Page 1

Affi-Gel®Hz
Immunoaffinity
Kit
Instruction
Manual
Catalog Number
153-6060
For Technical Service
Call Your Local Bio-Rad Office or
in the U.S. Call 1-800-4BIORAD
(1-800-424-6723)
Page 2

Table of Contents
Section 1 Introduction...............................................................1
1.1 Immunoaffinity Coupling and Usefulness of the
Immobilized Antibody.........................................................1
1.2 Hydrazide Coupling Chemistry...........................................2
1.3 Diagram of Kit Components ...............................................2
Section 2 Monoclonal Antibodies.............................................3
Section 3 Antibody Purification...............................................3
Section 4 Immobilization Protocol...........................................4
4.1 Buffer Exchange..................................................................4
4.2 Oxidation of IgG..................................................................6
4.3 Desalting Procedure.............................................................7
4.4 Coupling of Oxidized IgG to Affi-Gel Hz
Hydrazide Gel......................................................................7
Section 5 Applications of Affi-Gel Hz Immobilized IgG........9
5.1 Conditioning the Immunoaffinity Column..........................9
5.2 Sample Application ...........................................................10
5.3 Elution Suggestions...........................................................10
Section 6 Product Information...............................................11
6.1 Specifications.....................................................................11
6.2 Ordering Information.........................................................11
Page 3
Section 1
Introduction
The Affi-Gel Hz immunoaffinity kit is a unique approach to
IgG coupling to an agarose support matrix for affinity purif ication.
This kit achieves a more uniform orientation of coupled antibody than
currently available activated supports which couple via primary
amines. Affi-Gel Hz hydrazide gel is an agarose support which
reacts with the aldehydes of oxidized carbohydrates to form stable,
covalent hydrazone bonds. Immunoglobulin G is a glycoprotein
which contains approximately 3% carbohydrate localized on the
Fc region (heavy chain) of the antibody . Periodate oxidation of vicinal hydroxyls of the sugars of these carbohydrates forms aldehyde
groups for specific coupling to Affi-Gel Hz gel. This coupling
through the carbohydrate eliminates the loss of antibody activity
experienced in primary amino coupling at or near the antigen binding site by allowing the correct orientation of the antibody.
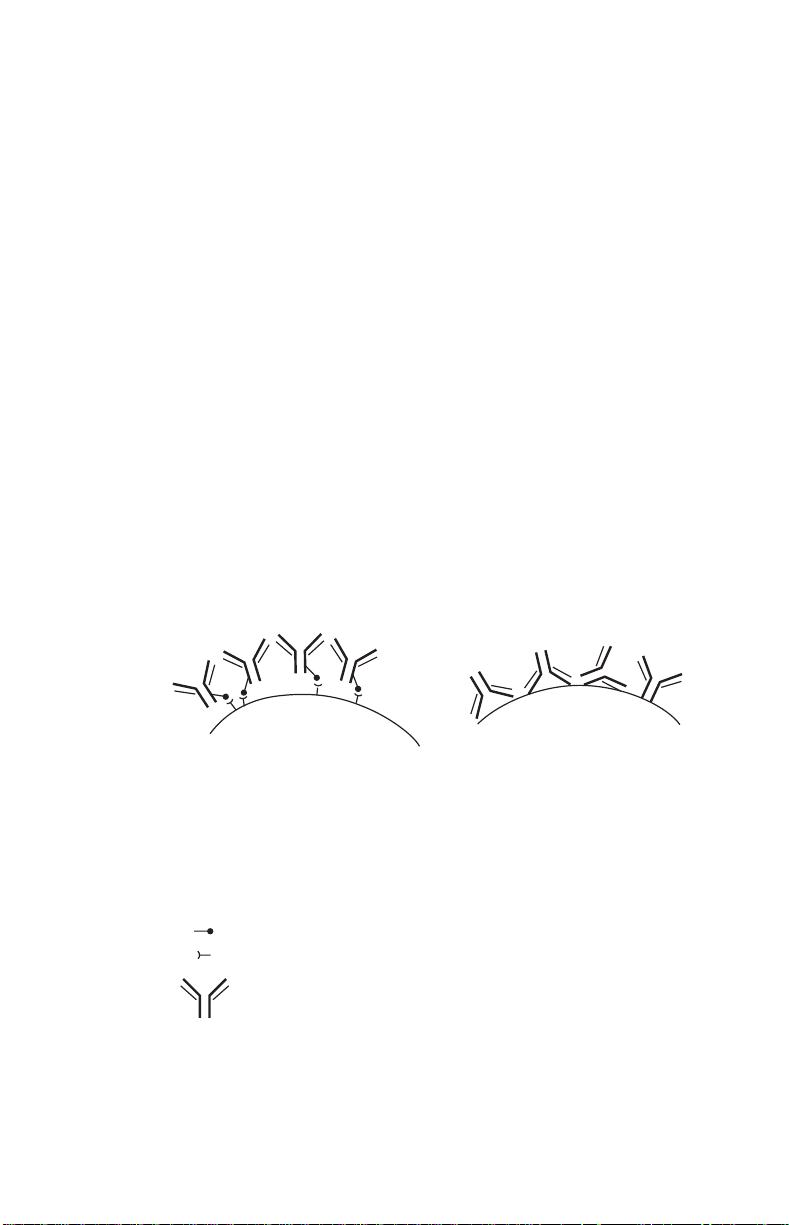
1.1 Immunoaffinity Coupling and Usefulness
of the Immobilized Antibody
Oriented coupling results in higher
antibody activity for greater antigen
binding capacity per coupled IgG.
IgG immobilized on Affi-Gel HZ
hydrazide gel through the carbohydrate of the Fc region.
Carbohydrate moieties on Fc region of IgG.
Hydrazide functional group on gel spacer arm.
Immunoglobulin G.
1
vs
Random coupling yields low antigen
binding due to attachment at or near
the binding side of the antibody. IgG
coupling directly to agarose support
via primary amine.
Page 4
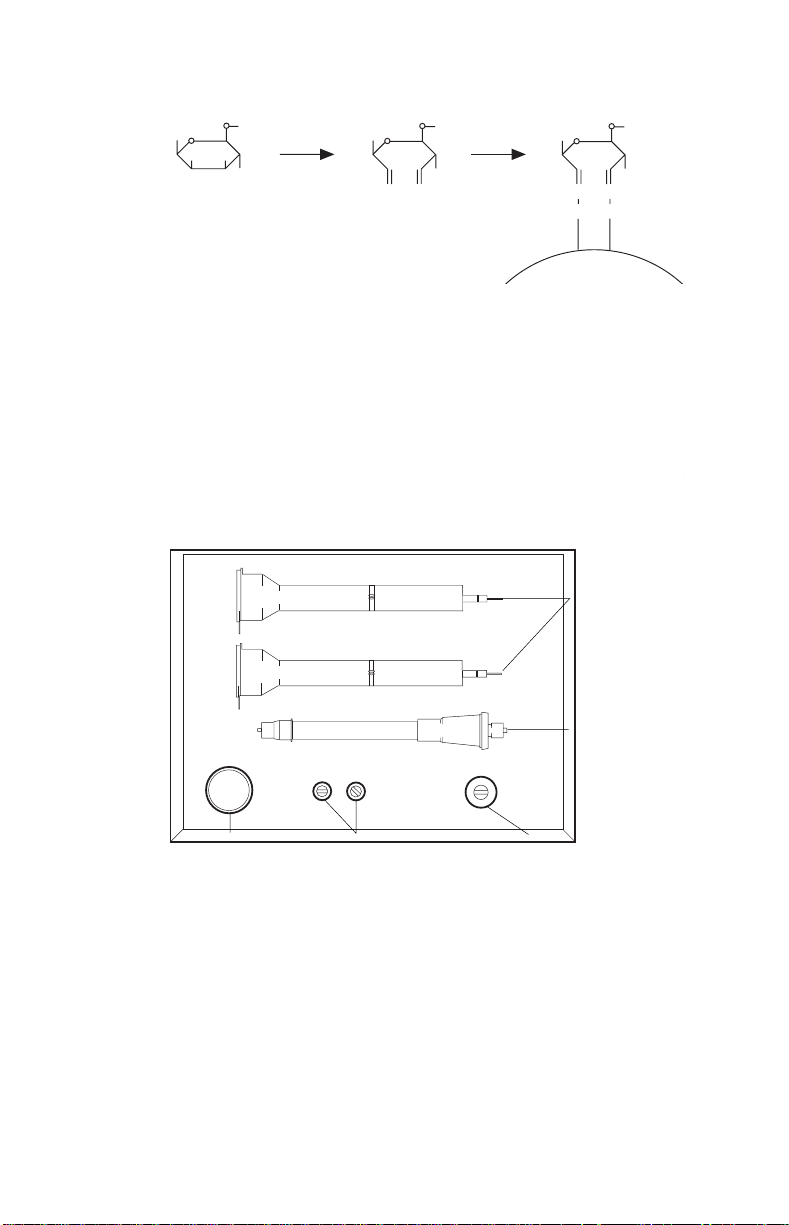
1.2 Hydrazide Coupling Chemistry
HOH
HOH2C
OH OH
OH
IgG
NalO
4
C
IgG
2
Affi-Gel Hz
OH
O
O
gel
HOH2C
NH
Affi-Gel Hz
gel
NH
IgG
OH
NN
Sugar residue of
carbohydrate on the
Fc region of IgG.
Periodate oxidation
of vicinal hydroxyls
to form aldehydes.
Oxidized IgG specific
coupling to Affi-Gel
Hz hydrazide gel.
1.3 Diagram of Kit Components
Econo-Pac
Desalting Columns
1 x 10 cm
Econo-Column
Chromatography
Column
Affi-Gel Hz 10 x
Coupling Buffer, 25 ml
Affi-Gel Hz Oxidizer,
25 mg NalO
4
each
Materials and Equipment Not Included in this Kit
Affi-Gel Hz Hydrazide
Gel, 5 ml
®
10DG
®
Essential
15 ml screw cap test tubes - polypropylene or polystyrene, leak-free
End-over-end rotating mixer
Transfer pipets
pH meter
UV-visible spectrophotometer
2
Page 5

Optional
Bio-Rad Protein Assay Kit I (catalog number 500-0001)
Section 2
Monoclonal Antibodies
Due to the unique specificity of monoclonal antibodies, coupling to Affi-Gel Hz gel may not be optimal for all monoclonal
antibodies. Loss of activity may occur depending on the individual
monoclonal. T o minimize loss of v aluable antibody preparations, BioRad highly recommends that a sample coupling experiment be performed to determine the efficiency of the coupling reaction. After
the results of the sample coupling are determined, the remaining
antibody can be coupled to the Affi-Gel Hz gel. If results are less than
adequate with the Affi-Gel Hz gel, use Af fi-Gel 10 gel to immobilize the monoclonal antibody.
Section 3
Antibody Purification
Prior to IgG coupling to Affi-Gel Hz hydrazide gel, the antibody must be at least partially purified. A high degree of purity will
insure that higher IgG concentrations are available for oxidation
and immobilization to the affinity support. This increased purity of
IgG will maximize coupling capacity and purification potential.
There are many approaches to antibody purification, and only a few
will be presented here.
For use with this kit, the purified antibody should be in a total
protein concentration of 1-5 mg/ml in a total maximum volume of
5 ml prior to buffer exchange. This recommended volume is necessary for rapid desalting and buffer exchange with the Econo-Pac
10DG columns. Regardless of the purification method(s) chosen, it
will be necessary to buffer exchange the purified antibody into the
Affi-Gel Hz coupling buffer prior to oxidation and coupling following the procedure in this manual. See Buffer Exchange, Section 4.1.
IgG from serum or ascites can be purified by using a combination of ion exchange with the Macro-Prep®high S support and
hydrophobic interaction chromatography with the Macro-Prep
t-butyl support (or a combination of affinity chromatography
with CM Affi-Gel blue gel and hydroxyapatite chromatography
with Macro-Prep CHT, type I support). DEAE Affi-Gel blue chro-
3
®
Page 6

matography may also be used to remove most serum components
except transferrin.
IgG in ascites fluid and serum may be purified by Aff i-Gel protein A agarose (MAPS®II kit) low pressure chromatography , or by
application to Affi-Prep®protein A medium to high pressure polymeric matrix. Protein A, a surface protein from Staphylococcus
aureus, binds the Fc region of man y mammalian IgG species. Protein
A purification will yield highly purified IgG for coupling to Aff i-Gel
Hz gel.
Hybridoma tissue culture supernatant containing dilute antibody (µg/ml concentrations) will necessitate a scheme to concentrate
and purify the immunoglobulin. Ammonium sulfate precipitation followed by dialysis and ion exchange chromatography, Bio-Gel®HT
hydroxyapatite, or Affi-Gel protein A gel are options that will yield
highly purified, concentrated antibody.
Some immunoglobulins are sensitive to high ammonium sulfate concentrations or low pH and, as a result, total antibody activity can be irreversibly reduced prior to coupling to Aff i-Gel Hz gel.
Care must be taken to minimize the duration of antibody exposure
to these conditions to retain activity essential to the applications of
the immunoaffinity matrix.
Section 4
Immobilization Protocol
4.1 Buffer Exchange
Prior to coupling to Affi-Gel Hz gel, it is necessary to exchange
the buffer in which the purified antibody appears. The Affi-Gel Hz
coupling buffer is optimized for antibody oxidation and immobilization to Affi-Gel Hz hydrazide gel. This buffer exchange can be
easily performed using the Econo-Pac 10DG desalting columns
provided.
Antibody immobilization to Affi-Gel Hz gel consists of buffer
exchange prior to the oxidation of the carbohydrate moieties on the
Fc region, desalting, and coupling. This oxidation forms aldehydes
for binding to the hydrazide functional group on the gel. Since the
carbohydrate constitutes a small percentage of the total glycoprotein
weight, the coupling reaction will progress slower than activ ated, selfcoupling gels. The bond formed is a stable hydrazone linkage that
is chemically resistant to many common elution conditions employed
4
Page 7

in affinity chromatography.
4.1A Dilution of Affi-Gel Hz 10x Coupling Buffer
1. Dilute Affi-Gel Hz 10x coupling buffer 1:10 with distilled,
deionized water and mix well.
2. Check the pH of the diluted coupling buffer with a pH meter. The
pH of the diluted buffer should be 5.5. If it is necessary to cor-
rect the pH of the diluted Affi-Gel Hz coupling buffer, use
1.0 M acetic acid or 1.0 M NaOH to bring the pH to 5.5.
Sodium azide, at a concentration of 0.02%, can be added to the
diluted coupling buffer for long-term storage. However, it is
best to dilute only the amount of 10x concentrate required.
4.1B Buffer Exchange with Econo-Pac 10DG Desalting
Columns
Buffer exchange with Econo-Pac 10DG columns gives a minimum dilution of the purified antibody . Sample v olumes may v ary,
but only 3.0 ml should be run at a time. These columns can be
regenerated and reused if adequately washed after protein collection.
1. Remove the upper cap from the Econo-Pac 10DG column and
pour off the excess buffer above the top frit.
2. Add 20 ml of the diluted Hz coupling buffer, pH 5.5 (f ill to the
30 ml mark), and snap off the bottom tip to start the column
flowing.
3. Allow the buffer to drain to the top frit. The column will not run
dry. Flow will stop when the buffer level reaches the top frit.
4. Add up to 3.0 ml of purified IgG sample to the Econo-Pac 10DG
column. Allow the sample to run completely into the column.
If applying a sample of less than 3.0 ml, add the difference in
diluted coupling buffer to the column, allowing it to run com-
pletely into the column (3.0 ml sample volume). Discard the
first 3.0 ml eluted from the column.
5. Add pH 5.5 coupling buffer (120% of the starting sample vol-
ume) to the top of the Econo-Pac 10DG column and collect 0.5
ml fractions. Monitor the absorbance of the fractions at 280
nm. Pool the fractions containing the purified antibody in Aff i-
Gel Hz coupling buffer.
5
Page 8

6. The Econo-Pac 10DG column should be washed with at least 20
ml of diluted coupling buffer, pH 5.5, to regenerate for desalt-
ing use after oxidation.
7. Place the yellow end cap over the column tip snugly to prev ent
leakage. Add 5 ml of diluted coupling buffer with 0.02% sodi-
um azide to the column and replace the top cap for column stor-
age.
8. The antibody is now ready for sodium periodate oxidation.
4.2 Oxidation of IgG
The oxidation of purified IgG will require the use of sodium
m-periodate (NaIO4).
Warning: Sodium periodate is a powerful oxidant. Avoid con-
tact and inhalation. May be harmful if swallowed. May react
violently with reducing agents, hydrides, and finely powdered
metals. Use only as directed in this manual. W ear glo ves and e ye
protection.
Affi-Gel Hz oxidizer is used as a stock solution and added to IgG
as required. Since sample volumes will vary, it is necessary that the
ratio of stock solution to sample be given, rather than actual volumes.
Sodium periodate is light sensitive; therefore, oxidation must be
performed in the dark. Maximum storage of the diluted sodium
periodate stock solution is 1 week at 4 °C, in the dark glass vial
provided. At the recommended sodium periodate concentrations,
oxidation of carbohydrate moieties is specific and does not alter
IgG activity.
1. Add 1.2 ml of distilled, deionized water to the sodium periodate
vial (25 mg), replace stopper, and vortex until dissolved.
2. Place the purified IgG sample (in diluted coupling buffer) in a
screw cap, polypropylene, or polystyrene tube. Make certain
that the tube selected will not leak.
3. Add the sodium periodate stock solution at one-tenth the volume
of purified IgG sample (e.g., 400 µ1 of NaIO4stock solution to
4.0 ml of purified IgG). For rabbit IgG, add the sodium perio-
date at three-tenths the volume of purified IgG (e.g., 1.2 ml
NaIO4to 4.0 ml purified IgG).
4. Secure cap and cover the tube with foil.
5. Rotate the antibody/sodium periodate mixture end-over-end for
l hour at room temperature.
6
Page 9

6. Immediately proceed to desalting, Section 4.3.
4.3 Desalting Procedure
Immediately after the 1 hour oxidation, it is necessary to remove
the sodium periodate from the IgG solution. Sodium periodate
remaining in the IgG sample will adversely affect coupling efficiency. This desalting procedure is the same as that for buffer
exchange (Section 4.1). It is important not to collect and pool fractions beyond the protein peak, since this will result in sodium periodate contamination of the oxidized IgG sample.
1. The Econo-Pac 10DG column(s) used in the buffer exchange
should be washed and equilibrated in diluted coupling buffer, pH
5.5.
2. Follow the buffer exchange procedure in Section 4.1. Remember
to limit the sample volume to 3.0 ml per column, per run. If a
larger sample volume requires multiple desalting runs, wash
each Econo-Pac 10DG column with at least 20 ml diluted cou-
pling buffer after each desalting run.
3. Reserve a small aliquot to determine starting IgG concentra-
tion. This aliquot will be used to calculate IgG coupling effi-
ciency. Measure the volume of oxidized IgG to be coupled.
4.4 Coupling of Oxidized IgG to Affi-Gel Hz
Hydrazide Gel
4.4A Washing Affi-Gel Hz Hydrazide Gel
Affi-Gel Hz hydrazide gel is supplied in isopropanol.
Warning: Isopropanol is poisonous and flammable. Keep away
from heat, sparks, and open flame. May cause eye burns and
skin irritation. Avoid breathing vapor as it irritates eyes, nose,
and throat. Wear gloves and eye protection.
Just prior to coupling, Affi-Gel Hz gel must be washed with
diluted coupling buffer, pH 5.5, to remove isopropanol.
1. With a pipet, transfer the gel/isopropanol slurry to a clear 15
ml tube and allow the gel to settle.
2. Remove isopropanol supernatant, add 10 ml of diluted coupling
buffer, pH 5.5, and mix well. Allow the gel to settle. Repeat.
3. Remove the supernatant above the gel. Add 5 ml of diluted cou-
pling buffer, pH 5.5.
7
Page 10

4. Transfer the gel buffer slurry to a coupling reaction tube. If less
than 5 ml of gel is to be used for coupling, wash only the vol-
ume of gel to be coupled. Unused gel should remain in iso-
propanol and be stored at 4 °C.
4.4B IgG Coupling
As previously recommended, the IgG concentration should be
between 1-5 mg/ml of gel. The total IgG sample volume limitation
of 5 ml is suggested to facilitate buffer e xchange and desalting in the
Econo-Pac 10DG columns. Slightly larger sample volumes will
exist at the time of coupling to Affi-Gel Hz gel.
1. Add oxidized, desalted IgG sample to gel in the reaction tube.
Cap securely and rotate end-over-end for 10-24 hours at room
temperature.
2. After coupling reaction is complete, pour gel/IgG slurry into
the 1.0 x 10 cm Econo-Column®chromatography column pro-
vided. Collect the column eluant and measure the volume.
3. Wash the Affi-Gel Hz immunoaffinity column with 1 column
volume of a suitable buffer containing 0.5 M NaCl ( e.g., PBS 0.5
M NaCl, pH 7.0). Collect the column eluant and save for effi-
ciency determination.
4. Wash the column with an application buffer containing 0.02%
sodium azide. If buffers other than PBS 0.5 M NaCl are to be
used, equilibrate the column in 10 volumes of this buffer. Place
yellow end cap securely onto bottom of the Econo-Column
chromatography column. Replace top cap and store column
with buffer above the gel bed at 4 °C until ready to use.
4.4C Calculation of IgG Coupling Efficiency
The efficiency of IgG coupling to Affi-Gel Hz gel can be calculated indirectly. Quantitation of the difference in IgG present
before and after coupling will enable efficiency determination. IgG
coupling can be calculated accurately for samples of high purity.
For samples contaminated with other glycoproteins, the percentage of IgG coupled cannot be calculated by total protein determination.
For Sample of High Purity
Measure the absorbance at 280 nm in a quartz cuvette against
an appropriate buffer blank. Dilute the IgG sample to obtain
absorbance values between 0.1 and 1.0.
8
Page 11

Abs. @ 280 nm
1.4
[total protein before coupling] - [total uncoupled protein (eluant + 0.5 M NaCl
wash)]
(total coupled protein)
(total protein before coupling)
= (mg IgG/ml) x dilution factor x sample volume = total IgG
= [total coupled protein]
x 100 = % protein coupled
The starting and final IgG solutions and 0.5 M NaCl wash can
be analyzed with the Bio-Rad Protein Assay to determine total protein as an alternative to the absorbance method or for IgG samples
of less purity.
Section 5
Applications of Affi-Gel Hz
Immobilized IgG
In this section, suggestions for sample application and elution
are presented. Conditions listed are commonly employed for elution
in affinity purif ication. These are by no means the only elution conditions which may be used. When choosing elution conditions for
your application, refer to Section 5.3 and Section 6 on precautions
and product specifications. Elution conditions should facilitate satisfactory purification without damaging the aff inity column or product.
5.1 Conditioning the Immunoaffinity Column
It is necessary to condition the column prior to applying the
sample mixture.
1. Remove column from 4 °C and allow it to reach room temper-
ature. Add 2-4 bed volumes of the buffer chosen for antigen
elution to the affinity column.
2. Regenerate the column with at least 5 bed volumes of applica-
tion buffer (such as PBS, pH 7.0). The immunoaf finity column
is now ready for sample application.
9
Page 12

5.2 Sample Application
1. Sample is applied to the immobilized IgG column. Samples
should be free of particulates. Complex samples should be dilut-
ed in application buffer and filtered if necessary. This will
enhance specific binding to the immobilized IgG and prolong
column life.
2. Wash column with 2 bed volumes of 0.5 M NaCl in application
buffer to remove any unbound protein.
3. Wash column with 1-2 bed volumes of application buffer of
lower NaCl concentration. The column is now ready for elu-
tion of bound antigen.
5.3 Elution Suggestions
The elution conditions necessary to break the antibody-antigen
bond vary according to bond strength. Elution conditions listed are
suggestions, and optimal conditions should be determined empirically. When choosing elution schemes for af f inity purif ication, select
conditions which give satisfactory purification without damaging the
matrix or the product. Very harsh conditions may denature the antibody coupled to Affi-Gel Hz gel and affect column performance.
Start with conservative rather than severe conditions and optimize
elution with slight modifications from run to run. In general, elution
should be carried out quickly. Request b ulletin 1099 for further discussion of elution schemes.
1. Add 2 bed volumes of eluant to affinity column.
2. Collect fractions and/or monitor elution profile with UV-visible
detector. The eluted antigen should be neutralized if eluted in lo w
pH, or precipitation may occur.
3. Allow the elution buffer to reach the top of the gel bed. Quickly
regenerate the column in application buffer containing 0.02%
sodium azide, and store at 4 °C until next use.
4. Quantitate purification yield.
Acid Elution
0.2 M glycine-HCl, pH 2.5
0.1 M acetic acid
0.15 M sodium citrate, pH 3.0
0.5 M formic acid
10
Page 13

Chaotropic Elution
4 M NaSCN
6 M urea
5 M guanidine-HC1
Elution Strategies
The preceding eluants generally do not denature antibodies.
Eluant must be compatible with the antigen. Several methods should
be tried in the following order:
1. Acid (pH 2-3.5) is common. May cause inactivity of some pro-
teins. Can reduce solubility of IgG.
2. Chaotropic salts (3.5 M NaSCN, 3-6 M GuHCl) are often effec-
tive. Usually used at neutral pH. SCN should not be used with
low pH.
*Minimize the exposure to harsh conditions by neutralizing acid quickly and diluting chaotropes.
Section 6
Production Information
6.1 Affi-Gel Hz Hydrazide Gel Product
Specifications
Operating temperature range 2-30 °C
Elution pH operating range 2-11
Particle size range 75-300 µm hydrated
*
6.2 Ordering Information
Catalog
Number Product Description
153-6060 Affi-Gel Hz Immunoaffinity Kit
153-6047 Affi-Gel Hz Hydrazide Gel, 25 ml
153-6054 Affi-Gel Hz 10x Coupling Buffer, 500 ml
153-6055 Affi-Gel Hz Oxidizer (NaIO
732-2010 Econo-Pac 10DG Desalting Columns, 30
153-7307 DEAE Affi-Gel Blue Gel, 100 ml
153-6159 Affi-Gel Protein A MAPS II Kit
156-0006 Affi-Prep Protein A Support, 5 ml
156-0005 Affi-Prep Protein A Support, 25 ml
11
), 250 mg
4
Page 14

Catalog
Number Product Description
130-0150 Bio-Gel HT Hydroxyapatite, 250 ml
156-0030 Macro-Prep High S Support, 100ml
156-0090 Macro-Prep t-Butyl HIC Support, 100ml
157-0040 Macro-Prep Ceramic Hydroxyapatite, type I, 100g
153-7304 CM Affi-Gel Blue Gel, 100ml
12
Page 15

Bio-Rad
Laboratories
U.S. (800) 4BIORAD California (510) 741-1000 Australia 02-9914-2800 Austria
(1) 877 89 01 Belgium 09-385 55 11 Canada (905) 712-2771 China (01)2046622
Denmark 39 17 9947 Finland 90 804 2200 France (1) 49 60 68 34 Germany 089
31884-0 India 91-11-461-0103 Italy 02-21609 1 Japan 03-5811-6270 Hong Kong
7893300 The Netherlands 0318-540666 New Zealand 09-443 3099 Singapore (65)
272-9877 Spain (91) 661 70 85 Sweden 46 (0) 735 83 00 Switzerland 01-809 55 55
United Kingdom 0800 181134
Life Science
Group
SIG 020996 Printed in USA
LIT101 Rev C
 Loading...
Loading...