Page 1

English
Bond™ Oracle™ HER2 IHC System
Instructions For Use
For use on Leica Biosystems’ BOND™ fully automated, advanced staining system.
Product Code TA9145 is designed to stain 60 tests (150 slides):
60 test slides with HER2 Primary Antibody
60 test slides with HER2 Negative Control
15 HER2 Control Slides with HER2 Primary Antibody
15 positive in-house tissue controls with HER2 Primary Antibody
IVD
Leica Biosystems Newcastle Ltd
Balliol Business Park
Benton Lane
Newcastle Upon Tyne NE12 8EW
United Kingdom
( +44 191 215 4242
Leica Biosystems Canada
71 Four Valley Drive
Concord, Ontario L4K 4V8
Canada
( +1 800 248 0123
Leica Biosystems Inc
1700 Leider Lane
Buffalo Grove IL 60089
USA
( +1 800 248 0123
Leica Biosystems Melbourne
Pty Ltd
495 Blackburn Road
Mt Waverly VIC 3149
Australia
( +61 2 8870 3500
Page 1 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 2

Contents
Intended Use .............................................................................................................................3
Summary and Explanation ......................................................................................................3
Background ..........................................................................................................................................3
Expression of HER2 .............................................................................................................................3
Clinical Concordance Summary ...........................................................................................................3
Principle of Procedure .............................................................................................................4
Components Provided ..........................................................................................................................4
Directions on Use .................................................................................................................................5
Storage and Stability ............................................................................................................................5
Specimen Preparation ..........................................................................................................................5
Warnings and Precautions ...................................................................................................................5
Procedure ..................................................................................................................................6
A. Reagents required but not supplied .................................................................................................6
B. Equipment required but not supplied ...............................................................................................6
C. Methodology ....................................................................................................................................6
D. Slide Layout .....................................................................................................................................6
E. Procedure Steps ..............................................................................................................................7
Quality Control ..........................................................................................................................9
HER2 Control Slide – HER2 Primary Antibody ..................................................................................10
In-house Positive Control Tissue – HER2 Primary Antibody ..............................................................10
In-house Negative Control Tissue Component – HER2 Primary Antibody .........................................10
Patient Tissue – HER2 Negative Control ..........................................................................................10
Patient Tissue – HER2 Primary Antibody ..........................................................................................10
Assay Verication ..............................................................................................................................10
Interpretation of Staining .................................................................................................................... 11
Slide Screening Order Rationale ..........................................................................................12
1. HER2 Control Slide – HER2 Primary Antibody ..............................................................................11
2. In-house Positive Control Tissue – HER2 Primary Antibody ..........................................................12
3. In-house Negative Control Tissue Component – HER2 Positive Control .......................................12
4. Patient Tissue – stained using the HER2 Negative Control ...........................................................12
5. Patient Tissue – stained using the HER2 Primary Antibody ...........................................................12
Limitations ..............................................................................................................................12
A. General Limitations ........................................................................................................................12
B. Product Specic Limitations ...........................................................................................................13
Cell Line Data ..........................................................................................................................14
Clinical Concordance of Bond Oracle HER2 IHC System to Dako HercepTest ................14
2x2 Concordance Results ..................................................................................................................15
3x3 Concordance Results ..................................................................................................................15
Clinical Concordance of Bond Oracle HER2 IHC System to PathVysion DNA HER-2 Probe
Kit.......................................................................................................................................................16
2x2 Concordance Results ..................................................................................................................16
Immunoreactivity – Normal Panel .........................................................................................17
Reproducibility Study ............................................................................................................18
Within and Between Precision Testing ...............................................................................................18
A. Within Run Precision Testing .........................................................................................................18
B. Between Run Precision Testing .....................................................................................................18
C. Lot-to-Lot Reproducibility ...............................................................................................................18
D. Between Laboratory Reproducibility ..............................................................................................19
E. Inter-Observer Reproducibility .......................................................................................................19
F. Between Instrument Precision (BOND-MAX vs BOND-III)..............................................................20
Troubleshooting .....................................................................................................................21
References ..............................................................................................................................22
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
English
Page 2 of 23
Page 3

Intended Use
For in vitro diagnostic use
Bond Oracle HER2 IHC System is a semi-quantitative immunohistochemical (IHC) assay to
determine HER2 (Human Epidermal Growth Factor Receptor 2) oncoprotein status in breast
English
cancer tissue processed for histological evaluation. The Bond Oracle HER2 IHC System is
indicated as an aid in the assessment of patients for whom Herceptin® (trastuzumab) treatment
is being considered (see Herceptin® package insert).
Note: All of the patients in the Herceptin® clinical trials were selected using an investigational
immunocytochemical Clinical Trial Assay (CTA). None of the patients in those trials were
selected using the Bond Oracle HER2 IHC System. The Bond Oracle HER2 IHC System has
been compared to the Dako HercepTest™ on an independent set of samples and found to
provide acceptably concordant results, as indicated in the Clinical Concordance Summary.
The actual correlation of the Bond Oracle HER2 IHC System to clinical outcome has not been
established.
Summary and Explanation
Background
The Bond Oracle HER2 IHC System contains the mouse monoclonal anti-HER2 antibody, clone
CB11. Clone CB11, originally developed by Corbett et al (1) and manufactured by Novocastra
Laboratories Ltd (now Leica Biosystems Newcastle Ltd), is directed against the internal domain
of the HER2 oncoprotein.
In a proportion of breast cancer patients, the HER2 oncoprotein is overexpressed as part of the
process of malignant transformation and tumor progression (2). Overexpression of the HER2
oncoprotein found in breast cancer cells suggests HER2 as a target for an antibody-based
therapy. Herceptin® is a humanized monoclonal antibody (3) that binds with high afnity to the
HER2 oncoprotein and has been shown to inhibit the proliferation of human tumor cells that
overexpress HER2 oncoprotein both in vitro and in vivo (4–6).
Since the rst immunoperoxidase technique, reported by Nakane and Pierce (7), many
developments have occurred within the eld of immunohistochemistry, resulting in increased
sensitivity. A recent development has been the use of polymeric labeling. This technology has
been applied to both primary antibodies and immunohistochemical detection systems (8). The
Compact PolymerTM detection system utilized by the Bond Oracle HER2 IHC System is part of
a family of novel, controlled polymerization technologies that have been specically developed
to prepare polymeric HRP-linked antibody conjugates. As this polymer technology is utilized in
the Oracle product range, the problem of nonspecic endogenous biotin staining, which may be
seen with streptavidin/biotin detection systems, does not occur.
Expression of HER2
The HER2 oncoprotein is expressed at levels detectable by immunohistochemistry in up to 20%
of adenocarcinomas from various sites. Between 10% and 20% of invasive ductal carcinomas
of the breast are positive for HER2 oncoprotein (9). 90% of cases of ductal carcinoma in situ
(DCIS) of comedo type are positive (10), together with almost all cases of Paget’s disease of
the breast (11).
Clinical Concordance Summary
The Bond Oracle HER2 IHC System was developed to provide an alternative to the investigational
Clinical Trial Assay (CTA) used in the Herceptin® clinical studies. The performance of the
Bond Oracle HER2 IHC System for determination of HER2 oncoprotein overexpression was
evaluated in an independent study comparing the results of the Bond Oracle HER2 IHC System
to the Dako HercepTest on 431 breast tumor specimens, of US origin. None of these tumor
specimens were obtained from patients in the Herceptin® clinical trials. The results indicated
a 92.34% concordance in a 2x2 analysis (95% condence intervals of 89.42% to 94.67%) and
86.54% in a 3x3 analysis (95% condence intervals of 82.95% to 89.62%) between the results
from the two assays.
Page 3 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 4
Principle of Procedure
The Bond Oracle HER2 IHC System contains components required to complete an
immunohistochemical staining procedure for formalin-xed, parafn-embedded tissues.
Following incubation with the ready-to-use HER2 Primary Antibody (clone CB11), this
system employs ready-to-use Compact Polymer technology. The enzymatic conversion of
the subsequently added chromogen results in the formation of a visible reaction product at
the antigenic site. The tissue sections may then be counterstained, dehydrated, cleared and
mounted. Results are interpreted using light microscopy. Control slides with four formalin-xed,
parafn-embedded human breast cancer cell lines are provided to validate staining runs. The
four cell lines demonstrate HER2 oncoprotein expression at 0, 1+, 2+ and 3+ intensities. The
staining intensity of these cell lines correlates to both HER2 oncoprotein receptor load per cell
and HER2 gene amplication status.
The Bond Oracle HER2 IHC System (product code TA9145) is for use on the Leica Biosystems’
BOND fully automated, advanced staining system.
Components Provided
The materials listed below (Table 1) are sufcient to stain 150 slides (60 test slides incubated with
HER2 Primary Antibody, 60 corresponding test slides incubated with HER2 Negative Control,
15 HER2 Control Slides incubated with HER2 Primary Antibody and 15 in-house positive tissue
controls incubated with HER2 Primary Antibody). The number of tests is based on the use of
a 150 µL automated dispense per slide. The kit provides materials sufcient for a maximum of
15 individual BOND staining runs.
English
HER2 Control Slides,
(x15)
Sections of formalin-xed, parafn-embedded, human breast cancer
cell lines that demonstrate HER2 oncoprotein expression at 0, 1+, 2+ and
3+ staining intensities when stained in accordance with the protocol
provided. These sections are fully adhered and do not require further
baking.
HER2 Primary
Antibody, 13.5 mL
HER2 Negative
Control, 9 mL
Peroxide Block,
22.5 mL
Post Primary, 22.5 mL
Polymer, 22.5 mL
DAB Part 1, 2.25 mL
DAB Part B (x2),
22.5 mL
Hematoxylin, 22.5 mL
Table 1. Bond Oracle HER2 IHC System components
Contains ready-to-use, afnity-puried, mouse monoclonal IgG antibody,
clone CB11 and 0.35% ProClin™ 950 .
Contains ready-to-use mouse IgG at an equivalent concentration to the
HER2 Primary Antibody and 0.35% ProClin™ 950.
Contains 3-4% hydrogen peroxide.
Rabbit anti-mouse IgG (<10 μg/mL) in Tris-buffered saline containing 10%
(v/v) animal serum and 0.09% ProClinTM 950.
Poly-HRP goat anti-rabbit IgG (<25 μg/mL) in Tris-buffered saline
containing 10% (v/v) animal serum and 0.09% ProClinTM 950.
Contains 66 mM 3,3’-diaminobenzidine tetrahydrochloride, in a stabilizer
solution.
Contains ≤0.1% (v/v) hydrogen peroxide.
Contains <0.1% hematoxylin.
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 4 of 23
Page 5

Directions on Use
All reagents supplied are formulated specically for use with this assay and lot numbers are
specic for each Bond Oracle HER2 IHC System. For the assay to be valid, no substitutions
should be made.
English
Storage and Stability
Store at 2–8 °C. Do not freeze. Return to 2–8 °C immediately after use. Any deviation from these
conditions will invalidate the assay. Ensure the Bond Oracle HER2 IHC System used is within its
designated expiry date. The signs indicating contamination and/or instability of the Bond Oracle
HER2 IHC System are: turbidity of the solutions, odor development, and presence of precipitate.
Storage conditions other than those specied above must be veried by the user.
Specimen Preparation
All specimens must be prepared to preserve the tissue for immunohistochemical staining.
Standard methods of tissue processing should be used for all specimens (12).
It is recommended that tissues are prepared in formalin-based xatives and are routinely
processed and parafn-embedded. For example, resection specimens should be blocked into
a thickness of 3–4 mm and xed for 18–24 hours in 10% neutral-buffered formalin. The tissues
should then be dehydrated in a series of alcohols and cleared through xylene, followed by
impregnation with molten parafn wax, held at no more than 60 °C. Tissue specimens should
be sectioned between 3–5 µm.
The slides required for HER2 oncoprotein evaluation and tumor verication should be prepared
at the same time. To preserve antigenicity, tissue sections mounted on slides (Leica BOND
Plus Slides – product code S21.2113) should be stained within 4–6 weeks of sectioning when
held at room temperature (20–25 °C). Following sectioning, it is recommended that slides are
incubated for 12–18 hours (overnight) at 37 °C. Sections which require additional adherence
may be incubated at 60 °C for a further hour.
In the USA, the Clinical Laboratory Improvement Act of 1988 requires in 42 CFR 493.1259(b)
that “The laboratory must retain stained slides for at least ten years from the date of examination
and retain specimen blocks at least two years from the date of examination”.
Warnings and Precautions
For professional users only.
One or more components in the product are hazardous.
As a rule, persons under 18 years of age are not allowed to work with this product. Users must
be carefully instructed in the proper work procedure, the hazardous properties of the product
and the necessary safety instructions.
Symptoms of overexposure to ProClin™ 950, the preservative used in the Oracle reagents, may
include skin and eye irritation and irritation to mucous membranes and upper respiratory tract.
The concentration of ProClin™ 950 in this product is up to a maximum of 0.35%. These solutions
do not meet the OSHA criteria for a hazardous substance. A Material Safety Data Sheet is
available upon request or from www.LeicaBiosystems.com.
Specimens, before and after xation, and all materials exposed to them, should be handled as
if capable of transmitting infection and disposed of with proper precautions.
Never pipette reagents by mouth and avoid contacting the skin and mucous membranes with
reagents and specimens. If reagents or specimens come into contact with sensitive areas, wash
with copious amounts of water. Seek medical advice. Consult federal, state or local regulations
for disposal of any potentially toxic components.
Minimize microbial contamination of reagents or an increase in nonspecic staining may occur.
Page 5 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 6

Procedure
A. Reagents required but not supplied
• BOND Dewax Solution (product code AR9222)
• BOND Epitope Retrieval Solution 1 (product code AR9961)
• BOND Wash Solution x10 Concentrate (product code AR9590)
• Standard solvents used in immunohistochemistry ( e.g. ethanol, absolute and graded)
• Xylene (or xylene substitutes)
• Mounting medium
• Distilled or de-ionized water
B. Equipment required but not supplied
• Leica Biosystems’ BOND-MAX and BOND-III fully automated, advanced staining
system(s)
• BOND Universal CovertilesTM (product code S21.2001 or S21.4583)
• BOND Mixing Stations (product code S21.1971)
• Drying oven, capable of maintaining 60 °C
• Light microscope (4–40x objective magnication)
• Slides (Leica BOND Plus Slides – product code S21.2113)
• Coverslips
• BOND Slide Label & Print Ribbon (product code S21.4564)
• BOND Aspirating Probe Cleaning System (product code CS9100)
C. Methodology
• Prior to undertaking this methodology, users must be trained in BOND fully automated
immunohistochemical techniques.
• Each test section to be stained with the HER2 Primary Antibody will require an identical
section for staining with the HER2 Negative Control. The negative control section allows
differentiation between specic and nonspecic staining at the antigen site. Each BOND
staining run should include a HER2 Control Slide. At the end of the staining protocol, if
the cell lines do not demonstrate the correct staining patterns (refer to Bond Oracle HER2
IHC Systems Interpretation Guide), the run should be regarded as invalid.
D. Slide Layout
A new BOND Universal Covertile (product code S21.2001 or S21.4583) should be used with
each slide. The use of BOND Universal Covertiles which have been previously utilized for
either immunohistochemical or in situ hybridization staining has not been validated with this
test.
The slide tray layout (Table 2) enables optimal performance of the Bond Oracle HER2 IHC
System and the full 60 tests to be obtained.
English
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 6 of 23
Page 7
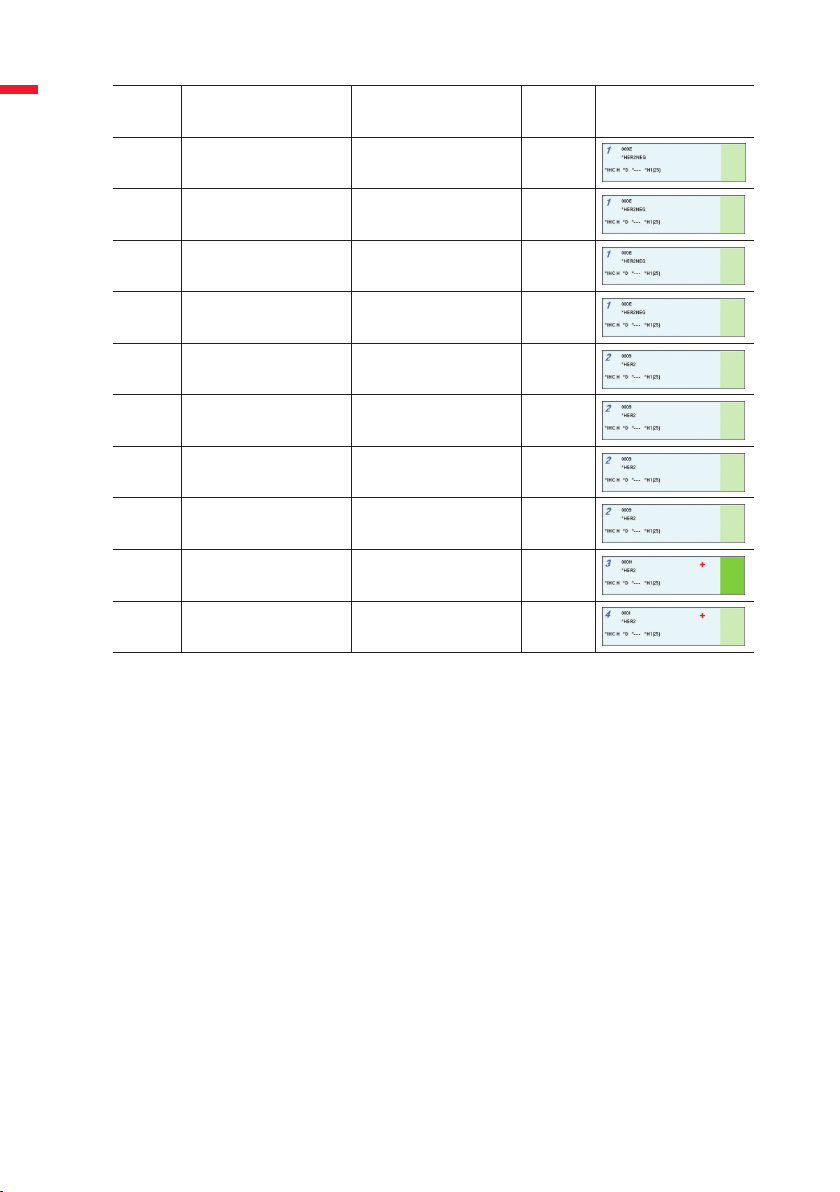
Slide
Slide Description Reagent Tissue
Position
English
1 Case 1 *HER2 Negative Control Test
2 Case 2 *HER2 Negative Control Test
3 Case 3 *HER2 Negative Control Test
4 Case 4 *HER2 Negative Control Test
5 Case 1 *HER2 Primary Antibody Test
6 Case 2 *HER2 Primary Antibody Test
7 Case 3 *HER2 Primary Antibody Test
8 Case 4 *HER2 Primary Antibody Test
9 HER2 Control Slide *HER2 Primary Antibody Positive
10 In-house Tissue Control *HER2 Primary Antibody Positive
Table 2. Slide tray layout, showing tissue type and reagent
Slide Icon
Type
E. Procedure Steps
Follow the steps below to set up a slide tray with the layout described in Table 2. These
instructions should be read in conjunction with the BOND System User Manual.
1. On the BOND instrument, ensure the bulk and hazardous waste containers have enough
capacity to perform the required staining runs.
2. Ensure there is adequate alcohol, distilled or de-ionized water, BOND Dewax Solution
(supplied as ready-to-use), BOND Epitope Retrieval Solution 1 (supplied as ready-to-use)
and BOND Wash Solution (supplied as x10 concentrate) in the bulk reagent containers to
perform the required staining runs.
3. Ensure that a clean BOND Mixing Station is installed.
4. Turn on the BOND fully automated, advanced staining system.
5. Turn on the BOND Controller attached to the BOND fully automated, advanced staining
system.
6. Open the BOND software.
7. For a new Bond Oracle HER2 IHC System, scan the reagent tray barcodes with the
handheld scanner to enter the system into the BOND reagent inventory.
8. Go to the Slide setup screen and click Add case.
Page 7 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 8

9. Enter details for the rst case. Ensure the dispense volume is set to 150 µL and the
preparation protocol is *Dewax. Click OK.
10. With the case highlighted in the Slide setup screen click Add slide.
11. First, add patient test slides. Ensure tissue type is set to Test tissue.
12. Conrm the dispense volume is 150 µL and the preparation protocol is *Dewax.
13. Select staining mode values Single and Oracle (do not click Oracle control).
14. Select process IHC.
15. Select *HER2 Negative Control from the marker list. The Protocols tab defaults to the correct
staining protocol (*IHC Protocol H) and HIER protocol (*HIER 25 min with ER1 (97)).
16. Click Add slide. The negative control reagent slide is created.
17. Still in the Add slide dialog, select *HER2 Primary Antibody from the marker list. Default
protocols and all other settings remain unchanged.
18. Click Add slide. The test slide is created.
19. Repeat steps 8 to 18 until all cases and patient test slides have been created.
20. Next, create the HER2 Control Slide. Add it to the last case or create a new case for
control slides, depending on your standard laboratory practises.
Important note: It is a requirement of the Bond Oracle HER2 IHC System that a HER2 Control Slide is
included in each run (ie slide tray) in order to validate the assay.
21. In the Add slide dialog set tissue type to Positive tissue.
22. Click Oracle control.
23. Select the lot number of the HER2 Control Slide in the Lot No list. The lot number is
inscribed on the label area of the slide.
Important note: The HER2 Control Slide must come from the same Bond Oracle HER2 IHC System that will
be used.
24. Select *HER2 Primary Antibody from the marker list. Retain dispense volume, staining
mode, process and protocol settings.
25. Click Add slide to add the HER2 Control Slide.
26. Finally, add a positive in-house tissue control slide.
27. Deselect Oracle control.
28. Select *HER2 Primary Antibody from the marker list. Retain dispense volume, staining
mode, and process and protocol settings. Tissue type remains Positive tissue.
29. Click Add slide. This completes slide creation.
30. Print slide labels. All Oracle slide labels have “OC” printed on them. The label for the
HER2 Control Slide also includes the Bond Oracle HER2 IHC System lot number.
31. Label slides appropriately.
32. Open the lids of all Bond Oracle HER2 IHC System containers and load the reagent tray
onto the BOND.
33. Place slides onto the slide tray in the order indicated in section D, Table 2. Apply new
Covertiles.
English
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 8 of 23
Page 9

34. Load the slide tray onto the BOND and press the Load/Unload button.
35. Conrm that the slides have been scanned and click the Run (Play) button on the System
status screen.
English
36. Ensure that the tray indicator eld displays Proc (OK) and batch number and nish time
are displayed.
37. When the run is completed press the Load/Unload button and remove the slide trays from
the BOND.
38. Remove Covertiles and rinse the slides in de-ionized water.
39. Dehydrate, clear and mount sections.
Quality Control
Differences in tissue xation, processing and embedding in the user’s laboratory may produce
signicant variability in results, necessitating regular performance of in-house controls in
addition to the HER2 Control Slides supplied by Leica Biosystems in the Bond Oracle HER2
IHC System. Consult the quality control guidelines of the College of American Pathologists
(CAP) Certication Program for Immunohistochemistry; see also CLSI (formerly NCCLS)
Quality Assurance for Immunocytochemistry, Approved Guideline (12) and Special Report:
Quality Control in Immunohistochemistry (13). In addition, refer to Table 3 below for the types of
immunohistochemical quality controls and their purposes.
Sample* Description HER2 Primary Antibody
Staining
HER2 Control
Slide
As supplied in the Bond Oracle
HER2 IHC System.
Controls staining procedure,
and indicates the validity of
HER2 Negative
Control Staining
the reagent performance.
In-house
Positive Control
Tissue
In-house
Negative
Control Tissue
Component
Tissue containing target
antigen. The ideal control
is weakly positive staining
tissue so as to define subtle
changes in primary antibody
sensitivity.
Tissues or cells expected
to be negative (could be
located in patient tissue or
positive/negative control
Controls all steps of the
analysis. Validates tissue
preparation and Bond Oracle
HER2 IHC System staining
performance.
Detection of nonspecific
antibody cross-reactivity
with cells/cellular
components.
Detection of
nonspecific
background
staining
tissue components).
*Fixed and processed as per patient sample
Table 3. Immunohistochemical quality controls and their purpose
Control tissue should be biopsy or surgical specimens, formalin-xed, processed and parafn-
embedded as soon as possible, and in the same manner as the patient sample(s). Specimens
must be handled appropriately to preserve the tissue antigenicity for immunohistochemical
staining. Standard methods of tissue processing should be employed for all specimens (12).
Page 9 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 10

HER2 Control Slide – HER2 Primary Antibody
Each of the supplied HER2 Control Slides contains four formalin-xed, parafn-embedded
human breast cancer cell line cores with staining intensity scores of 0, 1+, 2+ and 3+. One slide
must be included in each test run (ie slide tray). The correct evaluation of the HER2 Control
Slide supplied by Leica Biosystems indicates the validity of the test (refer to Bond Oracle HER2
IHC System Interpretation Guide). The HER2 Control Slides supplied with this system validate
reagent performance only and do not verify tissue preparation.
In-house Positive Control Tissue – HER2 Primary Antibody
If in-house positive control tissue components are used, they should be biopsy or surgical
specimens xed, processed and embedded as soon as possible in the same manner as the
patient sample(s). Positive tissue controls are indicative of correctly prepared tissues and
valid staining techniques. At least one positive control component for each test run should be
included. The positive control section should demonstrate weak positive staining so as to dene
subtle changes in primary antibody sensitivity.
Note: Known positive control tissue components should only be utilized for monitoring the correct
performance of processed tissues together with test reagents, NOT as an aid in formulating
a specic interpretation of patient samples. If the positive control tissue fails to demonstrate
appropriate positive staining, results obtained with patient specimens should be considered
invalid.
A multi tissue control block containing tumors representing all 4 HER2 grades may also be
effectively utilized as appropriate in-house control material.
In-house Negative Control Tissue Component – HER2 Primary Antibody
If in-house negative control components are used, they should be fresh biopsy or surgical
specimens xed, processed and embedded as soon as possible in the same manner as the
patient sample(s). Use of control tissue, known to be HER2 oncoprotein negative, with each
staining run veries the specicity of the primary antibody and provides an indication of any
nonspecic background staining. The variety of different cell types present in most tissue
sections offers internal negative control sites (this should be veried by the user). Normal breast
ducts unassociated with tumor may provide a reference to the validity of the assay. If specic
staining occurs in the internal negative control tissue, results with the patient specimens should
be considered invalid.
The use of multi-tissue control block representing all four HER2 grades may be utilized for the
purposes of negative and postive control tissues.
Patient Tissue – HER2 Negative Control
Use the supplied HER2 Negative Control in place of the HER2 Primary Antibody on a
corresponding section for each patient test to evaluate nonspecic staining and allow accurate
interpretation of specic HER2 oncoprotein staining at the antigenic site.
Patient Tissue – HER2 Primary Antibody
Positive staining intensity should be assessed within the context of any nonspecic background
staining with the HER2 Negative Control. As with any immunohistochemical test, a negative
result means that the antigen was not detected, not that the antigen was absent in the cells/
tissue assayed. Refer to Slide Screening Order Rationale, Limitations, Performance Evaluation
and Immunoreactivity for specic information regarding Bond Oracle HER2 IHC System
immunoreactivity.
Assay Verication
Prior to the initial use of any antibody or staining system in a diagnostic procedure, the user
should verify the antibody’s specicity by testing it on a series of in-house tissues with known
immunohistochemical positive and negative proles. Refer to Quality Control as previously outlined
and the quality control requirements of the CAP Certication Program for Immunohistochemistry
English
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 10 of 23
Page 11

and/or CLSI (formerly NCCLS) Quality Assurance for Immunocytochemistry, Approved
Guideline (12). These quality control procedures should be repeated for each new antibody lot,
or whenever there is a change in assay parameters. Human invasive (inltrating) ductal breast
carcinoma with known HER2 oncoprotein staining intensities from 0 to 3+ and other suitably
English
negative tissues are appropriate for assay verication.
Interpretation of Staining
For the determination of HER2 oncoprotein expression, only membrane staining pattern and
intensity should be evaluated using the scale presented in Table 4. A pathologist using a bright-
eld microscope should perform slide evaluation. For evaluation of the immunohistochemical
staining and scoring, an objective of 10x magnication is appropriate. The use of 20–40x
objective magnication should be used in the conrmation of the score. Cytoplasmic staining
should be considered as nonspecic staining and is not to be included in the assessment of
membrane staining intensity (14). To aid in the differentiation of 0, 1+, 2+, and 3+ staining, refer
to the Bond Oracle HER2 IHC System Interpretation Guide for representative images of the
staining intensities. Only specimens from patients with invasive breast carcinoma should be
scored. In cases with carcinoma in situ and invasive carcinoma in the same specimen, only the
invasive component should be scored.
Immunohistochemical Staining Pattern Score Assessment
No staining is observed or membrane staining is observed in less
than 10% of the tumor cells.
0 Negative
Faint/barely perceptible membrane staining is detected in more
than 10% of the tumor cells. The cells are only stained in part of
1+ Negative
their membrane.
Weak to moderate complete membrane staining is observed in
more than 10% of the tumor cells.
Strong complete membrane staining is observed in more than
10% of the tumor cells.
Table 4. Interpretation of HER2 staining
Equivocal
2+
(Weakly Positive)
3+ Strongly Positive
Bond Oracle HER2 IHC System staining results are interpreted as negative for HER2
oncoprotein expression with scores of 0 and 1+ staining intensity, equivocal (weakly positive)
with a score of 2+ staining intensity, and strongly positive with a score of 3+ staining intensity.
Bond Oracle HER2 IHC System is not intended to provide prognostic information to the patient
and/or physician and has not been validated for that purpose. For each staining assessment,
slides should be examined in the order presented below to determine the validity of the staining
run and enable semi-quantitative assessment of the staining intensity of the sample tissue.
Page 11 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 12

Slide Screening Order Rationale
Slides should be screened in the following order:
1. HER2 Control Slide – HER2 Primary Antibody
A valid assay with the Oracle HER2 Control Slide shows the following:
• Presence of strong brown, complete cell membrane staining in the 3+ Control Cell Line
SK-BR-3.
• Presence of weak to moderate brown, complete cell membrane staining in the 2+ Control
Cell Line, MDA-MB-453.
• Presence of faint/barely perceptible brown, incomplete cell membrane staining in the
1+ Control Cell Line, MDA-MB-175.
• No staining in the 0 Control Cell Line MDA-MB-231.
Important note: A feature of the MDA-MB-175 1+ control cell line is a distinct growth pattern in
which the cells form clusters. These clusters give rise to a continuous luminal brush border
region across the cell cluster. This brush border staining will be stronger than that of the rest
of the cell membrane. It is the faint/barely perceptible incomplete cell membrane staining that
is the correct HER2 oncoprotein 1+ staining pattern. Dot-like immunostaining of the Golgi
region in the cytoplasm may also be observed in this cell line.
2. In-house Positive Control Tissue – HER2 Primary Antibody
The PRESENCE of brown membrane staining should be observed corresponding to the
known HER2 oncoprotein status of the chosen positive control.
3. In-house Negative Control Tissue Component – HER2 Positive Control
The ABSENCE of membrane staining should be observed. A negative control tissue
component conrms the lack of detection system cross-reactivity to specically targeted cells/
cellular components. If membrane staining occurs in a negative control tissue component,
results with the patient specimen should be considered invalid.
4. Patient Tissue – stained using the HER2 Negative Control
The ABSENCE of membrane staining veries the specic labeling of the target antigen by the
primary antibody. Other brown staining occurring in the cytoplasm of the specimen treated
with the HER2 Negative Control, such as in connective tissue, leukocytes, erythrocytes,
or necrotic tissue, should be considered nonspecic background staining and should be
noted.
5. Patient Tissue – stained using the HER2 Primary Antibody
HER2 oncoprotein expression levels are determined by the criteria dened in both Table 4
and in the Bond Oracle HER2 IHC System Interpretation Guide.
English
Limitations
A. General Limitations
Immunohistochemistry is a laboratory based, multi-step technique, used to aid in the
interpretation and determination of histopathological characteristics. It is a technique which
requires specialized training in all aspects of procedure (including the selection of appropriate
reagents, tissue, xation, processing and IHC slide preparation) and interpretation.
Immunohistochemical staining of tissue is dependent on the handling, xation and
processing of the tissue prior to staining. Improper xation, freezing, thawing, washing,
drying, heating, sectioning or contamination with other tissues or uids may produce artifact,
antibody trapping, or false negative results. Inconsistent results may be due to variations in
xation, embedding methods, or to inherent irregularities within the tissue (15). Excessive or
incomplete counterstaining may also compromise correct interpretation of the results.
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 12 of 23
Page 13

Nonspecic staining, if present, usually has a diffuse appearance. Sporadic staining of
connective tissue may also be observed in sections from excessively formalin-xed tissues.
Use intact cells for interpretation of staining results. Necrotic or degenerated cells often stain
nonspecically (16). False-positive results may be seen due to non-immunological binding of
English
proteins or substrate reaction products. They may also be caused by endogenous enzymes
such as pseudoperoxidase (erythrocytes) or endogenous peroxidase (cytochrome C),
depending on the type of immunohistochemical stain used.
Tissues from patients infected with Hepatitis B virus and containing Hepatitis B virus surface
antigen (HBsAg) may exhibit nonspecic staining with horseradish peroxidase (17).
Unexpected immunohistochemical staining, or variations in the staining, may be as a result
of alterations in the expression levels of the encoding genes or antigens. Any change in
expected staining patterns should be interpreted in association with all other diagnostic
investigations.
The interpretation of immunohistochemical staining should be complemented by morphological
studies and the use of suitable control material, and should be evaluated within the context of
the patient’s clinical history and other any diagnostic tests by a qualied pathologist.
The performance of the assay (ie assessments of adequacy of both positive and negative
controls) and the interpretation of any immunohistochemical staining or its absence must
be carried out in an appropriately accredited/licensed laboratory under the supervision of a
suitably qualied and experienced pathologist, who is responsible for the overall assessment
of the immunohistochemical assay and its interpretation.
B. Product Specic Limitations
This product is not intended for use in ow cytometry. Performance characteristics have not
been determined for ow cytometry.
False negative results may be seen as a result of the degradation of antigens in the tissue
section. Slides required for HER2 oncoprotein evaluation and tumor verication should be
prepared at the same time. To preserve antigenicity, tissue sections mounted on slides
(Leica BOND Plus Slides – product code S21.2113) should be stained within 4–6 weeks
of sectioning when held at room temperature (20–25 °C). Following sectioning, slides are
recommended to be incubated for 12–18 hours at 37 °C. Sections which require further
adherence may be incubated at 60 °C for a further hour.
Minimal natural variation of immunohistochemical prole will be seen between growth batches
of cell lines utilized within the Bond Oracle HER2 IHC System. This natural variation is well
within acceptable tolerance levels of a biological entity and does not affect the interpretation
or performance of the system.
Characterization of the cell lines using both ow cytometry and in situ hybridization
as presented in Table 5 are also subject to natural biological variation. Technical and
interpretational variation of control cell lines as assessed by uorescent in situ hybridization
is also reported (18).
Assessment of the HER2 Control Slides should take into account all relevant expiry dates.
Store the Bond Oracle HER2 IHC System at 2–8 °C. Do not freeze. Return to 2–8 °C
immediately after use. Any deviations from these conditions will invalidate the assay.
Do not replace Bond Oracle HER2 IHC System reagents with any other components either
supplied by Leica Biosystems or by other manufacturers. To do so will invalidate the assay.
It is essential that all of the steps outlined in sections C to E (Procedure) are performed in the
prescribed order. Any deviation from this order will invalidate the assay.
It is essential that tissues xed only in formalin-based xatives be used in the assay. The use
of any other type of xative will invalidate the assay.
Tissue sections cut outside of the recommended thickness range have not been validated.
The use of any other section thickness may invalidate the assay.
Page 13 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 14

Cell Line Data
Cell Line BOND Oracle
HER2 IHC System
Prole
SK-BR-3 3+ 4.3x10
MDA-MB-453 2+ 1.4x10
MDA-MB-175 1+ 6.3x10
MDA-MB-231 0 9.3x10
*HER2 receptor load analysis as assessed by ow cytometry. + HER2 Gene Amplication Status as assessed by dual probe
(HER2:Chromosome 17) FISH.
Table 5. HER2 Control Slide prole
HER2
Receptor Load
per Cell*
5
5
4
3
HER2 Gene Amplication Status
HER2 Copy
Number
HER2:Chr17
Gene Ratio
13.35 3.55
5.73 2.05
3.33 1.20
3.15 1.13
+
Clinical Concordance of Bond Oracle HER2 IHC System v Dako
HercepTest
Part one of the study examined the suitability of the Bond Oracle HER2 IHC System for use
as an aid in determination of treatment with Herceptin® (trastuzumab) therapy. The study was
designed to examine the concordance between the Bond Oracle HER2 IHC System and the
Dako HercepTest, considered as the ‘gold standard’ for this assay. The acceptance criterion
was dened as greater than 75% overall concordance between the two tests with a 95%
condence interval (CI).
The study was conducted as a two-site, US based, blinded evaluation. Each investigational site
was supplied with formalin-xed, parafn-embedded breast cancer samples of known HER2
status. Cases were selected in reverse consecutive order from the clinical archives, representing
the consecutive ow of cases into a histopathology department for clinical testing, and tested
independently of other prognostic and/or predictive factors, with no bias introduced to the cohort.
Cohorts of 160 and 292 specimens were tested at Site 1 and Site 2 respectively. Each cohort
had an equal representation of equivocal/positive (2+, 3+) and negative (0, 1+) cases, based on
previously assigned HER2 IHC scores, resulting in a total study population of 452 samples.
Twelve samples were considered unsuitable, due to lack of sufcient invasive tumor and were
removed from the study. A further nine samples could not be scored as a result of tissue lifting
from the slide surface, resulting in a nal study population of 431 samples.
All cases were stained with the HercepTest according to the manufacturer’s instructions as
specied in the package insert. Sequential sections from each case were stained with the Bond
Oracle HER2 IHC System on board an automated Leica Biosystems BOND fully automated,
advanced staining system. All cases were de-linked from unique patient identifying information
and were accompanied by clinical data relating to tumor size, tumor stage, tumor grade and
estrogen receptor status.
All stained slides were masked and scored in a randomized fashion by trained observers at
two sites. For 2x2 concordance analysis, scores were interpreted as negative if the staining
intensity was 0 or 1+, and positive for scores of 2+ or 3+. For 3x3 concordance analysis, scores
were interpreted as negative if the staining was 0 or 1+, equivocal for scores of 2+ and positive
for scores of 3+. Data was then analyzed for positive staining agreement and negative staining
agreement.
English
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 14 of 23
Page 15

2x2 Concordance Results
In this primary analysis the test results from the two tests (Bond Oracle HER2 IHC System
and Dako HercepTest) are categorized as negative (0,1+) or positive (2+, 3+). The frequencies
of four possible combinations are displayed in a 2x2 table format (see Table 6). Then, the
English
overall concordance rate based on this 2x2 table was calculated accompanied by a 95% exact
condence interval (based on the binomial distribution).
The null hypothesis (H0), which the success criteria are set against, is that concordance is no
greater than 75%.
The observed agreement for 431 samples between the two tests in a 2x2 analysis show a
concordance of 92.34% (398/431) with a 95% CI of 89.42% - 94.67%. This data supports rejection
of the null hypothesis (H0) that agreement was no greater than 75% with a p-value<0.0001.
The percentage Positive Agreement (sensitivity) or the ability of Bond Oracle HER2 IHC System
to correctly identify HercepTest positive cases (the percentage of specimens scored positive
by both Bond Oracle HER2 IHC System and HercepTest out of all the HercepTest positive
cases) was 84.87% (129/152) with a 95% CI of 78.17%-90.16%. The percentage Negative
Agreement (specicity) or the ability of the test to correctly identify HercepTest negative cases
(the percentage of specimens scored negative by both Bond Oracle HER2 IHC System and
HercepTest out of all the HercepTest negative cases) was 96.42% (269/279) with a 95% CI of
93.51%-98.27%.
HercepTest
Negative Positive Totals
Bond Oracle HER2
IHC System
Negative
Positive
Totals
2x2 Concordance (95% CI) = 92.34% (89.42 to 94.67%); p<0.0001
Table 6. 2x2 concordance of Bond Oracle HER2 IHC System with HercepTest
269 23 292
10 129 139
279 152 431
3x3 Concordance Results
Data was grouped as negative (0 or 1+), equivocal (2+) or positive (3+) for 3x3 analysis and
showed a concordance of 86.54% (373/431) with a 95% CI of 82.95% to 89.62 %. Ther efore, the null
hypothesis (H0) that agreement was no greater than 75% was rejected with a p-value<0.0001.
The percentage Positive Agreement for 3+ (the percentage of specimens scored 3+ positive
by both Bond Oracle HER2 IHC System and HercepTest out of all the 3+ HercepTest positive
cases) in this study was 73.33% (66/90) with a 95% CI of 62.97% to 82.11%. The percentage
Negative Agreement was 96.42% (269/279) with a 95% CI of 93.51% to 98.27. See Table 7.
HercepTest
Negative
(0 or 1+)
Bond Oracle
HER2 IHC
System
Negative
(0 or 1+)
2+
3+
Totals
269 23 0 292
10 38 24 72
0 1 66 67
279 62 90 431
3x3 Concordance (95% CI) = 86.54% (82.95% to 89.62 %); p<0.0001
Table 7. 3x3 concordance of Bond Oracle HER2 IHC System with HercepTest
Page 15 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
2+ 3+ Totals
Page 16

In conclusion, the data generated in this study demonstrates that the Bond Oracle HER2 IHC
System can be used as an aid in determination of treatment for Herceptin® (trastuzumab)
therapy, based upon its high concordance with the HercepTest.
Clinical Concordance of Bond Oracle HER2 IHC System to PathVysion HER-2
DNA Probe Kit
Part 2 of the study was designed to examine the concordance between the Bond Oracle
HER2 IHC System and the Abbott Molecular PathVysion HER-2 DNA Probe Kit, considered
as the ‘gold standard’ for gene assessment reex assay used in conjunction with HER-2
immunohistochemistry.
This study was performed at the same investigational sites and used the same study cohort
as in Part 1. All cases were stained with the Abbott Molecular PathVysion HER-2 DNA Probe
Kit according to the manufacturers’ instructions as specied in the package insert. Sequential
sections from each case were stained with the Bond Oracle HER2 IHC System on board a
BOND fully automated, advanced staining system (from Part 1 of the clinical study). Of the 431
cases stained no result was obtained on three occassions due to insufcient probe hybridization
resulting in a total cohort of 428 cases.
All stained slides were scored by trained observers at two investigational sites. For 2x2
concordance analysis the scores were interpreted as negative if the HER2/CEP17 gene
amplication ratio was less than (<) 2.0 and positive if greater than or equal to (>) 2.0 following
a 20 tumor cell count.
2x2 Concordance Results
The observed agreement for 428 samples between the two tests in a 2x2 analysis show a
concordance of 87.6% (375/428) with a 95% CI of 84% to 90%.
The percentage Positive Agreement (sensitivity) or the ability of Bond Oracle HER-2 IHC System
to correctly identify PathVysion positive cases (the percentage of specimens scored positive by
both Bond Oracle HER2 IHC System and PathVysion out of all the PathVysion positive cases)
was 93.8% (61+30/97) with a 95% CI of 86.8% to 97.4%.
The percentage Negative Agreement (specicity) or the ability of the test to correctly identify
PathVysion negative cases (the percentage of specimens scored negative by both Bond Oracle
HER2 IHC System and PathVysion out of all the PathVysion negative cases) was 85.8%
(284/331) with a 95% CI of 81.6% to 89.2%. See Table 8.
English
PathVysion HER-2 DNA Probe Kit
Negative Positive Totals
Bond Oracle HER2
IHC System
0/1+
2+
3+
Totals
284 6 290
41 30 71
6 61 67
331 97 428
Overall Concordance (95% CI) = 87.6% (84 to 90%)
Table 8. 2x2 concordance of Bond Oracle HER2 IHC System staining v PathVysion HER-2 DNA Probe kit.
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 16 of 23
Page 17

Immunoreactivity – Normal Panel
Normal Tissue Type Staining Pattern
English
Adrenal Negative Negative
Brain, Cerebellum Negative Negative
Brain, Cerebrum Negative Negative
Breast Negative Negative
Bone Marrow Negative Negative
Colon Negative Negative
Esophagus Negative Negative
Eye Negative Negative
Hypophysis Moderate cytoplasmic staining
Kidney Negative Negative
Larynx Negative Negative
Liver Negative Negative
Lung Negative Negative
Mesothelium Negative Negative
Ovary Negative Negative
Pancreas Negative Negative
Parathyroid Negative Negative
Peripheral Nerve Negative Negative
Prostate Negative Negative
Salivary Gland Negative Negative
Skin Negative Negative
Small Intestine Negative Negative
Spleen Negative Negative
Stomach Weak cytoplasmic staining observed in
Striated Muscle Negative Negative
Testis Negative Negative
Thymus Negative Negative
Thyroid Negative Negative
Tonsil Negative Negative
Uterine Cervix Negative Negative
Uterus Negative Negative
Table 9. Normal Panel Staining
HER2 Primary Antibody HER2 Negative Control
observed in hypophyseal cells (1/3)
gastric glands (2/3)
Negative
Negative
Page 17 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 18

Reproducibility Study
Within and Between Precision Testing
Precision testing was performed at Leica Biosystems, Newcastle Ltd. The tissue used was a
formalin-xed, parafn-embedded composite tissue micro array (TMA) supplied by Isu Abxis
(Yonsei University Medical Center 134 Shinchon-dong, Seoul, 120-752 Korea), comprising
of 20, 4mm diameter invasive breast carcinoma tissue cores.The 20 cases were selected
based on previously assigned HER2 scores. On this basis, x5 cases of HER2 3+, x5 cases of
HER2 2+, x5 cases of HER2 1+ and x5 cases of HER2 0, were included.
A. Within Run Precision Testing
Within run precision testing of the Bond Oracle HER2 IHC Systems was evaluated on a
total of 40 consecutive sections from a TMA comprising of 20 invasive breast tumors and
40 HER2 Control Slides. All slides were stained with the Bond Oracle HER2 IHC System on
the BOND fully automated advanced staining system. Sections were stained during one
continuous period using a Bond Oracle HER2 IHC System from the same manufacturing
batch. Stained sections were blinded and assessed in a randomized fashion by a single
experienced observer to determine within run precision.
An evaluation of the slides from the within run investigation indicated that 733/800 (91.63%)
test data points could be interpreted. 40 data points were excluded due to presence of
DCIS only, and a further 27 data points could not be interpreted due to a loss of invasive
tumor (specic to 3 cores). Variation in staining occurred 61 (8.32%) out of a possible
733 staining events. On 37 occasions, variation from 3+ to 2+ (n = 20) and from 1+ to
0 (n = 17) was observed and would therefore not represent a change from clinically positive
to clinically negative or vice versa in a 2x2 data assessment. The remaining 24 (3.27%)
occasions represented a change from clinically negative (0 or 1+) to clinically positive
(2+ or 3+). Pass value = 96.7% (95% CI = 95.15% to 97.81%).
B. Between Run Precision Testing
Between run precision testing of the Bond Oracle HER2 IHC System was evaluated on a
total of 24 consecutive sections taken from a TMA comprising of 20 invasive breast tumors
and 24 HER2 Control Slides. All slides were stained with the Bond Oracle HER2 IHC
System on the BOND fully automated advanced staining system. The slides were evaluated
in 8 independent runs, performed within the same laboratory, on three separate occasions
using a Bond Oracle HER2 IHC System from the same manufacturing batch. Stained slides
were blinded and assessed in a randomized fashion by a single experienced observer to
determine between run precision.
An evaluation of the slides from the between run investigation indicated that 456/480
(95.00%) test data points could be interpreted. 24 data points could not be interpreted due
to a loss of invasive tumor (specic to 5 cores). Variation in staining occurred 42 (9.21%) out
of a possible 456 data points. On 30 occasions, variation from 3+ to 2+ (n = 10) and from
1+ to 0 (n = 20) were observed and would therefore not represent a change from clinically
positive to clinically negative or vice versa in a 2x2 data assessment. The remaining 12
(2.63%) represented a change from clinically negative (0 or 1+) to clinically positive
(2+ or 3+). Pass value = 97.37% (95% CI = 95.90% to 98.77%).
C. Lot-to-Lot Reproducibility
To determine Lot-to-Lot reproducibility, 3 lots of Bond Oracle HER2 IHC Systems were
manufactured under GMP on 3 separate occasions and evaluated on 24 breast tumor
sections (24 test data points) taken from four different formalin-xed, parafn-embedded
tissue blocks (representing 0, 1+, 2+ and 3+ HER2 staining intensities) and three HER2
Control Slides (12 control data points). Three independent runs were performed within the
same laboratory on three separate occasions, each using a separate manufacturing lot of
Bond Oracle HER2 IHC System. All slides were stained with the Bond Oracle HER2 IHC
System on board a BOND fully automated advanced staining system. Stained slides were
English
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 18 of 23
Page 19

masked and assessed in a randomized fashion by a single trained observer to determine Lotto-Lot reproducibility.
An evaluation of the slides (tests and controls) from the lot-to-lot investigation indicated that
English
36/36 data points could be interpreted. No variation in staining occurred in the 36 data points
between the three different manufacturing lots of the Bond Oracle HER2 IHC System. Staining
with the Bond Oracle HER2 IHC System is consistent across manufacturing batches.
D. Between Laboratory Reproducibility
Between laboratory reproducibility testing of the Bond Oracle HER2 IHC System was evaluated
at 3 sites, Leica Biosystems Newcastle Ltd (Site A), and two independent laboratories
(Sites B and C) on a total of 192 sections from a TMA comprising of 20 invasive breast
tumors and 24 HER2 Control Slides. Of the 192 TMA sections stained, 96 were stained with
the HER2 Primary Antibody and 96 with the HER2 Negative Control reagent. All slides were
stained with the Bond Oracle HER2 IHC System on the BOND fully automated advanced
staining system. The slides were evaluated in 8 independent runs performed within each of
the 3 different investigational sites using a Bond Oracle HER2 IHC System from the same
manufacturing batch. Stained slides were blinded and assessed in a randomized fashion by
a single experienced observer at Leica Biosystems, Newcastle Ltd to determine between
laboratory reproducibility.
An evaluation of the slides from the between laboratory reproducibility investigation indicated
that 1477/1920 (76.93%) test data points could be interpreted. 443 test data points could not
be interpreted due to:
a) Inadequate performance of the HER2 Control slide on 2/24 occasions resulting in 2 runs/160
test data points being removed. This event occurred once at Site A and once at Site B (80 data
test points per investigational site removed).
b) Deviation from the test plan at Site C, in which 24 slides in total were manually
counterstained with hematoxylin following Bond Oracle HER2 IHC System staining. This
resulted in excessive counterstaining of both HER2 control slides and TMA test data points
resulting in 240 data points being removed.
c) Loss of invasive tumor resulting in 23 test data points being removed. This event occurred
on 23 occasions at Site A and was a direct result of loss of tissue in the TMA block on production
of the 192 consecutive TMA sections required to complete this investigation.
d) Uninterpretable staining due to inadequate washing by the BOND fully automated advanced
staining system resulting in 20 data points being removed.
An evaluation of the interpretable slides in the between laboratory precision investigation
indicated that variation in staining occurred 79 (5.28%) out of a possible 1477 staining events.
Of these, 14/1477 (0.95%) occasions represented variations from 0 to 1+ or 2+ to 3+ and as
such did not represent a change from clinically positive to clinically negative or vice versa in a
2x2 data assessment. Pass value = 99.05% (95% CI = 98.42% to 99.46%). Of the 14 staining
events, 5/1477 (0.34%) staining events occurred at Leica Biosystems, Newcastle, Ltd (Site A),
8/1477 (0.54%) occurred at Site B and 1/1477 (0.07%) occured at Site C.
The remaining 65/1477 (4.40%) staining events showed variation from 2+ to 1+ or 2+ to 0
and therefore would represent a change from clinically positive to clinically negative or vice
versa in a 2x2 data assessment. Pass value = 95.6% (95% CI = 94.42% to 96.54%). Of the
65 clinically signicant changes, 11/65 (16.9%) occurred at Leica Biosystems, Newcastle,
Ltd (Site A), 24/65 (36.9%) occurred at Site B and 30/65 (46.1%) occured at Site C. Of the
clinically signicant changes on no occasions did a 3+ change to a negative (0 or 1+) result
or vice versa.
E. Inter-Observer Reproducibility
40 randomly selected invasive breast cancer cases, providing an equal distribution of each of the
HER2 IHC grades (resection specimens) were consecutively sectioned and provided to Leica
Biosystems, Newcastle Ltd (Site A), Site B and Site C for staining and interpretation. The sections
Page 19 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 20

were blinded and randomized at each site prior to scoring. Inter observer agreement between
the two independent clinical sites, Site B and Site C, was 87.5% (95% CI = 73.3% to 95.8%).
The agreement between Site B and Site C and Leica Biosystems Newcastle, Ltd was 92.5%
(95% CI = 79.6% to 98.4%) and 85% (95% CI = 70.1% to 94.29%) respectively. The analysis
of total concurrence between the three observers (A, B, C) is 82.50%.
F. Between Instrument Precision (BOND-MAX v BOND-III)
Between instrument precision testing using the Bond Oracle HER2 IHC System was
performed at a single independent European investigational site. The samples tested were
obtained from formalin-xed, parafn-embedded whole sections from ninety-nine invasive
breast cancer cases (needle core and resection specimens). Testing between instruments
was performed prospectively within the investigational site, staining consecutive sections on
the BOND-MAX and BOND-III platforms. Three cases were considered unsuitable due to
sample/tumor availability removed from the study.
Identical lot numbers of Bond Oracle HER2 IHC System and BOND Instrument ancillary
reagents were used across each instrument. Sections were stained prospectively over the
course of a 4-month period, with a maximum staining window of 5 days between staining
across both platforms to preserve antigenicity. Slides were interpreted at the investigational
site by a single experienced observer to determine between instrument precision.
An evaluation of the slides from between instrument precision showed a 2x2 concordance
between positive (2+, 3+) and negative (0, 1+) of 98.96% (95/96) with a 95% CI of 93.77 to
99.99% and a 3x3 concordance between positive (3+), equivocal (2+) and negative (0, 1+)
concordance of 96.88% (93/96) with a 95% CI of 90.83 to 99.32%.
BOND-MAX
Negative (0/1+) Positive (2/3+) Totals
Negative (0/1+)
BOND-III
Positive (2/3+)
Totals
Overall Concordance (95% CI) = 98.96% (93.77 to 99.99%)
Table 10. 2x2 concordance of Bond Oracle HER2 IHC System staining on BOND-MAX v BOND-III platforms.
72 0 72
1 23 24
73 23 96
English
Negative (0/1+) Equivocal (2+) Positive (3+) Totals
72 0 0 72
1 13 0 14
0 0 10 10
73 13 10 96
BOND-III
Negative (0/1+)
Equivocal (2+)
Positive (3+)
Totals
Overall Concordance (95% CI) = 98.96% (93.77 to 99.99%)
Table 11. 3x3 concordance of Bond Oracle HER2 IHC System staining on BOND-MAX v BOND-III platforms.
In conclusion, the data generated in this study demonstrates a high level of concordance
between the Leica Biosystems’ BOND-MAX and BOND-III Systems when evaluated using
the Bond Oracle HER2 IHC System.
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
BOND-MAX
Page 20 of 23
Page 21

Troubleshooting
Problem Probable Cause Remedial Action
English
No immunohistochemical
staining
Run aborted prior to
completion
Using BOND software, confirm the presence of
any reportable errors during the staining run and
address as instructed by the BOND software.
Weak specific
immunohistochemical
staining
Excessive specific
immunohistochemical
staining
Incorrect protocol selection
Ensure appropriate default to *IHC Protocol H in
the staining protocol field of the Add slide dialog.
Inadequate
deparaffinization of slides
Inappropriate bulk reagents
dispensed
Ensure *Dewax mode is selected in the
Preparation field of the Add slide dialog.
Ensure all BOND reagents have been allocated
to appropriate bulk containers and placed into
appropriate positions on the instrument.
Contamination of BOND Wash
Solution with sodium azide
Inappropriate epitope
retrieval
Use fresh BOND Wash Solution prepared to
appropriate working strength.
Ensure appropriate BOND Epitope Retrieval reagents
have been allocated into correct bulk containers, and
BOND software has defaulted to the appropriate
epitope retrieval protocol,
*HIER 25 min with *ER1 (97).
Inappropriate fixation
or processing of test
specimen
Bond Oracle HER2 IHC System
is being used outside its expiry
Ensure a formalin-based fixative is used and
that processing schedules are suitable for the
specimen undergoing testing.
Ensure the Bond Oracle HER2 IHC System used is
within its specified expiry date.
date
Inappropriate epitope
retrieval
Ensure appropriate BOND Epitope Retrieval
reagents have been allocated into appropriate bulk
containers, and the BOND software has defaulted
to *HIER 25 min with ER1 (97).
Variation in fixation Ensure a formalin-based fixative is used and
that processing schedules are suitable for the
specimen undergoing testing. If possible, retest
case using another block. If this is not possible,
assess the areas which show best fixation
patterns in conjunction with a corresponding
H&E stained section.
Page 21 of 23
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 22

Problem Probable Cause Remedial Action
Nonspecific background
staining
Inappropriate bulk reagents
dispensed
Inadequate deparaffinization
of slides
Nonspecific
immunohistochemical
Ensure all BOND reagents have been allocated
into appropriate bulk containers and placed into
appropriate positions on the instrument.
Ensure *Dewax is selected in the Preparation field
of the Add slide dialog.
Refer to Bond Oracle HER2 IHC System description
of normal tissue cross reactivity (refer to Table 9).
cross-reaction in tissue
Nonspecific
immunohistochemical
cross-reaction with areas
of tissue necrosis
Ensure a formalin-based fixative is used and
that processing schedules are suitable for the
specimen undergoing testing. If possible, retest
case using another block. If this is not possible,
assess in conjunction with a corresponding
H&E stained section, areas which show best
fixation patterns.
Drying artifact following
completion of a staining run
If slides are to be placed on an overnight run it
is recommended that the BOND delayed start
functionality is used. Ensure that there is an
adequate volume of distilled or de-ionized water
available to dispense on the slides for this period
to ensure the slides do not dry out.
Sections adhered to slides
with the aid of starch
Use unstarched slides (e.g. Leica BOND Plus
Slides – product code S21.2113).
additives
Tissue detached from
patient/control slide(s)
Use of incorrect type
of slides or inadequate
draining of section
Ensure appropriate slides are used for
patient/control sections (e.g. Leica BOND
Plus Slides – product code S21.2113).
Ensure slides receive adequate draining
and are incubated at 12–18 hours at 37 °C
(overnight). Sections which need further
adherence may be incubated at 60 °C for a
further hour.
Table 12. Bond Oracle HER2 IHC System Trouble Shooting Guide.
If any problems associated with the Bond Oracle HER2 IHC System fall outside the scope of the
troubleshooting guide (refer to Table 12) please contact your local Leica Biosystems’ Technical
Services Department or Distributor for assistance.
English
References
1. Corbett IP, Henry JA, Angus B et al. NCL-CB11, A new monoclonal antibody recognizing the internal
domain of the c-erbB-2 oncogene protein effective for use on formalin-xed, parafn-embedded
tissue. Journal of Pathology. 1990; 161:15-25.
2. Lonardo F, Di Marco E, King CR, Pierce JH, Segatto O, Aaronson SA, et al. The normal
erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence
of ligand. New Biologist 1990; 2: 992-1003.
3. Carter P, Presta L, Gorman CM, Ridgway JBB, Henner D, Wong WLT, et al. Humanization of an
anti-p185HER2 antibody for human cancer therapy. Proceedings of the National Academy of Science
USA 1992; 89: 4285-9.
4. Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal
antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor
necrosis factor. Molecular & Cell Biology 1989; 9: 1165-72.
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Page 22 of 23
Page 23

5. Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, et al. Differential responses of human
tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunology and Immunotherapy
1993; 37: 255-63.
6. Baselga J, Norton L, Albanell J, Kim Y-M, Mendelsohn J. Recombinant humanized anti-HER2
English
antibody (Herceptin®) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu
overexpressing human breast cancer xenografts. Cancer Research 1998; 58: 2825-31.
7. Nakane PK and Pierce GB. Enzyme labeled antibodies: Preparations and applications for the
localization of antigens. Journal of Histochemistry and Cytochemistry. 1967; 14: 929-931.
8. Tsutsumi Y, Serizawa A and Kawai K. Enhanced polymer one-step staining (EPOS) for proliferating
cell nuclear antigen and Ki-67 antigen-applications to intraoperative frozen diagnosis. Pathology
International. 1995; 45(2): 108-115.
9. Walker RA, Bartlett JMS Dowsett M, Ellis IO, Hanby AN, Jasani B, Miller K and Pinder SE. HER2
Testing in the UK- Further Update To Recommendations. Journal of Clinical Pathology 2008
10. Dickson, RB and Lippman, ME. Genes, Oncogenes, and Hormones. Boston, Kluwer Academic Publishers, 1992.
11. Keatings, L. et al. c-erbB-2 oncoprotein expression in mammary and extramammary Paget’s disease:
an immunohistochemical study. Histopathology. 1990; 17: 234-247.
12. The National Committee for Clinical Laboratory Standards (NCCLS). Quality assurance for
immunocytochemistry; Approved guideline. NCCLS document MM4-A (1-56238-396-5) NCCLS, 940 West
Valley Road, Suite 1400, Wayne, Pennsylvania 1999; 19087-1898: USA
13. Elias JM, Gown AM, Nakamura RM, Wilbur DC, Herman GE, Jaffe ES, et al. Special Report: Quality
control in immunohistochemistry. American Journal of Clinical Pathology 1989 ;92: 836-43.
14. Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal
human adult and fetal tissues. Oncogene 1990; 5: 953-62.
15. Nadji, M. and Morales, A. R. Immunoperoxidase, part I: the techniques and its pitfalls. Laboratory
Medicine 1983; 14: 767.
16. Jackson P. 2007. Quality Assurance in Immunohistochemistry. In: Immunohistochemistry, 2007
(ed. Renshaw S), PP 205-237. Scion Publishing Ltd.
17. Omata M, Liew C-T, Ashcavai M, Peters RL. Nonimmunologic binding of horseradish peroxidase to
hepatitis B surface antigen: a possible source of error in immunohistochemistry. American Journal of
Clinical Pathology 1980; 73: 626-32.
18. Bartlet JMS, Ibrahim M, et al External Quality Assurance of HER2 FISH Testing: Results of a UK
NEQAS Pilot Scheme. Journal of Clinical Pathology. 2006.
Amendments to previous issue
Branding, Additional Product Codes, Reproducibility Study.
Date of issue
18 June 2013
Symbol Identication
Batch Code
LOT
In vitro
IVD
diagnostic
medical device
Consult
instructions for
use
SN
Serial Number
HercepTest™ is a trademark of, and subject to, licences held by DakoCytomation, Denmark A/S
Herceptin® is a trademark of Genentech, Inc. and F. Hoffmann-La Roche Ltd.
Page 23 of 23
Storage
Manufacturer
Contains sufficient
REF
Catalog number
Fragile
Use by YYYY-MM-DD
for <n> tests
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
 Loading...
Loading...