Page 1

Service Guide
IntelliVue Patient Monitor
MP5/MP5T/MP5SC
Rel. L.x
Patient Monitoring
Page 2

Page 3
1Table of Contents
1 Introduction 7
Who Should Use This Guide 7
How to Use This Guide 7
Abbreviations 7
Responsibility of the Manufacturer 7
Passwords 8
Warnings and Cautions 8
2 Theory of Operation 9
Monitor Theory of Operation 9
3 Testing and Maintenance 25
Introduction 25
Terminology and Definitions 25
Recommended Frequency 26
When to Perform Tests 27
Testing Sequence 30
Visual Inspection 30
Safety Tests 31
System Test 55
Preventive Maintenance Procedures 66
Performance Assurance Tests 66
Reporting of Test Results 92
Other Regular Tests 95
Touchscreen Calibration 95
Disabling/Enabling Touch Operation 96
Printer Test Report 96
Battery Handling, Maintenance and Good Practices 97
After Installation, Testing or Repair 105
4 Troubleshooting 107
Introduction 107
How To Use This Section 107
Who Should Perform Repairs 107
Replacement Level Supported 107
Software Revision Check 108
Software Compatibility Matrix 108
Obtaining Replacement Parts 108
Troubleshooting Guide 108
3
Page 4

5 Repair and Disassembly 135
Who Should Perform Repairs 135
Tools required 136
Removing the Handle or Bedhanger 136
Removing the Predictive Temperature Assembly 137
Disassembling the Predictive Temperature Assembly 139
Removing/Exchanging the NBP Inlet 140
Separating the Front and Back of the Monitor 142
Removing the Recorder Slot Cover 146
Removing the Internal Quick Mount Solution 147
Removing the Short Range Radio (SRR) Interface 147
Removing the IntelliVue 802.11 Bedside Adapter Antenna or IIT Antenna (not for MP5T) 149
Removing the IntelliVue 802.11 Bedside Adapter (WLAN) (not for MP5T) 149
Removing the IntelliVue Instrument Telemetry (IIT) Module (not for MP5T and MP5SC) 153
Removing the IntelliVue 802.11 Bedside Adapter/IIT Holder 154
Removing the MSL Board (not for MP5T and MP5SC) 155
Removing the Backlight Inverter Board (Old NEC Display) 156
Removing the Backlight Converter Board (New NLT Display) 158
Removing the Power Supply 159
Removing the System Interface Board 160
Removing the Recorder Board 161
Removing the Microstream CO2 Assembly 163
Removing the Measurement Block 169
Removing the NBP Pump 173
Removing the Main Board 175
Removing the Touch Assembly 178
Removing the Loudspeaker 180
Removing the Power Button and LED Assembly 180
Removing the LCD Panel 181
Exchanging the Backlight (Old NEC Display only) 182
Modifying the Nurse Call Relay 183
6 Parts 187
MP5/MP5T/MP5SC Parts 187
External Display Part Numbers (not for MP5T) 201
Tympanic Thermometer Part Numbers 203
Smart Battery Charger Part Numbers 203
Test and Service Tools 204
7 Installation Instructions 207
Out-Of-Hospital Transport - Standards Compliance 207
Electromagnetic Emissions 209
Electromagnetic Interference (SRR) 209
Installation Checklist 209
Unpacking and Checking the Shipment 210
Installing the Predictive Temperature Probe 211
4
Page 5
Installing the Tympanic Thermometer 213
Installing the HS1-R Barcode Reader 216
Mounting the Monitor 226
Connecting the Monitor to AC Mains 233
Checking Out The Monitor 233
Loading Paper 236
Configuration Tasks 236
Network Configuration Tasks (Rev H.0 or higher) 239
Handing Over the Monitor 248
Installing Remote Devices (not for MP5T) 249
Clinical Network (Wired and Wireless) 251
Philips IntelliVue Information Center 251
IntelliVue Instrument Telemetry (IIT)(not for MP5T and MP5SC) 251
Short Range Radio 252
Connecting the MP5 to a Host Monitor (not for MP5T and MP5SC) 255
Nurse Call Relay (not for MP5T) 255
ECG Out Functionality (ECG Sync) (not for MP5T and MP5SC) 256
ECG Sync Pulse (not for MP5T and MP5SC) 256
8 Site Preparation 259
Introduction 259
Monitor Site Requirements 261
Remote Device Site Requirements 262
Remote Displays (M8031B) 264
Remote Displays - M8033C 264
Cabling Options and Conduit Size Requirements 265
Touch Cable 266
MSL Cables 266
Philips Medical LAN 267
LAN Interface 267
Telemetry Device (Patient Worn Device) cables 268
Nurse Call Relay Interface 268
ECG Out Interface 269
9 Gas Analyzers (not for MP5T and MP5SC) 271
10 Specifications 273
Essential Performance Characteristics 273
MDD Classification 275
Classification According to IEC 60601-1 275
Safety and Regulatory Information 275
Use Environment 275
Disconnecting from Power 276
Symbols 276
Physical Specifications 278
Environmental Specifications 278
5
Page 6

6
Page 7

1Introduction
This Service Guide contains technical details for the IntelliVue MP5/MP5T/MP5SC Patient Monitor
This guide provides a technical foundation to support effective troubleshooting and repair. It is not a
comprehensive, in-depth explanation of the product architecture or technical implementation. It offers
enough information on the functions and operations of the monitoring system so that engineers who
repair them are better able to understand how it works.
Who Should Use This Guide
This guide is for biomedical engineers or technicians responsible for installing, troubleshooting,
repairing, and maintaining Philips’ patient monitoring systems.
1
How to Use This Guide
Navigate through the table of contents at the left of the screen to select the desired topic. Links to
other relevant sections are also provided within the individual topics. You can also scroll through the
topics using the page up and page down keys.
Abbreviations
Abbreviations used throughout this guide are:
Name Abbreviation
IntelliVue MP5/MP5T/MP5SC Patient Monitor the monitor
Medical Information Bus MIB
Responsibility of the Manufacturer
Philips only considers itself responsible for any effects on safety, EMC, reliability and performance of
the equipment if:
• assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by Philips, and
• the electrical installation of the relevant room complies with national standards, and
7
Page 8

1Introduction
• the instrument is used in accordance with the instructions for use.
To ensure safety and EMC, use only those Philips parts and accessories specified for use with the
monitor. If non-Philips parts are used, Philips is not liable for any damage that these parts may cause to
the equipment.
This document contains proprietary information which is protected by copyright. All Rights Reserved.
Reproduction, adaptation, or translation without prior written permission is prohibited, except as
allowed under the copyright laws.
Philips Medizin Systeme Böblingen GmbH
Hewlett-Packard Str. 2
71034 Böblingen, Germany
The information contained in this document is subject to change without notice.
Philips makes no warranty of any kind with regard to this material, including, but not limited to, the
implied warranties or merchantability and fitness for a particular purpose.
Philips shall not be liable for errors contained herein or for incidental or consequential damages in
connection with the furnishing, performance, or use of this material.
Passwords
In order to access different modes within the monitor a password may be required. The passwords are
listed below.
CAUTION
Your hospital/organization is responsible that the passwords listed below are revealed to authorized
personnel only.
Monitoring Mode: No password required
Configuration Mode: 71034
Demo Mode: 14432
Service Mode: 1345
Consult the configuration guide before making any changes to the monitor configuration.
Warnings and Cautions
In this guide:
•A warning alerts you to a potential serious outcome, adverse event or safety hazard. Failure to
observe a warning may result in death or serious injury to the user or patient.
•A caution alerts you where special care is necessary for the safe and effective use of the product.
Failure to observe a caution may result in minor or moderate personal injury or damage to the
product or other property, and possibly in a remote risk of more serious injury.
8
Page 9
2Theory of Operation
Monitor Theory of Operation
The IntelliVue MP5/MP5T/MP5SC patient monitor is used for monitoring and recording multiple
physiological parameters of adults, pediatrics, and neonates. The monitor also generates alarms for the
measured parameters. The monitor is used by trained healthcare professionals in a hospital
environment.
The monitor stores data in trend, event, and calculation databases. You can see tabular trends (vital
signs) and document them on a printer. You can view measurement trend graphs, with up to three
measurements combined in each graph, to help you identify changes in the patient's physiological
condition. You can view fast-changing measurement trends with beat to beat resolution and see up to
four high resolution trend segments. Event surveillance enhances documentation and review of
physiologically significant events by automatically detecting and storing up to 50 user-defined clinical
events over a 24 hour period.
2
The monitor can be configured with various different measurement and interface capabilities.
The following comparison table shows in detail the differences between MP5, MP5T and MP5SC:
Functionality (including optional features) MP5 MP5T MP5SC
ECG yes no no
SpO2 yes yes yes
NBP yes yes yes
Predictive Temperature yes yes yes
Temperature yes no no
Invasive Pressure yes no no
Carbon Dioxide yes no no
Microstream CO
Direct Telemetry Connection yes yes no
ECG Output signal yes no no
LAN networking capability yes no yes*
WLAN networking capability yes no yes*
IntelliVue Instrument Telemetry networking capability yes no no
USB Interface yes no yes
Short Range Radio capability yes yes yes
2
yes no yes
9
Page 10
2 Theory of Operation
Functionality (including optional features) MP5 MP5T MP5SC
Severe Sepsis Screening yes no no
OxyCRG high resolution trend yes no no
Neonatal event review yes no no
Integrated recorder yes yes yes
Drug Calculator yes yes no
Gas monitor support yes no no
Connection to a host monitor (companion mode) yes no no
Connection to an external display yes no yes
Nurse call capability yes no yes
Spot Check yes no yes
Multi-Patient Spot Check no no yes
Early Warning Score yes no yes
* For MP5SC LAN and WLAN Networking capability are only available for HL7 export, not for IIC
support.
NOTE
The following descriptions may vary depending on the monitor option purchased.
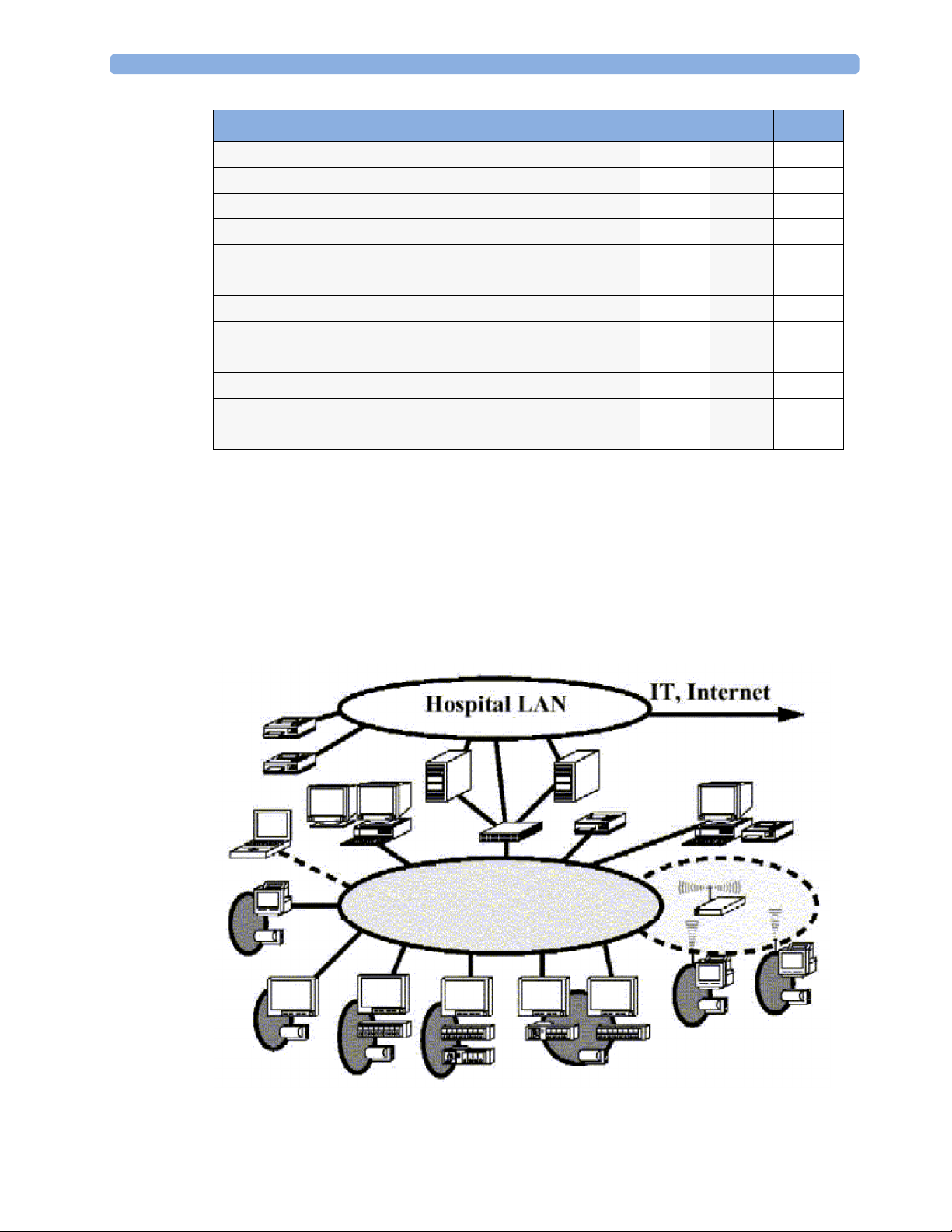
System Boundaries
The following diagram discusses specific boundaries within the overall system with respect to their
openness and real-time requirements:
10
System Boundaries
Page 11
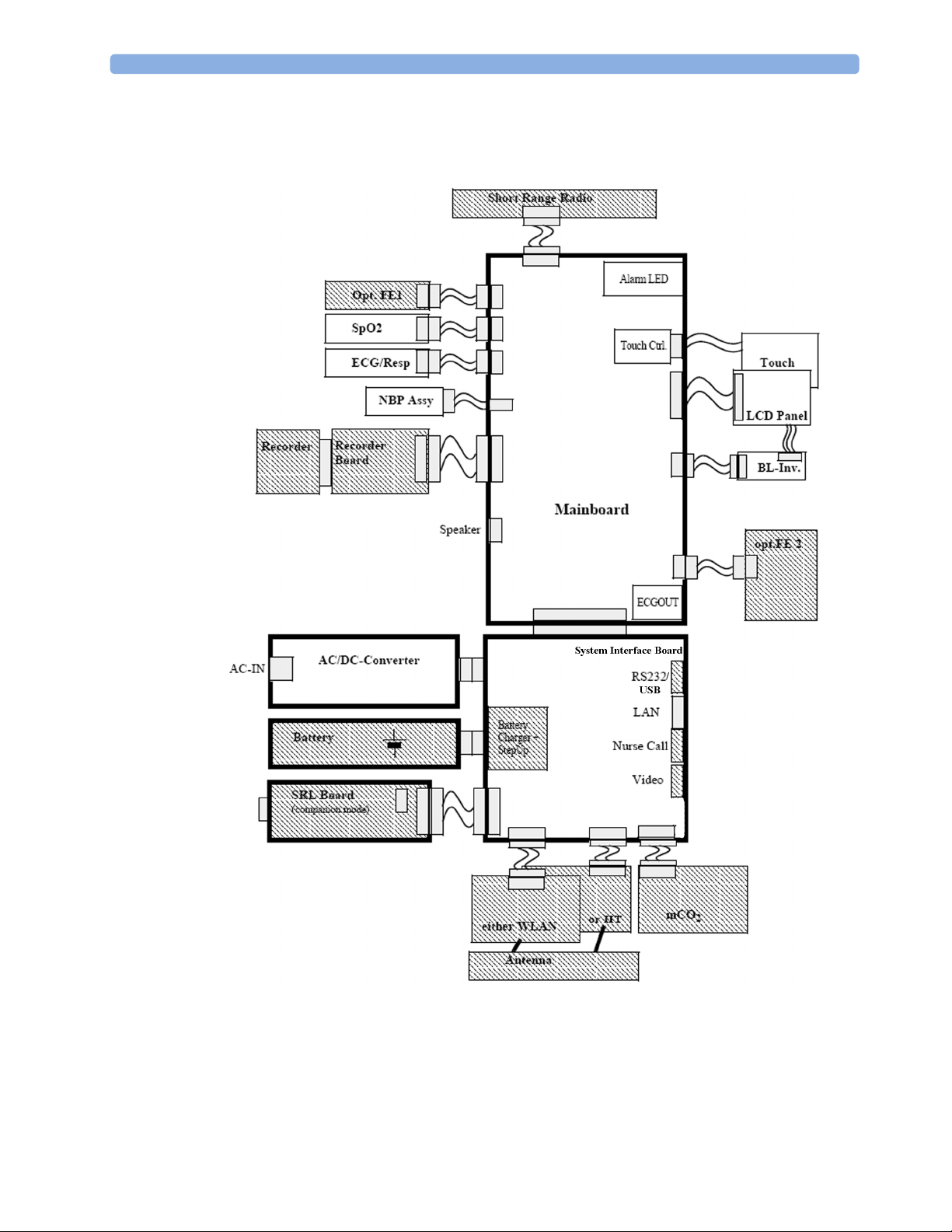
2 Theory of Operation
Measurement connections
Built-in measurement block
Philips Clinical Network (wired LAN)
connects multiple patient monitors, information centers,
application servers; closed system, only Philips qualified products
(tested and with regulatory approval) are connected, Philips is
responsible for guaranteed real-time functionality and performance
Philips Clinical Network (wireless)
like Philips Clinical Network (wired) LAN, however due to current
wireless technologies available it has reduced bandwidth, longer
latencies, reduced functionality
Hospital LAN, Internet
Standard Network, not under Philips control, no guaranteed
service, no real-time requirements
11
Page 12
2 Theory of Operation
Hardware Building Blocks
The following hardware building blocks make up the monitoring system. (Note that the MP5T and
MP5SC do not include all the hardware components shown below):
12
MP5/MP5T/MP5SC Hardware Building Blocks
Page 13
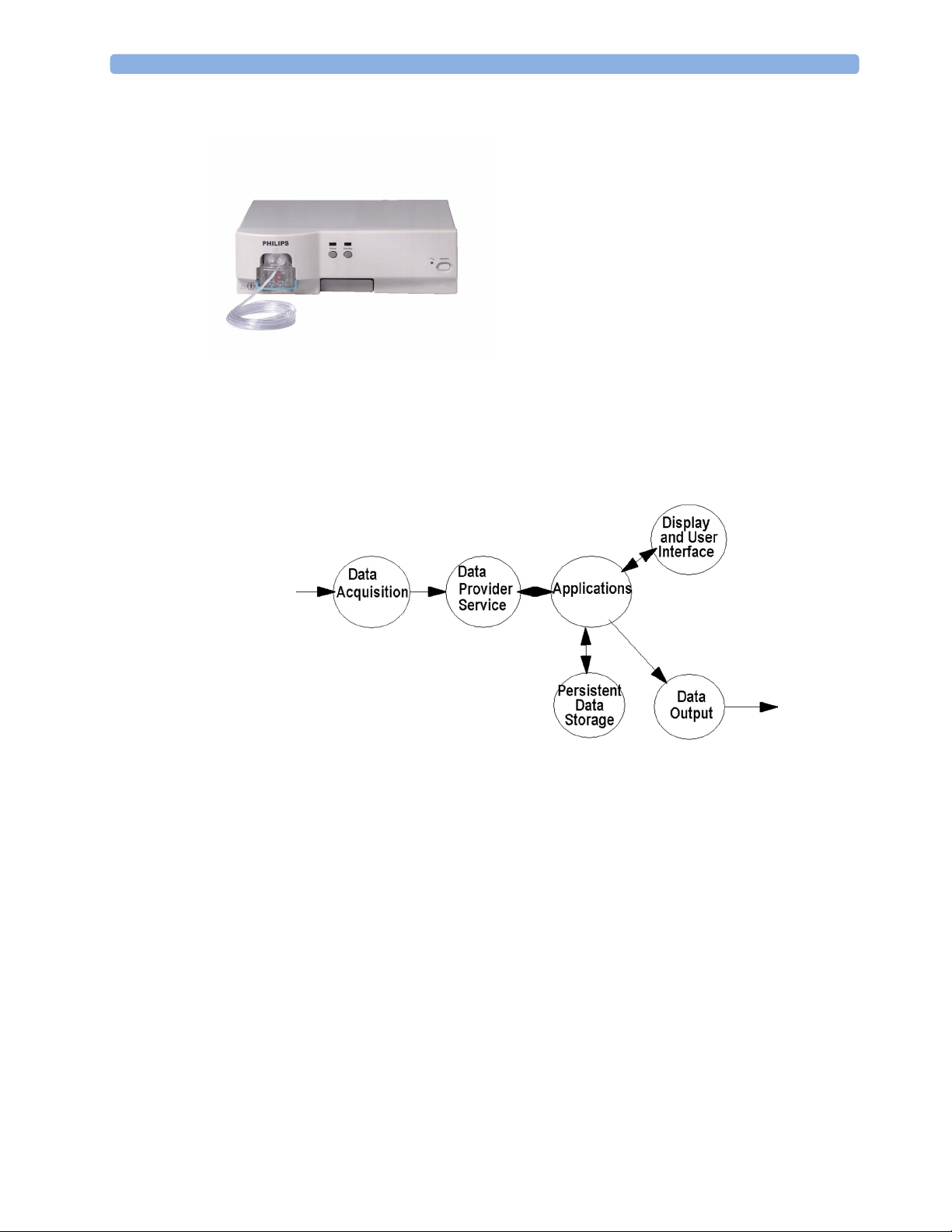
IntelliVue MP5/MP5T/MP5SC
The MP5/MP5T/MP5SC monitor:
• integrates the display and processing unit into a single package
• uses a 8.4” TFT SVGA color display
• uses the Touchscreen as input device
• integrates the measurement block (Front End 1 (FE1) and Front End 2 (FE2)) with optional
parameter sets
Optional Hardware
• One slot is provided for one of three available system interface boards. An optional built-in
wireless network interface IntelliVue 802.11 Bedside Adapter or IntelliVue Instrument Telemetry)
is supported. For further details regarding the wireless network please refer to the M3185A Philips
Clinical Network documentation.
• optional recorder
• optional battery
• optional MSL board
• optional Short Range Radio (SRR) board
2 Theory of Operation
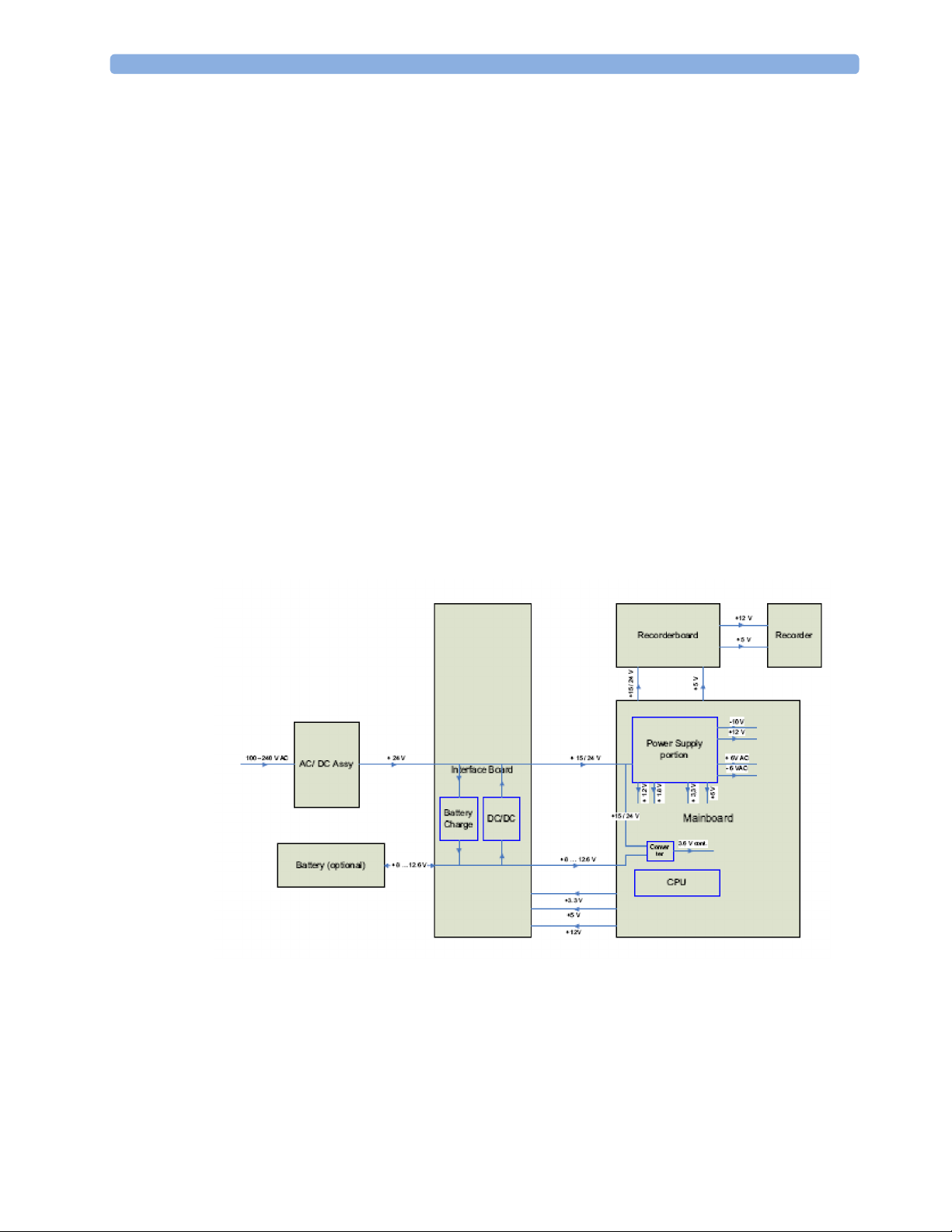
Power Distribution
Power Distribution Architecture
The AC/DC converter transforms the AC power (100-240 V AC range) coming from the power plug
into a 24 V / 50W DC source and isolates the monitoring system from the AC power mains.
The 24 V DC is distributed via the Interface Board to the optional battery charging circuit and to the
main- and recorder board.
13
Page 14

2 Theory of Operation
If the interface board contains the optional battery circuits, the power is used to charge the battery and
supply the monitoring system. As soon as the AC power source is disconnected, the optional battery
starts and keeps the system powered (battery mode). A DC/DC converter on the interface board
converts the 8-12.6 V DC power supplied by the battery into 15 V DC power, which is distributed to
the monitoring system.
The main board and recorder board contain power supply circuits, which convert the 24 /15 V DC
into several voltages supplying the particular components of the monitoring system.
The realtime clock and the buffered RAM is supplied with cont. 3.6 V DC power, provided either by
the 24 / 15 V DC system power or by the battery power and converted to 3.6 V DC.
The CPU board has an MPC852/62 MHz processor in the patient monitor that provides a number of
on-chip, configurable interfaces. An array of fast UARTS with configurable protocol options are
implemented in an ASIC (along with other system functions such as independent watchdogs, video,
etc.), providing interfacing capabilities to integrated measurements and System Interface Boards. The
serial interfaces can easily be electrically isolated. The main board contains additional video hardware.
The CPUs provide a LAN interface to connect to the Philips Clinical Network (Ethernet).
NOTE
An MP5 in companion mode does not receive its power from the host monitor via the MSL. MP5 is
always powered by AC power or battery.
System Interfaces
The following is a list of Interface boards which may be present in your monitor, depending on your
purchased configuration:
System Interface boards:
• Basic: LAN, Video #J01(no longer orderable)
• Battery: LAN, Battery Board, mCO
• Full: LAN, Battery, MIB/RS232, Video, Nurse Call, mCO
• Full USB: LAN, Battery, USB, Video, Nurse Call, mCO
Note that WLAN, IIT and MSL Interface require the full system interface board or the Full USB
system interface board.
The MP5T is delivered only with the Battery system interface board. The MP5SC is delivered only with
a Full USB system interface board.
The specifications for the above listed interfaces can be found in the technical data sheet for the
monitor and in the Installation and Specifications chapter of the Instructions for Use.
#J02
2
#J43
2
#J40
2
14
Page 15
Compatible Devices (not for MP5T and MP5SC)
IntelliVue G1/G5 Anesthetic Gas Module
Data Flow
The following diagram shows how data is passed through the monitoring system. The individual stages
of data flow are explained below.
2 Theory of Operation
Data Acquisition
Monitoring data (for example patient measurement data in the form of waves, numerics and alerts) is
acquired from a variety of sources:
• Measurement Block
The integrated measurements convert patient signals to digital data and apply measurement
algorithms to analyze the signals.
• External measurement devices
Data can be also acquired from devices connected to interface boards of the monitor. Software
modules dedicated to such specific devices convert the data received from an external device to
the format used internally. This applies to the IntelliVue G1/G5 Anesthetic Gas Module (not for
MP5T and MP5SC).
Data Flow
15
Page 16

2 Theory of Operation
• Server systems on the Philips Clinical Network
To enable networked applications such as the other bed overview, data can be acquired from server
systems attached to the Philips Clinical Network, for example a Philips Information Center
Data Provider System Service
All data that is acquired from integrated measurements or external measurement devices is temporarily
stored by a dedicated data provider system service. All monitor applications use this central service to
access the data in a consistent and synchronized way rather than talking to the interfaces directly.
This service makes the applications independent of the actual type of data acquisition device.
The amount of data stored in the data provider system service varies for the different data types. For
example several seconds of wave forms and the full set of current numerical values are temporarily
stored in RAM.
Persistent Data Storage System Service
Some applications require storage of data over longer periods of time. They can use the persistent data
storage system service. Dependent on the application requirements, this service can store data either in
battery backed-up (buffered) memory or in flash memory. The buffered memory will lose its contents
if the monitor is without power (not connected to mains) for an extended period of time. The flash
memory does not lose its contents.
The trend application for example stores vital signs data in a combination of flash memory and
buffered memory, while the system configuration information (profiles) is kept purely in flash
memory.
Display and User Interface Service
Applications can use high level commands to display monitoring data or status and command windows
on the internal LCD panel. These commands are interpreted by the display manager application. This
application controls the dedicated video hardware which includes video memory and a special
hardware in the ASIC.
User input is acquired from the touchscreen. The system software makes sure that the user input is
directed to the application which has the operating focus.
Monitor Applications
The monitor applications provide additional system functionality over the basic measurement and
monitoring capabilities. This includes for example trending, report generating, event storage or derived
measurements.
In general, the monitor applications use the data provider system service to access the measurement
data. Application interfaces to the other system services allow the application to visualize data, to store
data over extended periods of time or to output data to other devices.
Internal LAN (Measurement Link)
The MP5 communicates as a Multi-Measurement Module (MMS) in companion mode when
connected to a host monitor using an IEEE802.3/Ethernet LAN in the Measurement Link (MSL).
This network is used to distribute data between the the MP5 and the host monitor, for example:
16
• Digitized patient signals including wave data, numerical data and status information (typically from
the measurement server to a display unit)
Page 17

2 Theory of Operation
• Control data representing user interactions (typically from the display unit to a measurement
server)
• Shared data structures, for example representing patient demographical data and global
configuration items
The internal LAN allows plug and play configuration of the monitoring system. The system
automatically detects plugging or unplugging of measurement servers on the host monitor and
configures the system accordingly.
The components on the internal LAN are time-synchronized to keep signal data consistent in the
system. Dedicated hardware support for synchronization eliminates any latency of the network driver
software.
The integrated LAN provides deterministic bandwidth allocation/reservation mechanisms so that the
real-time characteristic of signal data and control data exchange is guaranteed. This applies to the data
flow from the measurement server to the monitor (for example measurement signal data) and the data
flow from the monitor to a measurement server (for example to feed data to a recorder module).
Integrated communication hubs in the monitor allow flexible cabling options (star topology, daisy
chaining of servers).
NOTE
The MP5 does not support any MMS on the MSL.
Microstream CO2
CO2 sample rate: 20 samples/second
Calculation of end tidal CO
The M3015A/B MMS Extensions use Microstream® non–dispersive infrared (NDIR) spectroscopy
to continuously measure the amount of CO2 during every breath, the amount of CO2 present at the
end of exhalation (etCO
rate. The displayed etCO
the Max Hold setting (configuration mode). It can be set to no peak picking (off), 10 seconds and 20
seconds.
Test method for respiration rate range:
A breath simulator system combined with CO
covering the specified range. The resulting end tidal CO
Differences between actual and expected end tidal CO
accuracy for the respective respiration rate, i.e. there was no effect of the respiration rate on the end
tidal CO
values beyond those limits.
2
Philips Clinical Network
The monitoring system may be connected to the Philips Clinical Network, for example to provide
central monitoring capabilities or other network services. This connection may be through a normal
wired connection or through a wireless connection.
(etCO2):
2
), the amount of CO2 present during inhalation (imCO2), and the respiratory
2
is the maximum etCO2 over the previous peak-picking interval as defined by
2
and N2 gases was used to simulate respiration rates
2
values were compared to the expected value.
2
values were within the limits of the specified
2
The monitor supports the connection of an internal wireless adapter, depending on the monitor model
(#J35, #J45, #J47). Switching between wired and wireless networks is automatically triggered by the
plugging or unplugging of the network cable.
17
Page 18

2 Theory of Operation
After configuration, the monitoring system sends the digitized patient signals including wave data,
numerical data and status information onto the network. Control data representing user interactions
can be exchanged between the monitoring system and a central station bi-directionally.
Additional protocols are supported for networked applications, for example for the other bed
overview function, which allows viewing of monitoring data from other patients on the network.
For plug and play operation, the monitoring system uses the standard BootP protocol to automatically
acquire a network address.
How does the Support Tool Work with the Monitor
The support tool is a Windows application typically installed on the laptop of a customer engineer or a
biomedical engineer working in the customer’s own service department.
The purpose of the support tool is to upgrade, configure and diagnose all monitoring components in
the system over the network.
The service protocol developed for this purpose uses a raw access to the devices without the need for
IP addresses etc. over a standard customer network installation, so that even defective devices can be
upgraded as long as the few kBytes of initial boot code are working. The boot code itself can also be
upgraded using the same protocol.
The tool allows access to internal service information and to serial numbers. It can be remotecontrolled, for example via a dial-up connection from a response center, provided the proper
infrastructure is in place.
For details see the Instructions for Use for the Support Tool.
18
Page 19
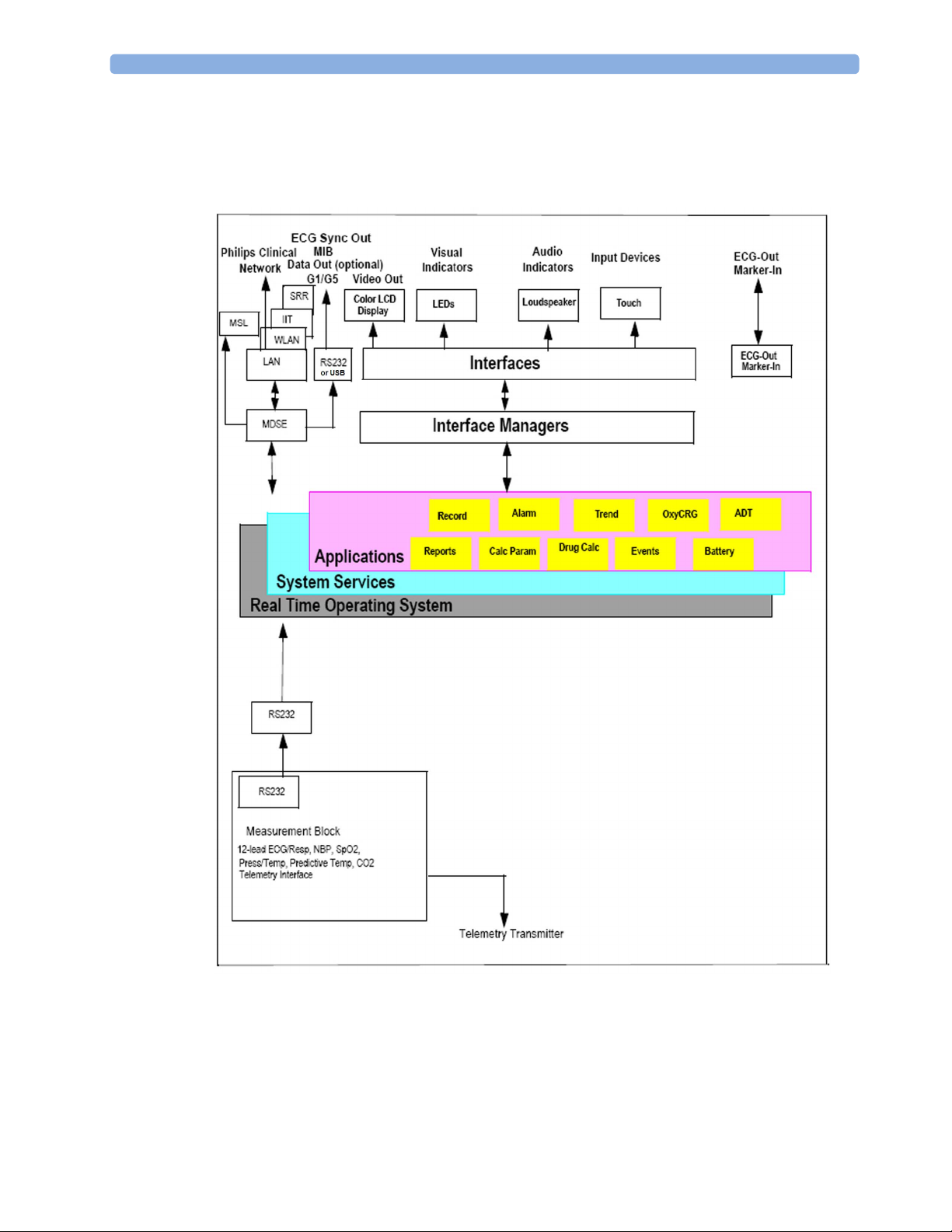
Monitor Software Block Diagram
The following shows the functional block diagram for the monitoring system. A legend explaining
terms and diagram elements follows. The information below varies depending on the purchased
monitor options.
2 Theory of Operation
IntelliVue Patient Monitoring System Functional Block Diagram
19
Page 20
2 Theory of Operation
Block Diagram Legend
Functional Block Description
Services
Operating System The Operating System (OS) provides a layer of isolation
System Services The System Services provide generic common system
Applications
Reports The Reports Service retrieves current and stored physiological
between the specific hardware implementation and the
application software. The OS performs system checks and
allocates resources to ensure safe operation when the system
is first started. This includes internal self-tests on several
hardware modules and configuration checks for validity of
configuration with the operating software. During normal
operation, the OS continues to run checks on system integrity.
If error conditions are detected the OS will halt monitoring
operations and inform the operator about the error condition.
services.
In particular:
They use a real-time clock component to track time. They
synchronize to network time sources and verify the accuracy
of the system time information. They are also responsible for
managing persistent user configuration data for all
Measurement parameters and IntelliVue Patient Monitoring
System software modules. User configuration data is stored in
a non-volatile read/write storage device
data and status data to format reports for printing paper
documentation. Examples of supported reports:
20
• Vital Signs Report
• Graphical Trend Report
• Event Review Report
• Event Episode Report
• ECG Report (12 Lead/Multi-Lead)
• Test Report
The Reports service generates report data which can be
printed on a central printer.
Record The Record Service retrieves current and stored physiological
data and status data to format a continuous strip recording. A
recording can be triggered manually by the operator or
automatically by an alarm condition. The Record Service uses
the services of the Recorder Interface to control a recorder.
The Record Service can also send data to a central recorder.
Page 21
2 Theory of Operation
Functional Block Description
Alarm The Alarm Service contains logic that prioritizes alarm
conditions that are generated by IntelliVue Patient Monitoring
System software modules. Visual alarm signals (messages) are
displayed at the top of the IntelliVue Patient Monitoring
System display and alarm sounds are generated by a
loudspeaker. Alarm conditions may be generated when a
physiological parameter exceeds preselected alarm limits or
when a physiological parameter or any other software module
reports an inoperative status (technical alarm, for example, the
ECG leads may have fallen off the patient). The Alarm service
manages the alarm inactivation states, for example suspension
of alarms, silencing of alarms, and alarm reminder. Alarm
signals may also be configured as latching (alarm signals are
issued until they are acknowledged by the operator, even
when the alarm condition is no longer true). The Alarm
service controls the visual alarm signals (alarm lamps).
Trend The Trend service stores the sample values of physiological
data and status data with a resolution of 12 seconds, 1 minute
or 5 minutes for a period of up to 48 hours. The data is kept
in battery buffered read/write storage and flash memory
devices to be preserved across power failures. The stored data
is protected via consistency checks and checksums. When a
new patient is admitted, the trend database erases all data of
the previous patient.
OxyCRG The OxyCRG (Oxygen CardioRespiroGram) service derives a
high-resolution trend graph from the Beat-to-Beat Heart
Rate, SpO2, and Respiration physiological data. The OxyCRG
is specialized for neonatal applications, allowing the operator
to identify sudden drops in Heart Rate (Bradycardia) and
SpO2 (Desaturation), and supporting the operator in
visualizing Apnea situations.
ADT The ADT (Admit/Discharge/Transmit) service maintains the
patient demographics information. The operator may admit a
new patient, discharge the old patient and enter or modify the
patient demographics.
Calc Param The Calc Param (Calculated Parameters) application performs
calculations on physiological numerical values to derive
calculated parameters like Temperature Difference.
Interface Managers
21
Page 22
2 Theory of Operation
Functional Block Description
MDSE The MDSE (Medical Data Service Element) Interface
Printer The Printer Interface Manager provides a high level interface
Display & Operator Interface The Display and Operator Interface Manager performs the
Manager is responsible for the exchange of real-time data
between the IntelliVue Patient Monitoring System display unit
and the Measurement parameters and other devices attached
to the network. MDSE establishes and maintains a data
communication link between the devices. It provides
configuration information about the remote device to
applications in the local device and it allows the exchange of
measurement data and status information between the
devices.
to a printer. It provides means to:
• establish a connection to the printer
• transfer data to the printer
• get status of the printer
• close connection to the printer
The Printer Interface Manager also supervises the connection
to the printer and whether the printer accepts data (for
example paper out). The Printer Interface Manager notifies
the operator in such cases.
following tasks:
Interfaces
• Screen presentation of real-time and stored physiological
measurement data, alarm condition data and status
information received from the MDSE interface manager,
the Alarm service or other IntelliVue Patient Monitoring
System modules
• Screen presentation of operating controls (control
windows)
• Processing of operating control commands received from
HIF Control interface. The module verifies and interprets
the received commands and forwards them to other
software modules of the IntelliVue Patient Monitoring
System display unit or measurement parameters.
• Sound generation (issues audible alarm signals and
generates audible information signals, for example QRS
and SpO2 tones, operator audible feedback)
22
Page 23
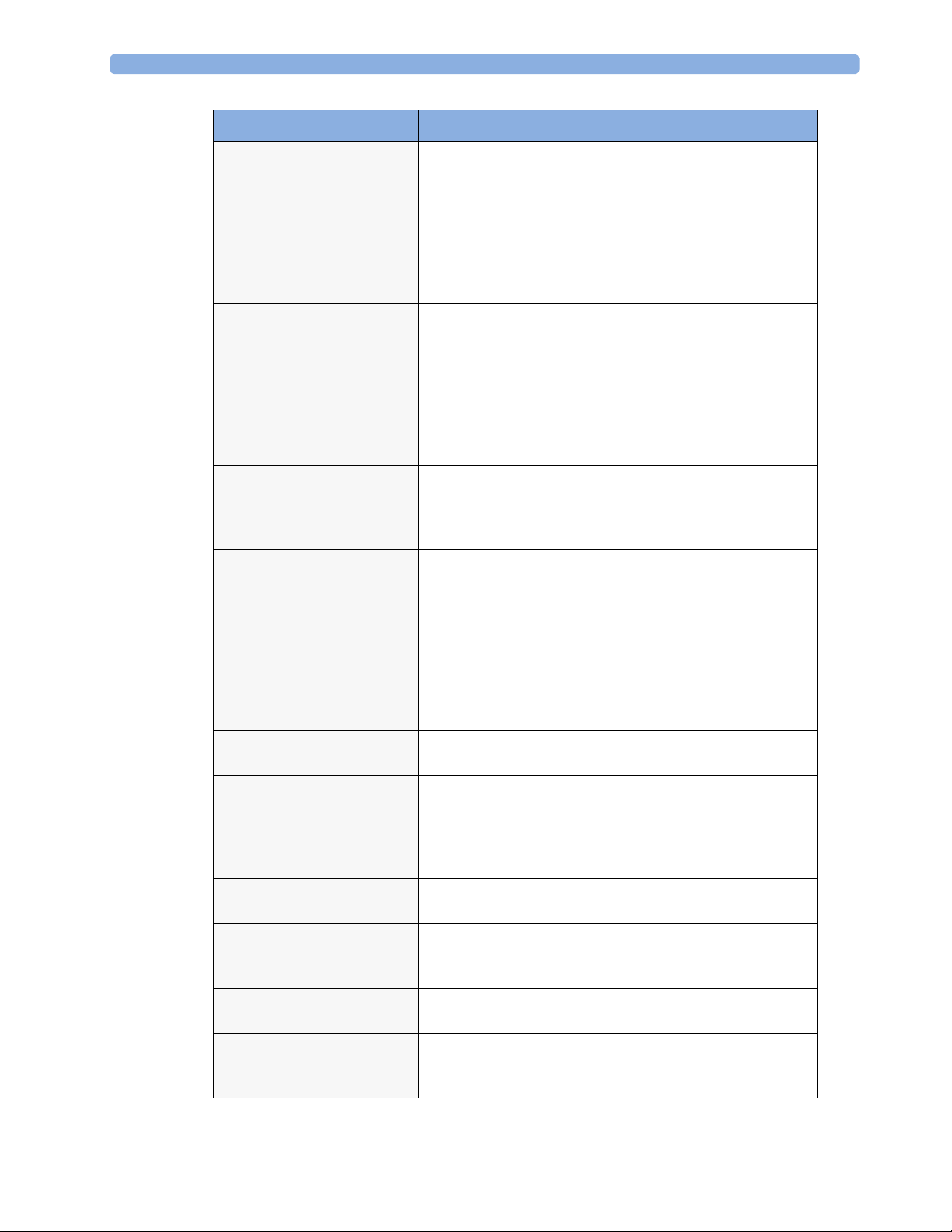
2 Theory of Operation
Functional Block Description
LAN The LAN interface implements the physical layer of IEEE
802.3. The LAN interface performs Manchester encoding/
decoding, receive clock recovery, transmit pulse shaping,
jabber, link integrity testing, reverse polarity detection/
correction, electrical isolation, and ESD protection.
Electronically separated interfaces are used for
communication to the Measurement parameters and to the
network.
Display Controller The Display Controller Interface consists of a video
controller, video RAM and the controlling software. The
Display Controller interface processes the high level display
commands (character and graphic generation, wave drawing)
and translates them into pixels, which are written into the
video RAM where the video controller chip generates the
video synchronization signals and the pixel stream for the
Color LCD Display.
HIF Control The HIF (Human Interface Control) interface scans the
Human Interface devices for operator controls (Touch
Screen), formats the collected data and sends it to the display
and Operating Interface.
ECG-Out Marker-In
(not for MP5T and MP5SC)
The ECG Out/Marker In interface receives the ECG
waveform directly from the ECG/Resp Arrhythmia STSegment physiological algorithm running on the main CPU
and converts the digital ECG signal to an analog ECG signal.
In addition, the ECG Out hardware receives from a
connected device the marker information and forwards this
data to the ECG/Resp Arrhythmia ST-Segment physiological
algorithm. The converted analog signal is used to synchronize
a connected device to the patient’s ECG
Nurse Call
(not for MP5T)
MIB
(not for MP5T and MP5SC)
The Nurse Call board contains a phone jack type connector
with a single close-on-alarm relay.
The MIB interface allows full-duplex, short-haul
asynchronous binary communication between the monitor
and an arbitrary (medical/non-medical) device using an eightpin RJ45 modular connector. Communication protocols using
this interface can be configured.
ECG Sync Out
(not for MP5T and MP5SC)
IIT
(not for MP5T and MP5SC)
A pulse signal is provided on the RS-232 interface to allow
synchronisation with other medical devices.
The built-in IIT adapter allows operation of the MP5
monitors within IntelliVue Instrument Telemetry
infrastructure.
WLAN
(not for MP5T)
The bulit-in WLAN interface allows wireless operation of the
MP5 monitors with the IntelliVue 802.11 Bedside Adapter.
SRR The built-in SRR interface allows wireless communication of
the MP5, MP5T and MP5SC monitors with an IntelliVue
Instrument Telemetry Transceiver.
23
Page 24
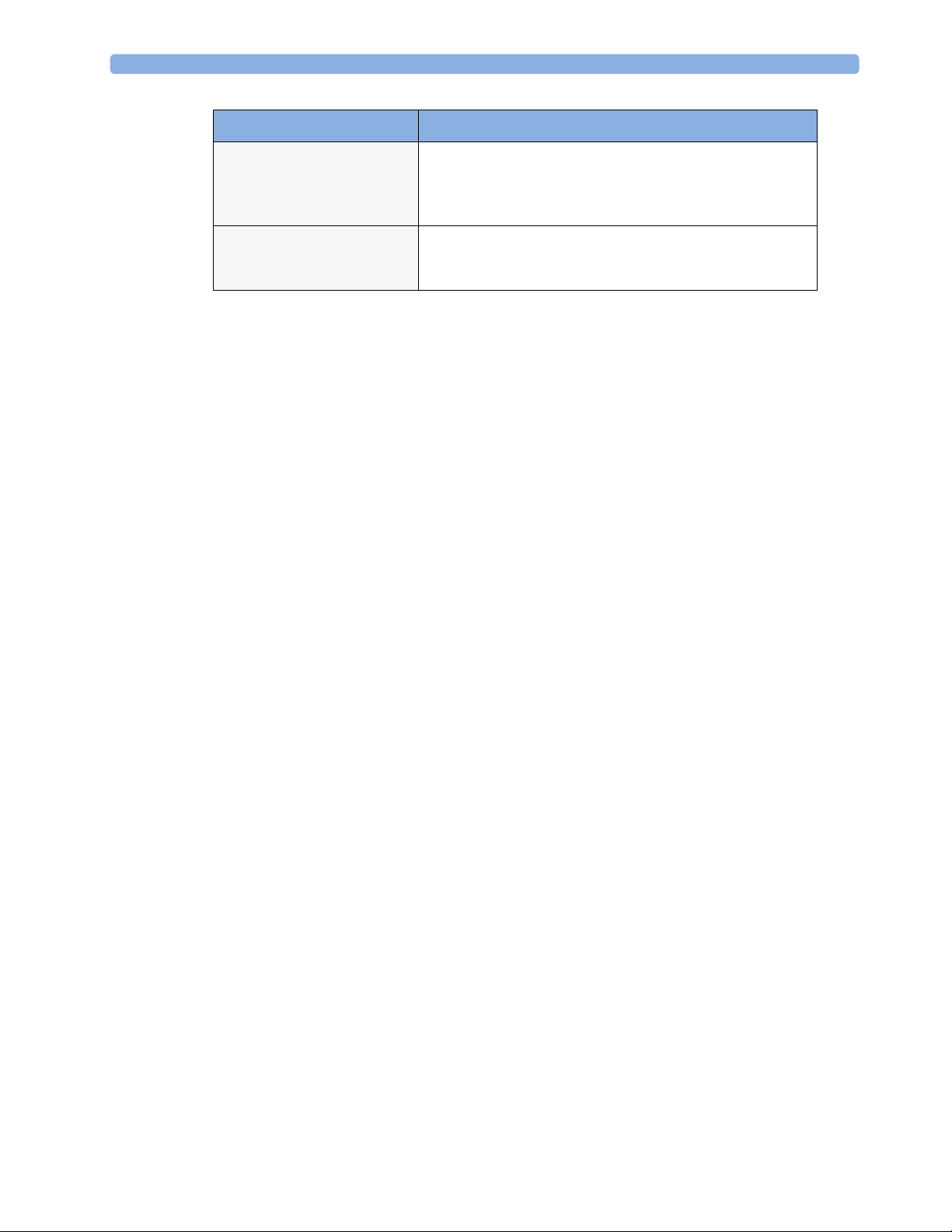
2 Theory of Operation
Functional Block Description
MSL
(not for MP5T and MP5SC)
USB Interface
(not for MP5T)
All components of the monitoring system communicate using
an IEEE802.3/ Ethernet LAN in the Measurement Link
(MSL). This network is used to distribute data between the
components
The USB interface allows connection of USB devices (Mouse,
Keyboard, Barcode Scanner) to the monitor. Note that USB
printers are not supported.
24
Page 25

3Testing and Maintenance
Introduction
This chapter provides a checklist of the testing and maintenance procedures to ensure the performance
and safety of the monitor.
These tests must be performed only by qualified personnel certified by the responsible organization.
Qualifications required are: training on the subject, knowledge, experience and acquaintance with the
relevant technologies, standards and local regulations. The personnel assessing safety must be able to
recognize possible consequences and risks arising from non-conforming equipment.
All recurring safety and performance assurance tests must be performed under equal environmental
conditions to be comparable.
Preventive Maintenance refers specifically to the series of tests required to make sure the measurement
results are accurate. The accuracy and performance procedures are designed to be completed as
specified in the following sections or when readings are in question.
3
For detailed instructions on the maintenance and cleaning of the monitor and its accessories, see Care
and Cleaning, Using Batteries and Maintenance and Troubleshooting in the monitor's Instructions for Use.
Terminology and Definitions
The following terms and definitions are used throughout this chapter and taken from the international
standards IEC 60601-1, IEC 60601-1-1 and IEC 62353.
• Medical System: a medical electrical system is a combination of at least one medical electrical
device and other electrical equipment, interconnected by functional connection or use of a
multiple portable socket-outlet.
• Patient Environment: any area in which intentional or unintentional contact can occur between
the patient and parts of the medical system or between the patient and other persons who have
had contact with parts of the medical system. The patient environment is defined anywhere within
1.5m (5 feet) of the perimeter of the patient's bed and 2.5m (8.2 feet) from the floor.
• Separation Device/Transformer: a component or arrangement of components with input parts
and output parts that, for safety reasons, prevent a transfer of unwanted voltage or current
between parts of a medical system.
• Multiple Portable Socket-Outlet: a combination of two or more socket-outlets intended to be
connected to or integrated with flexible cables or cords, which can easily be moved from one place
to another while connected to the power mains.
• Functional Connection: an electrical connection for transfer of signals and/or power.
25
Page 26
3 Testing and Maintenance
• Tests: Safety or Performance Assurance test procedures which may consist of several steps.
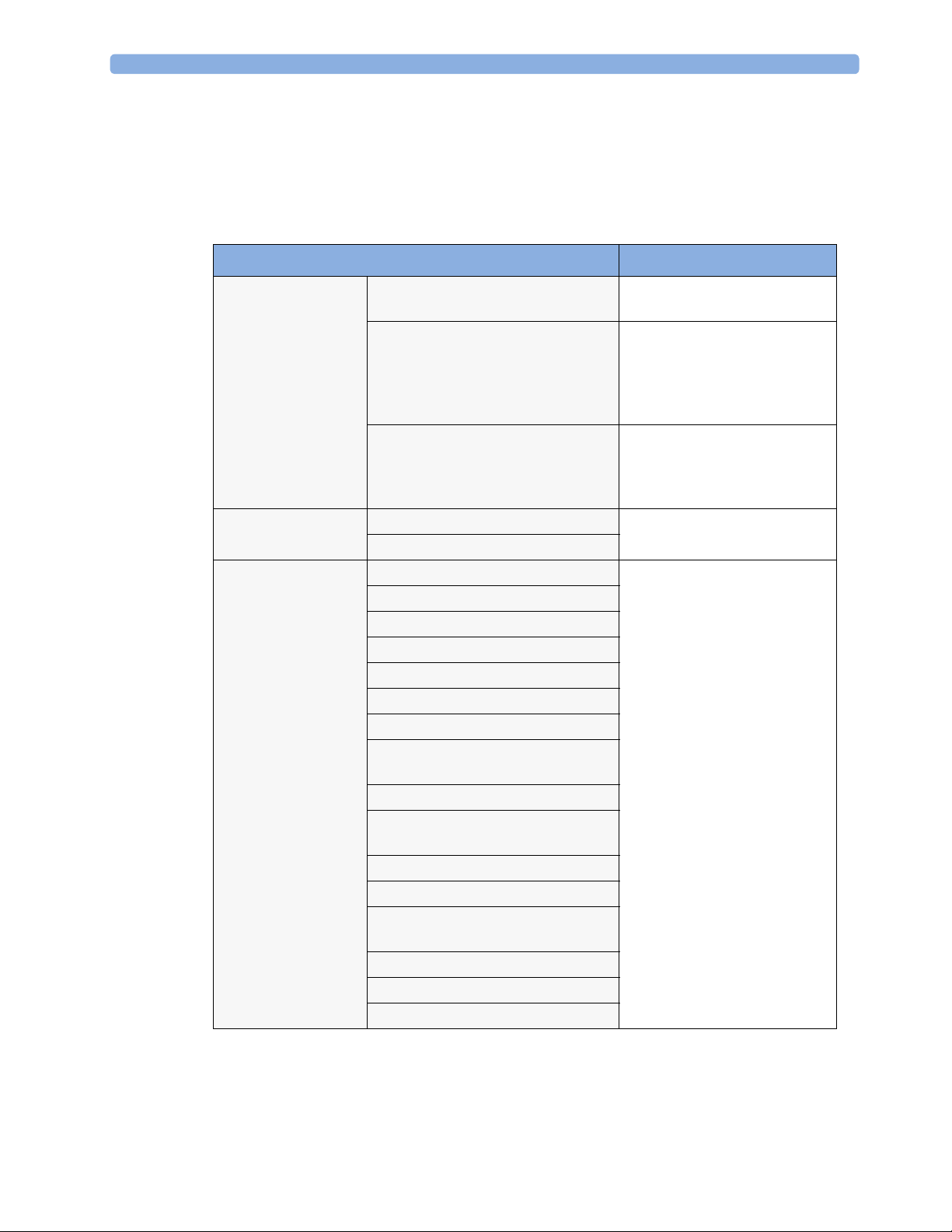
Recommended Frequency
Perform the procedures as indicated in the suggested testing timetable. These timetable
recommendations do not supersede local requirements.
Test s Frequency
Preventive
Maintenance
Other Regular Tests
Performance
Assurance Tests
NBP Performance Once every two years, or more
often if specified by local laws.
Microstream CO2 Calibration
1
Once a year or after 4000 hours
of continuous use and following
any instrument repairs or the
replacement of any instrument
parts.
Tympanic Temperature Calibration Once a year. If the unit is
dropped or damaged, check it
and calibrate it before further
use.
Visual Inspection Before each use.
Power On Test
ECG/Resp Performance Once every two years, or if you
ECG Out Sync Performance
ECG Sync Pulse Performance
SpO2 Performance
NBP Performance
Invasive Pressure Performance
Temperature Accuracy
1,2
1,2
1,2
1,2
suspect the measurement is
incorrect, except Mainstream
CO2 Accuracy Check,
Sidestream CO2 Accuracy Check
and Flow Check and Predictive
Temperature Accuracy Check required once a year.
Predictive Temperature Accuracy
Check
Mainstream CO2 Accuracy Check
1,2
Sidestream CO2 Accuracy Check and
Flow Check
Microstream CO2 Performance Test
Nurse Call Relay Performance
1,2
1
1
Power Loss Alarm Buzzer
Performance
MSL Assurance Test
1,2
Mounting Integrity Test
Battery Performance
26
Page 27

3 Testing and Maintenance
Test s Frequency
Safety
Test s
1
These tests do not apply for MP5T.
2
These tests do not apply for MP5SC
Visual
Electrical
Visual Inspection After each service event
Protective Earth Once every two years and after
Equipment Leakage Current
Patient Leakage Current
System Test Once every two years
When to Perform Tests
This table tells you when to perform specific tests.The corresponding test procedures are described in
the following sections All tests listed below must be performed on the monitor.
Service Event
(When performing...
Installation
Installation of a monitor in combination with a
medical or non-medical device connected to the
same multiple socket outlet.
Installation of a standalone monitor with no
display connected to the video output
Installation of a monitor with a medical display
specified by Philips
Installation of a monitor with an off-the-shelf
display (non-compliant with IEC60601-1)
Installation of a monitor with IntelliVue G1/
G5, connected to separate mains sockets.
Installation of monitor with IntelliVue
Instrument Telemetry (IIT)
Installation of a monitor with IT equipment e.g.
PC connected via a functional connection e.g.
Centronics or USB.
Installation of monitor with IntelliVue 802.11
Bedside Adapter
Installation of a monitor with Short Range
Radio (SRR)
Installation of networked monitor (LAN) Perform Visual Inspection and Power On Test
repairs where the power supply
has been removed or replaced or
the monitor has been damaged
by impact.
Tests Required
...Complete these tests)
Perform Visual Inspection, Power On and System
Tests
Perform Visual Inspection and Power On Test
Perform Visual Inspection and Power On Test
Perform Visual Inspection, Power On and System
Test
Perform Visual Inspection and Power On Tests
Perform Visual Inspection, Power On and IIT
communication test
Perform Visual Inspection, Power On and System
Tests
Perform Visual Inspection, Power On and
IntelliVue 802.11 Bedside Adapter
Communication Test
Perform Visual Inspection, Power On and SRR
communication test
27
Page 28
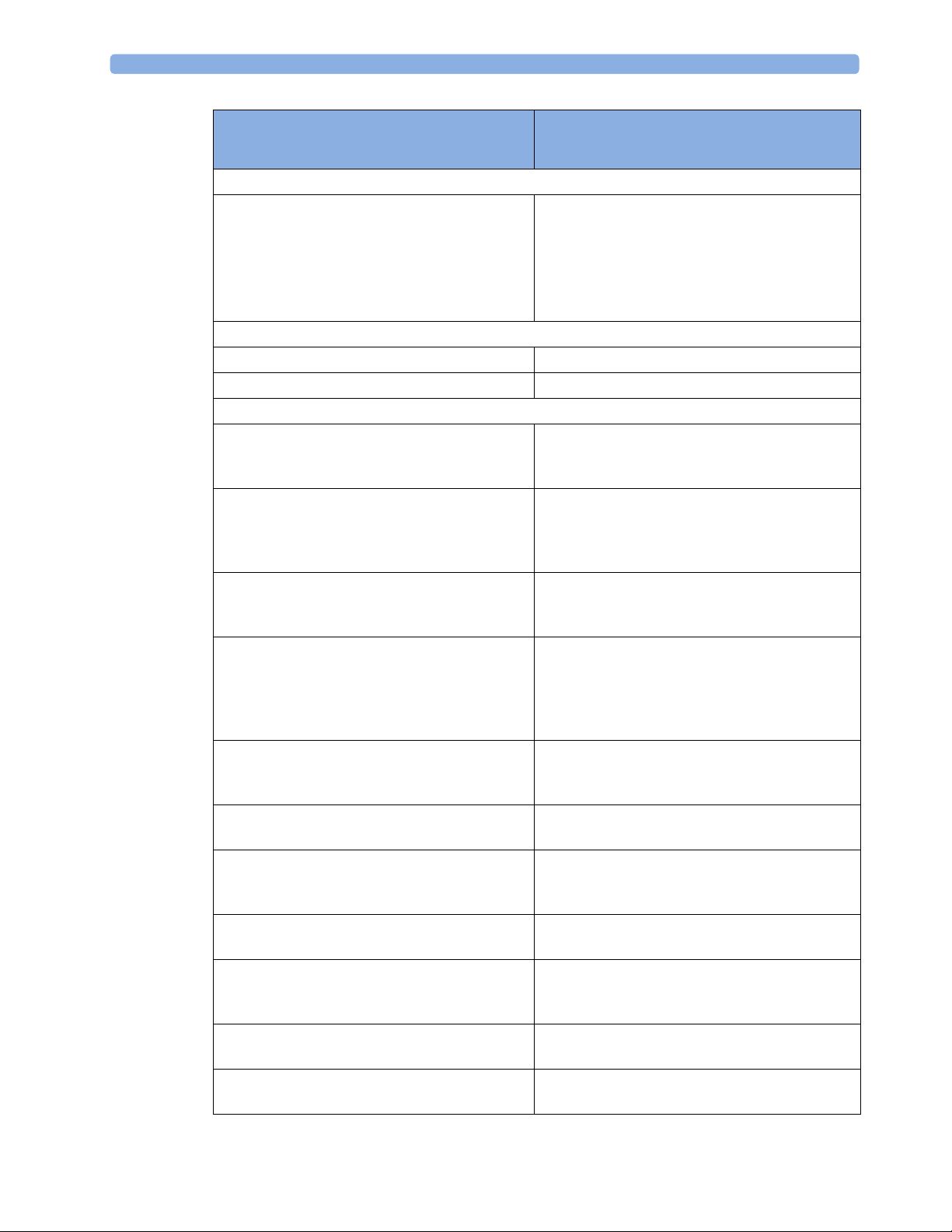
3 Testing and Maintenance
Service Event
(When performing...
Tests Required
...Complete these tests)
Preventive Maintenance
Preventive Maintenance* Perform preventive maintenance tests and
procedures:
• NBP calibration
• Microstream CO2 calibration
• Tympanic Temperature Calibration
Other Regular Tests and Tasks
Visual Inspection Perform Visual Inspection
Power On Test Perform Power On test
Repairs
Repairs where the monitor has been damaged by
impact, liquid ingression, fire, short circuit or
Perform Visual Inspection, Power On, all Safety
Tests and Full Performance Assurance Tests
electrical surge.
Repairs where the power supply, the mains
socket or an interface board is removed or
Perform Visual Inspection, Power On, all Safety
Tests and Basic Performance Assurance Test
replaced or the protective earth ground
connection is disrupted.
Repairs where the main board has been replaced. Perform Visual Inspection, Power On, Basic
Performance Assurance Test and NBP Accuracy
Test and Calibration.
Repairs where the measurement block has been
removed or replaced
Perform Visual Inspection, Power On, all Safety
Tests and Basic Performance Assurance Test.
If a certain parameter seems suspicious, perform
Full Performance Assurance Test for this
parameter.
Repairs where the NBP pump has been replaced Perform Visual Inspection, Power On, all Safety
Tests, Basic Performance Assurance Test and NBP
Performance Test and Calibration
Repairs of IntelliVue Instrument Telemetry (IIT)
Module
Perform Visual Inspection, Power On Test Block
and IIT communication test
Repairs of IntelliVue 802.11 Bedside Adapter Perform Visual Inspection, Power On and
IntelliVue 802.11 Bedside Adapter
Communication Test
Repairs of Short Range Radio (SRR) Interface Perform Visual Inspection, Power On and SRR
Communication Test
Repairs of the IntelliVue G1/G5 Perform Basic Performance Assurance Test. For
further testing requirements, see IntelliVue G1/
G5 Service Guide
Repairs where the Quick Mount has been
Perform Mounting Integrity Test
disassembled
All other IntelliVue Monitoring System repairs
(except when power supply is removed)
Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test
28
Page 29
3 Testing and Maintenance
Service Event
(When performing...
Tests Required
...Complete these tests)
Performance Assurance
Basic Performance Assurance Perform basic performance assurance tests for the
respective monitoring system component.
Full Performance Assurance Perform all accuracy and performance test
procedures listed in the following sections. If a
particular measurement is in question, perform the
measurement performance test only.
Upgrades
Software Upgrades Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test unless
otherwise specified in the Upgrade Installation
Notes shipped with the upgrade.
Hardware Upgrades Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test unless
otherwise specified in the Upgrade Installation
Notes shipped with the upgrade.
Hardware Upgrades where IntelliVue
Instrument Telemetry (IIT) is installed
Perform Visual Inspection, Power On Test, Basic
Performance Assurance Test and IIT
communication Test
Hardware Upgrades where IntelliVue 802.11
Bedside Adapter is installed
Perform Visual Inspection, Power On Test, Basic
Performance Assurance Test and IntelliVue 802.11
Bedside Adapter Communication Test
Hardware Upgrades where Short Range Radio
(SRR) is installed
Perform Visual Inspection, Power On Test, Basic
Performance Assurance Test and SRR
communication Test
Installation of Interfaces or Hardware Upgrades
where the power supply or parameter boards
Perform Visual Inspection, Power On Test, Basic
Performance Tests and all Safety Tests
need to be removed.
Combining or Exchanging System
Components (non-medical equipment
Perform the System Test for the respective system
components
connected to an IntelliVue monitor or medical
system equipment operated on a multiple socket
outlet)
NOTE
It is the responsibility of the facility operator or their designee to obtain reference values for recurring
safety and system tests. These reference values are the results of the first test cycles after an installation.
You may also purchase this service from Philips.
29
Page 30
3 Testing and Maintenance
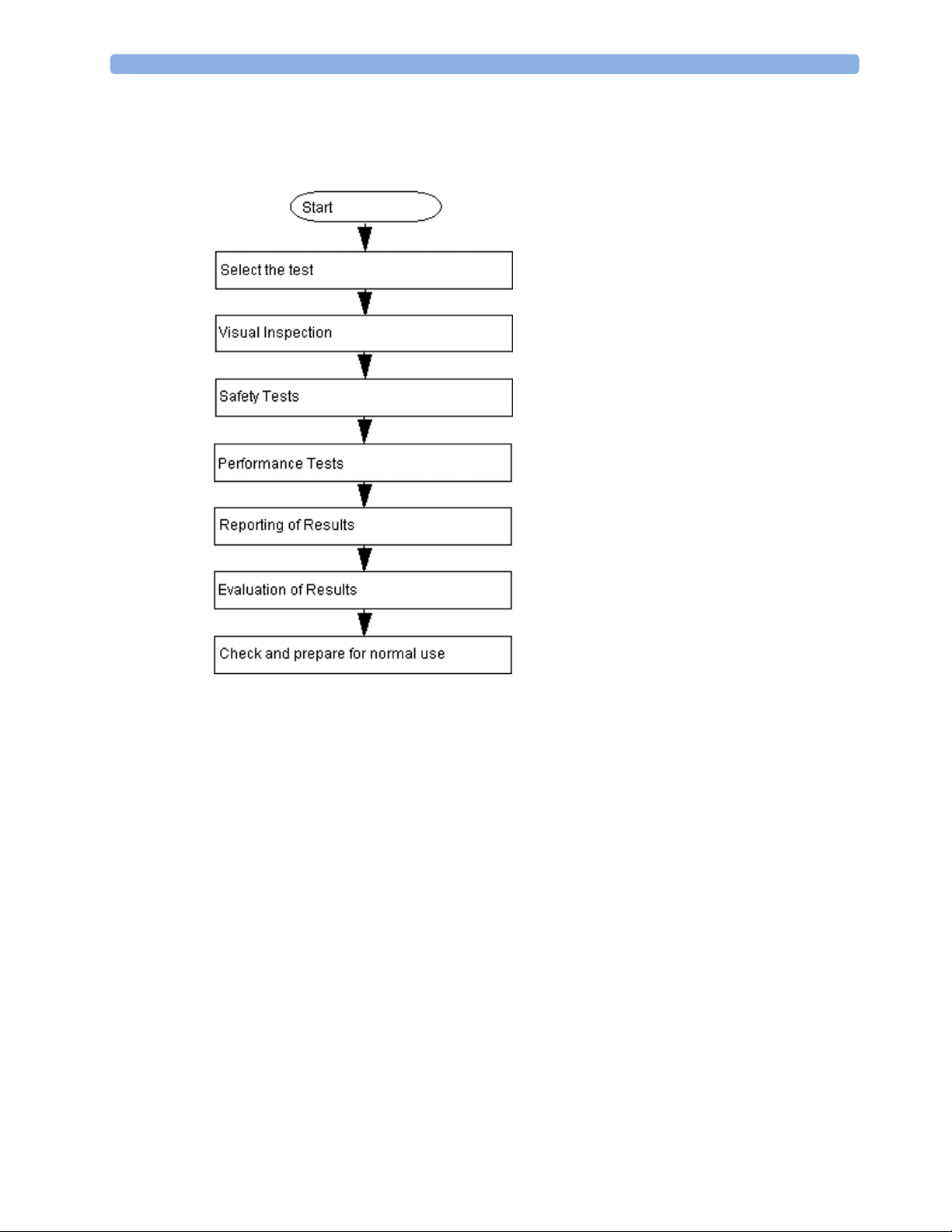
Testing Sequence
Summary of the recommended sequence of testing:
NOTE
If any single test fails, testing must be discontinued immediately and the device under test must be
repaired or labeled as defective.
Visual Inspection
Before Each Use
Check all exterior housings for cracks and damage. Check the condition of all external cables,
especially for splits or cracks and signs of twisting. If serious damage is evident, the cable should be
replaced immediately. Check that all mountings are correctly installed and secure. Refer to the
instructions that accompany the relevant mounting solution.
After Each Service, Maintenance or Repair Event
Ensure all fuses accessible from the outside comply with the manufacturer’s specification.
Check:
• the integrity of mechanical parts, internally and externally.
• any damage or contamination, internally and externally
30
Page 31

• that no loose parts or foreign bodies remain in the device after servicing or repair.
• the integrity of all relevant accessories.
Power On Test
1 Connect the monitoring system to mains and switch it on. This includes connected displays and
gas analyzers.
2 Make sure that all steps listed in the table Initial Instrument Boot Phase in the Troubleshooting section
are completed successfully and that an ECG wave appears on the screen.
The expected test result is pass: the monitor boots up and displays an ECG wave. The wave might be
a flat line if no simulator is attached.
Safety Tests
Safety tests are comprised of the following tests performed on the monitoring system:
• protective earth resistance
• equipment leakage current
• applied part leakage current
3 Testing and Maintenance
• system test (if applicable)
Safety test requirements are set according to international standards, their national deviations and
specific local requirements. The safety tests detailed in this Service Guide are derived from
international standards but may not be sufficient to meet local requirements. We recommend that you
file the results of safety tests. This may help to identify a problem early particularly if the test results
deteriorate over a period of time.
Each individual piece of equipment which has its own connection to mains or which can be connected
or disconnected from mains without the use of a tool must be tested individually. The monitoring
system as a whole must be tested according to the procedure described in “System Test” on page 55.
Accessories which can affect the safety of the equipment under test or the results of the safety test
must be included in the tests and documented.
Warnings, Cautions, and Safety Precautions
• These tests are well established procedures of detecting abnormalities that, if undetected, could
result in danger to either the patient or the operator.
• Disconnect the device under test from the patient before performing safety tests.
• Disconnect the device under test from mains before performing safety tests. If this is not possible,
ensure that the performance of these tests does not result in danger to the safety analyzer operator,
patients or other individuals.
• Test equipment (for example, a Safety Analyzer) is required to perform the safety tests. Please refer
to Annex C of IEC/EN 62353 for exact requirements for the measurement equipment and for
measurement circuits for protective earth resistance and leakage currents. Refer to the
documentation that accompanies the test equipment. Only certified technicians should perform
safety testing.
• The consistent use of a Safety Analyzer as a routine step in closing a repair or upgrade is
emphasized as a mandatory step to maintain user and patient safety. You can also use the Safety
31
Page 32

3 Testing and Maintenance
Analyzer as a troubleshooting tool to detect abnormalities of line voltage and grounding plus total
current loads.
• During safety testing, mains voltage and electrical currents are applied to the device under test.
Ensure that there are no open electrical conductive parts during the performance of these tests.
Avoid that users, patients or other individuals come into contact with touch voltage.
• For Europe and Asia/Pacific, the monitor complies with:
IEC 60601-1:1988 + A1:1991 + A2:1995(Ed.2); EN60601-1:1990 + A1:1993 + A2:1995(Ed.2);
IEC 60601-1-1:2001; EN 60601-1-1:2001; IEC 60601-1-2:2001+A1:2004; EN 60601-12:2001+A1:2006.
For USA, the monitor complies with:
UL60601-1:2003
For Canada, CAN/CSA C22.2#601.1-M90+S1+A2
• Local regulations supersede the testing requirements listed in this chapter.
• If a non-medical electrical device is connected to a medical electrical device, the resulting medical
electrical system must comply IEC 60601-1-1:2000/ EN 60601-1-1:2001 or IEC 60601-1:2005/
EN 60601-1:2006+A1:2012 (Ed.3) Section 16 "ME Systems"
• Perform safety tests as described on the following pages.
Safety Test Procedures
Use the test procedures outlined here only for verifying and recording the initial values prior to or at
installation, safe installation or service of the product, and for periodic recurrent testing. The setups
used for these tests and the acceptable ranges of values are derived from local and international
standards but may not be equivalent. These tests are not a substitute for local safety testing where it is
required for an installation or a service event. If using an approved safety tester, perform the tests in
accordance with the information provided by the manufacturer of the tester and in accordance with
your local regulations, for example IEC/EN 60601-1, UL60601-1 (US), IEC/EN 62353, and IEC/EN
60601-1-1. The safety tester should print results as detailed in this chapter, together with other data.
Please refer to Annex C of IEC/EN 62353 for requirements for the measurement equipment and for
measurement circuits for protective earth resistance and leakage currents.
The following symbols are used in the diagrams illustrating the safety tests:
Supply mains Protective earth
L, N Supply mains terminals PE Protective earth terminal
Mains part Applied part
32
Page 33

3 Testing and Maintenance
F-type applied part Measuring device
Resistance measuring device Connection to accessible
conductive parts
......... Optional connection
CAUTION
After each service, maintenance or repair event:
Ensure all fuses accessible from the outside comply with the manufacturer’s specification.
Check:
• the integrity of mechanical parts, internally and externally.
• any damage or contamination, internally and externally.
• that no loose parts or foreign bodies remain in the device after servicing or repair.
• the integrity of all relevant accessories.
Hints for Correct Performance of Safety Tests
• Perform a visual inspection on all detachable power cords used with the monitoring system and
include these in all safety test procedures.
• Connection lines such as data lines or functional earth conductors may appear to act like protective
earth connections. These may lead to incorrect measurements and need to be considered during
testing. If necessary, unplug these connections.
• During measurements, the device under test shall be isolated from earth (e.g. test on an insulated
work bench), except the protective earth conductor in the power supply cord.
• Position all cables and cords in such a manner that they do not influence the safety tests.
• Measurement of insulation resistance is not required.
• When testing a medical electrical system, where possible, test it such that potential ground voltage
variations are present as they may be during actual use.
Guideline for Performance of Safety Tests
This section introduces the general principle of performing recurrent safety tests. Product specific test
descriptions are described in the following sections.
Connect the detachable power cord of the device under test to the safety analyzer's test mains port.
Connect the enclosure test lead of the safety analyzer to the enclosure of the device under test, e.g. to
33
Page 34

3 Testing and Maintenance
the equipotential connector or unearthed conductive accessible parts where applicable during
Equipment Leakage Current Tests and Applied Part Leakage Current Tests. For testing the applied
part leakage current, connect all applied parts to the safety analyzer using the appropriate patient lead
or adapter cable. For the ECG parameter all ten ECG-leads need to be connected to the safety
analyzer. If necessary, use an adapter cable to connect all ten ECG-leads. If necessary, repeat the safety
test procedure until all available applied parts have been tested. Refer to the documentation that
accompanies the safety analyzer for further details on how to set up and perform the test.
Protective Earth Resistance Test - Setup Example
NOTE
The test lead needs to go to parts that require protective earthing. This may be a single connection or
several tested after each other
Equipment Leakage Current Test - Setup Example
NOTE
The test lead needs to go to the grounded enclosure parts, the ungrounded enclosure parts and all of
the applied parts connected together.
34
Page 35

Applied Part Current Test - Setup Example
NOTE
The above graphics resemble the Metron QA-90 setup and are protected by copyright. Copyright
owned by Fluke (Metron).
Safety Test Adapter Cable - Schematics
The following graphics provide schematics of safety test (patient lead) adapter cables which can be
used for electrical safety testing. These schematics can also be used as a guideline for making your own
safety test adapter cables. Alternatively, other methods to make safety test adapter cables can be used,
e.g. using a modified accessory cable.
3 Testing and Maintenance
NOTE
You may not need all of the cables displayed below for electrical safety testing of your respective
monitor.
35
Page 36

3 Testing and Maintenance
ECG
SpO2 (X2 #A01-#A04, M3001AL #A01-#A04, MP2/MP5 #SP1-#SP4)
36
Page 37

SpO2 (M1020A)
3 Testing and Maintenance
37
Page 38

3 Testing and Maintenance
Masimo rainbow SET (X2 #A05, M3001AL #A05, MP2/MP5 #SP5)
38
Page 39

Invasive Pressure
Invasive Pressure (M1006B #C01)
3 Testing and Maintenance
Temperature
39
Page 40

3 Testing and Maintenance
CO2 (MP5, M3014A)
CO2 (M1016A, M3016A)
40
4 = all resistors 120 KOhm
Page 41

Cardiac Output
3 Testing and Maintenance
BIS
Use Clamp Adapter Cable and BIS sensor simulator (P/N: M1034-61650, 453563233731).
41
Page 42

3 Testing and Maintenance
VueLink
4 = 220 Ohm
42
Page 43

IntelliBridge
3 Testing and Maintenance
EEG
43
Page 44

3 Testing and Maintenance
SvO2 (M1021A)
44
Page 45

ScVO2 (M1011A)
3 Testing and Maintenance
45
Page 46

3 Testing and Maintenance
tcpO2/tcpCO2
46
Page 47

MP5 Predictive Temperature
3 Testing and Maintenance
Tympanic Temperature
47
Page 48

3 Testing and Maintenance
MP5 TAAP
TcG10
48
Page 49

S(1): Protective Earth Resistance Test
Test to perform:
3 Testing and Maintenance
Measuring circuit for the measurement of Protective Earth Resistance in medical electrical
equipment that is disconnected from the supply mains.
This measures the impedance of the Protective Earth (PE) terminal to all exposed metal parts of the
Device under Test (DUT), which are for safety reasons connected to the Protective Earth (PE).
You can find metal parts of the device at the equipotential connector.
Measurements shall be performed using a measuring device capable to deliver a current of at least
200 mA into 500 mOhms with maximum open circuit voltage of 24V
This safety test is based on IEC/EN 62353.
Report the highest value (X1).
Test Expected test results
Protective Earth Resistance Test (with
X1 <= 300mOhms
mains cable)
NOTE
• If the protective earth resistance test fails, testing must be discontinued immediately and the device
under test must be repaired or labeled as defective.
• All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
• Flex the power cord during the protective earth resistance test to evaluate its integrity. If it does
not pass the test, exchange the power cord. Then repeat the test. If it still does not pass, follow the
instructions in the first bullet point of this note above.
49
Page 50

3 Testing and Maintenance
S(2): Equipment Leakage Current Test - Normal Condition
Test to perform:
Measuring circuit for the measurement of Equipment Leakage Current - Direct method
according to IEC/EN 62353.
This test measures leakage current of accessible conductive and non-conductive metal parts of the
monitor and the functional earth leakage current. It tests normal and reversed polarity. Perform the
test with S1 closed (Normal Condition).
There are no parts of the equipment that are not protectively earthed. Disconnect any data cables and
any connections that may provide an extraneous earth path. Test the device under test (DUT) on an
insulated surface. Do not touch the DUT during testing.
This safety test is based on IEC/EN 62353.
Report the highest value (X1).
Test Expected test results
Equipment Leakage Current Test
X1 <= 100μA
(Normal Condition - with mains cable)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
50
Page 51

S(3): Equipment Leakage Current Test - Single Fault Condition
Test to perform:
Measuring circuit for the measurement of Equipment Leakage Current - Direct method
according to IEC/EN 62353.
3 Testing and Maintenance
This test measures leakage current of accessible conductive and non-conductive metal parts of the
monitor and the functional earth leakage current. It tests normal and reversed polarity. Perform the
test with S1 open (Single Fault Condition).
There are no parts of the equipment that are not protectively earthed. Disconnect any data cables and
any connections that may provide an extraneous earth path. Test the device under test (DUT) on an
insulated surface. Do not touch the DUT during testing.
This safety test is based on IEC/EN 62353.
Report the highest value (X2).
Test Expected test results
Equipment Leakage Current Test (Single
Fault Condition - with mains cable)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
X2 <= 300μA
S(4): Applied Part Leakage Current - Mains on Applied Part
NOTE
During measurement of the Applied Part Leakage Current it is possible that the measured current can
exceed the allowed limit (per IEC/EN 60601-1 or IEC/EN 62353).
This can occur when the safety tester is connected to the invasive blood pressure and temperature
connectors at the same time during the applied leakage current measurement.
The connectors for the invasive blood pressure and temperature are independently functioning
connectors.
51
Page 52

3 Testing and Maintenance
Although there are individual connectors on the front end, internally those parameters use the same
electrical insulation interface and are hardwired to each other. This results in an electrical short of
those connectors during measurement if a test current is applied simultaneously. Therefore this should
be avoided.
Due to the combined insulation interface, it is sufficient to connect to only one parameter interface
(that is, Invasive Blood Pressure or Temperature) of the invasive blood pressure/temperature
measurement block. This avoids a short and the potential of exceeding the limit for the current.
Test to perform:
52
Measuring circuit for the measurement of Applied Part Leakage Current - Direct method
according to IEC/EN 62353.
This test measures applied part leakage current from applied part to earth caused by external main
voltage on the applied part. Each polarity combination possible shall be tested. This test is applicable
for ECG measurement inputs.
There are no parts of the equipment that are not protectively earthed.
This safety test is based on IEC/EN 60601-1, IEC/EN 62353, UL2601-1 Ed. 2/UL60601-1:2003 and
CSA 601.1-M90.
Report the highest value. (X1).
Test Expected test results
Applied Part Leakage Current Test
X1 <= 50μA (CF)
(Single Fault Condition - mains on
applied part)
Page 53

3 Testing and Maintenance
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
Applied Part Leakage Current - Mains on Applied Part for Tympanic Thermometer on Standalone Base Station
1 Remove the interface and the thermometer cable from the base station.
2 Insert the two safety test cables as shown below.
Cable A (IntelliBridge - 453564127781) and Cable B (Tympanic Temperature - 453564421601)
1 Connect to an appropriate safety tester (e.g. Fluke ESA 620) as shown below.
53
Page 54

3 Testing and Maintenance
2
Perform the test:
Measuring circuit for the measurement of Applied Part Leakage Current - Direct method
according to IEC/EN 62353
This test measures applied part leakage current from applied part to earth caused by external main
voltage on the applied part. Each polarity combination possible shall be tested. There are no parts of
the equipment that are not protectively earthed. This safety test is based on IEC/EN 60601-1, IEC/
EN 62353, UL2601-1 Ed. 2/UL60601-1:2003 and CSA 601.1-M90. Report the highest value. (X1).
Test Expected test results
Applied Part Leakage Current test (Single Fault
X1 <= 5000 μA (BF)
Condition - mains on applied part)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
Reference: Allowable Values for IEC 60601-1:1998 and UL 60601-1 Measurements
Protective Earth resistance (between the PROTECTIVE EARTH TERMINAL and any
ACCESSIBLE METAL PART which is PROTECTIVELY EARTHED, w/o power
cord):100mOhms
Protective Earth resistance of power cord: 100mOhms
Enclosure leakage current (IEC 60601-1 and UL60601-1): 100 A (N.C.)
Enclosure leakage current:(IEC 60601-1): 500 A (S.F.C)
Enclosure leakage current (UL 60601-1): 300 A (S.F.C)
Patient leakage current: (IEC 60601-1 and UL60601-1): 100 A (N.C.) for BF
54
Patient leakage current: (IEC 60601-1 and UL60601-1): 500 A (S.F.C.) for BF
Patient leakage current: (IEC 60601-1 and UL60601-1): 10 A (N.C.) for CF
Patient leakage current: (IEC 60601-1 and UL60601-1): 50 A (S.F.C.) for CF
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated
Page 55

Insulation Resistance
It is not recommended to perform measurements of the insulation resistance. Refer to IEC 62353 for
details about methods of the insulation resistance measurement.
System Test
After mounting and setting up a system, perform system safety tests according to IEC/EN 60601-1-1.
What is a Medical Electrical System?
A medical electrical system is a combination of at least one medical electrical piece of equipment and
other electrical equipment, interconnected by functional connection or use of a multiple portable
socket-outlet.
• Devices forming a medical electrical system must comply either with IEC/EN 60601-1-1 or IEC/
EN 60601-1+A1 Ed.3 clause 16.
• Any electrical device such as IT equipment that is connected to the medical electrical equipment
must comply either with IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16 and be
tested accordingly.
• Non-medical electrical equipment may require connection through a separating device (e.g. an
isolation transformer).
3 Testing and Maintenance
General Requirements for a System
After installation or subsequent modification, a system must comply with the requirements of the
system standard IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16. Compliance is checked
by inspection, testing or analysis, as specified in the IEC/EN 60601-1-1 or in this book.
Medical electrical equipment must comply with the requirements of the general standard IEC/EN
60601-1, its relevant particular standards and specific national deviations. Non-medical electrical
equipment shall comply with IEC safety standards that are relevant to that equipment.
Relevant standards for some non-medical electrical equipment may have limits for equipment leakage
currents higher than required by the standard IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3
clause 16. These higher limits are acceptable only outside the patient environment. It is essential to
reduce equipment leakage currents to values specified in IEC/EN 60601-1 when non-medical
electrical equipment is to be used within the patient environment.
55
Page 56

3 Testing and Maintenance
System Example
This illustration shows a system where both the medical electrical equipment and the non-medical
electrical equipment are situated at the patient’s bedside.
WARNING
• Do not use additional AC mains extension cords or multiple portable socket-outlets. If a multiple
portable socket-outlet is used, the resulting system must be compliant with IEC/EN 60601-1-1 or
IEC/EN 60601-1+A1 Ed.3 clause 16. Do not place multiple socket-outlets on the floor. Do not
exceed the maximum permitted load for multiple socket-outlets used with the system. Do not plug
additional multiple socket outlets or extension cords into multiple socket outlets or extension
cords used within the medical electrical system.
• Do not connect any devices that are not supported as part of a system.
• Do not use a device in the patient vicinity if it does not comply with IEC/EN 60601-1 or IEC
60601-1 edition 3 clause 16. The whole installation, including devices outside of the patient
vicinity, must comply with IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16. Any nonmedical device placed and operated in the patient’s vicinity must be powered via a separating
transformer (compliant with IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16) that
ensures mechanical fixing of the power cords and covering of any unused power outlets.
System Installation Requirements
• Ensure that the medical electrical system is installed in a way that the user achieves optimal use.
• Make sure the user is informed about the required cleaning, adjustment, sterilization and
disinfection procedures listed in the Instructions for Use.
• The medical electrical system must be installed in such a way that the user is able to carry out the
necessary cleaning, adjustment, sterilization and disinfection procedures listed in the Instructions
for Use.
56
Page 57

3 Testing and Maintenance
• Ensure that the medical electrical system is installed in a way that an interruption and restoration
of power to any part of the medical electrical system does not result in a safety hazard.
• We recommend using fixed mains socket outlets to power the medical system or parts thereof.
Avoid using multiple portable socket-outlets.
• Any multiple portable socket outlets used must be compliant with IEC 60884-1 and IEC/EN
60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16.
• Ensure that any part of the system connected to multiple portable socket-outlets is only removable
with a tool, i.e. the multiple portable socket-outlet provides a locking mechanism to prevent power
cords from being plugged or unplugged unintentionally. Otherwise, the multiple portable socketoutlet must be connected to a separation device. Multiple Socket Outlets used within the medical
electrical system must only be used for powering medical electrical equipment which is part of the
system.
• Ensure that any functional connections between parts of the medical electrical system are isolated
by a separation device according to IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16
to limit increased equipment leakage currents caused by current flow through the signal
connections where necessary (e.g. leakage current coming from non-medical electrical equipment
into medical electrical equipment or building ground voltage differences providing leakage current
through grounded data cables). This only works if the equipment leakage current of the respective
medical electrical system parts is not exceeded under normal conditions. This isolation is especially
important where the non-medical electrical equipment leakage currents can pass to the medical
electrical equipment in the system or building ground voltage differences can pass to the medical
electrical equipment via ground in a data cable connection in the system
• Avoid increase of equipment leakage currents when non-medical electrical equipment within the
medical electrical system is used. This only applies when if the equipment leakage current of the
respective medical electrical system parts is not exceeded under normal conditions. Use of an
additional protective earth connection, separation device or additional non-conductive enclosures
are options that can prevent a problem.
• Within the patient environment it is important to limit electrical potential differences between
different parts of a system. If necessary, use potential equalization equipment (equipotential cable)
or additional protective earth connections.
• Medical electrical equipment used in medical rooms must be connected to potential equalization
equipment (equipotential cable) to avoid electrical potential differences. Check your local
requirements for details.
Required Protective Measures at System Installation
For any IT equipment (IEC60950-1) operated in the patient environment ensure that the equipment
leakage current does not exceed the limits described in IEC 60601-1. Use a separation device to ensure
compliance. After installation of IT equipment in the patient environment, an equipment leakage
current test is required.
57
Page 58

3 Testing and Maintenance
Case 1: Medical Device Combined with Medical Device
If you combine a medical device with another medical device (incl. Philips specified displays) to form a
medical electrical system according to IEC60601-1-1 or IEC/EN 60601-1 edition 3 clause 16, no
additional protective measures are required. The medical electrical devices may be located in or outside
the patient vicinity in a medically used room. This is valid as long as the medical devices are connected
to separate mains outlets. No system test is required.
58
Page 59

3 Testing and Maintenance
If the combined medical devices are connected to the same multiple portable socket outlet an
enclosure leakage current test of the entire device combination on the multiple portable socket outlet is
required to ensure that the resulting protective earth leakage current and equipment leakage current
does not exceed the limits of IEC 60601-1-1 or IEC/EN 60601-1 edition 3 clause 16. Avoid using
multiple portable socket outlets. The medical electrical devices may be located in or outside the patient
vicinity in a medically used room. If the limits are exceeded, additional protective measures are
required, e.g. a separation device or the connection of each device to separate mains.
59
Page 60

3 Testing and Maintenance
Case 2: Medical Device Combined with a Non-Medical Device
If you combine a medical device with a non-medical device to form a medical electrical system
according to IEC60601-1-1 or IEC/EN 60601-1 edition 3 clause 16, additional protective measures
are required, e.g. usage of a separation device. The medical electrical devices or the IT equipment may
be located in or outside the patient vicinity in a medically used room. After system installation incl.
protective measures, a system test is required to ensure that the resulting equipment leakage current
and applied part leakage current does not exceed the limits of IEC 60601-1-1 or IEC/EN 60601-1
edition 3 clause 16.
For any IT equipment (IEC60950-1) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC 60601-1 or IEC/EN 60601-1 edition 3 clause 16.
Use a separation device to ensure compliance. After installation of IT equipment in patient vicinity, an
equipment leakage current test is required.
60
Page 61

3 Testing and Maintenance
If the combined devices forming the medical electrical system are connected to the same multiple
portable socket outlet, ensure that the resulting protective earth leakage current and equipment leakage
current do not exceed the limits of IEC 60601-1-1 or IEC/EN 60601-1 edition 3 clause 16. The
medical electrical devices or IT equipment may be located in or outside the patient vicinity in a
medically used room. Avoid using multiple portable socket outlets. If the limits of IEC 60601-1-1 or
IEC/EN 60601-1 edition 3 clause 16 are exceeded, additional protective measures are required, e.g. a
separation device or the connection of each device to separate mains.
61
Page 62

3 Testing and Maintenance
For any IT equipment (IEC60950-1) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC 60601-1 or IEC/EN 60601-1 edition 3 clause 16.
Use a separation device to ensure compliance. After installation of IT equipment in patient vicinity, an
equipment leakage current test is required.
62
Page 63

3 Testing and Maintenance
Case 3: Medical Device Combined with a Medical or Non-Medical Device with one Device in a Non-Medically-Used Room
If you combine a medical device with a medical or non-medical device to form a medical electrical
system according to IEC60601-1-1 or IEC/EN 60601-1 edition 3 clause 16 using a common
protective earth connection and one of the devices is located in a non-medically used room, additional
protective measures are required, e.g. usage of a separation device or additional protective earth
connection. The medical electrical devices or IT equipment may be located in or outside the patient
vicinity. After system installation incl. protective measures, a system test is required to ensure that the
resulting equipment leakage current does not exceed the limits of IEC 60601-1-1 or IEC/EN 60601-1
edition 3 clause 16.
63
Page 64

3 Testing and Maintenance
If you combine a medical device with a medical or non-medical device to form a medical electrical
system according to IEC60601-1-1 or IEC/EN 60601-1 edition 3 clause 16 using two separate
protective earth connections and one of the devices is located in a non-medically used room creating a
potential voltage difference, additional protective measures are required, e.g. usage of a separation
device or additional protective earth connection. The medical electrical devices or IT equipment may
be located in or outside the patient vicinity. After system installation incl. protective measures, a system
test is required to ensure that the resulting equipment leakage current does not exceed the limits of
IEC 60601-1-1 or IEC/EN 60601-1 edition 3 clause 16.
64
Page 65

3 Testing and Maintenance
System Test Procedure
If the medical electrical device has already been tested as a standalone device e.g. during factory safety
testing, an equipment leakage current test must only be performed once the device is connected to
another electrical device/system. If the medical electrical system has not been tested as a standalone
device, the device has to be tested as a standalone device (without connection to the system) and as
part of the system (with connection to the system).
Connect the detachable power cord of the device under test to the safety analyzer's test mains port.
Connect the enclosure test lead of the safety analyzer to the enclosure of the device under test as
described in the "Equipment Leakage Test" section . Refer to the documentation that accompanies the
safety analyzer for further details on how to set up the test.
Test Expected test results
Equipment Leakage Current Test
(Normal Condition)
Equipment Leakage Current Test (Single
Fault Condition)
After the testing of the device as a standalone device and as part of the system, check that the resulting
values (without connection and with connection to the system) do not differ by more than +/- 10%
from each other.
If the devices in the medical electrical system are connected to a multiple portable socket outlet the
resulting protective earth leakage current needs to be determined. All system components must be
connected to the multiple portable socket outlet and be switched on during this measurement.
Sys1 <= 100μA
Sys2 <= 300μA
Test Expected test results
Protective Earth Leakage Current of
Multiple Socket Outlets
Sys3 <= 300μA
65
Page 66

3 Testing and Maintenance
Refer to the documentation that accompanies the safety analyzer for further details on how to set up
the test.
Preventive Maintenance Procedures
Noninvasive Blood Pressure Measurement Calibration
Carry out the noninvasive blood pressure measurement performance tests at least every two years , or
as specified by local laws (whichever comes first).
Microstream CO2 Calibration
Carry out the Microstream CO2 calibration once a year or after 4000 hours of continuous use and
following any instrument repairs or the replacement of any instrument parts.
Tympanic Temperature Calibration
To verify the performance of the Tympanic Thermometer:
1 Purchase a Covidien calibration device, part number 303097. See the Covidien web site for
ordering information (www.covidien.com).
2 Follow the instructions provided with the calibration device to perform the test.
NOTE
The battery compartment in the Tympanic temperature probe is not functional.
CAUTION
After performing the calibration, check the body reference site selection of the Tympanic
Thermometer to make sure it matches the required settings for your hospital.
Performance Assurance Tests
Some of the following test procedures must be performed in service mode. To enter service mode
Operating Modes in the main menu. Then select Service Mode and enter the password.
select
If required, open the screen menu in the monitor info line at the top of the screen and select
access the service screen. This is required particularly for Anesthetic Gas Module testing procedures.
Basic Performance Assurance Test
This section describes the basic performance test procedure. Please refer to the section “When to
Perform Tests” on page 27 for detailed information on when which test procedure is required.
Service to
66
Procedure:
Power on the monitoring system and go into demo mode. Check that each parameter (incl. Gas
Analyzer) displays values.
Page 67

Full Performance Assurance Test
The following sections describe the full performance testing procedures i.e. detailed testing of each
parameter with a patient simulator or specified tools. Please refer to the section for information on
when which testing procedure is required.
ECG/Resp Performance Test
This test checks the performance of the ECG and respiration measurements.
Tools required: Patient simulator.
ECG Performance
1 Connect the patient simulator to the ECG/Resp connector on the monitor.
2 Configure the patient simulator as follows:
– ECG sinus rhythm.
– HR = 100 bpm or 120 bpm (depending on your patient simulator).
3 Check the displayed ECG wave and HR value against the simulator configuration.
4 The value should be 100bpm or 120 bpm+/- 2 bpm.
3 Testing and Maintenance
Respiration Performance
1 Change the Patient Simulator configuration to:
– Base impedance line 1500 Ohm.
– Delta impedance 0.5 Ohm.
– Respiration rate 40 rpm or 45 rpm.
2 The value should be 40 rpm +/- 2 rpm or 45 rpm +/- 2 rpm.
Test Expected test results
ECG Performance Test 100bpm +/- 2bpm or
120bpm +/- 2bpm
Respiration Performance Test 40 rpm +/- 2 rpm or
45 rpm +/- 2 rpm
ECG Out Sync Performance Test (not available via TAAP or SRR)
This test checks the performance of ECG synchronization between the monitor and a defibrillator. It
only needs to be performed when this feature is in use as a protocol at the customer site.
Tools required:
• Defibrillator with ECG Input.
• Patient simulator.
1 Connect the patient simulator to the ECG connector and the defibrillator to the ECG Sync
Output on the monitor with the ECG Sync cable.
2 Set the patient simulator to the following configuration:
– HR = 100 bpm or 120 bpm (depending on your patient simulator).
– ECG sinus rhythm.
3 Switch the defibrillator to simulation mode.
67
Page 68

3 Testing and Maintenance
4
Check that the ECG signal is displayed.
Test Expected test results
ECG Out Performance Test ECG Signal is displayed
ECG Sync Pulse Performance Test
This test checks the performance of the ECG sync pulse synchronization between the MP5 and
another medical electrical device. It only needs to be performed when this feature is used at the
customer site.
Tools required:
• other medical electrical device, e.g. CT Scanner etc.
•patient simulator
1 Connect the patient simulator to the ECG connector and the other medical electrical device to the
monitor.
2 Set the patient simulator to the following configuration:
– HR = 100 bpm or 120 bpm (depending on your patient simulator).
– ECG sinus rhythm.
3 Verify that the connected medical electrical device triggers accordingly.
Test Expected test results
ECG Sync Pulse Performance Test Connected medical electrical device
SpO2 Performance Test
This test checks the performance of the SpO2 measurement.
Procedure for Philips FAST SpO
Tools required: none
1 Connect an adult SpO
2 Measure the SpO
3 The value should be between 95% and 100%.
Test Expected test results
SpO
Performance Test 95% and 100%
2
Procedure for Nellcor OxiMax SpO
Nellcor recommends that the functionality of this parameter be verified using the SRC-MAX.
A possible performance assurance check requiring no tools would be:
1 Connect an adult SpO
2 Measure the SpO
3 The value should be between 95% and 100%.
value on your finger (this assumes that you are healthy).
2
value on your finger (this assumes that you are healthy).
2
triggers as expected
Technology:
2
transducer to the SpO2 connector.
2
Technology:
2
transducer to the SpO2 connector.
2
68
Test Expected test results
SpO
Performance Test 95% and 100%
2
Page 69

Procedure for Masimo rainbow SET Technology:
Tools required: none
3 Testing and Maintenance
1 Connect an adult SpO
2 Measure the SpO
3 The value should be between 95% and 100%.
transducer to the SpO2 connector.
2
value on your finger (this assumes that you are healthy).
2
Test Expected test results
SpO2 Performance Test 95% and 100%
In addition to the SpO
Performance Test procedure described above, the following tests are
2
recommended when using Masimo rainbow SET technology after a field repair or if the performance
of the Masimo rainbow SET board in the MMS is in question.
NOTE
The part numbers listed below are subject to change. All parts required for the tests described in this
section must be ordered directly from Masimo.
SET Tester and RRa Simulator:
1 Connect a dual patient cable (Masimo part number 3503) to the board.
2 Connect a Masimo SET tester (Masimo part number 3776) to M15 side of the dual cable.
3 Connect one end of an RRa simulator cable (Masimo part number EQ-12070) to M6 side of the
dual cable.
4 Connect the other end (3.5MM) of the RRa simulator cable (Masimo part number EQ-12070) to a
laptop or PC which contains TR19673A_Appendix_A.wav wave file.
5 Play the wave file and set up the computer per instructions in R-EQ-12070 document.
6 Verify that all enabled parameters are within specified range.
Sensor Port Test Tool:
1 Connect a round connector sensor port test tool (Masimo part number 3494) to the board.
2 Confirm that each LED turns on one at a time and then all LEDs turn on.
Shield Continuity:
1 Connect a shield continuity test cable (Masimo part number 3854) to the board.
2 Using a multimeter, measure the resistance across the red and black banana plugs. Verify that the
resistance is less than 5.
Measurement Validation
NOTE
A functional tester cannot be used to assess the accuracy of a pulse oximeter monitor or sensor.
However, it can be used to demonstrate that a particular pulse oximeter monitor reproduces a
calibration curve that has been independently demonstrated to fulfill a particular accuracy
specification.
69
Page 70

3 Testing and Maintenance
Philips FAST SpO2 Technology
The SpO
accuracy has been validated in human studies against arterial blood sample reference
2
measured with a CO-oximeter. In a controlled desaturation study, healthy adult volunteers with
saturation levels between 70% and 100% SaO
were studied. The population characteristics for those
2
studies were:
• about 50% female and 50% male subjects
• age range: 19 to 39
• skin tone: from light to dark brown
Pulse rate accuracy has been validated with an electronic pulse simulator.
Nellcor OxiMax Technology
Accuracy specifications are based on controlled hypoxia studies with healthy non-smoking adult
volunteers over the specified saturation SpO2 range(s). Pulse oximeter SpO2 readings were compared
to SaO2 values of drawn blood samples measured by hemoximetry. All accuracies are expressed as ±
"X" digits. Pulse oximeter equipment measurements are statistically distributed; about two-thirds of
pulse oximeter measurements can be expected to fall in this accuracy (ARMS) range. Because scatter
and bias of pulse oximeter SpO2 and blood SaO2 comparisons commonly increase as the saturation
decreases, and accuracy specifications are calculated from data spanning the stated range, different
accuracy values may result when describing partially overlapping ranges.
Subjects used to validate SpO2 measurement accuracies were healthy and recruited from the local
population. Comprised of both men and women, subjects spanned a range of skin pigmentations and
ranged in age from 18-50 years old.
Oxygen saturation accuracy can be affected by certain environmental, equipment, and patient
physiologic conditions (as discussed in the Instructions for Use for the monitor) that influence
readings of SpO2, SaO2, or both. Accordingly, observations of clinical accuracy may not achieve the
same levels as those obtained under controlled laboratory conditions.
Pulse rate accuracy has been validated with an electronic pulse simulator.
Masimo rainbow SET Technology:
The Masimo rainbow SET Technology with the designated sensors has been validated by Masimo
during motion and no motion conditions in human studies against an arterial blood sample reference
measured with a CO-oximeter and an ECG monitor. Pulse oximeter measurements are statistically
distributed, only about two-thirds of the measurements can be expected to fall within the specified
accuracy compared to CO-oximeter measurements.
, SpCO, and SpMet accuracy was determined by testing on healthy adult volunteers in the range
SpO
2
of 60-100% Sp0
, 0-40% SpCO, and 0-15% SpMet against a laboratory CO-Oximeter. SpO2 and
2
SpMet accuracy was determined on 16 neonatal NICU patients ranging in age from 7-135 days old and
weighing between 0.5-4.25 kg. Seventy-nine (79) data samples were collected over a range of 70-100%
and 0.5-2.5% MetHb with a resultant accuracy of 2.9% SpO2 and 0.9% SpMet.
SaO
2
The Masimo sensors have been validated for motion and no motion accuracy in human blood studies
on healthy adult male and female volunteers with light to dark skin pigmentation in induced hypoxia
studies in the range of 70-100% SpO
against a laboratory CO-oximeter and ECG monitor. This
2
variation equals plus or minus one standard deviation. Plus or minus one standard deviation
encompasses 68% of the population.
70
Masimo SET technology with LNOP, LNCS, and M-LNCS sensors has been validated for motion
accuracy in human blood studies on healthy adult volunteers in induced hypoxia studies while
performing rubbing and tapping motions, at 2 to 4 Hz at an amplitude of 1 to 2 cm and a non-
Page 71

3 Testing and Maintenance
repetitive motion between 1 to 5 Hz at an amplitude of 2 to 3 cm in induced hypoxia studies in the
range of 70-100% SpO
or minus one standard deviation, which encompasses 68% of the population
The Masimo SET Technology with LNOP Neo, LNCS Neo, and M-LNCS Neo sensors has also been
validated for neonatal motion accuracy in human blood studies on healthy adult volunteers in induced
hypoxia studies while performing rubbing and tapping motions, at 2 to 4 Hz at an amplitude of 1 to 2
cm and a nonrepetitive motion between 1 to 5 Hz at an amplitude of 2 to 3 cm in induced hypoxia
studies in the range of 70-100% SpO
The Masimo SET Technology has been validated for low perfusion accuracy in bench top testing
against a Biotek Index 2 simulator and Masimo's simulator with signal strengths of greater than 0.02%
and transmission of greater than 5% for saturations ranging from 70 to 100%. This variation equals
plus or minus one standard deviation which encompasses 68% of the population.
The Masimo sensors have been validated for pulse rate accuracy for the range of 25-240 bpm in bench
top testing against a Biotek Index 2 simulator. This variation equals plus or minus one standard
deviation which encompasses 68% of the population.
SpHb accuracy has been validated on healthy adult male and female volunteers and on surgical patients
with light to dark skin pigmentation in the range of 8-17 g/dl SpHb against a laboratory CO-oximeter.
This variation equals plus or minus one standard deviation which encompasses 68% of the population.
The SpHb accuracy has not been validated with motion or low perfusion.
against a laboratory co-oximeter and ECG monitor. This variation equals plus
2
against a laboratory CO-oximeter and ECG monitor.
2
For more details please refer to the sensor's directions for use (DfU).
NBP PerformanceTest
This section describes NBP test procedures.The monitor must be in service mode and the screen
“Service A” must be selected to perform these tests. The NBP Performance Test consists of:
• NBP Accuracy Test
• NBP Leakage Test
•NBP Linearity Test
•Valve Test
NBP Accuracy Test and Calibration
This test checks the performance of the non-invasive blood pressure measurement. Connect the
equipment as shown:
Tools required:
• Reference manometer (includes hand pump and valve), accuracy ±0.8mmHg.
• Expansion chamber (volume 250 ml +/- 10%)
71
Page 72

3 Testing and Maintenance
• Appropriate tubing.
In service mode, the systolic and diastolic readings indicate the noise of NBP channels 1 and 2
respectively. When static pressure is applied, the reading in NBP channel 1 should be below 50. The
value in parentheses indicates the actual pressure applied to the system.
1 Connect the manometer and the pump with tubing to the NBP connector and to the expansion
chamber.
2 In service mode, select the Setup NBP menu.
3 Select Close Valves: On
4 Raise the pressure to 280 mmHg with the manometer pump.
5 Wait 10 seconds for the measurement to stabilize.
6 Compare the manometer values with the displayed values.
7 Document the value displayed by the monitor (x1).
8 If the difference between the manometer and displayed values is greater than 3 mmHg or when
the NBP pump assembly has been changed, calibrate the NBP measurement. If not, proceed to
the leakage test.
9 To calibrate the NBP measurement, select Close Valves off then Calibrate NBP and wait for the
instrument to pump up the expansion chamber.Wait a few seconds after pumping stops until
EnterPrVal is highlighted and then move the cursor to the value shown on the manometer. If one of
the following prompt messages appears during this step, check whether there is leakage in the
setup:
– NBP unable to calibrate–cannot adjust pressure
– NBP unable to calibrate–unstable signal
10 Press Confirm.
If the INOP NBP Equipment Malfunction message occurs in monitoring mode, go back to service
mode and repeat the calibration procedure.
NBP Leakage Test
The NBP leakage test checks the integrity of the system and of the valve. It is required once every two
years and when you repair the monitor or replace parts.
1 If you have calibrated, repeat steps 2 to 6 from the accuracy test procedure so that you have 280
mmHg pressure on the expansion chamber.
2 Watch the pressure value for 60 seconds.
3 Calculate and document the leakage test value (x2).
x2 = P1 - P2
where P1 is the pressure at the beginning of the leakage test and P2 is the pressure displayed after
60 seconds.
The leakage test value should be less than 6 mmHg.
NOTE
The leakage test value of 6 mmHg applies for an expansion chamber of 250ml. When using a different
size of expansion chamber, the expected test result needs to be adapted accordingly. E.g for an
expansion chamber of 500ml, the leakage test value should be less than 3 mmHg. All other NBP
performance tests are independent of the expansion chamber size.
72
Page 73

NBP Linearity Test
1 Reduce the manometer pressure to 150 mmHg.
2 Wait 10 seconds for the measurement to stabilize.
3 After these 10 seconds, compare the manometer value with the displayed value.
4 Document the value displayed by the monitor (x3)
5 If the difference is greater than 3 mmHg, calibrate the MMS or X2 (see steps 9 to 10 in the
accuracy test procedure).
Valve Test
1 Raise the pressure again to 280 mmHg.
2 Select Close valves: Off.
3 Wait five seconds and then document the value displayed. The value should be less than 10 mmHg.
4 Document the value displayed by the monitor (x4).
Test Expected test results
Accuracy test x1 = 280 ± 3mmHg
Leakage test x2 = leakage test value
3 Testing and Maintenance
Difference ≤ 3mmHg
x2 < 6 mmHg (with 250ml
expansion chamber)
Linearity test x3 = 150 ± 3mmHg
Difference ≤ 3mmHg
Valve Test x4 = value < 10 mmHg
Invasive Pressure Performance Test
This test checks the performance of the invasive pressure measurement.
Tools required: Patient simulator.
1 Connect the patient simulator to the pressure connector.
2 Set the patient simulator to 0 pressure.
3 Make a zero calibration.
4 Configure the patient simulator as P(static) = 200 mmHg.
5 Wait for the display.
6 The value should be 200 mmHg ± 5 mmHg. If the value is outside these tolerances, calibrate the
Invasive Pressure measurement. If the measurement was calibrated with a dedicated reusable
catheter, check the calibration together with this catheter.
Test Expected test results
Invasive Pressure Performance Test 200 mmHg ± 5 mmHg
73
Page 74

3 Testing and Maintenance
Temperature Performance Test
This test checks the performance of the temperature measurement.
Tools required: Patient simulator (with 0.1°C or 0.2°F tolerance).
1 Connect the patient simulator to the temperature connector.
2 Configure the patient simulator to 40°C or 100°F.
3 The value should be 40°C ± 0.2°C or 100°F ± 0.4°F.
Test Expected test results
Temperature Performance Test 40°C ± 0.2°C or 100°F ± 0.4°F
Predictive Temperature Accuracy Check
Tools required:
• Calibration Key (CalKey) - Part No. 453564033691
The Calkey can be used to verify correct function of the module (in continuous mode). It does not test
the probe. The CalKey contains a known resistance which is converted to a specific temperature by the
module.
The monitor can stay in monitoring mode for this procedure.
Procedure:
1 Disconnect the probe and connect the CalKey.
2 Remove the probe from the holder. The monitor software switches to continuous mode
automatically.
3 Observe the displayed temperature. The value should read: 97.3°F ±0.2°F (36.3°C ±0.1°C).
Document whether the predictive temperature module passed or failed the accuracy check.
4 Disconnect the CalKey, connect the probe and return it to the holder.
5 The module test with the CalKey should be performed once a year.
Test Expected test results
Predictive Temperature Accuracy Test 97.3°F ±0.2°F (36.3°C ±0.1°C)
The 9600 Plus Calibration Tester from Welch Allyn provides a convenient way of testing the entire
thermometer system, module and probe. It is not intended for use by clinical users. Nevertheless,
biomeds or Philips field personnel may use it for probe verification. Currently, the tester is orderable
from Welch Allyn only. Follow the instructions provided with the tester.
Mainstream CO2 Accuracy Check
Tools Required:
• three airway adapters
74
• Verification Gas M2506A
• Gas cylinder regulator M2505A
You also need a local barometric pressure rating received from a reliable local source (airport, regional
weather station or hospital weather station) which is located at the same altitude as the hospital.
Page 75

Procedure:
3 Testing and Maintenance
1 Attach the M2501A CO
sensor to the patient monitor. Attach an airway adapter to the sensor.
2
Make sure that the sensor is disconnected from the patient circuit.
2 Switch on the patient monitor.
3 Enter the monitor’s Service Mode.
4 Using the sensor status provided in the M2501A Serial protocol, wait for the M2501A sensor to
warm up to its operating temperature.
5 The default setting for gas temperature is 22°C. If the gas temperature is significantly above or
below this value, correct the gas temperature setting.
6 Zero the sensor on the airway adapter being used in this test. Ensure Zero Gas is set to Room Air
7 Attach a regulated flowing gas mixture of 5% CO2, balance N2 to the airway adapter.
8 Set the gas correction to off.
9 Allow a few seconds for the gas mixture to stabilize and observe the CO2 value. The expected
value is 5% of the ambient pressure ±2mmHg
NOTE
Make sure that you follow the above steps correctly. If the sensor fails this check it must be exchanged.
The sensor cannot be calibrated.
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x1) Acceptance Range
Mainstream CO2 Accuracy
5% of 760 mmHg pressure ±2mmHg 36 mmHg - 40 mmHg
Test
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient pressure).
Sidestream CO2 Accuracy Check
Tools Required:
• Cal gas flow regulator M2267A
• Cal tube 13907A
• Verification Gas M2506A
• Straight Sample Line M2776A
You also need a local barometric pressure rating received from a reliable local source (airport, regional
weather station or hospital weather station) which is located at the same altitude as the hospital.
Procedure:
1 Attach the M2741A CO2 sensor to the patient monitor. Attach the sample line and the cal tube to
the sensor. Make sure that the sensor is disconnected from the patient circuit.
2 Switch on the patient monitor.
3 Enter the monitor’s Service Mode.
75
Page 76

3 Testing and Maintenance
4
Using the sensor status provided in the M2741A Serial protocol, wait for the M2741A sensor to
warm up to its operating temperature.
5 Zero the sensor. Ensure Zero Gas is set to Room Air
6 Attach a regulated flowing gas mixture of 5% CO2, balance N2 to the cal tube.
7 Set the gas correction to off.
8 Allow a few seconds for the gas mixture to stabilize and observe the CO2 value. The expected
value is 5% of the ambient pressure ±2mmHg
NOTE
Make sure that you follow the above steps correctly. If the sensor fails this check it must be exchanged.
The sensor cannot be calibrated
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x2) Acceptance Range
Sidestream CO2 Accuracy Test 5% of 760 mmHg pressure ±2mmHg 36 mmHg - 40 mmHg
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient pressure).
Sidestream CO2 Flow Check
Check the flow rate in the Sidestream CO2 extension as follows:
1 Connect the flowmeter to the sample line
2 Check on the flowmeter the flow that the Sidestream CO
ml/min ± 10 ml/min. If the value is not within tolerance check your setup again and perform
another flow check. If it fails again, the sensor must be replaced. The sensor cannot be calibrated.
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x3) Acceptance Range
Sidestream CO2 Flow Check 50 ml/min ±10 ml/min 40 ml/min - 60 ml/min
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient pressure).
Microstream CO2 Performance Test
Allow five seconds between individual service procedures to ensure stable equipment conditions.
When certain monitor procedures are running, service procedures are not possible and trying to start
them will result in a message
completes the current operation, then restart the service procedure.
Service Operation Failed in the monitor’s status line. Wait until the monitor
extension pump draws. It should be 50
2
76
This test checks the performance of the Microstream CO2 measurement. The Microstream CO2
measurement can either be integrated into the IntelliVue MP5 monitor or, for other IntelliVue
monitors, into the M3015A/B MMS Extensions. The Microstream CO2 performance test is required
Page 77

3 Testing and Maintenance
once per year or after 4000 hours of continuous use and when the instrument is repaired or when parts
are replaced.
This test uses calibration equipment that you can order (see the Parts section for the part number). The
procedure is summarized in the following steps. Refer to the documentation accompanying the
equipment for detailed instructions.
Tools Required:
• Standard tools, such as screwdriver, tweezers
• Electronic flowmeter, M1026-60144 or Mass Flowmeter 453564178121
• Digital Barometer ±2mbar or better
• Gas calibration equipment:
• Cal 1 gas 15210-64010 (5% CO
• Cal 2 gas 15210-64020 (10% CO
)
2
)
2
• Cal gas flow regulator M2267A
• Cal tube 13907A
• Calibration Line M3015-47301
• Leakage Test Kit M1013-64002 (451261014851) (only required for leakage test without M102660144 Flowmeter)
• Flexible Connecting Tube
You also need a local barometric pressure rating received from a reliable local source (airport, regional
weather station or hospital weather station) which is located at the same altitude as the hospital.
The CO2 calibration for the Microstream extension consists of the following steps:
• Leakage check, either with M1026-60144 Flowmeter or with 453564178121 Mass Flowmeter*
• Barometric pressure check and calibration, if required.*
• Flow check and calibration, if required
•Noise check
• CO2 Cal check and calibration, if required
•CO2 Cal verification
Perform all checks in the same session.
* Not applicable for all HW Revisions. See individual test sections for details.
NOTE
The M3015A/B HW Rev C is indicated as HW Rev. Q.xx.xx in the IntelliVue Revision Screen.
Leakage Check with M1026-60144 Flowmeter (only for M3015A with HW Rev. A and B and Firmware Revision < P.01.32)
The leakage check consists of checking the tubing between:
• the pump outlet and the mCO
• the pump inlet and calibration line inlet.
Check the user’s guide of the flowmeter for details on how to make a correct flow reading.
outlet and
2
77
Page 78

3 Testing and Maintenance
Part 1
1 Go into service mode and select Setup CO2 menu.
2 Connect a calibration line to the Microstream CO
3 Check the ambient pressure and the cell pressure shown in the monitor’s status line. The cell
input to start the pump running.
2
pressure should be approximately 20 mmHg lower than ambient pressure. (This test is only to
check that the pump starts and is running, which is also indicated by the noise generated by the
running pump.)
4 Connect the flowmeter outlet to the calibration line inlet using a flexible connecting tube.
5 Block the mCO
outlet using your fingertip and observe the flowmeter display. The value on the
2
flowmeter (x1) should decrease to between 0 and 4 ml/min, accompanied by an audible increase in
pump noise. If the value is within the tolerance limits, continue with part 2 of the leakage check.
6 If the value is outside the tolerance limits, there is a leakage between the pump outlet and the
outlet.
mCO
2
7 Open the MMS Extension or MP5 and check the tubing connections at the pump outlet and the
extension gas outlet. If the connections are good, then there is a leakage in the tubing and you
must exchange the MMS Extension or the mCO
Assembly of the MP5 respectively.
2
Part 2
1 Disconnect the flowmeter from the Part 1 setup and connect the flowmeter inlet to the M3015A
gas outlet or the MP5 mCO
2 Leave the calibration line connected to the M3015A inlet or the MP5 mCO
3 Block the inlet of the calibration line using your fingertip and observe the flowmeter display. The
gas outlet.
2
inlet..
2
value on the flowmeter (x2) should decrease to between 0 and 4 ml/min, accompanied by an
audible increase in pump noise. The cell pressure shown in the status line on the display should
decrease to between 300 and 500 mmHg. Do not block the inlet for longer than 25 seconds as this
will lead to an “Occlusion” INOP. If the value is within the tolerance limits, there are no leakages
and the leakage check is completed; proceed to the pump check.
78
4 If the value is not within the tolerance limits, there is a leakage between the calibration line inlet
and the pump inlet.
5 Check the calibration line connections and open the M3015A or MP5 to check the tubing
connections at the pump inlet and the M3015A or MP5 mCO
gas inlet. If the connections are
2
good, try replacing the calibration line and repeating the leakage check. If the situation remains,
there is a leakage in the tubing and the M3015A or the mCO
assembly of the MP5 must be
2
exchanged.
Test Expected test results
Leakage Check Parts 1 and 2 x1 = value of part 1 leakage check on flowmeter
(x1< 4.0 ml/min)
x2 = value of part 2 leakage check on flowmeter
(x2< 4.0 ml/min)
Page 79

3 Testing and Maintenance
Leakage Check for M3015B and M3015A with HW Rev C or M3015A with HW Rev. A/B without M1026-60144 Flowmeter
Preparation of Leakage Test Kit:
Remove two Luer connectors from the Leakage Test Kit, as shown in the following picture.
NOTE
These Luer connectors are not required for the actual Leakage Check. However, you should keep
them, as they are required for other tests (e.g. for the kit leak test as documented later in this section).
Test Setup:
1 Connect the Calibration Line (M3015-47301) to the inlet of the M8105A/M3015A/B (the
M8105A/M3015A/B must be switched off, either by disconnecting from the host monitor or by
switching off the monitor).
2 Connect the leakage test tubing to the outlet of the M8105A/M3015A/B, to the digital barometer,
to the calibration line, and the (empty) syringe as shown below. Make sure all connections have a
tight fit!
79
Page 80

3 Testing and Maintenance
Test Procedure:
1 Open the 3-way stopcock for all three limbs.
2 Switch on the digital barometer (the digital barometer should now display the actual ambient
pressure).
3 Now slowly draw at the syringe, as if filling the syringe, until the pressure (as displayed on the
digital barometer) drops to approximately 350 mbar below ambient pressure.Then close the line to
the syringe at the 3-way stopcock to syringe (circled in picture below).
4 Let the reading on the digital barometer stabilize for a moment and then perform the leakage
check: for 30 seconds the change of the pressure reading should be less than 20 mbar.
5 If the leakage test is NOT passed, check all connections once more and repeat the test.
Test Expected test results
Leakage Check Reading on the digital barometer
change is less than 20 mbar for 30
seconds (pass/fail)
NOTE
80
To ensure the integrity of the Leakage Test Kit (M1013-64002, 451261014851) the following Kit Leak
Test Procedure must be performed:
Page 81

3 Testing and Maintenance
a. Form a loop with the leakage test kit as shown in the picture below.
b. Connect the syringe to the 3-way stopcock and the digital barometer to the open tubing.
c. Draw at the syringe until the digital barometer shows approximately 350 mbar below ambient
pressure.
d. Close the 3-way stopcock to the syringe and wait 5 - 10 seconds. In this time, the overall
pressure should stabilize.
e. After 1 minute, check the pressure. The pressure should not increase more than 8 mbar in 1
minute for the test to pass.
f. If this test fails, exchange the leakage test kit.
Barometric Pressure Check and Calibration
NOTE
The M3015A with HW Rev C and the M3015B do not require calibration of the barometric pressure.
Therefore you will not be able to activate a barometric pressure calibration. If you are using a HW Rev
C M3015A or M3015B, perform the barometric pressure check as described below, making sure that
only a sample line is connected to the MMS Extension. If the pressure check fails, the M3015A/B
needs to be exchanged.
Check the barometric pressure value in the M3015A/B MMS Extension or the MP5 as follows:
1 Go into service mode and select Setup CO
2 Connect a calibration line to the Microstream CO
B MMS Extension or the MP5.
3 The status line at the bottom of the screen displays “CO
yyy” where xxx is the ambient pressure and yyy is the measured cell pressure. Check whether the
ambient pressure value (x3) matches (within the acceptable tolerance of ±12mm Hg) the reference
value you have received. If so, proceed to the leakage check.
If the value is not correct, calibrate as follows.
CO
a. Select
b. Select the value in the table which matches the reference value received from a reliable local source
(airport, regional weather station or hospital weather station). (The values are displayed with a
resolution of 2 mmHg up to 500 mmHg and a resolution of 1 mmHg from 500 mmHg to 825
mmHg.) Note: the selected value must be within ±10% of the current measured ambient pressure,
otherwise an error message will occur at restarting the monitor.
then select Barom.Press to activate a table of values.
2
menu.
2
input. This activates the pump in the M3015A/
2
pressure reading (ambient/cell) xxx/
2
81
Page 82

3 Testing and Maintenance
c. Confirm the barometric pressure setting.
d. Check that the ambient pressure displayed in the status line at the bottom of the screen is the same
as the value which you selected from the list in step b.
Test Expected test results
Barometric Pressure Check x3 = difference between the reference
Flow Rate Check and Calibration
Check the flow rate in the M3015A/B MMS Extension or the MP5 as follows:
pressure and the measured ambient
pressure displayed on the monitor
(x3<12 mmHg)
1 Connect the calibration line to the mCO
2 Check on the flowmeter the flow that the M3015A/B MMS Extension or MP5 mCO2 pump
inlet and the flowmeter outlet to the calibration line.
2
draws (x5). It should be 50 +15/-7.5 ml/min. If the value is within tolerance, proceed to the CO
Gas calibration check.
If the value is not within tolerance, calibrate as follows.
a. Adjust the flow in the instrument by selecting
Increase Flow or Decrease Flow until it is as close as
possible to 50 ml per minute as indicated on the flowmeter gauge.
b. When you are satisfied that the flow is set as close as possible to 50 ml per minute, select
Store Flow
and confirm the setting. If you do not store the adjusted flow within 60 seconds of the adjustment,
the old flow setting is restored.
c. If you cannot adjust the flow to within tolerance, replace the pump . If you still cannot make the
flow adjustment, this indicates a fault in the measurement extension, which must be replaced.
Note that the pump can only be replaced on M3015A with the old hardware Rev. A (i.e. Serial No.
DE020xxxxx)
Test Expected test results
Flow Rate Check Flow rate is 50 +15/-7.5 ml/min
2
82
Page 83

Noise Check
3 Testing and Maintenance
1 With the monitor in service mode, select Setup CO
2 Connect the calibration line, the cal tube, the flow regulator and the 5% calibration gas to the
inlet.
mCO
2
menu.
2
3 Open the valve to apply the 5% calibration gas and wait until the value is stable.
4 Check the noise index (x6) displayed next to the CO
of noise on the CO
wave). If the value exceeds 3 mmHg, replace the measurement extension.
2
value on the display (this indicates the level
2
Test Expected test results
Noise Check x6 = noise index displayed on monitor
(x6<3.0)
83
Page 84

3 Testing and Maintenance
CO2 Cal Check and Calibration
After switching the measurement extension on, wait at least 20 minutes before checking the
calibration. Check the calibration of the CO
1 Connect the calibration line, the cal tube, the flow regulator and the 5% calibration gas to the
inlet.
mCO
2
gas measurement as follows:
2
2 Calculate the expected measurement value in mmHg as follows:
0.05 x (ambient pressure) = value mmHg
for example 0.05 x 736 = 36.8 mmHg (with an ambient pressure of 736 mmHg)
3 Open the valve on the flow regulator to allow 5% CO
gas to flow into the extension. Allow the
2
value to stabilize.
4 Check that the value on the instrument (measurement value on the main screen, x7) matches the
calculated mmHg value ± 2.6 mmHg.
If the value is outside the tolerance, calibrate as described in step 8a to 8e below.
5 Disconnect the 5% calibration gas and connect the 10% calibration gas.
6 Calculate the expected measurement value and tolerance in mmHg as follows:
0.1 x (ambient pressure) = value mmHg
±0.07 x (value mmHg) = tolerance
for example 0.1 x 737 mmHg = 73.7 mmHg (with an ambient pressure of 737 mmHg)
±0.07 x 73.7 mmHg = ±5.16 mmHg tolerance
7 Open the valve on the flow regulator to allow 10% CO
gas to flow into the extension. Allow the
2
value to stabilize.
8 Check that the value on the instrument (x8) matches the calculated mmHg value within the
calculated tolerance. If so, the measurement extension is correctly calibrated.
If the value is outside the tolerance, calibrate as follows.
a. Keep the same setup and connect the 5% calibration gas.
84
b. Select
Cal. CO
.
2
c. Select the value for the calibration gas. (The default value is 5.0%.)
Page 85

d. Open the valve on the calibration gas to allow CO2 gas to flow into the extension. Allow the value
to stabilize before the start of the calibration. Leave the valve open until the instrument gives a
prompt that gas can be removed.
e. The extension calibrates and prompts when calibration is successful.
Test Expected test results
CO2 Cal Check x7 = calculated mmHg value ±2.6 mmHg
Calibration Verification
1 Keep the same setup as described in “CO2 Cal Check and Calibration” on page 84.
2 Reopen the 5% gas valve and allow the value to stabilize.
3 Check that the value displayed on the monitor is correct within the tolerance (see step above).
4 Disconnect the 5% calibration gas and connect the 10% calibration gas.
5 Open the valve on the flow regulator to allow 10% CO2 gas to flow into the extension. Allow the
value to stabilize.
6 Check that the value displayed on the monitor is correct within the tolerance (see step above).
3 Testing and Maintenance
x8 = calculated mmHg value within
calculated tolerance
If one or both values are not within tolerances, you must exchange the M3015A/B MMS Extension or
the MP5 mCO
Assembly.
2
Test Expected Test Results
Leakage Check parts
1 and 2*
x1 = value of part 1 leakage check on flowmeter
(x1< 4.0 ml/min)
x2 = value of part 2 leakage check on flowmeter
(x2< 4.0 ml/min)
Leakage Check
without Flowmeter
Barometric Pressure
Check
reading on the digital barometer change is less than 20
mbar for 30 seconds
x3 = difference between the reference pressure and the
measured ambient pressure displayed on the monitor
(x3<12 mmHg)
Flow Check x4 = difference between measured value and 50.0 ml/min
(x4 = 50+15/-7.5 ml/min)
Noise Check x5 = noise index displayed on monitor (x5<3.0)
Gas Calibration
CO
2
Check
x6 = difference between measured CO2 value and
calculated value, based on 5% CO
cal. gas. (x6 < 2.6
2
mmHg)
CO2 Cal Verification x7 = difference between measured CO2 value and
calculated value, based on 10% CO
cal. gas.
2
(x7 < ± {0.07 x value calculated})
* M3015A HW Rev. B and FW Revision < P.01.32 only
85
Page 86

3 Testing and Maintenance
Nurse Call Relay Performance Test
The nurse call relay performance test can be performed either at the phone jack type connector (this
only tests one relay) or at the multi-port nurse call connector (to test all three relays).
Phone Jack Type Connector Test (Traditional Nurse Call)
This test checks the operation of the traditional Nurse Call Relay. The Nurse Call Relay test is
recommended for customer sites where the nurse call is in use. The Nurse Call relay functions as
follows:
• Standard Operation—Relay open.
• Alarm Condition—Relay closed.
Tools required: Ohmmeter.
1 Plug a phono connector into the Nurse Call Relay connector.
2 Connect the ohmmeter.
3 If no alarm occurs, the relay contacts are open. When an alarm occurs, the relay contacts close.
4 The expected test result is: Alarm condition - Relay closed.
Test Expected test results
Nurse Call Relay Performance Test Alarm Condition—Relay closed
Modified MP5 Nurse Call Alarm Relay Test
Some customers may have an Open-On-Alarm relay instead of a Closed-On-Alarm for their Nursecall
system.
The modified Nurse Call relay functions as follows:
• Standard Operation—Relay closed.
• Alarm Condition—Relay open.
Tools required: Ohmmeter.
1 Plug a phono connector into the Nurse Call Relay connector.
2 Connect the ohmmeter and verify the above conditions.
3 If no alarm occurs, the relay contacts are closed. When an alarm occurs, the relay contacts open.
86
Page 87

4
The expected test result is: Alarm Condition - Relay open.
Test Expected test results
Nurse Call Relay Performance Test Alarm Condition—Relay open
MSL Assurance Test
Visually inspect all MSL connector sockets (cable/monitor/FMS/MMS).
1 Make sure that the pins of the connectors are not jolted.
2 Make sure that no pin is bent inwards or outwards.
3 Exchange connectors that show any evidence of damage or breakage.
3 Testing and Maintenance
Examples of damaged connectors
Test Expected test results
MSL Assurance Test Pins of connector not jolted/bent
(pass/fail)
Power Loss Alarm Buzzer Performance Test
1 Switch on the monitor.
2 Remove the battery and disconnect the monitor from AC power.
3 The Power Loss Alarm Buzzer should beep for about one minute.
4 To switch off the alarm sound, either press the power button, connect the monitor to AC power or
insert a battery
Test Expected test results
Power Loss Alarm Buzzer Performance
Test
Beep for one minute
Battery Performance Test
The lifetime of a Lithium Ion battery depends on the frequency and duration of use. When properly
cared for, the useful life is approximately 3 years or 500 charge-discharge cycles, whichever comes first.
In addition, experience indicates that the incidence of failure may increase with battery service life due
to the accumulated stresses of daily use.
The age of a lithium ion battery begins at the date of manufacture. To see the date of manufacture and
the number of charge-discharge cycles:
87
Page 88

3 Testing and Maintenance
1
Select the battery symbol on the patient monitor screen.
2 Select the appropriate Battery pop-up key.
3 If necessary, select the Battery pop-up key again to view the battery details.
The date of manufacture and the number of charge-discharge cycles are listed with other battery
data on the screen.
Provide customer with details about age of battery and recommend replacement, if necessary.
Test Expected test results
Battery Performance Test Battery age less than 3 years or 500 charge/discharge cycles
IntelliVue 802.11 Bedside Adapter Communication Test - Rev G.0 or lower (not for MP5T)
1 Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2 Go into Service Mode and select Main Setup -> Network -> Setup WLAN. In the Setup WLAN menu:
–set
–set
– set the
– set the Security Mode to match your installation.
– Enter the required keys/passwords.
Mode to either 802.11Ah, 802.11G, 802.11Bg (not recommended), Auto (not
recommended) or
None (this setting disables the wireless LAN functionality permanently), to
match your wireless infrastructure installation.
SSID to match your installation.
Country code to “1000”. Setting the country code to this value will automatically adjust
the regulatory domain to match the configuration of the infrastructure. Do not set the country
code to values other than “1000” unless otherwise instructed.
3 Select Main Setup -> WLAN Diagnostic to access the service window.
4 Proper installation of the IntelliVue 802.11 Bedside Adapter is assured by connecting to an access
point over the wireless link. Place the monitor with the IntelliVue 802.11 Bedside Adapter installed
in close proximity to the access point (e.g. if the access point is mounted on the ceiling, place the
monitor directly below). Wait until the
Conn.Status field in the service window shows Authenticatd
(for Rel. C.0 monitors) or Connected (for Rel D.0 or higher). Take the monitor approximately 5 m
away from the access point. There should be no walls or other obstacles between the monitor and
the access point. The following should apply:
– Observe the
RSSI value will fluctuate but should stay above 30 in a 5 m distance from the access point used.
The wireless link should be active, i.e. the
RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds. The
Conn.Status field should be Authenticatd (for Rel. C.0
monitors) or Connected (for Rel D.0 or higher), and the other fields should contain values. If the
RSSI value is significantly lower, check the distance to the access point and the antenna
orientation at the monitor. The antenna orientation should be vertical, but the physical
placement of the monitor or other equipment within its vicinity as well as walls or other
obstacles may influence the antenna orientation required to receive the best RSSI value.
5 If this test fails, retry in a different physical area with a different access point and/or check the
credential settings in the monitor.
Test Expected test results
IntelliVue 802.11 Bedside Adapter
RSSI value above 30
Performance Test
88
Page 89

3 Testing and Maintenance
IntelliVue 802.11 Bedside Adapter Communication Test - Rev H.0 or higher (not for MP5T)
1 Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2 Go into Service Mode and select Main Setup -> Network -> Setup WLAN. In the Setup WLAN menu:
–set
–set
– set the
– set the
– Enter the required keys/passwords.
3 Select Main Setup -> Network -> WLAN Diagnostic to access the WLAN Diagnostic window.
4 Proper installation of the IntelliVue 802.11 Bedside Adapter is assured by connecting to an access
point over the wireless link. Place the monitor with the IntelliVue 802.11 Bedside Adapter installed
in close proximity to the access point (e.g. if the access point is mounted on the ceiling, place the
monitor directly below). Wait until the
(for Rel. C.0 monitors) or Connected (for Rel D.0 or higher). Take the monitor approximately 5 m
away from the access point. There should be no walls or other obstacles between the monitor and
the access point. The following should apply:
– Observe the
Mode to either 802.11Ah, 802.11G, 802.11Bg (not recommended), Auto (not
recommended) or
None (this setting disables the wireless LAN functionality permanently), to
match your wireless infrastructure installation.
SSID to match your installation.
Country code to “1000”. Setting the country code to this value will automatically adjust
the regulatory domain to match the configuration of the infrastructure. Do not set the country
code to values other than “1000” unless otherwise instructed.
Security Mode to match your installation.
Conn.Status field in the service window shows Authenticatd
RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds. The
RSSI value will fluctuate but should stay above 30 in a 5 m distance from the access point used.
The wireless link should be active, i.e. the
Conn.Status field should be Authenticatd (for Rel. C.0
monitors) or Connected (for Rel D.0 or higher), and the other fields should contain values. If the
RSSI value is significantly lower, check the distance to the access point and the antenna
orientation at the monitor. The antenna orientation should be vertical, but the physical
placement of the monitor or other equipment within its vicinity as well as walls or other
obstacles may influence the antenna orientation required to receive the best RSSI value.
5 If this test fails, retry in a different physical area with a different access point and/or check the
credential settings in the monitor.
Test Expected test results
IntelliVue 802.11 Bedside Adapter
RSSI value above 30
Performance Test
IIT Communication Test - Rev G.0 or lower (not for MP5T)
1 Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2 Go into Configuration mode and, in the Network menu, set the RF Access Code in each profile to
match your installation.
3 Go into Service Mode. Select Main Setup -> Instr. Telemetry to access the Instrument Telemetry
Service window.
4 Proper installation of the IIT module is assured by connecting to an access point over the wireless
link. Place the monitor with the IIT module installed in close proximity to the access point (e.g. if
the access point is mounted on the ceiling, place the monitor directly below). Wait until the
89
Page 90

3 Testing and Maintenance
Conn.Status field in the Instrument Telemetry Service window shows Active. Take the monitor
approximately 5 m away from the access point. There should be no walls or other obstacles between the monitor and
the access point. The following should apply:
– Observe the
RSSI value should be around -50 ±10 in a 5 m distance from the access point used and the IIT
link should be active, i.e. the
contain values. If the
and the antenna orientation at both the monitor and the access point (both should be vertical).
– Remove the antenna. The
the connection could be unreliable. The
Seeking. If the difference between the
significantly lower, check the antenna and the antenna connector for damage and verify that
the cable from the IIT adapter to the antenna connector plate is connected properly.
5 If this test fails, retry in a different physical area with a different access point.
Error Conditions:
–The field MAC Instr. Tele should show a value unequal to 0000 0000 0000. If it does not, there
is a communication problem between the monitor and the IIT adapter.
– With an incorrect RF Access Code or an incorrect or defective antenna installation, the fields
IP Address,Server IP, Subnet Mask, and RSSI in the Instrument Telemetry Service window will
stay blank. The field
RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds. The
Conn.Status field should be Active and the other fields should
RSSI value is significantly lower, check the distance to the access point
RSSI value should be around -90 ±10. The IIT link may be active but
Conn. Status field may toggle between Inactive and
RSSI values measured with and without antenna is
Conn. Status will slowly toggle between Inactive and Seeking.
6 Perform the Access Point Controller (APC) test blocks as described in the Philips IntelliVue
Wireless Network Installation and Configuration Guide.
Test Expected test results
IIT Communication Test IIT Communication without
interference
IIT Communication Test - Rev H.0 or higher (not for MP5T and MP5SC)
1 Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2 Go into Service mode and, select Main Setup -> Network -> Setup IIT. In the Setup IIT menu, set
RF Access Code in each profile to match your installation.
the
3 Go into Service Mode. Select Main Setup -> Network -> IIT Diagnostic to access the Instrument
Telemetry Diagnostic window.
4 Proper installation of the IIT module is assured by connecting to an access point over the wireless
link. Place the monitor with the IIT module installed in close proximity to the access point (e.g. if
the access point is mounted on the ceiling, place the monitor directly below). Wait until the
Conn.Status field in the Instrument Telemetry Service window shows Active. Take the monitor
approximately 5 m away from the access point. There should be no walls or other obstacles between the monitor and
the access point. The following should apply:
– Observe the
RSSI value should be around -50 ±10 in a 5 m distance from the access point used and the IIT
link should be active, i.e. the
contain values. If the
and the antenna orientation at both the monitor and the access point (both should be vertical).
– Remove the antenna. The
the connection could be unreliable. The
RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds. The
Conn.Status field should be Active and the other fields should
RSSI value is significantly lower, check the distance to the access point
RSSI value should be around -90 ±10. The IIT link may be active but
Conn. Status field may toggle between Inactive and
90
Page 91

Seeking. If the difference between the RSSI values measured with and without antenna is
significantly lower, check the antenna and the antenna connector for damage and verify that
the cable fom the IIT adapter to the antenna connector plate is connected properly.
5 If this test fails, retry in a different physical area with a different access point.
Error Conditions:
–The field MAC IIT should show a value unequal to 0000 0000 0000. If it does not, there is a
communication problem between the monitor and the IIT adapter.
– With an incorrect RF Access Code or an incorrect or defective antenna installation, the fields
IP Address,Server IP, Subnet Mask, and RSSI in the Instrument Telemetry Service window will
stay blank. The field
6 Perform the Access Point Controller (APC) test blocks as described in the Philips IntelliVue
Conn. Status will slowly toggle between Inactive and Seeking.
Wireless Network Installation and Configuration Guide.
Test Expected test results
IIT Communication Test IIT Communication without
interference
Short Range Radio (SRR) Performance Test
1 Make sure that the short range radio interface is configured as follows: SRR On and appropriate
channel selected.
3 Testing and Maintenance
2 Assign a telemetry transceiver or IntelliVue CL Cableless Measurement device to the IntelliVue
Monitor according to the procedure described in the Instructions for Use of the patient monitor.
3 Check that the following conditions are fulfilled:
a. Place the telemetry transceiver or CL device close to the monitor.
b. The telemetry transceiver or CL device status is displayed on the monitor in the measurement
selection window.
c. Waves or numerics from the telemetry transceiver or CL device are displayed on the monitor.
There are no dropouts or gaps in waves or numeric transmission.
d. The battery status of the telemetry transceiver or CL device is displayed in the measurement
selection window.
e. The Signal Quality Indicator shows at least
4 Check that the data from the telemetry transceiver or CL device is transmitted to the monitor
within a 1m radius and that there are no dropouts or gaps in waves or numerics.
5 Check whether the connection remains stable within a 5m radius from the monitor.
6 Switch on all telemetry transceivers or CL devices used on the site and check that there are no
interferences between the transceivers and their assigned monitors.
7 Check and record the coverage area of the telemetry transceivers or CL devices and inform the
customer about this coverage area.
Test Expected test results
Short Range Radio (SRR) Performance
Test
SRR Communication without
interference within the coverage
91
Page 92

3 Testing and Maintenance
Mounting Integrity Test
Perform the Mounting Integrity Test
– whenever you have removed and reassembled a quick mount
– if one or more of the quick mount screws are loose
– if the monitor mounting is unstable
Remove the monitor from the mount and disassemble the quick mount. Ensure that the threaded
inserts of the MP5 chassis are not damaged.
If the quick mount is damaged, exchange the quick mount.
Ensure that all quick mount screws are tight (1.3 Nm). Test the quick mount by pressing the quick
release button. If it comes back out gradually and regularly, the quick mount is inserted correctly. If it
gets stuck, check whether the quick mount is assembled correctly.
Test Expected test results
Mounting Integrity Test All quick mount screws are tight. No
Reporting of Test Results
damage to quick mount. No damage
to threaded inserts of the MP5.
Philips recommends all test results are documented in accordance with local laws. Authorized Philips
personnel report the test result back to Philips. While hospital personnel (biomedical engineers or
technicians) do not need to report results to Philips, Philips recommends that they record and store the
test results in accordance with local laws.
The following table lists what to record after completing the tests in this chapter. Record the results in
the empty column in the Test and Inspection Matrix.
The following is a guide as to what your documentation should include:
• Identification of the testing body (for example, which company or department carried out the
tests).
• Name of the person(s) who performed the tests and the concluding evaluation.
• Identification of the device(s) and accessories being tested (serial number, etc.).
• The actual tests (incl. visual inspections, performance tests, safety and system tests) and
measurements required
• Date of testing and of the concluding evaluation.
• A record of the actual values of the test results, and whether these values passed or failed the tests.
• Date and confirmation of the person who performed the tests and evaluation.
The device under test should be marked according to the test result: passed or failed.
92
Page 93

Carrying Out and Reporting Tests
Test Report
3 Testing and Maintenance
Testing Organization:
Name of testing person:
Date:
Responsible Organization:
Device Under Test: ID-Number
Product Number: Serial No.:
Accessories:
Measurement Equipment (Manufacturer, Type, Serial
No., Calibration Date):
Safety Test Method used
Functional Test (parameters tested):
Mains voltage and frequency used during safety
testing:
(Check one of the following three options)
Test before putting into service (reference value)
Recurrent Test
Test after Repair
Test and Inspection Matrix
Test Test or Inspection
to be Performed
Expected Test Results Record the Results (mandatory for
Philips Personnel only)
What to record Actual Results
Visual
Inspection
Power On Power on the unit.
Noninvasive
Blood Pressure
Performance
Tests
Temperature
Performance
Test
Perfor m Visual
Inspection
Does the self-test
complete
successfully
Perfor m the
Accuracy Test
Performance
Leakage Test
Performance
Linearity Test
Performance Valve
Test
Perfor m the
Temperature
Performance Test
Pass or Fail V:P or V:F
If Yes, Power On test is passed PO:P or PO:F
X1 = value displayed by monitor
Difference <= 3mmHg
X2 = leakage test value
X2 < 6 mmHg
X3 = value displayed by monitor
Difference <= 3mmHg
X4 = value < 10 mmHg PN:P/X4 or
X1= 40°C ± 0.2°C or 100°F ±
0.4°F
PN:P/X1 or
PN:F/X1
PN:P/X2 or
PN:F/X2
PN:P/X3 or
PN:F/X3
PN:F/X4
PT: P/X1 or
PT: F/X1
93
Page 94

3 Testing and Maintenance
Test Test or Inspection
to be Performed
Expected Test Results Record the Results (mandatory for
Philips Personnel only)
What to record Actual Results
All other
performance
tests
Perfor m the
remaining
parameter
See expected results in test
procedures
P: P or
P: F
performance tests,
if applicable
Safety (1) Perform Safety Test
(1): Protective
Earth Resistance
Safety (2) Perform Safety Test
(2): Equipment
Leakage Current -
With mains cable:
Maximum impedance (X1):
<=300 mOhms
With mains cable:
Maximum leakage current
(X1):<= 100 μA
S(1):P/X1 or
S(1):F/X1
S(2): P/X1 or
S(2): F/X1
*
*
Normal Condition.
Safety (3) Perform Safety Test
(3): Equipment
Leakage Current -
With mains cable:
Maximum leakage current
(X2):<= 300 μA
S(3): P/X2 or
S(3): F/X2
*
Single Fault
Condition (Open
Earth)
Safety (4) Perform Safety Test
(4): Applied Part
Leakage Current -
Maximum leakage current (X1):
<=50 μA (CF)
S(4): P/X1 or
S(4): F/X1
*
Single Fault
Condition, mains
on applied part.
System
(Sys 1-2)
Perform the system
test according to
subclause 19.201 of
IEC/EN 60601-1-1
or IEC/EN 606011+A1 Ed.3 clause
Equipment Leakage Current:
Sys1 <= 100 μA (Normal
Condition)
Sys2 <= 300μA (Single Fault
Condition
Sys: PSys1/PSys2
or
Sys: FSys1/Fsys2
*
16, if applicable,
after forming a
system
System
(Sys 3)
Perform the system
test according to
subclause 19.201 of
IEC/EN 60601-1-1
or IEC/EN 606011+A1 Ed.3 clause
16, if applicable,
Protective Earth Leakage
Current if medical electrical
system components are
connected to the same Multiple
Portable Socket Outlet:
Sys3 <= 300 μA
Sys: PSys3
or
Sys: FSys3
*
after forming a
system
Key: P = Pass, F = Fail, X or Sys = test value to be recorded, * = Record the worst-case results and the associated
switch positions (e.g. normal/reverse polarity)
94
Page 95

NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
Evaluation
Safety and Functional Test passed
Repair required at a later date, safety and functional test passed
Device must be taken out of operation until repair and passed tests
Device failed and must be taken out of operation.
Notes:
Next Recurrent Test:
Name:____________________________________________________
Date/Signature:_____________________________________________
3 Testing and Maintenance
Yes No
Evaluation of Test Results
The evaluation of the test results must be performed by appropriately trained personnel with sufficient
product, safety testing and application knowledge.
If any test results are between 90% and 100% of the respective expected result, the previously
measured reference values must be taken into consideration for the assessment of the electrical safety
of the device under test. If no reference values are available, you should consider shorter intervals
between upcoming recurrent tests.
NOTE
If any single test fails, testing must be discontinued immediately and the device under test must be
repaired or labeled as defective. Be sure to inform the user about the test failure in writing.
Other Regular Tests
The care and cleaning requirements that apply to the monitor and its accessories are described in the
Instructions for Use. This section `details periodic maintenance procedures recommended for the
monitor and its accessories.
Touchscreen Calibration
To access the touchscreen calibration screen:
1 Enter service mode
2 Select Main Setup
3 Select Hardware
95
Page 96

3 Testing and Maintenance
4
Select Touch Calibration
Touchscreen Calibration Screen
Make sure you complete the calibration procedure without powering off the monitor mid-way. If the
monitor is powered off after the first point is touched, the touch panel will be deactivated until the
touch calibration is performed again.
If the touchscreen is accidentally mis-calibrated by selecting the wrong spot, you must use another
input device to re-enter calibration mode. If you have the Support Tool, you can initiate a touch
calibration from there.
Please refer to the documentation shipped with your selected display for further details on touchscreen
calibration procedures.
Disabling/Enabling Touch Operation
To temporarily disable touchscreen operation of the monitor, press and hold the Main Screen key. A
padlock symbol will appear on the key. Press and hold the
touchscreen operation.
Main Screen key again to re-enable
Printer Test Report
To verify your printer configuration you may want to print a test report.
To print a test report select
Your test report should look like this:
Main Setup -> Reports -> Setup Printers -> Print Test Rep.
96
Page 97

3 Testing and Maintenance
Battery Handling, Maintenance and Good Practices
This section provides some information on how to handle and maintain the battery in order to get the
best usage from them. Additionally, some good working practices are also given regarding the correct
disposal of the batteries. This section only applies if a system interface board with battery functionality
is installed in the monitor.
NOTE
If your monitor is connected to an IntelliVue Information Center, you should make sure that the IIC
uses the text catalog revision E.0 or later, otherwise battery INOPs may not display correctly on the
IIC. Consult your IIC documentation for instructions on upgrading the text catalog.
Battery Care
Battery care begins when you receive a new battery and continues throughout the life of the battery.
The table below lists battery care activities and when they should be performed.
Activity When to Perform
Perform a visual inspection Before inserting a battery into the
Charge the battery Upon receipt, after use, or if a low
Condition the battery When the "battery requires
monitor
battery state is indicated. To optimize
performance, a fully (or almost fully)
discharged battery should be charged
as soon as possible.
maintenance" symbol appears
97
Page 98

3 Testing and Maintenance
Activity When to Perform
Store the battery in a state of
charge in the range of 40% to
50%
Refer to your monitor's Instructions for Use for details on how to perform battery care activities,
including charging and conditioning. We recommend using the Philips Smart Battery Conditioner LG
1480 (865432)
Handling Precautions
Lithium ion batteries store a large amount of energy in a small package.Use caution when handling the
batteries; misuse or abuse could cause bodily injury and/or property damage.
• Do not short circuit - take care that the terminals do not contact metal or other conductive
materials during transport and storage
• Do not crush, drop or puncture - mechanical abuse can lead to internal damage and internal short
circuits which may not be visible externally
• Do not apply reverse polarity
• Do not expose batteries to liquids
• Do not incinerate batteries or expose them to temperatures above 60°C (140°F)
• Do not attempt to disassemble a battery.
If a battery has been dropped or banged against a hard surface, whether damage is visible externally or
not:
When not in use for an extended
period of time
Storage
• discontinue use
• dispose of the battery in accordance with the disposal instructions
If a battery shows damage or signs of leakage, replace it immediately. Do not use a faulty
battery in the monitor.
When storing batteries, make sure that the battery terminals do not come into contact with metallic
objects, or other conductive materials.
If batteries are stored for an extended period of time, they should be stored in a cool place, ideally at
15°C (60°F), with a state of charge of 20% to 40%. Storing batteries in a cool place slows the aging
process.
The batteries should not be stored at a temperature outside the range of -20°C (-4°F) to 60°C (140°F).
Do not store batteries in direct sunlight.
Stored batteries should be partially charged to 20% to 40% of their capacity every 6 months. They
should be charged to full capacity prior to use.
NOTE
Storing batteries at temperatures above 38°C (100°F) for extended periods of time could significantly
reduce the batteries’ life expectancy.
98
Page 99

Battery Lifetime Management
The lifetime of a Lithium Ion battery depends on the frequency and duration of use. When properly
cared for, the useful life is approximately 3 years or 500 full charge-discharge cycles, whichever comes
first. In addition, experience indicates that the incidence of failure may increase with battery service life
due to the accumulated stresses of daily use. We therefore strongly recommend that lithium ion
batteries be replaced after 3 years or 500 full charge-discharge cycles.
The age of a lithium ion battery begins at the date of manufacture. To see the date of manufacture and
the number of charge-discharge cycles:
1 Select the battery symbol on the patient monitor screen.
2 Select the appropriate Battery pop-up key.
3 If necessary, select the Battery pop-up key again to view the battery details.
The date of manufacture and the number of charge-discharge cycles are listed with other battery data
on the screen.
WARNING
The risk of battery failure increases with age, when a battery remains in use longer than 3 years or 500
full charge-discharge cycles. Such failures can result in overheating that in rare cases can cause the
battery to ignite or explode.
3 Testing and Maintenance
Disposal
Batteries should be disposed of in an environmentally-responsible manner. Consult the hospital
administrator or your local Philips representative for local arrangements.
Discharge the batteries and insulate the terminals with tape before disposal. Dispose of used batteries
promptly and in accordance with local recycling regulations.
About the Battery
The rechargeable Lithium-Ion batteries used in the monitor are regarded as Smart batteries because
they have built-in circuitry. (This circuitry communicates battery status information to the monitor.)
Capabilities of integrated battery charger: 12.6V, 5 Amps max.
Actual current/voltage: depends on smart battery request and monitor configuration. The approximate
charging time is 4 hours with the monitor switched off and up to 12 hours during monitor operation,
depending on the monitor configuration.
NOTE
Batteries will discharge within about 20 days if they are stored inside the monitor without AC power
connection.
Checking the Battery Status
When the Monitor is connected to the AC power supply, the battery charges automatically. The battery
can be charged remotely from the Monitor by using the battery charger. Use only the 865432 Smart
battery conditioner.
Battery status (level of charge) is indicated in several ways:
• LED on the front panel of the Monitor.
99
Page 100

3 Testing and Maintenance
• Battery gauge.
• Display of battery time below gauge.
• Battery status window.
• INOP messages.
The AC Power LED is only on when the power cord is connected and AC power is available to the
Monitor. In this case, the battery can be either charging or fully charged.
The battery LED can be green, yellow, or red depending on the following conditions:
Battery LED Colors If the monitor is connected to
AC power, this means
Green
Yellow
Red, flashing
Red, flashes intermittently
1 indicated by malfunction symbol and INOP
2 for further details see Troubleshooting section
battery full (≥90%)
battery charging
(battery power < 90%)
battery or charger malfunction1,2 battery or charger malfunction1,2
Battery Status on the Main Screen
Battery status information can be configured to display permanently on all Screens. It shows the status
of the battery and the battery power and battery time remaining. The battery time is only displayed
when the monitor is not running on AC power. Note that the battery status information may take a
few minutes after the monitor is switched on to stabilize and show correct values.
Battery power gauge:
If the monitor is running on
battery power, this means
≤ 10 minutes power remaining
100
This shows the remaining battery power. It is divided into sections, each representing 20% of the total
power. If three and a half sections are shaded, as in this example, this indicates that 70% battery power
remains. If no battery is detected, the battery gauge is greyed out.
Battery malfunction symbols:
If a problem is detected with the battery, these symbols are displayed. They may be accompanied by an
INOP message or by a battery status message in the monitor information line (if battery window is
open) providing more details.
Battery Status Symbols
Battery requires maintenance
Battery is empty
 Loading...
Loading...