Page 1

User Manual
[04]
95248
(01/2010)
EC REP
bioMérieux, Inc.
Box 15969
Durham, North Carolina 27704-0969 / USA
Tel. (1) 800-682-2666
bioMérieux
au capital de 12 029 370 €
673 620 399 RCS LYON
69280 Marcy l’Etoile / France
tél. 33 (0)4 78 87 20 00 / fax 33 (0)4 78 87 20 90
http://www.biomerieux.com
®
SA
Page 2

Algeria
bioMérieux Algérie EURL
Algéria Business Center
Les Pins Maritimes - Mohammadia
Alger
tel. (213) 21 89 14 81
fax (213) 21 89 14 82
Argentina
bioMérieux Argentina
Av. Congreso 1745
C1428BUE
Capital Federal Buenos Aires
tel. (54) 11 5555 6800
fax (54) 11 5555 6888
Australia
bioMérieux Australia P/L
Unit 25 - Parkview Business Centre
1, Maitland Place
Baulkham Hills NSW 2153
tel. (61) 2 8852 4700
fax (61) 2 8852 4777
Austria
bioMérieux Austria GmbH
Eduard-Kittenberger-Gasse 97
Top 3
A-1230 Wien
tel. (43) 186 50 650
fax (43) 186 50 661
Belgium
bioMérieux Benelux s.a./n.v.
Media Square
18–19 Place des Carabiniers
Bruxelles 1030
tel. (32) 2 743 01 70
fax (32) 2 733 55 97
Brazil
bioMérieux Brasil SA
Estrada Do Mapuá
491 Taquara - Jacarepaguá
CEP 22710 261
Rio de Janeiro RJ
tel. (55) 21 2444 1400
fax (55) 21 2445 6025
Canada
bioMérieux Canada, Inc.
7815, Henri-Bourassa West
Saint Laurent, QC
H4S 1P7
tel. (1) 514 336 7321
fax (1) 514 807 0015
Chile
bioMérieux Chile S.A.
Seminario 131
Providencia
Santiago
tel. (56) 2634 20 92
fax (56) 2634 20 93
China
bioMérieux China Limited
Room 1601-02B & 10
Est Ocean Centre
nº 24A Jiang Guo Men Nei Street
100004 Beijing
tel. (86) 10 6515 6963
fax (86) 10 6515 6993
bioMérieux China Limited
Room 2605, South Tower,
World Trade Center
371-375 Huan Shi Dong East Road
510095 Guangzhou
tel. (86) 20 8762 7010
fax (86) 20 8762 7015
Colombia
bioMérieux Colombia Ltda
Avenida 15 No. 100-43
Piso 2
Bogotá, D.C.
tel. (57) 1 520 0080
fax (57) 1 520 0088
(57) 1 520 0831
Czech Republic
bioMérieux CZ s.r.o.
Business Park Kosice
Jinonická 80
158 00 Praha 5
tel. (420) 2 57 290 623
(420) 2 57 290 232
fax (420) 2 57 290 964
Denmark
bioMérieux Danmark Aps
Smedeholm 13C
2730 Herlev
tel. (45) 70 10 84 00
fax (45) 70 10 84 01
Finland
bioMérieux Suomi Oy
Konalantie 47 C
FI-00390 Helsinki
tel. (358) 9 8545 6000
fax (358) 9 8545 6045
France
bioMérieux SA
69280 Marcy l’Etoile
tel. (33) (0)4 78 87 20 00
fax (33) (0)4 78 87 20 90
http://www.biomerieux.com
Germany
bioMérieux Deutschland GmbH
Weberstrasse 8
D 72622 Nürtingen
tel. (49) 7022 30070
fax (49) 7022 36110
Greece
bioMérieux Hellas S.A.
Papanikoli 70
15232 Halandri
Athens
tel. (30) 2 10 81 72 400
fax (30) 2 10 68 00 880
Hungary
bioMérieux Hungária Kft.
Fóto út. 56 (5. emelet)
H-1047 Budapest
tel. (36) 1 231 3050
fax (36) 1 231 3059
India
bioMérieux India Pvt. Ltd
A-32, Mohan Co-Operative Ind. Estate
New Delhi 110 024
tel. (91) 11 42 09 88 00
fax (91) 11 24 64 88 30
Indonesia
Representation Office
bioMérieux Indonesia
Enseval Building
Kawasan Industri Pulo Gadung JI. Pulo - Lentut No. 10
Jakarta Timur 13920
tel. (62) 21 461 51 11
fax (62) 21 460 41 07
Italy
bioMérieux Italia S.p.A.
Via Fiume Bianco, 56
00144 Roma
tel. (39) 06 523 081
fax (39) 06 523 08240
Ivory Coast
bioMérieux Afrique Occidentale
08 BP 2634
Avenue Joseph Blohorn
Abidjan 08
tel. (225) 22 40 93 93/22 40 41 40
fax (225) 22 40 93 94
Japan
Sysmex bioMérieux, Ltd.
Osaki Central Tower 8F
1-2-2 Osaki Shinagawa-ku
Tokyo 141-0032
tel. (81) 3 6834 2666
fax (81) 3 6834 2667
Korea
bioMérieux Korea Co., Ltd.
1st & 2nd Floor, Yoosung Building
# 830-67 Yeoksam-dong,
Kangnam-gu
Séoul 135-080
tel. (82) 2 2188 4700
fax (82) 2 547 6263
Mexico
bioMérieux México SA de CV
Chihuahua 88, col. Progreso
México 01080, D.F.
tel. (52) 55 5481 9550
fax (52) 55 5616 2245
Netherlands (The)
bioMérieux Benelux BV
Boseind 15
P.O. Box 23
5280 AA Boxtel
tel. (31) 411 65 48 88
fax (31) 411 65 48 73
i Manual Name
702358-4EN1 REV nn/nnnn
Page 3

New Zealand
bioMérieux New Zealand Ltd.
C/- Logical Freight Solutions
12C Rennie Drive, Airport Oaks
Auckland
tel. (64) 9 918 6354
fax (64) 9 918 6355
Norway
bioMérieux Norge AS
Økernveien 145
N-0513, Oslo
tel. (47) 23 37 55 50
fax (47) 23 37 55 51
Philippines (The)
Representation Office
bioMérieux Philippines
11th Floor, Pearlbank Centre
146 Valero Street, Salcedo Village
1227 Makati City
tel. (632) 817 7741
fax (632) 812 0896
Poland
bioMérieux Polska Sp. Z.o.o.
Ul. Zeromskiego 17
01-882 Warsaw
tel. (48) 22 569 85 00
fax (48) 22 569 85 54
Portugal
bioMérieux Portugal, Lda.
Av. 25 de Abril de 1974, nº 23-3º
2795-197 LINDA-A-VELHA
tel. (351) 21 415 23 50
fax (351) 21 418 32 67
Russia
o.o.o. bioMérieux
Derbenevskaya ul. 20, str. 11
115 114 Moscow
tel. (7) 495 221 10 79
fax (7) 495 221 10 79
Singapore
bioMérieux Singaporete. Ltd.
11 Biopolis Way, Helios, Block 11
#10-03 Singapore 138667
tel. (65) 6513 9554
fax (65) 6478 9501
South Africa
bioMérieux South Africa Pty
7 Malibongwe Drive
Randburg 2125
tel. (27) 11 801 91 10
fax (27) 11 791 24 19
Spain
bioMérieux España S.A.
Manual Tovar, 45–47
28034 Madrid
tel. (34) 91 358 11 42
fax (34) 91 358 06 29
Sweden
bioMérieux Sverige AB
Hantverksvägen 15
436 33 Askim
tel. (46) 31 68 84 90
fax (46) 31 68 48 48
Switzerland
bioMérieux Suisse s.a.
51, avenue Blanc
Case postale 2150
1211 Genève 2
tel. (41) 22 906 57 60
fax (41) 22 906 57 42
Taiwan
Representation Office
bioMérieux China Limited
Taiwan Branch
RM 608, No. 6-3 Ching Cheng Street
Taipei 105
tel. (886) 2 2545 2250
fax (886) 2 2545 0959
Thailand
bioMérieux Thailand Ltd
3195/9 Vibulthani Tower, 4th Floor
Rama IV Road, Klongton, Klongtoey
Bangkok 10110
tel. (66) 2 661 56 44
fax (66) 2 661 56 45
Turkey
bioMérieux Diagnostik A.S.
ğirmen Sok. Nida Plaza Kat:6
De
34742 Kozyata
tel. (90) 216 444 00 83
fax (90) 216 373 16 63
United Kingdom
bioMérieux UK Ltd
Grafton Way, Basingstoke
Hampshire RG22 6HY
tel. (44) 1256 461881
fax (44) 1256 816863
USA
bioMérieux, Inc.
100 Rodolphe Street
Durham NC 27712
tel. (1) 919 620 2000
Vietnam
Representation Office
bioMérieux Vietnam
Room 4A, 4th Floor
Green House Building
62A Pham Ngoc Thach Street, Ward 6
District 3
Ho Chi Minh City
tel. (84) 88 209 906
fax (84) 88 209 905
ği-Istanbul
Manual Name ii
702358-4EN1 REV nn/nnnn
Page 4
This product and its documentation complies with the In Vitro Diagnostic
Medical Device Directive 98/79/EC.
Liability Disclaimer
bioMérieux, Inc. makes no express or implied warranty regarding this
manual, its quality, performance, or appropriate use regarding any type of
specific procedure.
Furthermore, this manual may be modified by bioMérieux without notice and
without implying any obligation or liability on the part of the company.
Intellectual Property
bioMérieux, the blue logo, BacT/ALERT, BacT/LINK and MB/BacT are used,
pending, and/or registered trademarks of bioMérieux in the USA and other
countries.
CLSI is a registered trademark of Clinical and Laboratory Standards Institute,
Inc.
PSC and Quickscan are registered trademarks of PSC, Inc.
Zip is a registered trademark of Iomega Corporation.
© 2010 bioMérieux, Inc. All rights reserved.
No part of this publication may be reproduced, transmitted, transcribed,
stored in a retrieval system, or translated into any language (human or
computer) in any form, or by any means whatsoever, without the prior
express written permission of bioMérieux, Inc.
Page 5

Warranty
bioMérieux, Inc., (“Seller”) warrants the BacT/ALERT® instrument (the
“Instrument”) to the original purchaser for a period of one (1) year after date
of installation against defects in material and workmanship and defects
arising from failure to conform to specifications applicable on the date of
installation. Seller further agrees to correct, either by repair, or, at its election,
by replacement, any such defect found on examination to have occurred,
under normal use and service, during such one-year period, provided Seller
is promptly notified in writing upon discovery of such defect.
Seller shall not be liable under this Warranty for any defect arising from abuse
of the system, failure to operate and maintain the system in accordance with
the documentation included with the Instrument, including repair service,
alteration or modification of the system by any person other than service
personnel of bioMérieux, Inc., or use of modified, changed, or previously
used disposables.
The warranty of Seller set forth above and the obligations and liabilities
of Seller thereunder are exclusive and in lieu of all other remedies or
warranties, express or implied, arising by law or otherwise, with respect
to the system delivered hereunder (including without limitation any
obligation of Seller with respect to merchantability, fitness for particular
purpose and non-infringement). In no event shall Seller be liable for
incidental or consequential damages, however arising and whether or
not occasioned by Seller’s negligence.
This Warranty shall not be extended or altered except by written instrument
signed by Seller.
All of the product elements in the Seller’s Instrument and the total Instrument
are warranted to be new or equivalent to new for the full product warranty
period of one year. Disposables and replacement items with a normal life
expectancy of less than one (1) year, such as batteries and bulbs, are
excluded from this warranty.
Page 6

Page 7
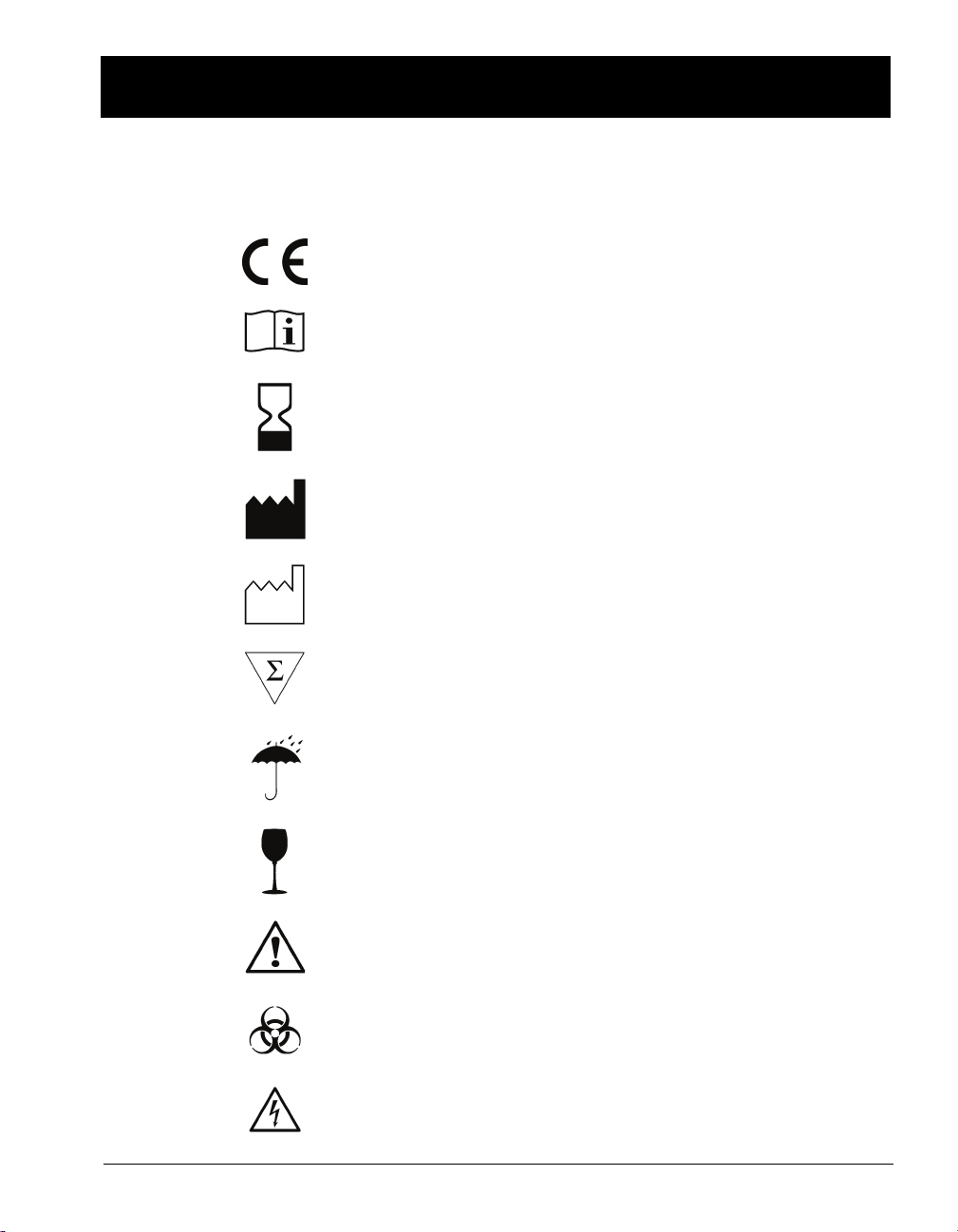
STANDARD SYMBOLS
The following table presents symbols that may appear in the instructions for
use or on the instrument, package inserts, or packaging.
CE-Marking of Conformity
Consult Instructions for Use
Use by
Manufacturer
Date of manufacture
Contains sufficient for <n> tests
Keep dry
Fragile, handle with care
Caution, consult accompanying documents
Biological risks
Electric shock warning
Page 8
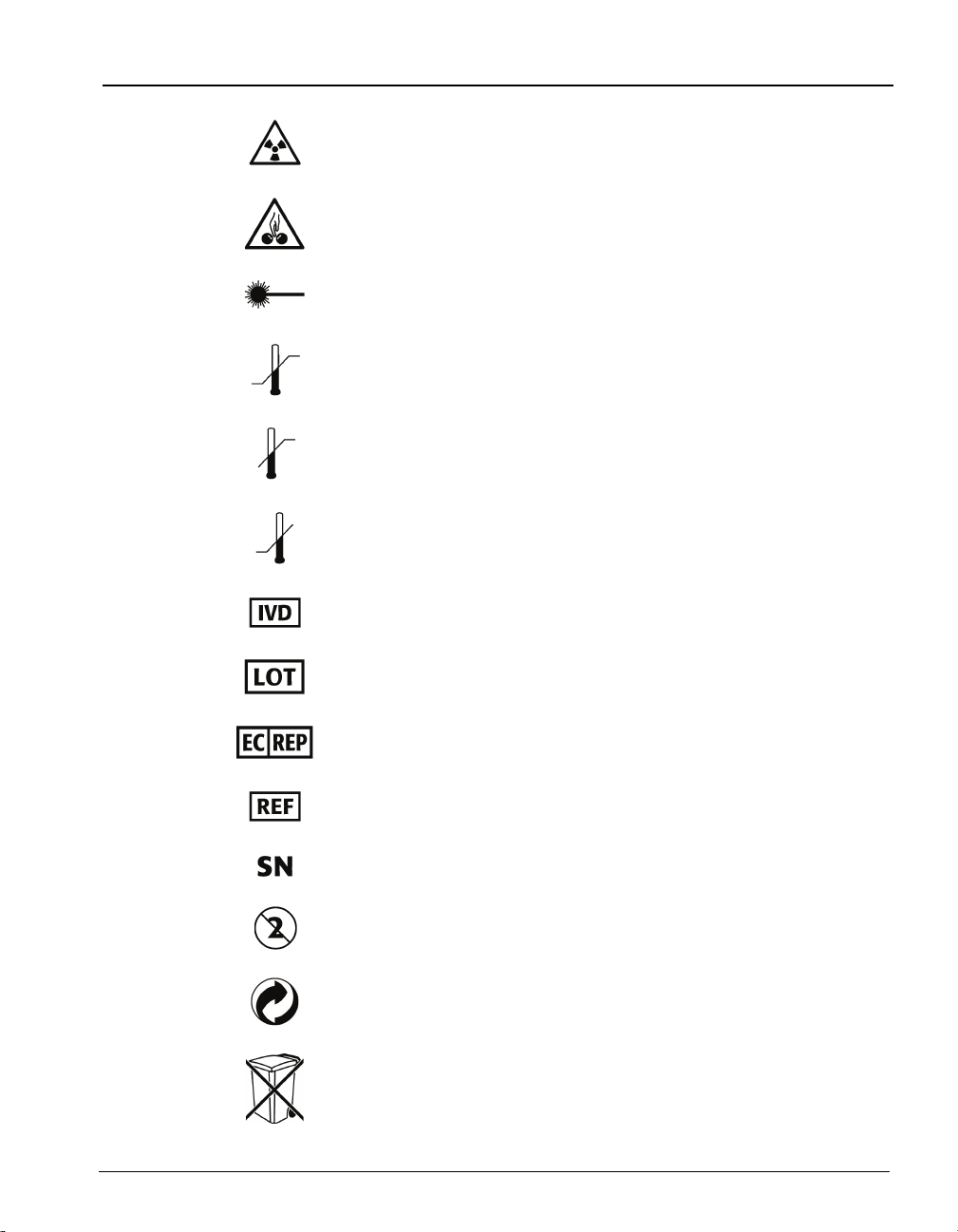
Radiation warning
Potential pinch-point warning
Laser
Temperature limitation
Upper limit of temperature
Lower limit of temperature
In Vitro Diagnostic Medical Device
Standard Symbols
Batch code
Authorized Representative in the European Community
Catalog number
Serial Number
Do not reuse
Recyclable
Separate collection for waste electrical and electronic
equipment
Page 9
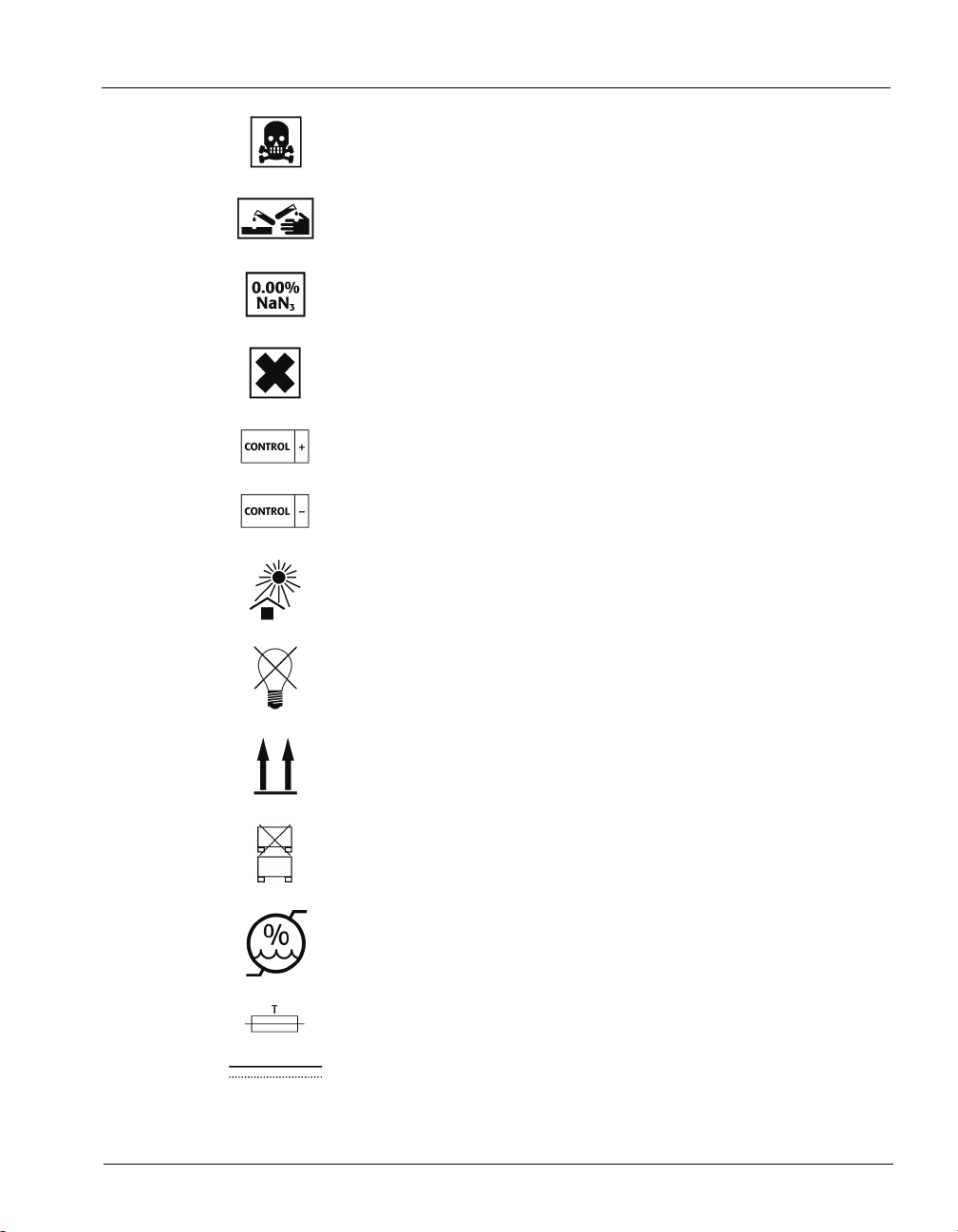
Standard Symbols
Very toxic
Corrosive
Sodium azide
Irritant
Positive control
Negative control
Keep away from sunlight
Protect from light
This way up
Do not stack
Humidity limitation
Fuse
Direct current
Page 10
Alternating current
Both direct and alternating current
Three-phase alternating current
Earth (ground) terminal
Protective conductor terminal
Frame or chassis terminal
Equipotentiality
ON (supply)
Standard Symbols
OFF (supply)
ON (only for a component of the system equipment)
OFF (only for a component of the system equipment)
Equipment protected throughout by double insulation or
reinforced insulation (Equivalent to Class II of IEC 536)
Potential tip over/crush hazard
Page 11

TABLE OF CONTENTS
Standard Symbols......................................................................................................vi
List of Figures ..........................................................................................................xiii
List of Tables ...........................................................................................................xvii
H
OW TO USE THIS MANUAL........................................................................................ 1-1
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
Specimen/Sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
Purpose of the BacT/ALERT
Additional Supplies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Purpose of This Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Manual Organization. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Chapter Organization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Finding Topics. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Typographic and Usage Conventions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
Name and Titles. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
Press . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
Procedural Steps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
Select. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
User Input . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
Warnings, Cautions, and Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
®
3D System . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
S
YSTEM OVERVIEW ..................................................................................................... 2-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
Hardware Configuration. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
Software Configuration Options. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
21 CFR Part 11 and HIPAA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Theory Of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Principle of Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Electrical Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Electrical Grounding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Electrical and Electronic Recycling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Fuse Replacement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
®
BacT/ALERT
3D Hardware. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Mycobacteria Drawers (Clinical Use). . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Controller Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-9
Operator Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Barcode Reader Aperture. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-9
BacT/ALERT® 3D User Manual i
95248
Page 12

Table of Contents
Backup Drive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Keyboard Drawer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Quick Reference Card . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Keyboard . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Accessing the Controller Module Keyboard . . . . . . . . . . . . . . . . . . . . . . . . . . .2-11
Printer Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
Audible Alarm Speaker. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
External Speaker Port. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Power Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Power Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Display Switch Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
CPU 1 Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
CPU 2 Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Module Ports (6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Monitor Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Modem Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
LIS Port (Diagnostic Port). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
COMM Port. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Controller Module Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-15
Electrical Power Services Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Power Consumed in Watts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Heat Dissipated . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Sound Emission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Instrument Dimensions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Controller Module Environmental Requirements . . . . . . . . . . . . . . . . . . . 2-15
Operating Temperature Range. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Storage Temperature Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Operating Humidity Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Storage Humidity Range. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Maximum Operating and Storage Altitude . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Pollution degree 2 in accordance with IEC 664 . . . . . . . . . . . . . . . . . . . . . . . .2-16
Overvoltage Category II per IEC 664 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Combination Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-16
Operator Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
Barcode Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
Keyboard . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
UPS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Backup Drive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Drawer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Rack . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Cell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Cell Indicator Lamp/Cell Indicator Light . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Cell Flag . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
Thermometer (Not Shown) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
Drawer Indicator Light — Yellow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
Drawer Indicator Light — Green. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
Printer Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
External Speaker Port. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
Power Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
ii BacT/ALERT® 3D User Manual
95248
Page 13

Table of Contents
Power Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-19
Display Switch Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-20
CPU 1 Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
CPU 2 Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
Module Port (3). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
Modem Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
LIS Port. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-20
Barcode Scanner Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
Keyboard Port. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-20
Monitor Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
COMM Port. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-20
Combination Module Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-20
Electrical Power Services Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-20
Power Consumed in Watts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Heat Dissipated . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Combination Module Dimensions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Sound Emission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Combination Module Environmental Requirements. . . . . . . . . . . . . . . . . 2-21
Operating Temperature Range. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-21
Storage Temperature Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Operating Humidity Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Storage Humidity Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-21
Maximum Operating and Storage Altitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-21
Pollution degree 2 in accordance with IEC 664 (Clinical Use) . . . . . . . . . . . . .2-21
Overvoltage Category II per IEC 664 (Clinical Use) . . . . . . . . . . . . . . . . . . . . .2-21
Incubation Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-22
Drawer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-22
Rack . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-22
Cell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-22
Cell Indicator Lamp/Cell Indicator Light . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-23
Cell Flag . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-23
Drawer Indicator Light — Yellow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-23
Drawer Indicator Light — Green. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-23
Controller Module Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-24
Power Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-24
Power Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-24
Incubation Module Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-25
Electrical Power Services Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Power Consumed in Watts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Heat Dissipated . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Instrument Dimensions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Sound Emission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Incubation Module Environmental Requirements . . . . . . . . . . . . . . . . . . 2-25
Operating Temperature Range. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-25
Storage Temperature Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Operating Humidity Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Storage Humidity Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-25
Maximum Operating and Storage Altitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-25
BacT/ALERT® 3D User Manual iii
95248
Page 14

Table of Contents
BASIC FUNCTIONS (CLINICAL USE).............................................................................. 3-1
Instrument Installation and Setup (Clinical Use) . . . . . . . . . . . . . . . . . . . . 2-26
Instrument Installation and Setup (INDUSTRY Use) . . . . . . . . . . . . . . . . 2-26
BacT/ALERT® 3D Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-27
Operator Panel Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-27
Common Screen Elements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-28
Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-28
Button . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-28
Common System Buttons. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-28
Scroll Button . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-29
Slidebar Switch. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-29
Anchor Display/Scroll Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-29
Text Entry Field. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-30
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Monitoring the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Main Screen Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Background Color. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-4
Instrument Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-4
Bottle Count Table . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-5
Viewing Faults. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Viewing the Cell Status Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Understanding the View Cell Status Screen Display . . . . . . . . . . . . . . . . . . . . .3-8
Text/Data Entry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Common Text Fields and Field Limit. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Using the Barcode Scanner to Enter Data . . . . . . . . . . . . . . . . . . . . . . . . 3-10
Manually Entering Text into a Data Entry Field (Keyboard) . . . . . . . . . . . 3-10
Loading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
Loading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
Changing the Maximum Test Time — Individual Bottles . . . . . . . . . . . . . 3-15
Handling Anonymous Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-16
Unloading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-16
Unloading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-17
Handling Unconfirmed Positive Bottles (False Positives) . . . . . . . . . . . . . 3-19
Accessing the Setup Screen Function Buttons . . . . . . . . . . . . . . . . . . . . . . . . 3-20
Accessing the Setup Screen. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-20
Inactivity Timeout for all Setup Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-21
Setup Screen Function Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-21
Viewing and Printing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-23
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-23
Viewing Bottle Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-23
Viewing/Printing Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-23
Viewing/Printing Graphs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-23
iv BacT/ALERT® 3D User Manual
95248
Page 15

Table of Contents
Using the Print Screen Function. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-24
Viewing, Printing, and Exporting Test Data . . . . . . . . . . . . . . . . . . . . . . . 3-24
Viewing and Printing Bottle Graphs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-31
Display Bottle Readings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-34
Sending/Requesting LIS Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-39
Sending Results to the LIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-39
Requesting Information from the LIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-39
BASIC FUNCTIONS (INDUSTRY USE) ......................................................................... 4-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Monitoring the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Main Screen Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Background Color. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-4
Instrument Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-4
Bottle Count Table . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-5
Viewing Faults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Viewing the Cell Status Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Understanding the View Cell Status Screen Display . . . . . . . . . . . . . . . . . . . . .4-8
Text/Data Entry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Common Text Fields and Field Limits . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Using the Barcode Scanner to Enter Data. . . . . . . . . . . . . . . . . . . . . . . . 4-10
Manually Entering Text into a Data Entry Field (Keyboard). . . . . . . . . . . 4-10
Loading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Loading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Changing the Maximum Test Time — Individual Bottles. . . . . . . . . . . . . 4-14
Handling Anonymous Bottles. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15
Unloading Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-16
Unloading Bottles. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-16
Handling Unconfirmed Positive Bottles (False Positives) . . . . . . . . . . . . 4-19
Accessing the Setup Screen Function Buttons. . . . . . . . . . . . . . . . . . . . . . . . 4-20
Accessing the Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-20
Inactivity Timeout for all Setup Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-21
Setup Screen Function Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-21
Viewing and Printing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-23
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-23
Viewing Bottle Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-23
Viewing/Printing Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-23
Viewing/Printing Graphs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-23
Using the Print Screen Function. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-24
Viewing, Printing, and Exporting Test Data . . . . . . . . . . . . . . . . . . . . . . . 4-24
Viewing and Printing Bottle Graphs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-31
Display Bottle Readings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-34
BacT/ALERT® 3D User Manual v
95248
Page 16

Table of Contents
E
Sending/Requesting LIS Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-39
Sending Results to the LIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-39
Requesting Information from the LIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-39
DITING TEST DATA (CLINICAL USE) ........................................................................... 5-1
Viewing/Editing Bottle Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Selecting Bottles Using the Edit Cell Contents Button . . . . . . . . . . . . . . . . 5-3
Selecting Bottles Using the Select Bottle to Edit/Graph Button . . . . . . . . . 5-4
Editing Bottle Details Using the Edit Bottle Detail Screen. . . . . . . . . . . . . . 5-5
Edit Bottle ID Field . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
View Accession Number Field . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
View Hospital ID Field. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
View Patient First Name Field . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
View Patient Last Name Field. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
Edit Load Status Slidebar Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
Edit Maximum Test Time Scroll Buttons. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
Edit Bottle Type Scroll Button . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
View Cell Location Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
View First Loaded Time Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
View Last Unloaded Time Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
View Time of Last Bottle Reading Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
View Test Time Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
View Test Result Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-9
Edit Test Result Button. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-9
Graph Bottle Readings Button . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-10
View Algorithm/Polynomial Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-10
View How Determined/Positivity Index Icon . . . . . . . . . . . . . . . . . . . . . . . . . . .5-11
Editing Data Relationships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-12
Initiating the Edit Data Relationships Function . . . . . . . . . . . . . . . . . . . . . 5-12
Editing Bottle ID to Accession Number Relationships. . . . . . . . . . . . . . . . 5-14
Attaching Bottle IDs without an Accession Number to an Accession Number .5-14
Moving a Bottle ID Association from one Accession Number to Another . . . . .5-15
Editing Accession Number to Hospital ID Relationships. . . . . . . . . . . . . . 5-16
Attaching Accession Numbers without a Hospital ID to a Hospital ID . . . . . . .5-16
Moving an Accession Number Association from one Hospital ID to Another . .5-17
Editing Hospital ID to Patient Name Relationships . . . . . . . . . . . . . . . . . . 5-17
E
DITING TEST DATA (INDUSTRY USE)....................................................................... 6-1
Viewing/Editing Bottle Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Selecting Bottles Using the Edit Cell Contents Button . . . . . . . . . . . . . . . . 6-3
Selecting Bottles Using the Select Bottle to Edit/Graph Button . . . . . . . . . 6-4
Editing Bottle Details Using the Edit Bottle Detail Screen. . . . . . . . . . . . . . 6-5
vi BacT/ALERT® 3D User Manual
95248
Page 17

Table of Contents
Edit Bottle ID Field . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
View Sample ID Field . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
View User Defined 1. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
View User Defined 2. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
View User Defined 3. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
Edit Load Status Slidebar Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-7
Edit Maximum Test Time Scroll Buttons. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Edit Bottle Type Scroll Button. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-7
View Cell Location Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
View First Loaded Time Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
View Last Unloaded Time Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
View Time of Last Bottle Reading Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-8
View Test Time Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
View Test Result Icon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
Edit Test Result Button. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
Graph Bottle Readings Button . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-10
View Algorithm/Polynomial Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-10
View How Determined/Positivity Index Icon . . . . . . . . . . . . . . . . . . . . . . . . . . .6-11
Editing Data Relationships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-12
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-12
Initiating the Edit Data Relationships Function . . . . . . . . . . . . . . . . . . . . 6-12
Editing Bottle ID to Sample ID Relationships. . . . . . . . . . . . . . . . . . . . . . 6-14
Attaching Bottle IDs without a Sample ID to a Sample ID . . . . . . . . . . . . . . . .6-14
Moving a Bottle ID Association from one Sample ID to Another . . . . . . . . . . .6-15
Editing Sample ID to User Defined 3 Relationships . . . . . . . . . . . . . . . . 6-16
Attaching Sample IDs without a User Defined 3 to a User Defined 3. . . . . . . .6-16
Moving a Sample ID Association from One User Defined 3 to Another . . . . . .6-17
Editing User Defined 3 to User Defined 1/User Defined 2
Relationships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-17
S
OFTWARE CONFIGURATION........................................................................................ 7-1
Setting the Maximum Test Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Setting the Audible Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
Priority of Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Terminating an Instrument Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Changing the System Password. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Initiating Manual Backup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7
Configuring Report Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-9
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-9
Entering Report Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-10
Configuring Report Contents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-13
Load Report Configuration Screen (Clinical Use). . . . . . . . . . . . . . . . . . . . . . .7-16
Status Report Configuration Screen (Clinical Use) . . . . . . . . . . . . . . . . . . . . .7-17
Unload Report Configuration Screen (Clinical Use) . . . . . . . . . . . . . . . . . . . . .7-18
Load Report Configuration Screen (INDUSTRY Use) . . . . . . . . . . . . . . . . . . . 7-19
Status Report Configuration Screen (INDUSTRY Use) . . . . . . . . . . . . . . . . . . 7-20
BacT/ALERT® 3D User Manual vii
95248
Page 18

Table of Contents
SYSTEM MAINTENANCE ............................................................................................... 8-1
Unload Report Configuration Screen (INDUSTRY Use). . . . . . . . . . . . . . . . . .7-21
Viewing and Printing Calibration Data . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-21
Viewing and Printing Calibration History. . . . . . . . . . . . . . . . . . . . . . . . . . 7-25
Hardware Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-2
Preventative Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-2
Safety Precautions and Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-2
General Precautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-3
Spill Cleanup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-3
Disinfection Procedure for Spills Onto the Instrument (Clinical Use) . . . . . . . . .8-4
Disinfection Procedure for Spills Onto the Instrument (INDUSTRY Use). . . . . .8-5
Disinfection Procedure for Spills Within the Instrument . . . . . . . . . . . . . . . . . . .8-5
Using the Keyboard in Place of the Operator Panel . . . . . . . . . . . . . . . . . . 8-8
UPS On/Off Button Location . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-9
Controller Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-9
Combination Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-10
Controller/Combination Module Reboot/Shutdown (BacT/ALERT® 3D
Select and SelectLink) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-10
Shutdown Method 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-11
Shutdown Method 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-11
Shutdown Method 3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-12
Shutdown Method 4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-13
Controller or Combination Module Startup . . . . . . . . . . . . . . . . . . . . . . . . 8-13
®
Full System Shutdown (BacT/ALERT
3D Signature) . . . . . . . . . . . . . . . 8-13
Full System Startup (BacT/ALERT® 3D Signature) . . . . . . . . . . . . . . . . . 8-14
Set Up an External Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-14
Restore Internal Monitor Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-14
Software Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-15
Restarting an Incubation Module or the Combination Module Drawers . . 8-15
Setting and Formatting the System Date and Time . . . . . . . . . . . . . . . . . 8-17
Enabling and Disabling Modules, Drawers, Racks, and Cells . . . . . . . . . 8-18
Relocating Bottles. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-21
Adjusting an Incubation or Combination Module’s Temperature . . . . . . . 8-21
Checking an Incubation or Combination Module’s Temperature . . . . . . . . . . .8-21
Setting the Optimal Temperature for an Incubation or Combination Module . .8-24
Calibrating an Incubation or Combination Module’s Temperature . . . . . . . . . .8-25
Calibrating an Instrument Cell. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-25
Locating a Cell Which Failed Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-26
Viewing a Cell’s Readings and/or Calibrating a Cell. . . . . . . . . . . . . . . . . . . . .8-26
Viewing Incubation Module Information . . . . . . . . . . . . . . . . . . . . . . . . . . 8-29
S
YSTEM TROUBLESHOOTING ....................................................................................... 9-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-2
viii BacT/ALERT® 3D User Manual
95248
Page 19

Table of Contents
Fault Codes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
Instrument Fault Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
Instrument Status Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-24
Operator Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-27
Bottle Problems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-39
User Output Device Problems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-39
21 CFR P
ART 11 MODE ........................................................................................... 10-1
Log In/Out of System — 21 CFR Part 11 Mode . . . . . . . . . . . . . . . . . . . . . . . 10-2
Logging In for the First Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-2
Logging In . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-5
Inactivity Timeout. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Login Errors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Change Password . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-8
Change Password Errors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-9
Logging Out . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-12
Configuring Users — 21 CFR Part 11 Mode. . . . . . . . . . . . . . . . . . . . . . . . . 10-12
Adding a User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-13
Deleting a User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-15
Deleting More Than One User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-16
Clearing a User Password . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-16
Clearing More Than One User Password. . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-17
Audit Trail . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-18
Accessing the Audit Trail . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-20
M
YCOBACTERIAL TESTING (CLINICAL USE)................................................................ 11-1
System Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
BacT/ALERT® 3D Instrument . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Circulation Fan . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-3
Rocker Clamp. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-3
Installation Procedures and Special Requirements . . . . . . . . . . . . . . . . . . . . .11-4
Barcodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-4
Load Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
Unload Positives and Negatives. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-5
Set Maximum Test Time. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
Positive Detection Algorithm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
Bottle Status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-5
System Startup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-6
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-6
MB Drawer Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-6
Limitations of the Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-7
Service and Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-7
BacT/ALERT® 3D User Manual ix
95248
Page 20

Table of Contents
I
NTERNATIONAL CHARACTER ENTRY ............................................................................A-1
B
Theory of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Principle of Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-7
Safety Features. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
General Precautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-9
Spill Cleanup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-9
Disinfection Procedure for Spills Within/Onto the Instrument . . . . . . . . . . . . . .11-9
Remove Drawer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-10
Device Comparison for Recovery of Mycobacteria. . . . . . . . . . . . . . . . . 11-10
International Character Entry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .A-2
Clinical Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-3
INDUSTRY Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-3
Entering International Characters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .A-4
OTTLE QUALITY CONTROL.........................................................................................B-1
BacT/ALERT
®
Culture Bottles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-2
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-2
Flip Cap . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Stopper/Seal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Bottle. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Volume Designations (Clinical Use) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Volume Designations (INDUSTRY Use). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Barcode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-3
Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-4
Limitations of the Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-4
Clinical Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-4
INDUSTRY Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-4
Quality Control of Growth Performance . . . . . . . . . . . . . . . . . . . . . . . . . . .B-4
Blood Culture Bottles (SA, SN, FA, FN, and PF) . . . . . . . . . . . . . . . . . . . . . . . B-5
Mycobacteria Culture Bottles (MB and MP) . . . . . . . . . . . . . . . . . . . . . . . . . . . B-5
Culture Bottles (iAST, iNST, and iLYM) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-5
BEST PRACTICES ........................................................................................................C-1
Best Practices for Preventing False Positives . . . . . . . . . . . . . . . . . . . . . . . . . .C-2
Preventing False Positives — User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-2
High Amount of White Blood Cells (Clinical Use) . . . . . . . . . . . . . . . . . . . . . . . C-2
Blood (or Sample) Volume too High . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-2
Large Bottle Loading and Unloading Events . . . . . . . . . . . . . . . . . . . . . . . . . . C-3
Temperature Changes in the Environment. . . . . . . . . . . . . . . . . . . . . . . . . . . . C-3
Anonymous Bottle Loading of BacT/ALERT
Bottle Not Completely Loaded into the Cell . . . . . . . . . . . . . . . . . . . . . . . . . . . C-4
®
FA and PF Bottles . . . . . . . . . . C-4
Preventing False Positives — Instrument . . . . . . . . . . . . . . . . . . . . . . . . . .C-5
Cell Noise . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-5
High Initial Value Readings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-7
False Positive MP Process Bottles Flagged by Delta Algorithm (Clinical Use) C-7
x BacT/ALERT® 3D User Manual
95248
Page 21

Table of Contents
GLOSSARY....................................................................................................GLOSSARY-1
I
NDEX .................................................................................................................. INDEX-1
BacT/ALERT® 3D User Manual xi
95248
Page 22

Table of Contents
xii BacT/ALERT® 3D User Manual
95248
Page 23

LIST OF FIGURES
Figure 1-1: Procedure Icon ...................................................................................................1-7
Figure 2-1: Electrical Grounding Requirements ...................................................................2-7
Figure 2-2: Instrument Front View ........................................................................................2-9
Figure 2-3: Removing the Barcode Reader Through the Barcode Reader Aperture .........2-10
Figure 2-4: Accessing the Keyboard and Reference Card .................................................2-12
Figure 2-5: Back View Controller Module ...........................................................................2-13
Figure 2-6: Front View Combination Module ......................................................................2-16
Figure 2-7: Back View Combination Module ......................................................................2-17
Figure 2-8: Cell Flag (Clip Removed) .................................................................................2-19
Figure 2-9: Front View Incubation Module (Right-Handed Configuration) ..........................2-22
Figure 2-10: Back View Incubation Module ..........................................................................2-24
Figure 2-11: Button Examples ..............................................................................................2-28
Figure 2-12: Scroll Button .....................................................................................................2-29
Figure 2-13: Slidebar Switch ................................................................................................2-29
Figure 2-14: Anchor Display/Scroll Buttons ..........................................................................2-30
Figure 3-1: Main Screen .......................................................................................................3-3
Figure 3-2: Combination Module with an Additional Incubation Module ...............................3-5
Figure 3-3: Controller Module with One Incubation Module .................................................3-5
Figure 3-4: Bottle Count Table/Unload Buttons ....................................................................3-6
Figure 3-5: View Cell Status Screen ....................................................................................3-7
Figure 3-6: Disabled Cell and Drawer ..................................................................................3-9
Figure 3-7: Main Screen — Load Mode .............................................................................3-12
Figure 3-8: Change Maximum Test Time Screen ...............................................................3-15
Figure 3-9: Main Screen — Unload Mode ..........................................................................3-17
Figure 3-10: Setup Screen ...................................................................................................3-20
Figure 3-11: Report Selection Screen ..................................................................................3-24
Figure 3-12: Sample Report Screen .....................................................................................3-26
Figure 3-13: Find Text Screen ..............................................................................................3-29
Figure 3-14: Save to File Screen ..........................................................................................3-30
Figure 3-15: Select Bottle to Edit/Graph Screen ..................................................................3-32
Figure 3-16: Graph Bottle Readings Screen ........................................................................3-33
Figure 3-17: Bottle Readings Screen ...................................................................................3-35
BacT/ALERT® 3D User Manual xiii
95248
Page 24

Figure 3-18: Find Text Screen ............................................................................................. 3-37
Figure 3-19: Save to File Screen ......................................................................................... 3-38
Figure 4-1: Main Screen ...................................................................................................... 4-3
Figure 4-2: Combination Module with an Additional Incubation Module .............................. 4-5
Figure 4-3: Controller Module with One Incubation Module ................................................ 4-5
Figure 4-4: Bottle Count Table/Unload Buttons ................................................................... 4-6
Figure 4-5: View Cell Status Screen .................................................................................... 4-7
Figure 4-6: Disabled Cell and Drawer ................................................................................. 4-9
Figure 4-7: Main Screen — Load Mode ............................................................................ 4-12
Figure 4-8: Change Maximum Test Time Screen .............................................................. 4-15
Figure 4-9: Main Screen — Unload Mode ......................................................................... 4-17
Figure 4-10: Setup Screen .................................................................................................. 4-20
Figure 4-11: Report Selection Screen ................................................................................. 4-24
Figure 4-12: Sample Report Screen .................................................................................... 4-26
Figure 4-13: Find Text Screen ............................................................................................. 4-29
Figure 4-14: Save to File Screen ......................................................................................... 4-30
Figure 4-15: Select Bottle to Edit/Graph Screen ................................................................. 4-32
Figure 4-16: Graph Bottle Readings Screen ....................................................................... 4-33
Figure 4-17: Bottle Readings Screen .................................................................................. 4-35
Figure 4-18: Find Text Screen ............................................................................................. 4-37
Figure 4-19: Save to File Screen ......................................................................................... 4-38
Figure 5-1: Edit Cell Contents Screen ................................................................................. 5-3
Figure 5-2: Select Bottle to Edit/Graph Screen ................................................................... 5-4
Figure 5-3: Edit Bottle Detail Screen ................................................................................... 5-5
Figure 5-4: Edit Test Result Screen .................................................................................... 5-9
Figure 5-5: Edit Data Relationships Screen ...................................................................... 5-13
Figure 5-6: Edit Bottle ID to Accession Number Relationships Screen ............................. 5-14
Figure 5-7: Edit Accession Number to Hospital ID Relationships Screen ......................... 5-16
Figure 5-8: Edit Hospital ID to Patient Name Relationships Screen .................................. 5-18
Figure 6-1: Edit Cell Contents Screen ................................................................................. 6-3
Figure 6-2: Select Bottle to Edit/Graph Screen ................................................................... 6-4
Figure 6-3: Edit Bottle Detail Screen ................................................................................... 6-5
Figure 6-4: Edit Test Result Screen .................................................................................... 6-9
Figure 6-5: Edit Data Relationships Screen ...................................................................... 6-13
xiv BacT/ALERT® 3D User Manual
95248
Page 25

Figure 6-6: Edit Bottle ID to Sample ID Relationships Screen ...........................................6-14
Figure 6-7: Edit Sample ID to User Defined 3 Relationships Screen .................................6-16
Figure 6-8: Edit User Defined 3 to User Defined 1/User Defined 2
Relationships Screen .......................................................................................6-18
Figure 7-1: Set Maximum Test Time Screen ........................................................................7-2
Figure 7-2: Setting the Audible Alarm Options Screen .........................................................7-4
Figure 7-3: Change Password Screen .................................................................................7-6
Figure 7-4: Padlock Icon (Full Open position) ......................................................................7-6
Figure 7-5: Padlock Icon (Half Open Position) .....................................................................7-7
Figure 7-6: Padlock Icon (Closed Position) ..........................................................................7-7
Figure 7-7: Backup Management Screen .............................................................................7-8
Figure 7-8: Backup In Progress Icon ....................................................................................7-9
Figure 7-9: Report Selection Screen ..................................................................................7-10
Figure 7-10: Report Label Entry Screen ...............................................................................7-11
Figure 7-11: Report Selection Screen ..................................................................................7-14
Figure 7-12: Load Report Configuration Screen (Clinical Use) ............................................7-16
Figure 7-13: Status Report Configuration Screen (Clinical Use) ..........................................7-17
Figure 7-14: Unload Report Configuration Screen (Clinical Use) .........................................7-18
Figure 7-15: Load Report Configuration Screen (INDUSTRY Use) .....................................7-19
Figure 7-16: Status Report Configuration Screen (INDUSTRY Use) ...................................7-20
Figure 7-17: Unload Report Configuration Screen (INDUSTRY Use) ..................................7-21
Figure 7-18: Report Selection Screen ..................................................................................7-22
Figure 7-19: Cell Calibration Report .....................................................................................7-23
Figure 7-20: Calibration Report ............................................................................................7-24
Figure 7-21: Report Selection Screen ..................................................................................7-25
Figure 7-22: Calibration History Screen ...............................................................................7-26
Figure 8-1: Front Access UPS Orientation .........................................................................8-10
Figure 8-2: Exit Software Button ........................................................................................8-12
Figure 8-3: View Cell Status Screen ..................................................................................8-16
Figure 8-4: Set Date/Time Screen ......................................................................................8-17
Figure 8-5: Enable/Disable Module, Drawer, Rack, or Cell Screen ....................................8-19
Figure 8-6: Reference Thermometer (Drawer B — Right-hand Configuration) ..................8-22
Figure 8-7: Digital Thermometer (Drawer A — Left-hand Configuration) ...........................8-22
Figure 8-8: Calibrate Module Temperature Screen ............................................................8-23
Figure 8-9: Calibrate Cell Screen .......................................................................................8-27
BacT/ALERT® 3D User Manual xv
95248
Page 26

Figure 8-10: View Incubation Module Information Screen ................................................... 8-30
Figure 9-1: Instrument Icon with Fault Codes ...................................................................... 9-3
Figure 9-2: Instrument Status Code 710 ........................................................................... 9-24
Figure 9-3: Operator Error Code 911 ................................................................................ 9-27
Figure 9-4: QuickScan
®
6000 Reset Barcode ................................................................... 9-40
Figure 9-5: Honeywell Reset Barcode ............................................................................... 9-40
Figure 9-6: Red Informational Warning Screen ................................................................. 9-42
Figure 10-1: Main Screen While Logged Out — 21 CFR Part 11 Mode .............................. 10-2
Figure 10-2: User Login Screen .......................................................................................... 10-3
Figure 10-3: User Login Screen with Change Password Fields .......................................... 10-4
Figure 10-4: Main Screen While Logged In — 21 CFR Part 11 Mode ................................. 10-5
Figure 10-5: User Login Screen .......................................................................................... 10-6
Figure 10-6: User Login Screen with Wrong Password Alert .............................................. 10-8
Figure 10-7: User Login Screen with Change Password Fields .......................................... 10-9
Figure 10-8: User Login Screen with Change Password Error .......................................... 10-10
Figure 10-9: User Login Screen with Wrong Password Alert ............................................ 10-11
Figure 10-10:User Configuration Screen ............................................................................ 10-13
Figure 10-11:Add User Screen ........................................................................................... 10-14
Figure 10-12:Delete User Screen ....................................................................................... 10-15
Figure 10-13:Clear Password Screen ................................................................................ 10-17
Figure 11-1: Instrument Icon for MB-Configured System .................................................... 11-3
Figure 11-2: Bottle Count Table and Unload Buttons .......................................................... 11-6
Figure 11-3: Metabolic Pathway for Mycobacterial CO
Figure B-1: Typical BacT/ALERT
®
Culture Bottle ................................................................B-2
...................................................... 11-8
2
Figure C-1: Example Cell Graph ..........................................................................................C-4
Figure C-2: Cell Graph Example — Electronics ...................................................................C-5
Figure C-3: Cell Graph Example — Reverts to Normal ........................................................C-6
Figure C-4: Cell Graph Example — Cyclic ...........................................................................C-6
Figure C-5: Cell Graph Example — High Initial Value Reading ...........................................C-7
xvi BacT/ALERT® 3D User Manual
95248
Page 27

LIST OF TABLES
Table 2-1: Common System Buttons ................................................................................2-28
Table 5-1: Bottle Specific Algorithms ................................................................................5-10
Table 5-2: Status Determination Codes ............................................................................5-11
Table 6-1: Bottle Specific Algorithms ................................................................................6-10
Table 6-2: Status Determination Codes ............................................................................6-11
Table 7-1: Report Field Descriptions .................................................................................7-12
Table 10-1: Audit Trail Events ...........................................................................................10-18
Table 11-1: Device Comparison for Recovery of Mycobacteria ........................................11-10
Table A-1: International Character Table ........................................................................... A-2
Table C-1: Bottle Volumes ..................................................................................................C-2
BacT/ALERT® 3D User Manual xvii
95248
Page 28

xviii BacT/ALERT® 3D User Manual
95248
Page 29

HOW TO USE THIS MANUAL
About This Chapter
This chapter gives you important information about the BacT/ALERT® 3D
system and how to use this manual. It is recommended that you read this
chapter first.
1
IMPORTANT: Read this manual carefully before you attempt to operate the
BacT/ALERT
®
3D system.
Chapter Contents
Intended Audience • 1-2
Specimen/Sample • 1-2
Purpose of the BacT/ALERT
Additional Supplies • 1-3
Purpose of This Manual • 1-4
Manual Organization • 1-4
Chapter Organization • 1-6
Finding Topics • 1-6
Typographic and Usage Conventions • 1-7
Name and Titles • 1-7
Press • 1-7
Procedural Steps • 1-7
References • 1-7
Select • 1-8
User Input • 1-8
Warnings, Cautions, and Information • 1-8
®
3D System • 1-2
BacT/ALERT® 3D User Manual 1-1
95248
Page 30
How To Use This Manual Intended Audience
Intended Audience
The BacT/ALERT® 3D system and this manual are intended for laboratory
use by trained, professional, clinical and INDUSTRY users.
Most material in the manual applies to both sets of users. If any information in
the manual is intended for clinical use only or industry use only, it is marked
Clinical Use or INDUSTRY Use.
Specimen/Sample
In this manual, you will find references to both “specimen” and “sample.”
“Specimen” refers to Clinical Use, and “sample” refers to INDUSTRY Use.
Purpose of the BacT/ALERT® 3D System
The BacT/ALERT® 3D Microbial Detection System is a totally automated test
system capable of incubating, agitating, and continuously monitoring aerobic
and anaerobic media inoculated with:
• patient specimens suspected of having bacteremia, fungemia, and/or
mycobacteremia (Clinical Use),
or
• samples to be monitored for bacterial or fungal contamination (INDUSTRY
Use).
CAUTION: bioMérieux shall not be liable as to any defect arising
from abuse of the instrument, failure to operate and maintain the
instrument in accordance with the User Manual, operation of the
instrument by a person who has not been trained in its operation
by bioMérieux, repair, service, alteration or modification of the
instrument by any person other than service personnel of
bioMérieux, or modification, change or reuse of the disposables
supplied by bioMérieux for use in the instrument.
CAUTION: This BacT/ALERT® 3D User Manual is only intended
for use with B.30 Software or higher. The software version B.30
(or higher) is displayed at the bottom of the instrument icon on
the Main screen.
1-2 BacT/ALERT® 3D User Manual
95248
Page 31

Additional Supplies How To Use This Manual
CAUTION: All figures depicting operator screens are examples
only. Actual screens may differ to the extent they are affected by
the actual data entered by the operator, or actual data
transmitted to the instrument over the DBMS or LIS interface, or
actual data generated by the instrument.
CAUTION: Regarding section "Entering Report Labels," the user
is solely responsible for the choice of customized report label
text and for validating that the intended label text appears in all
associated reports. bioMérieux shall not be liable for any
consequences resulting from misinterpretation of customized
report labels.
CAUTION: (INDUSTRY Use) The BacT/ALERT® system is
marketed for use in the detection of microorganisms in blood
and other normally sterile body fluids, and in the detection of
microorganisms in other sample matrices and for additional
specific indications for use, as specified in the package inserts,
user manuals, and labeling of the specific components of the
BacT/ALERT
BacT/ALERT
®
system. Customers who use the
®
system in testing of sample types or for
indications other than those described in the applicable package
inserts and user manuals do so at their own risk. Performance
characteristics for the BacT/ALERT
®
system for any use outside
the labeling, package insert, or user manual have not been
established.
Additional Supplies
Contact bioMérieux or your local vendor for laboratory supplies and
accessories.
BacT/ALERT® 3D User Manual 1-3
95248
Page 32

How To Use This Manual Purpose of This Manual
Purpose of This Manual
This manual focuses on the BacT/ALERT® 3D software application and how
you use it in your workflow. It contains step-by-step procedures for using your
BacT/ALERT
By using these procedures, you can perform all the functions required to
operate your system, including:
• accessing the BacT/ALERT
• system monitoring
• entering data (where applicable)
• loading and unloading bottles
• viewing and printing data (printing with the BacT/ALERT® 3D Signature is
performed at the data management computer)
• LIS interaction with the BacT/ALERT
• accessing the Setup screen
Note: Screens and figures are intended for illustrative purposes only and are not to
be construed as representations of actual test data, results, or components.
Screens and components are not shown to scale.
®
3D system.
®
3D software
®
3D SelectLink
Manual Organization
This manual is organized by chapters. Chapters are organized according to
the order of menu commands in the software application.
Chapter 1, How To Use This Manual — Provides an introduction to the
BacT/ALERT
that you read this chapter first.
Chapter 2, System Overview — This chapter gives you a complete
description of the different BacT/ALERT
configurations available. Also, the operator panel screens are listed along
with a description of common screen elements.
Chapter 3, Basic Functions (Clinical Use) — Introduces you to the Main
screen and shows you how to perform basic functions (ex. enter data, view
faults, view the Cell Status screen, load/unload bottles, etc.). This chapter
also introduces you to the Setup screen and the associated function buttons.
1-4 BacT/ALERT® 3D User Manual
®
3D system and how to use this manual. It is recommended
®
3D hardware and software
95248
Page 33

Manual Organization How To Use This Manual
Chapter 4, Basic Functions (INDUSTRY Use) — Introduces you to the
Main screen and shows you how to perform basic functions (ex. enter data,
view faults, view the Cell Status screen, load/unload bottles, etc.). This
chapter also introduces you to the Setup screen and the associated function
buttons.
Chapter 5, Editing Test Data (Clinical Use) — Explains how to access
bottle data for loaded and unloaded bottles using the Edit Bottle Detail
screen. This chapter shows you how to view and edit bottle data.
Chapter 6, Editing Test Data (INDUSTRY Use) — Explains how to access
bottle data for loaded and unloaded bottles using the Edit Bottle Detail
screen. This chapter shows you how to view and edit bottle data.
Chapter 7, Software Configuration — Explains how to configure the
software for your specific needs. This chapter shows you how to set the
maximum test time, set the audible alarms, change the system password,
initiate a manual backup, configure report screens, and view and print
calibration data.
Chapter 8, System Maintenance — This chapter provides you with
procedures on how to perform maintenance on the BacT/ALERT
®
3D
hardware and software.
Chapter 9, System Troubleshooting — Describes the different types of
instrument fault, instrument status and operator error codes, as well as bottle
and user output device problems, you may encounter when using the
instrument. Cause(s) and solutions(s) for each type of fault/error/problem are
also listed.
Chapter 10, 21 CFR Part 11 Mode — This chapter explains how to log in
and out of the instrument while in 21 CFR Part 11 mode, as well as
instructions for configuring users (ex. adding or deleting a user, or clearing a
user password). This chapter also describes the events recorded in the audit
trail and how to retrieve the audit trail.
Chapter 11, Mycobacterial Testing (Clinical Use) — This chapter provides
you with a complete description of the of the BacT/ALERT
®
3D instrument
when it is configured for Mycobacterial (MB) functions.
Appendix A, International Character Entry — Explains how to enter
international characters on the BacT/ALERT
®
3D instrument.
Appendix B, Bottle Quality Control — This chapter provides you with a
description of the BacT/ALERT® 3D culture bottle along with a quality control
procedure.
BacT/ALERT® 3D User Manual 1-5
95248
Page 34

How To Use This Manual Chapter Organization
Glossary — An alphabetized list of frequently used terms along with a
definition for each term.
Chapter Organization
All chapters include the following:
• About This Chapter — Brief description of the chapter’s content and
purpose.
• Chapter Contents — A table of contents for the chapter.
• Descriptions and/or Procedures — Chapters contain descriptions and
procedures appropriate to their subject matter. See the Manual
Organization section in this chapter for more information.
• Background Information, where applicable and useful.
Finding Topics
This manual uses several methods to help you find information and keep your
bearings.
Table of Contents — Located at the front of the manual. It contains the titles
of all chapters/appendices and their sections, and the page number of each
title and section.
List of Figures — Located at the front of the manual. It contains a list of all
figures in the manual and the page number of each figure.
List of Tables — Located at the front of the manual. It contains a list of all
tables in the manual and the page number of each table.
Chapter Contents — Located at the front of each chapter. It lists all sections
in the chapter and their page numbers.
Page Headers — Located at the top of each page. There are two parts to a
header: the chapter title and the primary section title.
Page Footers — Located at the bottom of each page. There are three parts
to a footer: the manual’s title, the chapter’s part number, and the page
number.
Index — Located at the back of the manual. It contains topical entries and
their page numbers.
1-6 BacT/ALERT® 3D User Manual
95248
Page 35

Typographic and Usage Conventions How To Use This Manual
Typographic and Usage Conventions
Name and Titles
Button, icon and field names are in Proper Case, bold.
Example: Press the Select Maximum Test Time button.
The names of windows and screens are in Proper Case, but are not bolded.
Example: The Set Maximum Test Time screen...
Press
This manual uses the word “press” to refer to pressing a button on the
Controller Module Operator Panel.
Example: Press the Load Bottles button.
We also use the word “press” to refer to pressing a key on the keyboard in
order to initiate action in the firmware.
Keyboard entries are in Proper Case and bolded (ex. Ctrl). If two keys are to
be pressed simultaneously, they will be separated with a plus sign (ex. press
Ctrl + Alt + Delete).
Procedural Steps
Steps in procedures are sequentially numbered. A bullet list in a step
indicates options.
Sections that contain procedures are denoted by the Procedure icon in the
margin.
Figure 1-1: Procedure Icon
References
References to chapter and section titles in this manual are in Proper Case.
Example: See Chapter 7, Software Configuration.
BacT/ALERT® 3D User Manual 1-7
95248
Page 36

How To Use This Manual Warnings, Cautions, and Information
References to other manuals are in Proper Case and italic.
Example: See the BacT/ALERT® 3D Service Manual.
Select
The word “select” is generally used for selecting user interface navigation.
Example: Select the appropriate bottle type from the Media Type scroll
button.
User Input
Instructions for user input begin with the word “type” or “enter.” This manual
uses bold for literal user input and italic for placeholders.
Example of a Literal User Input: Enter the Software Exit Password
24313124.
In this example, you are to type exactly what you see on the page (24313124
in this example).
Example of a Placeholder: Enter your password before you...
In this example, you are to type your assigned password.
Warnings, Cautions, and Information
This manual uses different types of symbols to alert you to important
information. Symbols and their associated information are labeled in text
where they occur and set off from surrounding paragraphs, as shown in the
following examples.
WARNING
Warning is a statement that alerts the user to the possibility of
injury, death, or other serious adverse reactions associated
with the use or misuse of a device.
1-8 BacT/ALERT® 3D User Manual
95248
Page 37

Warnings, Cautions, and Information How To Use This Manual
CAUTION: Caution is a statement that alerts the user to the
possibility of a problem with the device associated with its use
or misuse. Such problems include device malfunction, device
failure, damage to the device, or damage to other property.
Where applicable, a caution statement may include a precaution
that should be taken to avoid the hazard.
IMPORTANT: Important relates to content presented in this manual. It is used to
reinforce the importance of your understanding or remembering
something.
Note: Note supplies additional information about a topic.
BacT/ALERT® 3D User Manual 1-9
95248
Page 38

How To Use This Manual Warnings, Cautions, and Information
1-10 BacT/ALERT® 3D User Manual
95248
Page 39

SYSTEM OVERVIEW
About This Chapter
2
This chapter gives you a complete description of the different
BacT/ALERT
operator panel screens are listed along with a description of common screen
elements.
Note: Information in this chapter that is intended for clinical use only or industry use
only is marked Clinical Use or INDUSTRY Use.
Chapter Contents
Introduction • 2-2
Hardware Configuration • 2-2
Software Configuration Options • 2-2
21 CFR Part 11 and HIPAA • 2-3
Theory Of Operation • 2-4
Electrical Warnings • 2-4
Electrical Grounding • 2-6
Electrical and Electronic Recycling • 2-7
Fuse Replacement • 2-7
BacT/ALERT
Mycobacteria Drawers (Clinical Use) • 2-8
Controller Module • 2-9
Controller Module Specifications • 2-15
Controller Module Environmental Requirements • 2-15
Combination Module • 2-16
Combination Module Specifications • 2-20
Combination Module Environmental Requirements • 2-21
Incubation Module • 2-22
Incubation Module Specifications • 2-25
Incubation Module Environmental Requirements • 2-25
Instrument Installation and Setup (Clinical Use) • 2-26
Instrument Installation and Setup (INDUSTRY Use) • 2-26
BacT/ALERT
Operator Panel Screens • 2-27
Common Screen Elements • 2-28
®
®
®
3D hardware and software configurations available. Also, the
3D Hardware • 2-8
3D Software • 2-27
BacT/ALERT® 3D User Manual 2-1
95248
Page 40

System Overview Introduction
Introduction
The BacT/ALERT® 3D is the next generation of BacT/ALERT®
instrumentation with comparable sensitivity and specificity to the
BacT/ALERT
activated operator panel allows for a text-free user interface to direct rapid
loading and unloading of individual test samples.
Once placed in the unit, handling of a specimen/sample bottle is not required
until a result is obtained. Immediately upon detection, positive results are
indicated visually on the unit's monitor and, if desired, by an audible beep. If
no microbial growth is present after a specified time, a specimen/sample is
determined to be negative. The system will also indicate the negative
samples that are ready for removal when prompted. Because the system
handles bottles individually, testing of new specimens/samples may begin at
any time. The system also utilizes barcode technology to assist in specimen/
sample and data tracking.
The disposable culture bottles contain a liquid emulsion sensor that is
monitored continuously using solid-state photodetectors. In addition, the
bottles contain media and atmosphere which promote the recovery of a wide
variety of microorganisms without venting.
®
Classic systems. The system is non-invasive. A touch-
Hardware Configuration
The BacT/ALERT® 3D utilizes a flexible modular design in one of two
hardware configurations.
• A Controller Module that directs 1 to 6 Incubation Modules (each with 240
cells for bottle monitoring).
• A Combination Module that either stands alone (120 cells for bottle
monitoring), or directs up to 3 Incubation Modules.
Software Configuration Options
Both the Controller and Combination Modules supervise the reading of the
sensors and contain decision-making algorithms to determine which
specimens/samples are positive or negative. Both modules can be arranged
in one of three BacT/ALERT
®
• BacT/ALERT
Controller or Combination Module is not connected to either a bioMérieux
data management computer or a Laboratory Information System (LIS).
Limited data management is available through the system.
2-2 BacT/ALERT® 3D User Manual
3D Select configuration — The BacT/ALERT® 3D
®
3D software configurations:
95248
Page 41

Introduction System Overview
• BacT/ALERT® 3D SelectLink configuration — The BacT/ALERT® 3D
Controller or Combination module is connected directly to an LIS. Limited
data management is available through the system.
• BacT/ALERT
®
3D Signature configuration — The BacT/ALERT® 3D
Controller or Combination Module is connected directly to a bioMérieux
data management computer. Data generated by the BacT/ALERT
®
3D is
managed by extensive data management software produced by
bioMérieux. This data management software provides a high level of
flexibility. Data can easily be stored for a definable length of time, edited,
queried, sorted, and reported. Predefined queries and reports are
available, or they can be customized by the user. A detailed description of
the data management software is found in the appropriate data
management system operator manual.
The software configuration is listed at the top of all screens (except for the
Edit Cell Contents or View Cell Contents screen). The background color will
also indicate the configuration unless there is an error condition or a loaded
positive bottle (see Main Screen Introduction in Chapter 3, Clinical Use, and
Main Screen Introduction in Chapter 4, INDUSTRY Use):
• Blue — BacT/ALERT
• Green — BacT/ALERT® 3D SelectLink configuration
• Gray — BacT/ALERT® 3D Signature configuration
21 CFR Part 11 and HIPAA
The BacT/ALERT® 3D Version B.30 or higher product release provides
compatibility with 21 CFR Part 11 and Health Insurance and Portability and
Accountability Act (HIPAA) requirements.
When installed, the BacT/ALERT
operate in 21 CFR Part 11 mode. If 21 CFR Part 11 mode is enabled, you will
need to enter a user name and password to access all functions available to
the user. See Log In/Out of System — 21 CFR Part 11 Mode in Chapter 10.
To meet HIPAA requirements, a password is required to view and print test
data. These functions include access to the Print, Report Label Entry,
Report Configuration, Calibration Report, and Calibration History
buttons. These buttons are accessed via a Report Selection screen (see
Figure 3-11 for Clinical Use or Figure 4-11 for INDUSTRY Use). See the
topic, Configuring Report Screens in Chapter 7 for further information.
®
3D Select configuration
®
3D instrument can be configured to
BacT/ALERT® 3D User Manual 2-3
95248
Page 42

System Overview Introduction
Theory Of Operation
Principle of Detection
If microorganisms are present in the test sample, carbon dioxide is produced
as the microorganisms metabolize the substrates in the culture medium.
When growth of the microorganisms produces CO
the bottom of each culture bottle changes from dark to light.
A light-emitting diode (LED) projects light onto the sensor. The light reflected
is measured by a photodetector. As more CO
reflected. This information is compared to the initial sensor reading. If there is
a high initial CO
sustained production of CO
content, an unusually high rate of CO2 production, and/or a
2
, the sample is determined to be positive.
2
Mycobacterial growth in the BacT/ALERT® MP Bottles may also be
determined positive by the delta or a slow sustained change of CO
production (Clinical Use).
If the CO2 level does not change significantly after a specified number of days
at optimal conditions, the sample is determined to be negative.
, the color of the sensor in
2
is generated, more light is
2
2
CAUTION: Unloading or manipulating the bottles when not
indicated by the system may interfere with critical bottle
readings.
Electrical Warnings
The BacT/ALERT®3D has been designed and tested in accordance with the
standards listed below and has been supplied in a safe condition. A CB
Certification and Construction File have been established for the apparatus.
• UL 61010-1 (2nd Edition, 2001), Safety Requirements for Electrical
Equipment for Measurement, Control, and Laboratory Use; Part 1: General
Requirements
• IEC 61010-1 (2nd Edition, 2001), Safety Requirements for Electrical
Equipment for Measurement, Control and Laboratory Use
• IEC 61010-2-081 (1st Edition, 2001), Safety Requirements for Electrical
Equipment for Measurement, Control, and Laboratory Use –
Part 2-081: Particular requirements for automatic and semi-automatic
laboratory equipment for analysis and other purposes
• CAN/CSA-C22.2 No. 1010.1-92, Safety Requirements for Electrical
Equipment for Measurement, Control and Laboratory Use
2-4 BacT/ALERT® 3D User Manual
95248
Page 43

Introduction System Overview
WARNING
The user must adhere to the following warnings to ensure safe
operation and to maintain the apparatus in a safe condition:
• Ensure that the BacT/ALERT
®
3D instrument is configured for
the correct voltage at the instrument’s power entry port
before turning on.
• Intentional interruption of the protective conductor inside or
outside the apparatus, or disconnection of the protective
ground terminal, is prohibited.
• Disconnect the apparatus from all voltage sources before it is
opened for any adjustment, replacement, maintenance, or
repair.
• Do not perform any adjustments, maintenance, or repairs of
the opened apparatus while under voltage. If this is
unavoidable, maintenance must be carried out only by a
skilled person who is aware of the hazard involved.
• Whenever it is likely that the BacT/ALERT
®
3D module has
been impaired, it should be rendered inoperative and
secured against any unintended operation by disconnecting
the power cord. If the presence of moisture is evident, turn
off the machine at the breaker before removing the power
cord and fuse.
• Do not forcibly remove the Zip
Forcibly removing the Zip
®
Zip
disk or Zip® drive and may cause the system to lock up.
®
disk from the instrument.
®
disk may cause damage to the
BacT/ALERT® 3D User Manual 2-5
95248
Page 44

System Overview Introduction
WARNING
Contact your local bioMérieux Representative if any of the
following conditions occur:
• System shows visible damage.
• System fails to perform intended measurements.
• System has been subjected to storage under unfavorable
conditions (ex. above 90% humidity, extreme temperatures,
dusty environment, prolonged storage).
• System has been subjected to severe transport stresses.
Electrical Grounding
An electrical ground is required for this instrument. Before installing the
instrument, ensure that a grounded wall receptacle is available for each
module, which is factory equipped with a power supply cord that has the
appropriate grounding plug. It must be plugged into a mating grounding type
wall receptacle in accordance with the National Electrical Code and
applicable local codes and ordinances for this type of installation (see
Figure 2-1)..
WARNING
Do not remove the power cord’s ground prong under any
circumstances.
WARNING
Do not insert any object, other than a Zip® disk, into the Zip®
drive under any circumstances.
2-6 BacT/ALERT® 3D User Manual
95248
Page 45

Introduction System Overview
1 2
Figure 2-1: Electrical Grounding Requirements
1 — US Standard
2 — 220 Volt - European
Electrical and Electronic Recycling
WARNING
This statement only applies to European countries with regard
to the waste electrical and electronic equipment European
directive:
You can play an important role in contributing to reuse,
recycling and other forms of recovery of waste electrical and
electronic equipment. Sorting this type of waste significantly
reduces potential negative effects on the environment and
human health as a result of the presence of hazardous
substances in electrical and electronic equipment.
At the end of the life cycle of this product, do not dispose of the
product as unsorted municipal waste, even if it is
decontaminated. It is imperative that you contact bioMérieux to
assure for its appropriate disposal.
Fuse Replacement
There are no user-serviceable fuses in the BacT/ALERT® 3D system.
Contact your local bioMérieux Instrument Service Representative.
BacT/ALERT® 3D User Manual 2-7
95248
Page 46

System Overview BacT/ALERT® 3D Hardware
BacT/ALERT® 3D Hardware
Each BacT/ALERT® 3D system includes either a Controller Module with at
least one Incubation Module or a Combination Module. Each Controller
Module can direct up to six Incubation Modules. Each Combination Module
contains two incubation drawers in a right or left-handed configuration and
can direct up to three additional Incubation Modules.
Incubation Modules are available in right and left-handed configurations. A
right-handed Incubation Module is designed for placement on the right side of
the Controller or Combination Module and allows bottle access from the left
of an open drawer. Incubation Modules contain four drawers, each with the
capacity to hold up to 60 culture bottles. An Incubation Module can be
configured so one or more drawers are empty, allowing a 60, 120, 180, or
240-bottle capacity.
Mycobacteria Drawers (Clinical Use)
Mycobacteria (MB) and non-MB drawers can co-exist in the same Incubation
Module. MB drawers may be provided directly by bioMérieux or may be
converted from a non-MB configuration in the field. This is accomplished by
disengaging the agitation mechanism so the three racks within each drawer
remain immobile.
Each MB drawer handle has a red MB label also displaying the Incubation
Module number and letter corresponding to that drawer. The MB drawer
faces on the Instrument icon of the Main screen are also labeled.
Note: The BacT/ALERT
status of each drawer.
2-8 BacT/ALERT® 3D User Manual
®
3D software must also be configured to activate the MB
95248
Page 47

BacT/ALERT® 3D Hardware System Overview
Controller Module
1
2
3
4
1 — Operator Panel
2 — Barcode Reader
3 — Backup Drive (Located in Barcode Reader Aperture)
4 — Barcode Reader Strip
5 — Keyboard Drawer
Operator Panel
Displays bottle and system information. Includes a touchscreen overlay so
that the operator may input selections and data by touching the screen.
Barcode Reader Aperture
Houses a removable Barcode Reader that is used to scan bottle barcode
labels to identify bottles and accession/sample barcodes when loading or
unloading. The reader can be removed, if desired.
5
Figure 2-2: Instrument Front View
BacT/ALERT® 3D User Manual 2-9
95248
Page 48

System Overview BacT/ALERT® 3D Hardware
Figure 2-3: Removing the Barcode Reader Through the Barcode Reader Aperture
Backup Drive
Used for system backups and software upgrades. May be either a Zip® Drive
or a USB Port, depending on system configuration. Located in the Barcode
Reader Aperture.
Keyboard Drawer
Houses a pullout working surface, Quick Reference Card, and keyboard.
Quick Reference Card
Describes specific error codes. Located in the keyboard drawer beneath the
Barcode Reader Aperture. Located at the bottom of the Controller Module.
Keyboard
Provides an alternate means of input. Serves as a backup input device
should the Touchscreen or Barcode Reader fail.
2-10 BacT/ALERT® 3D User Manual
95248
Page 49

BacT/ALERT® 3D Hardware System Overview
Accessing the Controller Module Keyboard
There are two different designs of keyboard drawers, Design A and Design B
(see Figure 2-4).
To access the keyboard in a drawer of Design A:
1) Pull the keyboard drawer on the bottom of the Controller Module face out
as far as it will go. A cover is exposed which includes a pocket for the
Reference Card.
2) Grasp the sides of the exposed cover nearest the face of the Controller
Module.
3) Press the metal latches on each side of the drawer to extend fully.
4) Extend the drawer fully, and swivel the cover up.
5) Remove the keyboard, close the cover, and rest the keyboard on the
working surface.
To access the keyboard in a drawer of Design B:
1) Pull the keyboard drawer on the bottom of the Controller Module face out
as far as it will go. A cover is exposed which includes a pocket for the
Reference Card.
2) Grasp the sides of the exposed cover nearest you.
3) Press the metal latches on each side of the drawer to extend fully.
4) Swivel the cover up from the front to expose the keyboard.
5) Remove the keyboard, close the cover, and rest the keyboard on the
working surface.
BacT/ALERT® 3D User Manual 2-11
95248
Page 50

System Overview BacT/ALERT® 3D Hardware
Design A Design B
1
2
Figure 2-4: Accessing the Keyboard and Reference Card
1 — Notch for cord
2 — Press latch on each side to close
When keyboard entry is complete, the keyboard may be returned to its
original position beneath the cover plate. Close the drawer by depressing
both latches on each side of the drawer while pushing it closed.
2-12 BacT/ALERT® 3D User Manual
95248
Page 51

BacT/ALERT® 3D Hardware System Overview
1
2
3
4
5
6
Figure 2-5: Back View Controller Module
1 — Power Switch
2 — Power Connector
3 — Display Switch Assembly
4 — CPU 1 Port
5 — CPU 2 Port
6 — Module Ports (6)
7 — Fans
14
13
12
11
10
9
8
7
8 — Monitor Port
9 — Modem Port
10 — LIS Port (Diagnostic Port)
11 — Printer Port
12 — Comm Port
13 — External Speaker Port
14 — Audible Alarm Speaker
Printer Port
Interface port (parallel) for producing hard copy reports when the instrument
is not in the BacT/ALERT
BacT/ALERT® 3D User Manual 2-13
95248
®
Signature configuration.
Page 52

System Overview BacT/ALERT® 3D Hardware
Audible Alarm Speaker
Specified by the user to flag instrument failures, operator errors, and positive
bottle results.
External Speaker Port
Port for connecting external speakers that are available for order as a
separate kit.
Power Connector
Connector for alternating current (AC) power cord.
Power Switch
Turns the AC Power to the instrument On and Off.
Display Switch Assembly
Used to select the internal or external display device.
CPU 1 Port
Used to connect the bioMérieux data management computer to the Controller
Module (or Combination Module) or to connect two Controller Modules
together.
CPU 2 Port
Used to connect two Controller Modules (or Combination Modules) together.
Module Ports (6)
Connects an Incubation Module to the Controller Module.
Monitor Port
Used to connect an external monitor, if necessary.
Modem Port
Used to connect an external modem to the Controller Module. Allows remote
diagnosis of instrument problems. The external modem box should be in the
“OFF” position when not in use by a bioMérieux Representative.
LIS Port (Diagnostic Port)
Used to connect the Controller Module to a Laboratory Information System
(LIS). See Figure 2-5, Back View Controller Module, on page 2-13.
2-14 BacT/ALERT® 3D User Manual
95248
Page 53

BacT/ALERT® 3D Hardware System Overview
COMM Port
Reserved for future use.
Controller Module Specifications
Electrical Power Services Requirements
• 100/120 Volts (50-60 Hz)
• 220/240 Volts (50-60 Hz)
Power Consumed in Watts
• @115 Volts (72 Watts typical)
• @230 Volts (72 Watts typical)
Heat Dissipated
• 245 BTU/Hr. 2840 maximum
Sound Emission
• 46.4 dB
Instrument Dimensions
• Width — 14 inches (35.6 cm)
• Height — 36 inches (91.4 cm)
• Depth — 24.3 inches (61.7 cm)
• Weight — 91 pounds (57.2 Kg)
Controller Module Environmental Requirements
Operating Temperature Range
• 10°C to 30°C (50°F to 86°F)
Storage Temperature Range
• –17°C to 57°C (0°F to 135°F)
Operating Humidity Range
• 10% to 90% relative humidity, non-condensing.
Storage Humidity Range
• 10% to 90% relative humidity, non-condensing.
BacT/ALERT® 3D User Manual 2-15
95248
Page 54

System Overview BacT/ALERT® 3D Hardware
Maximum Operating and Storage Altitude
• 6562 feet (2000 meters)
Pollution degree 2 in accordance with IEC 664
Overvoltage Category II per IEC 664
Combination Module
4
23
1
Figure 2-6: Front View Combination Module
1 — Keyboard
2 — Printer
3 — Barcode Reader
4 — Operator Panel
5 — Combination Module
6 — Backup Drive
7 — UPS
8 — Modem
5
6
7
8
2-16 BacT/ALERT® 3D User Manual
95248
Page 55

BacT/ALERT® 3D Hardware System Overview
14
13
15
12
1
2
11
3
4
10
9
7
6
5
8
Figure 2-7: Back View Combination Module
1 — CPU 1 and CPU 2 Ports
2 — Power Switch
3 — Power Connector
4 — UPS Port
5 — Module 2 – 5 Ports
6 — Display Switch Assembly
7 — Modem Port
9 — Printer Port
10 — COMM Port
11 — Fan
12 — External Speaker Port
13 — Keyboard Port
14 — Barcode Reader Port
15 — LIS Port
8 — Monitor Port
Operator Panel
Provides a way of displaying information. Includes a touchscreen overlay so
that the operator may input selections and data by touching the screen.
Barcode Reader
The Barcode Reader is external to the Combination Module. The Barcode
Reader, used to scan bottle barcode labels to identify bottles and accession/
BacT/ALERT® 3D User Manual 2-17
95248
Page 56

System Overview BacT/ALERT® 3D Hardware
sample barcodes when loading or unloading, is on a stand separate from the
Combination Module.
Keyboard
The keyboard is external to the Combination Module. It provides an alternate
means of input. Serves as a backup input device should the Touchscreen or
Barcode Reader fail.
UPS
The UPS is external to the Combination Module.
Backup Drive
The Backup Drive allows system backups to be made to either a Zip® disk or
USB Flash Drive.
Drawer
Labeled A or B in the Combination Module. Each drawer contains 3 racks for
a drawer capacity of 60 culture bottles.
Rack
Each rack contains 20 cells.
Cell
Cells are designated with a number from 1 to 60. Each cell holds and
monitors one culture bottle.
Cell Indicator Lamp/Cell Indicator Light
Located next to each cell in the rack.
• Illuminates to indicate which cells are available for loading after the
operator selects the Load Bottles function.
• Illuminates to indicate which cells should be unloaded when the
operator selects one of the Unload Bottles functions.
• Blinks slowly or rapidly to indicate whether a bottle load or unload was
performed correctly or incorrectly, respectively.
Cell Flag
Secures bottles in cells and aids in cell diagnostics and bottle loading and
unloading determinations.
2-18 BacT/ALERT® 3D User Manual
95248
Page 57

BacT/ALERT® 3D Hardware System Overview
1
Figure 2-8: Cell Flag (Clip Removed)
1 — Clip
Thermometer (Not Shown)
Located in back of drawer.
Drawer Indicator Light — Yellow
• Illuminates when the drawer is open and turns off when the drawer is
closed.
• Flashes if the drawer remains open too long or if an error condition is
associated with the drawer.
Drawer Indicator Light — Green
• Illuminates if the drawer or its cells are involved in an operation the
user has selected.
• Flashes in conjunction with the yellow indicator if a drawer is left open
too long.
Printer Port
Interface port (parallel) for producing hard copy reports when the instrument
is not in BacT/ALERT
®
3D Signature configuration.
External Speaker Port
Port for connecting external speakers. External speakers are available for
order as a separate kit.
Power Connector
Connector for Alternating Current (AC) power cord.
Power Switch
Turns the AC power to the Combination Module on and off.
BacT/ALERT® 3D User Manual 2-19
95248
Page 58

System Overview BacT/ALERT® 3D Hardware
Display Switch Assembly
Used to select the internal or external display device.
CPU 1 Port
Used to connect the bioMérieux data management computer to the
Combination Module or to connect to a Controller Module.
CPU 2 Port
Used to connect to a Controller Module (or Combination Module).
Module Port (3)
Connects an Incubation Module to the Combination Module.
Modem Port
Used to connect an external modem to the Combination Module. Allows
remote diagnosis of instrument problems. The external modem box should be
in the “OFF” position when not in use by a bioMérieux Representative.
LIS Port
Used to connect the Combination Module to a Laboratory Information System
(LIS).
Barcode Scanner Port
Used to connect the barcode scanner.
Keyboard Port
Used to connect the keyboard.
Monitor Port
Used to connect an external monitor, if necessary.
COMM Port
Reserved for future use.
Combination Module Specifications
Electrical Power Services Requirements
• 100/120 Volts (50/60 Hz)
• 220/240 Volts (50/60 Hz)
2-20 BacT/ALERT® 3D User Manual
95248
Page 59

BacT/ALERT® 3D Hardware System Overview
Power Consumed in Watts
• @115 Volts — 256 Watts (idle), 640 Watts typical
• @230 Volts — 265 Watts (idle), 640 Watts typical
Heat Dissipated
• 904 BTU/Hr. 2840 maximum
Combination Module Dimensions
• Width — 19.5 inches (49.6 cm)
• Height — 30.8 inches (78.1 cm)
• Depth — 24.5 inches (62.2 cm)
• Unloaded Weight — 200 pounds (90.7 Kg)
• Loaded Weight — 216.5 (98.2Kg)
Sound Emission
• 55.5 dB
Combination Module Environmental Requirements
Operating Temperature Range
• 10°C to 30°C (50°F to 86°F)
Storage Temperature Range
• –17°C to 57°C (0°F to 135°F)
Operating Humidity Range
• 10% to 90% relative humidity, non-condensing.
Storage Humidity Range
• 10% to 90% relative humidity, non-condensing.
Maximum Operating and Storage Altitude
• 6562 feet (2000 meters)
Pollution degree 2 in accordance with IEC 664 (Clinical Use)
Overvoltage Category II per IEC 664 (Clinical Use)
BacT/ALERT® 3D User Manual 2-21
95248
Page 60

System Overview BacT/ALERT® 3D Hardware
Incubation Module
6
5
4
1
2
Drawer
Rack
Cell
3
Figure 2-9: Front View Incubation Module (Right-Handed Configuration)
1 — Green Indicator Lamp
2 — Yellow Indicator Lamp
3 — Drawer Label
4 — Digital Thermometer (located on the inner front panel of drawer B
in right-hand module and drawer A of left-hand module)
5 — Drawer
6 — Drawer Release Latch
Labeled A, B, C, or D in each Incubation Module. Contains 3 racks for a
drawer capacity of 60 culture bottles.
Each rack contains 20 cells.
Designated with a number from 1 to 60. Each cell holds and monitors one
culture bottle.
2-22 BacT/ALERT® 3D User Manual
95248
Page 61

BacT/ALERT® 3D Hardware System Overview
Cell Indicator Lamp/Cell Indicator Light
Located next to each cell in the rack.
• Illuminates to indicate which cells are available for loading after the
operator selects the Load Bottles function.
• Illuminates to indicate which cells should be unloaded when the
operator selects one of the Unload Bottles functions.
• Blinks slowly or rapidly to indicate whether a bottle load or unload was
performed correctly or incorrectly respectively.
Cell Flag
Secures bottles in cells and aids in cell diagnostics and bottle loading and
unloading determinations (see Figure 2-8, Cell Flag (Clip Removed), on
page 2-19).
Drawer Indicator Light — Yellow
• Illuminates when the drawer is open and turns off when the drawer is
closed.
• Flashes if the drawer remains open too long or if an error condition is
associated with the drawer.
Drawer Indicator Light — Green
• Illuminates if the drawer or its cells are involved in an operation the
user has selected.
• Flashes in conjunction with the yellow indicator if a drawer is left open
too long.
BacT/ALERT® 3D User Manual 2-23
95248
Page 62

System Overview BacT/ALERT® 3D Hardware
1
2
3
Figure 2-10: Back View Incubation Module
1 — Power Switch
2 — Power Connector
3 — Controller Module Port
Controller Module Port
Connects the Controller Module to an Incubation Module.
Power Connector
Connector for AC power cord.
Power Switch
Turns the AC power of the Incubation Module On and Off.
2-24 BacT/ALERT® 3D User Manual
95248
Page 63

BacT/ALERT® 3D Hardware System Overview
Incubation Module Specifications
Electrical Power Services Requirements
• 100/120 Volts (50/60Hz)
• 220/240 Volts (50-60 Hz)
Power Consumed in Watts
• @115 Volts — 256 Watts (idle), 640 Watts typical
• @230 Volts — 265 Watts (idle) 640 Watts typical
Heat Dissipated
• 904 BTU/Hr. 2840 maximum
Instrument Dimensions
• Width — 19.5 inches (49.6 cm)
• Height — 36 inches (91.4 cm)
• Depth — 24.3 inches (61.7 cm)
• Unloaded Weight — 262 pounds (118.8 Kg)
• Loaded Weight — 307 (133.8 Kg)
Sound Emission
54.7 dB
Incubation Module Environmental Requirements
Operating Temperature Range
• 10°C to 30°C (50°F to 86°F)
Storage Temperature Range
• –17°C to 57°C (0°F to 135°F)
Operating Humidity Range
• 10% to 90% relative humidity, non-condensing.
Storage Humidity Range
• 10% to 90% relative humidity, non-condensing.
Maximum Operating and Storage Altitude
• 6562 feet (2000 meters)
BacT/ALERT® 3D User Manual 2-25
95248
Page 64

System Overview BacT/ALERT® 3D Hardware
Instrument Installation and Setup (Clinical Use)
CAUTION: Initial installation and setup of the BacT/ALERT® 3D
Microbial Detection System, or of any added Incubation
Module(s) is to be performed only by a bioMérieux Service
Representative.
WARNING
Clinical Use: The BacT/ALERT® 3D has been designed to
minimize risks associated with MB testing. However, to further
reduce the risks of accidental exposure to infectious agents,
additional precautions should be taken. It is strongly
recommended that the instrument be placed in a laboratory
used for the routine culture of M. tuberculosis. For activities
involving the propagation and manipulation of M. tuberculosis
or Mycobacterium species grown in culture, Biosafety Level 3
Practice, Containment Equipment, and Facilities are required
as recommended by CDC and NIH guidelines.
At a minimum, the instrument should be placed in a contained environment
with controlled access which has a tuberculosis exposure control plan. The
locations should have surfaces which can be easily decontaminated using an
appropriate topical disinfectant. The instrument must not be placed in an
open corridor or hallway that is accessible to the general public or the patient
population.
If it is necessary to move or store the BacT/ALERT
modules, contact bioMérieux Service for assistance.
®
3D System or one of its
Instrument Installation and Setup (INDUSTRY Use)
CAUTION: Initial installation and setup of the BacT/ALERT® 3D
Microbial Detection System, or of any added Incubation
Module(s), is to be performed only by your local bioMérieux
Representative.
If it is necessary to move or store the BacT/ALERT® 3D System or one of its
modules, contact your local bioMérieux Representative for assistance.
2-26 BacT/ALERT® 3D User Manual
95248
Page 65

BacT/ALERT® 3D Software System Overview
BacT/ALERT® 3D Software
Operator Panel Screens
The Main screen is normally visible on the Operator Panel, but other screens
may be displayed to perform a variety of functions. Each screen has a screen
ID number in the upper left-hand corner to cross-reference it to the following
descriptions and instructions in this Manual. When selected, the Setup
screen replaces the Main screen.
Screen ID Numbers
• 1.0 Main screen
• 1.1 View Cell Status screen
• 1.2 Change Maximum Test Time screen
• 1.3 User Login screen (21 CFR Part 11 mode only)
• 2.0 Setup screen
• 2.1 Set Date/Time screen
• 2.2 Enable/Disable Module, Drawer, Rack, or Cell screen
• 2.3 Calibrate Module Temperature screen
• 2.4 Calibrate Cell screen
• 2.4.1 Cell Calibration Report Screen
• 2.7 Set Maximum Test Time screen
• 2.8 Set Audible Alarm Options screen
• 2.9 Change Password screen
• 2.11 Select Bottle to Edit/Graph screen
• 2.11.1 Edit Bottle Detail screen
• 2.11.1.1 Edit Test Result
• 2.11.2 Graph Bottle Readings screen
• 2.11.2.1 Bottle Readings screen
• 2.12 Edit Cell Contents screen (2.11.1, 2.11.1.1, 2.11.2 and 2.11.2.1 can
also be accessed from this screen)
• 2.13 View Incubation Module Information screen
• 2.14 Report Label Entry screen *
• 2.15 Report Configuration screen *
• 2.15.1 Report screen *
• 2.16 Backup Management screen
• 2.17 Edit Data Relationships screens
• 2.19 Viewing and Printing Calibration data screen
• 2.20 Bottle Type Customization screen
• 2.21 Report Selection screen*
• 2.21.1 Calibration History screen
• 2.23 User Configuration screen (21 CFR Part 11 mode only)
• 2.23.1 Add User screen (21 CFR Part 11 mode only)
• 2.23.2 Delete User screen (21 CFR Part 11 mode only)
BacT/ALERT® 3D User Manual 2-27
95248
Page 66

System Overview BacT/ALERT® 3D Software
• 2.23.3 Change User Password screen (21 CFR Part 11 mode only)
* Screen's availability dependent upon BacT/ALERT® 3D software
configuration.
Common Screen Elements
Icon
• Graphic symbols used instead of text to convey information and
concepts in the Operator Panel.
Button
• Appear as rectangular shapes which can be pressed to input choices
or activate functions.
• Button function is indicated by icon displayed on button face.
• If a button is gray, then it is disabled and its associated function is
unavailable.
12
Figure 2-11: Button Examples
1 — Enabled Button
2 — Disabled Button
Common System Buttons
Table 2-1: Common System Buttons
Check button – Accept changes, save data or
entries on that particular screen.
Cancel button – Discard changes or entries on
that particular screen to keep the original
values.
Previous Screen Button – Return to the
previous screen.
Next Screen Button – Move to the next
available screen.
2-28 BacT/ALERT® 3D User Manual
95248
Page 67

BacT/ALERT® 3D Software System Overview
Scroll Button
• Used for numeric entry and symbolic or media type selection.
• Consists of two small buttons and an area between them to display
the current value.
• Touching the top or bottom button of the scroll button displays the
next higher or lower value.
• Adjacent scroll buttons provide entry for multi-digit values.
Figure 2-12: Scroll Button
Slidebar Switch
• Turns a function On or Off. The specific function is represented by a
separate icon next to the slidebar.
• Touching the right half of the icon moves the slidebar to the right,
indicating an On selection (1).
• Touching the left half of the icon moves the slidebar to the left,
indicating an Off selection (0).
Figure 2-13: Slidebar Switch
Anchor Display/Scroll Buttons
• Anchor Display top or bottom buttons — Control whether the display
stays anchored on the first or last line of the diagnostic output.
• Line scroll up or down buttons — Move the display area one line at a
time (up or down). This function can also be performed by pressing the
↑ or ↓ key on the keyboard.
• Page scroll up or down buttons — Move the display area one page (up
or down). This function can also be performed by pressing the Page
Up or Page Down key on the keyboard.
• Home/End scroll buttons — Position the display area to the first or last
line of the diagnostic output. This function can also be performed by
pressing the Home or End key on the keyboard.
BacT/ALERT® 3D User Manual 2-29
95248
Page 68

System Overview BacT/ALERT® 3D Software
1 — Anchor Display Top Button
2 — Anchor Display Bottom Button
3 — Line Scroll Up Button
4 — Line Scroll Down Button
5 — Page Scroll Up Button
6 — Page Scroll Down Button
7 — Home Scroll Button
8 — End Scroll Button
Text Entry Field
A text entry field appears as a rectangular box that allows the user to enter
text either manually via the keyboard or by scanning a barcode (see Text/
Data Entry in Chapter 3). The Bottle ID field on the Main screen is an
example of a text field (see Figure 3-1, Main Screen).
1
2
Figure 2-14: Anchor Display/Scroll Buttons
3
4
5
6
7
8
2-30 BacT/ALERT® 3D User Manual
95248
Page 69

BASIC FUNCTIONS (CLINICAL USE)
About This Chapter
This chapter introduces you to the Main screen and provides you with
procedures on how to perform daily basic functions for clinical use.
Chapter Contents
Introduction • 3-2
Monitoring the System • 3-3
Main Screen Introduction • 3-3
Viewing Faults • 3-6
Viewing the Cell Status Screen • 3-7
Text/Data Entry • 3-9
Common Text Fields and Field Limit • 3-9
Using the Barcode Scanner to Enter Data • 3-10
Manually Entering Text into a Data Entry Field (Keyboard) • 3-10
Loading Bottles • 3-11
Loading Bottles • 3-11
Changing the Maximum Test Time — Individual Bottles • 3-15
Handling Anonymous Bottles • 3-16
Unloading Bottles • 3-16
Unloading Bottles • 3-17
Handling Unconfirmed Positive Bottles (False Positives) • 3-19
Accessing the Setup Screen Function Buttons • 3-20
Accessing the Setup Screen • 3-20
Setup Screen Function Buttons • 3-21
Viewing and Printing • 3-23
Introduction • 3-23
Viewing, Printing, and Exporting Test Data • 3-24
Viewing and Printing Bottle Graphs • 3-31
Display Bottle Readings • 3-34
Sending/Requesting LIS Information • 3-39
Sending Results to the LIS • 3-39
Requesting Information from the LIS • 3-39
3
BacT/ALERT® 3D User Manual 3-1
95248
Page 70

Basic Functions (Clinical Use) Introduction
Introduction
The basic functions are those tasks that may be performed during daily
workflow. They include:
• Monitoring system
• Entering data (where applicable)
• Loading and unloading bottles
• Viewing and printing data (printing with the BacT/ALERT® 3D Signature is
performed at the data management computer.)
• LIS interaction with the BacT/ALERT
• Accessing the Setup screen
®
3D SelectLink
3-2 BacT/ALERT® 3D User Manual
95248
Page 71

Monitoring the System Basic Functions (Clinical Use)
Monitoring the System
Main Screen Introduction
The BacT/ALERT® 3D system can be monitored from the Main screen (see
Figure 3-1).
10
1
2
3
4
5
6
7
Figure 3-1: Main Screen
1 — Screen ID Number
2 — Bottle Count Table
3 — Unload Buttons
4 — Manual Send Test Results button (SelectLink only)
5 — Manual Request Test Orders button (SelectLink only)
6 — Logout button (21 CFR Part 11 mode only)
7 — Load Bottles button
8 — Instrument Icon
9 — Current Date/Time
10 — Software Configuration
9
8
BacT/ALERT® 3D User Manual 3-3
95248
Page 72

Basic Functions (Clinical Use) Monitoring the System
CAUTION: If the current date/time displayed at the top of the
screen does not advance, contact bioMérieux Customer Service
immediately.
Background Color
The default background color is determined by the software configuration
(see Software Configuration Options in Chapter 2). The following conditions
will override default background colors:
• A yellow screen indicates that the instrument has detected a positive bottle.
• A red screen indicates that an instrument fault has occurred. Touching the
screen or pressing any key on the keyboard returns a red screen to yellow
or the configuration default color depending on whether positive bottles are
present. The fault code will remain on the screen until the error is cleared.
Instrument Icon
The following information is indicated on the Instrument icon:
• System ID numbers are assigned to both the Controller/Combination
Module and Incubation Modules.
• Combination Module has 2 System ID numbers.
• The programmed optimal temperature (ºC) is displayed for each Incubation
Module.
• Software version number for the BacT/ALERT
®
3D Controller/Combination
Module.
• Disabled or uninstalled components appear diagonally striped in gray on
the screen.
• An entire rack will appear diagonally striped if only one of its cells is
disabled.
3-4 BacT/ALERT® 3D User Manual
95248
Page 73

Monitoring the System Basic Functions (Clinical Use)
1
2
4
5
3
Figure 3-2: Combination Module with an Additional Incubation Module
1 — Combination Module Instrument Icon
2 — Combination Module ID
3 — Software Version
4 — Incubation Module ID
5 — Optimal Temp (ºC)
1
1 — Controller Module Instrument Icon
2 — Optimal Temp (ºC)
3 — Disabled Rack or Cell
4 — Controller ID
5 — Software Version
Bottle Count Table
Located just above the Instrument icon are the Unload buttons and a bottle
count table indicating the number of bottles of each type currently loaded in
the instrument.
2
4
3
5
Figure 3-3: Controller Module with One Incubation Module
BacT/ALERT® 3D User Manual 3-5
95248
Page 74

Basic Functions (Clinical Use) Monitoring the System
3
57
1
2
468
Figure 3-4: Bottle Count Table/Unload Buttons
1 — Total number of mycobacteria (MB) bottles loaded in the system.
2 — Total number of blood or sterile fluid culture (BC) bottles loaded in
the system.
3 — Total number of identified bottles with a positive test status.
4 — Unload Positive Identified Bottles Button
5 — Total number of bottles (identified and anonymous) with a
negative test status.
6 — Unload Negative Bottles Button
7 — Total number of anonymous bottles with a positive test status.
8 — Unload Positive Anonymous Bottles Button
9 — Total number of anonymous bottles with a negative-to-date or
negative test status.
10 — Unload Anonymous Negative or Negative-to-Date Bottles Button
9
10
Viewing Faults
Instrument faults are reported using a numeric code within a red diamond
shape. Fault codes are displayed on the Instrument icon where the fault
condition exists.
Note: Only high priority codes are displayed on the Instrument icon.
1) If the code appears on the Controller Module or the top half of the
Combination Module, then move to Step 4. If the fault code appears on
an Incubation Module or bottom half of the Combination Module, then
continue to Step 2.
2) Touch the Incubation or Combination Module on the Instrument icon
that contains the fault code.
3) The View Cell Status screen appears (see Viewing the Cell Status
Screen on page 3-7 and Figure 3-5).
• Drawer faults appear at the top of the screen.
• Rack faults appear at the left end of the rack display.
3-6 BacT/ALERT® 3D User Manual
95248
Page 75

Monitoring the System Basic Functions (Clinical Use)
• Cell faults appear in the cell display.
4) For a complete list and description of Instrument Fault Codes, see the
topic, Instrument Fault Codes in Chapter 9.
Viewing the Cell Status Screen
The View Cell Status screen is continuously updated with changes that occur
while the screen is active, such as the loading/unloading of bottles, new test
results, and the error status of the drawer, as indicated by fault codes
appearing or disappearing. To view the cell status screen:
1) Display the View Cell Status screen by touching the appropriate
Incubation Module on the Instrument icon (see Figure 3-1, Main Screen,
on page 3-3).
1011
1
2
3
45
Figure 3-5: View Cell Status Screen
1 — Incubation Module
Indicator
2 — Cell Fault Code
3 — Rack Fault Code
4 — Previous Screen Button
5 — Incubation Module
Selection Button
9
8
7
6
6 — Drawer Selection Button
7 — Rack
8 — Cell
9 — Restart Incubation Module
Button
10 — Drawer Fault Code
11 — Drawer Indicator
BacT/ALERT® 3D User Manual 3-7
95248
Page 76

Basic Functions (Clinical Use) Monitoring the System
2) If necessary, use the Incubation Module Selection and Drawer Selection
buttons to display the desired drawer.
Note: If a drawer is not installed, it is not displayed.
3) Press the Previous Screen button to return to the Main screen.
Understanding the View Cell Status Screen Display
For each cell, the cell identification number appears at the top of the circle.
A hollow circle indicates an empty cell, and a solid circle indicates a loaded
cell. The color of the solid circle indicates the bottle status or a cell that is
pending quality control check.
• Black — negative-to-date bottle
• Green — negative bottle
• Yellow — positive bottle
• White — cell is pending quality control check
Loaded cells also contain symbols to indicate the bottle's status:
+Positive
– Negative
* Negative-to-date
~ + Critical determination in progress. (Represents
a bottle that is presently undergoing a critical
determination as to whether it will turn positive
or remain negative or negative-to-date.)
Note: Bottles with a critical determination in progress status will be temporarily
removed from the bottle count table on the Main screen.
Cells loaded with an anonymous bottle contain a ?. If a loaded cell contains
no ?, then the bottle is identified.
Disabled racks and cells are indicated by gray diagonal stripes within their
borders. Disabled drawers are indicated by gray diagonal stripes within the
drawer indicator (see Enabling and Disabling Modules, Drawers, Racks, and
Cells in Chapter 8).
3-8 BacT/ALERT® 3D User Manual
95248
Page 77

Text/Data Entry Basic Functions (Clinical Use)
12
Figure 3-6: Disabled Cell and Drawer
1 — Disabled Cell
2 — Disabled Drawer
Note: If a drawer is disabled, its racks and cells are disabled and turn gray.
Text/Data Entry
Common Text Fields and Field Limit
Bottle ID field
Patient First Name field — may contain up to
20 characters
Patient Last Name field — may contain up to
31 characters
Accession Number field — may contain up to
16 characters
Hospital ID field — may contain up to 22
characters
Note: The field length and initial character type (alpha, numeric, or other) can be
configured for the Accession Number field. The initial character type can be
configured for the Bottle ID field. To configure these fields, contact your local
bioMérieux Representative.
Note: The Hospital ID, Patient First Name, and Patient Last Name fields do not
display with the BacT/ALERT
Note: All fields except the Bottle ID field may be hidden or disabled regardless of
software configuration. To hide or disable a field, contact your local
bioMérieux Representative.
®
3D Signature configuration.
BacT/ALERT® 3D User Manual 3-9
95248
Page 78

Basic Functions (Clinical Use) Text/Data Entry
Note: To change the order of the patient fields, contact your local bioMérieux
Representative.
Using the Barcode Scanner to Enter Data
To scan a bottle or accession barcode:
1) Before scanning the barcode, touch the desired field to place focus on
that field. The field should turn white indicating focus.
2) Rotate the bottle so the bottle ID or accession number barcode is on top.
3) Place the bottle over the barcode strip located in the Barcode Reader
Aperture below the Control Panel of the Controller Module (see
Figure 2-2, Instrument Front View) or at the base of the barcode reader
stand with the Combination Module (see Figure 2-6, Front View
Combination Module).
4) There will be two short beeps when the bottle ID is successfully scanned
into the Bottle ID field.
5) If the Accession Number field is enabled, there will be three short
beeps when the accession number is successfully scanned into the
Accession Number field.
If an Operator Error occurs, a series of beeps will alert the operator to view
the Operator Panel.
If the barcode is not read:
1) Verify the appropriate field has focus.
2) Move the bottle away from the barcode strip and initiate another scan.
Note: The Hospital ID field is a keyboard-entry field only.
Manually Entering Text into a Data Entry Field (Keyboard)
Field text, where applicable, can be entered using the keyboard (see
Figure 2-4, Accessing the Keyboard and Reference Card). If a barcode label
cannot be scanned successfully, the bottle ID or accession number can also
be entered using the keyboard.
See Appendix A for International character entry instructions.
Note: Before entering text into a field, touch the desired field to place focus on that
field. The field should turn white indicating focus.
1) Using the keyboard, enter the desired text.
2) Press the Tab key to move focus to the next field.
3-10 BacT/ALERT® 3D User Manual
95248
Page 79

Loading Bottles Basic Functions (Clinical Use)
Note: If desired, change the cursor location using the keyboard keys. Keys that
provide cursor positioning and editing functions are as follows:
LEFT ARROW Moves cursor left one position
Note: Entering text does not over-write existing text located to the right of the
insertion point.
Note: Entered text defaults to all-uppercase. To change the default, contact your
local bioMérieux Representative.
Loading Bottles
CAUTION: In order to preserve test data integrity, handle only
one bottle at a time. Completely load a bottle according to this
procedure before proceeding to the next bottle.
RIGHT
ARROW
HOME Moves cursor to the start of the text field
END Moves cursor to one position past end of text
DELETE Deletes the character at the current cursor position
BACKSPACE Deletes the character at the current cursor position
Moves cursor right one position
and moves the cursor left one position
Loading Bottles
1) From the Main screen (see Figure 3-1, Main Screen, on page 3-3), press
the Load Bottles button ( ).
BacT/ALERT® 3D User Manual 3-11
95248
Page 80

Basic Functions (Clinical Use) Loading Bottles
The Load Mode screen appears.
1
2
3
4
5
Figure 3-7: Main Screen — Load Mode
1 — Load Bottles Icon
2 — Change Maximum Test
Time Button
3 — Bottle Type Scroll Button
6
5 — Patient First Name Field*
6 — Accession Number Field
7 — Patient Last Name Field*
8 — Hospital ID Field*
7
8
4 — Bottle ID Field
*Available with Select and SelectLink only.
The number of available cells appears at the bottom of each drawer on
the Instrument icon.
Green indicators on Incubation or Combination Module drawers
illuminate drawers that contain available cells.
CAUTION: Inspect each bottle and sensor before loading:
If the sensor is yellow, treat the bottle as a positive culture. If the
bottle is cracked, do not load the bottle.
2) Verify the Bottle ID field appears white, then scan or manually enter the
bottle ID (see Text/Data Entry on page 3-9).
3-12 BacT/ALERT® 3D User Manual
95248
Page 81

Loading Bottles Basic Functions (Clinical Use)
If the field is left blank when a bottle is loaded, then the bottle is
considered anonymously loaded (see Handling Anonymous Bottles on
page 3-16).
3) Verify the correct bottle type is displayed on the Bottle Type scroll
button.
If the Bottle ID field contains data from a generic label, the bottle type
can be manually entered using the Bottle Type scroll button before
inserting the bottle to ensure proper testing of the bottle. The instrument
will also beep continuously to alert the operator that the bottle type needs
to be manually entered. The audible alert can be disabled by calling
bioMérieux for assistance. See Figure 3-7, Main Screen — Load Mode,
on page 3-12.
CAUTION: For best results, manually enter the bottle type when
“GENERIC” is the displayed bottle type. Otherwise, observe the
following:
When a bottle is loaded with a generic or anonymous bottle type,
care must be taken to load BacT/ALERT
®
MP bottles into
drawers labeled MB. The maximum test time assigned to a
generic bottle loaded into an MB drawer is the same time
assigned to the BacT/ALERT
®
MP media type in the Set
Maximum Test Time screen (see Setting the Maximum Test
Time in Chapter 7).
All non-MB bottles must be loaded into drawers labeled BC and
the maximum test time is set to the time specified for the
Unknown bottle type.
BacT/ALERT
media type or loaded anonymously. BacT/ALERT
®
MB Bottles should never be loaded with a Generic
®
MB must be
displayed on the Bottle Type scroll button before loading a
BacT/ALERT® MB bottle.
4) If the Accession Number field is enabled and blank, then continue to
Step 5. If the Accession Number field is disabled, then go to Step 7.
5) Verify the Accession Number field appears white, then scan or
manually enter the accession number.
6) If the fields are displayed and enabled, then manually enter the following
in the order listed: Hospital ID, Patient First Name, and Patient Last
Name.
BacT/ALERT® 3D User Manual 3-13
95248
Page 82

Basic Functions (Clinical Use) Loading Bottles
• To change the order of the Patient First Name and Patient Last
Name fields, contact your local bioMérieux Representative.
• Entries to the Patient First Name and Patient Last Name fields
cannot be made without the Hospital ID entry.
Note: The Hospital ID field is a keyboard-entry only field.
7) The default maximum test time is displayed above the Change Maximum
Test Time button. The scanned bottle's maximum test time can be
adjusted if desired. See Changing the Maximum Test Time — Individual
Bottles on page 3-15.
8) If all drawers are closed, slowly open a drawer with an illuminated
indicator. Available cells will have an illuminated cell indicator light.
9) Insert the bottle, sensor first, into a cell with an illuminated cell indicator
light.
WARNING
An erroneous test result (ex. false negative or false positive)
could occur if a bottle is not fully seated into a cell. When
inserting a bottle, ensure the bottle is fully seated into the cell.
See Appendix C, Best Practices for additional information on
preventing false positives.
10) The cell indicator light blinks slowly to acknowledge the bottle is loaded.
11) Verify that all text fields clear before proceeding.
12) Repeat Step 2 through Step 11 for each remaining bottle. Limit the bottle
load time to two minutes in one area to control entry of room temperature
bottles into racks. Close the drawer to allow temperature to equilibrate
before loading in that area again. Load bottles into different drawers (ex.,
If you have four incubators, use the drawers in all four incubators). See
Appendix C, Best Practices for loading bottles.
CAUTION: If a large number of bottles are loaded into the
Incubation Module at the same time and in the same areas, a
large heat mass loss within the racks may occur. This heat loss
may trigger the acceleration or rate algorithms to erroneously
flag positive.
13) When all bottles are loaded, ensure that all drawers are completely
closed, then press the Check button.
3-14 BacT/ALERT® 3D User Manual
95248
Page 83

Loading Bottles Basic Functions (Clinical Use)
If no operator or bottle loading activity has been recorded in a period of
two minutes, the instrument will terminate the Load Bottle operation.
Operator activity includes:
• pressing keys on the keyboard
• scanning barcodes
• touching the Operator Panel
• loading or unloading bottles
14) If applicable, enter the patient and accession data associated with the
loaded bottles into the bioMérieux data management computer.
Changing the Maximum Test Time — Individual Bottles
1) From the Load screen, press the Change Maximum Test Time button
( ) after scanning the bottle barcode.
The Change Maximum Test Time screen overlays and disables the Load
screen.
1
Figure 3-8: Change Maximum Test Time Screen
2
1 — Bottle ID Field
2 — Max Test Time Scroll Buttons
2) Verify the bottle ID matches that of the bottle for which you wish to
change the maximum test time.
3) Adjust the maximum test time in days using the Max Test Time scroll
buttons.
4) Press the Check button to accept the changes, or the Cancel button to
retain the original setting.
The system returns to the Load Mode screen.
BacT/ALERT® 3D User Manual 3-15
95248
Page 84

Basic Functions (Clinical Use) Unloading Bottles
Note: Changing the maximum test time of an individual bottle during loading does
not affect any other bottles of the same type.
Note: The maximum test time of an individual bottle can also be changed from the
Edit Bottle Detail screen after loading (see Viewing/Editing Bottle Data in
Chapter 5).
Handling Anonymous Bottles
Bottles loaded into the Incubation Module without accessing the Load Bottles
function on the Main screen are referred to as Anonymous bottles because
they are not associated with a bottle ID.
CAUTION: BacT/ALERT® MB (Mycobacteria Blood) Bottles
should never be loaded anonymously. Appropriate testing of
anonymous bottles occurs only when BacT/ALERT
are loaded into drawers labeled MB and non-MB bottles are
loaded into drawers labeled BC.
®
MP bottles
The maximum test time of anonymous bottles loaded into MB
drawers is the same time specified for the BacT/ALERT
media type on the Set Maximum Test Time screen.
Anonymous bottles loaded into BC drawers are assigned the
standard default algorithm.
Anonymous bottles should be removed and identified as specified in
Unloading Bottles on page 3-16, or identified using the Edit Bottle Detail
screen (see Editing Bottle Details Using the Edit Bottle Detail Screen in
Chapter 5).
Unloading Bottles
The BacT/ALERT® 3D signals which type of bottles are ready for unloading
by enabling the appropriate Unload button.
CAUTION: In order to preserve test data integrity, handle only
one bottle at a time. It is important to complete the procedure for
each bottle before proceeding to the next bottle.
®
MP
3-16 BacT/ALERT® 3D User Manual
95248
Page 85

Unloading Bottles Basic Functions (Clinical Use)
Unloading Bottles
1) Generate an Unload report (see Viewing, Printing, and Exporting Test
Data on page 3-24).
2) From the Main screen (see Figure 3-1, Main Screen, on page 3-3), press
the appropriate Unload button.
• The Unload Mode screen appears (see Figure 3-9).
• Green indicators illuminate on drawers containing bottles of the
selected unload type.
1
2
3
4
5
Figure 3-9: Main Screen — Unload Mode
1 — Unload Bottles Icon
2 — Unload Buttons
3 — Bottle Type Scroll Button
4 — Bottle ID Field
6
7
8
5 — Patient First Name Field
6 — Accession Number Field
7 — Patient Last Name Field
8 — Hospital ID Field
3) Open the indicated drawer. When the indicated drawer is open, cell
indicator lights light up next to all bottles in the selected category.
4) Remove one of the bottles indicated. Wait for the cell light to blink slowly
to acknowledge the removal of the bottle.
5) If the bottle was identified when loaded:
• The bottle ID and bottle type will appear on the Unload Mode screen;
however, the accession number, hospital ID, patient first name, and
BacT/ALERT® 3D User Manual 3-17
95248
Page 86

Basic Functions (Clinical Use) Unloading Bottles
patient last name will appear in disabled text fields, if information is
available.
a. It is not necessary to re-scan the bottle ID; however, doing so will
verify that bottle's identity.
b. If fields are blank or need editing, then use the Edit Data
Relationships function (see Editing Data Relationships in Chapter 5).
6) Go to Step 8.
7) If the bottle was anonymously loaded (the Bottle ID field is blank), scan
or manually enter the bottle ID.
a. Identify the bottle by entering the bottle ID, bottle type, accession
number, hospital ID, and patient first and last name as performed in
the procedure, Loading Bottles on page 3-11.
• Scanning the bottle ID successfully results in two short beeps.
• Scanning the accession number successfully results in three short
beeps.
• When identifying anonymous bottles, information entered in the Bottle
ID, Bottle Type, Accession Number, Hospital ID, Patient First
Name, and Patient Last Name fields, and with the Bottle Type scroll
button, is associated with the unloaded bottle once the next bottle is
unloaded or the Check button is pressed.
b. If the bottle is to be reloaded, immediately return the bottle to the cell
with the slowly blinking cell indicator light before unloading another
bottle.
WARNING
Bottles with a critical determination in progress will be
temporarily removed from the bottle count table on the Main
screen.
c. Do not reload the bottle if its status is negative, positive, or if it is
necessary to use the bottle label to enter information into the
bioMérieux data management computer.
CAUTION: Reloading bottles anonymously that were previously
loaded will result in duplicate bottle records.
3-18 BacT/ALERT® 3D User Manual
95248
Page 87

Unloading Bottles Basic Functions (Clinical Use)
8) Repeat Step 3 through Step 5 for the remaining bottles to be unloaded.
Limit the bottle unload time to no more than two minutes in one area.
Close the drawer to allow temperature to equilibrate before unloading
from the area again. See Appendix C, Best Practices for unloading
bottles and preventing false positives.
CAUTION: If a large number of bottles are unloaded from the
Incubation Module at the same time and in the same areas, a
large heat mass loss within the racks may occur. This heat loss
may trigger the acceleration or rate algorithms to erroneously
flag positive.
9) When finished unloading bottles, ensure that all drawers are completely
closed.
10) Press the Check button on the Unload Mode screen.
11) Verify that the bottles listed on the Unload report were unloaded.
12) Where applicable, enter patient and accession data associated with the
unloaded bottles into the bioMérieux data management computer.
13) Reload any previously Anonymous negative-to-date bottles.
If no operator or bottle loading activity has been recorded in a period of
two minutes, the instrument will terminate the Unload Bottle operation.
Operator activity includes:
• pressing keys on the keyboard
• scanning barcodes
• touching the Operator Panel
• loading or unloading bottles
Handling Unconfirmed Positive Bottles (False Positives)
If a smear of a positive bottle reveals no microorganisms, the bottle should be
subcultured and reloaded into the instrument via the Load Bottles function
(see Loading Bottles on page 3-11).
Note: If a bottle is reloaded into the instrument, its status will revert to negative-to-
date once a reading has been taken (maximum time – 10 minutes).
If growth appears on the subculture, edit the bottle's status to Positive on the
Edit Test Result screen which is accessed from the Edit Bottle Detail screen
(see Edit Test Result Button in Chapter 5), and unload the now positive
bottle.
BacT/ALERT® 3D User Manual 3-19
95248
Page 88

Basic Functions (Clinical Use) Accessing the Setup Screen Function Buttons
Note: Results that have been manually changed to negative or positive via the Edit
Bottle Detail screen (see Editing Bottle Details Using the Edit Bottle Detail
Screen in Chapter 5), will be marked on the report with a stick figure ( ).
Note: If a bottle is positive (set manually, or positive by any other reason) and then
is manually changed to negative-to-date, the stick figure ( ) will not appear.
Accessing the Setup Screen Function Buttons
To view and print data, print graphs, and perform all of the editing,
configuration, and maintenance functions of the system, you must first
access the Setup screen function buttons.
Accessing the Setup Screen
1) From the Main screen (see Figure 3-1, Main Screen, on page 3-3), press
the Next Screen button ( ).
The Setup screen appears.
1
2
Figure 3-10: Setup Screen
1 — Password Entry Keypad
2 — Function Buttons
3 — Key Symbol Button
4 — Padlock Icon
3-20 BacT/ALERT® 3D User Manual
4
3
95248
Page 89

Accessing the Setup Screen Function Buttons Basic Functions (Clinical Use)
2) Enter a valid password using the 1 – 4 buttons. Until a valid password is
entered and accepted, the Padlock icon will appear in the closed
position.
Note: Acceptable passwords consist of any combination of the numbers 1 to 4 and
have a maximum length of eight characters.
Note: Instruments are shipped with a password of 1234. For information on
changing the default password, see Changing the System Password in
Chapter 7.
3) Press the Key Symbol button to accept the password.
4) After a valid password is accepted, the Padlock icon changes to the full
open position and the function buttons become enabled.
Note: To correct an error made while entering a password, press the Key Symbol
button and re-enter the password.
Note: If more than 8 characters are entered, the Password Entry buttons will
become inactive and turn gray. Press the Key Symbol button and re-enter
the password.
Inactivity Timeout for all Setup Screens
While you are in the Setup screen (see Figure 3-10, Setup Screen) or one of
its sub-menus, an inactivity timeout will occur (within a period of time
configured by your bioMérieux Service Representative) if you do not perform
one of the following actions:
• press a screen or keyboard button
• scan a barcode
• load or unload a bottle
If an inactivity timeout occurs, the instrument display reverts from the
currently displayed screen to the Main screen. Any pending function is
cancelled as if the Cancel button on each successive screen was pressed.
Note: If a timeout occurs, it is possible that partially entered information will be lost.
Note: The inactivity timeout feature is disabled while a red operator error is
displayed on the screen.
Setup Screen Function Buttons
Set Date/Time button (see Setting and Formatting the System Date and Time in Chapter 8)
BacT/ALERT® 3D User Manual 3-21
95248
Page 90

Basic Functions (Clinical Use) Accessing the Setup Screen Function Buttons
Enable/Disable Module, Drawer, Rack, or Cell button (see Enabling and Disabling
Modules, Drawers, Racks, and Cells in Chapter 8)
Calibrate Module Temperature button (see Adjusting an Incubation or Combination
Module’s Temperature in Chapter 8)
Set Maximum Test Time button (see Setting the Maximum Test Time in Chapter 7)
Set Audible Alarm Options button (see Setting the Audible Alarms in Chapter 7)
Change Password button (see Changing the System Password in Chapter 7)
Select Bottle to Edit/Graph button (see Selecting Bottles Using the Select Bottle to Edit/
Graph Button in Chapter 5)
Edit Cell Contents button (see Selecting Bottles Using the Edit Cell Contents Button in
Chapter 5)
Calibrate Cell button (see Calibrating an Instrument Cell in Chapter 8)
View Incubation Module Information button (see Viewing Incubation Module
Information in Chapter 8)
Backup Management button (see Initiating Manual Backup in Chapter 7)
Edit Data Relationships button (see Initiating the Edit Data Relationships Function in
Chapter 5)
Report button — Select and SelectLink only (see Viewing, Printing, and Exporting Test
Data on page 3-24)
Configure Users button — Button only appears in 21 CFR Part 11 mode (see Configuring
Users — 21 CFR Part 11 Mode in Chapter 10)
Bottle Processing/Customization button (For use only with instruction from bioMérieux)
3-22 BacT/ALERT® 3D User Manual
95248
Page 91

Viewing and Printing Basic Functions (Clinical Use)
Viewing and Printing
Introduction
Viewing Bottle Data
The following bottle information can be viewed by accessing the Edit Bottle
Detail screen as described in Editing Test Data (see Viewing/Editing Bottle
Data in Chapter 5):
• Bottle ID
• Accession number
• Hospital ID (where applicable)
• Patient first and last name (where applicable)
•Cell ID
• Maximum test time
• Bottle type
• Date/time loaded
• Date/time unloaded
• Date/time of last bottle reading
•Test time
•Test result
• Algorithm type
• How determined/positivity index
Viewing/Printing Reports
With a BacT/ALERT® 3D Signature configuration, reports are viewed and
printed at the data management computer.
With the BacT/ALERT® 3D Select or SelectLink configurations, reports are
viewed and printed by using the Report button as described in the topic,
Viewing, Printing, and Exporting Test Data on page 3-24.
Viewing/Printing Graphs
Bottle Graphs can be viewed and printed on the BacT/ALERT® 3D Select
and SelectLink configurations as described in the topic, Viewing and Printing
Bottle Graphs on page 3-31. For the Signature configuration, graphics can be
viewed as described in the topic, Viewing and Printing Bottle Graphs on page
3-31, or at the data management computer. Since the BacT/ALERT
Signature configuration is not connected to a printer, you must use the data
management computer to print a graph.
Note: The print feature can be made unavailable regardless of software
configuration. When the feature is unavailable, the Print buttons do not
BacT/ALERT® 3D User Manual 3-23
95248
®
3D
Page 92

Basic Functions (Clinical Use) Viewing and Printing
display. To make the print feature unavailable, contact your local bioMérieux
Representative.
Using the Print Screen Function
You can print the current screen on the instrument by pressing Ctrl + P on
the keyboard.
Note: Select and SelectLink configurations only.
Viewing, Printing, and Exporting Test Data
Note: If you have a BacT/ALERT® 3D Signature configuration, you must use the
data management computer to view and print reports.
1) From the Setup screen (see Figure 3-10, Setup Screen, on page 3-20),
press the Report button ( ).
Note: The Report button is not available with the BacT/ALERT
configuration.
The Report Selection screen appears.
1
2
3
4
Figure 3-11: Report Selection Screen
1 — Display Report 1 Button
2 — Display Report 2 Button
4 — Previous Screen Button
5 — Check Button
3 — Display Report 3 Button
®
3D Signature
5
3-24 BacT/ALERT® 3D User Manual
95248
Page 93

Viewing and Printing Basic Functions (Clinical Use)
2) Press the Display Report 1, 2, or 3 button that is configured for the
information desired.
The Report screen will display. There are three default report
configurations:
• Display Report 1 button — Generates the Load Report screen with
1st Load Time as the primary sort, and Accession Number as the
secondary sort. Report has section breaks based on 1st Load Time.
• Display Report 2 button — Generates the Status Report screen with
Accession Number as the primary sort, and Bottle Type as the
secondary sort. Report has section breaks based on Accession
Number.
• Display Report 3 button — Generates the Unload Report screen with
Loaded as the primary sort, and Test Result as the secondary sort.
Report has section breaks based on Loaded and Test Result.
See Configuring Report Contents in Chapter 7 for examples of the Load,
Status, and Unload Report Configuration screens.
Note: Data from the last 1,920 bottles are displayed each time a Report screen is
accessed. The report screens come configured with default settings, but the
report configurations can be changed to display different data and to sort the
data differently (see Configuring Report Contents in Chapter 7).
BacT/ALERT® 3D User Manual 3-25
95248
Page 94

Basic Functions (Clinical Use) Viewing and Printing
21
20
19
1
2
3
4
5
6
7
8
9
10
Figure 3-12: Sample Report Screen
1 — Section Scroll Up Button
2 — Relative Record Scroll
Indicator
3 — Relative Record Scroll Bar
4 — Find Text Button
5 — Save Button
6 — Print Current Section
Button
7 — Print Report Button
8 — Cancel Print Button
9 — Previous Screen Button
10 — Section Scroll Down
Button
18
17
16
15
14
11 12 13
11 — Line Scroll Down Button
12 — Page Scroll Down Button
13 — End Scroll Button
14 — Gap Detection Indicator
15 — Report Data Lines
16 — Stick Figure
17 — Current Section Line
18 — Home Scroll Button
19 — Page Scroll Up Button
20 — Report Title
21 — Line Scroll Up Button
Note: Results that have been manually changed to negative or positive (see Editing
Bottle Details Using the Edit Bottle Detail Screen in Chapter 5) will be marked
on the report with a stick figure ( ).
Note: If a bottle is positive (set manually, or positive by any other reason) and then
is manually changed to negative-to-date, the stick figure ( ) will not appear.
3-26 BacT/ALERT® 3D User Manual
95248
Page 95

Viewing and Printing Basic Functions (Clinical Use)
Note: Bottles with an Instrument Fault Code 80 will be marked on the report with a
Gap Detection indicator (!) next to the result. If a negative bottle has an
Instrument Fault Code 80 while it is being unloaded, the Gap Detection
indicator will remain on the report (see Instrument Fault Codes in Chapter 9).
Note: The Current Group Line will always display the group that is associated with
the first displayed data record. It is always highlighted for easy reference. If
there are no section breaks in the displayed report, this line will become the
first line of data records displayed and reference highlighting will not occur.
3) To scroll up or down a group, press the appropriate Group scroll button.
Note: The Group scroll buttons are disabled if there are no section breaks in the
displayed report or if there are no available sections in the indicated direction.
4) To scroll up or down a data line, press the appropriate Line scroll button
or press the appropriate ↑ or ↓ key on the keyboard.
Note: The Line scroll buttons are disabled if there are no data lines in the indicated
direction.
5) To scroll up or down a data page, press the appropriate Page scroll
button or press the appropriate Page Up or Page Down key on the
keyboard.
Note: The Page scroll buttons are disabled if there are no data lines in the indicated
direction.
6) To scroll up to the oldest data line (the first data record in the report),
press the Home scroll button or press the Home key on the keyboard.
Note: The Home scroll button is disabled if there are no report data lines in the
indicated direction.
7) To scroll down to the newest data line (the last data record in the report),
press the End scroll button or press the End key on the keyboard.
Note: The End scroll button is disabled if there are no report data lines in the
indicated direction.
8) To move to a relative record position, press the Relative Record scroll
bar above/below the Relative Record Indicator.
Note: The Relative Record Indicator is sized proportionally to the amount of records
in the report.
Note: The Relative Record scroll bar is disabled if the Relative Record Indicator is
the same size as the scroll bar and all the data records in the report are
displayed.
BacT/ALERT® 3D User Manual 3-27
95248
Page 96

Basic Functions (Clinical Use) Viewing and Printing
9) To print the report, press the appropriate Print button:
• Pressing the Print Report button prints all records in the database
(with a maximum of 1,920 records).
• Pressing the Print Current Group button prints the Current Group
Line and all data records associated with the group.
Note: The Print buttons are only available if a printer is configured for the system.
To configure a printer for the system, contact your local bioMérieux
Representative.
10) While the report is printing:
• All Print and Save buttons are disabled.
• The operator may, however, view and scroll through the displayed
report, search for text in the displayed report, or exit the Report screen
and perform other operations.
•The Cancel Print button becomes available.
11) Press the Cancel Print button to stop sending data to the printer and to
empty the queue of any data waiting to be sent to the printer.
Note: Once the cancellation has been completed, the Print and Save buttons will
become enabled, and the Cancel Print button will be disabled.
12) To specify a text string and initiate a search of the report data for the
specified text, press the Find Text button. The Find Text screen will
appear.
3-28 BacT/ALERT® 3D User Manual
95248
Page 97

Viewing and Printing Basic Functions (Clinical Use)
1
Figure 3-13: Find Text Screen
1 — Find Text Field
Use the keyboard to enter the text you wish to seek, and press the
Check button to return to the Report screen. Pressing the Cancel button
will cancel the search request and return to the Report screen.
Note: The search will be performed upon returning to the report screen starting
from the top of the report. The record that contains the first occurrence of the
text will be scrolled to the first line of data displayed and the text itself will be
highlighted for reference.
If no occurrence of the specified text is found, the data displayed will not
change and no reference highlighting will occur.
13) To find the next instance of the specified text, press the F3 key on the
keyboard.
Note: If no new instance of the text is found the data displayed, and the highlighted
text will remain the same.
14) To save the displayed report to a text file, press the Save button.
BacT/ALERT® 3D User Manual 3-29
95248
Page 98

Basic Functions (Clinical Use) Viewing and Printing
The Save To File screen appears.
1
Figure 3-14: Save to File Screen
1 — File Name Field
Note: The default file name and path will appear automatically in the File Name
field. Use the keyboard to change the name of the file.
Note: All reports are saved to the pathname, D:\REPORTS, either using the default
file name or a specified file name. All reports are automatically given an
extension of .TXT.
15) Place the backup media in the backup drive.
Note: Backup media can either be a Zip
®
disk or a USB flash drive.
16) Press the Check button to initiate the save and return to the Report
screen. Pressing the Cancel button will cancel the save request and
return to the Report screen.
Note: The Print buttons will be disabled while the save is taking place. Once the
save has completed, the Print buttons will be enabled.
WARNING
Do not insert any object, other than a Zip® disk, into the Zip®
drive under any circumstances.
3-30 BacT/ALERT® 3D User Manual
95248
Page 99

Viewing and Printing Basic Functions (Clinical Use)
CAUTION: Do not forcibly remove the Zip® disk from the
instrument. Forcibly removing the Zip
to the Zip
®
disk or Zip® drive and may cause the system to lock
up.
17) Press the Previous Screen button to return to the screen from which the
Report screen was accessed.
Viewing and Printing Bottle Graphs
Note: If you have a BacT/ALERT® 3D Signature configuration, you must use the
data management computer to print a bottle graph since the
BacT/ALERT
1) Access the Setup screen and enter a valid password (see Accessing the
Setup Screen Function Buttons on page 3-20).
2) Press the Select Bottle to Edit/Graph button ( ).
®
3D Signature configuration is not connected to a printer.
®
disk may cause damage
BacT/ALERT® 3D User Manual 3-31
95248
Page 100

Basic Functions (Clinical Use) Viewing and Printing
The Select Bottle to Edit/Graph screen overlays and disables the Setup
screen.
9
1
8
2
7
3
4
Figure 3-15: Select Bottle to Edit/Graph Screen
5
1 — Incubation Module Scroll
Button
2 — Cell Scroll Buttons
3 — Setup Screen (Disabled)
4 — Previous Screen Button
6
6 — Check Button
7 — Graph Bottle Readings
Button
8 — Drawer Scroll Button
9 — Bottle ID Field
5 — Cancel Button
3) If you know the bottle ID of the bottle whose readings you wish to graph,
then enter the bottle ID in the Bottle ID field (see Text/Data Entry on
page 3-9) and go to Step 5. If you know the cell location but not bottle ID,
then continue to Step 4.
Note: Only the last 1,920 bottles loaded can be retrieved. Any other entry in the
Bottle ID field is invalid and will cause an Operator Error 940 (see Operator
Error Codes in Chapter 9).
4) Adjust the Incubation Module (1–6), Drawer (A–D), and Cell (1–60)
scroll buttons to select the cell location of the bottle whose readings you
wish to graph. The cell location defaults to Module 1, Drawer A, Cell 1
(1A01).
3-32 BacT/ALERT® 3D User Manual
95248
 Loading...
Loading...