Hach DR2010 User manual

49300-22
DR/2010
SPECTROPHOTOMETER
PROCEDURES MANUAL
© Hach Company, 1996–2000. All rights reserved. Printed in the U.S.A. |
ap/dk 12/99 7ed |
|
jnb/dk 10/00 rev2 |
2

TABLE OF CONTENTS |
|
INTRODUCTION .............................................................................................................. |
11 |
Sample Procedure Explained .................................................................................................. |
13 |
SECTION I CHEMICAL ANALYSIS INFORMATION......................................... |
17 |
Abbreviations .......................................................................................................................... |
17 |
Converting Chemical Species ................................................................................................. |
18 |
Hardness Conversion ........................................................................................................... |
19 |
Dissolved Oxygen................................................................................................................ |
20 |
Sample Collection, Preservation and Storage...................................................................... |
22 |
Collecting Water Samples ................................................................................................ |
25 |
Acid Washing Bottles ....................................................................................................... |
25 |
Correcting for Volume Additions ........................................................................................ |
26 |
Boiling Aids ......................................................................................................................... |
26 |
Sample Filtration.................................................................................................................. |
27 |
Temperature Considerations ................................................................................................ |
29 |
Sample Dilution Techniques................................................................................................ |
29 |
Sample Dilution and Interfering Substances .................................................................... |
30 |
Using Pipets and Graduated Cylinders ................................................................................ |
31 |
Using the TenSette Pipet...................................................................................................... |
32 |
Operating the TenSette Pipet ............................................................................................ |
32 |
Mixing Water Samples ........................................................................................................ |
33 |
Using Sample Cells.............................................................................................................. |
35 |
Orientation of Sample Cells.............................................................................................. |
35 |
Care of Hach 1-inch Sample Cells.................................................................................... |
35 |
Cleaning Sample Cells...................................................................................................... |
35 |
Sample Cell Matching ...................................................................................................... |
36 |
Volume Measurement Accuracy ...................................................................................... |
37 |
Using AccuVac Ampuls ...................................................................................................... |
37 |
Using Reagent Powder Pillows............................................................................................ |
38 |
Using PermaChem Pillows .................................................................................................. |
39 |
Using the Pour-Thru Cell..................................................................................................... |
39 |
Reagent and Standard Stability............................................................................................ |
40 |
Interferences ............................................................................................................................ |
41 |
pH Interference .................................................................................................................... |
41 |
Accuracy and Precision........................................................................................................... |
42 |
Standard Additions............................................................................................................... |
43 |
3

TABLE OF CONTENTS, continued |
|
Method Performance............................................................................................................... |
50 |
Estimated Detection Limit................................................................................................... |
50 |
Precision .............................................................................................................................. |
53 |
Estimating Precision......................................................................................................... |
53 |
Selecting the Best Wavelength ............................................................................................... |
54 |
Adapting HACH Procedures to Other Spectrophotometers................................................... |
57 |
Preparing a Calibration Curve ............................................................................................. |
57 |
%T Versus Concentration Calibration ............................................................................. |
57 |
Absorbance Versus Concentration Calibration ................................................................ |
59 |
USEPA Approved and Accepted Definitions......................................................................... |
60 |
SECTION II SAMPLE PRETREATMENT ............................................................... |
61 |
Digestion................................................................................................................................. |
61 |
EPA Mild Digestion with Hot Plate for Metals Analysis Only........................................... |
61 |
EPA Vigorous Digestion with Hot Plate for Metals Analysis Only ................................... |
62 |
General Digesdahl Digestion (Not USEPA accepted) ........................................................ |
63 |
Distillation .............................................................................................................................. |
63 |
SECTION III WASTE MANAGEMENT AND SAFETY........................................ |
65 |
Waste Management................................................................................................................. |
65 |
Waste Minimization ............................................................................................................ |
65 |
Regulatory Overview........................................................................................................... |
65 |
Hazardous Waste Definition................................................................................................ |
66 |
Characteristic Hazardous Waste Codes............................................................................... |
67 |
How to Determine if Waste is Hazardous ........................................................................... |
67 |
Examples of Hazardous Waste ............................................................................................ |
68 |
Hazardous Waste Disposal .................................................................................................. |
68 |
Management of Specific Wastes ......................................................................................... |
69 |
Special Considerations for Cyanide Containing Materials .............................................. |
69 |
Resources............................................................................................................................. |
70 |
Material Safety Data Sheets.................................................................................................... |
71 |
How to Obtain an MSDS..................................................................................................... |
71 |
Sections of an MSDS........................................................................................................... |
72 |
4

TABLE OF CONTENTS, continued |
|
Safety....................................................................................................................................... |
74 |
Material Safety Data Sheet .................................................................................................. |
74 |
Reading Labels Carefully .................................................................................................... |
74 |
Protective Equipment........................................................................................................... |
75 |
First Aid Equipment and Supplies ....................................................................................... |
75 |
General Safety Rules............................................................................................................ |
75 |
OSHA Chemical Hygiene Plan............................................................................................ |
76 |
SECTION IV PROCEDURES ........................................................................................ |
77 |
ALUMINUM, Aluminon Method........................................................................................... |
79 |
ALUMINUM, Eriochrome Cyanine R Method ...................................................................... |
87 |
ARSENIC, Silver Diethyldithiocarbamate Method ................................................................ |
95 |
BARIUM, Turbidimetric Method ......................................................................................... |
103 |
BENZOTRIAZOLE, UV Photolysis Method ....................................................................... |
111 |
BORON, Carmine Method.................................................................................................... |
117 |
BORON, Low Range, Azomethine-H Method .................................................................... |
121 |
BROMINE, DPD Method ..................................................................................................... |
129 |
CADMIUM, Dithizone Method............................................................................................ |
137 |
CHLORIDE, Mercuric Thiocyanate Method........................................................................ |
145 |
CHLORINE, FREE, DPD Method ....................................................................................... |
149 |
CHLORINE, FREE, DPD Rapid Liquid Method ................................................................ |
157 |
CHLORINE, FREE, HIGH RANGE, DPD Method............................................................. |
163 |
CHLORINE, FREE, DPD Test ‘N Tube™ Method ............................................................. |
169 |
CHLORINE, TOTAL, Ultra Low Range, DPD Method ...................................................... |
175 |
CHLORINE, TOTAL, Ultra Low Range, DPD Method ..................................................... |
183 |
CHLORINE, TOTAL, DPD Method .................................................................................... |
191 |
CHLORINE, TOTAL, DPD Rapid Liquid Method ............................................................. |
199 |
CHLORINE, TOTAL, HIGH RANGE, DPD Method ........................................................ |
205 |
CHLORINE, TOTAL, DPD Test ‘N Tube™ Method.......................................................... |
211 |
CHLORINE DIOXIDE, LR, Chlorophenol Red Method ..................................................... |
217 |
CHLORINE DIOXIDE, HR, Direct Reading Method.......................................................... |
221 |
CHLORINE DIOXIDE, DPD Method.................................................................................. |
223 |
5

TABLE OF CONTENTS, continued |
|
CHROMIUM, HEXAVALENT, 1,5-Diphenylcarbohydrazide Method.............................. |
233 |
CHROMIUM, TOTAL, Alkaline Hypobromite Oxidation Method ................................... |
239 |
COBALT, 1-(2-Pyridylazo)-2-Naphthol (PAN) Method ..................................................... |
245 |
COLOR, NCASI 253, Platinum-Cobalt Method.................................................................. |
249 |
COLOR, TRUE AND APPARENT, APHA Platinum-Cobalt Standard Method ............... |
253 |
COPPER, Bicinchoninate Method........................................................................................ |
257 |
COPPER, Porphyrin Method................................................................................................ |
265 |
COPPER, AUTOCATALYTIC, Colorimetric Method........................................................ |
271 |
CYANIDE, Pyridine-Pyrazalone Method ............................................................................ |
277 |
CYANURIC ACID, Turbidimetric Method ......................................................................... |
287 |
FLUORIDE, SPADNS Method............................................................................................ |
291 |
FORMALDEHYDE, MBTH Method .................................................................................. |
299 |
HARDNESS, Calcium and Magnesium; Calmagite Colorimetric Method.......................... |
303 |
HARDNESS, TOTAL, Ultra Low Range, Calcium and Magnesium Chlorophosphonazo |
|
Colorimetric Method ......................................................................................................... |
309 |
HARDNESS, TOTAL, Ultra Low Range, Calcium and Magnesium; Chlorophosphonazo |
|
Rapid Liquid Method ........................................................................................................ |
313 |
HYDRAZINE, p-Dimethylaminobenzaldehyde Method ..................................................... |
319 |
IODINE, DPD Method ......................................................................................................... |
325 |
IRON, FerroZine Method ..................................................................................................... |
333 |
IRON, FerroZine Rapid Liquid Method ............................................................................... |
339 |
IRON, FERROUS, 1,10 Phenanthroline Method ................................................................. |
345 |
IRON, TOTAL, FerroMo™ Method .................................................................................... |
349 |
IRON, TOTAL, FerroVer Method ....................................................................................... |
353 |
IRON, TOTAL, TPTZ Method............................................................................................. |
361 |
LEAD, Dithizone Method..................................................................................................... |
369 |
LEAD, LeadTrak™ Fast Column Extraction Method.......................................................... |
377 |
MANGANESE, HR, Periodate Oxidation Method .............................................................. |
387 |
MANGANESE, LR, PAN Method....................................................................................... |
391 |
MERCURY, Cold Vapor Mercury Concentration Method .................................................. |
397 |
MOLYBDENUM, MOLYBDATE, HR, Mercaptoacetic Acid Method.............................. |
413 |
MOLYBDENUM, MOLYBDATE, LR, Ternary Complex Method ................................... |
421 |
6

TABLE OF CONTENTS, continued |
|
NICKEL, 1-(2 Pyridylazo)-2-Naphthol (PAN) Method ....................................................... |
427 |
NICKEL, Heptoxime Method ............................................................................................... |
433 |
NICKEL, AUTOCATALYTIC, Photometric Method ......................................................... |
439 |
NITRATE, LR, Cadmium Reduction Method ...................................................................... |
443 |
NITRATE, MR, Cadmium Reduction Method..................................................................... |
449 |
NITRATE, HR, Cadmium Reduction Method...................................................................... |
457 |
NITRATE, HR, Chromotropic Acid Method, Test ‘N Tube™ ............................................ |
465 |
NITRITE, LR, Diazotization Method ................................................................................... |
471 |
NITRITE, LR, Diazotization, NED Rapid Liquid Method................................................... |
477 |
NITRITE, LR, Test ‘N Tube, Diazotization (Chromotropic Acid) Method ......................... |
483 |
NITRITE, HR, Ferrous Sulfate Method................................................................................ |
487 |
NITROGEN, TOTAL, HR, Test ’N Tube™, TNT Persulfate Digestion Method................ |
491 |
NITROGEN, AMMONIA, Nessler Method ......................................................................... |
499 |
NITROGEN, AMMONIA, Salicylate Method ..................................................................... |
505 |
NITROGEN, AMMONIA, High Range, Test ’N Tube, Salicylate Method......................... |
511 |
NITROGEN, AMMONIA, Low Range Test ‘N Tube, Salicylate Method .......................... |
517 |
NITROGEN, MONOCHLORAMINE and FREE AMMONIA, Salicylate Method............ |
523 |
NITROGEN, TOTAL, Test ’N Tube, TNT Persulfate Digestion Method ........................... |
531 |
NITROGEN, TOTAL KJELDAHL, Nessler Method .......................................................... |
539 |
NITROGEN, TOTAL INORGANIC, Test ‘N Tube, Titanium Trichloride Reduction ....... |
549 |
ORGANIC CARBON, TOTAL, Low Range, Direct Method.............................................. |
557 |
ORGANIC CARBON, TOTAL, High Range, Direct Method ............................................. |
565 |
ORGANIC MATTER, Dichromate Method......................................................................... |
573 |
OXYGEN, DISSOLVED, LR, Indigo Carmine Method ...................................................... |
579 |
OXYGEN, DISSOLVED, HR, HRDO Method ................................................................... |
583 |
OXYGEN, DISSOLVED, SHR, Super High Range Method ............................................... |
587 |
OXYGEN DEMAND, CHEMICAL, Reactor Digestion Method ........................................ |
591 |
Colorimetric Determination, 0 to 150 mg/L COD............................................................. |
593 |
Colorimetric Determination, 0 to 1,500 and 0 to 15,000 mg/L COD................................ |
595 |
OXYGEN DEMAND, CHEMICAL (COD), Dichromate Reflux Method .......................... |
601 |
Colorimetric Determination ............................................................................................... |
603 |
Buret Titration.................................................................................................................... |
605 |
OXYGEN DEMAND, CHEMICAL, Manganese III Digestion Method ............................. |
611 |
7

TABLE OF CONTENTS, continued |
|
OXYGEN DEMAND, CHEMICAL, Manganese III Digestion Method............................. |
615 |
OXYGEN SCAVENGERS, Iron Reduction Method for Oxygen Scavengers .................... |
623 |
OZONE, Indigo Method ....................................................................................................... |
627 |
PALLADIUM, N,N'-Dimethyldithiooxamide Method ........................................................ |
631 |
PCB IN SOIL, Immunoassay Method .................................................................................. |
635 |
PHENOLS, 4-Aminoantipyrine Method .............................................................................. |
645 |
PHOSPHONATES, Persulfate UV Oxidation Method ........................................................ |
651 |
PHOSPHORUS, REACTIVE, PhosVer 3 Method, Test ’N Tube Procedure...................... |
657 |
PHOSPHORUS, REACTIVE, (Also called Orthophosphate) Amino Acid Method ........... |
663 |
PHOSPHORUS, REACTIVE, (Also called Orthophosphate) Molybdovanadate Method .. |
669 |
PHOSPHORUS, REACTIVE, PhosVer 3 (Ascorbic Acid) Method.................................... |
677 |
PHOSPHORUS, REACTIVE, LOW RANGE, Ascorbic Acid Rapid Liquid Method........ |
685 |
PHOSPHORUS, REACTIVE, HIGH RANGE, Molybdovanadate Rapid Liquid Method . 691 |
|
PHOSPHORUS, REACTIVE, HR, Molybdovanadate Method, Test ’N Tube™ ............... |
697 |
PHOSPHORUS, TOTAL, Acid Persulfate Digestion Method............................................. |
703 |
PHOSPHORUS, TOTAL, PhosVer 3 with Acid Persulfate Digestion Test ‘N Tube.......... |
707 |
PHOSPHORUS, TOTAL, HR, Molybdovanadate Method with Acid Persulfate Digestion, |
|
Test ’N Tube™.................................................................................................................. |
715 |
PHOSPHORUS, ACID HYDROLYZABLE, PhosVer3 with Acid Hydrolysis, ................. 723 |
|
Test ’N Tube...................................................................................................................... |
723 |
PHOSPHORUS, ACID HYDROLYZABLE, Hydrolysis to Orthophosphate Method........ |
729 |
POLYACRYLIC ACID, Absorption-Colorimetric Method ................................................ |
733 |
POTASSIUM, Tetraphenylborate Method........................................................................... |
741 |
QUATERNARY AMMONIUM COMPOUNDS, Direct Binary Complex Method ........... |
747 |
SELENIUM, Diaminobenzidine Method ............................................................................. |
753 |
SILICA, HR, Silicomolybdate Method ................................................................................ |
761 |
SILICA, LR, Heteropoly Blue Method ................................................................................ |
767 |
SILICA, ULTRA LOW RANGE, Heteropoly Blue Method ............................................... |
773 |
SILICA, ULR, Heteropoly Blue Rapid Liquid Method ....................................................... |
779 |
SILVER, Colorimetric Method............................................................................................. |
785 |
SODIUM CHROMATE, Direct Colorimetric Method ........................................................ |
791 |
8

TABLE OF CONTENTS, continued |
|
SULFATE, SulfaVer 4 Method ............................................................................................ |
795 |
SULFIDE, Methylene Blue Method* ................................................................................... |
803 |
SURFACTANTS, ANIONIC, Crystal Violet Method.......................................................... |
807 |
SUSPENDED SOLIDS, Photometric Method...................................................................... |
811 |
TANNIN AND LIGNIN, Tyrosine Method ......................................................................... |
815 |
THM Plus™: Trihalomethanes, ........................................................................................... 819 |
|
TOXTRAK TOXICITY TEST, Colorimetric Method ......................................................... |
829 |
TPH IN SOIL, Immunoassay Method................................................................................... |
835 |
TPH IN WATER, Immunoassay Method ............................................................................. |
845 |
TURBIDITY, Attenuated Radiation Method (direct reading) .............................................. |
853 |
VOLATILE ACIDS, Esterification Method ......................................................................... |
857 |
ZINC, Zincon Method........................................................................................................... |
861 |
SECTION V GENERAL INFORMATION ............................................................... |
867 |
HOW TO ORDER............................................................................................................ |
869 |
REPAIR SERVICE .......................................................................................................... |
870 |
WARRANTY ..................................................................................................................... |
871 |
9
10

INTRODUCTION
This manual is divided into five sections:
Section I Chemical Analysis Information
This section applies to all the procedures. It provides background information and reference/review material for the technician or chemist. Commonly used techniques are explained in detail.
Section II Sample Pretreatment
This section provides a brief overview of sample pretreatment and three digestion procedures. Two are USEPA digestions. The Hach Digesdahl method is also included.
Section III Waste Management and Safety
Section 3 includes information an waste management, regulations, waste disposal and resources on waste management. The Safety portion covers reading an MSDS and general safety guidelines.
Section IV Procedures
Section 4 contains step-by-step illustrated instructions for measuring over 120 parameters. The steps also include helpful notes. Each procedure contains information on sample collection, storage and preservation, accuracy checks, possible interferences, summary of method and a list of the reagents and apparatus necessary to run the test.
Section V Ordering Information
This section provides information needed for ordering, shipping, return of items and Hach trademarks.
Before attempting the analysis procedures the analyst should read the instrument manual to learn about the spectrophotometer’s features and operation.
11

INTRODUCTION, continued
Hach Company Trademarks
AccuGrow® |
H2O University™ |
|
AccuVac® |
H2OU™ |
|
AccuVer™ |
Hach Logo® |
|
AccuVial™ |
Hach One® |
|
Add-A-Test™ |
Hach Oval® |
|
AgriTrak™ |
Hach.com™ |
|
AluVer® |
HachLink™ |
|
AmVer™ |
Hawkeye The Hach Guy™ |
|
APA 6000™ |
HexaVer® |
|
AquaChek™ |
HgEx™ |
|
AquaTrend® |
HydraVer® |
|
BariVer® |
ICE-PIC™ |
|
BODTrak™ |
IncuTrol® |
|
BoroTrace™ |
Just Add Water™ |
|
BoroVer® |
LeadTrak® |
|
C. Moore Green™ |
M-ColiBlue24® |
|
CA 610™ |
ManVer® |
|
CalVer® |
MolyVer® |
|
ChromaVer® |
Mug-O-Meter® |
|
ColorQuik® |
NetSketcher™ |
|
CoolTrak® |
NitraVer® |
|
CuVer® |
NitriVer® |
|
CyaniVer® |
NTrak® |
|
Digesdahl® |
OASIS™ |
|
DithiVer® |
On Site Analysis. |
|
Dr. F. Fluent™ |
Results You Can TrustSM |
|
OptiQuant™ |
||
Dr. H. Tueau™ |
||
OriFlow™ |
||
DR/Check™ |
||
OxyVer™ |
||
EC 310™ |
||
PathoScreen™ |
||
FerroMo® |
||
PbEx® |
||
FerroVer® |
||
PermaChem® |
||
FerroZine® |
||
PhosVer® |
||
FilterTrak™ 660 |
||
Pocket Colorimeter™ |
||
Formula 2533™ |
||
Pocket Pal™ |
||
Formula 2589™ |
||
Pocket Turbidimeter™ |
||
Gelex® |
||
|
Pond In Pillow™
PourRite®
PrepTab™
ProNetic™
Pump Colorimeter™
QuanTab®
Rapid Liquid™
RapidSilver™ Ratio™ RoVer® sension™
Simply AccurateSM
SINGLET™
SofChek™
SoilSYS™
SP 510™
Specê
StablCal®
StannaVer®
SteriChek™
StillVer®
SulfaVer®
Surface Scatter®
TanniVer®
TenSette®
Test ‘N Tube™
TestYES!SM
TitraStir®
TitraVer®
ToxTrak™
UniVer®
VIScreen™
Voluette®
WasteAway™
ZincoVer®
12
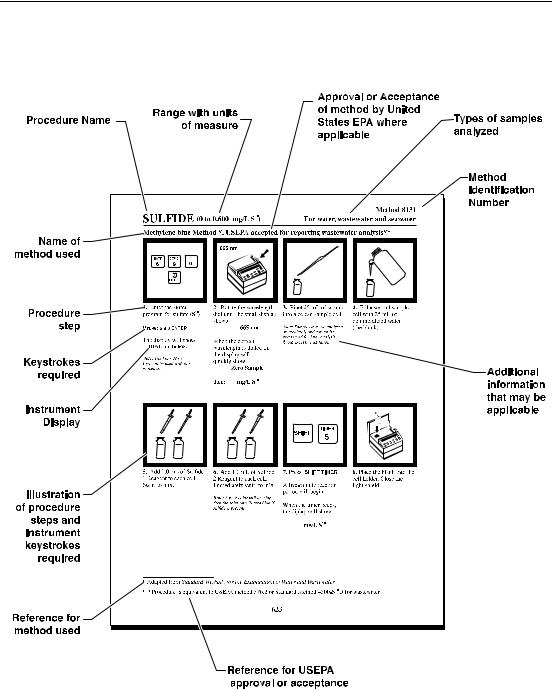
Sample Procedure Explained
13
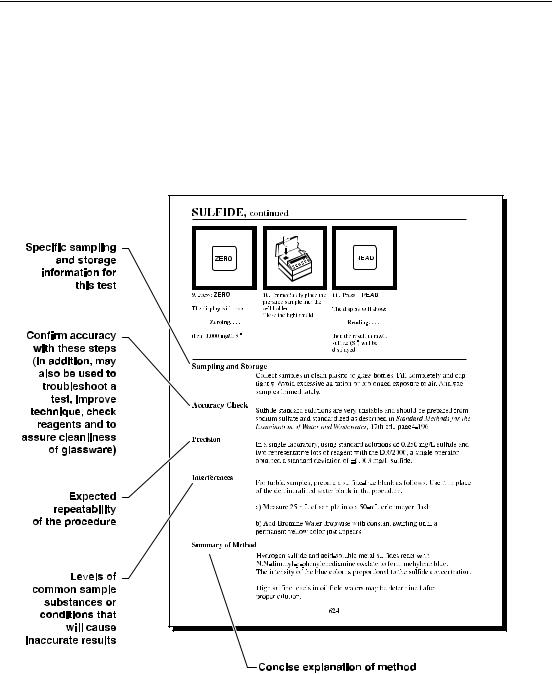
Sample Procedure Explained, continued
14

Sample Procedure Explained, continued
15
16

SECTION I CHEMICAL ANALYSIS INFORMATION
Abbreviations
The following abbreviations are used throughout the text of the procedure section:
Abbrev- |
Definition |
Abbrev- |
Definition |
|
iation |
iation |
|||
|
|
|||
|
|
|
|
|
°C |
degree(s) Celsius (Centigrade) |
HR |
high range |
|
|
|
|
|
|
°F |
degree(s) Fahrenheit |
kg/ha |
kilograms per hectare |
|
|
|
|
|
|
ACS |
American Chemical Society reagent |
l or L |
Liter. Volume equal to one cubic |
|
grade purity |
decimeter (dm3) |
|||
|
|
|||
|
Standard Methods for the Examination of |
lbs/Ac |
pounds per acre |
|
|
Water and Wastewater, published jointly by |
|
|
|
|
LR |
low range |
||
|
the American Public Health Association |
|||
|
|
|
||
|
MDL |
Method detection limit |
||
|
(APHA), the American Water Works |
|||
APHA |
Association (AWWA), and the Water |
MDB |
marked dropping bottle |
|
Environment Federation (WEF). Order from |
|
|
||
Standard |
mg/L |
milligrams per liter (ppm) |
||
Hach requesting Cat. No. 22708-00 or from |
||||
Methods |
|
|
||
the Publication Office of the American Public |
µg/L |
micrograms per liter (ppb) |
||
|
||||
|
Health Association. This book is the |
|
|
|
|
|
(milliliter)-approximately the same as a |
||
|
standard reference work for water analysis. |
|
||
|
ml or mL |
cubic centimeter (cc) or 1/1000 of a liter. |
||
|
Many procedures contained in this manual |
|||
|
|
Also known as a “cc”. |
||
|
are based on Standard Methods. |
|
||
|
|
|
||
|
|
|
|
|
AV |
AccuVac |
MR |
medium range |
|
|
|
|
|
|
Bicn |
bicinchoninate |
NIPDWR |
National Interim Primary Drinking |
|
Water Regulations |
||||
|
|
|
||
|
|
|
|
|
CFR |
Code of Federal Regulations |
NPDES |
National Pollutant Discharge |
|
Elimination System |
||||
|
|
|
||
|
|
|
|
|
conc |
concentrated |
P |
phosphorus |
|
|
|
|
|
|
DB |
dropping bottle |
PCB |
Poly chlorinated biphenyl |
|
|
|
|
|
|
EDL |
Estimated detection limit |
PV |
PhosVer® |
|
F&T |
free and total |
RL |
Rapid Liquid™ |
|
|
|
|
|
|
|
Formazin Attenuation Units. Turbidity unit |
|
|
|
FAU |
of measure based on a Formazin stock |
SCDB |
self-contained dropping bottle |
|
|
suspension. |
|
|
|
|
|
|
|
|
FM |
FerroMo® |
TNT |
Test ‘N Tube™ |
|
FV |
FerroVer® |
TPH |
Total petroleum hydrocarbons |
|
FZ |
FerroZine® |
TPTZ |
(2,4,6-Tri-(2-Pyridyl)-1,3,5-Triazine) |
|
g |
grams |
ULR |
Ultra low range |
|
|
|
|
|
|
gr/gal |
grains per gallon (1 gr/gal = 17.12 mg/L) |
USEPA |
United States Environmental |
|
Protection Agency |
||||
|
|
|
||
|
|
|
|
17

SECTION I, continued
Converting Chemical Species
Species conversion factors for many commonly used substances are preprogrammed into the DR/2010 (see Table 1). Conversions are method specific and are viewable after taking the reading by pressing CONC.
|
|
|
Table 1 |
Conversion Factors |
|
|
|
|
|
|
|
||
To Convert From... |
To... |
|
Multiply By... |
Conversion used in program # |
||
|
|
|
|
|
|
|
mg/L Al |
|
|
mg/L Al2O3 |
|
1.8895 |
9, 10 |
mg/L B |
|
|
mg/L H3BO3 |
|
5.7 |
45 |
mg/L Ca-CaCO3 |
mg/L Ca |
|
0.4004 |
220 |
||
mg/L CaCO3 |
|
mg/L Ca |
|
0.4004 |
227 |
|
mg/L CaCO3 |
|
mg/L Mg |
|
0.2428 |
227 |
|
µg/L Carbo. |
|
µg/L Hydro. |
|
1.92 |
182 |
|
|
|
|
|
|
|
|
µg/L Carbo. |
|
µg/L ISA |
|
2.69 |
182 |
|
|
|
|
|
|
|
|
µg/L Carbo. |
|
µg/L MEKO |
|
3.15 |
182 |
|
|
|
|
|
|
|
|
mg/L Cr6+ |
|
mg/L CrO42- |
|
2.231 |
90, 95 |
|
mg/L Cr6+ |
|
mg/L Na2CrO4 |
|
3.115 |
90, 95 |
|
mg/L Mg-CaCO3 |
mg/L Mg |
|
0.2428 |
225 |
||
mg/L Mn |
|
|
mg/L KMnO4 |
|
2.876 |
290, 295 |
mg/L Mn |
|
|
mg/L MnO4- |
|
2.165 |
290, 295 |
mg/L Mo6+ |
|
mg/L MoO42- |
|
1.667 |
315, 320, 322 |
|
mg/L Mo6+ |
|
mg/L Na2MoO4 |
|
2.146 |
315, 320, 322 |
|
mg/L N |
|
|
mg/L NH3 |
|
1.216 |
342, 343, 346, 347, 348 |
mg/L N |
|
|
mg/L NO3- |
|
4.427 |
346, 347, 348 |
mg/L Na |
CrO |
4 |
mg/L Cr6+ |
|
0.321 |
670 |
2 |
|
|
|
|
|
|
mg/L Na |
CrO |
4 |
mg/L CrO 2- |
|
0.72 |
670 |
2 |
|
4 |
|
|
|
|
mg/L NH2Cl-N |
mg/L Cl2 |
|
5.0623 |
386 |
||
mg/L NH2Cl-N |
mg/L NH2Cl |
|
3.6750 |
386 |
||
mg/L NH3-N |
|
mg/L NH3 |
|
1.216 |
380, 385, 387 |
|
mg/L NH |
-N |
|
mg/L NH + |
|
1.288 |
380, 385, 387 |
3 |
|
4 |
|
|
|
|
mg/L NO2- |
|
mg/L NaNO2 |
|
1.5 |
373 |
|
mg/L NO2- |
|
mg/L NO2--N |
|
0.3045 |
373 |
|
mg/L NO2--N |
|
mg/L NaNO2 |
|
4.926 |
345, 371, 375 |
|
µg/L NO2--N |
|
µg/L NaNO2 |
|
4.926 |
376 |
|
mg/L NO2--N |
|
mg/L NO2- |
|
3.284 |
345, 371, 375 |
|
µg/L NO2--N |
|
µg/L NO2- |
|
3.284 |
376 |
|
mg/L NO3--N |
|
mg/L NO3- |
|
4.427 |
344, 351, 353, 355, 359, 361 |
|
mg/L PO43- |
|
mg/L P |
|
0.3261 |
480, 482, 485, 490, 492, 535 |
|
µg/L PO43- |
|
µg/L P |
|
0.3261 |
488 |
|
mg/L PO43- |
|
mg/L P2O5 |
|
0.7473 |
480, 482, 485, 490, 492, 535 |
|
µg/L PO43- |
|
µg/L P2O5 |
|
0.7473 |
488 |
|
mg/L SiO2 |
|
mg/L Si |
|
0.4674 |
651, 656 |
|
µg/L SiO2 |
|
µg/L Si |
|
0.4674 |
645 |
|
18

SECTION I, continued
Hardness Conversion
Table 2 lists the factors for converting one unit of measure for hardness to another unit of measure. For example, to convert mg/L CaCO3 to German parts/100,000 CaO, multiply the value in mg/L x 0.056.
Table 2 Hardness Conversion Factors
|
|
British |
American |
French |
German |
|
|
|
|
Units of |
mg/L |
gr/gal |
parts/ |
Parts/ |
meq/L1 |
|
lbs./cu ft |
||
gr/gal (US) |
g/L CaO |
||||||||
Measure |
CaCO3 |
(Imperial) |
100,000 |
100,000 |
CaCO3 |
||||
CaCO3 |
|
|
|||||||
|
|
CaCO3 |
CaCO3 |
CaO |
|
|
|
||
|
|
|
|
|
|
||||
mg/L |
1.0 |
0.07 |
0.058 |
0.1 |
0.056 |
0.02 |
5.6x10-4 |
6.23x10-5 |
|
CaCO3 |
|
|
|
|
|
|
|
|
|
English |
14.3 |
1.0 |
0.83 |
1.43 |
0.83 |
0.286 |
8.0x10-3 |
8.9x10-4 |
|
gr/gal |
|
|
|
|
|
|
|
|
|
CaCO3 |
|
|
|
|
|
|
|
|
|
US gr/gal |
17.1 |
1.2 |
1.0 |
1.72 |
0.96 |
0.343 |
9.66x10-3 |
1.07x10-3 |
|
CaCO3 |
|
|
|
|
|
|
|
|
|
Fr. p/ |
10.0 |
0.7 |
0.58 |
1.0 |
0.56 |
0.2 |
5.6x10-3 |
6.23x10-4 |
|
100,000 |
|
|
|
|
|
|
|
|
|
CaCO3 |
|
|
|
|
|
|
|
|
|
Ger. p/ |
17.9 |
1.25 |
1.04 |
1.79 |
1.0 |
0.358 |
1x10-2 |
1.12x10-3 |
|
100,000 |
|
|
|
|
|
|
|
|
|
CaO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
meq/L |
50.0 |
3.5 |
2.9 |
5.0 |
2.8 |
1.0 |
2.8x10-2 |
3.11x10-2 |
|
g/L CaO |
1790.0 |
125.0 |
104.2 |
179.0 |
100.0 |
35.8 |
1.0 |
0.112 |
|
|
|
|
|
|
|
|
|
|
|
lbs./cu ft |
16,100.0 |
1,123.0 |
935.0 |
1,610.0 |
900.0 |
321.0 |
9.0 |
1.0 |
|
CaCO3 |
|
|
|
|
|
|
|
|
1‘epm/L, or ‘mval/L’
Note: 1 meq/L = 1N/1000
19

SECTION I, continued
Dissolved Oxygen
Table 3 lists the mg/L dissolved oxygen in water at saturation for various temperatures and atmospheric pressures. The table was formulated in a laboratory using pure water. The values given are only approximations for estimating the oxygen content of a particular body of surface water.
Table 3 Dissolved Oxygen Saturation in Water
|
|
|
|
|
Pressure in Millimeters and Inches Hg |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
mm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
775 |
760 |
750 |
725 |
|
700 |
675 |
650 |
625 |
|
|
|
|
|
|
|
|
|
|
||
|
Temp |
|
|
|
inches |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
°F |
|
°C |
30.51 |
29.92 |
29.53 |
28.45 |
|
27.56 |
26.57 |
25.59 |
24.61 |
|
|
|
|
|
|
|
|
|
|
|
|
32.0 |
|
0 |
14.9 |
14.6 |
14.4 |
13.9 |
|
13.5 |
12.9 |
12.5 |
12.0 |
|
|
|
|
|
|
|
|
|
|
|
|
33.8 |
|
1 |
14.5 |
14.2 |
14.1 |
13.6 |
|
13.1 |
12.6 |
12.2 |
11.7 |
|
|
|
|
|
|
|
|
|
|
|
|
35.6 |
|
2 |
14.1 |
13.9 |
13.7 |
13.2 |
|
12.9 |
12.3 |
11.8 |
11.4 |
|
|
|
|
|
|
|
|
|
|
|
|
37.4 |
|
3 |
13.8 |
13.5 |
13.3 |
12.9 |
|
12.4 |
12.0 |
11.5 |
11.1 |
|
|
|
|
|
|
|
|
|
|
|
|
39.2 |
|
4 |
13.4 |
13.2 |
13.0 |
12.5 |
|
12.1 |
11.7 |
11.2 |
10.8 |
|
|
|
|
|
|
|
|
|
|
|
|
41.0 |
|
5 |
13.1 |
12.8 |
12.6 |
12.2 |
|
11.8 |
11.4 |
10.9 |
10.5 |
|
|
|
|
|
|
|
|
|
|
|
|
42.8 |
|
6 |
12.7 |
12.5 |
12.3 |
11.9 |
|
11.5 |
11.1 |
10.7 |
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
44.6 |
|
7 |
12.4 |
12.2 |
12.0 |
11.6 |
|
11.2 |
10.8 |
10.4 |
10.0 |
|
|
|
|
|
|
|
|
|
|
|
|
46.4 |
|
8 |
12.1 |
11.9 |
11.7 |
11.3 |
|
10.9 |
10.5 |
10.1 |
9.8 |
|
|
|
|
|
|
|
|
|
|
|
|
48.2 |
|
9 |
11.8 |
11.6 |
11.5 |
11.1 |
|
10.7 |
10.3 |
9.9 |
9.5 |
|
|
|
|
|
|
|
|
|
|
|
|
50.0 |
|
10 |
11.6 |
11.3 |
11.2 |
10.8 |
|
10.4 |
10.1 |
9.7 |
9.3 |
|
|
|
|
|
|
|
|
|
|
|
|
51.8 |
|
11 |
11.3 |
11.1 |
10.9 |
10.6 |
|
10.2 |
9.8 |
9.5 |
9.1 |
|
|
|
|
|
|
|
|
|
|
|
|
53.6 |
|
12 |
11.1 |
10.8 |
10.7 |
10.3 |
|
10.0 |
9.6 |
9.2 |
8.9 |
|
|
|
|
|
|
|
|
|
|
|
|
55.4 |
|
13 |
10.8 |
10.6 |
10.5 |
10.1 |
|
9.8 |
9.4 |
9.1 |
8.7 |
|
|
|
|
|
|
|
|
|
|
|
|
57.2 |
|
14 |
10.6 |
10.4 |
10.2 |
9.9 |
|
9.5 |
9.2 |
8.9 |
8.5 |
|
|
|
|
|
|
|
|
|
|
|
|
59.0 |
|
15 |
10.4 |
10.2 |
10.0 |
9.7 |
|
9.3 |
9.0 |
8.7 |
8.3 |
|
|
|
|
|
|
|
|
|
|
|
|
60.8 |
|
16 |
10.1 |
9.9 |
9.8 |
9.5 |
|
9.1 |
8.8 |
8.5 |
8.1 |
|
|
|
|
|
|
|
|
|
|
|
|
62.6 |
|
17 |
9.9 |
9.7 |
9.6 |
9.3 |
|
9.0 |
8.6 |
8.3 |
8.0 |
|
|
|
|
|
|
|
|
|
|
|
|
64.4 |
|
18 |
9.7 |
9.5 |
9.4 |
9.1 |
|
8.8 |
8.4 |
8.1 |
7.8 |
|
|
|
|
|
|
|
|
|
|
|
|
66.2 |
|
19 |
9.5 |
9.3 |
9.2 |
8.9 |
|
8.6 |
8.3 |
8.0 |
7.6 |
|
|
|
|
|
|
|
|
|
|
|
|
68.0 |
|
20 |
9.3 |
9.2 |
9.1 |
8.7 |
|
8.4 |
8.1 |
7.8 |
7.5 |
|
|
|
|
|
|
|
|
|
|
|
|
69.8 |
|
21 |
9.2 |
9.0 |
8.9 |
8.6 |
|
8.3 |
8.0 |
7.7 |
7.4 |
|
|
|
|
|
|
|
|
|
|
|
|
71.6 |
|
22 |
9.0 |
8.8 |
8.7 |
8.4 |
|
8.1 |
7.8 |
7.5 |
7.2 |
|
|
|
|
|
|
|
|
|
|
|
|
73.4 |
|
23 |
8.8 |
8.7 |
8.5 |
8.2 |
|
8.0 |
7.7 |
7.4 |
7.1 |
|
|
|
|
|
|
|
|
|
|
|
|
75.2 |
|
24 |
8.7 |
8.5 |
8.4 |
8.1 |
|
7.8 |
7.5 |
7.2 |
7.0 |
|
|
|
|
|
|
|
|
|
|
|
|
77.0 |
|
25 |
8.5 |
8.4 |
8.3 |
8.0 |
|
7.7 |
7.4 |
7.1 |
6.8 |
|
|
|
|
|
|
|
|
|
|
|
|
78.8 |
|
26 |
8.4 |
8.2 |
8.1 |
7.8 |
|
7.6 |
7.3 |
7.0 |
6.7 |
|
|
|
|
|
|
|
|
|
|
|
|
80.6 |
|
27 |
8.2 |
8.1 |
8.0 |
7.7 |
|
7.4 |
7.1 |
6.9 |
6.6 |
|
|
|
|
|
|
|
|
|
|
|
|
20

SECTION I, continued
Table 3 Dissolved Oxygen Saturation in Water (continued)
|
|
|
|
|
Pressure in Millimeters and Inches Hg |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
mm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
775 |
760 |
750 |
725 |
|
700 |
675 |
650 |
625 |
|
|
|
|
|
|
|
|
|
|
||
Temp |
|
|
|
|
inches |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
°F |
|
°C |
30.51 |
29.92 |
29.53 |
28.45 |
|
27.56 |
26.57 |
25.59 |
24.61 |
|
|
|
|
|
|
|
|
|
|
|
|
82.4 |
|
28 |
8.1 |
7.9 |
7.8 |
7.6 |
|
7.3 |
7.0 |
6.7 |
6.5 |
|
|
|
|
|
|
|
|
|
|
|
|
84.2 |
|
29 |
7.9 |
7.8 |
7.7 |
7.4 |
|
7.2 |
6.9 |
6.6 |
6.4 |
|
|
|
|
|
|
|
|
|
|
|
|
86.0 |
|
30 |
7.8 |
7.7 |
7.6 |
7.3 |
|
7.0 |
6.8 |
6.5 |
6.2 |
|
|
|
|
|
|
|
|
|
|
|
|
87.8 |
|
31 |
7.7 |
7.5 |
7.4 |
7.2 |
|
6.9 |
6.7 |
6.4 |
6.1 |
|
|
|
|
|
|
|
|
|
|
|
|
89.6 |
|
32 |
7.6 |
7.4 |
7.3 |
7.0 |
|
6.8 |
6.6 |
6.3 |
6.0 |
|
|
|
|
|
|
|
|
|
|
|
|
91.4 |
|
33 |
7.4 |
7.3 |
7.2 |
6.9 |
|
6.7 |
6.4 |
6.2 |
5.9 |
|
|
|
|
|
|
|
|
|
|
|
|
93.2 |
|
34 |
7.3 |
7.2 |
7.1 |
6.8 |
|
6.6 |
6.3 |
6.1 |
5.8 |
|
|
|
|
|
|
|
|
|
|
|
|
95.0 |
|
35 |
7.2 |
7.1 |
7.0 |
6.7 |
|
6.5 |
6.2 |
6.0 |
5.7 |
|
|
|
|
|
|
|
|
|
|
|
|
96.8 |
|
36 |
7.1 |
7.0 |
6.9 |
6.6 |
|
6.4 |
6.1 |
5.9 |
5.6 |
|
|
|
|
|
|
|
|
|
|
|
|
98.6 |
|
37 |
7.0 |
6.8 |
6.7 |
6.5 |
|
6.3 |
6.0 |
5.8 |
5.6 |
|
|
|
|
|
|
|
|
|
|
|
|
100.4 |
|
38 |
6.9 |
6.7 |
6.6 |
6.4 |
|
6.2 |
5.9 |
5.7 |
5.5 |
|
|
|
|
|
|
|
|
|
|
|
|
102.2 |
|
39 |
6.8 |
6.6 |
6.5 |
6.3 |
|
6.1 |
5.8 |
5.6 |
5.4 |
|
|
|
|
|
|
|
|
|
|
|
|
104.0 |
|
40 |
6.7 |
6.5 |
6.4 |
6.2 |
|
6.0 |
5.7 |
5.5 |
5.3 |
|
|
|
|
|
|
|
|
|
|
|
|
105.8 |
|
41 |
6.6 |
6.4 |
6.3 |
6.1 |
|
5.9 |
5.6 |
5.4 |
5.2 |
|
|
|
|
|
|
|
|
|
|
|
|
107.6 |
|
42 |
6.5 |
6.3 |
6.2 |
6.0 |
|
5.8 |
5.6 |
5.3 |
5.1 |
|
|
|
|
|
|
|
|
|
|
|
|
109.4 |
|
43 |
6.4 |
6.2 |
6.1 |
5.9 |
|
5.7 |
5.5 |
5.2 |
5.0 |
|
|
|
|
|
|
|
|
|
|
|
|
111.2 |
|
44 |
6.3 |
6.1 |
6.0 |
5.8 |
|
5.6 |
5.4 |
5.2 |
4.9 |
|
|
|
|
|
|
|
|
|
|
|
|
113.0 |
|
45 |
6.2 |
6.0 |
5.9 |
5.7 |
|
5.5 |
5.3 |
5.1 |
4.8 |
|
|
|
|
|
|
|
|
|
|
|
|
114.8 |
|
46 |
6.1 |
5.9 |
5.9 |
5.6 |
|
5.4 |
5.2 |
5.4 |
4.8 |
|
|
|
|
|
|
|
|
|
|
|
|
116.6 |
|
47 |
6.0 |
5.9 |
5.8 |
5.6 |
|
5.3 |
5.1 |
4.8 |
4.7 |
|
|
|
|
|
|
|
|
|
|
|
|
118.4 |
|
48 |
5.9 |
5.8 |
5.7 |
5.5 |
|
5.3 |
5.0 |
4.8 |
4.6 |
|
|
|
|
|
|
|
|
|
|
|
|
120.2 |
|
49 |
5.8 |
5.7 |
5.6 |
5.4 |
|
5.2 |
5.0 |
4.7 |
4.5 |
|
|
|
|
|
|
|
|
|
|
|
|
122.0 |
|
50 |
5.7 |
5.6 |
5.5 |
5.3 |
|
5.1 |
4.9 |
4.7 |
4.4 |
|
|
|
|
|
|
|
|
|
|
|
|
21

SECTION I, continued
Sample Collection, Preservation and Storage
Correct sampling and storage are critical for accurate testing. For greatest accuracy, thoroughly clean sampling devices and containers to prevent carryover from previous samples. Preserve the sample properly; each procedure has information about sample preservation.
•The least expensive containers are polypropylene or polyethylene.
•The best and most expensive containers are quartz or PTFE (polytetrafluoroethylene, Teflon).
•Avoid soft glass containers for metals in the microgram-per-liter range.
•Store samples for silver determination in light absorbing containers, such as amber bottles.
Avoid contaminating the sample with metals from containers, distilled water or membrane filters. Thoroughly clean sample containers as described under Acid Washing Bottles.
Preservation slows the chemical and biological changes that continue after collection. These changes may change the amount of a chemical species available for analysis. Normally, analyze the samples as soon as possible after collection, especially when the analyte concentration is expected to be low. This also reduces the chance for error and minimizes labor.
Preservation methods include pH control, chemical addition, refrigeration and freezing. Table 4 gives the recommended preservation for various substances. It also includes suggested types of containers and the maximum recommended holding times for properly preserved samples.
Preserve aluminum, cadmium, chromium, cobalt, copper, iron, lead, nickel, potassium, silver and zinc samples for at least 24 hours by adding one Nitric Acid Solution Pillow 1:1 (Cat. No. 2540-98) per liter of sample. Check the pH with pH indicator paper or a pH meter to assure the pH is 2 or less. Add additional pillows if necessary. Adjust the sample pH prior to analysis by adding an equal number of Sodium Carbonate Anhydrous Powder Pillows (Cat. No. 179-98). Or raise the pH to 4.5 with Sodium Hydroxide Standard Solution, 1 N or 5 N.
22

SECTION I, continued
Table 4 Required Containers, Preservation Techniques and Holding Times1
|
Parameter No./Name |
Container2 |
Preservation3,4 |
|
|
Maximum |
|
|
|
Holding Time5 |
|||
|
|
|
|
|
|
|
Table 1A - Bacterial Tests |
|
|
|
|
|
|
|
|
|
|
|
|
|
1-4. Coliform, fecal and total |
P,G |
Cool, 4°C, 0.008%, Na |
S O |
6 |
6 hours |
|
|
|
|
2 |
2 |
3 |
|
5. Fecal streptococci |
P,G |
Cool, 4°C, 0.008%, Na |
S O |
6 |
6 hours |
|
|
|
|
2 |
2 |
3 |
|
Table 1B - Inorganic Tests |
|
|
|
|
|
|
|
|
|
|
|
|
|
1. Acidity |
P, G |
Cool, 4°C |
|
|
14 days |
|
|
|
|
|
|
|
|
2. Alkalinity |
P, G |
Cool, 4°C |
|
|
14 days |
|
|
|
|
|
|||
4. Ammonia |
P, G |
Cool, 4°C, H2SO4 to pH<2 |
28 days |
|||
9. Biochemical oxygen demand |
P, G |
Cool, 4°C |
|
|
48 hours |
|
(BOD) |
|
|
|
|
|
|
|
|
|
|
|
|
|
10. |
Boron |
P, PFTE or quartz |
HNO3 to pH<2 |
|
|
6 months |
11. |
Bromide |
P, G |
None required |
|
|
28 days |
|
|
|
|
|
|
|
14. |
Biochemical oxygen demand, |
P, G |
Cool, 4°C |
|
|
48 hours |
carbonaceous |
|
|
|
|
|
|
|
|
|
|
|
||
15. |
Chemical oxygen demand |
P, G |
Cool, 4°C, H2SO4 to pH<2 |
28 days |
||
16. |
Chloride |
P, G |
None required |
|
|
28 days |
|
|
|
|
|
|
|
17. |
Chlorine, total residual |
P, G |
None required |
|
|
Analyze immediately |
|
|
|
|
|
|
|
21. |
Color |
P, G |
Cool, 4°C |
|
|
48 hours |
|
|
|
|
|||
23-24. Cyanide, total and amenable |
P, G |
Cool, 4°C, NaOH to pH>12, 0.6 g |
14 days7 |
|||
to chlorination |
|
ascorbic acid6 |
|
|
|
|
25. |
Fluoride |
P |
None required |
|
|
28 days |
|
|
|
|
|
||
27. |
Hardness |
P, G |
HNO3 to pH<2, H2SO4 to pH<2 |
6 months |
||
28. |
Hydrogen ion (pH) |
P, G |
None required |
|
|
Analyze immediately |
|
|
|
|
|
||
31, 43. Kjeldahl and organic |
P, G |
Cool 4°C, H2SO4 to pH<2 |
|
28 days |
||
nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
Metals8 |
|
|
|
|
|
|
18. |
Chromium VI |
P, G |
Cool, 4°C |
|
|
24 hours |
|
|
|
|
|
|
|
35. |
Mercury |
P, G |
HNO3 to pH<2 |
|
|
28 days |
Metals, except boron, chromium VI |
|
|
|
|
|
|
and mercury: 3, 5-8, 12, 13, 19, 20, |
P, G |
HNO3 to pH<2 |
|
|
6 months |
|
22, 26, 29, 30, 32-34, 36, 37, 45, |
|
|
|
|
|
|
47, 51, 52, 58-60, 62, 63, 70-72, 74, |
|
|
|
|
|
|
759. |
|
|
|
|
|
|
38. |
Nitrate |
P, G |
Cool, 4°C |
|
|
48 hours |
|
|
|
|
|
|
|
39. |
Nitrate-nitrite |
P, G |
Cool 4°C, H2SO4 to pH<2 |
|
28 days |
|
40. |
Nitrite |
P, G |
Cool, 4°C |
|
|
48 hours |
|
|
|
|
|
||
41. |
Oil and grease |
G |
Cool, 4°C, HCl or H2SO4 to pH<2 |
28 days |
||
42. |
Organic Carbon |
P, G |
Cool, 4°C, HCl or H2SO4 or |
28 days |
||
|
|
|
H3PO4 to pH<2 |
|
|
|
44. |
Orthophosphate |
P, G |
Filter immediately; Cool, 4°C |
48 hours |
||
|
|
|
|
|
|
|
46a. Oxygen, dissolved probe |
G Bottle and top |
None required |
|
|
Analyze immediately |
|
|
|
|
|
|||
46b. Oxygen, dissolved, Winkler |
Do |
Fix on site and store in dark |
8 hours |
|||
|
|
|
|
|
|
|
48. |
Phenols |
G only |
Cool 4°C, H2SO4 to pH<2 |
|
28 days |
|
23

SECTION I, continued
Table 4 Required Containers, Preservation Techniques and Holding Times1 (continued)
|
Parameter No./Name |
Container2 |
Preservation3,4 |
Maximum |
|
Holding Time5 |
|||
|
|
|
|
|
49. |
Phosphorus, elemental |
G |
Cool, 4°C |
48 hours |
|
|
|
|
|
50. |
Phosphorus, total |
P, G |
Cool, 4°C, H2SO4 to pH<2 |
28 days |
53. |
Residue, total |
P, G |
Cool, 4°C |
7 days |
|
|
|
|
|
54. |
Residue, filterable |
P, G |
Cool, 4°C |
7 days |
|
|
|
|
|
55. |
Residue, Nonfilterable (TSS) |
P, G |
Cool, 4°C |
7 days |
|
|
|
|
|
56. |
Residue, Settleable |
P, G |
Cool, 4°C |
48 hours |
|
|
|
|
|
57. |
Residue, volatile |
P, G |
Cool, 4°C |
7 days |
|
|
|
|
|
61. |
Silica |
P, PFTE or quartz |
Cool, 4°C |
28 days |
|
|
|
|
|
64. |
Specific conductance |
P, G |
Cool, 4°C |
28 days |
|
|
|
|
|
65. |
Sulfate |
P, G |
Cool, 4°C |
28 days |
|
|
|
|
|
66. |
Sulfide |
P, G |
Cool 4°C, add zinc acetate plus |
7 days |
|
sodium hydroxide to pH>9 |
|
||
|
|
|
|
|
|
|
|
|
|
67. |
Sulfite |
P, G |
none required |
Analyze immediately |
|
|
|
|
|
68. |
Surfactants |
P, G |
Cool, 4°C |
48 hours |
|
|
|
|
|
69. |
Temperature |
P, G |
None required |
Analyze immediately |
|
|
|
|
|
73. |
Turbidity |
P, G |
Cool, 4°C |
48 hours |
|
|
|
|
|
1This table was adapted from Table II published in the Federal Register, July 1, 1997, 40 CFR, Part 136.3, pages 26-27. Organic tests are not included.
2Polyethylene (P) or glass (G).
3Sample preservation should be performed immediately upon sample collection. For composite chemical samples each aliquot should be preserved at the time of collection. When use of an automated sampler makes it impossible to preserve each aliquot, then chemical samples may be preserved by maintaining at 4°C until compositing and sample splitting is completed.
4When any sample is to be shipped by common carrier or sent through United States Mails, it must comply with the Department of Transportation Hazardous Material Regulations (49 CFR Part 172). The person offering such material for transportation is responsible for ensuring such compliance. For the preservation requirements of Table II, the Office of Hazardous Materials, Materials Transportation Bureau, Department of Transportation has determined that the Hazardous Materials Regulations do not apply to the following materials: Hydrochloric acid
(HCl) in water solutions at concentrations of 0.04% by weight or less (pH about 1.96 or greater); Nitric acid (HNO3) in water solutions at concentrations of 0.15% by weight or less (pH about 1.62 or greater); Sulfuric acid (H2SO4) in water solutions at concentrations of 0.35% by weight or less (pH about 1.15 or greater); and Sodium hydroxide
(NaOH) in water solutions at concentrations of 0.080% by weight or less (pH about 12.30 or less).
5Samples should be analyzed as soon as possible after collection. The times listed are the maximum times that samples may be held before analysis and still be considered valid. Samples may be held for longer periods only if the permitee, or monitoring laboratory, has data on file to show that the specific types of samples under study are stable for the longer time, and has received a variance from the Regional Administer under §136.3(e). Some samples may not be stable for the maximum time period given in the table. A permitee, or monitoring laboratory, is obligated to hold the sample for a shorter time if knowledge exists to show that this is necessary to maintain sample stability. See §136.3(e) for details. The term “analyze immediately” usually means within 15 minutes or less after sample collection.
6Should only be used in the presence of residual chlorine.
7Maximum holding time is 24 hours when sulfide is present. Optionally all samples may be tested with lead acetate paper before pH adjustments in order to determine if sulfide is present. If sulfide is present, it can be removed by the addition of cadmium nitrate powder until a negative spot test is obtained. The sample is filtered and then NaOH is added to pH 12.
8Samples should be filtered immediately on-site before adding preservative for dissolved metals.
9Numbers refer to parameter number in 40 CFR, Part 136.3, Table 1B.
24

SECTION I, continued
Collecting Water Samples
Obtain the best sample by careful collection. In general, collect samples near the center of the vessel or duct and below the surface. Use only clean containers (bottles, beakers). Rinse the container several times first with the water to be sampled.
Take samples as close as possible to the source of the supply. This lessens the influence the distribution system has on the sample. Let the water run long enough to flush the system. Fill sample containers slowly with a gentle stream to avoid turbulence and air bubbles. Collect water samples from wells after the pump has run long enough to deliver water representative of the ground water feeding the well.
It is hard to obtain a truly representative sample when collecting surface water samples. Obtain best results by testing several samples. Use samples taken at different times from several locations and depths. The results can be used to establish patterns for that particular body of water.
Generally, as little time as possible should elapse between collecting the sample and analyzing it.
Depending on the test, special precautions in handling the sample may be necessary. This prevents natural interferences such as organic growth or loss or gain of dissolved gases. Each procedure describes sample preservatives and storage techniques for samples that are held for testing.
Acid Washing Bottles
If a procedure suggests acid-washing, use the following procedure:
a)Clean the glassware or plasticware with laboratory detergent (phosphate-free detergent is recommended).
b)Rinse well with tap water.
c)Rinse with a 1:1 Hydrochloric Acid Solution or 1:1 Nitric Acid Solution. The nitric acid rinse is important for testing for lead.
d)Rinse well with deionized water. Up to 12-15 rinses may be necessary if chromium is being determined.
e)Air dry.
Use chromic acid or chromium-free substitutes to remove organic deposits from glass containers. Rinse containers thoroughly with water to remove traces of chromium.
25

SECTION I, continued
Wash glassware for phosphate determinations with phosphate-free detergents and acid-wash with 1:1 HCl. Thoroughly rinse the glassware with deionized water. For ammonia and Kjeldahl nitrogen, rinse with ammonia-free water.
Correcting for Volume Additions
If you use a large volume of preservative, correct for the volume of preservative added. This accounts for dilution due to the acid added to preserve the sample and the base used to adjust the pH to the range of the procedure. This correction is made as follows:
1.Determine the volume of initial sample, the volume of acid and base added, and the total final volume of the sample.
2.Divide the total volume by the initial volume.
3.Multiply the test result by this factor.
Example:
A one-liter sample was preserved with 2 mL of nitric acid. It was neutralized with 5 mL of 5 N sodium hydroxide. The result of the analysis procedure was 10.00 mg/L. What is the volume correction factor and correct result?
1.Total Volume = 1000 mL + 2 mL + 5 mL = 1007 mL
2. 1007
------------ = 1.007 = volume correction factor 1000
3.10.0 mg/L × 1.007 = 10.07 mg/L = correct result
Hach 1:1 Nitric Acid Pillows contain 2.5 mL of acid: correct for this volume. The addition of a Sodium Carbonate Power Pillow neutralizes the 1:1 Nitric Acid Pillow does not need to be corrected for.
Boiling Aids
Boiling is necessary in some procedures. Using a boiling aid such as boiling chips (Cat. no. 14835-31) reduces bumping. Bumping is caused by the sudden, almost explosive conversion of water to steam as it is heated. Avoid bumping; it may cause sample loss or injury.
Make sure the boiling aids will not contaminate the sample. Do not use boiling aids (except glass beads) more than once. Loosely covering the sample during boiling will prevent splashing, reduce the chances of contamination and minimize sample loss.
26
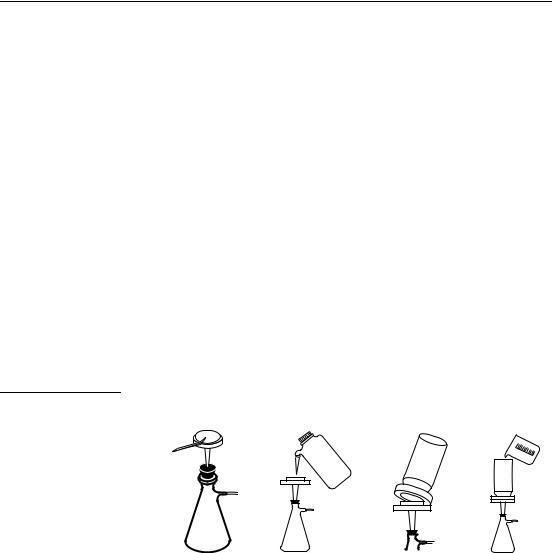
SECTION I, continued
Sample Filtration
Filtering separates particles from the aqueous sample. Filtration uses a medium, usually filter paper, to retain particles but pass solution. This is especially helpful when sample turbidity interferes with analysis. Two general methods of filtration are gravity and vacuum. Gravity filtration uses gravity to pull the sample though the filter paper. Vacuum filtration uses suction and gravity to move the sample through the filter. An aspirator or vacuum pump creates the suction. Vacuum filtration is faster than gravity filtration. Vacuum filter (see Figure 1) as follows:
1.Using tweezers, place a filter paper into the filter holder.
2.Place the filter holder assembly in the filtering flask. Wet the filter with deionized water to ensure adhesion to the holder.
3.Position the funnel housing on the filter holder assembly.
4.While applying a vacuum to the filtering flask, transfer the sample to the filtering apparatus.
5.Slowly release the vacuum from the filtering flask and transfer the solution from the filter flask to another container.
Figure 1 Vacuum Filtration
REQUIRED APPARATUS FOR VACUUM FILTRATION
Description |
Unit |
Cat. No. |
Filter Discs, glass 47 mm .................................................................. |
100/pkg |
................2530-00 |
Filter Holder, membrane ......................................................................... |
each.............. |
13529-00 |
Flask, filter, 500 mL ................................................................................ |
each.................. |
546-49 |
Pump, vacuum, hand operated ................................................................ |
each.............. |
14283-00 |
OR |
|
|
Pump, vacuum, portable, 115 V .............................................................. |
each.............. |
14697-00 |
Pump, vacuum, portable, 230 V .............................................................. |
each.............. |
14697-02 |
27
SECTION I, continued
Many of the procedures in this manual use gravity filtration. The only labware required is filter paper, a conical funnel and a receiving flask. This labware is included under Optional Equipment and Supplies at the end of a procedure. Gravity filtration is better for retaining fine particles. For faster filtering, add solution until the filter paper cone is three-fourths filled. Never fill the cone completely. Gravity filter (see Figure 2)
as follows:
1.Place a filter paper into the funnel.
2.Wet the filter with deionized water to ensure adhesion to the funnel.
3.Place the funnel into an erlenmeyer flask or graduated cylinder.
4.Pour the sample into the funnel.
Figure 2 Gravity Filtration
REQUIRED APPARATUS FOR GRAVITY FILTRATION
Description |
Unit |
Cat No. |
Cylinder, graduated, 100 mL ................................................................... |
each |
................. 508-42 |
Funnel, poly, 65 mm ................................................................................ |
each ............... |
1083-67 |
Filter Paper, 12.5 cm ................................................................................ |
each ............... |
1894-57 |
Flask, erlenmeyer, 125 mL ...................................................................... |
each ................. |
505-43 |
Testing for metals requires acid and heat to pretreat the sample. Since these conditions destroy filter paper, vacuum filtration with glass fiber filter discs is recommended. Also, glass filter discs, unlike paper, do not retain colored species.
28

SECTION I, continued
Temperature Considerations
For best results, most tests in this manual should be performed with sample temperatures between 20 °C (68 °F) and 25 °C (77 °F). If a test requires closer temperature control, notes in the procedure will indicate this.
Sample Dilution Techniques
Ten and 25 mL are the volumes used for most colorimetric tests. However, in some tests, the color developed in the sample may be too intense to be measured. Unexpected colors may develop in other tests. In both cases, dilute the sample to determine if interfering substances are present.
To dilute the sample easily, pipet the chosen sample portion into a clean graduated cylinder (or volumetric flask for more accurate work). Fill the cylinder (or flask) to the desired volume with deionized water. Mix well. Use the diluted sample when running the test.
To help with dilutions, Table 5 shows the amount of sample used, the amount of deionized water used to bring the volume up to 25 mL and the multiplication factor.
The concentration of the sample is equal to the diluted sample reading multiplied by the multiplication factor.
More accurate dilutions can be done with a pipet and a 100-mL volumetric flask (see Table 6 for more information). Pipet the sample and dilute to volume with deionized water. Swirl to mix.
Table 5 Sample Dilution Volumes
Sample |
mL deionized Water Used |
Multiplication |
Volume (mL) |
to Bring the Volume to 25 mL |
Factor |
|
|
|
25.0 |
0.0 |
1 |
|
|
|
12.5 |
12.5 |
2 |
|
|
|
10.01 |
15.0 |
2.5 |
5.01 |
20.0 |
5 |
2.51 |
22.5 |
10 |
1.01 |
24.0 |
25 |
0.2501 |
24.75 |
100 |
1For sample sizes of 10 mL or less, use a pipet to measure the sample into the graduated cylinder or volumetric flask.
29

SECTION I, continued
Table 6 Multiplication Factors for Diluting to 100 mL
Sample Volume (mL) |
Multiplication Factor |
|
|
1 |
100 |
|
|
2 |
50 |
|
|
5 |
20 |
|
|
10 |
10 |
|
|
25 |
4 |
|
|
50 |
2 |
|
|
Sample Dilution and Interfering Substances
Sample dilution may influence the level at which a substance may interfere. The effect of the interferences decreases as the dilution increases. In other words, higher levels of an interfering substance can be present in the original sample if it is diluted before analysis.
An Example:
Copper does not interfere at or below 100 mg/L for a 25.00 mL sample in a procedure. If the sample volume is diluted with an equal volume of water, what is the level at which copper will not interfere?
Total volume |
= Dilution factor |
Sample----------------------------------------volume |
|
----------25 = 2 |
|
12.5 |
|
Interference Level × Dilution Factor = Interference level in sample
100 × 2 = 200
The level at which copper will not interfere in the undiluted sample is at or below 200 mg/L.
30
 Loading...
Loading...