Page 1
Activated Immunoaff inity
Supports
Catalog Numbers
153-6046 Affi-Gel®10 Gel
153-6052 Affi-Gel 15 Gel
153-6098 Affi-Gel 10 and 15 Gel
Page 2
Table of Contents
Section 1 Introduction...................................... 1
Section 2 Coupling Chemistry......................... 2
Section 3 General Coupling Conditions......... 5
3.1 pH Dependence......................................... 5
3.2 Temperature .............................................. 12
3.3 Time .......................................................... 12
3.4 Ligand Concentration................................ 12
Section 4 Recommended Storage Conditions 16
Section 5 General Instructions........................ 16
5.1 Aqueous Coupling..................................... 16
5.2 Anhydrous Coupling................................. 19
Section 6 Monitoring For Protein Coupling.. 22
Section 7 Troubleshooting............................... 22
Section 8 Using the Coupled Support............. 25
i
Page 3

Section 9 Immunoaffinity Chromatography
with Affinity Supports..................... 26
9.1 Adsorption of the Sample.......................... 26
9.2 Removal of Unbound Solutes.................... 29
9.3 Elution Strategies ...................................... 30
9.4 Special Considerations for Labile Antigens34
9.5 Renaturation of Eluted Proteins ................ 35
Section 10 Ordering Information ..................... 36
Section 11 References......................................... 38
Section 1
Introduction
Affi-Gel 10 and Affi-Gel 15 affinity supports are activated immunoaffinity supports that offer rapid, high efficiency coupling for all ligands with a primary amino
group, including proteins throughout the entire range of
pIs and low molecular weight compounds such as peptides.1 Both Affi-Gel 10 and 15 supports are N-hydroxysuccinimide esters of a derivatized crosslinked agarose gel
bead support, and both couple to ligands spontaneously in
aqueous or non-aqueous solution.
The Affi-Gel 10 support, which contains a neutral 10atom spacer arm, has been used to couple a variety of
materials in affinity chromatography, immunoadsorption,
and other techniques. The Affi-Gel 15 support contains a
cationic charge in its 15-atom spacer arm which significantly enhances coupling efficiency for acidic proteins at
ii
1
Page 4
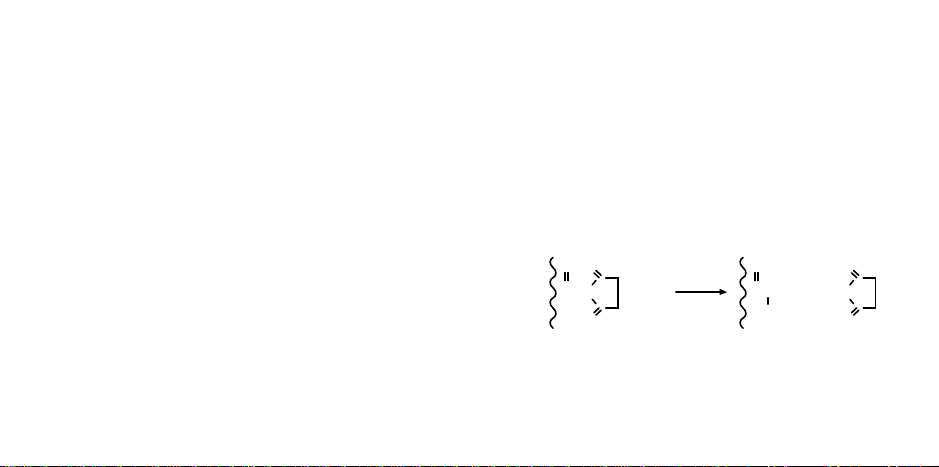
C-ON
O
O
O
+ R-NH
2
pH 6.5 to 8.5
Buffer
C-N-R
O
O
O
H
+ HO-N
physiological pH. Both Affi-Gel 10 and Affi-Gel 15 supports offer the following advantages:
• Covalent amide bonds couple the protein to the terminal carboxyl of the spacer arm
• Highly stable in chaotropic agents and from pH 2-11
• Rapid, gentle coupling within 4 hours
• Easy to use
• High capacity of up to 35 mg protein per ml
Section 2
Coupling Chemistry
Ligands with free alkyl or aryl amino groups will cou-
ple spontaneously with Affi-Gel 10 or 15 supports in
aqueous or non-aqueous solution (refer to Figure 1). Upon
addition of ligand, the N-hydroxysuccinimide is displaced, and a stable amide bond is formed. Since the reac-
2
tive ester immobilized on the gel is highly selective for
primary amino groups, spurious side reactions with the
ligand (i.e., cross-linking or other modifications in free
solution) are eliminated. Free sulfhydryls are among
functional groups other than primary amines known to
compete for coupling.
Affi-Gel 10 and Affi-Gel 15 supports are well suited
for coupling low molecular weight ligands. This can be
done in aqueous solution or, when solubility of the ligand
permits, in organic solvent.
Fig. 1. Coupling reaction of Affi-Gel supports with ligand
containing free amino groups.
3
Page 5
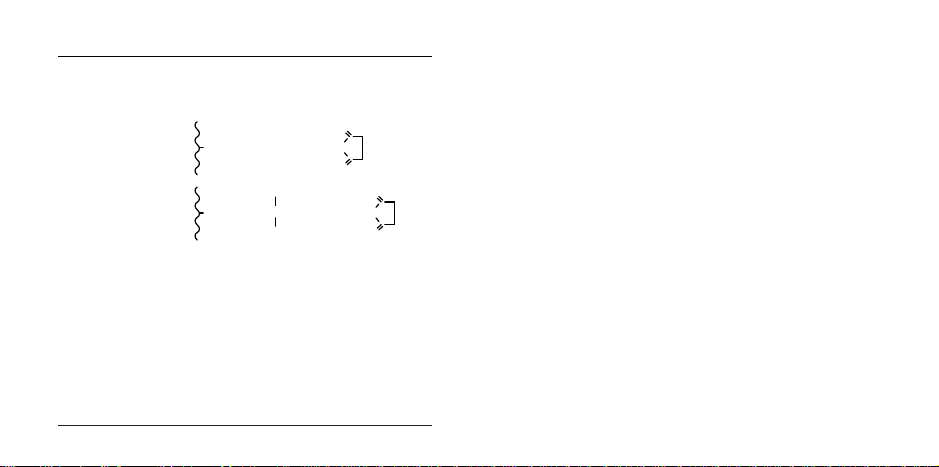
Product Description for Affi-Gel 10 and 15 gels
OCH2CONH(CH2)3N(CH2)3NHCO(CH2)2COON
O
O
CH
3
H
+
OCH2CONH(CH2)2NHCO(CH2)2COON
O
O
Matrix Bio-Gel A-5m agarose gel
Exclusion limit (M
Bead size 75-300 µm (50-200 mesh)
Spacer arm
Affi-Gel 10
Affi-Gel 15
Shipping medium 100% isopropanol
Capacity
Chemical capacity 15 µmoles/ml of gel
Protein capacity 35 mg/ml
Stability of unreacted support
Temperature -70 to 0 °C
pH range 3-10
Organic solvents stable in alcohols, DMSO, dioxane, formamide
Storage -20 °C 1 year
) 5,000,000
r
-70 °C 1.5 years
4
Section 3
General Coupling Conditions
3.1 pH Dependence
A major advantage of Affi-Gel 10 and 15 supports is
the mild conditions which will permit coupling. This is
particularly advantageous in applications which involve
sensitive enzymes or other proteins that irreversibly lose
biological activity when exposed to conditions outside
their physiological range. Coupling can be achieved with
Affi-Gel 10 and 15 supports between pH 3.0 to 10.0.
In order to maintain pH control, a minimum buffer
strength of 10 millimolar is recommended. Suitable
buffers include MES, MOPS, HEPES, POPSO, acetate,
and bicarbonate. Do not use buffers such as Tris or
glycine. They contain primary amino groups which will
couple to the gel, as will any primary amine-containing
compound which contaminates the ligand preparation.
5
Page 6

The Affi-Gel 10 support couples proteins best at a pH
near or below their isoelectric point, and the Affi-Gel 15
support couples proteins best near or above their isoelectric point. Therefore, when coupling at neutral pH (6.5-
7.5), the Affi-Gel 10 support is recommended for proteins
with isoelectric points of 6.5 to 11 (neutral or basic proteins), and the Affi-Gel 15 support is recommended for
proteins with isoelectric points below 6.5 (acidic proteins). See Table 1.
The difference in coupling efficiency of the Affi-Gel
10 and Affi-Gel 15 supports for acidic and basic proteins
can be attributed to interactions between the charge on the
protein and charge on the gel. Hydrolysis of some of the
active esters during aqueous coupling will impart a slight
negative charge to the Affi-Gel 10 support. This negative
charge will attract positively charged proteins (proteins
buffered at a pH below their isoelectric point) and enhance
their coupling efficiency. Conversely, the negative charge
will repel negatively charged proteins (proteins buffered
at a pH above their isoelectric point) and lower their coupling efficiency. The Affi-Gel 15 support, due to the tertiary amine incorporated into its arm, has a slight overall
positive charge, and the effects are reversed.
6
7
Page 7
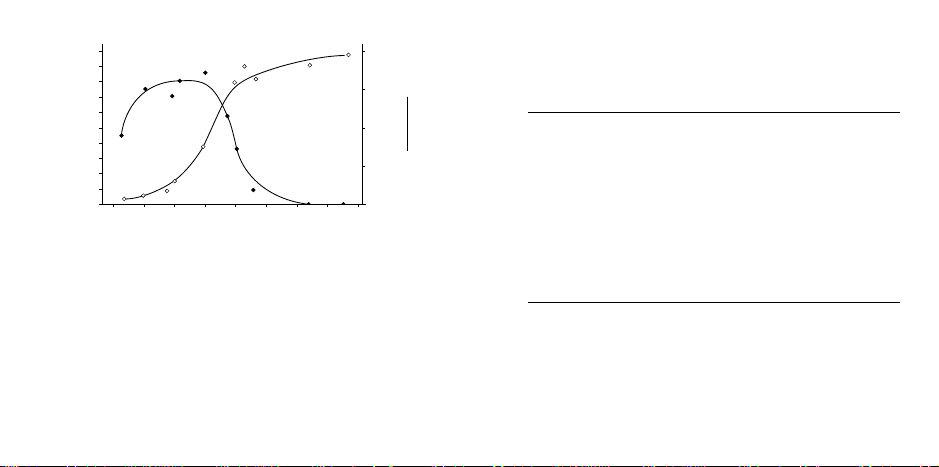
Fig. 2. Protein coupling with Affi-Gel and Affi-Gel 15 sup-
0
10
20
30
40
50
60
70
80
90
100
% Protein Coupled
0
5
10
15
20
mg coupled
ml gel
34567891011
pl
ports. Coupling conditions: Each protein solution (3 ml 0.1 M
MOPS, pH 7.5, containing 40 mg protein) was combined with
2 ml of Affi-Gel media. The gel slurry was mixed at 4 °C for 2
hours, and then stripped with 7 M urea containing 1 M NaCl.
The uncoupled protein was determined, using published
1cm
E
, by dilution of an aliquot of the urea effluent into 0.1 M
280
HCl and measurement of the absorbance at 280 nm (
Affi-Gel 15 gel; ●●—●● Affi-Gel 10 gel).
8
●—●
Table 1. Protein Coupling to Affi-Gel 10 and
Affi-Gel 15 Support
Protein pl Affi-Gel 15 Gel Affi-Gel 10 Gel
1. Fetuin 3.3 43 3.0
2. Alpha-1-antitrypsin 4.0 76 5.0
3. Ovalbumin 4.7 70 8.5
4. Bovine serum albumin 4.9 80 14
5. Human transferrin 5.9 87 36
6. Bovine hemoglobin 6.8 59 83
7. Human globulin 5.8-7.3 39 90
8. Myoglobin 6.8-7.8 10 85
9. Cytochrome c 9.3 0 90
10. Lysozyme 10-11 1 95
Coupling Efficiency (%)
9
Page 8

In addition to its effect on coupling, the slight charge
associated with each gel may sometimes be exploited in
the affinity separation itself, for example, it may be used
to enhance binding of weakly sorbed material, or elution
of strongly absorbed materials. In such cases, it may be
preferable to use the Affi-Gel 10 support to couple an
acidic protein, or the Affi-Gel 15 support to couple a basic
protein. Coupling efficiency can then be enhanced by
manipulating the coupling conditions in either of two
ways. Select the coupling pH so that the protein has a
charge opposite that of the gel, or add salt to the coupling
buffer to minimize charge interaction (80 mM CaCl2may
be useful for coupling acidic proteins to the Affi-Gel 10
support and 0.3 M NaCl may be useful when coupling
basic proteins to the Affi-Gel 15 support).2Examples of
these manipulations are shown in Table 2. The more basic
or more acidic the protein the larger the observed effects
will be.
Table 2. Coupling Efficiency of Acidic and
Neutral-to-Slightly-Basic Protein Under Various
Coupling Conditions
Affi-Gel 10 Affi-Gel 15
Coupling Buffer Efficiency (%) Efficiency (%)
Bovine Serum Albumin, pI 4.9
0.1 M MOPS, pH 7.5 14 80
0.1 M MOPS, pH 7.5 + 80 mM CaCl 90 ——
0.1 M MOPS, pH 7.5 + 0.3 M NaCl 22 47
0.1 M MES, pH 4.8 90 38
Human Globulin, pI 7.0 (average)
0.1 M MOPS, pH 7.5 83 40
0.1 M MOPS, pH 7.5 + 0.3 M NaCl 69 70
0.1 M NaHCO3, pH 8.5 80 70
Coupling Coupling
10
11
Page 9

3.2 Temperature
Coupling at 4 °C is recommended whenever possible.
The slower reaction rate at this temperature will afford a
greater measure of control, and many ligands will have a
greater stability at 4 °C than at 20 °C.
3.3 Time
Coupling to the Affi-Gel 10 and 15 supports is rapid.
As shown in Figure 3, for the Affi-Gel support, about 75%
of the maximum binding achieved with gamma globulin at
pH 7.5 takes place within 30 minutes at 4 °C. Ninety percent of the maximal coupling is achieved in an hour, and
within 4 hours the reaction is complete.
3.4 Ligand Concentration
The amount of protein coupled is proportional to the
amount of protein added to the gel up to about 30 mg
protein coupled per ml of gel (Figure 4). The efficiency of
coupling will vary with the protein and conditions of coupling (Figure 1). Greater than 30 mg protein/ml gel may
be coupled, but efficiency will taper off. When maximum
total capacity is desired, a high concentration of ligand
should be chosen (50 to 60 mg protein per ml of gel).
When maximum efficiency is the goal, as would be the
case with ligand preparations of limited quantity, the ligand concentration should be in the range of up to 25 mg
protein per ml of gel. Unbound sample may be recovered
and reused without further treatment. Optimum coupling
efficiency is achieved when the total reaction volume is
between 1.5 and 4.5 ml per ml of gel bed.
12
13
Page 10

mg protein coupled per ml gel
Conditions:
human gamma
globulin added
as shown
0.1 M MOPS, pH 7.5
10
20
30
40
10 20 30 40 50
mg protein added per ml gel
% protein coupled
Conditions:
15 mg human
gamma globulin
added per ml of gel
0.1 M HEPES, pH 8.0
4 °C
10
20
30
40
50
60
70
80
10 20 30 40 50 60
Time (minutes)
Fig. 3. Effect of time on protein coupling to Affi-Gel 10 gel.
A similar relationship is observed with Affi-Gel 15 gel.
14
Fig. 4. Effect of amount of protein added on protein coupling to Affi-Gel 10 gel. A similar coupling capacity is
observed with Affi-Gel 15 gel.
15
Page 11

Section 4
Recommended Storage Conditions
If Affi-Gel 10 gel is stored at -20 °C it retains at least
80% of original activity for at least 1 year. Storing at
-70 °C will extend the shelf life further.
Section 5
General Instructions
5.1 Aqueous Coupling
Material and equipment required for coupling ligands to
Affi-Gel 10 and Affi-Gel 15 gel under aqueous conditions.
1. Cold distilled water.
2. Coupling buffer of your choice without primary
amines or sulfhydryl groups; 10-100 mM HEPES,
MOPS, POPSO, acetate, or bicarbonate for proteins.
3. Closed container which holds at least four times the
volume of gel.
4. (Optional) rotating shaker.
5. (Optional) Buchner funnel.
Shake the vial, and make sure that you have a uniform
suspension. Transfer the desired quantity of slurry to a
Buchner funnel or glass fritted funnel. Drain the supernatant solvent, and wash the gel with three bed volumes of
cold 10 mM sodium acetate, pH 4.5, or cold (4 °C) deionized water. The wash can be facilitated, particularly when
working with larger amounts of gel, by applying a vacuum. Care should be taken, however, not to allow the gel
bed to go dry. For optimum coupling of ligands, the washing procedure should be completed and the gel combined
with the ligand solution within 20 minutes.
Transfer the moist gel cake to a test tube or flask and
add the cold ligand solution. Add at least 0.5 ml of ligand
16
17
Page 12

solution per ml of gel, and agitate sufficiently to make a
uniform suspension. Continue gentle agitation of the gel
slurry on a rocker, shaker, or wheel for 1 hour at room
temperature or 4 hours at 4 °C.
If the coupling time is short, or if the gel is to be used
immediately, we recommend a precautionary blocking of
any active esters. This can be accomplished by adding 0.1 ml
1 M glycine ethyl ester (pH 8) or 0.1 ml 1 M ethanolamine
HCl (pH 8) per ml gel. Allow 1 hour for completion of the
blocking reaction. Transfer the gel to a column and wash
with water or coupling buffer until the gel is free of reactants, as detected by O.D.
vents that will be used subsequently to elute substances
. Wash the gel with other sol-
280
specifically bound to the column. The column is now ready
for equilibration with starting buffer and application of sample. When not in use, store the columns at 4 °C, and in a
starting buffer containing 0.2% sodium azide.
5.2 Anhydrous Coupling
Coupling under anhydrous condition is the preferred
method when the solubility of the ligand permits. It is
ideal for peptides. Since there is no hydrolysis of active
esters in the absence of water, the only reaction which will
take place is the one between the ester and the ligand.
Material and equipment required for anhydrous coupling using Affi-Gel 10 and Affi-Gel 15 gel.
1. Cold (anhydrous) isopropanol.
2. Organic solvent of your choice; alcohol, dimethylsulfoxide (DMSO), dioxane, formamide free from free
amines, or mixtures of these solvents.
3. Closed container which holds four times the volume
of gel to be used.
4. (Optional) rotating shaker.
5. (Optional) Buchner funnel.
18
19
Page 13

Shake the vial, and make sure you have a uniform suspension. Transfer the desired amount to a Buchner funnel,
or glass fritted funnel. Drain the supernatant solvent, and
wash the gel with at least five bed volumes of cold isopropanol.
Transfer the moist gel to a test tube or flask, and add
the ligand solution. Add at least 0.5 ml of ligand solution
per ml of gel, and agitate to make a uniform suspension.
To obtain a quantitatively substituted gel with low
molecular weight ligands, it is necessary to add a slight
excess of ligand. The gel contains approximately 15
µmoles of active ester per ml of gel. In the absence of
hydrolysis, factors like time, concentration, and temperature, are less important. The reaction can be carried out at
any convenient volume at room temperature for several
hours. When using DMSO, conduct the reaction at 20 °C,
to avoid the unfavorable viscosity 4 °C.
20
Any unreacted groups that remain can be blocked by
addition of a slight excess of ethanolamine at the end of
the reaction. The resulting support will have the lowest
possible residual charge.
Summary of Coupling Conditions
Concentration of ligand
Protein 25 mg/ml of gel
Low MW ligand 15-20 µmoles/ml of gel
Optimum pH
Affi-Gel 10 gel near or below pI of ligand
Affi-Gel 15 gel near or above pI of ligand
Aqueous buffers MES, MOPS, HEPES, POPSO, acetate,
Organic solvents alcohols, DMSO, dioxane, acetone, for-
Temperature 4 °C recommended
Reaction time 1 - 4 hours
pH range 3-10
Reaction volume 1.5 - 4.5 ml per ml of gel bed
Other compatible buffer reducing agents such as 10 mM DTT or
components nonionic detergents
Blocking reagent ethanolamine
Suitable ligand must have primary amino group
bicarbonate (avoid Tris, glycine)
mamide
21
Page 14

Section 6
Monitoring for Protein Coupling
Soluble (unbound) protein remaining in the coupling
and wash buffers can be assayed by the Bio-Rad Protein
Assay (catalog number 500-0006) or by measuring
O.D.
. If measuring O.D.
280
sample should be lowered with 10 mM HCl. N-hydroxysuccinimide released during the coupling will absorb at
280 nm at neutral or basic pH. N-hydroxysuccinimide will
also interfere with the Lowry protein assay.
is preferred, the pH of the
280
Section 7
Troubleshooting
Occasionally, the ligand will not bind to Affi-Gel 10
or 15 affinity support. If the ligand does not bind, or if you
get a low capacity column, there are a number of possible
reasons.
• Affi-Gel support is more than 12 months old. Try new
material.
• The Affi-Gel support has been stored too warm.
• pH is not optimal. For Affi-Gel 10 gel, pH should be
near or below the pI of your ligand. For Affi-Gel 15
gel, it should be near or above the pI. Buffer concentration should be at least 10 mM to maintain optimum
pH. If pI is not known, try test coupling at a range of
different pHs.
• A primary amino group, other than the ligand is present; avoid Tris or glycine buffers.
• Ligand is not pure. For polyclonal IgG samples,
switch to Affi-Gel Hz support. For other samples,
increase purity of ligand by chromatography or
preparative electrophoretic methods.
• Aqueous coupling conditions provide less control
than anhydrous conditions. Switch to anhydrous conditions if ligand solubility permits.
22
23
Page 15

• Concentration of the ligand is too low. Protein concentrations of <25 mg/ml or small MW ligand concentrations of <15 mg/ml of gel will yield less than
optimum results.
• Ligand is too diluted. Volume of ligand should not
exceed 4.5 ml/ml of gel.
• Primary amino groups on ligand are sterically hindered; add nonionic detergent (up to 1%) or a
chaotrophic agent such as 1 M guanidine HCl.
• Ligand has a molecular weight greater than 500,000.
Affi-Gel 10 and 15 supports are not suitable for this
application. Try the Affi-Prep 10 support, which is
more porous.
Section 8
Using the Coupled Support
When the Affi-Gel 10 or 15 activated support has
been coupled to the ligand, it is ready to use.
General operating conditions
Flow rate 15-25 cm/h
Pressure limit 15 psi
Minimum buffer concentration 50 mM
Stability
Organic solvents alcohols
pH 2-11
Temperature autoclavable (ligand permitting)
Storage 1 year at 4 °C, add 0.02% NaN3or
other preservative to application or
starting buffer.
24
25
Page 16

Section 9
Immunoaffinity Chromatography
with Affinity Supports
9.1 Adsorption of the Sample
Preparing Antiserum for Antibody Purification
If an antibody from serum is to be affinity purified on
an immobilized antigen support, partial purification of the
antiserum is recommended. DEAE Affi-Gel blue gel and
CM Affi-Gel blue gel will remove complement factors
which bind immune complexes. They will also remove
protease which can destroy valuable antibody during sample storage or decrease column life by destroying the
immobilized protein. Antiserum should at least be heat
inactivated at 56 °C for 30 minutes to destroy the complement factors.
Optimizing Support and Sample Volumes
Use only the required amount of affinity support. If
excess support is used, sample elution becomes more difficult because the sample continues to bind and elute as it
passes down the column. Stronger elution conditions
become necessary, residence time is longer, the eluted
peak is broader, and there is a greater risk of denaturation
and poor recovery.
One method to insure that only the required amount of
affinity gel is used is to apply the sample to the top of the
column and elute using reverse flow (see Figure 5).
26
27
Page 17

flow
binding
elution
AB
Fig. 5. Use only the required amount of affinity support.
A) An excess of affinity support is used. During elution, sample
is exposed to excess capacity resulting in great dilution and a
broad peak. B) The sample is added to the top of the column,
then eluted using reverse flow. Only the required capacity is
used, resulting in minimal dilution and a sharper peak.
adaptor
binding
saturated gel
excess
capacity
28
elution
Another method is to titrate the gel with sample,
checking the supernatant for unbound sample after each
addition. This can be done either in a column or in a batch
mode. Continue until the gel is saturated. This method can
be used with a small amount of gel and sample to determine the capacity and the amount of gel required for the
purification.
9.2 Removal of Unbound Solutes
Proteins or other solutes which are not bound, or are
weakly bound by non-specific interactions, must be
washed off prior to elution. This can be done by washing
with mild chaotropic solutions (1 M NaSCN, 1 M guanidine hydrochloride, 1 M urea), with salts (1 M NaCl), or
with detergents (0.5% Triton®X-100). In many cases, the
elution buffer can be used, but at a lower concentration.
This frequently neglected wash step eliminates proteins
which may complicate final elution and helps yield a more
highly purified product.
29
Page 18

9.3 Elution Strategies
Elution is usually the most difficult step in
immunoaffinity chromatography. The objective is to
obtain high purity and high recovery of a stable and active
product. Attempting to maximize yields, elution conditions which denature the proteins are often chosen.
Antigens and antibodies are bound to each other by a
combination of ionic bonding, hydrogen bonding, and
hydrophobic interactions.2The strength of different antigen-antibody complexes varies widely. Other parameters
such as ligand density, steric orientation, and nonspecific
interactions can also be important. Many solvents have
been used as eluants in immunoaffinity chromatography,
and the choice of an effective eluant often appears to be
empirical. There is, however, a logical strategy, or
sequence of eluants to consider when approaching a new
immunoaffinity application.
1. Specific Elution with excess antigen or antibody
should be considered first, because, in theory, it will
always work. It is often impractical due to the cost
and availability of the specific eluant. Another disadvantage is that an antigen-antibody complex will be
eluted and the dissociation of this complex may be
necessary and difficult to achieve.
2. Acid Elution is the most commonly employed des-
orption method and is frequently very effective.
Eluants such as glycine-HCl, pH 2.5, 20 mM HCl,
and sodium citrate, pH 2.5, can be used to disrupt the
antigen-antibody interactions. Acid elution can give
low recoveries due to hydrophobic interactions
between the antigen and the antibody. An eluant such
as 1 M propionic acid, or the addition of 10% dioxane3or ethylene glycol to the acid eluant, is more
effective in dissociating such complexes.
30
31
Page 19

3. Base Elution is less frequently used than acid elution,
but, in some cases, it is more effective. Elution with
1M NH4OH or with 50 mM diethylamine, pH 11.5,
has been shown to be effective with membrane glyco-
proteins and with certain antigens which precipitate in
acid but are stable in base.3Organic solvents can also
be added to basic eluants as described above with acid
elution. An example in which an antibody to dinitro-
phenyl-bovine serum albumin (DNP-BSA) was cou-
pled to an affinity matrix is described in Reference 5.
DNP-BSA could not be eluted at all with acid or with
acid plus organic solvents. Base elution gave 60%
yield and base plus dioxane gave 95% yield of puri-
fied antigen.
4. Chaotropic Agents disrupt the tertiary structure of
proteins and, therefore, can be used to dissociate anti-
gen-antibody complexes. Chaotropic salts disrupt
ionic interactions, hydrogen bonding, and sometimes
hydrophobic interactions. Chaotropic anions are
effective in the order SCN->ClO
Chaotropic cations are effective in the order
-
>I>Br->Cl-.
4
guanidine>Mg2+>K+>Na+.7Eluants such as 8 M urea,
6 M guanidine hydrochloride, and 6 M NaSCN are
effective in disrupting most protein-protein interactions. The problem is that these strong chaotropes
may destroy the activity of the antigen and/or the antibody. Conditions as mild as possible should always
be used.
When the eluant has been chosen, the elution conditions should be refined by optimizing concentration, time,
temperature, and by combining the eluants described
above.
It is important to remove the eluted antigen or antibody from the eluant as quickly as possible to minimize
the chance of denaturation. If acid or base is used, the
6,7
32
33
Page 20

samples should be neutralized immediately following elution. If a chaotrope is used for elution, it can be rapidly
removed by desalting (Econo-Pac®10DG desalting
columns, Bio-Gel®P-6 DG desalting gel, Econo-Pac P6
desalting cartridges, or for very small volumes Bio-Spin
columns).
Alternative procedures have been published for eluting labile antigens from immobilized antibody columns.
Deionized water has been reported,
®
ally low. A method of increasing interest is electrophoretic
elution.1An electrical field is applied and the adsorbed
8,9
but yields are gener-
protein is electrophoresed away from the affinity matrix.
9.4 Special Considerations for Labile
Antigens
The stability of some antigens can be a problem.
Special considerations may be necessary. The mildest elution conditions possible are desirable, with rapid elution
and short exposure times being critical. For labile immobi-
lized antigen relatively mild elution conditions can be
used, and then a more complete regeneration with a
chaotropic salt can be done after every fourth or fifth use
of the column. This will increase the life of the column by
minimizing exposure to stringent conditions, and strip the
column of bound proteins to maintain the capacity.
34
9.5 Renaturation of Eluted Proteins
Proteins which have been denatured during elution can
often be renatured by the addition of a chaotropic agent
such as guanidine-HCl, followed by stepwise dialysis
against decreasing concentrations of the chaotrope. The
high concentration of guanidine-HCl puts the protein into a
random coil configuration. As the chaotrope is slowly
removed, the protein will return to its native form.
If you have any questions or suggestions regarding
the use of this or any of our other products, contact your
local Bio-Rad representative. Inside the United States, call
1-(800)-4BIORAD.
35
Page 21

Section 10
Ordering Information
Catalog
Number Product Description Comments
153-6099 Affi-Gel 10 Support, 25 ml
153-6051 Affi-Gel 15 Support, 25 ml
153-6046 Affi-Gel 10 Support, 4 x 25 ml
153-6052 Affi-Gel 15 Support, 4 x 25 ml
153-6098 Affi-Gel 10/15 Support, 25 ml of each
Purification of antibodies from serum
153-7304 CM Affi-Gel Blue Gel, Cibacron Blue F3GA dye
153-7307 DEAE Affi-Gel Blue Gel, Cibacron Blue F3GA dye
100 ml attached to CM Bio-Gel A
100 ml attached to DEAE Bio-Gel A
agarose gel; for rapid purification of IgG from serum.
agarose gel; for rapid purification of IgG from serum.
Catalog
Number Product Description Comments
Desalting and sample preparation
150-0738 Bio-Gel P-6DG Desalting Rapid protein and peptide
150-0739 Bio-Gel P-6DG Desalting
732-2010 Econo-Pac 10DG Prepacked Bio-Gel P-6DG;
732-0011 Econo-Pac P6 Cartridge, For desalting of up to 2 ml
732-6002 Bio-Spin 6 Chroma- For desalting of small sample
732-6006 Bio-Spin 30 Chroma- Same as Bio-Spin 6, but with
Gel, 100 g desalting
Gel, 1 kg
Desalting Columns, for desalting up to 3.3 ml
30 columns of 10 ml samples
5 ml samples
tography Columns, 25 volumes (<0.1 ml) with mini-
tography Columns, 25 an exclusion limit of 40,000.
mal dilution). Exclusion limit
6,000.
36
37
Page 22

Section 11
References
1. Prickett, K. S., et al., BioTechniques, 7, 580 (1989).
2. Frost, R., et al., Biochem. Biophys. Acta., 670, 163 (1981).
3. Izuta, S. and Saneyoshi, M., Anal. Biochem., 174, 318 (1988).
4. Rehm, H. and Lazdunski, M., Proc. Natl. Acad. Sci. USA, 85,
4919 (1988).
5. Wong, K. Y., et al., Biochem., 27, 375 (1988).
6. Elton, T. S., et al., Proc. Natl. Acad. Sci. USA, 85, 2518
(1988).
7. Strickland, D. K., et al., Biochem., 27, 1458 (1988).
8. Pejler, G., et al., J. Biol. Chem., 263, 5197 (1988).
9. Diaco, R., et al., J. Gen. Virol., 67, 345 (1986).
38
Bio-Rad Laboratories, 2000 Alfred Nobel Drive, Hercules, CA 94547
LIT156 Rev B
 Loading...
Loading...