Page 1

LS5
VACUUM & NON-VACUUM AUTOCLAVES
Instructions for use
1 10151
ST-IM61e
Page 2
Preliminary
Information
Safety notes
and cautions
Installation
Operation
Cleaning, care
and maintenance
Technical data
Read these Instructions before use
Keep these ‘Instructions for use’ in a safe convenient place for future reference. Read in
conjunction with the Publications detailed in Section 1.4 and 1.5.
Eschmann After Sales Service Department
The Eschmann After Sales Service Department is staffed and equipped to provide advice and
assistance during normal office hours. To avoid delays when making enquires, please quote the
Model and Serial Number of your Autoclave which is shown on the Serial Number plate, the
location of which is shown in Fig. 2 item 8. Please ensure you include all alpha and numeric digits
of the Serial Number.
For further information visit www.eschmann.co.uk
All correspondence relating to the after sales service of Eschmann Equipment to be addressed to :
UK Customers
Eschmann Equipment, Peter Road, Lancing, West Sussex BN15 8TJ, England.
T el: +44 (0) 1903 765040. Fax: +44 (0) 1903 875711.
Overseas Customers
Contact your local distributor. In case of doubt contact Eschmann Equipment.
Patents and Trade marks
The ESCHMANN name and logo are registered trade marks of Eschmann Holdings Limited.
“Eschmann Equipment” is a trading name of Eschmann Holdings Limited.
“Little Sister” and “LS5” are trade marks of Eschmann Holdings Limited.
Patents Pending plus: Pat. US5090033 and Pat. GB2238407.
Copyright © 2005 Eschmann Holdings Limited
All rights reserved. This booklet is protected by copyright. No part of it may be reproduced, stored in a
retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying,
recording or otherwise without written permission from Eschmann Holdings Limited.
The information in this publication was correct at the time of going to print. The Company, however,
reserves the right to modify or improve the equipment referred to.
The CE marking affixed to the product certifies that it complies with the
European Medical Devices Directive 93/42/EEC and related legislation.
Instructions for use
ST-IM61e May 2005
Page 3

VACUUM & NON-VACUUM AUTOCLAVES
Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2 Hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
3 Limitations on use . . . . . . . . . . . . . . . . . . . . . 5
4 Electrical safety . . . . . . . . . . . . . . . . . . . . . . . 5
5 General safety . . . . . . . . . . . . . . . . . . . . . . . 5
6 Conditions for safe use . . . . . . . . . . . . . . . . . 5
7 Warning markings . . . . . . . . . . . . . . . . . . . . . 5
8 Safety features . . . . . . . . . . . . . . . . . . . . . . . 6
9 Declaration . . . . . . . . . . . . . . . . . . . . . . . . . . 6
10 Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
11 Control and panel details . . . . . . . . . . . . . . . 6
Mains switch . . . . . . . . . . . . . . . . . . . . . . 6
Push-button . . . . . . . . . . . . . . . . . . . . . . 6
Digital displays . . . . . . . . . . . . . . . . . . . . 6
Indicators . . . . . . . . . . . . . . . . . . . . . . . . 7
12 Initial preparation for use . . . . . . . . . . . . . . . 7
13 Loading the autoclave . . . . . . . . . . . . . . . . . . 7
14 Running a cycle . . . . . . . . . . . . . . . . . . . . . . 8
15 Special programmes/Functions/Tests . . . . . . 9
16 Cleaning and care . . . . . . . . . . . . . . . . . . . . 10
17 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . 10
18 Printer instructions . . . . . . . . . . . . . . . . . . . 16
Installation . . . . . . . . . . . . . . . . . . . . . . 16
Output . . . . . . . . . . . . . . . . . . . . . . . . . 16
Paper roll renewal . . . . . . . . . . . . . . . . 16
Ribbon cartridge renewal . . . . . . . . . . . 17
Spares pack . . . . . . . . . . . . . . . . . . . . . 17
19 Technical data . . . . . . . . . . . . . . . . . . . . . . . 18
20 Accessories . . . . . . . . . . . . . . . . . . . . . . . . 18
LS5
Appendix 1 - Cycle B4 notes . . . . . . . . . . . . . . . 19
Appendix 2 - Safety checks . . . . . . . . . . . . . . . . 19
Appendix 3 - Daily record sheet . . . . . . . . . . . . 20
Table 1 Key to display A2 . . . . . . . . . . . . . . . . . 11
Table 2 Special functions . . . . . . . . . . . . . . . . . 12
Table 3 Error numbers and remedies . . . . . . . . 14
ST-IM61e P3/20
Page 4
1 Introduction
WARNING
Read these instructions before use and keep them in
a safe convenient place for future reference. The use
of this autoclave should be under the control of a
person with adequate sterilization training. The
operator remains responsible for ensuring that the
load is suitable for the process adopted. The autoclave
should only be used as specified by the manufacturer
and detailed within these instructions. Never tamper
with, bypass or interfere with any of the safety features.
There are no ‘operator’ serviceable parts inside the
autoclave. Eschmann Equipment is not responsible
for malfunction, or a reduced level of protection
provided by the equipment, if used in a manner not
specified by them. Only use in conjunction with
Eschmann accessories and mains lead.
RESERVOIR WATER QUALITY CAUTION
a. Eschmann recommend filling the reservoir with
‘Sterile Water for Irrigation’. This is low in
dissolved solids and has a low microbial count.
In the U.K. the Department of Health recommend
that ‘Sterile Water for Irrigation’ is used in benchtop Autoclaves (NHS Estates document
HTM2031).
b. If ‘Sterile Water for Irrigation’ is not being used
then Eschmann strongly recommend the use of
either distilled water, deionized water, purified
water or water treated by the reverse osmosis
process. These types of water are low in
dissolved solids and can help reduce the effects
of tap water detailed below.
c. DO NOT USE TAP WATER, this is high in
dissolved solids and can deposit lime scale,
block filters and cause damage to the pressure
vessel.
1.1 For Customers on the Mainland of England,
Scotland and Wales, or on the Isle of Wight ONLY, when
you receive your Autoclave ensure that you complete your
Service Guarantee and Registration Card, and then
FREEPOST it to Eschmann Equipment. Failure to return
this card means you will not benefit from our FREE
installation service, which will ensure your Autoclave is
correctly installed, plus a FREE service visit during the
following 12 months.
1.2 This manual applies to the following Autoclaves fitted
with software version 1.51 (see section 19.7) or later:-
SES Little Sister 5 Autoclave
(from Serial Number L5NC....) REF 87-060-01
SES Little Sister 5 Vacuum Autoclave
(from Serial Number L5SA3C1001) REF 87-060-25
It is also applicable to earlier models of the LS5 autoclave
that has been upgraded by Eschmann or their accredited
agents and has software version 1.51 (see section 19.7)
or later.
1.3 These Autoclaves are designed for the sterilization
of instruments, utensils and other items. Only the Vacuum
models can sterilize wrapped or pouched items and hollow
instruments. They operate automatically and have
programmes at 134°C and at 121°C (with and without
drying). For sterilization times, refer to section 19.
1.4 The Service Manual (ST-SM33) containing a
technical description, complete maintenance procedures
and an illustrated list of spare parts is available on request
from the Eschmann After Sales Service Department.
1.5 A Manual (ST-IM55) providing the ‘Manufacturers
recommended instructions for testing vacuum autoclaves’
to the applicable parts of HTM2010 and DB9804 is available
on request from the Eschmann After Sales Service
Department.
d. Eschmann also recommend changing the water
in the reservoir on a weekly basis, with the type
of water detailed in ‘a’ (or ‘b’) above. This will
reduce the build-up of contaminants in the water
that may cause blocked filters and/or damage
to the pressure vessel. Your local Health
Authority may suggest that you change the
reservoir water more frequently. Eschmann
advise you to follow your local Health Authority’ s
recommendations.
P4/20 ST-IM61e
Page 5
LS5
VACUUM & NON-VACUUM AUTOCLAVES
2 Hazards
2.1 The operator should be aware of the following
hazards:-
a. Burns to the operator caused by contact with hot
accessible surfaces of the load (use the ‘tray lifter’
to remove the sterilized load) and high temperature
internal surfaces of the chamber. Scalds to the
operator caused by hot water and/or steam from
the reservoir, from the chamber on opening the door
and from overfilling the reservoir.
b. The hazard of scalds and burns from contact with
hot surfaces are greater during validation when the
covers may be removed which also exposes the
risk of electric shock. V alidation should therefore only
be carried out by a qualified engineer.
c. Infection, caused by contact with water in the
reservoir if the reservoir is not drained and left to
dry as described in these instructions for use.
d. The autoclave indicates a successful cycle, but the
load remains non-sterile because the load is not
suitable for the cycle selected. All loads and loading
conditions should be validated. It is recommended that
the operator consults the instructions for use of the
items to be autoclaved and if in doubt consult a qualified
microbiologist (or in the UK an Authorized Person).
3 Limitations on use
5 General safety
IMPORTANT
The design of the autoclave pressure vessel is
approved by a third party to International Standards.
In order to ensure safety and to comply with UK
and/or International regulations, the vessel and
fittings should be inspected by a ‘competent person’
at intervals of no more than 14 months. This can be
carried out by an Eschmann trained engineer. Also
see Appendix 2.
5.1 The operator should follow these general safety
measures:-
a. Do not cover the ventilation ports at the rear.
b. Do not use abrasive powders, chemicals, or
solutions containing chlorine to clean the autoclave.
c. Do not attempt to open chamber door until unit has
cooled and internal pressure has fallen sufficiently to
allow the door to be opened, by pressing the
button.
d. If an error display appears during a cycle, do not
switch-off power until discharge of hot water or
steam into the reservoir has stopped.
6 Conditions for safe use
6.1 This autoclave has been designed for use in the
following conditions only:-
3.1 The operator should be aware of the following
limitations during use:
a. Never use trays or cassettes without perforations.
b. Do not process porous loads (e.g. drapes and
gowns) or fluids in this autoclave.
c. Items in pouches should only be processed as
detailed in these instructions.
d. Do not use in zones of risk associated with
flammable anaesthetics.
e. When sterilizing instruments not of solid metal
construction, the manufacturer of the instrument
must be consulted about its suitability for
autoclaving. (Note: The sterilizing temperature may
be exceeded during the drying phase).
4 Electrical safety
4.1 The operator should follow these electrical safety
measures:-
a. This equipment must be earthed.
b. Switch ‘off’ unit and disconnect from mains power
supply before renewing fuses, fitting the printer,
checking and cleaning the autoclave.
c. Do not attempt to service this equipment internally.
a. Indoor use.
b. Altitude from sea level up to 2000 metres.
c. Temperature 5°C to 40°C.
d. Maximum relative humidity 85% for the temperature
range above.
e. Mains supply voltage fluctuations not to exceed
±10% of the supply voltage.
Note: If it is required to operate this Autoclave outside of
these conditions, contact Eschmann Equipment at
the address given in these instructions.
7 Warning markings
7.1 The warning markings on this equipment have the
following meanings:
Caution; refer to accompanying documents
(i.e. these Instructions for Use).
Caution; hot surface. This symbol warns the
operator of high surface temperatures on the outside
of the equipment.
WARNING: DO NOT USE TAP WATER
(see CAUTION in section 1)
ST-IM61e P5/20
Page 6
8 Safety features
8.1 The autoclave incorporates these safety features:a. Door Interlock Switch and Door Catch Interlock
Switch. These prevent a programme starting if the
door and catch are not properly closed.
b. Pressure Relief Valve (safety valve). This valve
safely releases excess pressure up to a maximum
of 2.85+10%barG.
c. Microcomputer. The microcomputer constantly
monitors all key functions. If an error arises, it stops
the cycle, discharges pressure, and causes the
appropriate message to be displayed
d. Overheat Control. The microcomputer operates in
conjunction with an independent manual reset thermostat to protect the heating element from overheating.
e. Band Heater Thermostat (vacuum units only). This
prevents the band heater exceeding a preset
temperature.
9 Declaration
9.1 The design of the autoclave pressure vessel is
approved by a third party to International Standards. This
equipment is defined as:-
Installation Category 2 (Overvoltage Category 2)
Pollution Degree 2 (in accordance with IEC664).
10 Installation
10.1 The Autoclave should be installed by a competent
person. UK Customers should arrange for this equipment
to be installed by an Eschmann Trained Engineer before
use. (For free installation and service, see section 1.1). All
programmes and functions can be deactivated or reactivated
by an Eschmann Trained Engineer, on request. The ability
to achieve this is protected from tampering, access can only
be gained by use of a ‘password’. Note that a levelling kit
(part number 1 12713) is available for the feet.
Cycle temperature button. Each time the button
is pressed the cycle temperature will alternate between
134°C and 121°C. The temperature selected will be
indicated by the LED C adjacent to the temperature.
Unwrapped load (with drying) cycle button. Press
the button to select a cycle for an unwrapped load
with a drying phase after sterilization. The LED C
adjacent to the button will illuminate to show that
this cycle has been selected.
Unwrapped load (without drying) cycle button.
Press the button to select a cycle for an unwrapped
load without a drying phase after sterilization. The
LED C adjacent to the button will illuminate to
show that this cycle has been selected. (Also see
Appendix 1)
Programme ‘P’ button. This button can be used in
combination with other buttons to activate special
programmes, functions and extended sterilization
cycle times. For more details see Section 15.
Cycle start button. (Always press and hold) Pressing
the button will start the selected cycle.
Open door button. (Always press and hold) When
the cycle has been completed (indicated on the process
display chart ) press the button to
open the door.
Single wrapped load (with drying) cycle button.
(Vacuum autoclaves only) Press the button to
select a cycle for a single wrapped load with a drying
phase after sterilization. The LED C adjacent to the
button will illuminate to show that this cycle has
been selected. See section 15.5 for daily testing
information.
11 Control and display details
1 1.1 Open out the illustration page at the very back of this
manual to familiarise yourself with the controls and displays
shown in Fig. 1 and Fig. 2.
11.2 Mains switch
The mains switch is positioned at the front of the autoclave
(bottom left). The symbol ‘I‘ indicates ‘on’ and the symbol
‘0’ indicates ‘off’ (see 10, Fig. 2).
11.3 Push buttons (see Fig. 1)
Display change button. Button is disabled during
sterilization and drying phases. At any other time press
the button to display the cycle counter in display
D1. If the autoclave is running a cycle the display will
show the cycle number in progress, or, if a cycle is not
running the counter will show the number of cycles
completed (including any aborted). The display will
revert to show the time of day five seconds after the
button is released.
11.4 Digital displays (see Fig. 1)
D1 Time, cycle counter, sterilization or drying time
(remaining), or error number display. This display
normally shows the time of day (to adjust the time
display see section 15.4, Special Functions, F8).
Whilst pressing button this display will show the
cycle counter (see in section 11.3). During the
sterilization or drying phase of a cycle (if applicable)
the display will show the number of minutes remaining
for that phase of the cycle. In the event of an error
occurring it will display an error code. For more details
on the error codes see Table 3.
D2 Temperature display. This display normally shows
the actual chamber temperature. During special
functions it may display other details see details in the
‘Special functions table’.
D3 Pressure display. This display normally shows the
measured chamber pressure. During special functions
it may display other details see details in the ‘Special
functions table’.
P6/20 ST-IM61e
Page 7
LS5
VACUUM & NON-VACUUM AUTOCLAVES
11.5 Indicators (see Fig. 1)
A1 Low water indicator. When this indicator is
illuminated the water reservoir is low and should be
refilled before the next cycle is started (see section
12.5).
A2 Process display indicator. This indictor
has 19 LEDs that illuminate to show the stage reached
during a sterilization cycle. Each LED indicates a
stage and this is detailed in Table 1.
A3 Door unlocked indicator. This indicator is
illuminated when the door is unlocked.
A4 Door locked indicator. This indicator is illuminated
when the door is locked.
C Selection indicators. These indicators illuminate to
show which function has been selected (cycle or
temperature).
12 Initial preparation for use
12.1 When lifting or moving the Autoclave (with at least
two people), place the hands under the base at each side
of the unit. Place the autoclave on a flat, level, water and
heat resistant surface and ensure there is a working
clearance of 150 mm on the left hand side. Do not cover
the ventilation ports at the rear of the autoclave. The
autoclave does not require wall clearance at the back and
can be positioned flush against a wall (unless an extraction
unit is fitted see 12.6). Also see section 10.1.
12.5 With the door open fill the reservoir via the filling
cup (item 2, Fig. 2) up to the ‘MAX’ mark* engraved inside
the filling cup. Eschmann recommend the use of ‘Sterile
Water for Irrigation’ alternatively distilled or deionized water,
or water treated by reverse osmosis process can be used.
DO NOT USE TAP WATER. Close the door onto the
secondary catch only .
* Note: Only two litres of water are required to operate the
autoclave, the reservoir does not require filling to the ‘MAX’
mark if only a few cycles are to be run before the reservoir
is drained (see 16.2).
12.6 The autoclave has been designed to accept
standard 100mm domestic extraction ducting at the rear
of the unit. Before connecting the unit to an extraction
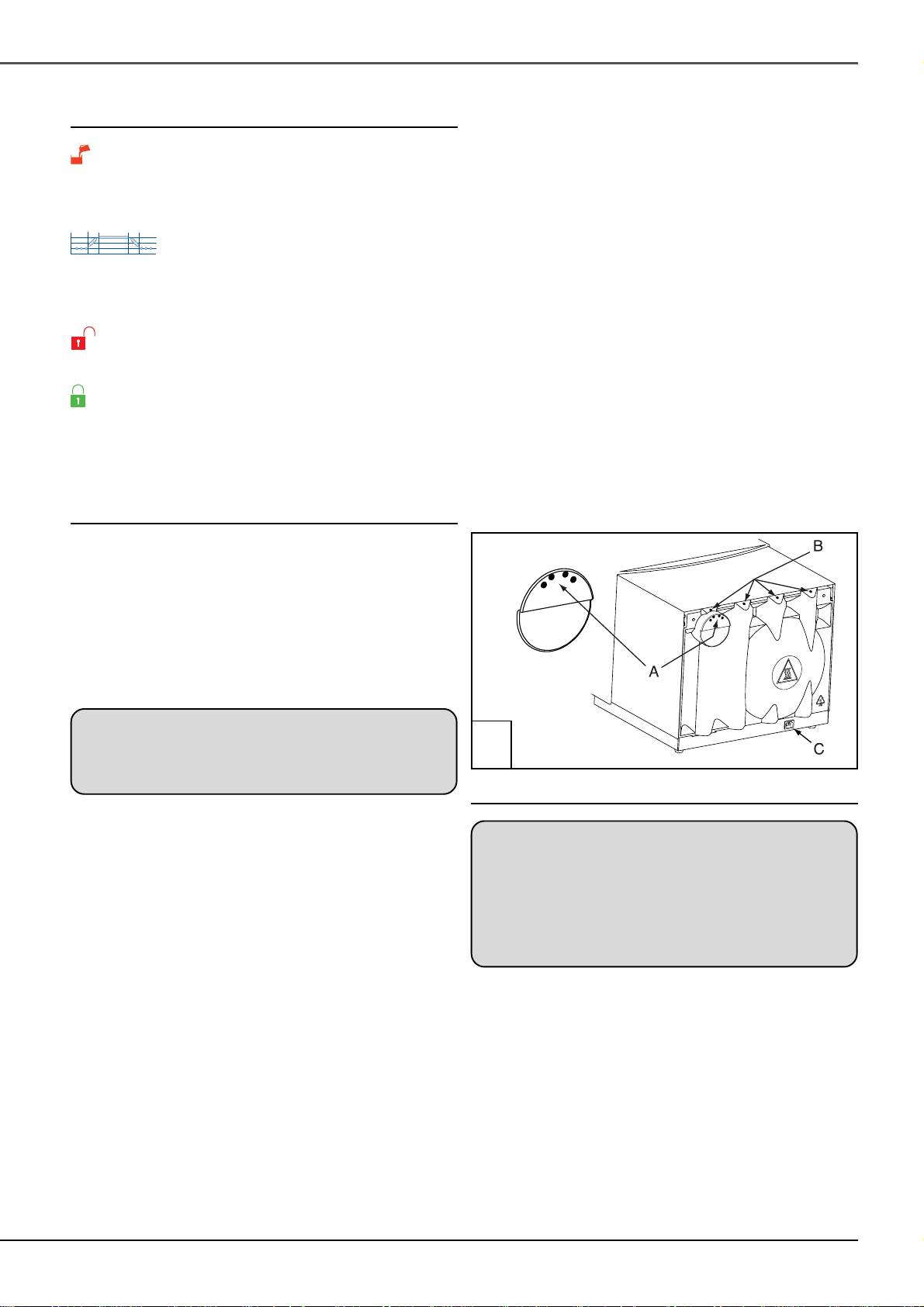
system (to remove exhaust steam) check which type of
reservoir is fitted. If it has 4 plugs (‘A’ in Fig.3) remove
them and reposition them in the holes marked ‘B’ in Fig. 3.
If the plugs are not provided block of the holes marked ‘B’
in Fig. 3 with suitable adhesive tape and carefully make
four holes (6mm diameter) as shown ‘A’ in Fig. 3. Do not
allow any swarf to enter the reservoir or make the holes
lower than indicated.
WARNING
This equipment must be earthed. Ensure that the
mains plug is easily accessible during use as this
is the means of disconnection.
12.2 The unit is supplied with an IEC mains input socket
at the rear (see C, Fig. 3) and a selection of mains input
leads. If the lead has a fused plug the correct fuse will be
installed. If an alternative plug is fitted ensure that the plug
is earthed and check that the fuse (if it is a fused plug) is
appropriate for the voltage of the equipment (see rating
plate on base of cabinet item 8, Fig. 2). Connect the
required ‘Eschmann’ lead to the rear socket, DO NOT use
equivalents.
12.3 Connect unit to mains power supply and switch on
by selecting unit power switch ‘0-I’ to ‘I’ (10, Fig. 2). V arious
displays will illuminate to show the autoclave has been
connected correctly , and is ‘on’.
12.4 T o open the chamber door (16, Fig. 2) press button
B7 (Fig. 1) and then push the secondary catch (item 15,
Fig. 2) to the left (when viewed from the front) to release
the door. Remove the tissue paper from the door and
accessories from within the chamber. Remove all packing
pieces carefully and dispose of these and the carton in
accordance with applicable recycling practices.
3
13 Loading the autoclave
CAUTION
When loading the autoclave take care that you do
not damage the door or front face of the chamber,
especially the door seal and mating face. Damage
to these parts can adversely affect performance.
When loading pouches ensure they do not make
‘paper cuts’ in the door seal.
13.1 Check you have read the limitations on use
(section 3) and the technical specification.
13.2 Operators should note that as load types and load
arrangements vary greatly, validation of particular load
arrangements is essential (e.g. wrapped cassettes and
maximum loads). Steam penetration can also be affected
by extremes of humidity. If in doubt, seek advice from a
qualified microbiologist or Authorised Person.
13.3 The autoclave is designed to sterilize instruments,
not to wash or clean them, therefore before loading
instruments into trays cassettes or pouches they should
be pre-washed. Preferably instruments should be washed
ST-IM61e P7/20
Page 8

in an ultrasonic cleaner or washer/disinfecter to remove
amalgam, debris, etc., then rinsed to remove all traces of
proprietary cleaners, chemicals or disinfectants. Residue
from these products could result in a blockage of the water
recycling system. If instruments are not cleaned this may
compromise the efficiency of the sterilization process.
13.4 When loading the autoclave note the guidelines that
follow for each type of autoclave:-
Vacuum and Non-Vacuum Units - Unwrapped solid
items, buttons and
Load unwrapped solid items loose on trays or into
cassettes. Do not overload trays or cassettes (see
TECHNICAL DATA for maximum tray loads). Avoid
‘bunching’ items together and ensure all items are
positioned so that they drain freely . Only use Eschmann
trays or cassettes and ensure that the load does not
block the holes in them. Loaded trays and cassettes
should then be placed in the sterilizing chamber. All items
must be positioned so that they drain freely and do not
trap rising air bubbles. (Also see Appendix 1)
Vacuum Units ONLY - Validated hollow instruments
including narrow lumens (e.g. dental handpieces),
single wrapped hollow or solid instruments
(see 2.1 d) button .
Sterilization of instruments, or other items in pouches
should only be done using the special pouch rack. A void
packing pouches too tightly and note that paper/film
pouches must be loaded such that the film sides of
adjacent pouches are face to face and sterilized using
this cycle with drying. Only use pouches recommended
for steam sterilization, see Accessories section 20. Items
should not be multiwrapped unless the load configuration
has been validated by a suitably qualified person (possibly
using an extended drying time). Also see section 15.5.
14 Running a cycle
14.1 Select mains ‘on/off’ switch to ‘I’ (on) (item 10, Fig. 2)
and open the door by releasing the secondary catch (item
15, Fig. 2). NOTE: If the door was locked closed when
switching ‘on’ indicator will flash and the button
should be pressed to unlock the door, the secondary catch
can then be released.
14.2 Load the autoclave with the items to be sterilized as
detailed in section 13.
14.3 Check that the low water level indicator ( , Fig. 1)
is not illuminated, if it is fill reservoir (see section 12.5).
14.4 Close the door and hold it firmly shut until the
automatic door lock operates. This is shown by indicator
switching off and indicator switching on. NOTE: A
cycle cannot be selected with the door open. When running
repeated cycles the display will retain the last cycle selected
(shown by the illumination of indicators C).
14.5 The autoclave will automatically select a 134°C
unwrapped cycle with drying for Non-vacuum
autoclaves or a 134°C wrapped cycle with drying for
Vacuum autoclaves (unless these cycles have been
disabled see section 10.1). If this is the required cycle pass
on to section 14.6.
T o select a different temperature press the button. Each
time is pressed the cycle temperature will alternate
between 134°C and 121°C. The temperature selected will
be indicated by the LED C adjacent to the temperature.
To select the required programme press the appropriate
button on the control panel ( , or ). The indicator C
for the selected programme will remain illuminated to show
which cycle has been selected. 134°C cycles can be
extended see section 15.
NOTE: Cycle temperature and programme can be selected
in either order and changed independently of each other
until the required ones have been selected. In the case of
low water a ‘beep’ will sound and the ‘low water indicator’
( Fig. 1) will flash.
14.6. Press the cycle start button . The autoclave can
be configured to have adjustable sterilization and drying
times (see section 10.1). The appearance of ‘ ’ in display
D2 indicates that the autoclave has been configured so
that these times can be adjusted. If these have been
enabled for a cycle (‘ ’ in display D2) follow section 14.6.2
‘i-iii’ before continuing with section 14.7; if they are not
enabled (‘ ’ does not appear in display D2) for that cycle
follow section 14.6.1 before continuing with section 14.7.
14.6.1 The sterilizing cycle will now proceed
automatically and the printer (if fitted) will commence
printing. Vacuum cycles may pause to cool the
chamber if it is above a preprogrammed limit. As the
cycle progresses indicator will show the
cycle stage reached (see Table 1) and the digital
displays D2 and D3 will display the actual chamber
temperature and pressure respectively. During the
sterilization or drying phase of a cycle (if applicable)
the display D1 will show the number of minutes
remaining for that phase of the cycle. If a printer is
fitted see section 17.5. Now pass on to section 14.7.
14.6.2 (i) As ‘ ’ has appeared in display D2 this cycle
allows the sterilization time to be increased. D2 will
show ‘ ’ and display D1 will flash and show the
current sterilization time in minutes and seconds.
T o increase this time press and hold the button,
to reduce this time press and hold the button,
until the sterilization time is the desired value. Press
button to accept this time. Pressing the
button at any point before the cycle starts causes
the autoclave to move back one stage in this
process.
14.6.2 (ii) If the cycle has a drying phase, ‘ ’ will
appear in display D2 to indicate that this cycle allows
the drying time to be increased. If ‘ ’ does not
appear in display D2 pass on to section 14.6 2 (iii).
Display D2 is showing ‘ ’ and display D1 will flash
and show the current drying time in minutes. To
increase this time press and hold the button, to
P8/20 ST-IM61e
Page 9

LS5
VACUUM & NON-VACUUM AUTOCLAVES
reduce this time press and hold the button, until
the drying time is the desired value. Press button
to accept this time. Pressing button at any
point before the cycle starts causes the autoclave
to move back one stage in this process.
14.6.2 (iii) The sterilizing cycle will now proceed
automatically and the printer (if fitted) will commence
printing (vacuum cycles may pause to cool the
chamber if it is above a preprogrammed limit). As
the cycle progresses indicator will show
the cycle stage reached (see T able 1) and the digital
displays D2 and D3 will display the actual chamber
temperature and pressure respectively. During the
sterilization or drying phase of a cycle (if applicable)
the display D1 will show the number of minutes
remaining for that phase of the cycle. If a printer is
fitted see section 17.5.
14.7. If a cycle “with drying” is selected then
commencement of the drying phase is indicated by
(see Table 1). On non-vacuum units the door
opens automatically (onto secondary catch) prior to the
start of drying. On vacuum units the door cannot be opened
until the drying phase has been completed.
14.8 On completion of a sterilizing cycle ‘without drying’,
indicated by (see Table 1) and an audible signal,
the door can be released by pressing . (Also see
Appendix 1).
14.9 Open the door fully and remove the load USING
THE TRAY LIFTER and leave the door open on the
secondary catch when not in use.
14.10 If during a cycle an error is detected display D1 will
display an error number (see section 17.3).
15 Special programmes/functions/tests
15.1 Extended sterilization time.
Pressing the ‘P’ button (indicator C adjacent to will
illuminate) before pressing the start button extends
the sterilization time from 3 minutes 5 seconds to 18
minutes. This extended cycle only works on the 134°C
cycles (not 121°C cycles). Pressing the ‘P’ button again
before starting the cycle will cancel the function (indicator
C will go out). Alternatively press the ‘P’ button (during
any cycle) when Phase 3 LEDs 2-7 are illuminated (see
Table 1) to increase the sterilization time by one sixth of
the selected sterilization time (one Phase 3 LED will go off
for each press, to indicate this).
15.2 Extended drying time.
The drying time can be extended in 5 minute steps to a
maximum of 50 additional minutes by pressing the ‘P’
button when ‘Phase 5 - LED1’ is illuminated (see T able
1). Each time the ‘P’ button is pressed the drying time will
be increased (by 5 minutes, or back to 0 after 50) and the
time added to the normal drying time will be displayed in
display D1. After a few seconds display D1 will revert to
the total remaining drying time. NOTE: Autoclave ‘beeps’
when ‘Phase 5 - LED1’ illuminates.
15.3 Accelerated condense.
The condensing time can be reduced by approximately 3
minutes (but more steam may be liberated by the
autoclave) if the ‘P’ button
LED1’ is illuminated (see Table 1). NOTE: Autoclave
‘beeps’ when ‘Phase 4 - LED1’ illuminates.
is pressed when ‘Phase 4 -
15.4 Special functions.
Switch ‘off’ the mains switch (item 10, Fig.2). Press and
hold in the ‘P’ button whilst switching the mains switch
back ‘on’ to make available several special functions as
detailed in the special functions table. When the autoclave
is switched on in this way display D1 only will be illuminated
(with other displays as required depending on which function
is selected). Initially display D1 will show F0, each time the
‘P’ button is pressed, this will increase from F0 up to F8
(F4, F5, F6 vacuum autoclave only and with door closed),
and then back to F0 again, until either the start button is
pressed to select that function, or, the autoclave is switched
‘off’ or is pressed (with the door open) to cancel special
function selection.
15.5 Daily Testing, Vacuum autoclaves
IMPORTANT:
The Eschmann LS5 Vacuum Type-S and QuickVac
Steam Penetration Test Device (hereafter called
‘Device’) has been developed and validated solely
for use in the Eschmann LS5/SES2555 Vacuum
T ype-S and Eschmann QuickV ac Steam Sterilizers.
Use of the ‘Device’ in any other sterilizer , or use with
any other type of indicator, may give dangerously
misleading results. The ‘Device’ must only be used
and stored as detailed in the ‘Instructions for Use’
supplied with the ‘Device’. Failure to replace the
‘Device’ after 100 uses could lead to failure of the
‘Device’ and dangerously misleading results.
15.5.1 In order to sterilize products and devices by
steam, steam must be able to penetrate through to and
contact all surfaces. Items such as packaging, pouches,
narrow or hollow instruments are not easy to penetrate
with steam. For this reason, the LS5/SES2555 vacuum
autoclave has been fitted with a pump to remove air and
thus allow steam to penetrate into the load. The Eschmann
LS5 V acuum Type-S and QuickV ac Steam Penetration T est
Device (order REF 87-040-71) has been designed to test
the steam penetration ability of the autoclave, by presenting
a defined challenge to the autoclave. The device consists
of a plastic tubular device with an indicator receptacle at
the end and is supplied with indicator strips. The device
must be replaced after 100 uses. This device satisfies the
requirement to perform a daily steam penetration test as
given in:
EN 554:1994 Sterilization of medical devices - Validation
and routine control of sterilization by moist heat, British
Standards Institution, clause 6.3.4
DB 2002(06) Device Bulletin, Benchtop Steam Sterilizers-
Guidance on purchase, Operation and Maintenance,
Medical Devices Agency, clause 5.3.1
ST-IM61e P9/20
Page 10

SN 2002(24) Safety Notice, Steam penetration tests in
vacuum benchtop sterilizers, Medical Devices Agency .
DB 2000(05) Device Bulletin, Guidance on the Purchase,
Operation and Maintenance of V acuum Benchtop Steam
Sterilizers, Medical Devices Agency, clause 5.3.1
DB 9804 Device Bulletin, The validation and periodic
testing of benchtop vacuum steam sterilizers, Medical
Devices Agency, clause 5.1
Note: Some of the above documents are available on
the Medical Devices Agency’ s website, www.medicaldevices.gov.uk
15.5.2 Frequency of use
To comply with the requirements of the regulatory
documents listed above, the steam penetration test must
be conducted at the commencement of each day that the
autoclave is to be used. This ‘verifies’ the suitability of
the autoclave’s air removal stage for the day ahead.
‘Appendix 3’ provides an example of a ‘Test Log’ that can
be photocopied and used to record the results. Eschmann
recommend the daily use of the device and the ‘T est Log’.
15.5.3 Conducting the test
Cycle (includes a drying phase) should be used to run
this test, or special function F6 (reduced cycle time as no
drying), if the autoclave is cool and dry before loading it
with the ‘Device’. Remember the ‘Device’ must be removed
within 10 minutes of the cycle end.
16 Cleaning and care
WARNING
Chlorine, even in the concentrations found in tap
water, can cause stainless steel to crack and could
damage the chamber. Disconnect from the mains
electrical supply before cleaning the Autoclave. Also
see Appendix 2.
16.1 Keep chamber trays, door seal and chamber face
clean. These should only be cleaned with a lint-free cloth.
Clean the outside of the autoclave by wiping down with a
cloth dampened with a 70% solution of industrial methylated
spirit (IMS) and water. Allow to dry by evaporation.
NOTE: Do not use abrasive powders, chemicals, or
solutions containing chlorine to clean the autoclave.
CAUTION
In common with other systems containing static
water reservoirs, water used in this unit can become
contaminated over a period of time, or following an
aborted cycle, and should be treated as a potential
risk of infection.
16.2 The reservoir should be drained weekly and allowed
to dry. To drain the reservoir release the reservoir tap
(item 12, Fig. 2) with the drain tube (item 11, Fig. 2) from
its clips (item 13, Fig. 2) at the bottom of the door and
open the tap by releasing the clip (see inset photographs
of open and closed tap in Fig. 2). Replace the drain tube
into its clips and ensure that it does not become trapped
when closing the door. Refill the reservoir (see section
12.5). Regular cleaning of the reservoir will reduce the
effects of excessive contaminants (e.g. handpiece
lubricant) which could be detrimental to the function of the
autoclave. For guidance on clean steam management and
HTM2031 contact Eschmann.
16.3 Attention to the following will increase the life of the
your Autoclave:-
a. Always leave the chamber door open on its
secondary catch when not in use.
b. At weekly intervals, lightly clean door seal and
chamber face with a lint-free cloth.
c. At weekly intervals remove any dirt or debris
that has collected around the gauze filter (item 7,
Fig. 2).
d. At monthly intervals remove any dirt or debris around
the gauze filter (item 7, Fig. 2) then gently pull it out
of its recess. Clean the recess and the filter carefully .
Replace the filter before using the autoclave again.
e. Replace the antibacterial filter on vacuum units (item 1,
Fig. 2) every three months. To remove the filter pull
gently whilst twisting. Replace by pushing a new one
into the rubber mount (small spigot facing out).
17 Maintenance
(ENSURE YOU HAVE READ SECTIONS 2-8)
17.1 Fuse Renewal
Fuses are fitted at the front of the autoclave (item 9, Fig. 2).
For fuse ratings refer to ‘TECHNICAL DAT A’. To extract a
fuse switch ‘off’ the unit and disconnect from the mains
power supply , insert a screwdriver or small coin in the slot
of the fuse holder and twist it counter-clockwise. After
inspecting or renewing a fuse, reverse the above procedure
to re-secure fuse holder.
17.2 Preventive Maintenance Agreement
We would strongly recommend that a ‘P.M.A.’ is taken out
on your Autoclave. Although the autoclave requires minimal
maintenance, it is important to have the autoclave checked
and calibrated at regular intervals. This ensures that the
exacting conditions necessary for sterilization are
maintained throughout the unit’s working life.
17.3 Errors and faults
a If, after switching ‘on’ power there is no visual display ,
first check power supply connections, both fuses at
the front of the unit (see Fuse Renewal) and the
fuse in the mains plug (if it is a fused plug).
b If an error occurs during a cycle (i.e. any time after
pressing the start button ), the cycle will abort
and provided power supply to the unit is maintained,
the error number will be indicated by the visual
display D1 (displays D2 and D3 will be continue to
show actual temperature and pressure).
c If power fails during a cycle, check supply
conditions and fuses at the front of the unit and in
the mains plug if fused, also check the mains lead
P10/20 ST-IM61e
Page 11

is correctly connected at both ends. Once power
is restored the display D1 will show the error
number (D2 and D3 will be blank) until cleared by
switching the autoclave ‘off’ and then ‘on’ whilst
depressing the start button . Release button
when the display appears.
d Most errors will require investigation by an
Eschmann trained engineer who will need to know
the model serial number (see the serial number plate
item 8, Fig. 2) and error number. To display the last
error number in display D1 press buttons and
together at any time.
e Errors that can be corrected by the operator are
detailed in the error table, see Table 3.
f Should the heating element reset thermostat
operate (in a nil water condition) this can be reset
by pressing the ‘reset button’ on the underside of
the autoclave (approximately 11cm in on the left
hand side underneath the unit as indicated on the
side of the unit).
17.4 Service
The Eschmann After Sales Service Department is staf fed
and equipped to provide advice and assistance during
normal office hours. T o avoid delays when making enquires,
please quote the Model and Serial Number of your
Autoclave (NOTE: For location of the serial number plate
see item 8, Fig. 2). If reporting an error please quote the
displayed error number. All correspondence relating to the
after sales service of Eschmann Equipment should be
addressed to :
LS5
VACUUM & NON-VACUUM AUTOCLAVES
TABLE 1
KEY TO DISPLAY A2
Phase 1
LED 1 Cycle started.
LED 2 Chamber minimum fill achieved.
LED 3 Chamber filled with water.
Phase 2
LED 1 Heater on.
LED 2 Approximately 90°C reached.
LED 3 Final heating phase started.
Phase 3
LED 1 Sterilization phase started.
LED 2-7 One sixth of the sterilization time
completed for each LED illuminated.
Phase 4
After Sales Service Department
Eschmann Equipment,
Peter Road, Lancing, West Sussex, BN15 8TJ
Tel: +44 (0) 1903 765040 Fax: +44 (0) 1903 875711
Also see Appendix 2.
17.5 Printer message
If a printer is fitted to your autoclave note that at specified
intervals it will print a message to remind you that ‘Service
and Certification’ is due. When this message appears
Eschmann recommend that you contact them to arrange
this as soon as possible (see contact details in section
17.4 above).
LED 1 Condensing stage started
LED 2 Water removed from chamber .
LED 3 Pressure approaching ambient.
Phase 5
LED 1 Start of drying phase.
LED 2 Drying phase 50% completed.
LED 3 Cycle completed (audible beep).
ST-IM61e P11/20
Page 12

TABLE 2 - SPECIAL FUNCTIONS
(See section 15.4 which provides instructions for accessing these special functions)
DISPLAY FUNCTION METHOD OF USE
F0 Protected functions To use this function requires the knowledge of the PIN code. This is normally
restricted to a trained service engineer who has access to this code and the
autoclave ‘Service Manual’. Therefore access to protected functions is not
available to the operator.
F1 Printer test (Note: The printer should be loaded with paper before entering this special
functions mode). With display D1 showing F1 press the start button to start
the printer test, a character set printout, display D1 shows F11. To stop the test
press button , the printer will stop at the end of the test cycle, display D1
shows F0. To resume normal use switch ‘off’ the mains switch (item 10, Fig. 2)
and then switch back ‘on’, or press with the door open.
F2 Cycle history With display D1 showing F2 press the start button to start printing a summary
of the last five cycles completed by the unit (cycle temperature, cycle type, cycle
count, date and time at start of cycle, date and time at end of cycle and the cycle
result). Display D1 returns to F0. To resume normal use switch ‘off’ the mains
switch (item 10, Fig. 2) and then switch back ‘on’, or press with the door open.
F3 Set delayed start With display D1 showing F3 press the start button to enter the delayed start
time setting mode and delayed start initiation, display D1 will show .
If the start hour displayed in D2 is correct (as set last time) and the minutes can
be assumed correct (as set last time) press the start button to programme
the autoclave to start automatically at this set time. The display D1 will change to
F0 when is pressed. Press with the door open to exit the setting mode.
If the start time is not correct when entering function F3 (display D1 showing
, display D2 showing the hour set in 24 hour clock mode) press to
increase the hour (or to reduce it). Press to confirm the hour when it is
correct. Display D1 will show , to increase the minutes (shown in D2)
press , or to reduce them. Press to confirm the minutes. The display
D1 will show F0. Press with the door open to exit the setting mode.
The autoclave should be left switched ‘on’ with the required load in place, the
door shut and the required cycle selected as follows. When the door is shut a ‘ ’
flashes ‘on and off’ after the time in display D1. This continues while the program
selection and any adjustments to it are made (see sections14.6.2 if ‘ ’ appears
in display D2). When cycle selection has been completed and is pressed to
confirm them, the ‘ ’ stops flashing. The cycle selected will be run automatically ,
starting at the time set, a ‘ ’ will be displayed in D1 after the time to show that a
delayed start time has been set. (Also see Appendix 1)
On completion of the cycle the autoclave is ready for the load to be removed and
normal use resumed without the need to exit from the special functions mode.
Note: If the same delayed start time is required each day the setting can be
entered quickly once display D1 shows F3, by pressing ( ).
F4 Leak Test With display D1 showing F0 close the autoclave door (if open), press until
display D1 shows F4, press . Indicator will flash, press to open the door,
(Vacuum Autoclave ONL Y)
Note. To conduct this test the
chamber temperature must be
below 50°C to avoid an Error 38,
and the door closed before
selecting F4.
indicator will illuminate. Close the door , will illuminate, press this starts
an automatic leak test which checks to see if the autoclave system is still pressure
tight. Display D3 will show the chamber pressure, after a few minutes display D2
will show the pressure rise to the nearest 0.1kPa.
T able 2 is continued over page
P12/20 ST-IM61e
Page 13

LS5
VACUUM & NON-VACUUM AUTOCLAVES
TABLE 2 (continued) - SPECIAL FUNCTIONS
DISPLAY FUNCTION METHOD OF USE
F4 Leak Test (continued) After a further 10 minutes if the pressure has not risen by more than 1.3kPa
display D2 will show indicating the autoclave has passed the test (or
to indicate the autoclave has failed the test) and an audible sound will be heard.
Press button and wait until display D2 and D3 go blank. To resume normal
use switch ‘off’ the mains switch (item 10, Fig. 2) and then switch back ‘on’, or
press with the door open.
F5 Air detection test For details on how to conduct this test consult manual ST-IM55 (issue ‘d’ or later).
(Vacuum Autoclave ONL Y)
F6 Steam penetration test. With display D1 showing F0 close the autoclave door (if open), press until
(Vacuum Autoclave ONL Y)
Note. To conduct this test the
door must be closed before
selecting F6. Also see 15.5.3.
display D1 shows F6, press . Indicator will flash, press to open the door,
indicator will illuminate. Place the Eschmann LS5 V acuum Type-S and QuickV ac
Steam Penetration T est Device in it’ s pouch (see test device instructions for use)
and into the chamber. Close the door , will illuminate, press the start button ,
this will start a special cycle (no drying), D2 and D3 will show temperature
and pressure as normal during the cycle. At the end of the cycle the normal
audible sound will be heard. Press to open the door , display D1 will be showing
F0. Remove the test pack and review the result within 10 mi nutes (see Test Device
instructions for use). T o resume normal use switch ‘off’ the mains switch (item 10,
Fig. 2) and then switch back ‘on’, or press with the door open. (NOTE: This
function can also be used to carry out HTM2010 Performance Qualification (PQ)
Tests in conjunction with the manuals detailed in sections 1.4 and 1.5).
F7 Disinfect function (NOTE: The ex-factory condition is for this function to be disabled. It can be enabled,
Note. To conduct this test the
door must be closed before
selecting F7.
F8 Adjust date and time With display D1 showing F8 press the start button to enter the adjust date
if required, during installation by an Eschmann trained engineer.) With display D1
showing F0 close the autoclave door (if open), press until display D1 shows
F7, press . Indicator will flash, press to open the door, indicator will
illuminate. Load the autoclave with the load to be disinfected and close the door,
will illuminate. Press the start button to start the disinfect function (a 2.5 minute
disinfect time at 1 10°C), D1 will continue to show F7 during the cycle and D2 and
D3 will show temperature and pressure. At the end of the cycle the normal audible
sound will be heard. Press to open the door and remove the load. Display D1
will be showing F0. To resume normal use switch ‘off’ the mains switch (item 10,
Fig. 2) and then switch back ‘on’, or press with the door open.
and time function. Display D1 will show and D2 will show the year, to
increase the year press (or to reduce it) when correct press button to
confirm the year. The display D1 will show and D2 will show the month in
numerical format, to increase the month press (or to reduce it) when
correct press button to confirm. The display D1 will show and D2 will
show the day in numerical format, to increase the day press (or to reduce
it) when correct press button to confirm. The display D1 will show and
D2 will show the hour, to increase the hour press (or to reduce it) when
correct press button to confirm. The display D1 will show and D2 will
show the minute, to increase the minute press (or to reduce it) when
correct press button , display D1 shows F0 display D2 and D3 will be blank.
The time and date are now set (seconds were set to zero when was pressed).
Press with the door open to continue normal use.
ST-IM61e P13/20
Page 14
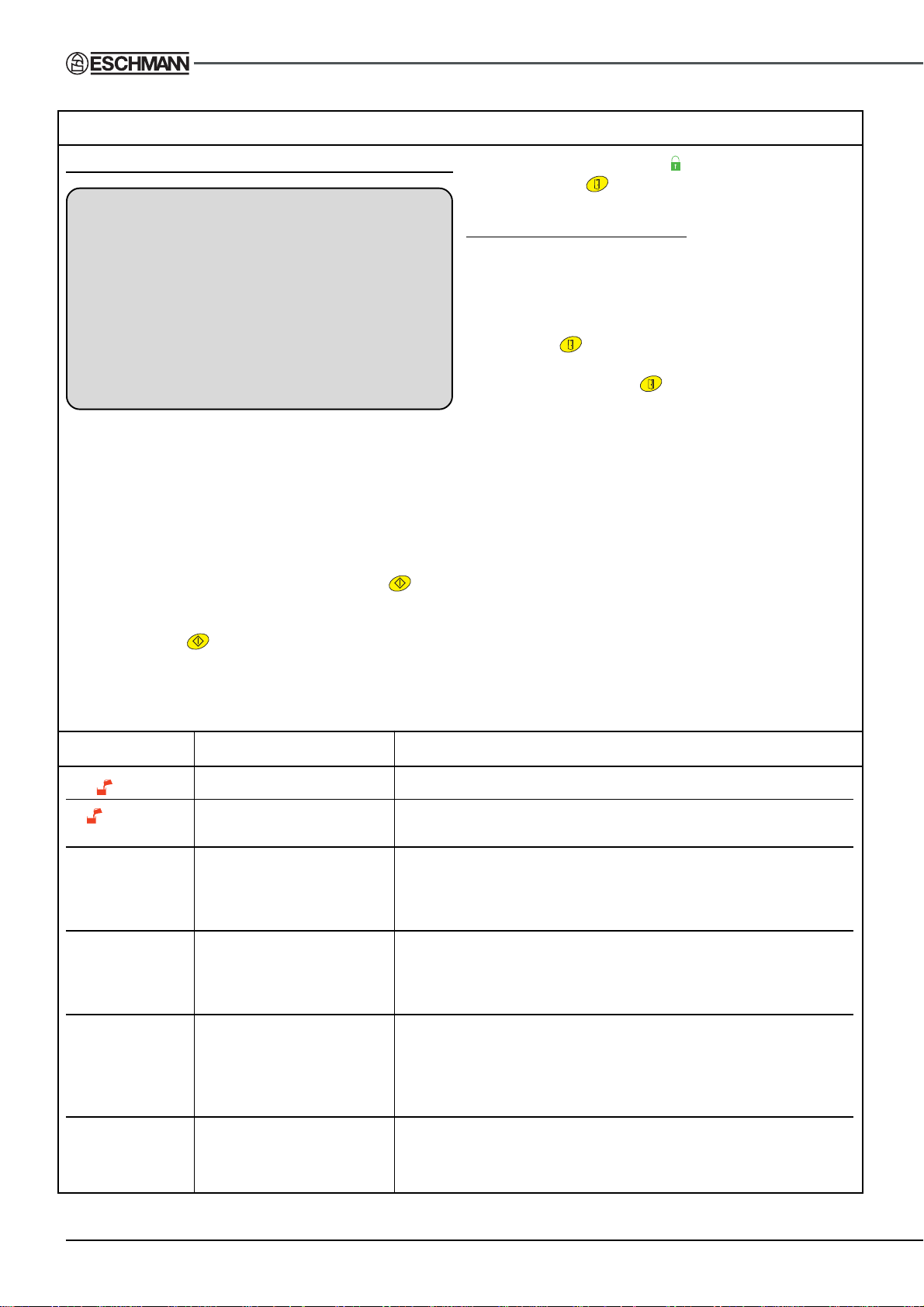
TABLE 3 - ERROR NUMBER DETAILS AND REMEDIES
Clearing error display and restarting a cycle
WARNING
If an error display appears during a cycle, do not
attempt to open the chamber door until the unit has
cooled (for safety below 50°C) and internal pressure
has fallen sufficiently to release the door, see below
‘3 Check pressure’. Where a cycle is aborted and/or
an error display is indicated the load should be treated
as non-sterile. The load should be re-wrapped if
wrapped and then sterilized by running the cycle again.
Also note the Caution after section 16.1 concerning
the contamination of water in the reservoir.
1 Record error number : For future reference record
the error number before clearing the display . In the event
of mains power failure, power must be restored to the
autoclave to achieve this. (Also see 17.3d)
2 Clear error and reset autoclave : T o clear the error
display and reset the autoclave wait until any audible
discharge of water and steam has stopped and switch OFF
the autoclave with the mains switch (item 10 Fig. 2). Wait
10 seconds, press-and-hold-in the ‘Start button’ ( Fig.1)
whilst switching back ON with the mains switch (item 10
Fig. 2), immediately the normal display appears release
the ‘Start button’ ( Fig.1).
3 Check pressure : Before attempting to open the
door note the pressure reading, this should be below
104kPa before the door is opened.
4 Open the door : Note will be flashing, open the
door by pressing . If the door fails to open see NOTE
below, there may still be water in the autoclave.
NOTE: Door opening problems. Although unlikely, there
may be up to a litre of water (which could be HOT) still in
the chamber. Be prepared to collect this water in a suitable
container. A built-in safety feature inhibits door opening
unless the following procedure is adopted. Switch OFF at
mains switch (item 10 Fig. 2). Press-and-hold-in the ‘Door
open button’ ( Fig.1) whilst switching back ON with the
mains switch (item 10 Fig. 2) wait two seconds and then
before releasing button (which will open the door)
hold a container in place under the door to collect any
(HOT) water.
5 Check and prepare the aborted load : Loads must
be considered non-sterile if an error occurs and should be
resterilized. All aborted wrapped loads should be dried,
placed in new pouches and reprocessed.
6 Check chamber before starting another cycle :
The chamber should be allowed to dry and cool before
starting another cycle. To start another cycle select the
cycle and start the autoclave in the normal manner.
7 If the error persists or an error number occurs that
is listed at the end of this table then call the Eschmann
After Sales Service Department (see section 17.4) or
contact your approved service engineer. Do not attempt to
solve an error using tools or tampering with the autoclave.
DISPLAY CAUSE REMEDY
is on Water level too low . Fill reservoir as detailed in section 12.5
flashing Cycle started and water. Fill reservoir as detailed in section 12.5
level is too low
Err01 Mains supply failure a. Check local supply conditions and all fuses.
during a cycle b. Check supply plug wiring and power cable for possible breaks.
c. Check if Autoclave has been switched OFF accidentally .
d. Follow ‘Clearing error display and restarting a cycle’.
Err03 Fill error Follow ‘Clearing error display and restarting a cycle’ but before
starting a new cycle check the reservoir water fill level and dry the
water level sensors (item 17, Fig. 2). If door fails to open see
section 4 of ‘Clearing error display and restarting a cycle’.
Err04 Low water a. Check the autoclave is not over loaded.
b. Check that the load cannot collect water (e.g. a kidney dish
not inverted) re-position load if required so that it can drain.
c. Dry the water level sensors (item 17, Fig. 2).
d. Follow ‘Clearing error display and restarting a cycle’.
Err10, 13, 14, A programmed test has Follow ‘Clearing error display and restarting a cycle’ but before
23, 24 and 25 detected a possible fault starting a new cycle check door seal and chamber sealing face
and aborted the cycle. are clean and not damaged. Damaged seals need replacing.
(continued over page)
P14/20 ST-IM61e
Page 15

LS5
VACUUM & NON-VACUUM AUTOCLAVES
TABLE 3 - ERROR NUMBER DETAILS AND REMEDIES (continued from page 14)
DISPLAY CAUSE REMEDY
Err26 Water sensing error a. Check load is not touching water sensors (item 17, Fig. 2) and
early in cycle re-position load if required.
b. Dry the water level sensors (item 17, Fig. 2).
c. If door fails to open see section 4 of ‘Clearing error display
and restarting a cycle’.
d. Follow ‘Clearing error display and restarting a cycle’.
Err27, 28 Heating error Follow ‘Clearing error display and restarting a cycle’ but check
29 and 30 the reset button before starting a new cycle, see section 17.3 ‘f’.
Err 33 Water sensing error a. Gauze filter blocked, see section 16.3 ‘c’ and ‘d’.
later in cycle b. Check load is not touching water sensors (item 17, Fig. 2) and
re-position load if required.
c. Dry the water level sensors (item 17, Fig. 2).
d. If door fails to open see section 4 of ‘Clearing error display
and restarting a cycle’.
e. Follow ‘Clearing error display and restarting a cycle’.
Err35 Door failed to open Follow ‘Clearing error display and restarting a cycle’ but note
during drying phase that it may be required to assist opening the
door manually .
Err36 Door unlocked during cycle a. Follow ‘Clearing error display and restarting a cycle’.
b. Check that when door is closed, green LED (item A4 Fig. 1) is
illuminated. If red LED (item A3 Fig. 1) is illuminated contact
Eschmann Equipment (see section 17.4).
Err38, 39, 40 Chamber temperature Follow ‘Clearing error display and restarting a cycle’ but
or pressure error allow the autoclave to cool down before starting another cycle.
Err 44, 45 Water level sensor error a. Check load is not touching water sensors (item 17, Fig. 2) and
re-position load if required.
b. Dry the water level sensors (item 17, Fig. 2).
c. If door fails to open see section 4 of ‘Clearing error display
and restarting a cycle’.
d. Follow ‘Clearing error display and restarting a cycle’.
Err46 Door not opening a. Try gentle assistance to open the door.
after several attempts b. T emperature etc. may be too high, wait 5-10 minutes then
follow ‘Clearing error display and restarting a cycle’.
c. If door still fails to open see section 4 of ‘Clearing error
display and restarting a cycle’.
Any other Miscellaneous Follow ‘Clearing error display and restarting a cycle’ and if
error code problem repeats contact Eschmann Equipment (see section 17.4).
ST-IM61e P15/20
Page 16
18 Operating instructions for printer
IMPORTANT OPERATOR NOTES
Follow the notes and cautions below when changing
the paper roll and ink ribbon cartridge. Also do not
pull on the paper whilst the printer is in operation,
it may damage the print head. Using knives, etc. to
remove a paper jam will also damage the print head.
Always open the front panel and use the paper feed
button (item 15 Fig. 4.3) to clear the jam.
18.1 Printer installation (REF 87-066-66)
Install the printer unit in the autoclave as follows:
a Remove the printer from its packaging and remove
the mains power supply from the autoclave.
b T o gain access to the printer compartment open the
printer door (13, Fig. 4.2 and 4.4) by turning the door
latch (17, Fig. 4.4) anticlockwise with a small coin
or suitable screwdriver.
c Remove the printer connector (2, Fig. 4.1) from its
storage pocket (3, Fig. 4.1) and gently pull out the
ribbon cable (1, Fig. 4.1) from within the autoclave.
(Note. Do not connect the printer at this stage as it
is possible to connect it the wrong way round and
twist the ribbon cable).
d Orientate the printer unit as shown in Fig. 4.2 and
locate the hole in the left hand printer bracket arm
(10, Fig. 4.2) over the spring pivot pin (4, Fig. 4.1).
Gently push the printer into place so that the right
hand spring pivot pin (5, Fig. 4.1) clicks into place in
the hole in the right hand printer bracket arm (11,
Fig. 4.2). When correctly located the printer should
hinge freely about the pivot pins and when positioned
vertically the printer door should close easily without
interfering with the printer.
e Push the printer connector (2, Fig. 4.1) into place
on the pins at the back of the printer unit without
twisting the ribbon cable (1, Fig. 4.1).
f Ensure that the printer ribbon (16, Fig. 4.2) is still in
place (if not replace it as detailed in section 18.4c)
and insert a paper roll as detailed in sections
18.3 b-e. Then carry out a printer test as detailed in
section 15.4 referring to Table 2, section F1.
18.2 Printer Output
The printer output gives the following information:
Manufacturer’s name - Eschmann Equipment
Machine type - e.g. Little Sister 5 N
Serial number - an alpha numeric code
Cycle type - e.g. 134C - wrapped
Date and Time started - dd:mm:yy hh:mm:ss
Counter reading - Five digits with leading zeros
Cycle information - Cycle stage code and time,
temperature and pressure every 20 secs. Maximum
and minimum sterilization temperatures.
Cycle ended message - ‘Cycle Complete’ & time or ,
*** CYCLE F AILED*** , dd:mm:yy hh:mm:ss followed
by the error number
If an error occurs during the cycle, it is recorded with date
and time on the printout and the message ‘CYCLE FAILED’.
Errors are designated by error codes as Table 3.
Cycle stage codes on the printout are:
TPV* - End of vacuum pulses
TPP* - End of first pressure pulse
TSS - Start of sterilization
TSE - End of sterilization
TDS - Start of drying phase
TDE - End of drying phase
(* Vacuum Autoclaves ONLY)
18.3 Paper Roll Renewal
CAUTION
DO NOT USE ALTERNATIVE PAPER ROLLS
The quality and size of paper rolls used in the printer,
can only be supplied by Eschmann Equipment.
T o gain access to the printer compartment open the printer
door (13, Fig. 4.2 and 4.4) by turning the door latch (17,
Fig. 4.4) anticlockwise with a small coin or suitable
screwdriver. Replace the printer paper roll as follows:
a. Gently rotate the printer unit down from its vertical
position as shown in Fig. 4.2, to horizontal as shown
in Fig. 4.3. If any paper remains in the printer, cut it
free with scissors where it enters the back of the
paper feed slot (12, Fig. 4.2) and gently ease it from
the front of the printer whilst pressing the paper feed
button (15, Fig. 4.3) on the back of the printer unit.
(Note. DO NOT pull the paper through the printer
and NEVER pull the paper backwards).
b. Remove the paper roll holder (19, Fig. 4.3) with the
old roll core from the recess (6, Fig. 4.1) behind the
printer. Take a new roll of paper and separate the
end from the roll. Remove any paper that is damaged
or has adhesive on it, then using scissors, trim the
paper end to approximately 60° as shown in Fig. 4.3.
c. Locate the new paper roll onto the roll holder in the
direction of arrow A in Fig. 4.3 with the roll unwinding
from the bottom towards you as shown in Fig. 4.3.
Replace the roll holder with the new roll of paper
back into the recess (6, Fig. 4.1) behind the printer
unit by moving it in the direction of arrow B as shown
in Fig. 4.3. If the roll is not in the correct orientation
paper will not feed through the printer smoothly .
d. Insert the cut end of the paper into the rear of the
paper insert slot (12, Fig. 4.2). Gently push the paper
through the slot whilst pressing the paper feed button
(15, Fig. 4.3) until the printer mechanism grips the
paper and pulls it through to the front of the printer,
DO NOT pull the paper. Check that the printer ribbon
lies flat and not twisted.
e. Turn the paper roll by hand to take up any slack
paper and then carefully return the printer unit to
the vertical position. Pass the paper from the front
of the printer through the slot in the printer door
(7, Fig. 41 and 4.4) and close the door. To lock the
latch (17, Fig. 4.4), press and rotate until it snaps
P16/20 ST-IM61e
Page 17

locked. Gently pull paper showing through the door
feed slot to take up any slack, DO NOT PULL HARD
and then tear off against the cutting edge of the slot.
18.4 Ribbon Cartridge Renewal
CAUTION
DO NOT USE ALTERNATIVE RIBBONS
Only use ribbons that have been supplied by
Eschmann Equipment. Replace the ribbon cartridge
before it becomes dry. They contain a protective
lubricant for the print head. Replace during every
third paper roll replacement.
Renew the ribbon cartridge as follows:
LS5
VACUUM & NON-VACUUM AUTOCLAVES
4.1
a. Open the printer door, remove the old paper roll and
spring clip (18, Fig. 4.2).
b. Support the printer in the vertical position and grip
the end of ribbon cartridge at the end marked ‘PUSH’
(Fig. 4.2, item 9). Remove the cartridge by pulling it
towards you gently .
c. Install new cartridge with faceted disc (item 14,
Fig. 4.2) facing out and to the right, by inserting the
right hand side onto its drive shaft first, and then
CAREFULLY press cartridge into place where
marked ‘PUSH’ (Fig. 4.2, item 9). Replace spring
clip (18, Fig. 4.2).
d. Ensure ribbon is taut. If necessary tighten ribbon by
turning faceted disc (Fig. 4.2, item 14) on cartridge,
clockwise, using finger or fingernail.
e. Replace paper roll and close printer door.
18.5 Spares Pack
A spares pack is available comprising five paper rolls and
two ribbon cartridges (see accessories section 20).
Key to Fig. 4
1 - Printer ribbon cable
2 - Printer connector
3 - Connector storage pocket
4 - Spring pivot pin (left)
5 - Spring pivot pin (right)
6 - Printer roll recess
7 - Printer door paper feed slot
8 - Printer unit
9 - ‘PUSH’ indication
10 - Left hand printer bracket arm
1 1 - Right hand printer bracket arm
12 - Paper feed slot
13 - Printer door
14 - Faceted disc
15 - Paper feed button
16 - Printer ribbon
17 - Door latch
18 - Cartridge ribbon spring clip
19 - Paper roll holder
4.2
4.3
4.4
ST-IM61e P17/20
Page 18

19 Technical data
20 Accessories
19.1 Electrical
Supply 230V 50/60 Hz a.c.
For use with alternating current
Loading at 230V 2.0kW 10A (max.)
Fuses 12.5A (Part.No. 112474) (x2)
T3.15A (Part.No.696207) (x1)
- not operator replaceable.
19.2 Sterilizing data
Sterilizing time at 134/137°C is 3min.5sec., at 121/
124°C is 15min. and at 134/137°C (extended, see
section 15.1) is 18min.
Drying time (if selected) is from 17 minutes (but can
be extended, see section 15.2).
Operating pressure at 121°C is 1.03 bar, at 124°C
is 1.23 bar, at 134°C is 2.03 bar and at 137°C is
2.32 bar.
Note: If the autoclave is configured to have
adjustable sterilization and drying times the above
times can be altered during cycle selection (see
section 14.6).
19.3 Maximum Loads
Trays (set of 4 different colours) . . . . . . REF 87-040-11
Tray lifter . . . . . . . . . . . . . . . . . . . . . . . . REF 87-040-90
Chiropody tray . . . . . . . . . . . . . . . . . . . . REF 87-041-12
Cassette (tray with lid) . . . . . . . . . . . . . . REF 87-040-87
Cassette furniture . . . . . . . . . . . . . . . . . REF 87-041-44
Universal cassette holder . . . . . . . . . . . REF 87-040-92
Vertical cassette holder . . . . . . . . . . . . .REF 87-040-96
Bacterial filter (pack of 10) . . . . . . . . . . . REF 82-961-68
Printer . . . . . . . . . . . . . . . . . . . . . . . . REF 87-066-66
Printer spares pack . . . . . . . . . . . . . . . . REF 87-034-05
Pouch Rack . . . . . . . . . . . . . . . . . . . . . . REF 87-040-66
Pouch (small) . . . . . . . . . . . . . . . . . . . . REF 87-033-10
Pouch (medium) . . . . . . . . . . . . . . . . . . REF 87-033-18
Pouch (large) . . . . . . . . . . . . . . . . . . . . . REF 87-033-29
Steam penetration test device* . . . . . . . REF 87-040-71
* Contains one device and 100 test strips
Tray - 1.5kg maximum per tray
Cassette - 2.0kg maximum per cassette
Chamber - 8.0kg maximum total load
19.4 Water reservoir capacity
8.0 litre
19.5 Weight (approx.)
Non-Vacuum Net = 42.5kg
Non-V acuum Shipping = 55.5kg.
For V acuum add 6.5kg.
19.6 Safety standards
EN 61010-1 (1993) inc. Amendment 2 (1995)
EN 61010-2-041 (1996)
19.7 Software version
The version of software loaded onto this autoclave
is briefly displayed each time the autoclave is
switched on in display D1 (e.g. 1.51).
P18/20 ST-IM61e
Page 19

LS5
VACUUM & NON-VACUUM AUTOCLAVES
APPENDIX 1
Special notes when using non-drying cycle B4
Section 14.8 states that the door can be opened when
the cycle ‘without drying’ has been completed (indicated
both visually and audibly, see 14.8). Opening the door
allows the load to dry and avoids excessive
condensation build up in the chamber. If the door is not
opened at the end of the cycle a pool of water may form
in the chamber (from trapped condensation) and the
load will be wet. This pool of water may then come out
of the chamber when the door is eventually opened.
Eschmann therefore recommend the following:-
1 When running the cycle ‘without drying’ always open
the door immediately the cycle has finished and
unload the chamber as soon as possible.
2 If the cycle ‘without drying’ is to be run overnight
using the special function F3, always ensure the
cycle is timed to finish when personnel have returned
at the start of the next day (to open the door and
unload the chamber).
3 Do not start a cycle ‘without drying’ at the end of the
day if personnel are not going to be present when
the cycle has finished (to open the door and unload
the chamber).
4 If in doubt use either of the cycles ‘with drying’ to
eliminate the potential problem.
NB. Eschmann do not recommend running a cycle
‘without drying’ (B4) if the chamber door is not
opened shortly after the cycle has been completed
and the load removed as soon as possible.
APPENDIX 2
SAFETY CHECKS
Operators should ensure that the following safety checks
are carried out.
Weekly
i Check that the door opens and closes easily.
ii Check the door seal for any signs of damage.
iii Check the secondary door catch latches effectively.
iv Check for any obvious escape of steam or water
during a cycle (apart from the normal escape via
reservoir vents).
Any of the above defects should be attended to by a
‘Competent Person’ immediately and the autoclave
should not be used until repair has been effected (see
note below).
Annually
i Check that the pressure relief valve operates
correctly at the set pressure.
ii Inspect the pressure system for integrity .
iii Check door microswitches and interlocks.
iv Check door locking mechanism for integrity
v Check pressure indicators for correct operation.
NOTE:
Annual inspections should only be undertaken by a
‘Competent Person’. Eschmann can provide
comprehensive service contracts which cover
preventive maintenance to ensure trouble free operation
of your autoclave as well as six monthly and annual
inspections of the pressure system to satisfy the
requirements of the Pressure Systems Safety
Regulations 2000.
ST-IM61e P19/20
Page 20

APPENDIX 3
LS5/SES2555 VACUUM AUTOCLAVE DAILY RECORD SHEET
AUTOCLAVE DETAILS: Serial No.: Dept.: Site:
TEST STRIP DETAILS
DETAILS
LOT
CYCLE *
& NUMBER
PASS or
FAIL ** SIGNATURE
DATE &
ATTACH DEVICE TEST STRIP HERE
* Cycle (should be B8 or special function F6), press B1 for Cycle Number.
* * If cycle fails record error code number in the column.
P20/20 ST-IM61e
Page 21

Page 22

Eschmann Equipment, Peter Road, Lancing, West Sussex, BN15 8TJ, England.
Tel: +44 (0) 1903 753322. Fax: +44 (0) 1903 875789. www.eschmann.co.uk
Page 23

KEY:-
1
3
4
˚C
1
2
1
˚
C
˚C
A1 Low water indicator.
A2 Process display indicator.
A3 Door unlocked indicator.
A4 Door locked indicator.
B1 Display change button.
B2 Cycle temperature button.
B3 Unwrapped load (with
drying) cycle button.
B4 Unwrapped load (without
drying) cycle button.
B5 Programme ‘P’ button.
B6 Cycle start button.
B7 Open door button.
B8 Single wrapped load (with
drying) cycle button.
C Selection indicators.
D1 Time / cycle counter
& Sterilization / drying time (remaining)
& Error number display.
D2 Temperature display.
D3 Pressure display.
1
KEY:-
1 Filter
2 Water filling cup
3 Remote computer connector
4 Printer
5 Printer door
6 Paper feed slot
7 Chamber filter
8 Serial label
9 Mains fuses
10 Mains ‘on/off’ switch
11 Drian tube
12 Drain tube tap (open and closed)
13 Drain tube retaining clips
15 Secondary catch
16 Autoclave door
17 Water level sensors
Open
Closed
2
ST-IM61e
 Loading...
Loading...