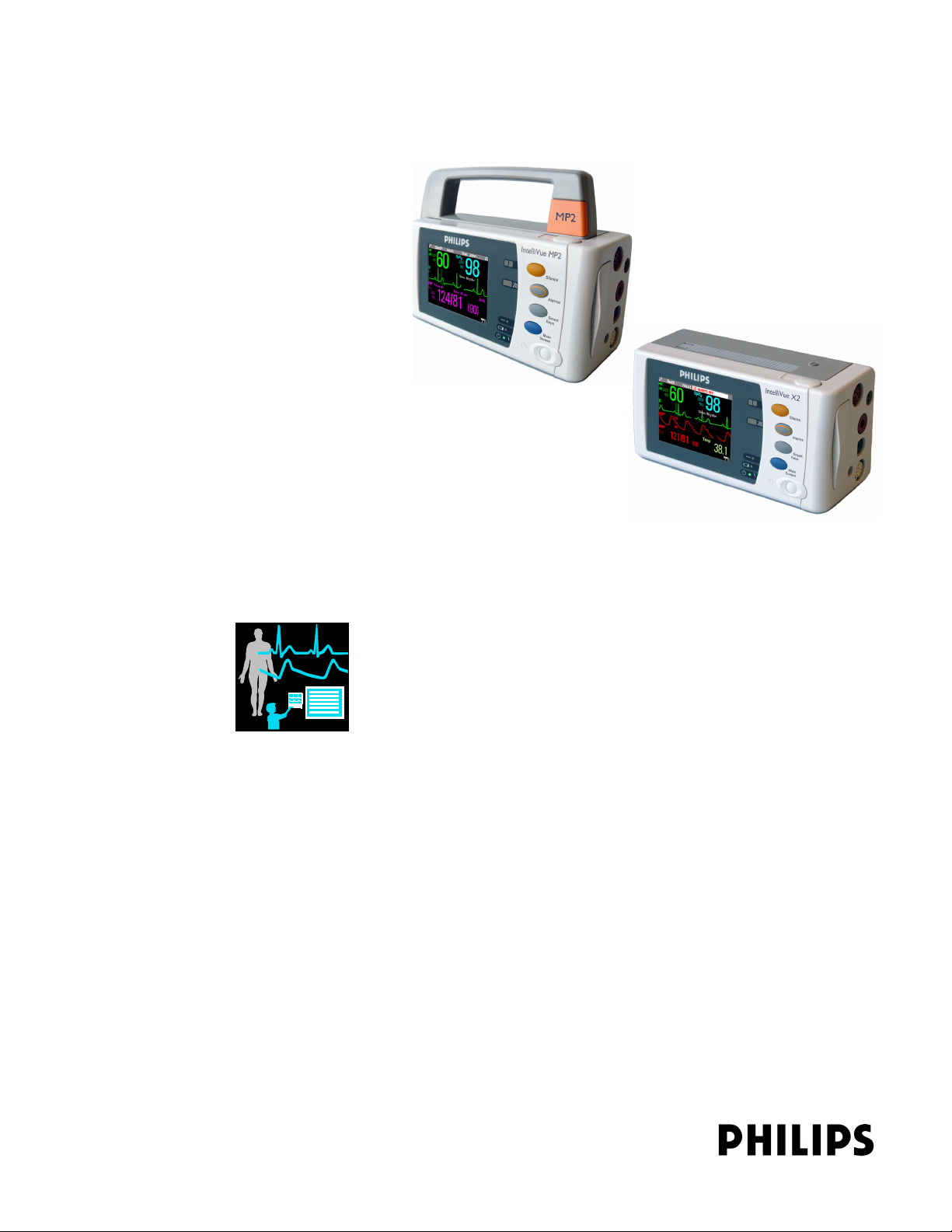
IntelliVue MP2/X2
Service Guide
IntelliVue Patient Monitor
MP2/X2
Patient Monitoring

Part Number M3002-9301B
4535 641 12541
*M3002-9301B*
Contents
1 Introduction 9
Who Should Use This Guide 9
How to Use This Guide 9
Responsibility of the Manufacturer 10
Passwords 10
Warnings and Cautions 11
2 Theory of Operation 13
Monitor Theory of Operation 13
System Boundaries 14
Hardware Building Blocks 15
Data Flow 19
How does the Support Tool Work with the Monitor 22
Monitor Software Block Diagram 22
Block Diagram Legend 23
3 Testing and Maintenance 27
Introduction 27
Terminology and Definitions 28
Recommended Frequency 29
When to Perform Tests 30
Testing Sequence 34
Visual Inspection 35
Before Each Use 35
After Each Service, Maintenance or Repair Event 35
Power On Test 35
Safety Tests 36
Warnings, Cautions, and Safety Precautions 37
Safety Test Procedures 38
System Test 62
What is a Medical Electrical System? 62
General Requirements for a System 62
System Example 63
System Installation Requirements 64
Required Protective Measures at System Installation 65
System Test Procedure 75
Preventive Maintenance Procedures 76
Noninvasive Blood Pressure Measurement Calibration 76
Performance Assurance Tests 76
Basic Performance Assurance Test 76
3

Full Performance Assurance Test 77
ECG/Resp Performance Test 77
ECG Sync Performance Test 78
SpO2 Performance Test 78
NBP PerformanceTest 79
Invasive Pressure Performance Test 81
Temperature Performance Test 82
M3014A Capnography Extension Performance Tests 82
Microstream CO2 Performance Test 85
Cardiac Output Performance Test 90
Power Loss Alarm Buzzer Performance Test (only if Multi-Port Nurse Call Connector Board is installed) 92
IntelliVue 802.11 Bedside Adapter Communication Test 92
IIT Communication Test 93
Short Range Radio (SRR) Performance Test 94
Reporting of Test Results 95
Carrying Out and Reporting Tests 95
Evaluation of Test Results 97
Other Regular Tests 98
Locking/Unlocking Touch Operation 98
Battery Handling, Maintenance and Good Practices 98
About the Battery 98
Checking the Battery Status 99
Battery Status on the Main Screen 100
Battery Status Window 101
Checking Battery Charge 103
Replacing a Battery 103
Optimizing Battery Performance 104
Battery Safety Information 108
After Installation, Testing or Repair 108
4 Troubleshooting 109
Introduction 109
How To Use This Section 109
Who Should Perform Repairs 109
Replacement Level Supported 110
Software Revision Check 110
Software Compatibility Matrix 110
Compatibilty with MMS 111
Compatibility with Information Center 111
Obtaining Replacement Parts 111
Troubleshooting Guide 112
Checks for Obvious Problems 112
Checks Before Opening the Instrument 112
Troubleshooting Tables 114
4
Status Log 130
List of Error Codes 131
Troubleshooting with the Support Tool 131
Troubleshooting the Individual Measurements or Applications 131
5 Repair and Disassembly 133
Who Should Perform Repairs 133
Tools required 133
Removing the Battery 134
Removing the Handle 134
Removing the Side Cover 135
Removing the Display/Exchangi ng the SR R B oar d 136
Reassembly of the Display 138
Removing the NBP Pump Assembly 138
Reassembling the NBP pump chassis 140
Exchanging the NBP Pump 142
Exchanging the NBP Airguide / IIT or WLAN Assembly 145
Reassembly Procedure 147
Exchanging the Loudspeaker 150
Reassembly Procedure 151
Removing the Power Board 152
Reassembly Procedure 154
Removing the ECG Sync Pulse Out Connector 155
Removing the Main Board 157
Removing the Rear Housing 158
Removing the Measurements 160
Exchanging the Main Housing 161
Exchanging the Silicon Pads 162
MMS Extensions - Exchanging the Top Cover, MSL Flex Cable and the Dual Link Bar 165
Exchange Procedures 166
Disassembly Procedures for the M3015A MMS Extension (HW Rev. A) 177
Removing the Front Cover 177
Refit Procedures for the MMS Extension 181
Smart Battery Charger LG1480 (M8043A) 183
Cleaning the Air Filter Mats 183
Replacing the Fan 183
6 Parts 187
Exchange and Replacement Parts 189
MMS Extension Parts (M3012A, M3014A, M3015A and M3016A) 192
MMS Extension Part Numbers - Release Mechanisms 192
MMS Extension Part Numbers - Top Cover, Flex Cable and Link Bar 193
MMS Extension Part Numbers - Front Bezels 193
Exchange Parts List 195
5

Smart Battery Charger Part Numbers
197
7 Installation Instructions 199
Out-Of-Hospital Transport - Standards C om pli ance 199
Electromagnetic Interference (SRR) 201
Installation Checklist 201
Unpacking and Checking the Shipment 201
Initial Inspection 202
Claims for Damage 202
Repacking 202
Mounting the Monitor 203
Mounting the Monitor using the Anti-slip Pad 203
Mounting the Monitor using the MMS Mount and Mounting Clamp 205
Connecting the Monitor to AC Mains 209
Host Monitor as Power Source 209
External Power Supply M8023A(Standard with MP2, Optional with X2) 210
Checking Out the Monitor 211
Configuration Tasks 212
Checking Country-Specific Default Settings 212
Setting Altitude, Line Frequency, ECG Cable Colors and Height & Weight Units 213
Configuring the Equipment Label 213
Configuring IP Address, Subnet Mask and Default Gateway 214
Configuration Settings for CSCN Routed Bedside Monitors (RBM) 214
Configuring Routed Bedside Monitors Support 215
Setting the Date and Time 215
Handing Over the Monitor 216
Philips Clinical Network (Wired) 216
Philips IntelliVue Information Center 216
IntelliVue Instrument Telemetry (IIT) 217
Short Range Radio 218
Configuring SRR Channels 218
ECG Sync Pulse 221
MSL Cable Termination 222
8 Site Preparation 225
Introduction 225
Site Planning 225
Roles & Responsibilities 226
Monitor Site Requirements 228
Space Requirements 228
Environmental Requirements 228
Electrical and Safety Requirements (Customer or Philips) 229
Connecting Non-Medical Devices 230
Philips Medical LAN 230
6

9 MP2/X2 Product Structure 231
Upgrades 238
10 Default Settings Appendix 241
Country-Specific Default Settings 241
11 Index 251
7

8

1
1Introduction
This Service Guide contains technical details for the IntelliVue MP2 Patient Monitor and the
IntelliVue X2.
This guide provides a technical foundation to support effective troubleshooting and repair. It is
not a comprehensive, in-depth explanation of the product architecture or technical
implementation. It offers enough information on the functions and operations of the monitoring
system so that engineers who repair them are better able to understand how it works.
Who Should Use This Guide
This guide is for biomedical engineers or technicians responsible for installing, troubleshooting,
repairing, and maintaining Philips’ patient monitoring systems.
How to Use This Guide
This guide is divided into eight sections. Navigate through the tab le of conte nts at the left of the
screen to select the desired topic. Links to other relevant sections are also provided within the
individual topics. In addition, scrolling through the topics with the page up and page down keys
is also possible.
9

1 Introduction Responsibility of the Manufacturer
Responsibility of the Manufacturer
Philips only considers itself responsible for any effects on safety, EMC, reliability and
performance of the equipment if:
- assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by Philips, and
- the electrical installation of the relevant room complies with national standards, and
- the instrument is used in accordance with the instructions for use.
To ensure safety and EMC, use only those Philips parts and accessories specified for use with
the monitor. If non-Philips parts are used, Philips is not liable for any damage that these parts
may cause to the equipment.
This document contains proprietary information which is protected by copyright. All Rights
Reserved. Reproduction, adaptation, or translation without prior written permission is prohibited,
except as allowed under the copyright laws.
Philips Medizin Systeme Böblingen GmbH
Hewlett-Packard Str. 2
71034 Böblingen, Germany
The information contained in this document is subject to change without notice.
Philips makes no warranty of any kind with regard to this material, including, but not limited to,
the implied warranties or merchantability and fitness for a particular purpose.
Philips shall not be liable for errors contained herein or for incidental or consequential damages
in connection with the furnishing, performance, or use of this material.
Passwords
In order to access different modes within the monitor a password may be required. The
passwords are listed below.
Monitoring Mode: No password required
Configuration Mode: 71034
Demo Mode: 14432
Service Mode: 1345
Consult the configuration guide before making any changes to the monitor configuration.
10

Warnings and Cautions 1 Introduction
Warnings and Cautions
In this guide:
- A warning alerts you to a potential serious outcome, adverse event or safety hazard. Failure
to observe a warning may result in death or serious injury to the user or patient.
- A caution alerts you where special care is necessary for the safe and effecti ve use of the
product. Failure to observe a caution may result in minor or moderate personal injury or
damage to the product or other property, and possibly in a remote risk of more serious
injury.
11

1 Introduction Warnings and Cautions
12 13

2Theory of Operation
Monitor Theory of Operation
The IntelliVue MP2/X2 Patient Monitor:
- displays real-time data
- alarms in the case of patient or equipment problems
- offers limited data storage and retrieval (trending)
- interfaces to the Philips Clinical Network and other equipment
2
NOTE
The monitor can be configured with various different measurement and interface capabilities.
The following descriptions may vary depending on the monitor option purchased.
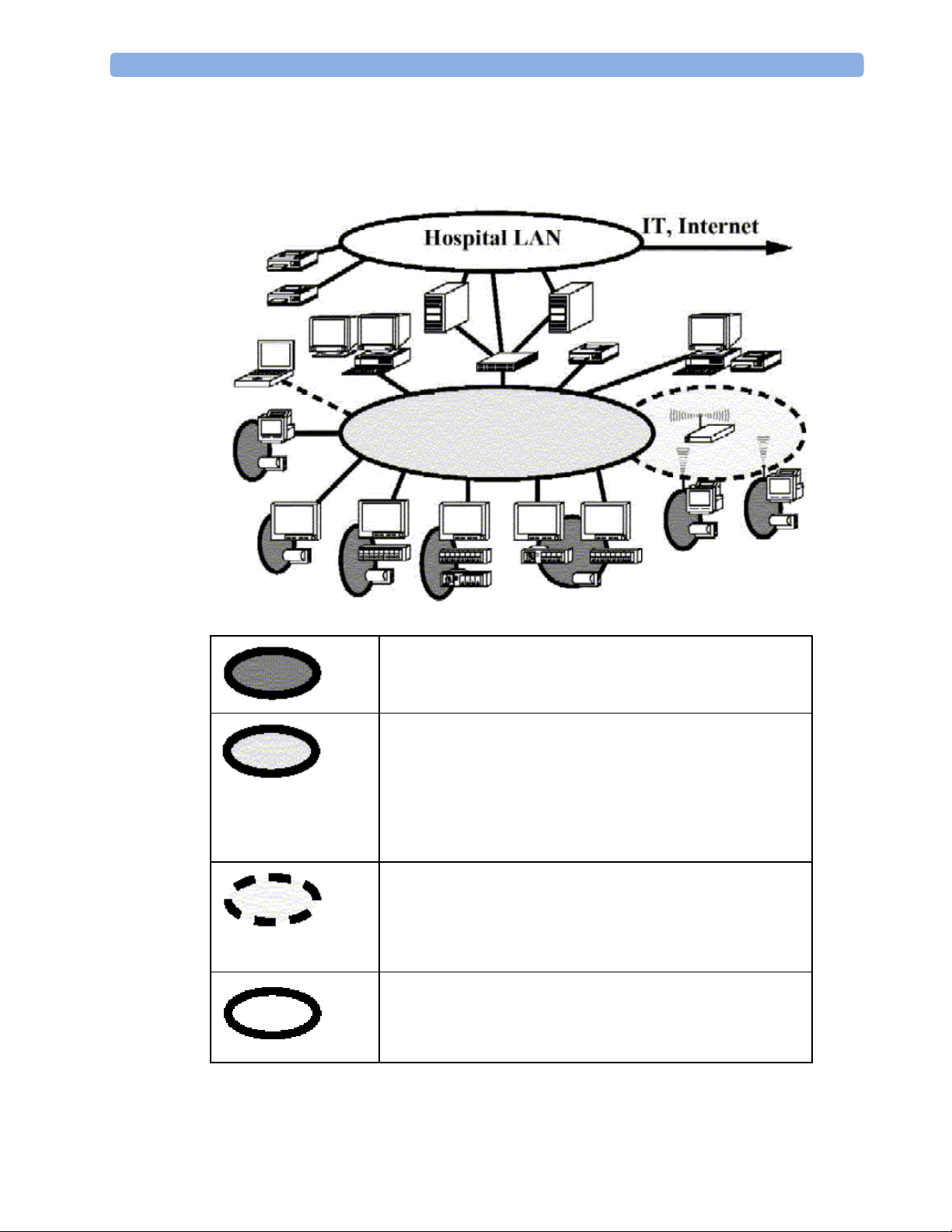
2 Theory of Operation Monitor Theory of Operation
System Boundaries
The following diagram discusses specific boundaries within the overall system with respect to
their openness and real-time requirements:
System Boundaries
Measurement connections
Built-in measurement block
Philips Clinical Network (wired LAN)
connects multiple patient monitors, information centers,
application servers; closed system, only Philips qualified
products (tested and with regulatory approval) are connected,
Philips is responsible for guaranteed real-time functionality and
performance
Philips Clinical Network (wireless)
like Philips Clinical Network (wired) LAN, however due to
current wireless technologies available it has reduced
bandwidth, longer latencies, reduced functionality
Hospital LAN, Internet
Standard Network, not under Philips control, no guaranteed
service, no real-time requirements
14
Monitor Theory of Operation 2 Theory of Operation
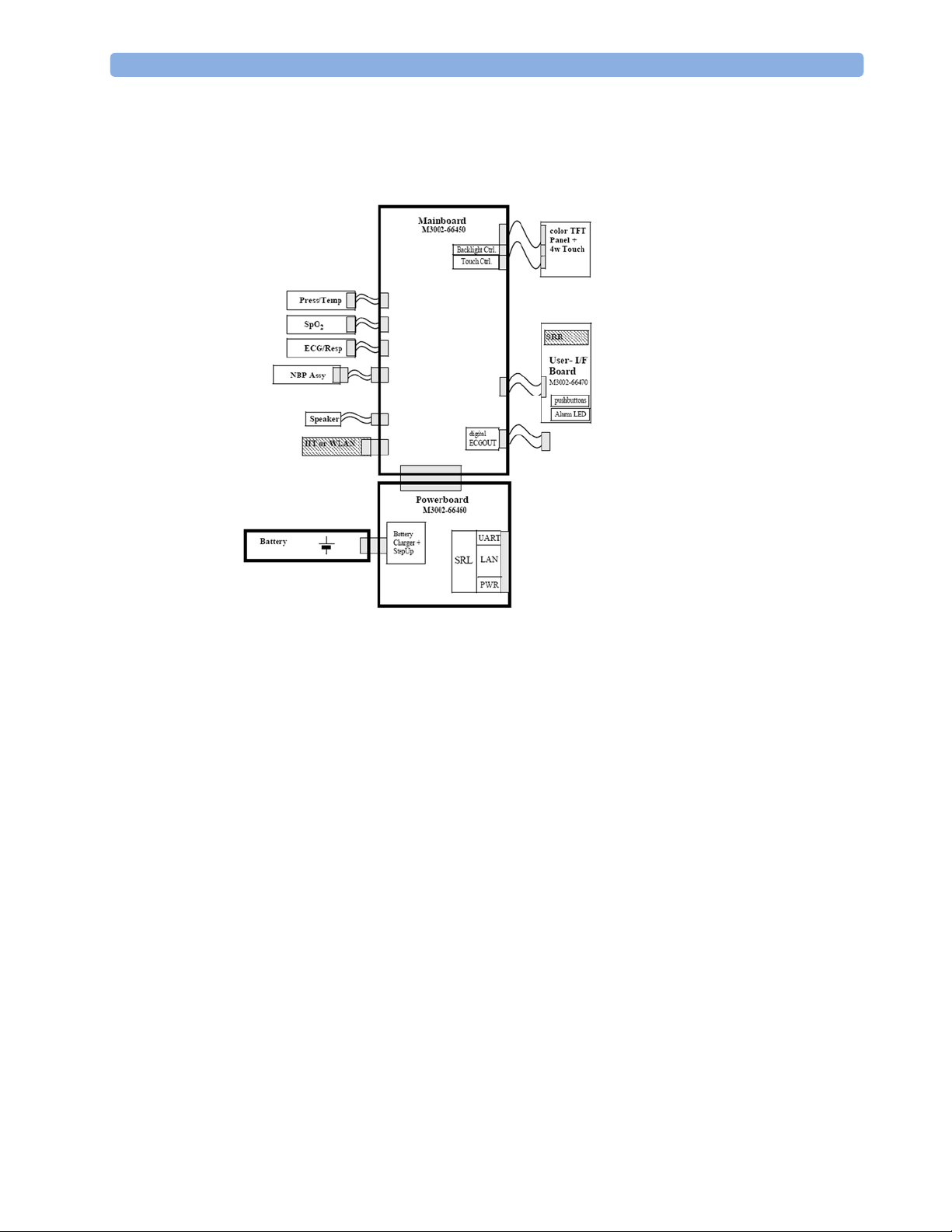
Hardware Building Blocks
The following hardware building blocks make up the monitoring system:
IntelliVue MP2
MP2/X2 Hardware Building Blocks
The MP2 monitor:
- integrates the display and processing unit into a single package
- uses a 3.5” color TFT display
- uses the Touchscreen as input device
- integrates the measurement block with optional parameter sets
- has an internal battery
- standalone patient monitor
15
2 Theory of Operation Monitor Theory of Operation
IntelliVue X2
The IntelliVue X2:
- integrates the display and processing unit into a single package
- uses a 3.5” color TFT display
- uses the Touchscreen as input device
- integrates the measurement block with optional parameter sets
- has an internal battery
- can be used as a Multi-Measurement Module or as a standalone patient monitor
Optional Hardware
- An optional built-in wireless network interface (IntelliVue 802.11 Bedside Adapter or
IntelliVue Instrument Telemetry) is supported. For further details regarding the wireless
network please refer to the M3185A Philips Clinical Network documentation.
- Integrated Short Range Radio (SRR)
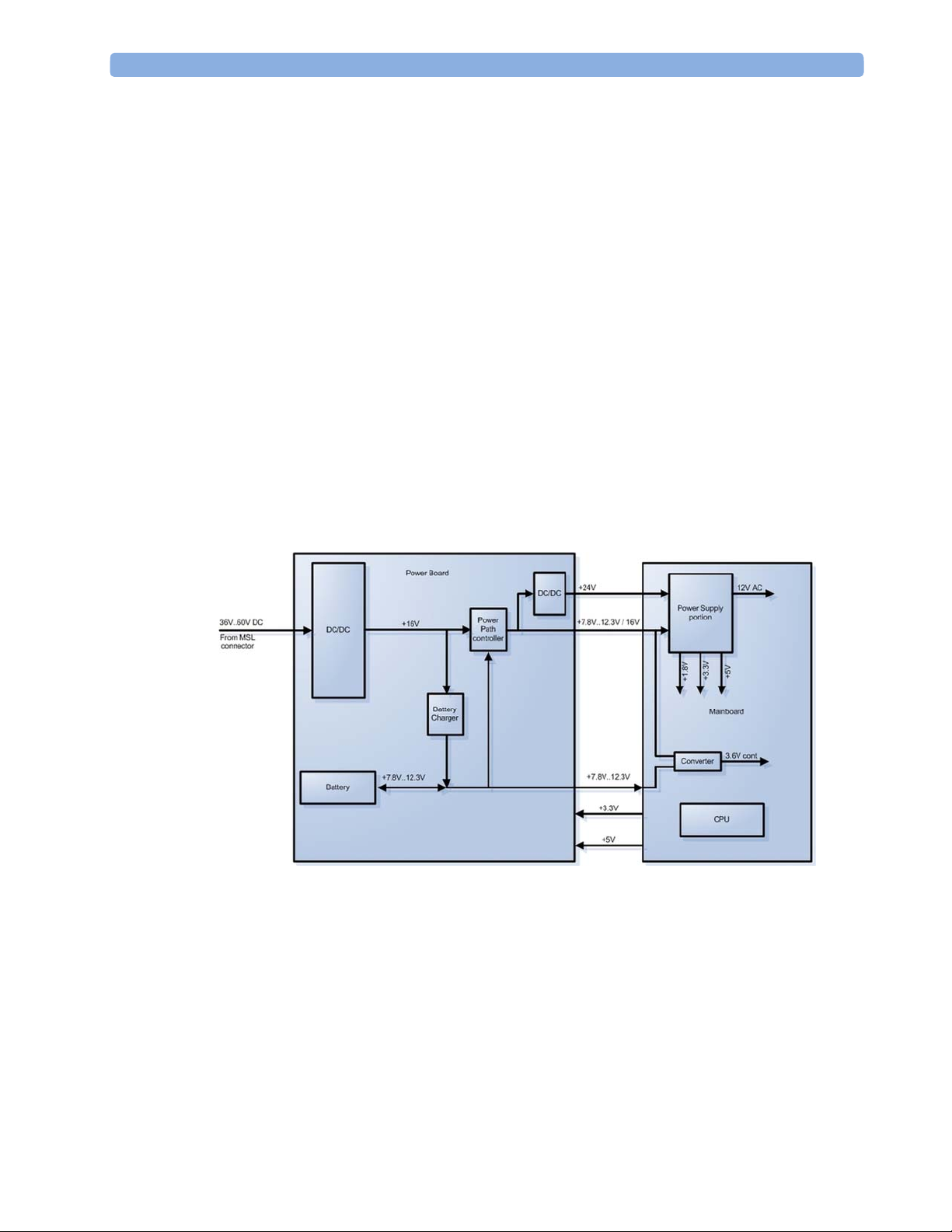
Power Distribution
Power Distribution Architecture
16

Monitor Theory of Operation 2 Theory of Operation
The DC/DC converter transforms the DC power (36-60 V DC range) coming from the MSL plug
into a 16 V DC source and isolates the monitoring system from the DC MSL.
The 16V DC is distributed via the Power Board to the battery charging circuit and to the main
board.
The power is used to charge the battery and supply the monitoring system. As soon as the DC
power source is disconnected, the battery starts and keeps the system powered (battery mode).
The main board contains power supply circuits, which convert the 16 V DC into several voltages
supplying the particular components of the monitoring system.
The realtime clock and the buffered RAM is supplied with cont. 3.6 V DC power, provided
either by the 16 V DC system power or by the battery power and converted to 3.6 V DC.
The CPU board has an MPC852 MHz processor in the patient monitor that provides a number of
on-chip, configurable interfaces. An array of fast UARTS with configurable protocol options are
implemented in an ASIC (along with other system functions such as independent watchdogs,
video, etc.), providing interfacing capabilities to integrated measurements. The main board
contains additional video hardware.
The CPU provides a LAN interface to connect to the Philips Clinical Network (Ethernet).
System Interfaces
The LAN interface on the Measurement Link (MSL) is used as the network interface.
17

2 Theory of Operation Monitor Theory of Operation
Compatible Devices
NOTE
M3012A, M3014A, M3015A, M3016A MMS Extensions
The MMS Extensions are not supported if the IntelliVue MP2/X2 is powered from the internal
battery. Although they can still be attached, they will not function in this case.
18
Monitor Theory of Operation 2 Theory of Operation
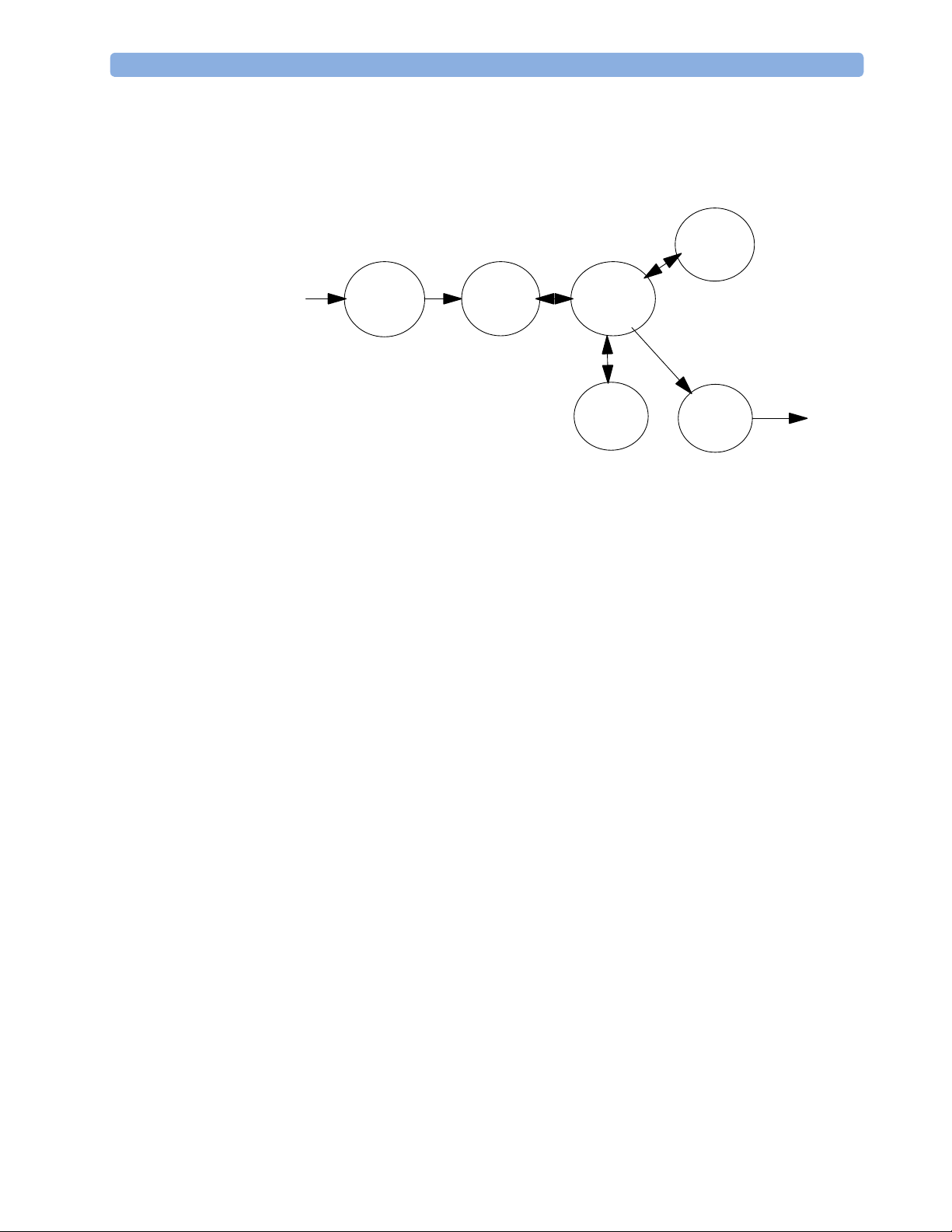
Data Flow
The following diagram shows how data is passed through the monitoring system. The individual
stages of data flow are explained below.
Display
and User
Interface
Data
Acquisition
Data
Provider
Service
Applications
Data Flow
Data Acquisition
Monitoring data (for example patient measurement data in the form of waves, numerics and
alerts) is acquired from a variety of sources:
- Measurement Block
- External measurement devices
- Server systems on the Philips Clinical Network
Persistent
Data
Storage
Data
Output
The integrated measurements convert patient signals to digital data and apply measurement
algorithms to analyze the signals.
Data can be also acquired from devices connected to the monitor. Software modules
dedicated to such specific devices convert the data received from an external device to the
format used internally.
To enable networked applications, data can be acquired from server systems attached to the
Philips Clinical Network, for example a Philips Information Center
19

2 Theory of Operation Monitor Theory of Operation
Data Provider System Service
All data that is acquired from integrated measurements or external measurement devices is
temporarily stored by a dedicated data provider system service. All monitor applications use this
central service to access the data in a consistent and synchronized way rather than talking to the
interfaces directly.
This service makes the applications independent of the actual type of data acquisition device.
The amount of data stored in the data provider system service varies for the different data types.
For example several seconds of wave forms and the full set of current numerical values are
temporarily stored in RAM.
Persistent Data Storage System Service
Some applications require storage of data over longer periods of time. They can use the
persistent data storage system service. Dependent on the application requirements, this service
can store data either in battery backed-up (buffered) memory or in flash memory. The buffered
memory will lose its contents if the monitor is without power (not connected to mains) for an
extended period of time. The flash memory does not lose its contents.
The trend application for example stores vital signs data in a combination of flash memory and
buffered memory, while the system configuration information (profiles) is kept purely in flash
memory.
Display and User Interface Service
Applications can use high level commands to display monitoring data or status and command
windows on the internal LCD panel. These commands are interpreted by the display manager
application. This application controls the dedicated video hardware which includes video
memory and a special hardware in the ASIC.
User input is acquired from the touchscreen. The system software makes sure that the user input
is directed to the application which has the operating focus.
Monitor Applications
The monitor applications provide additional system functionality over the basic measurement
and monitoring capabilities. This includes for example trending, report generating, event storage
or derived measurements.
In general, the monitor applications use the data provider system service to access the
measurement data. Application interfaces to the other system services allow the application to
visualize data, to store data over extended periods of time or to output data to other devices.
20

Monitor Theory of Operation 2 Theory of Operation
Internal LAN (Measurement Link)
The MP2/X2 communicates using an IEEE802.3 Ethernet LAN in the Measurement Link
(MSL). This network is used to distribute data between the components, for exam ple:
- Digitized patient signals including wave data, numerical data and status information
(typically from the measurement server to a display unit)
- Control data representing user interactions (typically from the display unit to a measurement
server)
- Shared data structures, for example representing patient demographical data and global
configuration items
The internal LAN allows plug and play configuration of the monitoring system. The system
automatically detects plugging or unplugging of measurement servers and configures the system
accordingly.
The components on the internal LAN are time-synchronized to keep signal data consistent in the
system. Dedicated hardware support for synchronization eliminates any latency of the network
driver software.
The integrated LAN provides deterministic bandwidth allocation/reservation mechanisms so that
the real-time characteristic of signal data and control data exchange is guaranteed. This applies
to the data flow from the X2 to the host monitor (for example measurement signal data) and the
data flow from the host monitor to an X2 (for example to feed data to a recorder module).
Philips Clinical Network
The monitoring system may be connected to the Philips Clinical Network, for example to
provide central monitoring capabilities or other network services. This connection may be
through a normal wired connection.
After configuration, the monitoring system sends the digitized patient signals including wave
data, numerical data and status information onto the network. Control data representing user
interactions can be exchanged between the monitoring system and a central station
bi-directionally.
For plug and play operation, the monitoring system uses the standard BootP protocol to
automatically acquire a network address.
21
2 Theory of Operation Monitor Theory of Operation
How does the Support Tool Work with the Monitor
The support tool is a Windows application typically installed on the laptop of a customer
engineer or a biomedical engineer working in the customer’s own service department.
The purpose of the support tool is to upgrade, configure and diagnose all monitoring componen ts
in the system over the network.
The service protocol developed for this purpose uses a raw access to the devices without the
need for IP addresses etc. over a standard customer network installation, so that even defective
devices can be upgraded as long as the few kBytes of initial boot code are working. The boot
code itself can also be upgraded using the same protocol.
The tool allows access to internal service information and to serial numbers. It can be remotecontrolled, for example via a dial-up connection from a response center, provided the proper
infrastructure is in place.
For details see the Instructions for Use for the Support Tool.
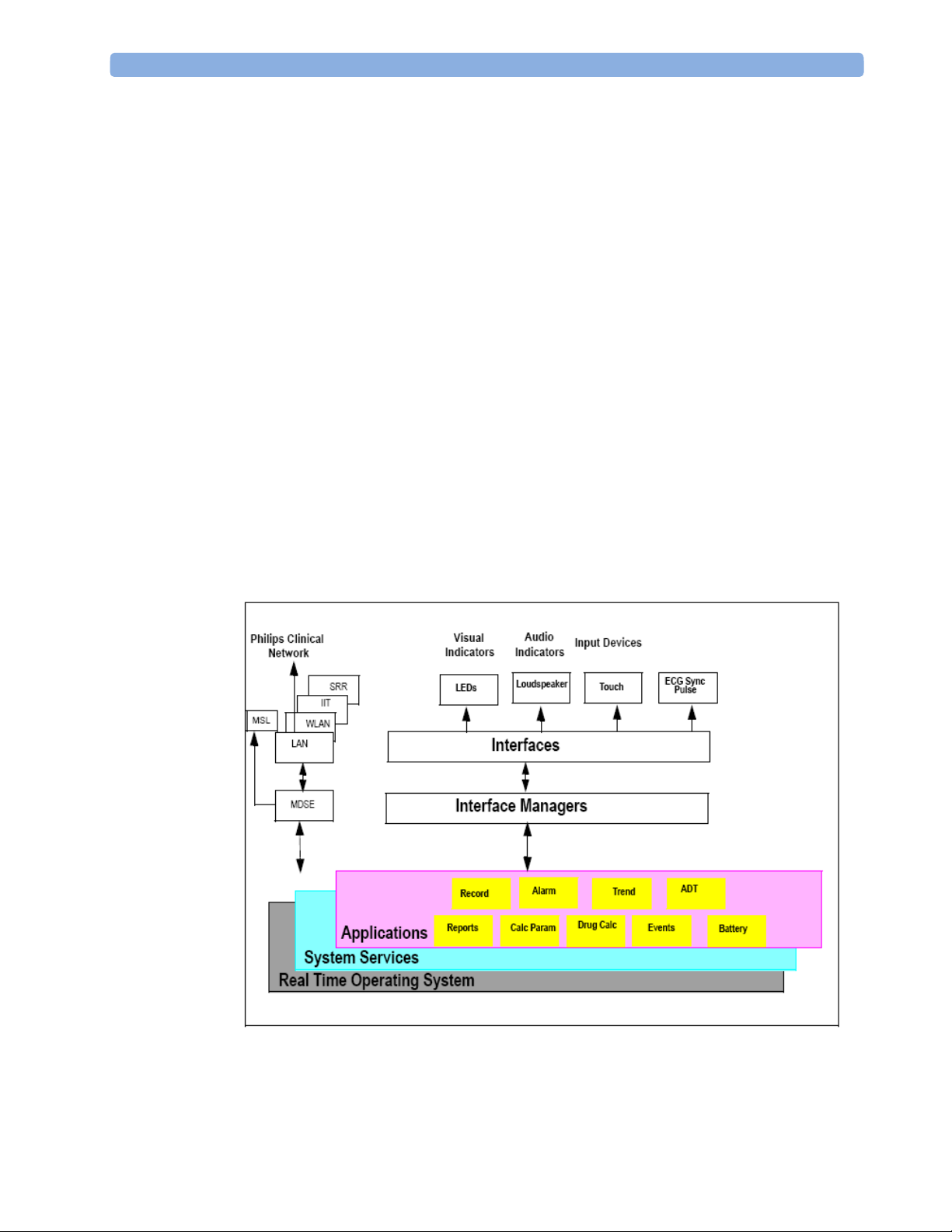
Monitor Software Block Diagram
The figure below shows the functional block diagram for the monitoring system. A legend
explaining terms and diagram elements follows. The information below varies depending on the
purchased monitor options.
Functional Block Diagram
22

Monitor Theory of Operation 2 Theory of Operation
Block Diagram Legend
Functional Block Description
Services
Operating System The Operating System (OS) provides a layer of isolation between the specific
hardware implementation and the application software. The OS performs system
checks and allocates resources to ensure safe operation when the system is first
started. This includes internal self-tests on several hardware modules and
configuration checks for validity of configuration with the operating software.
During normal operation, the OS continues to run checks on system integrity. If
error conditions are detected the OS will halt monitoring operations and inform
the operator about the error condition.
System Services The System Services provide generic common system services.
In particular:
They use a real-time clock component to track time. They synchronize to
network time sources and verify the accuracy of the system time information.
They are also responsible for managing persistent user configuration data for all
Measurement parameters and IntelliVue Patient Monitoring System software
modules. User configuration data is stored in a non-volatile read/write storage
device
Applications
Reports The Reports Service retrieves current and stored physiological data and status
data to format reports for printing paper documentation. Examples of supported
reports:
- Vital Signs Report
- Graphical Trend Report
- Event Review Report
- Event Episode Report
- ECG Report (12 Lead/Multi-Lead)
- Test Report
The Reports service generates report data which can be printed on a central
printer.
23
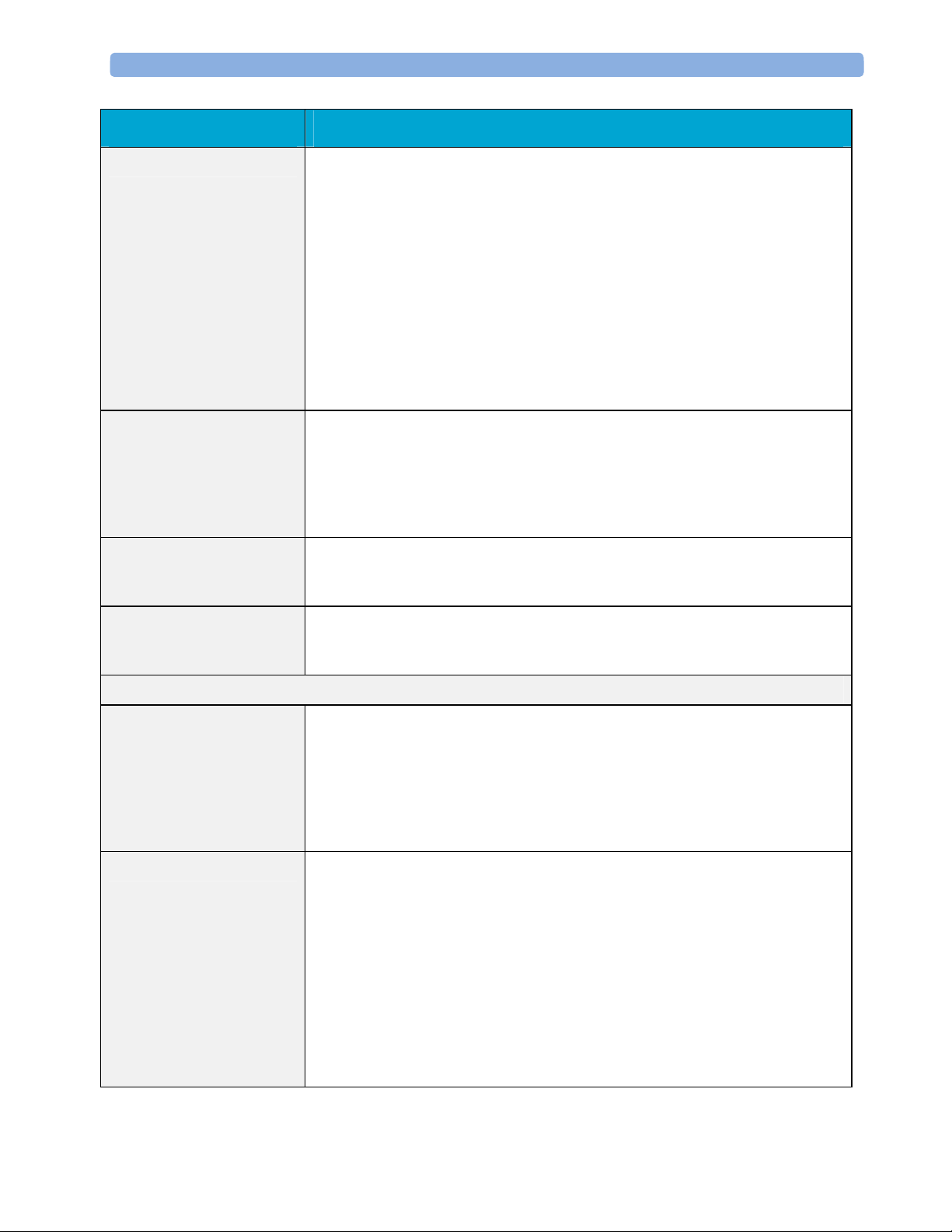
2 Theory of Operation Monitor Theory of Operation
Functional Block Description
Alarm The Alarm Service contains logic that prioritizes alarm conditions that are
generated by IntelliVue Patient Monitoring System software modules. Visual
alarm signals (messages) are displayed at the top of the IntelliVue Patient
Monitoring System display and alarm sounds are generated by a loudspeaker.
Alarm conditions may be generated when a physiological parameter exceeds
preselected alarm limits or when a physiological parameter or any other software
module reports an inoperative status (technical alarm, for example, the ECG
leads may have fallen off the patient). The Alarm service manages the alarm
inactivation states, for example suspension of alarms, silencing of alarms, and
alarm reminder. Alarm signals may also be configured as latching (alarm signals
are issued until they are acknowledged by the operator, even when the alarm
condition is no longer true). The Alarm service controls the visual alarm signals
(alarm lamps).
Trend The Trend service stores the sample values of physiological data and status data
with a resolution of 12 seconds, 1 minute or 5 minutes for a period of up to 48
hours. The data is kept in battery buffered read/write storage and flash memory
devices to be preserved across power failures. The stored data is protected via
consistency checks and checksums. When a new patient is admitted, the trend
database erases all data of the previous patient.
ADT The ADT (Admit/Discharge/Transmit) service maintains the patient
demographics information. The operator may admit a new patient, discharge the
old patient and enter or modify the patient demographics.
Calc Param The Calc Param (Calculated Parameters) application performs calculations on
physiological numerical values to derive calculated parameters like Temperature
Difference.
Interface Managers
MDSE The MDSE (Medical Data Service Element) Interface Manager is responsible
for the exchange of real-time data between the IntelliVue Patient Monitoring
System display unit and the Measurement parameters and other devices attached
to the network. MDSE establishes and maintains a data communication link
between the devices. It provides configuration information about the remote
device to applications in the local device and it allows the exchange of
measurement data and status information between the devices.
Printer The Printer Interface Manager provides a high level interface to a printer. It
provides means to:
- establish a connection to the printer
- transfer data to the printer
- get status of the printer
- close connection to the printer
The Printer Interface Manager also supervises the connection to the printer and
whether the printer accepts data (for example paper out). The Printer Interface
Manager notifies the operator in such cases.
24
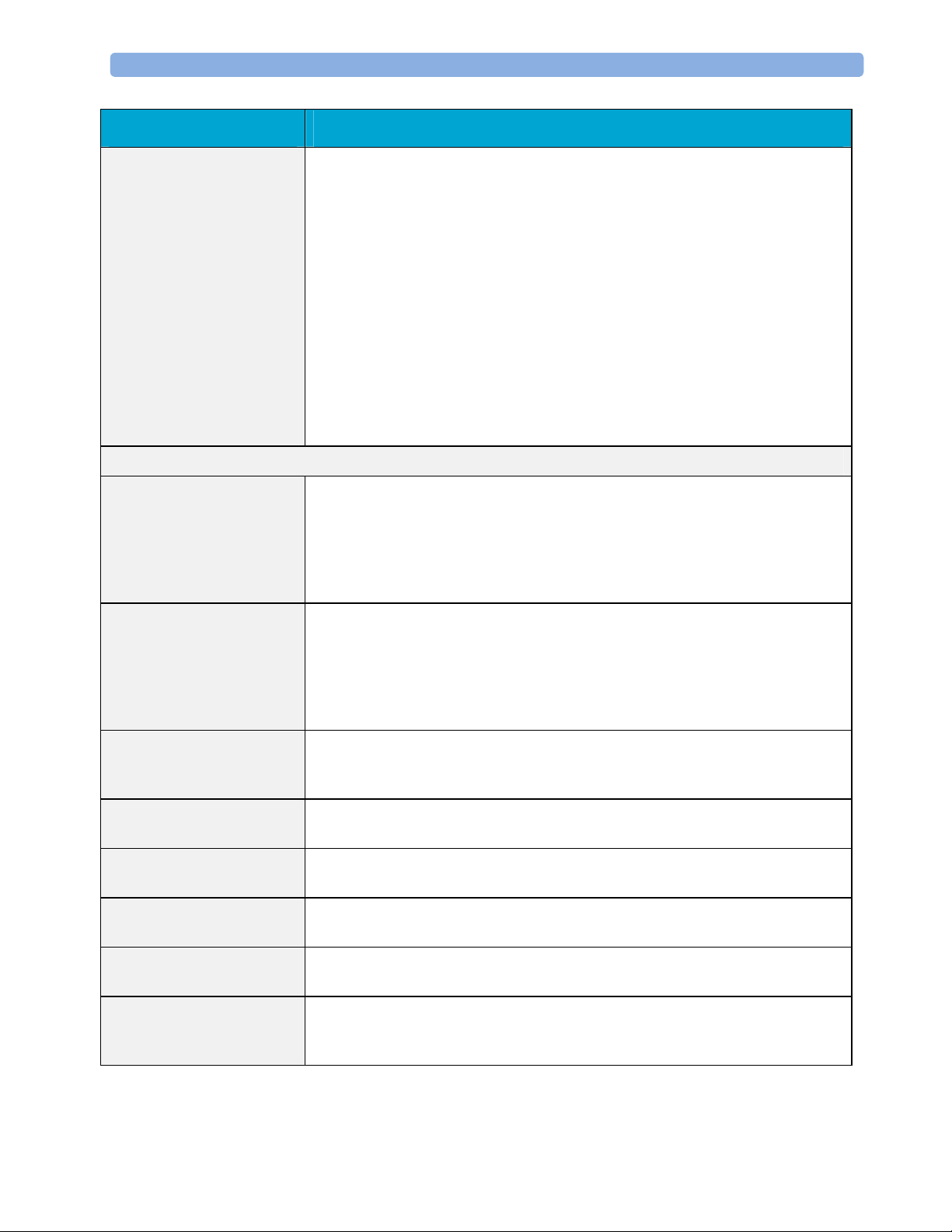
Monitor Theory of Operation 2 Theory of Operation
Functional Block Description
Display & Operator
Interface
The Display and Operator Interface Manager performs the following tasks:
- Screen presentation of real-time and stored physiological measurement data,
alarm condition data and status information received from the MDSE
interface manager, the Alarm service or other IntelliVue Patient Monitoring
System modules
- Screen presentation of operating controls (control windows)
- Processing of operating control commands received from HIF Control
interface. The module verifies and interprets the received commands and
forwards them to other software modules of the IntelliVue Patient
Monitoring System display unit or measurement parameters.
- Sound generation (issues audible alarm signals and generates audible
information signals, for example QRS and SpO2 tones, operator audible
feedback)
Interfaces
LAN The LAN interface implements the physical layer of IEEE 802.3. The LAN
interface performs Manchester encoding/decoding, receive clock recovery,
transmit pulse shaping, jabber, link integrity testing, reverse polarity
detection/correction, electrical isolation, and ESD protection. Electronically
separated interfaces are used for communication to the Measurement parameters
and to the network.
Display Controller The Display Controller Interface consists of a video controller, video RAM and
the controlling software. The Display Controller interface processes the high
level display commands (character and graphic generation, wave drawing) and
translates them into pixels, which are written into the video RAM where the
video controller chip generates the video synchronization signals and the pixel
stream for the Color LCD Display.
HIF Control The HIF (Human Interface Control) interface scans the Human Interface devices
for operator controls (Touch Screen), formats the collected data and sends it to
the display and Operating Interface.
Sync Out (ECG) A pulse signal is provided on the Sync Out connector to allow synchronisation
with other medical devices.
IIT The built-in IIT module allows operation of the MP2/X2 monitors within
IntelliVue Instrument Telemetry Infrastructure.
WLAN The built-in WLAN interface allows wireless operation of the X2/MP2 monitors
with the IntelliVue 802.11 Bedside Adapter
SRR The built-in SRR interface allows wireless communication of the MP2/X2
monitors with an IntelliVue Instrument Telemetry Transceiver.
MSL All components of the monitoring system communicate using an IEEE802.3/
Ethernet LAN in the Measurement Link (MSL). This network is used to
distribute data between the components
25


3Testing and Maintenance
Introduction
This chapter provides a checklist of the testing and maintenance procedures to ensure the
performance and safety of the monitor and the MMS Extensions. For testing of the host monitor
and the Flexible Module Rack (FMS), see the Service Guide of the host monitor.
3
These tests must be performed only by qualified personnel certified by the responsible
organization. Qualifications required are: training on the subject, knowledge, experience and
acquaintance with the relevant technologies, standards and local regulations. The personnel
assessing safety must be able to recognize possible consequences and risks arising from
non-conforming equipment.
All recurring safety and performance assurance tests must be performed under equal
environmental conditions to be comparable.
Testing of the MP2/X2 may be performed either on the MP2/X2 (with external power supply)
directly or (for the X2) on the host monitor.
Preventive Maintenance refers specifically to the series of tests required to make sure the
measurement results are accurate. The accuracy and performance procedures are designed to be
completed as specified in the following sections or when readings are in question.
For detailed instructions on the maintenance and cleaning of the monitor and its accessories, see
Care and Cleaning, Using Batteries and Maintenance and Troubleshooting in the monitor's
Instructions for Use.
27

3 Testing and Maintenance Terminology and Definitions
Terminology and Definitions
The following terms and definitions are used throughout this chapter and taken from the
international standards IEC 60601-1, IEC 60601-1-1 and IEC 62353.
- Medical System: a medical electrical system is a combination of at least one medical
electrical device and other electrical equipment, interconnected by functional connection or
use of a multiple portable socket-outlet.
- Patient Vicinity: any area in which intentional or unintentional contact can occur between
the patient and parts of the medical system or between the patient and other persons who
have had contact with parts of the medical system. The patient vicinity is defined anywhere
within 1.5m (5 feet) of the perimeter of the patient's bed and 2.5m (8.2 feet) from the floor.
- Separation Device/Transformer: a component or arrangement of components with input
parts and output parts that, for safety reasons, prevent a transfer of unwanted voltage or
current between parts of a medical system.
- Multiple Portable Socket-Outlet: a combination of two or more socket-outlets intended to
be connected to or integrated with flexible cables or cords, which can easily be moved from
one place to another while connected to the power mains.
- Functional Connection: an electrical connection for transfer of signals and/or power.
- Tests: Safety or Performance Assurance test procedures which may consist of several steps.
28
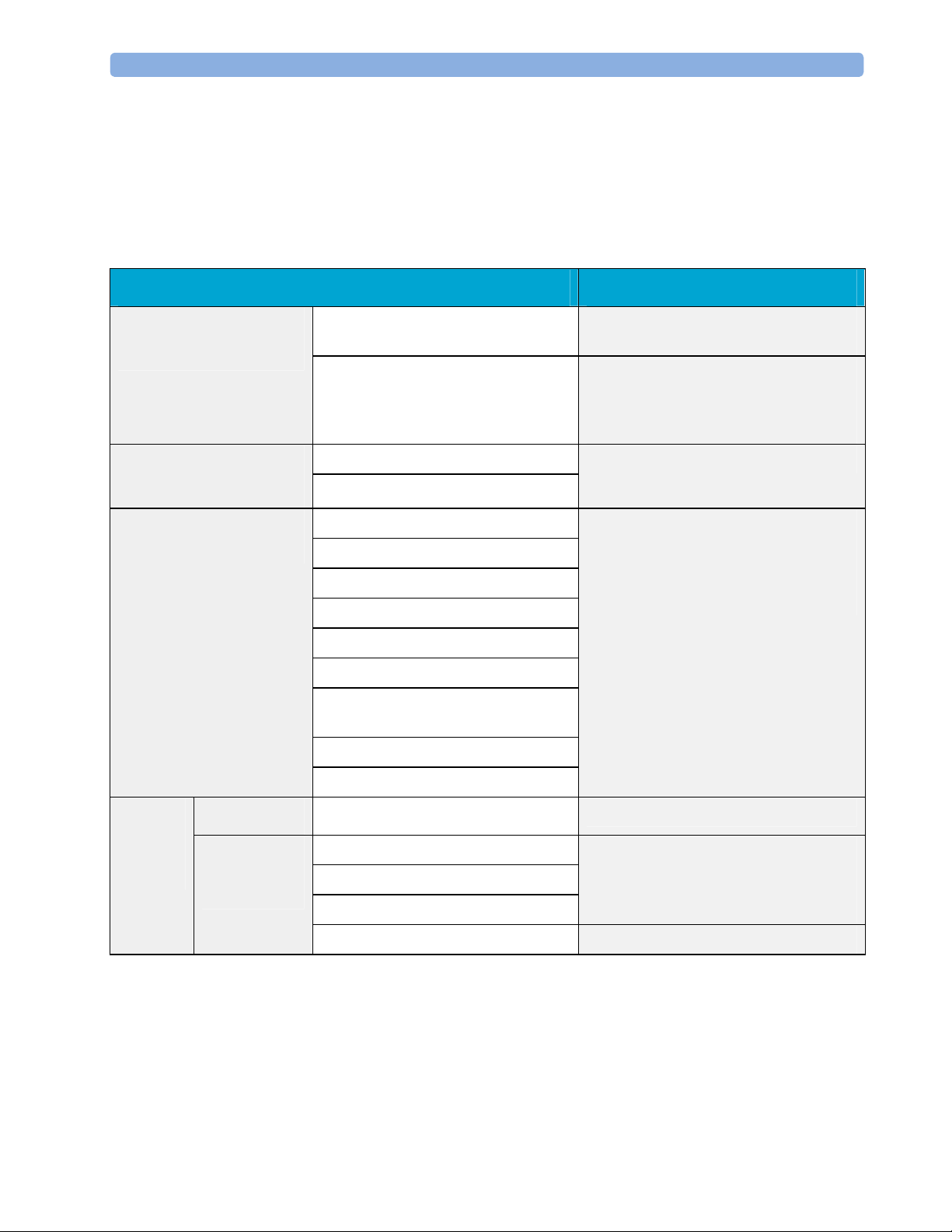
Recommended Frequency 3 Testing and Maintenance
Recommended Frequency
Perform the procedures as indicated in the suggested testing timetable. These timetable
recommendations do not supersede local requirements.
Tests Frequency
Preventive Maintenance*
Table 1: Suggested Testing Timetable
NBP Performance Once every two years, or more often if
specified by local laws.
Microstream CO2 Calibration Once a year or after 4000 hours of
continuous use and following any
instrument repairs or the replacement of
any instrument parts.
Other Regular Tests
Performance Assurance
Tests
Safety
Tests
Visual
Electrical
Visual Inspection
Before each use.
Power On Test
ECG/Resp Performance
ECG Sync Pulse Performance
SpO2 Performance
NBP Performance
Once every two years, or if you suspect
the measurement is incorrect, except
Mainstream CO2 Accuracy Check,
Sidestream CO2 Accuracy Check and
Flow Check - required once a year.
Invasive Pressure Performance
Temperature Accuracy
M3014A Capnography Extension
Performance Tests
Microstream CO2 Performance Test
C.O. Performance Test
Visual Inspection After each service event.
Protective Earth
Equipment Leakage Current
Patient Leakage Current
Once every two years and after repairs
where the power supply has been
removed or replaced or the monitor has
been damaged by impact.
System Test Once every two years
*M3015A with the old hardware Rev. A (i.e. Serial No. DE020xxxxx) also require the CO2
pump/CO
scrubber replacement procedure. This is required every three years or after 15000
2
operating hours.
29
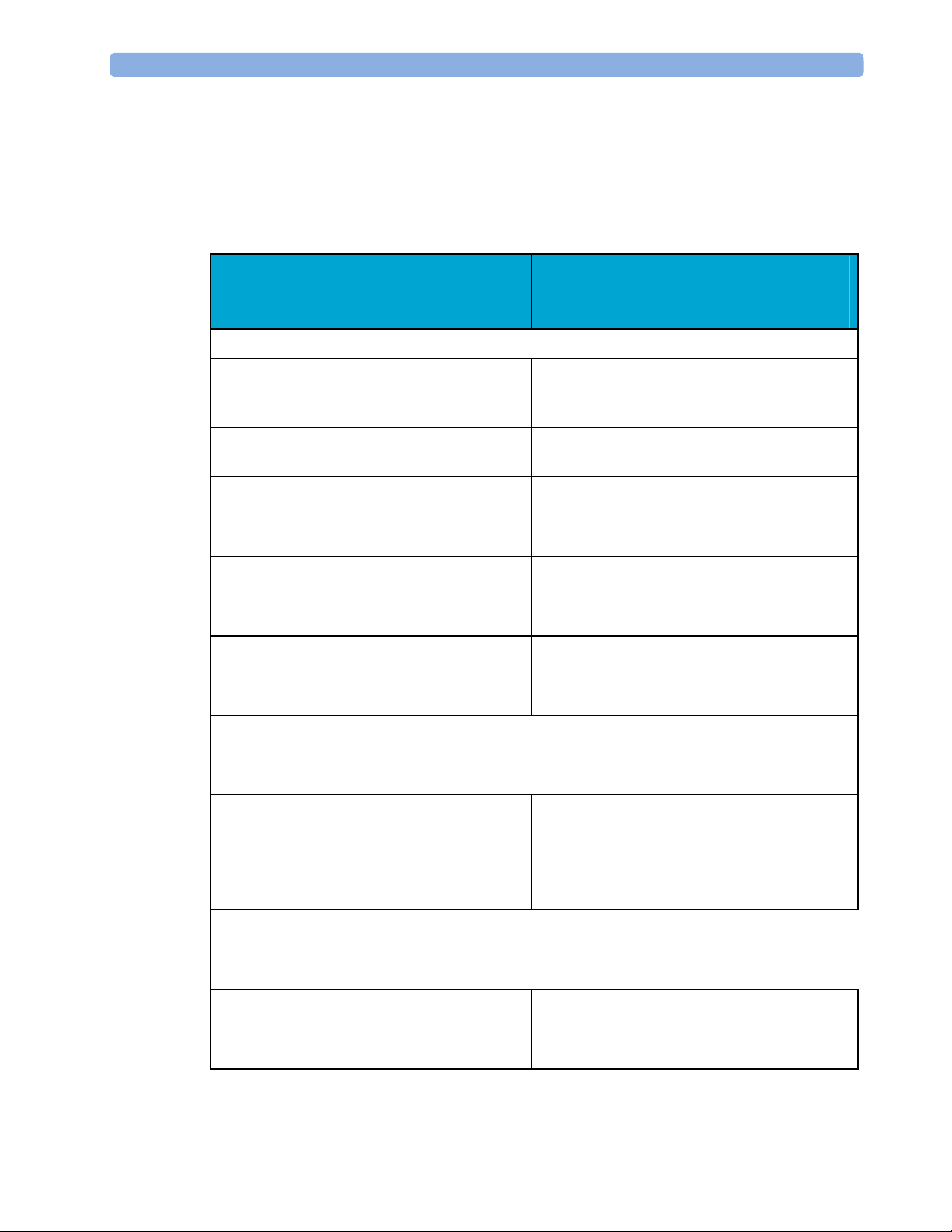
3 Testing and Maintenance When to Perform Tests
When to Perform Tests
This table tells you when to perform specific tests.The corresponding test procedures are
described in the following sections All tests listed below must be performed on the monitor
itself and its host monitor.
When to perform tests
Service Event
(When performing...
Installation
Installation of a monitor in combination with
a medical or non-medical device connected to
the same multiple socket outlet.
Installation of monitor with IntelliVue
Instrument Telemetry (IIT)
Installation of monitor with IntelliVue
802.11 Bedside Adapter
Installation of a monitor with Short Range
Radio (SRR)
Installation of networked monitor (LAN) Perform Visual Inspection and Power On Test
Preventive Maintenance
Tests Required
...Complete these tests)
Perform Visual Inspection, Power On and
System Tests
Perform Visual Inspection, Power On and IIT
communication test
Perform Visual Inspection, Power On and
IntelliVue 802.11 Bedside Adapter
Communication Test
Perform Visual Inspection, Power On and SRR
communication test
Preventive Maintenance*
Other Regular Tests and Tasks
Visual Inspection
30
Perform preventive maintenance tests and
procedures:
- NBP calibration
- Microstream CO2 calibration
Perform Visual Inspection test block

When to Perform Tests 3 Testing and Maintenance
Service Event
(When performing...
Power On Test
Repairs
Repairs where the monitor has been damaged
by impact, liquid ingression, fire, short circuit
or electrical surge.
Repairs where the MSL power board is
removed or replaced
Repairs where the main board has been
replaced.
Tests Required
...Complete these tests)
Perform Power On test block
Perform Visual Inspection, Power On, all
Safety Tests and Full Performance Assurance
Tests
Perform Visual Inspection, Power On, all
Safety Tests and Basic Performance Assurance
Test
Perform Visual Inspection, Power On, Basic
Performance Assurance Test and NBP
Accuracy Test and Calibration.
Repairs where the measurement block has
been removed or replaced
Repairs of IntelliVue Instrument Telemetry
(IIT) Module
Repairs of IntelliVue 802.11 Bedside
Adapter
Repairs of Short Range Radio (SRR)
Interface
Repairs where the rear housing has been
removed or replaced.
Repairs where the NBP pump has been
replaced
Perform Visual Inspection, Power On, all
Safety Tests and Basic Performance Assurance
Test.
If a certain parameter seems suspicious,
perform Full Performance Assurance Test for
this parameter.
Perform Visual Inspection, Power On Test
Block and IIT communication test
Perform Visual Inspection, Power On and
IntelliVue 802.11 Bedside Adapter
Communication Test
Perform Visual Inspection, Power On and SRR
Communication Test
Perform Visual Inspection, Power On, all
Safety Tests and Basic Performance Assurance
Test.
Perform Visual Inspection, Power On, all
Safety Tests, Basic Performance Assurance
Test and NBP Performance Test and
Calibration
Repairs of the M3015A MMS Extension Perform Visual Inspection, Power On, all
Safety Tests, Basic Performance Assurance
Test
31

3 Testing and Maintenance When to Perform Tests
Service Event
(When performing...
All other IntelliVue Monitoring System
repairs (except when MSL power board is
Tests Required
...Complete these tests)
Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test
removed)
Performance Assurance
Basic Performance Assurance Perform basic performance assurance tests for
the respective monitoring system component.
Full Performance Assurance Perform all accuracy and performance test
procedures listed in the following sections. If a
particular measurement is in question, perform
the measurement performance test only.
Upgrades
Software Upgrades Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test unless
otherwise specified in the Upgrade Installation
Notes shipped with the upgrade.
Hardware Upgrades Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test unless
otherwise specified in the Upgrade Installation
Notes shipped with the upgrade.
Hardware Upgrades where IntelliVue
Instrument Telemetry (IIT) is installed
Perform Visual Inspection, Power On Test,
Basic Performance Assurance Test and IIT
communication Test
Hardware Upgrades where IntelliVue 802.11
Bedside Adapter is installed
Perform Visual Inspection, Power On Test,
Basic Performance Assurance Test and
IntelliVue 802.11 Bedside Adapter
Communication Test
Hardware Upgrades where Short Range
Radio (SRR) is installed
Perform Visual Inspection, Power On Test,
Basic Performance Assurance Test and SRR
communication Test
Installation of Interfaces or Hardware
Upgrades where the power supply or
Perform Visual Inspection, Power On Test,
Basic Performance Tests and all Safety Tests
parameter boards need to be removed.
Combining or Exchanging System
Components
Perform the System Test for the respective
system components
*M3015A with the old hardware Rev. A (i.e. Serial No. DE020xxxxx) also require the pump and
scrubber replacement procedures.
32

When to Perform Tests 3 Testing and Maintenance
NOTE
It is the responsibility of the facility operator or their designee to obtain reference values for
recurring safety and system tests. These reference values are the results of the first test cycles
after an installation. You may also purchase this service from Philips.
33

3 Testing and Maintenance Testing Sequence
Testing Sequence
Summary of the recommended sequence of testing:
Start
Select the test
Visual Inspection
Safety Tests
Performance Tests
Reporting of Results
Evaluation of Results
See When to Perform Tests
See Visual Test (see "
page
35).
Before Each Use" on
See Safety Test Procedures (on page
38).
See Performance Assurance Tests (on page
76).
See Reporting of Test Results (on page
95)
See Evaluation of Test Results (on page
97)
NOTE
Check and prepare for normal use
If any single test fails, testing must be discontinued immediately and the device under test must
be repaired or labeled as defective.
34

Visual Inspection 3 Testing and Maintenance
Visual Inspection
Before Each Use
Check all exterior housings for cracks and damage. Check the condition of all external cables,
especially for splits or cracks and signs of twisting. If serious damage is evident, the cable should
be replaced immediately. Check that all mountings are correctly installed and secure. Refer to
the instructions that accompany the relevant mounting solution.
After Each Service, Maintenance or Repair Event
Ensure all fuses accessible from the outside comply with the manufacturer’s specification.
Check:
- the integrity of mechanical parts, internally and external ly .
- any damage or contamination, internally and externally
- that no loose parts or foreign bodies remain in the device after servicing or repair.
- the integrity of all relevant accessories.
Power On Test
1. Connect the monitoring system to mains and switch it on. This includes connected displays
2. Make sure that all steps listed in the table Initial Instrument Boot Phase in the
The expected test result is pass: the monitor boots up and displays an ECG wave. The wave
might be a flat line if no simulator is attached.
and MMS Extensions.
Troubleshooting section are completed successfully and that an ECG wave appears on the
screen.
35

3 Testing and Maintenance Safety Tests
Safety Tests
The following safety test needs to be performed on the monitoring system:
- applied part leakage current
- system test (if required)
Safety test requirements are set according to international standards, their national deviations and
specific local requirements. The safety tests detailed in this Service Guide are derived from
international standards but may not be sufficient to meet local requirements. We recommend that
you file the results of safety tests. This may help to identify a problem early particularly if the
test results deteriorate over a period of time.
Each individual piece of equipment of the monitoring system which has its own connection to
mains or which can be connected or disconnected from mains without the use of a tool must be
tested individually. The monitoring system as a whole must be tested according to the System
Test (on page
Accessories of the monitoring system which can affect the safety of the equipment under test or
the results of the safety test must be included in the tests and documented.
61) procedure.
Electrical safety tests for MP2/X2 can be performed either on the individual device (MP2 or X2)
connected to the external power supply or on a connected host monitor (e.g. MP20-90). Note that
if the electrical safety tests are performed with a host monitor the protective earth resistance and
equipment leakage current come mainly from the host monitor. The earthing of MP2/X2 is for
functional purposes and does not provide protection against electric shock. The protection
against electric shock in this device is provided by double and/or reinforced insulation. The
protective earth resistance and equipment leakage current measurements for MP2/X2 are
optional.
36

Safety Tests 3 Testing and Maintenance
Warnings, Cautions, and Safety Precautions
- These tests are well established procedures of detecting abnormalities that, if undetected,
could result in danger to either the patient or the operator.
- Disconnect the device under test from the patient before performing safety tests.
- Disconnect the device under test from mains before performing safety tests. If this is not
possible, ensure that the performance of these tests does not result in danger to the safety
analyzer operator, patients or other individuals.
- Test equipment (for example, a Safety Analyzer) is required to perform the safety tests.
Please refer to Annex C of IEC/EN 62353 for exact requirements for the measurement
equipment and for measurement circuits for protective earth resistance and leakage currents.
Refer to the documentation that accompanies the test equipment. Only certified technicians
should perform safety testing.
- The consistent use of a Safety Analyzer as a routine step in closing a repair or upgrade is
emphasized as a mandatory step to maintain user and patient safety. You can also use the
Safety Analyzer as a troubleshooting tool to detect abnormalities of line voltage and
grounding plus total current loads.
- During safety testing, mains voltage and electrical currents are applied to the device under
test. Ensure that there are no open electrical conductive parts during the performance of
these tests. Avoid that users, patients or other individuals come into contact with touch
voltage.
- For Europe and Asia/Pacific, the monitor complies with:
IEC60601-1:1988 + A1:1991 + A2:1995 = EN60601-1:1990 +A1:1993 + A2:1995
IEC60601-1-1:2000
For USA, the monitor complies with:
UL60601-1
For Canada, CAN/CSA C22.2#601.1-M90
- Local regulations supersede the testing requirements listed in this chapter.
- If a non-medical electrical device is connected to a medical electrical device, the resulting
medical electrical system must comply with IEC/EN 60601-1-1.
- Perform safety tests as described on the following pages.
37

3 Testing and Maintenance Safety Tests
Safety Test Procedures
Use the test procedures outlined here only for verifying safe installation or service of the
product. The setups used for these tests and the acceptable ranges of values are derived from
local and international standards but may not be equivalent. These tests are not a substitute for
local safety testing where it is required for an installation or a service event. If using an approved
safety tester, perform the tests in accordance with the information provided by the manufacturer
of the tester and in accordance with your local regulations, for example IEC/EN 60601-1,
UL60601-1 (US), IEC/EN 62353, and IEC/EN 60601-1-1. The safety tester should print results
as detailed in this chapter, tog ether with other data.
Please refer to Annex C of IEC/EN 62353 for requirements for the measurement equipment and
for measurement circuits for protective earth resistance and leakage currents.
The following symbols are used in the diagrams illustrating the safety tests:
CAUTION
Supply mains
Protective earth
L, N Supply mains terminals PE Protective earth terminal
Mains part
F-type applied part
Applied part
Measuring device
Connection to accessible
conductive parts
.........
Resistance measuring
device
Optional connection
After each service, maintenance or repair event:
Ensure all fuses accessible from the outside comply with the manufacturer’s specification.
Check:
- the integrity of mechanical parts, internally and external ly .
- any damage or contamination, internally and externally.
- that no loose parts or foreign bodies remain in the device after servicing or repair.
- the integrity of all relevant accessories.
38

Safety Tests 3 Testing and Maintenance
Hints for Correct Performance of Safety Tests
- Perform a visual inspection on all detachable power cords used with the monitoring system
and include these in all safety test procedures.
- Connection lines such as data lines or functional earth conductors may appear to act like
protective earth connections. These may lead to incorrect measurements and need to be
considered during testing. If necessary, unplug these connections.
- Position all cables and cords in such a manner that they do not influence the safety tests.
- Measurement of insulation resistance is not required.
Guideline for Performance of Safety Tests
Connect the detachable power cord of the device under test to the safety analyzer's test mains
port. For testing the detachable power cord protective earth, use the setup pr ov ided with your
safety analyzer. For testing the equipment leakage current and the applied part leakage current,
connect all applied parts to the safety analyzer using the appropriate patient lead or adapter
cable. For the ECG parameter all ten ECG-leads need to be connected to the safety analyzer. If
necessary, use an adapter cable to connect all ten ECG-leads. If necessary, repeat the safety test
procedure until all available applied parts have been tested. Refer to the documentation that
accompanies the safety analyzer for further details on how to set up and perform the test.
NOTE
39
Detachable Power Cord Protective Earth Test - Setup Example
Equipment Leakage Current and Applied Part Current Test - Setup Example
The above graphics resemble the Metron QA-90 setup and are protected by copyright. Copyright
owned by Fluke (Metron).

3 Testing and Maintenance Safety Tests
Safety Test Adapter Cable - Schematics
The following graphics provide schematics of safety test (patient lead) adapter cables which can
be used for electrical safety testing. These schematics can also be used as a guideline for making
your own safety test adapter cables. Alternatively, other methods to make safety test adapter
cables can be used, e.g. using a modified accessory cable.
NOTE
You may not need all of the cables displayed below for electrical safety testing of your
respective monitor.
ECG:
40

Safety Tests 3 Testing and Maintenance
SpO2 (MP2/X2, MP5, M3001A & M1020B #A01, #A02, #A03):
41

3 Testing and Maintenance Safety Tests
SpO2 (M1020A):
42

Safety Tests 3 Testing and Maintenance
Invasive Pressure:
43

3 Testing and Maintenance Safety Tests
M1006B #C01:
Temperature:
44

Safety Tests 3 Testing and Maintenance
CO2 (MP5, M3014A):
45

3 Testing and Maintenance Safety Tests
CO2 (M1016A, M3016A):
4 = all resistors 120 KOhm
46

Safety Tests 3 Testing and Maintenance
Cardiac Output:
47

3 Testing and Maintenance Safety Tests
BIS:
Use Clamp Adapter Cable and M1034-61650 BIS sensor simulator.
48

Safety Tests 3 Testing and Maintenance
VueLink:
4 = 220 Ohm
49

3 Testing and Maintenance Safety Tests
IntelliBridge:
50

Safety Tests 3 Testing and Maintenance
EEG:
51

3 Testing and Maintenance Safety Tests
SvO2 (M1021A):
52

Safety Tests 3 Testing and Maintenance
ScvO2 (M1011A):
53

3 Testing and Maintenance Safety Tests
tcpO2/tcpCO2:
54

Safety Tests 3 Testing and Maintenance
MP5 predicitive Temperature:
55

3 Testing and Maintenance Safety Tests
MP5 TAAP:
56

Safety Tests 3 Testing and Maintenance
S(1): Detachable Power Cord Protective Earth Test (optional)
This test can be performed upon request by the customer.
Test to perform:
Use an Ohmmeter to measure the earth wire resistance of the detachable power cord.
This safety test is based on IEC/EN 60601-1, IEC/EN 62353, UL2601-1 Ed. 2/UL60601-1:2003
and CSA 601.1-M90.
Report the highest value (X1).
Test Expected test results
Protective Earth Resistance Test X1 <= 100mOhms
NOTE
- If the protective earth resistance test fails, testing must be discontinued immediately and the
device under test must be repaired or labeled as defective.
- Flex the power cord during the protective earth resistance test to eva luate its integrity. If it
does not pass the test, exchange the power cord.
- The functional earth conductor is required for EMC purposes. It has no protective function
against electrical shock. The protection against electrical shock is provided by double and/or
reinforced insulation.
S(2): Equipment Leakage Current Test - Normal Condition (optional)
This test can be performed upon request by the customer.
Test to perform:
57

3 Testing and Maintenance Safety Tests
Measuring circuit for the measurement of Equipment Leakage Current - Direct method
according to IEC/EN 62353.
This test measures the functional earth leakage current. It tests normal and reversed polarity.
Perform the test with S1 closed (Normal Condition). There is no exposed metal part or functional
earth connector which can be used to attach a test lead.
This safety test is based on IEC/EN 60601-1, IEC/EN 62353, UL2601-1 Ed. 2/UL60601-1:2003
and CSA 601.1-M90.
Report the highest value (X1).
Test Expected test results
Equipment Leakage Current Test
X1 <= 100μA
(Normal Condition - with mains
cable)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise
stated.
S(3): Equipment Leakage Current Test - Single Fault Condition (optional)
This test can be performed upon request by the customer.
Test to perform:
58

Safety Tests 3 Testing and Maintenance
Measuring circuit for the measurement of Equipment Leakage Current - Direct method
according to IEC/EN 62353.
This test measures the functional earth leakage current. It tests normal and reversed polarity.
Perform the test with S1 open (Single Fault Condition). There is no exposed metal part or
functional earth connector which can be used to attach a test lead.
This safety test is based on IEC/EN 60601-1, IEC/EN 62353, UL2601-1 Ed. 2/UL60601-1:2003
and CSA 601.1-M90.
Report the highest value (X2).
Test Expected test results
Equipment Leakage Current Test
X2 <= 300μA
(Single Fault Condition - with mains
cable)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise
stated.
59

3 Testing and Maintenance Safety Tests
S(4): Applied Part Leakage Current - Mains on Applied Part
NOTE
During measurement of the Applied Part Leakage Current it is possible that the measured current
can exceed the allowed limit (per IEC/EN 60601-1 or IEC/EN 62353).
This can occur when the safety tester is connected to the invasive blood pressure and
temperature connectors at the same time during the applied leakage current measurement.
The connectors for the invasive blood pressure and temperature are independently functioning
connectors.
Although there are individual connectors on the front end, internally those parameters use the
same electrical insulation interface and are hardwired to each other. This results in an electrical
short of those connectors during measurement if a test current is applied simultaneously .
Therefore this should be avoided.
Due to the combined insulation interface, it is sufficient to connect to only one parameter
interface (that is, Invasive Blood Pressure or Temperature) of the invas ive bl ood
pressure/temperature measurement block. This avoids a short and the potential of exceeding the
limit for the current.
Test to perform:
60

Safety Tests 3 Testing and Maintenance
Measuring circuit for the measurement of Applied Part Leakage Current - Direct method
according to IEC/EN 62353.
This test measures applied part leakage current from applied part to earth caused by external
main voltage on the applied part. Each polarity combination possible shall be tested. This test is
applicable for ECG measurement inputs. There is no exposed metal part or functional earth
connector which can be used to attach a test lead.
This safety test is based on IEC/EN 60601-1, IEC/EN 62353, UL2601-1 Ed. 2/UL60601-1:2003
and CSA 601.1-M90.
For measurement limits and test voltage, refer to test block Safety (4), Test and Inspection
Matrix.
Report the highest value. (X1).
Test Expected test results
Applied Part Leakage Current Test
X1 <= 50μA
(Single Fault Condition - mains on
applied part)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise
stated.
61

3 Testing and Maintenance System Test
System Test
After mounting and setting up a system, perform system safety tests according to IEC/EN
60601-1-1.
What is a Medical Electrical System?
A medical electrical system is a combination of at least one medical electrical piece of
equipment and other electrical equipment, interconnected by functional connection or use of a
multiple portable socket-outlet.
- Devices forming a medical electrical system must comply with IEC/EN 60601-1-1.
- Any electrical device such as IT equipment that is connected to the medical electrical
equipment must comply with IEC/EN 60601-1-1 and be tested accordingly.
General Requirements for a System
After installation or subsequent modification, a system must comply with the requirements of the
system standard IEC/EN 60601-1-1. Compliance is checked by inspection, testing or analysis, as
specified in the IEC/EN 60601-1-1 or in this book.
Medical electrical equipment must comply with the requirements of the general standard IEC/EN
60601-1, its relevant particular standards and specific national deviations. Non-medical electrical
equipment shall comply with IEC safety standards that are relevant to that equipment.
Relevant standards for some non-medical electrical equipment may have limits for equipment
leakage currents higher than required by the standard IEC/EN 60601-1-1. These higher limits are
acceptable only outside the patient environment. It is essential to reduce equipment leakage
currents to values specified in IEC 60601-1 when non-medical electrical equipment is to be used
within the patient environment.
62

System Test 3 Testing and Maintenance
System Example
This illustration shows a system where both the medical electrical equipment and the
non-medical electrical equipment are situated at the patient’s bedside.
WARNING
- Do not use additional AC mains extension cords or multiple portable socket-outlets. If a
multiple portable socket-outlet is used, the resulting system must be compliant with IEC/EN
60601-1-1. Do not place multiple socket-outlets on the floor. Do not exceed the maximum
permitted load for multiple socket-outlets used with the system. Do not plug additional
multiple socket outlets or extension cords into multiple socket outlets or extension cords
used within the medical electrical system.
- Do not connect any devices that are not supported as part of a system.
- Do not use a device in the patient vicinity if it does not comply with IEC/EN 60601-1. The
whole installation, including devices outside of the patient vicinity, must comply with
IEC/EN 60601-1-1. Any non-medical device placed and operated in the patient’s vicinity
must be powered via a separating transformer (compliant with IEC/EN 60601-1-1) that
ensures mechanical fixing of the power cords and covering of any unused power outlets.
63

3 Testing and Maintenance System Test
System Installation Requirements
- Ensure that the the medical electrical system is installed in a way that the user achieves
optimal use.
- Make sure the user is informed about the required cleaning, adjustment, sterilization and
disinfection procedures listed in the Instructions for Use.
- The medical electrical system must be installed in such a way that the user is able to carry
out the necessary cleaning, adjustment, sterilization and disinfection procedures listed in the
Instructions for Use.
- Ensure that the medical electrical system is installed in a way that an interruption and
restoration of power to any part of the medical electrical system does not result in a safety
hazard.
- We recommend using fixed mains socket outlets to power the medical system or parts
thereof. Avoid using multiple portable socket-outlets.
- Any multiple portable socket outlets used must be compliant with IEC 60884-1 and IEC
60601-1-1.
- Ensure that any part of the system connected to multiple portable socket-outlets is only
removable with a tool, i.e. the multiple portable socket-outlet provides a locking mechanism
to prevent power cords from being plugged or unplugged unintentionally. Otherwise, the
multiple portable socket-outlet must be connected to a separation device. Multiple Socket
Outlets used within the medical electrical system must only be used for powering medical
electrical equipment which is part of the system.
- Ensure that any functional connections between parts of the medical electrical system are
isolated by a separation device according to IEC 60601-1-1 to limit increased equipment
leakage currents caused by current flow through the signal connections. This only works if
the equipment leakage current of the respective medical electrical system parts is not
exceeded under normal conditions.
- Avoid increase of equipment leakage currents when non-medical electrical equipment within
the medical electrical system is used. This only works if the equipment leakage current of
the respective medical electrical system parts is not exceeded under normal conditions. Use
additional protective earth connection, separation device or additional non-conductive
enclosures.
- Within the patient environment it is important to limit electrical potential differences
between different parts of a system. If necessary, use potential equalization equipment
(equipotential cable) or additional protective earth connections.
- Medical electrical equipment used in medical rooms must be connected to potential
equalization equipment (equipotential cable) to avoid electrical potential differences. Check
your local requirements for details.
64

System Test 3 Testing and Maintenance
Required Protective Measures at System Installation
For any IT equipment (IEC60950) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC 60601-1. Use a separation device to ensure
compliance. After installation of IT equipment in patient vicinity, an enclosure leakage current
test is required.
65

3 Testing and Maintenance System Test
Case 1: Medical Device Combined with Medical Device
If you combine a medical device with another medical device (incl. Philips specified displays) to
form a medical electrical system according to IEC60601-1-1, no additional protective measures
are required. The medical electrical devices may be located in or outside the patient vicinity in a
medically used room. This is valid as long as the medical devices are connected to separate
mains outlets. No system test is required.
66

System Test 3 Testing and Maintenance
If the combined medical devices are connected to the same multiple portable socket outlet an
enclosure leakage current test of the entire device combination on the multiple portable socket
outlet is required to ensure that the resulting protect ive earth leakage current and equipment
leakage current does not exceed the limits of IEC 60601-1-1. Avoid using multiple portable
socket outlets. The medical electrical devices may be located in or outside the patient vicinity in
a medically used room. If the limits are exceeded, additional protective measures are required,
e.g. a separation device or the connection of each device to separate mains.
67

3 Testing and Maintenance System Test
Case 2: Medical Device Combined with a Non-Medical Device
If you combine a medical device with a non-medical device to form a medical electrical system
according to IEC60601-1-1, additional protective measures are required, e.g. usage of a
separation device. The medical electrical devices or the IT equipment may be located in or
outside the patient vicinity in a medically used room. After system installation incl. protective
measures, a system test is required to ensure that the resulting equipment leakage current and
applied part leakage current does not exceed the limits of IEC 60601-1-1.
68

System Test 3 Testing and Maintenance
For any IT equipment (IEC60950) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC 60601-1. Use a separation device to ensure
compliance. After installation of IT equipment in patient vicinity, an enclosure leakage current
test is required.
69

3 Testing and Maintenance System Test
If the combined devices forming the medical electrical system are connected to the same
multiple portable socket outlet, ensure that the resulting protective earth leakage current and
equipment leakage current do not exceed the limits of IEC 60601-1-1. The medical electrical
devices or IT equipment may be located in or outside the patient vicinity in a medically used
room. Avoid using multiple portable socket outlets. If the limits of IEC 60601-1-1 are exceeded,
additional protective measures are required, e.g. a separation device or the connection of each
device to separate mains.
70

System Test 3 Testing and Maintenance
For any IT equipment (IEC60950) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC 60601-1. Use a separation device to ensure
compliance. After installation of IT equipment in patient vicinity, an enclosure leakage current
test is required.
71

3 Testing and Maintenance System Test
Case 3: Medical Device Combined with a Medical or Non-Medical Device with one Device in a Non-Medically-Used Room
If you combine a medical device with a medical or non-medical device to form a medical
electrical system according to IEC60601-1-1 using a common protective earth connection and
one of the devices is located in a non-medically used room, additional protective measures are
required, e.g. usage of a separation device or additional protective eart h connec tion. The medical
electrical devices or IT equipment may be located in or outside the patient vicinity. After system
installation incl. protective measures, a system test is required to ensure that the resulting
equipment leakage current does not exceed the limits of IEC 60601-1-1.
72

System Test 3 Testing and Maintenance
73

3 Testing and Maintenance System Test
If you combine a medical device with a medical or non-medical device to form a medical
electrical system according to IEC60601-1-1 using two separate protective earth connections and
one of the devices is located in a non-medically used room creating a potential voltage
difference, additional protective measures are required, e.g. usage of a separation device or
additional protective earth connection. The medical electrical devices or IT equipment may be
located in or outside the patient vicinity. After system installation incl. protective measures, a
system test is required to ensure that the resulting equipment leakage current does not exceed the
limits of IEC 60601-1-1.
74

System Test 3 Testing and Maintenance
System Test Procedure
If the medical electrical device has already been tested as a standalone device e.g. during factory
safety testing, an equipment leakage current test must only be performed once the device is
connected to the LAN network. If the medical electrical system has not been tested as a
standalone device, the device has to be tested as a standalone device (without connection to the
system) and as part of the system (with connection to the system).
Connect the detachable power cord of the device under test to the safety analyzer's test mains
port. Connect the enclosure test lead of the safety analyzer to the enclosure of the device under
test, e.g. to the equipotential connector. Refer to the documentation that accompanies the safety
analyzer for further details on how to set up the test.
Test Expected test results
Equipment Leakage Current Test
(Normal Condition)
Equipment Leakage Current Test
(Single Fault Condition)
Sys1 <= 100μA
Sys2 <= 300μA
75

3 Testing and Maintenance Preventive Maintenance Procedures
After the testing of the device as a standalone device and as part of the system, check that the
resulting values (without connection and with connection to the system) do not differ by more
than +/- 10% from each other.
If the devices in the medical electrical system are connected to a multiple portable socket outlet
the resulting protective earth leakage current needs to be determined. All system components
must be connected to the multiple portable socket outlet and be switched on during this
measurement.
Test Expected test results
Protective Earth Leakage Current of
Sys3 <= 300μA
Multiple Socket Outlets
Refer to the documentation that accompanies the safety analy z er for further detai ls on how to s et
up the test.
Preventive Maintenance Procedures
Noninvasive Blood Pressure Measurement Calibration
Carry out the noninvasive blood pressure measurement performance tests at least every two
years , or as specified by local laws (whichever comes first).
Performance Assurance Tests
Some of the following test procedures must be performed in service mode. To enter service
mode select Operating Modes in the main menu. Then select Service Mode and enter the
password.
If required, open the screen menu in the monitor info line at the top of the screen and select
Service to access the service screen. This is required particularly for Anesthetic Gas Module
testing procedures.
Basic Performance Assurance Test
This section describes the basic performance test procedure. Please refer to the section When to
Perform Tests for detailed information on when which test procedure is required.
Procedure:
Power on the monitoring system and go into demo mode. Check that each connected parameter
(integrated, module, MMS, Gas Analyzer, Vuelink connected device) displays values.
76

Performance Assurance Tests 3 Testing and Maintenance
Full Performance Assurance Test
The following sections describe the full performance testing procedures i.e. detailed testing of
each parameter with a patient simulator or specified tools. Please refer to the section When to
perform Tests for information on when which testing procedure is required.
ECG/Resp Performance Test
This test checks the performance of the ECG and respiration measurements.
Tools required: Patient simulator.
ECG Performance
1. Connect the patient simulator to the ECG/Resp connector.
2. Configure the patient simulator as follows:
- ECG sinus rhythm.
- HR = 100 bpm or 120 bpm (depending on your patient simulator).
3. Check the displayed ECG wave and HR value against the simulator configuration.
4. The value should be 100bpm or 120 bpm+/- 2 bpm.
Respiration Performance
1. Change the Patient Simulator configuration to:
- Base impedance line 1500 Ohm.
- Delta impedance 0.5 Ohm.
- Respiration rate 40 rpm or 45 rpm.
2. The value should be 40 rpm +/- 2 rpm or 45 rpm +/- 2 rpm.
Test Expected test results
ECG Performance Test 100bpm +/- 2bpm or
Respiration Performance Test 40 rpm +/- 2 rpm or
120bpm +/- 2bpm
45 rpm +/- 2 rpm
77

3 Testing and Maintenance Performance Assurance Tests
ECG Sync Performance Test
This test checks the performance of ECG synchronization between the monitor and a
defibrillator. It only needs to be performed when this feature is in use as a protocol at the
customer site.
Tools required:
- Defibrillator with ECG Sync and Marker Output.
- Patient simulator.
1. Connect the patient simulator to the ECG connector and the defibrillator to the ECG Sync
Output on the monitor.
2. Set the patient simulator to the following configuration:
- HR = 100 bpm or 120 bpm (depending on your patient simulator).
- ECG sinus rhythm.
3. Switch the defibrillator to simulation mode.
4. Check that the marker pulse is displayed before the T-wave begins.
Test Expected test results
ECG Sync Performance Test Marker pulse is displayed before
SpO2 Performance Test
This test checks the performance of the SpO2 measurement.
Tools required: none
1. Connect an adult SpO2 transducer to the SpO2 connector.
2. Measure the SpO
3. The value should be between 95% and 100%.
Test Expected test results
SpO2 Performance Test 95% and 100%
the T-wave begins
value on your finger (this assumes that you are healthy).
2
78

Performance Assurance Tests 3 Testing and Maintenance
Measurement Validation
The SpO2 accuracy has been validated in human studies against arterial blood sample reference
measured with a CO-oximeter. In a controlled desaturation study, healthy adult volunteers with
saturation levels between 70% and 100% SaO2 were studied. The population characteristics for
those studies were:
- about 50% female and 50% male subjects
- age range: 18 to 45
- skin tone: from light to black
NOTE
A functional tester cannot be used to assess the accuracy of a pulse oximeter monitor. However,
it can be used to demonstrate that a particular pulse oximeter monitor reproduces a calibration
curve that has been independently demonstrated to fulfill a particular accuracy specification.
NBP PerformanceTest
This section describes NBP test procedures.The monitor must be in service mode and the screen
“Service A” must be selected to perform these tests. The NBP Performance Test consists of:
- NBP Accuracy Test
- NBP Leakage Test
- NBP Linearity Test
- Valve Test
NBP Accuracy Test
This test checks the performance of the non-invasive blood pressure measurement. Connect the
equipment as shown:
79

3 Testing and Maintenance Performance Assurance Tests
Tools required:
- Reference manometer (includes hand pump and valve), accuracy 0.2% of reading.
- Expansion chamber (volume 250 ml +/- 10%)
- Appropriate tubing.
In service mode, the systolic and diastolic readings indicate the noise of NBP channels 1 and 2
respectively. When static pressure is applied, the reading in NBP channel 1 should be below 50.
The value in parentheses indicates the actual pressure applied to the system.
1. Connect the manometer and the pump with tubing to the NBP connector on the MMS and to
the expansion chamber.
2. In service mode, select the Setup NBP menu.
3. Select Close Valves: On
4. Raise the pressure to 280 mmHg with the manometer pump.
5. Wait 10 seconds for the measurement to stabilize.
6. Compare the manometer values with the displayed values.
7. Document the value displayed by the monitor (x1).
8. If the difference between the manometer and displayed values is greater than 3 mmHg,
9. To calibrate the MMS, select Close Valves off then Calibrate NBP and wait for the
10. Press Confirm.
If the INOP NBP Equipment Malfunction message occurs in monitoring mode, go back to
service mode and repeat the calibration procedure.
NBP Leakage Test
The NBP leakage test checks the integrity of the system and of the valve. It is required once
every two years and when you repair the monitor or replace parts.
1. If you have calibrated, repeat steps 2 to 6 from the accuracy test procedure so that you have
calibrate the MMS. If not, proceed to the leakage test.
instrument to pump up the expansion chamber.Wait a few seconds after pumping stops until
EnterPrVal is highlighted and then move the cursor to the value shown on the
manometer. If one of the following prompt messages appears during this step, check
whether there is leakage in the setup:
- NBP unable to calibrate–cannot adjust pressure
- NBP unable to calibrate–unstable signal
280 mmHg pressure on the expansion chamber.
2. Watch the pressure value for 60 seconds.
3. Calculate and document the leakage test value (x2).
x2 = P1 - P2
where P1 is the pressure at the beginning of the leakage test and P2 is the pressure displayed
after 60 seconds.
The leakage test value should be less than 6 mmHg.
80

Performance Assurance Tests 3 Testing and Maintenance
NBP Linearity Test
1. Reduce the manometer pressure to 150 mmHg.
2. Wait 10 seconds for the measurement to stabilize.
3. After these 10 seconds, compare the manometer value with the displayed value.
4. Document the value displayed by the monitor (x3)
5. If the difference is greater than 3 mmHg, calibrate the MMS (see steps 9 to 10 in the
accuracy test procedure).
Valve Test
1. Raise the pressure again to 280 mmHg.
2. Select Close valves: Off.
3. Wait five seconds and then document the value displayed. The value should be less than 10
mmHg.
4. Document the value displayed by the monitor (x4).
Expected Test Results for NBP Accuracy Test, Leakage Test, Linearity Test & Valve Test
Test Expected test results
Accuracy test x1 = value displayed by monitor
Difference ≤ 3mmHg
Leakage test x2 = leakage test value
x2 < 6 mmHg
Linearity test x3 = value displayed by monitor
Difference ≤ 3mmHg
Valve Test x4 = value < 10 mmHg
Invasive Pressure Performance Test
This test checks the performance of the invasive pressure measurement.
Tools required: Patient simulator.
1. Connect the patient simulator to the pressure connector.
2. Set the patient simulator to 0 pressure.
3. Make a zero calibration.
4. Configure the patient simulator as P(static) = 200 mmHg.
5. Wait for the display.
81

3 Testing and Maintenance Performance Assurance Tests
6. The value should be 200 mmHg ± 5 mmHg. If the value is outside these tolerances, calibrate
the Invasive Pressure measurement. If the measurement was calibrated with a dedicated
reusable catheter, check the calibration together with this catheter.
Table 4:
Test Expected test results
Invasive Pressure Performance Test 200 mmHg ± 5 mmHg
Temperature Performance Test
This test checks the performance of the temperature measurement.
Tools required: Patient simulator (with 0.1°C or 0.2°F).
1. Connect the patient simulator to the temperature connector.
2. Configure the patient simulator to 40°C or 100°F.
3. The value should be 40°C ± 0.2°C or 100°F ± 0.4°F.
Table 2:
Test Expected test results
Temperature Performance Test 40°C ± 0.2°C or 100°F ± 0.4°F
M3014A Capnography Extension Performance Tests
The procedures below describe the mainstream and sidestream CO2 performance tests for the
M3014A Capnography Extension.
Mainstream CO2 Accuracy Check
Tools Required:
- three airway adapters
- Verification Gas M2506A
- Gas cylinder regulator M2505A
You also need a local barometric pressure rating received from a reliable local source (airport,
regional weather station or hospital weather station) which is located at the same altitude as the
hospital.
Procedure:
1. Attach the M2501A CO
sensor to the patient monitor. Attach an airway adapter to the
2
sensor. Make sure that the sensor is disconnected from the patient circuit.
2. Switch on the patient monitor.
3. Enter the monitor’s Service Mode.
82

Performance Assurance Tests 3 Testing and Maintenance
4. Using the sensor status provided in the M2501A Serial protocol, wait for the M2501A
sensor to warm up to its operating temperature.
5. The default setting for gas temperature is 22°C. If the gas temperature is significantly above
or below this value, correct the gas temperature setting.
6. Zero the sensor on the airway adapter being used in this test. Ensure Zero Gas is set to Room
Air
7. Attach a regulated flowing gas mixture of 5% CO2, balance N2 to the airway adapter.
8. Set the gas correction to off.
9. Allow a few seconds for the gas mixture to stabilize and observe the CO2 value. The
expected value is 5% of the ambient pressure ±2mmHg
NOTE
Make sure that you follow the above steps correctly. If the sensor fails this check it must be
exchanged. The sensor cannot be calibrated.
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Table 6:
Test Expected test results (x1) Acceptance Range
Mainstream CO2 Accuracy
Test
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient
pressure).
Sidestream CO2 Accuracy Check
Tools Required:
- Cal gas flow regulator M2267A
- Cal tube 13907A
- Verification Gas M2506A
- Straight Sample Line M2776A
You also need a local barometric pressure rating received from a reliable local source (airport,
regional weather station or hospital weather station) which is located at the same altitude as the
hospital.
Procedure:
5% of 760 mmHg pressure ±2mmHg
36 mmHg 40 mmHg
1. Attach the M2741A CO2 sensor to the patient monitor. Attach the sample line and the cal
tube to the sensor. Make sure that the sensor is disconnected from the patient circuit.
2. Switch on the patient monitor.
3. Enter the monitor’s Service Mode.
83

3 Testing and Maintenance Performance Assurance Tests
4. Using the sensor status provided in the M2741A Serial protocol, wait for the M2741A
sensor to warm up to its operating temperature.
5. Zero the sensor. Ensure Zero Gas is set to Room Air
6. Attach a regulated flowing gas mixture of 5% CO2, balance N2 to the cal tube.
7. Set the gas correction to off.
8. Allow a few seconds for the gas mixture to stabilize and observe the CO2 value. The
expected value is 5% of the ambient pressure ±2mmHg
NOTE
Make sure that you follow the above steps correctly. If the sensor fails this check it must be
exchanged. The sensor cannot be calibrated
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x2) Acceptance Range
Sidestream CO2 Accuracy
Test
5% of 760 mmHg pressure ±2mmHg
36 mmHg 40 mmHg
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient
pressure).
Sidestream CO2 Flow Check
Check the flow rate in the Sidestream CO2 extension as follows:
1. Connect the flowmeter to the sample line
2. Check on the flowmeter the flow that the Sidestream CO2 extension pump draws. It should
be 50 ml/min ± 10 ml/min. If the value is not within tolerance check your setup again and
perform another flow check. If it fails again, the sensor must be replaced. The sensor cannot
be calibrated.
84

Performance Assurance Tests 3 Testing and Maintenance
Microstream CO2 Performance Test
Allow five seconds between individual service procedures to ensure stable equipment conditions.
When certain monitor procedures are running, service procedures are not possible and trying to
start them will result in a message Service Operation Failed in the monitor’s status line.
Wait until the monitor completes the current operation, then restart the service procedure.
This test checks the performance of the Microstream CO2 measurement. The Microstream CO2
measurement can either be integrated into the IntelliVue MP5 monitor or, for other IntelliVue
monitors, into the M3015A MMS Extension. The Microstream CO2 performance test is required
once per year and when the instrument is repaired or when parts are replaced.
This test uses calibration equipment that you can order (see the Parts section for the part
number). The procedure is summarized in the following steps. Refer to the documentation
accompanying the equipment for detailed instructions.
Tools Required:
- Standard tools, such as screwdriver, tweezers
- Electronic flowmeter, M1026-60144
- Gas calibration equipment:
- Cal 1 gas 15210-64010 (5% CO
- Cal 2 gas 15210-64020 (10% CO
)
2
)
2
- Cal gas flow regulator M2267A
- Cal tube 13907A
- Calibration Line M3015-47301
You also need a local barometric pressure rating received from a reliable local source (airport,
regional weather station or hospital weather station) which is located at the same altitude as the
hospital.
The CO2 calibration for the Microstream extension consists of the following steps:
- Leakage check
- Barometric pressure check and calibration, if required.
- Pump check
- Flow check and calibration, if required
- Noise check
- CO2 Cal check and calibration, if required
- CO2 Cal verification using 2nd cal gas
Perform all checks in the same session.
85

3 Testing and Maintenance Performance Assurance Tests
Leakage Check
The leakage check consists of checking the tubing between:
- the pump outlet and the mCO
outlet and
2
- the pump inlet and FilterLine inlet.
Check the user’s guide of the flowmeter for details on how to make a correct flow reading.
Part 1
1. Go into service mode and select Setup CO2 menu.
2. Connect a FilterLine to the Microstream CO
input to start the pump running.
2
3. Check the ambient pressure and the cell pressure shown in the monitor’s status line. The cell
pressure should be approximately 20 mmHg lower than ambient pressure.
4. Connect the flowmeter outlet to the FilterLine inlet using a flexible connecting tube.
5. Block the mCO
outlet using your fingertip and observe the flowmeter display. The value on
2
the flowmeter (x1) should decrease to between 0 and 4 ml/min, accompanied by an audible
increase in pump noise. If the value is within the tolerance limits, continue with part 2 of the
leakage check.
6. If the value is outside the tolerance limits, there is a leakage between the pump outlet and the
mCO
outlet.
2
7. Open the MMS Extension or MP5 and check the tubing connections at the pump outlet and
the extension gas outlet. If the connections are good, then there is a leakage in the tubing and
you must exchange the MMS Extension or the mCO
Assembly of the MP5 respectively.
2
Part 2
1. Disconnect the flowmeter from the Part 1 setup and connect the flowmeter inlet to the
M3015A gas outlet or the MP5 mCO
2. Leave the Filterline connected to the M3015A inlet or the MP5 mCO
gas outlet.
2
inlet..
2
3. Block the inlet of the FilterLine using your fingertip and observe the flowmeter display. The
value on the flowmeter (x2) should decrease to between 0 and 4 ml/min, accompanied by an
audible increase in pump noise. The cell pressure shown in the status line on the display
should decrease to between 300 and 500 mmHg. Do not block the inlet for longer than 25
seconds as this will lead to an “Occlusion” INOP. If the value is within the tolerance limits,
there are no leakages and the leakage check is completed; proceed to the pump check.
4. If the value is not within the tolerance limits, there is a leakage between the FilterLine inlet
and the pump inlet.
5. Check the FilterLine connections and open the M3015A or MP5 to check the tubing
connections at the pump inlet and the M3015A or MP5 mCO
gas inlet. If the connections
2
are good, try replacing the FilterLine and repeating the leakage check. If the situation
remains, there is a leakage in the tubing and the M3015A or the mCO
must be exchanged.
assembly of the MP5
2
86

Performance Assurance Tests 3 Testing and Maintenance
Barometric Pressure Check and Calibration
Check the barometric pressure value in the M3015A MMS Extension or the MP5 as follows:
Pump Check
1. Go into service mode and select Setup CO
2. Connect a FilterLine to the Microstream CO
menu.
2
input. This activates the pump in the M3015A
2
MMS Extension or the MP5.
3. The status line at the bottom of the screen displays “CO
pressure reading (ambient/cell)
2
xxx/yyy” where xxx is the ambient pressure and yyy is the measured cell pressure. Check
whether the ambient pressure value (x3) matches (within the acceptable tolerance of ±12mm
Hg) the reference value you have received. If so, proceed to the leakage check. If the value
is not correct, calibrate as follows.
a. Select CO
then select Barom.Press to activate a table of values.
2
b. Select the value in the table which matches the reference value received from a reliable local
source (airport, regional weather station or hospital weather station). (The values are
displayed with a resolution of 2 mmHg up to 500 mmHg and a resolution of 1 mmHg from
500 mmHg to 825 mmHg.) Note: the selected value must be within ±10% of the current
measured ambient pressure, otherwise an error message will occur at restarting the monitor.
c. Confirm the barometric pressure setting.
d. Check that the ambient pressure displayed in the status line at the bottom of the screen is the
same as the value which you selected from the list in step b.
1. Connect the flowmeter inlet to the mCO
gas outlet.
2
2. Connect the FilterLine to the mCO
3. Block the inlet of the FilterLine using your fingertip and observe the cell pressure on the
monitor display. The cell pressure (x4) should be more than 120 mmHg below the ambient
pressure shown. If the pressure difference is less than 120 mmHg, the pump is not strong
enough and you should replace it, irrespective of the Pump OpTime.
Flow Rate Check and Calibration
Check the flow rate in the M3015A MMS Extension or the MP5 as follows:
1. Connect the flowmeter to the CO
2. Check on the flowmeter the flow that the M3015A MMS Extension or MP5 mCO2 pum p
draws (x5). It should be 50 ml/min ± 7.5 ml/min. If the value is within tolerance, proceed to
the CO
Gas calibration check. If the value is not within tolerance, calibrate as follows.
2
3. Adjust the flow in the instrument by selecting Increase Flow or Decrease Flow until
it is as close as possible to 50 ml per minute as indicated on the flowmeter gauge.
4. When you are satisfied that the flow is set as close as possible to 50 ml per minute, select
Store Flow and confirm the setting. If you do not store the adjusted flow within 60
seconds of the adjustment, the old flow setting is restored.
inlet.
2
FilterLine.
2
87

3 Testing and Maintenance Performance Assurance Tests
5. If you cannot adjust the flow to within tolerance, replace the pump . If you still cannot make
the flow adjustment, this indicates a fault in the measurement extension, which must be
replaced.
Note that the pump can only be replaced on M3015A with the old hardware Rev. A (i.e.
Serial No. DE020xxxxx
Noise Check
1. With the monitor in service mode, select Setup CO
2. Disconnect the flowmeter and connect the 5% calibration gas and flow regulator in its place.
3. Open the valve to apply the 5% calibration gas and wait until the value is stable.
4. Check the noise index (x6) displayed next to the CO
level of noise on the CO
extension.
wave). If the value exceeds 3 mmHg, replace the measurement
2
CO2 Gas Measurement Calibration Check
After switching the measurement extension on, wait at least 20 minutes before checking the
calibration. Check the calibration of the CO
1. Check that the 5% calibration gas and flow regulator are connected.
2. Calculate the expected measurement value in mmHg as follows:
0.05 x (ambient pressure) = value mmHg
for example 0.05 x 736 = 36.8 mmHg (with an ambient pressure of 736 mmHg)
3. Open the valve on the flow regul ator to allow 5% CO
the value to stabilize.
4. Check that the value on the instrument (measurement value on the main screen, x7) matches
the calculated mmHg value ± 2.6 mmHg. If the value is outside the tolerance, calibrate as
described in step in this procedure onwards.
menu.
2
value on the display (this indicates the
2
gas measurement as follows:
2
gas to flow into the extension. Allow
2
5. Disconnect the 5% calibration gas and connect the 10% calibration gas.
6. Calculate the expected measurement value and tolerance in mmHg as follows:
0.1 x (ambient pressure) = value mmHg
±0.07 x (value mmHg) = tolerance
for example 0.1 x 737 mmHg = 73.7 mmHg (with an ambient pressure of 737 mmHg)
±0.07 x 73.7 mmHg = ±5.16 mmHg tolerance
7. Open the valve on the flow regulator to allow 10% CO
gas to flow into the extension.
2
Allow the value to stabilize.
8. Check that the value on the instrument (x8) matches the calculated mmHg value within the
calculated tolerance. If so, the measurement extension is correctly calibrated. If the value is
outside the tolerance, calibrate as follows.
9. If not already connected, connect the 5% calibration gas.
10. Select Cal. CO
.
2
11. Select the value for the calibration gas. (The default value is 5.0%.)
88

Performance Assurance Tests 3 Testing and Maintenance
12. Open the valve on the calibration gas to allow CO2 gas to flow into the extension. Allow the
value to stabilize before the start of the calibration. Leave the valve open until the instrument
gives a prompt that gas can be removed.
13. The extension calibrates and prompts when calibration is successful.
Calibration Verification
1. Reopen the 5% gas valve and allow the value to stabilize.
2. Check that the value displayed on the monitor is correct within the tolerance (see step
above).
3. Disconnect the 5% calibration gas and connect the 10% calibration gas.
4. Open the valve on the flow regulator to allow 10% CO2 gas to flow into the extension.
Allow the value to stabilize.
5. Check that the value displayed on the monitor is correct within the tolerance (see step
above).
If one or both values are not within tolerances, you must exchange the M3015A MMS Extension
or the MP5 mCO
Assembly.
2
Test Expected Test Results
Leakage Check
parts 1 and 2
x1 = value of part 1 leakage check on flowmeter
(x1< 4.0 ml/min)
x2 = value of part 2 leakage check on flowmeter
(x2< 4.0 ml/min)
Barometric
Pressure Check
x3 = difference between the reference pressure and the
measured ambient pressure displayed on the monitor
(x3<12 mmHg)
Pump Check x4 = difference in pressure between cell pressure and
ambient pressure displayed on the monitor during
occlusion (x4 >120 mmHg)
Flow Check x5 = difference between measured value and 50.0
ml/min (x5<7.5 ml/min)
Noise Check x6 = noise index displayed on monitor (x6<3.0)
CO2 Gas
Calibration Check
x7 = difference between measured CO2 value and
calculated value, based on 5% CO
cal. gas. (x7 < 2.6
2
mmHg)
CO2 Cal
Verification
x8 = difference between measured CO2 value and
calculated value, based on 10% CO
cal. gas.
2
(x8 < ± {0.07 x value calculated})
89

3 Testing and Maintenance Performance Assurance Tests
Reset Time Counters
NOTE
This procedure only applies to M3015A with the old hardware Rev. A (i.e. Serial No.
DE020xxxxx
You must check the time counters on the Microstream CO
instrument. As well, when parts are replaced, the appropriate counters must be reset to zero.
The counters for CO
pump, IR Src and Last Cal are displayed in the status line. The values are
2
updated when entering the Setup CO2 menu.
Observe the following guidelines:
- When calibrating the CO
extension, if no parts have been replaced, check the displayed
2
values of Reset PumpOpTime and Reset IRSourceTime selections to make sure that
they are within suggested guidelines for use (15, 000 hours of continuous use). If the counter
time is greater than 15, 000 hours, replace the appropriate part. See Repair and Disassembly
for details.
- When calibrating the CO
extension, if parts have been replaced, reset the appropriate values
2
using the Reset PumpOpTime and Reset IRSourceTime selections. See Repair and
Disassembly for details.
Resetting the PumpOpTime generate s the INOP: “CO
must perform a flow check and store the flow in service mode (select Store Flow).
CO2 Pump / CO2 Scrubber Replacement
NOTE
This procedure only applies to M3015A with the old hardware Rev. A (i.e. Serial No.
DE020xxxxx
extension before calibrating the
2
OCCLUSION”. To clear this INOP you
2
Refer to the Repair and Disassembly section for the replacement procedures.
Cardiac Output Performance Test
These tests check the performance of the cardiac output measurement.
1. Connect the patient simulator to the C.O. module using the patie nt cab le.
2. Configure the patient simulator as follows:
Injection temperature: 2 °C
Computation Const: 0.542
(Edward's Catheter)
Flow: 5 l/min
3. Check displayed value against the simulator configuratio n.
4. Expected test result: C.O. = 5 +/– 1 l/min.
Test Expected test results
Cardiac Output Performance Test C.O. = 5 +/– 1 l/min
90

Performance Assurance Tests 3 Testing and Maintenance
Service Tool Procedure, Version 1
This procedure applies for Service Tool M1012-61601 in combination with C.O. modules
without option C10 and M3012A MMS extensions with option C05.
1. In monitoring mode, connect the C.O. interface cable to the module.
2. Connect one side of the service tool to the injectate receptacle of C.O. interface cable and
the other side to catheter cable receptacle.
3. Enter the C.O. Procedure window and check the results. The expected test result is:
o
Tblood = 37.0
C +/- 0.1oC
Test Expected test results
Cardiac Output Service Tool
Tblood = 37.0oC +/- 0.1oC
Procedure Version 1
Service Tool Procedure, Version 2
This procedure applies only for Service Tool M1012-61601 in combinatio n wit h C.O . m odules
with option C10 and for the M3012A MMS Extension with option C10.
1. In monitoring mode, connect the C.O. interface cable to the module.
2. Connect one side of the service tool to the injectate receptacle of the C.O. interface cable
and the other side to the catheter cable receptacle.
3. Enter C.O. Procedure window and check results for:
- Method of measurement
- Arterial Catheter constant
- Tblood
The expected results are:
- Transpulmonary
- 341
o
- Tblood = 37.0
C +/- 0.1oC
4. Make sure the main alarms are switched on.
5. Disconnect the Catheter cable receptacle from the service tool
6. Enter the Setup C.O Window and change the method of measurement to “Right Heart”
7. Enter the C.O. Procedure window and check t he Tinj val ue. The expected res ul t is:
Tinj = 0.0
o
C +/- 0.1oC
Test Expected test results
Cardiac Output Service Tool
Procedure Version 2
Tinj = 0.0oC +/- 0.1oC
91

3 Testing and Maintenance Performance Assurance Tests
Power Loss Alarm Buzzer Performance Test (only if Multi-Port Nurse Call Connector Board is installed)
1. Switch on the monitor.
2. Remove the battery and disconnect the monitor from AC power.
3. The Power Loss Alarm Buzzer should beep for about one minute.
4. To switch off the alarm sound, either press the power button, connect the monitor to AC
power or insert a battery
Test Expected test results
Power Loss Alarm Buzzer
Beep for one minute
Performance Test
IntelliVue 802.11 Bedside Adapter Communication Test
1. Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2. Go into Service Mode and select Main Setup -> Network -> Setup WLAN. In the
Setup WLAN menu:
- set Mode to either 802.11Ah, 802.11G, 802.11Bg (not recommended), Auto (not
recommended) or None (this setting disables the wireless LAN functionality
permanently), to match your wireless infrastructure installation.
- set SSID to match your installation.
- set the Country code to “1000”. Setting the country code to this value will
automatically adjust the regulatory domain to match the configuration of the
infrastructure. Do not set the country code to values other than “1000” unless otherwise
instructed.
- set the Security Mode to WPA(PSK) and enter the WPA password (string between
8 and 63 characters).
3. Select Main Setup -> WLAN Diagnostic to access the service window.
4. Proper installation of the IntelliVue 802.11 Bedside Adapter is assured by connecting to an
access point over the wireless link. Place the monitor with the IntelliVue 802.11 Bedside
Adapter installed in close proximity to the access point (e.g. if the access point is mounted
on the ceiling, place the monitor directly below). Wait until the Conn.Status field in the
service window shows Authenticatd (for Rel. C.0 monitors)or Connected (for Rel D.0 or
higher). Take the monitor approximately 5 m away from the access point. There should be
no walls or other obstacles between the monitor and the access point. The following should
apply:
92

Performance Assurance Tests 3 Testing and Maintenance
- Observe the RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds.
The RSSI value wil fluctuate but should stay above 30 in a 5 m distance from the access
point used. The wireless link should be active, i.e. the Conn.Status field should be
Authenticatd (for Rel. C.0 monitors)or Connected (for Rel D.0 or higher), and the other
fields should contain values. If the RSSI value is significantly lower, check the distance
to the access point and the antenna orientation at the monitor. The antenna orientation
should be vertical, but the physical placement of the monitor or other equipment within
its vicinity as well as walls or other obstacles may influence the antenna orientation
required to receive the best RSSI value.
5. If this test fails, retry in a different physical area with a different access point.
6. Perform the Wireless Switch test blocks as described in the Philips IntelliVue 802.11 a/g
Infrastructure Installation and Configuration Guide.
Test Expected test results
IntelliVue 802.11 Bedside Adapter
RSSI value above 30
Performance Test
IIT Communication Test
1. Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2. Go into Configuration mode and, in the Network menu, set the RF Access Code in each
profile to match your installation.
3. Go into Service Mode. Select Main Setup -> Instr. Telemetry to access the
Instrument Telemetry Service window.
4. Proper installation of the IIT module is assured by connecting to an access point over the
wireless link. Place the monitor with the IIT module installed in close proximity to the
access point (e.g. if the access point is mounted on the ceiling, place the monitor directly
below). Wait until the Conn.Status field in the Instrument Telemetry Service window
shows Active. Take the monitor approximately 5 m away from the access point. There should
be no walls or other obstacles between the monitor and the access point. The following
should apply:
- Observe the RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds.
The RSSI value should be around -50 ±10 in a 5 m distance from the access point used
and the IIT link should be active, i.e. the Conn.Status field should be Active and the
other fields should contain values. If the RSSI value is significantly lower, check the
distance to the access point and the antenna orientation at both the monitor and the
access point (both should be vertical).
- Remove the antenna. The RSSI value should be around -90 ±10. The IIT link may be
active but the connection could be unreliable. The Conn. Status field may toggle
between Inactive and Seeking. If the difference between the RSSI values measured with
and without antenna is significantly lower, check the antenna and the antenna connector
for damage and verify that the cable fom the IIT adapter to the antenna connector plate
is connected properly.
5. If this test fails, retry in a different physical area with a different access point.
93

3 Testing and Maintenance Performance Assurance Tests
Error Conditions:
- The field MAC Instr. Tele should show a value unequal to 0000 0000 0000. If it
does not, there is a communication problem between the monitor and the IIT adapter.
- With an incorrect RF Access Code or an incorrect or defective antenna installation, the
fields IP Address,Server IP, Subnet Mask, and RSSI in the Instrument
Telemetry Service window will stay blank. The field Conn. Status will slowly toggle
between Inactive and Seeking.
6. Perform the Access Point Controller (APC) test blocks as described in the Philips IntelliVue
Wireless Network Installation and Configuration Guide.
Short Range Radio (SRR) Performance Test
1. Make sure that the short range radio interface is configured as follows: SRR On and
appropriate channel selected.
2. Assign a telemetry transceiver to the IntelliVue Monitor according to the proc edure
described in the Instructions for Use of the patient monitor.
3. Check that the following conditions are fulfilled:
a. Place the telemetry transceiver close to the monitor.
b. The telemetry transceiver status is displayed on the monitor in the measurement
selection window.
c. Waves or numerics from the telemetry transceiver are displayed on the monitor. There a
re no dropouts or gaps in waves or numeric transmission.
d. The battery status of the telemetry transceiver is displayed in the measurement selection
window.
e. The Signal Quality Indicator shows at least
4. Check that the data from the telemetry transceiver is transmitted to the monitor within a 1m
radius and that there are no dropouts or gaps in waves or numerics.
5. Check whether the connection remains stable within a 5m radius from the monitor.
6. Switch on all telemetry transceivers used on the site and check that there are no interferences
between the transceivers and their assigned monitors.
7. Check and record the coverage area of the telemetry transceivers and inform the customer
about this coverage area.
94

Reporting of Test Results 3 Testing and Mainten ance
Reporting of Test Results
Philips recommends all test results are documented in accordance with local laws. Authorized
Philips personnel report test result back to Philips to add to the product development database.
While hospital personnel (biomedical engineers or technicians) do not need to report results to
Philips, Philips recommends that they record and store the test results in accordance with local
laws.
The following table lists what to record after completing the tests in this chapter. Record the
results in the empty column in Table 16.
The following is a guide as to what your documentation shou ld incl ud e:
- Identification of the testing body (for example, which company or department carried out the
tests).
- Name of the person(s) who performed the tests and the concluding evaluation.
- Identification of the device(s) and accessories being tested (serial number, etc.).
- The actual tests (incl. visual inspections, performance tests, safety and system tests) and
measurements required
- Date of testing and of the concluding evaluation.
- A record of the actual values of the test results, and whether these values passed or failed the
tests.
- Date and confirmation of the person who performed the tests and evaluation.
The device under test should be marked according to the test result: passed or failed.
Carrying Out and Reporting Tests
Testing Organization:
Name of testing person:
Responsible Organization:
Device Under Test: ID-Number
Product Number: Serial No.:
Accessories:
Test Report
Test before putting into service (reference value)
Recurrent Test
Test after Repair
Measurement Equipment (Manufacturer, Type,
Serial No.):
Functional Test (parameters tested):
95

3 Testing and Maintenance Reporting of Test Results
Test Block
Visual
Inspection
Test and Inspection Matrix
Test or
Inspection to be
Performed
Perform Visual
Inspection
Power On Power on the
unit. Does the
self-test complete
successfully
Noninvasive
Blood
Perform the
Accuracy Test
Pressure
Performance
Tests
Performance
Leakage Test
Performance
Linearity Test
Record the Results (mandatory for
Expected Test Results
Philips Personnel only)
What to record Actual
Results
Pass or Fail V:P or V:F
If Yes, Power On test is
PO:P or PO:F
passed
X1 = value displayed by
monitor
PN:P/X1 or
PN:F/X1
Difference <= 3mmHg
X2 = leakage test value
X2 < 6 mmHg
X3 = value displayed by
monitor
PN:P/X2 or
PN:F/X2
PN:P/X3 or
PN:F/X3
Difference <= 3mmHg
Performance
Valve Test
Temperature
Performance
Test
All other
performance
tests
Perform the
Temperature
Performance Test
Perform the
remaining
parameter
performance
tests, if applicable
Safety (4) Perform Safety
Test (4): Patient
Leakage Current
- Single Fault
Condition, mains
on applied part.
System
(Sys 1-2)
Perform the
system test
according to
subclause 19.201
of IEC/EN
60601-1-1, if
applicable, after
forming a system
X4 = value < 10 mmHg PN:P/X4 or
PN:F/X4
X1= 40°C ± 0.2°C or 100°F ±
0.4°F
See expected results in test
procedures
Maximum leakage current
(X1): <=50 μA
Equipment Leakage Current:
Sys1 <= 100 μA (Normal
Condition)
PT: P/X1 or
PT: F/X1
P: P or
P: F
S(4): P/X1 or
S(4): F/X1
Sys: PSys1/PSys2
or
Sys: FSys1/Fsys2
Sys2 <= 300μA (Single Fault
Condition
96

Reporting of Test Results 3 Testing and Mainten ance
Test or
Test Block
System
(Sys 3)
Inspection to be
Performed
Perform the
system test
according to
subclause 19.201
of IEC/EN
60601-1-1, if
applicable, after
forming a system
Expected Test Results
Protective Earth Leakage
Current if medical electrical
system components are
connected to the same
Multiple Portable Socket
Outlet:
Sys3 <= 300 μA
Key: P = Pass, F = Fail, X or Sys = test value to be recorded
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise
stated.
Evaluation:
Safety and Functional Test passed
Repair required at a later date, safety and functional test passed
Record the Results (mandatory for
Philips Personnel only)
What to record Actual
Results
Sys: PSys3
or
Sys: FSys3
Yes No
Device must be taken out of operation until repair and passed tests
Device failed and must be taken out of operation.
Notes:
Next Recurrent Test:
Name:____________________________________________________
Date/Signature:_____________________________________________
Evaluation of Test Results
The evaluation of the test results must be performed by appropriately trained personnel with
sufficient product, safety testing and application knowledge.
If any test results are between 90% and 100% of the respective expected result, the previously
measured reference values must be taken into consideration for the assessment of the electrical
safety of the device under test. If no reference values are available, you should consider shorter
intervals between upcoming recurrent tests.
NOTE
If any single test fails, testing must be discontinued immediately and the device under test must
be repaired or labeled as defective. Be sure to inform the user about the test failure in writing.
97

3 Testing and Maintenance Other Regular Tests
Other Regular Tests
The care and cleaning requirements that apply to the monitor and its accessories are described in
the Instructions for Use. This section details periodic maintenance procedures recommended for
the monitor and its accessories.
Locking/Unlocking Touch Operation
To temporarily disable touchscreen operation of the monitor, press and hold the Main Screen
key. The message “Touch disabled, press Main Screen” will appear on the screen. Press and hold
the Main Screen key again to re-enable touchscreen operation.
Battery Handling, Maintenance and Good Practices
This section provides some information on how to handle and maintain the battery in order to get
the best usage from it. Additionally, some good working practices are also given regarding the
correct disposal of the battery.
NOTE
If your MP2/X2 is connected to an IntelliVue Information Center, you should make sure that the
IIC uses the text catalog revision F.0 or later, otherwise battery INOPs may not display correctly
on the IIC. Consult your IIC documentation for instructions on upgrading the text catalog.
About the Battery
When monitoring a patient, one Philips M4607A rechargeable Lithium Ion battery must always
be inserted into the battery compartment on the right side of the MP2/X2. This applies even
when you are running the MP2/X2 external power, either via the external power supply or when
connected to a host monitor. The battery has the effect of sealing the battery compartment,
thereby preventing the ingress of fluids or foreign bodies. A severe yellow INOP (!!INSERT
BATTERY) will be issued if the monitor is connected to AC mains without a battery fully
inserted in the battery compartment. This INOP will persist until a battery is loaded.
98

Battery Handling, Maintenance and Good Practices 3 Testing and Maintenan ce
To use the MP2/X2 with battery power, disconnect it from the host monitor or the external
power supply (M8023A).
The rechargeable Lithium-Ion Mangan battery used in the MP2/X2 is regarded as a Smart
battery because it has built-in circuitry. (This circuitry communicates battery-status information
to the Monitor.)
To get the most out of the battery, observe the following guidelines:
- Condition the battery only upon maintenance request prompt on display.
- If a battery shows damage or signs of leakage, replace it immediately. Do not use a
faulty battery in the MP2/X2.
- Capabilities of integrated battery charger: 10.8 V, 1 mAh (typ.)
Actual current / voltage: depends on smart battery request and monitor configuration
The approximate charging time is 2 hours with the monitor switched off and up to 12 hours
or more during monitor operation, depending on the monitor configuration.
Checking the Battery Status
When the MP2/X2 is connected to the external power supply (M8023A), the battery charges
automatically. The battery can be charged remotely from the MP2/X2 by using the battery
charger. Use only the M8043A Smart battery charger with the additional adapter.
Battery status (level of charge) is indicated in several ways:
- LED on the front panel of the Monitor.
- Battery gauge.
- Display of battery time below gauge.
- Battery status window.
- INOP messages.
The AC Power LED is only on when the power cord is connected and AC power is available to
the Monitor. In this case, the battery can be either charging or fully charged.
The battery LED can be yellow, or red depending on the following conditions:
Battery LED Colors If the MP2/X2 is connected
to a host monitor or
If the monitor is running on
battery power, this means
external power supply , this
means
battery charging
Yellow
Red, flashing
Red, flashes
intermittently
1
indicated by malfunction symbol and INOP
2
for further details see Troubleshooting section
malfunction
battery or charger
1,2
≤ 10 minutes power remaining
battery or charger
malfunction
1,2
99

3 Testing and Maintenance Battery Handling, Maintenance and Good Practices
Battery Status on the Main Screen
Battery status information can be configured to display permanently on all Screens. It shows the
status of the battery, with the battery power remaining and, when the battery is not charging, an
estimate of the monitoring time this represents.
Battery power gauge:
This shows the remaining battery power. It is divided into sections, each representing 20% of the
total power. If three sections are filled, as in this example, this indicates that 60% battery power
remains. If no battery is detected, a blank battery gauge marked with a flashing red X is
displayed. If no data is available from the battery, a question mark is shown in the gauge.
Battery status/malfunction indicator:
Normal battery function is indicated by the battery power gauge, together with the remaining
operating time, on the Main Screen. You are informed of problems or changes in the status of the
battery by the battery status/malfunction indicator. This consists of a blank battery gauge
containing a symbol. If the symbol is red, this indicates a critical situation. You can check the
specific cause of the problem by looking at the symbol(s) displayed in the Battery Status
window.
Battery status indicator Battery malfunction indicator
Alternates with the battery gauge on the Main
!
Screen.
Check in the
window to see which status symbol is dis pl ay e d
to identify the cause.
Battery Status
!
X
The red ! flashes. Critical battery
situation or malfunction. Check in the
Battery Status window
to see which malfunction ind icator is
displayed, or refer to the INOP, to
identify the cause.
Indicator for missing battery (flashing
red X). An INOP is issued when the
battery compartment is empty, and the
MP2/X2 is connected to external power
(a host monitor or the optional external
power supply). This
BATTERY
15 seconds while the monitor is
connected to AC mains power,
allowing you sufficient time to load a
new battery. After silencing, the INOP
reappears every 10 seconds until a
battery is loaded.
!!INSERT
INOP is suppressed for
100
 Loading...
Loading...