Page 1
PSS World Medical, Inc.
4345 Southpoint Blvd.
Jacksonville, FL 32216
Select Medical Products
Artwork Template
Aneroid
Sphygmomanometer
Doc. # T-GS-003
R ev. 1
Eective Date: 10-08-2007
Author: Meghan Little
Blood pressure measurements determined with this device are equivalent
to those obtained by a trained observer using the auscultatory method, within
the limits prescribed by:
• American National Standard ANSI/AAMI SP9, 1994, Non-automated
sphygmomanometers.
• American National Standard ANSI/AAMI SP10, 2002, Manual, electronic,
or automated sphygmomanometers (FlexiPort cuff only).
Introduction
Intended Use
Aneroid sphygmomanometers are used by professional healthcare providers
and individuals trained in auscultatory blood pressure technique to determine
systolic and diastolic blood pressure in humans and animals.
Contraindications
Aneroid sphygmomanometers are contraindicated for neonate use. Do not
use with neonatal cuffs or neonate patients.
Warnings
A warning statement in this manual identifies a condition or practice which, if
not corrected or discontinued immediately, could lead to patient injury, illness,
or death.
WARNING: U.S. Federal law restricts this device to sale by or on the order of a
physician.
WARNING: If luer lock connectors are used in the construction of tubing, there
is a possibility that they might be inadvertently connected to intravascular fluid
systems, allowing air to be pumped into a blood vessel. Immediately consult a
physician if this occurs.
WARNING: Do not allow a blood pressure cuff to remain on patient for more
than 10 minutes when inflated above 10 mm Hg. This may cause patient
distress, disturb blood circulation, and contribute to the injury of peripheral
nerves.
WARNING: Safety and effectiveness with neonate cuffs (sizes from neo 1 to
neo 5) is not established.
WARNING: Use only Welch Allyn manufactured blood pressure cuffs and
accessories; substitution might result in measurement error.
WARNING: Only use the cuff when visible artery index marker falls within the
range markings indicated on the cuff, otherwise erroneous readings may
result.
WARNING: Do not apply cuff to areas on patient where skin is delicate or
damaged. Check cuff site frequently for irritation.
WARNING: Allow space for 1 to 2 fingers between patient and cuff.
WARNING: Do not apply cuff to limbs used for IV infusion.
WARNING: Minimize cuff movement and limb motion during readings.
WARNING: Ensure an airtight seal at all connection points prior to use.
WARNING: Intravenous Systems (IV) - Do not connect cuffs with luer lock
connectors to intravenous fluid systems or air may enter patient.
Cautions
A caution statement in this manual identifies information within the manual to
avoid equipment failure.
CAUTION: Do not press cuff with a hot iron.
CAUTION: Do not allow foreign debris to ingress into tubes or port on cuff.
CAUTION: Do not use steam or heat to sterilize the cuff, or tubing.
CAUTION: Intravenous Systems (IV) - Do not connect cuffs with luer lock
connectors to intravenous fluid systems or fluid may enter the cuff.
CAUTION: Do not inflate the cuff unless the hook and loop is closed.
Directions for Use
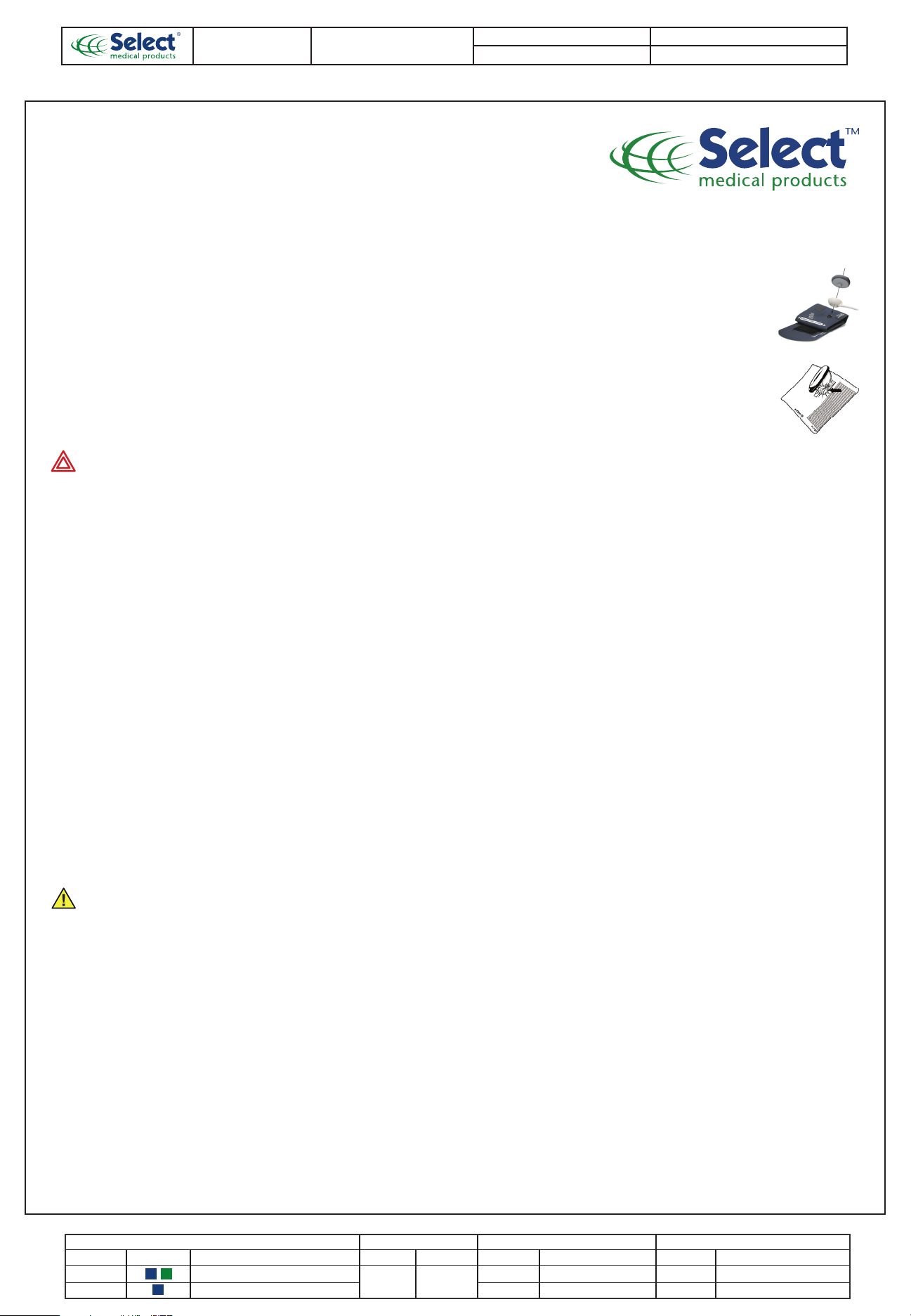
Connections
PSS-44
1. Attach the inflation bulb to the tube (if needed).
Use alcohol to facilitate this.
2. Align and press the Durashock gauge with the FlexiPort
adapter onto the cuff port.
3. Verify an airtight seal is achieved at all connection points.
PSS-45
1. Push the gauge stem into the adapter port until you feel
it engage.
2. Connect the short cuff tube to the barb on the adapter.
3. Connect the barb on the inflation bulb valve to the long cuff tube.
4. Make certain an airtight seal is achieved at all connection points.
Operation
Blood pressure measurements can be affected by the position of the patient
and their physiologic condition. Before beginning a measurement, ensure that
the patient rests for at least five minutes, has support of their back and feet,
and does not cross their legs. Passively support the patient’s lower arm and
keep the upper arm at heart level. The procedure needs to take place in a
quiet environment with no talking. Failure to follow these recommendations
can result in inaccurate blood pressure measurements.
1. Select cuff size appropriate for the patient’s arm circumference.
The applicable range, in centimeters, is printed on each cuff.
NOTE: The “Artery Index Marker” on the cuff should fall within the “Range”
indicated on the cuff. If the artery index marker falls short of the
range, use a larger cuff to ensure accurate results. If the artery
index marker is past the range, use a smaller cuff to ensure
accurate results.
2. Wrap the cuff around the arm with the artery index marker located over the
brachial artery and with the lower edge of the cuff 2.5 cm above the bend
in the elbow.
3. Inflate cuff rapidly to a level 30 mm Hg above estimated (or palpatory)
systolic pressure.
4. Partially open the valve to allow deflation at a rate of 2 to 3 mm Hg per
second. As the pressure falls, note systolic pressure and diastolic pressure
detected with your stethoscope.
5. Rapidly release the remaining pressure and record measurements
immediately. After a minimum of 30 seconds, repeat the above steps for a
second reading.
Specifications
The aneroid sphygmomanometer is accurate to +/-3 mm Hg.
This product will maintain the safety and performance characteristics specified
at temperatures ranging from 0° C to 46° C at a relative humidity level not to
exceed 85%.
Standards
• American National Standard ANSI/AAMI, SP9: 1994, Non-automated
sphygmomanometers.
• American National Standard ANSI/AAMI SP10, 2002, Manual,
electronic, or automated sphygmomanometers (except for section
4.2.2a for size 13 disposable cuffs)(FlexiPort cuff only).
• European Standard EN 1060-1: 1995, Non-invasive sphygmomanometers
- Part 1: General Requirements (except for section 9.3a for sizes 6, 7, 8, 9
disposable and reusable cuffs).
• European Standard EN 1060-2: 1996, Non-invasive sphygmomanometers
- Part 2: Supplementary requirements for mechanical sphygmomanometers.
• European Standard EN 1060-3: 1997, Non-invasive sphygmomanometers
- Part 3: Supplementary requirements for electro-mechanical blood pressure
measuring systems (FlexiPort cuff only).
Wav e
Logo
Text
PMS Colors
PMS 281 C Blue, 356 C Green
N/A
Revisions
Draft 3
Working Draft
05-30-08
Label
Information
Multiple Sku’s
BP Cus
1:1 Proportion, 8.5 x 11”
Re v.
Description
Date
Artwork Approvals
Drawn By
K
Checked By
Meghan Little
Description
Scale; Dimensions
Page 2

Maintenance
Cleaning
Aneroid Gauge, Inflation Bulb, and Valve: Wipe the aneroid gauge, inflation
bulb, and valve with slightly dampened cloth or alcohol pad.
Reusable One-Piece Cuff: Use one or more of the following methods and allow
to air dry:
• Wipe with mild detergent and water solution (1:9 solution). Rinse.
• Wipe with Enzol ® per manufacturer’s instructions. Rinse.
• Wipe with 0.5% bleach and water solution. Rinse.
• Wipe with 70% isopropyl alcohol.
• Launder with mild detergent in warm water (60°C/140°F maximum), normal
wash cycle. Cuff is compatible with 5 wash cycles (Reusable only). Close
port cuff with laundering plug (REF 5082-159).
Warranty
Your Select
original defects in material and workmanship and to perform in accordance
with manufacturer’s specifications under normal use and service. The warranty
period* begins from the date of purchase from the company or its authorized
distributors. The company’s obligation is limited to the repair or replacement of
components determined by the company to be defective within the warranty
period. These warranties extend to the original purchaser and cannot be
assigned or transferred to any third party. This warranty shall not apply to any
damage or product failure determined by the company to have been caused
by misuse, accident (including shipping damage), neglect, improper
maintenance, modification, or repair by someone other than the company or
one of its authorized service representatives.
*Gauge Warranty
TM
Medical product, when new, is warranted to be free from
Two-Piece Cuff and Bladder: Safely clean the cuffs with a damp cloth or wash
in warm water (60°C/140°F maximum) with mild detergent. DO NOT PRESS
WITH HOT IRON.
Before laundering the cuff:
1. Remove the bladder from the two-piece cuffs.
2. Place the hook and loop fasteners in the closed position.
3. Machine launder using gentle cycle, warm water and mild detergent.
4. Air dry completely and reassemble components.
Low-level disinfection procedure (FlexiPort Reusable cuffs only)
Prepare Enzol ® enzymatic detergent according to manufacturer's instructions. Apply port-cap (REF 5082-159) to cuff. Spray detergent solution liberally
onto cuff and use a sterile brush to agitate the detergent solution over entire
cuff surface for five minutes. Rinse continuously with distilled water for five
minutes. To disinfect, first follow the cleaning steps above, then spray cuff with
10% bleach solution until saturated, agitate with a sterile brush over entire cuff
surface for five minutes. Rinse continuously with distilled water for five minutes.
Wipe off excess water with sterile cloth and allow cuff to air dry.
Calibration Check of Aneroid Sphygmomanometer
Quick Check of Calibration:
At zero pressure, make certain the pointer is within the oval surrounding the
zero-pressure gradation on the dial. Although an unpressurized reading of zero
does not guarantee accuracy at all scale points, failure of the pointer to
indicate zero (± 3 mm Hg) is an obvious sign of error.
Full Check of Calibration:
The company recommends a full check of calibration at least every two years
or according to local law and after maintenance and repair. Use the following
procedure:
1. Connect the instrument under test to a high quality, known pressure
standard and a 150 to 500 cc test volume using a T-connector.
2. Pressurize gauge to slightly above 300 mm Hg.
3. Bleed pressure down no faster than 10 mm Hg per second, stopping to
check the pressure at 300, 250, 200, 150, 100, 50 (60 for US) and 0 mm Hg.
NOTE: Your ability to measure the accuracy of a gauge depends upon the
sensitivity of the pressure standard you use for the calibration
procedure.
Should the aneroid sphygmomanometer deviate from the +/-3 mm Hg
accuracy specification during the warranty period, the company will
recalibrate the sphygmomanometer at no charge. For questions, contact your
TM
Medical Products representative for assistance.
Select
Gauge: Model PSS-45: Ten-year warranty
Model PSS-44: Three-year warranty
*Accessory Warranty: Inflation Bulb and Valve: One year
FlexiPort One-piece Blood Pressure Cuff: Three years
Two-piece Blood Pressure Cuff: Two years
These express warranties are in lieu of any and all other warranties, express or
implied, including the warranties of merchantability and fitness for a particular
purpose, and no other person has been authorized to assume for the
company any other liability in connection with the sale of the product. The
company shall not be liable for any loss or damages, whether direct,
incidental, or consequential, resulting from the breach of any express
warranty, except as set forth herein.
© 2008 Welch Allyn, Inc. All rights reserved. No one is permitted to reproduce
or duplicate, in any form, this manual or any part thereof without permission
from Welch Allyn. Welch Allyn assumes no responsibility for any injury to
anyone, or for any illegal or improper use of the product, that may result from
failure to properly use the product in accordance with the instructions,
cautions, warnings, or statement of intended use published in this manual.
Welch Allyn, FlexiPort™, and Durashock™ are trademarks of Welch Allyn, Inc.
ENZOL is a trademark of Advanced Sterilization Products.
Questions or Comments about Select Medical Products? 800-777-4908
Manufactured for PSS World Medical, Inc.
4345 Southpoint Blvd., Jacksonville, FL 32216
Material # 711395 Ver. A
U.S. Patents 6,578,428; 6,036,718; and additional patents pending.
If using a manometer (mercury column or aneroid gauge) rated at +/-3.0 mm
Hg, an undetectable error of up to 6.0 mm Hg is possible. If using a device
(e.g., digital pressure standard) rated at +/-0.1 mm Hg, an undetectable error
of up to only 3.1 mm Hg is possible.
The company recommends using as sensitive as possible a pressure standard
when performing calibration checks. A Setra Pressure Meter (REF 2270-01),
which is calibrated for +/-0.1 mm Hg, or Netech (REF 200-2000IN), which is
calibrated for +/-1.0 mm Hg, works well for this application. Each meter is
available. Contact your local PSS distributor.
 Loading...
Loading...