STERIS TruSystem 7000 User manual
TruSystem 7000 Operating Table
Service Manual

Translation of the original German service manual
CE mark/Conformity: This is a Class I medical device according to the Council Directive 93/
42/EEC concerning medical devices and is compliant with the Directive version currently in force
at the time of product sale. The manufacturer declares the conformity of this product with the
essential requirements of the Council Directive 93/42/EEC concerning medical devices
according to Appendix I, as well as the implementation of an assessment procedure required for
Class I product conformity under Appendix VII and documents this with the CE mark.
ETL mark: Intertek tested the product for the USA and Canada. ETL classification regarding risk
of electric shock and fire, as well as mechanical hazard in accordance with ULSTD 60601-1;
CAN/CSA STD C22.2 NO.601.1.
Manufacturer and
distributor
Sales TRUMPF Medizin Systeme GmbH + Co. KG
Telephone +49 89 80907–0
Fax +49 89 80907–40020
Technical Customer
Service
Telephone +49 3671 586–41199
Fax +49 3671 586–41175 +49 3671 586–41175
E-mail Service@trumpfmedical.com Service.wwo@trumpfmedical.com
TRUMPF Medizin Systeme GmbH + Co. KG
Carl–Zeiss–Straße 7–9
07318 Saalfeld
Germany
www.trumpfmedical.com
Benzstraße 26
82178 Puchheim
Germany
Germany other countries
+49 3671 586–41911
(24 hr service hotline)
We at TRUMPF Medical are constantly improving our products.
Therefore, we reserve the right to make changes to the format, equipment, and technology at any time.
Reprinting, copying or translating this document, in whole or in part, is forbidden without the express written
permission of TRUMPF Medizin Systeme GmbH + Co. KG.
TRUMPF Medizin Systeme GmbH + Co. KG expressly reserves all rights under copyright law.
Within the bounds of the legal requirements, the manufacturer is responsible for the technical safety characteristics
of this apparatus only if the maintenance, repairs, and modifications to this apparatus are performed by him or
by someone appointed by him and in accordance with his instructions.
© TRUMPF Medizin Systeme GmbH + Co. KG Revision level: 2016–10–06

This service manual applies to the following sales units:
Product name Mat. no.
TruSystem 7000 1841046
TruSystem 7000 V 1841050
Operating table TruSystem 7000 U 1604788
TruSystem 7000 (MBW) 1841048
TruSystem 7000 (MBW) V 1841082
Operating table TruSystem 7000 U (MB) 1604786
TruSystem 7000 (dV) 1841049
TruSystem 7000 (dV) V 1841083
Operating table TruSystem 7000 U (dV) 1723633
Cable remote control TS7000 U 1767067
Cable remote control TS7000 U (dV) 1798326
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Contents
Contents
1 Important Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
2 Safety information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
2.1 Safety during repair work . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
2.2 Liability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
2.3 Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
2.4 Explanation of Symbols. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
2.5 Glossary. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
2.6 Operating Table Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3 Remote control ▸System◂ (secured) menu . . . . . . . . . . . . . . . . . . . . . . . 19
4 Test procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
5 Safety information for the batteries (#1533137) . . . . . . . . . . . . . . . . . 24
6 Tools, measuring equipment and auxiliary tools . . . . . . . . . . . . . . . . . . 26
7 Preparing the Operating Table . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
8 Open and close the lower column cover . . . . . . . . . . . . . . . . . . . . . . . . 30
9 Open and close the top column cover . . . . . . . . . . . . . . . . . . . . . . . . . . 31
10 Disconnecting and reconnecting the internal power supply on the
operating table . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
11 Emergency adjustment for the lift . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
12 Mechanical parts of the table top . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
12.1 Table top . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
12.2 Pad plate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
12.3 Center box cover . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
12.4 Leg section gear box. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
12.4.1 Operating table without side rail at the joint . . . . . . . . . . . . . . . . . . . . . . . . . . 42
12.4.2 Operating table with side rail at the joint. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
12.5 Back section gear box . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
12.6 Energy chain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
12.7 Linear guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
12.8 Toothed rack . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
12.9 Longitudinal travel toothed belt. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
12.10 Retrofit kit for the side rail of the leg section joint . . . . . . . . . . . . . . . . . . . . . . . 54
13 Electrical parts of the table top . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
13.1 Motor controller circuit board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
13.2 Communications controller circuit board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
13.3 Battery on the communications controller circuit board. . . . . . . . . . . . . . . . . . 60
13.4 Drive unit for leg section . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 5

Contents
13.5 Leg section motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .63
13.6 Back section drive unit (back section bar) . . . . . . . . . . . . . . . . . . . . . . . . . . . . .65
13.7 Back section motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .67
13.8 Longitudinal travel motor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .69
13.9 CAN distributor circuit board (center box) . . . . . . . . . . . . . . . . . . . . . . . . . . . .71
13.10 CAN distributor circuit board (bar) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .72
13.11 Endolight interface circuit board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .74
14 Mechanical parts of the column . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
14.1 Column cover . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .75
14.2 Metal panel with the column keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .77
14.3 Bellows . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .78
14.4 Frame (connection ring) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .79
14.5 Toothed belt from the Trendelenburg drive . . . . . . . . . . . . . . . . . . . . . . . . . . . .81
14.6 Toothed belt from the tilt right drive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .84
14.7 Toothed belt from the tilt left drive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .87
14.8 Main cardan . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .90
14.9 Ratchet brace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .91
14.10 Telescopic spindle. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .93
14.10.1 Telescopic spindle - Variant 1. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .93
14.10.2 Telescopic spindle - Variant 2. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .96
14.11 Toothed belt from the main drive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100
14.12 Toothed washer of the telescopic spindle . . . . . . . . . . . . . . . . . . . . . . . . . . . 103
15 Electrical parts of the column . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 106
15.1 Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 106
15.2 Power supply unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 109
15.3 Power supply socket . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 111
15.4 Column keypad. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
15.5 Speaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 114
15.6 Lift motor assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 115
15.7 Trendelenburg assembly. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118
15.8 Tilt right assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 122
15.9 Tilt left assembly. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 125
15.10 Trendelenburg motor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
15.11 Tilt right motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
15.12 Tilt left motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 132
15.13 Connector / power supply / relay board (power supply socket). . . . . . . . 134
15.14 Column controller circuit board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 135
15.15 Cardan with sensor (Trendelenburg drive) . . . . . . . . . . . . . . . . . . . . . . . . . . 137
15.16 ISM module circuit board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 139
15.16.1 Option A . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 139
15.16.2 Option B . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 139
15.17 Retrofit ISM module (Mat. No. 2064680) . . . . . . . . . . . . . . . . . . . . . . . . . . 141
16 Mechanical parts of the table base . . . . . . . . . . . . . . . . . . . . . . . . . . . 143
16.1 Wheel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 144
16.2 Hinged foot on wheel (floor lock) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 146
6 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Contents
17 Electrical parts of the table base . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 148
17.1 Drive unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 149
17.2 Control module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 152
17.3 Line power socket . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 154
17.4 Power input fuses. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155
17.5 Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 156
18 Hydraulic parts of the table base. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 158
18.1 Information on the hydraulics system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 158
18.2 Refilling oil in the assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 160
18.3 Bleeding the hydraulic system. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 161
18.4 Coil on the pump assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163
18.5 Locking cylinder with hose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
18.6 Hose #1 through #4 with coupling (on the locking cylinder). . . . . . . . . . . . 169
18.7 Assembly with valve block . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 174
19 Cabling overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 177
20 Table top cables. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 180
20.1 Ground wire_24 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 181
20.2 Cable W174 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 183
20.3 Cable W165 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 184
20.4 Cable W166 and W167 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 185
20.5 Cable W168 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 187
20.6 Cable W169 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 188
20.7 Cable W170 and W171 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 189
20.8 Coupling point sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 191
20.8.1 Operating table without side rail at the joint . . . . . . . . . . . . . . . . . . . . . . . . . 191
20.8.2 Operating table with side rail at the joint. . . . . . . . . . . . . . . . . . . . . . . . . . . . 192
20.9 OR sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 195
20.10 Cable W193 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 196
20.11 Cable W190/W191 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 198
21 Column cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 199
21.1 Cable W104 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 200
21.2 Cable W159 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 203
21.3 Cable W160 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 205
21.4 Cable W161 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 207
21.5 Cable W164 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 209
21.6 Cable W134 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 213
21.7 Cable W172 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 215
21.8 Cable W173 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 217
21.9 Cable W107 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 219
21.10 Cable W108 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 221
21.11 Cable W109 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 223
21.12 Cable W110 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 225
21.13 Reset switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 227
21.14 Power sensor on the Trendelenburg drive . . . . . . . . . . . . . . . . . . . . . . . . . . . 229
21.15 Cable adapter. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 230
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 7

Contents
21.15.1 Option A . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 230
21.15.2 Option B . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 230
22 Table base cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 232
22.1 Cable W150 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 233
22.2 Cable W151 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 236
22.3 Cable W152 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 238
22.4 Cable W153 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 240
22.5 Cable W154 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 242
22.6 Cable W155 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 244
22.7 Cable W177 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 247
22.8 Cable W156 and W158 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 249
22.9 Cable W157 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 251
22.10 Cable W175 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 253
22.11 Cable W162 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 256
22.12 Ground wire _25 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 258
23 Quick-connect coupling on the assembly . . . . . . . . . . . . . . . . . . . . . . . 260
24 Adjustment and calibration tasks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 262
24.1 Adjusting the spindle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 262
24.2 Align the bars . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 265
24.3 Calibrate leg section motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 266
24.4 Calibrate back section motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 266
24.5 Calibrate longitudinal travel motor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 267
24.6 Calibrate tilt / Trendelenburg motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 267
24.7 Calibrate lift motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 268
25 Control units. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 269
26 Technical assistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 270
26.1 Operating conditions in battery-operated mode. . . . . . . . . . . . . . . . . . . . . . 270
26.2 Audio signals operating status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 271
26.3 Visual error signals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 272
26.4 Status display on the battery LED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 274
26.5 Monitoring tilt and Trendelenburg position angles . . . . . . . . . . . . . . . . . . . . 275
27 Components with serial numbers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 276
28 Fuse overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 279
29 Torque overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 280
30 Maintenance and repair . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 281
30.1 Maintenance log content . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 281
30.2 List of high-wear parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 283
31 Lubrication schedule. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 284
31.1 Column . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 284
31.2 Table top . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 284
8 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Contents
31.3 Table base. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 285
31.4 Components (leg section for example) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 285
32 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 286
33 Functional overview of electrical components . . . . . . . . . . . . . . . . . . . 291
34 Circuit diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 295
35 Hydraulics Diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 299
36 Service Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 303
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 9

Contents
10 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
1Important Information
This manual and any repair steps specified herein must be observed
and followed. Repairs may be performed only by service
technicians from TRUMPF Medizin Systeme GmbH + Co. KG or by
personnel authorized, trained, and certified by Trumpf Medical.
Only the repairs and settings listed in this guide or known to you
from the appropriate service training by our technical support
service may be performed! Repairs not covered in these instructions
may be performed only after consulting with the TRUMPF Medical
Technical Service department or by Trumpf Medical itself.
Unauthorized or provisional repairs are not permitted even if a
customer so requests. All laws, legal regulations and standards must
be observed and adhered to. Only use original spare parts
designated by Trumpf Medical as spare parts. Defective parts that
are not in proper working order must be replaced with original
spare parts, even if it is beyond the scope of the repair order! After
repairs, return cables to their original installed positions and avoid
any shearing or crushing. After each repair, recheck the electrical
connections against the circuit diagram before performing a
functional test. After all repairs or adjustments, a functional test has
to be performed in accordance with these instructions or relevant
standards. Recheck the position of the cables during and following
all equipment positions!
Important Information
Germany
Only deliver the device to the customer in the tested state with
full functionality! Hand-over to the customer must be effected in
writing with confirmation from the customer. The functionality
must be demonstrated to the customer!
Proper use of and instructions on how to operate the TruSystem
7000 operating table can be found in the instruction manual.
The Service Center will be grateful for any corrections and tips for
more efficient repairs. For questions or feedback please contact:
TRUMPF Medizin Systeme GmbH + Co. KG
Technical Customer Service
Carl-Zeiss-Str. 7–9
07318 Saalfeld
Germany
Telephone +49 3671 586–41199
(24 hr service hotline)
Fax 03671 586 –41175
E-mail Service@trumpfmedical.com
Internet www.trumpfmedical.com
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 11

Important Information
Other countries
Telephone +49 3671 586–41911
Fax + 49 3671 586–41175
E-mail Service.wwo@trumpfmedical.com
Internet www.trumpfmedical.com
12 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

2 Safety information
2.1 Safety during repair work
• Important: use only stainless-steel screws of strength class 70 on
the operating table! Comply with the torque specified for
tightening screws.
•For all screws ≥ M4 without split lock washers, use a mediumstrength screw locking agent. Notice, the agent can be
loosened again by heating the screws with a hair dryer.
• Protective work clothing:
Do not wear clothing which can become caught in the
equipment. Shirt and jacket sleeves should be buttoned or rolled
up.
Tie or put up long hair.
Tuck the ends of scarves, ties or shawls into your clothing or pin
them down. Loose clothing can be a hazard!
For activities that are moderately dangerous for the eyes, wear
protective glasses (e.g., for soldering work or when removing
taut springs and fasteners, when hammering in or hammering out
pins or similar parts).
Safety information
Protection against
infection
• Do not perform any activity that may put other people in danger
or that can make the device a source of danger!
• Store removed housing and other machine parts in a safe place
while working. Always store tools or removed parts in such a
way that no one can stumble and fall over them. Keep the area
around the device clean and tidy during and after maintenance
work.
• Do not let any screws, nuts or other parts fall into the column.
Immediately remove any parts that fell in. Parts that have not
been removed can damage other components or cables when
the OR table is adjusted. Important! The operating table may not
be released to the customer if parts fell in and were not removed.
• After performing work, reattach protective devices and replace
them if necessary. This includes, for example, covers, cable ties,
cable mounts, cable shields, ground and potential conductor
connections, and power connections. Check connection of
equipotential bonding conductor/grounding cable in
accordance with the applicable standards.
• Risk of infection throughout the entire hospital! Follow all safety
measures, behavioral rules and hygiene requirements. Follow
the requirements of the medical facility for protecting against
infection.
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 13

Liability
• Perform maintenance and repair work only on disinfected
operating tables! Disinfection is performed by the medical
facility.
• Perform work in the OR area only with appropriate
authorization.
• Immediately seek a physician in the event of a complaint of
possible infection. Inform the doctor you were working in a
hazardous area - even if this was several months prior.
Prevention against
infection
2.2 Liability
2.3 Disposal
• Ask the doctor about possible risks and discuss how to avoid
them in your work as a medical service technician.
• Get a hepatitis B inoculation if advised by a physician. A booster
shot is required every 3 to 5 years. The incubation period for
hepatitis B is 1 to 6 months!
TRUMPF Medizin Systeme GmbH + Co. KG IS LIABLE ONLY for
reliable and proper functionality of the operating table IF
• installation, modification, and repairs are performed by
Trumpf Medical service technicians or by personnel authorized,
trained, and certified by Trumpf Medical.
• the operating table is used properly in accordance with the
instruction manual.
The products must be recycled in an environmentally friendly
manner. Disposal, including that of individual parts, must be
environmentally friendly, i.e., in accordance with the legal
regulations currently in force. For information on proper disposal of
old equipment, please contact either Technical Service at
Trumpf Medical, your local sales representative, or the appropriate
national agency Trumpf Medical will take back your old equipment
or products that are defective or no longer used. For detailed
information, contact Technical Service.
When decommissioning an operating table, the lithium ion batteries
have to be removed by a Trumpf Medical service technician or a
person trained and authorized by Trumpf Medical. Return removed
and unusable batteries in suitable packaging to Trumpf Medical
Technical Service. Important: the returns must be declared as
hazardous materials of class 9/UN3480! Trumpf Medical will
take responsibility for the environmentally proper disposal of the
battery.
14 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
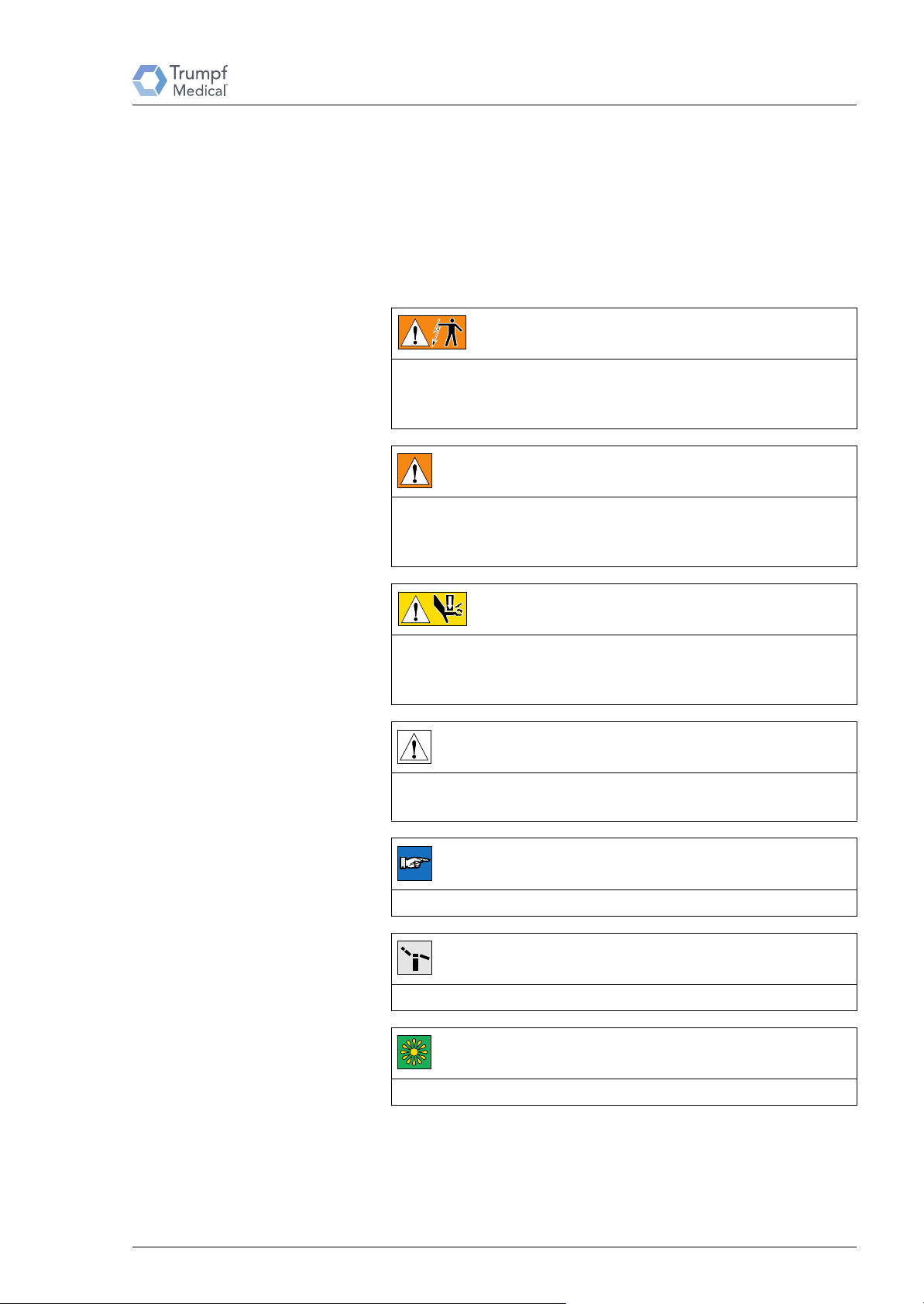
2.4 Explanation of Symbols
Important information in these repair instructions is marked with
symbols and keywords. Keywords such as DANGER, WARNING
or CAUTION indicate the level of danger involved. The symbols
emphasize the message visually. Additional symbols can indicate
injury hazards or danger to life and limb. The measures to prevent
hazards must be observed.
Refers to a directly imminent danger that will result in death or
serious injuries if the appropriate precautionary measures are not
taken.
Refers to a directly imminent danger that can result in death or
serious injuries if the appropriate precautionary measures are not
taken.
Explanation of Symbols
DANGER
Risk of death (for example, electric shock)!
WARNING
Risk of death!
CAUTION
Risk of injury (for example, crushing)!
Refers to a possible danger that can lead to slight to moderate
injury or damage to the equipment if the appropriate precautionary
measures are not taken.
CAUTION
Risk of material damage!
Refers to a possible danger that can lead to equipment damage if
the appropriate precautionary measures are not taken.
NOTE
Additional useful information and tips.
TEST
Performing functional tests, measurements and tests
ENVIRONMENT
Information on environmentally friendly disposal.
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 15

Glossary
2.5 Glossary
The following terms and abbreviations are used in these repair
instructions:
Abbreviation Explanation
#Material number
Trumpf Medical TRUMPF Medizin Systeme GmbH + Co. KG
Service Center Technical Service at Trumpf Medical
16 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
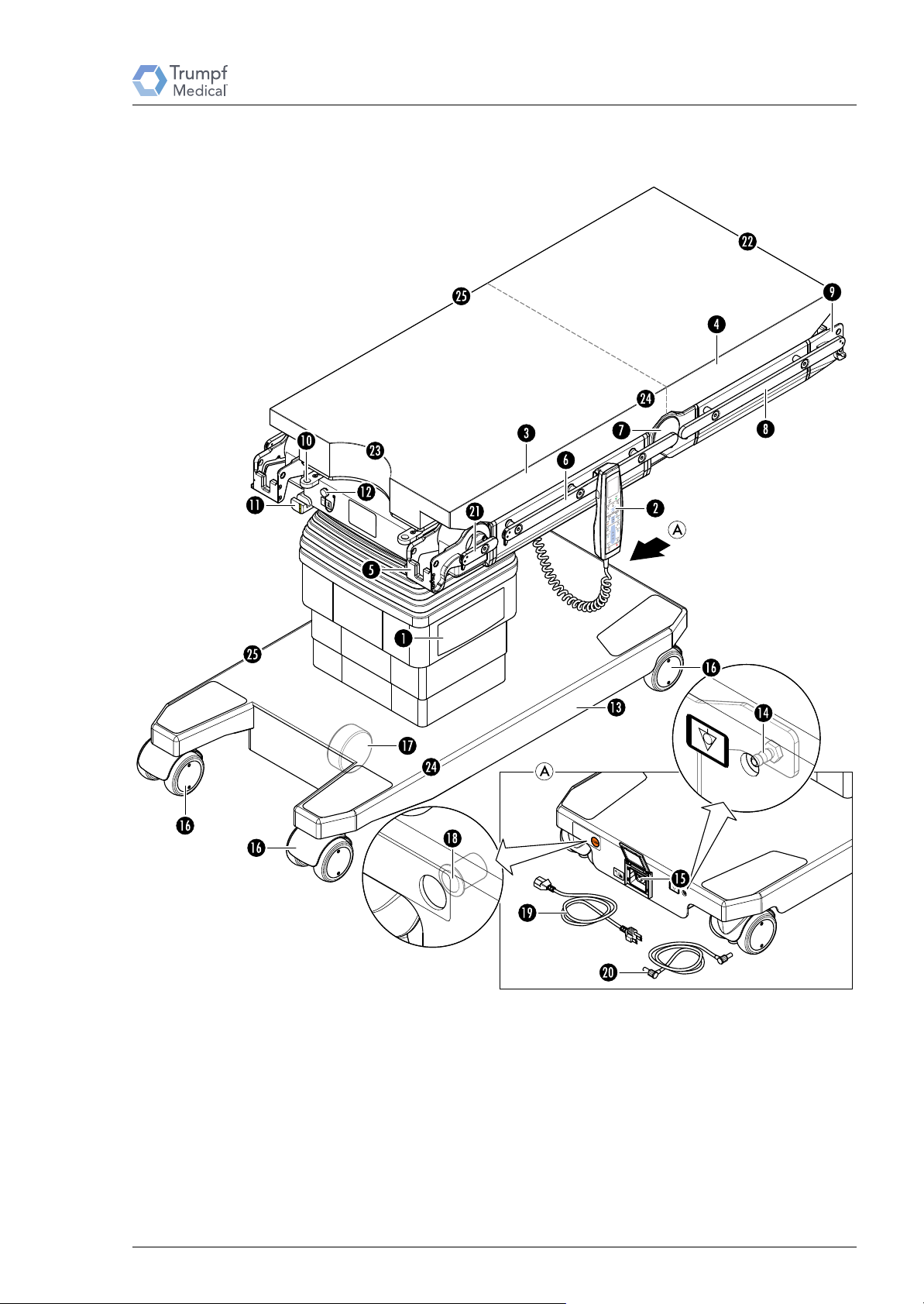
2.6 Operating Table Overview
Operating Table Overview
Key:
[1] Column keypad
[2] Remote control
[3] Seat section
[4] Back section
[5] Motorized leg section joint with fixture L
[6] Side rail for seat section
[7] Motorized back section joint
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 17

Operating Table Overview
Key:
[8] Side rail for back section
[9] Fixture S
[10] Insertion opening for extension adapter
[11] Bayonet locking mechanism for extension adapter
[12] Control unit connector socket (head and foot end)
[13] Table base
[14] Connector pin for equipotential bonding cable
[15] Connector socket for power cable
[16] wheel
[17] Wheel for table base support (directional travel/drive mode)
[18] Key for emergency release of the table 's base (under the
label)
[19] Power cable
[20] Equipotential bonding cable
[21] Side rail for leg section joint
*1
[22] Head end
[23] Foot end
[24] Left side
[25] Right side
*1
not available on all operating table variants
18 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Remote control ▸System◂ (secured) menu
3Remote control ▸System◂ (secured) menu
The ▸System◂ menu and its functions are available only with the
Cable remote control TS7000 U (#1767067).
The ▸System◂ (secured) submenu is located under the ▸Settings◂
menu item on the touch screen. This menu item is passwordprotected and accessible only to trained Trumpf Medical service
technicians. The password, which is set at the factory, is available
from the Service Center.
The individual numbers (0 to 9) of the password (code) are set
using the arrow keys [i16]/[i17]/[i47] and [i48]. The cursor
flashes at the currently active position. After it is entered, confirm the
password with the OK [i31] key.
The ▸System◂ menu contains the following displays and settings:
–1. ▸Display of HW/SW states◂
–2. ▸Block RC on Key Error◂
–3. ▸Show Key Error Status◂
–4. ▸Component Test◂
–5. ▸Motion Sensor Test◂
–6. ▸Key test FB◂
–7. ▸LED test FB◂
–8. ▸Start Flash update◂
–9. ▸Error memory exp.◂ (export)
– 10. ▸End emergency mode◂
– 11. ▸Reset or table◂
– 12. ▸Factory defaults◂
– 13. ▸Network address◂
– 14. ▸Set IR code◂
– 15. ▸Change password◂
– 16. ▸Acceleration sensor active◂
– 17. ▸Display battery state◂
– 18. ▸ISM parameters◂
– 19. ▸SD Card Info◂
– 20. ▸Show all messages◂
– 21. ▸Touch sensors inactive◂
– 22. ▸Test touch sensors◂
– 23. ▸Request service mode◂
NOTE
Standby mode is switched off for most of the settings in the
▸Settings◂ menu.
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 19
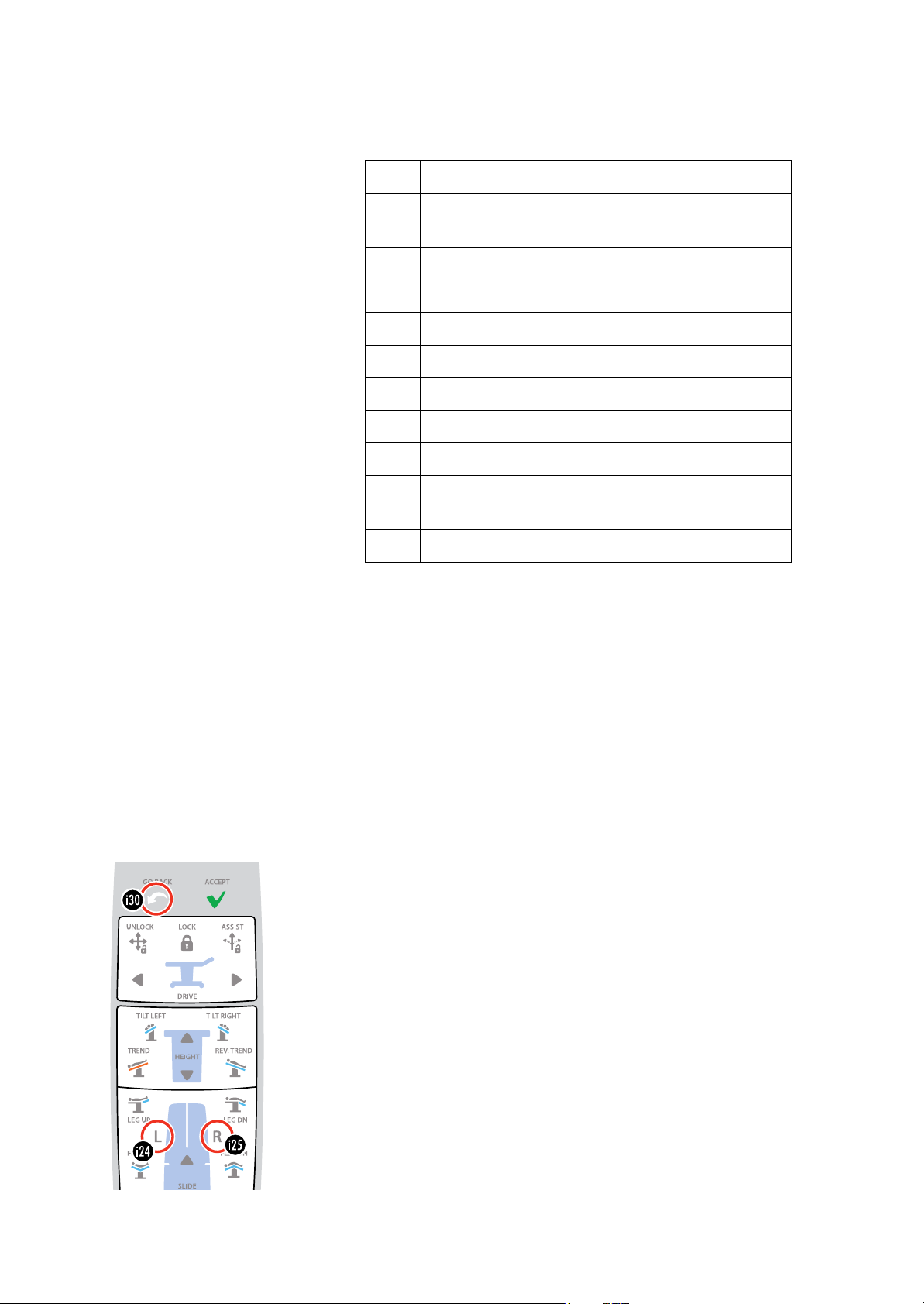
Remote control ▸System◂ (secured) menu
1. ▸Display of HW/SW states◂
Line Meaning
1 Hardware state and software version of the
application
2 Serial number
3 Graphics version
4Text version
5 Character set version
6 Upper shell software version
7 Boot loader version
8 Software package version
9 Motor controller and communication processor
version (presently without function)
10 Radio module software version
2. ▸Block RC on Key Error◂
This function can be used to block remote control if a keyboard
error was determined.
3. ▸Show Key Error Status◂
The display shows which key has an error.
4. ▸Component Test◂
The menu item delivers a function test for all hardware
components (touch, motion sensor). Do not make any settings
using this menu item.
5. ▸Motion Sensor Test◂
Tilt sensor test
6. ▸Key test FB◂
The key test function lets you check the functionality of the
individual keys on the remote control, except for the
ANCEL [i30] key. The key functions are not executed. When
C
the key is pressed, the (hexadecimal) key code is shown on the
display. No key code is shown for the keys that select joints
(L [i24]/R [i25]).
7. ▸LED test FB◂
During the remote control LED test, the background illumination
of the individual keys is tested in sequence.
8. ▸Start Flash update◂
No function at this time.
20 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
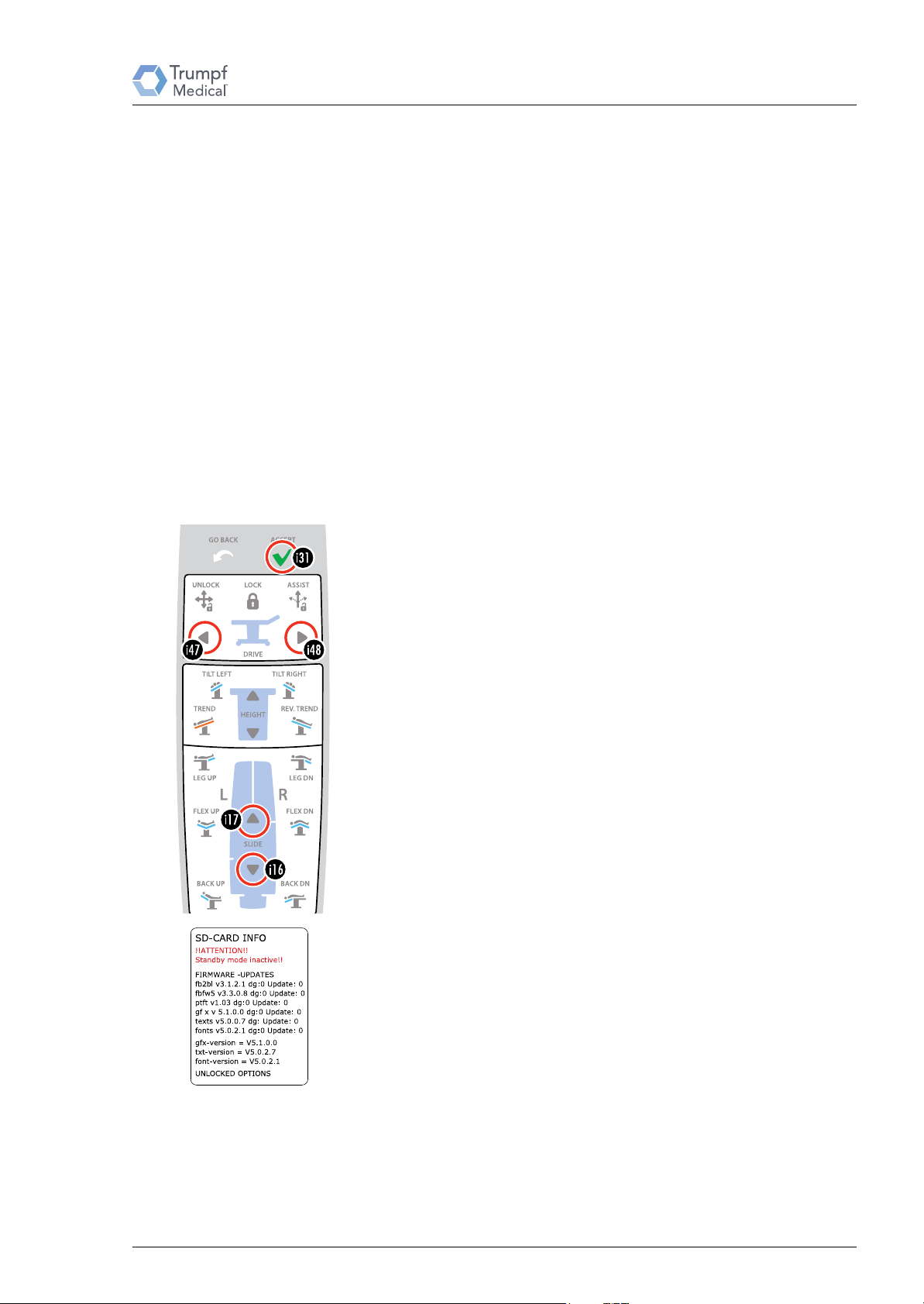
Remote control ▸System◂ (secured) menu
9. ▸Error memory exp.◂ (export)
No function at this time.
10. ▸End emergency mode◂
No function at this time.
11. ▸Reset or table◂
No function at this time.
12. ▸Factory defaults◂
No function at this time.
13. ▸Network address◂
The LAN and W-LAN IP address is shown.
Important: the IP address must be read from right to left. For
example, 1.2.3.4 is shown on the display. The correct IP
address is 4.3.2.1.
14. ▸Set IR code◂
No function at this time.
15. ▸Change password◂
You can change the password that has been set at the factory.
The individual numbers (0 to 9) of the password are set using
the arrow keys [i16]/[i17]/[i47] and [i48]. The cursor flashes
at the currently active position. After it is entered, confirm the
password with the O
K [i31] key.
16. ▸Acceleration sensor active◂
The motion sensor can be switched off if frequent shaking in the
area prevents standby mode. The sensor for activating the
remote control from the standby mode is active when the
display is marked by an * (asterisk). To activate or deactivate
the sensor press the OK [i31] key.
17. ▸Display battery state◂
Function is not active.
18. ▸ISM parameters◂
Function is not active.
19. ▸SD Card Info◂
Menu item delivers information on the version of the text and
graphics files.
20. ▸Show all messages◂
The operating table transmits system information in an
encrypted code to the remote control. The most important
system information (e.g., error messages, status reports) are
stored in plain text messages and are displayed on the remote
control. Upon activating the ▸Show all messages◂ function, all
system information is displayed. The ▸Show all messages◂ is
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 21

Remote control ▸System◂ (secured) menu
21. ▸Touch sensors inactive◂
22. ▸Test touch sensors◂
23. ▸Request service mode◂
identified by an * (asterisk). To activate or deactivate (normal
state) the messages press the O
K [i31] key.
Function is not active.
Touch screen fields are shown on the display. When one of
the individual fields is touched, the respective active field is
marked yellow.
Activate service mode
22 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
4 Test procedures
Test procedures
As the medical products from Trumpf Medical are distributed
worldwide, uniform guidelines for electrical retesting should be
used.
According to the IEC 60601-1 standard, the limit values according
to the CF classification apply when testing applied parts. Based on
the design type, the Trumpf Medical electric operating tables only
include one applied part of Class B.
Furthermore, the Trumpf Medical product-specific prescribed retests
and safety checks are obligatory.
Germany International
In Germany, initial testing and
retesting of electrical devices
are governed by the stipulations
of the accident prevention
regulation BGV A3.
VDE 0751-1 is the standard that
In countries with different
national legislation, the countryspecific standards and
guidelines for the retesting of
electrical medical devices are
mandatory.
serves as the basis for retesting
of electrical medical devices.
Therefore in accordance with
the VDE 0751-1 standard noted
above, the specifications based
on IEC 60601-1 are definitive
for Trumpf Medical medical
devices.
All maintenance work must be carried out with the help of the
maintenance protocol and take into consideration the spare or
wearing parts list and the lubrication plan (see page 284).
Conduct a complete functional test after service work is completed.
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 23
Safety information for the batteries (#1533137)
5 Safety information for the batteries (#1533137)
The TruSystem 7000 operating table contains two lithium ion
batteries. Because of their size (number of individual cells plugged
in, amount of lithium contained), they are classified as a hazardous
material. There is a risk of explosion in case of fire. As a result, there
are legal ordinances regarding the handling, deployment, and
installation of this type of battery.
The lithium ion batteries were specially developed by
Trumpf Medical. An electronic protective circuit on a circuit board
in the housing monitors the threshold values of the current / voltage
data. The electrical connection outward is provided through a
7W2 D-Sub socket.
Technical parameters Threshold values
Nominal voltage (3.7 V/cell) 40.7 V
Nominal capacity (2.4 Ah/cell) 4.8 Ah
Handling on/in
operating table
Working voltage range 37 V…45.1 V
Shutdown undervoltage (2.4 V / cell) approx. 26 V
Shutdown overvoltage (4.35 V / cell) approx. 48 V
Shutdown overcurrent 20 A
Self-discharge current with active battery 180 μA
Max. discharge current over 2 min. 8 A
Max. continuous discharge current 4.8 A
Charging time with 3 A charge current Approx. 3 hrs
Storage temperature range – 40 °C to 80 °C
Max. internal battery temperature during
discharge
Max. internal battery temperature during
charge
Battery Position
1on power supply
60 °C
40 °C
2 on lift motor
The battery is connected using cable W134 (#1538234) for
battery 1 and W172 (#1538235) for battery 2. The battery is
directly connected or disconnected using the corresponding
connection cable in the operating table. There are no special
measures, such as a connection sequence on the power supply. A
24 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Safety information for the batteries (#1533137)
battery is inserted or removed using the carrying strap. The column
base has two diagonal guide pins that position the battery housing.
Battery 1 is secured in place by a special plate (battery
bracket_power supply). Battery 2 is secured by the spiral cable
holder_rear.
Storage For extended battery storage, the manufacturer recommends a
charge level of approx. 40 %. For storage, as a guide
Trumpf Medical recommends a no-load voltage of approx. 43 V
for the battery charge level. Batteries should be stored in a cool, dry
place.
Charging/
discharging
The batteries are usually charged in the operating table by the
power supply. During external charging, the maximum charge
current equals 2.4 A. The maximum charge voltage of 45.1 V may
not be exceeded. A rechargeable battery is full (stops charging)
when the charging current falls below 100 mA for at least 1 minute.
The maximum discharge current for a period of no more than 2
minutes may not exceed 8 A. Important: there is no temperature
monitoring when the battery is used as an external energy source.
The threshold temperatures may not be exceeded.
To prevent hazards, the battery state is monitored automatically in
the operating table.
Transport All batteries must undergo a Trumpf Medical certified safety test
before being released for general use. To ship a battery, use the
manufacturer's special transport packaging (reusable packaging).
Disposal Return removed and unusable batteries in suitable packaging to
Trumpf Medical Technical Service. Important: the returns must be
declared as hazardous materials of class 9/UN3480!
Trumpf Medical will take responsibility for the environmentally
proper disposal of the battery.
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 25

Tools, measuring equipment and auxiliary tools
6 Tools, measuring equipment and auxiliary tools
Tools
Basic tools and equipment
Allen wrench set
Open wrench set
Philips head screwdriver
Slotted screwdriver
Side cutting pliers
Nylon mallet
Thickness gauge
Retainer ring pliers
Circlip pliers with check screw
Slide hammer
Special tools Mat. no.
Torque wrench 2 Nm to 150 Nm
Measurement
equipment
(T) Hook wrench
for removing the leg section and back section motor
Product name Mat. no.
Multimeter
Cable tester
Electronic spirit level
Secutest SIII
(T) TS7000 lifting drive gauge
for setting the level position on the lifting motor
(T) TS7500 additional drives gauge
for setting the level position on the auxiliary drive motor
(T) Belt tension meter
to check the toothed belt tension on the Trendelenburg
and longitudinal travel motor
1484981
1729967
1525774
1798264
Spring balance
to check the toothed belt tension on the Trendelenburg
and tilt motor
26 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016

Tools, measuring equipment and auxiliary tools
Product name Mat. no.
Auxiliary tools
(T) Adjustment gauge TS7000
1817764
for aligning the two bars
(T) Overlay for joint setting TS7500
1525777
Support point for the spirit level (for example when
setting the level position on the back section motors).
At least two overlays are required.
Product name Mat. no.
(T) Multifunction tool
1553708
for placing the operating table on its side
(T) Star wheel
1800121
for placing the operating table on its side
(T) Spring balance bracket
1814183
to set the toothed belt tension on the Trendelenburg
and tilt motor
M6x60 retaining screw
Lubricants and
additives
for securing the column cover
M5 clamping screw
to set the toothed belt tension on the Trendelenburg
and tilt motor
2 M8x60 retaining screws
for securing the telescopic spindle during emergency
adjustment of the lift
Cable tie
Product name Mat. no.
Terostat MS 939 adhesive 4150068
Loctite 5203 sealant 4150054
Loctite 5699 sealant 4150065
omniFIT 100 M screw locking agent 4150018
omniFIT 200 M screw locking agent 4150020
Thread-locking adhesive UHU weak fast 4150046
Thermal grease 4150060
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 27

Tools, measuring equipment and auxiliary tools
Product name Mat. no.
THERMOPLEX® ALN 250 EP low-viscosity gear
1483750
grease
®
TURMOGREASE
LI 802 EP special grease 1473385
TURMOPLEX® L220 lubricant 4150047
PROFI-TURBO-GREASE® spray 4150050
Klüberlectric KR 44-120 1557674
Castrol Hyspin DSP 32
1864008
Mineral oil (viscosity of 32) for refilling the assembly
Care and cleaning kit 4159014
Alcohol (degreasing agent)
28 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
7 Preparing the Operating Table
Before beginning repair work, prepare the operating table in
accordance with the following work steps:
Preparing the Operating Table
1.
CAUTION
Danger of infection!
Note to hygienic conditions at the site, and clarify any
questions concerning protection against infection with the
doctor in charge. Before beginning work, have the medical
facility confirm that all necessary measures for protection
against infection have been implemented. Perform repairs
only after protection of personnel against infection is ensured.
2. Lock operating table (brake)
3. Move to the level position on the operating table.
4. Remove all table components and accessories from the
operating table and store them in a safe place.
5. Move the operating table to the most elevated position.
6. Disconnect the operating table from the external power supply
(first remove the power cable plug from the insulated contact
receptacle and then from the connection socket on the table
base).
7. Remove the pad from the table top so that it is not damaged
during repair.
During repair work, do not make any adjustments to the operating
table except those explicitly specified.
Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016 29
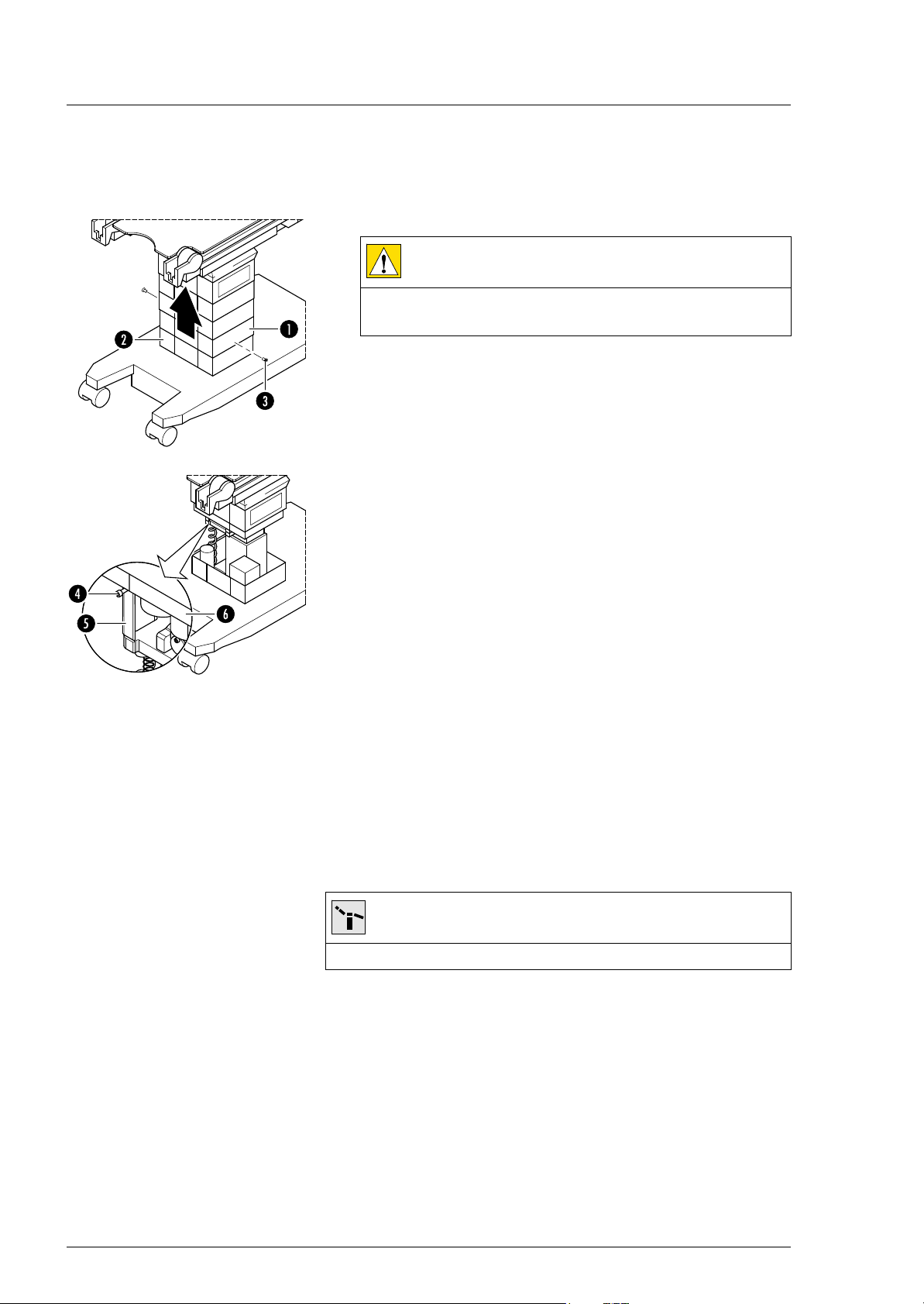
Open and close the lower column cover
8 Open and close the lower column cover
Open 1. Prepare the operating table (see chapter 7 on page 29).
2. Turn off the operating table.
CAUTION
Risk of injury due to capacitor voltage.
Switch off repeatedly if needed. No LEDs may be illuminated
on the column keypad!
3. Disconnect the lowest column cover [1] from the pot [2] (2
screws [3]).
4. Carefully slide the column cover upward, tighten the retaining
screw [4] on the Trendelenburg assembly [5], and place the
column cover [6] on the retaining screw.
Close 1. Grasp the column cover, remove the holding screw from the
Trendelenburg assembly and guide the cover carefully
downward.
2. Install the lowest column cover on the pot (2 screws).
3. Put on the pad.
4. Switch on the operating table at the column keypad.
5. Connect the operating table to the external power supply.
TEST
Perform a function test.
30 Service Manual TruSystem 7000 Operating Table – 1 764 986 – 10/2016
 Loading...
Loading...