Page 1

Model 3520
pH/mV/Temperature Meter
Operating Manual
352 050/REV A/03-03
Page 2

Safety
Please read this information carefully prior to installing or using this equipment.
1. The unit described in this manual is designed to be operated only by trained personnel. Any
adjustments, maintenance and repair must be carried out as defined in this manual, by a person
qualified to be aware of the hazards involved.
2. It is essential that both operating and service personnel employ a safe system of work, in addition to
the detailed instructions specified in this manual.
3. References should always be made to the Health & Safety data supplied with any chemicals used.
Generally accepted laboratory procedures for safe handling of chemicals should be employed.
4. If it is suspected that safety protection has been impaired in any way, the unit must be made
inoperative and secured against any intended operation. The fault condition should immediately be
reported to the appropriate servicing authority.
352 050/REV A/03-03
Page 3

Model 3520 pH/mV/Temperature Meter
Operating Manual
Contents
Section 1 Introduction
Instrument Description 1.1
Instrument Specification 1.2
Section 2 Installation
Unpacking 2.1
Installation 2.2
Displays/Controls 2.3
Inputs/Outputs 2.4
Section 3 Operation
Theory of pH measurement 3.1
pH Measurement 3.2
Preparation of Buffer Solution 3.3
Solution Temperature Values 3.4
Good Practice Guidelines 3.5
Set-Up Parameters 3.6
pH Calibration 3.7
Error Codes 3.8
mV Mode 3.9
Performing Measurements 3.10
Status Page 3.11
Results Storage and Display 3.12
GLP Functions 3.13
Section 4 Maintenance
General 4.1
Cleaning/Re-conditioning of Glass Electrodes 4.2
Section 5 Optional Accessories
Optional Accessories 5.1
Spares 5.2
Section 6 Interfacing
Analogue 6.1
RS232 6.2
Keypad Emulation 6.3
Printing 6.4
Section 7 Troubleshooting
Troubleshooting 7.1
Functional Checks 7.2
Reset Procedure 7.3
EC Declaration of Conformity
350 050/REV A/03-03
Page 4

Section 1
Introduction
1.1 Instrument Description
The Model 3520 is a fully specified laboratory pH/mV/Temperature meter that includes full support
for good laboratory practices (GLP). The meter supports 1, 2 or 3 point pH calibration on either
manually entered pH buffers or automatically temperature compensated buffers to DIN, JIS and
NIST standards. Powerful data logging capabilities are included with the ability to store up to 500
readings either manually, at timed intervals or on alarm events.
1.2 Instrument Specification
pH (1, 2 or 3 point cal)
Range: -2.000 to 20.000pH
Resolution: 0.001 / 0.01 / 0.1pH
Accuracy: ±0.003pH
mV (Absolute or Relative)
Range: -1999 to +1999mV
Resolution: 0.1mV
Accuracy: ±0.2mV
Input Impedance: >1012ohms
Temperature Measuring
Ranges: -10 to +105°C / 14 to 221°F
Resolution: 0.1°C / 1°F
Accuracy: ±0.5°C / ±1°F
ATC Range: 0 to 100°C / 32 to 212°F
Manual Temp. Compensation: 0 to 100°C / 32 to 212°F
Auto Buffer Selection: Jenway (2.00, 4.00, 7.00, 9.20 and 10.00)
DIN (3.06, 4.65, 6.79, 9.23, 12.75)
NIST (1.68, 4.01, 6.87, 9.18, 12.45)
JIS (1.68, 4.01, 6.87, 9.18, 12.45) or manually entered buffers
Calibration: User selectable 1, 2 or 3 point
Outputs: Analogue 1mV per 0.01pH
RS232 serial and IrDA printer interface
Alarm - open collector
Clock: 24 hours, hrs/min/sec or day of month/month/year, leap year
corrected (European and American formats)
GLP: Calibration reminder interval (1-999 hours)
Alarm outputs (open collector and audible)
Security code protected user data
Display: Back lit 1/8 VGA monochrome LCD
Languages: English, French, German, Italian, Spanish, Portuguese
Power: Power Supply 9Vac
Size: 275(l)x240(w)x150(d)mm
Weight: 850g
1
352 050/REV A/03-03
Page 5
Section 2
Installation
2.1 Unpacking
Remove the Model 3520 from the packaging and ensure the following items are included:
1. Model 3520 pH/mV/Temperature Meter
2. Glass bodied combination pH electrode (924 005)
3. ATC probe (027 500)
4. Electrode holder
5. 4, 7 and 10pH buffer sachets
6. BNC shorting plug (009 146)
7. Power Supply (as specified at time of ordering the product)
8. Condensed operating instructions (352 051)
9. Operating Manual (352 050)
Any shortages or damage should be reported immediately to the manufacturer or your local
distributor.
2.2 Installation
The Model 3520 is supplied ready to use. Connect the ATC (if required) and the pH electrode to the
rear panel Temp and pH sockets.
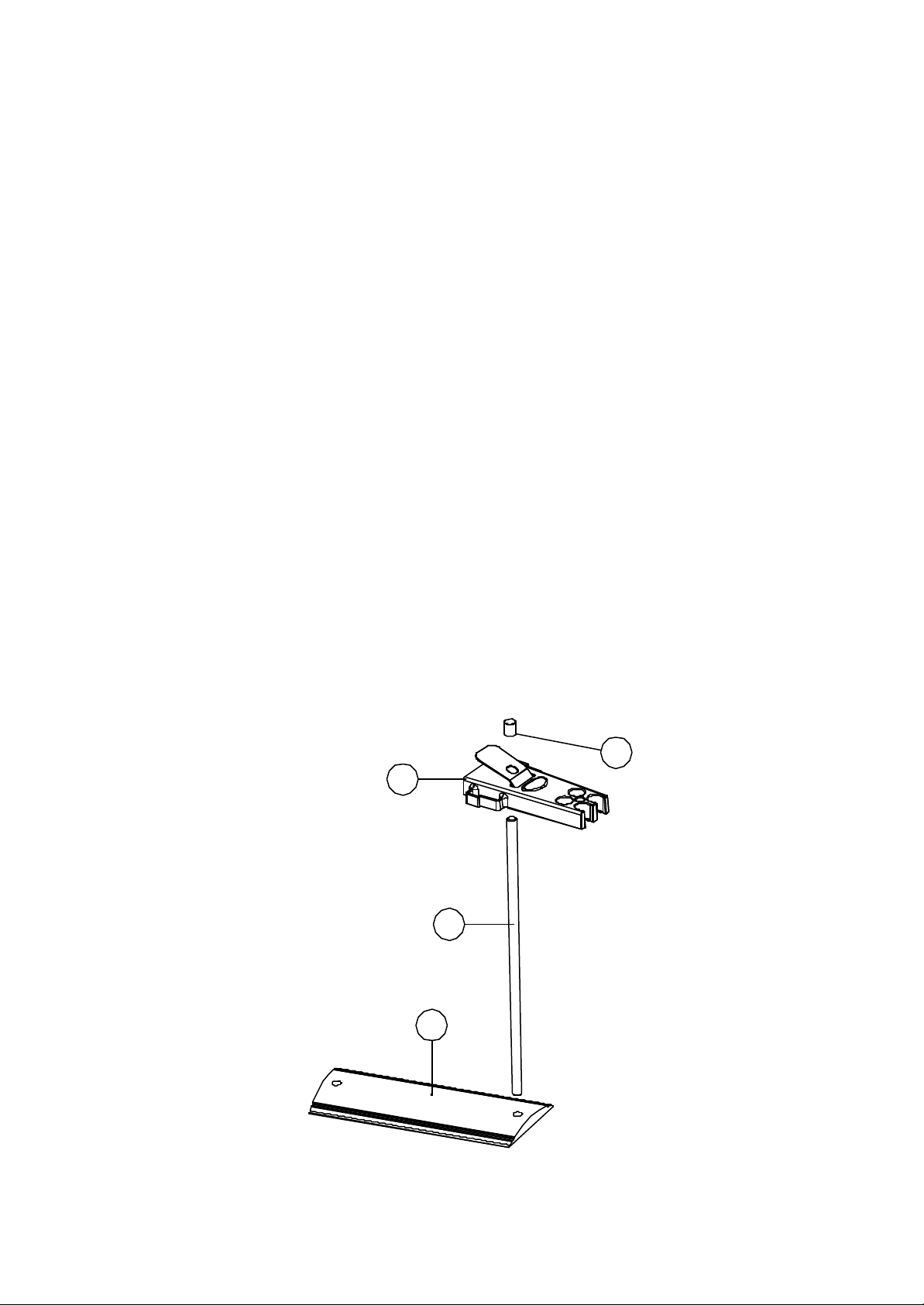
The electrode stand requires minimal assembly (refer to the diagram below).
Fig. 2.2.1 Electrode Holder Assembly
4
1
2
3
2
352 050/REV A/03-03
Page 6
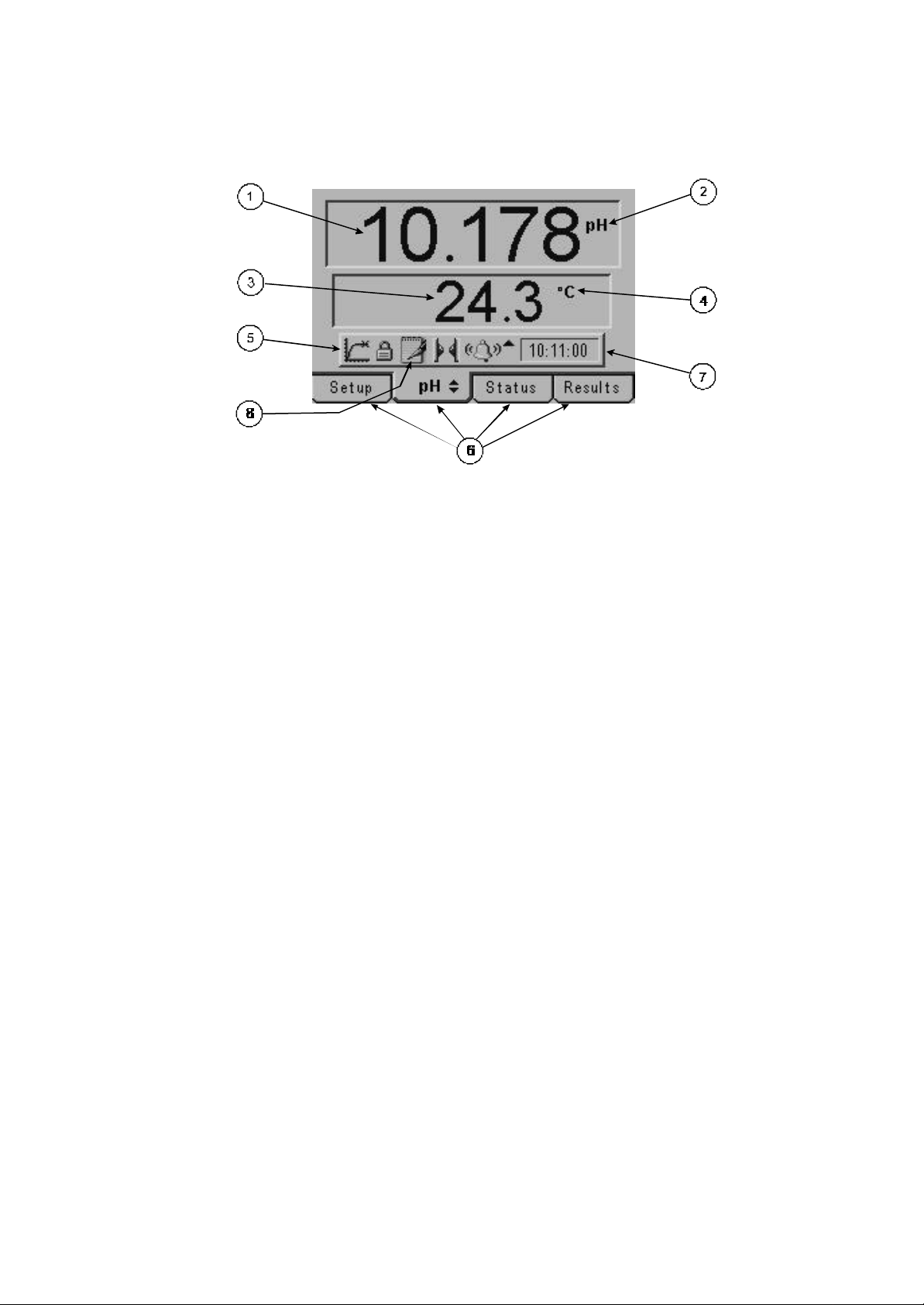
2.3 Display
Fig. 2.3.1 – Display
1. Primary display – 4½ digit. Provides direct readout in pH and millivolts of samples and
standards.
2. Mode annunciators – shows selected measurement mode; pH, mV (Absolute and Relative).
3. Secondary display – 3½ digit display. Provides direct readout of automatic or manual
temperature.
4. Mode annunciators – indicates temperature in °C or °F and whether the measurements are
manually temperature compensated (MAN symbol).
5. Endpoint symbol – this symbol is displayed when an endpoint has been detected.
6. Mode tags – Each mode tag is highlighted when selected; SETUP, MODE (pH or mV),
STATUS or RESULTS. If a double headed arrow symbol is present this indicates that the
mode can be changed to an alternative option (pH/mV).
7. Real time clock - will display either date or time.
8. The following symbols will appear along the display:
Padlock - Set up parameters security locked
Notepad symbol - data logging to internal memory
IrDA status
Alarm indication - an Up arrow refers to Hi alarm / a Down arrow refers to Low alarm
3
352 050/REV A/03-03
Page 7
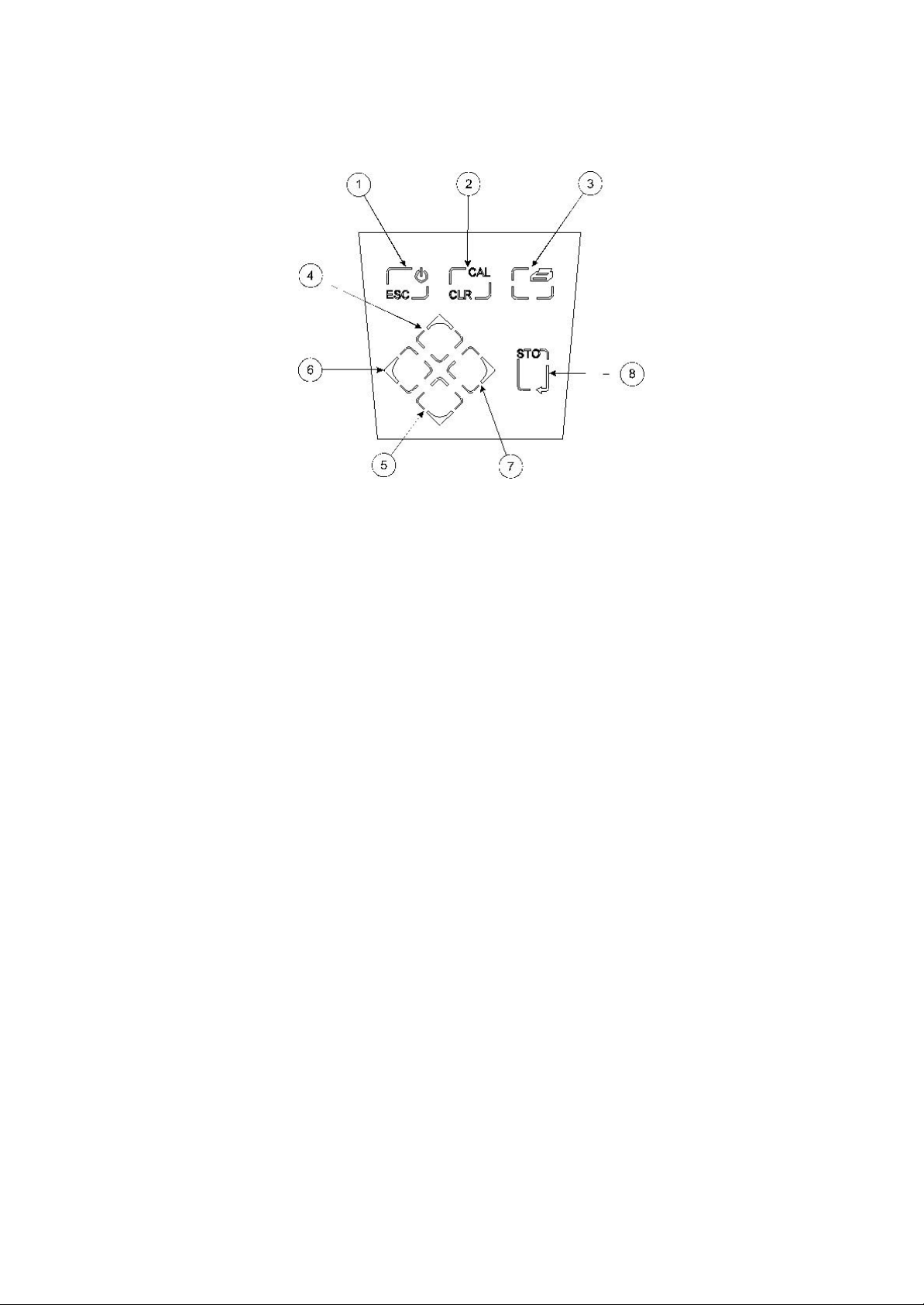
2.4 Keypad
2.4.1 Keypad
1. ESC used to switch the instrument on and to place into standby mode (only if power
supply lead remains connected to the instrument). Also used to escape/exit a
mode.
2. CAL / CLR used to select and perform a calibration sequence. This key is also used to clear
readings from Memory. Used to select Abs/Rel mV in mV mode.
3. Print key used to initiate a print.
4. Up Arrow used for adjustment during set up, to scroll results and to toggle between mV and
pH modes.
Down Arrow used for adjustment during set up, to scroll results and to toggle between mV and
pH modes.
Left Arrow used for adjustment during set up and to move between mode tags.
Right Arrow used for adjustment during set up and to move between mode tags.
STO used to accept an entered value in set-up mode and to instigate a stored reading.
This key can also be used as a CAL key during calibration.
4
352 050/REV A/03-03
Page 8
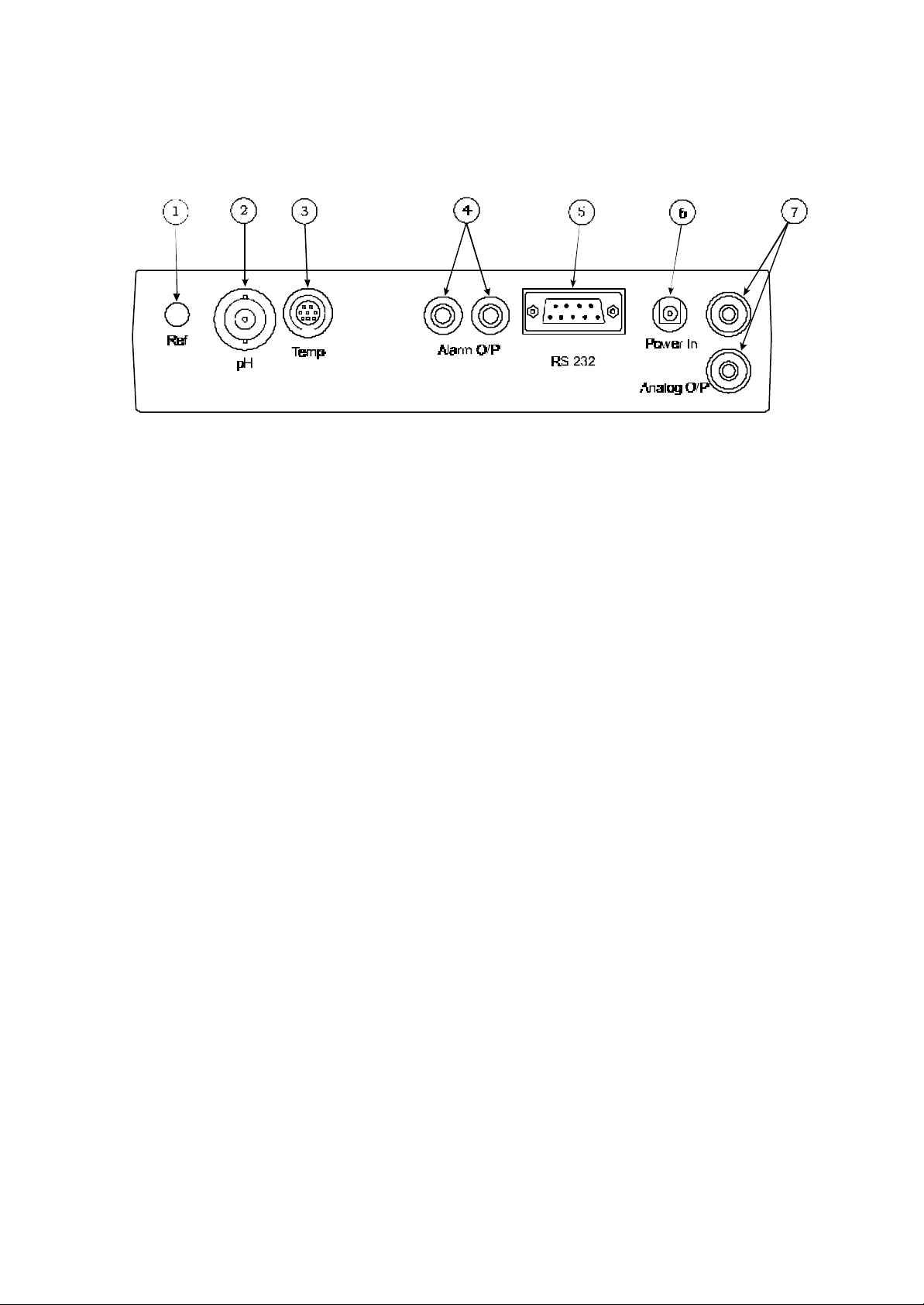
2.5 Inputs/Outputs
Fig. 2.5.1 – Rear panel layout
1. Ref Socket 2mm pin socket. Connection socket for separate reference electrode. When
performing measurements with some pH and ion selective electrodes a separate
reference electrode is needed.
2. pH Socket BNC type socket which allows combination pH or redox electrodes to be used.
3. Temp Socket 8 pin mini-DIN socket. This allows the optional Automatic Temperature
Compensation (ATC) probe to be connected.
4. Alarm Output 2 x 4mm sockets. Open collector alarm outputs.
Red for Hi / Black for Low.
5. Printer Socket 9 way socket for RS232 and IrDA connection.
5. Power In AC 9V I/P socket. 2.1 x 5.5mm socket allowing the power supply to be connected
to the instrument.
7. Analog Out 2x4mm sockets. Analogue output (buffered electrode potential).
5
352 050/REV A/03-03
Page 9

Section 3
Operation
3.1 Theory of pH Measurement
pH is a unit of measurement which defines the degree of acidity or alkalinity of a solution. It is
measured on a scale of 0 to 14. The pH value quantifies the degree of hydrogen ion activity of an
acid or a base.
The internationally accepted symbol, pH, is derived from “p”, the mathematical symbol of the
negative logarithm and “H”, the chemical symbol for Hydrogen. The pH value is the negative
logarithm of Hydrogen ion activity as shown in the mathematical relationship pH= -log[H+].
The pH value of a substance is directly related to the ratio of the Hydrogen ion [H+] and the Hydroxyl
ion [OH-] concentrations. If the concentration of H+ is greater than OH-, the material is acidic and
has a pH value of less than 7. Conversely, if the concentration of OH- is greater than H+ the material
is basic, with a pH value greater than 7. If the concentrations of H+ and OH- are equal the material is
neutral with a pH value of 7.
It can, therefore, be seen that pH is a measurement of both acidity and alkalinity, even though by
definition it is a selective measurement of hydrogen ion activity. The logarithmic relationship
between hydrogen ion concentration and the pH unit means that a change of one pH unit
represents a ten-fold change in hydrogen ion concentration.
3.2 pH Measurement
pH can be measured by using either pH papers/indicators or a pH meter, dependent on the level of
accuracy required. pH papers or indicators change colour as the pH level varies. These can be
used as a guide to the pH level, but can be limited in accuracy and difficult to interpret correctly in
murky or coloured samples.
For greater accuracy the use of a high impedance pH meter is recommended, together with a pH
measuring electrode and reference electrode.
Each component part of the measurement system can be described as follows:
a) the pH meter – is a high impedance amplifier used to accurately measure the minute electrode
voltages produced. The pH meter will display the results directly in pH units on either an analogue
or digital display. Voltages can also be read for special applications, ORP (Oxidation-Reduction
Potential) measurements or with Ion Selective Electrodes.
b) the pH electrode – is a hydrogen ion sensitive glass bulb, with a millivolt output that varies with
the changes in the relative hydrogen ion concentration inside and outside of the bulb. The pH
electrode has very high internal resistance, making the voltage change with pH difficult to measure.
The input impedance of the pH meter and leakage resistances are therefore important factors.
c) the reference electrode – these cells consist of an internal element, usually a silver/silver
chloride wire, electrolyte (KCl) and a liquid junction. The liquid junction provides a leak path for the
internal electrolyte to “weep” into the sample chamber and provide an electrical contact with the
liquid to be measured. If the liquid junction is inefficient then measurement will be inaccurate. It is
common for the reference electrode to be incorporated into the pH electrode. It is then called a
combination electrode. The Model 3520 is supplied with a combination electrode.
6
352 050/REV A/03-03
Page 10

The voltage developed by each individual pH electrode in the presence of a known hydrogen ion
concentration is theoretically predictable, but in practise deviations from the theoretical value can be
expected. These deviations will change slowly during the life of an electrode. It is therefore essential
to routinely calibrate the system using solutions with a known and constant pH value. These
solutions are called buffers.
3.3 Preparation of Buffer Solutions
Care must be taken in the preparation of all buffer solutions. The correct quantity of distilled or
deionised water should be used when preparing the solutions. For accurate and repeatable results
it is essential to follow the manufacturers instructions carefully.
3.4 Solution Temperature Values
The value of all buffer solutions varies with solution temperature. For accurate calibration of
electrodes using buffer solutions, it is necessary to measure the temperature of the buffer solution
being used. The unit should then be calibrated to the corrected pH value. Manufacturers of buffer
powders and solutions will provide a table of values at varying temperatures for their buffers.
Note: Buffer solutions will contaminate with exposure to air and should be stored in airtight
containers when not in use. Used solution should be discarded and not returned to the container as
this will cause contamination.
For best results fresh solutions should be prepared prior to calibration.
7
352 050/REV A/03-03
Page 11

3.5 Good Practice Guidelines
The types of electrodes are many and various. For the majority of tests carried out on aqueous
solutions, with a reasonable ionic strength; at ambient temperatures and with limited use in strongly
acidic or alkaline solutions, the standard glass or epoxy bodied combination electrode is ideal.
For other applications a more suitable pH/reference electrode pair may be required; details or
advice supplied on request.
The following general guidelines indicate the care and maintenance required for the three main
groups of electrodes (Combination, Reference and pH). For more detailed advice on specific
electrodes contact the electrode manufacturer.
1) After use Rinse thoroughly with deionised water
Short term storage Immerse in pH 4 buffer (all types)
Long term storage Fit wetting cap filled with 3M KCl adjusted to pH 4.
2) Electrodes should be stored a) away from direct sunlight
b) in a vertical position
c) within their specified temperature range
3) Always ensure the electrode is used within its specified temperature range. Degradation of
electrodes used above their specified temperature is rapid and irreversible.
4) Ensure the level of fill solution is above the internal elements in the electrode and that this
level remains above the sample in use.
5) DO NOT touch the sensitive glass pH membrane or reference junction during use. Excess
droplets of solution may be removed by gently blotting with filter paper or tissue. DO NOT rub
the electrode as this may induce an electrostatic charge.
6) Ensure no air bubbles are trapped at the bottom of the electrode. Removal of air bubbles is
possible by holding the electrode vertically and gently tapping the electrode body. Larger
bubbles may be removed by shaking the electrode in a downward direction.
7) During use ensure the electrode is rinsed in deionised water between each measurement to
eliminate risk of contamination of solutions.
8) Ensure that the side port/inlet if present is uncovered, especially during a long run of tests.
9) For samples such as blood, serum or any measurements of Tris buffer solutions the junction
may become badly clogged. For these measurements it is recommended that the Tris buffer
electrode is used (924 030).
10) For applications associated with the measurement of food extracts, it is recommended that
the Food electrode is used (924 051). This will reduce the risk of blockage from fat proteins,
will be easy to clean and is perfect for measurements in agar media. This electrode is also
recommended for measurements where deposits on the electrodes are likely. The flat
surface is easy to clean and robust.
11) For low ionic strength applications the Environmental electrode (924 050) is recommended.
8
352 050/REV A/03-03
Page 12

3.6 Set Up Parameters
The following section details the set-up modes available to the user on the main set up menu
screen:
3.6.1 Instrument Setup
This option will allow the following parameters to be set:
Exit allows the user to exit this menu.
pH Resolution allows the user to select the preferred resolution for the samples under test.
Use the Up/Down arrows on the keypad to scroll between 0.001pH, 0.01pH
and 0.1pH.
mV Resolution allows the user to select the preferred resolution. Use the Up/Down arrows
on the keypad to scroll between 0.1mV and 1mV.
Language enables the selection of the appropriate language – English, French,
German, Italian, Spanish or Portuguese. Use the Up/Down keys to scroll
through thelanguage options.
LCD Brightness (%) enter the value using the keypad. Press the STO key to accept the value.
9
352 050/REV A/03-03
Page 13

3.6.2 Temperature Setup...
Select the Temperature Setup sub menu by highlighting the option and pressing the STO key. The
following menu will be shown:
Temp units allows selection of the preferred unit of measurement (either °C or °F) using
the Up/Down keys which toggle between the two units.
Manual temperature allows the manual temperature value to be set. Press the STO key to
accept the value.
3.6.3 Calibration Set up …
This option allows the following parameters to be set:
Exit enables the user to exit the Setup menu and return to the main set up
screen.
Cal Buffers Used allows selection of 1, 2 or 3 point calibration.
Cal Buffer Set allows selection to be made from the choice of buffer types available:
Auto-Buffer (Jenway), Manual Entry, Auto-Buffer (JIS), Auto-Buffer (NIST) or
Auto-Buffer (DIN).
Manual Cal 1 allows Cal 1 manual buffer value to be set using the Up/Down keys. The
Left/Right arrow keys allow forward and backward movement along the
row of digits. Press the STO key to accept the value.
Manual Cal 2 allows Cal 2 manual buffer value to be set using the Up/Down keys. The
Left/Right arrow keys allow forward and backward movement along the
row of digits. Press the STO key to accept the value.
Manual Cal 3 allows Cal 3 manual buffer value to be set using the Up/Down keys. The
Left/Right arrow keys allow forward and backward movement along the
row of digits. Press the STO key to accept the value.
10
352 050/REV A/03-03
Page 14

3.6.4 Alarms Setup...
Exit enables the user to exit the Setup menu and return to the main
set up screen.
Alarm Outputs can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Audible Alarm Warning can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
pH Alarm High allows the user to set the high alarm limit up to 20.000 pH.
pH Alarm Low allows the user to set the low alarm limit down to -2.000 pH.
mV Alarm High allows the user to set the high alarm limit up to +1999.9mV.
mV Alarm Low allows the user to set the low alarm limit -1999.9 mV.
3.6.5 G.L.P Setup …
Exit enables the user to exit the Setup menu and return to the main
set up screen.
Cal Reminder can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Cal Reminder Interval can be set within the limits of 001 to 999 hours by using the Up/Down
keys.The Left/Right arrow keys allow forward and backward
movement along the row of digits. Press the STO key to accept the
value.
Cal Reminder Audible Alarm can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
User ID up to a 4 digit code can be set using the Up/Down keys. The Left/
Right arrow keys allow forward and backward movement along the
row of digits. Press the STO key to accept the value
11
352 050/REV A/03-03
Page 15

Batch ID up to a 3 digit code can be set by using the Up/Down keys.The Left/
Right arrow keys allow forward and backward movement along the
row of digits. Press the STO key to accept the value.
3.6.6.Security Setup …
Select the Security Setup sub menu buy highlighting the option and pressing the STO key. The
following menu will be shown:
Exit enables the user to exit the Set Up menu and return to the previous
set up screen.
Data Entry Security can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Security Code up to a 3 digit code can be set by using the Up/Down keys.The Left/
Right arrow keys allow forward and backward movement along the
row of digits. Press the STO key to accept the value.
3.6.7 Data Logging Set up …
Exit enables the user to exit the Set Up menu and return to the main
set up screen.
Data Log Event can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Data Log To allows the data to be sent to Memory or to the external Printer.
Selection is made via the Up/Down keys which toggle between the
two settings.
352 050/REV A/03-0312
Page 16

Data Log Interval can be set between 00:00:01 and 23:59:59.
Memory Full gives the user to select the Stop (cease storing results and not to
overwrite existing stored information) or Overwrite (overwrite existing
results) options when the memory is full. Selection is made via the
Up/Down keys which toggle between thetwo settings.
Prompt Before Deleting can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Clr Key toggles between Deletes Results Before, Deletes All Results and
Deletes Results Since. Selection is made via the Up/Down keys
which toggle between the four settings.
3.6.8 Printer Setup …
Select the Printer Setup sub menu buy highlighting the option and pressing the STO key. The
following menu will be shown:
Exit enables the user to exit the Setup menu and return to the main
instrument display.
Printer Interface toggles between Infrared and Serial. Selection of the preferred option
can be made using theUp/Down keys which toggle between the two
settings.
Serial Printer Baudrate toggles between 9600 and 1200. Selection of the preferred option can
be made using the Up/Down keys which toggle between the two
settings. (Refer Section 6.2).
13
352 050/REV A/03-03
Page 17

3.6.9 Endpoint Detection Set up …
Exit enables the user to exit the Set Up menu and return to the main
instrument display.
Endpoint detection can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Endpoint Audible Alarm can be enabled or disabled by using the Up/Down keys which toggle
between the two settings.
Endpoint Stability (Sec) can be set within the limits of 001 to 999 seconds.
3.6.10 Clock Set up …
Select the Clock Setup sub menu buy highlighting the option and pressing the STO key. The
following screens will be shown:
Exit enables the user to exit the Setup menu and return to the main
instrument display.
Display toggles between Time and Date. Selection of the preferred option
can be made using theUp/Down keys which toggle between the two
settings.
Date Format toggles between European (DD/MM/YY) and American (MM/DD/YY)
formats. Selection of the preferred option can be made using the Up/
Down keys which toggle between the two settings.
Time allows time to be set (hrs/min/sec) using the Up/Down
keys. The Left/Right arrow keys allow forward and backward
movement along the row of digits. Press the STO key to accept the
setting.
Date allows date to be set (in previously selected format – European or
American) using the Up/Down keys. The Left/Right arrow keys allow
forward and backward movement along the row of digits. Press the
STO key to accept the setting.
352 050/REV A/03-0314
Page 18

3.7 pH Calibration
3.7.1 Calibration with pH Buffers - Manual Temperature Compensation
To exit the calibration sequence at any time press the ESC key. This will cancel the pH calibration
and return the instrument to the MODE menu.
Note: Buffer solutions should be carefully prepared as per the manufacturers instructions.
When using manual temperature compensation (no ATC probe fitted) the solution
temperature should be measured and the value entered in the set up menu prior to
calibrating the instrument (refer 3.6.5). The buffer solutions should all be at the same
temperature.
1. Select the pH measuring mode using the Up/Down arrows which toggle between pH and mV
modes. Press the CAL key.
The primary display will show the current pH reading.
The secondary display will show the manually set temperature reading in °C or °F. When manual
temperature compensation is being used the annunciator will indicate MAN.
2. Immerse the electrode(s) in the first buffer solution and allow the instrument to stabilise. When
no pH change of less than 0.005pH is detected over a five second period the endpoint symbol will
be displayed. The symbol display will show:
where 1/3 is the number of calibration points selected in the Calibration Setup menu.
Value should show the manually temperature corrected value of the buffer used.
Press the CAL or STO key.
The display will then show the next part of the calibration sequence if a 2 and/or 3 point calibration
has been selected. If a 1 point calibration only is required the instrument will display CAL OK and
the Eo value and then return to the main measuring screen. The current calibration information,
including the Eo value can be found on the Status screens.
Rinse the electrode(s) in deionised water.
15
352 050/REV A/03-03
Page 19

3. CAL 2 Immerse the electrode(s) in the second buffer solution and allow the instrument to
stabilise. When no pH change of less than 0.005pH is detected over a five second period the
endpoint symbol will be displayed.
The symbol display will show:
where 2/3 is the number of calibration points selected.
Value should show the manually temperature corrected value of the buffer used.
Press the STO or CAL key.
The display will then show the next part of the calibration sequence if a 2 and/or 3 point calibration
has been selected. If a 2 point calibration only is required the instrument will display CAL OK, the
Eo value and the slope efficiency and then return to the main measuring screen. The current
calibration information, including the Eo value can be found on the Status screens.
Rinse the electrode(s) in deionised water.
3. CAL 3 Immerse the electrode(s) in the second buffer solution and allow the instrument to
stabilise. When no pH change of less than 0.005pH is detected over a five second period the
endpoint symbol will be displayed.
The symbol display will show:
where 3/3 is the number of calibration points selected.
Value should show the manually temperature corrected value of the buffer used.
Press the STO or CAL key.
The instrument will display CAL OK, the Eo value and the slope efficiency.
The instrument will return to the main measuring screen. The current calibration information,
including the Eo value can be found on the Status screens.
16
Page 20

Rinse the electrode(s) in deionised water.
Once a successful calibration has been completed the instrument will return to the measuring
mode.
The instrument is then ready to undertake the measurement of unknown solutions. If the tempera-
ture of the unknown solution differs from the buffer, the Temperature Set up menu should be used
to set the instrument display to the temperature of the unknown solution.
If the instrument fails a calibration the error message ERROR Eo OUTSIDE ±30mV LIMITS will
scroll along the secondary display. (refer Section 3.8 for details of Error Codes).
3.7.2 Calibration with pH Buffers - Automatic Temperature Compensation
To exit the calibration sequence at any time press the ESC key. This will cancel the pH calibration
and return the instrument to the MODE menu.
Note: Buffer solutions should be carefully prepared as per the manufacturers instructions.
The buffer solutions should all be at the same temperature.
1. Select the pH measuring mode using the Up/Down arrows which toggle between pH and mV
modes. Press the CAL key.
The primary display will show the current pH reading. The main display annunciators will indicate
CAL 1 and the buffer type being used.
The secondary display will show the ATC temperature in °C or °F.
2. Immerse the electrode(s) in the first buffer solution and allow the instrument to stabilise. When
no change in the least significant display digit is detected over a five second period the endpoint
symbol will be displayed. Press the CAL or STO key.
The display will show:
where 1/3 is the number of calibration points selected in the Calibration Setup menu.
Value should show the STC value of the buffer used.
Press the CAL or STO key.
The display will then show the next part of the calibration sequence if a 2 and/or 3 point calibration
has been selected. If a 1 point calibration only is required the instrument will display CAL OK and
the Eo value and then return to the main measuring screen. The current calibration information,
including the Eo value can be found on the Status screens.
17
352 050/REV A/03-03
Page 21

Rinse the electrode(s) in deionised water.
3. CAL 2 Immerse the electrode(s) in the second buffer solution and allow the instrument to
stabilise. When no pH change of less than 0.005pH is detected over a five second period the
endpoint symbol will be displayed.
The symbol display will show:
where 2/3 is the number of calibration points selected.
Value should show the ATC value of the buffer used.
Press the STO or CAL key.
The display will then show the next part of the calibration sequence if a 2 and/or 3 point calibration
has been selected. If a 2 point calibration only is required the instrument will display CAL OK, the
Eo value and the slope efficiency and then return to the main measuring screen. The current
calibration information, including the Eo value can be found on the Status screens.
Rinse the electrode(s) in deionised water.
3. CAL 3 Immerse the electrode(s) in the second buffer solution and allow the instrument to
stabilise. When no pH change of less than 0.005pH is detected over a five second period the
endpoint symbol will be displayed.
The symbol display will show:
where 3/3 is the number of calibration points selected.
Value should show the ATC value of the buffer used.
Press the STO or CAL key.
The screen will display CAL OK, the Eo value and the slope efficiency.
The instrument will return to the main measuring screen. The current calibration information,
including the Eo value can be found on the Status screens.
18
Page 22

Rinse the electrode(s) in deionised water.
Once a successful calibration has been completed the instrument will return to the measuring
mode.
The instrument is then ready to undertake the measurement of unknown solutions. If the tempera-
ture of the unknown solution differs from the buffer, the Temperature Set up menu should be used
to set the instrument display to the temperature of the unknown solution.
If the instrument fails a calibration the error message ERROR Eo OUTSIDE ±30mV LIMITS will
scroll along the secondary display. (refer Section 3.8 for details of Error Codes).
3.8 Error Codes
ERROR EO OUTSIDE ±30mV LIMITS
This error message will be displayed when the mV value for a buffer is more or less than 30mV
from the ideal Nernstian value.
ERROR SLOPE OUTSIDE 75% -125%LIMITS
This error message is displayed when the slope value is outside the range of 75 - 125%.
UNABLE TO RECOGNISE BUFFER
This error message will be displayed when the buffer value is more than 0.5 pH units from the set
value.
19
Page 23

3.9 Millivolt Mode
Absolute Millivolts
When this mode is selected the unit will display the actual voltage developed by the electrode when it
is immersed in a solution containing ions to which the electrode is sensitive.
The electrode may be a combination type or a suitable sensing/reference pair, depending on the
specific test being carried out.
pH, Redox and Ion Selective electrodes can all be used in this mode. Most of these determinations will
require the preparation of calibration curves or other analytical methods to enable the mV reading to
be converted to a concentration unit. For further information on these determinations refer to the
electrode instructions, which will normally give details of calibration solutions, interferences and the
limits of the methodology.
A very useful application of the Absolute mV range is for monitoring the performance of standard pH
electrodes. Using accurate and fresh buffers at a constant temperature, the millivolt output of the
electrode should be noted and compared to the theoretical ideal. As the electrode ages, becomes
contaminated or dirty, these values will drift, indicating that corrective action should be taken.
Recording these values as part of a routine Quality Control program can give a good indication of the
condition of the electrode.
Relative Millivolts
This mode is suitable for determinations using Redox and Ion Selective Electrodes and has the
additional benefit of being able to zero any offset voltage developed by the electrode in a blank solution,
i.e; a solution that has none of the ions to be measured, but has all the other characteristics of the
unknown samples. A blank solution would normally have its ionic strength and pH adjusted as required
for the electrode in use.
As the display is zeroed automatically when the Relative millivolt mode is selected, it is necessary to
immerse the electrode in the blank solution with the Absolute mV mode selected. When the reading
has stabilised the Relative mV mode should then be selected. The display will be set to zero, thereby
removing any offset voltage.
Sample measurement is then carried out by using a variety of well tried analytical methods; from
simple calibration curves through titrations, to single and multiple addition methods.
Select the mV mode using the Up/Down arrows which toggle between pH and mV modes.
The CAL/CLR key switches between Absolute and Relative mV. Relative mV is indicated by REL on
the display.
20
Page 24

3.10 Performing Measurements
To perform measurements in pH, mV or temperature modes the following should be carried out:
1. mV Measurement
a) Connect the electrode to the unit via the BNC socket on the rear panel. If a separate
reference electrode is to be used, this should be connected to the Ref socket.
b) Select mV mode using the Up/Down arrows. The display will show the electrode output
directly in mV.
2. Temperature Measurement (using ATC)
a) Connect the ATC probe to the unit via the Temp socket on the rear panel.
b) Select °C or °F via the Set up menu.
The secondary display will show ATC probe temperature directly in °C or °F.
3. Temperature Measurement (Manual)
a) If Manual temperature compensation is being used, the preferred measurement range
should be selected via the Set Up menu.
b) Immerse the electrode into the solution and set the to the solution temperature via the Set
Up menu.
4. pH Measurement
a) Perform a calibration sequence using manual or automatic temperature compensation.
b) Immerse the electrode(s) into the solution to be measured and note the results once the
reading has stabilised.
NOTE: Ensure the pH/Reference probe combination are compatible with the samples being
measured. Non-compatibility may be indicated by drifting readings, noise or shortened
electrode life. During use the electrode must be rinsed between each measurement to
eliminate contamination of solutions. Excess droplets of solution may be removed by
gently blotting with filter paper or tissue.
For further details refer to Section 3.5-Good Practice Guidelines.
21
Page 25

3.11 Status Page
The Status page displays the current calibration information.
If no valid calibration data is stored (e.g. after a reset or a failed calibration) the warning screen:
will be shown.
The status page will show the calibration data in the order it was carried out. Date, time,
temperature, mV and pH readings will be displayed for each buffer.
The Eo value and slope efficiency are displayed at the bottom of the calibration information.
22
Page 26

3.12 Results storage and display
The Model 3520 has a variety of options relating to the storage of data.
To initiate data logging the Data Log Event setting should be ENABLED.
By default this option is MANUAL.
The settings available are: Manual, Timed Interval, Endpoint Detection, Timed After Endpoint, Alarm
Set, Alarm Clear, Alarm Set & Clear and Disabled. Press the Up/Down arrows to cycle through
them.
Manual - logs results on pressing the STO key.
Timed Interval - logs on time and interval set by Data Log Interval in Hr:Min:Sec.
Endpoint Detection - logs data when the endpoint is detected.
Timed After Endpoint - logs at the Data Log Interval after the endpoint.
Alarm Set - data logs when the alarm set point is reached.
Alarm Clear - data logs when the alarm is cleared.
Alarm Set & Clear - data logs at the alarm set point and when the alarm condition clears.
Disabled - no data logging is possible when this option is selected.
The 3520 can log to either the memory or the printer. The memory can hold 500 data points which
can be accessed via the Results screen.
When the memory is full there are two options available regarding any additional logging. The
default is STOP.
Stop - when the memory is full no further data logging can occur until some
locations are deleted.
Overwrite overwrites data from the earlier storage point.
To prevent the accidental erasure of data the "Prompt Before Deleting" option can be either Enabled
or Disabled.
The function of the CLR key can also be set in this menu. The options define the use of the delete
key.
23
Page 27

Disabled - no manual deleting of results is possible.
Deletes Results Before - deletes all results before the selected data point.
Delete Results Since - deletes all results since the selected data points.
Deletes All Results - deletes all stored results.
3.12.1 Accessing Stored Results
To access results which have been stored use the Right arrow key to select results.
The most recently stored results will be displayed on the screen. If more results are stored than
can fit on a single screen then a series of data screens are available. The current screen and total
number of screens are shown in the bottom right hand corner of the screen.
Other screens of data can be accessed using the Up/Down arrow keys.
The CLR key will delete data according to the set mode.
If the PROMPT BEFORE DELETING warning is set then a second CLR is required to delete
results.
The PRINT key will print all stored data.
24
Page 28

3.13 GLP Functions
A variety of GLP functions are available via the GLP Setup menu.
A reminder that calibration is due can be set via the Cal Reminder option. Once this option is set a
valid calibration is required to use the 3520 after the time limit has elapsed. This option is
DISABLED by default.
The interval time is set in hours on this menu.
NOTE: A calibration should be performed immediately after the setting of this value to
reset the clock. This should be done as soon as set up is complete.
The on screen reminder can be accompanied by an audible alarm. This is set using the audible
alarm setting on this menu.
NOTE: The use of the Calibration Reminder feature will prevent the user from
performing measurements with the 3520 until a valid calibration is carried out.
The user and batch ID can be used to identify sets of samples and a specific user. This information
is printed when data is output to the printer. The batch number is also stored in the results memory.
3.13.1 Security
To control access to set up options and data manipulation functions a security code can be set
using this menu. When enabled a password is required to make any changes to the set up menus.
When the code is ENABLED a user can measure a sample, log data and calibrate the unit, but
cannot change settings within the set up screens until a valid password is entered.
25
Page 29

Section 4
Maintenance
4.1 General
The Model 3520 is designed to give optimum performance with minimum maintenance. It is only
necessary to keep the external surfaces clean and free from dust. To give added protection when
not in use the unit should be switched off and covered with the optional dust cover.
4.2 Cleaning/Re-conditioning of Combination Electrodes
For general purpose use, combination electrodes can be cleaned with a mild detergent solution or a
commercial glass cleaning solution (provided these are not strongly acidic). The electrode surface
should be wiped with a clean cloth soaked in the cleaning agent, and/or allow the membrane to
stand in the solution until clean. Rinse and repeat as necessary. Electrodes which have been
allowed to dry out should be soaked overnight in warm distilled water.
Table of Cleaning Agents for Glass Electrodes
Deposit
General deposits
Inorganic coatings
Metal compounds
Oil/Grease
Resins/Lignins
Proteins (blood, etc)
Stubborn deposits
Cleaning Agent
Genklene or mild detergent solution
Commercial glass cleaning solution (not strongly acidic)
Acid solution, not stronger than 1M
Complexing agent (EDTA) or suitable solvent
Acetone, alcohol or detergent (not strongly acidic)
Enzyme solutions (e.g; pepsin in 0.1M HCl)
Hydrogen peroxide, sodium hypochlorite or domestic bleach
Note: Solvents such as carbon tetrachloride, trichloroethylene, petroleum, ether, etc,
MUST NOT be used for cleaning electrodes that have a plastic body or a plastic protective
skirt.
26
352 050/REV A/03-03
Page 30

Section 5
Optional Accessories
5.1 Optional Accessories
The following list of items are available as optional accessories for use with the Model 3520:
060 406 Dust cover
037 701 IrDA printer supplied with roll of thermal paper, serial connection lead, power supply,
power connection lead (UK) and pouch
037 801 Interface cable kit
050 002 Serial communication software (3½” disk)
pH electrodes
924 001 General purpose, epoxy bodied combination, 12mm diameter. For liquids.
924 005 General purpose, glass bodied combination, 12mm diameter. For liquids.
For a complete listing of all available electrodes please contact your local distributor.
Buffer Solutions Redox Standards
025 163 2.00 pH buffer (500ml) 025 157 200mV @ 25°C (500ml)
025 037 4.00 pH buffer (500ml) 025 158 300mV @ 25°C (500ml)
025 038 7.00 pH buffer (500ml) 025 159 465mV @ 25°C (500ml)
025 162 9.22 pH buffer (500ml)
025 039 10.05 pH buffer (500ml)
025 179 pH 4 buffer sachets (pack of 10)
025 180 pH 7 buffer sachets (pack of 10)
025 181 pH 10 buffer sachets (pack of 10)
Miscellaneous
025 160 3M KCl Electrode Fill Solution (100ml)
025 161 Electrode Cleaning Solution (500ml)
5.2 Spares
924 005 pH combination electrode (glass bodied)
027 500 ATC probe
009 146 BNC shorting plug
037 702 Paper roll, thermal
021 030 UK 230V power supply
021 031 European 230V power supply
021 032 US 115V power supply
021 033 230V leaded power supply
352 050/REV A/03-0327
Page 31

Section 6
Interfacing
6.1 Analogue
All units are provided with 2 x 4mm sockets, marked as ANALOG OUT, on the rear panel. An analogue
output voltage of 1mV per least significant digit is available from these sockets. Recorder output
±2000mV, proportional to displayed reading:
1mV per 0.01pH (pH measurement and calibration modes)
1mV per 1mV (mV measurement mode)
6.2 RS232
The Bi-directional RS232 interface is available on the rear panel 9 way D type connector.
The connections are as follows:
DCD 1 - LINKED TO DTR AND DSR
RXD 2 - INPUT TO 3520
TXD 3 - OUTPUT FROM 3520
DTR 4 - LINKED TO DCD AND DSR
GND 5
DSR 6 - LINKED TO DCD AND DTR
RTS 7 - OUTPUT FROM 3520
CTS 8 - INPUT TO 3520
Suggested interconnections are detailed below:
3520 IBM PC XT (25 way “D”)
1 DCD DCD 8
2 RXD RXD 3
3 TXD TXD 2
4 DTR DTR 20
5 GND GND 7
6 DSR DSR 6
7 RTS RTS 4
8 CTS CTS 5
9
3520 IBM PC XT (9 way “D”)
1 DCD 1 DCD
2 RXD 2 RXD
3 TXD 3 TXD
4 DTR 4 DTR
5 GND 5 GND
6 DSR 6 DSR
7 RTS 7 RTS
8 CTS 8 CTS
9 9
NOTE: Interface Cable (Order Code: 013 203) is required.
28
352 050/REV A/03-03
Page 32

Interfacing (continued)
The RS232 communications parameters on the computer or printer need to be set to match those of
the Model 3520, as detailed below:
1200 Baud 9600 Baud
7 Data Bits 8 data bits
Odd Parity OR No parity
1 Stop Bit 1 stop bit
Setting of these options is detailed in Section 3.6.6.
The Model 3520 supports both hardware (CTS/RTS) flow control and software XON/XOFF flow con-
trol.
Pressing the PRINT key outputs from the RS232 interface.
Sending an ASCII “D” to the 3520 causes a printout of the current displayed reading plus sample
number.
Sending an ASCII “C” causes a printout of the last calibration parameters.
Sending an ASCII "P" causes a printout of the stored readings.
6.3 Keypad Emulation
Keypad remote control using RS232 interface:
7 - Instrument On / Standby / Escape
1 - Calibrate / Memory Clear
9 - Print
8 - Up Arrow
2 - Down Arrow
4 - Left Arrow
6 - Right Arrow
3 or 5 - Enter / Store
29
352 050/REV A/03-03
Page 33

6.4 Printing
A 32 column serial printer (037 701) is available for use with the Model 3520.
There are two methods of connecting the serial printer to the Model 3520:
a) IrDA - the IrDA interface is a line of sight, wireless communication protocol. The IrDA sensor
(located on the front left hand corner of the printer) should be in line with the Ir window on the side of
the 3520. The Ir icon on the symbol display indicates whether the units are attempting to connect
(single icon flashing) or connected (two icons).
b) Connect serial cable supplied with the printer to the 9 way socket located on the rear panel of the
instrument.
To intiate a print out of data press the print key.
When the first print is performed a header section will be printed showing:
Instrument name
Time and Date
Spacing for entry of Operator & User ID
Operator ID number
Most recent calibration information
Eo value
Slope efficiency
Buffer type
This will be followed by results data in either pH or mV dependent on mode selected. Details will
also be given on temperature. Time and date of the stored readings will be displayed.
An asterisk (*) indicates that manual temperature compensation is being used.
A reading in the Relative mV mode will be indicated by an R.
Each reading will be identified by a batch number.
A calibration will reset the printout and the header information will be re-printed.
To obtain a print out of stored readings, enter the RESULTS MODE and press the print key. A print
out of all filled memory locations will then be generated.
6.5 Alarm Outputs
The 3520 provides two alarm outputs. These can be accessed using the two 4mm connectors on
the rear of the 3520. They are open collector outputs. To set the alarm limts at which these are
activated, please refer to section 3.6.4.
To use the alarm outputs, they should be ENABLED in the Alarms Setup screen see section 3.6.4
They will operate in both pH measurement and absolute millivolts. The alarm outputs will remain
active until the alarm condition is no longer evident or the alarm limits are reset in the Alarm Set up
menu.
For the Hi alarm output please use the Red 4mm phone connector.
For the Low alarm output please use the Black 4mm Phono connector.
For further information please contact your local distributor or the manufacturer.
30
352 050/REV A/03-03
Page 34

Section 7
Troubleshootiing and functional checks
7.1 Troubleshooting
Fault Possible Cause Action
No display Check power supply Check that correct 9V ac power supply
is connected and switched on.
Erratic display Check power supply Unit must be used with supplied 9V
acpower supply. Usage of other units will
cause the 3520 not to operate.
Drifting erratic readings Electrode fault Use BNC cap to test 3520 (see 7.2)
Replace electrode.
Cannot calibrate Electrode Fault Use BNC cap to test 3520 (see 7.2)
Replace electrode.
ERROR EO ... Buffer problem Use freshly prepared buffers.
ERROR SLOPE .... Electrode problem Use BNC cap to test 3520 (see 7.2)
Replace electrode.
Unable to recognise Using correct buffer set Is the buffer type correct? Use AUTO for
buffers Jenway supplied buffers.
Replace buffers
Use BNC cap to test 3520 (see 7.2)
Replace electrode.
Temperature readings Temp probe faulty Check 3520 using section 7.2
fluctuating Replace temperature probe.
Temperature readings Temp probe faulty Check 3520 using section 7.2
incorrect Replace temperature probe.
Manual temp not set Set meter to read °C and set
temperature against a calibrated
thermometer.
Will not print IrDA Connection Line up units or alternatively use
broken supplied RS232 connector.
Paper out The feed light on the printer will flash if
the unit requires paper.
Battery flat Connect ac power supply.
If the above does not answer your query try the FAQ section on the www.Jenway.com Website.
31
352 050/REV A/03-03
Page 35

7.2 Functional check
The measurement function of the meter can be checked using the enclosed BNC shorting cap
(009 146).
1) Remove the ATC probe if connected.
2) Set Manual temperature compensation to 25°C.
3) Remove pH probe and replace with BNC shorting cap.
4) Select mV mode the display should read ±1.
If the mV reading is greater than ±1mV perform a reset (refer Section 7.3).
To make measurements from this point refit the ATC probe and pH probe and calibrate the 3520
using fresh buffer solutions (see section 3.6).
Temperature input check.
Remove the temperature probe and apply a 10Kohm resistor across the pins of the temp input as
described in fig 7.2.1
Fig 7.2.1 Temperature input with connection detail
7.3 Reset Procedure
NOTE: Performing a reset will return all options to the default values. It will not delete
stored data.
1. Remove AC power connector from the rear panel socket.
2. Press and hold the STO key.
3. Replace the AC power connector into the rear panel socket. The secondary display will
momentarily show E2 RST.
4. If this does not resolve the problem please contact the manufacturer or your local distributor.
352 050/REV A/03-0332
Page 36

EC Declaration of Conformity
Jenway Model 3520 pH/mV/Temperature Meter complies with the following European Standards:
EN 50081-1:1992 Electromagnetic compatibility – Generic emission standard
EN 50082-1:1992 Electromagnetic compatibility – Generic immunity standard
EN 61010-1:2001 Safety requirements for electrical equipment for measurement, control and
laboratory use
Following the provision of:
EMC Directive – 89/336/EEC and Low Voltage Directive – 73/23/EEC
Martyn J. Fall
Managing Director, Jenway
Gransmore Green, Felsted, Dunmow,
Essex, CM6 3LB, England
33
352 050/REV A/03-03
 Loading...
Loading...