Agfa CR75, CR85 Service manual

HEALTHCARE
Imaging Services
Document No: DD+DIS378.05E
nd
Edition
2
Service Manual
CR 85-X
Type 5148/100
CR 75.0
Type 5146/105
(as of SN ≥ 6000)
CR 85-X
Type 5148/100
Use, dissemination, distribution or reproduction of this document by unauthorized personnel is not permitted and may be unlawful.
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
02-2007 printed in Germany
Agfa Company Confidential
CR 75.0
Type 5146/105
(as of SN ≥ 6000)
CONFIDENTIALITY NOTE:
DOCUMENT CONTROL NOTE:
Document Node ID: 10028251
eq_00_about manual_e_template_v02
Copyright © 2007 Agfa-Gevaert HealthCare

DD+DIS378.05E
► Manufacturer
Agfa-Gevaert HealthCare GmbH
Tegernseer Landstraße 161
D - 81539 München
Germany
About this Manual
WARNING:
Improper operation or service activities may cause damage or injuries.
INSTRUCTION:
(1) Read the "Generic Safety Directions" document
(see MEDNET GSO => General Info => Agfa HealthCare => Publications =>
Service Manual) prior to attempting any operation, repair or maintenance task on
the equipment.
(2) Strictly observe all safety directions within the "Generic Safety Directions" and on
the product.
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
DOCUMENT CONTROL NOTE:
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 2
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential

DD+DIS378.05E
► Purpose of this document
This document provides information on the structure and contents of the Service Manual.
► Document History
About this Manual
Edition.
Revision
Release
Date
2.0 02-2007
Changes
compared to previous version 1.0
• Only layout changes as this Service Documentation is
also valid for CR 75.0 Type 5146/105 (as of SN ≥ 6000).
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
DOCUMENT CONTROL NOTE:
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 3
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential

DD+DIS378.05E
► Chapter Overview
Chapter
Order List
0
About this Manual
Controls, Connections and Set Up Procedures
1
Site and System Data / Installation Report
2 Functional Description
3
Repair and Service
3.1 Machine specific Safety and Repair Information
3.2 Machine specific Tools, Software Tools and Auxiliary Equipment
About this Manual
3.3 Troubleshooting
3.4 Electrical and Mechanical Codes, Fuses, LEDs
3.5 Replacement of Parts
3.6 Adjustments and Calibrations
3.7 Software Menus and Setting
3.8 Software Releases, Patches
3.9 FAQ - Frequently Asked Questions
4 Reference and Circuit Diagrams
5 Spare Parts List
6 Accessories
7 Field Modifications
8 Manufacturing Standard Modifications
9 Maintenance
10 Service Bulletins
11 Installation Planning
123
12 Glossary
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
DOCUMENT CONTROL NOTE:
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 4
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential
DD+DIS378.05E
► Explanation of notes
This documentation uses:
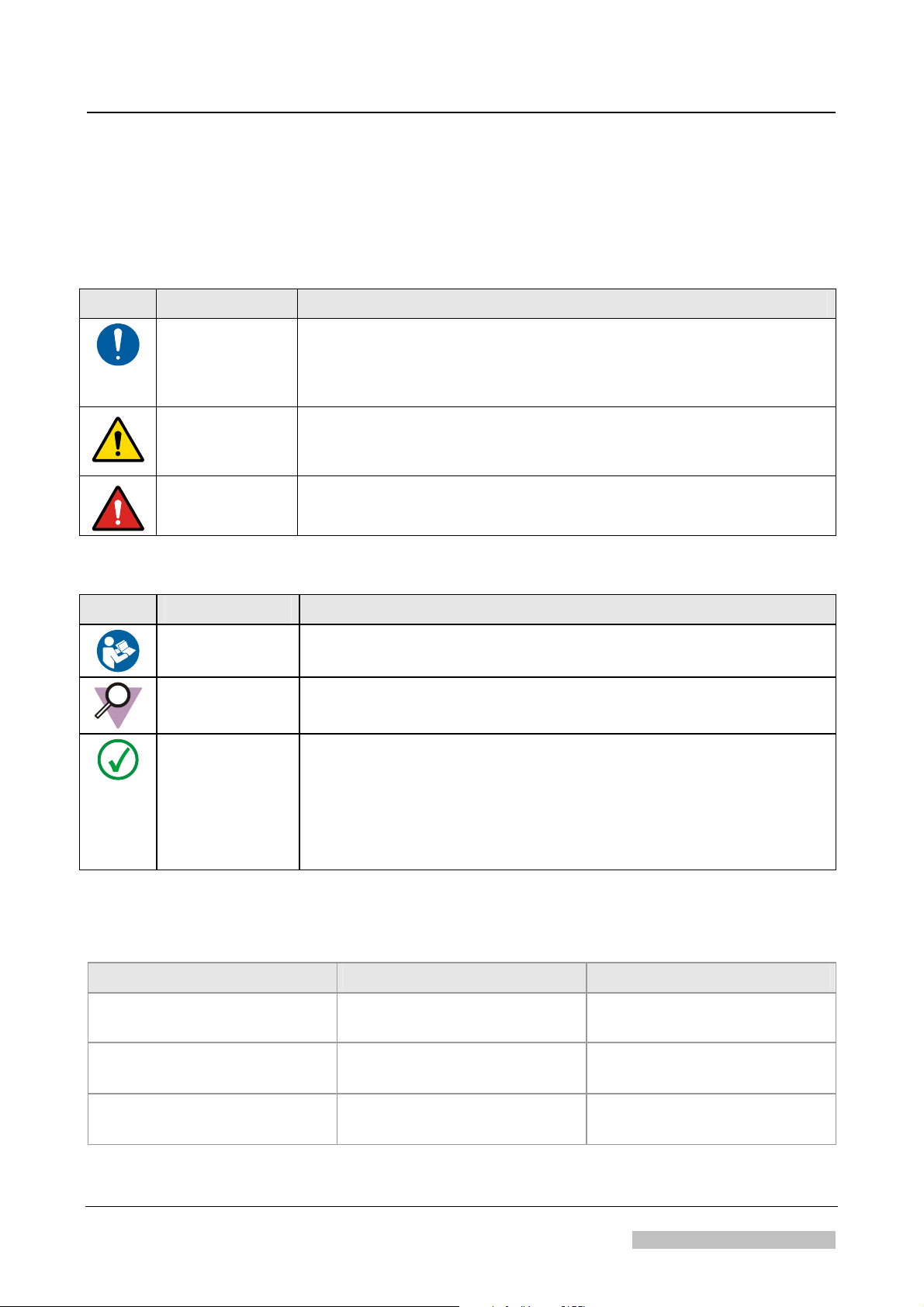
Safety relevant notes
Icon Signal Word Situation
About this Manual
CAUTION:
Possible dangerous situation: Light injuries or damage to the
equipment described in the manual and/or damage to any other
equipment or goods and/or environmental pollution can be the
consequence.
WARNING:
Dangerous situation: Potential serious injury to a user, engineer,
patient or any other person and possible mistreatment of patients
DANGER:
can be the consequence.
Direct, immediate danger: Death or serious injuries can be the
consequence.
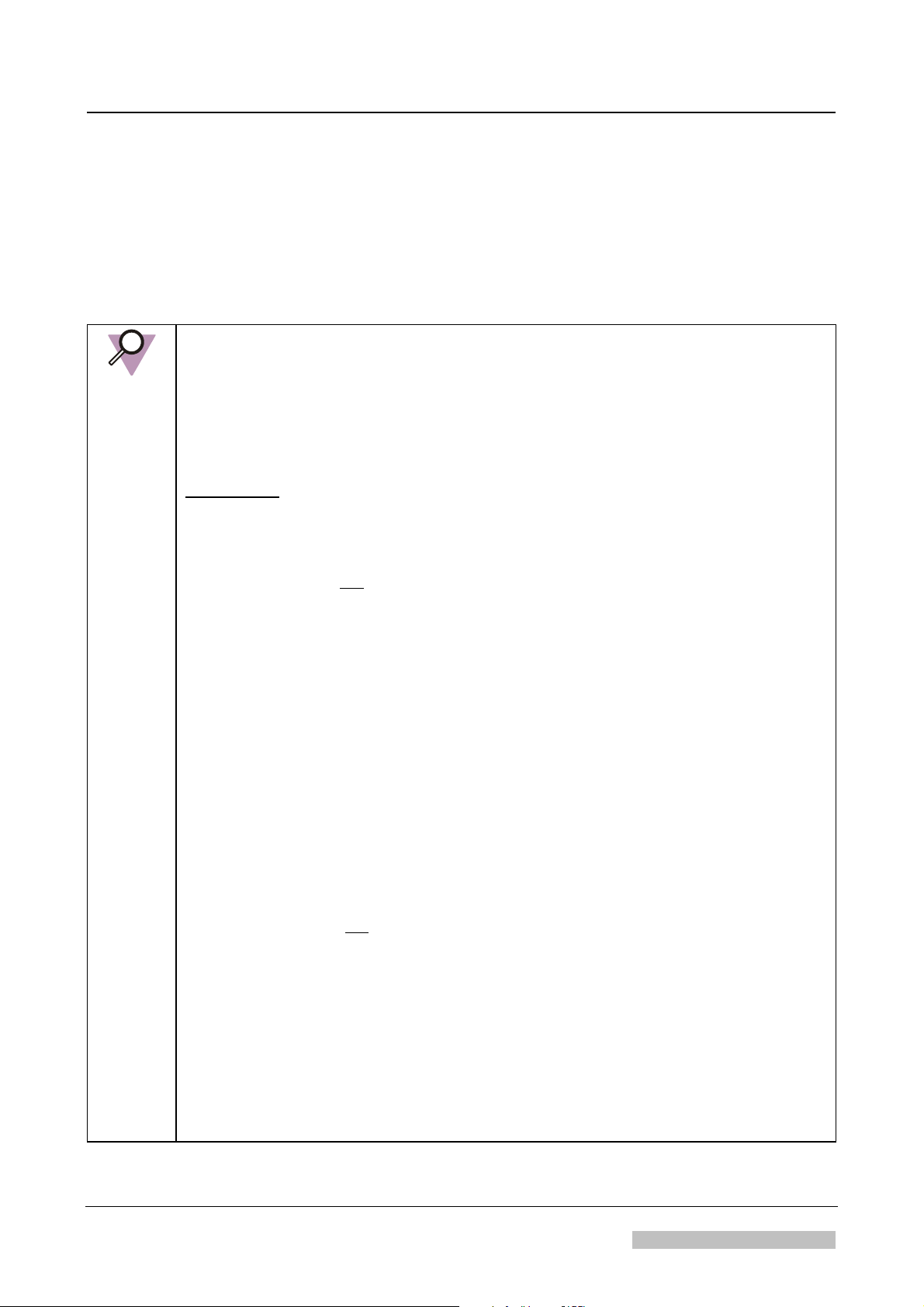
Not-safety relevant notes
Icon Name Type of Information
INSTRUCTION: Indicates an instruction where it is important to follow literally the
described actions.
IMPORTANT: Highlights very important actions which have to be carried out to
NOTE:
prevent malfunction.
• Indicates advice to facilitate the following step or action without
having a direct influence on the step or action.
• Highlights unusual points
• Indicates background information
• Can be used to explain or highlight displays of the graphical user
interface.
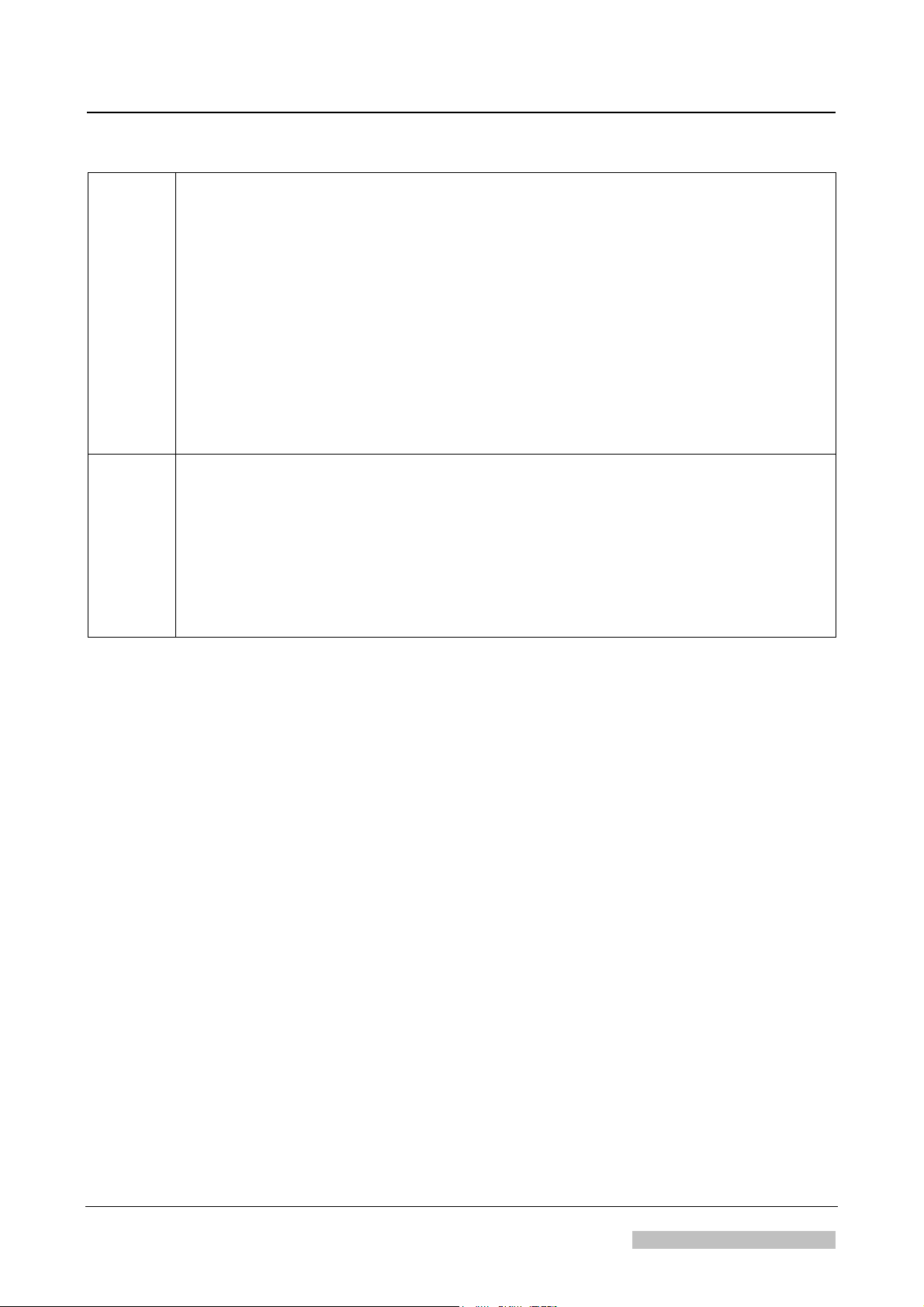
► Conventions
Actions Way of writing Sample
Action,
explanation
Action with the mouse or the
"Return" key
Required text input via the
keyboard
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 5
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential
Switch the machine on Switch the machine on
<omni-cd.exe>
Double-click the
<omni-cd.exe> icon
vips
Enter vips and click on
<Continue>
DOCUMENT CONTROL NOTE:
DD+DIS378.05E
1 About this Manual
2nd Edition CR 85-X Type 5148/100 – CR 75.0 Type 5146/105
Service Documentation (DD+DIS378.05E)
IMPORTANT:
nd
The 2
CR 85-X Type 5148/100 and CR 75.0 Type 5146/105 , DD+DIS378.05E is valid for:
• CR 85-X – Type 5148/100
• CR 75.0 – Type 5146/105 (as of SN ≥ 6000)
Edition of the Service Documentation for
About this Manual
Explanation:
The improved detector unit (light guide and photomultiplier) from CR 85-X Type
5148/100 is integrated in CR 75.0 and hence the new subtype CR 75.0 Type
5146/105 has been created.
The improvement is not
available for:
• ADC Compact Plus Type 5146/100
• CR 75.0 Type 5146/101
• Centricity CR MP3510 Type 5146/200 and 201
The Digitizer CR 75.0 Type 5146/105 has been introduced in production and can be
distinguished from ADC Compact Plus Type 5146/100, CR 75.0 Type 5146/101 and
Centricity CR MP3510 Type 5146/200 and 201 by the type label:
• Type: 5146/105
• Serial Number SN: ≥ 6000
Note that the CR 85-X / CR 75.0 Type 5146/105 Service Documentation
(DD+DIS378.05E) is not
valid for:
• ADC Compact Plus Type 5146/100
• CR 75.0 Type 5146/101
• Centricity CR MP3510 Type 5146/200 and 201
For the ADC Compact Plus Type 5146/100, CR 75.0 Type 5146/101 and
Centricity CR MP3510 Type 5146/200 and 201 only the
CR 75.0 Service Documentation, DD+DIS002.04E and Spare Part List,
DD+DIS007.04M must be used.
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
DOCUMENT CONTROL NOTE:
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 6
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential

DD+DIS378.05E
Reason
for the
nd
Edition
2
The 2nd Edition of this Documentation has been released due to the fact that the
CR 85-X Service Documentation from now on is also valid for
CR 75.0 Type 5146/105.
Only the layout of the Service Documentation and product specific safety notes have
been adapted. No other changes of the content have been made.
Product
Description
The CR 85-X Type 5148/100 digitizer is the follow-up model of the
CR 75.0 Type 5146/100 digitizer.
It has been designed for General Radiology environments and, particularly, for the
CR Mammography 1C Solution.
The main changes between CR 75.0 Type 5146/100 and
CR 85-X Type 5148/100 are:
• Acrylic light guide with glued PMT (PhotoMultiplier Tube) is used instead of
About this Manual
optical fibers.
Download
from
MedNet
This Service Documentation is available on the MedNet GSO Library.
Path: Computed Radiography / CR Digitizers / CR 85-X
Features of
the
Digitizer
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 7
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential
The CR 85-X Type 5148/100 (CR 75.0 Type 5146/105) scans the exposed
CR image plate, converts the information into digital data and automatically transfers
the image to the image processing station for further processing and visualization.
The digitizer requires but little manual interaction. All you have to do, after exposure
and identification of the cassette, is to place it in the input buffer of the digitizer. You
can deposit up to 10 cassettes of different sizes simultaneously in the input buffer.
The Digitizer takes in the cassettes one by one. The Digitizer reads the demographic
data and routing information from the memory chip in the cassette, opens the
cassette, removes the image plate and scans the latent image by means of a
sweeping laser beam.
Once the image is digitized, the cassette is returned to the output buffer to be used
for new exposures. After a full Digitizer cycle, the plate has turned 180° in the
cassette.
Depending on the X-ray intensity which has affected the phosphor during the
exposure, more or less light will be emitted during laser scanning. The light is
converted into an electrical signal. This signal is then converted into a digital bit
stream.
Once converted into digital form, the digitized image is transferred to the image
processing station for further processing and visualization.
DOCUMENT CONTROL NOTE:
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
DD+DIS378.05E
Further features of the digitizer include:
• The digitizer permits assigning the status “emergency” to an image. An
• The digitizer permits re-erasing an image plate before re-using it. In specific
Intended
Use of the
Digitizer
This device must only be used to scan exposed X-ray cassettes, containing an
erasable image plate (IP).
This device is part of a system, consisting of X-ray cassettes with erasable phosphor
image plates, an identification station for the cassettes and a workstation where the
resulting digital image information is further processed and routed.
It is intended that this device is only operated in a radiological environment by
qualified staff.
About this Manual
emergency image will be given priority by the image processing station.
cases, this is necessary to prevent ghost images caused by previous
exposures or stray radiation from interfering with the image of interest. You can
erase a batch of
up to 9 image plates.
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
DOCUMENT CONTROL NOTE:
Edition 2, Revision 0 CR 85-X Type 5148/100 Chapter 0 / 8
02-2007 CR 75.0 Type 5146/105 (as of SN ≥ 6000) Agfa Company Confidential


Document No: DD+DIS378.05E
Copyright 2007 Agfa-Gevaert HealthCare
All rights reserved.
Technical modifications reserved.
Published by
Agfa-Gevaert HealthCare GmbH
Tegernseer Landstraße 161
D - 81539 München
Germany
AGFA and the Agfa-Rhombus are trademarks of Agfa-Gevaert NV
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
Agfa Company Confidential
DOCUMENT CONTROL NOTE:
HEALTHCARE
Imaging Services
Document No: DD+DIS238.06E
► Purpose of this Document
Generic Safety Directions
for HealthCare Imaging Products
This Generic Safety Directions document comprises the general safety relevant information including
relevant environmental and occupational safety instructions for the Service Engineer.
It is valid for all Agfa HealthCare Imaging Products and part of each Service Documentation as well as
Installation Planning document.
The latest version is available via MedNet, GSO Library path:
General Info => Agfa HealthCare => Publications => Service Manual
Generic Safety Directions
► Document History
Edition.
Revision
1.3 07-2009
Release
Date
Changes
compared to previous Revision 1.2:
• Updated table with laser classification to latest changes of the
corresponding standard. See section 3.3.
• Added section Environmental and occupational Safety Instructions.
See section 9.
• Added safety note concerning inroom installations of CR equipment
and corresponding X-ray shielding. See section 17.
• Added laser safety note and safety note concerning electrical checks
after repairs. See section 19.
• Added treatment for Lithium batteries in sections 19 and 21.
• Updated information concerning the recycling pass. See section 24.
► Referenced Documents
Document Title
Not applicable Not applicable
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
Edition 1, Revision 3
07-2009 printed in Germany
Agfa Company Confidential
DOCUMENT CONTROL NOTE:
Document Node ID: 11849633
eq_generic_safety_directions_e_template_v01
Copyright © 2009 Agfa HealthCare N.V.

DD+DIS238.06E
► Manufacturer
Agfa HealthCare N.V.
Publisher
Agfa-Gevaert HealthCare GmbH
Tegernseer Landstraße 161
D - 81539 München
Germany
Copyright © 2009 Agfa HealthCare N.V.
All rights reserved.
Technical modifications reserved.
AGFA and the Agfa-Rhombus are trademarks of Agfa HealthCare N.V.
Generic Safety Directions
WARNING:
Improper operation or service activities may cause damage or injuries.
INSTRUCTION:
(1) Read the "Generic Safety Directions" document
(see MEDNET GSO => General Info => Agfa HealthCare => Publications =>
Service Manual) prior to attempting any operation, repair or maintenance task on the
equipment.
(2) Strictly observe all safety directions within the "Generic Safety Directions" and on
the product.
The controlled version of this document resides on MedNet. Any printed copy of this document is uncontrolled.
Edition 1, Revision 3 Generic Safety Directions for HealthCare Imaging Products Page 2 of 28
07-2009 Agfa Company Confidential
DOCUMENT CONTROL NOTE:
 Loading...
Loading...