Page 1

3B SCIENTIFIC3B SCIENTIFIC
3B SCIENTIFIC®
3B SCIENTIFIC3B SCIENTIFIC
U14330 Leclanché cell
Instruction sheet
8/05 ALF
PHYSICSPHYSICS
PHYSICS
PHYSICSPHYSICS
1
2
3
4
®
... going one step further
1 Carbon electrode with 4-mm socket
2 4-mm socket
3 Lid
4 Zinc electrode
5 Glass vessel
1. Safety instructions
• Caution! Heavy metal salts are toxic.
• Safety goggles are a must when working with acids or
alkalis.
• Students must always be thoroughly informed about
the hazards of the chemicals used.
• Leaking fluid can cause permanent stains and holes
in clothing.
• The apparatus must be thoroughly cleaned after the
experiment.
• Applicable regulations must be strictly adhered to
when disposing of the chemicals.
5
2. Description, technical data
The model for a dry cell battery was invented around
1860 by French chemist Georges Leclanché. The cell named
after him, which continues to be the most common battery even today, uses manganese dioxide and is not rechargeable. The Leclanche cell consists of a cylindrical
zinc electrode, a carbon electrode, a ceramic cell and a
glass vessel. The cell as supplied has no filling. When
filled, the Leclanche cell provides a voltage of
approx. 1.5 V.
Connections: via 4-mm sockets
Dimensions: 175 mm high, 65 mm Æ
3
Page 2
2.1 Scope of delivery
1 Glass vessel
1 Clay cylinder
1 Lid
1 Zinc electrode with socket
1 Carbon electrode with socket
3. Principle
The combination of two half-cells for the purpose of
converting chemical energy into electrical energy is called
a galvanic cell. In a Leclanche cell, a zinc electrode forms
the negative pole and a carbon rod with the manganese
dioxide (MnO2) coating forms the positive pole. In the
space between, ammonium chloride is used as an electrolyte. The ensuing chemical reaction chiefly results from
the oxidation of zinc and reduction of manganese dioxide.
Oxidation:
+
Zn+2 NH Zn NH 2e 2H
Reduction:
2 MnO 2 H 2 e Mn O H O
Redox reaction:
Zn+2 NH 2 Mn O Zn NH Mn O H O
→++
43
++→ +
2232
+
42 3 232
()
−
+
+→ ++
+
2
2
()
+
−
+
2
2
The reactions shown here are simplified. They are far
more complicated in reality. The reaction ceases when
the manganese dioxide has been used up.
4. Operation
• To construct a Leclanché cell requires the following:
Ammonium chloride solution (NH4Cl), approx. 20%
Manganese dioxide (powder) (MnO2)
Graphite (powder)
• Mix the manganese dioxide powder and some graphite powder in a beaker. Then add the ammonium
chloride solution and stir the mixture to form a paste.
• Position the zinc electrode into the glass vessel and
place the ceramic cylinder inside.
• Position the carbon electrode in the centre of the
ceramic cylinder and fill up all remaining space with
the manganese dioxide paste.
• Fill up the glass vessel with the 20% ammonium chloride solution and cover it with the lid.
• The apparatus and electrodes must be thoroughly
cleaned immediately after the experiment.
• Chemicals which cannot be reused must be stored in
special vessels and disposed of in an orderly fashion
afterwards, strictly adhering to applicable regulations.
4
5
6
7
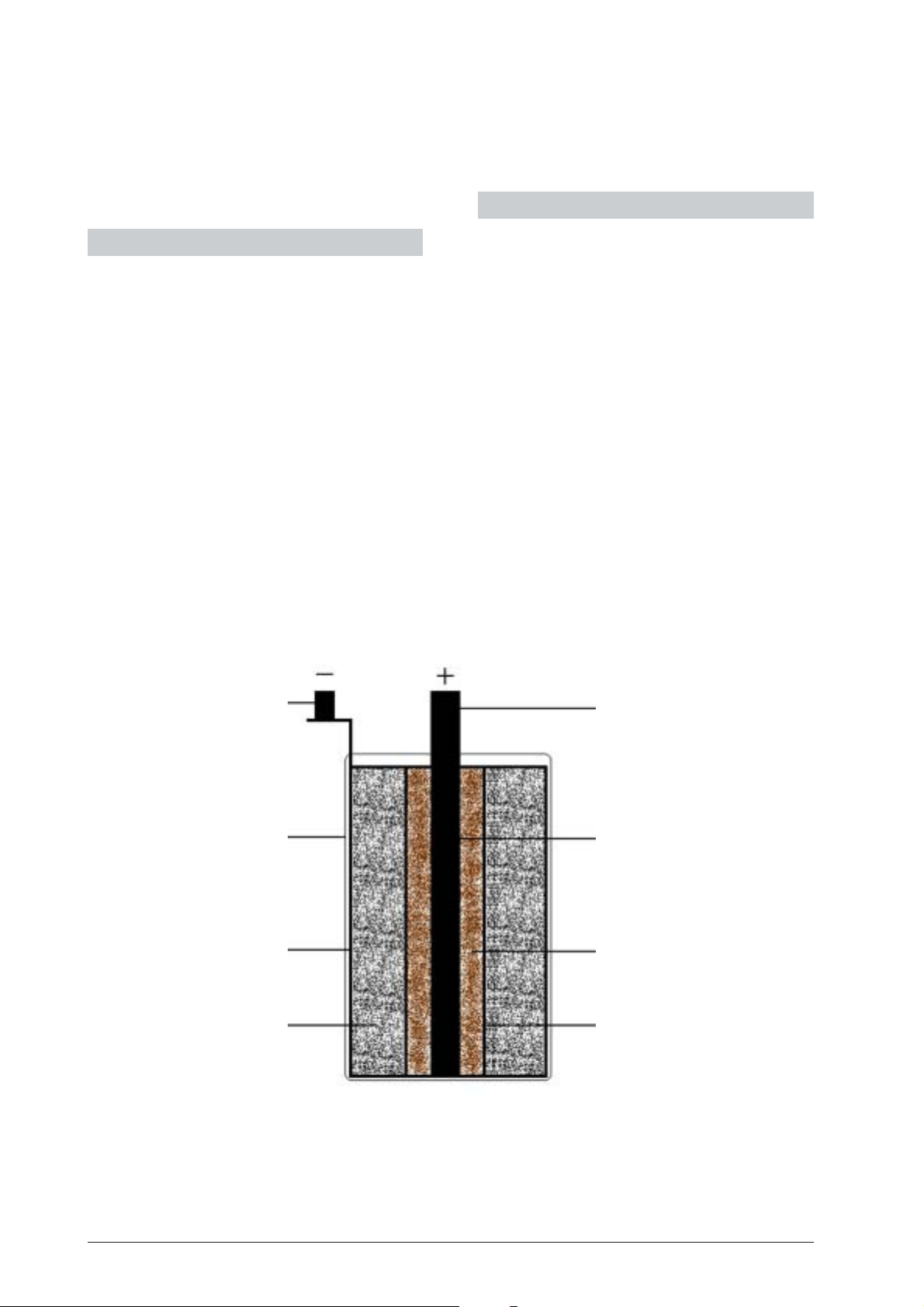
Fig. 1: Leclanché cell
4
3
2
1
1 Ceramic cylinder
2 Manganese dioxide coating
3 Carbon electrode
4 4-mm socket
5 Glass vessel
6 Zinc cylinder
7 Ammonium chloride
solution
3B Scientific GmbH • Rudorffweg 8 • 21031 Hamburg • Germany • www.3bscientific.com • Technical specifications subject to change
4
 Loading...
Loading...