
Phonak RemoteControl
User Guide
Welcome
Congratulations on choosing the Phonak RemoteControl.
Please read the user guide carefully to make sure that you
understand and get the best out of your remote control.
For more information about features and benets, simply
contact your hearing care professional.
! Compatibility information:
Check your hearing aid compatibility with your
hearing care professional.
Phonak – life is on
www.phonak.com
CE mark applied: 2019
2 3

Content
1. Description 6
2. Using phonak RemoteControl 7
2.1 Activating the Phonak RemoteControl 7
2.2 Switching On/O 7
2.3 Understanding the indicator light 7
2.4 Pairing the Phonak RemoteControl with
your hearing aid 8
2.5 Changing hearing aid volume 9
2.6 Changing hearing aid program 10
3. Inserting a new battery 11
3.1. Open the battery compartment 11
3.2. Remove the battery from the battery
compartment 11
3.3. Insert the new battery 11
3.4. Close the battery compartment 12
4. Reset the Phonak RemoteControl 13
5. Troubleshooting 14
4 5
6. Compliance information 16
7. Information and description of symbols 22
8. Important safety information 26
8.1 Hazard warnings 26
8.2 Information on product safety 29
8.3 Other important information 31
9. Service and warranty 32
9.1 Local warranty 32
9.2 International warranty 32
9.3 Warranty limitation 33
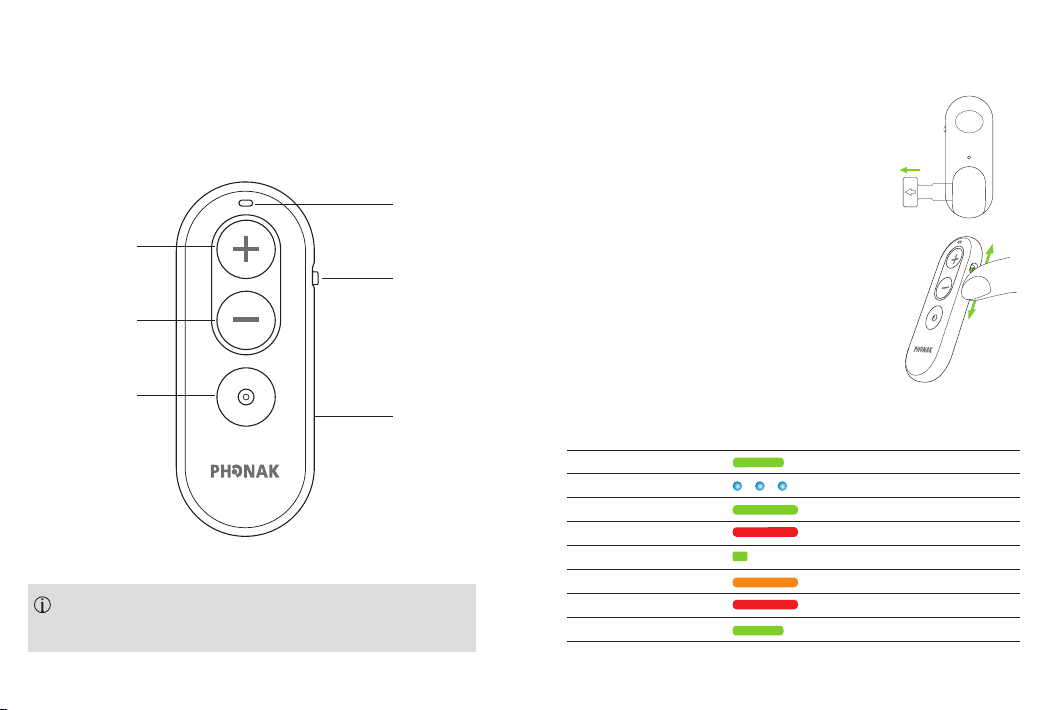
1. Description
2. Using Phonak RemoteControl
The intended user of the Phonak RemoteControl is to
enable changes of your hearing aid programs and volume.
2.1 Activating the Phonak RemoteControl
Pull out the battery protective tab to activate
your Phonak RemoteControl.
Light indicator
Volume up
On/O slider
Volume down
Program change
Battery door
The Phonak RemoteControl must be paired with your
hearing aids before you can use it (chapter 2.4).
6 7
2.2 Switching On/O
Use the slider to switch on or o.
2.3 Understanding the indicator light
Solid green for 3 seconds Switched on, ready to use
Blinking blue Paring mode
Solid green for 5 seconds Pairing successful
Solid red for 5 seconds Paring failed
Solid green for 1 second Conrmation of push button press
Solid orange Battery warning
Solid red General device error
Solid green for 3 seconds Reset successful
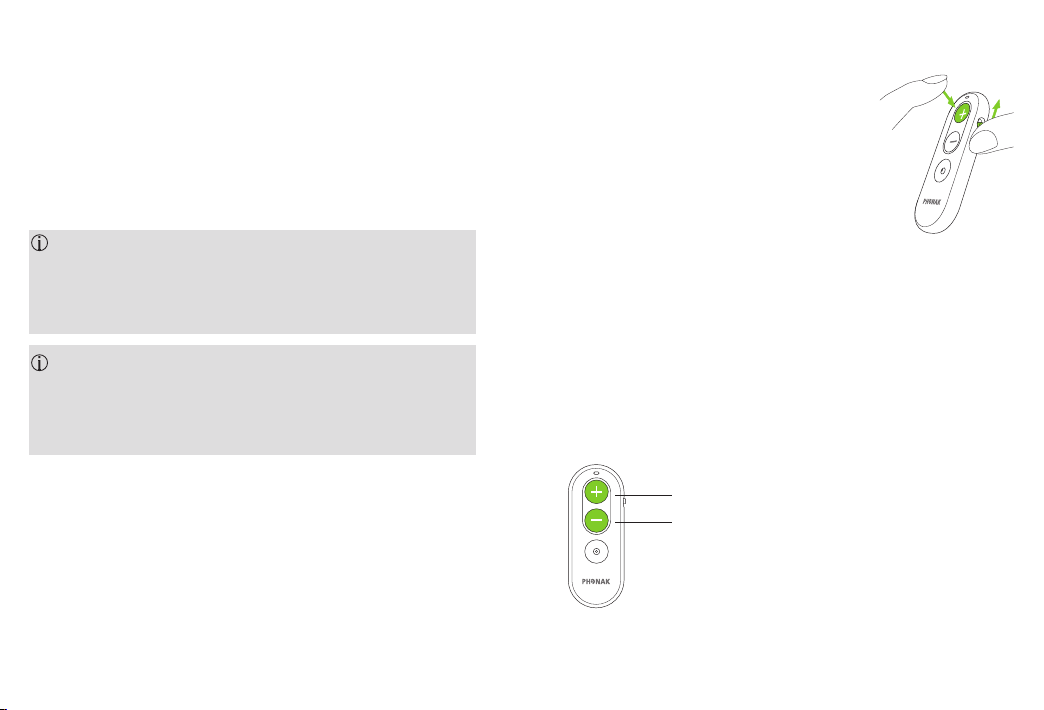
2.4 Pairing the Phonak RemoteControl with your
hearing aid
To use your Phonak RemoteControl with your hearing
aids, you must rst pair the hearing aids.
It is only necessary to perform the pairing procedure
once with your hearing aids. After the initial pairing,
your Phonak RemoteControl will connect automatically
with your hearing aids.
The Phonak RemoteControl will be automatically in the
pairing mode if switched on for the rst time. This is
indicated by the blinking blue light indicator on your
Phonak RemoteControl.
1. Press and hold the volume
1
up “+” button
2. Switch on your Phonak RemoteControl
whilst still pressing the
volume up “+” button
3. Release the volume up ”+” button
when the light indicator starts to blink blue
4. The Phonak RemoteControl and your hearing aids
will now pair automatically
5. After a successful pairing the light indicator is
solid green for 5 seconds and you may hear a
notication beep in your hearing aid
6. You can now use your Phonak RemoteControl
2.5 Changing hearing aid volume
2
Set your hearing aids into the pairing mode by switching
them on. You now have 3 minutes to pair your Phonak
RemoteControl with your hearing aids.
8 9
Press “+” to increase volume
Press “–” to increase volume
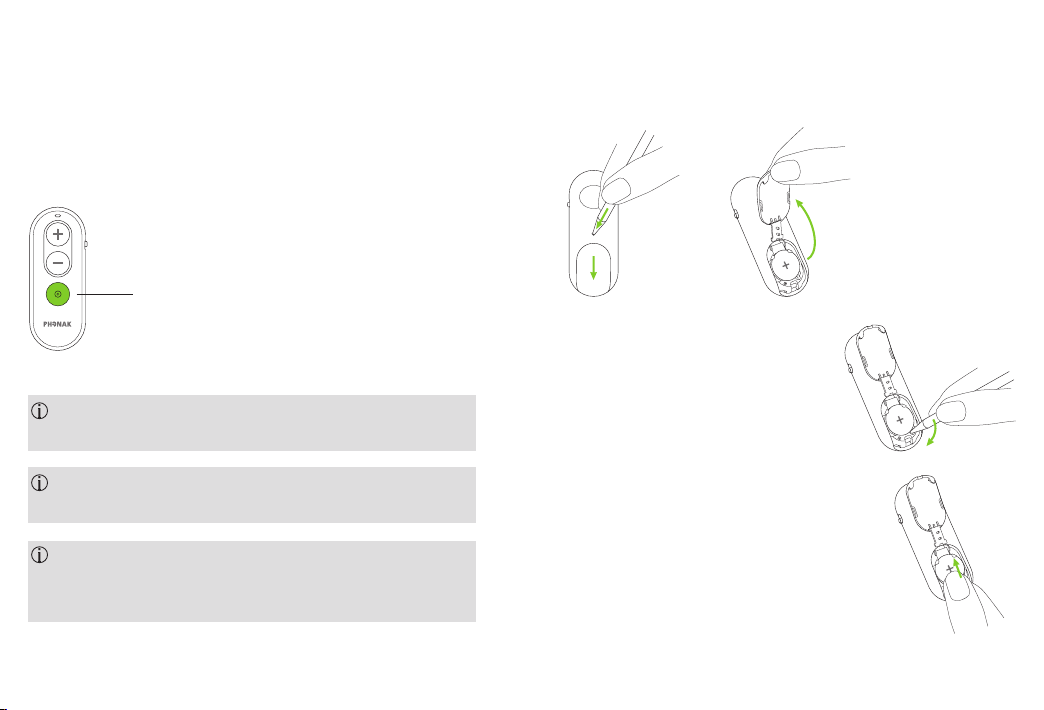
3. Inserting a new battery
2.6 Changing hearing aid program
Each press of the program button will switch your hearing
aids to the next available program.
Press to change the hearing aid program
Hearing aids may beep to conrm selection, depending
on your hearing aid conguration.
Changes are applied to both hearing aids at the same
time.
For information about your personal settings, ask your
hearing care professional to print your Phonak
RemoteControl instructions.
10 11
3.1. Open the battery compartment
1 2
3.2. Remove the battery from
the battery compartment
3.3. Insert the new battery

4. Reset the Phonak RemoteControl
The Phonak RemoteControl requires a lithium button
cell (CR2032)
3.4. Close the battery compartment
12 13
A reset of your Phonak RemoteControl will remove the
pairing to your hearing aids.
Press the volume up “+” button and the program change
button for more than 10 seconds.

5. Troubleshooting
Problem
No indicator light when a button
is pressed
Hearing aids do not respond to
the RemoteControl commands
Solid red light indicator
Causes
Remote control is switched o
Batter is empty
Hearing aids are switched o
Hearing aids are not paired with
your RemoteControl
Remote control is switched o
Battery is empty
General device error
What to do
Switch on your remote control (chapter 2.2)
Change battery (chapter 3)
Switch on your hearing aids (see hearing aid user guide)
Pair the RemoteControl (chapter 2.4)
Switch on your remote control (chapter 2.2)
Change battery (chapter 3)
Reset your RemoteControl (chapter 4) and pair your hearing aids
(chapter 2.4)
If the problem persists, contact your hearing care
professional for assistance.
14 15

6.
Compliance information
Declaration of conformity
Hereby Phonak AG declares that this Phonak product is
in compliance with the essential requirements of the
Medical Devices Directive 93/42/EEC as well as the
Radio Equipment Directive 2014 / 53 / EU. The full text
of the declaration of conformity can be obtained from
the manufacturer or the local Phonak representative
whose address can be taken from the list on http://
www.phonak.com (worldwide locations).
Australia/New Zealand:
Indicates a device’s compliance with applicable
Radio Spectrum Management’s (RSM) and
Australian Communications and Media Authority
(ACMA) regulatory arrangements for the legal sale
in New Zealand and Australia.
The compliance label R-NZ is for radio products
supplied in the New Zealand market under
conformity level A1.
16 17
The following model is certied under:
Phonak RemoteControl
USA FCC ID: KWC-RC1
Canada IC: 2262A-RC1
Notice 1:
This device complies with Part 15 of the FCC Rules and
with RSS-210 of Industry Canada. Operation is subject
to the following two conditions:
1) this device may not cause harmful interference, and
2) this device must accept any interference received,
including interference that may cause undesired
operation.
Notice 2:
Changes or modications made to this RemoteControl
not expressly approved by Phonak may void the FCC
authorization to operate this device.

Notice 3:
This device has been tested and found to comply with the
limits for a Class B digital device, pursuant to Part 15 of
the FCC Rules and ICES-003 of Industry Canada. These
limits are designed to provide reasonable protection
against harmful interference in a residential installation.
This device generates, uses and can radiate radio
frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful
interference to radio communications.
However, there is no guarantee that interference will not
occur in a particular installation. If this device does
cause harmful interference to radio or television
reception, which can be determined by turning the
equipment o and on, the user is encouraged to
try to correct the interference by one or more of the
following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the device and
receiver.
• Connect the device into an outlet on a circuit
different from that to which the receiver is
connected.
• Consult the dealer or an experienced radio/TV
technician for help.
Notice 4:
Japanese Radio Law and Japanese
Telecommunications Business Law Compliance
This device is granted pursuant to the Japanese Radio Law
(電波法) and the Japanese Telecommunications Business
Law (電気通信事業法). This device should not be
modied (otherwise the granted designation number will
become invalid).
XXXXXXXX
R
T
XXXXXXXX
Radio information of your wireless hearing aid
Antenna type
Operation frequency
Modulation
Radiated power
Monopole antenna
2.4 GHz – 2.48 GHz
GFSK
< 2.8mW
Bluetooth®
Range
Bluetooth
Proles supported
~1m
4.2
BLE (GATT)
18 19

Compliance with emission and immunity standards
Emission standards EN 60601–1-2:2015
IEC 60601–1-2:2014
EN 55011:2009+A1
CISPR11:2009/AMD1:2010
CISPR22:1997
CISPR32:2012
ISO 7637-2:2011
CISPR25:2016
EN 55025:2017
Immunity standards EN 60601-1-2:2015
IEC 60601-1-2:2014
EN 61000-4-2:2009
IEC 61000-4-2:2008
EN 61000-4-3:2006+A1+A2
IEC 61000-4-3:2006+A1+A2
EN 61000-4-4:2012
IEC 61000-4-4:2012
EN 61000-4-5:2014
IEC 61000-4-5:2014
EN 61000-4-6:2014
IEC 61000-4-6:2013
EN 61000-4-8:2010
IEC 61000-4-8:2009
EN 61000-4-11:2004
IEC 61000-4-11:2004
IEC 60601-1 (§ 4.10.2):2005
ISO 7637-2:2011
20 21

7. Information and description
of symbols
With the CE symbol, Sonova AG conrms that
this product – including accessories – meets the
requirements of the Medical Devices Directive
93/42/EEC as well as the Radio Equipment
Directive 2014/53/EU. The numbers after the
CE symbol correspond to the code of certied
institutions that were consulted under the
above-mentioned directives.
This symbol indicates that the products
described in these user instructions adhere to
the requirements for an applied part of Type B
of EN 60601-1. The surface of the hearing aid
is specied as an applied part of Type B.
Indicates the medical device manufacturer, as
dened in EU Directive 93/42/EEC.
22 23
This symbol indicates that it is important for
the user to read and take into account the
relevant information in these user guides.
This symbol indicates that it is important for
the user to pay attention to the relevant warning
notices in these user guides.
Important information for handling and
product safety.
This symbol confers that the electromagnetic
interference from the device is under limits
approved by the US Federal Communications
Commission.
The Bluetooth® word mark and logos are
registered trademarks owned by Bluetooth SIG,
Inc. and any use of such marks by Sonova AG is
under license. Other trademarks and trade
names are those of their respective owners.

Japanese mark for certied
radio equipment.
Humidity during storage: 0% to 70%, if not in
use.
Indicates the manufacturer’s serial number so
that a specic medical device can be identied.
Indicates the manufacturer’s catalogue number
Atmospheric pressure during transportation
and storage: 200 hPA to 1500 hPa
During transportation keep dry.
so that the medical device can be identied.
The symbol with the crossed-out garbage bin
This symbol indicates that it is important for
the user to read and take into account the
relevant information in this user guide.
is to make you aware that this device may not
be thrown away as normal household waste.
Please dispose of old or unused device, at waste
disposal sites intended for electronic waste, or
Temperature during transportation
and storage: –20° to +60° Celsius
(–4° to +140° Fahrenheit).
24 25
give your device to your hearing care
professional for disposal. Proper disposal
protects the environment and health.

8. Important safety information
Please read the information on the following pages before
using your device
Changes or modications to the device that were not
explicitly approved by Sonova are not permitted.
Such changes may damage your device.
8.1 Hazard warnings
If problems occur which cannot be resolved by
Your device operate between 2.4 GHz–2.48 GHz
frequency range. When ying please check if ight
operator requires devices to be switched o.
The intended use of the remote control is to enable
changes of the hearing aid programs and volume.
following the remedy guidelines in the
troubleshooting section of this user guide, consult
your hearing care professional.
Batteries are toxic if they are swallowed! Keep out of
the reach of children, individuals with cognitive
impairment, and pets. If batteries are swallowed,
Keep this device out of reach of children and mentally
consult your physician immediately!
challenged people or pets.
This device is not for children below 36 months. The
This remote control uses low-power, digitally coded
transmission to communicate to your hearing system.
Although unlikely, interference with medical devices
(i.e. pacemakers, debrillators, etc.) is possible.
Therefore users must not keep this remote control in
or near a breast pocket.
usage of this device by children and individuals with
cognitive impairment should be supervised at all
times to ensure their safety. It is a small device and
contains small parts. Do not leave children and
individuals with cognitive impairment unsupervised
with this device. If swallowed, consult a physician or
hospital immediately as the device or its parts can
cause choking!
26 27

Remove the battery if you are not using the device
for a long period of time.
Dispose of electrical components in accordance with
your local regulations by Sonova AG.
8.2 Information on product safety
Protect the device from excessive moisture (bathing,
swimming), heat (radiator, car dashboard) or direct
skin contact when sweating (workout, tness, sport).
External devices may only be connected if they have
been tested in accordance with corresponding
Do not drop the device. Dropping onto a hard
surface can damage your device.
IECXXXXX standards.
Special medical or dental examination including
Do not use the device in explosive areas (ammable
anesthetics, mines or industrial areas with a danger
of explosions), oxygen rich environments or where
electronic equipment is prohibited.
radiation described below, may adversely aect the
correct functioning of your device. Remove and keep
it outside the examination room/area before
undergoing:
• Medical or dental examination with X-ray
(also CT scan).
• Medical examinations with MRI/NMRI scans,
generating magnetic elds.
Protect the device sockets and battery slot from dirt
and debris.
28 29
Clean the device using a damp cloth. Never use
household cleaning products (washing powder, soap,
etc.) or alcohol to clean the device. Never use a
microwave or other heating devices to dry the device.
The digitally-coded, transmission technology used in
the device is highly reliable and experiences virtually
no interference from other devices. It should be
noted, however, that when operating the hearing
system near computer equipment, larger electronic
installations or other strong electromagnetic elds, it
may be necessary to be at least 60 cm (24”) away
from the interfering device to ensure proper
operation.
Keep the device at least 10 cm away from any kind of
magnets.
When the device is not in use, turn it o and store it
safely.
Do not use your device in areas where electronic
equipment is prohibited.
30 31
8.3 Other important information
High-powered electronic equipment, larger electronic
installations and metallic structures may impair and
signicantly reduce the operating range.
If the hearing aids do not respond to the device
because of an unusual eld disturbance, move away
from the disturbing eld.

9. Service and warranty
9.1 Local warranty
Please ask the hearing care professional, where you
purchased your devices, about the terms of the local
warranty.
9.2 International warranty
Sonova oers a one year limited international warranty,
9.3 Warranty limitation
This warranty does not cover damage from improper
handling or care, exposure to chemicals or undue stress.
Damage caused by third parties or non-authorized service
centers renders the warranty null and void. This warranty
does not include any services performed by a hearing care
professional in their office.
valid as of the date of purchase. This limited warranty
covers manufacturing and material defects. The warranty
Serial number:
is valid only if proof of purchase is shown.
Date of purchase:
The international warranty does not aect any legal rights
that you might have under the local warranty or the
applicable national legislation governing sale of consumer
goods.
32 33
Authorized hearing care professional
(stamp/signature):

Your hearing care professional:
7 613389 288407
Manufacturer:
Sonova AG
Laubisrütistrasse 28
CH-8712 Stäfa
Switzerland
www.phonak.com
029-0782-02/V1.00/2019-02/na © 2019 Sonova AG All rights reserved
 Loading...
Loading...