Page 1

Agilent MassHunter Workstation –
Data Acquisition for 6400 Series Triple
Quadrupole LC/MS
Familiarization Guide
For In Vitro Diagnostic Use
Before you begin 3
Prepare your system 3
Prepare to acquire data 4
Exercise 1 – Develop an acquisition method 6
Task 1. Enter acquisition parameters and acquire data 6
Task 2. Determine precursor ion masses 11
Task 3. Find optimum fragmentor voltage for maximum response 14
Task 4. Determine product ion masses 24
Task 5. Find optimum collision energy for MRM acquisition 30
Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition
data file or an MRM method 33
Task 1. Create a batch file from an existing MRM data file 33
Task 2. Print a report in the Quantitative Analysis program 36
Task 3. Create a Dynamic MRM method using Update dMRM 38
Task 4. Create a Dynamic MRM method from an MRM method 40
Exercise 3 – Create a Triggered Dynamic MRM acquisition method 42
Task 1. Create a Triggered Dynamic MRM method from a Dynamic
MRM method manually 42
Task 2. Add/Modify compounds in an existing database 45
Task 3. Create a Triggered Dynamic MRM method from an existing
database 55
Exercise 4 – Optimize Acquisition parameters 59
Task 1. Use Optimizer to optimize acquisition parameters 59
Page 2

2 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Use the exercises in this guide to learn how to use the Agilent 6460 Triple
Quad Mass Spectrometer (Model K6460). You can do these exercises with the
demo data files, SulfaDrugs, shipped with the system (in the Data folder of
your Qualitative Analysis installation disk), or with data you acquire.
In Exercise 1, you learn how to determine the best acquisition settings for
analyzing your compounds of interest. These instructions help you understand
not only how to set up a worklist to optimize instrument parameters for best
sensitivity in acquisition, but also how to use the Agilent MassHunter
Qualitative Analysis program to identify parameter values producing optimum
signal response. You can also learn about the Qualitative Analysis program by
using the Qualitative Analysis Familiarization Guide or the Qualitative
Analysis online Help.
In Exercise 2, you learn how to use either an acquired data file or the
Quantitative Analysis report results to update a Dynamic MRM method. This
method allows you to easily set up a Dynamic MRM method.
In Exercise 3, you learn how to create a triggered Dynamic MRM method.
In Exercise 4, you learn how to use two programs to optimize parameters.
Agilent MassHunter Optimizer helps you optimize acquisition parameters.
Specifically, it automates the selection of the best precursor ion and the
fragmentor voltage for the most abundant precursor ion, selection of the best
product ions, and optimization of collision energy values for each transition
for a list of compounds you specify.
Each task is presented in a table with three columns:
• Steps – Use these general instructions to proceed on your own to explore
the program.
• Detailed Instructions – Use these if you need help or prefer to use a
step-by-step learning process.
• Comments – Read these to learn tips and additional information about each
step in the exercise.
NOTE
See the Concepts Guide to learn more about how the triple quadrupole mass spectrometer
works and why the fragmentor and collision energy voltages are important. For
background information, see Chapter 3, “Triple Quadrupole MS and Sensitivity”, in the
Concepts Guide. See the online Help for detailed information on how the program works.
Page 3

Before you begin
Prepare your system
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 3
Before you begin
Before you begin, you need to check that your system is ready. If you plan to
acquire data, you also need to set up the instrument.
Prepare your system
1 Check that:
• The Data Acquisition program has been installed.
• The LC modules and the K6460 Mass Spectrometer have been configured.
• The performance has been verified.
• The system has been turned on.
If these actions have not yet been done, see the Installation Guide for your
instrument.
2 Copy the data files to your PC.
Copy the folder named SulfaDrugs in the Data folder on your Qualitative
Analysis installation disk to any location on your hard disk. This folder
contains all the data files needed for this exercise.
NOTE
Do not re-use the sulfa drug data files already on your system unless you know that you
copied them from the originals on the disk and you are the only one using them. Data files
that are already on the system may contain processed results, leading to different behavior
during the exercises in this guide.
Page 4

Before you begin
Prepare to acquire data
4 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Prepare to acquire data
If you do not intend to acquire data but want to learn how to use the
Qualitative Analysis program for method development, you can skip this step,
which tells you how to prepare the demo sample. You then do those tasks that
show you how to use the Qualitative Analysis program with the sulfa drug
data files shipped with the system.
Parts List The exercise in this guide uses this equipment and materials:
• Agilent K1260 Infinity LC modules: well-plate sampler, binary pump,
thermostatted column compartment, DAD
• Zorbax column (see Table 1 on page 4)
• A 1 ng/μL concentration of the sulfa mix sample (prepared in this step)
1 Prepare the LC solvents.
For the A channel, add 1 mL of 5M ammonium formate to a 1-liter reservoir
filled with HPLC-grade water.
For the B channel, add 1 mL of 5M ammonium formate to a 1-liter reservoir
filled with 90:10 acetonitrile and HPLC-grade water.
2 Prepare the sample.
a Add 10 μL of the sulfa mix from one of the ampoules (500 μL) to 990 μL
of solvent A in a 2 mL glass sample vial so that the final concentration is
1 ng/μL.
b Cap the vial and place in a sample location in the autosampler.
3 Set up the LC column.
Use the column from Table 1. Other columns and instrument parameters
may be used in these exercises, but some parameters may need adjustment,
and the results will differ.
Table 1 Zorbax column
Triple Quadrupole Column Description Particle
Size
Pore Size Part
Number
6460 RRHD Eclipse Plus
C18.2.1 mm x 50 mm
1.8 µm 95Å 959757-902
Page 5

Before you begin
Prepare to acquire data
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 5
4 Set the column temperature to 60C. Lower temperatures may be used;
however, the retention times will be longer, and the pump pressure may
exceed the limit of some LC systems.
The Electrospray LC Demo Sample (P/N 59987-20033) contains five ampoules
with 100 ng/μL each of sulfamethizole (M+H)+ = 271, sulfamethazine (M+H)+ =
279, sulfachloropyridazine (M+H)+ = 285, and sulfadimethoxine (M+H)+ = 311.
Sulfamethizole Sulfamethazine Sulfachloropyridazine Sulfadimethoxine
NOTE
Determining optimal parameter values for acquiring sample compound data requires that
the Triple Quadrupole instrument already be tuned on the Tuning Mix calibrant ions. Before
proceeding with this exercise, make sure you have used Checktune or Autotune to verify
that calibrant ions each have the proper mass assignment, peak width, and signal
intensity.
See the Quick Start Guide, Installation Guide or online Help for instructions on tuning the
instrument.
Page 6

Exercise 1 – Develop an acquisition method
Task 1. Enter acquisition parameters and acquire data
6 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Exercise 1 – Develop an acquisition method
For this exercise you analyze a mixture of four sulfonamide compounds. These
tasks show you how to manually select the acquisition parameters including
using the Qualitative Analysis program to analyze the data files. You can
instead use the automated process to select some of the acquisition
parameters. See “Exercise 4 – Optimize Acquisition parameters” on page 59 to
learn how to automate this process.
Task 1. Enter acquisition parameters and acquire data
In this exercise, you enter the conditions for the analysis of the sulfa drug mix.
l
Steps Detailed Instructions Comments
1 Enter LC parameters appropriate
for sulfa drug mix.
See Table 2.
a Double-click the Data Acquisition
icon.
b Make sure that Acquisition appears
as the selection in the Context text
box.
If Tune is the selection, click
Acquisition from the Context
dropdown menu in the Combo bar.
c Enter the LC parameters listed in the
Table 2.
• The Data Acquisition window
appears. See Figure 1 on page 8.
Table 2 LC parameters for sulfa drug mix
Parameter LC Parameter
PUMP
• Flowrate 800 µL/min
• Solvent A 5 mM ammonium formate in water
• Solvent B 5 mM ammonium formate in 90:10 acetonitrile:water
• Gradient (min - %B) 0 min - 13%
1.80 min - 60%
2 min - 60%
Page 7

Exercise 1 – Develop an acquisition method
Task 1. Enter acquisition parameters and acquire data
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 7
• Stop Time 2.5 min
• Post Time 3.0 min
INJECTOR
• Inj. Vol. 2.0 µL
• Injection Standard
• Draw Position 0.0 mm
UV DETECTOR
• Ch A 254 nm (4 nm BW on DAD)
• REF A (DAD only) 400 nm (80 nm BW)
COL THERM
• Temp 60 °C for the K6460 with an AJS ESI source
40 °C for ESI source
Table 2 LC parameters for sulfa drug mix (continued)
Parameter LC Parameter
Page 8

Exercise 1 – Develop an acquisition method
Task 1. Enter acquisition parameters and acquire data
8 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Figure 1 MassHunter Workstation – Data Acquisition window
Steps Detailed Instructions Comments
2 Enter MS parameters appropriate
for sulfa drug mix and save the
method as iiiMS2Scantest.m,
where iii are your initials.
See Table 3 on page 9.
a Click the QQQ tab in the Method
Editor window.
b Select MS2 Scan from the Scan Type
list in the Time Segments table.
c Enter the other MS parameters as
listed in Table 3. These parameters are
in either the Acquisition or the Source
tabs.
d Save the method as
iiiMS2Scantest.m, where iii are your
initials.
Page 9

Exercise 1 – Develop an acquisition method
Task 1. Enter acquisition parameters and acquire data
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 9
Figure 2 Select Scan Type of MS2 Scan in the QQQ tab
Table 3 MS parameters for sulfa drug mix
Parameter Value (ESI) Value (AJS ESI)
• Inlet ESI (positive polarity) AJS ESI (positive polarity)
• Scan Type MS2 Scan MS2 Scan
• Delta EMV (+) 400 V 200 V
• Mass Range 100 to 400 100 to 400
• Cell Accelerator Voltage 7 V 7 V
• Gas Temp 350 °C 350 °C
• Gas Flow 12 L/min 10 L/min
• Nebulizer 50 psi 35 psi
• Sheath Gas Temperature not applicable 400 °C
• Sheath Gas Flow not applicable 12 L/min
• Nozzle Voltage not applicable 0 V
• Capillary Voltage positive 4000 V 4000 V
• Fragmentor 100 V 100 V
Page 10

Exercise 1 – Develop an acquisition method
Task 1. Enter acquisition parameters and acquire data
10 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Steps Detailed Instructions Comments
3 Acquire data (optional).
• Set up a one-line worklist with
the method you just created.
• Name the data file
iiisulfamix01.d, where iii are
your initials.
• Designate a directory path to
hold your data files and method.
a If necessary, click View > Worklist to
display the Worklist window.
b Click Worklist > Worklist Run
Parameters. Verify that the
parameters are set properly. Click OK.
c Click Worklist > Add Multiple
Samples.
d Type iii
sulfamix01.d as the
data file name
e Select iiiMS2Scantest.m as the
method name.
f Click the Sample Position tab.
g Select the Autosampler, Well-plate or
Vial Tray.
h In the graphic, select a single position.
Click OK.
i In the Worklist window, mark the
check box to the left of the sample.
j Click the Start Worklist Run icon in
the main toolbar, the Run Worklist
icon in the Worklist toolbar or click the
Worklist > Run command.
• The Worklist window is tabbed with
the Method Editor window by
default. Click the Worklist tab to
show the Worklist window.
• The Number of samples is set to 1.
• You have just acquired a full scan
MS data file to see what ions are
being formed from the sample.
• This step is optional because you
can perform the next step with an
example data file that comes with
the program. If you prefer, you can
create your own data file as
described in this step.
Page 11

Exercise 1 – Develop an acquisition method
Task 2. Determine precursor ion masses
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 11
Task 2. Determine precursor ion masses
In this exercise, you determine the precursor ions for each of the sulfa drugs
in the acquired data file.
Steps Detailed Instructions Comments
1 Open the acquired data file.
• In the Qualitative Analysis
program, open either the
example file, sulfamix01.d, or
the data file you created in “Task
1. Enter acquisition parameters
and acquire data” on page 6.
a Double-click the Qualitative Analysis
icon.
The program displays the “Open Data
File” dialog box.
• When you open the sulfa drug
directory after installation, the Load
result data (lower left corner)
check box is grayed out.
• If you see the check box marked,
this means that the data file(s)
already contains results. Clear this
check box before opening the file.
Page 12

Exercise 1 – Develop an acquisition method
Task 2. Determine precursor ion masses
12 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
b Do one of the following:
• Select the example data file
sulfamix01.d, and click Open.
• Select the data file you created in
“Task 1. Enter acquisition
parameters and acquire data” on
page 6, and click Open.
By default, the system displays the
Total Ion Chromatogram (TIC).
• The figure below shows the default
layout.
• The Qualitative Analysis program
displays a newly opened data file
with the same layout and display
settings used for the previous data
file. Therefore, you MUST make
sure to return to the default
settings for this exercise.
Before you begin, make sure that all
previous settings are returned to their
default values:
• Restore default layouts
• Click Configuration > Window
Layouts > Restore Default
Layout.
• Make sure the method is default.m.
(see title bar)
• Click Method > Open.
• Select default.m, and click Open.
• Return display options to default
settings.
• In the Configuration menu, click
each of the Display Options
commands.
• Click Default, and then click OK.
Or...
• Restore the General layout.
• Click Configuration > Configure
for Workflow > General.
• Click OK.
• (optional) Save method changes
if needed.
• Return display options to default
settings.
• In the Configuration menu, click
each of the Display Options
commands.
• Click Default, and then OK.
Steps Detailed Instructions Comments
Page 13

Exercise 1 – Develop an acquisition method
Task 2. Determine precursor ion masses
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 13
2 Determine precursor ion masses
for all four peaks.
• You have determined them
correctly if you find the values
are similar to those shown in
this table:
• If you acquired the data file
using the Agilent Jet Stream
Technology, the retention times
may be different.
• The sulfamix01.d data file was
acquired with a different column
so your retention times are
different.
• Close the data file after finding
the precursor ion masses.
a In the Chromatogram Results window,
make sure that the Range Select icon
in the toolbar is on.
b Click the left mouse button and drag
the cursor across the first peak to
produce a shaded region, as in the
figure below.
c Right-click the shaded area, and click
Extract MS Spectrum from the
shortcut menu.
.
• The system displays an averaged
spectrum across the peak in the
MS Spectrum Results window.
• The precursor mass of the first
compound, sulfamethizole, is
determined to be m/z 270.9.
• To obtain a single scan, doubleclick the apex of the peak.
d Repeat step a through step c for the
other compounds.
The precursor ion masses should
match those in the table in step 2.
e Click File > Close Data File.
f When asked if you want to save the
results, click No.
• Some compounds form sodium
(Na) and/or potassium (K) adducts
as well, corresponding to M + 23
and M + 39 masses respectively.
Seeing these masses along with
the M + H can make for an easy
confirmation of which ion is the
pseudo-molecular ion (M + H)+.
Steps Detailed Instructions Comments
Page 14

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
14 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Task 3. Find optimum fragmentor voltage for maximum response
Task 3 shows you how to carry out the optimization for fragmentor voltage by
creating selected ion-monitoring experiments for each compound within a
method and setting up multiple methods with varying fragmentor voltages.
Steps Detailed Instructions Comments
1 Set up six methods for six different
fragmentor voltages.
• Change to a SIM experiment.
• Use 60, 80, 100, 140, 180 and
220 volts as the fragmentor
voltages for the six methods.
• Save the methods as
iiiMS2SIMxxx.m, where iii are
your initials and xxx is the
voltage.
a In the Scan Type dropdown list, click
MS2 SIM.
Page 15

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 15
b In the Acquisition tab, enter the
Compound Name and Mass
(precursor ion mass) for
sulfadimethoxine (311 m/z).
c Right-click anywhere in the Scan
segments section, and click Add Row.
d Type the Compound Name and the
Mass for sulfachloropyridazine (285
m/z).
e Repeat steps c and d for
sulfamethazine (279 m/z) and
sulfamethizole (271 m/z).
f Save the method as iiiMS2SIM140.m,
where iii are your initials.
g Change the fragmentor voltage to 60,
and save the method as
iiiMS2SIM060, where iii are your
initials.
h Repeat step g for voltages 80, 100, 180
and 220, saving the methods as
iiiMS2SIM080, iiiMS2SIM100,
iiiMS2SIM180 and iiiMS2SIM220,
where iii are your initials.
• With the MS2SIM Scan Type set, a
different set of columns appears in
the Acquisition window.
• The Instrument Control and Data
Acquisition program creates a SIM
experiment for each compound
mass, starting with a default
fragmentor voltage of 140. See the
example below.
Steps Detailed Instructions Comments
Page 16

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
16 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
2 Set up and run the worklist
(optional).
• Set up six samples with Sample
Name SulfaDrugMix to inject 1ul
from vials 1-6 or the ones you
choose.
• Specify the data files as
iiiSulfaSIMxxx.d, where iii are
your initials and xxx is the
voltage.
a Click the Worklist icon if necessary to
make sure the worklist is visible.
b Click Worklist > New to start a new
worklist. You do not need to save the
last worklist.
c To set up the run, right-click the upper
left corner of the worklist, and click
Worklist Run Parameters.
d Type the paths for the method and
data files.
e Type the information for the 60 voltage
run.
f Click Worklist > Add Sample.
Another sample is added to the
Worklist. Add five samples to the
worklist for voltages 80-220.
g Mark the checkbox to the left of the
Sample Name for each of the six
samples.
• This step is optional because you
can use data files shipped with the
system to perform many of the
tasks in this exercise.
h Start the worklist.
• Click Worklist > Run.
• Click the icon in the main
toolbar.
• Click the icon in the worklist
toolbar.
• Note that the program only runs
those samples that are marked with
a checkmark.
• You can also run the worklist in
locked mode by clicking the lock
button in the main toolbar (the icon
looks like this icon when it is
locked).
Steps Detailed Instructions Comments
Page 17

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 17
3 Set up a qualitative method to
view the EIC data automatically.
• Open the data file
Sulfa_SIM60.d or your own
iiiSulfa_SIM60.d, where iii are
your initials.
• In the Method Editor, add in the
EICs corresponding to the
precursor ion masses of 271,
279, 285, and 311.
• Save the method as iiiExercise1,
where “iii” are your initials.
a Click File > Open Data File.
The system displays the Open Data
File dialog box
b Select either Sulfa_SIM60.d or
iiiSulfa_SIM60.d, and click Open.
c Click Method > Method Editor or
View > Method Editor.
The system displays the Method
Editor window.
• The Qualitative Analysis program
should be open. If not, see
“Double-click the Qualitative
Analysis icon.” on page 11.
• By default, the Method Editor is a
floating window. The window is
docked for these images. See the
online Help for more information on
floating and docking windows.
Steps Detailed Instructions Comments
Page 18

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
18 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
d If necessary, click Define
Chromatograms in the Chromatogram
section of the Method Explorer.
e To delete the BPC chromatogram, click
Delete in the Method Editor window.
f Select EIC for the Chromatogram
Definition Type,
g In the MS Chromatogram tab, make
sure MS Level is set to All and Scans
is set to All scan types.
h Clear the Do cycle sum check box.
i Type
271 as the m/z value.
j Click Add.
k Repeat steps i and j for the other
precursor ions,
279, 285 and
311.
l Click Method > Save As. The system
opens the Save As dialog box
m Save the method as iiiExercise 1.m.
n Click Save.
• The default Method Editor list
selection after installation is
Integrate (MS).
• You can also select Define
Chromatograms from the Method
Items list in the Method Editor
window.
• When you are defining
chromatograms, instead of deleting
the Defined chromatogram, you can
select EIC. Then, you enter the m/z
value and click the Change button.
Steps Detailed Instructions Comments
Page 19

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 19
4 Extract the chromatogram for the
data file and view the results.
• Make sure you can see all five
chromatograms, the TIC and
four EICs.
a Click the Run button on the Method
Editor toolbar to run the Extract
Defined Chromatogram command.
b To see the TIC and four EICs, click the
arrow next to the Maximum Number
of List Panes icon in the
Chromatogram Results toolbar, as
shown in the example below.
c Select 5 to view five chromatograms
simultaneously.
The system displays chromatogram
results as shown below.
• You can also click the
Chromatograms > Extract Defined
Chromatograms command to
extract the defined chromatograms.
Steps Detailed Instructions Comments
Page 20

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
20 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
5 Extract the remaining ion
chromatograms automatically.
• Extract Defined Chromatograms
should be the default action for
Assign File Open Actions.
• Open the remaining data files,
Sulfa_SIM80.d through
Sulfa_SIM220.d.
• Close the Method Explorer.
a Select File Open Actions from the
General section in the Method
Explorer.
b Make sure that Actions to be run list
only contains Extract Defined
Chromatograms.
c Click File > Open Data File.
The system displays the Open Data
File dialog box.
d Select the data files to be opened,
Sulfa_SIM80.d through
Sulfa_SIM220.d.
e Mark the Run ‘File Open’ actions
from selected method check box.
(lower left corner)
• The Qualitative Analysis Method
Editor lets you define actions to be
performed automatically upon
opening a data file(s).
Steps Detailed Instructions Comments
Page 21

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 21
f Click Open.
The Qualitative Analysis program
displays all the EICs for all the data
files selected.
g To close the Method Explorer, Method
Editor and MS Spectrum Results
windows, click the X in the upper right
corner of each window.
• You can instead click View >
Method Explorer, View > Method
Editor, and View > MS Spectrum
Results.
Steps Detailed Instructions Comments
Mark this check box.
Page 22

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
22 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Steps Detailed Instructions Comments
Page 23

Exercise 1 – Develop an acquisition method
Task 3. Find optimum fragmentor voltage for maximum response
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 23
6 Select the fragmentor voltage that
produces the maximum response
for each of the precursor ions.
• Close the data files after you
determine the optimum voltage.
a In the Data Navigator window,
highlight the EICs for 271.0 m/z.
b Click the Show only the highlighted
items icon, .
Only the 271 m/z check boxes are
now marked.
c Look at the relative intensities of each
peak to determine which fragmentor
voltage setting will be best to use for
the 271 precursor.
d Repeat step a through step c for the
other three base peaks or precursor
ions.
e Click File > Close All.
f Click No in the Save dialog box.
• You press the Ctrl key to be able to
select multiple objects from the
Data Navigator window.
• You press the Shift key to be able to
select a group of objects.
• A fragmentor voltage of 100 should
be sufficient for each precursor ion.
• You can now determine the product
ions that are available for the
multiple-reaction monitoring
experiments to maximize sensitivity
for the analysis.
• You click Method > Save or
Method > Save As to save the
method changes.
• Click an EIC in the Data Navigator
window to change which chromatogram
is labeled in the Chromatogram Results
window. When the chromatogram label
color matches the color of the
chromatogram that has the highest
intensity, you use the Fragmentor
voltage that was used for that file.
Steps Detailed Instructions Comments
You can overlay the
chromatograms by
clicking the Overlaid
mode icon in the
Chromatogram Results
toolbar.
Page 24

Exercise 1 – Develop an acquisition method
Task 4. Determine product ion masses
24 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Task 4. Determine product ion masses
In this part of the method development, we will use three collision energies to
determine the best fragment ions to use for the eventual Multiple Reaction
Monitoring (MRMs) acquisition.
Steps Detailed Instructions Comments
1 Set up three product ion
acquisition methods and acquire
data.
• Use the MS parameters in the
example below, but change the
Fragmentor voltage to the
optimum voltage you determined
in the previous task.
• Save methods as iiiSulfamix
PI_xx.m, where iii are your
initials and xx is the collision
energy.
a Click the QQQ tab in the Method
Editor pane.
b Select
Product Ion in the Scan Type
combo box to scan each precursor ion
for all its product ions.
c Enter all MS parameters as listed in
the example below, making sure the
Collision Energy is set to
15 and the
Fragmentor voltage is set to the
optimum voltage determined in Task 3.
d Save the method as iiiSulfamix
PI_15.m.
e Repeat step c and step d for collision
energies of 30 and 45.
• When you change the Scan Type in
the Time Segments table, the Scan
segments table is reset. If you want
to copy the Scan segments to the
new Scan segments table,
highlight all of the lines in the Scan
segments table and then right-click
the Scan segments table and click
Copy. After you select a new Scan
Type, right-click the Scan segments
table and click Paste from
Clipboard.
• You cannot copy and paste the
Scan segments table between all
Scan Types.
2 Set up and run the worklist
(optional).
• Specify the data files as
iiiSulfamix PI_xx.d, where iii
are your initials and xx is the
collision energy.
a Click the Worklist tab.
b Add three samples to the worklist for
collision energies 15, 30 and 45.
c Mark the check box to the left of the
Sample Name for each sample you are
adding.
d Click Worklist > Run.
• This step is optional because you
can determine the product ion
masses from the data files shipped
with the system.
• Use the instructions in Step 2 of
Task 3 to set up the worklist.
Page 25

Exercise 1 – Develop an acquisition method
Task 4. Determine product ion masses
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 25
3 Set up a qualitative method to
integrate and extract product ion
spectra.
• Use the data files
SulfamixPI_xx.d, where xx is
the collision energy, or your own
data files, iiiSulfamixPI_xx.d.
• Open Method Explorer and
Method Editor.
• Use TICs set up for MS/MS,
product ion and each of the
precursor ions 271, 279, 285,
311.
• Make sure the MS/MS
integrator has been selected and
the maximum number of peaks
has been limited to the largest
100 peaks.
• Add the ability to integrate and
extract peak spectra to the file
actions run upon data opening.
• Save the changes to the current
method.
a Click the Open Data File icon in the
toolbar.
b Select SulfamixPI_15.d.
c Clear the Run File Open Actions from
Specified Method check box, and
click Open.
d Make sure the Method Explorer and
the Method Editor windows are
displayed; otherwise, click the Method
Explorer and then Method Editor
icons.
e In the Chromatogram section in the
Method Explorer window, select
Define Chromatograms.
f Delete any existing chromatograms in
the Defined Chromatograms list.
g Select TIC from the Type list in the
Define chromatograms section.
h For MS level, select MS/MS.
i Mark the Do cycle sum check box.
j For Scans, select Product ion.
k For Precursor ion m/z, type
271.
l Click the Add button.
m Repeat steps j and k for each ion.
• The Qualitative Analysis program
should already be open and contain
iiiexercise 1.m as the method.
Steps Detailed Instructions Comments
Page 26
Exercise 1 – Develop an acquisition method
Task 4. Determine product ion masses
26 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
n From the Method Explorer in the
Chromatogram section, click
Integrate (MS/MS).
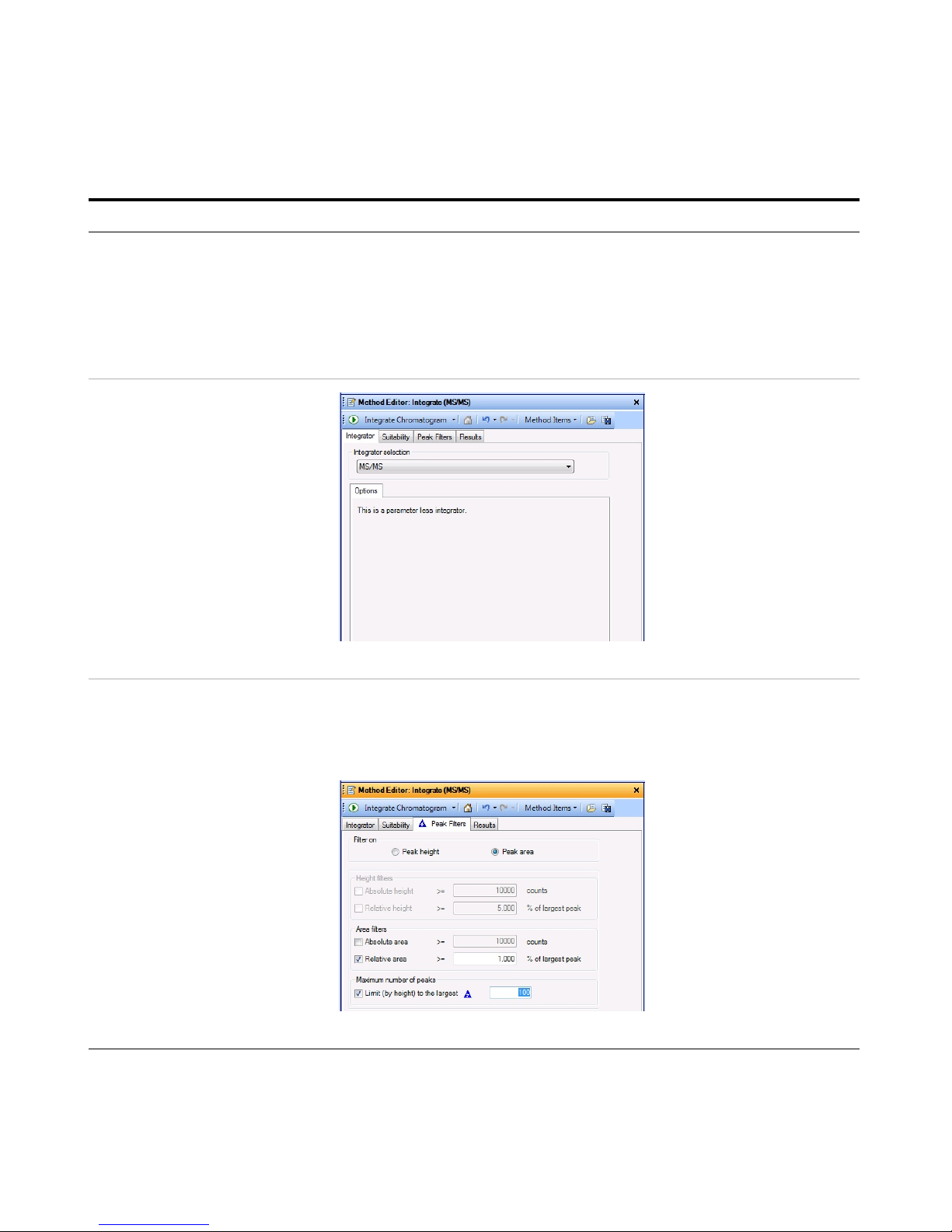
o Select MS/MS as the Integrator
selection, if necessary.
• These data files contain MS/MS
data, so you need to modify the
parameters in the Integrate
(MS/MS) section. If the data file
contained only MS data, you would
need to modify the parameters in
the Integrate (MS) section.
Figure 3 Integrate (MS/MS) > Integrator Tab
p Click the Peak Filters tab. Make sure
that the Limit (by height) to the
largest check box is marked and set to
the value
100 as shown below.
Figure 4 Integrate (MS/MS) > Peak Filters tab
Steps Detailed Instructions Comments
Page 27

Exercise 1 – Develop an acquisition method
Task 4. Determine product ion masses
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 27
q Click General in Method Explorer, and
then click File Open Actions.
r Select Integrate and extract peak
spectra from the Available actions list
and click to add this to
Actions to be run.
Figure 5 General > File Open Actions tab
s To apply the changes to the current
method, iiiexercise1.m, click the Save
Method icon. You can also click
Method > Save.
4 Run the qualitative method on the
current data file.
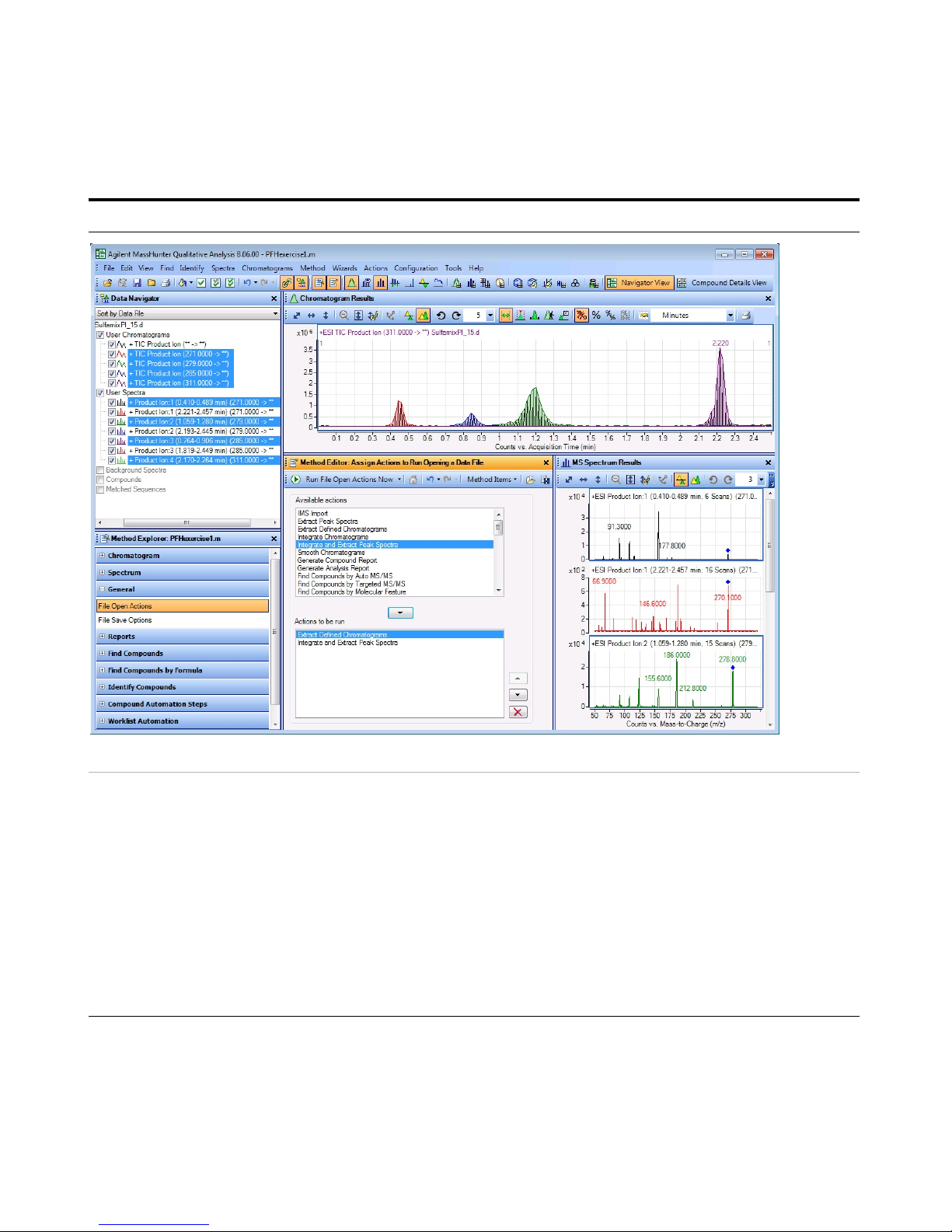
• In the Method Editor toolbar, click the
Run button, . When the Assign
Actions to Run Opening A Data File
section is displayed, the Actions to be
run list is run.
• The program first extracts the
product ion chromatograms for
each precursor ion in the data file.
• Next, it finds the largest peak in the
total ion chromatograms, and
integrates and extracts peak
spectra from each integrated peak.
• See Figure 6 on page 28.
Steps Detailed Instructions Comments
Page 28
Exercise 1 – Develop an acquisition method
Task 4. Determine product ion masses
28 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Figure 6 Results for integration and extraction of peak spectra.
5 Run the ‘File Open’ actions on the
remaining product ion data files.
• Use either the example files,
Sulfamix PI_xx.d, or the data
files you acquired in step 2.
a Click File > Open Data File.
The system displays the Open Data
File dialog box.
b Hold the Ctrl key and do one of these:
• Select the two data files Sulfamix
PI_30.d, and Sulfamix PI_45.d.
• Select the data files you acquired in
step 2.
c Mark the Run ‘File Open’ actions
from selected method check box in
the Open Data File dialog box, and
click Open.
• After the data files open, the
Qualitative Analysis method first
extracts the product ion
chromatograms for each precursor
ion.
• Next, it integrates each total ion
chromatogram and extracts peak
spectra from each integrated peak.
Steps Detailed Instructions Comments
Page 29
Exercise 1 – Develop an acquisition method
Task 4. Determine product ion masses
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 29
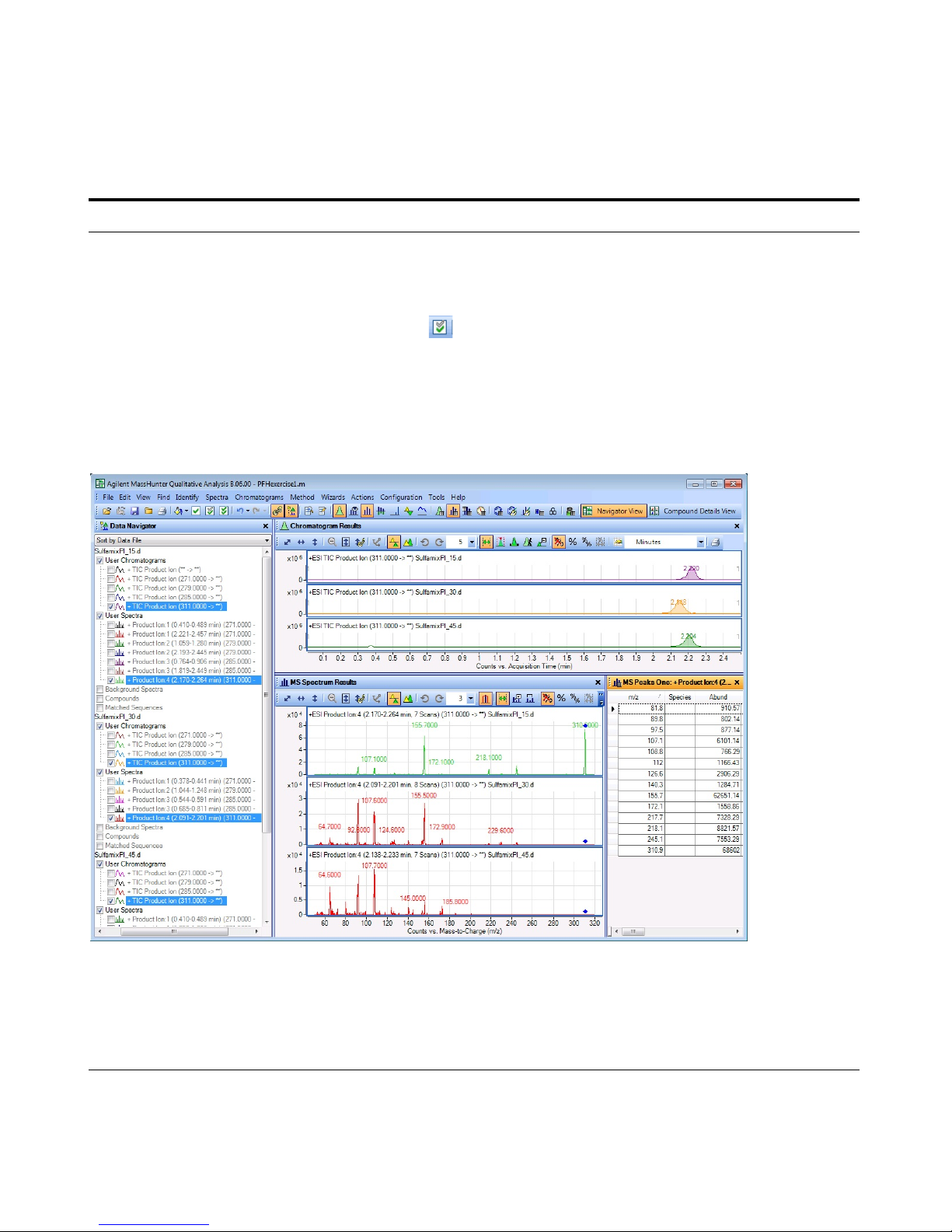
6 Identify product ions.
• View each set of TICs and
spectra individually (e.g., 271
m/z first).
• Close the data files.
a In the Data Navigator, select the TICs
and spectra for the 271 m/z precursor
ion.
b Click the Show only the highlighted
items icon, .
c Click View > MS Spectrum Peak List 1.
d Examine the spectra to see which
fragment ions are produced at which
collision energies.
e Repeat steps a to d until all the
product ions are identified.
f Click the Close Data File icon in the
main toolbar, and click Close when the
dialog box containing the list of data
files pops up.
• The m/z 155.7 product ion is the
most abundant of any product ion
and the highest signal is recorded
at 15 V. This means that a good
choice for the MRM for
sulfamethizole would be 271.0 >
155.7 when the collision energy is
around 15 V.
• The peak may not be labeled if the
peak is too wide.
•
• The product ions appear to be:
Sulfamethizole-271.0 > 155.7
Sulfamethazine-279.0 > 185.7
Sulfachloropyridazine-285.0 > 155.7
Sulfadimethoxine-311.0 > 155.7
Steps Detailed Instructions Comments
Page 30

Exercise 1 – Develop an acquisition method
Task 5. Find optimum collision energy for MRM acquisition
30 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Task 5. Find optimum collision energy for MRM acquisition
In this task, you set up MRM acquisition methods for the sulfa drugs for
different collision energies. By examining the spectra and comparing peak
intensities, you determine the optimal collision energy settings for the
compounds.
Steps Detailed Instructions Comments
1 Set up three MRM acquisition
methods.
• Use all the MS parameters in the
example below except for the
collision energy value.
• Use collision energies of 10, 15
and 20.
• Save methods as iiiSulfamix
MRM_xx.m, where iii are your
initials and xx is the collision
energy.
a Click the QQQ tab.
b Set Scan Type to MRM.
c Enter all MS parameters shown in the
example below except for the collision
energy value.
d In the collision energy column, type
10 for each compound.
e Save the method as iiiSulfamix
MRM_10.m.
f Repeat step d and step e for collision
energies of 15, 20, 25, 30 and 35
saving the methods as iiiSulfamix
MRM_xx.m, where iii are your initials
and xx is the collision energy.
• Because the largest peaks were
produced with a collision energy of
15 in the previous exercise, you will
look at only those collision energies
to either side of 15.
Page 31
Exercise 1 – Develop an acquisition method
Task 5. Find optimum collision energy for MRM acquisition
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 31
2 Set up and run the worklist
(optional).
• Specify the data files as
iiiSulfamix MRM_xx.d, where
iii are your initials and xx is the
collision energy.
a Click the Worklist tab to make the
worklist visible.
b Add six samples to the worklist for
collision energies 10, 15, 20, 25, 30, 35.
c Mark the check box to the left of the
Sample Name for each of the three
samples.
d Click Worklist > Run.
• This step is optional because you
can use the six example data files
in the next step.
3 Compare the compound transition
intensities at different collision
energies.
• Open the MRM data files:
SulfamixMRM_10.d
SulfamixMRM_15.d
SulfamixMRM_20.d
SulfamixMRM_25.d
SulfamixMRM_30.d
SulfamixMRM_35.d
• Set the MRM chromatogram
extraction parameters as shown
at right for all transitions.
• Disable the TICs for clarity and
examine the peak intensities.
• Compare the intensities of each
compound transition obtained at
one collision energy with the
same compound transition
obtained at another collision
energy. (Do this in Overlaid
Mode with all the MRM
chromatograms.)
• Close the data files but don’t
save results.
• Refer to Table 4 on page 32 for
optimal method settings for
each compound.
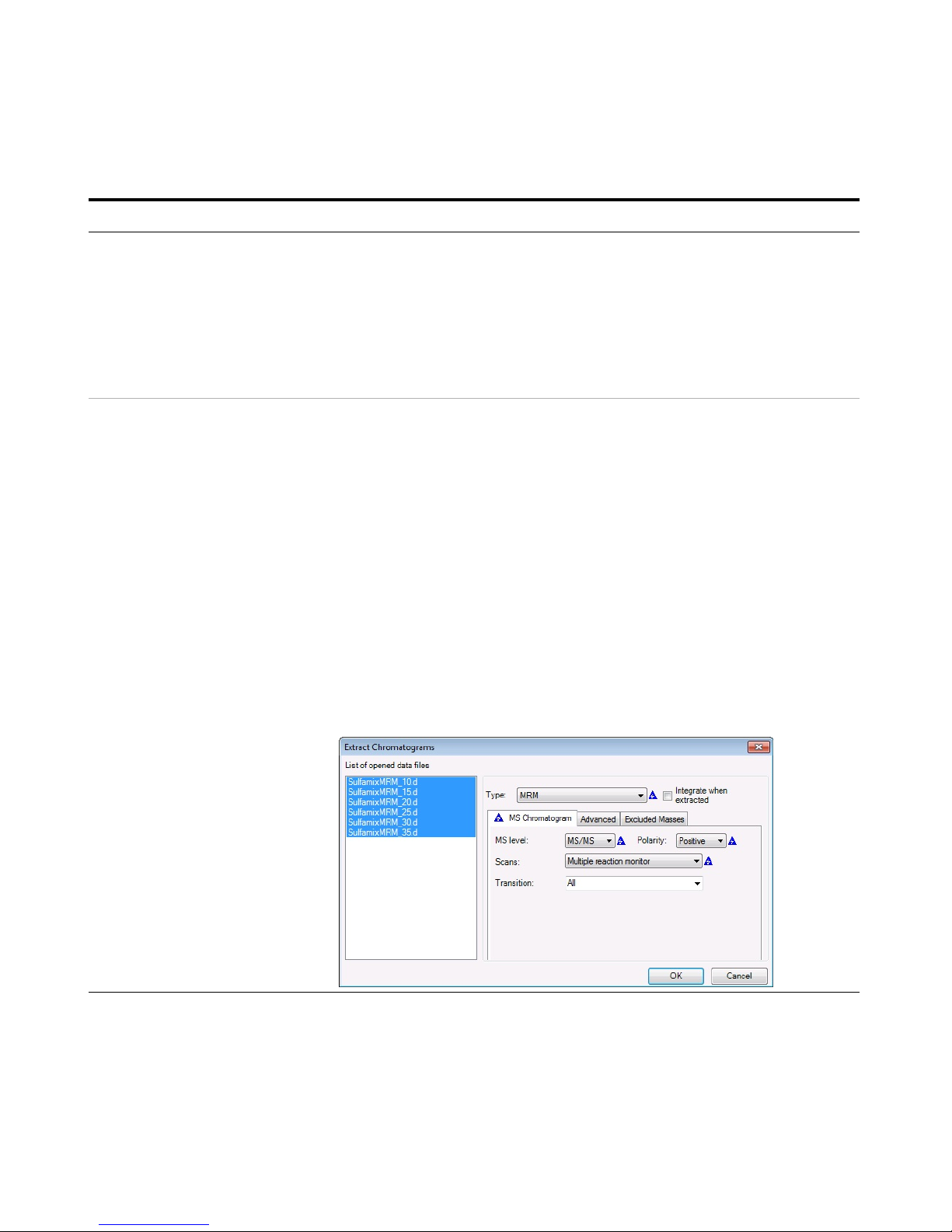
a Open the Qualitative Analysis
program.
b Clear the Run ‘File Open’ actions...
check box.
c Open the MRM data files in the
Qualitative Analysis program.
d Right-click the Chromatogram Results
window, and click Extract
Chromatograms from the shortcut
menu.
e To select all data files, click the last
file while holding down the Shift key.
f Enter the parameters as listed in the
example below, and click OK.
g Clear the TIC check boxes to make the
MRM chromatograms easier to view.
• Why a spectrum for MRM? It’s a
feature of the program to show
spectra even for MRM experiments
and can be quite handy for
comparing relative intensities of
product ions generated from the
same precursor.
• You can also click Chromatograms
> Extract Chromatograms to start
this dialog box.
Steps Detailed Instructions Comments
Page 32

Exercise 1 – Develop an acquisition method
Task 5. Find optimum collision energy for MRM acquisition
32 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
h Click the Overlaid Mode icon, .
i Compare peak intensities for each
compound transition in each data file
in the Chromatogram Results window.
• Compare the colors shown in
Chromatogram Results with the
color next to the MRM transition
name in the Data Navigator.
• You can also right-click the
Chromatogram Results window
header and compare the colors of
the chromatograms to the colors of
the titles in the shortcut menu.
Unless you decide to acquire MRMs at
lower collision energies, you should
find that the optimal method settings
are as shown in Table 4.
j Click the Close Data File icon in the
main toolbar, and click Close when the
Close Data File dialog box appears.
• You now have all the information
you need to do an MRM acquisition
experiment of the sulfa drug
mixture. Consider doing at least
one more run with those settings.
Steps Detailed Instructions Comments
Table 4 Compounds and Collision Energy
Compounds MRM Transition Collision Energy (V)
Sulfamethizole 271.0 > 155.8 10
Sulfamethazine 279.0 > 185.7 15
Sulfachloropyridazine 285.0 > 155.7 10
Sulfadimethoxine 311.0 > 155.7 15
Page 33

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 1. Create a batch file from an existing MRM data file
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 33
Exercise 2 – Develop a Dynamic MRM method from an MRM
acquisition data file or an MRM method
The purpose of this exercise is to create a Dynamic MRM method from an
acquired MRM data file for sulfamix_MRM data files with the correct retention
times for Dynamic MRM using the Quantitative Analysis program.
For this exercise, you have three main tasks:
• “Task 1. Create a batch file from an existing MRM data file” on page 33
• “Task 2. Print a report in the Quantitative Analysis program” on page 36
• “Task 3. Create a Dynamic MRM method using Update dMRM” on page 38
You can easily create a Dynamic MRM method from an existing MRM method.
• “Task 4. Create a Dynamic MRM method from an MRM method” on page 40
Task 1. Create a batch file from an existing MRM data file
In this exercise, you create a batch and a method from an existing MRM data
file.
Steps Detailed Instructions Comments
1 Open the Quantitative Analysis
program and create a batch file
with one sample file,
SulfamixMRM_10.d.
• Copy the data file
SulfamixMRM_10.d from the
installation disk to the
\MassHunter\Data\MRM_to_
DMRM folder.
a Double-click the QQQ Quantitative
Analysis icon or the Drug Quant
(QQQ) icon.
b Click File > New Batch.
c Navigate to the \MassHunter\Data\
MRM_to_DMRM folder.
d Type
MRM_to_DMRM in the File
name text box.
e Click Open.
f If the Add Samples dialog box does
not open, click File > Add Samples.
g Select the file SulfamixMRM_10.d.
h Click OK.
• The file SulfamixMRM_10.d is on
the installation disk in the
\Support\Data folder. Copy this
entire folder to the
\MassHunter\Data\
MRM_to_DMRM folder.
Page 34

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 1. Create a batch file from an existing MRM data file
34 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
2 Create a method for that batch
using MRM data.
a Click Method > New > New Method
from Acquired MRM Data.
b Select the SulfamixMRM_10.d data
file.
c Click Open.
3 Set the Concentration Setup,
Qualifier Setup, and Calibration
Curve Setup.
• Add calibration level 1 with a
concentration of 10000.
• Set the Uncertainty to Relative
for all qualifiers.
• Set the Curve Fit to Linear.
• Set the Curve Fit Origin to
Include.
• Set the Curve Fit Weight to
None.
a Select Concentration Setup in the
Manual Setup Tasks section in the
Method Tasks pane.
b Select the first compound in the table.
c Right-click the compound row and
click New Calibration Level from the
shortcut menu.
d Enter
A1 in the Level column and 10
in the Conc. column.
e Right-click in the Level box and click
Copy Calibration Levels To.
f Click Select All. Click OK.
g Select Qualifier Setup in the Manual
Setup Tasks section in the Method
Tasks pane.
h Verify that the Uncertainty is Relative.
i Select Calibration Curve Setup in the
Manual Setup Tasks section in the
Method Tasks pane.
j Set Curve Fit to Linear for all
compounds.
k Set CF Origin to Include for all
compounds.
l Set CF Weight to None for all
compounds.
• Refer to the online Help in the
Quantitative Analysis program for
additional help on these tasks.
• You can also click Method > Copy
Calibration Levels To to display the
Copy Calibration Levels To dialog
box.
• To enter the same value in all cells
in a column, you can change the
value in the first row, and then
right-click that value in the first row
and click Fill Down.
• The compound names in
Quantitative Analysis need to
exactly match the Compound Name
in the QQQ Acquisition program. If
you capitalized the Compound
Name in the Data Acquisition
program, then you need to make
sure that the Name in the
Quantitative Analysis program is
also capitalized.
Steps Detailed Instructions Comments
Page 35

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 1. Create a batch file from an existing MRM data file
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 35
•
4 Verify method and then save the
method and apply the method to
the batch.
a Click Method > Validate.
b Click OK on the message box. Fix any
errors, if necessary.
c Click Method > Save As.
d Enter
MRM_to_DMRM.
e Click the Save button.
f Click Method > Exit.
g (optional) Click Analyze in the Apply
Method dialog box.
h Click Yes to apply the method to the
batch.
• In the Apply Method dialog box,
you can click Analyze to
automatically start additional batch
processing.
5 Analyze and save the batch. a Click Analyze > Analyze Batch.
b Click File > Save Batch.
Steps Detailed Instructions Comments
Page 36

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 2. Print a report in the Quantitative Analysis program
36 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Task 2. Print a report in the Quantitative Analysis program
In this task, you print a report using any template.
You can update a Dynamic MRM method using either a data file or a
quantitation report folder, so this task creates the quantitation report folder.
Steps Detailed Instructions Comments
1 Print a report using the template
MRM_to_DMRM.xltx.
a Click File > Save Batch.
b Click Report > Generate.
The system displays the Generate
Report dialog box.
c Select the Report folder. This folder
name will be used in the next task.
d Select the Report method.
e Click Edit. The Report Method Edit
dialog box opens.
f Click the Results tab. Click Yes
Always generate results file.
g Click Save & Exit.
h Mark All samples.
i Mark All compounds.
j Click OK.
• Copy the MRM_to_DMRM.xltx
template from the \Support\Data
folder on the installation disk.
• For this report, you do not need to
print the report.
• If you have not created a Report
Method, see the online Help for
Quantitative Analysis for
instructions on how to create a
report method.
• The Data Acquisition program uses
the results file in the Quant reports
folder to update the method, so you
must generate this file if you want
to update your method with it.
2 Check the status of the report
using the Queue Viewer program.
a Click Report > Queue Viewer.
b Wait for the report to finish printing.
c Close the Task Queue Viewer
program.
Page 37

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 2. Print a report in the Quantitative Analysis program
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 37
Steps Detailed Instructions Comments
Page 38

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 3. Create a Dynamic MRM method using Update dMRM
38 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Task 3. Create a Dynamic MRM method using Update dMRM
You can create a Dynamic MRM method from an MRM data file or a
Quantitative Analysis method. You first set the Scan Type to Dynamic MRM,
and then you use the Update MRM Method dialog box.
Steps Detailed Instructions Comments
1 Open the method iiiSulfamix
MRM_10.m and save it to a new
name with the format iiiSulfamix
dMRM.m, where iii are your
initials.
a Click File > Open > Method.
b Select the iiiSulfamix MRM_10.m
method. Click OK.
c Click Method > Save As.
d Type the new method name with the
format iiiSulfamix_dMRM.m.
• In this example, the batch is in the
\MassHunter\Data\
MRM_to_DMRM folder.
2 Change the method to a dynamic
MRM method with the same
compounds. You can either use a
data file or the report that was
generated in the last task.
a Click the Acquisition tab in the QQQ
tab in the Method Editor window.
b Right-click the Scan segments table
and click Update DMRM Method. The
Dynamic MRM Update Options dialog
box opens.
c Select the folder containing the
report.results.xml file or the data file
iiiSulfamix MRM_10.d.
d Mark Add new compound/transition.
e Mark Update retention time.
f Mark Update trigger window.
g Click OK.
• The Dynamic MRM Update tool
automatically sets the Scan type to
Dynamic MRM.
• You can select either a data file that
was acquired with a Scan Type of
MRM or a Quant Report folder as
the input to this dialog box. The
Scan segments are created from
one of these two input sources.
You can update the compounds in the Scan
segments table by using a QQQ data file or a
Quantitative analysis report folder.
If you select a Quantitative analysis report
folder, you need to make sure to generate the
results file. See “Task 2. Print a report in the
Quantitative Analysis program” on page 36 to
learn how to always generate the results file.
Page 39

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 3. Create a Dynamic MRM method using Update dMRM
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 39
•
h Verify that each row has a Compound
Name. A blank Compound Name is
not allowed.
i Click Method > Save.
Steps Detailed Instructions Comments
The compounds from the data file or quantitation report are automatically added to the Scan segments table.
Page 40

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 4. Create a Dynamic MRM method from an MRM method
40 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Task 4. Create a Dynamic MRM method from an MRM method
You can create a Dynamic MRM method directly from an MRM method by
using the Paste from Clipboard command from the shortcut menu. You need
to manually enter the retention time.
Steps Detailed Instructions Comments
1 Open the method iiiSulfamix
MRM_10.m and save it to a new
name with the format iiiSulfamix
dMRM2.m, where iii are your
initials.
a Click File > Open > Method.
b Select the iiiSulfamix MRM_10.m
method.
c Click OK.
d Click Method > Save As.
e Type the new method name with the
format iiiSulfamix_dMRM2.m.
f Click the Save button.
2 Copy all compounds from the Scan
segments table in the MRM
method.
a Click the Acquisition tab in the QQQ
tab in the Method Editor.
b Select all of the rows in the Scan
segments table.
c Right-click the Scan segments table
and click Copy.
• To select all of the rows in the Scan
segments table, you select the first
row in the table, Then, you scroll to
the last row in the Scan segments
table. Press the Shift key and select
the last row in the table.
3 Change the Scan Type to Dynamic
MRM and paste the rows into the
new Scan segments table.
a Select Dynamic MRM for the Scan
Type.
b Right-click the Scan segments table
and click Paste from Clipboard.
c Click Method > Save.
• To combine multiple Time
Segments into one Dynamic MRM
Time Segment, you paste the Scan
segments into Excel and create one
long list. Then, you copy all of the
Scan segments in Excel.
4 Enter the retention times and
delete the original compound.
a Type the value for the Ret. Time (min)
for Sulfachloropyridazine.
b Type the value for the Ret. Time (min)
for Sulfadimethoxine.
c Type the value for the Ret. Time (min)
for Sulfamethazine.
d Type the value for the Ret. Time (min)
for Sulfamethizole.
e Select the original compound in the
Scan segments table.
f Right-click and click Delete Row.
g Click Method > Save.
• Retention time is different for
different systems and columns. For
the example data files, enter the
following values:
• Sulfachloropyridazine: 0.65
minutes
• Sulfadimethoxine: 2.03 minutes
• Sulfamethazine: 0.98 minutes
• Sulfamethizole: 0.37 minutes
Page 41

Exercise 2 – Develop a Dynamic MRM method from an MRM acquisition data file or an MRM method
Task 4. Create a Dynamic MRM method from an MRM method
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 41
•
Steps Detailed Instructions Comments
Page 42
Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 1. Create a Triggered Dynamic MRM method from a Dynamic MRM method manually
42 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Exercise 3 – Create a Triggered Dynamic MRM acquisition
method
For this exercise you analyze a mixture of four sulfonamide compounds.
Task 1. Create a Triggered Dynamic MRM method from a Dynamic
MRM method manually
You can create a Triggered Dynamic MRM method directly from a Dynamic
MRM method. In a Triggered Dynamic MRM method, you specify some of the
transitions to be primary transitions. These transitions are acquired for the
entire retention time window. Some of these primary transitions are also
marked as triggers. As the data is acquired, the program checks whether or
not the abundances of the trigger transitions are higher than the threshold. If
the abundances are higher than the thresholds and other additional
conditions are met, then the secondary transitions are acquired. These other
conditions are described in the Concepts Guide.
Steps Detailed Instructions Comments
1 Open the method iiiSulfamix
dMRM2.m, where iii are your
initials.
a Click File > Open > Method.
b Select the iiiSulfamix_dMRM2.m
method.
c Click OK.
d Click Method > Save As.
e Type the new method name with the
format
iiiSulfamix_TriggeredDMRM.m.
f Click the Save button.
• A Triggered Dynamic MRM method
is a type of Dynamic MRM method.
The Scan Type for both methods is
Dynamic MRM.
• The Dynamic MRM method is the
template method for the
optimization.
Page 43

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 1. Create a Triggered Dynamic MRM method from a Dynamic MRM method manually
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 43
2 Change the method to a triggered
dynamic MRM method.
a Click the Acquisition tab in the QQQ
tab in the Method Editor.
b Mark the Triggered check box in the
Triggered MRM section. This section
is only available if the Scan Type is
Dynamic MRM.
c Select whether to automatically mark
the highest product ion as the Primary.
d Enter the value for Repeats.
• Several columns are added to the
Scan segments table. These
columns only apply to a triggered
dynamic MRM method.
• The value Repeats is the number of
times to acquire each of the
secondary transitions when the
triggering conditions are met.
3 Add additional transitions. See the
next image.
a Select the first compound. Right-click
and click Insert Row. Repeat to insert
another row
b Modify the information in these rows
to match the information in the next
image.
c Repeat for each compound.
• For each compound, we are going
to add additional transitions.
4 Select the transitions that are the
Primary transitions. See the next
image.
a For each transition, mark the Primary
check box if it is a Primary transition.
b Verify that you have marked at least
one transition as the Primary
transition for each Compound Name.
• You can select multiple transitions
from each compound to be Primary
transitions. If a transition has the
same Compound Name, then it is
part of the same compound. You
must mark at least one transition as
a Primary transition for each
compound.
• Typically, the most abundant ion is
the Trigger transition.
Steps Detailed Instructions Comments
Page 44

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 1. Create a Triggered Dynamic MRM method from a Dynamic MRM method manually
44 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
5 Select the transitions that are the
Trigger transitions and set the
trigger conditions.
a For each compound, mark the Trigger
check box if it is a Trigger transition.
b (optional) Mark a second Trigger
transition.
c Enter the Threshold value for each
Trigger transition.
d Enter the Trigger Entrance for each
Trigger transition.
e Enter the Trigger Delay for each
Trigger transition.
f Enter the Trigger Window for each
Trigger transition.
• For each compound, you can have
two Trigger transitions.
• If the Trigger transition has an
abundance over the Threshold,
then that triggering condition is
met.
• By default, the Trigger Entrance,
the Trigger Delay and the Trigger
Window are set to 0. If these
values are 0, then these triggering
conditions are not enabled.
• The threshold is established when
the method is updated using a data
file. The other values are also
selected based on results. You must
collect data at different settings to
establish what works best. These
values are very important to the
success of the method.
•
Steps Detailed Instructions Comments
Page 45
Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 45
Task 2. Add/Modify compounds in an existing database
You can also manually add compounds to a database and modify the
compounds in the database. In the next task, you create a Triggered Dynamic
MRM method from the compounds in the database.
Steps Detailed Instructions Comments
1 Review the
iiiSulfamix_dMRM2.m, where iii
are your initials.
a Click File > Open > Method.
b Select the iiiSulfamix_dMRM2.m
method.
c Click OK.
d Review the parameters.
• A Triggered Dynamic MRM method
is a type of Dynamic MRM method.
The Scan Type for both methods is
Dynamic MRM.
2 Start the MassHunter Optimizer
program.
• Double-click the Optimizer icon. . • If you are optimizing peptides, use
the Optimizer for Peptides program.
3 Set parameters on the Optimizer
Setup tab.
a Click the Optimizer Setup tab.
b Click the Injection (with or without
column) button.
c Set the CE range from
4 to 48.
d Set the Cell Accelerator Voltage to
7.
e Right-click the table and click Add
Method.
f Select the iiiSulfamix_dMRM2.m
method.
• To create low mass product ions
from a precursor ion near 300 m/z,
you need fairly high collision
energies.
4 Set parameters on the Precursor
Ion Selection tab.
a Click the Precursor Ion Selection tab.
b Verify that +H is marked for the
Positive ions (with priorities) list.
5 Set parameters on the Product Ion
Selection tab.
a Click the Product Ion Selection tab.
b Click the Mass (m/z) button under
Low mass cut-off.
c Enter
60 for the low mass cut-off.
• On the Product Ion Selection, you
can automatically add up to 4
product ions per compound (for
instance, 2 primaries and 2
secondaries). You want 8 to 10
peaks in the composite spectrum to
prove that this is indicative of the
compound, so you need to add at
least some of the product ions
manually.
Page 46
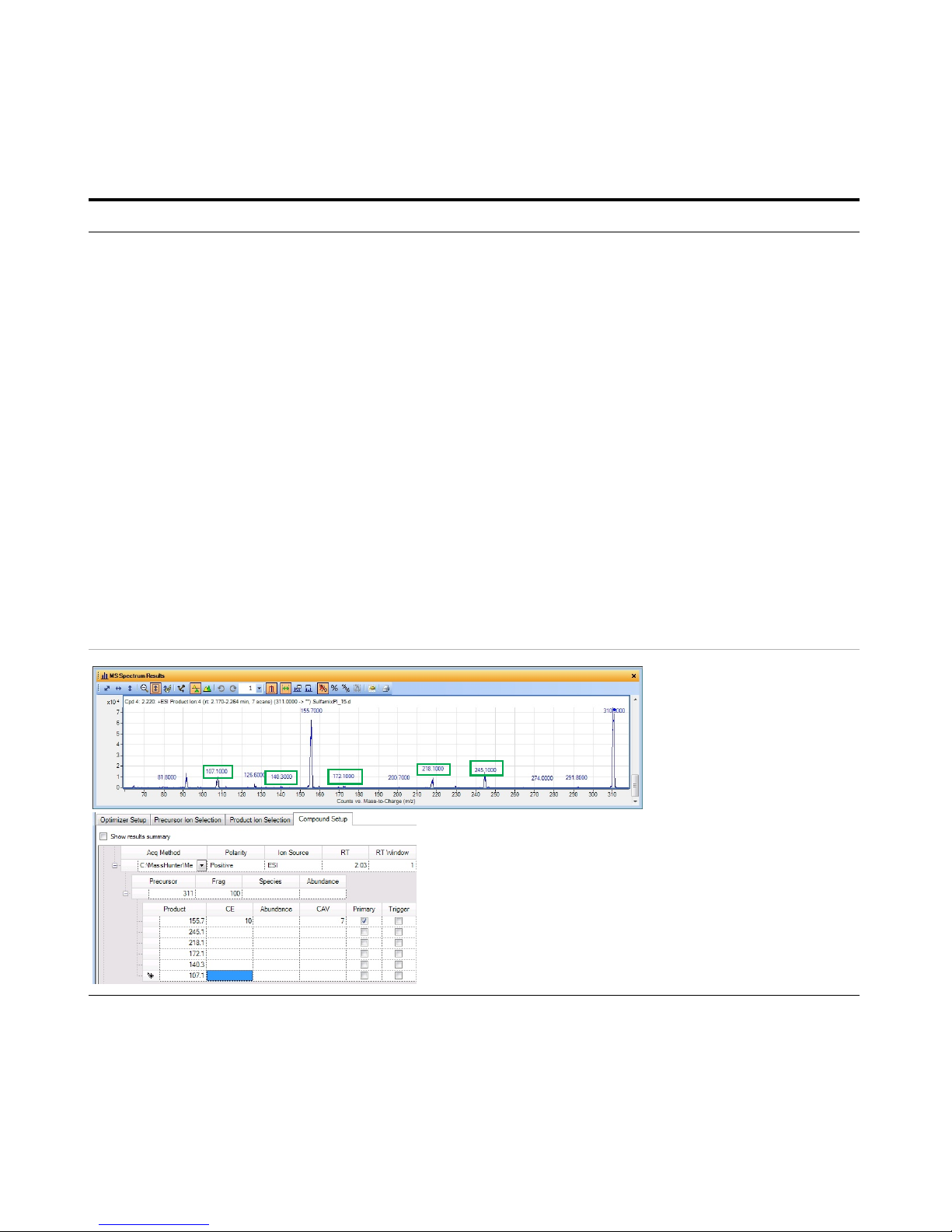
Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
46 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
6 Set parameters on the Compound
Setup tab and add additional
transitions.
• For Precursor ion 311, add the
following product ions: 245.1,
218.1, 172.1, 140.3, 107.1
• For Precursor 285, add the
following product ions: 108.1,
92.1, 80.1, 65.1, 39.2
• For Precursor 279, add the
following product ions: 185.7,
155.6, 107.7, 92.1
• For Precursor 271, add the
following product ions: 177.8,
115, 91.3, 80.1, 64.8
a Click the Compound Setup tab.
b Click the Import/Export > Import
from Acquisition Methods command.
c Select the iiiSulfamix_dMRM2.m
method and click Open.
d (optional) Right-click the tab and click
Expand/Collapse All Rows.
e Select one of the Product rows for
one of the compounds. In this
example, select the Product row 155.7
for Precursor 311.
f Right-click the Product row and click
Add Product Ion. In this example, you
add 5 product ion rows.
g Enter the Product in each of the
product ion rows that were added. See
“To determine product ions in the
Qualitative Analysis program:” on
page 47.
h Add product ions for the other three
compounds.
• For each compound, we are going
to add additional transitions.
• In the Qualitative Analysis program,
you examine Product Ion data files
which you acquired previously to
determine additional transitions to
add. See “Task 4. Determine
product ion masses” on page 24.
• You can use the arrow keys to move
between rows in the Product table.
• Some of the product ions were
determined from the Veterinarian
Drugs tMRM database.
• See Table 5 on page 54 for values to
use in the method.
Steps Detailed Instructions Comments
This product ion scan has a
precursor mass of 311. You
examine the MS spectrum to
determine the product ions to
add to the Product ion section
of the Compound Setup table.
The product ions that are manually added as
additional Product ions in Optimizer are shown in
the MS Spectrum Results window. The green
boxes were added in this guide to show which
product ions were used.
Page 47
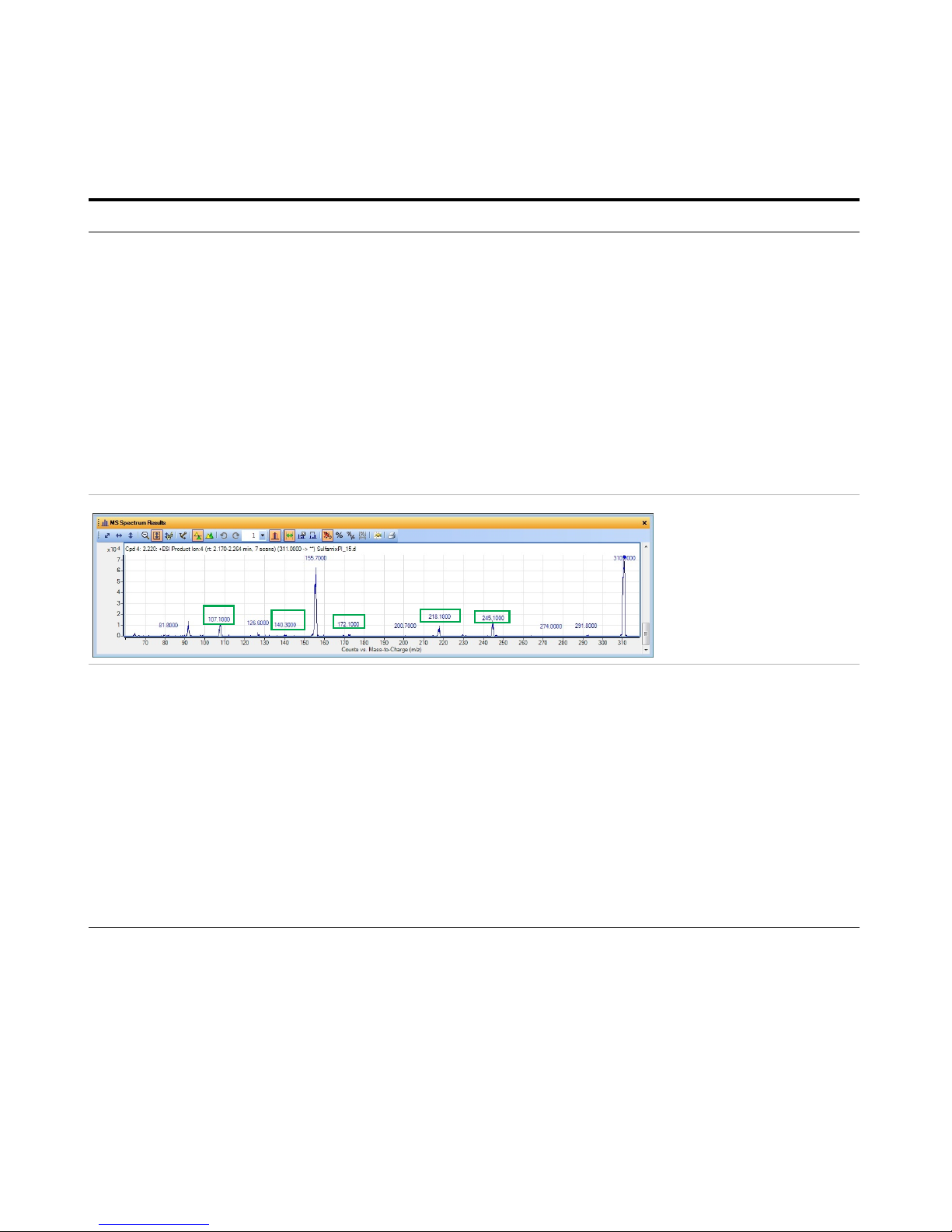
Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 47
• To determine product ions in the
Qualitative Analysis program:
a Open the SulfamixPI_15.d from “Task
4. Determine product ion masses” on
page 24.
b Click Find > Find Compounds by
Targeted MS/MS.
c Close the Compound List window.
d Select a compound in the Data
Navigator window. For this example,
click Cpd 4.
e Click the Autoscale Y-axis icon in the
MS Spectrum Results toolbar.
f Right-click and drag to zoom in on the
MS spectrum.
• If possible, rearrange the windows
on the screen so you can see the
Optimizer program and the
Qualitative Analysis program at the
same time.
• See Table 5 on page 54 for values to
use in the method.
7 Set other parameters in the
Compound Setup tab and start the
optimization.
• You cannot perform a
multi-compound run.
• You have to mark each row in
the table to use.
a Mark the check box in the left column
at the top of the table. The check box
for every row in the table is marked.
b Clear the Perform multi-compound
run check box in the right column.
c Click the Start Optimization button in
the Optimizer toolbar.
• You cannot perform a
multi-compound run with the
number of transitions that were
added. If you mark this check box,
then the Expected peak width
(base) is automatically set to
almost 80 seconds wide. If you
clear this check box, then the
Expected peak width is calculated
to be around 9 seconds which is
more appropriate.
• See Table 5 on page 54 for values to
use in the method.
Steps Detailed Instructions Comments
This product ion scan has a
precursor mass of 311. You
examine the MS spectrum to
determine the product ions
to add to the Product ion
section of the Compound
Setup table.
Page 48

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
48 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
8 Examine the Optimizer Report. a Examine the Collision Energy for each
Product Ion.
b Print or save the report.
9 Save the compounds. • Click the File > Save Compounds
command.
10 Import compounds from a
database.
• Click the Import/Export > Import from
Database command. The Database
Browser program opens.
• You can also import compounds
that were distributed as part of a
database.
Steps Detailed Instructions Comments
As a general rule, as the Product Ions get smaller,
the optimal Collision Energy gets larger. However,
when you also examine the abundance, you can
see that if the Collision Energy is set to 48 for the
smallest product ion, the smallest product ion can
become the dominant peak. The collision energies
are further adjusted later in this task.
This report was generated with a different set of
transitions.
Page 49
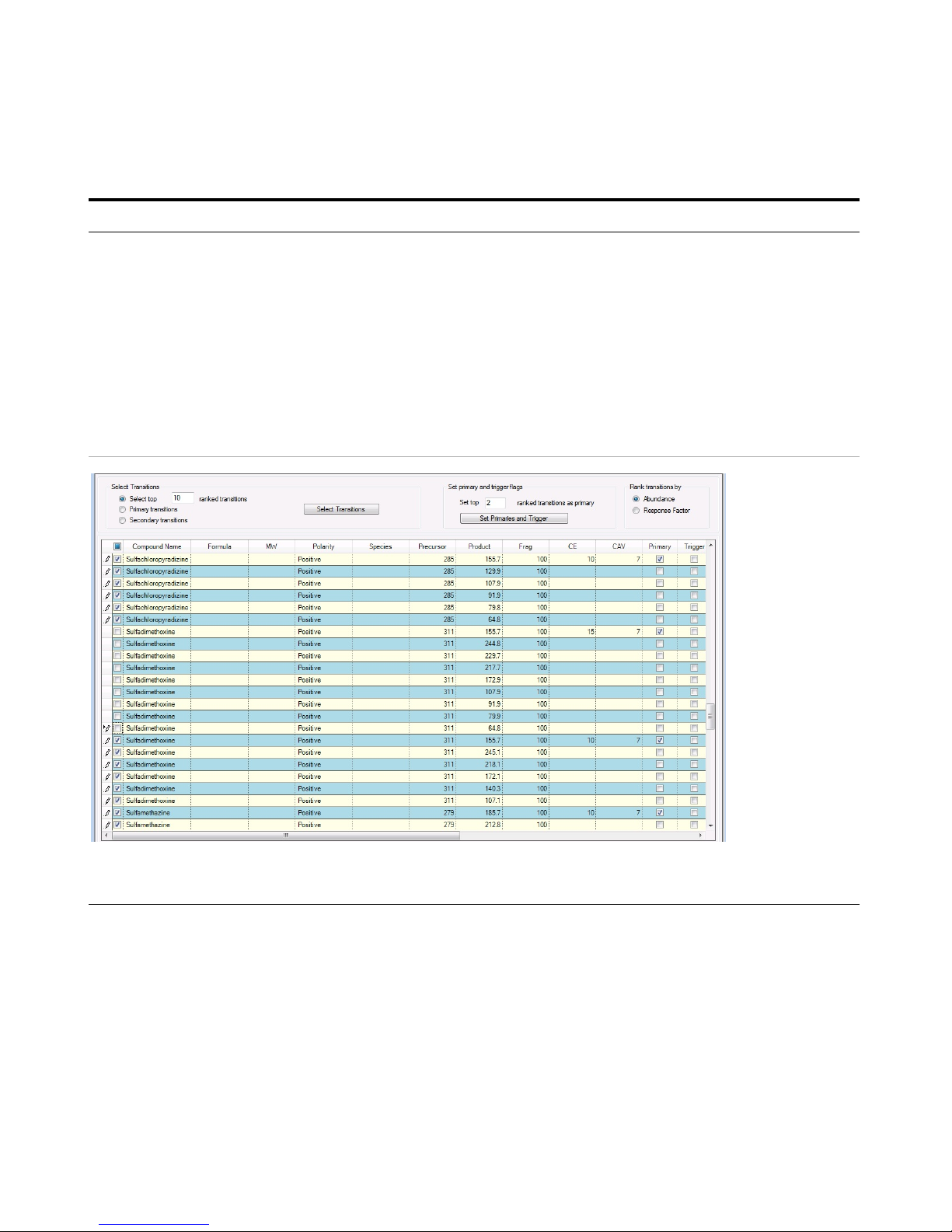
Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 49
11 In the Database Browser program,
select the transitions.
a Mark the Show All Records check
box.
b Click the Select top button under
Select Transitions.
c Type
10 for the ranked transitions.
d Click the Select Transitions button.
• All the transitions that you typed in
are visible.
• The tools to allow you to set up
Primary transitions and Secondary
transitions are available in this
program.
• If any of the compounds have two
collision energies, clear the check
boxes for one of these values.
• See Table 5 on page 54 for values to
use in the method.
Steps Detailed Instructions Comments
Page 50

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
50 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
12 In the Database Browser program,
automatically select the Primary
transitions and Trigger transition.
a In the Set top ranked transitions as
primary box, enter 2.
b Click the Set Primaries and Trigger
button.
• The program automatically selects
the two most abundant transitions
as the Primary transitions.
• The program also selects the most
abundant transition as the Trigger.
• You can manually select a second
Trigger transition.
• See Table 5 on page 54 for values to
use in the method.
Steps Detailed Instructions Comments
You examine the Primary column and the Trigger column to determine which transitions are selected. You can select
one or two Trigger transitions. You can select multiple Primary transitions.
Page 51

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 51
13 Review the Primary transitions and
Trigger transitions.
• For sulfachloropyridazine, select
285 m/z -> 155.7 m/z transition
as the Primary and Trigger
transition.
• For sulfadimethoxine, select 311
m/z -> 155.7 m/z transition as
the Primary and Trigger
transition.
• For sulfamethazine, select 279
m/z -> 185.7 m/z transition as
the Primary and Trigger
transition.
• For sulfamethizole, select 271
m/z -> 155.8 m/z transition as
the Primary and Trigger
transition.
• Review each compound. Change the
Primary and Trigger transitions to the
transitions listed in the left column.
• Change the other Primary transitions
as shown below.
• The program selected the most
abundant transitions which in this
example often had a low m/z for
the Product Ion. A very abundant
low m/z ion may be unsuitable as a
Primary transition.
• You can select two Primary
transitions as triggers for a
compound.
• In the example below, all of the
columns have values. The
Fragmentor voltages and collision
energy values were set in
Optimizer.
• See Table 5 on page 54 for values to
use in the method.
Steps Detailed Instructions Comments
Page 52

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
52 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
14 Review the Import List table on
the Import List tab.
a Click the Add to Import List button.
b Click the Import List tab.
c Review the Import List table.
• In this example, you are importing
from the database to the Import
List. Then, you are importing from
Database Browser to Optimizer.
15 Review the Compound Setup table
in Optimizer. You replace all
compounds with the compounds
from the Database Browser
program.
a Click the Import button.
b Click the Yes to All button.
c In the Compound Setup tab in
Optimizer, review the compounds.
• The compounds in Optimizer are
overwritten by the compounds that
you updated in the Database
Browser program.
Steps Detailed Instructions Comments
Page 53

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 53
16 Save the new compound
parameters to the database.
• Click the File > Save Compounds
command to save all of the changes to
the database.
• You cannot see these results by
default, but the Primary and Trigger
transitions are updated in the
project.
• The Primary column, Trigger
column, Trigger Entrance Delay
column, Trigger Delay column,
Trigger Window column and
Trigger MRM Threshold column
are available in the Compound
Setup tab. They may be hidden.
Steps Detailed Instructions Comments
Page 54

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 2. Add/Modify compounds in an existing database
54 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
You select the most abundant transition as the Trigger. The threshold is set
automatically when any acquisition method is updated. You can set the
threshold manually, also.
Table 5 Information for TMRM method for sulfa drugs
Compound Precursor Product Ion Primary Trigger Threshold Collision
Energy
Sulfachloropyridazine 285 155.7 Yes Yes 500 12
Sulfachloropyridazine 285 92.1 Yes No 24
Sulfachloropyridazine 285 108.1 No No 24
Sulfachloropyridazine 285 80.1 No No 60
Sulfachloropyridazine 285 65.1 No No 60
Sulfadimethoxine 311 155.7 Yes Yes 500 16
Sulfadimethoxine 311 107.1 Yes No 28
Sulfadimethoxine 311 245.1 No No 16
Sulfadimethoxine 311 218.1 No No 16
Sulfadimethoxine 311 172.1 No No 32
Sulfadimethoxine 311 140.3 No No 40
Sulfamethazine 279 185.7 Yes Yes 500 12
Sulfamethazine 279 92.1 Yes No 32
Sulfamethazine 279 155.6 No No 16
Sulfamethazine 279 107.7 No No 28
Sulfamethizole 271 155.8 Yes Yes 500 10
Sulfamethizole 271 91.3 Yes No 40
Sulfamethizole 271 177.8 No No 10
Sulfamethizole 271 115 No No 20
Sulfamethizole 271 80.1 No No 40
Sulfamethizole 271 64.8 No No 40
Page 55

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 3. Create a Triggered Dynamic MRM method from an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 55
Task 3. Create a Triggered Dynamic MRM method from an existing
database
You can create a Triggered Dynamic MRM method from a database such as the
Pesticide Triggered MRM Database and Library, the Forensics and
Toxicology Triggered MRM Database and Library, or the Veterinary Drug
Triggered MRM Database and Library. These databases can be purchased
from Agilent. You can also copy the information from an Excel spreadsheet,
but that method is not described in this guide.
Steps Detailed Instructions Comments
1 In the Data Acquisition program,
you now import the updated
compounds from the database.
These compounds have optimized
collision energies and also Primary
and Trigger transitions marked.
a Switch to the Data Acquisition
program.
b Open the iiiSulfamix_dMRM2.m
method.
c In the QQQ tab, click the Acquisition
tab. The Scan segments table
contains four rows which are deleted
later.
d Right-click the Scan Segments table
and click Import from Database
Browser. The Database Browser
program opens.
e Mark the Show All Records check
box.
f Mark all of the transitions for the four
sulfa drug compounds. Clear the
check boxes next to any unwanted
compounds.
g Click the Add to Import List button.
h Click the Import List tab.
i Review the Import List table.
j Click the Import button.
k Delete the original compounds from
the Scan segments table.
l Mark the Triggered check box under
Triggered MRM.
• Before you import compounds from
Database Browser, the Scan
segments table contains at least
one row. After importing
compounds from the Database
Browser, you need to remove any
original rows.
• The Scan segments table always
has to have at least one row.
• The triggering information is loaded
from the Database Browser
program even if the Triggered check
box is clear.
• See the online Help for the Data
Acquisition program and the QQQ
Concepts Guide for an explanation
of the other triggering conditions:
Trigger Entrance, Trigger Delay,
and Trigger Window.
Page 56

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 3. Create a Triggered Dynamic MRM method from an existing database
56 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
2 Save the method to a new method
name, iiiSulfas_TriggerOpt.m,
where iii are your initials.
a Click the Method > Save Method
command.
b Type iiiSulfas_TriggerOpt.m.
c Click the Save button.
Steps Detailed Instructions Comments
Page 57

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 3. Create a Triggered Dynamic MRM method from an existing database
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 57
3 Review the method in the Dynamic
MRM Viewer dialog box.
a Right-click the Scan segments table
and click Edit DMRM Method. The
Dynamic MRM Viewer dialog box is
opened.
b Type
200 for the Cycle time. This
value is shown in the Acquisition tab.
c Click between the Primaries only
button and the All transitions button
if the Dynamic MRM Statistics
information is not updating. Then,
click the All transitions button.
d Click Close.
• The compounds in Optimizer were
overwritten by the compounds that
you updated in the Database
Browser program.
• You can modify the Cycle time and
see how the Minimum Dwell Time
is changed. If the Minimum Dwell
Time is less than 5 ms, and
especially if it is less than 2 ms,
then signal-to-noise is poor.
• A Dwell Time of 8 ms per transition
is fine.
Steps Detailed Instructions Comments
Page 58

Exercise 3 – Create a Triggered Dynamic MRM acquisition method
Task 3. Create a Triggered Dynamic MRM method from an existing database
58 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
4 Review the Trigger Thresholds to
verify that they are appropriate.
a Do an injection to make sure that the
Trigger Thresholds are set properly.
b Right-click the Scan segments table
and click Update DMRM Method.
c In the MRM Update Options dialog
box, select True for Update threshold.
d Enter the value for the Percent of
Height for the Trigger Threshold.
e Select the data file that you just
acquired.
f Click OK.
Steps Detailed Instructions Comments
Page 59

Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 59
Exercise 4 – Optimize Acquisition parameters
For this exercise you optimize a mixture of four sulfonamide compounds.
Task 1. Use Optimizer to optimize acquisition parameters
Optimizer helps you optimize acquisition parameters. Specifically, it
automates the selection of the best precursor ions, the optimization of the
fragmentor voltage for each precursor ion, selection of the best product ions,
and optimization of collision energy values for each transition for a list of
compounds you specify.
To do this task, you first need to create the method iiiSulfamix MRM_10.m in “Task
5. Find optimum collision energy for MRM acquisition”
on page 30. You do not need to
acquire the data file.
Steps Detailed Instructions Comments
1 Start the MassHunter Optimizer
program.
• Double-click the Optimizer icon. . • If you are optimizing peptides, use
the Optimizer for Peptides program.
Page 60
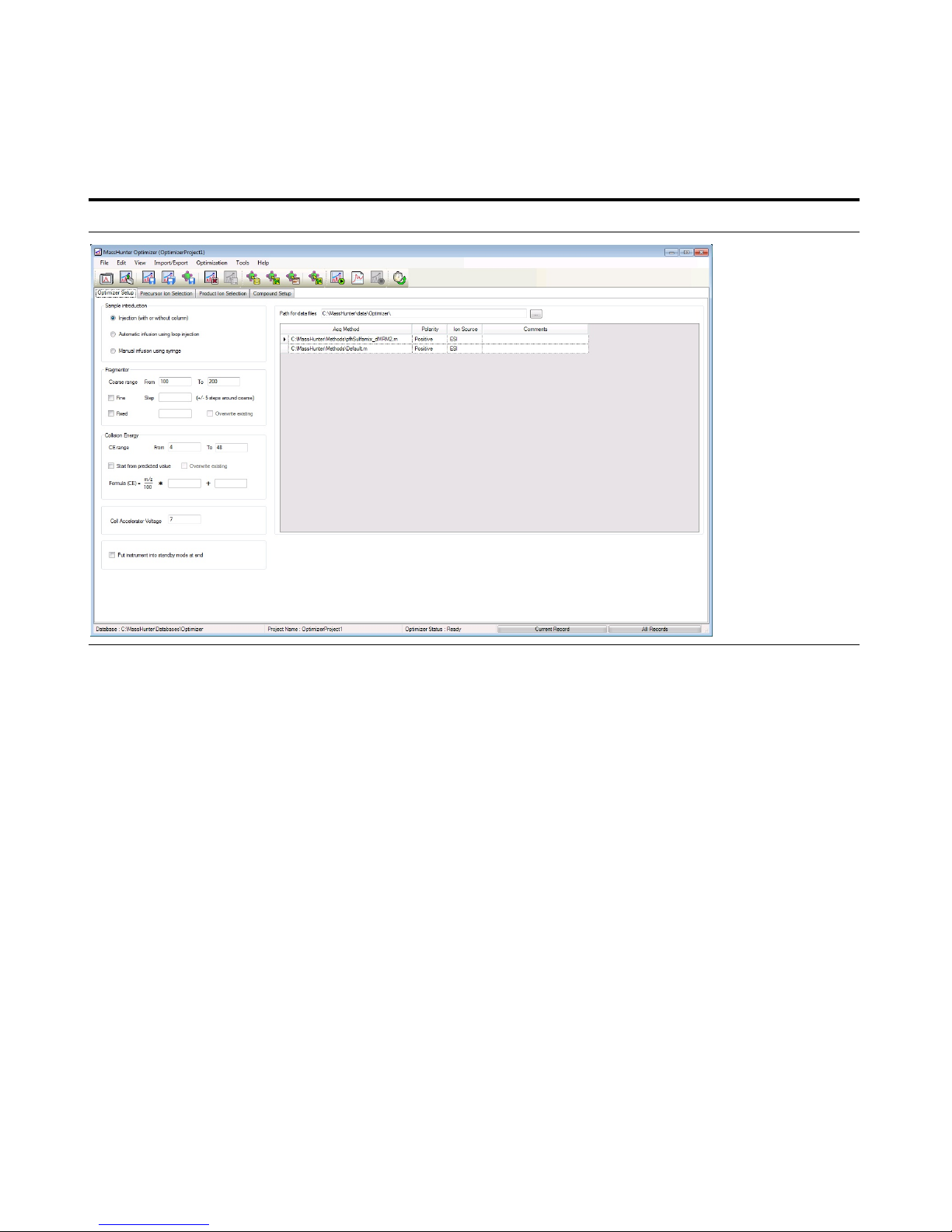
Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
60 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
Steps Detailed Instructions Comments
Page 61

Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 61
2 Set the optimization parameters. a Click the Optimizer Setup tab.
b Set the Sample introduction method
to Injection (with or without
column).
c Set the range for ramping the Collision
Energy from 0 to 40 V.
d Select a Path for data files to store the
optimization run data.
e Right-click the table on the right and
select Add Method from the shortcut
menu.
f Click the button on the right side of
the Acq Method cell to open the Open
Method dialog box.
g Select the method created in the
previous exercise iiiSulfamix
MRM_10.m and click OK. The Polarity
and Ion Source will be filled in from
the values set in the selected method.
h Check to make sure that the Ion
Source from the method matches the
physical configuration of your
instrument.
i Repeat step e to step h to select
additional methods.
• Fine optimization refines the coarse
ramping values and provides better
optimization but takes longer to
run.
• The data can be displayed later
with MassHunter Qualitative
Analysis program.
Steps Detailed Instructions Comments
Page 62
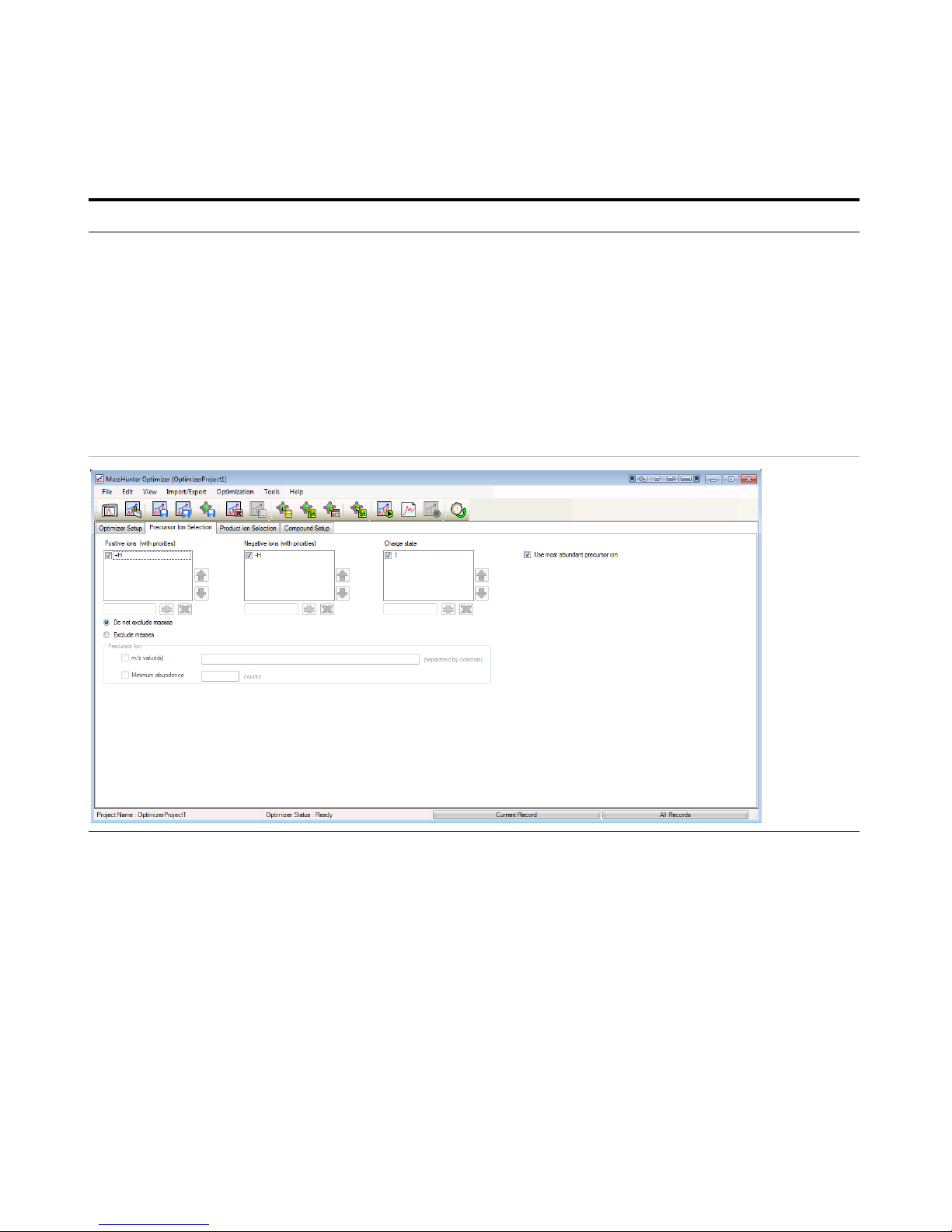
Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
62 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
3 Select the precursor ions a Click the Precursor Ion Selection tab.
b Select the Positive ions +H adduct.
c Select the Charge state of 1.
d Set the search priority of the precursor
ions.
e (optional) To exclude certain masses
from consideration, click Exclude
masses at the bottom of the screen.
Enter the m/z Values to exclude
separated by commas and/or enter a
Minimum abundance value in counts.
• Mark the Use most abundant
precursor ion check box to use the
most abundant precursor ion.
• Clear the Use most abundant
precursor ion check box and use
the Up and Down arrow buttons to
set the search order (ions at the top
of the list are given more priority).
• You can also enter Neutral Losses
to exclude (for example H
2
O).
••
Steps Detailed Instructions Comments
Page 63
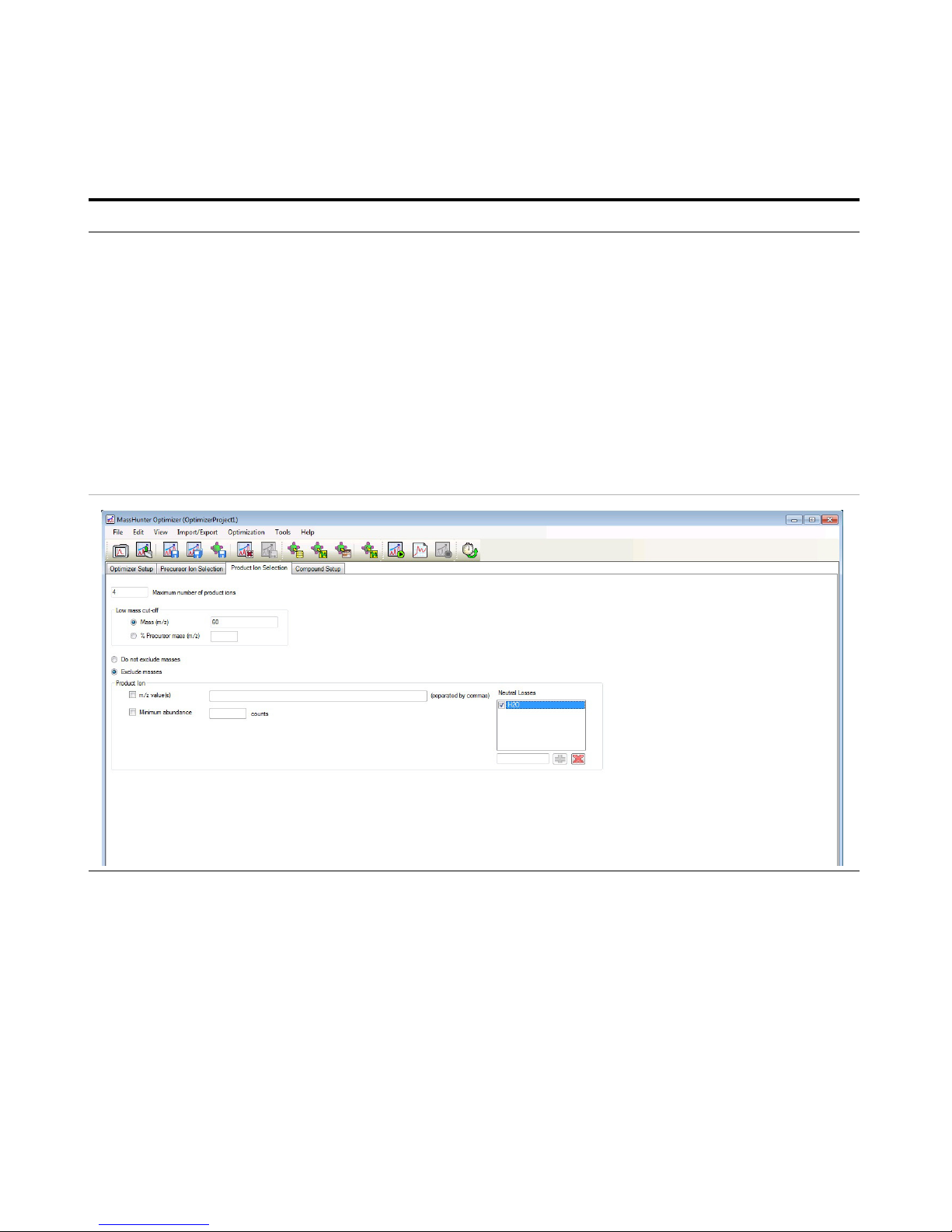
Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 63
4 Select the product ions a Click the Product Ion Selection tab.
b Enter a Low mass cut-off value.
Select Mass (m/z) of 60 m/z.
c To exclude certain masses from
consideration, click Exclude masses
option at the bottom of the screen.
Enter the m/z Values to exclude
separated by commas and/or enter a
Minimum abundance value in counts.
d If desired, you can also enter Neutral
Losses to exclude, for example H
2
0.
Enter a formula in the box and click
the button to add it to the list.
• You want to set the Low mass
cut-off value because you do not
want to optimize low mass
non-specific ions. For the sulfa
drugs, significant ions are above 60
m/z.
Steps Detailed Instructions Comments
Page 64

Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
64 Agilent 6400 Series Triple Quad LC/MS Familiarization Guide
5 Set up a compound list. The
formula for the four Sulfa Drugs
are:
• Sulfamethizole
C
9H10O2N4S2
• Sulfamethazine
C
12H14O2N4
S
• Sulfachloropyridazine
C
10H9O2N4
SCl
• Sulfadimethoxine
C
12H14O4N4
S
a Click the Compound Setup tab.
b Clear the Show results summary
check box above the table while you
set up the compound list.
c Right-click the table and select Add
Compound from the shortcut menu to
add a row to the end of the table.
d Enter
Sulfamethizole as the
Compound Name.
e Enter
Sulfa drugs as the group
name in the Groups column.
f Enter
C9H10O2N4S2 as the
Formula of the compound. The mass
is calculated.
g Enter the Sample Position for the new
compound.
h (optional) Enter an Optimization dwell
time value to set longer or shorter
cycle times.
i Repeat the steps above to add the
other three sulfa drugs to the table.
j Mark the Select columns for the
compounds (rows) to use for
optimization.
k Save the compound list to the
database or to the current project.
• Compounds are global to all
projects. Compound information
such as name, group, formula, and
mass in one project will be
reflected in the entire database.
• If no methods or ions are specified
here, then optimization for the
compound uses the methods from
the Optimizer Setup tab and
information from the Precursor Ion
Selection and Product Ion Selection
tabs to generate the ions.
• You can also enter the
monoisotopic mass in the Mass
column instead of the Formula.
Steps Detailed Instructions Comments
Page 65
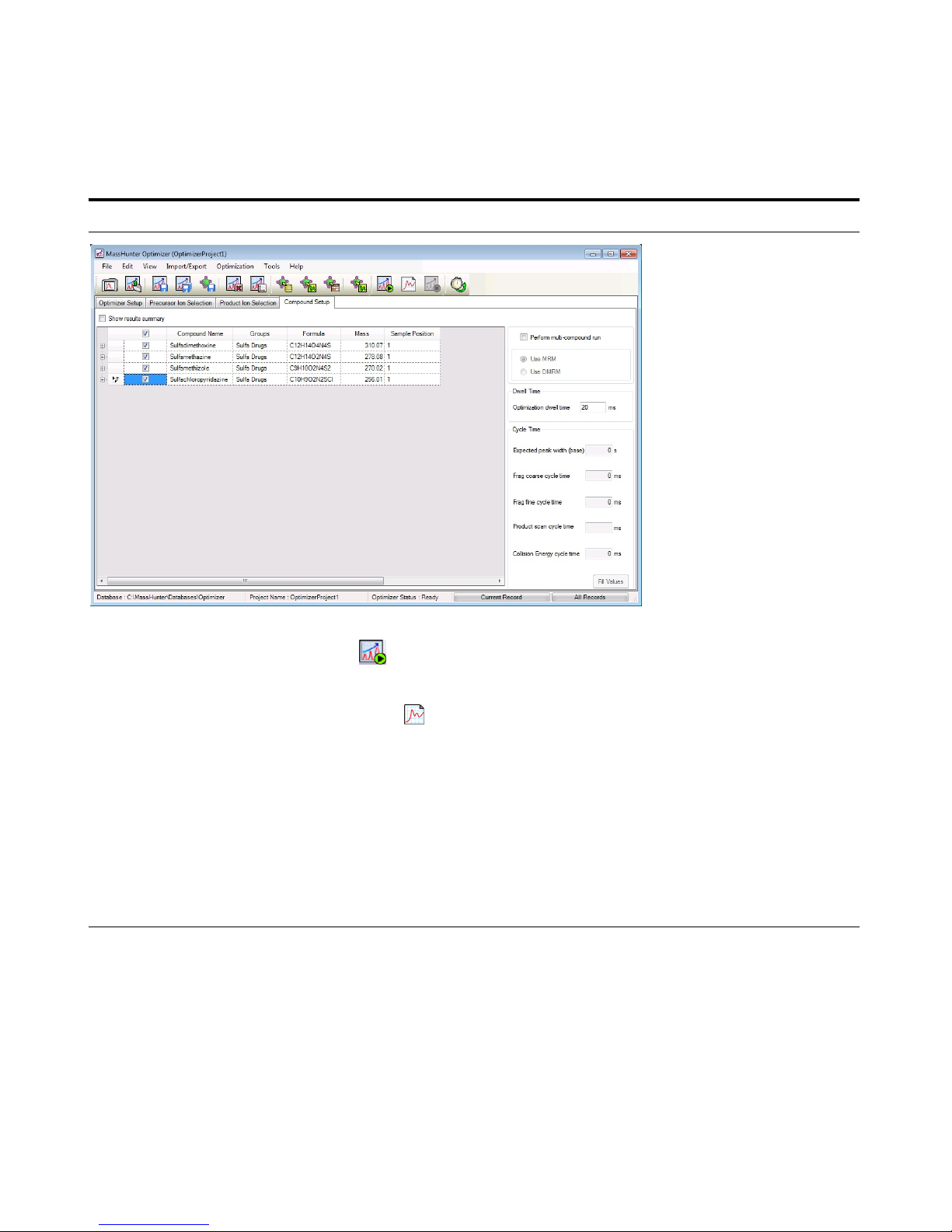
Exercise 4 – Optimize Acquisition parameters
Task 1. Use Optimizer to optimize acquisition parameters
Agilent 6400 Series Triple Quad LC/MS Familiarization Guide 65
6 Start the optimization process. • Click the Start Optimization button
( ) on the toolbar
or
• Click the Ion Breakdown Profile
button ( ) on the toolbar.
7 Review results. a Click the Compound Setup tab.
b Mark the Show results summary
check box above the table.
c Review the following values for each
transition ion in the Compound Table:
• Fragmentor
• Collision Energy
d Review the printed optimization
report.
• (optional) Use the MassHunter
Workstation Qualitative Analysis
program to look at the data.
• See the online Help for Optimizer or
the Optimizer Quick Start Guide to
learn how to import optimization
results to acquisition for MRM time
segments.
Steps Detailed Instructions Comments
Page 66

Agilent Technologies, Inc. 2016
Printed in USA
Revision A, August 2016
*K3335-90207*
K3335-90207
www.agilent.com
In This Book
The exercises in this guide
help you learn to use the
Agilent 6460 Triple Quad
Mass Spectrometer (Model
K6460) system. In this guide,
you acquire data and then
analyze the results using the
MassHunter Qualitative
Analysis program to learn
how to develop an
acquisition method.
For In Vitro Diagnostic Use
The K6460 mass spectrometer is
intended to be used to identify
inorganic or organic compounds in
human specimens by ionizing the
compounds and separating the
resulting ions by means of electrical
field according to their mass.
 Loading...
Loading...