Page 1

OPERATOR’S MANUAL
40
-1
Disposable Electrode
Vitrode V0654-000707O First Edition: 29 Aug 2002 Sixteenth Edition: 09 Nov 2016
The Vitrode V disposable electrode is designed for ECG monitoring and impedance
Small type Standard type
method respiration monitoring.
The standard type is for adults or children and the small type is for neonates or
children. Both the electrode and the lead are made of radiolucent carbon ber.
DIN type
Ø3 jack
Ø3 jack
DIN type
Please read this manual thoroughly prior to operation to ensure optimum
performance.
The Vitrode V disposable electrode is radiolucent. The electrode passes X-rays easily without making a shadow on the X-ray.
NOTE
Depending on the irradiated time and site, the electrode might make a slight shadow. The electrode does not perfectly pass X-rays.
Qty Electrode Size Connector Type Lead Color Application
40 (4 × 10 packages)
50 (5 × 10 packages) DIN 5 colors red, yellow, green, black, white
60 (6 × 10 packages) DIN, Ø3 jack 6 colors red, yellow, green, brown, black, purple
Small type
20 × 20 mm
120 (3 × 40 packages)
90 (3 × 30 packages)
50 (5 × 10 packages)
40 (4 × 10 packages) 4 colors red, yellow, green, black
Standard type
45 × 25 mm
DIN, Ø3 jack 4 colors red, yellow, green, black
DIN 3 colors red, yellow, green
5 colors red, yellow, green, black, white
DIN
Ø3 jack
60 (6 × 10 packages) 6 colors red, yellow, green, brown, black, purple
Carbon lead
ECG monitoring for
neonates or children
ECG monitoring for
adults or children
Symbol Description Symbol Description
Caution Non-reusable, single use
Lot number Expiration date
Temperature limits Manufacturer
0
Keep away from sunlight
European representative
The CE mark is a protected conformity mark of the European
Community. Products marked with this symbol comply with the
requirements of the Medical Device Directive 93/42/EEC.
(Background
color: blue)
Follow instructions for
use
WARNING
• Do not use this electrode during MRI examination because it may
cause skin burn on the electrode placement area.
• When performing defibrillation, discharge as far as possible from
electrodes on the chest of the patient. If there is a possibility
that the defibrillator paddle could touch electrodes, remove the
electrodes from the patient. If the defibrillator paddle directly
contacts electrodes, the discharged energy may cause skin burn to
the patient.
CAUTION
• For the following patients and skin sites, redness and skin erosion
may occur and occasionally result in scar.
- Patients with weak skin (patient with allergy, neonate or low birth
weight infant)
- Delicate or weak skin
• Do not attach the electrode on a wound or inflamed skin. Redness
or irritation may occur on the skin.
• If the skin gets irritated or redness appears on the skin from the
electrode, change the attachment site or stop using the electrode.
Take extreme care for the patients with delicate skin.
• Iodine may cause discoloration of the electrode. If the skin was
disinfected with iodine, wipe the skin with ethanol before placing
the electrode.
• When removing the electrode from the patient, remove it carefully
and slowly from the edge. If you remove it forcefully, the skin may
tear.
• Take extreme care to prevent the patient from swallowing the
electrode. The electrode is chemically harmless to people but the
adhesive gel on the Vitrode V is highly expansive. If the electrode
is swallowed, the adhesive gel absorbs gastric juice and swells
several to several dozen times in size and stays in the stomach.
Swelling adhesive gel in stomach will cause the patient to vomit
and be unable to eat and drink.
After removing the electrode, check there is no adhesive gel left on
the patient.
• To prevent electrodes from being swallowed, make sure that the
number of electrodes to be disposed of is the same as the number
of electrodes that were taken out of the package.
• Be careful not to touch the adhesive gel on the small type
electrodes for neonates or children (20 × 20 mm). The gel contains
a bitter flavor to prevent the patient from swallowing the electrode.
Wash your hands if you touch the gel.
1
Page 2
MEDICAL ELECTRONIC INSTRUMENT CORP.
Phone +86 21-5743-6998 Fax +86 21-5743-6939
NIHON KOHDEN CORPORATION
1-31-4 Nishiochiai, Shinjuku-ku, Tokyo 161-8560, Japan
Phone +81 3-5996-8036 Fax +81 3-5996-8100
http://www.nihonkohden.com/
• Dispose of the connector holder after use. Take extreme care to
prevent the patient from swallowing the connector holder. The
connector holder is a part which is used to connect electrode leads
to the ECG connection cord.
• The Vitrode V electrode should not be used in conjunction with
other types of electrodes because offset voltage becomes high and
it may affect ECG recording.
• After opening the package, use the contents soon to prevent the
gel from drying. Store the rest of the electrodes by folding the
top of the package twice and sealing it with tape to prevent the
electrodes from drying.
• When noise interferes with the ECG or the electrode starts to come
off, replace the electrode with a new one.
• Do not use electrodes which are past the expiration date.
• Do not use this electrode for electric stimulation.
• Do not alter the shape of electrodes. ECG may not be recorded.
• When removing the electrode from a neonate, remove it carefully
and slowly, because neonatal skin is sensitive.
• Do not reuse the disposable electrode.
• Do not get liquid on the electrode. The adhesive becomes weak
and correct measurement might be impossible.
• The electrode cannot be sterilized. Sterilizing the electrode may
damage it.
• Black spots may appear on the silver surface of the electrode.
These are caused by variation in the Ag and AgCl coating
thickness and have no effect on the quality or performance of the
electrode.
• When using remover for removing adhesive gel from the patient’s
skin, refer to the remover operator’s manual.
• When using remover, wipe the remover completely before
attaching a new electrode.
• When separating the small type electrodes with 3 color leads,
separate them slowly to avoid twisting the leads. Otherwise the
covering may peel off the wire and the electrodes may become
unusable.
• Dispose of Nihon Kohden products according to your local laws
and your facility’s guidelines for waste disposal. Otherwise, it may
affect the environment. If there is a possibility that the product may
have been contaminated with infection, dispose of it as medical
waste according to your local laws and your facility’s guidelines for
medical waste. Otherwise, it may cause infection.
• United States law restricts this product to sale by or on the order of
a physician.
Composition
Electrode: Ag/AgCl applied carbon
Adhesive gel: Acrylic hydrophilic high polymer, glycerin, water
Electrode lead: Carbon, PVC
Attaching Electrodes
1. Determine the electrode site. Do not place the electrode on a wound,
inamed, wrinkled or uneven skin surface.
2. For stable ECG monitoring, clean the electrode site with a cotton pad
moistened with alcohol.
3. For more stable ECG monitoring, rub the sites with skinPure skin
preparation gel (YZ-0019).
NOTE
This preparation is not needed for child or neonate use.
4. Thoroughly dry the skin with a clean cotton pad. Residual sweat,
moisture or skinPure will reduce the adhesive properties of the
electrode.
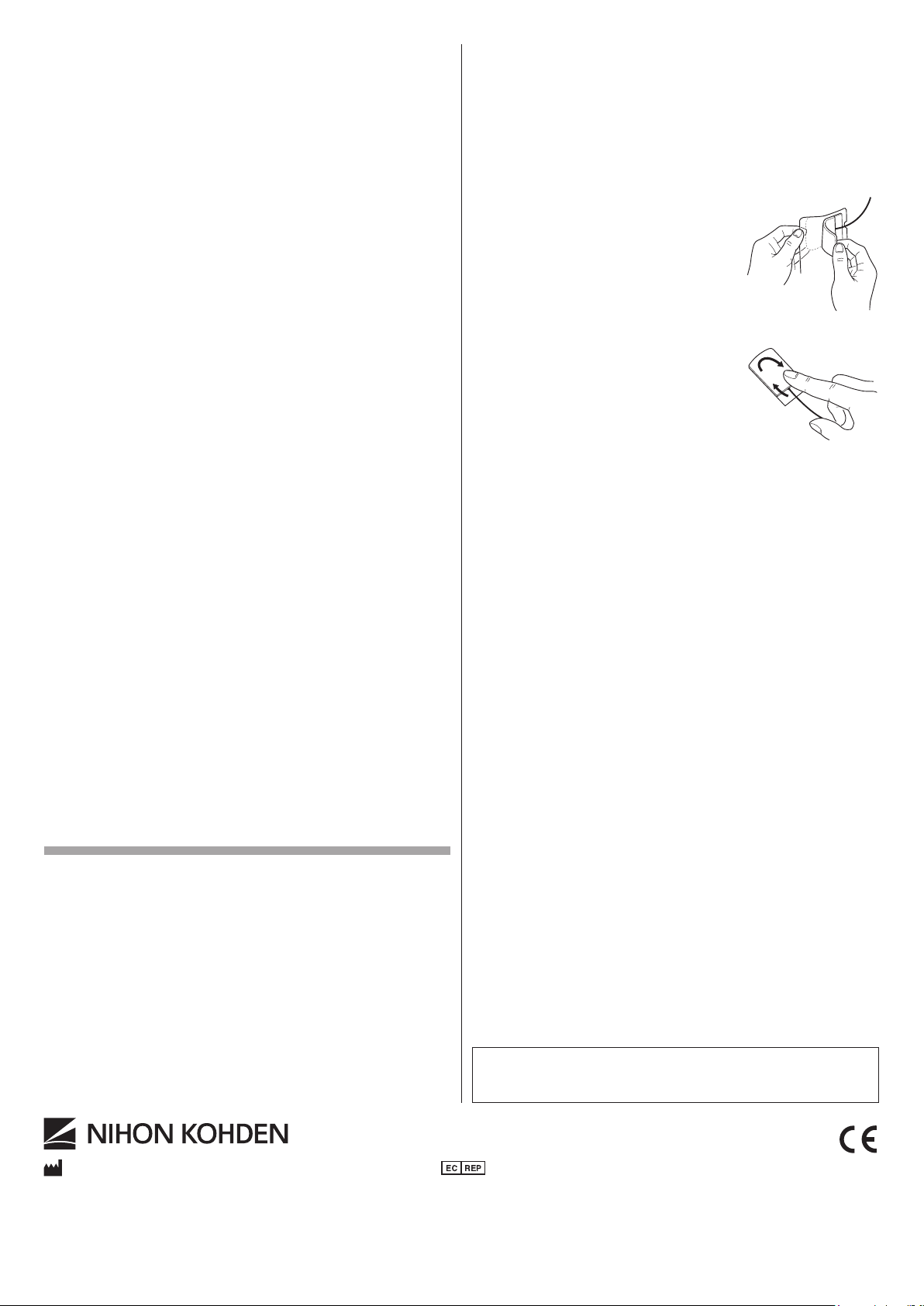
5. Open the package and take out the required number of electrodes.
6. Remove the electrode from the plastic lm carefully.
NOTE
• Avoid touching the adhesive surface.
• Do not pull the lead when you remove
the electrode from the plastic film. It
may break the lead.
7. Press one edge of the electrode down on the
site, then continue to apply the rest of the
electrode in the same manner.
8. Press the tape down rmly with a nger for
secure contact as shown right.
9. Connect the electrode lead to the
corresponding input. If necessary, fasten the
electrode lead to the skin with surgical tape.
NOTE
• Do not connect the electrode connector directly to the
transmitter. The signals are not transmitted properly and the
monitor cannot receive the signals. Use the JC-933P ECG
connection cord.
• Make sure that there is enough slack in the electrode lead. This
prevents body movement from peeling the electrode off and
breaking the electrode lead.
Replacement
Replace the electrode about every 24 hours.
Storage
Store electrodes in a cool and dry place away from high temperatures or
direct sunlight.
Specifications
Operating conditions
Temperature: 10 to 40°C (50 to 104°F)
Humidity: 30 to 95%
(When attaching to a place where the skin surface
is not wet)
Storage conditions
Temperature: −10 to +40°C (14 to 104°F)
Humidity: 15 to 95%
Lifetime
The lifetime of the electrode is printed on the package. The lifetime is
24 months (including the month of manufacture) after manufacture.
(tested by Nihon Kohden)
Copyright Notice
The entire contents of this manual are copyrighted by Nihon Kohden.
All rights are reserved.
Manufacturer
NIHON KOHDEN AMERICA, INC.
15353 Barranca Parkway, Irvine, CA 92618, U.S.A.
Toll-free +1-800-325-0283
Phone +1 949-580-1555 Fax +1 949-580 -1550
European Representative
NIHON KOHDEN EUROPE GmbH
Raiffeisenstrasse 10, D-61191 Rosbach, Germany
Phone + 49 6003-827-0 Fax + 49 6003-827- 599
2
SHANGHAI KOHDEN
567 Huancheng Bei Road
Shanghai Comprehensive Industrial Development Zone
Shanghai 201401, China
 Loading...
Loading...