Hach 16900 User manual

16900-08
Digital Titrator
Model 16900
© Hach Company, 1980-2006. Digital Titrator manufactured under U.S. patent 4,086,062. |
jk/dk June 2006 24 ed |
All rights reserved. |
|
2

TABLE OF CONTENTS |
|
SPECIFICATIONS.................................................................................................................... |
7 |
OPERATION.......................................................................................................................... |
9 |
GENERAL DESCRIPTION ................................................................................................... |
11 |
1.1 Introduction ....................................................................................................................... |
11 |
1.1.1 Following a Procedure for the First Time................................................................ |
12 |
1.2 Step-By-Step ..................................................................................................................... |
13 |
1.3 Helpful Hints..................................................................................................................... |
18 |
1.3.1 To Reuse a Partially Emptied Cartridge .................................................................. |
18 |
1.3.2 To Calculate Titrant Volume Used........................................................................... |
18 |
1.3.3 To Fill Your Own Titration Cartridges..................................................................... |
18 |
1.3.4 Verifying Technique................................................................................................. |
19 |
1.4 Adapting a Buret Titration to the Digital Titrator ............................................................. |
22 |
1.5 Using PermaChem® Powder Pillows ................................................................................ |
23 |
1.6 Safety................................................................................................................................. |
24 |
TITRATION PROCEDURES .......................................................................................... |
27 |
ACID-BASE |
|
Acid Determination................................................................................................................. |
29 |
Base Determination................................................................................................................. |
31 |
ACIDITY |
|
Methyl Orange Method ........................................................................................................... |
35 |
Phenolphthalein (Total) Method.............................................................................................. |
37 |
ALKALINITY |
|
Phenolphthalein and Total Method ......................................................................................... |
41 |
AMMONIA, HIGH RANGE (Ammonium Hydroxide) |
|
Ammonia Titration Procedure................................................................................................. |
49 |
CARBON DIOXIDE |
|
Using Sodium Hydroxide........................................................................................................ |
55 |
CHELANT, FREE |
|
Using Magnesium Chloride .................................................................................................... |
59 |
CHELANT, TOTAL |
|
Using Bismuth Nitrate............................................................................................................. |
63 |
3

TABLE OF CONTENTS, continued |
|
CHLORIDE |
|
Mercuric Nitrate Method ........................................................................................................ |
67 |
Silver Nitrate Method ............................................................................................................. |
68 |
CHLORINE, FREE AND TOTAL |
|
DPD-FEAS Method................................................................................................................ |
75 |
CHLORINE, TOTAL |
|
Iodometric Method (1 to 400 mg/L as Cl2 Using Sodium Thiosulfate) ................................. |
79 |
Iodometric Method (20 to 70,000 mg/L as Cl2 Using Sodium Thiosulfate) .......................... |
82 |
CHLORINE, FREE |
|
Amperometric Forward Titration............................................................................................ |
87 |
CHLORINE, TOTAL |
|
Amperometric Back Titration ................................................................................................. |
93 |
CHLORINE, TOTAL |
|
Amperometric Forward Titration.......................................................................................... |
105 |
CHROMATE |
|
Using Sodium Thiosulfate .................................................................................................... |
113 |
HARDNESS DECISION TREE........................................................................................... |
119 |
HARDNESS, CALCIUM |
|
Using EDTA.......................................................................................................................... |
121 |
HARDNESS, TOTAL |
|
Using EDTA.......................................................................................................................... |
127 |
HARDNESS, TOTAL, SEQUENTIAL |
|
Sequential Titration Procedure (Limited Sample) ................................................................ |
135 |
HYPOCHLORITE (Bleach) |
|
Iodometric Method ............................................................................................................... |
143 |
IRON |
|
Using the TitraVer® Titration Cartridge ............................................................................... |
147 |
NITRITE |
|
Using Ceric Standard Solution ............................................................................................. |
151 |
4

TABLE OF CONTENTS, continued |
|
OXYGEN, DISSOLVED |
|
Azide Modification of Winkler Method................................................................................ |
155 |
Using a 300-mL BOD Bottle ................................................................................................ |
155 |
Using a 60-mL BOD Bottle .................................................................................................. |
157 |
SALINITY |
|
Using Mercuric Nitrate.......................................................................................................... |
161 |
SULFITE |
|
Using Iodate-Iodide............................................................................................................... |
163 |
TURBIDITY STANDARDS |
|
Preparing Turbidity-Free Water ............................................................................................ |
167 |
VOLATILE ACIDS |
|
Using Sodium Hydroxide...................................................................................................... |
173 |
APPENDIX A |
|
ACCURACY CHECK AND STANDARD ADDITIONS.................................................... |
177 |
GENERAL INFORMATION ......................................................................................... |
189 |
REPLACEMENT PARTS AND ACCESSORIES................................................................ |
191 |
HOW TO ORDER................................................................................................................. |
193 |
REPAIR SERVICE................................................................................................................ |
194 |
WARRANTY ........................................................................................................................ |
195 |
5
6

SPECIFICATIONS
Digital Titrator
Delivery: 800 digits/mL or 0.00125 mL/digit
Accuracy*: ± 1% for readings over 100 digits. (Uncertainty of readings is 1 digit. Most samples require more than 100 digits.)
Weight: 132 g (4.7 oz.)
Cartridges for the Digital Titrator
Volume: 13 mL
Number of tests: Most reagents are formulated to provide 100 typical titrations; the number may vary depending on sample concentration.
Weight (full): 56.75 g (2 oz.)
* Overall method accuracy includes, in addition to the Digital Titrator, other sources of error controlled by the analyst. The other sources of error include: sampling, sample volume, dilution (if required), end point detection, reagent quality, and interferences.
7
8

OPERATION
DANGER
Handling chemical samples, standards, and reagents can be dangerous. Review the necessary Material Safety Data Sheets and become familiar with all safety procedures before handling any chemicals.
DANGER
La manipulation des échantillons chimiques, étalons et réactifs peut être dangereuse. Lire les Fiches de Données de Sécurité des Produits (FDSP) et se familiariser avec toutes les procédures de sécurité avant de manipuler tous les produits chimiques.
PELIGRO
La manipulación de muestras químicas, estándares y reactivos puede ser peligrosa. Revise las fichas de seguridad de materiales y familiarícese con los procedimientos de seguridad antes de manipular productos químicos.
GEFAHR
Das Arbeiten mit chemischen Proben, Standards und Reagenzien ist mit Gefahren verbunden.
Es wird dem Benutzer dieser Produkte empfohlen, sich vor der Arbeit mit sicheren Verfahrensweisen und dem richtigen Gebrauch der Chemikalien vertraut zu machen und alle entsprechenden Materialsicherheitsdatenblätter aufmerksam zu lesen.
PERIGO
A manipulação de amostras, padrões e reagentes químicos pode ser perigosa. Reveja a folha dos dados de segurança do material e familiarize-se com todos os procedimentos de segurança antes de manipular quaisquer produtos químicos.
9
10
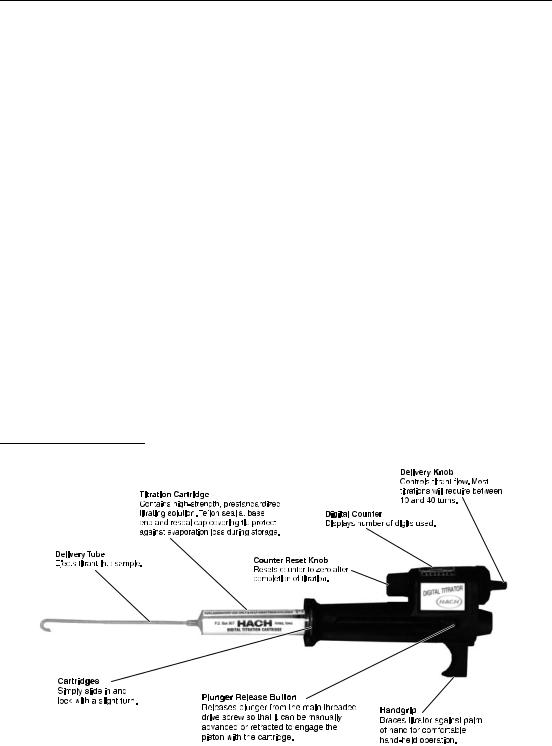
GENERAL DESCRIPTION
1.1 Introduction
Hach’s Digital Titrator is a new concept in titrimetric analysis. It is a precision dispensing device fitted with compact cartridges that contain concentrated titrants. Accurate titrations are made without the bulk and fragility of conventional burets.
A main drive screw in the Digital Titrator controls a plunger which forces the concentrated titrant from a titration cartridge in a carefully regulated flow. The titrator body is constructed of precision-molded, heavy-duty, chemicaland impact-resistant acetal plastic. Accuracy is rated at ± 1% or better for a titration of more than 100 digits. For titrations less than 100, accuracy is ± 1 digit.
Titration solutions (titrants) are packaged in disposable polypropylene or Kynar® containers with Teflon-covered neoprene seals and polyethylene resealable closures to cover the cartridge tips. Each cartridge contains approximately 13 mL of titrating solution, enough for 50–100 average titrations. Titrant solutions are typically controlled to ± 0.5% concentration with normality and tolerances listed on the label. Titrant concentrations are designed for titrations of 10 to 40 turns
(100 to 400 digits) of the delivery knob. For the most commonly used concentration ranges, the digits appearing in the counter window correspond to the sample concentration.
Figure 1 Hach Digital Titrator
11

GENERAL DESCRIPTION, continued
Both portable and fixed-position titrations are possible with the Digital Titrator. The instrument has a grip for hand-held operation or it can be clamped to a TitraStir® Stir Plate or laboratory stand for stationary setups. See Figure 1.
Each Digital Titrator comes with five delivery tubes and a methods manual, which covers the most commonly tested parameters and the corresponding titrant cartridges. Right-angle (ninety-degree) delivery tubes for stationary setups are available as an optional accessory.
1.1.1Following a Procedure for the First Time
Each method is divided into five sections: Procedure, Accuracy Check, Interferences, Summary of Method, and Reagents and Apparatus. For more information about how to select a procedure or for answers to chemical questions, see Hach’s Water Analysis Handbook (literature 8376). For more information about chlorine measurement, also see the technical booklet titled, Current Technology of Chlorine Analysis for Water and Wastewater
(literature 7019).
The Procedure details how to perform the method step-by-step. To select the appropriate sample volume and titration cartridge based on expected sample concentration, use the tables provided in each procedure. If the expected sample concentration is not known, start with one of the smaller sample volumes and determine its approximate concentration. Retest with the appropriate sample size.
The ranges in the table overlap to offer more flexibility. In most procedures, the number of digits used for each concentration range will be 100 to 400 digits.
To determine the actual concentration of the sample, use the correct digit multiplier for the sample volume and titration cartridge used.
Throughout the procedure, the notes will provide additional information.
The Accuracy Check provides a way to verify the results and determine if interferences are present. It also provides a method for checking the performance of reagents, the Digital Titrator and the operator’s technique. Further information is provided in
Appendix A, Accuracy Check and Standard Additions.
12

GENERAL DESCRIPTION, continued
The Interferences section identifies common interferences causing inaccurate results and describes how to eliminate their effects. The interference levels are based on the sample volume that has 1.0 as the digit multiplier. Higher interference levels may be tolerated if a smaller sample is used.
The Summary of Method section discusses the chemical reaction taking place and information that applies to the entire procedure.
The Reagents and Apparatus list concludes the procedure. All the items required to perform the test are listed first and are available from Hach. The items listed in the notes or interferences sections are included in the optional listings.
1.2 Step-By-Step
1.Select a sample volume and titration cartridge corresponding to the expected sample concentration from the table given in each procedure.
If the expected sample concentration is not known, start with one of the smaller sample volumes and determine its approximate concentration. Retest with the appropriate sample size.
2.Slide the cartridge into the titrator receptacle and lock in position with a slight turn. See Figure 2.
Figure 2 Sliding the Cartridge into Place
3.Remove the polyethylene cap and insert a clean delivery tube into the end of the cartridge until it is tight. See Figure 3. Use
a straight tube with a hook at the end for hand-held titrations; use a 90° tube with a hook at the end for stationary setups.
13
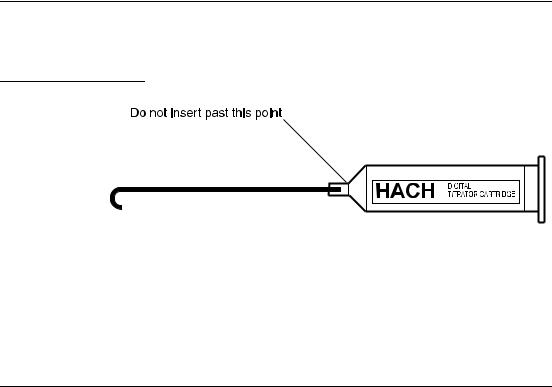
GENERAL DESCRIPTION, continued
Do not insert tube past cartridge extension; see illustration below. In some instances, it might be necessary to remove a small burr on the leading edge of the tube before insertion.
Figure 3 Inserting the Delivery Tube
4.For stationary titrations, use a TitraStir Stir Plate or a clamp holder and clamp to attach the titrator to a laboratory stand. See Figure 4 and Figure 5.
The TitraStir Stir Plate holds the Digital Titrator during the titration and also stirs the sample at a constant speed, leaving the analyst free to detect the end point. When a TitraStir Stir Plate is used, substitute or add the following
Optional Apparatus.
APPARATUS
Quantity Required |
|
|
|
Description |
Per Test |
Unit |
Cat. No. |
Delivery Tubes, 90° with hook for TitraStir® Stir Plate |
...........1 ............. |
5/pkg.......... |
41578-00 |
Flask, Erlenmeyer, 125 mL....................................................... |
1 ............... |
each.............. |
505-43 |
Flask, Erlenmeyer, 250 mL....................................................... |
1 ............... |
each.............. |
505-46 |
Stir Bar, 28.6 x 7.9 mm............................................................. |
1 ............... |
each.......... |
20953-52 |
TitraStir® Stir Plate, 115 Vac.................................................... |
1 ............... |
each.......... |
19400-00 |
TitraStir® Stir Plate, 230 Vac.................................................... |
1 ............... |
each.......... |
19400-10 |
5.To start titrant flowing and flush the delivery tube, hold the tip of the cartridge up. Advance the plunger release button to engage the piston with the cartridge (push the button in and toward the cartridge). Do not expel solution when pushing the piston toward the cartridge. Turn the delivery knob until air is expelled and several drops of solution flow from the tip. As you turn the knob a drive screw pushes a piston against the cartridge seal and forces liquid out through the delivery tube. Then use the counter reset knob to turn the digital counter back to zero and wipe the tip. The tip can be rinsed with deionized water rather than wiped, if desired.
14
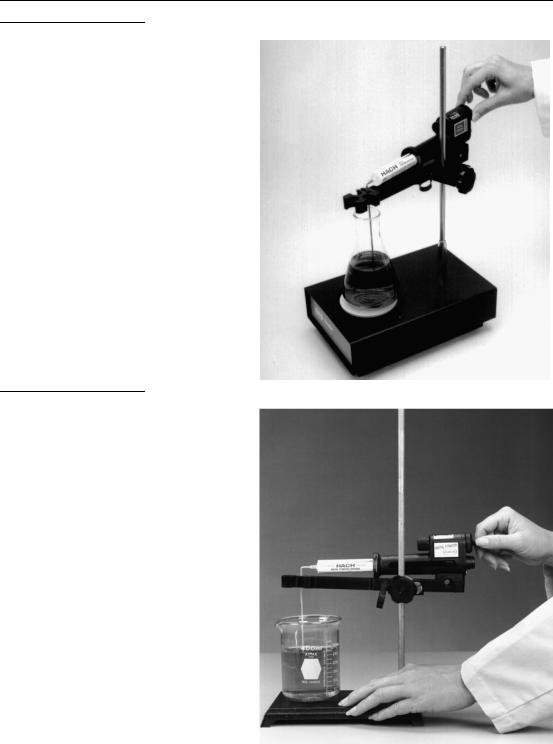
GENERAL DESCRIPTION, continued
Figure 4 Using the TitraStir® Stir Plate
Figure 5 Using a Laboratory Stand
15
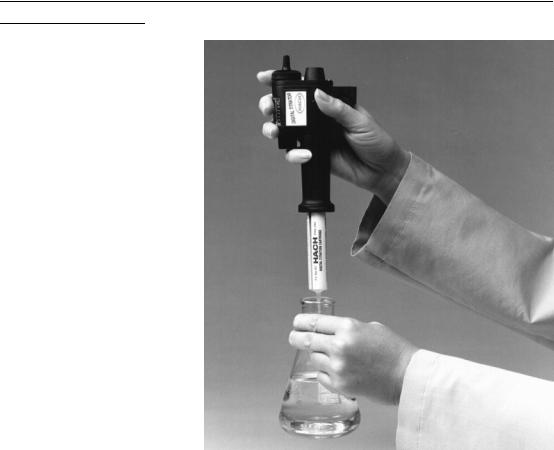
GENERAL DESCRIPTION, continued
Figure 6 Titrating the Sample
6.Use the smallest appropriate graduated cylinder or pipet to measure the sample volume from the given table. Transfer the sample into a 125-mL or 250-mL Erlenmeyer flask. Dilute to the appropriate total volume with deionized water
if necessary.
Note: Sample volume measurements and dilutions (if required) must be made accurately. However, final total volume of titrated solution is not critical.
7.Add the necessary reagents to the sample and swirl to mix.
8.Immerse the delivery tube tip in the solution and swirl the flask while titrating. Titrate by turning the delivery knob. Keep turning the knob and swirling the sample until the end point is reached. Record the number of digits that appear in the digital counter window. See Figure 6.
16

GENERAL DESCRIPTION, continued
Note: The number of digits required will usually range from 100 to 400. In nearly all of the procedures if the digits required is less than 100 or more than 400, an alternate sample volume or titrant cartridge should be used.
Note: Inaccurate results will occur if the delivery tube tip is held out of the solution rather than under the solution surface.
9.Calculate the concentration of your sample by using the following formula:
Digits Required × Digit Multiplier = Sample Concentration
Where:
Digits Required = the number that appeared in the digital counter window in Step 8.
Digit Multiplier = the number from the table given in the procedure.
It takes into account the sample dilution and titrant strength.
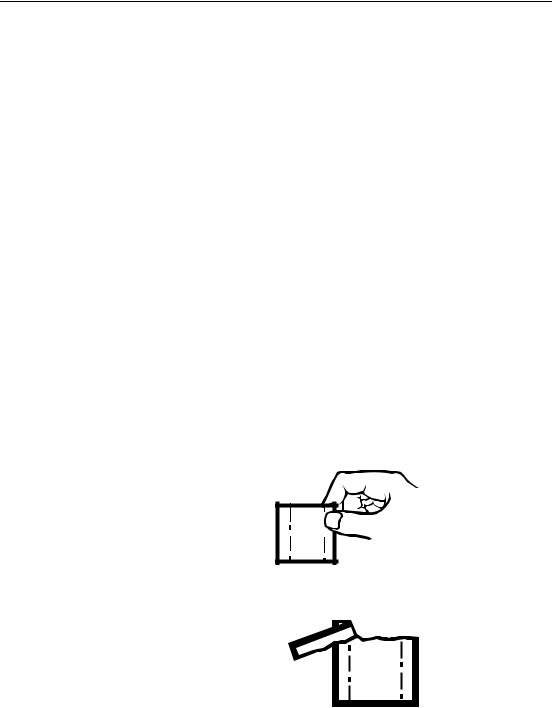
10.After completing testing for the day, press the plunger release button and manually retract the plunger into the body of the titrator. Remove the cartridge. Remove the delivery tube and reseal the cartridge with the polyethylene cap. See Figure 7.
Figure 7 Retracting the Plunger
11.Discard or clean the delivery tube immediately after use. To clean, force water, then air, into the tube opening with a syringe or wash bottle.
17

GENERAL DESCRIPTION, continued
1.3 Helpful Hints
1.3.1To Reuse a Partially Emptied Cartridge
1.With the plunger fully retracted, attach cartridge to the titrator.
2.Press the plunger release; then manually push the plunger against the cartridge seal.
3.Attach a delivery tube. Hold the tip of the cartridge up. Eject air and a few drops of titrant, zero the counter, and wipe
the tip.
4.Titrate as usual.
1.3.2To Calculate Titrant Volume Used
Normalities of many Hach titration cartridge solutions have been designed so that the number of digits used in a titration corresponds to the sample concentration in mg/L. To determine the volume used in mL, divide the Digital Titrator reading by 800.
1.3.3 To Fill Your Own Titration Cartridges
Cartridges may be cleaned and refilled, or new empty cartridges, Cat. No. 14495-01, can be purchased from Hach Company. See Figure 8. When preparing to refill old cartridges, push the cartridge seal out of the cartridge with air pressure applied through the tip. Cap the tip, fill with solution and reinsert the cartridge seal using care to avoid wrinkling the Teflon sheath. Filling also can be accomplished at the tip with a syringe.
Figure 8 Digital Titrator Cartridges
18

GENERAL DESCRIPTION, continued
1.3.4Verifying Technique
Whenever procedures are changed or new equipment is used, it is helpful to run a sample of known concentration. This technique will confirm the operator is following the procedure correctly and the new equipment is working properly. One objective important to Hach Company is making our tests self-verifying. This means Hach makes the tools available so the operator can check their own work for accurate results without relying on an outside lab or chemist.
For most of the tests in this manual, Table 1 on page 20 lists each procedure, the suggested standard, the volume of standard needed, the titration cartridge used, and the number of expected digits when the test is performed correctly. The suggested standards are Voluette® or PourRite™ Ampules whenever possible because of their superior accuracy and stability.
To use titration standards follow these steps:
1.Select the procedure of interest and order the appropriate standard. Use the given catalog numbers.
2.Measure the volume of standard to be used as the sample in the procedure using a TenSette® Pipet or Class A pipet.
3.Perform the procedure as written, adding deionized water as necessary.
4.After titrating, the required number of digits should approximately equal the expected digits.
Call Hach Technical and Customer Service (1-800-227-4224) for additional help.
19

GENERAL DESCRIPTION, continued
.
Table 1 Titration Standards
Procedure |
Standard Description |
Volume of |
Titration Cartridge |
Expected |
|
Standard |
|||||
(Parameter) |
(Cat. No.) |
(Cat. No.) |
Digits |
||
(mL) |
|||||
|
|
|
|
||
|
|
|
|
|
|
Acid-Base: |
0.500 N H2SO4 (2121-26) |
1.0 |
1.600 N NaOH |
250 |
|
Acid |
|
|
(14379-01) |
|
|
|
|
5.0 |
8.00 N NaOH |
250 |
|
|
|
|
(14381-01) |
|
|
|
|
|
|
|
|
Base |
0.500 N Na2CO3 |
1.0 |
1.600 N H2SO4 |
250 |
|
|
(14278-10) |
|
(14389-01) |
|
|
|
|
5.0 |
8.00 N H2SO4 |
250 |
|
|
|
|
(14391-01) |
|
|
Acidity |
0.500 N H2SO4 (2121-26) |
0.1 |
0.1600 N NaOH |
250 |
|
|
|
|
(14377-01) |
|
|
|
|
1.0 |
1.600 N NaOH |
250 |
|
|
|
|
(14379-01) |
|
|
|
|
|
|
|
|
Alkalinity |
0.500 N Na2CO3 (14278-10) |
0.1 |
0.1600 N H2SO4 |
250 |
|
|
|
|
(14388-01) |
|
|
|
|
1.0 |
1.600 N H2SO4 |
250 |
|
|
|
|
(14389-01) |
|
|
Calcium*: |
10,000 mg/L CaCO3 |
0.1 |
0.0800 M EDTA |
100 |
|
mg/L CaCO3 |
(2187-10) |
1.0 |
(14364-01) |
100 |
|
|
|
0.800 M EDTA |
|||
|
|
|
(14399-01) |
|
|
G.d.h. |
10,000 mg/L CaCO3 |
0.2 |
0.1428 M EDTA |
112 |
|
|
(2187-10) |
|
(14960-01) |
|
|
|
|
1.0 |
0.714 M EDTA |
112 |
|
|
|
|
(14959-01) |
|
|
|
|
|
|
|
|
Carbon |
10,000 mg/L CO2 (14275-10) |
0.2 |
0.3636 N NaOH |
100 |
|
Dioxide |
|
|
(14378-01) |
|
|
|
|
2.0 |
3.636 N NaOH |
|
|
|
|
|
(14380-01) |
|
|
|
|
|
|
|
|
Chloride |
12,500 mg/L Cl (14250-10) |
0.1 |
0.2256 N Hg(NO3)2 |
125 |
|
|
|
0.1 |
(14393-01) |
|
|
|
|
0.2256 N AgNO3 |
125 |
||
|
|
|
(14396-01) |
|
|
|
|
1.0 |
1.128 N AgNO3 |
250 |
|
|
|
|
(14397-01) |
|
|
|
|
1.0 |
2.256 N Hg(NO3)2 |
125 |
|
|
|
|
(921-01) |
|
20

GENERAL DESCRIPTION, continued
Table 1 Titration Standards (Continued)
Procedure |
Standard Description |
Volume of |
Titration Cartridge |
Expected |
||
Standard |
||||||
(Parameter) |
(Cat. No.) |
(Cat. No.) |
|
Digits |
||
(mL) |
|
|||||
|
|
|
|
|
||
|
|
|
|
|
||
Chlorine |
~50 mg/L Cl2 |
2.0 |
0.02256 N Na2S2O3 |
varies** |
||
|
(14268-20) |
|
(24091-01) |
|
|
|
|
(see certificate) |
|
|
|
|
|
|
~25 mg/L Cl2 |
0.5 |
0.00564 N FEAS |
varies*** |
||
|
(26300-20) |
|
(22923-01) |
|
|
|
Chromate |
1000 mg/L Cr |
1.0 |
0.2068 N Na2S2O3 |
223 |
||
|
(2231 mg/L CrO4) |
|
(22676-01) |
|
|
|
|
(14664-42) |
|
|
|
|
|
Hardness: |
|
0.1 |
0.0800 M EDTA |
100 |
||
mg/L CaCO3 |
10,000 mg/L CaCO3 (2187-10) |
0.1 |
(14364-01) |
|
100 |
|
|
|
0.0800 M CDTA |
||||
|
|
1.0 |
(14402-01) |
|
|
|
|
|
0.800 M EDTA |
100 |
|||
|
|
1.0 |
(14399-01) |
|
|
|
|
|
0.800 M CDTA |
100 |
|||
|
|
|
(14403-01) |
|
|
|
G.d.h. |
10,000 mg/L CaCO3 (2187-10) |
0.2 |
0.1428 M EDTA |
112 |
||
|
|
|
(14960-01) |
|
|
|
|
|
1.0 |
0.714 M EDTA |
112 |
||
|
|
|
(14959-01) |
|
|
|
|
|
|
|
|
||
Iron |
50 mg/L Fe |
10.0 |
0.0716 M TitraVer |
200 |
||
|
(14254-10) |
|
(20817-01) |
|
|
|
|
1000 mg/L Fe |
10.0 |
0.716 M TitraVer |
100 |
||
|
(2271-42) |
|
(20818-01) |
|
|
|
|
|
|
|
|
||
Oxygen, |
10 mg/L as DO |
100 |
0.2000 N Na2S2O3 |
500 |
||
Dissolved**** |
(401-11) |
|
(22675-01) |
|
|
|
|
|
200 |
2.00 N Na2S2O3 |
100 |
||
|
|
|
(14401-01) |
|
|
|
Sulfite |
5000 mg/L SO (22674-10) |
1.0 |
0.3998 N KIO |
–KI |
250 |
|
|
3 |
|
3 |
|
|
|
|
|
|
(14961-01) |
|
|
|
*One to two drops of Magnesium Standard Solution (10 g/L as CaCO3) must be added to get a sharp end point. These added drops will not change the results.
**The expected digits equal the volume of standard times the concentration on the certificate (e.g., 2 mL x 50 mg/L = 100 digits).
***The expected digits equals the volume of standard times the concentration on the certificate times the constant, 4. (Example: 0.5 mL x 50 mg/L x 4 = 100 digits)
****Add one Sulfamic Acid Powder Pillow to the volume of standard and follow Steps 10 to 12 in the Dissolved Oxygen Procedure. It is not necessary to add the first two reagents.
21

GENERAL DESCRIPTION, continued
1.4 Adapting a Buret Titration to the Digital Titrator
Adapt any standard titration procedure using a buret to the Digital Titrator by using the following procedure.
1.Determine the approximate number of digits required. The Digital Titrator dispenses 1 mL per 800 digits on the counter. Using the following equation, determine the digits required for your buret method.
Nt × mLt × 800
Digits Required = --------------------------------------
Nc
Where:
Nt = Normality of buret titrant
mLt = milliliters of buret titrant required for an average titration Nc = Normality of Digital Titrator cartridge
2.If the number of digits required is within the range of 70 to 350, you can use the procedure as written, substituting the Digital Titrator directly for the buret. Or, if the number of digits is outside of this range, make the
following modifications:
a.If the number of digits required is more than 350, reduce the sample size to save titrant.
b.If the number of digits required is less than 70, increase the sample size to increase precision.
c.If the sample size is altered, adjust the amount of buffering or indicating reagents by the same proportion.
3.When using the Digital Titrator for your buret method, note the number of digits required for a sample titration. To convert the digits required to the equivalent number of milliliters if the buret method was used, calculate:
× Nc Equivalent Buret Milliliters = Digits Required ----------------------
800 x Nt
If the sample size was changed, adjust the equivalent buret milliliters accordingly. If the sample size was increased, reduce the equivalent buret milliliters; if the sample size was reduced increase the equivalent buret milliliters. Multiply the equivalent
22
GENERAL DESCRIPTION, continued
buret milliliters by any normally used factors to calculate concentration in oz/gal, g/L, etc.
Example: Adapt a buret procedure, which normally requires about 20 mL of a 0.4 N titrant, to the Digital Titrator. Try an 8.0 N titration cartridge. The first equation above gives:
0.4 × 20 × 800
Digits Required = -------------------------------------= 800 digits 8.0
Because this would use excessive titrant, reduce the sample size to one fourth its normal size to reduce the digits required to 200, well within the recommended range.
Upon completion of the titration using the smaller sample size, calculate the equivalent buret milliliters by the second equation above. If 205 were the digits required:
205 × 8.0 Equivalent Buret Milliliters = ------------------------= 5.13 mL
800 × 0.4
Multiply the 5.13 mL by 4 to account for the reduction in sample size to give the true equivalent buret milliliters of 20.5 mL. If the buret method called for multiplying the number of milliliters of titrant by a factor to calculate the concentration of a sample component, then multiply 20.5 by that factor.
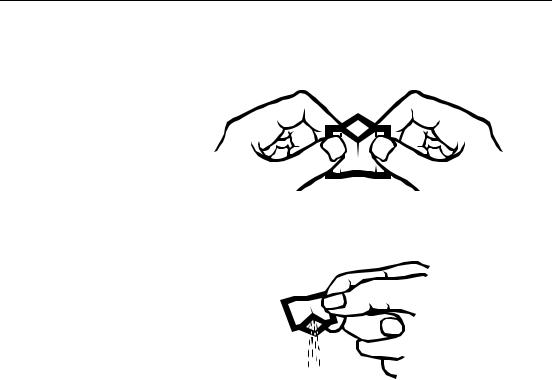
1.5Using PermaChem® Powder Pillows
1.Tap the PermaChem on a hard surface to collect the powdered reagent in the bottom.
2.Tear across on the dotted pillow line marked “TEAR” holding the pillow away from your face.
23
GENERAL DESCRIPTION, continued
3.Using two hands, Push both sides toward each other until thumbs and forefingers form a diamond. Make sure to Crease the foil pack, so that it forms a spout.
4.Pour the pillow contents into the sample. The polyfilm lining is specially formulated to deliver all the powder necessary for accurate results (no tapping on the vessel edge is necessary).
1.6 Safety
Safety is the responsibility of each individual when performing analysis procedures, and the analyst must develop and maintain good safety habits. Because many of the procedures in this methods handbook use potentially hazardous chemicals and apparatus, it is important that the analyst practice good laboratory techniques to minimize accidents. The following paragraphs present several techniques applicable to water analysis in the laboratory and in the field. They are not all inclusive, of course, nor do they apply only to the procedures provided in this handbook. They are general in nature but emphasize practices that are often key factors in personal injury incidents.
•Read labels carefully. Never remove the label from a reagent container. When preparing a reagent or standard solution, be sure to label the container clearly and date it.
•A Material Safety Data Sheet (MSDS) comes with each reagent. This sheet contains helpful information on first aid,
24

GENERAL DESCRIPTION, continued
spill and disposal procedures, and precautionary measures and should be read before using the product.
•Warning labels also appear on some of the apparatus used with the test procedures.
•Wear protective clothing when handling chemicals that cause irritation or burns. Eye protection in particular is important to guard against spattering and splashes from accidental spills when caustic materials are being used.
•Use tongs or finger cots when transferring apparatus that is hot.
•Use mechanical pipetters: Mouth pipetting could result in accidentally ingesting dangerous chemicals. Make a habit of using mechanical pipet fillers for all pipetting. This will avoid mistakes that could cause serious injury.
•Use special care with dangerous chemicals and apparatus.
•Follow the test procedure steps carefully and observe all precautionary measures. It is good practice to read the entire procedure carefully before beginning the procedure. Use safety equipment, such as pipet fillers, protective clothing, and ventilating hoods, appropriate for the test being conducted. Wipe up all spills promptly. Do not smoke or eat in an area where toxic or irritating chemicals are used. Use reagents and apparatus only as they were meant to be used and use them only as directed in the test procedure. Do not use damaged labware and malfunctioning equipment.
25
26

TITRATION PROCEDURES
27
28
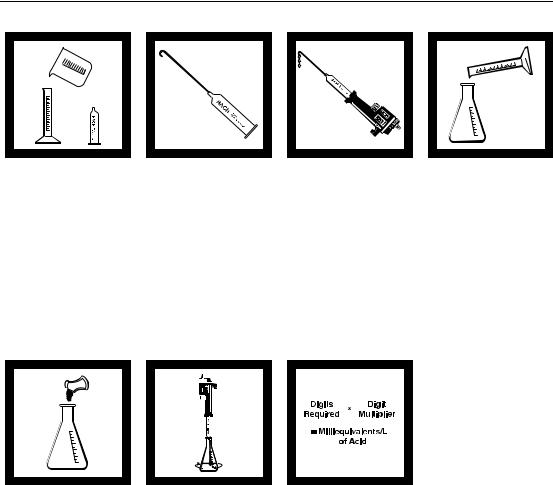
Method 8200
ACID-BASE (10 to 4000 mg/L as meq/L)
Acid Determination
1. Select the sample volume corresponding to the expected acid concentration in milliequivalents (meq)/L or normality (N) from
Table 1.
Note: See Sampling and Storage following these steps.
5. Add the contents of one Phenolphthalein Indicator Powder Pillow and swirl to mix. The solution should be colorless.
Note: Four drops of Phenolphthalein Indicator Solution may be substituted for the Phenolphthalein Indicator Powder Pillow.
2. Insert a clean delivery tube into the appropriate Sodium Hydroxide Titration Cartridge. Attach the cartridge to the titrator body. See
General Description, Step-by-Step, for assembly instructions.
6. Place the delivery tube tip into the solution and swirl the flask while titrating with sodium hydroxide until a light pink color forms and persists for 30 seconds. Record the number of digits required.
3. Flush the delivery tube by turning the delivery knob to eject a few drops of titrant. Reset the counter to zero and wipe the tip.
Note: For added convenience use the TitraStir® Stir Plate. See General Description, Step 3 in Step-by-Step.
7. Calculate:
Digits Required x
Digits Multiplier = Milliequivalents per Liter of Acid
Note: To determine the normality of the sample, divide the milliequivalents per liter obtained by 1000.
4. Use a graduated cylinder or pipet to measure the sample volume from
Table 1. Transfer the sample into a clean 250-mL Erlenmeyer flask. Dilute to about the 100-mL mark with deionized water, if necessary.
29

ACID-BASE, continued
Table 1
Range meq/L |
Range N |
Sample |
|
Titration |
Catalog |
Digit |
Volume (mL) |
|
Cartridge |
Number |
Multiplier |
||
|
|
|
||||
|
|
|
|
|
|
|
1-4 |
0.001-0.004 |
100 |
1.6 N NaOH |
14379-01 |
0.02 |
|
|
|
|
1.6 N H2SO4 |
14389-01 |
|
|
4-10 |
0.004-0.01 |
50 |
1.6 N NaOH |
14379-01 |
0.04 |
|
|
|
|
1.6 N H2SO4 |
14389-01 |
|
|
10-40 |
0.01-0.04 |
100 |
8 |
N NaOH |
14381-01 |
0.1 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
20-80 |
0.02-0.08 |
50 |
8 |
N NaOH |
14381-01 |
0.2 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
50-200 |
0.05-0.2 |
20 |
8 |
N NaOH |
14381-01 |
0.5 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
100-400 |
0.1-0.4 |
10 |
8 |
N NaOH |
14381-01 |
1.0 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
200-800 |
0.2-0.8 |
5 |
8 |
N NaOH |
14381-01 |
2.0 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
500-2000 |
0.5-2 |
2 |
8 |
N NaOH |
14381-01 |
5.0 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
1000-4000 |
1-4 |
1 |
8 |
N NaOH |
14381-01 |
10.0 |
|
|
|
8 N H2SO4 |
14391-01 |
|
|
|
|
|
8 |
N HCl |
14390-01 |
|
30
 Loading...
Loading...