Page 1

Tec 7 Vaporizer
User’s Reference Manual
Page 2

User Responsibility
w
CAUTION
This Product will perform in conformity with the description thereof contained
in this User’s Reference manual and accompanying labels and/or inserts,
when assembled, operated, maintained, and repaired in accordance with the
instructions provided. This Product must be checked periodically. A defective
Product should not be used. Parts that are broken, missing, plainly worn,
distorted, or contaminated should be replaced immediately. Should repair or
replacement become necessary, Datex-Ohmeda recommends that a
telephonic or written request for service advice be made to the nearest
Datex-Ohmeda Customer Service Center. This Product or any of its parts
should not be repaired other than in accordance with written instructions
provided by Datex-Ohmeda and by Datex-Ohmeda trained personnel. The
Product must not be altered without the prior written approval of
Datex-Ohmeda. The user of this Product shall have the sole responsibility for
any malfunction which results from improper use, faulty maintenance,
improper repair, damage, or alteration by anyone other than Datex-Ohmeda.
U.S. Federal law restricts this device to sale by or on the order of a
licensed medical practitioner. Outside the U.S.A, check local laws
for any restriction that may apply.
Datex-Ohmeda products have unit serial numbers with coded logic which
indicates a product group code. The year of manufacture and a sequential
unit number for identification.
AAA A 12345
T
his alpha character indicates the year of product
manufacture and when the serial number was
assigned; “C” = 1999, “D” = 2000, “E” = 2001,
etc. “I” and “O” are not used.
Page 3

1 Introduction
2 Description
3 Setup and Mounting Procedure
Table of Contents
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is a Tec 7 Vaporizer? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Control dial . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Safety interlocks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Vaporizer identification label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Vaporizer mounting procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Mounting the vaporizer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
4 Operating Instructions
Checking the vaporizer for correct mounting . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Removing the vaporizer from a manifold . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Setting the dial . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Filling and draining the vaporizer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Filling procedure with funnel filler . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Draining procedure with funnel filler . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
™
Filling procedure with Easy-Fil
Draining procedure with Easy-Fil . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Filling procedure with Quik-Fil™ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Draining procedure with Quik-Fil . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
1175-0013-000
i
Page 4

Tec 7 Vaporizer
5 Maintenance
User maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Maintenance intervals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
External cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Internal contamination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Output concentration check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Checking the calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Analytical techniques . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Service Policy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
6 Principle of Operation
7 Specifications
Interlock mechanism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Delivery of gas/agent vapor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Bypass circuit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Vaporizing chamber circuit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Weight and dimensions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Flow characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
Effects of variables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
Anesthetic agent consumption . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
Barometric pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
Ambient temperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-9
Back pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-9
Carrier gas composition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-10
Warranty
ii
Time out of service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-10
Effects of variables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-10
1175-0013-000
Page 5

1 Introduction
Precautions
ww
ww
WARNING Do not fill the vaporizer with any agent other than the agent specified on
the front label. The vaporizer is designed for that agent only. If any
substance other than that specified is used, patient injury could occur.
United States (U.S.) Federal law restricts this device to sale by or on the
order of a licensed medical practitioner. Outside the U.S., check local
laws for any restrictions that may apply.
Do not attempt to use a vaporizer that has been dropped. A dropped
vaporizer MUST be sent to the nearest Datex-Ohmeda Field Operations
Unit for servicing.
Do not use malfunctioning equipment. Make all necessary repairs or
have the equipment serviced by an authorized Datex-Ohmeda service
center. After repair, test the equipment to ensure that it is functioning
properly in accordance with the manufacturer’s published
specifications.
1175-0013-000
1-1
Page 6
y
Tec 7 Vaporizer
Symbols
Important
ww
ww
WARNING Warnings tell about a condition that can cause injury to the operator or
European Standard EN 740 that an appropriate gas monitor is used to monitor the concentration of anesthetic
agent vapor in the inspiratory gas when the vaporizer is in operation in order to provide
protection against hazardous output in the event of a device malfunction.
Datex-Ohmeda strongly recommends the use of anesthesia gas monitoring with this
equipment. Refer to local standards for mandatory monitoring.
Requests for servicing facilities, advice or assistance must be addressed to a local
Datex-Ohmeda office.
Additional copies of this manual, can be requested from a local Datex-Ohmeda Field
Operations Unit or a Datex-Ohmeda Authorized Distributor.
Datex-Ohmeda strongly recommends that you keep all relevant documentation,
including this manual and accompanying labels, immediately available to all users.
Warnings and Cautions tell you about conditions that can occur if you do not follow all
instructions in this manual.
the patient.
Anesthetic Workstations and Their Modules
requires
onl
SEV
ISO
ENF
HAL
ww
ww
CAUTION Cautions tell about a condition that can cause damage to the
equipment. Read and follow all warnings and cautions.
Caution: federal law prohibits dispensing
without prescription.
Sevoflurane
Isoflurane
% v/v
OFF symbol/OFF setting
Percentage of anesthetic vapor per total volume.
Lock
z
Enflurane
Unlock
Z
Halothane
Maximum Agent Level
Minimum Agent Level Stock number
Systems with this mark agree with the European
Council Directive (93/42/EEC) for Medical
Devices when they are used as specified in their
User’s Reference manuals. The xxxx is the
certification number of the Notified Body used
by Datex-Ohmeda's Quality Systems.
w
NN
NN
Caution or Warning
Direction of flow
Serial number
1-2
1175-0013-000
Page 7

2 Description
What is a Tec 7 Vaporizer?
The Tec 7 Vaporizer is designed for use in continuous flow techniques of inhalation
anesthesia. Each vaporizer is agent specific and is clearly labeled with the anesthetic
agent that it is designed for.
The vaporizer is temperature, flow and pressure compensated so that its output
remains relatively constant despite cooling due to evaporation, variations in inlet flow
and fluctuating pressures as described in Section 7, Effects of Variables.
The vaporizer is designed to be used on Selectatec
vaporizer can be installed on other Selectatec Manifolds but the interlock system is
designed to function on Selectatec Series Mounted Manifolds only. Mounting a Tec 7
Vaporizer on a Selectatec 7 Compatibility Block is not recommended.
®
Series Mounted Manifolds. The
1175-0013-000
2-1
Page 8

Tec 7 Vaporizer
AB80008
Figure 2-1 • Tec 7 Vaporizer
w WARNING Improper use may result in patient injury.
This manual and its associated documentation must be studied before
any attempt is made to install, operate or clean any part of the Tec 7
Vaporizer.
The performance of the anesthesia machine and vaporizer can be
degraded if the machine and vaporizer are mis-matched.
Only operate the vaporizer with dry medical gases.
If a vaporizer containing agent in the sump has been inverted, connect it
to a gas scavenging system, set the dial to 5% and purge the vaporizer
with the carrier gas at 5 liters/minute for 5 minutes.
ww
ww
CAUTION The vaporizer is intended to be operated in its upright position.
Turn the vaporizer to when it is not in use.
2-2
1175-0013-000
Page 9

Components
2 Description
Control dial
Safety interlocks
w WARNING Earlier versions of the Selectatec Series Mounted Manifold that provide
A single control dial with a concentration scale calibrated in percentage of anesthetic
agent vapor per total volume (% v/v) sets the desired concentration of the anesthetic
agent.
A dial release in the dial assembly helps prevent accidental displacement of the
control dial from the position. To select an ON setting, squeeze the dial release
and simultaneously rotate the dial counter-clockwise.
The dial and dial release are designed to enable an ON setting to be selected using
only one hand.
The vaporizer incorporates an interlock mechanism. This mechanism also interfaces
with the Selectatec
• The vaporizer must be locked onto the manifold before it can be turned ON.
• Only one vaporizer at a time can be turned ON when two or more vaporizers are
fitted on a Selectatec
• The gas flow enters only the vaporizer that is turned ON.
• Any unwanted anesthetic trace vapor is minimized after a vaporizer is turned to
.
®
Series Mounted Manifold to help satisfy the following criteria:
®
Series Mounted Manifold.
mounting positions for three vaporizers require that if only two vaporizers
are fitted, then the center position must be occupied. If the center
position is not occupied, the interlock that helps ensure that only one
vaporizer at a time can be turned ON is ineffective.
Vaporizer identification
label
1175-0013-000
Later versions of the Selectatec Series Mounted Manifold that provide mounting
positions for three vaporizers incorporate an additional interlock that helps ensure
that only one vaporizer at a time can be turned ON even if the center position is not
occupied.
A vaporizer identification label is affixed to the back panel of the vaporizer as
illustrated on Fig. 2-2.
An anesthesia system fitted with a vaporizer identification unit uses this label to
identify the vaporizer type.
2-3
Page 10

Tec 7 Vaporizer
w WARNING Do not affix any additional labels or markings to the back panel. They
may adversely affect the operation of the vaporizer identification unit.
1
H
A
L
O
T
H
A
N
E
1. Vaporizer identification label
Figure 2-2 • Vaporizer identification label
AB80011
2-4
1175-0013-000
Page 11

3 Setup and Mounting Procedure
Vaporizer mounting procedure
The vaporizer is designed to be used on Selectatec Series Mounted Manifolds. The
vaporizer can be installed on other Selectatec Manifolds but the interlock system is
designed to function on Selectatec Series Mounted Manifolds only.
Mounting a Tec 7 Vaporizer on a Selectatec 7 Compatibility Block is not
recommended.
ww
ww
WARNING Do not lift or support the vaporizer by holding the control dial. Handle the
vaporizer with care at all times.
Before mounting a vaporizer onto the Selectatec Series manifold,
ensure that each manifold port valve O-ring is intact and that there is no
foreign matter around the mating surfaces. A damaged O-ring and/or
foreign matter around the mating surfaces can cause leaks.
Earlier versions of the Selectatec Series Mounted Manifold that provide
mounting positions for three vaporizers require that if only two vaporizers
are fitted, then the center position must be occupied. If the center
position is not occupied, the interlock that helps ensure that only one
vaporizer at a time can be turned ON is ineffective.
Do not use a vaporizer if the liquid level decreases below the minimum
level.
1175-0013-000
Before using a vaporizer allow it to attain the ambient temperature of the
location in which it has to be used.
3-1
Page 12
Tec 7 Vaporizer
Mounting the vaporizer
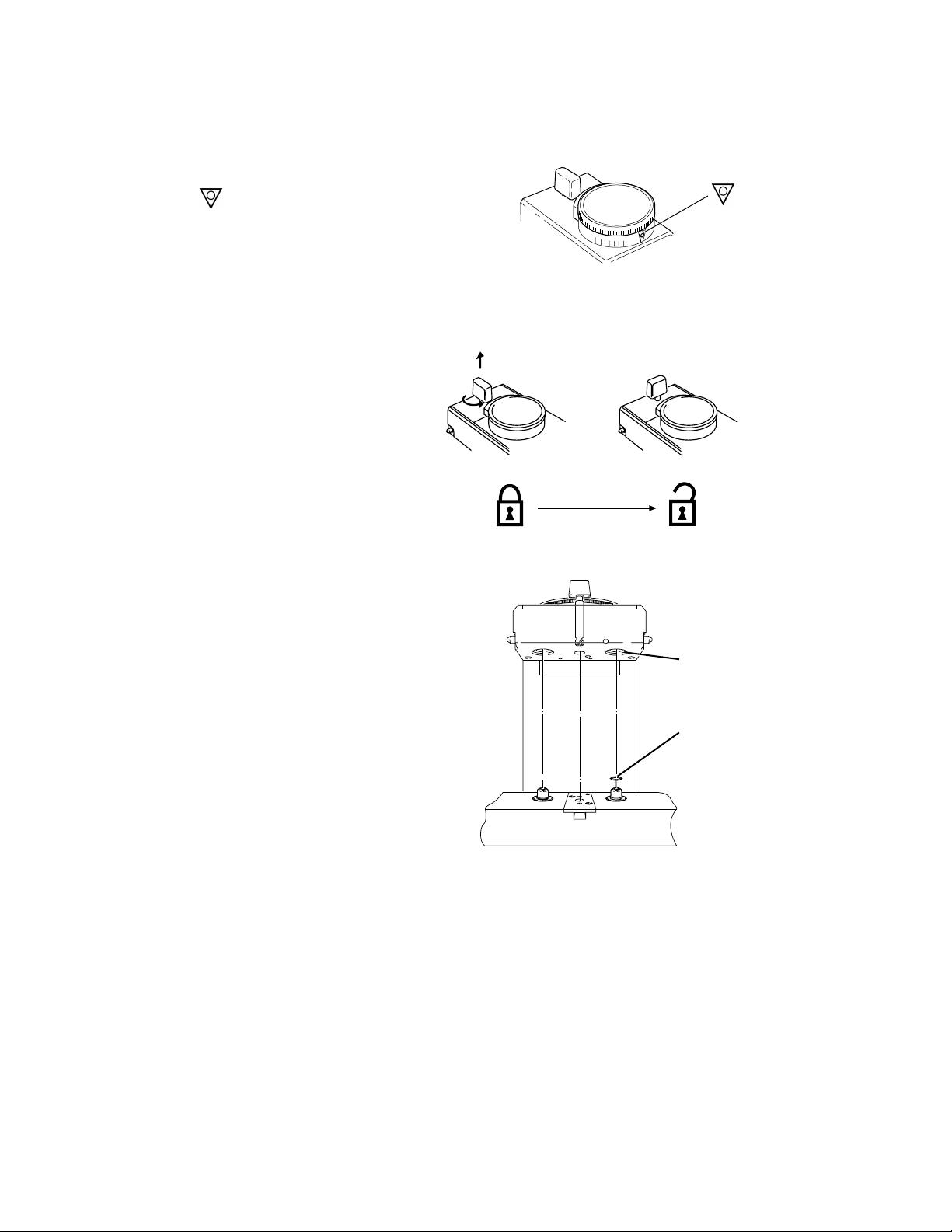
Step 1
Set the dial to .
Step 2
Unlock the locking lever.
• Turn the lever counter-clockwise.
• Make sure the lever releases.
Step 3
Prepare the manifold.
• Remove any plugs fitted to the
vaporizer interlock block ports.
• Verify that each manifold port
valve O-ring is intact. If
necessary, remove the existing
O-rings and fit one new O-ring to
each port valve, as described in
the relevant anesthesia system
User’s Reference Manual.
Replacement O-rings are
supplied with each vaporizer.
n
e
b
Å
Figure 3-1 • Setting the concentration dial
Figure 3-2 • Unlocking the locking lever
AB80001
AB80002
1
2
AA13052
3-2
1. Vaporizer Interlock Block Port - ensure plugs removed
2. Replace Manifold Port Valve O-ring, if necessary
Figure 3-3 • Readying the manifold
1175-0013-000
Page 13
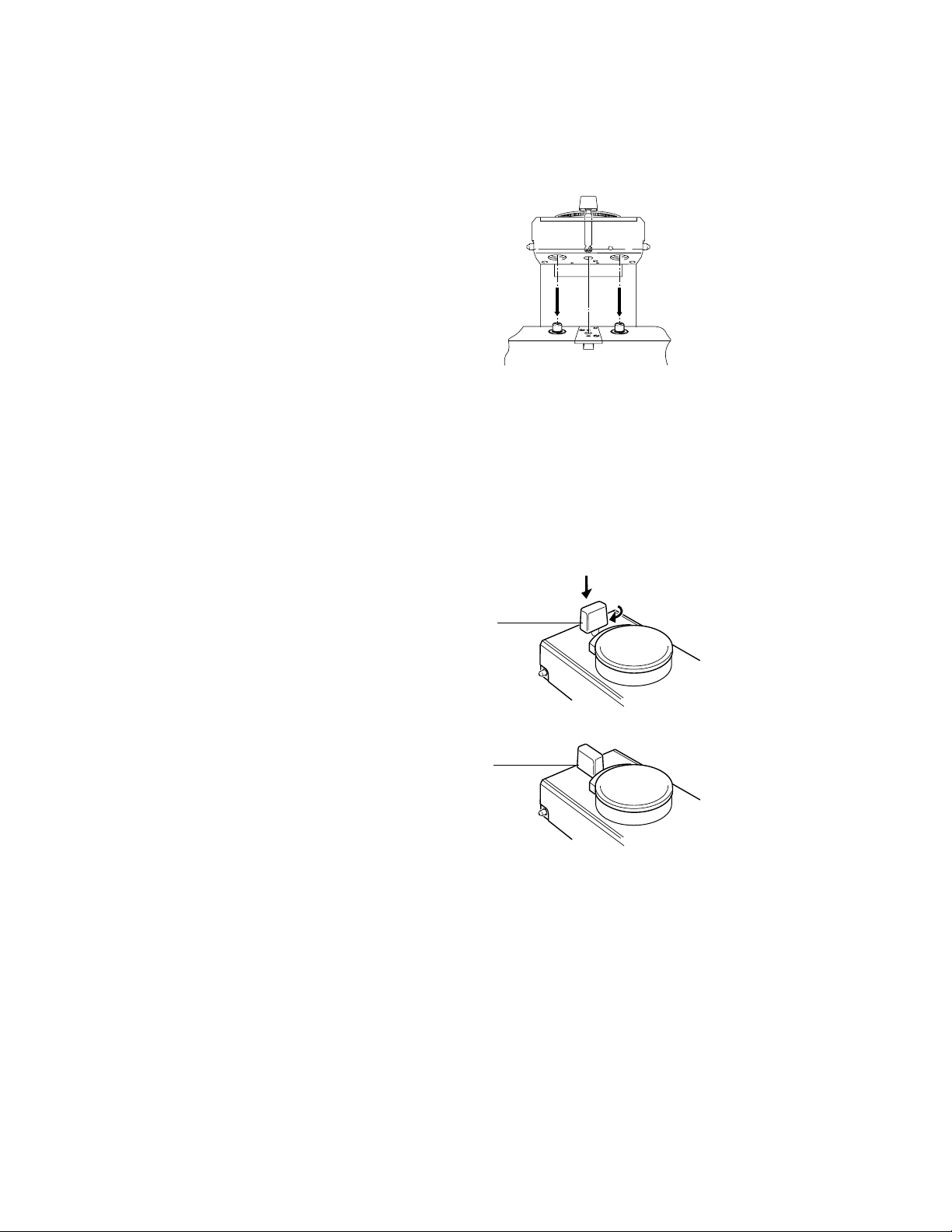
Step 4
Install the vaporizer onto the manifold.
• Hold the main body of the
vaporizer in an upright position
with both hands
• Lower the vaporizer onto the
manifold, ensuring that the
vaporizer interlock block ports
engage correctly with the
manifold port valves.
.
ww
ww
CAUTION Push the locking lever all the way down before turning it. The mechanism
3 Setup and Mounting Procedure
AB80018
Figure 3-4 • Installing the vaporizer
can be damaged if an attempt is made to turn the lever before it is
pushed all the way down.
Step 5
Lock the vaporizer onto the manifold.
• Push the locking lever all the way
down.
• Turn it clockwise to the locked
position to lock the vaporizer
onto the manifold.
Step 6
Ensure that the vaporizer is correctly
mounted (see instructions on the next
page).
1
1
AB80004
1. Locking lever
Figure 3-5 • Locking the vaporizer onto a manifold
1175-0013-000
3-3
Page 14
Tec 7 Vaporizer
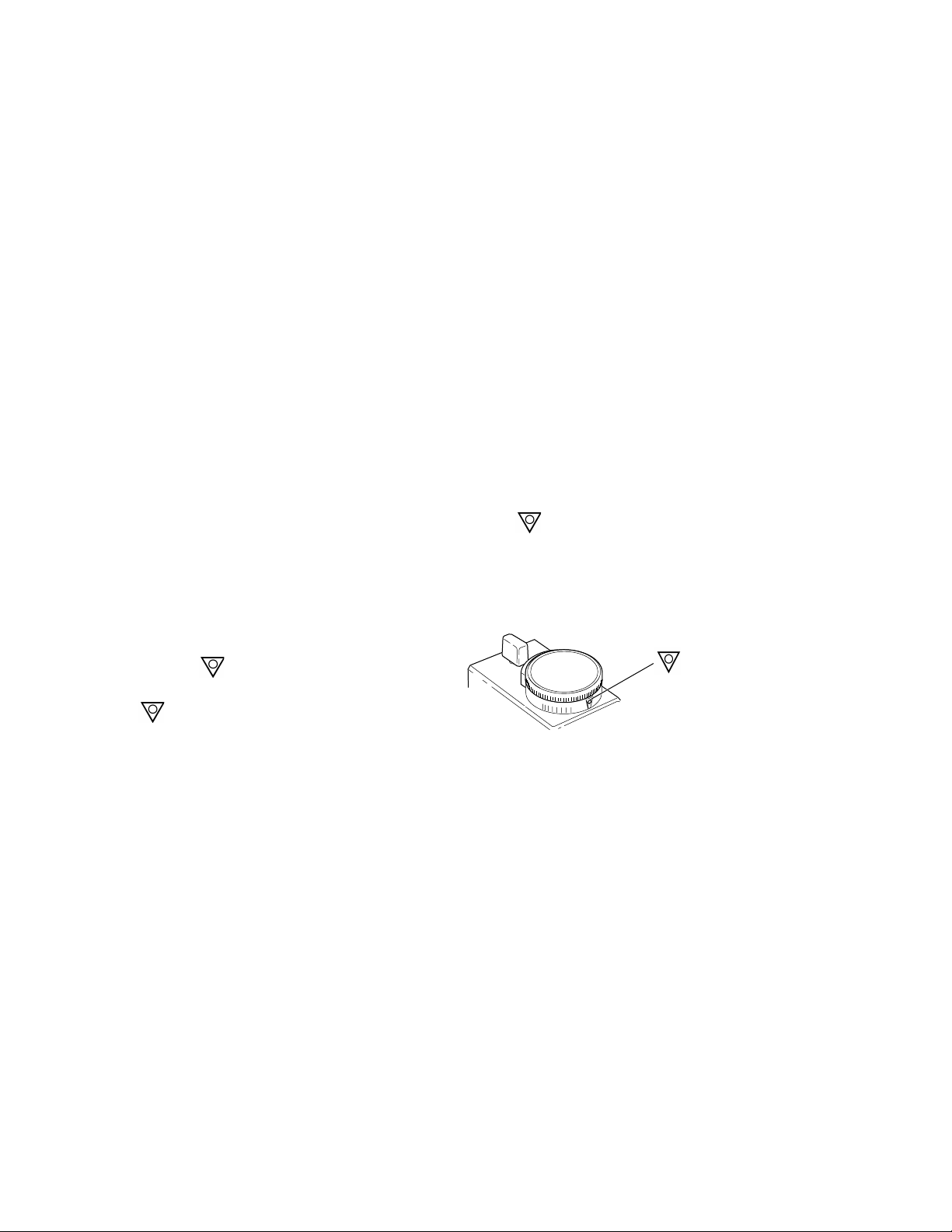
Checking the vaporizer for correct mounting
ww
ww
WARNING To help ensure correct operation, do not use a vaporizer that is either
visibly out of line on the manifold or that can be lifted off the manifold
when the locking lever is in the locked position.
If more than one vaporizer is fitted, visually check to make sure that the tops of the
vaporizers are level. If the vaporizer is visibly out of line, perform steps 2 and 3 as
described in Removing the vaporizer from a manifold and remount it correctly.
When the vaporizer appears to be level and the locking lever is in the locked position,
attempt to lift the vaporizer straight up from the manifold. If the vaporizer can be lifted
off the manifold, it is not correctly mounted. Remount the vaporizer (see Vaporizer
mounting procedure ).
Verify that the interlock rods are in alignment by making sure that only one vaporizer at
a time can be turned ON.
Check the anesthesia system for leaks in accordance with the relevant User’s
Reference Manual with the vaporizer dial turned to 0% and then repeat the check with
the vaporizer dial turned to .
Removing the vaporizer from a manifold
Step 1
Set the dial to .
If the dial is not completely turned to
OFF
the position the vaporizer cannot
be released from the manifold.
OFF
Figure 3-6 • Setting the dial
n
e
b
Å
AB80001
3-4
1175-0013-000
Page 15

Step 2
Unlock the locking lever.
• Turn the locking lever counterclockwise.
• Release the locking lever and
check that the locking lever
springs up to the unlocked
position to release the vaporizer
from the manifold.
3 Setup and Mounting Procedure
Locked
1
2
Unlocked
1. Locking lever
2. Dial
Figure 3-7 • Unlocking the locking lever
1
2
AB80003
Step 3
Carefully lift the vaporizer up from the
manifold.
AB80019
Figure 3-8 • Lifting the vaporizer
1175-0013-000
3-5
Page 16

Tec 7 Vaporizer
3-6
1175-0013-000
Page 17

4 Operating Instructions
Setting the dial
ww
ww
Important
WARNING High percent dial settings combined with low gas flows may lead to
hypoxic mixtures in the breathing circuit. Datex-Ohmeda strongly
recommends the use of oxygen monitoring.
The dial release must be operated to turn the dial from the
OFF
setting.
Do not turn the dial if the vaporizer is not properly locked onto the
manifold.
European Standard EN 740 that an appropriate gas monitor is used to monitor the concentration of anesthetic
agent vapor in the inspiratory gas when the vaporizer is in operation in order to provide
protection against hazardous output in the event of a device malfunction.
Datex-Ohmeda strongly recommends the use of anesthesia gas monitoring with this
equipment. Refer to local standards for mandatory monitoring.
Anesthetic Workstations and Their Modules
requires
1175-0013-000
4-1
Page 18

Tec 7 Vaporizer
Step 1
Press the dial release and turn the dial
in a counter-clockwise direction from
the setting.
Note that it is not possible to turn on
the vaporizer if an adjacent Tec series,
(except Tec 3) is turned on.
Step 2
Åben
AB80020
The vaporizer should not be used
between and the first graduation
mark.
To avoid inadvertent delivery of small
concentrations, turn the control dial to
when the vaporizer is not in use.
Figure 4-1 • Releasing the dial
4-2
1175-0013-000
Page 19

Filling and draining the vaporizer
ww
ww
WARNING Do not fill the vaporizer with any agent other than the agent specified on
the front label. The vaporizer is designed for that agent only. If any
substance other than that specified is used, patient injury could occur.
Only fill the vaporizer when it is in an upright position. Failure to do so
may result in the vaporizer being overfilled.
To avoid explosive hazards, flammable anesthetic agents such as Ether
and Cyclopropane must not be used in or with this vaporizer. Only
anesthetic agents that comply with the requirements for non-flammable
anesthetic agents in the IEC 60601-2-13 Standard,
Requirements for the Safety of Anesthesia Machines
use in the presence of this vaporizer.
4 Operating Instructions
Particular
, are suitable for
As this vaporizer is not suitable for use with flammable anesthetic agents
such as Ether or Cyclopropane, the use of antistatic breathing tubes and
face masks is not necessary. The use of antistatic or electrically
conductive breathing tubes when using high frequency electric surgery
equipment may cause burns and is therefore not recommended in any
application of this vaporizer.
Do not fill the vaporizer unless the control dial is in the position.
Do not turn the dial ON during filling or attempt to fill beyond the ¥
mark.
Do not drain the agent into any container other than a properly marked
drug container.
Ensure that the filler cap is tightened prior to use.
1175-0013-000
4-3
Page 20

Tec 7 Vaporizer
When filling the Tec 7 Vaporizer, observe the following:
• Periodically check the agent level. The vaporizer should be refilled at appropriate
intervals. The vaporizer is designed to function according to specification as long
as there is agent visible above the
mark.
• The vaporizer must be filled and used in an upright position. Small deviations
from the upright position do not affect either the output or the safety of the
vaporizer.
• Every two weeks, preferably when the agent level is low, drain the contents of the
vaporizer into an appropriately marked container and discard the agent. Less
frequent intervals may be used when the anesthetic agent does not contain
additives or stabilizing agents, but the procedure must be performed at least
once every year.
• The following steps should be taken for Halothane vaporizers:
— Drain the vaporizer every two weeks
— If Halothane is used infrequently the vaporizer should be drained after use.
— The decomposition of halothane causes the release of halides, which may
corrode metal components particularly in the presence of moisture. Also a
preservative added to halothane by its manufacturers to impede
decomposition can leave a residue, which may cause vaporizer
components to stick.
• If the vaporizer is not upright, check the agent level more frequently to avoid a
misleading impression of the amount of agent in the vaporizer.
Step 1
Turn the vaporizer dial clockwise to the
OFF
position.
Åben
AB80021
Figure 4-2 • Turning the vaporizer OFF
4-4
1175-0013-000
Page 21

Filling procedure with
funnel filler
w WARNING Before filling a vaporizer equipped with a funnel filler, turn the cap slowly
Step 1
Remove the filler cap by turning it
counter-clockwise. Ensure that the
drain plug is closed by tightening it
with the tool end of the filler cap.
4 Operating Instructions
to allow any pressure to gradually vent.
Ensure that the drain plug screw, located on the lower front of the
vaporizer, is correctly tightened to help prevent loss of liquid agent.
Step 2
Verify that the anesthetic agent is the
same as that specified on the
vaporizer front label. Observe the
agent level through the sight glass
indicator on the side of the filler body.
Pour the agent slowly into the filling
port, as illustrated on Fig. 4-3, until
the level reaches the
level may decrease slightly as the
wicks absorb the agent. To help
prevent overfill, ensure the agent level
is at or below the fill line.
¥
mark. The
Step 3
Replace and tighten the filler cap to
minimize leaks.
AB80022
Figure 4-3 • Filling a vaporizer that incorporates a funnel filler
AB80013
Figure 4-4 • Replacing the filler cap
1175-0013-000
4-5
Page 22

Tec 7 Vaporizer
Draining procedure
with funnel filler
ww
ww
CAUTION Do not allow the container to become completely full during draining
Step 1
Remove the filler cap. Insert the tool
end of the cap into the drain plug
below the filling port on the filler body
as illustrated in Fig. 4-5.
Step 2
Position a properly marked container
under the drain spout.
Step 3
Unscrew, but do not remove, the drain
plug to allow the vaporizer contents to
pour from the drain spout into the
container.
The vaporizer must only be drained into a properly marked container.
procedures.
AB80023
Figure 4-5 • Draining a vaporizer with a funnel filler
Step 4
After draining is complete, tighten the
drain plug to help minimize leaks.
Step 5
Replace and tighten the filler cap to
help minimize leaks.
AB80013
Figure 4-6 • Replacing the filler cap
4-6
1175-0013-000
Page 23

Filling procedure
with Easy-Fil
™
4 Operating Instructions
wwwwWARNING
Step 1
Align the notches on the bottle
adapter to the bottle collar and tighten
the adapter onto the agent bottle.
Step 2
Remove the filler cap. Align the bottle
adapter keys with the index slots in the
filler block as illustrated in Fig. 4-7.
Insert the bottle nozzle into the filler
block.
Step 3
Press the agent bottle fully into the
vaporizer filler block. Allow the liquid
to flow into the vaporizer until the
maximum level mark ¥ is reached. To
help prevent overfill, ensure the agent
level is at or below the fill line. Pay
particular attention to the level in the
sight glass and the air return bubbles
flowing into the bottle.
Step 4
Ensure that the drain plug screw, located on the lower front of the
vaporizer, is correctly tightened to help prevent loss of liquid agent.
The filling system consists of three elements:
• the bottle collar
• the bottle adapter
• the filler block
The vaporizer must only be filled using the correct agent specific filling system
3
2
1
1. Filler cap
2. Index slot
3. Bottle adapter
Figure 4-7 • Filling a vaporizer with Easy-Fil
.
AB80015
AB80013
Release the bottle when the vaporizer
is full and the continuous stream of
bubbles ceases.
Step 5
Remove the bottle from the vaporizer
filler. Replace the filler cap and the
cap on the agent bottle. Ensure that
the filler cap is tightened to help
minimize leaks.
1175-0013-000
Figure 4-8 • Replacing the filler cap
4-7
Page 24

Tec 7 Vaporizer
Draining procedure
with Easy-Fil
Step 1
ww
ww
CAUTION Do not allow the container to become completely full during draining
procedures.
The vaporizer must only be drained into a properly marked container.
Remove the cap from the vaporizer
filler.
Step 2
Place the empty container opening
under the drain nozzle as shown in
Fig. 4-9.
Step 3
Unscrew the drain plug with the tool
attached to the filler cap. Drain the
vaporizer until empty.
Step 4
After draining, tighten the drain plug to
help minimize leaks.
Step 5
Replace and tighten the filler cap to
minimize leaks.
AB80014
Figure 4-9 • Unscrewing the drain plug
AB80013
4-8
Figure 4-10 • Replacing the filler cap
1175-0013-000
Page 25

Filling procedure with
Quik-Fil
ww
ww
WARNING Ensure that the drain plug screw, located on the lower front of the
Step 1
Remove the protective cap from the
anesthetic agent bottle filler, checking
that the bottle and filler mechanism
are not damaged.
4 Operating Instructions
™
vaporizer, is correctly tightened to help prevent loss of liquid agent.
3
AB80015
Step 2
Remove the filler cap. Insert the bottle
nozzle into the filler block. Rotate the
bottle to align the bottle filler nozzle
keys with the index slots in the filler
block as illustrated on Fig. 4-11.
Step 3
Press the agent bottle fully into the
vaporizer filler block. Allow the liquid
to flow into the vaporizer until the
maximum level mark ¥ is reached.
Pay particular attention to the level in
the sight glass and the air return
bubbles flowing into the bottle.
Step 4
Release the bottle when the vaporizer
is full and the continuous stream of
bubbles ceases. To help prevent
overfill, ensure the agent level is at or
below the fill line.
Step 5
2
1
1. Filler cap
2. Index slot
3. Nozzle key
Figure 4-11 • Filling a vaporizer with a Quik-Fil
AB80013
Remove the bottle from the vaporizer
filler. Replace the filler cap and the
cap on the agent bottle. Ensure that
the filler cap is tightened to help
minimize leaks.
1175-0013-000
Figure 4-12 • Replacing the filler cap
4-9
Page 26

Tec 7 Vaporizer
Draining procedure
with Quik-Fil
Step 1
ww
ww
CAUTION Do not allow the container to become completely full during draining
procedures.
The vaporizer must only be drained into a properly marked container.
Remove the cap from the vaporizer
filler.
Step 2
Place the empty container under the
drain nozzle as shown in Fig. 4-13.
Screw bottle onto drain spout.
Step 3
Unscrew the drain plug with the tool
attached to the filler cap. Drain the
vaporizer until empty.
Step 4
After draining, tighten the drain plug to
help minimize leaks.
Step 5
Replace and tighten the filler cap to
minimize leaks.
AB80026
Figure 4-13 • Unscrewing the drain plug
AB80013
4-10
Figure 4-14 • Replacing the filler cap
1175-0013-000
Page 27

5 Maintenance
User maintenance
ww
ww
WARNING Do not modify, tamper with, or disassemble the vaporizer. Doing so can
Maintenance intervals
Note
damage the unit and alter the graduation accuracy.
Prior to performing any maintenance procedures or returning to a service center for
repairs, clean and disinfect the vaporizer.
Every two weeks: When the agent is low, drain the contents of the vaporizer into an
appropriately marked container and discard the agent. For Halothane vaporizers
check the output of anesthetic agent periodically with an agent monitor. See note
below.
Three years from
purchase date and
every six months
thereafter:
The decomposition of Halothane causes the release of halides, which may corrode
metal components particularly in the presence of moisture. Also a preservative added
to Halothane by its manufacturers to impede decomposition can leave a residue,
which may cause vaporizer components to stick. If Halothane is used infrequently the
vaporizer should be drained after use.
Planned safety inspections together with the anesthesia
system by qualified personnel.
Inspect and perform output concentration check.
1175-0013-000
5-1
Page 28

Tec 7 Vaporizer
Cleaning
ww
ww
WARNING Do not put water or any other solvent into a vaporizer. A vaporizer should
be filled with the specified anesthetic agent only.
Do not immerse the vaporizer in water or any other liquid.
Do not autoclave the vaporizer.
External cleaning
Internal contamination
Output concentration
check
To clean external surfaces, use a moist cloth and neutral detergent (pH 7 to 10.5).
Never allow cleaning agents to accumulate either in the filler, the gas inlet and outlet
ports or around the control dial.
If the vaporizer is filled or partly filled with an incorrect volatile agent or other
contaminant (such as water), proceed as follows:
1. Remove the vaporizer from service immediately and label the vaporizer stating
that it is contaminated. Discard all liquid.
2. Return the vaporizer to a Datex-Ohmeda Authorized Service Center stating that
the vaporizer is contaminated and, if possible, the type of contaminant in the
vaporizer.
Connect the Tec 7 to an Anesthesia Machine.
1. Set the oxygen output of the anesthesia machine to a flow of 5± 0.5 L/min.
2. Ensure that the fresh gas output is connected to a gas scavenging system.
3. Measure the concentration at the fresh gas outlet, using an agent monitor which
is calibrated to measure the specific agent.
4. Allow the readings to stabilize and check that the readings are within specified
tolerances.
5. Document and maintain the test results, including the date, person performing
the test, and serial number of the unit tested.
5-2
1175-0013-000
Page 29

5 Maintenance
The accuracy of the measuring equipment must be considered when
obtaining the readings!
Sevoflurane Dial Setting Min Vol% Max Vol%
1% .6 1.40
3% 2.55 3.45
5% 4.25 5.75
Checking the calibration
Enflurane, Halothane or
Isoflurane Dial Setting
1% .75 1.25
3% 2.55 3.45
5% 4.25 5.75
The performance of most vaporizers that are in clinical use can be confirmed by observing
patient signs and consumption of anesthetic agents. Some users may, however, wish to
employ analyzers either as a routine procedure or as part of an investigation to determine
whether any abnormalities of performance have developed.
To help to achieve the reliability and consistency of performance of the Tec 7 Vaporizer,
Datex-Ohmeda uses closely specified test conditions, test methods and detailed protocol
in conjunction with training, experience and quality auditing systems. For these reasons,
the full program necessary to help to ensure that a vaporizer complies with Datex-Ohmeda
specifications cannot practicably be carried out in a field situation.
The following points must be considered when any measurements are being carried out on
vaporizers to assist in determining whether any abnormalities of performance have
developed.
Min Vol% Max Vol%
1175-0013-000
1. To predict the concentration that the vaporizer can be expected to deliver, the
detailed nominal performance data and the preceding comments must be taken
into account.
2. The method of test used must not be such that it bears little relation to normal
conditions of clinical use.
3. Any sampling techniques used must be such as to ensure the following:
a. The sample is fully representative of the vaporizer output, which may not be a
homogeneous mixture at the vaporizer outlet.
b. The absorption of agent by any connecting tubing is negligible.
4. If a number of vaporizers are being examined at the same time the probability of all
of them being consistently in error is so remote as to be negligible and the cause of
any apparent error probably lies in the test method employed.
5. Consistent and reproducible analytical techniques must be used.
6. If unexpected results are obtained, it is a wise precaution to repeat the observation
because the vaporizer may be more reliable than the techniques used to observe its
performance.
5-3
Page 30

Tec 7 Vaporizer
7. If unexpected results occur, it is also worthwhile checking for sources of error
such as the flowmeter, leaks or absorption by adjacent components.
8. Full account must be taken of any extraneous effects on the analyzer that may
arise from changes in the carrier gas composition.
9. If the anesthetic machine on which the vaporizer is fitted is left for a period of
time with no gases flowing, sensitive analyzers may detect small concentrations
of agent for a short time at the machine outlet after the gas flow is turned ON with
the vaporizer turned to . This is a normal machine characteristic caused by
residual vapor left in the machine from previous use.
10. When the vaporizer is turned from to 0% or above after a period out of use,
an increased concentration may occur that rapidly stabilizes to the set
concentration within approximately 10 seconds at 5 liters/minute flow.
11. At the 0% setting it is normal for small steady concentrations to be observable on
sensitive analyzers.
Analytical techniques
For field checking of the state of calibration, many techniques and analyzers are
available. Datex-Ohmeda would not recommend any one technique or analyzer in
preference to another, but the calibration and reliability of analyzers must be
realistically considered and account must be taken of errors of use.
The following method of checking can be used where special equipment is not
available or where a secondary check of analyzers is desired. The characteristics of the
vaporizer are such that, if the vaporizer is satisfactory at one dial setting, it should be
satisfactory at all other graduations.
1. Ensure that the vaporizer is at least half full and has been at an ambient
temperature of 21 ± 2° C for at least three hours.
2. With the vaporizer securely mounted, drain the vaporizer as detailed in Section 4
and, after draining, ensure that either the drain plug and the filler cap are both
securely tightened or the port valve is fully closed and the locking clamp is in the
up position, as appropriate.
3. Check that the control dial is turned to and then carefully and quickly pour a
measured 50 milliliters of agent into the vaporizer without spilling.
4. Leave the vaporizer at a nominal temperature of 21 ± 2° C for one hour to help to
ensure that the temperature has stabilized.
5. Set the flowrate to 5 liters/minute oxygen.
6. Turn the control dial to 2%, note the time and check that the flowrate is still 5
liters/minute. Readjust the flowrate as necessary.
7. Leave the vaporizer at this setting for 30 minutes. Periodically check and adjust
the flowrate as necessary. Turn the vaporizer to and turn the oxygen OFF.
5-4
1175-0013-000
Page 31

5 Maintenance
8. Drain the vaporizer as detailed previously in Instruction 2 and measure the
amount of liquid drained off. The amount of liquid consumed should be as
follows:
Enfluratec 15.5 milliliters
Fluotec 13.5 milliliters
Isotec 15.5 milliliters
Sevotec 16.6 milliliters
Appropriate action must be taken to handle the exhaust gases and spillage.
Service Policy
Note
This method is designed to be a quick and easy check of vaporizer operation and,
therefore , it is somewhat imprecise. However, it is unusual for the measured liquid
consumption to vary by more than 25% of the values listed above.
Repairs and service procedures must be performed at a Datex-Ohmeda Authorized
Service Center. Contact your Datex-Ohmeda Service Representative or Datex-Ohmeda
Authorized Distributor for information on maintenance and shipping.
1175-0013-000
5-5
Page 32

Tec 7 Vaporizer
5-6
1175-0013-000
Page 33

6 Principle of Operation
Interlock mechanism
The vaporizer locking lever is interlocked with the vaporizer percentage control dial so
that the control dial release, located at the rear of the dial, cannot be actuated until
the vaporizer locking lever is in the locked position.
With the vaporizer locking lever in the locked position, the dial release can be pressed
in toward the dial to operate the interlock mechanism, which allows the manifold port
valves to open, prevents an adjacent vaporizer from being turned on, and allows the
vaporizer to operate.
Turning the control dial to automatically reverses the operating sequence, which
allows the dial release to move out to lock the dial in the position, closes the
manifold port valves and vents the vaporizer gas connecting ports, and allows an
adjacent vaporizer to be turned ON.
Turning the locking lever to the unlocked position releases the vaporizer allowing it to
be removed from the manifold.
Delivery of gas/agent vapor
Overview
The output concentration of the Tec 7 Vaporizer is regulated by the ‘variable flow-split’
method described in the following text and shown in figures 6-1 and 6-2.
A total flow of fresh gas from upstream flowmeters enters the vaporizer from the
flowmeter where it is immediately split into two streams. One stream flows into the
fresh gas bypass circuit and the other stream flows through the vaporizing chamber
where it is enriched with the vapor of the liquid anesthetic agent.
1175-0013-000
6-1
Page 34

Tec 7 Vaporizer
Bypass circuit
1
The bypass circuit includes the gas transfer manifold and also a thermostat assembly
that is located at the base of the vaporizer.
The fresh gas flows through the bypass circuit vertically downwards across the sump
base through the thermostat and back up the gas transfer manifold to the common
gas outlet as shown in Figure 6-1.
The thermostat deflects according to its temperature to control the resistance offered
to the flow of gas through it. This deflection varies the relative proportions of gas
flowing through the bypass and vaporizing chamber circuits.
9
6
7
3
8
4
AB80.039
52
Vaporizing chamber
circuit
1. Gas from flowmeter
2. Sump base
3. Thermostat
4. Gas transfer manifold
5. To common gas outlet
6. Shut-off open
7. Vaporizing chamber
8. Flow control (vapor channel)
9. Rotary valve
Figure 6-1 • Bypass circuit
The fresh gas flow through the vaporizing chamber, as shown on Figure 6-2, flows from
the flowmeter across the sump cover where it is diverted through the central cavity of
the rotary valve and back through the Intermittent Positive Pressure Ventilation (IPPV)
compensating assembly.
Gas now flows from the IPPV assembly down through the tubular wick assembly where
it picks up anesthetic vapor and then flows across the base of the vaporizing chamber
above the liquid agent.
From the base of the vaporizing chamber the gas/agent mixture flows through the
sump cover to the proportional radial drug control groove of the rotary valve and then
back into the sump cover where it combines with the fresh gas from the bypass circuit.
The combined total flow then flows out from the vaporizer and via the Selectatec
circuitry to the anesthesia gas delivery system.
6-2
1175-0013-000
Page 35

6 Principle of Operation
12
11
10
1
2
3
4
5
9
8
7
1. Rotary valve
2. Enriched fresh gas out
3. Combined fresh gas and enriched gas out
4. Fresh gas bypass
5. Fresh gas out
6. Thermostat
7. Vaporizing chamber
8. Wick assembly
9. IPPV compensating assembly
10. Sump cover
11. Vapor control channel
12. Shown in ON position
1175-0013-000
AB80005
6
Figure 6-2 • Vaporizer schematic
6-3
Page 36

Tec 7 Vaporizer
6-4
1175-0013-000
Page 37

7 Specifications
Note All specifications are nominal and subject to change without notice.
Calibration
Check the calibration certificate that is included with your Tec 7 Vaporizer.
Vaporizers are calibrated at 21°C using an oxygen carrier gas at a flow of 5 liters/
minute and they are temperature, flow and pressure compensated within the
specified operating range.
w WARNING The Tec 7 Vaporizer can only be calibrated at a Datex-Ohmeda
Authorized Service Center.
1175-0013-000 7-1
Page 38

Tec 7 Vaporizer
Performance
Weight and dimensions
Accuracy at 5 liters/
min O
21 ± 2 °C
2
Liquid capacity To fully charge a vaporizer with dry wicks: 300 ml (nominal)
Flow resistance at
5 liters/minute of
O
at 21 ± 2 °C
2
Operating
temperature range
Storage temperature
range
Vaporizer 5%: ± 0.25% of delivered agent or ± 15% dial setting
(whichever is greater)
Vaporizer 8%: ± 0.4% of delivered agent or ± 15% dial setting
(whichever is greater)
Retained by wick system: 75 ml (nominal)
To fill from minimum to maximum mark:
5% Vaporizer: 170 ml (nominal)
8% Vaporizer (Sevotec):
137 ml (nominal)
10 - 15 cm H2O with Vaporizer setting ON +0%
18 °C to 35 °C (64 °F to 95°F)
–40 °C to 65 °C (–40 °F to 149°F)
Note: Protect the vaporizer packaging from condensation.
Weight 7 kg 15 lb 6 oz (empty)
Depth 210 mm 8.25 inches
Width 110 mm 4.375 inches
Height 250 mm 9.875 inches (in the unlocked position)
7-2 1175-0013-000
Page 39

Flow characteristics
Isotec 5%
5
4
7 Specifications
AB80027
5
4
3
% ISOFLURANE
2
1
0
0.2 1 5 10 15
Flowrate (liters/minute oxygen)
Figure 7-1 • Effect of Flowrate at 21 ± 2° C with oxygen flowing
5
4
3
3
2
1
0.6
0.2
DIAL SETTING
5
4
3
AB80028
2
% ISOFLURANE
1
0
15 20 25 30
2
1
0.6
0.2
35
DIAL SETTING
Temperature °C
Figure 7-2 • Effect of temperature at 5 liters/minute with oxygen flow
1175-0013-000 7-3
Page 40

Tec 7 Vaporizer
Fluotec 5%
AB80029
5
5
4
3
% HALOTHANE
2
1
0
0.2 1 5 10 15
4
3
2
1
0.6
0.2
DIAL SETTING
Flowrate (liters/minute oxygen)
Figure 7-3 • Effect of Flowrate at 21 ± 2° C with oxygen flowing
5
5
AB80030
4
3
% HALOTHANE
2
1
0
15 20 25 30
4
3
2
1
0.6
0.2
35
Temperature °C
Figure 7-4 • Effect of temperature at 5 liters/minute with oxygen flow
7-4 1175-0013-000
DIAL SETTING
Page 41

Sevotec 5%
5
4
7 Specifications
AB800031
5
4
3
% SEVOFLURANE
2
1
0
0.2 1 5 10
Flowrate (liters/minute oxygen)
3
DIAL SETTING
2
1
0.6
0.2
15
Figure 7-5 • Effect of Flowrate at 21 ± 2° C with oxygen flowing
5
5
4
4
AB80032
3
% SEVOFLURANE
2
1
0
15 20 25 30
Temperature °C
3
DIAL SETTING
2
1
0.6
0.2
35
Figure 7-6 • Effect of temperature at 5 liters/minute with oxygen flow
1175-0013-000 7-5
Page 42

Tec 7 Vaporizer
Enfluratec 5%
5
4
AB80035
5
4
3
% ENFLURANE
2
1
0
0.2 1 5 10
Flowrate (liters/minute oxygen)
15
3
2
1
0.6
0.2
DIAL SETTING
Figure 7-7 • Effect of Flowrate at 21 ± 2° C with oxygen flowing
5
5
4
4
AB80036
3
% ENFLURANE
2
1
0
15 20 25 30
Temperature °C
3
DIAL SETTING
2
1
0.6
0.2
35
Figure 7-8 • Effect of temperature at 5 liters/minute with oxygen flow
7-6 1175-0013-000
Page 43

Sevotec 8%
8
7
6
7 Specifications
AB80033
5
4
% SEVOFLURANE
3
2
1
0
0.2 1 5 10
Flowrate (liters/minute oxygen)
Figure 7-9 • Effect of Flowrate at 21 ± 2° C with oxygen flowing
8
7
6
5
4
% SEVOFLURANE
3
15
8
7
6
5
4
3
8
7
6
5
4
3
2
1
0.6
0.2
DIAL SETTING
AB80034
DIAL SETTING
2
1
0
15 20 25 30
2
1
0.6
0.2
35
Temperature °C
Figure 7-10 • Effect of temperature at 5 liters/minute with oxygen flow
1175-0013-000 7-7
Page 44

Tec 7 Vaporizer
Effects of variables
Anesthetic agent
consumption
Isoflurane, Halothane
and Enflurane
Sevoflurane The rate of consumption of anesthetic agent depends primarily on flowrate and vapor
The rate of consumption of anesthetic agent depends primarily on flowrate and vapor
output concentration. As an approximate working figure, 1 milliliter of liquid
anesthetic is required to provide 200 milliliters of vapor.
The approximate hourly consumption of anesthetic agents can be expressed as
3 x % x F, where % represents the setting of the vaporizer output percentage and F
represents the input flowrate in liters/minute.
Example: If a vaporizer is set to deliver 2% at 6 liters/minute total gas
input flowrate then the approximate rate of consumption
= 3 x 2 x 6 = 36 ml/hour.
The figures are approximate and are intended for general guidance only.
output concentration. As an approximate working figure, 1 milliliter of liquid
anesthetic is required to provide 200 milliliters of vapor.
The approximate hourly consumption of anesthetic agents can be expressed as 3.3 x
% x F, where % represents the setting of the vaporizer output percentage and F
represents the input flowrate in liters/minute.
Example: If a vaporizer is set to deliver 2% at 6 liters/minute total gas
input flowrate then the approximate rate of consumption
= 3.3 x 2 x 6 = 39.6 ml/hour.
The figures are approximate and are intended for general guidance only.
Barometric pressure The Tec 7 Vaporizer is calibrated in percent v/v at 760 mmHg. If the ambient pressure
changes the % v/v changes, so that at an ambient pressure P mmHg the delivered
percentage (D% v/v) is calculated as follows:
% x 760
D =
To obtain a consistent depth of anesthesia when gross changes of barometric
pressure occur, the % v/v must be changed in inverse proportion to the barometric
pressure.
The vaporizer automatically makes this % v/v change and for practical clinical
purposes the effects of barometric pressure can be ignored.
7-8 1175-0013-000
where % is the nominal setting of the vaporizer.
P
Page 45

7 Specifications
Ambient temperature The effects of variation in temperature are normally negligible at commonly used
combinations of dial setting and ambient temperature.
If the vaporizer temperature is above the range shown on the performance curves, the
vaporizer output may be unpredictably high, particularly if the temperature of the
agent approaches the agent boiling point specified by the agent manufacturer.
If the vaporizer temperature is below the range shown on the performance curves, the
vaporizer output may be lower than expected.
To help avoid inaccuracies due to extreme temperatures, before using the vaporizer it
must be allowed to attain a temperature within the range shown on the performance
curves.
Back pressure
wwwwWARNING Pressures in excess of 400 mmHg may overcome the internal pressure
balance and cause a variation in output.
Steady back pressure The vaporizer cannot distinguish between pressures at the outlet due to barometric
pressure and pressures in excess of barometric due to steady back pressures applied
by downstream components. The equation given in the section Barometic Pressure
therefore applies with the term P now being the absolute pressure at the outlet, that is,
barometric pressure plus back pressure. Steady back pressure reduces the % v/v.
Currently, it is unlikely that the steady back pressure imposed by commonly used
downstream components, other than some ventilators, exceeds 30 mmHg at
commonly used flowrates. Back pressures as high as 30 mmHg would reduce the
delivered % v/v, at 760 mmHg barometric pressure, to the following:
760
= 0.96 of what would otherwise be expected.
--------790
790
Under normal clinical circumstances effects of this magnitude can be ignored.
Fluctuating back pressure Fluctuating back pressure may be imposed on the vaporizer by downstream
components and/or assisted or controlled ventilation to the patient. These fluctuating
back pressures can affect the vaporizer and increase the concentration by
intermittently altering the pressures, and consequently the flow distribution, within the
vaporizer.
The greatest effects are observed at combinations of very low flowrates and low dial
setting with large and rapid pressure fluctuations. The effects become progressively
less important as the dial setting and flowrate increase and the magnitude and rate of
cycling of the pressure fluctuations decrease.
1175-0013-000 7-9
Page 46

Tec 7 Vaporizer
Carrier gas composition
ww
ww
WARNING Only operate the vaporizer with dry medical gases.
Time out of service
Effects of variables
Small output decreases can occur when the carrier gas composition is changed from
100% oxygen.
When either air or nitrous oxide is employed as the carrier gas, the output is lowered
compared to the output when oxygen is the carrier gas. This effect is the greatest (up
to 20% of setting) at low flows when nitrous oxide is employed, but using nitrous oxide
reduces the required inspired concentrations of volatile agent that can, depending
upon the proportion, mitigate the decreases in output from the vaporizer.
If the anesthesia machine on which the vaporizer is fitted is left for a period of time
with no gases flowing, small concentrations may be detected at the machine outlet
immediately after the gas flow is turned ON. This is a normal machine characteristic
and is caused by residual vapor left in the machine from previous use.
When the vaporizer is turned from the setting after a period out of use, a brief
high concentration may occur that rapidly stabilizes to the set concentration within
approximately 10 seconds at 5 liters/minute.
These phenomena are normal characteristics of vaporizers. In use the volume of vapor
involved is small compared to the volume of the breathing circuit.
Ambient temperature, input flowrate and duration of use can affect delivered
concentration, particularly when the vaporizers are used at extremes of the usual
clinical range.
Note
Use of the vaporizer at high gas flows and high dial concentrations may affect the
accuracy of delivered concentrations. Refer to Performance Curves in this chapter for
full information.
The valve design and temperature compensation system of Tec 7 Vaporizers reduces
the effects to levels such that, under most clinical conditions, their effect on vaporizer
performance is not clinically significant.
7-10
1175-0013-000
Page 47

Warranty
This Product is sold by Datex-Ohmeda under the warranties set forth in the
following paragraphs. Such warranties are extended only with respect to the
purchase of this Product directly from Datex-Ohmeda or Datex-Ohmeda’s
Authorized Dealers as new merchandise and are extended to the Buyer
thereof, other than for the purpose of resale.
For a period of 36 months from the date of original delivery to Buyer or to
Buyer’s order, but in no event for a period of more than three years from the
date of original delivery by Datex-Ohmeda to a Datex-Ohmeda Authorized
Dealer, this Product, other than its expendable parts, is warranted against
functional defects in materials and workmanship and to conform to the
description of the Product contained in this User’s Reference Manual and
accompanying labels and/or inserts, provided that the same is properly
operated under the conditions of normal use, that regular periodic
maintenance is performed and that replacements and repairs are made in
accordance with the instructions provided. This same warranty is made for a
period of thirty (30) days with respect to expendable parts. The foregoing
warranties shall not apply if the Product has been repaired other than by
Datex-Ohmeda or in accordance with written instructions provided by
Datex-Ohmeda, or altered by anyone other than Datex-Ohmeda, or if the
Product has been subject to abuse, misuse, negligence, or accident.
Datex-Ohmeda’s sole and exclusive obligation and Buyer’s sole and exclusive
remedy under the above warranties is limited to repairing or replacing, free of
charge, at Datex-Ohmeda’s option, a Product, which is telephonically
reported to the nearest Datex-Ohmeda Customer Support Center and which, if
so advised by Datex-Ohmeda, is thereafter returned with a statement of the
observed deficiency, not later than seven (7) days after the expiration date of
the applicable warranty, during normal business hours, transportation
charges prepaid, and which, upon Datex-Ohmeda’s examination, is found not
to conform with above warranties. Datex-Ohmeda shall not be otherwise
liable for any damages including but not limited to incidental damages,
consequential damages, or special damages.
There are no express or implied warranties which extend beyond the
warranties hereinabove set forth. Datex-Ohmeda makes no warranty of
merchantability or fitness for a particular purpose with respect to the product
or parts thereof.
Page 48

CCCCoooorrrrppppoooorrrraaaatttteeee OOOOffffffffiiiiccccee
Datex-Ohmeda Division
Instrumentarium Corp.
PO Box 900
FIN-00031 Helsinki
Finland
Tel 358 10 394 11
Fax 358 9 146 3310
NNNNoooorrrrtttthhhh AAAAmmmmeeeerrrriiiiccccaa
UUUUnnnniiiitttteeeedddd SSSSttttaaaatttteeeess
CCCCuuuussssttttoooommmmeeeerrrr SSSSeeeerrrrvvvviiiicccceeee,,,, TTTTeeeecccchhhhnnnniiiiccccaaaallll SSSSuuuuppppppppoooorrrrtttt aaaannnndddd
DDDDiiiissssttttrrrriiiibbbbuuuuttttiiiioooonnnn CCCCeeeennnntttteeeerr
Datex-Ohmeda, Inc.
PO Box 7550
Madison, WI 53707-7550, USA
Tel 1 800 345 2700
Fax 1 608 221 4384
EEEEqqqquuuuiiiippppmmmmeeeennnntttt SSSSeeeerrrrvvvviiiicccceeee CCCCeeeennnntttteeeerr
Datex-Ohmeda, Inc.
1315 West Century Drive
Louisville CO 80027-9560, USA
Tel 1 800 345 2700
Fax 1 303 373 1607
CCCCaaaannnnaaaaddddaa
Datex-Ohmeda (Canada) Inc.
1093 Meyerside Drive, Unit 2
Mississauga, Ontario
L5T 1J6
Canada
Tel 1 800 268 1472
Tel 1 905 565 8572
Fax 1 905 565 8592
AAAAssssiiiiaaaa////PPPPaaaacccciiiiffffiiiicc
aa
CCCChhhhiiiinnnnaa
Datex-Ohmeda Pte. Ltd.
Room B416, COFCO Plaza
8 Jianguomennei Avenue
Beijing 100005, PR China
Tel 86 10 6526 9773
Fax 86 10 6526 0653
Datex-Ohmeda Pte. Ltd.
Room 1708, Yunlong Mansion
No. 122 Luoguo Street
Chengdu 610017, PR China
Tel 86 28 661 4424
Fax 86 28 676 2703
ee
aa
ss
rr
rr
aa
cc
Datex-Ohmeda Pte. Ltd.
403 Huan Shi Dong Road
Room 1602, GIE Tower
Guangzhou, 510095, P R China
Tel 86 20 8732 2521
Fax 86 20 8732 2518
Datex-Ohmeda Pte. Ltd.
Room 2509 Lippo Plaza
No. 222 Huaihai Road (M)
Shanghai 200021, P.R. China
Tel 8621 5382 5657
Fax 8621 5382 1691
Datex-Ohmeda Pte. Ltd.
Room 809, Truroll Plaza
Wusheng Road
Wuhan 430033, P R China
Tel 86 27 8571 2536
Fax 86 27 8571 2655
aa
IIIInnnnddddiiiiaa
Datex-Ohmeda (India) Pvt. Ltd.
Block EP & GP, Sector V
Plot XI-16, Salt Lake City
Calcutta 700091
India
Tel 91 33 3574002
Fax 91 33 3574001
aa
IIIInnnnddddoooonnnneeeessssiiiiaa
Datex-Ohmeda Pte. Ltd.
Wisma Danamon Aetna Life 19th Floor
Jln. Jend Sudirman Kav. 45-46 Jakarta
12930, Indonesia
Tel 62 21 575 0864
Fax 62 21 575 0865
nn
JJJJaaaappppaaaann
Datex-Ohmeda K. K.
TRC Annex 9F
6-1-1 Heiwajima
Ohta-ku, Tokyo 143-0006
Japan
Tel 81 3 5763 6801
Fax 81 3 5763 6838
Datex-Ohmeda K. K.
Technical Center
TRC A Bldg. AE 4-8
6-1-1 Heiwajima
Ohta-ku, Tokyo 143-0006
Japan
Tel 81 3 5763 6850
Fax 81 3 5763 6852
aa
KKKKoooorrrreeeeaa
Datex-Ohmeda Pte. Ltd.
10th Floor, Sam Sung Building
36 - 1, Yoido-Dong, Youngdeungpo-Ku
Seoul, Korea
Tel 82 2 786 7421
Fax 82 2 786 7420
aa
MMMMaaaallllaaaayyyyssssiiiiaa
Datex-Ohmeda Pte. Ltd.
Level 2 Bangunan O'Connor
13 Jalan 223
46100 Petaling Jaya
Selangor, West Malaysia
Tel 60 3 754 7872
Fax 60 3 757 6948
ee
SSSSiiiinnnnggggaaaappppoooorrrree
Datex-Ohmeda Pte. Ltd.
152 Beach Road
#12-05/07 Gateway East
Singapore 189721
Tel 65 391 8618
Fax 65 291 6618
TTTThhhhaaaaiiiillllaaaannnndddd
Datex-Ohmeda Pte. Ltd.
12th Floor (Unit F) Grand Amarin Tower
1550 New Petchburi Road, Makasan, Rajathevi,
Bangkok 10320, Thailand
Tel 66 2 2071012/13
Fax 66 2 207 1014
TTTTaaaaiiiiwwwwaaaannnn aaaannnndddd PPPPhhhhiiiilllliiiippppppppiiiinnnneeeess
Datex-Ohmeda Pte. Ltd.
2nd Floor, No. 85, Chien-Kuo North Road,
Sec. 2
Taipei, Taiwan
Republic of China
Tel 886-2 2515-0457
Fax 886-2 2501-9136
mm
VVVViiiieeeettttnnnnaaaamm
Datex-Ohmeda Pte. Ltd.
522G Nguyen Tri Phuong St.
Ho Chi Minh City, Dist. 10 Vietnam
Tel 848 865 5875
Fax 848 862 5501
aa
AAAAuuuussssttttrrrraaaalllliiiiaa
Datex-Ohmeda Pty. Ltd.
Units 1 & 2
149 Arthur Street
P O Box 356
Homebush
NSW 2140
Australia
Tel 61 132 229
Fax 61 297 461796
ee
EEEEuuuurrrrooooppppee
ss
CCCCIIIISSSS////BBBBaaaallllttttiiiiccccss
Datex-Ohmeda
Regional Head Office
PO Box 70071
GR-16610 Glyfada - Athens
Greece
Tel +30 10 9625136-7
Fax +30 10 9623687
ss
ee
FFFFrrrraaaannnnccccee
Datex-Ohmeda S.A.S.
ZAC de Sans-Souci
1211 Chemin de la Bruyère
F-69760 Limonest
France
Tel 33 (0) 4 78 66 62 10
Fax 33 (0) 4 78 43 26 58
yy
GGGGeeeerrrrmmmmaaaannnnyy
Datex-Ohmeda GmbH
Dr. Alfred-Herrhausen-Allee 24
D-47228 Duisburg
Germany
Tel 49 2065 691-0
Fax 49 2065 691-236
yy
IIIIttttaaaallllyy
Datex-Ohmeda S.p.A.
Via Cassanese 100
20090 Segrate, Milan
Italy
Tel 39 2 21693431
Fax 39 2 26926226
NNNNeeeetttthhhheeeerrrrllllaaaannnnddddss
Datex-Ohmeda B.V.
Kantemarsweg 18
Post Box 22
3870 CA Hoevelaken
Netherlands
Tel 31 33 253 5404
Fax 31 33 253 7223
SSSSppppaaaaiiiinn
Datex-Ohmeda S.L.
C/Manuel Tovar 26
28034 Madrid
Spain
Tel 34 1 334 26 00
Fax 34 1 358 12 84
UUUUnnnniiiitttteeeedddd KKKKiiiinnnnggggddddoooomm
Datex-Ohmeda Ltd.
Ohmeda House
71 Great North Road
Hatfield Hertfordshire
AL9 5EN England
Tel 44 1707 263570
Fax 44 1707 260191
LLLLaaaattttiiiinnnn AAAAmmmmeeeerrrriiiiccccaaaa,,,, CCCCaaaarrrriiiibbbbbbbbeeeeaaaann
Datex-Ohmeda
9155 South Dadeland Blvd.
Suite 1218
Miami, FL 33156, USA
Tel 1 305 670 8540
Fax 1 305 670 2316
MMMMiiiiddddddddlllleeee EEEEaaaasssstt
Datex-Ohmeda
Regional Head Office
PO Box 70071
GR-16610 Glyfada - Athens
Greece
Tel +30 10 9625136-7
Fax +30 10 9623687
ss
nn
mm
nn
tt
The addresses listed on this cover are current as of 4/02. For any location changes,
please visit our website at www.datex-ohmeda.com and click on the Contacts button.
Datex-Ohmeda, Inc.
PO Box 7550
Madison WI 53707-7550
USA
Tel 608 221 1551
Fax 608 222 9147
www.datex-ohmeda.com
Tec 7 vaporizer
User’s Reference Manual, English
1175 0013 000
06 02 A 18 05 02
Printed in USA
©Datex-Ohmeda, Inc. All rights reserved
 Loading...
Loading...