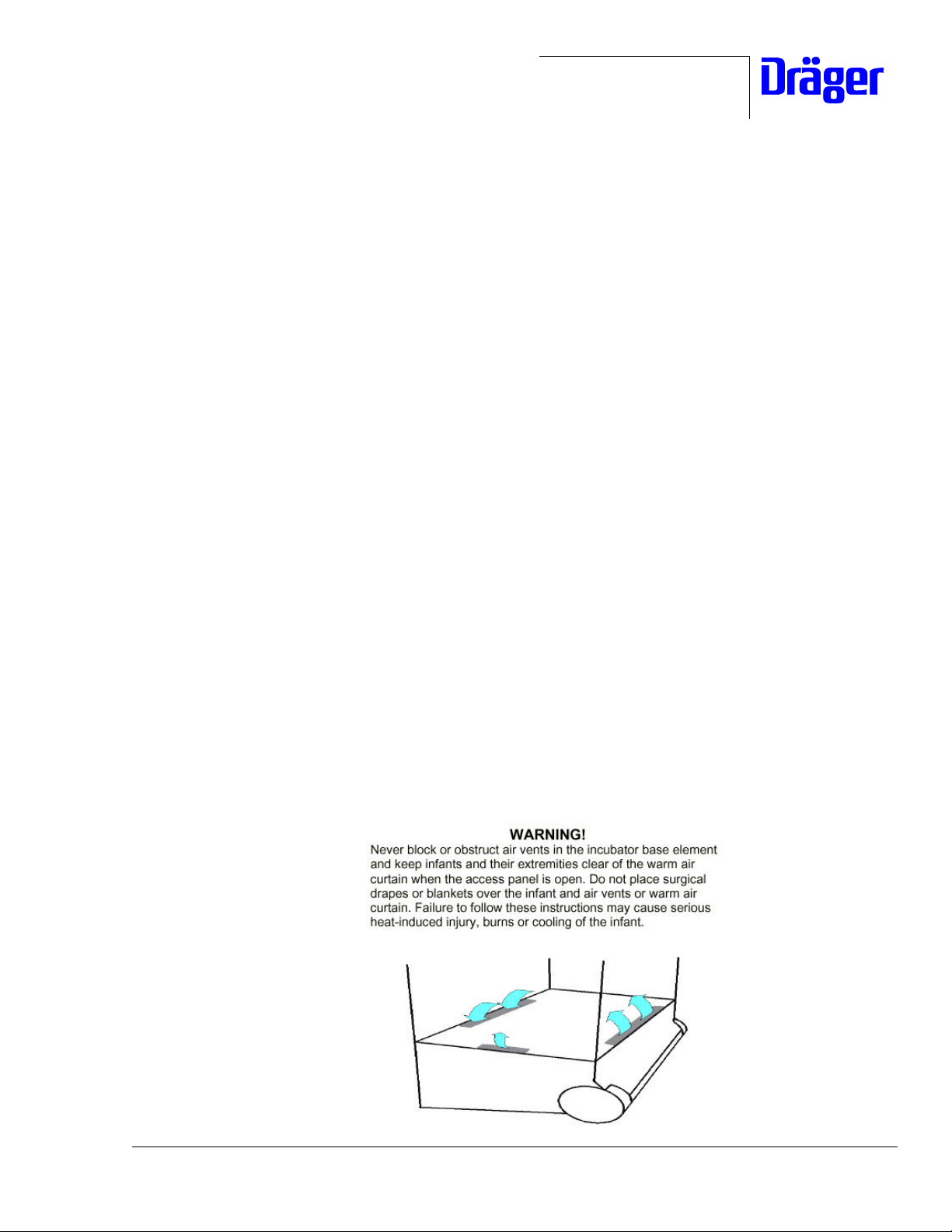
Reason: Patient Safety and Incubator Performance may be seriously
compromised if air flow passages are not kept clear of obstruction
(blankets, surgical drapes, stuffed animals, etc.) during clinical use.
Additionally, when the access panel is open, a curtain of warm air
flows along the front of the mattress towards the top of the hood.
Because the temperature of this air curtain is higher than the typical
incubator air temperature, the infant and all its extremities must be
kept clear of this warm air path.
Solution: The following warning shall be added to the Operating Instructions
and labeling of the Incubator 8000 IC, Incubator 8000 SC, and
Incubator 8000 NC:
1. The letter "Important Information for Users of Dräger Incubator
8000 IC/SC/NC" is to be filed in the Operating Instructions of
each unit.
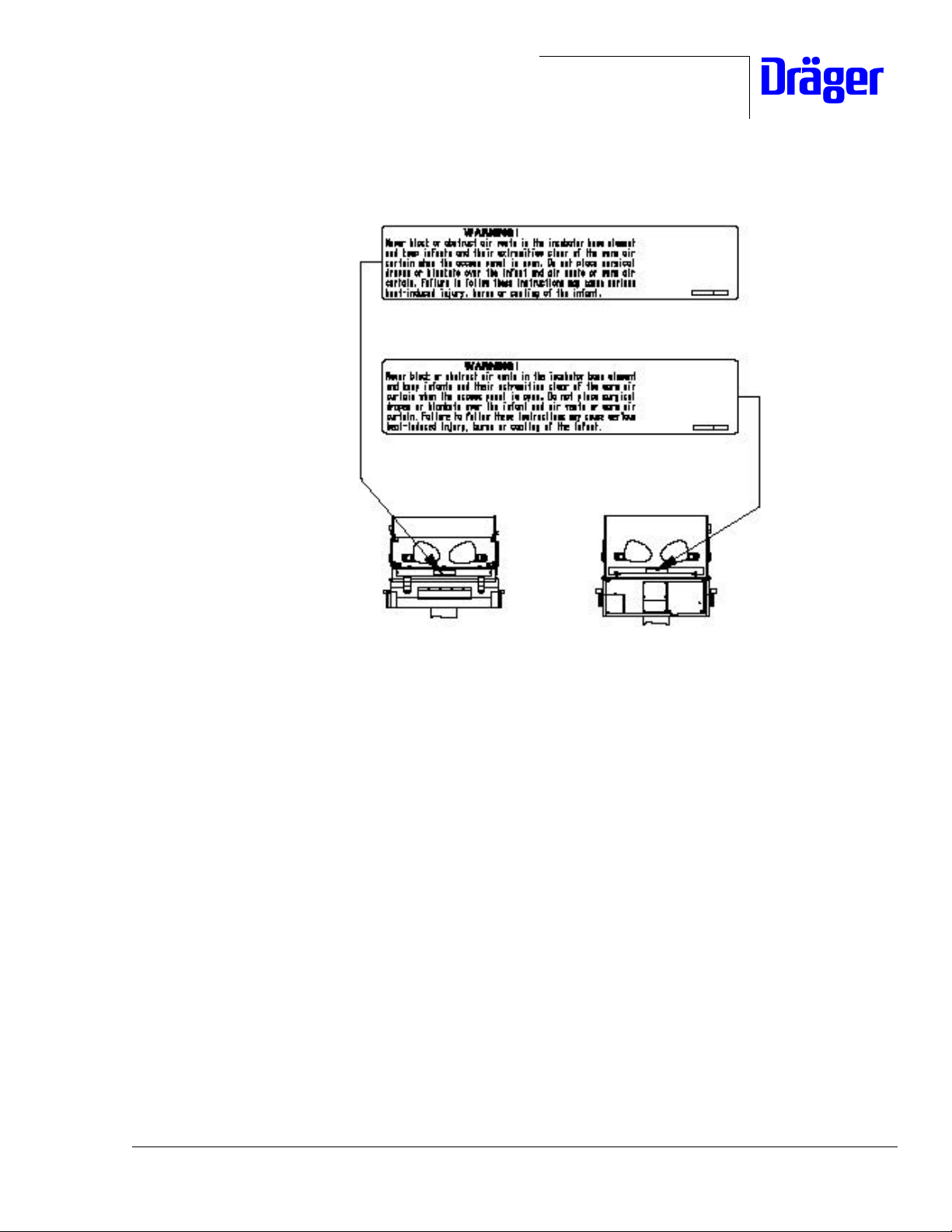
2. A warning label is to be placed on the front and on the back face
of the mattress stretcher according to the following Instruction
"Adhesive Statement 2M22363".
6141.22X Incubator 8000 IC / SC/ NC TSB No. 19 May 2000 1/2
Page 2
Dräger Medical, Inc.
Devices affected: All units in the USA and Canada delivered prior May 2000.
When: At next Service Call or Preventive Maintenance.
Additional Info: As always, it is essential that the Customers train each potential user so
that all are adept in properly and safely using the incubator.
Cost: Labels free of charge.
Ordering Info: For one Incubator order 2 pieces:
Warning label Incubator P/N 2M 22 351
Distribution: Dräger Service Personnel and Authorized Service Organizations for
CCS products.
If you have any questions, please contact Technical Support by
phone at 1-800-543-5047 or by fax at 1-215-721-5789
Dräger Medical, Inc.
Technical Product Manager
6141.22X Incubator 8000 IC / SC/ NC TSB No. 19 May 2000 2/2
 Loading...
Loading...