Page 1
INSTRUCTIONS
BX61WI
FIXED-STAGE MOTORIZED
UPRIGHT MICROSCOPE
This instruction manual is for the Olympus Fixed-Stage Motorized Upright Microscope Model BX61WI.
To ensure the safety, obtain optimum performance and to familiarize yourself fully with the use of this
microscope, we recommend that you study this manual thoroughly before operating the microscope.
Retain this instruction manual in an easily accessible place near the work desk for future reference.
A X 6 1 1 8
Page 2

This device complies with the requirements of directive 98/79/EC concerning in vitro diagnostic medical devices. CE marking means the conformity to the directive.
NOTE: This equipment has been tested and found to comply with the limits for a Class A digital device,
pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection
against harmful interference when the equipment is operated in a commercial environment. This
equipment generates, uses, and can radiate radio frequency energy and, if not installed and used in
accordance with the instruction manual, may cause harmful interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful interference in which case
the user will be required to correct the interference at his own expense.
FCC WARNING: Changes or modifications not expressly approved by the party responsible for compliance
could void the user’s authority to operate the equipment.
Page 3
BX61WI
CONTENTS
Correct assembly and adjustments are critical for the microscope to exhibit its full performance. If you are going to
assemble the microscope yourself, please read Chapter 9, “ASSEMBLY” (pages 42 to 49) carefully. For the modules
provided with instruction manuals, also read the assembly procedures in their instruction manuals.
IMPORTANT — Be sure to read this section for safe use of the equipment. —
1 MODULE NOMENCLATURE
2 CONTROLS
TRANSMITTED LIGHT BRIGHTFIELD OBSERVATION PROCEDURE
3
4 USING THE CONTROLS
4-1 Microscope Frame .................................................................................................................................................................. 14, 15
1 Voltage Indication 2 Transmitted/Reflected Light Switch
Light Preset Switch
3
4-2 Focusing Block............................................................................................................................................................................. 15, 16
Replacing the Focus Adjustment Knob
1
3 Objective Up/Down Buttons
Using the Frost Filter Insertion/Removal Lever
5
4-3 Stage (IX-SVL2) .............................................................................................................................................................................. 17, 18
1 Placing the Specimen 2 Moving the Specimen
3 Setting the Grounding 4
Using the Light Shielding Sheet
5
4-4 Revolving Nosepiece .................................................................................................................................................................... 18
1 Switching the Objectives
4-5 Observation Tube .................................................................................................................................................................... 19, 2 0
1 Adjusting the Interpupillary Distance 2 Adjusting the Diopter
3 Using the Eye Shades
Selecting the Light Path of Trinocular Tube
5
4-6 Condenser ........................................................................................................................................................................................ 21 ,22
1 Centering the Condenser 2 Oblique Illumination (WI-OBCD)
4-7 Water Immersion Objectives.............................................................................................................................................. 23
1 Using Water Immersion Objectives (Water Immersion Cap)
Using the Filter Turret
4
2 F/C Button
Objective Escape/Return Button
4
Adjusting the X-Axis/Y-Axis Knob Rotation Tension
Lowering the Stage Height
6
Using Eyepiece Micrometer Disks
4
1-3
4
5-11
12, 13
14-23
Page 4
5 OTHER OBSERVATION METHODS
5-1 Differential Interference Contrast Observation.............................................................................. 24-28
1 Attaching the Analyzer 2 Attaching the Polarizer
3 Attaching the DIC Prisms (for Revolving Nosepiece)
Attaching the DIC Prisms (for Condenser)
4
Adjusting the Polarizer Position (except the U-UCD8)
5
Observation Method
6
5-2 Reflected Light Fluorescence Observation................................................................................................... 29
5-3
Infrared Light (IR)/Differential Interference Contrast (DIC) Observation
1 Introduction 2 Attaching the IR Modules
3 DIC Observation Using IR
5-4 Macro Reflected Light Fluorescence Observation ................................................................. 33-35
1 Introduction 2 Attaching the Modules
3 Filter Characteristics of Fluorescence Mirror Units
4 Fabricating Optional Mirror Units
24-35
............... 29-32
6 TROUBLESHOOTING GUIDE
7 SPECIFICATIONS
8 OPTICAL CHARACTERISTICS
9
ASSEMBLY
—
See this section for the replacement of the light bulb. —
10 LAMP HOUSING INSPECTION SHEET
36-38
39, 40
41
42-49
50
Page 5

IMPORTANT
This microscope employs a UIS2 (UIS) (Universal Infinity System) optical design, and should be used only
with modules designed for the BX2 series (which belong to the Olympus BX series).
For the applicable modules, please consult Olympus or the latest catalogues. Less than optimum performance may result if inappropriate module combinations are used.
Configuration of Instruction Manuals
Since this microscope is expandable to a variety of systems, separate instruction manuals are prepared so
that the user has to read only the manuals according to the user’s own system.
Manual Name Main Contents
BX61WI Observation procedures including transmitted light brightfield,
DIC and IR observations
BX-UCB/U-HSTR2
BX2 Software for PC (CD-ROM)
* BX2-BSW Ver. 03.01 or higher
TH4 External halogen lamp power supply
BX-RFAA/BX-URA2/BX-RFA Reflected light fluorescence observation procedure
U-FWT/FWR/FWO Motorized filter wheels (The U-FWT cannot be used with this
WI-DPMC Information on the dual-port magnification changer tube
WI-XYM/XYS Information on the XY mover and bridge stage
WI-SSNP Information on the swing-slide revolving nosepiece
Functions of the Control Box (incorporating the power supply)
and Hand Switch
Methods of PC control of functions
* Normal operation is not available unless the specified software is used.
microscope.)
BX61WI
SAFETY PRECAUTIONS
1. After the equipment has been used in an observation of a specimen that
2. Culture liquid or water spilt on the stage, condenser or microscope may dam-
3. If a foreign object is caught during motorized focusing operation, there will
4. Emergency stop of focus operation is possible by turning the focus adjustment
is accompanied with a potential of infection, clean the parts coming in
contact with the specimen to prevent infection.
· Moving this product is accompanied with the risk of dropping the
specimen. Be sure to remove the specimen before moving this product.
· In case the specimen is damaged by erroneous operation, promptly take
the infection prevention measures.
· The product becomes unstable if its height is increased by an accessory
mounted on it. In this case, take anti-toppling measures to prevent the
specimen from being dropped when the product topples down.
age the equipment. Immediately wipe the liquid or water off if it is spilt on them.
be an error in the focusing block and the motorized focusing operation will
be suspended.
Recovery procedure
· If there is no error in motorized operation, the caught object can be
removed by turning the focusing knob.
· If there is an error in motorized operation, the focusing knob becomes
inoperable. Disassemble the relevant modules to remove the caught
object. Replace the relevant modules afterward.
· Turn off the power and then on again. The system will restart unless there
is a malfunction in the motor.
knob (or dial) on the microscope frame or on the U-FH (in either direction),
or by pressing the FOCUS control button ( , , F/C or ESC), after focus
operation has been activated (except when data is being downloaded to a
PC).
When the BX-UCB control box’s main switch is set to “ I ” (ON), the focus
operates automatically (the objective raises once and then returns to the
original position) for initialization. (It takes about one minute.)
If the above emergency procedure is performed during this automatic focus
operation, the microscope stops operating. Should this happen, set the main
switch to “ ” (OFF) and then “ I ” (ON) again.
1
Page 6
1
Fig. 1
2
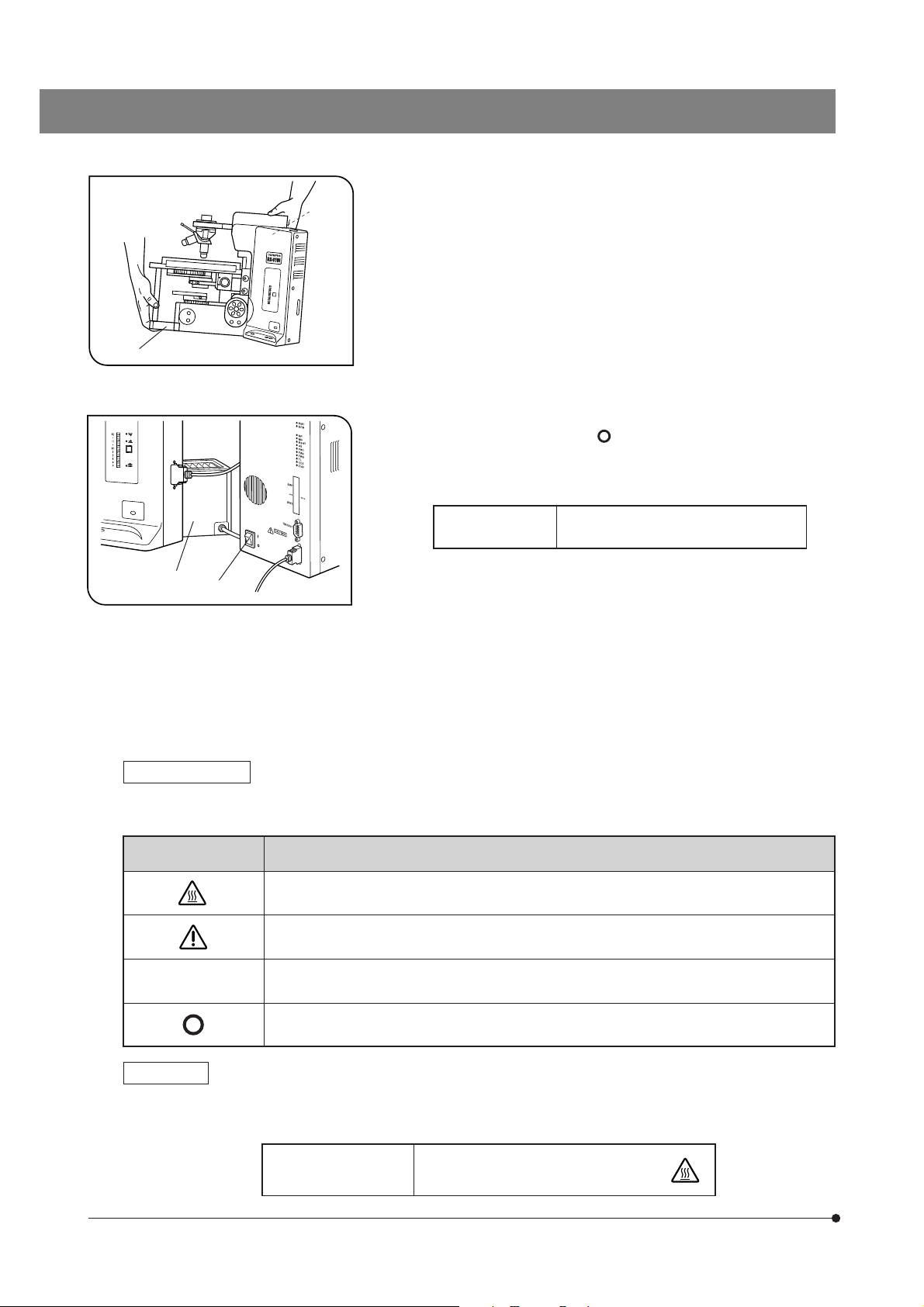
5. When moving the microscope, disconnect the reflected light illuminator,
observation tube and transmitted light lamp housing and carefully carry
the microscope by the base (front edge) 1 and the grasping part on the
rear of the arm 2 as shown in Fig. 1. (Weight: approx. 15 kg.)
Also be careful against slipping of hands during carrying.
#Damage to the microscope will occur if you grasp it by other parts
including the stage, focus adjustment knob, etc.
6. The surfaces of the lamp housing on the rear of the microscope will
become extremely hot during operation. When installing the microscope,
make sure to allow ample free space (10 cm or more) around and in
particular above the lamp housing.
7. When installing the microscope, route the power cord away from the
lamp housing. Should the power cord come in contact with the hot lamp
housing, the power cord could melt and cause electric shock.
8 To avoid potential shock hazards and burns when replacing the light
bulb, set the main switch ³ to “ ” (OFF) then disconnect the power cord
from the wall outlet in advance. Whenever you replace the bulb during
use or right after use, allow the lamp housing | and bulb to cool before
touching. (Fig. 2)
4
3
Fig. 2
Designated
Bulbs
# The microscope also incorporate a fuse (this should be
replaced by the manufacturer or an Olympus-authorized
agent).
9. Always use the power cord provided by Olympus. If the proper power
cord is not used, product safety performance cannot be warranted.
Always ensure that the grounding terminal of the microscope and that
10.
of the wall outlet are properly connected. If the equipment is not grounded,
Olympus can no longer warrant the electrical safety performance of the
equipment.
Never insert metallic objects into the air vents of the microscope frame
11.
as this could result in electrical shock, personal injury and equipment
damage.
12V100WHAL (PHILIPS 7724)
12V50WHAL-L (LIFE JC)
Safety Symbols
The following symbols are found on the microscope. Study the meaning of the symbols and always use the equipment
in the safest possible manner.
Symbol Explanation
Indicates that the surface becomes hot, and should not be touched with bare hands.
Before use, carefully read the instruction manual. Improper use could result in personal injury to
the user and/or damage to the equipment.
2
l
Indicates that the main switch is ON.
Indicates that the main switch is OFF.
Warnings
Warning engraving is placed at parts where special precaution is required when handling and using the microscope.
Always heed the warnings.
Warning engraving
position
Lamp housing (U-LH100-3/U-LH100IR)
(Warning against high temperature)
Page 7
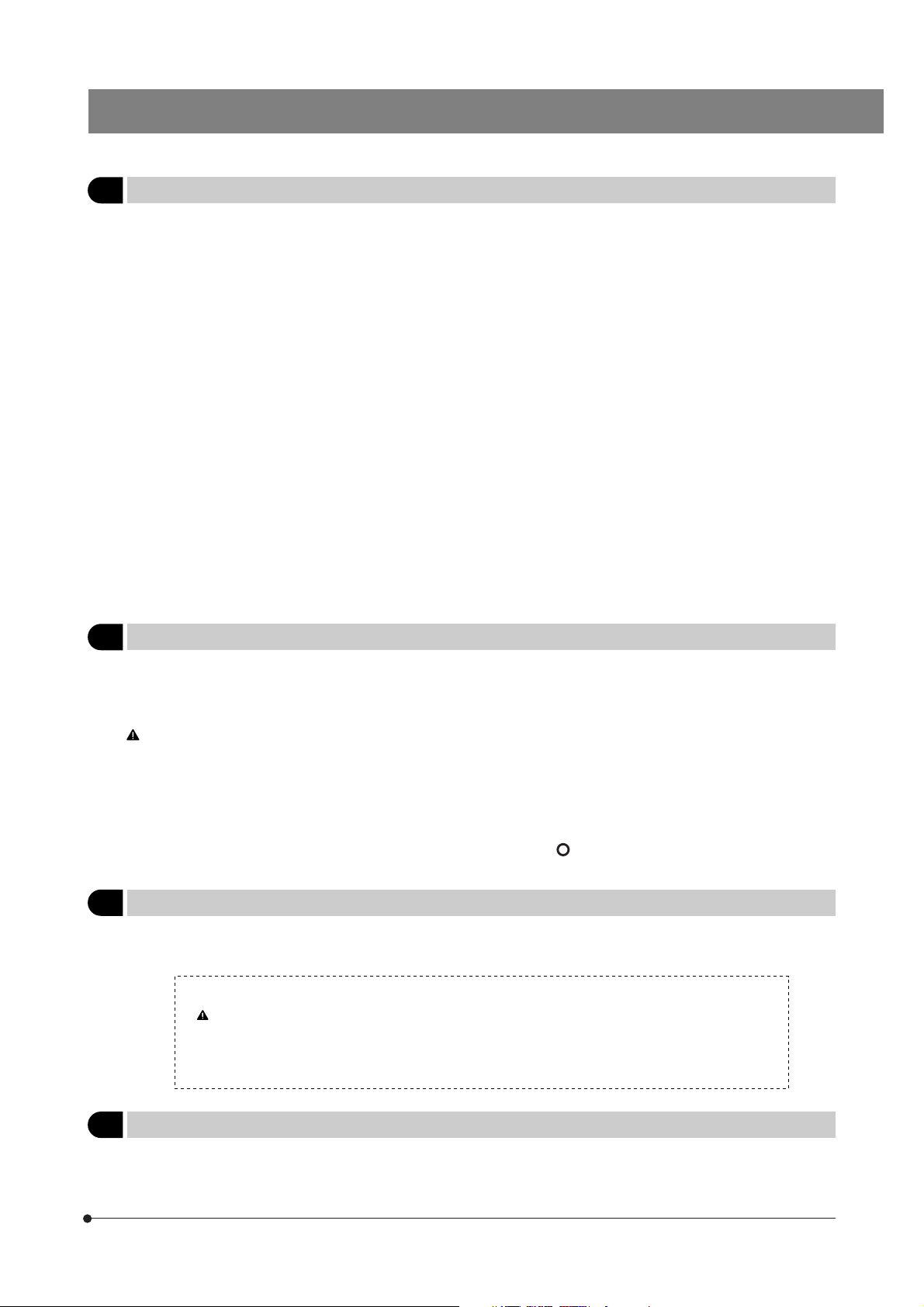
1 Getting Ready
1. A microscope is a precision instrument. Handle it with care and avoid subjecting it to sudden or severe impact.
2. The U-SWTR-3 super-widefield observation tube (FN 26.5) cannot be used with the BX61WI microscope.
3. The BX61WI microscope can be used with an intermediate attachment (such as a BX-RFAA, BX-URA2 or BX-RFA reflected light illuminator, U-ECA or U-CA magnification changer, etc.).
Two intermediate attachments can be used only in the following conditions:
· The U-CA or U-ECA magnification changer or U-FWO filter wheel can be mounted as the second attachment.
· When a TV adapter with 1X or higher power is used, 2/3-inch CCD TV observation is possible.
· The peripheral areas of the field of view may be obscured or cut off in binocular observation using the U-TR30-2, U-ETR3
or U-TR30IR (FN 22) super-widefield observation tube.
4. In IR (infrared) observation, the U-CA or U-ECA magnification changer can be used only when the U-ETR3 or U-TR30IR
observation tube is used.
5. In photomicrography with visible light, correct exposure may be impossible if the microscope is set for IR observation.
Be sure to engage the provided IR cut filter (light blue) before photomicrography.
6. When the XLUMPlanFLN20XW objective is used, only the U-TV1X, U-TVCAC, U-PMTVC2XIR or U-PMTVC4XIR TV adapter
can be used.
7. Do not attempt to remove or loosen the click springs and screws. Otherwise, Olympus can no longer warrant the
performance of the microscope.
The clicking force of the revolving nosepiece has been set weak in order to reduce vibrations during objective switching.
To reproduce the correct click position, switch the objectives gently by operating the lever.
8. Caution for use of the U-ETR3 upright trinocular tube:
When the aperture stop of the condenser is reduced using a reflected light fluorescence illuminator and the LUMPlanFl60XW
objective, part of the observed field of view may be obscured slightly. This is due to the reduction of the light intensity in
the field of view due to the narrow aperture and is not due to a defective optical adjustment of the microscope.
This phenomenon does not affect the photomicrography or TV camera light path.
BX61WI
2 Maintenance and Storage
1. To clean the lenses and other glass components, simply blow dirty away using a commercially available blower and
wipe gently using a piece of cleaning paper (or clean gauze).
If a lens is stained with fingerprints or oil smudges, wipe it gauze slightly moistened with commercially available absolute
alcohol.
Since the absolute alcohol is highly flammable, it must be handled carefully.
Be sure to keep it away from open flames or potential sources of electrical sparks --- for example, electrical
equipment that is being switched on or off, which could cause ignition of a fire.
Also remember to always use it only in a well-ventilated room.
2. Do not attempt to use organic solvents to clean the microscope components other than the glass components. To clean
them, use a lint-free, soft cloth slightly moistened with a diluted neutral detergent.
3. Never attempt to disassemble any part of the microscope.
4. When not using the microscope, make sure to set the main switch to “ ” (OFF), confirm that the lamp housing is cool
enough and cover the microscope with the provided dust cover.
3 Caution
If the microscope is used in a manner not specified by this manual, the safety of the user may be imperiled. In addition,
the equipment may also be damaged. Always use the equipment as outlined in this instruction manual.
The following symbols are used to set off text in this instruction manual.
: Indicates that failure to follow the instructions in the warning could result in bodily harm to the
user and/or damage to equipment (including objects in the vicinity of the equipment).
# : Indicates that failure to follow the instructions could result in damage to equipment.
} : Indicates commentary (for ease of operation and maintenance).
Intended use
4
This instrument has been designed to be used to observe magnified images of specimens in routine and research
applications.
Do not use this instrument for any purpose other than its intended use.
3
Page 8
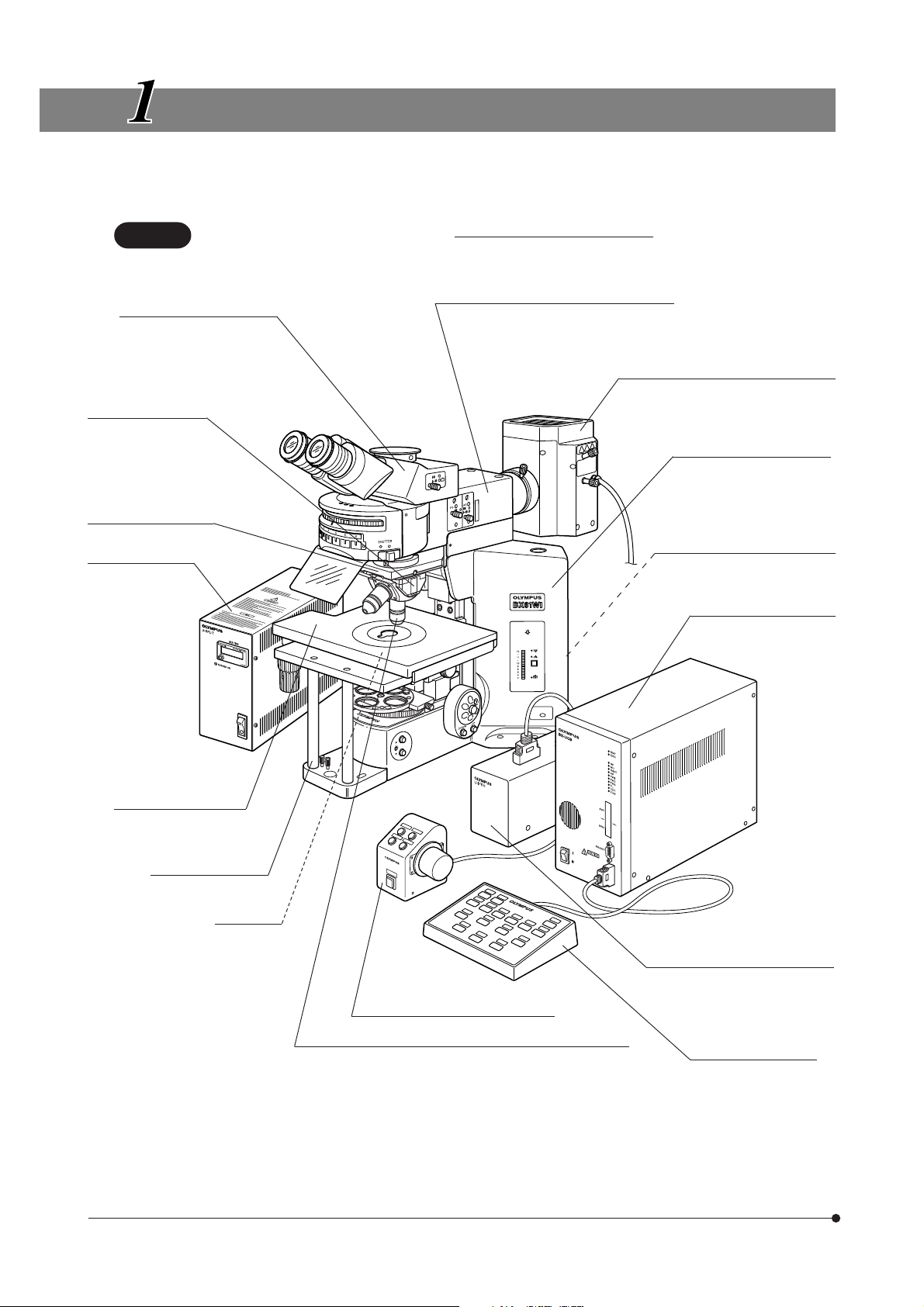
MODULE NOMENCLATURE
}The modules shown below are only the representative modules. As there are other modules which can be combined with
the microscope but are not shown below, please also refer to the latest Olympus catalogues or your dealer.
CAUTION
Trinocular Observation Tube
· U-TR30-2 (FN 22)
· U-TR30IR (FN 22)
· U-ETR3 (FN 22)
Revolving Nosepiece
· WI-SRE3
· WI-SNPXLU2
· U-SLRE
· WI-SSNP
(with revolving arm)
Revolving Arm WI-NPA
Power Supply Unit
U-RFL-T
The Z board combined with the BX61WI must always be the U-ZPCB(T2). Also note that the DIP switch
setting should be changed when the Z boar
d is used (see page 44).
Transmitted Arm BX-ARM
or Reflected Light Fluorescence Illuminator
· BX-RFAA
· BX-URA2
· BX-RFA
Reflected Light Mercury Lamp Housing
· U-LH100HG
· U-LH100HGAPO
Microscope Frame BX61WIF
Transmitted Light Lamp Housing
· U-LH100-3
· U-LH100IR
Control Box BX-UCB
The U-ZPCB(T2) Z Board
can be mounted here.
4
Cross Stage IX-SVL2
or bridge stage WI-XYS
Fixed-Stage Adapter
WI-FSH
Condenser
· WI-UCD
· WI-DICD
· WI-OBCD
· U-UCD8
· U-SC3
· U-AAC
· U-AC2
Focus Adjustment Knob Unit U-FH
Objectives
· MPLN5X
· UMPlanFLN10XW/20XW
· LUMPlanFLN40XW/60XW
· LUMFLN60XW
· LUMPlanFl100XW
· XLUMPlanFLN20XW (exclusively for use with the WI-SNPXLU2)
· XLFluor2X/340 (exclusively for use with the U-SLRE)
· XLFluor4X/340 (exclusively for use with the U-SLRE)
Focus Adjustment Knob Interface
U-IFFH
Hand Switch U-HSTR2
Page 9
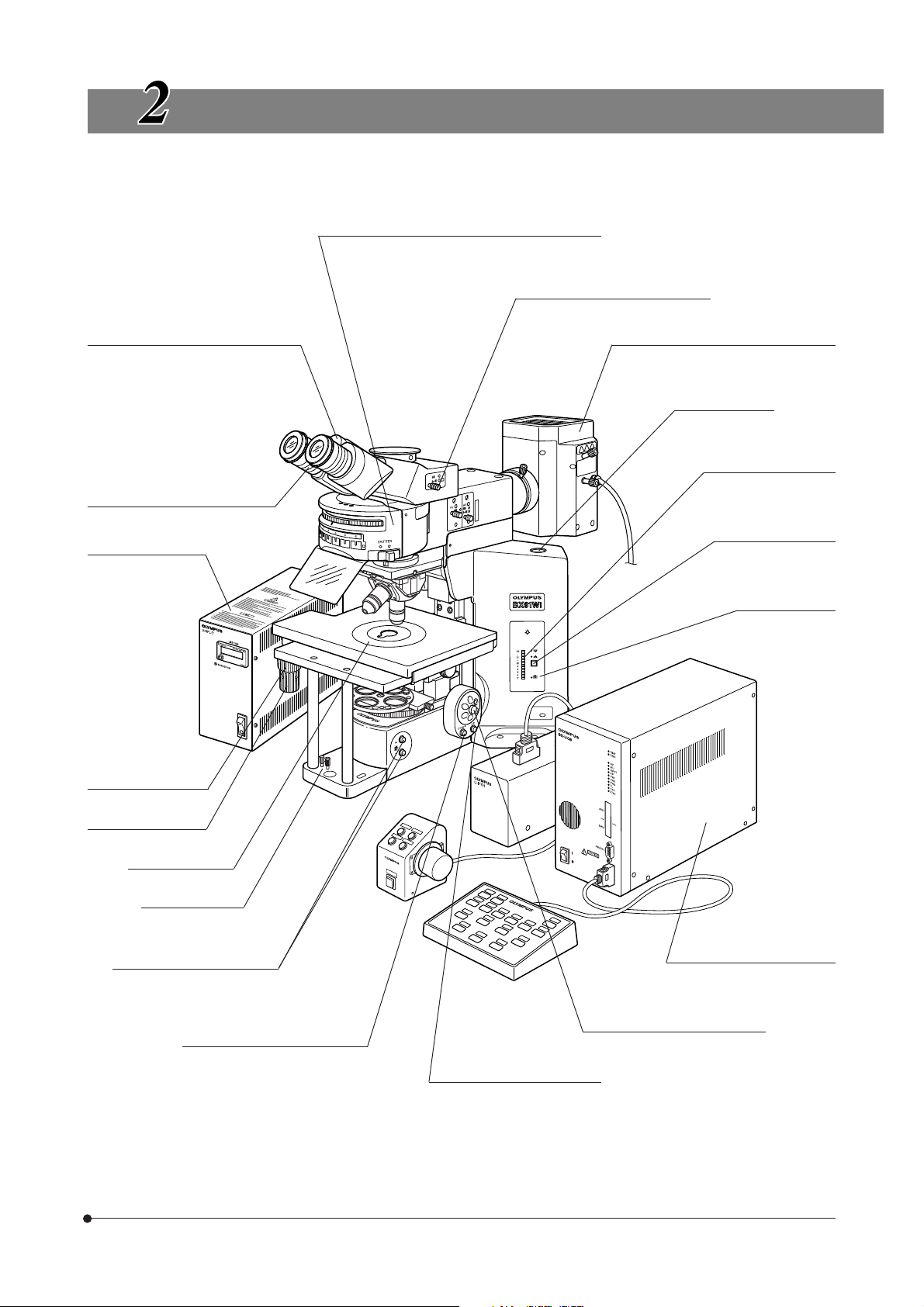
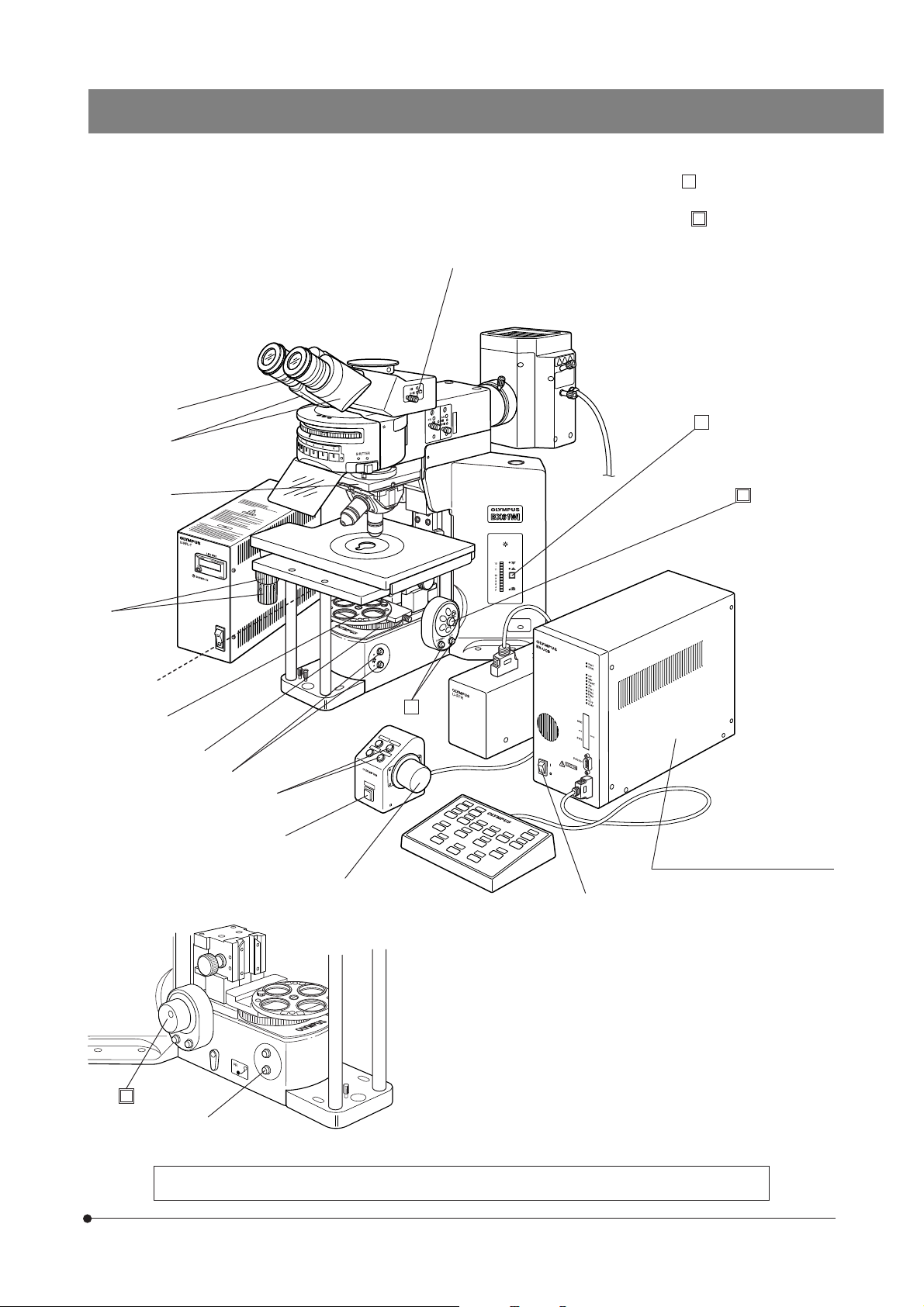
CONTROLS
}If you have not yet assembled the microscope, read Chapter 9, “ASSEMBLY” (pages 42 to 49).
Reflected light fluorescence illuminator
(See the separately provided instruction manual.)
Light path selector knob (Page 20)
BX61WI
Interpupillary distance scale (Page 19)
Diopter adjustment ring (Page 19)
Mercury lamp power
supply unit
(See the separately
provided instruction
manual.)
Y-axis knob (Page 17)
Reflected light mercury lamp housing
(See the separately provided instruction
manual.)
Allen screwdriver
(Storage position)
Lamp voltage indicator
(Page 14)
Transmitted/reflected
light switch (Page 14)
(For halogen bulb)
PHOTO indicator
(Page 14)
X-axis knob (Page 17)
Stage center plate
Centering knobs
(Storage position)
Light intensity control buttons
}The descriptions on the left side of the microscope frame, filter turret, revolving nosepiece, condenser, etc. will be given in
the subsequent pages.
(Page 14)
Objective down button (Page 16)
(See the separately provided
instruction manual.)
Focus adjustment dial (Page 15)
Objective up button (Page 16)
Control Box BX-UCB
5
Page 10
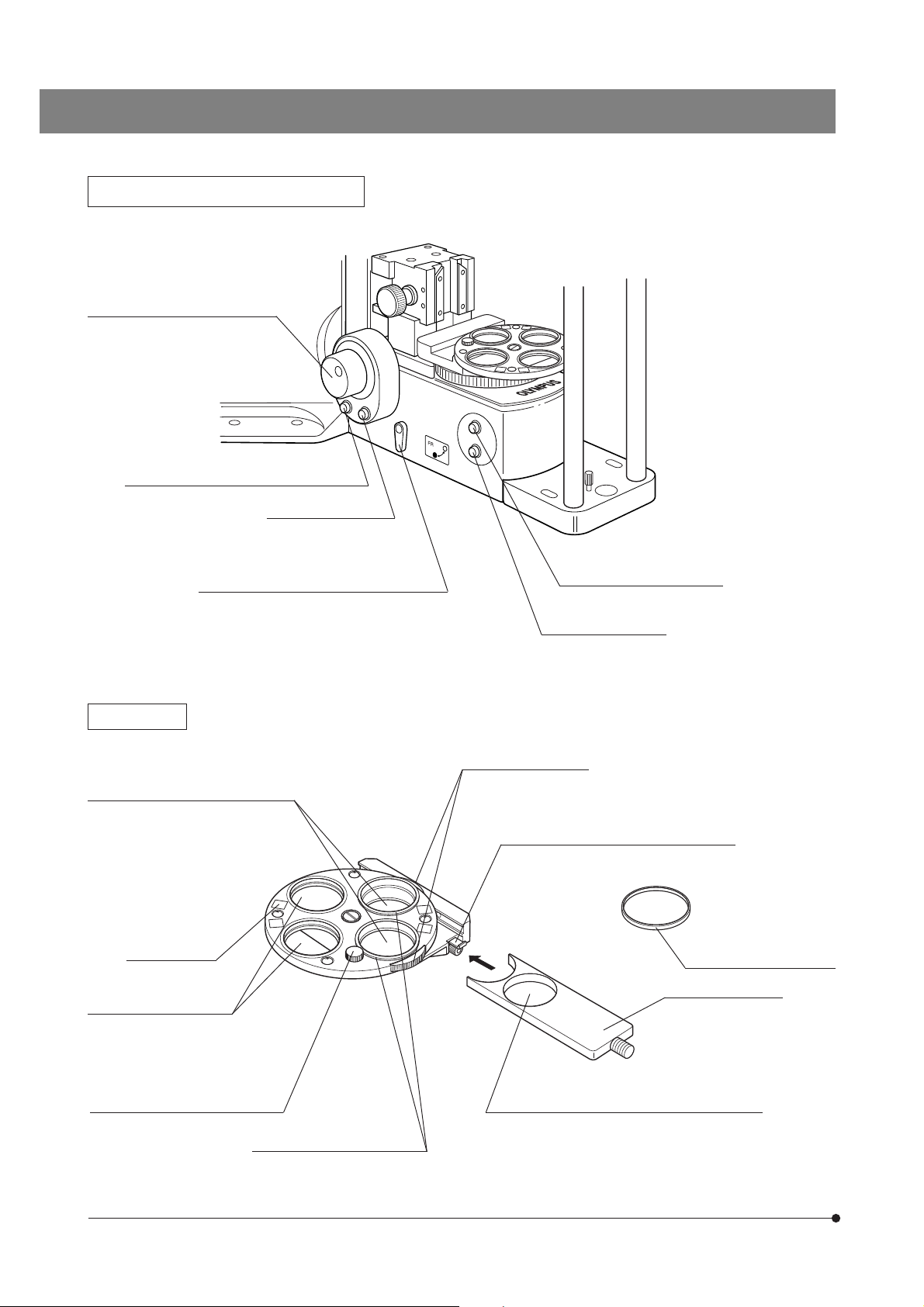
Left Side View of Microscope Frame
Focus adjustment knob (Page 15)
Objective escape/return button (Page 16)
F/C button (Page 16)
F: Fine adjustment
C: Coarse adjustment
Frost filterinsertion/removal lever (Page 16)
Filter Turret
Polarizer positions* (Page 24)
Accepts a 32PO or 32POIR polarizer.
Indication label
attaching frame
Filter positions
Accepts 32 mm filters with
thickness of no more than
6 mm.
Light Preset switch (Page 14)
Lamp ON-OFF switch
Polarizer rotating dials
(Page 28)
Slider insertion/removal stopper (Page 15)
* Accepts the combination of a
polarizer push ring and a 32 mm
filter. The thickness should be no
more than 6 mm.
Filter frame reinforcing ring
(Page 46)
Filter slider (Page 15)
Polarizer clamping knob (Page 24)
6
Polarizer push rings (Page 15)
Made of white plastic
IR filter insertion position
Accepts a 32BP775, 32IR900 or a 32 mm filter with
thickness of no more than 5.3 mm.
Page 11
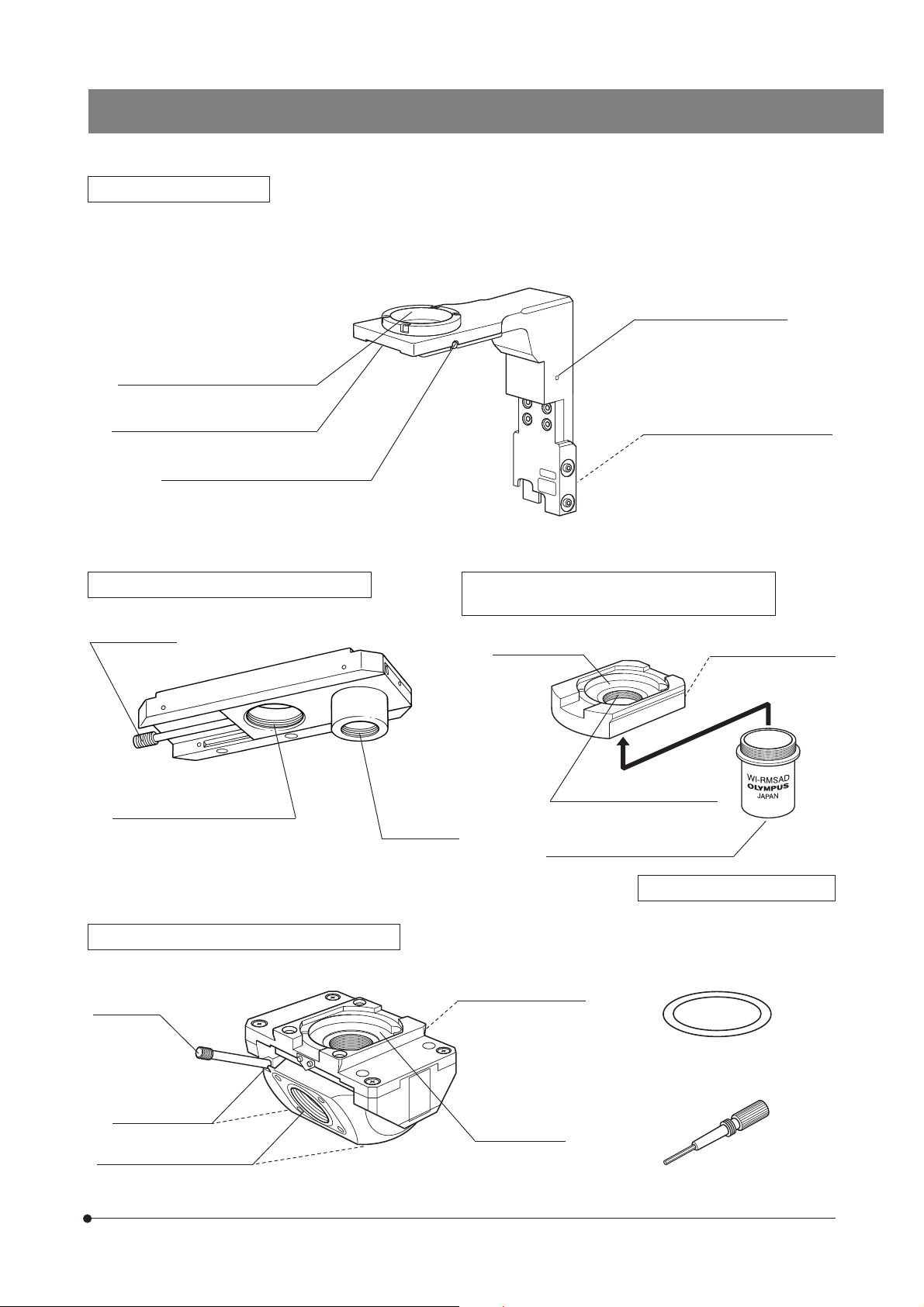
BX61WI
Revolving Arm WI-NPA
#Note that the revolving arm can be mounted only before the reflected light illuminator or transmitted light arm and
the IX-SVL2 stage are mounted (page 45).
}This revolving arm accepts the U-SLRE, WI-SNPXLU2 or WI-SRE3 revolving nosepiece.
Grounding screw hole (M3)
Light shielding tube retaining screw
Revolving nosepiece mount dovetail
Revolving nosepiece clamping screw
Sliding Revolving Nosepiece U-SLRE
Switching lever
XLFluor objective mount screw
M 34 mm, pitch 1 mm
UIS2 (UIS)
objective
mount screw
Microscope frame mount dovetail
Single-Position Revolving Nosepiece XLU
WI-SNPXLU2
DIC mount hole
(Page 25)
XLU objective mount screw
M 25 mm, pitch 0.75 mm
UIS2 (UIS) objective mount screw
Drop prevention screw
Swinging Revolving Nosepiece WI-SRE3
Swing lever
Centering screws
UIS objective mount screws
RMS Adapter WI-RMSAD
Confocality correction washer
Drop prevention screw
(Thickness 10, 30 and 50 μm, x 3 each)
DIC mount hole
(Page 25)
Centering knobs
7
Page 12
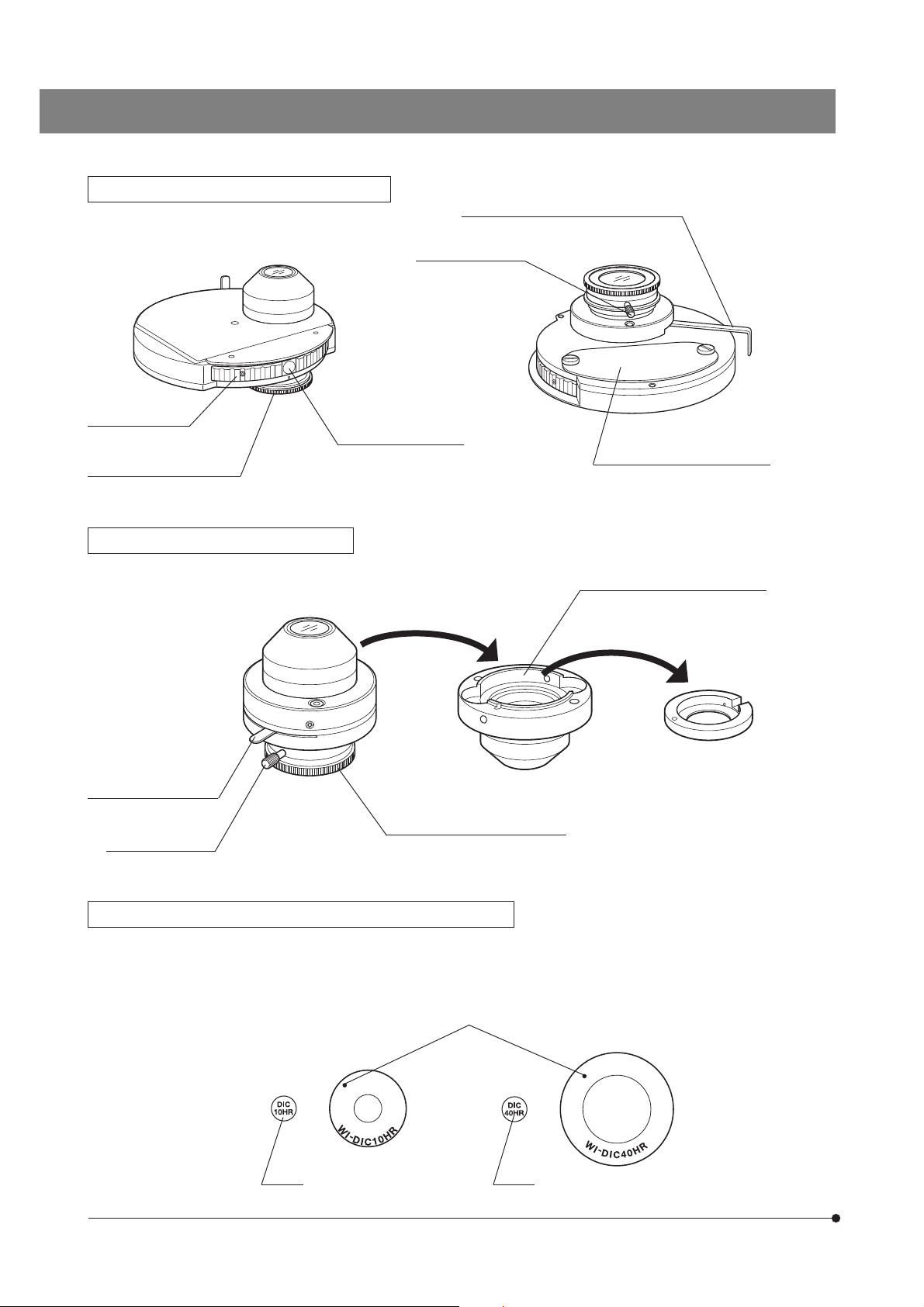
Long-WD Universal Condenser WI-UCD
Aperture iris diaphragm lever (Page 22)
Quarter-wave plate
clamping knob
Turret (4 positions)
Quarter-wave plate rotation
ring (Page 28)
Long-WD DIC Condenser WI-DICD
Aperture iris diaphragm
lever (Page 22)
Quarter-wave plate
clamping knob
Optical element index
mount (Page 26)
DIC prism replacement cover
DIC prism (Large) mount position
DIC prism (Small) adapter
Quarter-wave plate rotation ring
(Page 28)
Differential Interference Contrast Prisms (For Condenser)
}The WI-UCD condenser accepts two large and two small DIC prisms while the WI-DICD condenser accepts one large or
small DIC prism.
When selecting the brightfield (BF) light path using the WI-UCD, leave one DIC prism (large) mount position empty.
Positioning indices
· WI-DIC10HR
· WI-DIC20HR
Index
8
· WI-DIC40HR
· WI-DIC60HR
· WI-DICXLU20HR
Index
Page 13
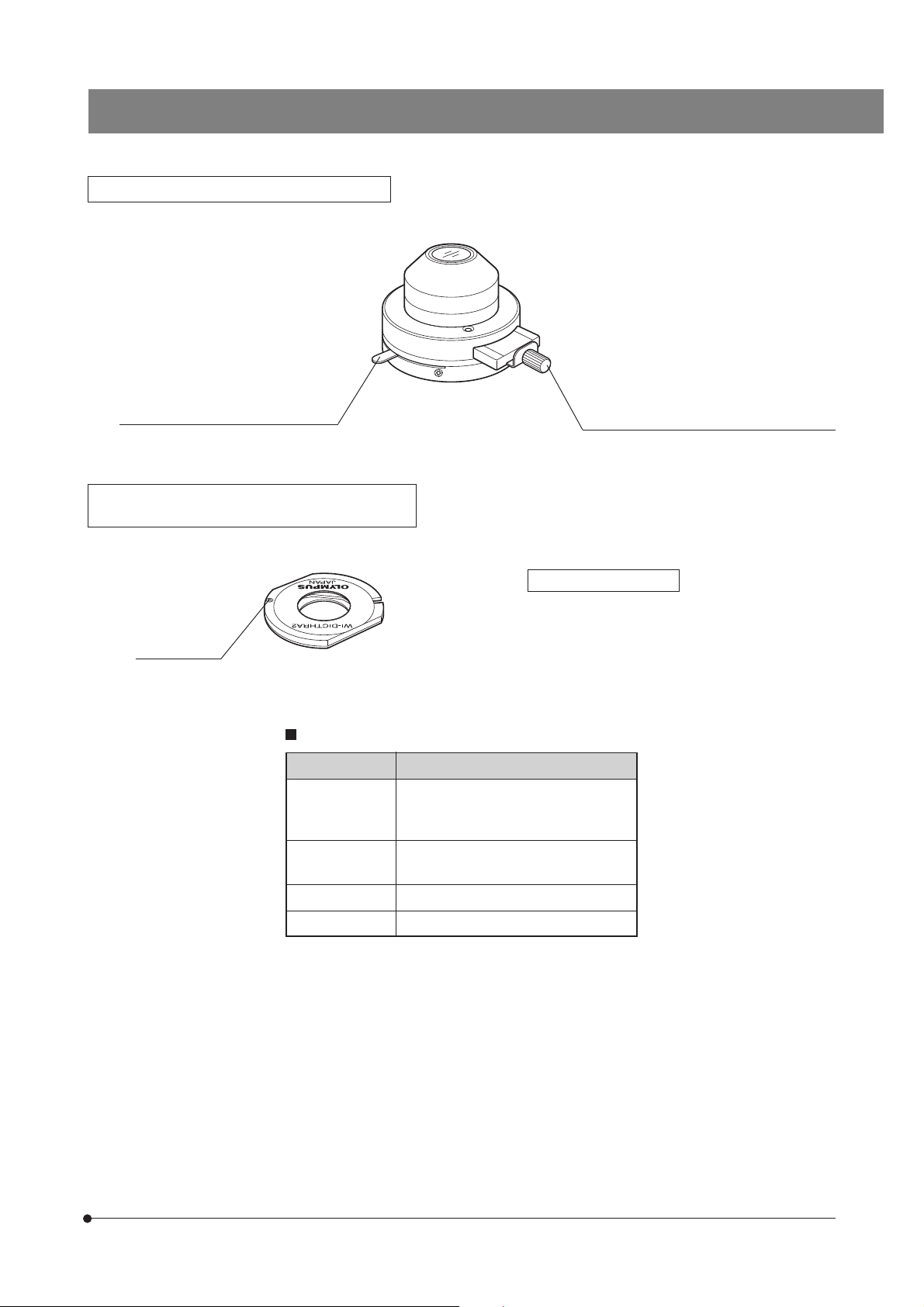
Long-WD Oblique Condenser WI-OBCD
BX61WI
Aperture iris diaphragm lever (Page 22)
High-Resolution DIC Prism A WI-DICTHRA2
DIC Prism WI-DICT2
Positioning pin
Condensers and Applicable Objective Magnifications
Condenser
WI-UCD
WI-DICD
WI-OBCD
U-UCD8
U-SC3
U-AAC 10X or more
Oblique iris insertion/removal knob (Page 22)
}This prism can be mounted in the DIC prism position
of the WI-SNPXLU2 or WI-SRE3.
Applicable condensers
WI-DICTHRA2: WI-UCD, W-DICD
WI-DICT2: U-UCD8
Applicable Objective Magnification
5X or more
2X or more
U-AC2 5X or more
9
Page 14
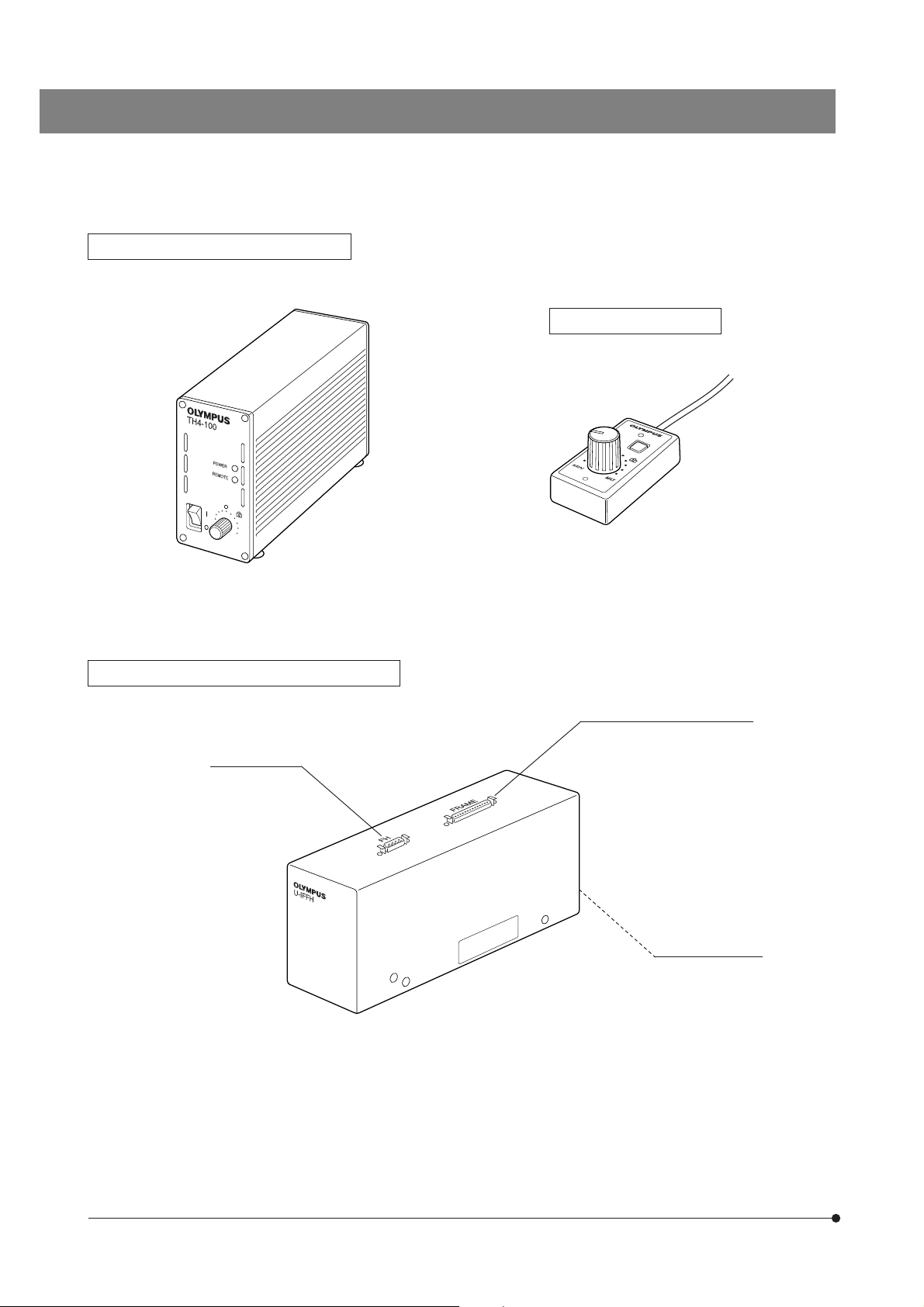
}The following modules are required to eliminate electrical noise, which may affect the potential measurement data during
patch clamping.
Halogen Lamp Power Supply TH4
Focus Adjustment Knob Interface U-IFFH
(See the separately provided instruction manual for details.)
Hand Switch TH4-HS
U-FH connector
Microscope FRAME connector
BX-UCB connector
10
Page 15
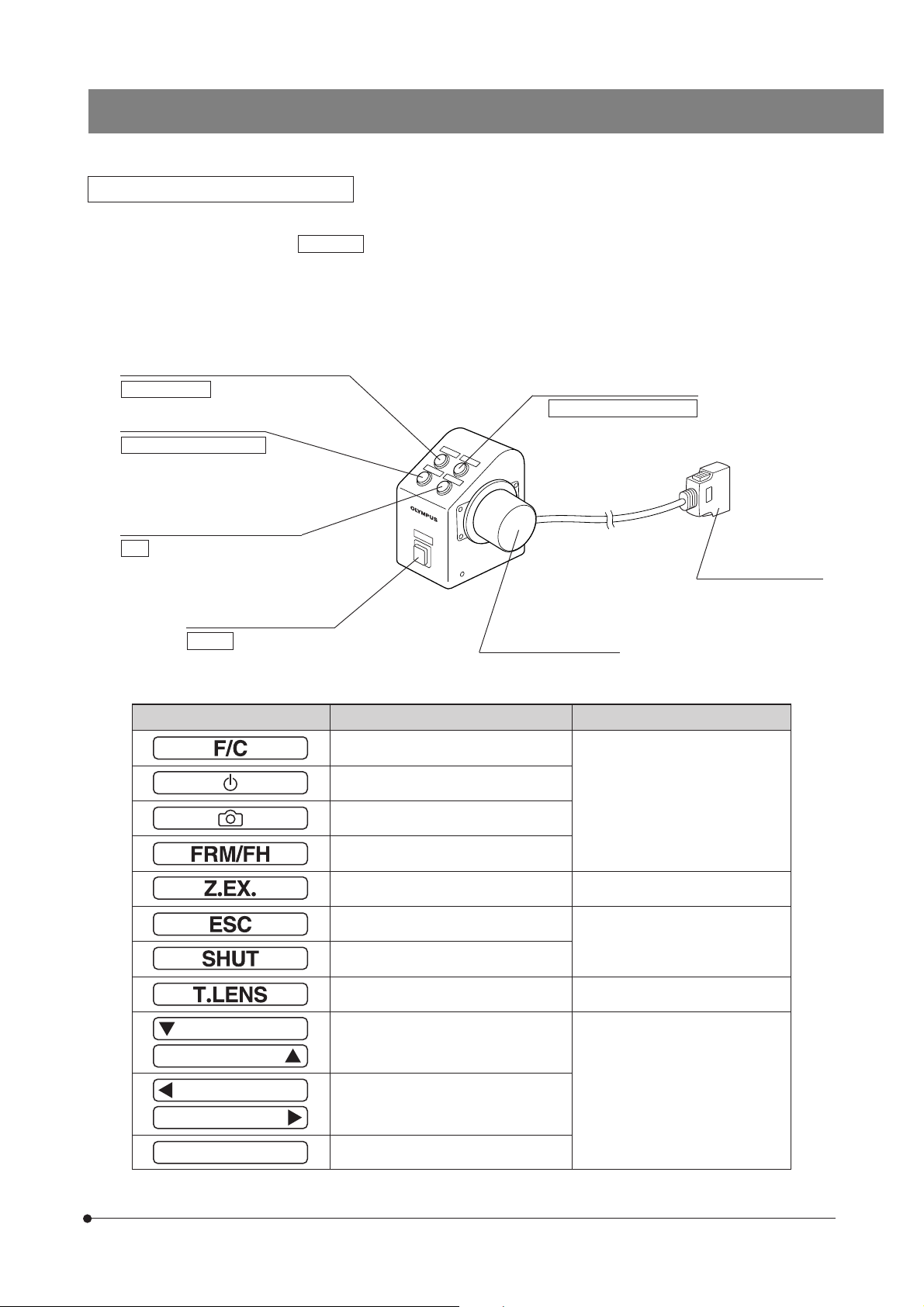
Focus Adjustment Knob Unit U-FH
The button functions described below are those when the microscope is used stand-alone.
The buttons functions inside are the initial setups for PC control (remote operation).
The button functions can also be assigned as required by the user. For the assignment, refer to the instructions for the
BX2-BSW BX Software (Ver. 03.01 or higher).
After setting up the button functions, attach the provided stickers near the buttons. For the function abbreviations and
symbols on the stickers, see the following table.
Objective escape/return button (Page 16)
Lamp ON/OFF
F/C button (Page 16)
Focusing motor ON/OFF
Objective down button (Page 16)
F/C
Transmitted/reflected light
switch button (Page 14)
Option
Objective up button (Page 16)
Objective escape/shelter
Connect to the U-IFFH.
Focus adjustment knob
BX61WI
Connector
Abbreviations & Symbols Function Note
Fine/Coarse switching
Lamp ON/OFF
Set/cancel photo voltage
Focus adjustment knob FRAME/U-FH
Z-focusing motor ON/OFF OFF: Electrical noise reduction
Escape/return objective
Shutter IN/OUT
Condenser top lens IN/OUT Not used with the BX61WI.
Up/down operation for brightness adjustment, objective, etc.
Left/right operation of mirror unit, filter
wheel, etc.
The function name can be written
in the blank area using an oil-ink
pen.
11
Page 16
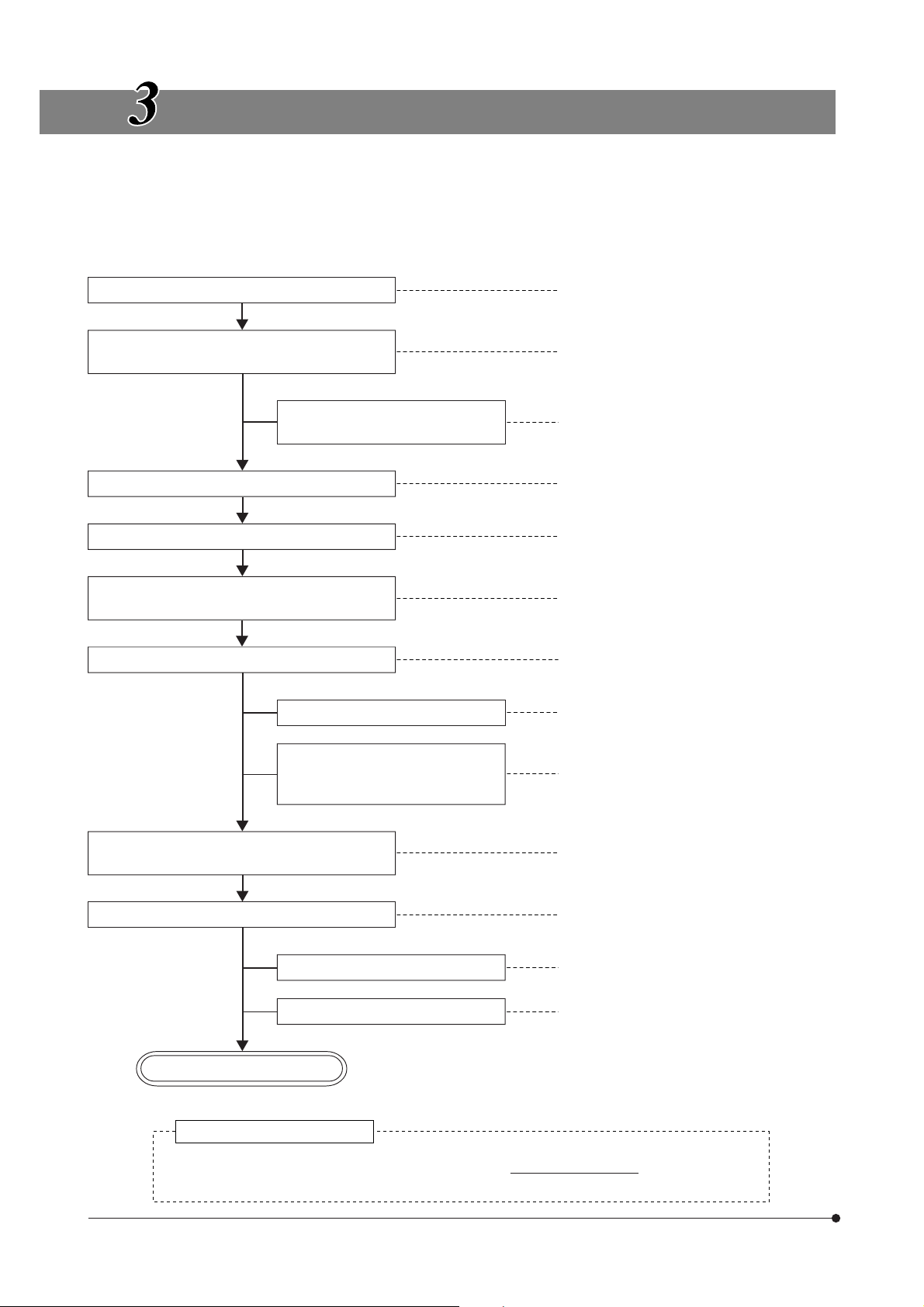
TRANSMITTED LIGHT BRIGHTFIELD
OBSERVATION PROCEDURE
}The following flow shows the operating procedure for the transmitted light brightfield observation which is the basic
observation method of this microscope. The operating procedures for DIC observation, fluorescence DIC observation and
IR DIC observation will be described separately in Chapter 5, “OTHER OBSERVATION METHODS” on page 24.
(Controls Used)
Set the main switch to “ I ” (ON).
Select the transmitted light illumination and
adjust its brightness.
Disengage the filter from the light
path.
Select the binocular light path. …Light path selector knob (P. 20)
Place the specimen on the stage. 7X-axis and Y-axis knobs (P. 17)
Engage a low-magnification objective in the
light path.
Bring the specimen in focus.
@Main switch
²Lamp ON-OFF switch
³Transmitted/reflected light switch (P. 14)
|Light intensity control buttons (P. 14)
5Filter turret (P. 15)
8Swing lever (P. 18)
9Objective up/down buttons, focus
adjustment knob/dial
(Page)
(P. 16)
Adjust the brightness. |Light intensity control buttons (P. 14)
Adjust the interpupillary distance.
Adjust the diopter.
Adjust the light axis.
Engage the objective to be used in the light
path and bring the specimen in focus.
Adjust the aperture iris and field iris diaphragms.
Engage the required filters.
Adjust the brightness.
Start observation.
Tip for microscope operation
aBinocular tube (P. 19)
bDiopter adjustment ring (P. 19)
cCondenser height adjustment knob (P. 21)
dCondenser centering knobs (P. 21)
eField iris diaphragm ring (P. 21)
‡Swing lever (P. 18)
9Objective up/down buttons, focus
adjustment knob/dial
fAperture iris diaphragm lever (P. 22)
eField iris diaphragm ring (P. 21)
5Filter turret (P. 15)
|Light intensity control buttons (P. 14)
(P. 16)
12
In patch-clamp testing, switch the microscope controls cautiously and gently so that the patch
electrodes do not slip off.
Page 17
BX61WI
(Note) Controls with numbers inside can perform the same
functions as the controls with numbers inside ¦.
However, the function of control is not available when
the U-FH is in use.
6
9
7
cdf
a
8
5
b
e
4
9
3
9
3
3
9
9
Control Box BX-UCB
* The U-ZPCB (T2) Z board can
1
be mounted here.
See page 44 for the setup and
installation of the Z board.
9
2
} Make a photocopy of the observation procedure pages and post it near your microscope.
13
Page 18

USING THE CONTROLS
4-1 Microscope Frame
5
3
2
Fig. 3
Fig. 4
1
4
6
1 Voltage Indication
1. Press the light intensity control button 1 to increase the voltage and
make illumination brighter.
Pressing the button 2 makes the illumination darker.
2. The numerals to the left of the lamp voltage indicator LEDs 3 indicate
the reference values of the voltages.
#The LEDs may turn off (temporarily) when the motor is driven, but the
illumination intensity even if this occurs.
2 Transmitted/Reflected Light Switch
}The same effect as this switch can also be obtained using the switch on
the U-FH focus adjustment knob unit (see page 15).
The illumination can be switched between the transmitted light and
reflected light by pressing the transmitted/reflected light switch 4.
: Reflected light illumination
: Transmitted light illumination
The LED indicator of the selected illumination lights.
3 Light Preset Switch
}The Light Preset switch sets the light intensity voltage to a voltage suitable
for color photography* (the factory default is 9 V) regardless of the current
setting of the light intensity control buttons.
* Achieved by engaging the LBD filter.
Setting the Desired Brightness (Figs. 3 & 4)
1. Press the Light Preset switch 5. The PHOTO indicator LED 6 lights up.
2. Press one of the light intensity control buttons 1 and 2 to set the desired
brightness.
3. Press the Light Preset switch again. The LED turns off, the brightness
returns to the original brightness but the setting made above is stored in
memory.
4. Hereafter, pressing the Light Preset switch sets the brightness to the
intensity value set in step 2 above.
#Be sure to return to the 9 V setting when performing color photogra-
phy.
}When the Light Preset switch 5 is pressed, an announcement tone (short
beep) is generated at the 9 V position of the switch.
}When the external TH-4 power supply unit is used instead of the
microscope’s built-in power supply circuitry, refer to the instruction manual
provided with the TH4.
(Fig. 3)
(Fig. 3)
(Fig. 4)
14
Page 19

BX61WI
(Fig. 5)
3
5
1
Fig. 5
2
4
4 Using the Filter Turret
}Filters with a diameter of 32 mm can be inserted in positions 1 to 4.
1. Positions 1 and 2 are rotatable. When the 32PO or 32POIR polarizer is
placed in either position, the polarizer can be fixed by means of the push
ring (made of white plastic).
}When filter position 1 is engaged in the light path, the polarizer clamping
knob 5 comes at the front where the operation is easy.
2. Filter position 3 accepts any type of 32 mm filter.
# When using two filters together, the thickness of the lower filter should
be no more than 2 mm. Otherwise, the upper filter may drop during
rotation.
3. Filter position 4 accepts the 32BP775 or 32IR900 IR filter. As the filter
cannot be inserted unless the filter slider is removed, remove it by releasing
the insertion/removal stopper below the slider and loosening the slider
clamping screw using the provided Allen screwdriver.
4-2 Focusing Block
}The same effect as the focus adjustment knob on the microscope frame can also be obtained using the U-FH focus
adjustment knob unit. However, when the microscope is used stand-alone while the cable to the U-FH is connected, the
focus adjustment is available only from the focusing knob on the U-FH.
3
Objective escape/return button
F/C button
Transmitted/reflected
light switch button
Focus Adjustment Knob Unit U-FH
4
2
Fig. 6
1
Objective up button
Objective down button
Focus adjustment knob
1 Replacing the Focus Adjustment Knob
}The focus adjustment knob is installed on the right side of the microscope
when it is shipped from the factory. (Detachable)
1. Loosen the clamping screw 1 with the Allen screwdriver and remove the
focus adjustment knob 2.
2. Remove the seal from the focus adjustment knob screw hole on the
other side and attach the knob by reversing the removal procedure.
3. Attach a provided seal on the screw hole 4 of the removed focus adjustment knob 3.
(Fig. 6)
15
Page 20

1
2
Fig. 7
2 F/C Button
}The F/C button switches the function of the focus adjustment knob 1
and focus adjustment dial between F (fine) and C (coarse). (For safety, the
F/C button is set automatically to F at the moment the main switch of the
BX-UCB control box is set to “ I ” (ON).)
· Each press of the F/C button 2 switches F and C alternately.
Objective fine movement: 0.1 mm per turn
Objective coarse movement: 0.3 mm per turn
(Fig. 7)
2
1
1
Fig. 8
Fig. 9
3 Objective Up/Down Buttons
#When the objective is lowered, be careful not to let it collide with the
specimen.
· Press the objective up button 1 to raise the objective and press the
objective down button 2 to lower the objective.
· The stroke is 25 mm.
4 Objective Escape/Return Button
When replacing the specimen, press the Objective Escape/Return button
1 The objective will rise by 5 mm (in 1 sec.). Pressing the button again
returns the objective to the original height.
#Even after escaping, care is still required during objective switching,
for the objective may still interfere with the edge of the container.
If this happens, raise the objective higher using the objective up
button.
(Fig. 8)
(Fig. 9)
16
1
Fig. 10
5
Using the Frost Filter Insertion/Removal Lever
}Low observation light can be brightened by turning the frost filter insertion/
removal lever 1 which controls the built-in frost filter, in the direction of the
arrow. However, although the brightness is increased, irregularity in lighting
may also increase.
(Fig. 10)
Page 21

4-3 Stage (IX-SVL2)
BX61WI
1
2
Fig. 11
Fig. 12
1 Placing the Specimen
1. Place the specimen on the center of the stage.
}The optional stage center plate (IX-CP50) makes it possible to observe a
wide range of a big petri dish, etc. (Central hole diameter: 50 mm)
2 Moving the Specimen
1. The specimen can be moved by turning the X-axis knob 1 and Y-axis
knob 2.
The movement strokes are 50 mm (X-axis) x 43 mm (Y-axis).
(Fig. 11)
(Fig. 12)
Fig. 13
Fig. 14
1
2
3 Setting the Grounding
}In case of electrical physiological experiment, etc., the specimen can be
1
grounded from the stage.
Prepare a grounding wire 1 and M4 screw 2 and attach grounding as
shown in Fig. 13.
# The screw hole may sometimes be stuck by paint, etc. In such a case,
screw in the M4 screw a few times to expose the metallic thread inside the screw hole and improve the contact before attaching the
grounding wire firmly.
4
Adjusting the X-Axis/Y-Axis Knob Rotation Tension
}The rotation tension of the X-axis and Y-axis knobs can be adjusted inde-
pendently.
1. Loosen the 2 set screws 1 of a knob using the provided Allen wrench,
hold the stage so that it will not move, then turn the knob to adjust the
tension. Turning it in the direction of the arrow increases the tension and
turning in the opposite direction decreases the tension.
2. After adjustment, tighten the set screws firmly.
# If the tension of a knob is too heavy or too light, skipping or returning of
image may occur during the stage movement.
(Fig. 13)
(Fig. 14)
17
Page 22

(Fig. 15)
Fig. 15
1
5 Using the Light Shielding Sheet
# The light shielding sheet provided with the reflected light fluorescence
illuminator is too small to be used with the BX61WI. Always use the
light shielding sheet provided with the BX61WI.
}During fluorescence observation using a low-magnification objective, the
fluorescence image may be deteriorated due to light reflected from the
condenser or the surroundings. In this case, use the light shielding sheet.
1. Lower the condenser to the lower limit position using the condenser
height adjustment knob.
2. Insert the light shied sheet 1 all the way into the gap between the upper
and lower stages on the side of the stage (IX-SVL2).
# If the condenser is lowered insufficiently, the sheet cannot be inserted
into the normal position and the light shielding effect cannot be obtained.
6 Lowering the Stage Height
The stage can be lowered by 50 mm by removing the condenser holder.
See page 49 for details.
4-4 Revolving Nosepiece
If the petri dish in use is filled with liquid, it may splash when the objective is switched. As such liquids are sometimes
toxic, be sure to move the revolving nosepiece away from the petri dish before switching the objective.
Even after the revolving nosepiece has been moved, re-focusing is easy by making use of the objective escape/return
button (page 16).
1
1
Fig. 16
Fig. 17
1 Switching the Objectives
}The clicking force of the revolving nosepiece has been set weak in order
to reduce vibrations during objective switching.
To reproduce the correct click position, switch the objectives gently by
operating the lever.
Sliding Revolving Nosepiece U-SLRE
Switch the objective by holding the objective switching lever 1 and
gently moving it back and forth.
}By attaching the objective switching lever 1 on the opposite side, a UIS
objective can be positioned on the front side of the microscope.
Swinging Revolving Nosepiece WI-SRE3
Switch objectives by gently puling up or pushing down the swing lever 1.
Handle the lever gently till it contacts a stopper on the revolving nosepiece.
(Figs. 16 & 17)
18
Page 23

4-5 Observation Tube
BX61WI
2
Fig. 18
Fig. 19
1
1 Adjusting the Interpupillary Distance
While looking through the eyepieces, adjust for binocular vision until the
left and right fields of view coincide completely. The index dot · indicates
the interpupillary distance.
}Note your interpupillary distance so that it can be quickly duplicated.
2 Adjusting the Diopter
1. Looking through the eyepiece without the diopter adjustment ring, adjust
the focusing using the objective up/down buttons to bring the specimen
into focus.
2. Looking through an eyepiece sleeve with the diopter adjustment ring 1
turn only the ring to focus on the specimen. (Fig. 19)
(Figs. 19 & 20)
(Fig. 18)
Fig. 20
Fig. 21
Using Finder Eyepieces
1. Looking through the right eyepiece with your right eye, turn the top of
the eyepiece 2 until a clearly defined double crosslines can be seen in
the field of view. (Figs. 19 & 20)
2. Looking through the right eyepiece, adjust the focusing using the objective up/down buttons to bring the specimen and double crosslines into
simultaneous focus.
3. Looking through the left eyepiece with your left eye, turn the diopter adjustment ring 1 to focus on the specimen.
3 Using the Eye Shades
When Wearing Eyeglasses
Use with the eye shades in the normal, folded-down position. This will
prevent the eyeglasses from being scratched.
When Not Wearing Eyeglasses
Extend the folded eye shades in the direction of the arrow to prevent
extraneous light from entering between the eyepieces and eyes.
(Fig. 21)
19
Page 24

Fig. 22
1
2
4 Using Eyepiece Micrometer Disks
Eyepiece micrometer disks can be inserted into the WHN10X-H (or
WHN10X) eyepieces.
Use 24 mm dia. x 1.5 mm micrometer disks.
Following Fig. 22, remove the micrometer mounting frame 2 from the
eyepiece and place a micrometer disk 1 into the mounting frame so
that the surface with the model indication faces downward.
Re-attach the micrometer mounting frame in the original position.
(Fig. 22)
Fig. 23
1
5 Selecting the Light Path of Trinocular Tube
Slide the light path selector knob 1 to select the desired light path.
Trinocular
Tube
U-TR30-2 Binocular 100%
U-ETR3 Binocular 100% TV/photo 100%
U-TR30IR Binocular 100% Shuttered TV/photo 100%
Pushed In
Light Path Selector Position
Intermediate Pulled Out
Binocular 20%,
TV/photo 80%
TV/photo 100%
(Fig. 23)
20
Page 25

4-6 Condenser
BX61WI
4
1
Fig. 24
Fig. 25
2
3
1 Centering the Condenser
1. Set the aperture iris diaphragm lever 1 to the open position. (Fig. 25)
2. Set the field iris diaphragm ring 2 to the open position ( ¦). (Fig. 24)
3. Focus on the specimen using the 10X objective.
4. Close the field iris diaphragm ring 2 so that the diaphragm image comes
inside the field of view.
5. Manipulate the condenser height adjustment knob 3 to focus on the
diaphragm image.
6. Turn the two condenser centering knobs 4 on the condenser holder to
move the iris diaphragm image to the center of the field of view. (Fig. 24,
Fig. A Fig. B).
7. Gradually open the field iris diaphragm. The condenser is properly
centered if the iris image is centered and inscribed in the field of view
(Fig. B Fig. C).
}During actual use, open the field diaphragm slightly until its image cir-
cumscribes the field of view.
(Figs. 24 to 26)
Fig. A Fig. B Fig. C
Field Iris Diaphragm
The field iris diaphragm restricts the diameter of the beam of light entering
the objective and thus excludes extraneous light, improving image
contrast. The diameter of the field iris should be adjusted for objective magnification to the extent that it just circumscribes the field of view.
21
Page 26

Aperture iris
diaphragm image
Objective pupil
Fig. 26
70-80%
30-20%
Aperture Iris Diaphragm
· The aperture iris diaphragm determines the numerical aperture of the
illumination system. Matching the numerical aperture of the illumination
system with that of the objective provides better image resolution and
contrast, and also increases the depth of focus.
· Since the contrast of microscope specimens is ordinarily low, setting the
condenser aperture iris diaphragm to between 70% and 80% of the NA
of the objective in use is usually recommended. If necessary, adjust the
ratio by removing the eyepieces and looking into the eyepiece sleeve
while adjusting the aperture iris diaphragm lever 1 until the image shown
in Fig. 26 is seen. (Fig. 25)
2
Fig. 27
Fig. 28
1
2 Oblique Illumination (WI-OBCD)
}The shading and 3D feeling of the specimen can be adjusted by varying
the width and orientation of the area subjected to oblique illumination.
This is possible with objectives from 5X to 100X.
#When the XLUMPlanFI20XW objective is used, oblique illumination
cannot provide a satisfactory effect due to the high NA (0.95) of the
objective.
IMPORTANT
1. Push in the oblique iris insertion/removal knob 1.
2. Turn the knob 1 to adjust the width of the area illuminated by oblique
illumination. (Fig. 28)
3. Adjust the orientation of the oblique illumination by turning the top part
2 of the condenser.
}Pull out the oblique iris insertion/removal knob when using the condenser
as usual.
The width of the oblique illumination area is maintained even after the
insertion/removal knob 1 has been pulled out, so the same condition
can be reproduced the next time the knob is pushed in.
The effect of oblique illumination assumes that the
field iris image is focused correctly.
Before proceeding to the following, pull out the oblique iris insertion/removal knob 1 and bring the field
iris image in focus (this is the same operation as that
described on page 21).
(Figs. 27 & 28)
22
Page 27

4-7 Water Immersion Objectives
BX61WI
2
Fig. 29
Fig. 30
1
2
1
1 Using Water Immersion Objectives
}When the UMPlanFLN series, LUMPlanFLN series or XLUMPlanFl20XW
objective is used, cultured tissue specimens which are often very thick
can be observed by immersing the specimen, objective front lens and
manipulator extremity in a medium (water) with the same refractive index.
# The electrically insulated area and immersion depth of the objective
1 are shown by the range of 2.
CAUTION
Water Immersion Cap for XL Objectives XL-CAP
In photometering with a film potential-sensitive fluorochrome, the water
surface fluctuations can be reduced and S/N can be improved by fitting
this cap 2 onto the top of the objective 1 (XLFuor2X/340 or XLFluor4X/
340).
Do not immerse the entire objective, for this will cause
malfunction.
After every immersed use, be sure to clean the front lens
with neutral detergent.
(Figs. 29 & 30)
23
Page 28

OTHER OBSERVATION METHODS
5-1 Differential Interference Contrast Observation
# The normal optical performance of DIC observation cannot be manifested if a plastic petri dish is used.
}DIC prisms (for revolving nosepiece and condenser), an analyzer and a polarizer are required for DIC observation.
When the reflected light fluorescence illuminator is not used, the U-KPA intermediate tube is required to attach the analyzer.
1
2
1
4
Fig. 31
Fig. 32
3
2
3
1 Attaching the Analyzer
U-ANT Analyzer
}Drop the U-ANT analyzer in the dummy slider of the U-KPA intermediate
tube.
1. Place the U-ANT 1 with the side with indications facing up, align the
indices and drop the analyzer into the dummy slider 2 (the analyzer will
be absorbed by magnet).
2. Set the dummy slider 2 back into the U-KPA 3 and tighten the clamping
knob 4.
U-AN Analyzer
}Insert the U-AN into the analyzer insertion slot of the reflected light fluo-
rescence illuminator. (Refer also to the instruction manual of your
reflected light fluorescence illuminator.)
2 Attaching the Polarizer
}The performance of a polarizer deteriorates after it has been subjected to
light for a lobe period (about 500 hours of continuous use). Replace the
polarizer when it has been used for a long period.
Drop the polarizer into the filter insertion position with a push ring 1 or 2,
and clamp with the push ring.
}It is recommended to insert the polarizer in insertion position 1. This is
because the polarizer rotation clamping knob 3 comes on the front of
the microscope when the insertion position 1 is engaged in the light
path.
}When the 32PO polarizer is used, adjustment is easier than with the
32POIR since images are brighter with the 32PO.
Besides, removal of the IR filter (32BP775 or 32IR900) makes images
brighter during adjustment although infrared light observation is to be
performed.
(Figs. 32 & 33)
(Fig. 31)
24
1
Fig. 33
When Using the U-UCD8
#The polarizer built into the U-UCD8 is not necessary with this micro-
scope. Remove it as described below.
1. Using a Phillips precision screwdriver, remove the 6 clamping screws 1
retaining the polarizer cover at the bottom side of the condenser. (Fig. 33)
2. Remove the cover to expose the polarizer and remove it together with
the frame. (Retain the removed polarizer carefully for future use with a
microscope other than the BX61WI.
3. Attach the polarizer cover to the original position.
Page 29

BX61WI
3
3
4
Fig. 34
2
1
4 Attaching the DIC Prisms (for Condenser)
}DIC prisms can be inserted in three types of condensers including the WI-UCD, WI-DICD and U-UCD8*.
* Do not use the U-UCDTP530 one-wave plate for the U-UCD8 but use the exclusive WI-TP137 quarter-wave plate.
List of DIC System Combinations
Condenser
DIC prism
(for revolving nosepiece)
Objective
Attaching the DIC Prisms (for Revolving Nosepiece)
}The DIC prisms for use in the revolving nosepiece include the W-
DICTHRA2 (high-resolution type) and WI-DICT2 (middle-contrast type). The
revolving nosepieces in which a DIC prism can be inserted are the WISRE3 and WI-SNPXLU2.
The DIC prisms (WI-DICT or WI-DICTHRA) cannot be attached to the revolving nosepieces (WI-SRE3 or WI-SNPXLU2) because of their shapes
and sizes.
1. Remove the revolving nosepiece from the revolving arm, then fully loosen
the drop prevention screw 1 with a Phillips precision screwdriver.
2. Hold a DIC prism 2 with the side with indications facing up, and insert it
by aligning the positioning pin 3 with the groove 4 on the revolving
nosepiece.
After the insertion, tighten the drop prevention screw 1 securely.
3. Attach the revolving nosepiece onto the revolving arm.
(Figs. 35 to 38)
Shearing Amount
Small (High resolution) Medium (Middle contrast)
WI-UCD
WI-DICD
WI-DICTHRA2 WI-DICT2
DIC prism (for condenser)
U-UCD8
(with WI-TP137)*
(Fig. 34)
10X WI-DIC10HR (small) DIC10
20X WI-DIC20HR (small)
Magnification
CCD observation
Application
** The actual view is equivalent to the middle-contrast type because of lower magnification and higher NA than usual.
(Surface to deep)
Binocular observation
(Surface layer only)
40X WI-DIC40HR (large) DIC40
60X DIC60
100X DIC100
XLU20X **WI-DICXLU20HR
Observation of relatively shallow area
Optimum for surface layer observation.
WI-DIC60HR (large)
(0 to 100 μm)
Observation of relatively deep area
(50 to 150 μm)
Less suitable for surface layer observation than the high-resolution type.
25
Page 30

1
2
With the WI-UCD Condenser (Figs. 35 - 37)
}When selecting the brightfield (BF) light path using the WI-UCD, leave
one DIC prism (large) mount position empty.
1. Remove the WI-UCD condenser from the microscope frame.
2. Remove the condenser cover 1 by loosening the retaining screws 2
using a coin, etc.
Fig. 35
7
4
3
5
6
8
Fig. 36
B
A
Fig. 37
3. Attach the suitable DIC prism for the objective in use as described below.
· Using the dedicated knob provided with the condenser, loosen the two
DIC prism clamping screws 3 until the rotatable limits.
· Rotate the turret by 90° counterclockwise, and drop in the DIC prism by
aligning its positioning pin 5 with the positioning groove 6 in the hole of
the turret 4 (Fig. 36).
#Be careful not to touch the prism inside the frame.
A. Rotate the turret 4 by 90° clockwise (Fig. 37) and tighten the two DIC
prism clamping screws 3 uniformly using the dedicated knob provided
with the condenser. (Figs. 36 & 37)
#Do not tighten the screws too much, or the prism frame may be
deformed.
B. Rotate the turret 4 by 90° clockwise (Fig. 37), and attach the index sticker
7 provided with the DIC prism onto the side 8 of the condenser turret 4
so that the index sticker is upside down. (Figs. 36 & 37)
4. After attaching all of the required DIC prisms, attach the cover 1 and
tighten the retaining screws 2. (Fig. 35)
5. Attach the condenser back onto the microscope frame.
26
Page 31

1
4
Fig. 38
3
2
BX61WI
With the WI-DICD Condenser (Fig. 38)
}The WI-DICD should be attached after completing the polarizer position
as described in item
1. Remove the WI-DICD condenser from the microscope frame.
2. Remove the two clamping screws 1 using the Allen screwdriver provided
with the microscope, then place the top of the condenser upside down.
3. When the DIC prism for use with the objective in use is a small DIC
prism, drop it in by aligning the positioning groove 2 on the adapter
located on the inner side with the pin 3 of the prism.
When the DIC prism for use with the objective is a large DIC prism, remove the adapter and drop in the DIC prism.
}Retain the adapter for future possible use.
4. Tighten the clamping screws 4 with the knob provided with the condenser.
5. Attach the condenser on the microscope again.
5 .
With the U-UCD8
}Attach the DIC prism by referring to the instruction manual provided with
the U-UCD8.
Adjusting the Polarizer Position
5
(except the U-UCD8)
# This adjustment is not necessary when the U-UCD8 is used. How-
ever, be sure to insert the WI-TP137 quarter-wave plate in a position
where the U-UCDTP530 one-wave plate for the U-UCD8 is otherwise
inserted.
}This adjustment is possible without removing the DIC prism (for revolving
nosepiece). However, it is not possible if a DIC prism for condenser is
engaged in the light path. Remove or disengage the DIC prism for condenser as described below.
· WI-DICD:Remove the DIC prism.
· WI-UCD :Rotate the turret to engage a position without DIC prism.
When the U-LH100IR Lamp Housing Is Used
Be sure to take the following measure to protect your eyes from
the IR rays.
}Insert the IR cut filter (light blue) provided with the microscope into
the filter slider 1 then push it in to engage it.
(Fig. 39)
3
Fig. 39
2
1
1. Remove the condenser from the microscope.
2. Remove an objective and engage the position without the objective in
the light path.
3. Engage the polarizer and analyzer in the light path (page 24) and turn
the transmitted light on.
27
Page 32

4. Remove the eyepiece from the eyepiece sleeve, look into the sleeve, turn
the polarizer rotation dial 2 so that the black interference stripe (Fig. 40)
is darkest, and tighten the clamping knob 3.
5. Engage an objective (as low-magnification as possible) in the light path,
attach the condenser and bring the specimen surface into focus.
2
Fig. 40
Fig. 41
1
IMPORTANT
}With the WI-DICD, do not attach the DIC prism.
With the WI-UCD, engage a position without DIC prism in the light path.
6. If the condenser has not been centered yet, center it (page 21).
}The interference stripe is not visible clearly if the field iris is focused insuf-
ficiently.
7. Turn the quarter-wave plate rotation ring 1 so that the black interference stripe seen at the center of the eyepiece sleeve’s field of view,
then tighten the clamping knob 2. Ignore the short interference stripes
in the surroundings in this adjustment.
Since this adjustment renders the field of view dark, observation cannot
be started unless the observation method described in the next item is
employed.
Now the adjustment is complete.
· Attach the eyepiece and objective again to the microscope frame.
· With the WI-DICD, remove it and mount the required DIC prism.
· When an IR cut filter is used, remove it and mount the required filter.
The interference stripe is less clearly visible when the
specimen is thick. In this case, it is recommended to
bring a scratch or like on the bottom of the petri dish
to facilitate the subsequent adjustment operation.
6 Observation Method
(Fig. 42)
28
2
Fig. 42
1
1. Engage the objective to be used in the light path.
2. When the WI-UCD or U-UCD8 condenser is used, engage the DIC prism
matching the objective in the light path by rotating the turret.
3. Place the specimen on the stage and bring the specimen into focus.
}The contrast may be improved by stopping down the aperture iris dia-
phragm to an optimum aperture.
4. Rotate the polarizer dial 1 on the filter turret to obtain optimum contrast
for the specimen. Tighten the clamping knob 2 if required.
Page 33

BX61WI
5-2 Reflected Light Fluorescence Observation
}Refer to the instruction manual of your reflected light fluorescence system. If you are using a reflected light illuminator which
is not motorized, it is recommended to use the WI-RSH illuminator shutter.
Illuminator Shutter WI-RSH
}The shock during observation can be reduced by using this optional shutter in place of using the shutter built into the
BX-URA2 or BX-RFA reflected light illuminator.
Idle hole
Shutter position
To be used by inserting in the 6-position filter/polarizer
insertion slot of the reflected light illuminator.
5-3 Infrared Light (IR)/Differential Interference Contrast (DIC) Observation
}The IR rays (775 or 900 nm) transmit the specimen by about 4 or 5 times more than visible light (550 nm). Therefore, the IR
observation is suitable for observing deep areas of a thick brain slice or optic nerve specimen.
1 Introduction
1. Since the IR wavelength used is 775 or 900 nm, the TV camera in use should be sensitive in the wavelength used.
(Example: C2741-79 CCD camera mfd. by Hamamatsu Photonics)
The IR light is harmful to your eyes. Avoid visual observation and use the TV monitor whenever possible. Should
visual observation be used, mount the IR cut filter (light blue) provided with the filter turret and engage the IR cut
filter in the light path.
2. To reduce the influence of heat on the specimen, stop down the field iris diaphragm of the BX61WI microscope as small
as possible. However, the contrast may sometimes be improved by circumscribing the field iris diaphragm with the field
of view.
29
Page 34

3. To enable IR observation, the following modules should be replaced with those based on the IR specifications.
IR TV camera
TV adapter
Trinocular tune
U-TR30IR
U-ETR3
U-TR30-2
(Note)
Video contrast device such as
the ARGUS10.20 by Hamamatsu
Photonics, monochrome video
monitor
Objectives
· MPLN5X
· UMPlanFLN10XW/20XW
· LUMPlanFLN40XW/60XW
· LUMFLN60XW
· LUMPlanFl100XW
· XLUMPlanFLN20XW
Objectives enclosed in are
usable with 900 nm IR rays.
Polarizer
32POIR
IR filter
32BP775 or 32IR900
Analyzer
WI-ANIR or
WI-ANTIR
(to be used together with the U-KPA)
Lamp housing
U-LH100IR
Thermal reflection filter
45SCF
(Note) Notes for combination of the TV adapter, intermediate attachment and observation tube
When an observation tube other than the U-TR30IR is used, select the TV adapter by referring to combinations a) to c)
below.
# With IR observation, the combination with the U-PMTVC or U-DPT cannot manifest full performance.
a) Combination for observing a wide field (direct image 1X)
Direct image adapter
U-TV1X
Mount adapter
One of 3 models*
* The mount adapter should be one of: 1) U-CMAD3 mount adapter; 2) U-BMAD bayonet mount adapter; 3) U-SMAD Sony
camera mount adapter.
Note) When the contrast is enhanced rather excessively by an image processor, the central area of the monitored image
may be made bright and noticeable.
TV camera
(IR specification model)
30
b) Combination using C-mount adapter IR system (visible light to 1000 nm)
C-mount adapter IR system
U-TVCAC
U-PMTVC2XIR
C-mount TV camera
(IR specification model)
U-PMTVC4XIR
c) Combination using U-CA or U-ECA intermediate tube
One of these intermediate tubes can be used only in combination with the U-ETR3 or U-TR30IR trinocular tube. The TV
adapter used in this combination should be one of that used in a) or b).
Page 35

BX61WI
1
2
4
3
Fig. 43
Fig. 44
2 Attaching the IR Modules
Thermal Reflection Filter 45SCF
1. Remove the collector lens of the U-LH10IR lamp housing 1 by loosening the 3 clamping screws 2 with an Allen wrench (width across flats of
2.5 mm).
2. While positioning the 45SCF filter 3 so that the arrow on its frame points
in the opposite direction of the lamp housing, insert the filter in the lamp
housing, and clamp by tightening the ring spring 4 provided with the
filter.
3. Attach the collector lens to the original position.
(Figs. 43 & 44)
IR Filter 23BP775 or 32IR900
}Be sure to insert the 32BP775 or 32IR900 IR filter in the filter slider below
the filter turret. (For the mounting method, see page 15.)
# If the IR filter is inserted above the polarizer in the filter turret, the
polarizer will be burnt.
Other IR Modules
Also replace other required modules with the IR modules (see page 30).
31
Page 36

3 DIC Observation Using IR
Since IR light is harmful to your eyes, use the monitor observation whenever possible even in adjustments.
1. First, perform adjustments for DIC observation without using IR.
}Do not mount the IR filter and see Section 5-1, “Differential Interference Contrast Observation” on page 24.
IMPORTANT
· Focus the field iris diaphragm image (page 21).
Be sure to perform this adjustment accurately because it determines the visual performance
using the IR light.
· With DIC observation using IR, do not stop down the aperture iris diaphragm lever 1 but leave
the diaphragm open. Since the contrast can be enhanced by the video enhancement function of the CCD camera controller, the diaphragm should be left open here so that the system
can manifest full performance.
1
2. Then engage the IR filter (32BP775 or 32IR900) in the light path by pushing in the filter slider.
3. While observing the monitor, perform DIC observation using IR.
a) Turn the condenser turret (other than the WI-DICD) to select the DIC prism matching the objective to be used.
b) Engage the objective to be used in the light path.
# Penetration of air bubbles inside the front lens of objective will deteriorate the view. To prevent this by removing
the bubbles, turn the revolving nosepiece slightly to move the immersed objective to the left and right for a few
times.
c) Bring the specimen into focus by moving the objective up and down.
4. Turning the 32POIR polarizer varies the density of the background. Set the polarizer to obtain optimum contrast for the
specimen.
32
Page 37

BX61WI
5-4 Macro Reflected Light Fluorescence Observation
}The macro reflected light fluorescence observation makes possible bright, low-magnification fluorescence observation by
combining low-magnification fluorescence mirror units and fluorescence objective.
1 Introduction
1. For the low-magnification fluorescence observation, use a low-magnification fluorescence mirror units.
The increased observation beam diameter of the fluorescence mirror units brightens the fluorescence by about 25%.
However, due to the large size of the fluorescence mirror units, they can be mounted only in every other position when the
BX-URA2 or BX-RFA reflected fluorescence illuminator is used (a total of 3 units can be mounted on each illuminator).
2. When performing transmitted light brightfield observation using a low-magnification fluorescence objective (2X or 4X),
also use the U-SC3 or U-UCD8 swinging condenser. If other condenser is combined, it will not be possible to illuminate
the entire field of view.
3. During low-magnification fluorescence observation, objective switching or stage movement, be careful so that the UIS2
(UIS) objective does not interfere with the specimen or culture container.
4. The low-magnification fluorescence objectives have been designed to manifest performances with no-covered dry
specimens to specimens located 5 mm below water surface level.
As a result, with water immersed specimens, the focused positions of these objectives are different from UIS2 (UIS)
objectives.
5. To enable macro reflected light fluorescence observation, the following modules should be replaced.
Low-magnification fluorescence
mirror units
U-MGFP/XL
U-MGFPA/XL
U-MF/XL
Sliding revolving
nosepiece
U-SLRE
Low-magnification fluorescence objective
XLFluor2X/340
XLFluor4X/340
Water immersion cap
for XL objective
XL-CAP
UIS2 objectives
UMPlanFLN-W series
LUMPlanFLN-W series
LUMFLN60XW
UPlanSApo10X to 40X
UPlanFLN10X to 40X only
Condenser
U-SC3
U-UCD8
33
Page 38

2 Attaching the Modules
Low-Magnification Fluorescence Mirror Units
}Select the suitable mirror units for purpose of observation by referring to
page 35.
}If you want to fabricate optional mirror units, see page 35.
· Mount the mirror units as indicated in the instruction manual of your
reflected light fluorescence system.
Note that mirror units can be mounted only in every other positions.
(Figs. 45 & 46)
6
1
5
3
2
Fig. 45
Fig. 46
4
Objective
1. Screw a UIS2 objective 2 into the position on the deeper side of the
U-SLRE sliding revolving nosepiece 1.
2. Screw a XLFluor2X/340 or XLFluor4X/340 low-magnification fluorescence
objective 3 into the position on the shallower side of the U-SLRE.
Sliding Revolving Nosepiece U-SLRE
1. Raise the revolving nosepiece mount fully.
2. Loosen the revolving nosepiece mount screw 4 on the microscope frame
using the Allen screwdriver provided with it.
3. Align the mount dovetail 5 of the sliding revolving nosepiece with the
revolving nosepiece mount dovetail and gently slide the sliding revolving
nosepiece all the way in from the front as shown in the figure.
4. Clamp the revolving nosepiece by tightening the revolving nosepiece
mount screw 4.
34
Swinging Condenser U-SC3/U-UCD8
#Attach the U-SC3 with the top lens swung out.
The condenser top lens should be swung out when using the 2X or
4X objective.
Page 39

3 Filter Characteristics of Fluorescence Mirror Units
BX61WI
Excitation
Method
IB
1. IB excitation (wide bandwidth) U-MGFP/XL 2. IB excitation (wide bandwidth) U-MGFPA/XL
Transmittance (%)
Mirror Unit Dichroic Mirror Excitation Filter Barrier Filter Application
U-MGFP/XL
DM505 BP460-490
U-MGFPA/XL
Wavelength (nm) Wavelength (nm)
BA510IF
BA510-550
Transmittance (%)
For EGFP, S65T, RSGFP.
(U-MGFPA/XL is for fluorochrome
separation.)
4 Fabricating Optional Mirror Units
}An optional mirror unit can be fabricated by attaching the custom-order barrier filter, excitation filter and dichroic mirror to
the U-MF/XL.
Dimension Conditions of Optical Components of Mirror Unit
· Barrier filter: 32
· Excitation filter: 25
· Dichroic mirror: See figure on the right.
Dichroic mirror
(made to custom order)
# When replacing the dichroic mirror, take special care not to stain it by leaving fingerprints, etc.
-0.1/-0.2
mm, max. thickness 4 mm
-0.1/-0.2
mm, max. thickness 6 mm
Barrier filter (made to custom order)
Interference mirror surface
Thickness: 1.5 ±0.05 mm
Excitation filter
(made to custom order or
ommercially marketed product)
UP
35
Page 40

TROUBLESHOOTING GUIDE
Under certain conditions, performance of the microscope may be adversely affected by factors other than defects. If problems
occur, please review the following list and take remedial action as needed.
If you cannot solve the problem after checking the entire list, please contact your local Olympus representative for assistance.
Problem
1. Optical System
a) The bulb does not light. The power cord of the BX-UCB is un-
plugged.
The main switch of the BX-UCB is not
ON.
The Lamp ON/OFF switch on the BX61WI
is not ON.
The bulb is burnt out.
The transmitted/ reflected light switch is
set to reflected light ( ).
b) The bulb lights but the field of view
is dark.
c) Field of view is obscured or not
evenly illuminated.
d) Dirt or dust is visible in the field of
view.
The aperture or field iris diaphragm is
opened in sufficiently.
The condenser is in too low a position.
The light path selector knob of is set to
position .
The light path selector knob is in an intermediate position.
The revolving nosepiece is not in a click
position.
The condenser is installed incorrectly. Re-install it. 47
The revolving nosepiece is installed incorrectly.
The filter turret or filter slider is incorrectly
engaged in the light path.
The frost insertion/removal lever is set to
an intermediate position or OUT.
An objective outside the illumination
range of the condenser is in use.
The condenser is not centered. Adjust the centering. 21
The field iris diaphragm is closed too
much.
The lamp bulb is not installed correctly. Push the halogen bulb terminals all
Dirt/dust on eyepieces.
Dirt/dust on condenser top lens.
Cause Remedy Page
Plug the power cord into a power outlet.
Set the main switch to “ I ” (ON).
Set the Lamp ON/OFF switch to ON.
Replace the bulb.
Set the switch so that the LED indicating transmitted light ( ) lights.
Open the aperture and field iris diaphragms.
Adjust the condenser height.
Set the light path selector knob to position or .
Set the light path selector knob to a
click position according to the purpose..
Set it in a click position.
Secure it by pushing in the sliding
dovetail all the way until the stopper.
Engage them correctly in the light path.
Engage the frost filter correctly in the
light path.
Use an appropriate condenser for the
purpose.
Open it until it circumscribes the field
of view.
the way into stop position.
Clean thoroughly.
–
–
–
48
14
21/22
21
20
20
18
45
15
16
9
21
48
3
36
Dirt/dust on specimen.
Page 41

BX61WI
Problem
e) Visibility of observed image is poor.
· Image is not sharp.
· Contrast is poor.
· Details are poorly visible
f ) One side of image is blurred. The revolving nosepiece is installed in-
g) Image appears to waver.
h) Focusing is lost when the objective
is switched (with the WI-SRE3).
i ) The field of view becomes only
slightly brighter when the voltage is
raised.
The objective in use is not designed for
UIS2 (UIS) series.
The condenser is set to too low a position.
The aperture iris diaphragm is closed too
much.
The objective is engaged incorrectly in
the light path
Air in the objective front lens. Remove the air.
The specimen such as a brain slice is
fixed poorly.
Bubbles attached to the objective front
lens.
Too small quantity of solution in the petri
dish.
The petri dish is tilted. Place the petri dish correctly on the
Dirt/dust on the objective front lens. Clean it thoroughly using neutral de-
Dust/dirt on the condenser. Clean it thoroughly. 3
correctly.
The objective is engaged incorrectly in
the light path.
The objective is placed incorrectly (may
be loose) in the revolving nosepiece
position.
The stage center plate is tilted. Correct the tilt.
The revolving nosepiece is installed incorrectly.
The objective is engaged incorrectly in
the light path.
The objective is placed incorrectly (may
be loose) in the revolving nosepiece position.
The condenser is centered incorrectly. Center it correctly. 21
The confocality is adjusted incorrectly. Adjust it correctly.
The condenser is centered incorrectly. Center it correctly. 21
The condenser is in too low a position. Adjust the condenser height. 21
Cause Remedy Page
Replace with a specified objective for
UIS2 (UIS) series.
Adjust the condenser height.
Open it sufficiently.
Make sure that revolving nosepiece
clicks into place correctly.
Fix it correctly.
Remove the bubbles.
Supply sufficient solution in the petri
dish.
stage.
tergent.
Secure it by pushing in the sliding
dovetail all the way until the stopper.
Make sure that revolving nosepiece
clicks into place correctly.
Insert the objective all the way into the
revolving nosepiece position until it is
stopped.
Secure it by pushing in the sliding
dovetail all the way until the stopper.
Make sure that revolving nosepiece
clicks into place correctly.
Insert the objective all the way into the
revolving nosepiece position until it is
stopped.
41
21
22
18
32
–
32
–
17
3
45
18
–
–
45
18
–
45/46
37
Page 42

Problem
2. Electrical System
a) The bulb intermittently lights and
goes out.
b) The lamp bulb burns out soon after
lighting.
c) The brightness cannot be varied
with the light intensity control.
d) The brightness cannot be varied
with the light intensity control.
e) The BX-UCB’s Z/AF gets into the
error status immediately after the
control box is turned on, and no operation is possible.
3. Observation Tube
a) The field of view of one eye does
not match that of the other.
4. Stage
a) Stage travel in the horizontal (X-axis)
direction stops in the middle.
b)The X-axis and/or Y-axis stage
knobs are too light or too heavy to
rotate.
Cause Remedy Page
The bulb is nearly burnt out.. Replace the bulb. 48
A cord or connector is not properly connected.
The bulb in use is not the specified lamp. Replace with a standard bulb.
No lamp bulb is installed. Attach a lamp bulb. 48
The lamp bulb is burnt out. Replace the lamp bulb. 48
The lamp housing output connector is
unplugged.
The lamp bulb is burnt out. Replace the lamp bulb.
Emergency stop is activated because the
focus adjustment knob is operated during initialization.
The interpupillary distance is incorrect.
Incorrect diopter adjustment.
Different eyepieces are used on the left
and right.
You are not accustomed to parallel optical axis.
The specimen is set incorrectly. Place the specimen correctly.
The X-axis and/or Y-axis rotation tension
is not adjusted properly.
Connect cords and plugs securely.
48
Plug the lamp housing output connector.
48
Turn the control box off and then on
again to perform the initialization operation.
Adjust interpupillary distance. 19
Adjust diopter.
Change one eyepiece to match the
other so that both sides are of the
same type.
When looking into eyepieces, do not
stare at image from the beginning but
see the overall field of view. It is sometimes recommended to turn your eyes
away from eyepieces, look far off and
look into eyepieces again.
Adjust the knobs to optimum tension.
19
17
17
–
–
1
–
–
38
Page 43

SPECIFICATIONS
Item Specification
1. Optical system UIS2 (UIS) (Universal Infinity System) optical system (Infinity correction)
2. Illumination system Transmitted Kohler illumination built in (FN 22)
12 V, 100 W long-life halogen bulb (pre-centered) 12V100WHAL-L (PHILIPS 7724) or 12 V, 50 W
long-life halogen bulb (precentered) 12V50WHAL-L (LIFE JC)
Average life time: Approximately 2000 hr. when used as directed.
Light intensity voltage range: Below 2.0 up to 12.0 V DC (continuously variable).
With a light intensity preset switch (Voltage variation range: Below 2.0 up to 12.0 V DC)
External power supply rating (BX-UCB): AC 100-120/220-240 V , 50/60 Hz, 3.5/1.5 A
Power consumption: 50 to 300 W (variable depending on the number of connected modules.) (Example – About 200 W with the 100 W halogen bulb and motorized focusing)
3. Focusing system Drive system: Motorized focusing using a stepping motor and ball screw.
Revolving nosepiece height movement by cross-roller guide
Finest adjustment scale: 1 μmm (fine adjustment sensitivity of 1 μm)
Resolution: 0.01 μm
Maximum stage speed: 3 mm/sec.
Stroke per rotation: 0.1 mm (fine), 1 mm (coarse)
Full stroke range: 25 mm
4. Revolving nosepiece Model
Attachable modules
5. Observation tube Model U-TR30-2
Field number 22
WI-SRE3
Swinging Revolving
Nosepiece
DIC prisms mountable DIC prisms mountable
Widefield Trinocular
U-SLRE
Sliding Revolving
Nosepiece
Widefield, Upright-Image Trinocular
Revolving Nosepiece
U-ETR3
BX61WI
WI-SNPXLU2
Single-Position
Tube tilting 30° 25°
Interpupillary
distance adjustment
Light path
selection
6. Stage Model IX-SVL2
X/Y movement
mechanism
7. Long-WD condenser Model
N.A. 0.8
Working distance
Aperture iris Variable aperture iris diaphragm
Turret 4-position
DIC prisms
Other Quarter-wave plate built in
3-step switching: 1Binocular 100%
2Binocular 20%
TV/Photo 80%
3TV/photo 100%
X-axis/Y-axis knob tension adjustable
Movement range: 50 mm (X-axis) x 43 mm (Y-axis)
WI-UCD
Universal Condenser
Max. 4 prisms can be mounted. Only 1 prism can be mounted.
50 mm to 76 mm
2-step switching: 1Binocular 100%
Stage with Bottom-Side Knobs
WI-DICD
DIC Condenser
5.7 mm
2TV/photo 100%
WI-OBCD
Oblique Condenser
Variable oblique iris built in
39
Page 44

Item Specification
8. Operating environment · Indoor use
· Altitude: Max. 2000 m
· Ambient temperature: 10° to 40°C (50°F to 104°F)
· Maximum relative humidity: 80% for temperatures up to 31°C (88°F), decreasing linearly
through 70% at 34°C (93°F), 60% at 37°C (99°F), to 50% relative humidity at 40°C (104°F)
· Supply voltage fluctuations: ±10%
· Pollution degree: 2 (in accordance with IEC60664)
· Installation (overvoltage) category: II (in accordance with IEC60664)
40
Page 45

OPTICAL CHARACTERISTICS
UIS series objectives not listed here can also be combined with this microscope.
The following table shows the optical characteristics of combinations of eyepieces and objectives. The figure on the right
shows the performance data engraved on the objectives.
NOTE
Refer to the latest catalogue or consult your local Olympus
representative for the updated information on the eyepieces
and objectives that can be combined with this microscope.
Magnification
Mechanical tube length
Water immersion
indicator (white)
BX61WI
Objective type
Number of aperture
FN (Field number)
Color band
Objective
MPLN
UIS2
Plan Achromat
series
(FN 22)
UMPlanFLN-W
Water Immersion
Universal Plan SemiApochromat
(FN 26.5)
LUMPlanFLN-W
Long-WD Water
Immersion Universal
Plan SemiApochromat
(FN 26.5)
LUMFLN-W
Long-WD Water
Immersion Universal
Semi-Apochromat
(FN 26.5)
XLUMPlanFLN-W
High-NA Water
Immersion
(FN 22)
LUMPlanFl-W
UIS
series
Long-WD Water
Immersion Universal
Plan SemiApochromat
(FN 26.5)
XLFluor
Low-Power
Fluorescence
(FN 22)
Optical
character
Eyepiece
Total
Power
WHN10X (FN22)
Focal
Depth
(μm)
Actual
Field
Power
5X 0.1 20.0 3.36 50X 98 4.4
10XW 0.30 3.50 1.10 100X 20 2.2
20XW 0.50 3.50 0.67 200X 6.1 1.1
40XW* 0.80 3.30 0.42 400X 2.0 0.55
60XW* 1.00 2.00 0.34 600X 1.3 0.37
60XW* 1.10 1.50 0.31 600X 0.7 0.37
20XW* 1.00 2.00 0.34 200X 0.83 1.1
100XW* 1.00 1.50 0.34 1000X 0.83 0.22
2X/340 0.14 20.0 2.40 20X 132 11
4X/340 0.28 28.4 1..20 40X 33.0 5.5
N.A.
W.D.
(mm)
Resolution
(μm)
Remark
Water immersion
impossible
*Usable with IR
900 nm.
Correction
collar
Water immersion
impossible
Water immersion
impossible
NOTE
Be sure to clean the extremity of water immersion objective using neutral detergent.
If the extremity is left without cleaning, contamination remains and the objective performance will deteriorate.
41
Page 46

ASSEMBLY
9-1 Assembly Diagram
The diagram below shows the sequence of assembly of the modules. The numbers indicate the order of assembly.
The module numbers shown in the following diagram are merely the typical examples. For the modules with which the
module numbers are not given, please consult your Olympus representative or the latest catalogues.
# When assembling the microscope, make sure that all parts are free of dust and dirt, and avoid scratching any parts
or touching glass surfaces.
Assembly steps enclosed in will be detailed on the subsequent pages.
}All assembly operations are possible by using the Allen screwdriver ( ) provided with the microscope. However,
the reflected light illuminator should be attached using the Allen wrench provided with the illuminator to clamp the internal
screws. (To assure the performance, please have your dealer assemble the illuminator.)
The filter turret and cross stage are to be clamped respectively using the special tools provided with them.
(Note) For the detailed assembly procedures of the reflected light fluorescence system, the BX-UCB control box and the TH4
power supply unit, refer to their instruction manuals.
Eyepieces
WHN10X (FN 22)
35WHN10X (FN 22)
Revolving arm
Revolving
nosepiece
Objectives
(See next page.)
Intermediate
attachment
U-ECA
U-FWO
U-KPA, etc.
Trinocular tube
U-TR30-2 (FN 22)
U-ETR3 (FN 22)
Transmitted light arm
BX-ARM
Reflected light
fluorescence
illuminator
BX-RFAA
BX-URA2
BX-RFA
Reflected light mercury bulb
lamp housing
U-LH100HG
U-LH100HGAPO
Cross stage
IX-SVL2
Fixed stage
adapter
WI-FSH
Condenser
WI-UCD
WI-DICD
WI-OBCD
U-UCD8
U-SC3
Stage center plate
Condenser clamping knob
Focusing adjustment
knob unit
U-FH
Hand switch
U-HSTR2
Microscope
frame
BX61WIF
Filter turret
Filter frame
reinforcing
ring
Power supply unit
U-RFL-T
Halogen lamp housing
U-LH100-3
U-LH100IR
Lamp socket
clamping screw
Focusing adjustment
knob interface
U-IFFH
Control box
BX-UCB
42
Page 47

Revolving Arm, Revolving Nosepiece, Objectives
BX61WI
Light shielding tube
Turr et
Light shielding tube
(Transmitted light
arm adapter)
Transmitted light arm
BX-ARM
The revolving nosepiece clamping
screw is not used.
Sliding revolving nosepiece
U-SLRE
Objective
XLFluor2X/340
XLFluor4X/340
Swinging
revolving
nosepiece
WI-SRE3
Reflected light fluorescence
illuminator
BX-RFAA
BX-URA2
BX-RFA
Light shielding tube
Revolving arm
WI-NPA
Revolving nosepiece clamping screw
Clamping screws
XLU single-position
revolving nosepiece
WI-SNPXLU2
RMS adapter
WI-RMSAD
Objective
XLUMPlanFLN20XW
UIS2 (UIS) objective
5 Waterproof covers (x 3): BIorepellent polyehtylene
Magnets
Magnetic support plates
(with double-side adhesive tape)
Clamping band
Microscope frame
BX61WIF
43
Page 48

9-2 Detailed Assembly Procedures
# Be always sure to use the U-ZPCB(T2) Z board which is designed to be compatible with the BX61WI.
The following phenomena will occur with the U-ZPCB even though the correct setup is performed.
· The rotation direction of the focus adjustment knob is opposite to the actual movement direction of the objectives.
· Initialization may not be possible depending on the position of the focusing block. (The microscope may not start up.)
Setting Up and Mounting the Z Board
}The on-board DIP switches on the Z board have been designed for use
with the BX61 or BX62 microscope at the factory (i.e. all of the S1, S2 and
S3 switches at the OFF positions).
Change the setup of the DIP switches to enable the use of the Z board
with the BX61WI.
2
S1 S2 S3
Fig. 47
1
3
Fig. 48
Changing the On-Board DIP Switch Setting (Fig. 47)
#Set all other switches than those listed below to the OFF positions.
· S2 Set No. 2 and No. 3 to ON.
· S3 Set No. 2, No. 4 and No. 5 to ON.
#If the setting is not correct, the objective may lower and hit the speci-
men during initialization.
Mounting the Z Board (Fig. 48)
}Set the main switch of the BX-UCB control box 1 to “ ” (OFF) before
mounting the Z board.
1. Loosen the 6 knobs clamping the 2 option slot covers on the rear of the
BX-UCB and remove the knobs and covers.
2. Align the connector of the Z board 2 with that inside the BX-UCB and
insert the board along the board rails.
3. Clamp the Z board 2 using the clamping knobs removed above. Attach
the other cover 3 in the same way.
}Retain the cover removed for mounting the Z board carefully.
44
Page 49

BX61WI
}When performing observation on a desktop, attach the provided rubber feet (x 4) to the front (x 2) and rear (x 2) parts on the
bottom of the base. Note that the rubber feet are not necessary when installing the microscope on an anti-vibration bench.
2
4
3
2
Fig. 49
1
Fig. 50
1
1 Attaching the Revolving Arm
1. Loosen the 2 clamping screws 2 of the revolving arm 1 using an Allen
screwdriver and fit the arm into the mount dovetail on the microscope
frame along the direction of arrow from to .
2. Push the revolving arm all the way until it is stopped, then tighten the
clamping screws.
3. Screw in the light shielding tube 3 into the retaining screw 4.
1 3
2 Attaching the Revolving Nosepiece
}The attaching procedure is common for the U-SLRE, WI-SRE3 and
WI-SNPXLU2 revolving nosepieces, except that the WI-SNPXLU2 2 should
be inserted so that the round end comes on the front.
CAUTION
1. Loosen the revolving nosepiece clamping screw 1 using an Allen screwdriver, and slide in the revolving nosepiece 2 along the mount dovetail.
2. Push the revolving nosepiece all the way in and tighten the clamping
screw.
In DIC observation using the WI-SRE3 or WI-SNPXLU2
revolving nosepiece, attach the DIC prisms before attaching the revolving nosepiece (see page 25).
(Fig. 49)
(Fig. 50)
Fig. 51
1
2
WI-SRE3 Only
}When the microscope has been assembled, the confocality adjustment
and centering of the two objectives can be performed.
Adjusting the Confocality of Objective (Figs. 51 & 52)
}To maintain the accurate focusing even after the objective is switched,
the height of the objective with the higher focal point is corrected by
attaching a washer.
Nine washers with three kinds of thickness (10, 30 and 50 μm), three per
kind, are provided with the microscope.
1. Engage the objective on the front side in the light path. Use the coarse/
fine adjustment knobs on the front side to adjust focusing.
}Accurate focus cannot be achieved if the coarse/fine adjustment knobs
on the back side are used.
2. To find the confocality difference, read the scale indication of the fine
adjustment dial 2 (1 scale graduation: 1 μm).
45
Page 50

3
4
5
6
Fig. 52
3. Engage the objective on the back side in the light path.
Adjust focusing, read the scale indication of the fine adjustment knob
and obtain the difference from the value read in step 2 above.
4. The objective to use the washer is determined by the rotation direction of
the fine adjustment dial 2. (Fig. 51)
· Direction of the arrow: Objective on the front side.
· Opposite direction to the arrow: Objective on the back side.
5. Remove the objective 3, fit the washer 5 with the required thickness on
the screw 4, and then attach the objective again.
6. Switch the objective and confirm that confocality is implemented.
#The confocality adjustment may sometimes be unable to implement
perfect confocality.
Centering the Objective (Fig. 52)
}The centering mechanism is provided only for the objective on the front
side.
1. Adjust focusing using the objective on the front side, then move the
target region in the specimen on the center of the field.
2. Switch the objective to that on the front side.
3. Insert the centering knobs into the threaded holes 6 and turn them to
move the target in the objective on the center.
}After completing centering, store the centering knobs in the accommo-
dation position on the front side of the microscope (page 5) so as not to
lose them.
3
2
1
Fig. 53
3 Attaching the Filter Turret
}When a bridge stage or a stage with similar design is used, the filter slider
1 can be attached so that it faces toward the front of the microscope.
1. Drop in the filter frame reinforcing ring 2 into the filter holder on the
microscope frame.
2. Loosen the 2 filter turret clamping screws 3 using the provided Allen
wrench.
3. Fit the filter turret on the filter holder and tighten the clamping screws 3
lightly to a degree at which the filter turret will not rotate.
4. For the insertion of the polarizer and filters, see page 15.
(Fig. 53)
46
Page 51

BX61WI
4
3
2
3
Fig. 54
Fig. 55
1
2
2
3
1
4 Attaching the Condenser
# When attaching a condenser other than the WI-UCD, remove the
upper limit stopper screw 1 of the condenser holder with an Allen
screwdriver.
}In DIC observation, attach the DIC prism (for condenser) before attaching
the condenser onto the microscope frame (page 25). However, with the
WI-DICD condenser, the DIC prism should be attached after adjusting the
polarizer position.
1. Rotate the condenser height adjustment knob 2 to raise the condenser
holder to an optimum height.
2. Fully loosen the condenser clamping knob 3.
3. Slide in the condenser 4 from the front along the mount dovetail all the
way until it is stopped.
# When the microscope frame has a positioning pin on the rear posi-
tion of the condenser, align the condenser with the groove on the
condenser holder.
4. Tighten the condenser clamping knob and lower the condenser holder
to the lowest limit position.
5 Attaching the Waterproof Cover
}Attach the waterproof cover onto the condenser if required.
The waterproof cover is applicable only to the WI-UCD, WI-DICD and
WI-OBCD condensers.
1. Fit the hole of the waterproof cover 1 in the extremity of the condenser
and clamp with the clamping band.
CAUTION
In DIC observation, the condenser has to be removed
and attached during adjustments. Therefore, in this case,
do not attach the clamping band but just fit the hole of
the waterproof cover in the extremity of the condenser
and attach the clamping band after completing the adjustments.
(Fig. 54)
(Fig. 55)
2. To hold the skirt of the waterproof cover, attach the pieces of doublesided adhesive tape on the magnetic support plates 2 on both side of
the microscope frame and press the tape firmly against the microscope
frame.
}The magnetic support plates 2 are most effective when they are at-
tached symmetrically at positions by about 15 mm above the
indication.
3. Fix the waterproof cover using the magnets 3.
}When the cross stage is used, the stage mounting screw holes (x 4 on
the front and rear) are hidden by the waterproof cover. However, this does
not pose problem because the screws can later be attached by passing
through the waterproof cover.
BX61WI
47
Page 52

6
Attaching the Halogen Lamp Housing
(Figs. 56 to 59)
2
1
Fig. 56
Fig. 57
5
3
6
Attaching the Halogen Bulb
}The applicable lamp bulb model is the 12V100WHAL-L (PHILIPS 7724) or
the 12V50WHAL-L (LIFE JC).
1. Fully loosen the clamping screw 1 at the top of the lamp housing using
the Allen screwdriver provided with the microscope frame.
2. Remove the lamp housing 2 by lifting it up.
3. Tilt the bulb socket 3 by 90° in the direction of the arrow.
4. While pushing down the bulb clamping lever 4 down, hold the halogen
bulb 5 with gloves or a piece of gauze, insert the bulb pins 6 straight
and fully into the pin sections 7 on the lamp socket.
Then return the lamp clamping lever gently back to the original position
to clamp the bulb.
To prevent reduced bulb life or cracking, do not touch the bulb with
bare hands. If fingerprints are accidentally left on the bulb, wipe the
bulb with a soft cloth.
5. Fit the lamp housing from up and tighten the clamping screw 1 by
applying downward pressure. (Fig. 56)
1
4
Fig. 58
2
Fig. 59
Caution for Bulb Replacement During or Right after Use
The bulb, lamp housing and areas near these will be extremely hot
during and right after use.
Set the main switch to “ ” (OFF), disconnect the power cord from
the wall outlet, then allow the old bulb and lamp housing to cool
before replacing the bulb with a new of the designated type.
7
Attaching the Halogen Lamp Housing (Fig. 59)
1. Loosen the bulb socket clamping screw 1 using the Allen screwdriver.
2. Push in the halogen lamp housing 2 with bulb and tighten the clamping
screw 1.
48
Page 53

BX61WI
1
2
4
3
6
Fig. 60
Fig. 61
7
5
7 Attaching the Cross Stage
}When using a WI-XYS bridge stage, attach it by referring to its instruction
manual.
1. Align the two WI-FSH fixed stage adapters 1 with the front of the microscope frameand clamp the adapters by tightening the hex-socket screws
from the bottom side using the Allen wrench provided with the microscope frame.
2. Lower the condenser, align the mounting holes 3 and 5 of the IX-SVL2
cross stage 2 with the mounting screw holes 4 and 6, and clamp the
cross stage by tightening the hex-socket screws with the Allen wrench
provided with the microscope frame.
}When the waterproof cover is used, attach hex-socket screws to the screw
holes 4 and 6 by passing through the waterproof cover.
(Figs. 60 & 61)
Fig. 62
Lowering the Stage Height
When no condenser is used, the stage height can be lowered by 50 mm
by loosening the 2 holder clamping screws (7 in Fig. 60) and removing
the condenser holder.
In this case, however, the length of the WI-FSH fixed stage adapter becomes excessive. To deal with this, order custom fabrication of two support
pillars as shown in Fig. 62 or fabricate them by yourself.
49
Page 54

10
{Study the instruction manual for the lamp housing before inspection.
{For safe use of the lamp housing, we recommend performing the following inspection periodically (every time you replace
the lamp bulb and at least every 6 months).
{The table below identifies the check items to be observed. Put (X) if not applicable or ( ) if applicable.
{If there is any ( ) mark noted, immediately stop use of the product, and contact Olympus for detailed inspections or replace
the lamp housing.
{If you detect an abnormality other than that listed below or with other Olympus product, also stop the use of the product and
contact Olympus for detailed inspections.
{Note that the service, replacement and detailed inspections are charged after expiration of the warranty period.
1. More than 8 years have passed since original purchase or the total power ON
time has exceeded 20,000 hours.
2. Lamp does not light sometimes even though the main switch is set to on.
(Except discharge burners*
3. Illumination flickers when you move the lamp cable or lamp housing.
4. Lamp cable is unusually hot to the touch.
5. Scorching or burning odor is produced during use.
6. Illumination still flickers after replacement with a new lamp bulb.
(Except discharge burners*
7. Deformation, backlash, or looseness, etc. when you assemble the lamp housing. (Impossibility of removing the top section of lamp housing when you attempt to replace the lamp bulb, etc.)
8. Extreme discoloration of the lamp housing connection terminal or lamp
socket. Uneven discoloration of the left and right sections of these parts.
(Except discharge burners*
9. Discoloration, deformation or cracking of the lamp housing.
10. Melting, crack, deformation or solidification of the lamp cable or a wiring part.
11. Increased frequency of servicing compared to similar devices put into use at
the same time as the lamp housing.
LAMP HOUSING INSPECTION SHEET
If you have any questions, please contact Olympus.
Check results (Date)
Check items // / /
1
.)
1
.)
1
.)
50
* When the Check Result columns become insufficient, copy this sheet.
1
*
Discharge burners: Mercury burner / Xenon burner / Metall halide burner
Page 55

Page 56

EC REP
Shinjuku Monolith, 3-1, Nishi Shinjuku 2-chome, Shinjuku-ku, Tokyo, Japan
Wendenstraße 14-18, 20097 Hamburg, Germany
3500 Corporate Parkway, P.O. Box 610, Center Valley, PA 18034-0610, U.S.A.
One Corporate Drive, Orangeburg, NY 10962, U.S.A.
491B River Valley Road, #12-01/04 Valley Point Office Tower, Singapore 248373
31 Gilby Road, Mount Waverley, VIC., 3149, Australia
Blue Lagoon Drive, Suite 290 Miami, FL 33126, U.S.A.
5301
01/10
 Loading...
Loading...