Page 1
SES LS3
A UTOCLAVE
Instructions for use
490346
ST-IM29h
Page 2

Introduction
Warnings and
information
Preparation
and loading
Sterilizing
and drying
Cleaning and care
Maintenance
Technical data
Read these Instructions before use
Keep these ‘Instructions for use’ in a safe convenient place for future reference.
Eschmann After Sales Service Department
The Eschmann After Sales Service Department is staffed and equipped to provide advice and
assistance during normal office hours. To avoid delays when making enquires, please quote the
Model and Serial Number of your Autoclave (NOTE: For location of the Serial Number Plate
see Fig.1. Item 19).
For Customers on the Mainland of England, Scotland and Wales, or on the Isle of Wight :When you receive your Little Sister 3 Autoclave ensure that you complete your Service Guarantee
and Registration Card, and then FREEPOST it to Eschmann Equipment. Failure to return this
card means you will not benefit from our FREE installation service, which will ensure your Autoclave
is correctly installed, plus two FREE service visits during the following 12 months.
For further information visit www.eschmann.co.uk
All correspondence relating to the after sales service of Eschmann Equipment to be addressed to :
UK Customers
Eschmann Equipment, Peter Road, Lancing, West Sussex BN15 8TJ, England.
T el: +44 (0) 1903 765040. Fax: +44 (0) 1903 762006.
Overseas Customers
Contact your local distributor. In case of doubt contact Eschmann Equipment.
Patents and Trade marks
The ESCHMANN name and logo are registered trade marks of Eschmann Holdings Limited .
“Eschmann Equipment” is a trading name of Eschmann Holdings Limit ed.
“Little Sister”, “SES”, and “LS3” are trade marks of Eschmann Holdings Limited.
Patents : Patents Pending plus - Pat. US5090033 and Pat. GB2238407
Copyright © 2002
All rights reserved. This booklet is protected by copyright. No part of it may be reproduced, stored in a
retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying,
recording or otherwise without written permission from Eschmann Holdings Limited.
The information in this publication was correct at the time of going to print. The Company, however,
reserves the right to modify or improve the equipment referred to.
If the CE mark is affixed to the product, it indicates compliance with Council Directive
93/42/EEC of 14 June 1993 concerning medical devices.
Instructions for use
ST-IM29h October 2003
Page 3
SES LS3
AUTOCLAVE
CONTENTS
INTRODUCTION
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Water quality caution . . . . . . . . . . . . . . . . . . . . . . . 3
WARNINGS
Hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Limitations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Electrical safety . . . . . . . . . . . . . . . . . . . . . . . . . . 4
General safety . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
User notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
INFORMATION
Safety Features . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Declaration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Conditions of safe use . . . . . . . . . . . . . . . . . . . . . 5
Warning markings. . . . . . . . . . . . . . . . . . . . . . . . . 5
INITIAL PREPARATION FOR USE
Initial preparation for use . . . . . . . . . . . . . . . . . . . . 6
LOADING THE AUTOCLAVE
Loading the autoclave . . . . . . . . . . . . . . . . . . . . . . 6
STERILIZING AND DRYING
Sterilizing and drying . . . . . . . . . . . . . . . . . . . . . . . 6
CLEANING AND CARE
Cleaning and care . . . . . . . . . . . . . . . . . . . . . . . . . 7
SAFETY CHECKS
Weekly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Annually . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
UK Guidance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
MAINTENANCE
Fuse renewal . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Preventative Maintenance Agreement . . . . . . . . . 7
Error Correction . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Overheating . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Cleaning Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
TABLE 1
Fault finding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
OPERATING INSTRUCTIONS FOR PRINTER
Printer output . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Front Panel Controls. . . . . . . . . . . . . . . . . . . . . . 10
Paper Roll Renewal . . . . . . . . . . . . . . . . . . . . . . 10
Ribbon Cartridge Renewal . . . . . . . . . . . . . . . . . 10
Spares pack . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Setting Time and Date . . . . . . . . . . . . . . . . . . . . . 11
Illustrations for Printer. . . . . . . . . . . . . . . . . . . . . . 11
TECHNICAL DATA
Technical data . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
INTRODUCTION
There are four versions of the Little Sister 3 Autoclave,
Short and Long chamber both with and without printer. For
instructions on how to use the printer (if fitted) see the
special section towards the end of this booklet.
These instructions apply to the following Autoclaves:SES LITTLE SISTER 3 STANDARD
(from Serial Number LCB8B0000, or LSK8B0000 for non-CE).
without printer : 87-020-05 87-022-01
87-022-17
with printer : 87-020-13 87-022-09
87-022-25
SES LITTLE SISTER 3 LONG
(from Serial Number LLCB8B0000, or LLSB8B0000 for non-CE).
without printer : 87-020-21 87-022-49
87-022-66
with printer : 87-020-29 87-022-58
87-022-74
The Accessories listed below are available:-
Tray . . . . . . . . . . . . . . . . . . . . . . . . REF 87-040-07
Tray lifter . . . . . . . . . . . . . . . . . . . . . REF 87-040-90
Chiropody tray . . . . . . . . . . . . . . . . . REF 87-041-12
Tray - Long . . . . . . . . . . . . . . . . . . . . REF 87-040-19
Cassette (tray with lid) . . . . . . . . . . . REF 87-040-87
Printer spares pack . . . . . . . . . . . . . REF 87-034-05
Service Manual
A Service Manual containing technical description,
complete maintenance procedures and an illustrated list
of spare parts is available on request from the Eschmann
After Sales Service Department.
Reservoir Water Quality Caution
a. Eschmann recommend filling the reservoir with ‘Sterile
Water for Irrigation’. This is low in dissolved solids and
has a low microbial count. In the U.K. the Department
of Health recommend that ‘Sterile Water for Irrigation’
is used in bench-top Autoclaves (NHS Estates
document HTM2031).
b. If ‘Sterile Water for Irrigation’ is not being used then
Eschmann strongly recommend the use of either
distilled water, deionized water, purified water or
water treated by the reverse osmosis process. These
types of water are low in dissolved solids and can help
reduce the effects of tap water detailed below.
c. DO NOT USE TAP WATER, this is high in dissolved
solids and can deposit lime scale, block filters and
cause damage to the pressure vessel.
d. Eschmann also recommend changing the water in the
reservoir on a weekly basis, with the type of water
detailed in ‘a’ (or ‘b’) above. This will reduce the build-up
of contaminants in the water that may cause blocked
filters and/or damage to the pressure vessel. Your local
Health Authority may suggest that you change the
reservoir water more frequently. Eschmann advise you
to follow your local Health Authority’s recommendations.
ST-IM29h P3/12
Page 4
WARNING
READ NOTES (1-5) BEFORE USE
The use of this autoclave should be under the
control of a responsible Person with sterilization
training. The user remains responsible for ensuring
that the load is suitable for the process adopted.
Note 1.
regarding the maximum temperature the instrument
can withstand. This is necessary as the sterilizing
temperature for 121ºC cycles may be exceeded
(typically to peaks of 130°C) during the drying phase
under certain conditions such as small loads.
g. The Autoclave will discharge steam during and at the
end of some cycles. Ensure the autoclave is not
positioned close to heat or smoke detectors as these
may be activated by the release of steam.
THE USER SHOULD BE A WARE OF THE FOLLOWING
POSSIBLE HAZARDS
a. Burns to the user caused by hot accessible surfaces
of the load, burns to the user caused by high
temperature internal surfaces of the chamber, scalds
to the user caused by hot water and/or steam from the
reservoir, scalds to the user caused by hot water from
the chamber on opening the door, scalds to the user
caused by hot water and/or steam from overfilling the
reservoir.
b. The hazard of scalds and burns from contact with hot
surfaces are greater during validation with the cover
removed which also exposes the risk of electric
shock. Validation should therefore only be carried out
by a qualified engineer.
c. Infection, caused by contact with water in the reservoir
if the reservoir is not drained, left to dry and recharged
at regular intervals as described in these instructions
for use (see paragraph 22) to prevent possible
contamination.
d. The autoclave indicates a successful cycle, but the
load remains non-sterile because the load is not
suitable for the steam sterilization cycle. All loads and
loading conditions must be validated. It is
recommended that the user consults the instructions
for use of the items to be autoclaved and if in doubt
consult a qualified microbiologist or Authorized Person.
Note 3.
THE USER SHOULD FOLLOW THESE ELECTRICAL
SAFETY MEASURES
a. This equipment must be earthed.
b. Switch ‘off’ unit and disconnect from mains power
supply before renewing fuses.
c. Switch ‘off’ unit and disconnect from mains power
supply before checking and cleaning the autoclave.
d. Do not attempt to service this equipment internally.
Note 4.
THE USER SHOULD FOLLOW THESE GENERAL
SAFETY MEASURES
a. Do not fill the reservoir during a sterilizing cycle.
b. Do not cover the ventilation louvres.
c. Do not use abrasive powders, chemicals, or solutions
containing chlorine to clean the autoclave.
d. Always allow unit to cool before attempting to open
the chamber door.
e. If an error display appears during a cycle, do not
switch-off power until discharge of hot water or steam
into the reservoir has stopped. Do not attempt to open
chamber door until unit has cooled and internal
pressure has fallen sufficiently to release pressure
safety bolt. Also see ‘Safety Checks’ page 7.
Note 2.
THE USER SHOULD BE A WARE OF THE FOLLOWING
LIMITATIONS DURING USE
a. Never use trays or cassettes without perforations as
this will result in a non-sterile load.
b. Do not process wrapped, sealed or porous goods or
fluids in this autoclave.
c. Not to be used in zones of risk associated with
flammable anaesthetics.
d. The Little Sister 3 is designed to sterilize instruments,
NOT TO WASH THEM. If instruments are not cleaned
this may compromise the efficiency of the sterilization
process.
e. These Autoclaves are not suitable for instruments or
items with narrow lumens.
f. When sterilising instruments not of solid metal
construction, the manufacturer of the instrument must
be consulted about its suitability for autoclaving,
P4/12 ST-IM29h
Note 5.
GENERAL POINTS TO REMEMBER DURING USE
a. Close the chamber door properly before selecting the
required programme.
b. Always leave the chamber door slightly open after
use.
c. Never use tap water see section 6 and ‘Reservoir
Water Quality Caution’ on page 3.
d. Ensure instruments are free from debris before placing
them in sterilizer chamber.
e. Use the ‘tray lifter’ to remove the sterilized load.
f. Keep chamber trays and chamber face clean.
g. Clean door seal and chamber face with a lint-free
cloth at weekly intervals.
h. Regular maintenance (every three months is
recommended) is required to ensure continued safety
and reliability
Page 5
SES LS3
AUTOCLAVE
GENERAL INFORMATION NOTES
IMPORTANT
The design of the autoclave pressure vessel is
approved by a third party to PD5500 Cat.3. The
pressure vessel is constructed and tested to BS3970
Parts 1 and 4. In order to ensure safety and to
comply with UK regulations, the vessel and fittings
should be inspected by a ‘competent person’ at
intervals of no more than 14 months. This can be
carried out by an Eschmann trained engineer.
The Little Sister 3 Autoclave (Standard and Long) is designed
for the sterilization of unwrapped instruments, utensils and
other items. It operates automatically at the touch of a single
programme selector touch button, and has four programmes
134°C and 121°C, both with and without drying (see Important
Note below right). The Autoclave is capable of 4 cycles per
hour when the ‘134°C Without drying’ programme is selected.
For sterilization cycle times, refer to the ‘TECHNICAL DA T A ’
section on page 12.
Little Sister 3 Autoclave, safety features:-
a. Pressure Door Lock. The door is mechanically locked
at pressures above 0.14 bar.
b. Door Interlock Switch. This prevents programme
starting if door is not properly closed.
c. Electrical Door Lock. This prevents the door being
opened inadvertently by the operator once the cycle
has started and holds the door closed until the end of
the cycle. It will also keep the door closed under all
fault conditions.
d. Pressure Relief Valve (safety valve). This valve safely
releases excess pressure (i.e. above 2.6 bar).
e. Microcomputer. The microcomputer constantly
monitors all key functions. If an error arises, it
immediately stops the cycle, discharges pressure,
and causes the appropriate message to be displayed
(See Error Correction para. 27).
f. Overheat Control. The microcomputer operates in
conjunction with an independent manual reset
thermostat to protect the heating element from
overheating. The autoclave is also fitted with a fusible
link which, should overheating occur, will cut all power
to the heating element and microcomputer and operate
the manual reset thermostat, leaving only the red
overheat warning lamp illuminated on the front panel.
Declaration
The design of the autoclave pressure vessel is approved
by a third party to PD5500 Cat.3. The pressure vessel is
constructed and tested to BS3970 Parts 1 and 4.
Conditions of Safe Use:-
a. Indoor Use.
b. Altitude up to 2000 metres.
c. Temperature 5°C to 40°C.
d. Maximum relative humidity 80% for temperatures up
to 31°C, decreasing linearly to 50% relative humidity
at 40°C.
e. Mains supply voltage fluctuations not to exceed ±10%
of the normal voltage.
f. No other voltage fluctuations specified by the
manufacturer.
Note: If it is required to operate this Autoclave outside of
these conditions, contact Eschmann Equipment at the
address given in these instructions.
IMPORT ANT NOTE:
UK Customers should arrange for this equipment
to be installed by an Eschmann Trained Engineer
before use. (For free installation and service details
see page 2). Also see ‘Safety Checks’ page 7.
The preset sterilizing temperature for your Little Sister 3
Autoclave is displayed on a temporary label affixed to the
front of the unit. All programmes can be deactivated or
reactivated by an Eschmann Trained Engineer , on request.
WARNING MARKINGS
The warning markings on this equipment have the
following meanings
Caution; refer to accompanying documents
(i.e. these Instructions for Use).
This symbol, adjacent to the door knob, warns
the user of the possible escape of steam and
hot water when the door is opened.
Caution; refer to accompanying documents
(i.e. these Instructions for Use).
This symbol, adjacent to the overheat light at
the bottom of the control panel, warns the user
of overheating of the sterilizing chamber.
Caution; hot surface.
This symbol warns the user of high surface
temperatures on the outside of the equipment.
Caution; refer to accompanying documents
(i.e. these Instructions for Use).
This symbol on the serial number label (rating
plate), and on the lid of the unit, warns the user
that it is necessary to read the Instructions for
Use before using the equipment.
Note: This equipment is defined as:
Installation Category 2 (Overvoltage Category 2)
Pollution Degree 2 (in accordance with IEC664).
ST-IM29h P5/12
Page 6

INITIAL PREPARATION FOR USE
1. When lifting or moving the Little Sister 3 Autoclave
(preferably with two people), place the hands under
the base at each side of the unit. Place the autoclave
on a flat, level surface and ensure there is a working
clearance of 150 mm all round for adequate ventilation.
Do not cover the ventilation louvres. Also see Note 2
part ‘g’ on page 4.
2. Check that the plug fuse is appropriate for the voltage
of the equipment (see rating plate on back of cabinet).
3. Connect unit to mains power supply (Warning this
equipment must be earthed) and switch on by
selecting unit power switch ‘0-I’ to ‘I’ (Fig. 1). Display
will go to ‘LS3’ followed by cycle count then door/test.
Note: If the door knob is turned to the fully closed
position the chamber door cannot be opened until
power is switched on.
4. Open chamber door (Fig. 2). Remove tissue paper
from the door; autoclaving trays, tray lifter; reservoir
lid, drip tray and feet adjustment pack. Discard all
packing pieces.
5. Fit reservoir cover and place plastic drip tray at front
of unit, below door. Fit feet adjustment pack
components as follows:
a. Plastic cups to protect work surface from rubber
feet marks.
b. If work surface is not level, place spacers, as
required, inside cup(s).
11. Do not overload trays, and avoid ‘bunching’ items
together.
12. Do not use trays or cassettes without holes and
ensure that the load does not block the holes in the
perforated trays and cassettes.
13. Load trays and place them in the sterilizing chamber
(Fig. 4 and 4a). All items must be positioned so that
they drain freely and do not trap rising air bubbles.
14. Close the chamber door by first ensuring the door
handle is turned fully open (counter-clockwise) before
shutting the door and locking by turning the handle
180° clockwise (Fig. 2).
STERILIZING & DRYING THE LOAD
15. Select required programme by pressing the
appropriate button on control panel (Fig. 5). The
indicator lamp for the selected programme remains
illuminated. The sterilizing cycle will now proceed
automatically and the printer (if fitted) will commence
printing. As the cycle progresses various displays will
appear in the display window (Fig. 1 item 3) to indicate
programme status.
Note: Select only from the programmes at the preset
sterilizing temperature (see Important Note, page 5).
16. The display sequence during the sterilizing cycle is:
Display Interpretation
6. Remove reservoir lid (Fig. 1) and fill reservoir up to
‘MAX’ mark (Fig. 3) with ‘Sterile Water for Irrigation’.
DO NOT USE TAP WATER, see ‘Reservoir Water
Quality Caution’ on page 3. Refit the reservoir lid.
LOADING THE AUTOCLAVE
7. Check you have read the limitations on use page 4
and technical Specification page 12.
8. Check that water level in reservoir is between ‘MAX’
and ‘MIN’ marks (Fig. 3). DO NOT FILL DURING
CYCLE.
9. With chamber door open (para. 3, Note), select mains
‘on/off’ switch to ‘I’ (on) (Fig. 1). This will initiate a single
audible signal followed by the display ‘LS3’, then the
number of cycles, the time of day (see note below) and
the flashing green indicators for the preset cycles.
Note: For units without a printer ‘rEAdy’ is displayed
not the time of day.
10. Before loading instruments into trays or cassettes
they should be pre-washed, preferably in an ultrasonic
cleaner to remove amalgam, debris, etc., then rinsed
to remove all traces of proprietary cleaners, chemicals
or disinfectants, before being placed in the sterilizer
chamber. Residue from these products could result in
a blockage of the water recycling system.
door/tESt Test door interlock switch (para. 14)
(Flashing
alternately)
door Sterilizing chamber door open
time of day Programme can now be selected
(e.g. 1 1-45) Note: for units without a printer
‘rEAdy’ is displayed.
FiLL Chamber being filled
HEAt Heater temperature below 92°C
92 to 136 Heating to sterilizing stage
S135.5 ‘S’ flashing, indicates sterilizing begun
and timing started
cond Steam being discharged and
condensed
dry Load being dried
End Cycle complete
Ser Service set to display at 1500 cycles.
This will not inhibit the use of the
autoclave, but, do arrange a service.
P6/12 ST-IM29h
Page 7
SES LS3
AUTOCLAVE
17. If a cycle “With Drying” is selected then commencement
of the drying phase is indicated by the display ‘dry’
accompanied by a rapid intermittent audible signal.
When this signal is heard, maximum drying will be
achieved if the chamber door is opened halfway and
then closed until it touches the metal catch on the front
fascia panel, leaving a small gap (approx. 1cm) for
ventilation. Instruments can be taken out any time
during the drying phase, but best results will be
achieved by waiting until the automatic cycle is
complete.
18. On completion of a sterilizing cycle ‘without drying’,
the display ‘End’ will appear, accompanied by a brief
audible signal. When the door is opened to remove
load the display will change to ‘door’.
19. On completion of a sterilizing cycle ‘with drying’ (where
chamber door is opened slightly during the drying
phase), the display ‘End’ will appear briefly
accompanied by an audible signal and followed, after
a few seconds by ‘door’.
20. Open door fully and remove load USING THE TRAY
LIFTER and leave the door open slightly when not in
use.
AUTOCLAVE CLEANING & CARE
SAFETY CHECKS
24. Users should ensure that the following periodic safety
checks are carried out at the stated intervals:-
Weekly
¬ Check that the door opens and closes easily
¬ Check the door seal for any signs of damage
¬ Check that the secondary door catch latches
effectively
¬ Check for any obvious escape of steam or water
during a cycle (i.e. other than is normal from the top
of the reservoir)
CAUTION
Annual inspections should only be
undertaken by a Competent Person.
Annually
¬ Check the pressure relief valve operates freely
and at the set pressure
¬ Inspect the Pressure System for integrity
¬ Check Door micro-switches and interlocks
¬ Check Door locking mechanism for integrity
¬ Check Pressure indicators for correct operation
¬ Check overheat devices for function
UK Guidance
WARNING
Chlorine, even in the concentrations found in tap
water, can cause stainless steel to crack and could
damage the chamber. Disconnect from the mains
electrical supply before cleaning the Autoclave.
21. Keep chamber trays, door seal and chamber face
clean. These should only be cleaned with a lint-free
cloth. Clean the outside of the autoclave by wiping
down with a cloth dampened with a 70% solution of
industrial methylated spirit (IMS) and water. Allow to
dry by evaporation.
Note: Do not use abrasive powders, chemicals, or
solutions containing chlorine to clean the autoclave.
22. The reservoir must be drained weekly and be allowed
to dry (Fig. 6). When refilling see section 6 and
‘Reservoir Water Quality Caution’ on page 3, DO
NOT USE TAP WATER. Regular cleaning of the
reservoir will reduce the effects of excessive handpiece
lubricant which could be detrimental to the function of
the autoclave.
23. Attention to the following will increase the life of the
your Little Sister 3 Autoclave:
a. After use leave chamber door slightly open.
b. At weekly intervals, lightly clean door seal and
chamber face with a lint-free cloth.
Inspections can be arranged by contacting the Eschmann
After Sales Service Department, see inside cover for contact
details. Eschmann can also provide comprehensive service
contracts, which cover preventive maintenance to ensure
trouble free operation of your autoclave as well as an annual
inspection of the pressure system to satisfy the requirements
of the Pressure Systems Safety Regulations 2000.
MAINTENANCE
(Ensure you have read pages 3-5)
Fuse Renewal
25. Fuses are fitted at the back of the autoclave (Fig. 1,
detail). For fuse ratings refer to ‘TECHNICAL DATA’.
To extract a fuse switch ‘off’ the unit and disconnect
from the mains power supply, insert a screwdriver or
small coin in the slot of the fuse holder and twist it
counter-clockwise. After inspecting or renewing a fuse,
reverse the above procedure to re-secure fuse holder.
Preventive Maintenance Agreement
26. We would strongly recommend that a ‘P.M.A.’ is taken
out on your Little Sister 3 Autoclave. Although the
autoclave requires minimal maintenance, it is important
to have the autoclave checked and calibrated at
regular intervals. This ensures that the exacting
conditions necessary for sterilization are maintained
throughout the unit’s working life.
ST-IM29h P7/12
Page 8

Error Correction
27. If, after switching ‘on’ power there is no visual display,
or audible tone, first check power supply connections.
Also check all 3 fuses at rear of unit (see Fuse Renewal).
If any error occurs during a cycle (i.e. any time after
selecting a programme), the cycle will abort and provided
power supply to the unit is maintained, the error will be
indicated by one of the visual displays shown in Table 1.
If power fails during a cycle, check supply conditions,
fuses, and connections as indicated in this paragraph.
Overheating
28. In the unlikely event of overheating, the red overheat
warning lamp (Fig. 1) will illuminate. If this happens,
first allow approximately 10 to 15 minutes for the
autoclave to cool, then check the water level in
reservoir. When the water level is correct, press the
button marked ‘PRESS TO RESET’ at the rear of the
cabinet (Fig.1, detail) and re-start the cycle in the
normal way. If the fault persists, switch off the unit and
call an Eschmann trained engineer.
Note: Should the ‘press to reset’ thermostat operate
in a nil water condition, the chamber temperature is
below 138°C. The maximum chamber temperature
on overheat, to melt the thermal fuse is 250°C.
Cleaning the Filter
29. The Little Sister 3 Autoclave is fitted with a filter which
will require cleaning by a qualified service engineer.
This should be carried out at least annually.
P8/12 ST-IM29h
Page 9

SES LS3
AUTOCLAVE
Table 1 Fault Finding
WARNING
If an error display appears during a cycle, do not switch-off power until discharge of hot water or steam
into reservoir has stopped. Do not attempt to open the chamber door until the unit has cooled and internal
pressure has fallen sufficiently to release the pressure safety bolt. Loads whose cycles are aborted by one
of these error displays should be treated as non-sterile.
DISPLAY CAUSE REMEDY
a. Check local supply conditions
b. Check supply plug wiring and power cable for possible breaks.
c. Because there can be no guarantee that the load has been sterilised,
‘ELECt’ will continue to be displayed after power has been restored.
ELECt Temporary failure in T o re-start cycle:
(+ audible the mains supply to i. Wait until discharge of water or steam into reservoir has stopped.
signal) unit. ii. Switch ‘of f’ power and wait approximately 10 seconds.
iii. Push and hold-in any one of the four programme selection
buttons and, at the same time, switch ‘on’ power .
iv . Release programme selection button as soon as ‘LS3’ appears.
v . Select required programme to re-start cycle.
ERR2 Door switch faulty a. T o re-start cycle see remedy for ELECt.
(+ audible or maladjusted. b. If ‘ERR2’ persists call an Eschmann trained engineer .
signal)
WARNING: Do Not Open Door
a. For safety reasons, switch ‘off’ power.
LoH2O Insufficient water b. Check water level in reservoir is above ‘MIN’ mark.
(+ audible in reservoir If water is above the ‘MIN’ mark, do not fill to ‘MAX’ mark
signal) during a cycle.
c. To re-start cycle see remedy for ELECt.
d. If ‘LoH2O’ persists call an Eschmann trained engineer .
WARNING: Do Not Open Door
ERR3 Water failed to a. Allow unit to cool, there may be hot water in the chamber.
enter chamber To re-start cycle see remedy for ELECt.
from reservoir b. If these errors persist call an Eschmann trained engineer.
Water level in a. Wait for any discharge of water or steam into reservoir to cease.
ERR4 chamber has b. Switch ‘off’ power and carry out water level check, as for ‘LoH2O’.
(+ audible dropped slightly . c. To re-start cycle see remedy for ELECt.
signal) d.If ‘H2O’ persists call an Eschmann trained engineer .
ERR5 Heater not working a. Check RESET button (see para 28).
(+ audible b. If ‘ERR5’ persists call an Eschmann trained engineer .
signal)
ERR6,7,8,9 Temperatures outside a. Allow unit to cool. To re-start cycle see remedy for ELECt.
ERR8 pre-programmed b. If these errors persist call an Eschmann trained engineer.
ERR9 limits for sterilization
(+ audible
signal)
door/tESt Door closed with
Flashing power on. a. Leave door open slightly when not in use before removing power.
alternately
ST-IM29h P9/12
Page 10

OPERATING INSTRUCTIONS
FOR PRINTER
Printer Output
The printer output gives the following information:
¬ Manufacturer’s name Eschmann Equipment
¬ Machine type SES Little Sister 3
¬ Serial number Alpha numeric code
¬ Cycle type e.g. 134°C no drying
¬ Time and date started hh:mm:ss on dd:mm:yy
¬ Counter reading Fiv e di gits wit h leading zeros
¬ Time/temp information Time every 22 secs
(approx.) + temp. + graph
of temperature.
¬ Cycle ended message The ‘cycle end’ is defined
as the end of the
sterilizing phase.
¬ Time and date ended hh:mm:ss on dd:mm:yy
If an error occurs during the cycle, it is recorded with date
and time on the printout and the message ‘CYCLE FAILED’.
Errors are designated by error codes as follows:
ERR1 -Faulty temperature sensor/channel
detected (‘ELECt’ on display)
ERR2 -Door open during cycle
ERR3 -Chamber did not fill with water
ERR4 -Water loss early in cycle
ERR5 -No heat
ERR6 -Control temperature low
ERR7 -Control temperature high
ERR8 -Monitor temperature low
ERR9 -Monitor temperature high
These error codes will generally require investigation by
an Eschmann trained engineer.
Front Panel Controls
All controls for the printer are on the front of the panel.
a. Door Latch Button - To open door (Fig. 7,1) push the
door latch (Fig. 7, 2) sideways, in the direction of
arrow, and pull the door open to expose paper roll
(Fig. 7,4).
b. Paper Feed Button - Ensure the sterilizer mains ‘on/
off’ switch is in the ‘I’ (on) position. Press lower part of
paper feed button (Fig. 7,3) to activate paper feed,
which will continue for as long as the button is
depressed.
Paper Roll Renewal
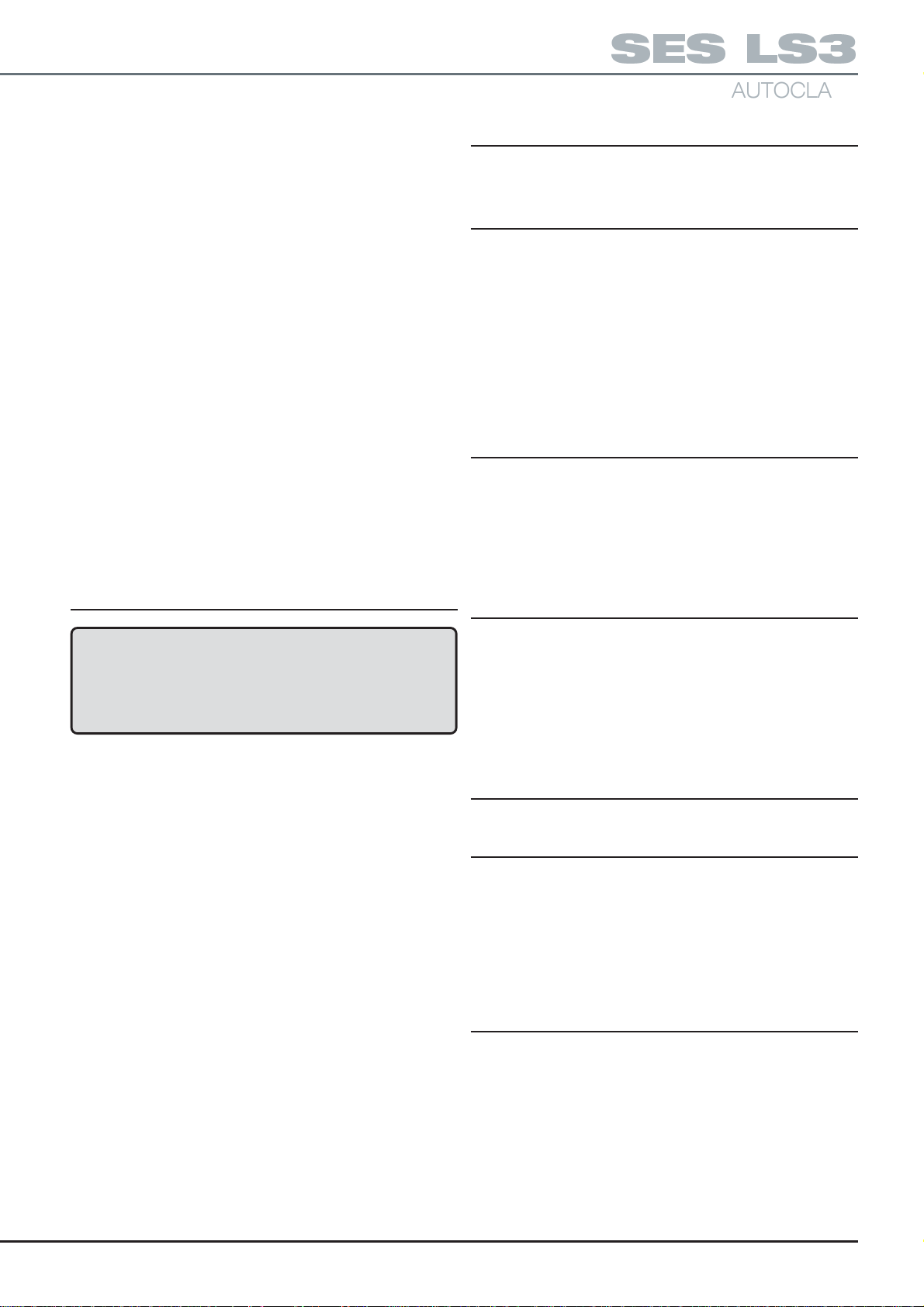
Renew paper roll as follows:
a. Open autoclave chamber door to provide more access.
b. Push door latch button (Fig. 7,2) sideways, in direction of
arrow and pull door open to expose paper roll (Fig. 7,4).
c. If any paper remains in the printer, tear end off against
paper tear bar (Fig. 7,8) and carefully withdraw the
remaining paper backwards from the rear of printer
mechanism (Fig. 7,5).
d. Compress paper roll spring-loaded retainer button
(Fig. 7,6) and withdraw empty paper spool from spindle.
e. Take new roll of paper, separate paper end from the roll.
Remove any damaged or gummed part of the paper.
f. Compress paper roll spring-loaded retainer button
(Fig. 7,6) and locate new paper roll on spindle with
paper unrolling counter-clockwise as seen from open
end of spindle (Fig 7).
g. Using scissors, trim end of paper roll at an angle of
approximately 30° (Fig. 8) and insert this end of the
paper into the paper insert slot (Fig. 8,1). Press paper
feed actuator arm (Fig. 7,7) until mechanism grips
paper and pulls it through to front of printer door.
h. Turn paper roll by hand to take up any slack paper
i. Close the printer door. Check paper feed by pressing
paper feed button (Fig. 7,3) until the end of the
trimmed paper is clear of the tear bar. Ensure that the
paper feeds freely from the printer.
Ribbon Cartridge Renewal
Renew ribbon cartridge as follows:
a. Remove paper roll.
b. Support door with left hand and press upwards with
right hand on bottom of printer mechanism chassis
(Fig. 9) and separate chassis from door.
CAUTION:
Do not pull printer mechanism chassis from
door until catch has been released.
c. Leaving printer door fully open, expose printer
mechanism and ribbon cartridge (Fig. 10,1).
d. Pinch the end of ribbon cartridge marked ‘PUSH’
(Fig. 10,1) and remove cartridge.
e. Install new cartridge, checking that left hand side of
cartridge is correctly located on drive shaft, and
CAREFULLY press cartridge into place.
f. Ensure ribbon is taut. If necessary tighten ribbon by
turning faceted disc (Fig. 10,2) on cartridge, clockwise,
using finger or fingernail.
g. Fit paper roll as described above.
Spares Pack
CAUTION:
DO NOT USE ALTERNATIVE PAPER ROLLS
The quality and size of paper rolls used in the printer,
can only be supplied by Eschmann Equipment.
A spares pack is available comprising:
¬ Five paper rolls
¬ Two ribbon cartridges
The spares pack is available from Eschmann Equipment
under catalogue number 87-034-05.
P10/12 ST-IM29h
Page 11
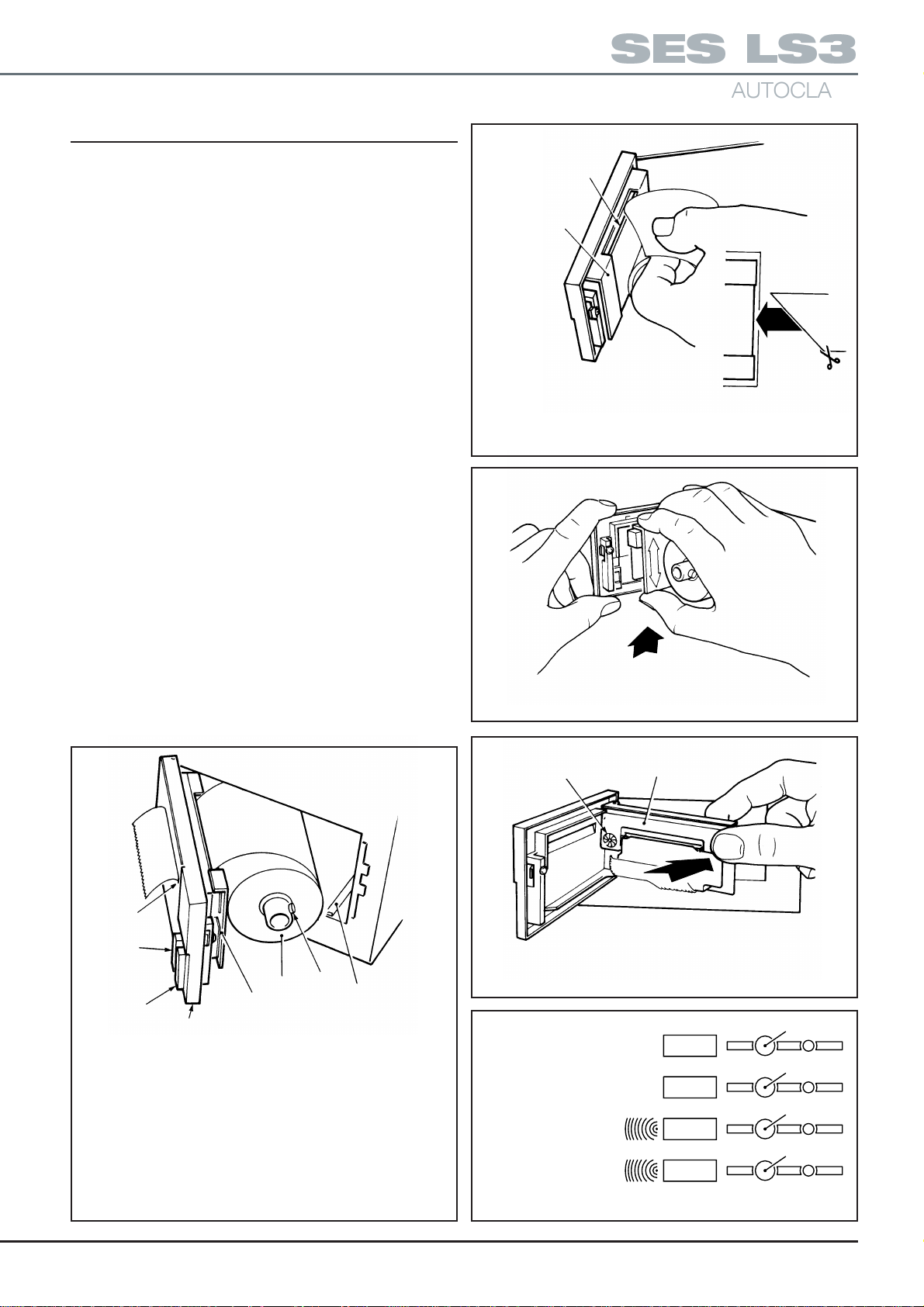
Setting Date and Time
SES LS3
AUTOCLAVE
1. To change the clock setting (e.g. from GMT to BST or
the reverse) proceed as follows:
a. Select mains ‘on/off switch to ‘0’ (off).
b. Refer to Fig. 11, push and hold down buttons
SW1 and SW4 simultaneously then select ‘on/
off’ switch to ‘I’ (on).
c. The display will read ‘01’.
d. Then proceed as described in paragraph 2 (a)
to (g).
2. To set the date and time proceed as
a. If closed, open chamber door.
b. Display reads ‘01’. Push and hold down button
SW1 (Fig.1 1). Observe that number changes to
02,03 etc. through to 12. Also note that pushing
and holding SW2 makes the number decrease.
Use SW1 and SW2 to set the number corresponding to the month (01=January etc.). Push
SW3 to retain selection.
c. Display reads ‘dy01’. Again use SW1 and/or
SW2 to display the day of the month (push SW3
to retain the day).
Note: The computer will prevent entry of dates
such as 31st November.
d. Display reads ‘yr00’. Use SW1/SW2 to set any year
from 00 to 99 e.g. 97. Then push SW3 to retain it.
e. Display reads ‘hr00’. Use SW1/SW2 to set the
hour - the system utilises a 24 hour clock. Push
SW3 to retain.
f. Display reads ‘in00’ (minutes). Use SWI/SW2 to
show current minutes and push SW3 to retain it.
g. The clock is now set.
1
2
1. Paper Insert Slot
2. Printer Mechanism Chassis
Fig. 8. Paper Feed
Fig. 9. Ribbon Cartridge
8
3
2
1. Printer Door
2. Door Latch
3. Paper Feed Button
4. Paper Roll
5. Printer Mechanism
6. Spring-Loaded Retainer Button
7. Paper Feed Actuator Arm
8. Paper T ear Bar
Fig. 7. Printer
2
1. Ribbon Cartridge
2. Faceted Disc
6
4
5
1
7
Fig. 10. Ribbon Cartridge in Position
Programmes
without drying
Programmes
with drying
Fig. 11. Setting Date and time
{
{
1
SW1
134°C
SW2
121°C
SW3
134°C
SW4
121°C
ST-IM29h P11/12
Page 12

TECHNICAL DATA
ELECTRICAL
Supply
220V/230V,
50/60 Hz a.c.
For use with alternating current
Loading
Volts Standard Long
220V 1.9kW 2.4kW
230V 2.0kW 2.6kW
Fuses
Standard 220V - 13A (Pt.No.380002) (x2)
230V - 10A (Pt.No.380003) (x2)
Long 230V - 15A (Pt.No.301871) (x2)
220V - 15A (Pt.No.301871) (x2)
All versions F400mA (Pt.No.696181) (x1)
SAFETY STANDARDS
IEC 1010-1 (1990) - Standard version only
IEC 601-1 (1977) BS5724:Part 1 (1979)
ESCHLE (Second Edition 1986)
EN 61010-1 (1993) inc. Amendment 2 (1995)
EN 61010-2-041 (1996)
STERILIZING
Time
At 134/137°C - 3min 20sec
At 121/124°C - 15min
Typical overall cycle times without drying at 2kW
134°C - 13min
121°C - 24min
Drying time when selected
up to 17 minutes after sterilizing cycle
Operating pressure
137°C - 2.32 bar
134°C - 2.03 bar
124°C - 1.25 bar
121°C - 1.03 bar
Maximum Load per Tray
1.5kg Standard 3.5kg Long
WATER RESERVOIR
Capacity 2.0 litre
WEIGHT (approx.)
Net 27.5kg Standard 35.5kg Long
Shipping 32.0kg Standard 40.0kg Long
P12/12 ST-IM29h
Page 13

Page 14

Page 15

OPEN THIS PAGE OUT FROM
THE OTHER SIDE TO GAIN
EASY ACCESS TO THE
ILLUSTRATIONS WHILST
READING THE TEXT.
ST-IM29h
OPEN HERE
TO GAIN
ACCESS TO
ILLUSTRATIONS
Page 16
19
13
14
15
1
2
3
4
16
5
6
10
1112
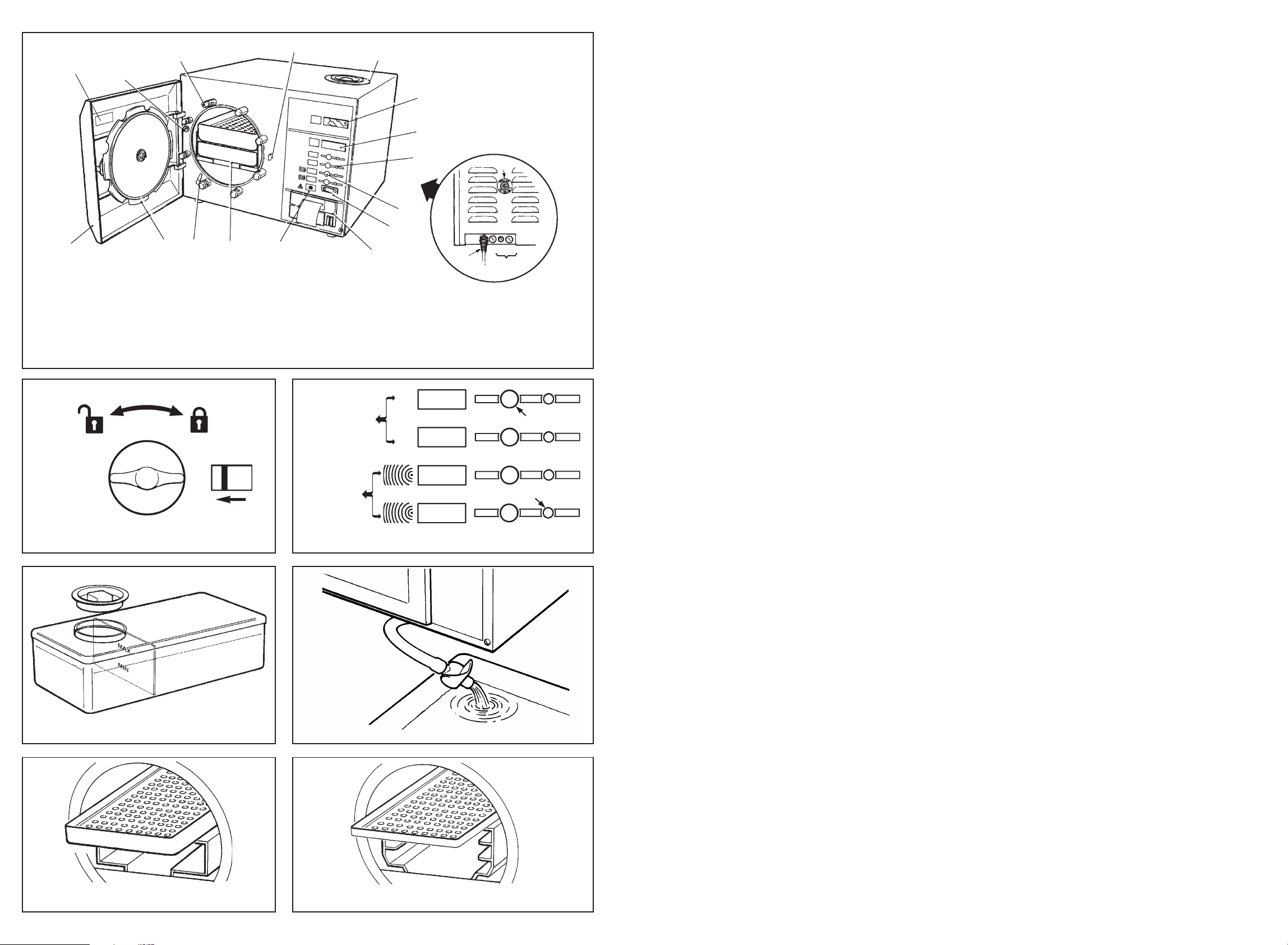
1. Reservoir lid 6. Mains on/off Switch 12. Chamber Door
2. Analog Pressure Gauge 7. Printer (if fitted) 13. Electric Door Bolt
3. Display Window 8. Overheat Warning Lamp 14. Pressure Door Bolt 18. Fuses
4. Programme Indicator 9. Sterilising Trays (4 on 1 5 . Door Safety Catch 1 9. Serial Number Plate
Lamps Standard, 2 on Long) 16. Overheat Reset Button
5. Programme Selection 10. Door Interlock Switch 17. Power Supply Cable
Touch Buttons 11. Door Seal
9
8
7
17
18
Fig. 1. Little Sister 3 Autoclave
(Long Version + Printer)
Unlocked Locked
Programmes
without drying
134°C
Programme Selection Touch Buttons
121°C
Programmes
with drying
To Open
134°C
Programme Indicator Lamps
121°C
Fig. 2. Door Controls
Fig. 3. Water Reservoir
Fig. 5. Control Panel
Fig. 6. Drain T ap
Fig. 4. Sterilising Chamber Long
Fig. 4a. Sterilising Chamber Standard
 Loading...
Loading...