Page 1

ABPM 7100
Ambulatory Blood Pressure Monitor
Directions for Use
Page 2

2
© 2014 Welch Allyn, Inc. To support the intended use of the product described in this publication, the
purchaser of the product is permitted to copy this publication for internal distribution only, from the media
provided by Welch Allyn.
Caution: Federal US law restricts sale of the device identified in this manual to, or on the order of, licensed
physicians.
Welch Allyn does not accept liability for injuries or unlawful or improper use of the product which may result
from the fact that the product is not used in accordance with the instructions, cautions and warnings, as well
as the indications for use published in this manual.
Welch Allyn is a registered trademark of Welch Allyn, Inc., ABPM 7100 and CardioPerfect are trademarks of
Welch Allyn, Inc.
The copyright for the firmware in this product remains with the manufacturer of this device. All rights
reserved. The firmware may not be read out, copied, decompiled, redeveloped, disassembled or brought into
any human-readable format. This does not pertain to the sales of firmware or a firmware copy. All usage and
ownership rights to the Software remain with I.E.M. GmbH.
Welch Allyn Technical Support:
http://www.welchallyn.com/about/company/locations.htm
I.E.M. GmbH
Cockerillstr. 69
52222 Stolberg
Germany
Manufactured for Welch Allyn
Ende der Liste für Textmarke Copyright === Pos: 4 /_Steuerung/Inhaltsverzeichnis @ 0\ mod_1402574713889_6.docx @ 5059 @ 1 @ 1
Page 3

Table of contents - 3
Table of contents
Symbols 4
Introduction 5
Preliminary note 5
About these directions for use 5
Clinical trials 5
CE Mark 5
Content 6
Direction for use 6
Intended use 6
Indications for use 6
Improper use 6
Essential Performance 7
Side effects of 24-hour blood pressure monitoring 7
Product description 7
Introduction 7
The ABPM 7100 8
Technical Data 10
Preparing the ABPM 7100 11
Safety instructions 11
Inserting the batteries 12
Activating the device 13
Setting the time / date 13
Clearing the memory 13
Transferring patient data (ID) 14
Setting measurement logs 14
Selecting a suitable cuff 15
Applying the ABP Monitor and cuff 15
Connecting the cuff tubing to the ABPM 7100 17
Positioning the patient for measurement 18
Measurement process 18
Safety instructions 18
Initial measurement 20
24-hour measurement 20
Performing a measurement 20
Cancelling a measurement 21
Unsuccessful measurement 21
Care and Maintenance 21
Cleaning and disinfection 21
Maintenance plan 22
Troubleshooting 23
Basic error sources 23
Transmission error 23
Checklist 23
Error codes 24
Limited Warranty 27
Service Policy 27
EMC Guidelines and Manufacturer Declaration 28
Patient Information - operation of the ABPM 7100 31
=== Ende der Liste für Textmarke TOC ===
Pos: 6 /Sammel/01_Einführung @ 0\mo d_1398690513744_6.docx @ 4730 @ 122222233 @ 1
Page 4
4 - Symbols
Symbols
Documentation symbols
WARNING The warning statement
identifies an immediate threat. Nonadherence may lead to the most severe
injuries and to death
CAUTION The caution statement
identifies a possible hazard. Nonadherence may lead to minor or
moderate injuries
Attention
The attention statement marks possible
material damage. Non-adherence may
lead to damage to the device or its
accessories
Note
The note statement marks further
information on the ABPM 7100 or its
accessories
INTERNAL REFERENCE Marks
references within the document to further
information
EXTERNAL REFERENCE Marks
references to external documents
containing further optional information
Mandatory – Consult Directions for Use
Meets essential requirements of
European Medical Device Directive
93/42/EEC
Consult Directions for Use, Electronic
version available at Welchallyn.com, or
Hard copy DFU available from Welch
Allyn within 7 days.
Power symbols
Battery symbol indicates the type of
power supply
Nonionizing electromagnetic radiation
Connectivity symbols
FCC ID and IC
Bluetooth Connectivity
Shipping, storing and environment symbols
Separate the device from other
disposables for recycling.
See www.welchallyn.com/weee
Miscellaneous symbols
Manufacturer
yyyy-mm
Date of manufacture
Reference/Model number
Serial number
Reorder/Catalog number
Batch code
Global Trade Item Number
Protection class
Defibrillation-proof type BF applied part
Page 5

Introduction - 5
Introduction
Preliminary note
With the ABPM 7100 24-hour blood pressure measuring device, you now have an Ambulatory Blood
Pressure Monitoring System (ABPM System) at your disposal.
The ABPM 7100, also specified as ABP Monitor, can be prepared for a new patient in just a few minutes.
This permits the optimum use of the ABP Monitor and allows you to process one 24-hour profile per day.
The ABPM 7100 can therefore be quickly integrated into everyday practice life. The recorded blood pressure
values must be evaluated with the intended software.
In combination with the Hypertension Management Software and an appropriate license, the ABPM 7100 is
also able to process a haemodynamic analysis of the recorded pulse waves.
About these directions for use
These directions for use will familiarize you with the use of the ABPM 7100 and its accessories.
The software CardioPerfect Workstation (CPWS) is used for measurement evaluation.
Upgrades for haemodynamic evaluation may also be purchased from Welch Allyn. Please contact Welch
Allyn for further information.
With reference to specific version characteristics, only the parts relevant for your respective version will
apply.
Please refer to the CPWS directions for use for software operating instructions.
For the upgrades please refer to the respective directions for use to operate the
Hypertension Management Software (HMS), version 5.0 and above.
Note
These directions for use explain the ABPM 7100 and its accessories in the sequence in which
you setup the device for a blood pressure measurement, followed by the installation, initial
operation, measurement preparation, placement on the patient and the evaluation. Individual
functions are only explained when they are needed. You will therefore be familiarized with the
ABPM 7100 on a step-by-step basis.
This directions for use must be kept with the product for later use!
Clinical trials
The blood pressure measuring device ABPM 7100 fulfills the requirements of the ESH (European Society of
Hypertension).
The blood pressure measuring device ABPM 7100 fulfills the requirements of the BHS (British Hypertension
Society).
CE Mark
The ABPM 7100 fulfills the requirements of the following directives:
Directive 93/42/EEC (MDD)
Directive 1999/5/EC (R&TTE)
Directive 2011/65/EU (RoHS)
The ABPM 7100 bears the CE mark.
Page 6

6 - Direction for use
Content
Standard
1. ABPM 7100 Monitor
2. Pressure Cuff – Size “Adult”
3. Carrying Pouch
4. PC Interface Cable
5. 4x AA Alkaline Batteries
6. ABPM 7100 Directions For Use
7. Calibration Notice
8. CPWS Software (dependent on set)
9. Pressure Cuff – Size “Adult Plus” (dependent on set)
HMS Option
1. HMS Software
2. Bluetooth®-Dongle
3. Quick Start Guide (dependent on upgrade
option)
4. Version dependent 16 digit License Code
(dependent on upgrade option)
Warning
Risk of injury posed by the use of other accessories. The use of unapproved accessories may
lead to incorrect measurement results.
Only use accessories approved and distributed by the manufacturer and Welch Allyn.
Check the accessories regarding the manufacturer’s information before first use.
Pos: 8 /Sammel/02_Gebrauchshinweise @ 0\mod_1398692778439_6.docx @ 4732 @ 1 2222 @ 1
Direction for use
Intended use
The ABPM 7100 is intended for clarifying the blood pressure status and for use as a diagnostic aid for an
individual adult patient (in the patient’s environment). The ABPM 7100 may only be used under medical
supervision and after detailed instruction has been provided by the doctors or healthcare professionals. The
ABPM 7100 in combination with the Hypertension Management Software (HMS) provides a derived
ascending aortic blood pressure wave form and a range of central arterial indices.
Indications for use
The ABPM 7100 is an automated microprocessor controlled ambulatory blood pressure monitor (ABPM)
which monitors, accumulates and stores: heart beat (rate), systolic and diastolic data of an individual
adult patient (in the patient’s environment) for a session which may last 24 hours.
The ABPM 7100 in combination with the Hypertension Management Software (HMS) provides a derived
ascending aortic blood pressure wave form and a range of central arterial indices.
It is used with a standard cuff for blood pressure measurement.
It is used in those patients where information related to the ascending aortic blood pressure is desired
but in the opinion of the physician, the risk of cardiac catheterization procedure or other invasive
monitoring may outweigh the benefits.
Improper use
The ABPM 7100 may not be used for any purpose other than for the blood pressure monitoring
procedures described here!
Due to the strangulation risk posed by tubing and cuff, the ABPM 7100 may not be used on patients
with limited cognitive abilities, patients under anesthetics and must not be placed within reach of
unsupervised children.
The ABPM 7100 must not be used on neonates and children under the age of 3 years. The device has
not been tested with pregnant women, including preeclamptic patients.
The ABPM 7100 must not be used for blood pressure monitoring purposes during surgeries.
The ABPM 7100 is not intended for alarm-triggering monitoring purposes in intensive care units.
Page 7

Product description - 7
Essential Performance
The main performance features are defined as blood pressure measurement with:
Error tolerances of the pressure gauge and measurement results are within required limits (IEC
80601-2-30).
Maximum change value in blood pressure determination is as specified in IEC 80601-2-30.
Cuff pressurization remains within specified limits (IEC 80601-2-30).
An error is issued in the event that successful blood pressure measurement is impossible.
The ABPM 7100 does not issue ALARMS pursuant to IEC 60601-1-8 and is not intended for use in
connection with HF surgical equipment or to clinically monitor patients in intensive care units.
Basic safety means that the patient cannot be endangered by any automatic device procedure. During any
unclear conditions, the ABPM 7100 must therefore transfer into the safe Standby mode, during which the
ABPM 7100 cannot automatically inflate the cuff, while this can be manually triggered by pushing the START
button.
In this context, any interruption of a measurement or in automatic operation by an external influence, or the
ability of the ABPM 7100 to test error conditions, is regarded as the retention or restoration of basic safety,
and not as non-adherence to the main performance features.
Pos: 9 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_1399967957 149_6.docx @ 4804 @ @ 1
Side effects of 24-hour blood pressure monitoring
24-hour blood pressure monitoring is a commonly practiced, accepted and valued measurement
technique, used in daily diagnostics and treatment monitoring.
When considering 24-hour blood pressure monitoring, always check whether the patient displays
coagulation disorders or is being treated with anticoagulants. As with occasional blood pressure
measurements, petechial haemorrhage (bleeding) may occur on the measured arm despite a correctly
seated cuff. The innate patient-dependent risk resulting from treatment with anticoagulants or in patients
with coagulations disorders arises irrespective of the type of monitoring device.
Pos: 10 /Sammel/03_Sicherheit @ 0\ mod_1398780831924_6.docx @ 4755 @ 222 @ 1
Pos: 11 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Product description
Introduction
The ABPM 7100 System consists of two main components:
The ABPM 7100 with cuffs and accessories
Patient management software for the doctor to evaluate the measurement results
With the CPWS software the ABPM 7100 can be prepared for measurement, transfer stored measurement
results to the PC, display transferred measurements on the screen in various formats such as graphics, lists
and statistics and print out measurement results. Optional is the possibility to evaluate the measurement
results with the HMS and its upgrades.
The ABPM 7100 can be prepared immediately for the next patient. With little practice this procedure can be
completed in just a few minutes. This allows the doctor to use the ABPM 7100 around the clock on each
work day.
The ABPM 7100 is designed to allow recording and display of a blood pressure profile throughout the day
and at night. Additional parameters such as nocturnal values and blood pressure fluctuations are recognized.
This permits the doctor to prescribe optimal medical treatment for each individual.
Measurement with the ABPM 7100 can be either automated or be manually controlled by the user. In order
to start a series of automatic measurement, the user must initiate the first measurement by pressing the
START button and the doctor should check the reliability of the first measurement.
During the first measurement, the cuff is inflated in increments, to determine the cuff pressure required to
measure the systolic blood pressure value. The maximum required inflation pressure is stored and applied
by direct inflation during the subsequent automatic measurements. This procedure is called AFL – Auto
Feedback Logic.
Page 8

8 - Product description
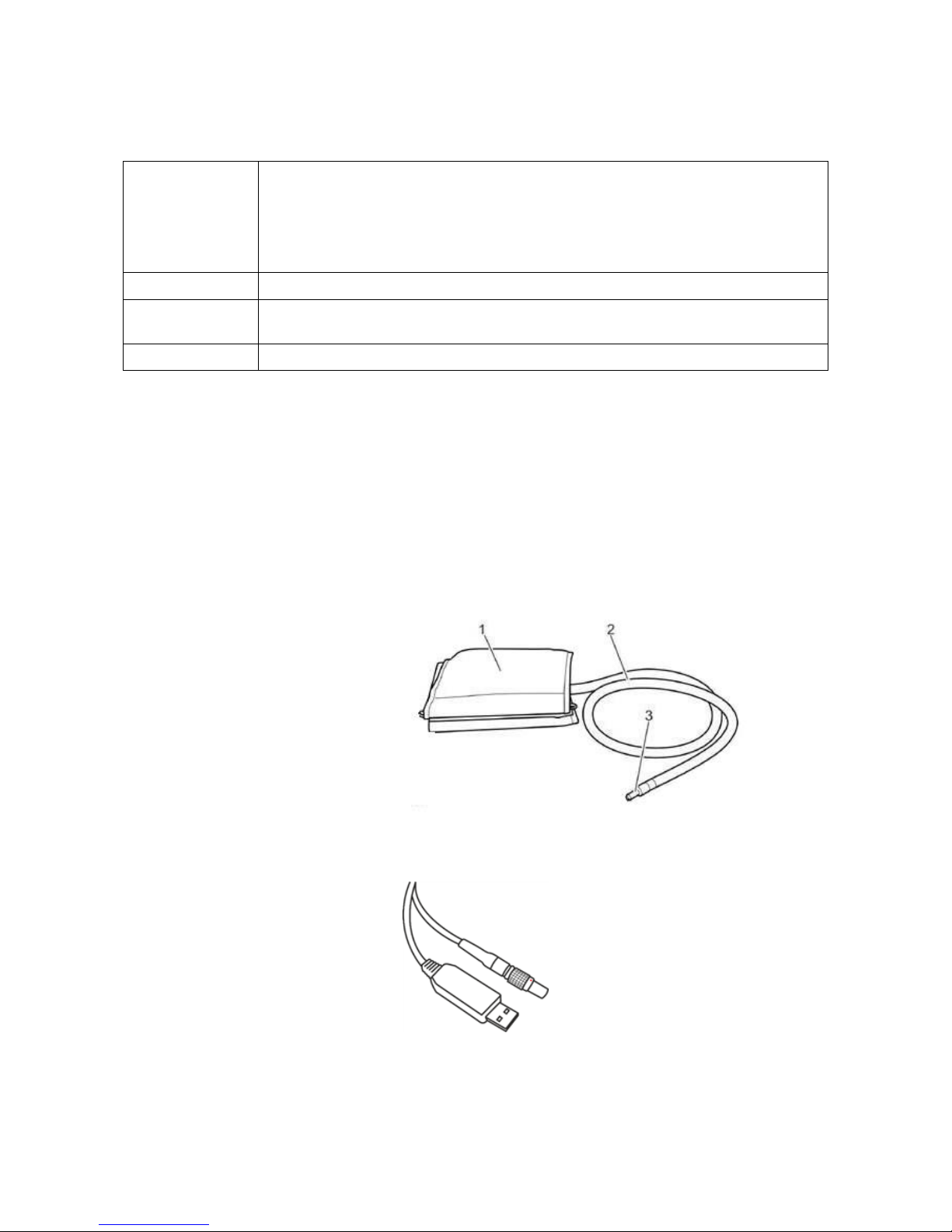
The ABPM 7100
Components
1
2
3
4
5
6
7
Cuff connection
ON/OFF button
LCD-Display
START button
DAY/NIGHT button
EVENT button
PC Interface cable port
The Buttons
ON/OFF
The ON/OFF button turns the ABPM 7100 on and off. To prevent unintended activation,
the ABPM 7100 turns on or turns off only when the button is pressed for more than 2
seconds.
START
The START button serves to
initiate a manual measurement to ascertain whether the ABPM 7100 is working correctly.
initiate a 24-hour measurement.
perform a measurement outside the specified measurement cycle.
DAY/NIGHT
The DAY/NIGHT button is used to differentiate between waking and sleeping phases
during the measurement, which is important for statistics and the graphic displays.
The patient is instructed to press the DAY/NIGHT button upon going to bed and again,
when getting up in the morning. This individually adapts the measurement interval to the
patient and assists you in the analysis of the blood pressure profile.
EVENT
The patient uses the EVENT button to document the time of medication or to record any
events which may cause the blood pressure to rise or fall. Pressing the button will trigger a
measurement, the patient should note the reason for pressing the EVENT button in the
event log.
LCD Display
The LCD display is located on the front of the ABPM 7100 casing. It displays useful information for the doctor
and the patient regarding measurement data, monitor settings and measurement errors. When the START
button is pressed, the number of previously registered measurements will be shown before starting a manual
measurement.
Page 9
Product description - 9
Audible signals
Individual or multiple beeps of audible signals are used. The following table explains the meaning of the
beeps:
1 beep
Switching ON/OFF
Starting and ending a measurement (except at night intervals)
Removal of the interface cable
Establishing and ending Bluetooth® communication
Measurement errors
3 beeps
System errors
Continuous beeps
Severe system errors (e.g. cuff pressure is higher than 15 mmHg for longer than
10 seconds outside the measurement)
Combined beeps
Manual deletion of measurement, 1 beep followed by 5 beeps 2 seconds later
Cuff connection
The cuff connection is located at the top of the ABPM 7100 casing.
The cuff is connected to the ABPM 7100 via a metal connector.
Attention
Measurement errors
The cuff connection must always engage with an audible “CLICK“. A poor connection between the
ABPM 7100 and cuff will result in measurement errors.
The Arm Cuff
1
2
3
The arm cuff
Air tube
Air tube connection
PC Interface Cable
In order to read data from the ABPM 7100, the interface cable must be connected to an USB slot on a PC.
Page 10
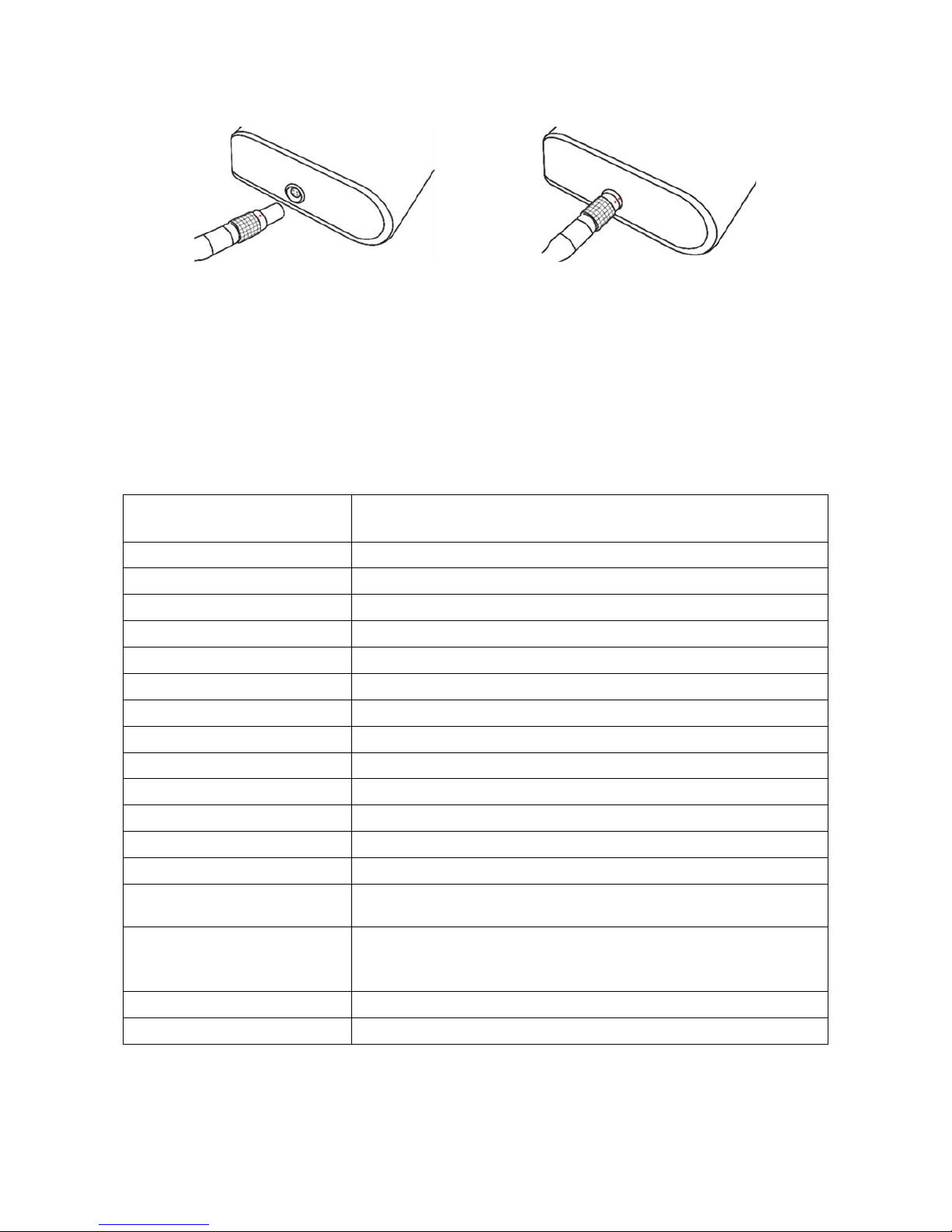
10 - Product description
PC Interface Cable Port
The connecting port for the PC interface cable is located at the bottom of the ABPM 7100 casing.
The red dot on the plug must align with the red dot on the port before plugging.
To disconnect, pull on the knurled ring of the connector.
Connecting the ABPM 7100 to the PC
To transfer the data from the ABPM 7100, ensure that the interface cable is connected correctly to an USB
port on the PC and to the interface cable port on the device.
Technical Data
Measurement pressure range:
Systolic 60 to 290 mmHg
Diastolic 30 to 195 mmHg
Accuracy:
+/- 3 mmHg in display range
Static pressure range:
0 to 300 mmHg
Pulse range:
30 to 240 beats per minute
Procedure:
oscillometric
Measurement intervals:
0, 1, 2, 3, 4, 5, 6, 10, 12, 15, 20 or 30 measurements per hour
Measurement logs:
4 adjustable interval groups
Memory capacity:
300 Measurements
Battery capacity:
> 300 Measurements
Operating temperatures:
+5°C to +40°C
Operating humidity:
15% to 93%
Storage environment:
-25°C to +70°C and 15% to 93% humidity
Dimensions:
121 x 80 x 33 mm
Weight:
approx. 220 g excluding batteries
Power supply:
2 Ni-MH batteries with 1,2 V each and min. 1500 mAh (AA, Mignon) or
2 Alkali 1,5 V batteries (AA, Mignon, LR6)
Interfaces:
USB-Interface cable
Bluetooth® (Class 1 / 100 m and max. 100 mW with 2,402 GHz to
2,480 GHz) only available with optional HMS software
Expected operational device life:
5 Years
Expected operational cuff life:
6 Months
Page 11

Preparing the ABPM 7100 - 11
Preparing the ABPM 7100
Safety instructions
Warning
Risk of strangulation posed by the shoulder strap and cuff tubing.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Do not place the shoulder strap and cuff tubing around the patient’s neck.
Always place the cuff tubing under the outer clothing (even at night).
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Measurement can be interrupted at any stage by pushing any of the buttons. This deflates the cuff
and the device can be removed.
Cautio n
Risk of injury caused by incorrect application of the device.
The doctor must ensure that, due to the patient’s medical condition, the use of the device does not
result in impaired blood circulation in the arm.
If the patient has limited cognitive abilities, the device may only be used under supervision.
While it is still attached to a patient, the device may never be connected to a PC or other device.
Instruct the patient to place the device in such a way that, while the cuff is inflated, the tubing is not
compressed or kinked, especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Risk of injury caused by incorrect application of the cuff.
The doctor must ensure that, due to the patient’s medical condition, the use of the device does not
result in impaired blood circulation in the arm.
If the patient is has limited cognitive abilities, the device may only be used under supervision.
It is imperative that you instruct the patient in the correct seating of the cuff.
Inform the patient that the cuff may only be used on the upper arm.
Ensure that neither the shoulder strap nor the cuff tubing can ever wrap around the patient’s neck.
Always place the cuff tubing und the outer clothing (even at night).
Instruct the patient to place the device in such a way that, while the cuff is inflated, the tubing is not
compressed or kinked, especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Intolerances caused by the use of disinfectants.
Wash to remove residues.
Wash the cuff sleeve with a mild detergent in the washing machine at max. 30°C without spinning.
Page 12

12 - Preparing the ABPM 7100
Inserting the batteries
Attention
Damage to device
Do not wear the ABPM 7100 while showering! If you suspect that liquid has entered the device while
cleaning or using it, the device shall no longer be used on the patient.
If the device was exposed to moisture, switch off the device and remove the batteries.
Inform your service immediately and send the device in for inspection.
The device may not be operated around MRI scanners or in the immediate vicinity of other medical
electrical equipment.
During a defibrillator discharge, the device shall not be in contact with the patient. Such a discharge
may damage the ABPM 7100 and cause it to display incorrect values.
The ABPM 7100 is not suitable for simultaneous use with HF surgical equipment.
Measurement can be interrupted at any stage by pushing a random button. This deflates the cuff and
the device can be removed.
Attention
Device function
Although zinc-carbon batteries may indicate sufficient voltage during a battery test, their output is
frequently insufficient to perform 24-hour measurements.
To conduct measurements over 48 hours, you will need 2 additional batteries as replacement after
24 hours.
Attention
Damage to device
Do not open the casing. Once the device is opened, all warranties will lapse.
Open the batteries compartment on the rear of the ABPM 7100 casing to insert the batteries into the ABPM
7100 according to the battery polarities ( + / - ) and close the compartment.
Note
Always use fully charged batteries for a new measurement
Only use undamaged batteries
Please remove the batteries if the device has not been used for a longer period
When inserting the batteries, please ensure correct polarity
Page 13

Preparing the ABPM 7100 - 13
Activating the device
Attention
Damage to device
Do not wear the ABPM 7100 while showering. If you suspect that liquid has entered the device while
cleaning or using it, the device shall no longer be used on the patient.
In the device was exposed to moisture, switch off the device and remove the batteries.
Inform your service immediately and send the device in for inspection.
The device may not be operated around MRI scanners or in the immediate vicinity of other medical
electrical equipment.
During a defibrillator discharge, the device shall not be in contact with the patient. Such a discharge
may damage the ABPM 7100 and cause it to display incorrect values.
The ABPM 7100 is not suitable for simultaneous use with HF surgical equipment.
Measurement can be interrupted at any stage by pushing a random button. This deflates the cuff and
the device can be removed.
Attention
Hygiene
Ensure hygiene in accordance with the maintenance schedule.
Always check the condition of the ABPM 7100 by observing the initial display shown on the device shortly
after turning it on and before handing it to a patient. The ABPM 7100 performs a self-test. In addition, a beep
sounds to check the speaker. The following should be displayed in this sequence:
Test
Display
Comment
Battery condition (volts)
2.85
(At least 2.75 volts for NiMH batteries and at least 3.10 volts for
alkaline batteries)
Display Segment Test
999:999
to
000:000
The display of the figures (999:999 to 000:000) is accompanied by all
other symbols of the LCD in succession. Check whether all segments
are correctly and fully displayed (the complete program code is
checked for correctness in the background)
Current 24h time
21:45
hh:mm
If the internal test detects an error, the ABPM 7100 will indicate “E004” on the display and emit an audible
signal. For safety reasons, the use of the ABPM 7100 will be locked. The faulty ABPM 7100 unit should be
send back immediately for repairs to your dealer or to Welch Allyn.
Setting the time / date
The ABPM 7100 has an internal buffer battery allowing the time to continue even if the batteries have been
removed. Nevertheless the time and the date should be checked before every measurement series.
The time and date can be set automatically with the patient management software.
Alternatively the time and date can be set manually. Press and hold the START button and then press the
EVENT buttons to enter the Set Time mode. Use the START button to select the appropriate item and use
the EVENT button to jump to the next display item.
Clearing the memory
The device memory must be cleared before every measurement series, i.e. blood pressure data form the
previous patient must not remain in the memory.
If there are existing data, the memory can be cleared with the delete function of the analysis software.
Alternatively the data can be cleared manually. Press and hold the START button for a minimum of 5
seconds until “cLr” is displayed. Within the next 5 seconds press and hold the EVENT button for at least 2
seconds to confirm the deletion of the stored measurements. The device emits a single beep to indicate that
the memory is cleared.
Page 14

14 - Preparing the ABPM 7100
Transferring patient data (ID)
The ABPM 7100 must be prepared by transferring patient data (ID) with the help of the patient management
software, so that correct data allocation is possible when it is read out after measurement.
Please refer to the respective patient management software manual for how to transfer patient data (ID) to
the ABPM 7100.
Setting measurement logs
In the patient management software you can optionally choose between eleven (1-11) logs. A log serves to
set the measurement intervals. As soon as you have conducted a measurement, the log can only be
changed once you have fully deleted all data.
Manual log settings
For manual log setting, press and hold the DAY/NIGHT button while simultaneously pressing the EVENT
button. Use the START button to change the log and confirm with the EVENT button.
Setting the logs via software
To set the logs via software please refer to the respective patient management software manual.
Note
Logs 1, 2, 10 and 11 are set by default but can be changed via the patient management
software.
Log 5 is suitable for nighttime activities (night shift).
Log 9 is designated as “Schellong-Test”.
Log 10 automatically sends the measurement values to your doctor’s PC via Bluetooth®.
Bluetooth® communication is not supported with the CPWS software.
Log 11 is only available to upgraded ABPM 7100 systems in connection with HMS from
version 5.0. Blood pressure measurement intervals and the 24h PWA can be set
separately here. Please contact Welch Allyn for further information.
Log
Day-Time
Night-Time
Measurements
per hour
Audible signal
Display of measured values
1
08:00
23:59
4
YES
YES
00:00
07:59
2
NO 2
08:00
22:59
4
YES
YES
23:00
07:59
1
NO 3
07:00
21:59
4
YES
NO 22:00
06:59
2
NO 4
08:00
23:59
4
YES
NO
00:00
07:59
2
NO 5
18:00
09:59
4
YES
YES
10:00
17:59
2
NO 6
07:00
23:59
4
YES
YES
00:00
06:59
2
NO 7
06:00
22:59
4
YES
NO 23:00
05:59
2
NO 8
07:00
08:59
6
YES
YES
09:00
23:59
4
YES
00:00
06:59
2
NO 9
09:00
08:59
30
NO
YES
10
08:00
07:59
30
YES
NO
11
08:00
23:59
4
YES
YES
00:00
07:59
2
NO
Page 15

Preparing the ABPM 7100 - 15
Selecting a suitable cuff
Cautio n
Risk of injury caused by incorrect application of the cuff.
The doctor must ensure that, due to the patient’s medical condition, the use of the device does not
result in impaired blood circulation in the arm.
If the patient is has limited cognitive abilities, the device may only be used under supervision.
It is imperative that you instruct the patient in the correct seating of the cuff.
Inform the patient that the cuff may only be used on the upper arm.
Ensure that neither the shoulder strap nor the cuff tubing can ever wrap around the patient’s neck.
Always place the cuff tubing und the outer clothing (even at night).
Instruct the patient to place the device in such a way that, while the cuff is inflated, the tubing is not
compressed or kinked, especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Intolerances caused by the use of disinfectants.
Wash to remove residues.
Wash the cuff sleeve with a mild detergent in the washing machine at max. 30°C without spinning.
The correct cuff size is important for correct blood pressure measurement. To obtain reproducible
measurements, standardized measuring conditions are needed. Measure the circumference of the upper
arm and select the appropriate cuff:
Welch Allyn Size Number
Upper Arm Circumference
Cuff
10
20 – 24 cm
Small Adult
11
24 – 32 cm
Adult
11L
32 – 38 cm
Adult Plus
12
38 – 55 cm
Large Adult
Applying the ABP Monitor and cuff
Warning
Risk of strangulation posed by the shoulder strap and cuff tubing.
If the patient is has limited cognitive abilities, the device may only be used under supervision.
Do not place the shoulder strap and cuff tubing around the patient’s neck.
Always place the cuff tubing under the outer clothing (even at night).
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Measurement can be interrupted at any stage by pushing any of the buttons. This automatically
deflates the cuff and the device can be removed.
Warning
Poor circulation caused by continuous cuff pressure.
Do not kink the connecting tubing.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Ensure the correct placement of the shoulder strap and cuff tubing.
Always place the cuff tubing under the outer clothing (even at night).
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Page 16

16 - Preparing the ABPM 7100
Warning
Placement and inflation of the cuff over a wound may lead to further injuries.
Placement and inflation of the cuff on any limb with an intravascular access or under
intravascular treatment or an arteriovenous (A-V) shunt may lead to temporary interruption of
circulation and therefore to further patient injury.
Placement and inflation of the cuff on the arm at the side of a breast amputation may lead to
further injury.
Examine the patient for wounds, bandages, etc.
Question the patient regarding previous treatments.
Observe the patient closely.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Intolerances caused by the use of disinfectants.
Wash to remove residues.
Wash the cuff sleeve with a mild detergent in the washing machine at max. 30°C without spinning.
Cautio n
Risk of injury caused by incorrect application of the cuff.
The doctor must ensure that, due to the patient’s medical condition, the use of the device does not
result in impaired blood circulation in the arm.
If the patient has limited cognitive abilities, the device may only be used under supervision.
It is imperative that you instruct the patient in the correct seating of the cuff.
Inform the patient that the cuff may only be used on the upper arm.
Ensure that neither the shoulder strap nor the cuff tubing can ever wrap around the patient’s neck.
Always place the cuff tubing und the outer clothing (even at night).
Instruct the patient to place the device in such a way that, while the cuff is inflated, the tubing is not
compressed or kinked, especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Risk of injury caused by incorrect application of the device.
The doctor must ensure that, due to the patient’s medical condition, the use of the device does not
result in impaired blood circulation in the arm.
If the patient has limited cognitive abilities, the device may only be used under supervision.
While it is still attached to a patient, the device may never be connected to a PC or other device.
Instruct the patient to place the device in such a way that, while the cuff is inflated, the tubing is not
compressed or kinked, especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Page 17

Preparing the ABPM 7100 - 17
Applying the ABP Monitor and cuff:
1. Position the carrying pouch on the right side of the patient. By varying the length of the pouch strap, it
can be worn around the hips or around the shoulders.
2. Alternatively a normal belt matching the clothes can be used.
3. Fit the cuff onto the patient.
The correct cuff seating is very important for correct blood pressure measurement.
4. Align the cuff so that no part of the cuff tubing is kinked. In this regard, the tube connection on the cuff
must face upwards.
5. Align the cuff so that the lower edge of the cuff is approximately 2 cm above the inside of the patient’s
elbow.
6. Tighten the cuff around the upper arm until one finger can be introduced under the cuff.
7. It is imperative that the artery symbol is positioned on the brachial artery. If you have aligned the cuff
correctly, the metal bar will lie on the outside of the upper arm (on the elbow side), whereby the cuff
sleeve must cover the skin under the metal bar.
8. Guide the tubing through the shirt’s row of buttons and out of the clothing, behind the nape of the neck
to the ABPM 7100 on the right side of the body.
9. The cuff can be worn on the naked upper arm or over a thin shirt sleeve.
10. The placement of the pressure tube must guarantee the upper arm’s free motion.
Connecting the cuff tubing to the ABPM 7100
1. Push the tube firmly onto the connection, with the cuff tubing engaging with an audible “CLICK” (to
detach, simply pull back the knurled ring).
2. Before measurement, check to ensure that tubing, ABPM 7100 and cuff are positioned correctly. The
ABPM 7100 is ready for measurement only once this is ensured.
Page 18

18 - Measurement process
Positioning the patient for measurement
The patient should take the following position during blood pressure measurement:
Comfortably seated
Legs uncrossed
Feet flat on the ground
With support to the back and the arms
With the cuff center at one level with the right atrium
Note
During measurement, the patient should be as relaxed as possible and may not speak
unless he wants to report any discomfort!
Allow for 5 minutes of relaxation before recording the first measurement value.
Blood pressure measurements can be influenced by the patient’s position (standing,
sitting, lying), by exertion or the patient’s physiological state. Exclude these influencing
factors to the greatest possible degree!
Measurement process
Safety instructions
Warning
Risk of strangulation posed by the shoulder strap and cuff tubing.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Do not place the shoulder strap and cuff tubing around the patient’s neck.
Always place the cuff tubing under the outer clothing (even at night).
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Measurement can be interrupted at any stage by pushing any of the buttons. This automatically
deflates the cuff and the device can be removed.
Warning
Poor circulation caused by continuous cuff pressure.
Do not kink the connecting tubing.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Ensure the correct placement of the shoulder strap and cuff tubing.
Always place the cuff tubing under the outer clothing (even at night).
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Warning
Poor circulation due to overly frequent measurements.
Check the date of the last measurement.
Inform the patient about this warning.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Observe the patient closely.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Page 19

Measurement process - 19
Warning
If the patient is wearing an additional ME device on the same limb for monitoring purposes, the
placement and inflation of the cuff may trigger the temporary loss of the existing ME device’s
function.
The operation and use of the automated non-invasive blood pressure monitoring device may lead
to longer impaired blood circulation in the patient or respective limb.
Examine the patient.
Question the patient regarding previous treatments.
Observe the patient closely.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Risk of injury caused by incorrect application of the cuff.
The doctor must ensure that, due to the patient’s medical condition, the use of the device does not
result in impaired blood circulation in the arm.
If the patient has limited cognitive abilities, the device may only be used under supervision.
It is imperative that you instruct the patient in the correct seating of the cuff.
Inform the patient that the cuff may only be used on the upper arm.
Ensure that neither the shoulder strap nor the cuff tubing can ever wrap around the patient’s neck.
Always place the cuff tubing und the outer clothing (even at night).
Instruct the patient to place the device in such a way that, while the cuff is inflated, the tubing is not
compressed or kinked, especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Instruct the patient to switch off and remove the device immediately and to inform the doctor in the
event of experiencing any pain.
Cautio n
Intolerances caused by the use of disinfectants.
Wash to remove residues.
Wash the cuff sleeve with a mild detergent in the washing machine at max. 30°C without spinning.
Attention
Damage to device
Do not wear the ABPM 7100 while showering. If you suspect that liquid has entered the device while
cleaning or using it, the device shall no longer be used on the patient.
In the device was exposed to moisture, switch off the device and remove the batteries.
Inform your service immediately and send the device in for inspection.
The device may not be operated around MRI scanners or in the immediate vicinity of other medical
electrical equipment.
During a defibrillator discharge, the device shall not be in contact with the patient. Such a discharge
may damage the ABPM 7100 and cause it to display incorrect values.
The ABPM 7100 is not suitable for simultaneous use with HF surgical equipment.
Measurement can be interrupted at any stage by pushing a random button. This deflates the cuff and
the device can be removed.
Attention
Hygiene
Ensure hygiene in accordance with the maintenance schedule.
Page 20

20 - Measurement process
Attention
Measurement errors
The use of components not included in the scope of delivery may lead to measurement errors. Only
use the accessories offered by Welch Allyn.
Although the ABPM 7100 fulfills all EMC standard requirements, it should not be exposed to any
strong electromagnetic fields, as this may lead to malfunctions outside the limit values.
Medical electrical equipment is subject to special EMC precautions. Please observe the directives
attached.
The cuff tubing between the ABPM 7100 and the cuff may not be knotted, compressed or pulled
apart.
The cuff connection must always engage with an audible “CLICK”. A loose connection between the
tubing and the device leads to measurement errors.
Initial measurement
24-hour measurement
1. Ensure sufficient battery voltage. At least 2.6 volts for NiMH batteries and at least 3.10 volts for
alkaline batteries!
2. The doctor must go through these instructions together with the patient before a 24-hour
measurement.
3. The doctor must explain the possible hazards in detail on the basis of the warnings above!
4. Ensure that the patient has understood all functions and observable points!
Safety:
For your own safety during the following steps, please observe the safety instructions at the
start of this chapter, as well as the functional overview.
Performing a measurement
1. To trigger a measurement, press the START button.
The number of stored measurements will be shown on the LCD display.
An audio beep will announce the upcoming measurement.
Manual measurement will start.
2. The patient should stay calm during the measurement process, until the measurement is completed.
Allow your arm to hang loose, or place your lower arm loosely on the table or on a support whilst
sitting. Avoid any movement!
3. Doctor: Please check the values of the first measurement for plausibility, so that subsequent automatic
measurements can be processed correctly and correct cuff position is ensured.
4. In the event of an error measurement, please follow the instructions in sections Measurement
preparations and Troubleshooting.
Note
Severe malfunctions are indicated by a continuous beep.
In the event of a continuous beep, switch off the device, remove the cuff and inform your
doctor.
Hand the data sheet “Patient information – operation of the ABPM 7100” to each patient.
The data sheet is attached as a copy template.
Portable and mobile HF communication equipment may influence medical electrical devices.
Extreme temperatures, humidity or air pressure can influence measurement accuracy.
Please observe the operating conditions.
The pulse wave analysis provides additional indicators for possible risks, but is not
permissible as a sufficient indicator for individual illness or as a treatment recommendation.
Note
An initial measurement is required for starting the measurement log. The initial measurement
must be checked by a physician for plausibility!
Page 21

Care and Maintenance - 21
Cancelling a measurement
A measurement will be cancelled by pressing any buttons during the measurement process. The LCD
display will then show -StoP- and the ABPM 7100 will beep 5 times. This cancellation will be stored in the
measurement value table under Cancel.
Unsuccessful measurement
1. If the display shows errors, reexamine the correct procedure during set-up and positioning of the
device.
2. Dismiss the patient only after a successful manual measurement!
Inform the patient sufficiently in order to explain the situation!
3. Repeat the measurement.
4. If the display still shows errors, repeat the initial operation process.
5. For further troubleshooting measures and faults removal, please refer to the Troubleshooting section.
Note
Severe malfunctions are indicated by a continuous beep.
In the event of a continuous beep, switch off the device, remove the cuff and inform
your doctor.
Pos: 13 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Pos: 14 /Sammel/05_Wartung und Pflege @ 0\mod_1399377131629_6.docx @ 4772 @ 1 2233233 @ 1
Care and Maintenance
To ensure the optimal functionality of the ABPM 7100 regular care and maintenance of the unit is required.
Attention
Damage to device
Do not open the casing. Once the device is opened, all warranties will lapse.
Cleaning and disinfection
The user (doctor) decides whether and when the cuff sleeve should be disinfected for hygienic reasons (e.g.
after every use).
The following agents are recommended for disinfecting the cuff sleeve:
Terralin Liquid (Manufacturer: Schülke & Mayr)
Promanum N (Manufacturer: B. Braun)
The use of disinfectants not recommended by Welch Allyn shall render the user responsible for proof of safe
application.
Note
It is imperative that you observe the manufacturer’s information regarding the use of these
products. Allow the agents to dry off completely.
Cautio n
Intolerances caused by the use of disinfectants: Some patients display intolerance (e.g. allergies)
to disinfectants or their components.
Never use disinfectants which leave residues on the product or which are not suitable for contact
with the skin.
Carefully wash the cuff to remove residues.
Page 22

22 - Care and Maintenance
Cleaning the ABP Monitor and carrying pouch
Attention
Damage to the ABP Monitor and carrying pouch caused by the use of solvents
Only use a moistened cotton cloth to clean the ABPM 7100 Monitor.
Do not use strong or solvent-based additives.
Ensure that no liquid enters the device.
If liquid does penetrate the device, switch if off immediately and return it to your Welch Allyn
specialist for inspection.
1. Use only a moistened cotton cloth to clean the ABPM 7100.
2. Read the safety instructions carefully and observe them closely during cleaning.
Cleaning the cuff sleeve, bladder and tubing
Attention
Damage to the cuff caused by disinfectants
Do not submerge the cuff sleeve in disinfectants.
Avoid disinfecting the cuff bladder and connected rubber tubing.
Attention
Damage to the cuff sleeve during washing
Always close the Velcro strip before washing!
Wash the cuff sleeve with a mild detergent in the washing machine at max. 30°C. Do not spin.
Do not use fabric softeners or other washing aids (e.g. hygiene rinses, textile deodorants). These
agents can leave behind residues and damage the material.
The cuff sleeve is not suitable for drying in a dryer.
Attention
Damage to the bladder and tubing caused by disinfectants
Before washing, carefully remove the bladder and tubing from the cuff sleeve.
Avoid disinfecting the cuff bladder and connected rubber tubing.
The bladder and tubing can be damaged by disinfectants.
Wipe down the bladder with lukewarm water and add a mild detergent, if necessary.
Ensure that no water enters the tube opening.
1. When cleaning the cuff sleeve, bladder and tubing, use only mild detergents in lukewarm water
without fabric softener.
2. Read the safety instructions carefully and observe them closely during cleaning.
Maintenance plan
Attention
Damage to device
Do not open the casing. Once the device is opened, all warranties will lapse.
Page 23

Troubleshooting - 23
Weekly Maintenance
Analysis review:
1. Review the print-out of your measurement analysis for:
Correctly entered times and intervals in accordance with the log.
Times of day/night transitions.
Correct standard values (nocturnal decrease).
2. Check the device, cuff and the cuff tubing for superficial soiling and clean it as specified in the
Cleaning section.
3. Check the cuff and the cuff tubing for superficial damage. In the event of damages return it to your
Welch Allyn specialist for inspection.
Checking battery voltage:
Always use fully charged or new batteries.
The battery voltage appears on the display of the ABPM 7100 for approximately 3 seconds after the device
is switched on. The battery voltage must be at least 2.6 volt to ensure a 24-hour measurement.
Maintenance every 2 years
As proof of continuous compliance to “Basic Requirements” pursuant to Directive 93/42/EEC, the ABPM
7100 must be subjected to metrological checks every two years. In certain countries, this requirement may
be regulated by national laws or regulations.
Welch Allyn offers to provide metrological checks and the servicing comprising of the following:
Metrological monitoring.
Software updates (if required)
Functional check: Electronics, pump and pneumatic circuit.
Pos: 15 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Pos: 16 /Sammel/06_Fehlersuche @ 0\ mod_1399377587130_6.docx @ 4774 @ 1222223 3 @ 1
Troubleshooting
Attention
Damage to device
Do not open the casing. Once the device is opened, all warranties will lapse.
Basic error sources
The following may cause error measurements or unintended events:
The patient’s arm movement during measurement
Device deactivation (e.g. at night)
Incorrect cuff size
Cuff displacement while wearing it
Omitted successful initial measurement by the doctor
Medication not taken
Wrong log set by the user
Empty, incorrectly charged or outdated batteries
Kinked or knotted cuff tubing
Transmission error
The ABPM 7100 reviews the transmitted data to prevent errors. If an error occurs, “E004” will be shown on
the display.
Checklist
Please review the following checklist for any errors occurring during the operation of the ABPM 7100. Many
errors have simple causes:
Check to see that all cables are connected correctly.
Check to see whether the ABPM 7100 and the computer are switched on.
Check to see whether the batteries have sufficiently voltage.
Note
Some errors are combined with a continuous alarm for safety reasons. The continuous alarm
can be cancelled by pressing any button. If there is residual pressure inside the cuff, open the
cuff immediately.
Page 24

24 - Troubleshooting
Error codes
Error description of the ABPM 7100
Error symptom
Possible cause
Remedy
Time and date are not
updated following a
longer period without
power supply from power
packs or batteries.
The internal buffer battery is
depleted.
Date and time can be reset after every
power pack or battery replacement.
Send the device to your Welch Allyn
specialist.
Measurement data can
no longer be called
up/displayed.
An error occurred during patient
data storage.
Delete the respective patient (menu bar) and
recreate it.
The connection between
the ABPM 7100 and the
PC is faulty.
The incorrect COM interface is
set.
Set the correct interface in the service
programs.
Cable plug or socket is defective.
Inspect the plug and the socket on the
ABPM 7100. Ensure that the pins are
straight to guarantee contact.
The ABPM 7100 is not in
transmitting mode (the displays
shows the time).
Switch the ABPM 7100 off and then on
again without removing the connecting
cable.
No patient number.
The ABP Monitor is not
initialized, i.e. the patient number
was not transferred during the
preparation for a 24-hour
measurement.
The patient number can also be transmitted
after the measurement. This does not
influence the measurement data.
No measurements were
conducted during the
nocturnal phase.
The battery packs or batteries
were prematurely depleted.
The power packs or batteries may be
defective (please contact your Welch Allyn
specialist).
The patient has switched off the
ABPM 7100.
Draw the patient’s attention to the urgency of
a complete 24-hour measurement.
The display does not
show “co” or “bt”.
You are not in transmitting mode.
Communication via cable: Switch the ABPM
7100 off and then on again without pulling
the plug.
Communication via BT: Press and hold the
START button and then press the
DAY/NIGHT button. Select “bt” using the
START button.
No automatic
measurements will be
performed.
No manual measurements
performed after application.
Valid manual measurement must always be
performed after the device has been
positioned.
Incorrect log set.
Set log 1 or 2.
The measurement
interval does not meet
your expectations.
Incorrect log set.
The programmed log does not correspond
with the set log in the ABPM 7100. Check
the log manually on the device.
No manual measurements
performed after application.
Conduct manual measurement to activate
the set log
Page 25

Troubleshooting - 25
Error symptom
Possible cause
Remedy
Err 1
The patient displays severe
arrhythmia.
ABP Monitor not applicable.
The arm was moved during
measurement.
Keep the arm still during measurement.
Insufficient valid pulse rate detected.
Place the cuff on your arm again.
Err 2
The arm was moved during
measurement.
Keep the arm still during measurement.
Cuff does not fit the arm snugly.
Check the seating of the cuff and that of the
device.
Err 3
Blood pressure beyond the
measurement range.
Permanent notification renders the ABP
Monitor unsuitable for the patient.
Strong arm movements.
Keep the arm still during measurement.
Problems with the pneumatics.
If the error persists permanently, send the
device to your Welch Allyn specialist.
Err 4
Data transmission cable incorrectly
inserted in ABP Monitor.
Insert the cable into the ABP Monitor
correctly.
Pins in the plug of the data
transmission cable are mechanically
damaged.
Check the plug to see whether the pins on
the inside are damaged. If they are, contact
your Welch Allyn specialist.
Measurement value was not
correctly transmitted.
Restart the transmission.
Err 5
bAtt
Power pack or battery voltage too
low.
Replace the power packs or batteries.
Power packs or batteries are
defective.
The power pack or battery voltage is correct
but “bAtt” is displayed during cuff inflation.
Replace the power packs.
Battery contacts are corroded.
Clean the battery contacts with a cotton
cloth and a little alcohol.
Err 6 +
Possible continuous
alarm until a button is
pressed.
Build-up 25 fair.
Check the cuff for a build-up of air or a kink
in the tubing. If the cuff tubing is kinked,
straighten the tubing. Otherwise send the
device in for inspection immediately.
Blood pressure cuff incorrectly
connected.
Connect the cuff to the device.
Leaky points in the cuff or the cuff
tubing.
If necessary, replace the cuff.
Err 7
The memory of the blood pressure
measurement device is full. (a
maximum of 300 measurements and
events can be stored)
Delete the data in the ABP Monitor but
ensure that the data was stored on your PC
first.
Err 8
Measurement cancelled with a
pressed button.
Err 9 +
Possible continuous
alarm until a button is
pressed.
Residual pressure inside the cuff
Wait for the cuff to deflate completely.
Zero point comparison was
unsuccessful.
Send the device to your specialist for
inspection immediately or directly to your
Welch Allyn specialist.
Page 26

26 - Troubleshooting
Error symptom
Possible cause
Remedy
Err 10 +
Continuous alarm
until a button is
pressed.
Severe error caused by accumulated
pressure outside the measurement
process.
Send the device to your specialist for
inspection and repair immediately or
directly to your Welch Allyn specialist.
These error messages all show a
severe error in the program code.
The analysis unit
does not react to data
transmission but the
display shows “co”.
Data transmission cable not correctly
inserted in the PC. (also refer to Err 4)
Check whether the 9-pin plug of the data
transmission cable is securely seated in
the device’s interface socket. (also refer to
Err 4)
The ABPM 7100
measures every two
minutes.
Log 9 is set in the ABPM 7100.
Set log 1 or 2.
The desired log
cannot be set with the
button combination.
The last patient’s measurement
values are still contained in the
memory.
Delete the data in the ABP Monitor but
ensure that the data was stored first.
The ABP Monitor
cannot be switched
on.
The battery packs or batteries were
incorrectly inserted.
Reinsert either power packs or batteries
and ensure correct polarity.
Power pack or battery voltage too low.
Replace the power packs or batteries.
Defective display.
Send the device to your specialist for
repair or directly to your Welch Allyn
specialist.
An error occurs during
the first
measurement.
The cuff size is not suitable for the
patient’s arm circumference.
Measure the patient’s arm circumference
and compare this with the imprint on the
cuff. You may require a different cuff size.
Communication error ABPM 7100 Bluetooth Interface
Error symptom
Possible cause
Remedy
Code 1
Bluetooth® interface of the ABPM 7100 was not
started correctly.
Possible hardware fault.
Send the device to your Welch Allyn
specialist for inspection.
Code 2
Bluetooth® interface of the ABPM 7100 could
not be configured correctly.
(Communication problem between ABPM 7100
and the Bluetooth® module.)
Try again. If the error persists, send
the device to your Welch Allyn
specialist for inspection.
Code 3
The status of the Bluetooth® interface of the
ABPM 7100 could not be determined.
(Communication problem between ABPM 7100
and the Bluetooth® module.)
Try again. If the error persists, send
the device to your Welch Allyn
specialist for inspection.
Code 4
The Bluetooth® interface of the ABPM 7100 has
not yet been paired with the analysis software.
Reconnect the device via Bluetooth®.
Code 5
The Bluetooth® interface of the ABPM 7100
could not connect to the Bluetooth dongle on
the computer.
Try again. If the error persists, send
the device to your Welch Allyn
specialist for inspection.
Code 6
The measurement value memory of the ABPM
7100 contains unsent blood pressure values.
These will be sent once further
measurements have been performed.
Code 7
The ABPM 7100 is paired with a cell phone or
GSM modem, which is technically incapable of
transmitting measurement values, is outside the
network range or is incorrectly configured.
Try again. If the error persists, contact
your Welch Allyn specialist.
Pos: 17 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Pos: 18 /Sammel/07_Gewährleistungs- un d Reparaturbedingungen @ 0\mod_139946418 1122_6.docx @ 4784 @ 1 @ 1
Page 27

Limited Warranty - 27
Limited Warranty
Welch Allyn warrants the product to be free of defects in material and workmanship and to perform in
accordance with manufacturer's specifications for the period of one year from the date of purchase from Welch
Allyn or its authorized distributors or agents.
The warranty period shall start on the date of purchase. The date of purchase is: 1) the invoiced ship date if
the device was purchased directly from Welch Allyn, 2) the date specified during product registration, 3) the
date of purchase of the product from a Welch Allyn authorized distributor as documented from a receipt from
said distributor.
This warranty does not cover damage caused by: 1) handling during shipping, 2) use or maintenance contrary
to labeled instructions, 3) alteration or repair by anyone not authorized by Welch Allyn, and 4) accidents.
The product warranty is also subject to the following terms and limitations: Accessories are not covered by the
warranty. Refer to the directions for use provided with individual accessories for warranty information.
Shipping cost to return a device to a Welch Allyn Service center is not included.
A service notification number must be obtained from Welch Allyn prior to returning any products or
accessories to Welch Allyn's designated service centers for repair. To obtain a service notification number,
contact Welch Allyn Technical Support.
THIS WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING BUT
NOT LIMITED TO THE IMPLIED WARRANTIES OF MERCHANTABILTY AND FITNESS FOR A
PARTICULAR PURPOSE. WELCH ALLYN’S OBLIGATION UNDER THIS W ARRANTY IS LIMITED TO
REPAIR OR REPLACEMENT OF PRODUCTS CONTAINING A DEFECT. WELCH ALLYN IS NOT
RESPONSIBLE FOR ANY INDIRECT OR CONSEQUENTIAL DAMAGES RESULTING FROM A PRODUCT
DEFECT COVERED BY THE WARRANTY.
Service Policy
All repairs on products under warranty must be performed by Welch Allyn or by a service provider authorized
by Welch Allyn. Unauthorized repairs will void the warranty. In addition, whether or not covered under
warranty, any product repair should be performed exclusively by Welch Allyn or by a service provider that
has been authorized by Welch Allyn.
If the product fails to function properly - or if you need assistance, service, or spare parts - contact the
nearest Welch Allyn Technical Support Center.
Before contacting Welch Allyn, try to duplicate the problem, and check all accessories to ensure that they are
not causing the problem. When calling, please be prepared to provide:
• Product name, model number, and serial number of your product.
• Complete description of the problem.
• Complete name, address and phone number of your facility.
• For out-of-warranty repairs or spare parts orders, a purchase order (or credit card) number.
• For parts orders, the required spare or replacement part numbers.
If your product requires warranty, extended warranty, or non-warranty repair service, please call first the
nearest Welch Allyn Technical Support Center. A representative will assist you troubleshooting the problem
and will make every effort to solve it over the phone, avoiding potential unnecessary return of your product.
In case a return cannot be avoided, the representative will record all necessary information and will provide a
Return Material Authorization (RMA) number, as well as the appropriate return address. An RMA number
must be obtained prior to any return.
If you have to return your product for service, follow these recommended packing instructions:
• Remove all hoses, cables, sensors, power cords, and other accessories (as appropriate) before
packing, unless you suspect they are associated with the problem.
• Wherever possible use the original shipping carton and packing materials.
• Include a packing list and the Welch Allyn Return Material Authorization (RMA) number.
It is recommended that all returned goods be insured. Claims for loss or damage to the product must be
initiated by the sender.
Pos: 19 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Page 28

28 - EMC Guidelines and Manufacturer Declaration
Pos: 20 /Sammel/08_Anhang @ 0\mod_1 400228500241_6.docx @ 4869 @ 123333 @ 1
EMC Guidelines and Manufacturer Declaration
Table 1 – Guidelines and Manufacturer Declaration
Electromagnetic emission for all ME devices and ME systems
Guidelines and Manufacturer Declaration - Electromagnetic emissions
The ABPM 7100 is intended for operation in an electromagnetic environment as specified below. The
customer or user of the ABPM 7100 should ensure its operation in such an environment.
Emission measurement
Compliance
Electromagnetic Environment Guideline
HF Emissions according to CISPR 11
Group 1
The ABPM 7100 utilizes HF power for its
internal function only. Its HF emission is
therefore very low and it is improbable that
neighbouring electronic device experience any
interference.
HF Emissions according to CISPR 11
Class B
The ABPM 7100 is suitable for use in other
facilities than the living area and those
immediately connected to the public supply
network, which also supplies buildings used for
residential purposes.
Emission of harmonics
according to IEC 61000-3-2
Not applicable
Emission of voltage fluctuations/
flickers according to IEC 61000-3-3
Not applicable
Table 2 – Recommended safety distances between portable and mobile HF communication devices
and the ME device or ME system for ME devices or ME systems, which are not life-supporting
Recommended safety distances between portable and mobile HF telecommunication devices and
the ABPM 7100
The ABPM 7100 is intended for operation in an electromagnetic environment, in which disturbance variables
are controlled. The customer or user of the ABPM 7100 can assist in preventing electromagnetic
interferences by adhering to the minimum distance between portable and mobile HF telecommunication
devices (transmitters) and the ABPM 7100 – depending on the output power of the device, as specified
below.
The transmitter’s nominal
capacity
W
Safety distance, depending on transmission frequency
m
150 kHz to 80 MHz
d = 1.2
80 MHz to 800 MHz
d = 1.2
800 MHz to 2.5 GHz
d = 2.3
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters whose nominal capacity is not covered by the table above, the recommended safety
distance d can be calculated in meters (m) using the formula given in the respective column, whereby P is
the maximum nominal capacity of the transmitters in Watts (W) as per the information given by the
transmitter’s manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not be applicable in all cases. The distribution of electromagnetic
variables is influenced by the absorption and reflection of buildings, objects and people.
Page 29

EMC Guidelines and Manufacturer Declaration - 29
Table 3 – Guidelines and Manufacturer Declaration
Electromagnetic immunity – for all ME devices and ME systems
Guidelines and Manufacturer Declaration - Electromagnetic immunity
The ABPM 7100 is intended for operation in an electromagnetic environment as specified below. The
customer or user of the ABPM 7100 should ensure its operation in such an environment.
Immunity tests
IEC 60601-test
levels
Compliance levels
Electromagnetic Environment -
Guidelines
Electrostatic
discharge (SD)
according to IEC
61000-4-2
± 6 kV
Contact discharge
± 8 kV
Air discharge
± 6 kV
Contact discharge
± 8 kV
Air discharge
Floors should consist of wood or
cement or ceramic tiles. If the floor
consists of synthetic materials, relative
humidity must be at least 30%.
Rapid transient
electrical
disturbance/bursts
according to IEC
61000-4-4
± 2 kV
for mains cables
± 1 kV
for input and output
lines
Not applicable
± 1 kV
for input and output
lines
The ABPM 7100 does not have an AC
power supply.
Surges
according to IEC
61000-4-5
± 1 kV
Line-to-line voltage
± 2 kV
Line-to-earth voltage
Not applicable
Not applicable
The ABPM 7100 does not have an AC
power supply.
Voltage drops,
short interruptions
and fluctuations in
supply voltage
according to IEC
61000-4-11
< 5% UT
(> 95% drop in UT)
for a 1/2 period
Not applicable
The ABPM 7100 does not have an AC
power supply.
40% UT
(60% drop in UT)
for 5 periods
Not applicable
70% UT
(30 % drop in UT)
for 25 periods
Not applicable
< 5% UT
(> 95% drop in UT)
for 5 seconds
Not applicable
Magnetic field in
supply frequency
(50/60 Hz)
according to IEC
61000-4-8
3 A/m
3 A/m
Magnet fields in network frequency
should correspond with the typical
values found in business and hospital
environments.
NOTE UT is the AC voltage before the application of the test levels.
Page 30

30 - EMC Guidelines and Manufacturer Declaration
Table 4 – Guidelines and Manufacturer Declaration
Electromagnetic immunity for ME devices or ME systems that are not life-supporting
Guidelines and Manufacturer Declaration - Electromagnetic immunity
The ABPM 7100 is intended for operation in an electromagnetic environment as specified below. The
customer or user of the ABPM 7100 should ensure its operation in such an environment.
Immunity tests
IEC 60601-test
levels
Compliance
level
Electromagnetic Environment - Guidelines
Portable and mobile RF communications equipment
should be used no closer to any part of the ABPM
7100, including cables, than the recommended
safety distance calculated from the equation
applicable to the frequency.
Recommended safety distance:
Conducted
disturbance
variables
according to
IEC 61000-4-6
Effective value
3 V
150 kHz to
80 MHz
3 V
d = 1.2
Radiated
disturbance
variables
according to
IEC 61000-4-3
3 V/m
80 MHz to
2.5 GHz
3 V/m
d = 1.2 for 80 MHz to 800 MHz
d = 2.3 for 800 MHz to 2.5 GHz
with P as the transmitter’s nominal value in watt (W)
according to the transmitter manufacturer’s
information and d as the recommended safety
distance in meters (m).
The field intensity of stationary radio transmitters, as
determined by an electromagnetic on-sitea survey,
should be less than the compliance level in each
frequency range.b
Interference may occur in the vicinity of equipment
marked with the following symbol.
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not be applicable in all cases. The distribution of electromagnetic
variables is influenced by the absorption and reflection of buildings, objects and people.
a
Theoretically, the field intensity of stationary transmitters such as base stations of cellular telephones and
mobile landline devices, amateur radio stations, AM and FM radio and TV stations, cannot be accurately
determined in advance. To assess the electromagnetic environment regarding the stationary transmitter, a
study on the location’s electromagnetic phenomena should be considered. If the measured field strength in
the location in which the ABPM 7100 is used exceeds the applicable RF compliance level above, the
ABPM 7100 should be monitored to verify normal function. If abnormal performance is observed,
additional measures may be necessary, such as re-orienting or relocating the ABPM 7100.
b
Over the frequency range of 150 kHz to 80 MHz, field intensity should be less than 3 V/m.
Page 31

Patient Information - operation of the ABPM 7100 - 31
Pos: 21 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Pos: 22 /Sammel/09_Bedienung für den Pa tienten **KOPIERVORLAGE** @ 0\mod_139 8849974787_6.docx @ 4762 @ 122222 @ 1
Patient Information - operation of the ABPM 7100
Safety instructions
Warning
Risk of strangulation posed by the shoulder strap and cuff tubing.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Do not place the shoulder strap and cuff tubing around the patient’s neck.
Always place the cuff tubing under the outer clothing (even at night).
Switch off and remove the device immediately and to inform the doctor in the event of experiencing
any pain.
Measurement can be interrupted at any stage by pushing a random button. This deflates the cuff and
the device can be removed.
Warning
Poor circulation caused by continuous cuff pressure.
Do not kink the connecting tubing.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Ensure the correct placement of the shoulder strap and cuff tubing.
Always place the cuff tubing under the outer clothing (even at night).
Switch off and remove the device immediately and to inform the doctor in the event of experiencing
any pain.
Warning
Placement and inflation of the cuff over a wound may lead to further injuries.
Placement and inflation of the cuff on any limb with an intravascular access or under
intravascular treatment or an arteriovenous (A-V) shunt may lead to temporary interruption of
circulation and therefore to further patient injury.
Placement and inflation of the cuff on the arm at the side of a breast amputation may lead to
further injury.
Switch off and remove the device immediately and to inform the doctor in the event of experiencing
any pain.
Warning
If the patient is wearing an additional ME device on the same limb for monitoring purposes, the
placement and inflation of the cuff may trigger the temporary loss of the existing ME device’s
function.
The operation and use of the automated non-invasive blood pressure monitoring device may lead
to longer impaired blood circulation in the patient or respective limb.
Switch off and remove the device immediately and to inform the doctor in the event of experiencing
any pain.
Warning
Poor circulation due to overly frequent measurements.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Switch off and remove the device immediately and to inform the doctor in the event of experiencing
any pain.
Page 32

32 - Patient Information - operation of the ABPM 7100
Cautio n
Risk of injury caused by incorrect application of the cuff.
If the patient has limited cognitive abilities, the device may only be used under supervision.
Ensure that neither the shoulder strap nor the cuff tubing can ever wrap around the patient’s neck.
Always place the cuff tubing und the outer clothing (even at night).
Place the device in such a way that, while the cuff is inflated, the tubing is not compressed or kinked,
especially during sleep.
Petechiae, haemorrhages or subcutaneous haematoma may occur in some patients.
Switch off and remove the device immediately and inform the doctor in the event of experiencing any
pain.
Attention
Damage to device
Do not open the casing. Once the device is opened, all warranties will lapse.
Attention
Damage to device
Do not wear the ABPM 7100 while showering. If you suspect that liquid has entered the device while
cleaning or using it, the device shall no longer be used on the patient.
In the device was exposed to moisture, switch off the device and remove the batteries.
The device may not be operated around MRI scanners or in the immediate vicinity of other medical
electrical equipment.
During a defibrillator discharge, the device shall not be in contact with the patient. Such a discharge
may damage the ABPM 7100 and cause it to display incorrect values.
Measurement can be interrupted at any stage by pushing a random button. This deflates the cuff and
the device can be removed.
Attention
Measurement errors
Although the ABPM 7100 fulfills all EMC standard requirements, it should not be exposed to any
strong electromagnetic fields, as this may lead to malfunctions outside the limit values.
The cuff tubing between the ABPM 7100 and the cuff may not be knotted, compressed or pulled
apart.
The cuff connection must always engage with an audible “CLICK”. A loose connection between the
tubing and the device leads to measurement errors.
Note
Severe malfunctions are indicated by a continuous beep.
In the event of a continuous beep, switch off the device, remove the cuff and inform
your doctor.
24-hour measurement
1. Before a 24-hour measurement, go through these instructions together with your doctor.
2. Let your doctor explain possible hazards in detail on the basis of the warnings above.
3. Ensure that you have understood all functions and observable points.
Safety:
For your own safety during the following steps, please observe the safety instructions at the
start of this chapter.
Page 33

Patient Information - operation of the ABPM 7100 - 33
Position of the cuff
It is imperative that the artery symbol is positioned on the brachial artery. If the cuff is aligned correctly, the
metal bar will lie on the outside of the upper arm (on the elbow side), whereby the fabric bag must cover the
skin under the metal bar.
The Buttons
ON/OFF
The ON/OFF button turns on and off the ABPM 7100 when the button is pressed for more
than 2 seconds.
START
The START button serves to
initiate the automatic protocol.
trigger a measurement in addition to the automatic protocol.
DAY/NIGHT
The DAY/NIGHT button is used to differentiate between waking and sleeping phases
during the measurement. Press the DAY/NIGHT button immediately before going to bed
and upon waking.
EVENT
Press the EVENT button to record an event which may affect the blood pressure and to
trigger an additional measurement. Note the reason for pressing the EVENT button in the
event log.
Measurement process
During the first measurement, the cuff is inflated in increments, to determine the cuff pressure required to
measure the systolic blood pressure value. This maximum required inflation pressure is stored and applied
by direct inflation during the subsequent automatic measurements. The patient should stay calm during the
measurement process, until the measurement is completed. Allow your arm to hang loose, or place your
lower arm loosely on the table or on a support whilst sitting. Avoid any movement! In the event of a failed
measurement a new measurement is performed automatically according to the measurement process
described above.
Cancelling a measurement
A measurement will be cancelled by pressing any buttons during the measurement process causing the cuff
to be quickly deflated automatically. The LCD display will then show “-StoP-” and the ABPM 7100 will beep 5
times. This cancellation will be stored in the measurement value table under Cancel.
Pos: 23 /_Steuerung/==========SEITENUM BRUCH========== @ 0\mod_139996795 7149_6.docx @ 4804 @ @ 1
Ende der Liste für Textmarke Inhalt ===
Pos: 25 /_Steuerung/Index @ 0\mod_1402 573435838_6.docx @ 5054 @ 1 @ 1
=== Ende der Liste für Textmarke Index ===
os: 27 /_Steuerung/Rückseite @ 0\mod_ 1401873508600_6.docx @ 5014 @ @ 1
Page 34

Reorder No.
106169
Material No.
722592
DIR
80019691 Ver. A
=== Ende der Liste für Textmarke Rüc kseite ===
 Loading...
Loading...