Page 1

High-Speed Processing of Large
TM
Specimens on the Peloris
Dual
Retort Tissue Processor
Geoffrey Rolls
Leica Microsystems, Biosystems Division, Melbourne, Australia
Page 2

High-Speed Processing of Large Specimens on the
Peloris
TM
Dual Retort Tissue Processor
Geoffrey Rolls
Leica Microsystems, Biosystems Division, Melbourne, Australia
This paper presents the results of comparative evaluations carried out during field trials in two large
public hospitals, that set out to examine whether the design features of the Peloris dual retort tissue
processor led to reduced processing times for large specimens without compromising quality. In the
context of a busy histopathology laboratory, we demonstrate that the introduction of Peloris processing
would allow the processing of large, dense specimens in 6 hours leading to the completion of more runs
in a working day and the reduction of turnaround times.
Ten sequential six hour and nine hour processing runs were undertaken on a Peloris processor at either
45 °C or 55 °C and the resultant blocks and sections were evaluated for quality using a comprehensive
scoring system. Peloris results were compared to those achieved on a matched panel of specimens
processed using a processor that represents the industry standard (the Tissue-Tek
show that for large, dense specimens Peloris can produce results of an equivalent standard to an
“overnight” schedule (13 hours) run on a VIP using much shorter schedules. Results also indicate that
there is some advantage in processing at higher temperature (55 °C) for both the six and nine hour
schedules.
®
VIP™). The results
Introduction
A fundamental requirement in the histopathology laboratory is to
safely and effectively process specimens. Sectioning of paraffin
blocks should be straightforward with the resultant sections
being of a high standard demonstrating excellent morphological
detail. This applies to the complete range of specimen types and
sizes, ranging from tiny, delicate fragments to sizeable wedges
of dense tissue. In busy laboratories these requirements must be
balanced against increasing demands to reduce turnaround times,
to process greater numbers of specimens and to complete more
runs in the working day.
The traditional view embraces a conservative approach to
processing of all specimen types. A survey of standard histology
texts published in the last twenty years reveals an average
processing time of 15.7 hours for “routine overnight processing”
using xylene as a clearing agent. A survey of local and international
laboratories shows that the average duration of a “routine
overnight” schedule on an enclosed processor using xylene and
excluding any additional fixation steps is 10.3 hours (see Table 1 for
details). Of course these laboratories do use shorter schedules for
special purposes, where particular types of specimens are to be
processed (eg. endoscopic biopsies), or where specimens falling
within strict size limits are included.
These field trials were conducted by Vision BioSystems (VBS).
Vision Biosystems has since formed part of the Biosystems Division
of Leica Microsystems. What is needed then, is a processor which
can effectively process a full load of cassettes, say 200 – 300 per
retort, containing the complete range of tissues normally included
in a “routine overnight run”, but doing so in a much shorter time,
preferably within the six hour threshold that would allow them
to be completed within one working day. The Peloris dual retort
tissue processor has been designed with these requirements in
mind. Compared to processors representing the current industry
standard it has a much faster and more even heating response in
the retorts, fast fill and drain actions, a basket design allowing better
fluid exchange with reduced reagent carryover, and more effective
agitation. These features are designed to reduce processing times.
The purpose of this paper is to present the results of comparative
evaluations carried out during field trials that set out to show that
the design features of Peloris lead to reduced processing times
without compromising quality.
/ 1
Page 3
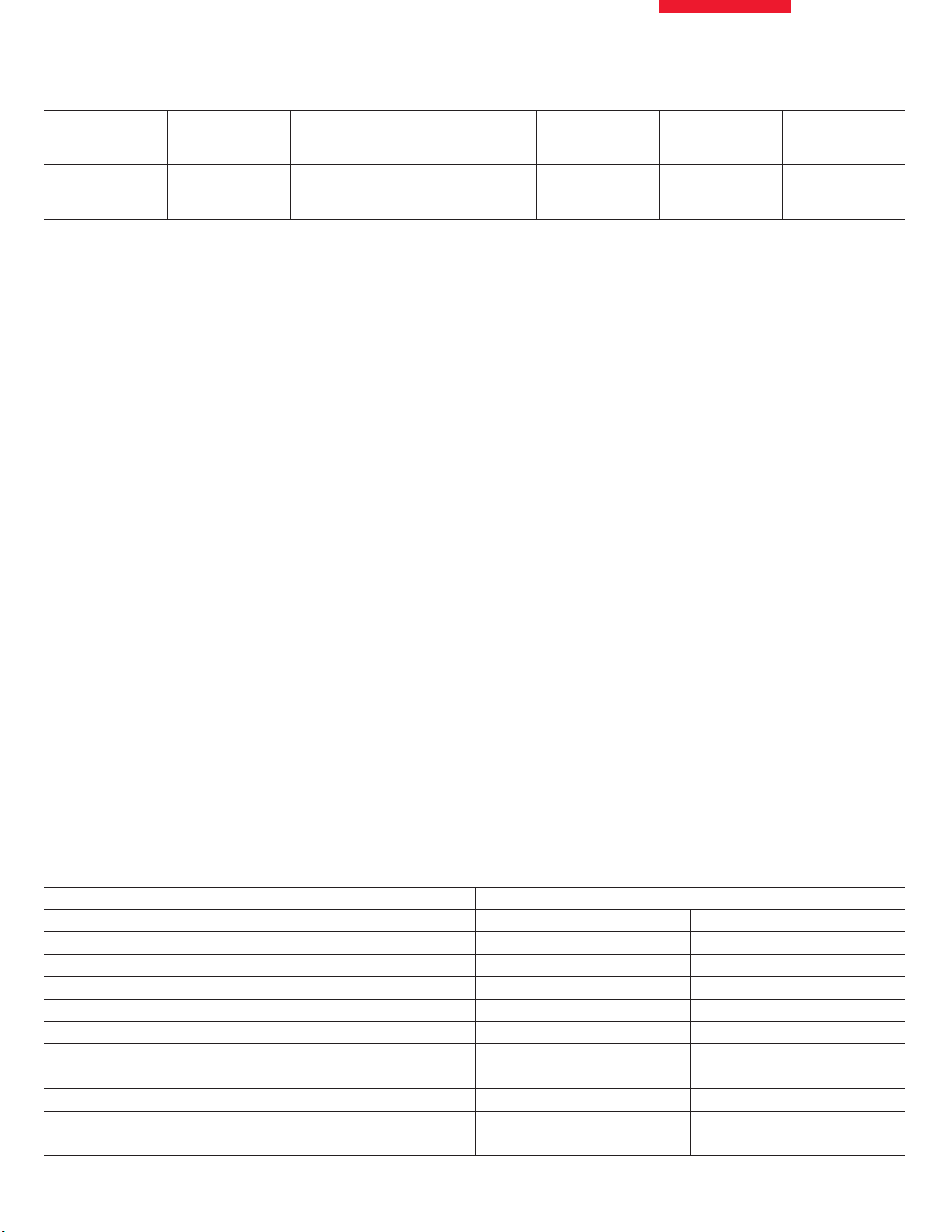
Number of Labs Types of Processor
(number)
25 Leica TP1050 (20)
VIP (4)
Shandon (1)
Table 1. Routine overnight processing schedules
Schedule Description Average Number
of Steps
Routine overnight 13 12 620 694
Average Number
of Steps Excluding
Fixation
Average Total Time
Excluding Fixation
(minutes)
Average Total Time
(minutes)
Independent field trials were carried out in two large public
teaching hospitals in Melbourne, Australia, during 2003 and 2004.
They were conducted in the Anatomical Pathology laboratories
of the Royal Melbourne Hospital (RMH) (1200 beds) and at Austin
Health (AH) (840 beds). The evaluations relating to processing speed
were conducted in a similar fashion during both field trials. They
involved senior histology scientists from both external laboratories
together with scientists from Vision BioSystems, in the assessment
of processed blocks and stained sections from a number of
processing runs. Using duplicate sets of matched specimens of
a variety of dimensions and types, multiple processing runs were
carried out using Peloris and an “industry standard” processor
in each laboratory (in both cases a Tissue-Tek VIP). Results from
Peloris rapid schedules run at 45 °C and 55 °C were compared with
VIP standard “routine overnight schedules” currently used in each
laboratory which served as a normal control.
Processing at elevated temperatures is widely accepted as a
means of accelerating processing by increasing diffusion rates
in specimens (1, 2) although some authors believe that this can
cause additional shrinkage and staining problems (2, 3). We have
certainly not found this to be the case during extensive testing of
Peloris. These trials provided an opportunity to compare results of
rapid processing at 45 °C and 55 °C and to confirm that there were
no adverse effects from using the higher temperatures.
The assessments were carried out independently with staff at
the external sites using slightly different scoring methods, with
the assessors being unaware of the schedules used to process
the specimens. The scoring system used by VBS staff has been
extensively used throughout the development and testing of Peloris
to evaluate the quality of tissue processing and as a mechanism for
optimizing standard processing protocols. A score is calculated by
assessing 23 parameters and is expressed as a percentage. The
complete details are provided elsewhere(4).
Method
Testing throughout the RMH and AH trials was conducted such
that all processing results were compared directly to those of their
existing tissue processors (RMH - Tissue-Tek VIP 4, AH Tissue-Tek
VIP 5). As far as possible specimens for assessment were kept
identical in terms of size, fixation and source on both instruments
for each processing run. All specimens were thoroughly fixed. Not
every specimen in each run was evaluated. Any specimens that
were not for evaluation but loaded into retorts on Peloris to provide
a representative case load comparable to the VIP, consisted of pig
tissue supplied by VBS. Typical specimens used in assessment of
processing and their approximate dimensions are shown in Table 2.
At each laboratory for each of 10 sequential working days, three
processing runs were carried out. Two rapid schedules were run
on Peloris using the two retorts. Retort A was used for the 6 hour
schedule and Retort B for the 9 hour. Runs were carried out daily
at either 45 °C or 55 °C. For each 6 and 9 hour run on Peloris a
routine overnight run was completed on a VIP containing a normal
diagnostic specimen load together with a set of the duplicate test
specimens (200 – 250 cassettes). These served as our normal
control group. Fresh reagents were provided for run 1 and not
changed for the 10 runs on both Peloris and the respective VIP.
For each Peloris run, in addition to the test specimens, cassettes
containing various pig tissues were included to take the specimen
number to 228, which provided an equivalent specimen load to that
in the respective VIP processor (75% capacity of each retort). The
processing schedules used are shown in Tables 3 and 4. Figure
1 illustrates the difference in step times between the various
schedules used. Note that the total pump and drip times in the
Peloris schedules are considerably shorter than those of the VIP.
Royal Melbourne Hospital (RMH) Austin Hospital (AH)
Tissue Dimensions (mm) Tissue Dimensions (mm)
Intestine 15 x 10 x 5 Intestine 30 x 8 x 5
Liver (pig) 25 x 15 x 5 Intestine 20 x 15 x 5
Spleen 15 x 10 x 5 Liver (pig) 20 x 20 x 5
Lung 25 x 15 x 5 Liver 30 x 25 x 5
Kidney (pig) 15 x 10 x 5 Lung 20 x 20 x 5
Heart (pig) 20 x 15 x 5 Lung 20 x 15 x 5
Thyroid 20 x 10 x 5 Kidney (pig) 20 x 15 x 5
Skin 30 x 25 x 5 Kidney 15 x 15 x 5
Breast 30 x 25 x 5 Heart (pig) 20 x 20 x 5
Prostate Chips Heart 20 x 15 x 5
Table 2. Typical specimens used in assessment of processing
/ 2
Page 4

Schedule P1 P2
9 Hour Xylene 6 Hour Xylene
Step No. Reagent Time (minutes) Time (minutes) Drip Time
(seconds)
1 Formalin 0 45 10 45 or 55 Off Med
2 70% ethanol 5 10 10 45 or 55 Off Med
3 90% ethanol 10 10 10 45 or 55 Off Med
4 100% ethanol 15 15 10 45 or 55 Off Med
5 100% ethanol 40 20 10 45 or 55 Off Med
6 100% ethanol 50 20 10 45 or 55 Off Med
7 100% ethanol 50 35 10 45 or 55 Off Med
8 xylene 35 10 10 45 or 55 Off Med
9 xylene 40 25 10 45 or 55 Off Med
10 xylene 50 35 10 45 or 55 Off Med
11 Paraffin wax 35 25 10 60 Vac Med
12 Paraffin wax 50 35 10 60 Vac Med
13 Paraffin wax 65 45 10 60 Vac Med
Total step time 505 330
Total processing
time
Table 3. Peloris processing schedules
531
(8.9 hours)
356
(5.9 hours)
Temp º C P/V Stir
Schedule V1 (RMH) Schedule V2 (AH)
13 Hour Xylene 13 Hour Xylene
Step No. Reagent Time (minutes) Temp ºC P/V Reagent Time (minutes) Temp ºC P/V
1 Formalin 30 40 Yes Formalin 120 45 Yes
2 Formalin 30 40 Yes 70% Ethanol 30 40 Yes
3 70% Ethanol 30 40 Yes 90% Ethanol 30 40 Yes
4 95% Ethanol 45 40 Yes 100% Ethanol 60 40 Yes
5 100% Ethanol 45 40 Yes 100% Ethanol 60 40 Yes
6 100% Ethanol 75 40 Yes 50/50 Eth/Xyl 60 40 Yes
7 50/50 Eth/Xyl 90 40 Yes 100% Ethanol 60 40 Yes
8 100% Ethanol 75 40 Yes Xylene 30 40 Yes
9 Xylene 60 40 Yes Xylene 60 40 Yes
10 Xylene 90 40 Yes Xylene 60 40 Yes
11 Wax 60 58 Yes Wax 30 58 Yes
12 Wax 60 58 Yes Wax 30 58 Yes
13 Wax 60 58 Yes Wax 60 58 Yes
14 Wax 0 58 Yes Wax 60 58 Yes
Total step time 750 750
Total processing
time
Table 4. Tissue-Tek VIP processing schedules
810
(13.5 hours)
810
(13.5 hours)
/ 3
Page 5

Schedule and Processor
Peloris 6 hr at 55 ºC
Peloris 6 hr at 45 ºC
Peloris 9 hr at 55 ºC
Peloris 9 hr at 45 ºC
VIP 13 hr control RMH
VIP 13 hr control AH
Step Times in Minutes
Fixation Dehydration Clearing Infiltration Pumping and drip time
0 200 400 600 800 1,000
Figure 1. Routine overnight processing schedules
After each processing run the specimens were embedded,
sections cut and stained with H&E using the standard methods of
each laboratory. During microtomy all blocks were assessed for
Specimens scoring between 65% and 75% are considered to be of
a good standard for diagnosis. Any specimen scoring above 80% is
considered to be of exceptional quality.
ease of sectioning and other parameters (4). All test sections were
screened on-site by hospital scientists to check that they were of
an overall satisfactory standard. Sections were deemed to be satisfactory if they scored 1 or 2 on a simple three point scale (0, 1, or 2).
A representative sample of blocks and slides from the test group
of at least 4 per run, were scored by VBS staff according to the
full VBS protocol (4). In using this protocol any specimen which
scores <50% in any single parameter is considered a “fail”.
Schedule Number of Runs Number Satisfactory/Number of
Test Slides Screened (RMH & AH)
RMH Evaluation
Peloris 6hr at 45 °C 5 60/60 26/26 73%
Peloris 9hr at 45 °C 5 60/60 26/26 72%
Peloris 6hr at 55 °C 5 60/60 28/28 75%
Peloris 9hr at 55 °C 5 60/60 28/28 75%
VIP 13 hr control 10 88/90 49/51 73%
AH Evaluation
Peloris 6hr at 45 °C 5 52/54 21/22 68%
Peloris 9hr at 45 °C 5 54/54 22/22 69%
Peloris 6hr at 55 °C 5 54/54 22/22 78%
Peloris 9hr at 55 °C 5 54/54 22/22 79%
VIP 13 hr control 10 78/78 24/24 75%
Combined results RMH and AH
Peloris 6hr at 45 °C 10 112/114 47/48 70%
Peloris 9hr at 45 °C 10 114/114 48/48 71%
Peloris 6hr at 55 °C 10 114/114 50/50 77%
Peloris 9hr at 55 °C 10 114/114 50/50 77%
VIP 13 hr control 20 196/198 103/105 74%
Table 5. Combined results from 20 processing runs
Results
Table 5 shows the combined results from 20 processing runs (10
runs at RMH and 10 runs at AH). Figure 2 is included to demonstrate
the consistent quality of processed blocks over 10 days for five
consecutive runs, at either 45 °C or 55 °C, in comparison with the
VIP control. Figures 3 and 4 are micrographs showing typical fields
in the processed tissues.
Number Passed/Number Test
Slides Scored (VBS)
Average Score
/ 4
Page 6

VBS Score
100%
80%
60%
40%
20%
Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Day 9 Day 10
Processing Runs by Working Day
Peloris 6 hour 45 ºC mean score Retort A Peloris 6 hour 55 ºC mean score Retort A VIP 13 hour mean score
Peloris 9 hour 45 ºC mean score Retort A Peloris 9 hour 55 ºC mean score Retort A
Figure 2. Graph showing the consistency of results of sequential runs for 10 days.
A
Figure 3. A comparison of typical H&E stained sections of pig liver produced using different processing schedules on three matched specimens from the same case. A Peloris 6 hour
at 55 °C, B Peloris 9 hour at 55 °C and C VIP overnight control schedule. Note the well-preserved lobules showing minimal shrinkage with no cracking or separation from the portal
connective tissue in each case. There is no discernable difference in morphological detail or staining quality between the 6 and 9 hour 55 °C runs and the 13 hour control.
A
B
B
C
C
Figure 4. A comparison of typical H&E stained sections of small intestine produced using different processing schedules on three matched specimens from the same case. A
Peloris 6 hour at 55 °C, B Peloris 9 hour at 55 °C and C VIP overnight control schedule. Note the well-preserved intestinal glands and well-defined nuclei in each. There is no
discernable difference in morphological detail or staining quality between the 6 hour and 9 hour runs at 55 °C and the 13 hour control.
/ 5
Page 7

Discussion
The results show that Peloris can process large specimens to
an equivalent standard to an “overnight” 13 hour schedule run
on an industry-standard processor such as the VIP using shorter
schedules – of either 6 or 9 hours. As can be seen in Table 2 the
specimens used were relatively large, having characteristics that
would mean in most laboratories they would be processed on
on “overnight” run. The results also indicate that there is some
advantage in processing at higher temperature (55 °C) for both
the 6 hour and 9 hour schedules. It is likely that for even larger
and denser specimens than those used here, this improvement in
processing quality would be more pronounced.
We consider the overall quality of the sections produced in these
field trials to be satisfactory. When looking at the magnitude of the
VBS scores, it must be remembered that, as well as reflecting the
quality of processing achieved, they are limited by the initial quality
of specimen fixation. As is always the case in histopathology
laboratories some of the specimens showed sub-optimal fixation
particularly noticeable in some larger pieces of tissue that were
intentionally chosen for these trials. However, because we used
matched sets of specimens for assessment we believe our results
are unbiased.
Unlike other processors, in Peloris the chosen processing
temperature in the retort is achieved very rapidly. This is
particularly important if accelerated processing is to be achieved
using short step times. It may explain why, in some processors,
higher temperatures appear not to shorten processing times to the
extent that is possible with Peloris.
Figure 2 indicates that rapid processing on Peloris produces very
consistent results overall, at least the equivalent of the VIP. These
results were achieved without changing reagents on Peloris. It
should also be noted that processing on Peloris was done using
the two retorts. Both the 6 and 9 hour schedules were run at the
same time, the end-points being timed so that the embedding could
be done sequentially. Figure 2 shows the consistency in the results
achieved in each retort. This clearly demonstrates the versatility of
the two-retort design that allowed the processing of at least twice
as many specimens as the VIP in a shorter time.
Conclusion
The results of comparative evaluations carried out during field trials
clearly show that the design features of Peloris lead to reduced
processing times without compromising quality. Tissues processed
at both 45 °C and 55 °C produced consistent, high-quality results
with evidence that the higher temperature is an advantage for
both the 6 hour and 9 hour schedules. The trials were completed
efficiently, causing little disruption in the participating laboratories,
due in large part to the versatility of Peloris in possessing two
retorts that could be used simultaneously.
In the context of a busy histopathology laboratory, our results
indicate that the introduction of Peloris processing would allow
large specimens to be processed in six hours, leading to the
completion of more runs in a working day and the reduction of
turn-around times.
Acknowledgements
The Biosystems Division of Leica Microsystems would like to thank
Mr Terry Cass and the staff of the Anatomical Pathology Department,
Division of Laboratory Medicine, Austin Health, Victoria, Australia
and Mr Richard Lau and the staff of the Anatomical Pathology
Department, Melbourne Health Pathology, Victoria, Australia for
their valuable assistance in conducting the trials described in this
paper and in the preparation of this manuscript. Thanks are also
due to Mr David Roche (Biosystems Division) for coordinating the
project and collecting and collating the data.
References
1. Winsor L. Tissue processing. In: Woods A, Ellis R, editors. Laboratory
histopathology. New York: Churchill Livingstone; 1994. p. 4.2-23.
2. Anderson G, Gordon KC. Tissue processing, microtomy and paraffin sections.
In: Bancroft JD, Stevens A, editors. Theory and practice of histological
techniques. 4th ed. New York: Churchill Livingstone; 1996.
3. Carson FL. Histotechnology. 2nd ed. Chicago: ASCP Press; 1997.
4. Vision BioSystems. Assessing the quality of tissue processing and the
performance of Peloris™ using the VBS scoring system. Melbourne: Vision
BioSystems Ltd May 2005.
08/2007 95.7753 Rev A
Leica Biosystems Melbourne Pty Ltd, 495 Blackburn Road, Mount Waverley VIC 3149, Australia
Telephone +61 3 9211 7400 Fax +61 3 9211 7401 ABN 72 008 582 401
/ 6
 Loading...
Loading...