STERIS Amsco Service Manual

OPERATING INSTRUCTIONS
Amsco® Century
®
Gravity and Prevacuum Sterilizers
(11/13/06) P129367-408
STERIS Mexico
Avenida Avante 790
Parque Industrial Guadalupe
Guadalupe, Nuevo Leon, Mexico C.P. 67190
www.steris.com
© 2006– STERIS Corporation. All rights reserved. Printed in U.S.A
Table of Contents Operating Instructions 129367-408

A WORD FROM STERIS CORPORATION
This manual contains important information on proper use of this sterilizer. All
operators and department supervisors are urged to carefully review and
become familiar with the warnings, cautions and instructions contained herein.
This sterilizer is specifically designed to process goods using only the cycles
as specified in this manual. If there is any doubt about a specific material or
product, contact the manufacturer of the product for the recommended
sterilization technique.
STERIS carries a complete line of accessories for this unit to simplify, organize
and assure sterility of the sterilization process. Instrument trays, pouches and
biological/chemical monitoring systems are all available to fulfill your facility’s
processing needs. A STERIS representative will gladly review these with you.
Advisory
Service
Information
Indications for
Use
A summary of the
servicing this equipment can be found in Section 1 of this manual. Do not
operate or service the equipment until you have become familiar with this
information.
Any alteration of the sterilizer not authorized or performed by STERIS which
could affect its operation will void the warranty, could adversely affect
sterilization efficacy, could violate federal, state and local regulations and
jeopardize your insurance coverage.
A thorough preventive maintenance program is essential to safe and proper
sterilizer operation. You are encouraged to contact STERIS concerning our
Preventive Maintenance Agreement. Under terms of this agreement, preventive maintenance, adjustments, and replacement of worn parts are done on a
scheduled basis to assure equipment performance at peak capability and to
help avoid untimely or costly interruptions. STERIS maintains a nationwide staff
of well-equipped, factory-trained technicians to provide this service, as well as
expert repair services. Contact STERIS for details.
The Amsco® Century® Steam Sterilizer is designed for efficient, sterilization of
non-porous and porous, heat and moisture-stable materials used in healthcare
facilities. The Century Steam Sterilizer is available in the following configurations:
Safety Precautions
to be observed when operating and
16" x 16" x 26" Single Door Gravity
16" x 16" x 26" Double Door Gravity
20" x 20" x 38" Single Door Gravity
20" x 20" x 38" Double Door Gravity
Table of Contents Operating Instructions 129367-408
16" x 16" x 26" Single Door Prevacuum
16" x 16" x 26" Double Door Prevacuum
20" x 20" x 38" Single Door Prevacuum
20" x 20" x 38" Double Door Prevacuum
i
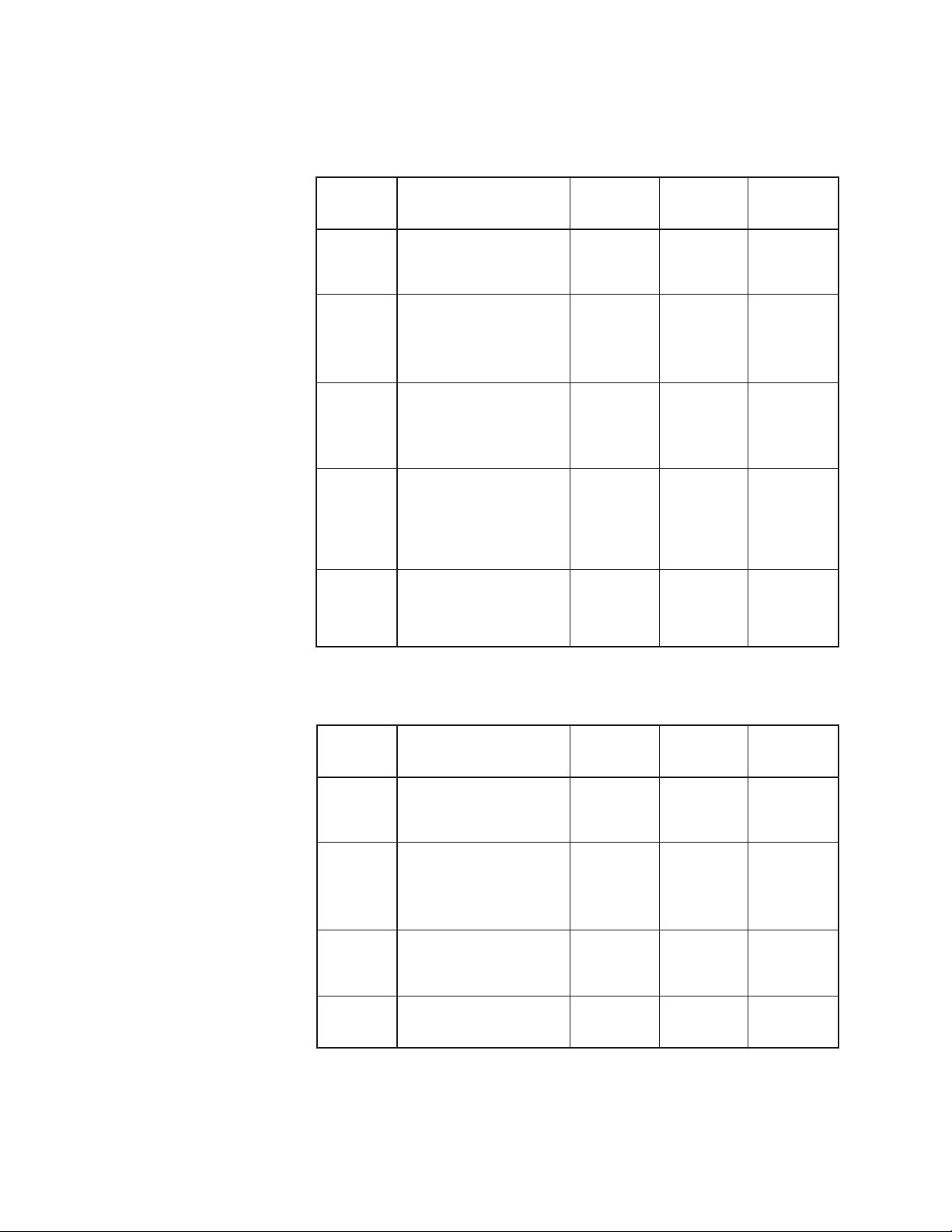
The Amsco Century Steam Sterilizer is equipped with the following factoryprogrammed sterilization cycles and cycle values:
PREVACUUM CONFIGURATION
CYCLES RECOMMENDED STERILIZE STERILIZE DRY TIME
LOADS TEMP. TIME
FLASH Unwrapped 270°F 3.0 minutes 1.0 minute
Instrument tray with (132°C)
a single instrument.
FLASH Unwrapped instrument 270°F 10 minutes 1.0 minute
tray with non-porous (132°C)
multiple instruments,
maximum weight 17lbs.
EXPRESS Single wrapped 270°F 4.0 minutes 3.0 minutes
instrument tray with (132°C)
a single instrument.
Non-porous goods only.
PREVAC Up to two double 270°F 4.0 minutes 20 minutes
1
wrapped instrument (132°C)
trays, maximum
weight 17 lbs. Up
to six fabric packs.
PREVAC Up to two double 275°F 3.0 minutes 16 minutes
wrapped instrument (135°C)
trays, maximum
weight 17 lbs.
1
Five minute Dry Time can be used for processing a single fabric pack.
GRAVITY CONFIGURATION
CYCLES RECOMMENDED STERILIZE STERILIZE DRY TIME
LOADS TEMP. TIME
FLASH Unwrapped 270°F 3.0 minutes 1.0 minute
Instrument tray with (132°C)
a single instrument.
FLASH Unwrapped instrument 270°F 10 minutes 1.0 minute
tray with non-porous (132°C)
multiple instruments,
maximum weight 17lbs.
GRAVITY Up to two double wrapped 270°F 15 minutes 30 minutes
trays, maximum (132°C)
weight 17 lbs.
GRAVITY Up to six Fabric Packs. 250°F 30 minutes215 minutes
(121°C)
2
A 270°F (132°C) cycle adjusted to 25 minute Sterilize Time can be used for
processing fabric packs.
ii
129367-408 Operating Instructions Table of Contents

TABLE OF CONTENTS
Section
Title
A WORD FROM STERIS CORPORATION ...................................... i
1 SUMMARY OF SAFETY PRECAUTIONS ............................ 1-1
2 STERILIZATION TECHNIQUES ......................................... 2-1
2.1 General .............................................................................................................. 2-1
2.2 Special Information Regarding the Express Cycle .............................................. 2-1
2.2.1 Preparing Instruments for Express Cycle Sterilization .................................. 2-2
2.2.2 Unloading ..................................................................................................... 2-2
2.3 Special Information Regarding The Flash Cycle ................................................. 2-3
2.4 Control Measures For Verifying Sterilization Process ......................................... 2-4
2.4.1 Biological Monitors ....................................................................................... 2-4
2.4.2 Testing for Prevacuum Efficiency ................................................................ 2-4
2.5 Dart (Bowie-Dick) Test ....................................................................................... 2-5
2.6 Vacuum Leak Test ............................................................................................. 2-5
2.7 Sterilization Process Recommendations .......................................................... 2-5
3 COMPONENT IDENTIFICATION ........................................ 3-1
3.1 General .............................................................................................................. 3-2
3.2 Main Sterilizer and Cycle Controls ...................................................................... 3-2
3.3 Control Displays ................................................................................................. 3-3
3.4 Alarm Displays ................................................................................................... 3-4
3.5 Operating End Control Panel .............................................................................. 3-5
3.6 Cycle Selection Touch Screen Pads .................................................................. 3-6
3.6.1 Values Touch Screen Pads .......................................................................... 3-6
3.6.2 Abort Touch Screen Pad .............................................................................. 3-7
3.7 Printer ................................................................................................................ 3-7
3.8 Printouts ............................................................................................................. 3-8
3.9 Power Door Operation ........................................................................................ 3-9
3.10 Emergency Door Opening Procedure .............................................................. 3-10
3.11 Optional Electric Steam Generator ................................................................. 3-11
Page
4 STERILIZER OPERATION ................................................. 4-1
4.1 Before Operating the Sterilizer............................................................................ 4-1
4.2 Preparing Loads for Sterilization Cycles ............................................................ 4-2
4.3 Guidelines for Placement of Various Loads ........................................................ 4-3
4.4 Unloading the Sterilizer ....................................................................................... 4-4
4.5 Loading Car Instructions: Loading ...................................................................... 4-5
4.6 Loading Car Instructions: Unloading ................................................................... 4-6
4.7 Loading/Unloading Sterilizer Equipped With Rack and Shelves .......................... 4-6
4.8 Sterilizer (Factory) Cycle Settings ...................................................................... 4-7
4.9 270°F Flash Cycle .............................................................................................. 4-8
4.10 Gravity Cycle ................................................................................................. 4-11
4.11 270°F Express Cycle ...................................................................................... 4-14
4.12 270°F Prevac Cycle........................................................................................ 4-17
4.13 275°F Prevac Cycle........................................................................................ 4-20
4.14 DART (Bowie-Dick) Test (Prevacuum Sterilizer only) .................................... 4-23
4.15 Vacuum Leak Test (Prevacuum Sterilizer only) .............................................. 4-25
Section 4 Contents Continued On Next Page
Table of Contents Operating Instructions 129367-408
iii

TABLE OF CONTENTS (Cont'd)
Section
4.16 Optional Liquid Cycle ...................................................................................... 4-27
4.17 Sterilization Techniques for Optional Liquid Cycle .......................................... 4-30
4.18 Recommendations for Sterilizing Liquids ........................................................ 4-31
4.19 Aborting Cycles .............................................................................................. 4-32
4.20 Cycle Graphs ................................................................................................. 4-33
Title
5 STERILIZER CYCLES AND CYCLE VALUES ...................... 5-1
5.1 Sterilizer (Factory) Cycle Settings ...................................................................... 5-2
5.2 Change Values ................................................................................................... 5-3
5.3 Change Cycle Values ......................................................................................... 5-4
5.3.1 Overview ...................................................................................................... 5-4
5.3.2 Step by Step ................................................................................................ 5-4
5.4 Adjusting Sterilize Time And Dry Time ............................................................... 5-7
5.5 Change Time and Date ....................................................................................... 5-8
5.6 Change Machine Setup .................................................................................... 5-10
5.7 Access Code .................................................................................................... 5-11
5.8 Lockout ............................................................................................................ 5-13
5.9 Utilities Control ................................................................................................. 5-14
5.10 Language ........................................................................................................ 5-17
5.11 Machine Number ............................................................................................ 5-18
5.12 Time Format ................................................................................................... 5-19
5.13 Print Format ................................................................................................... 5-20
5.14 Audible Signals .............................................................................................. 5-21
5.15 Units ............................................................................................................... 5-22
5.16 Date Format ................................................................................................... 5-22
5.17 Duplicate Print ................................................................................................ 5-23
5.18 Leaving Change Values .................................................................................. 5-23
Page
LIST OF TABLES
Table
2-1 Cycle Availability ...................................................................................................................... 2-1
2-2 Item Processing Guidelines For Express Cycle ........................................................................ 2-2
3-1 Required Feed Water Quality for Carbon Steel Steam Generators .......................................... 3-12
4-1 Factory-Set Cycles and Cycle Values ....................................................................................... 4-7
4-2 Liquid Cycle Parameters ........................................................................................................ 4-30
5-1 Cycle Availability ...................................................................................................................... 5-1
5-2 Factory-Set Cycles and Cycle Values ....................................................................................... 5-2
5-3 Change Machine Setup .......................................................................................................... 5-10
iv
129367-408 Operating Instructions Table of Contents
Title
Page

!
!
!
!
!
!
!
!
!
!
!
SUMMARY OF SAFETY PRECAUTIONS 1
Following is a list of the
indicate the potential for danger to personnel, and CAUTIONS indicate the potential for damage to equipment. These
precautions are repeated (in whole or in part), where applicable, throughout the manual. This is a listing of all
Precautions
appearing in the manual. Carefully read them before proceeding to use or service the unit.
Safety Precautions
which must be observed when operating this equipment. WARNINGS
Safety
WARNING - BURN HAZARD:
When sterilizing liquids, to prevent personal injury or property damage resulting from bursting bottles and hot
fluid, you must observe the following procedures:
• Use Liquid cycle only; no other cycle is safe for processing liquids.
• Use only vented closures; do not use screw caps or rubber stoppers with crimped seals.
• Use only Type I borosilicate glass bottles; do not use ordinary glass bottles or any container not designed for
sterilization.
• Do not allow hot bottles to be jolted; this can cause hot-bottle explosions. Do not move bottles if any boiling
or bubbling is present.
It is inappropriate for a healthcare facility to sterilize liquids for direct patient contact.
Sterilizer, rack/shelves, and loading car will be hot after cycle is run. Always wear protective gloves and
apron when removing a processed load. Protective gloves and apron must be worn when reloading sterilizer
following the previous operation.
Before daily flushing of the generator, generator must be at zero psig and cooled to room temperature.
Do not attempt to open the sterilizer door if a WATER IN CHAMBER ALARM condition exists. Call a qualified
service technician before attempting to use sterilizer further.
Sterilizer operator may be severely burned by scalding water if the water level control malfunctions. The
steam generator level control may malfunction if the supply water exceeds 26,000 ohms/cm (38.5 conductivity
min.). Do not connect to treated water (e.g., distilled, reverse osmosis, deionized) unless water resistivity is
determined to be acceptable. If water exceeds 26,000 ohms/cm, contact STERIS Engineering Service for
information concerning modifications required to the generator control system.
After manual exhaust, steam may remain inside the chamber. Always wear protective gloves, apron and a
face shield when following emergency procedure to unload sterilizer. Stay as far back from the chamber opening
as possible when opening the door.
Allow sterilizer to cool to room temperature before performing any cleaning or maintenance procedures.
Failure to shut off the steam supply when cleaning or replacing strainers can result in serious injury. Jacket
pressure must be 0 psig before beginning work on the steam trap.
Proper testing of the safety valve requires the valve to be operated under pressure. Exhaust from the safety
valve is hot and can cause burns. Proper safety attire (gloves, eye protection, insulated overall) as designated
by OSHA, is required. Testing is to be performed by qualified service personnel only.
Steam may be released from the chamber when door is opened. Step back from the sterilizer each time the
door is opened to minimize contact with steam vapor.
1-1
Summary of Warnings and Cautions Operating Instructions 129367-408
WARNING – ELECTRIC SHOCK AND BURN HAZARD:
!
!
!
!
!
!
!
!
!
!
!
!
!
Disconnect all utilities to sterilizer before servicing. Do not service the sterilizer unless all utilities have been
properly locked out. Always follow OSHA Lockout-Tagout and electrical safety-related work practice standards.
(See CFR 1910.147 and .331 through .335.)
WARNING – PERSONAL INJURY HAZARD:
Avoid personal injury from bursting bottles. Liquid sterilization cycle must only be used for liquids in
borosilicate (Pyrex) flasks with vented closures.
Door must be locked and the key retained prior to entering chamber for servicing. Always follow appropriate
Lockout-Tagout and electrical safety-related work practice standards. Emergency stop switch can be depressed
and key retained on sliding door units.
WARNING - EXPLOSION HAZARD:
This sterilizer is not designed to process flammable compounds.
WARNING - SLIPPING HAZARD:
To prevent falls keep floors dry by immediately wiping up any spilled liquids or condensation in sterilizer loading
or unloading area.
WARNING – PERSONAL INJURY AND/OR EQUIPMENT DAMAGE HAZARD:
Regularly scheduled preventive maintenance is required for safe and reliable operation of this equipment.
Contact your STERIS service representative to schedule preventive maintenance.
When closing the chamber door, keep hands and arms out of the door opening and ensure opening is clear
of obstructions.
Repairs and adjustments to this equipment must be made only by fully qualified service personnel.
Maintenance performed by inexperienced, unqualified persons or installation of unauthorized parts could cause
personal injury or result in costly equipment damage.
WARNING - STERILITY ASSURANCE HAZARD:
Load sterility may be compromised if the biological indicator or air leak test indicates a potential problem.
If these indicators show a potential problem, refer the situation to a qualified service technician before using the
sterilizer further.
According to AAMI standards, a measured leak rate greater than 1.0 mm Hg/minute indicates a problem
with the sterilizer. Refer the situation to a qualified service technician before using the sterilizer further.
The Express cycle is only intended for use with a single instrument in a single wrapped instrument tray.
The Express cycle is not intended for processing porous items (except the tray wrapper).
The Flash cycle is not intended for processing porous items.
1-2
129367-408 Operating Instructions Summary of Warnings and Cautions

CAUTION - POSSIBLE EQUIPMENT DAMAGE:
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
Gasket must be fully retracted prior to operating sterilizer door.
Failure to flush generator daily could result in malfunction of the generator. Warranty on the generator will be voided
unless flushed daily.
Before flushing generator, ensure generator drain valve is fully open to prevent generator heaters from turning
on during flush phase.
If zero dry time is selected, sterilizer automatically initiates a vapor removal phase in place of drying. This phase
can still draw a vacuum to 5.0 inHg. Consult device manufacturer's recommendations to ensure devices being
processed can withstand this depth of vacuum.
Lifting the chamber float switch when cleaning the chamber may cause the sterilizer control to initiate a “Chamber
Flooded” alarm. If this alarm condition occurs, the operator must turn the control power OFF then ON to clear the
alarm. The control power switch is located in the mechanical area at the side of the sterilizer. Placing the sterilizer
in standby does not clear this alarm.
Never use a wire brush, abrasives, or steel wool on door and chamber assembly. Do not use cleaners containing
chloride on stainless-steel surfaces. Chloride-based cleaners will deteriorate stainless steel, eventually leading
to failure of the vessel.
Do not use cleaners containing chlorides on loading cars. Chloride-based cleaners will deteriorate the loading car
metal.
Sterilization of chloride-containing solutions (e.g., saline) can cause chamber corrosion and is not recommended
by the manufacturer. If, however, chloride-containing solutions must be processed, clean the chamber after each
use.
Allow thermostatic traps to cool down to room temperature before removing cover. Since there is nothing to limit
expansion, the bellows may rupture or fatigue if trap is opened while hot.
Actuation at less than 75% of rated pressure can allow debris to contaminate the seat and cause the safety valve
to leak. A leaking safety valve must be replaced.
Insufficient service clearance will make repairs more difficult and time-consuming.
Piping sized too small may cause water hammer, resulting in damage to the sterilizer.
After installation, it is mandatory to brace piping at the drain funnel so that it will not move vertically.
Make sure door opening is clear of any obstruction before closing the door(s).
Do not attempt to open sterilizer door during manual operation unless chamber is at 0 psig.
During manual operation, gasket must be fully retracted prior to operating sterilizer door.
Immediately wipe up saline solution spills on loading car, to prevent damage to stainless steel.
1-3
Summary of Warnings and Cautions Operating Instructions 129367-408
STERILIZATION TECHNIQUES 2
The information in this section is intended as a general guide to steam
2.1 General
WARNING – BURN HAZARD: It is inappropriate for
a health care facility to sterilize liquids for direct patient contact.
sterilization techniques. Also recommended is reference to the standards of the
Association for the Advancement of Medical Instrumentation (AAMI ST-46,
Steam Sterilization and Sterility Assurance, 3rd Edition
).
• Prior to sterilization, all materials and articles must be thoroughly cleaned.
• After sterilization, goods should be stored in conditions that will not compro-
mise the barrier quality of their wrapping materials.
• Always carefully segregate items which have been sterilized using conventional cycles from those sterilized using Flash or Express cycles.
IMPORTANT: The sterilization cycles listed in Table 2-1 have been validated
using techniques documented in AAMI ST-8 and AAMI ST-37. If different cycle
parameters (sterilize time and dry time only) other than those in Table 2-1 are
required, it is the responsibility of the health care facility to validate the cycle.
Reference AAMI guidelines/standards for a guide to validating sterilization
cycles and to ensure that proper sterility assurance level (SAL) as well as
moisture retention acceptance criteria are met.
NOTE: Contact your customer service representative for information on a wide
range of education/training programs designed to meet the educational needs of
health care industries.
The E xpress cycle
only
on prevacuum sterilizers, and is not provided on gravity sterilizers.
is an abbreviated prevacuum cycle that is intended for use
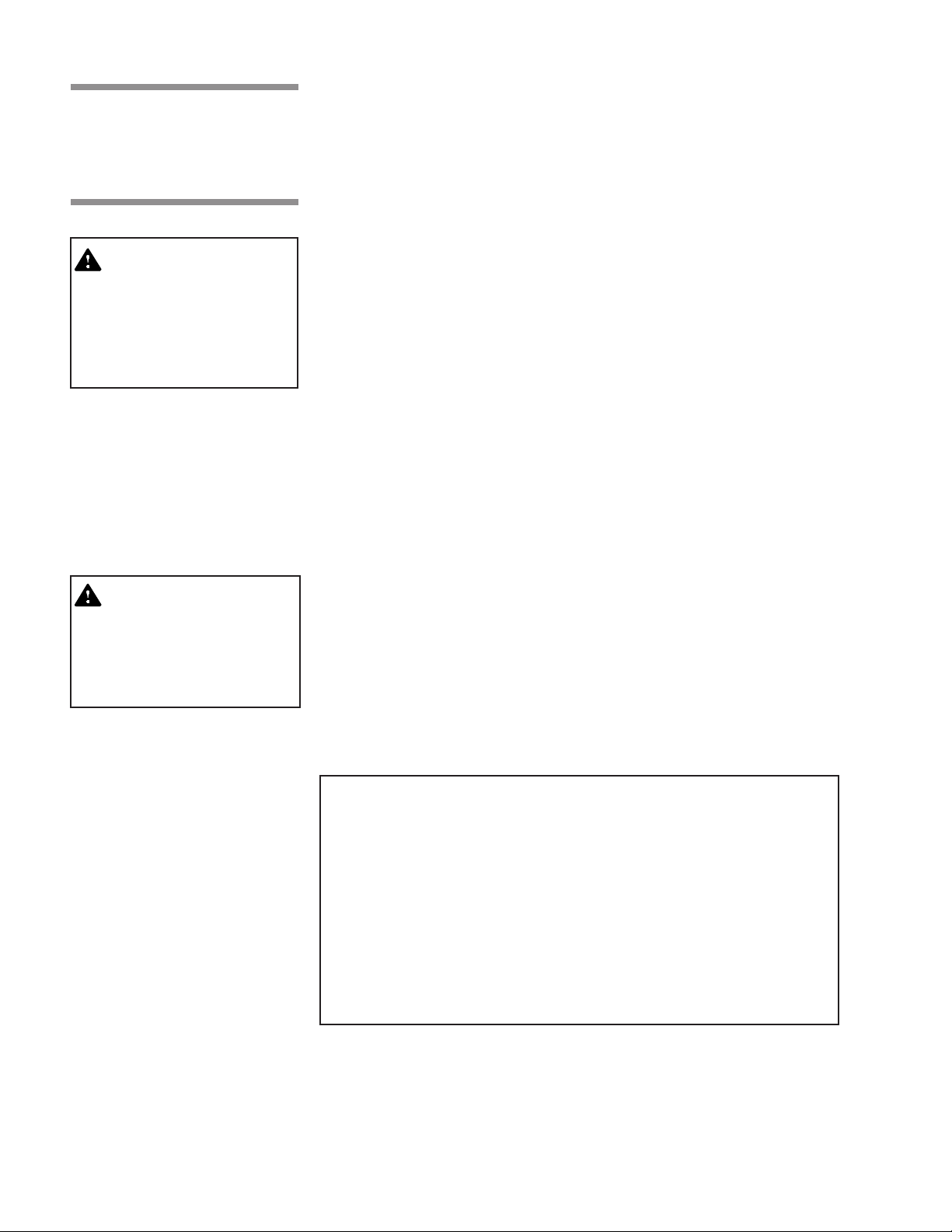
Table 2-1. Cycle Availability
Cycle Sterilize Sterilize Dry Prevac Gravity
Type Load Temperature Time Time Default Optional Default Optional
Gravity* Full Load Fabric Packs 270°F 25 min 15 min X X
Gravity* Full Load Fabric Packs 250°F 30 min 15 min X X
Gravity* Full Load Instrument Trays 270°F 15 min 30 min X X
Gravity* Full Load Instrument Trays 250°F 30 min 30 min X X
Liquid* Three 1000ml Bottles 250°F 45 min N/A X X
Prevac* Single Fabric Pack 270°F 4 min 5 min X N/A N/A
Prevac* Full Load Instrument Trays 270°F 4 min 20 min X N/A N/A
Prevac* Full Load Instrument Trays 275°F 3 min 16 min X N/A N/A
Flash** Unwrapped, Non-porous
Express** Single-wrapped
Flash Unwrapped, Non-porous
DART* Bowie-Dick Test Pack 270°F 3 1/2 min 1 min X N/A N/A
Leak*
Test None N/A N/A N/A X N/A N/A
Instrument Tray 270°F 3 min 1 min X X
Instrument Tray 270°F 4 min 3 min X N/A N/A
Instrument Tray 270°F 10 min 1 min X X
*Cycles qualified to AAMI ST-8
**Cycles qualified to AAMI ST-37
2-1
Sterilization Techniques Operating Instructions 129367-408
2.2 Special
Information
Regarding the
Express Cycle
Appropriate parameters for sterilization are preset by STERIS. It is designed
to permit sterilization using a single instrument in a single
or textile, but not a peel pouch) on the instrument tray.
Rationale: The single wrapper serves to confine and contain the sterilized item
from environmental contaminants that may be encountered enroute from the
sterilizer to the point of use. A single wrapped item sterilized with the Express
cycle does not have a shelf life.
wrapper (non-woven
WARNING – STERILITY
ASSURANCE HAZARD:
The Express cycle is only
intended for use with a
single instrument in a
single wrapped instrument tray.
2.2.1 Preparing
Instruments for
Express Cycle
Sterilization
WARNING – STERILITY
ASSURANCE HAZARD:
The Express cycle is not
intended for processing
porous items (except the
tray wrapper).
The Express cycle is useful in providing quick turnaround of an instrument
using the wrapped technique for transport from the sterilizer to the point of use.
Instrument trays processed using the Express cycle are intended for immediate
use.
Decontaminate and then prepare a surgical instrument (open and/or disassembled), and place in a perforated or mesh bottom instrument tray. Only nonporous items (except for the wrapper) should be processed using this cycle.
Items with lumens (e.g., needles for injection and diagnostics or metal suction
cannulae) are considered porous items, and must not be processed using this
cycle.
The Express cycle has fewer prevacuum pulses so air removal and subsequent
steam contact within lumens may be difficult to achieve.
Table 2-2. Item Processing Guidelines for Express Cycle
Examples of non-porous items that can be processed using the
Express cycle:
• Forceps,
• Needle Holders,
• Scissors and other routine metal instruments.
Examples of porous items that CANNOT be processed using the
Express cycle:
• Towels,
• Rubber or Plastic Items,
• Items with Lumens,
• Items with sliding parts that prevent sterilant contact with surfaces.
2-2
129367-408 Operating Instructions Sterilization Techniques
2.2.2 Unloading Use instrument processed in an Express cycle immediately. Do not store for
later use.
When opening and unloading the sterilizer at the conclusion of the Express
cycle always use aseptic techniques. Sterile towels and sterile gloves are used
to remove the tray from the sterilizer. The still-wrapped tray is transported to the
point of use, being careful not to contact any unsterile surfaces. The tray is
placed on a previously draped sterile surface field and the wrapper opened by
the circulating nurse. The draping material used must be impervious to liquids
and able to withstand contact with hot surfaces.
To shorten the sterilization process, drying time has been reduced in the
Express cycle. Thus, the single wrapper will appear dry at the conclusion of
the cycle, but the content of the package will, in all probability, still be wet with
condensation formed during sterilization. Water will penetrate the wrapper,
especially on the under side of the tray because of gravity. The wrapper only
serves the function of protecting the tray and its content from particulate matter
encountered on the way from the sterilizer to the point of use.
Any moisture present can strike through the wrapper, so procedures should be
developed to avoid contamination by contact with unsterile surfaces. For
example:
• Wear sterile gloves and use sterile towels as “pot holders” when removing
the goods from the sterilizer.
• Never place the wrapped tray on an unsterile surface.
2.3 Special
Information
Regarding The
Flash Cycle
WARNING – STERILITY
ASSURANCE HAZARD:
The Flash cycle is not intended for processing porous items.
The Flash Cycle
Amsco® Century® prevacuum and gravity configurations. The Flash cycle has
been designed to sterilize an unwrapped item at sterilization parameters preset
by STERIS. There is no storage or shelf life of flash sterilized items.
Rationale: The Flash Cycle is intended for sterilizing an unwrapped item
intended for immediate use (e.g. a dropped instrument). In any method of
sterilization, it is important to adhere to good processing practices. This is
particularly important in flash sterilization.
Preparing Instruments For Flash Cycle Sterilization
As prescribed in AAMI ST-37, prior to flash sterilization of a dropped
instrument, ensure the item is free of soil by the appropriate decontamination
procedure. The flash sterilized item then must be transferred immediately,
using aseptic technique, from the sterilizer to the actual point of use, usually
the sterile field in an ongoing sterile procedure.
Use items processed in a Flash Cycle immediately. Do not store the processed
items for later use.
is an abbreviated gravity cycle
and is provided on both the
2-3
Sterilization Techniques Operating Instructions 129367-408
2.4 Control
Measures For
Verifying
Sterilization
Process
2.4.1 Biological Monitors
As part of the operator's verification of the sterilization process,
biological indicators may be used to demonstrate that sterilization
conditions have been met.
NOTE: Contact STERIS for information on specific biological indicators
recommended for use with this sterilizer.
A live spore test utilizing
form of biological monitoring. This type of product utilizes controlled
populations of a controlled resistance, so that survival time and kill
time can be demonstrated.
To verify the process, insert the biological indicator in a test pack and
place pack on the bottom shelf. Run test pack through a typical cycle.
On completion, forward test pack and monitor to appropriate personnel
for evaluation. Refer to AAMI guidelines to conduct routine biological
monitoring.
G. stearothermophilus
is the most reliable
2.4.2 Testing for
Prevacuum Efficiency
WARNING – STERILITY
ASSURANCE HAZARD:
Load sterility may be compromised if the biological
indicator or air leak test
indicates a potential problem. If these indicators
show a potential problem,
refer the situation to a
qualified service technician before using the sterilizer further.
Run a Dart® (Bowie-Dick test) cycle daily before processing any loads. The
first prevacuum cycle of each day should be used to test the adequacy of
air removal from the chamber and load, so that steam can penetrate the
load. It is not a test for adequate exposure to heat in terms of time-attemperature.
Tests such as the Bowie-Dick or the Dart® (Daily Air Removal Test)* are
designed to document the removal of residual air from a sample challenge
load.
In the case of these tests, following exposure in a prevacuum sterilizing
cycle, the pack is opened, the indicator examined and conclusions are
drawn as to the pattern of residual air, if any, that remained in the pack
during the sterilizing cycle. Any indication of a malfunction must be
reported to the supervisor. Sterilizer should not be used during this time.
* Available from STERIS.
.
2-4
129367-408 Operating Instructions Sterilization Techniques
2.5 Dart (BowieDick) Test
2.6 Vacuum Leak Test
Conduct a residual air test (e.g., Bowie-Dick test) at the beginning of each day
according to the AAMI standard ST-46. STERIS can provide a product called
Dart® (Daily Air Removal Test), designed to be as sensitive as the standard
AAMI Bowie-Dick test pack in detecting air leaks. Refer to instructions for
running Dart test given in Section 4 of this manual. If a Dart is not available,
construct Bowie-Dick test package in accordance with instructions given in
AAMI standard ST-8.
Run the Vacuum Leak test cycle daily or weekly. This test measures the
integrity of the sealed pressure vessel and associated piping to assure air is
not being admitted to the sterilizer during the vacuum draw downs. Refer to
appropriate cycle description in Section 4 of this manual.
WARNING – STERILITY
ASSURANCE HAZARD:
According to AAMI standards, a measured leak
rate greater than 1.0 mm
Hg/minute indicates a
problem with the sterilizer.
Refer the situation to a
qualified service technician before using the sterilizer further.
2.7 Sterilization
Process
Recommendations
After running a vacuum leak test, a value or leak rate will be printed on the printer
tape. This value will help define a trend over a period of time if the integrity of
the system begins to deteriorate (i.e., allowing air to enter the system). By
running a vacuum leak test cycle daily or weekly, the operator or maintenance
personnel can always monitor the air tightness of the system and make repairs
or adjustments when necessary.
NOTE: A leak rate of greater than 1.0 mmHg per minute indicates a problem with
the sterilizer that must be addressed.
Saturated steam is a well controlled, reliable method for processing items which
can withstand the temperatures and pressures associated with steam sterilization. The requirements for achieving reproducible results are well known by
many users, but are not always understood by all users.
The condition most likely to result in sterilization problems is a failure to remove
all of the air from the items being processed. For example, placing an empty
beaker or bowl in an upright position in a gravity displacement sterilizer may
result in the object not being sterilized, or may require exceptionally long
sterilization times. This problem is because air has almost twice the density as
does saturated steam under the same conditions. Thus, the air sits in the
bottom of the container, and the steam forms a stable layer over the air. This
effect is similar to oil forming a stable layer over water. As long as there is no
mechanism for actively mixing the two, the bottom of the container will only see
dry heat, which is not an effective sterilization method at the temperatures
typically used in steam processes.
There are two traditional methods for enhancing the sterilization of solid bottom
containers in gravity displacement cycles. These are:
• Place 1.0 mL of water for each liter of volume in the bottom of each container.
The expansion of the water into steam as the product is heated will force most
of the air out of the object, thus allowing steam to reach all surfaces and effect
sterilization.
• The better, more reliable method is to orient all objects in a manner which
would allow water to flow out. When the steam enters the chamber, it will tend
to layer over the air. However, the object is now oriented so the air can flow
out. As the air flows out of the container, it will be replaced by the steam. The
steam can now reach all surfaces and effect sterilization.
2-5
Sterilization Techniques Operating Instructions 129367-408
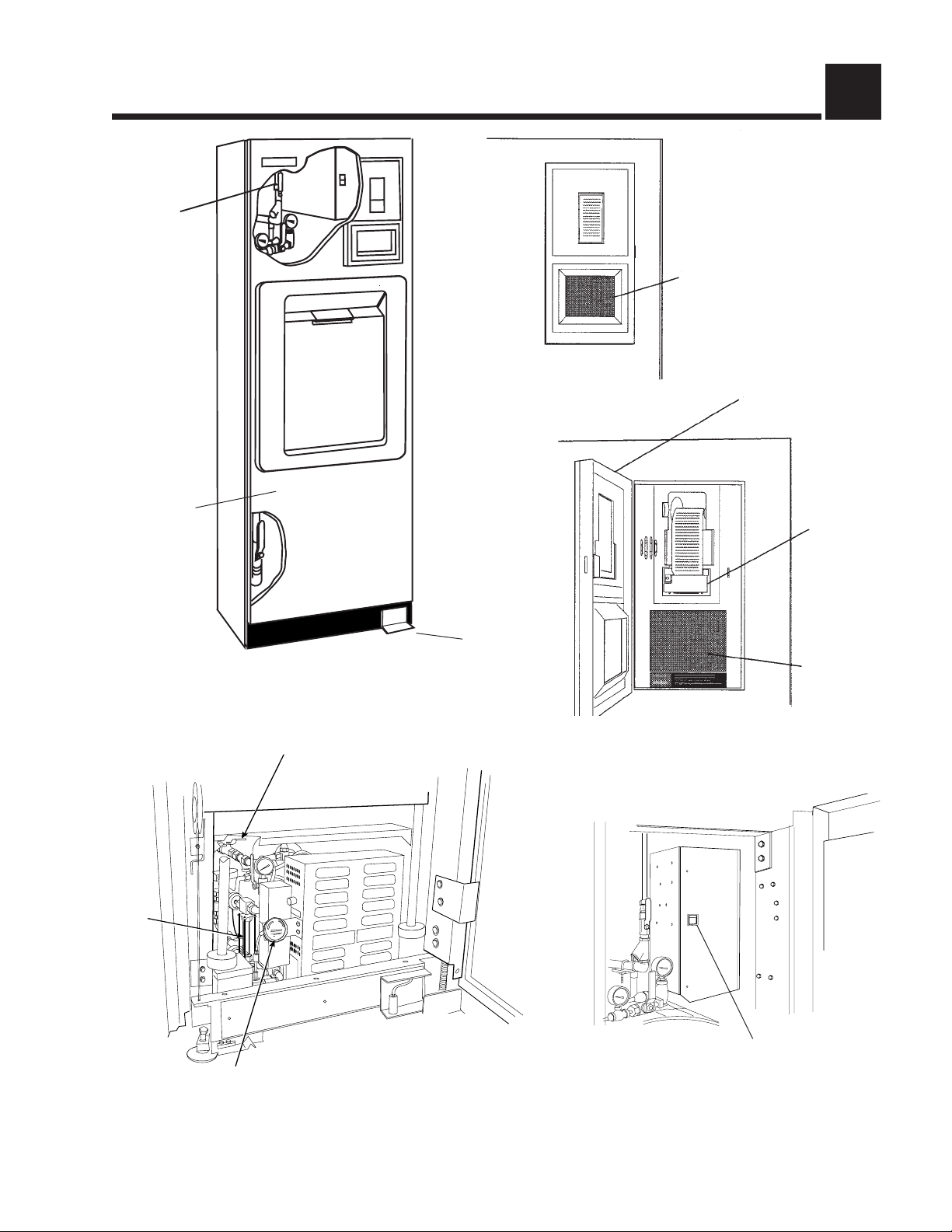
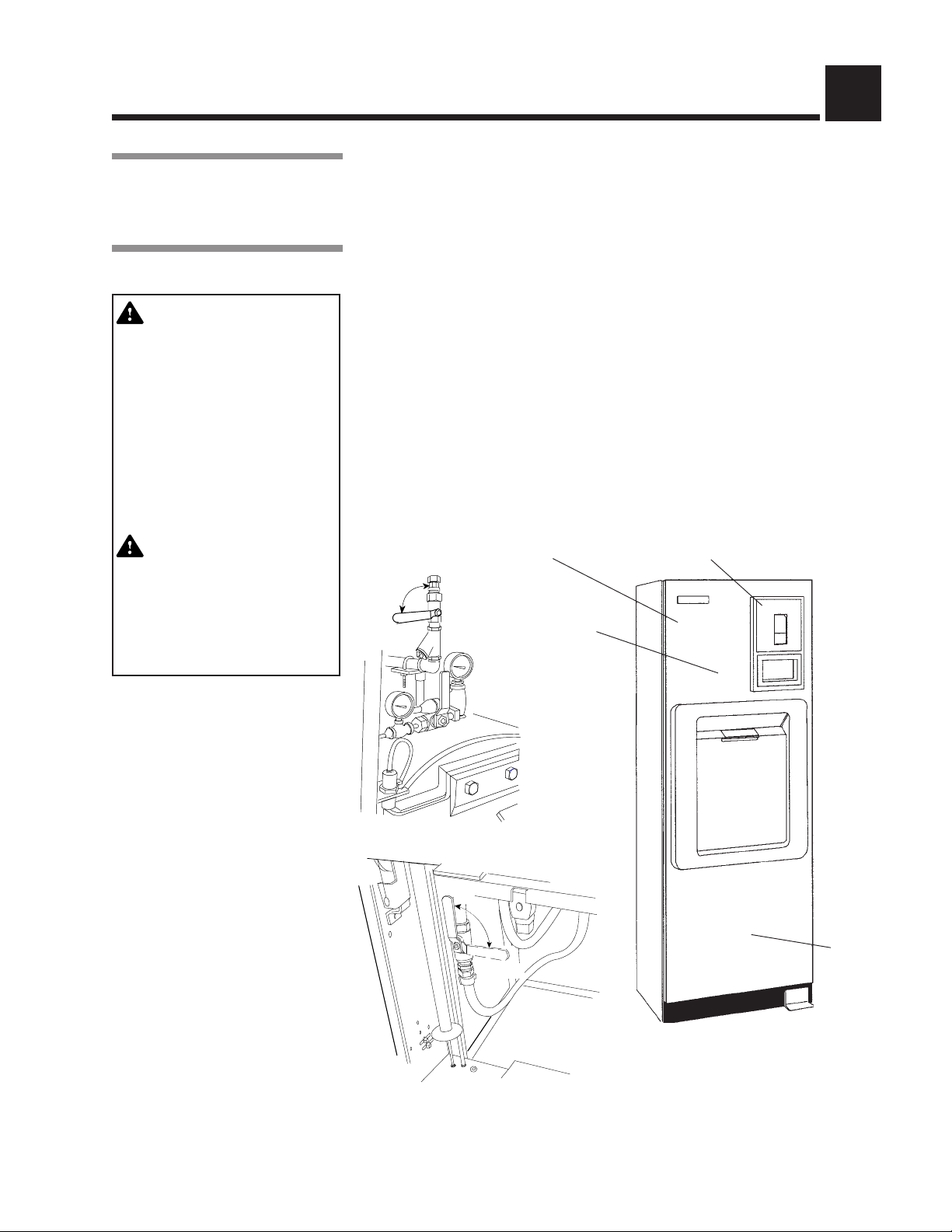
COMPONENT IDENTIFICATION 3
Steam
Supply
Valve
Touch Screen
Access Panel
(Front panel of
unit is hinged
for service
access)
Century Sterilizer
Generator Water
Supply Valve
Foot Pedal
Century Control Panel
Century Control Panel w/Door Open
Control Access
Door
Printer
Touch Screen
Sight
Glass
JACKET
CHAMBER
Optional Electric
Steam Generator
Generator Drain Valve
Main Power Disconnect Switch.
This should be left on at all times.
Figure 3-1. Amsco® Century® Sterilizer Components
Component Identification Operating Instructions 129367-408
3-1
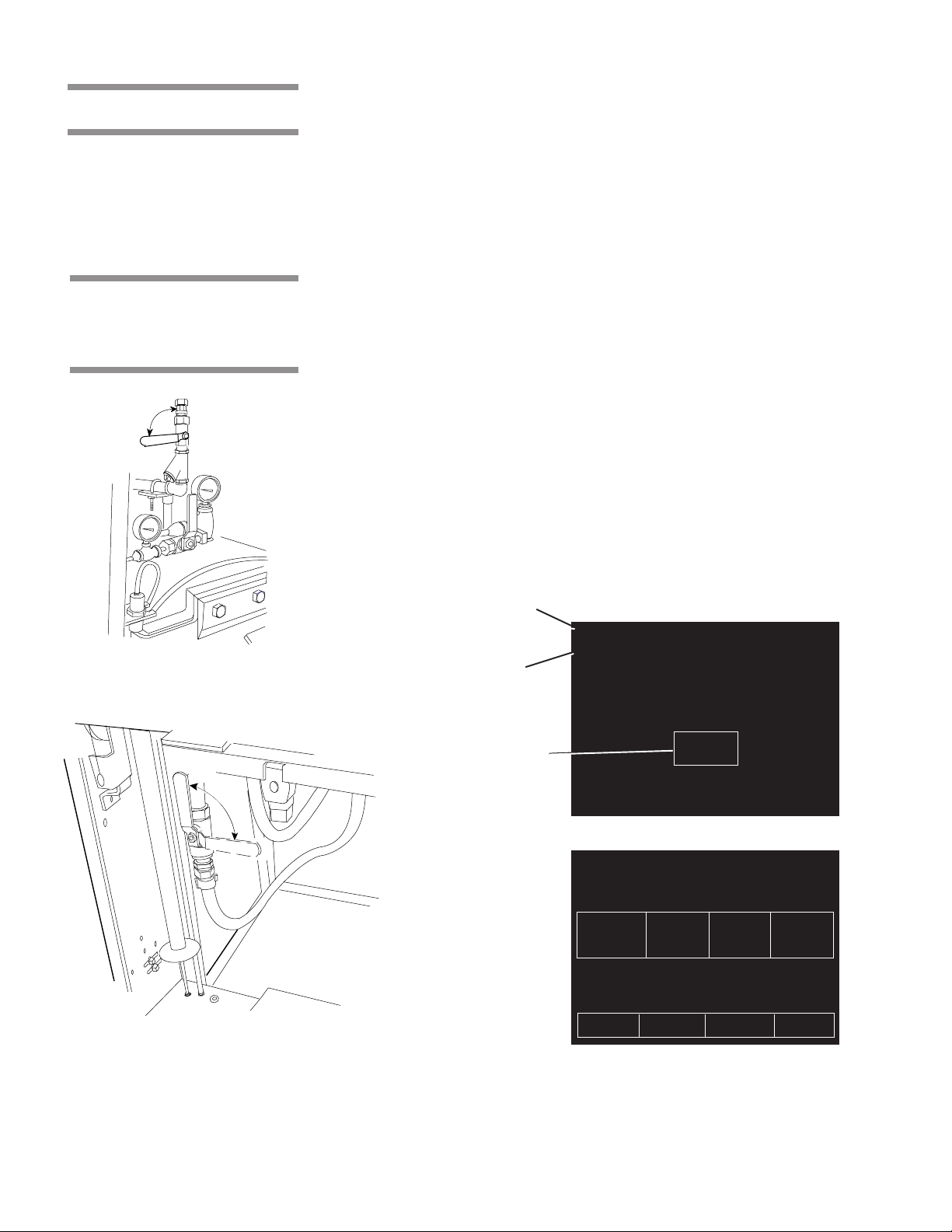
3.1 General
Use this manual to become familiar with control locations and functions before
operating the sterilizer (refer to Fig. 3-1). The controls for this sterilizer are
contained within the control touch screen. Control touch pads appear on the
screen as needed during each operation. Available controls change as the
sterilizer steps through different operations.
3.2 Main Sterilizer
and Cycle
Controls
Open
Closed
JACKET
CHAMB ER
Steam Supply Valve
••
• Main Power Disconnect Switch
••
(refer to Fig. 3-1) – Located behind the
access door, on the main control box, this switch disconnects power to the
control. Under normal operation, this switch is left in the open position at
all times.
••
• Steam Supply Valve – This is located behind the front access door, above
••
the chamber door. Refer to Figure 3-2. Ensure this is in the open position
before trying to operate the sterilizer.
••
• Water Supply Valve – This is located behind the front access door, below
••
the chamber door. Refer to Figure 3-2. Ensure this is in the open position
before trying to operate the sterilizer.
••
• Steam Generator Control – The optional integral electric steam generator
••
(if present) is located in the space below the sterilizer chamber. The
generator is automatically turned on by the sterilizer’s control when the
sterilizer is turned on. Refer to the instructions later in this section before
operating a sterilizer equipped with an integral steam generator.
Sterilizer Status
STATUS ............ STANDBY 0
TIME .................. 00:00 AM
Current
Time & Date
DATE ................. 00-0 0-00
Open
Touch Screen
Pad—Press to Turn
the Sterilizer ON
ON
Standby Screen
STATUS ............ DOOR OPEN 1
Closed
Water Supply Valve
TEMP ................. 000F
PRESS ............... 00 PSIG
12 34
FLASH FLASH EXPRESS PREVAC
270F 270F 270F 270F
S=03M S=10M S=04M S=04M
D=01M D=01M D=03M D=20M
00:00 AM 00-00-00
PAPER MORE
FEED CYCLES
MENU STANDBY
ON Screen
Figure 3-2. Main Sterilizer and Cycle Controls
3-2
129367-408 Operating Instructions Component Identification
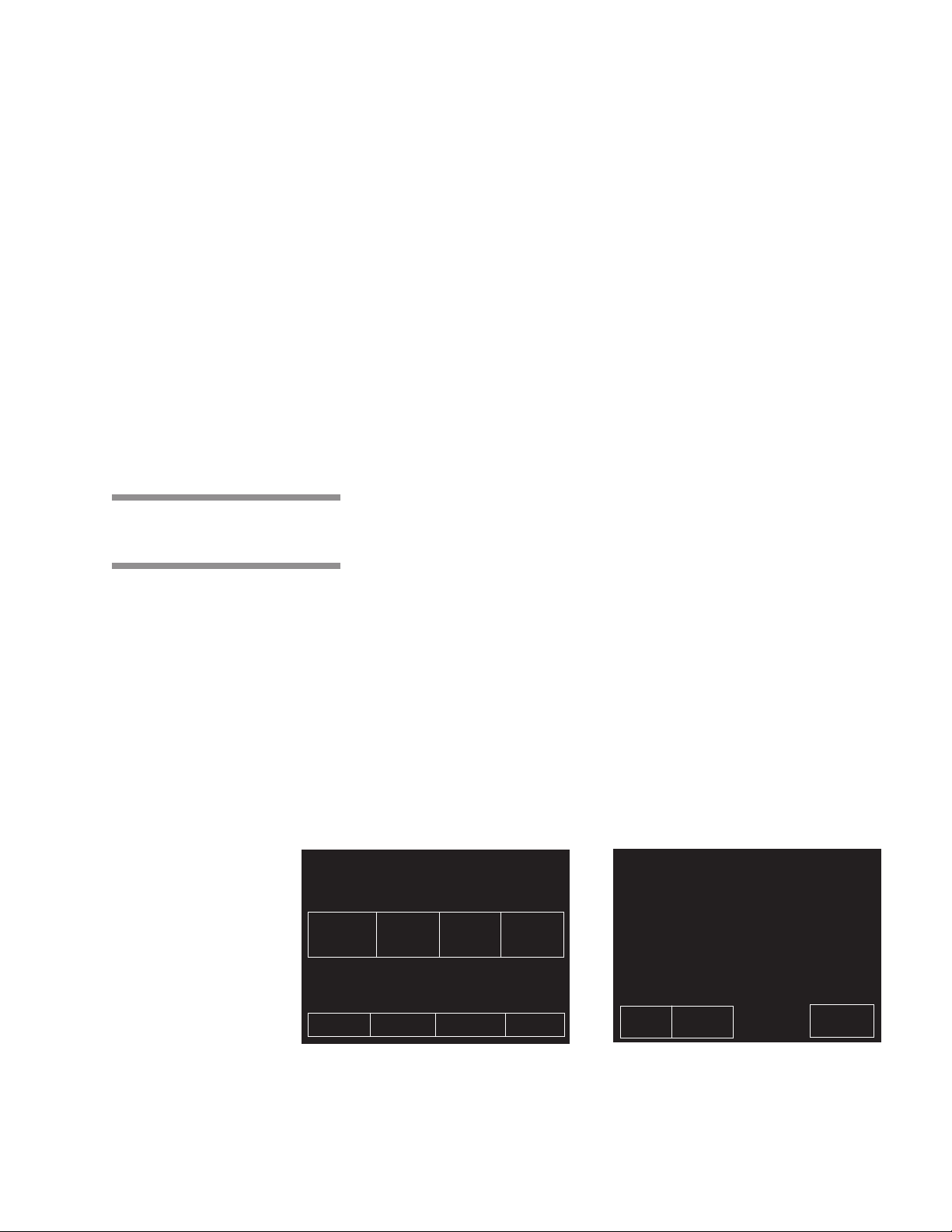
3.3 Control Displays
» Generator Water Supply Valve (refer to Fig. 3-1) – This valve should
always be open. Ensure it is open before attempting to operate the
sterilizer.
» Generator Drain Valve (refer to Fig. 3-1) – This should be opened once
a day, while generator is at room temperature, to flush the generator of
residual solids (present in most water) that may have accumulated in
the generator’s boiler. After the boiler has been flushed ensure the
drain valve is then closed. Refer to instructions later in this section.
••
• Sterilizer Control Touch Pad
••
––
– This is visible on the control touch screen
––
whenever the sterilizer is in Standby mode. Refer to Figure 3-2.
NOTE: Screen touch pads respond to very slight pressure, and only need to be
pressed lightly.
The sterilizer enters operating mode when the ON touch pad is pressed.
This touch pad switches the sterilizer control between Standby and Ready
conditions (Standby mode is usually used at night when the sterilizer is not
being operated—steam is turned off and machine cools, saving energy).
A screen reference number appears in the upper right corner of each display.
Numbers are used for reference only, and do not relate to the operating
sequence of the screen.
Control displays can be divided into two categories, those occurring when the
sterilizer is “out-of-cycle” and those occurring when the sterilizer is "in-cycle".
Typical out-of-cycle and in-cycle displays are shown in Figure 3-3.
• Out-of-cycle displays are used to start cycles, or set up and adjust
sterilizer operation. With the exception of the cycle starting displays, most
out-of-cycle displays will only be used occasionally. Detailed instructions
for adjusting the sterilizer operating parameters are in Section 5 of this
manual.
• Generally, when the sterilizer is in-cycle, displays appear automatically
and, unless an abnormal conditions occurs, require no special attention or
instructions. In-cycle displays tell the operator at what temperature and
pressure the sterilizer chamber is operating, show the current cycle phase
and indicate when the processing cycle is complete. For more details
about operating cycles, refer to Section 4 of this manual.
STATUS ............ DOOR OPEN 1
TEMP ................. 000F
PRESS ............... 00 PSIG
12 34
FLASH FLASH EXPRESS PREVAC
270F 270F 270F 270F
S=03M S=10M S=04M S=04M
D=01M D=01M D=03M D=20M
00:00 AM 00-00-00
PAPER MORE
FEED CYCLES
MENU STANDBY
Out-of-Cycle In-Cycle
STATUS ............ STERILIZE 00:00 4
TEMP ................ 000 F
PRESS ............. 00 PSIG
CYCLE .............. 1, FLASH, 270F, S=03M, D=01M
PROJECTED CYCLE COMPLETION TIME:
0:00
MINUTES SECONDS
PAPER STATUS
FEED PRINT
ABORT
Figure 3-3. Typical In and Out-Of-Cycle Display
3-3
Component Identification Operating Instructions 129367-408

3.4 Alarm Displays
Alarm displays tell operators and technicians when the sterilizer is experiencing an abnormal condition. Alarm conditions can be caused by failure of utility
supplies or sterilizer components. Section 5 of ROUTINE MAINTENANCE
MANUAL (P129367-410), details the steps an operator can take to solve most
alarm conditions. Typical alarm displays are shown in Figure 3-4.
When an alarm occurs during cycle operation, a display appears on the screen,
accompanied by an audible tone. This display indicates the problem as
determined by control sensors, and lists a brief troubleshooting list. The
operator should follow the instructions on the screen, if possible. If these
instructions fail to clear the alarm, consult your departmental supervisor or a
trained service technician before using the sterilizer further.
STATUS ............ ALARM! 205
→ CHAMBER: 70.0 F 00.0 psig
STERILIZER WILL:
→ AUTOMATICALLY TRY TO COMPLETE CYCLE
OPERATOR INSTRUCTIONS:
1. SILENCE ALARM
2. CHECK STEAM SUPPLY VALVE
3. IF ALARM RECURS, CALL SERVICE
SILENCE
STATUS ............ SERVICE INFORMATION: 206
→→
→ CHAMBER DID NOT REACH STERILIZE
→→
CAUSES AND CORRECTION:
1. STEAM PRESSURE LESS THAN 50 PSIG
2. STEAM REGULATOR MALFUNCTION
2. SOLENOID VALVE MALFUNCTION
3. CONTROL OUT OF CALIBRATION
TOO LONG IN CHARGE
→→
→ IF CLOSED, OPEN VALVE
→→
ABORT
ALARM PRINT FEED HELP
TEMPERATURE WITHIN ALLOTTED TIME
→→
→ CHECK STEAM SUPPLY PIPING
→→
→→
→ REPAIR
→→
→→
→ REPAIR S09
→→
→→
→ REPAIR S02
→→
→→
→ RECALIBRATE (CONTACT QUALIFIED
→→
SERVICE PERSON)
ABORT
STATUS PAPER
TOO LONG IN CHARGE
PAPER
FEED
SERVICE
EXIT
Figure 3-4. Typical Alarm Displays
3-4
129367-408 Operating Instructions Component Identification
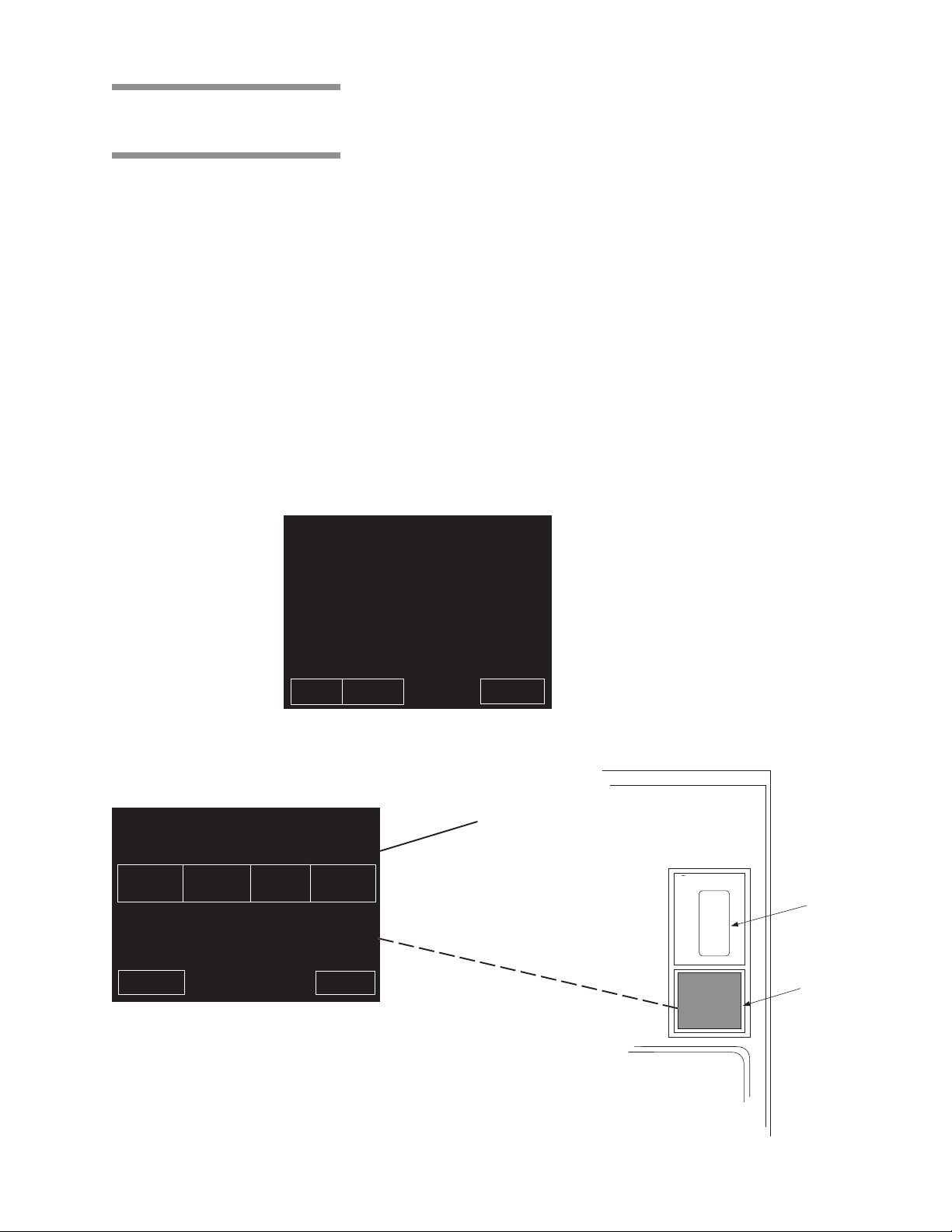
3.5 Operating End Control Panel
STATUS ............ STERILIZE 00:00 4
TEMP ................ 000 F
PRESS ............. 00 PSIG
CYCLE .............. 1, FLASH, 270F, S=03M, D=01M
A sterilizer equipped with two doors, will also be equipped with two control
panels. The control panel at the loading door of the sterilizer is referred to as
the "operating end control," the control panel located at the unloading door is
referred to as the "non-operating end control."
A single-door sterilizer is equipped with an "operating end control," only.
NOTE: Except for the presence of the printer (which is only present at the
operating end of the unit), control panels at both ends of the unit are similar and
each can be used to start or abort the sterilizer.
The operating end control panel (see Fig. 3-5) is used to:
••
• Select and start cycles.
••
••
• Abort cycles.
••
••
• Set cycles and cycle values.
••
••
• Obtain status printouts (see “Printer” paragraph later in this section).
••
The operating end control includes a printer for cycle documentation.
Cycle status and control messages are shown on a 30 column x 40 line graphics
display. Cycles can be started or aborted using the touch screen pads. Cycles
and cycle values can be set using the Change Values procedure (accessible
from the sterilizer MENU screen). If changing cycle values becomes necessary, refer to Section 5 of this manual.
PROJECTED CYCLE COMPLETION TIME:
PAPER STATUS
FEED PRINT
When in-cycle, the display shows a status of the sterilizer,
status of the cycle, and large numbers indicate the
approximate time remaining in the current cycle.
STATUS ............ MENU 2
TEMP ................. 128F
PRESS ............... 0 PSIG
CHANGE
TIME
DATE
PAPER
FEED
CHANGE CHANGE DUPLICATE
&
CYCLE MACHINE PRINT
VALUES SETUP
EXIT
Menu Screen
0:00
MINUTES SECONDS
ABORT
Typical Menu Screen
AMSCO Time
00:00:00
AMSCO Time
00:00:00
AMSCO Time
00:00:00
AMSCO Time
00:00:00
AMSCO Time
00:00:00
AMSCO Time
00:00:00
Cycle
Cycle
Printout
Printout
Touch
Touch
Screen
Screen
Figure 3-5. Operating End Control Panel
3-5
Component Identification Operating Instructions 129367-408
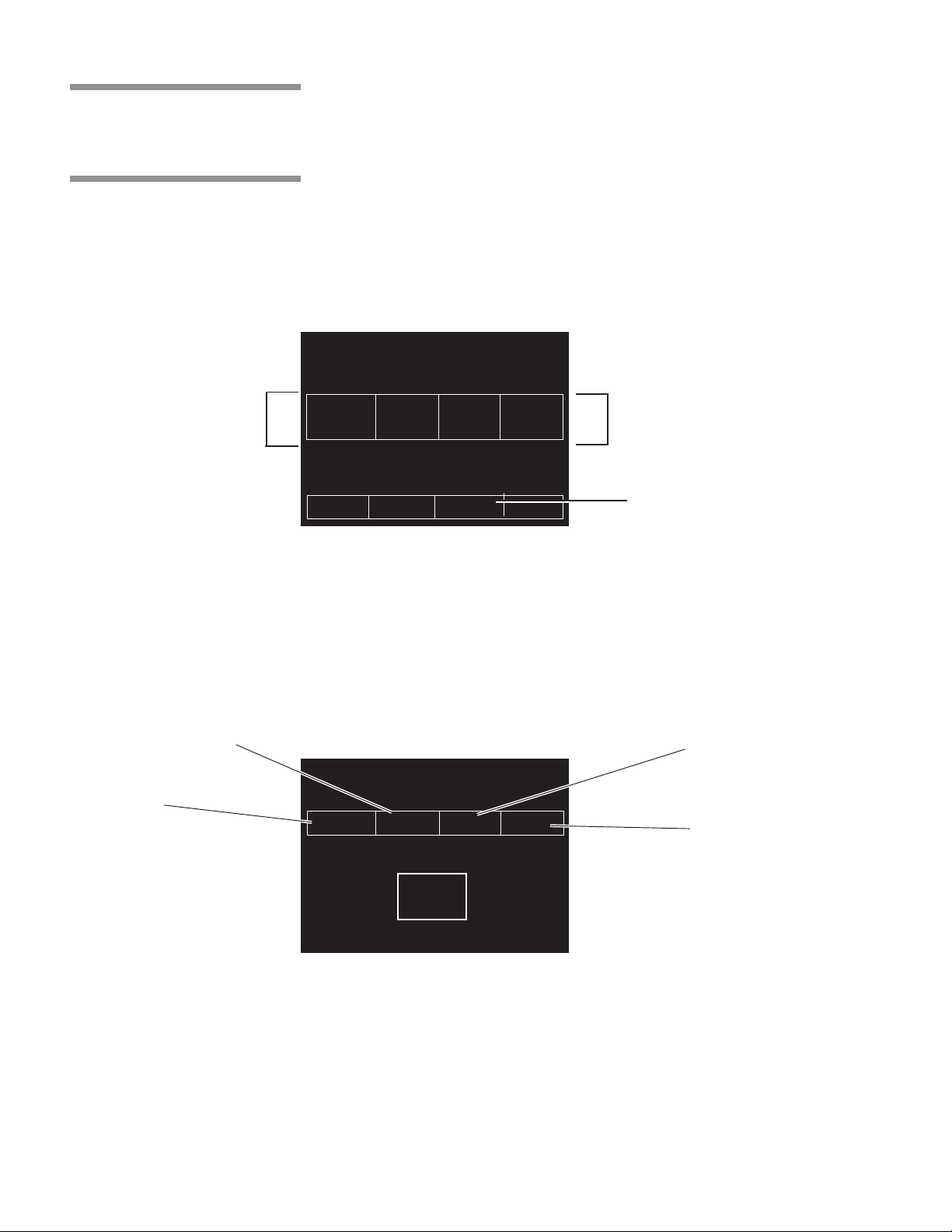
3.6 Cycle
Selection Touch
Screen Pads
Press to StartPress to Start
Press to Start
Press to StartPress to Start
(Select) Cycles(Select) Cycles
(Select) Cycles
(Select) Cycles(Select) Cycles
Four cycle selection touch pads are shown on the screen in Figure 3-6. These
pads display the basic parameters of the cycle (cycle name, sterilization
exposure temperature, sterilization exposure time and dry time), additional
cycles may be selected by pressing MORE CYCLES. Details on individual
cycles are in Section 4.8.
Up to seven additional cycles can be programmed and displayed. It is the
responsibility of the healthcare facility to validate the cycle. Reference AAMI
for guidelines and standards for a guide to validating sterilization cycles and
to ensure that proper sterility assurance level (SAL) as well as moisture retention
acceptance criteria are met.
STATUS ............ DOOR OPEN 1
TEMP ................. 000F
PRESS ............... 00 PSIG
12 34
FLASH FLASH EXPRESS PREVAC
270F 270F 270F 270F
S=03M S=10M S=04M S=04M
D=01M D=01M D=03M D=20M
00:00 AM 00-00-00
PAPER MORE
FEED CYCLES
MENU STANDBY
Press to StartPress to Start
Press to Start
Press to StartPress to Start
(Select) Cycles(Select) Cycles
(Select) Cycles
(Select) Cycles(Select) Cycles
Press for More CyclesPress for More Cycles
Press for More Cycles
Press for More CyclesPress for More Cycles
3.6.1 Values Touch Screen Pads
Sterilization ExposureSterilization Exposure
Sterilization Exposure
Sterilization ExposureSterilization Exposure
TemperatureTemperature
Temperature
TemperatureTemperature
Cycle NameCycle Name
Cycle Name
Cycle NameCycle Name
Figure 3-6. Cycle Selection Touch Pads
These touch screen pads are accessed through the MENU screen by pressing
CHANGE CYCLE VALUES. The values touch pads are used for changing the
operating values used in cycles, changing the cycles displayed on the cycle
selection menus, and for changing the operating settings of the sterilizer.
Instructions for changing sterilizer cycle parameters are in Section 5 of this
manual.
Sterilization ExposureSterilization Exposure
Sterilization Exposure
Sterilization ExposureSterilization Exposure
TimeTime
Time
TimeTime
Dry TimeDry Time
Dry Time
Dry TimeDry Time
SELECT ITEM TO CHANGE
1 TEMP STER DRY
FLASH 270 F 03 M 01 M
EXIT
11
Figure 3-7. Values Touch Screen Pads
3-6
129367-408 Operating Instructions Component Identification
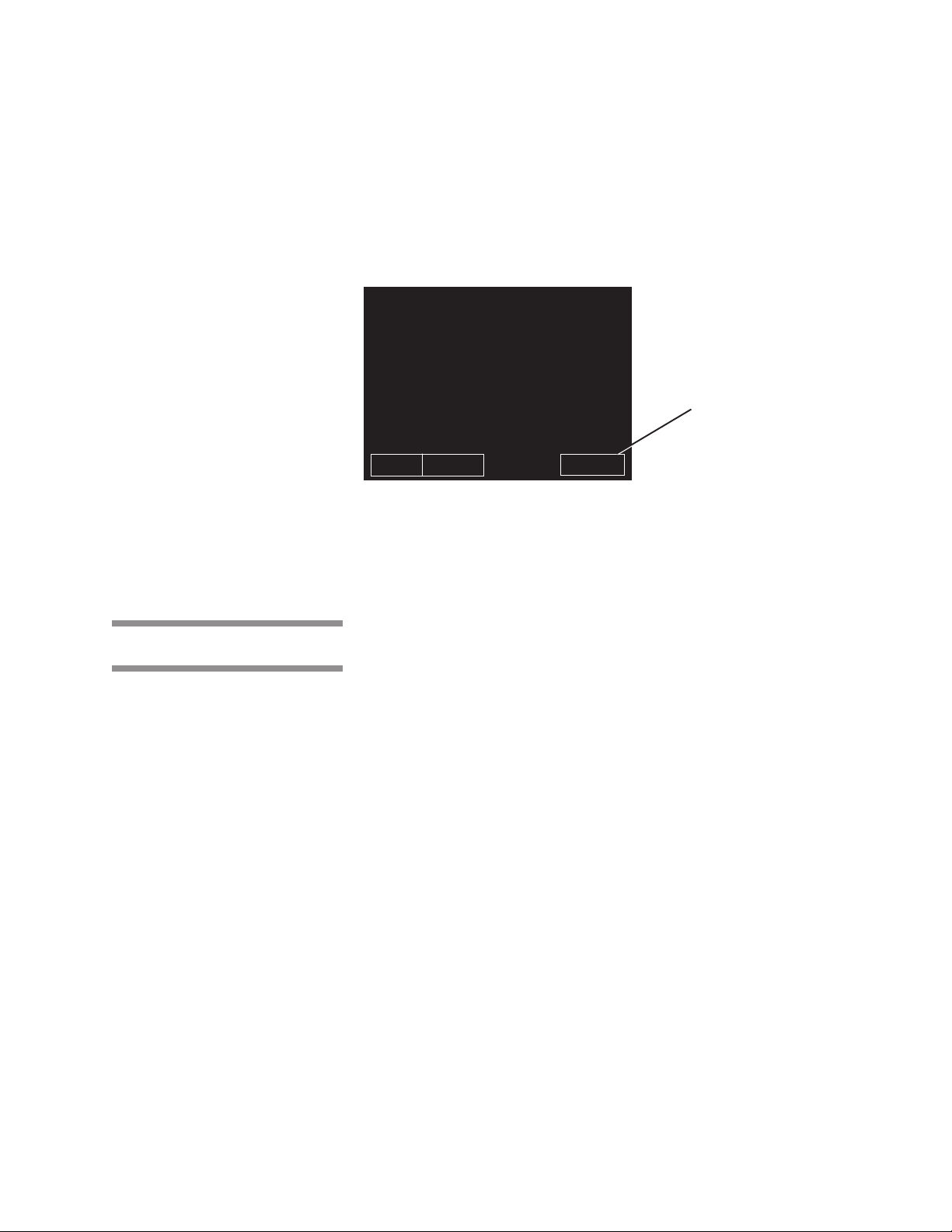
3.6.2 Abort Touch Screen Pad
The ABORT touch screen pad is used to end a cycle before it finishes normally.
A cycle only needs to be aborted if an abnormal condition or a control problem
develops during the cycle. Pressing ABORT causes the sterilizer chamber to
depressurize (if pressurized), or Air Break (if in vacuum), the door seal
deactivates, the control prompts the operator to open the door, and the
sterilizer returns to its normal out-of-cycle state. If an abnormal condition
persists after fully aborting the cycle, contact your supervisor or a qualified
service technician before trying to operate the sterilizer further.
STATUS ............ STERILIZE 00:00 4
TEMP ................ 000 F
PRESS ............. 00 PSIG
CYCLE .............. 1, FLASH, 270F, S=03M, D=01M
PROJECTED CYCLE COMPLETION TIME:
Figure 3-8. Cycle AbortTouch Screen Pad
3.7 Printer
PAPER PRINT
FEED STATUS
0:00
MINUTES SECONDS
ABORT
Abort Touch
Screen Pad
Refer to Figure 3-1.
Printer records all cycle data on 2-1/4 inch wide single-ply paper. See ROUTINE
MAINTENANCE MANUAL
(P129367-410)
for paper changing procedure.
Printer functions controlled by touch screen pads are as follows:
• Paper Feed — Press to feed out paper from the roll stored inside the
control. Accessible during all phases of operation, including alarm
conditions. Press and hold for continuous feed.
• Duplicate Print
— Press to obtain a complete duplicate printout of the last
previously run cycle (when unit is not in cycle). This touch pad is only visible
on the screen during Complete and Change Values menu. The Duplicate
Print touch screen pad is not visible upon first power-up of the day.
• Status Print — Press to obtain a printout of current cycle phase and
conditions (when unit is in cycle). This touch pad is only visible during cycle
operation.
• Print Values — Press to obtain a printout of all currently set cycles and
cycle values. Only accessible when the unit is not in cycle. This touch
screen pad appears on Change Values menu only.
3-7
Component Identification Operating Instructions 129367-408
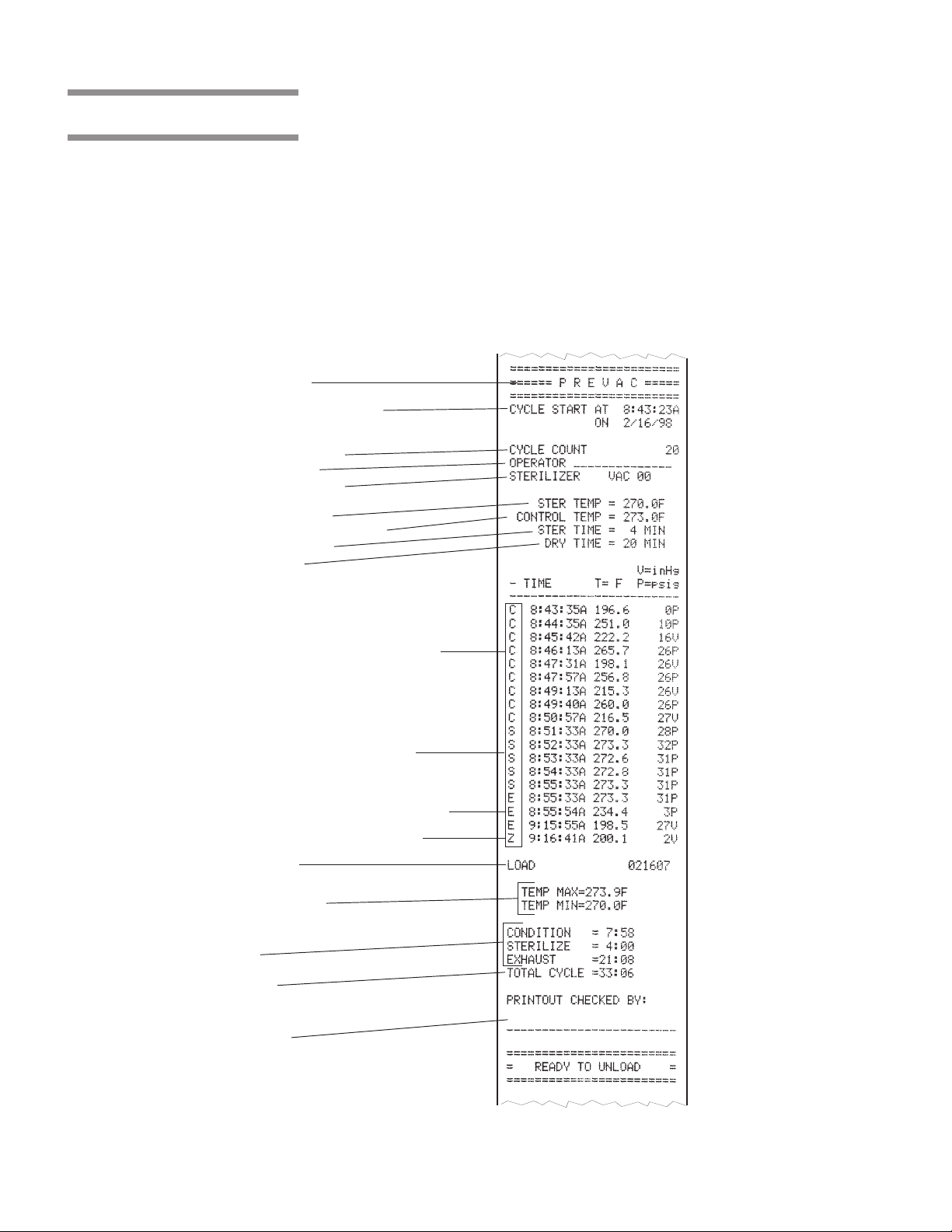
3.8 Printouts
Cycle Type
Cycle Start Time & Date
Total Cycle Count
Operator I.D.
Machine Number
Sterilize Temp.
Control Overdrive Temp.
Sterilize Time
Dry Time
Refer to Figure 3-9.
The printout reports useful information about each cycle the sterilizer runs. This
includes the load number, which is a unique identifying code. Each load
number is printed in the following format: a two digit month (e.g., January = 01),
a two digit day (e.g., second day of any given month = 02) and a two digit daily
cycle count (e.g., third cycle of the day = 03). In our examples the complete
load number would then be 010203.
During the cycle, status lines on the printouts show the time the line was printed,
chamber temperature and the level of vacuum or pressure in the chamber. Each
status line also begins with a letter code. This code indicates during which
cycle phase the print line occurred, or what kind of event caused the print line
to occur.
Refer below to see other features of the printout.
Additional Status Print Codes:
F = Alarm (Failure)
L = Leak Test (Vacuum or Hold)
D = Demand Print (Print Status)
Load Number
Sterilize Temp. Min./Max.
Time in Phase
Total Cycle Time
Review and verify critical cycle
parameters were achieved during
processing, then sign printout to
indicate verification.
Conditioning
• Charge
• Vacuum Pulses
Sterilize
Exhaust & Dry
Complete
3-8
129367-408 Operating Instructions Component Identification
Figure 3-9. Typical Printout

3.9 Power Door Operation
The sterilizer door is operated at the foot pedal (refer to Fig. 3-10).
• Pressing the foot pedal while the door is in the closed (up) position causes
the door to open (lower).
• Pressing the foot pedal while the door is in the open (lowered) position,
causes the door to close (by raising).
NOTE: The foot pedal only provides control of the visible door. For double-door
units, the foot pedal cannot be used to control the door at the opposite end of
the sterilizer.
Figure 3-10. Foot Pedal
WARNING - PERSONAL INJURY HAZARD: When clos-
ing the chamber door keep
hands and arms out of the
door opening and ensure
opening is clear of any
obstructions.
Important:
Operation of Power Door if Motor Fails
If the power door cannot be operated using the foot pedal (such as during a
power failure or if the drive mechanism is damaged), it is still possible to
operate the door manually.
Keep the door closed when the unit is not in use.
Using hand pressure, pull up or push down on the handle to operate the door.
Increased effort is required on the part of the operator to slide the door. Do not
try to raise or lower door rapidly as fast operation may damage the door drive
mechanism.
NOTE: If the power fails, and pressure remains in the sterilizer chamber, refer
to Section 3.10, Emergency Door Opening Procedure.
3-9
Component Identification Operating Instructions 129367-408
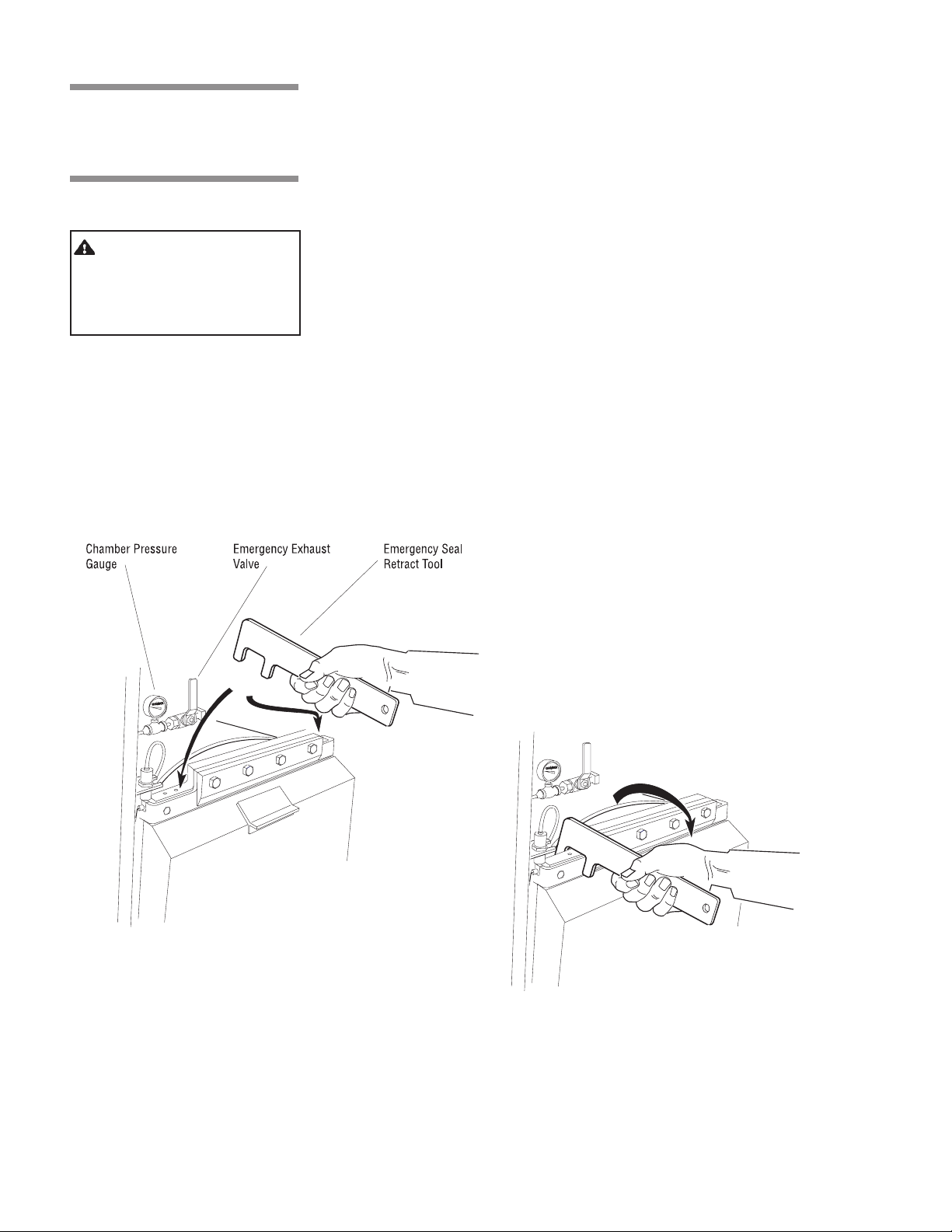
3.10 Emergency
Door Opening
Procedure
WARNING – BURN HAZARD: Do not attempt to
open sterilizer door if a
water in chamber alarm
condition exists.
This procedure should only be used when pressure remains in the sterilizer
chamber, and the door cannot be opened normally because the sterilizer has
lost either electrical or water utilities. This emergency door opening procedure
can be used to retrieve a load in the chamber. This procedure requires pushing
on the door cover to retract the door seal into the groove, then pushing the door
downward manually.
Procedure:
1. Swing open the access panel of the sterilizer. Open the emergency exhaust
hand valve until chamber exhausts to 0.0 psig. See Figure 3-11.
2. Using the door release tool provided, press on the upper left hand and right
hand corners of the chamber door (see Fig. 3-11). The door should give
inward slightly, indicating that the seal has been pushed into the groove.
The door must “bottom out” against the metal end-frame of the sterilizer
chamber.
3. Close the access panel of the sterilizer.
4. Press down on the door handle to lower the door. The door will slide stiffly,
so be prepared to press down hard.
5. Once the door is open, do not use the sterilizer until the unit has been
examined by a qualified service technician. Further use without attention
may damage the sterilizer.
6. Close the emergency exhaust valve.
Figure 3-11. Emergency Door Opening Procedure
3-10
129367-408 Operating Instructions Component Identification
3.11 Optional
Electric Steam
Generator
CAUTION – POSSIBLE
EQUIPMENT DAMAGE:
Failure to flush generator
on a daily basis could result in generator malfunction.
If a building steam source is not available, the sterilizer may be equipped with
an electric steam generator. The generator automatically converts water to
steam using electric heat. The steam created is then used to power the
sterilizer.
Steam generators are highly susceptible to mineral scaling if the supplied water
has any level of hardness. Refer to Table 3-1 for water quality requirements.
IMPORTANT: Regardless of the hardness level of supplied water, the generator must be flushed every day before use to prevent mineral scaling or carryover
of debris into the chamber.
ATTENTION: Warranty on this steam generator will be voided unless daily
flushing procedures are performed.
Daily Generator Start Up Procedure
1. Press the ON button on the sterilizer touch screen (screen #0). Display
advances to screen #72. Instructions on how to flush the generator are listed
on screen #72.
Pressing STOP TIMER stops flush
phase and resets timer to three
minutes.
Pressing CANCEL bypasses flush
phase and advances display to
screen #1. Do not press CANCEL
unless generator has already been
flushed that day.
WARNING – BURN HAZARD: Before daily flushing
of the generator, generator must be at zero psig
and cooled to room temperature.
CAUTION – POSSIBLE
EQUIPMENT DAMAGE:
Before flushing generator,
ensure generator drain
valve is fully open.
FLUSH GENERATOR
THE GENERATOR SHOULD BE FLUSHED
EVERY DAY.
TO FLUSH GENERATOR:
1. VERIFY GENERATOR PRESSURE
IS 0 psig.
2. OPEN GENERATOR MANUAL DRAIN VALVE.
3. PRESS START TIMER TO BEGIN
FLUSH PHASE.
4. ALLOW GENERATOR TO FLUSH FOR 5
MINUTES.
FLUSH TIMER: 5:00
START
TIMER
STOP
TIMER
CANCEL
72
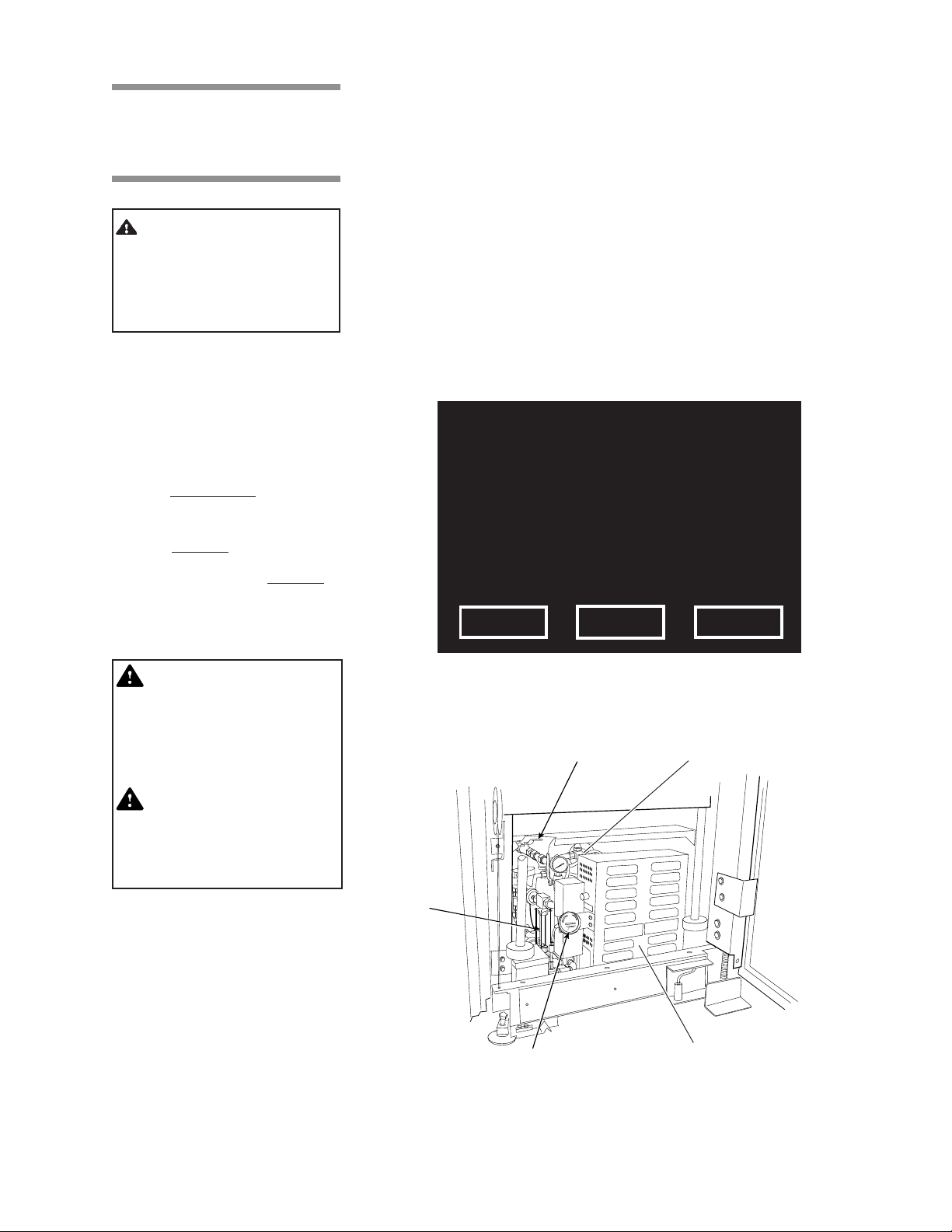
2. Check generator pressure gauge (see Fig. 3-12). Generator must be at
0.0 psig and room temperature before flushing.
Water Supply Valve to
the Generator
Water-level
Sight Glass
Generator Pressure Gauge
Generator Drain Valve
Generator Electric Box
Figure 3-12. Optional Steam Generator
3-11
Component Identification Operating Instructions 129367-408
NOTE: If generator is not at 0.0 psi, the Flush can be bypassed by pressing
CANCEL, however the flush should not be bypassed on a continuous basis or
else damage to the generator may occur. Failure to flush the generator on a daily
basis will void the generator warranty.
To ensure generator is at 0.0 psig the sterilizer can be shut off at end of the day
and by next morning the unit will be able to be flushed. Approximately seven
hours are required for generator to cool down to less than 140°F.
3. Open drain valve on the side of the generator electric box (see Fig. 3-12).
4. Verify that the water supply valve to the sterilizer is open.
5. Ensure the water supply valve to the generator is open (see Fig. 3-12).
6. Press START TIMER on screen #72. Water automatically flushes through
the generator and out the drain for 3.0 minutes. Flush timer on screen #72
counts down time remaining in the flush phase.
7. After 5.0 minutes, alarm buzzer sounds and display advances to screen
#73. Instructions on how to restart the generator are listed on screen #73.
73
WARNING – BURN HAZARD:
FLUSHING OF GENERATOR COMPLETE
TO RESTART GENERATOR:
1. CLOSE GENERATOR MANUAL
DRAIN VALVE.
2. PRESS CONTINUE TO START THE
GENERATOR AND ADVANCE TO
THE MAIN MENU.
CONTINUE
Sterilizer operator may be severely burned by scalding water if the water level control
malfunctions. The steam generator level control may malfunction if the supply water
exceeds 26,00 ohms/cm
(38.5 micromhos conductivity min.). Do not connect to
8. Close the generator drain valve.
9. Press CONTINUE on screen #73. Generator automatically fills to the proper
level and starts to heat. Display screen advances to the Main Menu screen
(#1). Allow ten minutes warm-up time once generator starts to fill.
10. Close front cabinet panel.
NOTE: The generator must be flushed every day before use.
treated water (e.g., distilled,
reverse osmosis, deionized)
unless water resistivity is determined to be acceptable. If
water exceeds 26,000 ohms/
cm, contact STERIS for information concerning modifications required to the generator control system.
Total Hardness as CaCO3* 0-17 mg/L 130 mg/L
Total Dissolved Solids 50-150 mg/L 250 mg/L
Total Alkalinity as CaCO
Resistivity - ohms/cm 2000-6000 26000
*17.1 mg/L = 1 grain hardness
Table 3-1. Required Feed Water Quality for
Carbon Steel Steam Generators
Nominal Maximum
Condition Conditions Conditions
Temperature as supplied 140° F (60° C)
3
pH 6.8-7.5 6.5-8.5
Total Silica 0.1-1.0 mg/L 2.5 mg/L
50-100 mg/L 180 mg/L
3-12
129367-408 Operating Instructions Component Identification
STERILIZER OPERATION 4
Operate sterilizer by referring to the appropriate cycle description in this
4.1 Before
Operating the
Sterilizer
WARNING – BURN HAZARD: Sterilizer, rack/
shelves, and loading car
will be hot after cycle is
run. Always wear protective gloves and apron
when removing a processed load. Protective
gloves and apron must be
worn when reloading sterilizer following the previous operation.
WARNING – SLIPPING
HAZARD: To prevent falls
keep floors dry by immediately wiping up any
spilled liquids or condensation in sterilizer loading
or unloading area.
section. The steps described on the next three pages are general instructions
that apply to all cycle operations.
1. Press ON touch screen pad on the sterilizer control display.
• The printer records the time and date that the power is turned ON.
2. Open chamber door
a. Check that drain strainer is clean and in place and that chamber
interior is clean. See ROUTINE MAINTENANCE MANUAL
(P129367-410)
if cleaning is necessary.
b. Close chamber door.
3. Open access door on the operating end of the sterilizer — refer to
Figure 4-1. Turn on steam (Fig. 4-1a) and water (Fig. 4-1b) supplies. Close
access door.
• Steam enters jacket and begins to warm chamber.
4. Open control access door.
Control
Access
Door
Closed
Open
JACKET
Access
Panel
Steam
Supply
Valve
(4-1a)
CHAMB ER
4-1a Steam Supply Valve
Open
Water
Supply
Closed
Valve
(4-1b)
4-1b Water Supply Valve
Figure 4-1. Steam Valves
Sterilizer Operation Operating Instructions 129367-408
4-1
 Loading...
Loading...