Page 1

Technical
Publications
Direction 2291859-100
Rev. 1
LOGIQ 7
GE Medical Systems
0459
Quick Guide
Copyright© 2002 By General Electric Co.
Operating Documentation
Page 2

Regulatory Requirement
GE Medical Systems
GE Medical System: Telex 3797371
P. O. Box 414, Milwaukee, Wisconsin 53201 U.S.A.
(Asia, Pacific, Latin America, North America)
GE Ultraschall TEL: 0130 81 6370 toll free
Deutschland GmbH & Co. KG TEL: (49)(0) 212.28.02.207
Beethovenstraße 239, Postfach 11 05 60, D-42655 Solingen
GERMANY
Page 3

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 A
Revision History
REV
0
1
PAGE REVISION
NUMBER HISTORY
Title Rev. 1
A and B Rev. 1
1-54 Rev. 1
DATE
November 1, 2001
November 15, 2002
List of Effective Page
REASON FOR CHANGE
Initial Release
R2.1.0 Release
Please verify that you are using the latest revision of this document. Information pertaining to this document is maintained on GPC (GE Medical
Systems Global Product Configuration). If you need to know the latest revision, contact your distributor, local GE Sales Representative or in the
USA call the GE Ultrasound Clinical Answer Center at 1-800-682-5327 or 262-524-5698.
Page 4
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 B
CAUTION
FOR USA ONLY
“United Sates law restricts this device to sale or use by or on the order of a physician” if sold in the United States.
Page 5
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 1
System Power
Power On
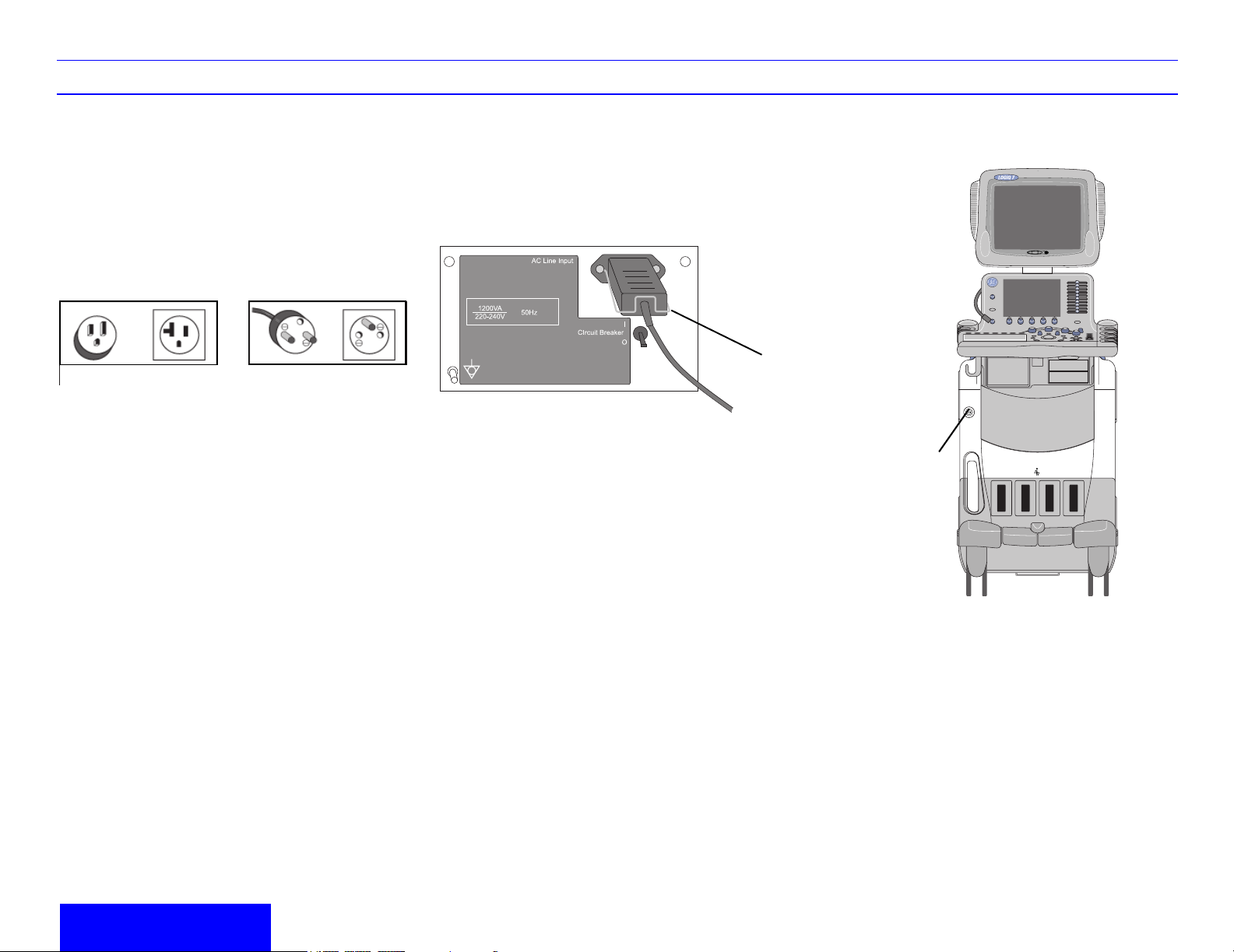
To connect the system to the electrical supply:
1. Ensure that the wall outlet is of the appropriate
type.
12
ab
a
Figure 1-1. Example Plug and Outlet
Configurations
1. 100-120 VAC, 1200 VA
a, b Plug and Outlet Configuration
2. 220-240 VAC, 1200 VA
a, b Plug and Outlet Configuration
2. Ensure that the power switch is turned off.
b
3. Unwrap the power cable. Make sure to allow
sufficient slack in the cable so that the plug is
not pulled out of the wall if the system is moved
slightly.
4. Attach the power plug to the system and secure
it in place by using the retaining clamp.
a
Figure 1-2. Power Plug
a. Retaining clamp for power plug
CAUTION: Ensure that the retaining clamp for the
power plug is fixed firmly.
Use caution to ensure that the power cable does
not disconnect during system use. If the system is
accidentally unplugged, data may be lost.
Press the Power switch to turn the power on. The
circuit breaker must also be in the on position.
a
Preparing for an Exam
Figure 1-3. Power Switch Location
a. Power Switch Location
Page 6
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 2
Power Off
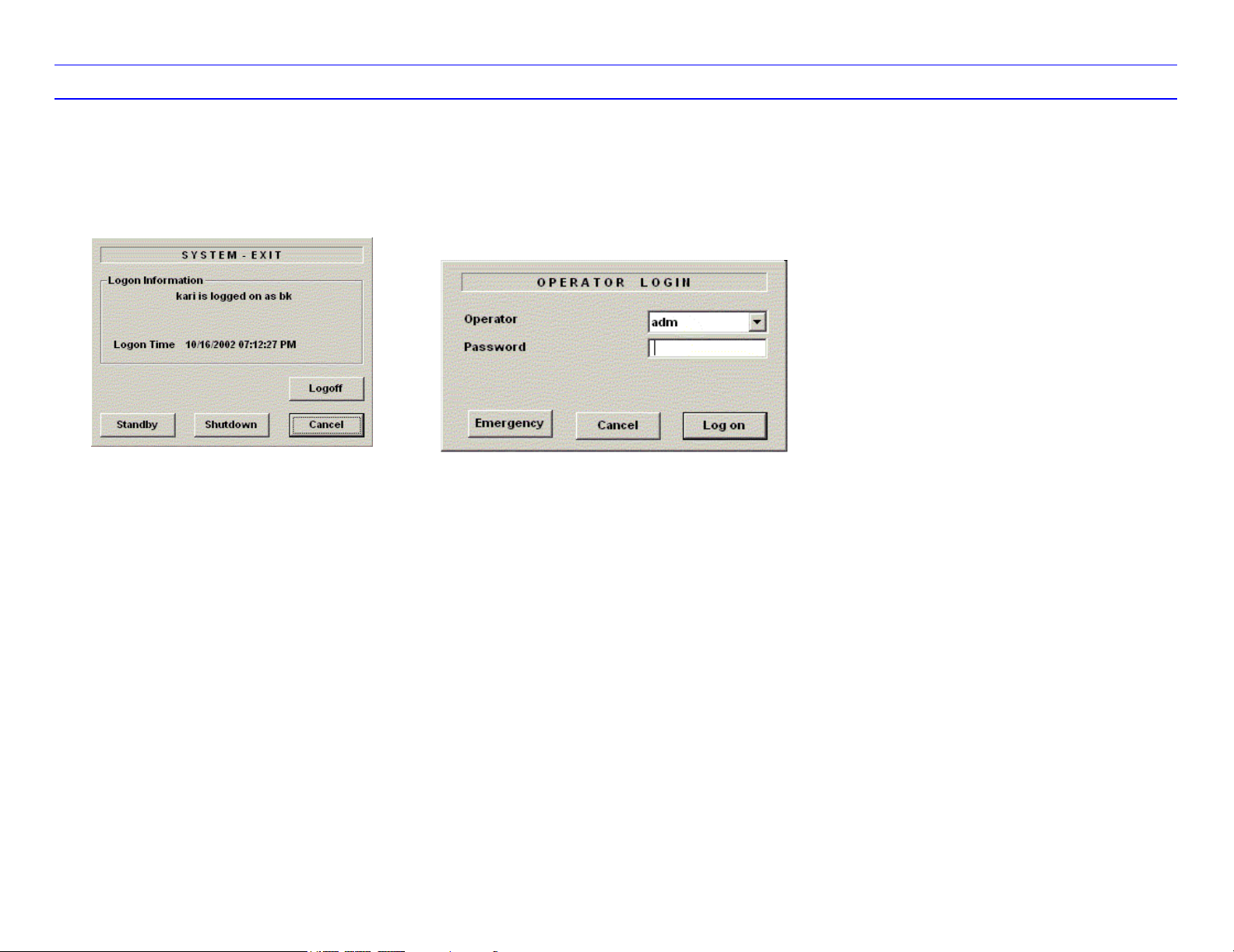
To power down the system:
1. Press the Power switch at the front of the
system once.
2. The System-Exit window is displayed.
3. Using the Trackball or Tab key, select
Shutdown.
The shutdown process takes about 30 seconds
(the Power On light is green) and is completed
when the control panel illumination is turned off
(the Power On light is amber).
4. Disconnect the probes.
Clean or disinfect all probes as necessary.
Store them in their shipping cases to avoid
damage.
Starting an Exam
You need to select a pre-configured dataflow that
sets up the ultrasound system to work according to
the services associated to the dataflow.
1. Select your Operator Login and type in your
Password:
2. Press Log on.
3. Fill in the New Patient menu as described on
Page 3.
OR,
If the patient name is on the patient record list,
1. Trackball to the patient’s name to highlight the
name, (or perform a search to locate the
patient) then press Select Patient.
Page 7
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 3
Starting an Exam
New Patient
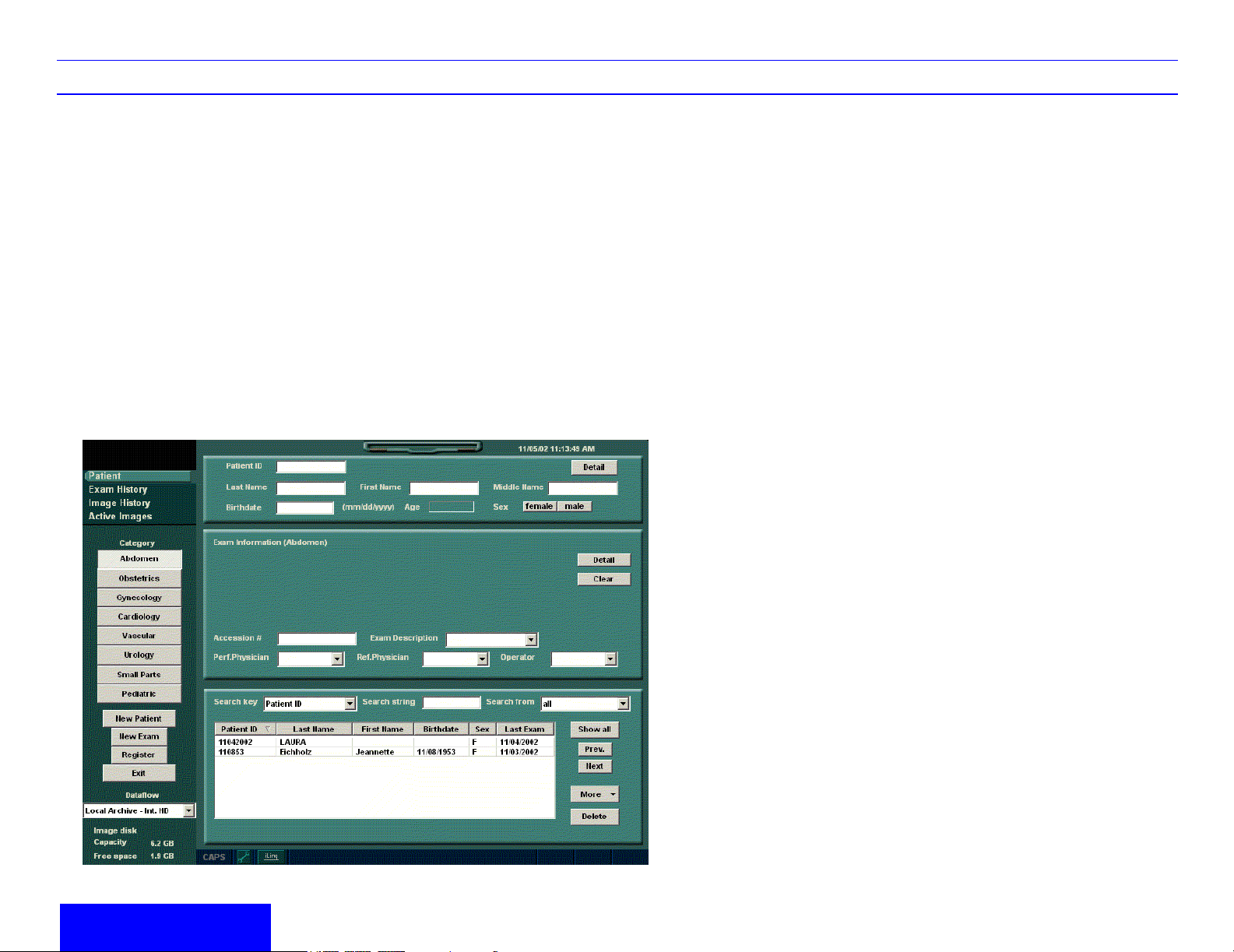
To start a new patient’s exam,
1. Press Patient. Press the New Patient button on
the Patient menu.
2. Select the Exam Category.
3. Type the Patient ID, Patient Name, Birthdate,
etc.
4. Press the Register button on the Patient menu
(DO NOT press Register if you are
automatically generating a patient ID).
5. Press Scan, B-Mode, Esc, or Exit. Select the
probe from the Touch Panel.
1
2
3
4
Probe Selection
Select a probe from the Touch Panel (the system
automatically selects the last-used application for
this probe).
Patient Entry Menu
Image Management Window [1]
Access to this patient’s exam history and image
management features.
5
1
3
1
1
2
1
6
6
2
5
1
7
3
7
1
4
Category Selection Window [2]
Select the appropriate exam application.
Function Selection Window [3]
New Patient is used to clear the patient entry
screen to input a new patient’s data into the
database. New Exam is used to create a new exam
on a current patient. Register is used to enter new
patient information into the database prior to the
actual exam being performed. Exit returns you to
scanning.
Dataflow [4]
Selects this exam’s dataflow preference.
Patient Information and Detail Windows [5]
Patient ID, Name, Birthdate, Age, Sex, Patient’s
Address, Telephone Number and Comments
Exam Information and Detail Windows [6]
Contains pertinent exam specific information.
Patient List Window [7]
Lists the patients in the database. “Search key”
enables searching list by Patient ID, Last Name,
First Name, Birthdate, Sex and Last Exam date.
“Search string” and “Search from” fields help define
the search parameters. More gives you options like
moving images and DICOM properties. Delete is
use to remove the selected patient from the
database.
Preparing for an Exam
Page 8
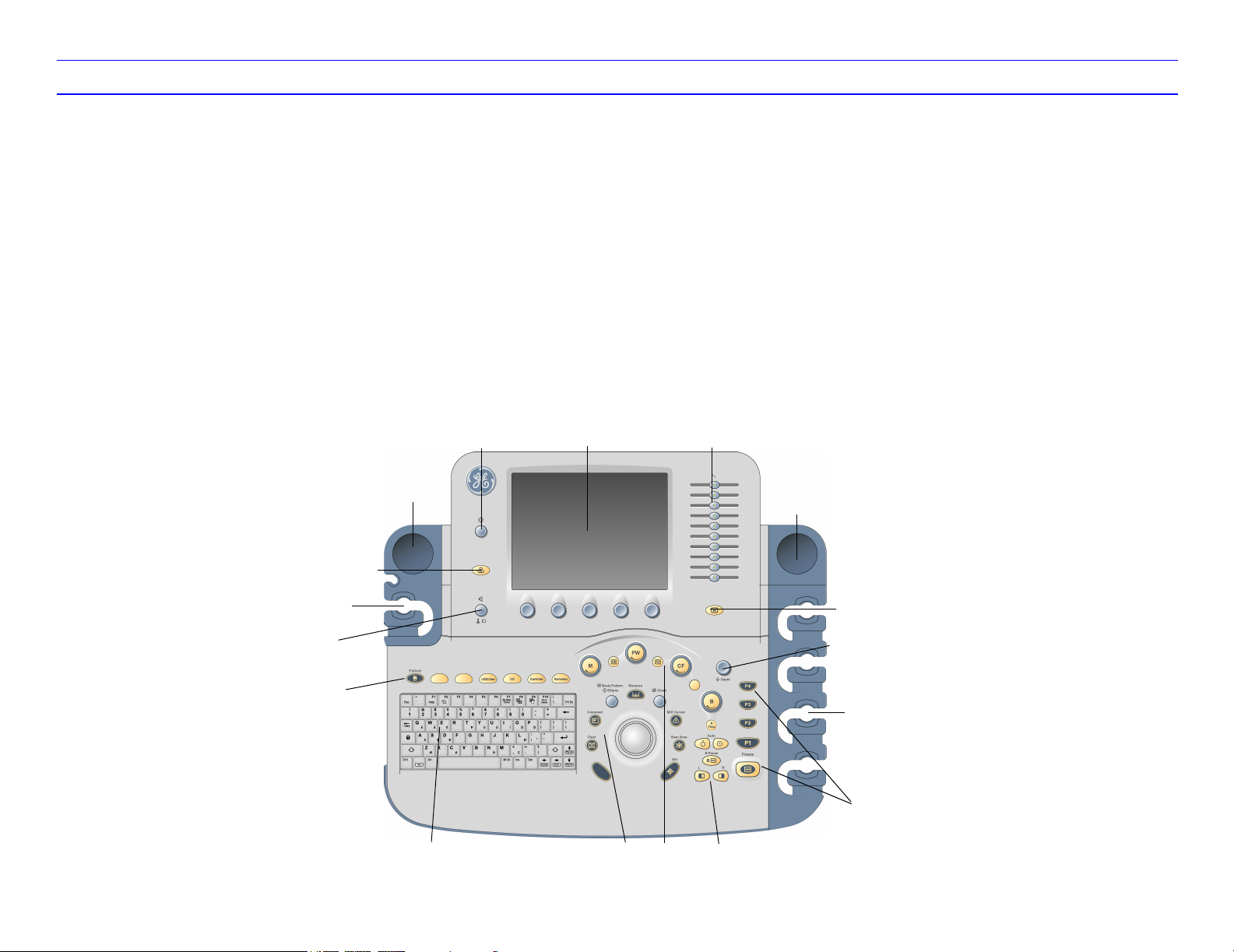
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 4
LOGIQ 7 Control Panel Tour
1. Touch Panel. Touch the Touch Panel to adjust
controls.
At the bottom of the Touch Panel, there are five
combination rotary dials/push buttons. The
functionality of these keys changes, depending
upon the currently displayed menu. Press the
button to switch between controls (as with
Focus Position/Number), or rotate the dial to
adjust the value.
2. Touch Panel Brightness. Rotate to adjust.
3. Video. Press to control the VCR.
4. Audio Volume. Press to turn microphone on/off;
rotate to adjust speaker volume.
5. TGC. Move slide pots left/right to adjust TGC.
6. Reverse. Press to invert the image left/right.
7. Additional Feature Keys. Patient, LOGIQView,
3D, Contrast, Harmonics. Press to activate
these controls.
8. Keyboard. Use the keyboard to enter patient
information and annotations.
9. Mode/Gain Keys: M Mode, Pulsed Wave
Doppler (PW) Modes, Power Doppler Imaging
(PDI) Mode, Color Flow (CF) Mode, B Mode,
and B Flow. Press these key to activate the
mode; rotate the key to adjust the Gain.
5
15
2
1
10. Imaging/Measurement Keys: Clear, Comment,
Body Pattern, Ellipse, Measure, Zoom, M/D
Cursor, Scan Area, Set. Press or rotate these
keys, as necessary.
11. Depth. Rotate to adjust the Depth.
12. Imaging Feature Keys: Auto Optimize On/Off, B
Pause, Multi Image Left/Right Select. Press
these keys to activate/deactivate these
functions.
13. Freeze and Print Keys. Press Freeze to
freeze the image; press the P keys to archive,
print, or send the image.
14. Probe Holder.
15. Gel Holder.
15
14
4
3
6
11
7
14
13
8
10
9
12
Page 9
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 5
LOGIQ 7 Touch Panel Tour
In general, the key status is indicated at the top of
the key. There are different types of Touch Panel
keys:
1. Press to toggle control on/off.
2. Progression keys are used to assess the
impact of the control on the image
progressively.
1
2
3. Progress/Select keys are used for controls that
have three or more choices.
4. Rotate the knob below the Touch Panel to set
values.
5. Press knob below the Touch Panel to select
additional control, then rotate the knob to set
values.
6. Press to move to the next Touch Panel page.
6
3
Control Panel/
Touch Panel Tour
4
4
5
Page 10
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 6
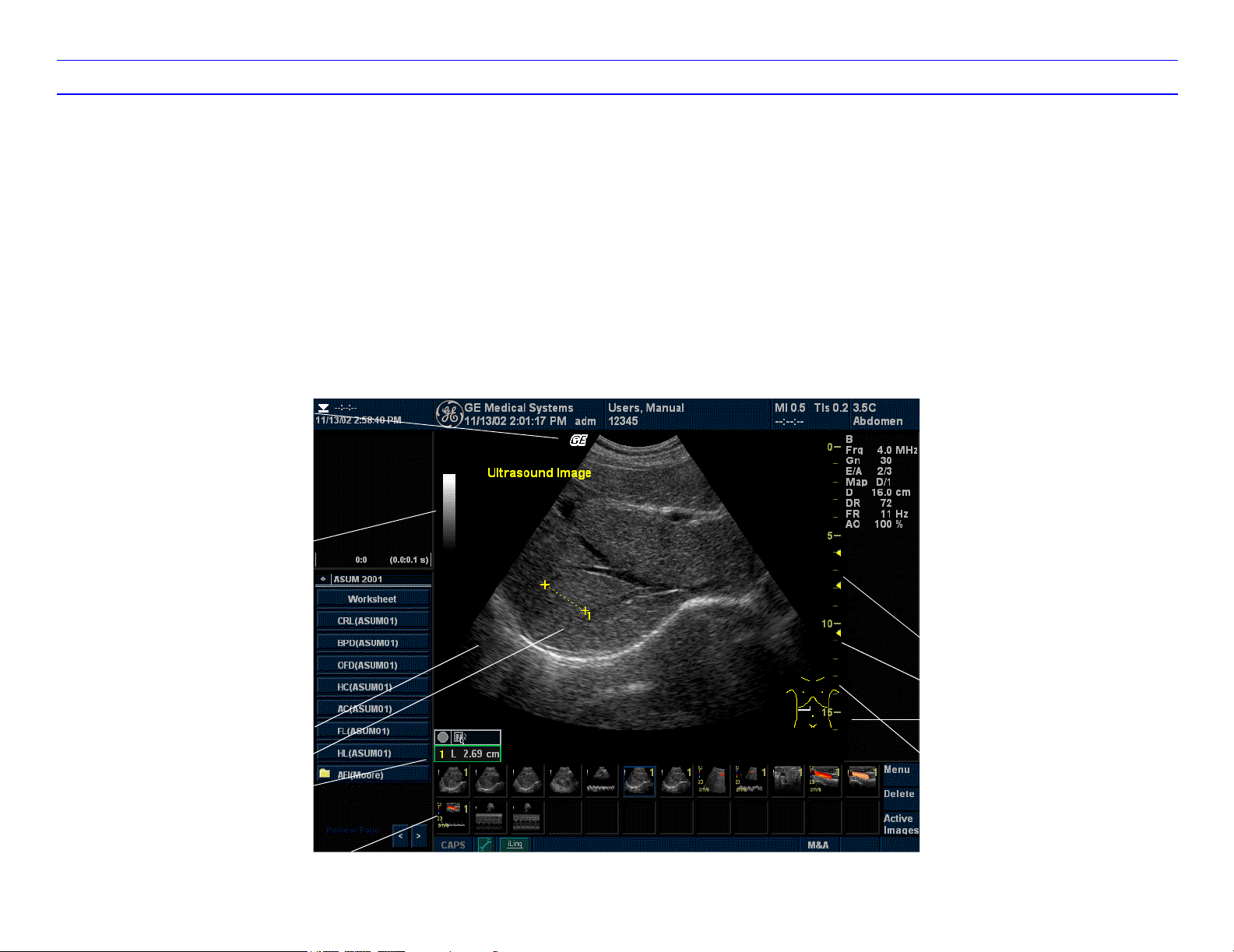
LOGIQ 7 Monitor Display Tour
1. Institution/Hospital Name, Date, Time, Operator
Identification.
2. Patient Name, Patient Identification.
3. Acoustic Output Readout
4. GE Symbol (Probe Orientation Marker). The
symbol is reversed on flipped images.
5. Image Preview, zoom reference box: .
6. Gray/Color Bar.
7. Cine Gauge. CINE frame/Total # of CINE
frames (28/51), Frame time/total loop time.
4
5
6
7
8. Measurement Summary Window.
9. Image.
10. Measurement.
11. Measurement Results Window.
12. Probe Identifier. Exam Study.
13. Imaging Parameters by Mode.
14. Focal Zone.
15. TGC would display here if preset (not shown on
the image).
16. Body Pattern.
132
17. Depth Scale.
18. Image Management Menu: Menu, Delete, and
Active Images.
19. Image Clipboard.
20. Caps Lock: On or Off.
21. Service Browser icon (wrench), iLinq icon, and
system messages display.
22. Trackball Functionality Status: Scroll,
M&A (Measurement and Analysis), Position,
Size, Scan Area Width and Tilt.
12
13
10
11
8
14
15
9
19
222120
16
17
18
Page 11
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 7
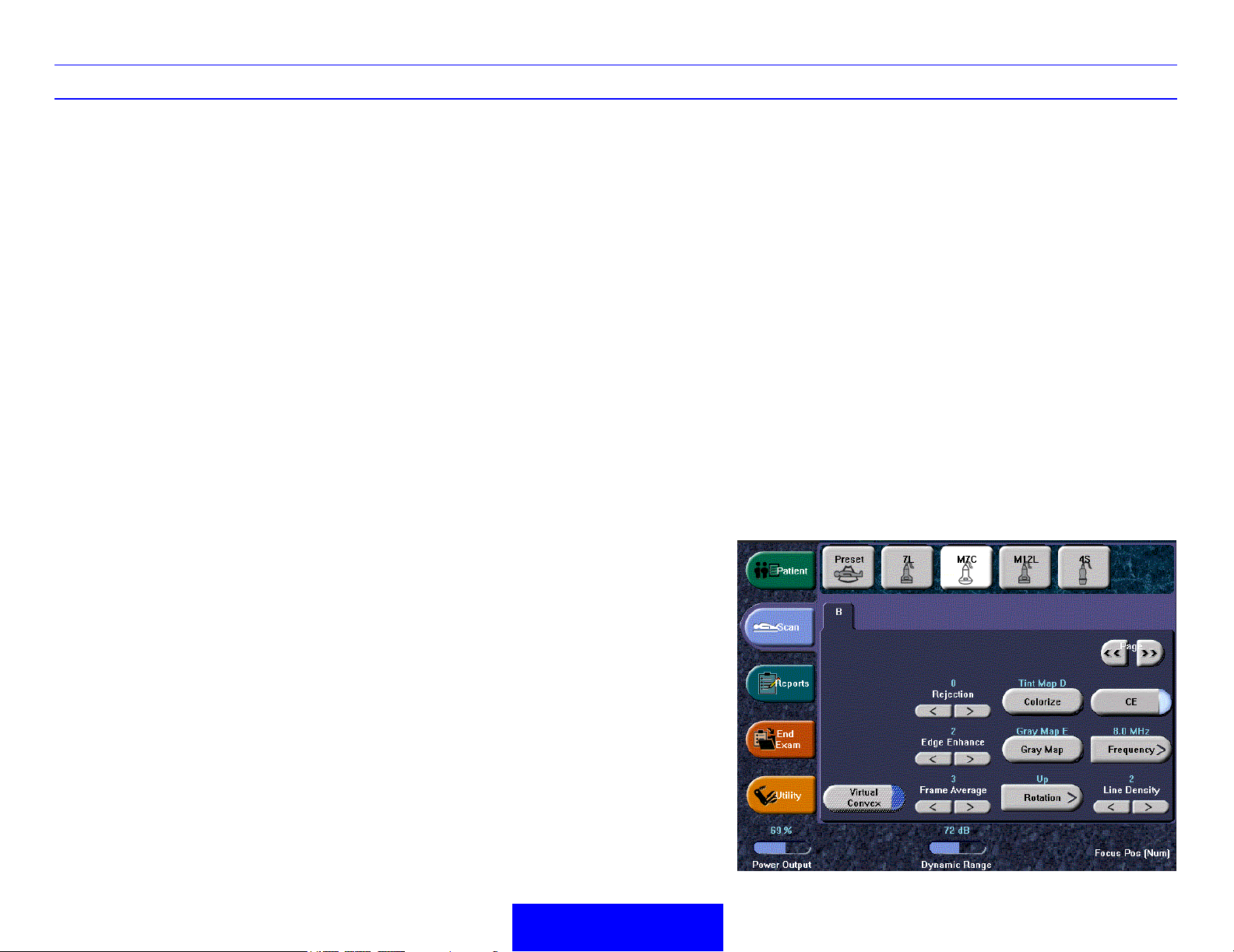
B/M-Mode Image Optimize
Power Output
Optimizes image quality and allows user to reduce
beam intensity. 10% increments between 0-100%.
Values greater than 0.1 are displayed.
Dynamic Range
Dynamic Range controls how echo intensities are
converted to shades of gray, thereby increasing the
adjustable range of contrast.
Focus Number and Position
Increases the number of transmit focal zones or
moves the focal zone(s) so that you can tighten up
the beam for a specific area. A graphic caret
corresponding to the focal zone position(s) appears
on the right edge of the image.
NOTE: Push key to toggle between Focus Number
and Focus Position.
Edge Enhance
Edge Enhance brings out subtle tissue differences
and boundaries by enhancing the gray scale
differences corresponding to the edges of
structures. Adjustments to M Mode's edge
enhancement affects the M Mode only.
Colorize
Enables gray scale image colorization. To
deactivate, reselect a Gray Map.
Gray Map
Determines how the echo intensity levels received
are presented as shades of gray.
Rotation (Up/Down)
Rotates the image by selecting the value from the
pop up menu.
Coded Excitation (CE)
B Softener
Affects amount of lateral smoothing.
Suppression
Eliminates low-level echoes associated with
acoustic/electrical noise.
B Flow Image Optimize
Provides intuitive representation of non-quantitative
hemodynamics in vascular structures.
Sensitivity/PRI
Adjusts the sample rate for the flow signal.
Background On/Off
Background On views the anatomy roadmap;
Background Off views flow information only.
Virtual Convex
On Linear and Sector probes, provides a larger field
of view in the far field.
Rejection
Selects a level below which echoes will not be
amplified (an echo must have a certain minimum
amplitude before it will be processed).
Frame Average
Temporal filter that averages frames together. This
has the effect of presenting a smoother, softer
image.
Improves image resolution and
penetration in the far field.
Frequency
Multi Frequency mode lets you
downshift to the probe's next lower
frequency or shift up to a higher
frequency.
Line Density
Optimizes B Mode frame rate or spatial
resolution for the best possible image.
B/M Mode
Image Optimize
Page 12
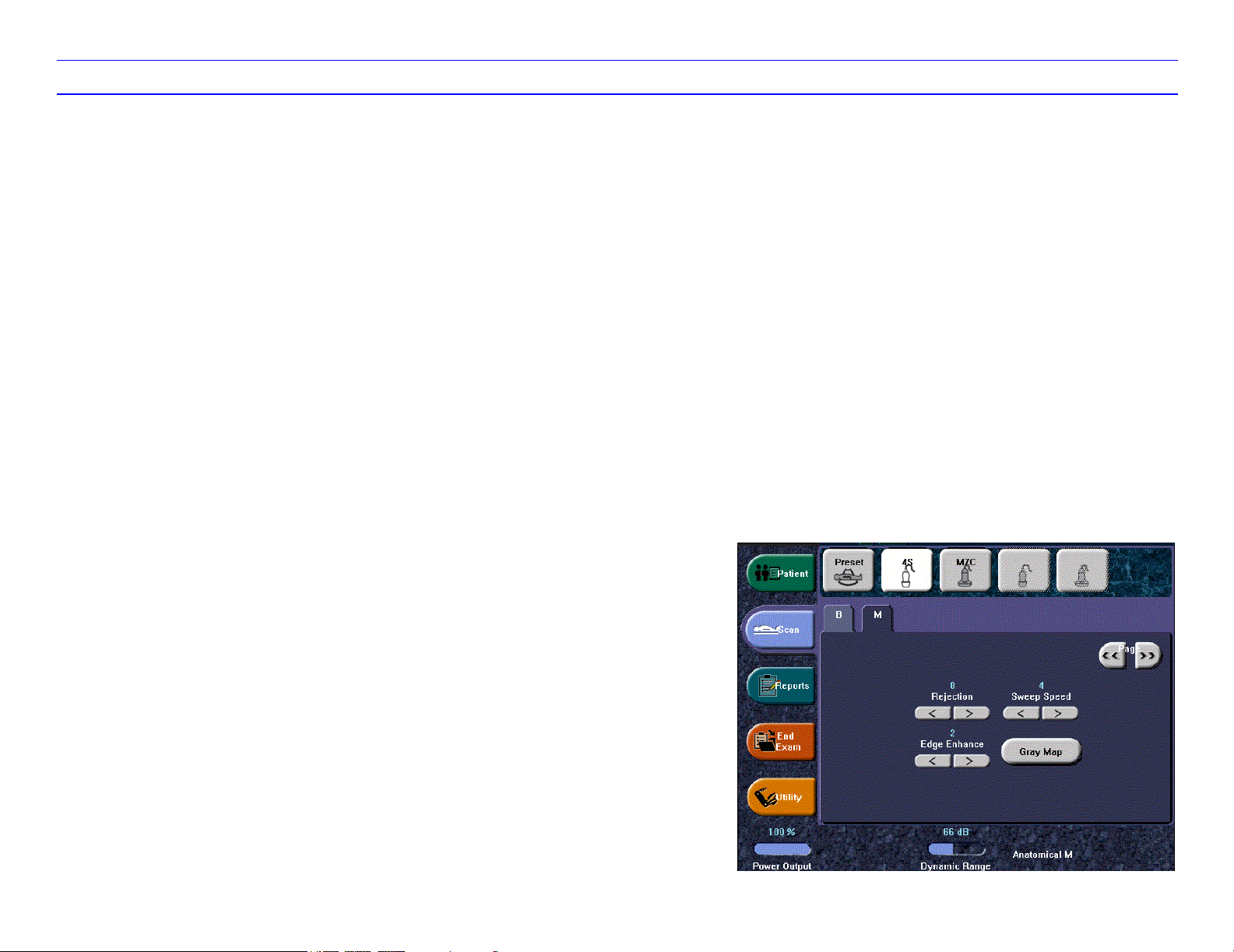
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 8
B/M-Mode Image Optimize (continued)
Anatomical M Mode
Allows you to rotate or move the M line in order to
image difficult-to-reach anatomy.
Sweep Speed
Changes the speed at which the timeline is swept.
Full Timeline
Expands display to full timeline display.
Display Format
Changes the horizontal/vertical display layout
between B-Mode and M-Mode.
B-Mode Control Panel Controls
Auto Optimize
Automatic Tissue Optimization optimizes the image
based upon a specified Region of Interest (ROI) or
anatomy within the display.
Reverse
Flips the image left/right. The GE symbol flips
accordingly.
Multi Image
Press L to activate Multi Image; press R/L to toggle
between live image.
B/M Mode Scanning Hints
Auto Optimize. Improves imaging performance
while reducing optimization time.
Coded Harmonics. Enhances near field resolution
for improved small parts and OB/GYN imaging as
well as far field penetration.
B Flow. Provides a more intuitive
representation of non-quantitative
hemodynamics in vascular
structure.
Frequency. Changes system parameters to best
optimize for a particular patient type.
Maps. There is an inter-dependency between gray
maps, gain, and dynamic range. If you change a
map, revisit gain and dynamic range settings.
Dynamic Range. Affects the amount of gray scale
information displayed.
Edge Enhance. Better delineates the amount of
border sharpness.
Frame Average. Smooths the image by averaging
frames. Affects the amount of speckle reduction.
Zoom
Magnifies a zoom region of interest, which is
magnified to approximately the size of a full-sized
image. An un-zoomed reference image is displayed
adjacent to the zoom window. The system adjusts
all imaging parameters accordingly. Press to
activate/deactivate; rotate to increase/decrease
zoom factor. Use the Trackball to position the
Zoom ROI.
Page 13
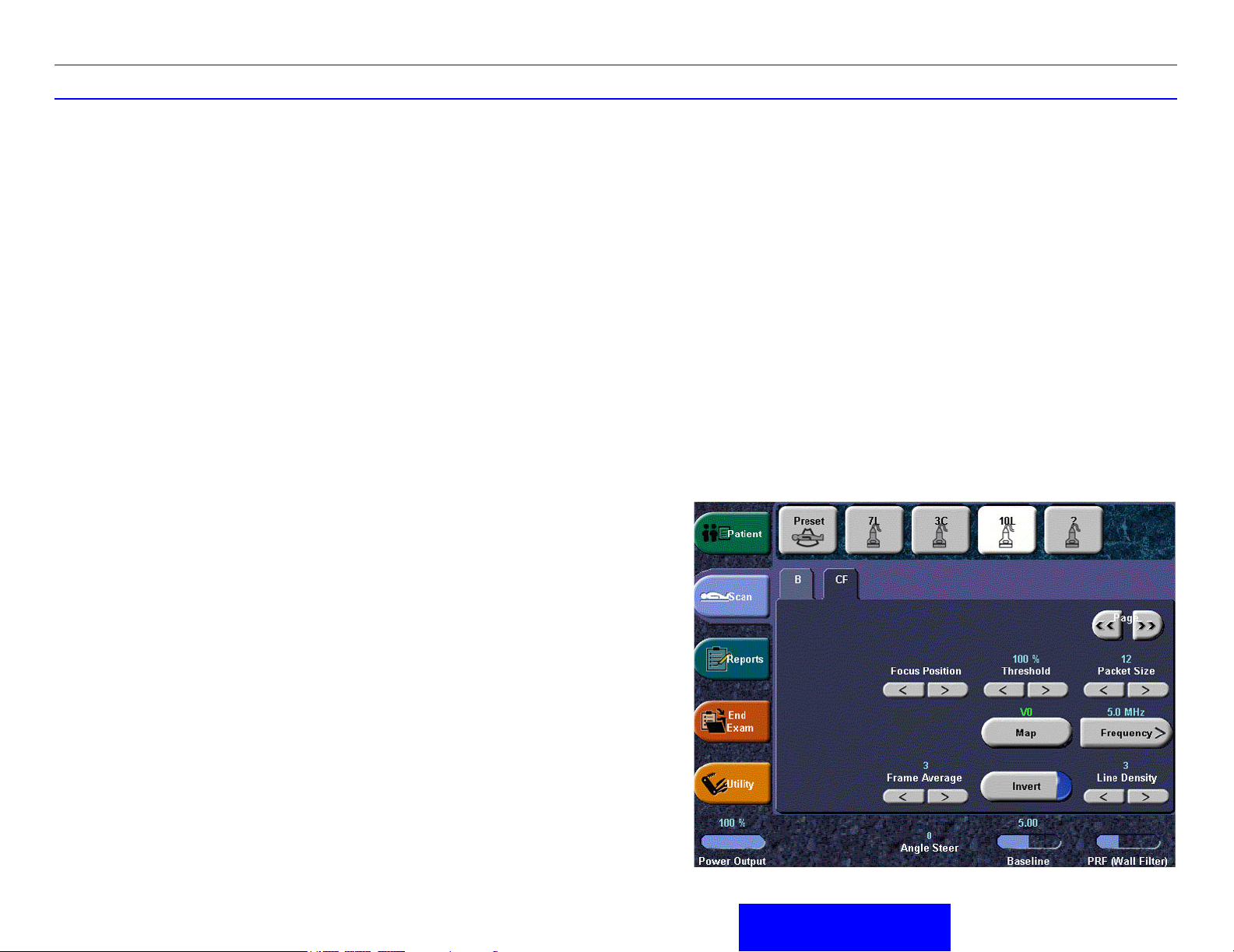
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 9
Color Flow/PW Doppler Image Optimize
Angle Correct
Estimates the flow velocity in a direction at an angle
to the Doppler vector by computing the angle
between the Doppler vector and the flow to be
measured.
Angle Steer/Fine Angle Steer
Angle Steer slants the Color Flow region of interest
or the Doppler M line to obtain a better Doppler
angle. Press Angle Steer to access Fine Angle
Steer. Fine Angle Steer allows you to steer the
Doppler cursor left/right 10 and 20 degrees.
Available from the Doppler Mode Touch Panel.
Baseline
Adjusts the baseline to accommodate faster or
slower blood flows to eliminate aliasing.
PRF/Wall Filter
Velocity scale determines pulse repetition
frequency. If the sample volume gate range
exceeds single gate PRF capability, the system
automatically switches to high PRF mode. Multiple
gates appear, and HPRF is indicated on the display.
Wall Filter insulates the Doppler signal from
excessive noise caused from vessel movement.
NOTE: Push key to toggle between PRF and Wall
Filter.
Threshold
Threshold assigns the gray scale level at which
color information stops.
Map
Allows a specific color map to be selected. After a
selection has been made, the color bar displays the
resultant map.
Invert
Allows blood flow to be viewed from a different
perspective, i.e. red away (negative velocities) and
blue toward (positive velocities). The real-time or
frozen image can be inverted.
Packet Size
Controls the number of samples gathered for a
single color flow vector.
Color Flow/Doppler Mode
Image Optimize
Page 14
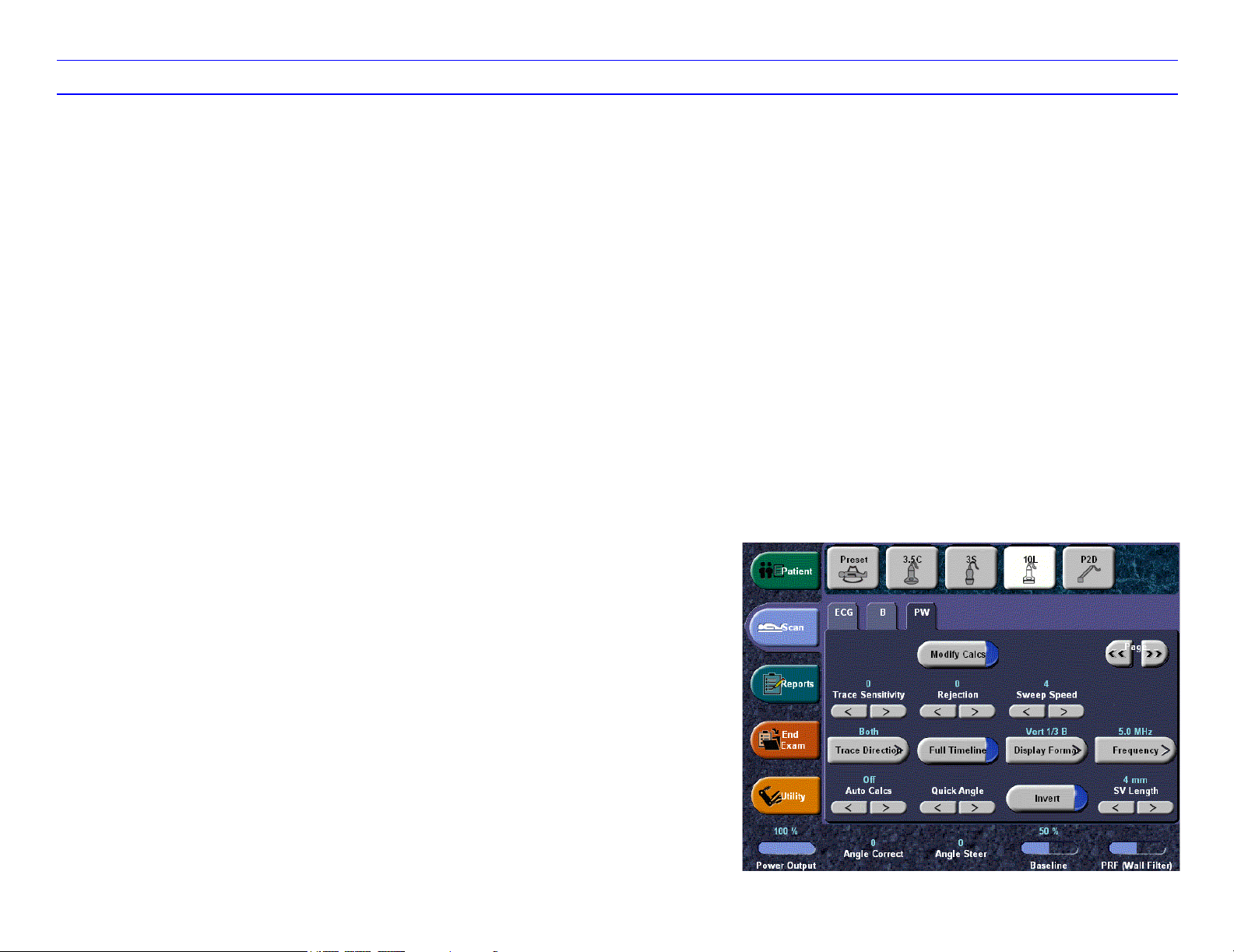
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 10
Color Flow/PW Doppler Image Optimize (continued)
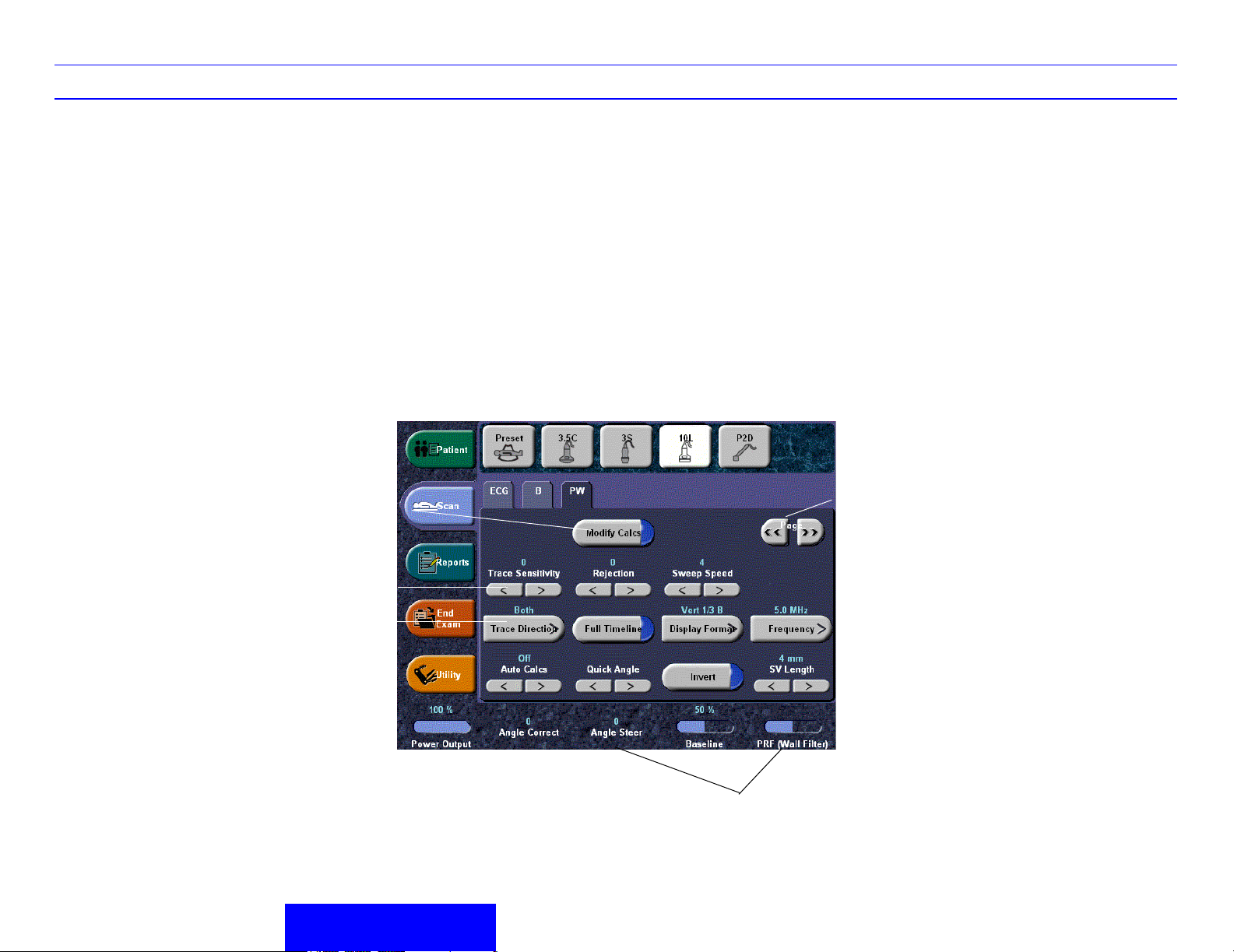
Modify Auto Calcs
Press to select desired Auto Calcs.
Trace Sensitivity
In Auto Calcs, increase to pick up more signal or
decrease to pick up less signal.
Trace Direction
Select Above, Below, or Both.
Auto Calcs
Specify Auto Calcs Off/Live/Frozen.
Quick Angle
Quickly adjusts the angle by 60 degrees.
Sample Volume Gate Length
Sizes the sample volume gate.
Trace Method
Specify Max, Mean, or Off.
Duplex
Duplex on: simultaneous B-Mode and PW Mode;
Duplex off: toggle live B-Mode and PW via B Pause.
PW/CF Ratio
Adjusts the ratio between Spectral Doppler and
Color Flow.
Compression
Increase to make frozen image appear more
contrasty; decrease to make frozen image appear
softer.
Time Resolution
Lower makes the image appear smoother; higher
makes the image appear sharper.
Color Flow Control Panel Control
Scan Area. Toggles between the CFM ROI window
size and position.
M/D Cursor. Activates the Doppler cursor.
Scanning Hints
Line Density. Trades frame rate for
sensitivity and spatial resolution. If the
frame rate is too slow, reduce the size
of the region of interest, select a different
line density setting, or reduce the packet
size.
Wall Filter. Affects low flow sensitivity
versus motion artifact.
To improve sensitivity. Increase Gain,
decrease PRF, increase Power Output,
adjust Line Density, decrease Wall Filter,
increase Frame Averaging, increase
Packet Size, reduce ROI to the smallest
reasonable size, and position the Focal
Zones properly.
To decrease motion artifact. Increase the PRF,
and increase the Wall Filter.
To eliminate aliasing. Increase the PRF and lower
the Baseline.
For venous imaging. Ensure that you have
selected the vascular exam category, select a
venous application, select the appropriate probe for
very superficial structure, select two focal zones,
adjust the depth to the anatomy to be imaged,
maintain a low gain setting for gray scale, activate
Color Flow, maintain the PRF at a lower setting, and
increase Frame Averaging for more persistence.
Page 15
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 11
Basic Measurements
NOTE: The following instructions assume that you
first scan the patient and then press Freeze.
Distance and Tissue Depth Measurements
1. Press Measure once; an active caliper
displays.
2. To position the active caliper at the start point
(distance) or the most anterior point (tissue
depth), move the Trackball.
3. To fix the start point, press Set. The system
fixes the first caliper and displays a second
active caliper.
4. To position the second active caliper at the end
point (distance) or the most posterior point
(tissue depth), move the Trackball.
5. To complete the measurement, press Set. The
system displays the distance or tissue depth
value in the measurement results window.
NOTE: Before you complete a measurement:
To toggle between active calipers, press
Measure.
To erase the second caliper and the current
data measured and start the measurement
again, press Clear once.
NOTE: To rotate through and activate previously
fixed calipers, turn Cursor Select.
NOTE: After you complete the measurement, to
erase all data that has been measured to this point,
but not data entered onto worksheets, press Clear.
Circumference/Area (Ellipse) Measurement
1. Press Measure once; an active caliper
displays.
2. To position the active caliper, move the
Trackball.
3. To fix the start point, press Set. The system
fixes the first caliper and displays a second
active caliper.
4. To position the second caliper, move the
Trackball.
5. Turn the Ellipse control; an ellipse with an
initial circle shape appears.
NOTE: Be careful not to press the Ellipse control
as this activates the Body Pattern.
6. To position the ellipse and to size the measured
axes (move the calipers), move the Trackball.
7. To increase the size, turn the Ellipse control in
a clockwise direction. To decrease the size, turn
the Ellipse control in a counterclockwise
direction.
8. To toggle between active calipers, press
Measure.
9. To complete the measurement, press Set. The
system displays the circumference and area in
the measurement results window.
NOTE: Before you complete a measurement:
To erase the ellipse and the current data
measured, press Clear once. The original
caliper is displayed to restart the
measurement.
To exit the measurement function without
completing the measurement, press Clear
a second time.
Circumference/Area (Trace) Measurement
1. Press Measure twice; a trace caliper displays.
2. To position the trace caliper at the start point,
move the Trackball.
3. To fix the trace start point, press Set. The trace
caliper changes to an active caliper.
4. To trace the measurement area, move the
Trackball around the anatomy. A dotted line
shows the traced area.
NOTE: To erase the dotted line but not the trace
caliper, press Clear once. To clear the trace caliper
and the current data measured, press Clear twice.
NOTE: To erase the line (bit by bit) back from its
current point, move the Trackball or turn the
Ellipse control counterclockwise.
5. To complete the measurement, press Set. The
system displays the circumference and the area
in the measurement results window.
NOTE: Before you complete a measurement:
To erase the line (bit by bit) back from its
current point, move the Trackball or turn the
Ellipse control counterclockwise.
To erase the dotted line but not the trace
caliper, press Clear once.
To clear the trace caliper and the current
data measured, press Clear twice.
Basic Measurements/
Calculations
Page 16
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 12
Volum e
1. To make a volume calculation, do one of the
following:
• Make one distance measurement.
• Make two distance measurements.
• Make three distance measurements.
NOTE: Three distances should be done in the
dual format mode (side by side images). One
measurement is usually made in the sagittal
plane and two measurements in the axial plane.
• Make one distance and one ellipse
measurement.
• Make one ellipse measurement.
2. Select Volume.
Time Interval Measurement
1. Press Measure twice; and active caliper with a
vertical dotted line displays.
2. To position the active caliper at the start point,
move the Trackball.
3. To fix the start point, press Set. The system
fixes the first caliper and displays a second
active caliper.
4. To position the second caliper at the end point,
move the Trackball.
5. To complete the measurement, press Set. The
system displays the time interval between the
two calipers in the measurement results
window.
Velocity Measurement
1. Press Measure; an active caliper with a vertical
dotted line displays.
2. To position the caliper at the desired
measurement point, move the Trackball.
3. To complete the measurement, press Set. The
system displays the velocity measurement in
the measurement results window.
PI, RI, S/D Ratio, D/S Ratio or A/B Ratio
Select PI, RI, S/D Ratio, A/B Ratio or D/S Ratio
from the Doppler Touch Panel. Perform velocity
measurements.
1. The first caliper is the start point on the Doppler
waveform. This would be V
velocity for RI, systole for S/D ratio, "A" velocity
for A/B ratio or diastole for D/S ratio.
2. The second caliper is the end-point caliper to
the end point of the Doppler waveform. This
would be V
diastole for S/D ratio, "B" velocity for A/B ratio
or systole for D/S ratio.
NOTE: For the PI calculation, if Trace Auto is not
selected, manually trace the waveform between
V
and Vd.
MAX
NOTE: For the PI calculation, if Trace Auto is on,
the system automatically traces the waveform when
Set is pressed to fix V
for PI, minimum velocity for RI,
d
.
d
for PI, peak
MAX
Worksheets
Measurement/Calculation worksheets are available
to display and edit measurements and calculations.
There are generic worksheets as well as
Application specific worksheets. The worksheets
are selected from the Measurement Touch Panel.
Reports
This feature is not currently available.
Page 17
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 13
Using Probes
Connecting a probe
1. Place the probe's carrying case on a stable
surface and open the case.
2. Carefully remove the probe and unwrap the
probe cable.
3. DO NOT allow the probe head to hang free.
Impact to the probe head could result in
irreparable damage.
4. Turn the connector locking handle clockwise.
5. Align the connector with the probe port and
carefully push into place.
6. Turn the connector locking handle clockwise to
secure the probe connector.
7. Carefully position the probe cable in the probe
cord holder spot so it is free to move, but not
resting on the floor.
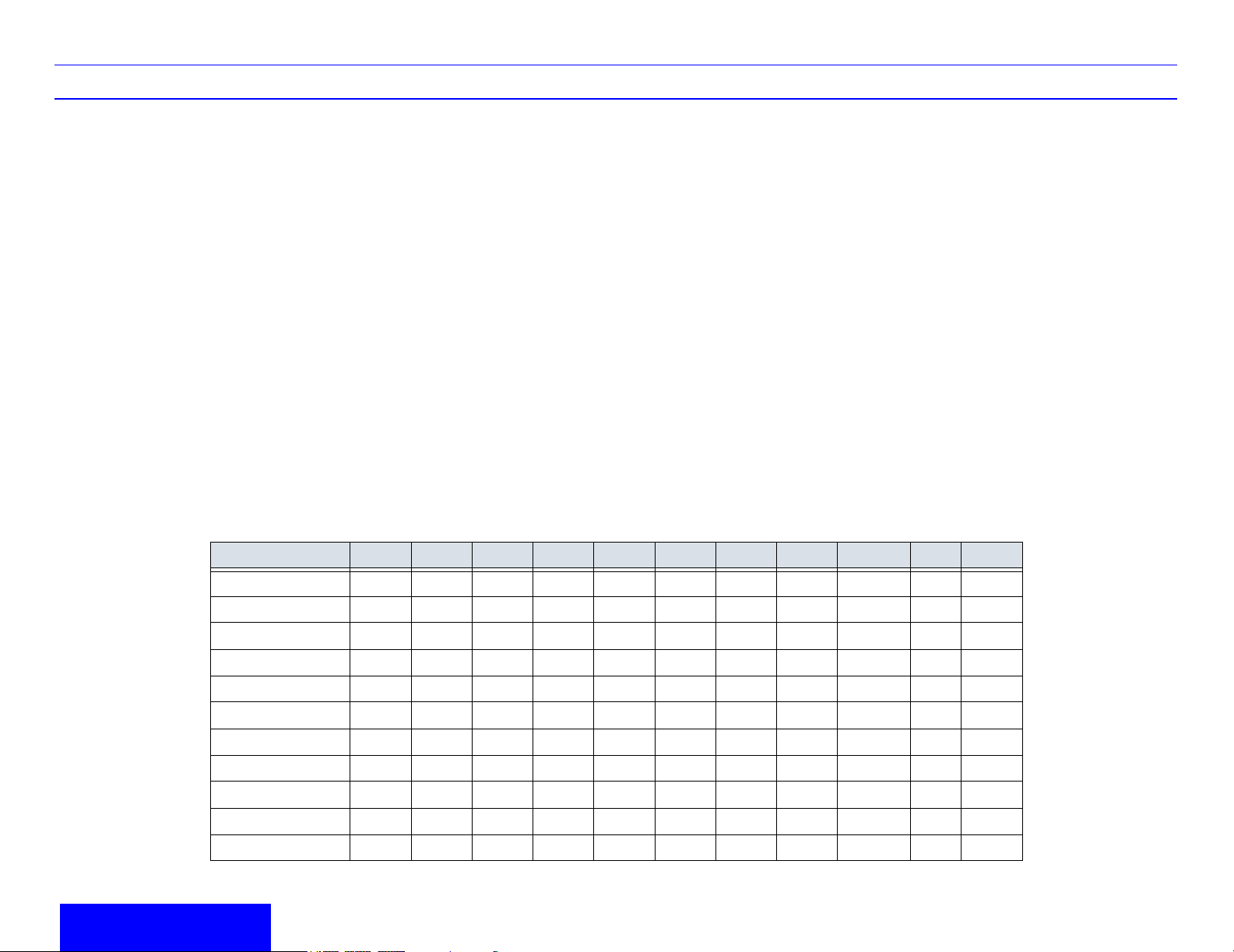
Probe Application 3C 3.5C 5C M7C E8C 7L 10L i12L M12L 3S 10S
Abdomen XXXX XX XX
Small Parts XXX XXX X X
Periph. Vasc. X X X X X X
Activating the probe
Select the appropriate probe from the probe
indicators on the Touch Panel.
The probe activates in the currently-selected
operating mode. The probe's default settings for the
mode and selected exam are used automatically.
Deactivating the probe
When deactivating the probe, the probe is
automatically placed in standby mode.
1. Press the Freeze key.
2. Gently wipe the excess gel from the face of the
probe.
3. Carefully slide the probe around the right side
of the keyboard, toward the probe holder.
Table 1-1: Probe Indications for Use
Ensure that the probe is placed gently in the
probe holder.
Disconnecting the probe
Probes can be disconnected at any time. However,
the probe should not be selected as the active
probe.
1. Move the probe locking handle
counterclockwise. Pull the probe and connector
straight out of the probe port.
2. Carefully slide the probe and connector away
from the probe port and around the right side of
the keyboard. Ensure the cable is free.
3. Be sure that the probe head is clean before
placing the probe in its storage box.
Using Probes
OB/GYN XXXXX X
Pediatrics X X X X X X X
Neonatal X X X X
Urology XXXXX XX
Surgery XX
Endocavity X
Transcranial X
Cardiac XX
Page 18
LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 14
Probe Features
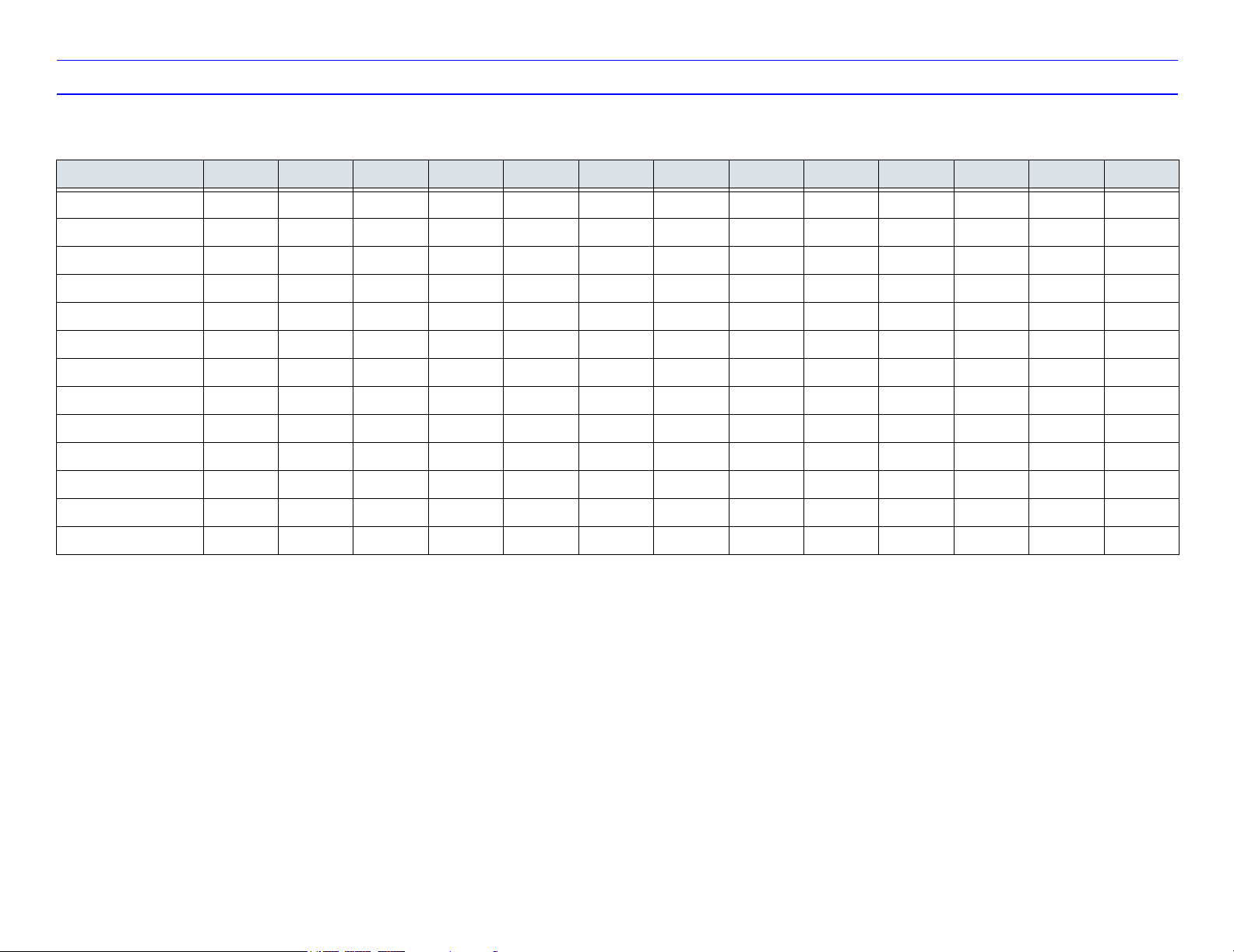
Table 1-2: Probe Features
Probe Feature 3C 3.5C 5C M7C E8C 7L 10L i12L M12L 3S 10S P2D P6D
Coded Excitation X
Coded Harmonics X X X X X X X
B-Flow X
Coded Contrast X
LOGIQ View XXXXXXXXXXX
Virtual Convex XXXX X
Easy 3D XXXXXXXXXXX
Advanced 3D XXXXXXXXXXX
Anatomical M ModeXXXXXXXXXXX
M Color Flow XXXXXXX XXX
TruAccess XXXXXXXXXXX
Biopsy XXXXXXX X
Non-Imaging CW XX
Page 19

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 15
Biological
Probe Cleaning and Disinfection Instructions
Probe Safety
WARNING
Hazard
CAUTION
Ultrasound probes are highly sensitive medical instruments that can easily be damaged by improper handling. Use care when handling and protect from damage when not
in use. DO NOT use a damaged or defective probe. Failure to follow these precautions can result in serious injury and equipment damage.
Ultrasound transducers can easily be damaged by improper handling and by contact with certain chemicals. Failure to follow these precautions can result in serious injury
and equipment damage.
• Do not immerse the probe into any liquid beyond the level specified for that probe. Never immerse the transducer connector or probe adapters into any liquid.
• Avoid mechanical shock or impact to the transducer and do not apply excessive bending or pulling force to the cable.
• Transducer damage can result from contact with inappropriate coupling or cleaning agents:
• Do not soak or saturate transducers with solutions containing alcohol, bleach, ammonium chloride compounds or hydrogen peroxide
• Avoid contact with solutions or coupling gels containing mineral oil or lanolin
• Avoid temperatures above 60°C.
• Inspect the probe prior to use for damage or degeneration to the housing, strain relief, lens and seal. Do not use a damaged or defective probe.
Adequate cleaning and disinfection are necessary to prevent disease transmission. It is the responsibility of the equipment user to verify and maintain the effectiveness of the
infection control procedures in use. Always use sterile, legally marketed probe sheaths for intra-cavitary and intra-operative procedures.
For neurological intra-operative procedures, use of a legally marketed, sterile, pyrogen free probe sheath is REQUIRED. Probes for neuro surgical use must not be sterilized
with liquid chemical sterilants because of the possibility of neuro toxic residues remaining on the probe.
A defective probe or excessive force can cause patient injury or probe damage:
• Observe depth markings and do not apply excessive force when inserting or manipulating intercavity probes.
• Inspect probes for sharp edges or rough surfaces that could injure sensitive tissue.
In order for liquid chemical germicides to be effective, all visible residue must be removed during the cleaning process. Thoroughly clean the probe, as described on the
following page before attempting disinfection.
Electrical
Hazard
Using Probes
CREUTZFIELD-JACOB DISEASE
Neurological use on patients with this disease must be avoided. If a probe becomes contaminated, there is no adequate disinfecting means.
The probe is driven with electrical energy that can injure the patient or user if live internal parts are contacted by conductive solution:
• DO NOT immerse the probe into any liquid beyond the level indicated by the immersion level diagram. Never immerse the probe connector or probe adaptors into any liquid.
• DO NOT drop the probes or subject them to other types of mechanical shock or impact. Degraded performance or damage such as cracks or chips in the housing may
result.
• Inspect the probe before and after each use for damage or degradation to the housing, strain relief, lens, and seal. A thorough inspection should be conducted during the
cleaning process.
• DO NOT kink, tightly coil, or apply excessive force on the probe cable. Insulation failure may result.
• Electrical leakage checks should be performed on a routine basis by GE Service or qualified hospital personnel. Refer to the service manual for leakage check procedures.
Page 20

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 16
Probe Cleaning, After Each Use
1. Disconnect probe from ultrasound console and
remove all coupling gel from probe by wiping
with a soft cloth and rinsing with flowing water.
2. Wash the probe with mild soap in lukewarm
water. Scrub the probe as needed using a soft
sponge, gauze, or cloth to remove all visible
residue from the probe surface. Prolonged
soaking or scrubbing with a soft bristle brush
(such as a toothbrush) may be necessary if
material has dried onto the probe surface.
3. Rinse the probe with enough clean potable
water to remove all visible soap residue.
4. Air dry or dry with a soft cloth.
Probe Disinfection, After Each Use
1. Prepare the germicide solution according to the
manufacturer's instructions. Be sure to follow all
precautions for storage, use and disposal.
2. Place the cleaned and dried probe in contact
with the germicide for the time specified by the
germicide manufacturer. High-level disinfection
is recommended for surface probes and is
required for endocavitary and intraoperative
probes (follow the germicide manufacturer's
recommended time).
Probes for neuro surgical intra-operative use
must NOT be sterilized with liquid chemical
sterilants because of the possibility of neuro
toxic residues remaining on the probe.
Neurological procedures must be done with the
use of legally marketed, sterile, pyrogen free
probe sheaths.
3. After removing from the germicide, rinse the
probe following the germicide manufacturer's
rinsing instructions. Flush all visible germicide
residue from the probe and allow to air dry.
Probe Immersion Levels
M12L
M7C
3S
10S
E8C
3C
3.5C
5C
7L
10L
123
1. Fluid Level
2. Aperture
3. Contact face within patient environment
i12L
P2D
P6D
Probe Disinfection Agents
The following high level disinfectant agents have
been approved for use with all probes:
• Cidex OPA
• Cidex
Cidex Plus has been approved for the 8C probe.
Sporox II high level disinfectant has been approved
for the 7L, 10L, 12L, 4S, 8C, and E8C probes.
Pera Safe high level disinfectant has been
approved for the 7L, 10L, 12L, M7C, M12L, and
E8C probes.
The following low level disinfect agents have been
approved for use with all probes:
• Ster Bac Blu
• Sani-Cloth HB (Wipes)
T-Spray and T-Spray II low level disinfectant has
been approved for the 7L, 10L, 12L, M7C, M12L,
4S, and E8C probes.
Virex II 256 low level disinfectant has been
approved for the 7L, 10L, 12L, M7C, M12L, and
E8C probes.
Page 21

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 17
Image Management
Clipboard
As images are saved by pressing any of the print
keys (P1, P2, P3, or P4), the images appear at the
bottom of the display on the clipboard as
thumbnails of the images saved during the exam.
These images remain on the clipboard until the end
of the exam.
Printing Images
Press the appropriate print key (P1, P2, P3, or P4).
For more information on programming the Print
buttons, See “Buttons” on page 21.
Browsing an Exam’s Stored Images
‘Mouse over’ the image in the clipboard, then press
Set to view an enlarged thumbnail image.
Managing an Exam’s Stored Images
From the Display, press Active Images; from the
New Patient menu, open Active Images.
Deleting an Image
Select the image on the clipboard, then press the
onscreen Delete shortcut.
Or, go to Active Images (lower, right-hand portion
of the display). Highlight all the images that need to
be deleted and press Delete All Temp Images
from the Touch Panel
Formatting a CD/MOD
1. Insert the backup media. Format the backup
media, CD-ROM or MOD. Select the Utility tab
on the Touch Panel. Select Connectivity, then
Tools. Label the media appropriately. Press
Format.
2. The Ultrasound system displays a pop-up menu
when the formatting has been completed. Press
Ok to continue. Verify that the format was
successful.
Backing Up Patient Information
Format a CD/MOD prior to following these steps.
1. Select the Utility tab on the Touch Panel. Select
System, then Backup/Restore.
2. Select everything under Backup by placing a
check mark in front of Patient Archive, Report
Archive, and User Defined Configuration. Then
press Backup.
3. Answer ‘Ok’ to the Back-Up pop-up message
as many times as the number of items you are
backing up.
NOTE: The detailed section of this menu
decouples the user defined configuration above.
This allows you to selectively restore what you want
to restore across multiple machines.
Image/ Patient Management
and Connectivity
Page 22

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 18
Saving Images as JPEG files and CINE Loops as AVI files
Format a CD/MOD prior to following these steps.
1. Press Menu (on the lower, right-hand portion of
the display) and select Save As. The SAVE AS
menu appears.
2. Specify Compression and Save As Type and
press Save. The image is saved to the CD/
MOD.
3. When you have saved all the images you want
on the CD/MOD, remove the media (press F3
to eject the CD-ROM).
Moving (Archiving) Images
Format a CD/MOD prior to following these steps.
1. Insert the backup media. Press Patient. Press
More, then select Move Images. The Move
Images pop-up appears.
2. Fill in the From Date, then press Recalculate.
Specify to Keep days together. Check that you
have enough disk space for the images you
want to move. If not, revise the move criteria.
Select Move Images, then press OK. An inprogress message appears. The archive
operation is complete when you receive this
message.
4. Finalize the CD by selecting Yes. The CD-ROM
is ejected from the system.
NOTE: If you want to add more images to the CD,
select “No” and do not finalize the CD.
Page 23

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 19
Exporting Patient Data
Format a CD/MOD prior to following these steps.
1. Press Patient. Deselect any selected patient(s)
in the search portion of the Patient screen.
Press More (located at the lower, right-hand
corner of the Patient menu).
2. Select Export. Specify the type of removable
media (MOD or CD-ROM) on the Export popup. Press OK. Then, please wait until the
Patient menu is visible.
3. In the patient list at the bottom of the Patient
menu, select the patient(s) you want to export.
NOTE: You can use Windows commands to
select more than one patient.
NOTE: You need to use your best judgment
when moving patients’ images. If there are lots
of images or loops, then only move a few
patients at a time.
Importing Patient Data/Images
1. At another Ultrasound system, insert the MOD
or CD-ROM.
2. Press Patient, press More, then Import. The
Import From pop-up message appears. Press
OK.
3. The Patient menu just shows the patients
available for import from the removable media
you just loaded onto the system.
4. Select the patients to be imported.
5. Press Copy Patient from the Select All/Copy
Patient menu.
6. Please wait for the patient information to be
copied to this Ultrasound system. Informational
messages appear while the import is taking
place.
7. Press F3 to eject the media.
Connectivity
Connectivity on the LOGIQ 7 is based on the
Dataflow concept.
Dataflow Concept
A dataflow is a set of pre-configured services. For
example, DICOM services may be for storage,
worklist, verify, etc. In addition, there are other
service types like video print, standard color print,
storage to local hard drive, select patient from local
database, etc.
4. Once you have selected all of the patients to
export, press Copy Patient from the Select All/
Copy Patient Menu.
5. Informational status messages appear as the
copy is taking place. A final status report popup message appears. Press OK.
6. Press F3 to eject the CD or press the MOD
eject button. Specify that you want to finalize
the CD-ROM.
Image/ Patient Management
and Connectivity
Page 24

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 20
Configuring Connectivity
Login as Administrator. Press the left Utility tab.
Select the Connectivity tab. Configure the menus
from right to left, starting with TCP/IP first.
TCP/IP
Type in the Computer’s Name (better known as the
AE Title). Identify the Ultrasound system to the rest
of the network by filling in its IP Address, Subnet
Mask, and Gateway (if applicable). Press Save.
Services (better known as Destinations)
1. Select the Server from the pull-down menu.
2. Press Add.
3. Select all the services for this device from the
pull-down menu to the right.
4. Press Add.
5. At the bottom of the menu, fill in the appropriate
criteria for this service. Repeat this step for
each selected service for this device.
Page 25

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 21
Buttons
You can assign print buttons (P1-P4) to a device or
to a dataflow.
NOTE: You can configure each print key to multiple
output devices/workflows.
Dataflow
Creates a Dataflow, (‘WL-LA-DServ -- Worklist,
Local Archive, DICOM Server, for example).
1. Name the Dataflow (select from pull-down
menu or add a new dataflow).
2. Configure the flow in the Services section of the
screen. Select the Service from the pull-down
menu and press Add.
NOTE: Remember to specify each service Role as
Primary or Secondary. The primary service
specifies what always happens first to incoming or
outgoing data. Only one primary role is available.
1
Image/ Patient Management
and Connectivity
2
Page 26

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 22
Verifying a Device
Select the device, press DICOM Verify.
Views
Views a snapshot of this ultrasound system’s
connectivity architecture (onboard network +
external network + dataflows).
Pinging a Device (DICOM Echo)
Select the device, press Check.
Page 27

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 23
DICOM Status
To check the status of all DICOM jobs or redirect
DICOM jobs, press F4.
Image/ Patient Management
and Connectivity
Page 28

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 24
Using CINE
Activating CINE
Press Freeze, then roll the Trackball to activate
CINE. To start CINE Loop playback, press Run/
Stop. To stop CINE Loop playback. press Run/Stop.
To activate Timeline CINE, press Freeze, press
Scan Area, then roll the Trackball to activate
CINE.
Quickly Move to Start/End Frame
Press First to move to the first CINE frame; press
Last to move to the last CINE frame.
Start Frame/End Frame
Turn the Start Frame dial to the left to move to the
beginning of the CINE Loop. Turn the dial to the
right to move forward through the CINE Loop.
Turn the End Frame dial to the right to move to the
end of the CINE Loop. Turn the dial to the left to
move backward through the CINE Loop.
Adjusting the CINE Loop Playback Speed
Turn the Loop Speed dial clockwise/counterclockwise to increase/decrease the CINE Loop
playback speed.
Disconnecting B-Mode CINE from Timeline CINE
To review the B-Mode CINE Loop only, press Cine
Mode Selection and select B Only.
To review the Timeline CINE Loop only, press Cine
Mode Selection and select TL Only.
To return to linked B-Mode and Timeline CINE Loop
review, press Cine Mode Selection and select
B/TL.
Moving through a CINE Loop Frame By Frame
Turn Frame by Frame to move through CINE
memory one frame at a time.
Page 29

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 25
Easy 3D
Acquiring a 3D Scan
1. Optimize the B-Mode image. Ensure even gel
coverage.
2. Press the 3D control panel key. Two screens
appear.
3. To start acquiring the 3D image, press ‘L’ (the
left split screen key).
4. To perform a parallel scan, scan evenly. To
perform a sweep (fan) scan, rock the probe
once. Note the distance of the scan.
5. The 3D volume of interest is generated on the
right side of the screen in real time.
NOTE: If the image stops before you’re done
scanning, start acquiring the 3D volume of interest
again.
6. To stop the 3D scan, press ‘R’ (the right split
screen key).
Manipulating the 3D Scan
Imagine you are able to manipulate the 3D volume
of interest (VOI) in your hand.
You can rotate it left to right or right to left. You can
rotate it forward/backward (white hand).
Then, imagine that you can view the volume of
interest one slice at a time through the anatomy
(red hand).
Also imagine that you are able to pull back tissue to
view specific portions of anatomy (yellow and green
hands).
The 3D volume of interest is a tangible anatomical
object that you can see and manipulate easily using
the Trackball and Set control panel keys.
Practice positioning the pointer at different places
within the 3D volume of interest. Highlight different
colors, press Set to select this volume for
manipulation. Use the hand to move the 3D volume.
Adjusting the 3D Volume of Interest
You can colorize the 3D volume of interest.
You can resize the VOI by adjusting the scan
distance.
Performing a Surface Render
From the 3D Touch Panel, press 3D, then press
Texture on the next Touch Panel to add a
photorealistic/clay-like quality to the render.
Adjust the opacity and density via Threshold/
Opacity (press the key to adjust opacity). This
adjusts what ‘grays’ the system recognizes,
allowing you to emphasize/de-emphasize grays as
necessary.
Scalpel
To cut away portions of the anatomy,
1. Press Scalpel. A caliper appears on the 3D
VOI.
2. Press Set to set the caliper. Trackball around
the portion to be cut away.
3. Double click and apply the scalpel.
4. Change the projection and scalpel again.
NOTE: You can undo one scalpel, then check
apply on side of monitor.
3DView Scanning Hints
Set the appropriate values for the 3D Acq Mode
and Scan Plane.
Post Processing
It is advisable to set the scan distance before the
scan begins.
Page 30

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 26
Contact Information
INTERNET
http://www.gemedicalsystems.com
USA
GE Medical Systems
Ultrasound Service Engineering
4855 W. Electric Avenue
Milwaukee, WI 53219
TEL: (1) 800-437-1171 or FAX: (1) 414-647-4090
Clinical Questions
For information in the United States, Canada,
Mexico and parts of the Caribbean, call the
Customer Answer Center:
TEL: (1) 800-682-5327 or (1) 262-524-5698
In other locations, contact your local Applications,
Sales or Service Representative.
Service Questions
For service in the United States, call GE CARES
TEL: (1) 800-437-1171
Accessories Catalog Requests
To request the latest GE Accessories catalog or
equipment brochures in the United States, call the
Response Center: TEL: (1) 800-643-6439
In other locations, contact your local Applications,
Sales or Service Representative.
Placing an Order
To place an order, order supplies or ask an
accesory-related question in the United States, call
the GE Access Center: TEL: (1) 800-472-3666
In other locations, contact your local Applications,
Sales or Service Representative.
OTHER COUNTRIES
NO TOLL FREE
TEL: international code + 33 1 39 20 0007
CANADA
GE Medical Systems
Ultrasound Svc Engineering TEL: (1) 800-664-0732
4855 W. Electric Avenue
Milwaukee, WI 53219
Customer Answer Center TEL: (1) 262-524-5698
LATIN & SOUTH AMERICA
GE Medical Systems
Ultrasound Svc Engineering TEL: (1) 305-735-2304
4855 W. Electric Avenue
Milwaukee, WI 53219
Customer Answer Center TEL: (1) 262-524-5698
EUROPE
GE Ultraschall
Deutschland GmbH & Co. KG
Beethovenstraße 239
Postfach 11 05 60
D-42655 Solingen -- TEL: 0130 81 6370 toll free
TEL: (49) 212.28.02.207 -- FAX: (49) 212.28.02.431
ASIA
GE Medical Systems Asia
Asia Support Center
67-4 Takakura cho, Hachiouji-shi
Tokyo, 192-0033
TEL: (81) 426-48-2940 -- FAX: (81) 426-48-2905
ARGENTINA
GEME S.A.
Miranda 5237
Buenos Aires - 1407
TEL: (1) 639-1619 -- FAX: (1) 567-2678
AUSTRIA
GE GesmbH Medical Systems Austria
Prinz Eugen Strasse 8/8
A-1040 WIEN
TLX: 136314
TEL: 0660 8459 toll free -- FAX: +43 1 505 38 74
BELGIUM
GE Medical Systems Benelux
Gulkenrodestraat 3
B-2160 WOMMELGEM
TEL: 0 800 11733 toll free
FAX: +32 0 3 320 12 59
TLX: 72722
BRAZIL
GE Sistemas Médicos
Av Nove de Julho 5229
01407-907 São Paulo SP
TEL: 0800-122345 -- FAX: (011) 3067-8298
Page 31

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 27
DENMARK
GE Medical Systems
Fabriksparken 20
DK-2600 GLOSTRUP
TEL: +45 4348 5400 -- FAX: +45 4348 5399
FRANCE
GE Medical Systems
738 rue Yves Carmen
F-92658 BOULOGNE CEDEX
TEL: 05 49 33 71 toll free -- FAX: +33 1 46 10 01 20
GERMANY
GE Ultraschall
Deutschland GmbH & Co. KG
Beethovenstraße 239
Postfach 11 05 60
D-42655 Solingen
TEL: 0130 81 6370 toll free
TEL: (49) 212.28.02.207 -- FAX: (49) 212.28.02.431
GREECE
LUXEMBOURG
TEL: 0800 2603 toll free
MEXICO
GE Sistemas Médicos de Mexico S.A. de C.V.
Rio Lerma #302, 1º y 2º Pisos
Colonia Cuauhtémoc
06500-México, D.F.
TEL: (5) 228-9600 -- FAX: (5) 211-4631
NETHERLANDS
GE Medical Systems Nederland B.V.
Atoomweg 512
NL-3542 AB UTRECHT
TEL: 06 022 3797 toll free -- FAX: +31 304 11702
POLAND
GE Medical Systems Polska
Krzywickiego 34
P-02-078 WARSZAWA
TEL: +48 2 625 59 62 -- FAX: +48 2 615 59 66
SPAIN
GE Medical Systems España
Hierro 1 Arturo Gimeno
Poligono Industrial I
E-28850 TORREJON DE ARDOZ
TEL:900 95 3349 free -- FAX: +34 1 675 3364
TLX: 22384 A/B GEMDE
SWEDEN
GE Medical Systems
PO-BOX 1243
S-16428 KISTA
TEL: 020 795 433 toll free -- FAX: +46 87 51 30 90
TLX: 12228 CGRSWES
SWITZERLAND
GE Medical Systems (Schweiz) AG
Sternmattweg 1
CH-6010 KRIENS
TEL: 155 5306 -- FAX: +41 41 421859
TURKEY
GE Medical Systems Hellas
41, Nikolaou Plastira Street
G-171 21 NEA SMYRNI
TEL: +30 1 93 24 582 -- FAX: +30 1 93 58 414
ITALY
GE Medical Systems Italia
Via Monte Albenza 9
I-20052 MONZA
TEL: 1678 744 73 toll free -- FAX: +39 39 73 37 86
TLX: 3333 28
PORTUGAL
GE Medical Systems Portuguesa S.A.
Rua Sa da Bandeira, 585
Apartado 4094 TLX: 22804
P-4002 PORTO CODEX
TEL: 05 05 33 7313 toll free - FAX: +351 2 2084494
RUSSIA
GE VNIIEM
Mantulinskaya UI. 5A
123100 MOSCOW
TEL: +7 095 956 7037 -- FAX: +7 502 220 32 59
TLX: 613020 GEMED SU
Contact Information
GE Med. Sys. Turkiye A.S.
Mevluk Pehliran Sodak
Yilmaz Han, No 24 Kat 1
Gayretteppe
ISTANBUL
TEL: +90 212 75 5552 -- FAX: +90 212 211 2571
UNITED KINGDOM
GE Medical Systems
Coolidge House
352 Buckingham Avenue
SLOUGH
Berkshire SL1 4ER
TEL: 0800 89 7905 toll free -- FAX: +44 753 696067
Page 32

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 28
Paperless Documentation
Introduction
Documentation is being provided via:
• Release Notes (supplied on paper)
• Quick Guide (supplied on paper)
• Online Help (on the Ultrasound Scanner via F1)
• CD-ROM. You can view user documentation on
a PC or on the Ultrasound Scanner via the
Customer Documentation CD-ROM, which
includes:
• Basic User Manual
• Advanced Reference Manual
• Quick Guide
• Quick Card(s)
• Release Notes and Workarounds
• Basic Service Manual
NOTE: All user documentation is provided in
multiple languages on the CD-ROM.
Using Online Help Via F1
Online Help is available via the F1 key.
The Help screen is divided into three sections:
• navigational tools on the top, left portion of the
screen (Hide, Back, Forward)
• help book navigational tools on the left portion
of the screen (Contents, Index, Search,
Favorites)
• content portion on the right side of the screen
where help topics are displayed
Navigating Through Help
Online Help is organized like a manual, with
individual chapters, sections, and pages.
Click on the plus (+) sign next to MANUAL to open
up the book.
Click on the plus sign next to the chapter you want
to view to open up that chapter.
Click on the plus sign next to the chapter you want
to view to open up that section.
Click to open up the page to view that page’s
information.
The blue, underlined text links you to related topics.
Click on the link to move to the new topic.
To go back to the previous screen, press Back. To
return to the link, press Forward.
Page 33

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 29
Help Links
After you click on a blue, underlined portion of text,
the screen updates with this link’s content.
Searching for a Topic in Help
To search for a specific topic, click on the Search
tab. Type in the topic name in the Type in the
keyword to find: field. Topics with the word or
phrase you typed appear in the Select Topic to
display: area. Either double click on the topic you
want to view or highlight the topic and press the
Display button to view this topic.
Creating a List of Favorite Topics in Help
You may find that there are topics you need to refer
to often. In this case, it’s a good idea to save these
topics as Favorites. To save a topic as a favorite,
press the Favorites tab, highlight the topic in the
Topics window, and press the Add button. You can
now view this topic quickly by going to the Favorites
help tab.
Using the Help Index
Or, you can look for topics by using the Index. Press
the Index tab, then use the scroll bar to look up a
topic.
Other Help Features
To hide the left side of the screen, press the Hide
icon at the upper, left-hand portion of the screen. To
view the left side of the screen again, press the
Show icon at the upper, left-hand portion of the
screen.
To size the Help window, position and hold down
the cursor at the corner of the screen while moving
the Trackball.
To move the Help window to the Touch Panel
display, position and hold down the cursor at the
very top of the Help window while moving the
Trackball to the Touch Panel display.
Exiting Online Help
To exit Online Help, press the ‘X’ in the upper, righthand corner of the Online Help window.
Electronic Documentation
Page 34

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 30
Accessing Documentation Via a PC
To view user documentation on a PC,
1. Insert the CD into the CD drive.
2. Open the CD drive on your desktop.
3. Double click on the ‘gedocumentation.html’
document.
4. Select the item you want to view (click on the
blue, underlined link in the File Name column).
To close the window, click on the ‘X’ in the upper,
right-hand corner of the browser window.
NOTE: If your PC does not have the Adobe
Acrobat Reader, the PC version is supplied on the
CD. Open the CD and double click on
‘ar505enu.exe. Follow the prompts to install Adobe
Acrobat Reader on your PC.
Accessing Documentation on the Ultrasound Scanner Via the CD-ROM
To access documentation via the CD-ROM,
1. Logon as ‘Operator’ next to Select User Level.
Enter the following password: ‘uls’. Press Okay.
2. Press Utilities and insert the CD-ROM.
3. Select Scanner Utilities.
4. Select Scanner Documentation Interface.
5. Scroll to find the document, double click on the
document, and open it.
NOTE: You can search through a document, use
hyperlinks in the Table of Contents and Index to
locate topics, and navigate via bookmarks.
NOTE: In addition to viewing documentation on the
Ultrasound system, the Documentation CD can be
read on any PC.
To exit, press the ‘X’ in the upper, right-hand corner
of the documentation window.
Page 35

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 31
DANGER
G
Precaution Levels
Icon description
Various levels of safety precautions may be found on the equipment and different levels of concern are identified by one of the following flag words and icons which
precede the precautionary statement .
Indicates that a specific hazard is known to exist which through inappropriate conditions or actions will cause:
• Severe or fatal personal injury
• Substantial property damage.
WARNIN
CAUTION
NOTE: Indicates precautions or recommendations that should be used in the operation of the ultrasound system, specifically:
• Maintaining an optimum system environment
• Using this Manual
• Notes to emphasize or clarify a point.
Indicates that a specific hazard is known to exist which through inappropriate conditions or actions may cause:
• Severe personal injury
• Substantial property damage.
Indicates that a potential hazard may exist which through inappropriate conditions or actions will or can cause:
• Minor injury
• Property damage.
Safety
Page 36

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 32
Biological
A
Explosi
Hazard Symbols - Icon Description
Potential hazards are indicated by the following icons:
Table 1-1: Potential Hazards
Icon Potential Hazard Usage Source
Hazard
Electrical
Hazard
Moving
Hazard
coustic
Output
Hazard
on
Hazard
Smoke &
Fire Hazard
• Patient/user infection due to contaminated equipment. • Cleaning and care instructions
• Sheath and glove guidelines
• Electrical micro-shock to patient, e.g., ventricular • Probes
• ECG
• Connections to back panel
• Console, accessories or optional storage devices that can fall on patient, user, or others.
• Collision with persons or objects result in injury while maneuvering or during system
transport.
• Injury to user from moving the console.
• Patient injury or tissue damage from ultrasound radiation. • ALARA, the use of power output following
• Risk of explosion if used in the presence of flammable anesthetics. • Flammable anesthetic
• Patient/user injury or adverse reaction from fire or smoke.
• Patient/use injury from explosion and fire.
• Moving
• Using brakes
• Transporting
the as low as reasonably achievable
principle
• Replacing fuses
• Outlet guidelines
ISO 7000
No. 0659
Page 37

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 33
G
Important Safety Considerations
The following topic headings (Patient Safety, and Equipment and Personnel Safety) are intended to make the equipment user aware of particular hazards
associated with the use of this equipment and the extent to which injury can occur if precautions are not observed. Additional precautions may be provided
throughout the manual.
CAUTION
Improper use can result in serious injury. The user must be thoroughly familiar with the instructions and potential hazards involving
ultrasound examination before attempting to use the device. Training assistance is available from GE Medical Systems if needed.
The equipment user is obligated to be familiar with these concerns and avoid conditions that could result in injury.
Patient Safety
Related Hazards
WARNIN
Patient identification
Always include proper identification with all patient data and verify the accuracy of the patient's name or ID numbers when entering such data. Make sure correct
patient ID is provided on all recorded data and hard copy prints. Identification errors could result in an incorrect diagnosis.
Diagnostic information
Equipment malfunction or incorrect settings can result in measurement errors or failure to detect details within the image. The equipment user must become
thoroughly familiar with the equipment operation in order to optimize its performance and recognize possible malfunctions. Applications training is available through
the local GE representative. Added confidence in the equipment operation can be gained by establishing a quality assurance program.
The concerns listed can seriously affect the safety of patients undergoing a diagnostic ultrasound examination.
CAUTION
Safety
The system’s acoustic output remains transmitting when the user controls are being used. Allowing the system to transmit acoustic output
with the probe not in use (or in its holder) can cause the probe to build up heat. Always turn off acoustic output or freeze the image when
not in use.
Page 38

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 34
Related Hazards (continued)
Mechanical hazards
The use of damaged probes or improper use and manipulation of intracavity probes can result in injury or increased risk of infection. Inspect probes often for sharp,
pointed, or rough surface damage that could cause injury or tear protective barriers. Never use excessive force when manipulating intracavity probes. Become
familiar with all instructions and precautions provided with special purpose probes.
Electrical
Hazard
CAUTION
CAUTION
CAUTION
Training
It is recommended that all users receive proper training in applications before performing them in a clinical setting. Please contact the local GE representative for
training assistance.
ALARA training is provided by GE Application Specialists. The ALARA education program for the clinical end-user covers basic ultrasound principles, possible
biological effects, the derivation and meaning of the indices, ALARA principles, and examples of specific applications of the ALARA principle.
A damaged probe can also increase the risk of electric shock if conductive solutions come in contact with internal live parts. Inspect probes
often for cracks or openings in the housing and holes in and around the acoustic lens or other damage that could allow liquid entry.
Become familiar with the probe's use and care precautions outlined in Probes and Biopsy.
Ultrasound transducers are sensitive instruments which can easily be damaged by rough handling. Take extra care not to drop
transducers and avoid contact with sharp or abrasive surfaces. A damaged housing, lens or cable can result in patient injury or serious
impairment or operation.
Ultrasound can produce harmful effects in tissue and potentially result in patient injury. Always minimize exposure time and keep
ultrasound levels low when there is no medical benefit. Use the principle of ALARA (A
only when needed to obtain diagnostic image quality. Observe the acoustic output display and be familiar with all controls affecting the
output level. See the Bioeffects section of the Acoustic Output chapter in the Advanced Reference Manual for more information.
Do not use with Defibrillator.
This equipment does not have a defibrillator approved applied part.
s Low As Reasonably Achievable), increasing output
Page 39

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 35
G
G
DANGER
Explosi
Equipment and Personnel Safety
Related Hazards
WARNIN
WARNIN
on
Hazard
Electrical
Hazard
This equipment contains dangerous voltages that are capable of serious injury or death.
If any defects are observed or malfunctions occur, stop operating the equipment and perform the proper action for the patient. Inform a
qualified service person and contact a Service Representative for information.
There are no user serviceable components inside the console. Refer all servicing to qualified service personnel only.
Only approved and recommended peripherals and accessories should be used. All peripherals and accessories must be securely
mounted to the LOGIQ 7.
The concerns listed below can seriously affect the safety of equipment and personnel during a diagnostic ultrasound examination.
Risk of explosion if used in the presence of flammable anesthetics.
To avoid injury:
• Do not remove protective covers. No user serviceable parts are inside. Refer servicing to qualified service personnel.
• To assure adequate grounding, connect the attachment plug to a reliable (hospital grade) grounding outlet.
• Never use any adaptor or converter of a three-prong-to-two-prong type to connect with a mains power plug. The protective earth
connection will loosen.
• Do not place liquids on or above the console. Spilled liquid may contact live parts and increase the risk of shock.
Safety
• Plug any peripherals into the LOGIQ 7 AC power outlet.
Page 40

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 36
Biological
Related Hazards (continued)
CAUTION
Smoke &
Fire Hazard
Hazard
CAUTION
CAUTION
Do not use this equipment if a safety problem is known to exist. Have the unit repaired and performance verified by qualified service
personnel before returning to use.
The system must be supplied from an adequately rated electrical circuit. The capacity of the supply circuit must be as specified in
Chapter 3 of the Basic User Manual.
For patient and personnel safety, be aware of biological hazards while performing invasive procedures. To avoid the risk of disease
transmission:
• Use protective barriers (gloves and probe sheaths) whenever possible. Follow sterile procedures when appropriate.
• Thoroughly clean probes and reusable accessories after each patient examination and disinfect or sterilize as needed. Refer to
Probes and Biopsy in the Basic User Manual for probe use and care instructions.
• Follow all infection control policies established by your office, department or institution as they apply to personnel and equipment.
Contact with natural rubber latex may cause a severe anaphylactic reaction in persons sensitive to the natural latex protein. Sensitive
users and patients must avoid contact with these items. Refer to package labeling to determine latex content and FDA’s March 29, 1991
Medical Alert on latex products.
The system is equipped with an Auto Freeze feature which disables acoustic output and freezes the image when the sytem is not in use.
Take care when deactivating this feature.
CAUTION
Never put any device onto the monitor.
Page 41

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 37
Related Hazards (continued)
CAUTION
CAUTION
CAUTION
Archived data is managed at the individual sites. Performing data backup (to any device) is recommended on a daily basis.
Do not unpack the LOGIQ 7. This must be performed by qualified service personnel only.
Do not use the LOGIQ 7 Ultrasound system ECG wave for diagnosis and monitoring.
Safety
Page 42

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 38
Device Labels
Label Icon Description
The following table describes the purpose and location of safety labels and other important information provided on the equipment.
Table 1-2: Label Icons
Label/Icon Purpose/Meaning Location
Identification and Rating Plate Manufacture’s name and address
Date of manufacture
Model and serial numbers
Electrical ratings (Volts, Amps, phase, and frequency)
Type/Class Label Used to indicate the degree of safety or protection.
IP Code (IPX8) Indicates the degree of protection provided by the enclosure per IEC60 529. Can be used in operating room
environment.
Type BF Applied Part (man in the box) symbol is in accordance with IEC 878-02-03. Probe connectors and PCG connector
Type CF Applied Part (heart in the box) symbol is in accordance with IEC 878-02-03. ECG marked Type CF
“ATTENTION” - Consult accompanying documents” is intended to alert the user to refer to the operator manual
or other instructions when complete information cannot be provided on the label.
!
“CAUTION” - Dangerous voltage” (the lightning flash with arrowhead) is used to indicate electric shock
hazards.
“Mains OFF” indicates the power off position of the mains power breaker. Refer to Chapter 3 in the Basic User Manual for
See “Label Location (b)” on page 52.
Foot Switch
Var ious
Inside of console
location information.
“Mains ON” indicates the power on position of the mains power breaker. Refer to Chapter 3 in the Basic User Manual for
location information.
Page 43

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 39
Table 1-2: Label Icons
Label/Icon Purpose/Meaning Location
“ON” indicates the power on position of the power switch.
CAUTION: This Power Switch DOES NOT ISOLATE Mains Supply.
“Standby” indicates the power standby position of the power switch.
CAUTION: This Power Switch DOES NOT ISOLATE Mains Supply.
“Protective Earth” indicates the protective earth (grounding) terminal. Internal
“Equipotentiality” indicates the terminal to be used for connecting equipotential conductors when
interconnecting (grounding) with other equipment.
Refer to Chapter 3 in the Basic User Manual for
location information.
Rear of console
Safety
Page 44

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 40
Classifications
Type of protection against electric shock
Class I Equipment (*1)
Degree of protection against electric shock
Type BF Applied part (*2)
Type CF Applied part (*3)
(for PCG, Probes marked with BF symbol)
(for ECG, Probes marked with CF symbol)
Continuous Operation
System is Ordinary Equipment (IPX0)
Footswitch is IPX8
*1. Class I EQUIPMENT
EQUIPMENT in which protection against electric shock does not rely on BASIC INSULATION only, but includes an earth ground. This additional safety precaution
prevents exposed metal parts from becoming LIVE in the event of an insulation failure.
*2. Type BF APPLIED PART
TYPE BF APPLIED PART providing a specified degree of protection against electric shock, with particular regard to allowable LEAKAGE CURRENT.
Table 1-3: Type BF Equipment
Normal Mode Single fault condition
Patient leakage current Less than 100 microA Less than 500 microA
*3. Type CF APPLIED PART
Type CF Applied Part providing a degree of protection higher than that for TYPE BF Applied Part against electric shock particularly regarding allowable LEAKAGE
CURRENTS.
Table 1-4: Type CF Equipment
Normal Mode Single fault condition
Patient leakage current Less than 10 microA Less than 50 microA
Page 45

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 41
EMC (Electromagnetic Compatibility)
NOTE: This equipment generates, uses and can radiate radio frequency energy. The equipment may cause radio frequency interference to other medical and nonmedical devices and radio communications. To provide reasonable protection against such interference, this product complies with emissions limits for a Group 1,
Class A Medical Devices Directive as stated in EN 60601-1-2. However, there is no guarantee that interference will not occur in a particular installation.
NOTE: If this equipment is found to cause interference (which may be determined by turning the equipment on and off), the user (or qualified service personnel)
should attempt to correct the problem by one or more of the following measure(s):
• reorient or relocate the affected device(s)
• increase the separation between the equipment and the affected device
• power the equipment from a source different from that of the affected device
• consult the point of purchase or service representative for further suggestions.
NOTE: The manufacturer is not responsible for any interference caused by using other than recommended interconnect cables or by unauthorized changes or
modifications to this equipment. Unauthorized changes or modifications could void the users’ authority to operate the equipment.
NOTE: To comply with the regulations on electromagnetic interference for a Class A FCC Device, all interconnect cables to peripheral devices must be shielded
and properly grounded. Use of cables not properly shielded and grounded may result in the equipment causing radio frequency interference in violation of the FCC
regulations.
NOTE: Do not use devices which intentionally transmit RF Signals (cellular phones, transceivers, or radio-controlled products) in the vicinity of the equipment as it
may cause performance outside the published specifications. Keep the power to these types of devices turned off when near this equipment.
The medical staff in charge of this equipment is required to instruct technicians, patients, and other people who maybe around this equipment to fully comply with the
above requirement.
Safety
Page 46

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 42
(continued)
EMC Performance
All types of electronic equipment may characteristically cause electromagnetic interference with other equipment, either transmitted through air or connecting
cables. The term EMC (Electromagnetic Compatibility) indicates the capability of equipment to curb electromagnetic influence from other equipment and at the same
time not affect other equipment with similar electromagnetic radiation from itself.
Proper installation following the service manual is required in order to achieve the full EMC performance of the product.
The product must be installed as stipulated in 4.2, Notice upon Installation of Product.
In case of issues related to EMC, please call your service personnel.
The manufacturer is not responsible for any interference caused by using other than recommended interconnect cables or by unauthorized changes or modifications
to this equipment. Unauthorized changes or modifications could void the users’ authority to operate the equipment.
CAUTION
Do not use devices which intentionally transmit RF signals (cellular phones, transceivers, or radio controlled products) in the vicinity of this
equipment as it may cause performance outside the published specifications. Keep the power to these type devices turned off when near
this equipment.
Keep power to these devices turned off when near this equipment.
Medical staff in charge of this equipment is required to instruct technicians, patients and other people who may be around this equipment
to fully comply with the above regulation.
Portable and mobile radio communications equipment (e.g. two-way radio, cellular/cordless telephones, wireless computer networks) should be used no closer to
any part of this system, including cables, than determined according to the following method:
Table 1-5: Portable and mobile radio communications equipment distance requirements
Frequency Range: 150 kHz - 80 MHz 80 MHz - 800 MHz 800 MHz - 2.5 GHz
Calculation Method: d=[3.5/V1] square root of P d = [3.5/E1] square root of P d = [7/E1] square root of P
Where: d= separation distance in meters, P = rated power of the transmitter, V
If the maximum transmitter power in
watts is rated The separation distance in meters should be
5 2.6 2.6 5.2
20 5.2 5.2 10.5
100 12.0 12.0 24.0
=compliance value for conducted RF, E1 = compliance value for radiated RF
1
Page 47

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 43
Notice upon Installation of Product
Separation distance and effect from fixed radio communications equipment: field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast, and TV broadcast transmitter cannot be predicted theoretically with accuracy. To
assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the ultrasound system is used exceeds the applicable RF compliance level as stated in the immunity declaration, the ultrasound system should be
observed to verify normal operation. If abnormal operation is observed, additional measures may be necessary, such as re-orienting or relocating the ultrasound
system or using an RF shielded examination room may be necessary.
1. Use either power supply cords provided by GE Medical Systems or ones designated by GE Medical Systems. Products equipped with a power source plug
should be plugged into the fixed power socket which has the protective grounding conductor. Never use any adaptor or converter to connect with a power
source plug (i.e. three-prong-to-two-prong converter).
2. Locate the equipment as far away as possible from other electronic equipment.
3. Be sure to use only the cables provided by or designated by GE Medical Systems. Connect these cables following the installation procedures (i.e. wire power
cables separately from signal cables).
4. Lay out the main equipment and other peripherals following the installation procedures described in the Option Installation manuals.
General Notice
1. Designation of Peripheral Equipment Connectable to This Product.
The equipment indicated on Chapter 15 of the Basic User Manual can be hooked up to the product without compromising its EMC performance.
Avoid using equipment not designated in the list. Failure to comply with this instruction may result in poor EMC performance of the product.
2. Notice against User Modification
The user should never modify this product. User modifications may cause degradation in EMC performance.
Modification of the product includes changes in:
a. Cables (length, material, wiring, etc.)
b. System installation/layout
c. System configuration/components
d. Securing system parts (cover open/close, cover screwing)
3. Operate the system with all covers closed. If a cover is opened for some reason, be sure to shut it before starting/resuming operation.
4. Operating the system with any cover open may affect EMC performance.
Safety
Page 48

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 44
Peripheral Update for EC countries
The following is intended to provide the users in EC countries with updated information concerning the connection of the LOGIQ 7 to image recording and other
devices or communication networks.
The LOGIQ 7 has been verified for overall safety, compatibility and compliance with the following on-board image recording devices:
• Sony UP 895 MDW B&W Video Printer
• Sony UP-21 MD Color Video Printer
• Sony UP-51MDU Color Video Printer
• Mitsubishi 91W Color Video Printer
• Sony SVO-9500 MD2 S-VHS Video Cassette Recorder\
• Sony UP-D895 B&W Digital Printer
• Sony UP-D21MD Color Digital Printer
The LOGIQ 7 has also been verified for compatibility, and compliance for connection to a local area network (LAN) via the rear panel Ethernet connection, provided
the LAN components are IEC/EN 60950 compliant.
Connection may also be made to a CE Marked and IEC/EN 60950 compliant modem using one of the serial ports at the rear panel.
The LOGIQ 7 may also be used safely while connected to devices other than those recommended above if the devices and their specifications, installation, and
interconnection with the system conform to the requirements of IEC/EN 60601-1-1.
General precautions for installing an alternate on-board device would include:
1. The added device must have appropriate safety standard conformance and CE Marking.
2. The total power consumption of the added devices, which connect to the LOGIQ 7 and are used simultaneously, must be less than or equal to the rated supply
of the LOGIQ 7.
3. There must be adequate heat dissipation and ventilation to prevent overheating of the device.
4. There must be adequate mechanical mounting of the device and stability of the combination.
5. Risk and leakage current of the combination must comply with IEC/EN 60601-1.
6. Electromagnetic emissions and immunity of the combination must conform to IEC/EN 60601-1-2.
Page 49

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 45
Peripheral Update for EC countries (continued)
General precautions for installing an alternate off-board, remote device or a network would include:
1. The added device(s) must have appropriate safety standard conformance and CE Marking.
2. The added device(s) must be used for their intended purpose having a compatible interface.
3. Signal or mains isolation devices and additional protective earth may be needed to assure compliance with IEC/EN 60601-1-1.
CAUTION
The connection of equipment or transmission networks other than as specified in the user instructions can result in an electric shock
hazard or equipment malfunction. Substitute or alternate equipment and connections requires verification of compatibility and conformity to
IEC/EN 60601-1-1 by the installer. Equipment modifications and possible resulting malfunctions and electromagnetic interference are the
responsibility of the owner.
Declaration of Emissions
This system is suitable for use in the following environment. The user must assure that it is used only in the electromagnetic environment as specified.
Table 1-6: Declaration of Emissions
Emission Type Compliance Electromagnetic Environment
CISPR 11
RF Emissions
Group 1
Class A
This system uses RF energy only for its internal function. Therefore, RF emissions are very low and are not likely to cause any interference in nearby electronic
equipment. It is suitable for use in all establishments, other than domestic establishments and those directly connected to the public low-voltage power supply
network that supplies buildings used for domestic purposes. Note: Select only one underlined word(s) according to CISPR Class A/B.
Safety
Page 50

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 46
Declaration of Immunity
This system is suitable for use in the following environment. The user must assure that the system is used according to the specified guidance and only in the
electromagnetic environment listed.
Table 1-7: Declaration of Immunity
Immunity Type Test Level Compliance EMC Environment and Guidance
IEC 61000-4-2
Static discharge
(ESD)
IEC 61000-4-4
Electrical fast
transient/burst
IEC 61000-4-5 Surge Immunity ± 1.5 kV differential
IEC 61000-4-11
Voltage dips, short interruptions and
voltage variations on mains supply
IEC 61000-4-8
Power frequency (50/60 Hz) magnetic
field
IEC 61000-4-6
Conducted RF
IEC 61000-4-3
Radiated RF
NOTE: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people.
± 8 kV contact
± 8 kV air
± 1.5 kV for mains ± 2 kV for mains
± 2.5 kV common
< 5% U
TBD 3 A/m
TBD 3 V
3 V/m
80 MHz - 2.5 GHz
(> 95% dip) for 0.5 cycle; < 5% UT (> 95% dip) for 0.5 cycle;
T
± 6 kV contact
± 8 kV air
± 1 kV for SIP/SOP
± 1 kV differential
± 2 kV common
40% U
(60% dip) for 5 cycles;
T
70% U
(30% dip) for 25 cycles;
T
< 5% U
150 kHz - 80 MHz
3 V/m
80 MHz - 2.5 GHz
(>95% dip) for 5 sec
T
RMS
Floors should be wood, concrete, or ceramic tile. If floors are
covered with synthetic material, the relative humidity should
be at least 30%.
Mains power quality should be that of a typical commercial
and/or hospital environment. If the user requires continued
operation during power mains interruptions, it is
recommended that the system be powered from an
uninterruptable power source (UPS).
NOTE: UT is the a.c. mains voltage prior to application of the
test level.
Power frequency magnetic fields should be at levels
characteristic of a typical location in a typical commercial and/
or hospital environment.
Separation distance to radio communication equipment must
be maintained according to the Table 1-5 on page 42.
Interference may occur in the vicinity of equipment marked
with the symbol
Page 51

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 47
Patient Environmental Devices
1. Peripheral Device (Signals I/O Port, Power In)
2. Front Panel (Signal I/O Port, Power Out)
3. Non-Imaging Probes
4. Imaging Probes
5. Probe Port
6. ECG Cable
7. PCG Sensor
8. Physio-Signal Input Panel
9. Rear Panel
1
6
7
24
25
2
13
8
20
3
5
4
9
10
11
12
18
14
15
16
17
19
21
22
23
Figure 1-1. Patient Environmental Devices
10. Signals I/O Port
11. Power Out
12. Signals I/O Port
13. Footswitch Connector
14. Power In
15. Peripheral Devices
16. Signals I/O Port
17. Power In
18. InSite Modem (Signal I/O Port)
19. Power Telephone Line
20. Footswitch
21. Power Line (AC~)
22. Ground Line
23. Power Cable with Protective Earth
24. MO Drive
25. CD-RW
Safety
Page 52

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 48
Acceptable Devices
The devices shown in “Patient Environmental Devices” on page 1-47 are specified to be suitable for use within the PATIENT ENVIRONMENT.
CAUTION
DO NOT connect any probes or accessories without approval by GE within the PATIENT ENVIRONMENT.
See “Peripheral Update for EC countries” on page 44.
Unapproved Devices
CAUTION
Unapproved devices shall not be used in the patient environment.
If devices are connected without the approval of GE, the warranty will be INVALID.
Any device connected to the LOGIQ 7 must conform to one or more of the requirements listed below:
1. IEC standard or equivalent standards appropriate to devices.
2. The devices shall be connected to PROTECTIVE EARTH (GROUND).
Accessories, Options, Supplies
CAUTION
Unsafe operation or malfunction may result. Use only the accessories, options and supplies approved or recommended in these
instructions for use.
Page 53

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 49
Acoustic Output
Located on the upper right section of the system display monitor, the acoustic output display provides the operator with real-time indication of acoustic levels being
generated by the system. See the Acoustic Output chapter in the Advanced Reference Manual for more information. This display is based on NEMA/AIUM
Standards for Real-time Display of Thermal and Mechanic Acoustic Output Indices on Diagnostic Ultrasound Equipment.
Acoustic Output Display Specifications
The display consists of three parts: Thermal Index (TI), Mechanical Index (MI), and a relative Acoustic Output (AO) value. Although not part of the NEMA/AIUM
standard, the AO value informs the user of where the system is operating within the range of available output. Depending on the examination and type of tissue
involved, the TI parameter will be one of three types:
• Soft Tissue Thermal Index (TIS). Used when imaging soft tissue only, it provides an estimate of potential temperature increase in soft tissue.
• Bone Thermal Index (TIB). Used when bone is near the focus of the image as in the third trimester OB examination, it provides an estimate of potential
temperature increase in the bone or adjacent soft tissue.
• Cranial Bone Thermal Index (TIC). Used when bone is near the skin surface as in transcranial examination, it provides an estimate of potential temperature
increase in the bone or adjacent soft tissue.
The TI and MI is displayed at all times. The MI and TI displays start at a value of 0.0 and increments in steps of 0.1. Display precision is ± 0.1, and accuracy is ±
50%.
Controls Affecting Output
The potential for producing mechanical bioeffects (MI) or thermal bioeffects (TI) can be influenced by certain controls.
The Acoustic Output control has the most significant effect on Acoustic Output.
Indirect effects may occur when adjusting other controls. Controls that can influence MI and TI are detailed under the Bioeffects portion of each control in the Modes
chapter of the Basic User Manual.
Always observe the acoustic output display for possible effects.
Safety
Page 54

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 50
HINTS
G
A
Best practices while scanning
Raise the Acoustic Output only after attempting image optimization with controls that have no effect on Acoustic Output, such as Gain and
TGC.
NOTE: Refer to the Optimization sections of the Modes chapter for a complete discussion of each control.
WARNIN
coustic
Output
Hazard
Be sure to have read and understood control explanations for each Mode used before attempting to adjust the Acoustic Output control or
any control that can effect Acoustic Output.
Use the minimum necessary acoustic output to get the best diagnostic image or measurement during an examination. Begin the exam with
the probe that provides an optimum focal depth and penetration.
Acoustic Output Default Levels
In order to assure that an exam may not start at a high output level, the LOGIQ 7 may initiate scanning at a reduced default output level. This reduced level is preset
programmable and depends upon the exam category and probe selected. It takes effect when the system is powered on or New Patient is selected.
Page 55

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 51
Warning Label Locations
Console Labels
Table 1-8: Label Location Explanations
(1)
1. Possible shock hazard. Do not remove covers or panels. No user serviceable parts are inside. Refer
(2)
(3)
(4)
(5)
(6)
(7)
Japan/USA/Asia Console(100V)
servicing to qualified service personnel.
2. Do not use the following devices near this equipment: cellular phone, radio receiver, mobile radio
transimitter, radio controlled toy, etc. Use of these devices near this equipment could cause this equipment
to perform outside the published specifications. Keep power to these devices turned off when near this
equipment.
3. The equipment weighs approximately 225 kg (496 lbs). To avoid possible injury and equipment damage
when transporting from one area of use to another:
- Be sure the pathway is clear
- Limit movement to a slow careful walk.
- Use two or more persons to move the equipment on inclines or long distance.
4. Prescription Device (For U.S.A. Only)
5. The CE Mark of Conformity indicates this equipment conforms with the Council Directive 93/42/EEC.
6. CISPR
CAUTION: The LOGIQ 7 conforms to the CISPR11, Group 1, Class A of the international standard for
Electromagnetic disturbance characteristics.
7. Voltage Range (Indication label)
8. Signal ground point label
CAUTION: This is only for “FUNCTIONAL GROUNDING”, NOT “PROTECTIVE EARTH”.
9. Power (Indication label)
10. Grounding reliability can only be achieved when this equipment is connected to a receptacle marked
“Hospital Only” or “Hospital Grade”.(For U.S.A., Canada, Japan)
(8)
(9)
(10)
100-120VÅ`
220-240VÅ`
Figure 1-2. Label Location (a)
Safety
Page 56

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 52
Console Labels (continued)
1.ETL Label: NRTL Listing and Certification Mark is used to designate conformance to nationally recognized product safety
standards. The Mark bears the name and/or logo of the testing laboratory, product category, safety standard to which conformity
is assessed, and a control number.
2.Identification and Rating Plate–USA/Asia 120V Console
3.Identification and Rating Plate–Europe/Asia/USA 220V Console
4.Identification and Rating Plate–Japan 120V Console
(1)
ETL LISTED
CONFORMS TO
UL STD. 2601-1
L
L
R
CERTIFIED TO
R
D
I
C
E
S
T
D
I
E
S
T
46477
CAN/CSA C22.2 NO. 601.1
ETL TESTING LABORATORIES INC.
CORTLAND, NEW YORK 13043
MADE FOR GE MEDICAL SYSTEMS
MILWAUKEE, WISCONSIN BY
GE YOKOGAWA MEDICAL SYSTEMS LTD.
TOKYO, JAPAN
MODEL
SERIAL
MANUFACTURED
VOLTS
POWER
FREQUEMCY
LOGIQ 7
120Vac
1200VA
60Hz
CLASS I
1PHASE
(2) (4)(3)
~
GE Yokogawa Medical Systems
Figure 1-3. Label Location (b)
Page 57

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 53
Fax Back Form
You can order printed documentation by faxing this form to Coakley-Tech.
Customer Name Telephone Number Mailing Address
Quantity Publication Direction Number
Basic User Manual 2286866-100, 101, 106, 127
Quick Guide 2291859-100, 101, 106, 127
Advanced Reference Manual 2291860-100
Basic Service Manual 2286865
Fax To: Fax Number: Attention:
Coakley-Tech (414) 389-9130 Norm Keene
Page 58

LOGIQ 7 Quick Guide Direction 2291859-100 Rev. 1 54
This page intentionally left blank.
 Loading...
Loading...