Page 1

Bio-Scale™Mini
Profinity™GST
Cartridges, 1 and 5 ml
Instruction Manual
Catalog #
732-4620
732-4622
732-4624
Page 2

Table of Contents
Section 1...Introduction ........................................1
Section 2 Product Information............................2
Section 3 Connection to Low-Pressure
Chromatography Systems..................6
Section 4 Connection to Medium- and High-
Pressure Chromatography Systems .10
Section 5...Buffers and Methods........................12
Section 6 Quick Solubility Screening
Protocols..........................................13
Section 7 Preparation of E. coli Lysates ...........17
Section 8 Preparing a Cartridge, and
Subsequent Purification....................19
Section 9 Scaling Up........................................23
Section 10 Regenerating, Cleaning,
Sanitizing, and Storage.....................24
Section 11 Troubleshooting Guide......................26
Page 3

Section 12 Ordering Information.........................29
Section 13 References .......................................31
Section 14 Legal Notices ...................................32
Page 4

Section 1
Introduction
Bio-Scale Mini GST cartridges are convenient,
disposable, prepacked low-pressure chromatographic
cartridges. Bio-Scale Mini cartridges offer both
increased run-to-run reproducibility and high purity
of protein through a patent-pending column design
and novel resin technology. Compatible with aqueous
buffers most commonly used for protein purification,
Bio-Scale Mini cartridges offer improved performance for protein separation needs.
Profinity glutathione support is based on Bio-Rad's
proprietary UNOsphere
™
technology (US patent
6,423,666) for capture and purification of glutathione
S-transferase (GST)-tagged proteins. Its ligand
density has been optimized for maximum capture of
target proteins. Ideal for scale-up, the Profinity
glutathione support’s open pore structure is ideal for
purifying proteins of a wide molecular weight range.
(Smith DB et al., 1988)
Bio-Scale Mini GST cartridges are packed with
1
Page 5

Bio-Rad's innovative Profinity GST resin. Structural
characteristics such as the polymeric nature,
optimized ligand density, and open pore structure of
the Profinity GST bead result in superb mechanical
strength and performance of the prepacked
cartridges.
Section 2
Product Information
Bio-Scale Mini cartridges are disposable, easy-to-use,
prepacked chromatographic cartridges supplied
ready for use in convenient 1 ml and 5 ml sizes.
Cartridges are available for a variety of
chromatographic techniques, including desalting,
ion exchange (IEX), and affinity (AC) chromatography.
See Ordering Information for a listing of the complete
Bio-Scale Mini cartridge product line.
Bio-Scale Mini cartridges are quickly connected to
liquid chromatography systems or luer-end syringes.
The cartridges can be used with any liquid
chromatography system capable of setting a high
pressure limit of 45 psi (equivalent to 3 bar or
2
Page 6

300 kPa). Alternatively, luer fittings offer convenient
connection directly to a Luer-Lok syringe for quick,
one-step purification.
Table 1 Bio-Scale Mini GST cartridge
specifications
Sizes 1 ml and 5 ml bed volumes
Dimensions 1 ml: 40 mm length x 5.6 mm inner
diameter
5 ml: 40 mm length x 12.6 mm
inner diameter
Maximum pressure tolerance 45 psi
Recommended flow rates 1 ml: 1.0–2.0 ml/min (240–480 cm/hr)
5 ml: 5–10 ml/min (240–480 cm/hr)
Maximum flow rate 1 ml: 6 ml/min (1,440 cm/hr)
5 ml: 20 ml/min (963 cm/hr)
Fittings Female luer inlet and
male luer outlet
Column material Polypropylene
Frit material Polyethylene (HDPE)
Shipping conditions 20% ethanol
Storage recommendations 20% ethanol or 2% Benzyl Alcohol
Autoclavability Not autoclavable
3
Page 7

Table 2. Profinity GST Resin Specifications
Functional ligand Glutathione Derivative
Base bead UNOsphere
Particle size range 45–90 µm
Mean particle size 70 µm
Functional group density ≥60 µmol/g
Dynamic binding capacity* >11 mg/ml
Recommended linear flow rate <600 cm/hr at 25°C
Maximum operating pressure ≥43 psi
Chemical compatibility See table 3
Storage 4–8°C
Shelf life in 20% EtOH >1 year at 4–8°C
Operational temperature 4–40°C
* 60% Breakthrough for a purified 51 kD GST fusion protein at 0.5 ml/min.
Note: Dynamic binding capacity is a function of a number of factors
including pH, flow rate, and sample temperature.
Profinity GST cartridges are compatible with aqueous
buffers most commonly used with GST purification
techniques.
4
Page 8

Table 3. Buffer and Chemical Compatibilities for
Profinity GST cartridges
Reagent Stability
Buffer reagents
Tris 50 mM
HEPES 50 mM
MOPS 50 mM
Sodium or potassium phosphate 50 mM
Chelating agents
EDTA, EGTA 5 mM
Sulfhydryl reagents
β−Mercaptoethanol 30 mM
DTT 5 mM
TCEP 10 mM
Detergents
Nonionic detergents (Triton, Tween, NP-40) 5%
Cationic detergents (CTAB) 1%
Zwitterionic detergents (CHAPS, CHAPSO) 5%
Anionic detergents (SDS, Sarkosyl) 1%
Denaturing agents (for cleaning only)
Guanidine-HCl 6 M
Urea 8 M
Other additives
NaCl 2 M
MgCl
2
100 mM
CaCl
2
10 mM
Glycerol 20%
Ethanol 20%
Citrate 80 mM
5
Page 9

Section 3
Connection to
Low-Pressure
Chromatography Systems
Bio-Scale Mini cartridges are ideal for use with
Bio-Rad's BioLogic™LP chromatography system,
Econo™Gradient pump, the patented* Model EP-1
Econo pump, and all low-pressure chromatography
instruments. Bio-Scale Mini cartridges can be
conveniently connected directly to the system using
the luer fittings on the cartridge.
1. Install 1.6 mm inner diameter (ID) tubing in the
pumphead. Adjust the platen pressure screw
(on pumphead) using a screwdriver or coin.
Turn the screw counterclockwise as far as it will
go, then turn clockwise three full turns.
Assemble with fittings and lock rings as shown
in Figure 1.
* US patent 5,135,658
6
Page 10
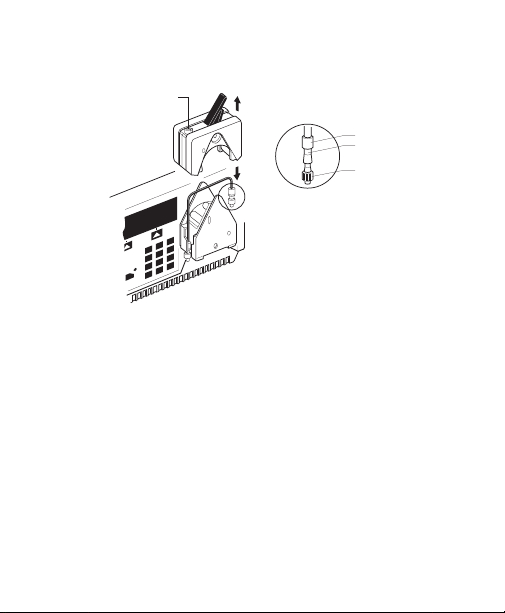
(Use orange lock rings and medium size barb fittings
with 1.6 mm tubing.)
Fig. 1. BioLogic LP system setup.
2. To maximize gradient accuracy and to apply
samples efficiently, install 1.6 mm ID tubing
from the pump to the MV-6 sample inject valve
(if available). If using the MV-6 sample inject
valve, turn the knob counterclockwise as far as
it will go so it will now correspond to the printed
diagram on the valve (see Figure 2).
7
Platen pressure
screw
Lock-ring
Tubing
Luer fittimg
See detail
23
1
9
456
78
0
C .
Alarm
Page 11
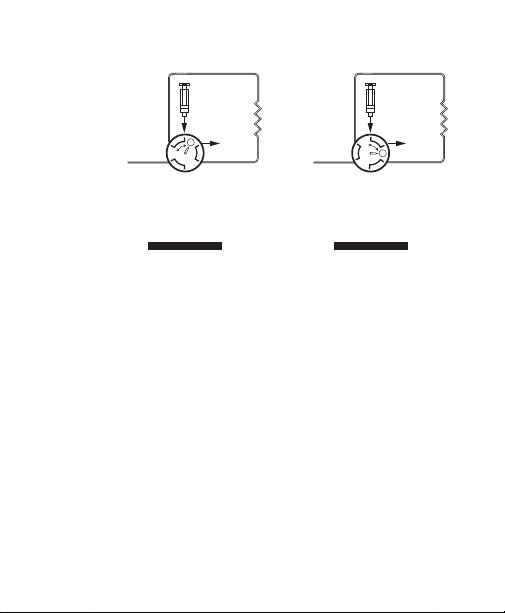
Fig. 2. Connecting to an MV-6 valve.
3. Connect the inlet of the cartridge to the male
luer fitting on the MV-6 sample inject valve (see
Figure 2). If not using the MV-6 sample inject
valve, connect a barb to male luer fitting on the
1.6 mm ID tubing, then connect to the top of
the female luer on the Bio-Scale Mini cartridge.
For optimum performance, a cartridge should
be mounted vertically with the arrow on the
cartridge pointing downward (see Figure 3).
BIOLOGIC LP
8
SYSTEM OR ECONO
GRADIENT PUMP
SAMPLE LOOP
INJECT
PORT
MV-6
TO
INJECT
VALVE
FILL
TO
COLUMN
WASTE
BIOLOGIC LP
SYSTEM OR ECONO
GRADIENT PUMP
TO
MV-6
INJECT
VALVE
SAMPLE LOOP
INJECT
PORT
FILL
WASTE
TO
COLUMN
"INJECT" POSITION"FILL" POSITION
Page 12

4. Connect the cartridge outlet to the 1.6 mm ID
tubing leading to the BioLogic LP system
optics module or to the Model EM-1 Econo UV
monitor. It is recommended to use the shortest
length (approximately 10 cm) of 1.6 mm ID
tubing. Connect a barb to female luer to the
1.6 mm ID tubing, then connect to the
bottom of the male luer on the Bio-Scale Mini
cartridge.
Fig. 3. Cartridge and fittings. Luer fittings and column: a
cartridge should be mounted vertically with the arrow on the
cartridge pointing downward.
9
Page 13

Section 4
Connection to Medium
and High-Pressure
Chromatography Systems
Bio-Scale Mini cartridges can be connected to any
liquid chromatography system, provided that the
maximum pressure limit (3 bar, 45 psi, or 300 kPa)
of the cartridges is not exceeded. It is recommended
that the system pressure limit be set according to
the cartridge pressure limit. Pressures in excess of
3 bar are usually caused by restrictions in tubing or
detector cells downstream from the cartridge.
Bio-Rad offers two fitting kits for easy connection of
a Bio-Scale Mini cartridge to a BioLogic DuoFlow,
HPLC, or FPLC-type system.
4.1 BioLogic DuoFlow Systems
The luer to BioLogic system fittings kit (catalog
#732-0113) includes 1/4-28 female to male luer and
1/4-28 female to female luer to connect one Bio-Scale
Mini cartridge to the BioLogic DuoFlow system. (see
Figure 4)
10
Page 14

Fig. 4. Luer to 1/4-28 adaptor.
4.2 HPLC Systems
The luer to 10-32 adaptor fittings kit (catalog
#732-0112) provides fittings necessary to connect
the Bio-Scale Mini cartridge to nut and ferrule type
fittings found on most HPLC systems. Alternatively,
the cartridge can be connected to HPLC systems
via a low dead-volume 1/16 inch union with a new
piece of stainless-steel tubing attached to the
union. Simply slip a short length of the 0.8 mm ID
tubing over the 1/16 inch OD stainless-steel tubing
to a distance of 1 cm.
4.3 FPLC Systems
The luer to M6 adaptor fittings kit (catalog #732-0111)
provides fittings necessary to connect the Bio-Scale
Mini cartridge to the M6 fittings found on FPLC or
related systems. Alternatively, connection can be
made by using one GE Healthcare Union Luerlock
female to M6 female fitting (GE 18-1027-12) and
11
Page 15

one Upchurch P-686, female slip luer to male M6 fitting or GE 18-1027-62, Union luerlock female to M6
male fitting. To prevent tubing or cartridge failure, do
not exceed the maximum recommended flow rate of
the cartridge.
* Fittings kit ordering information can be found within the Ordering Information section of this
manual.
Section 5
Buffers and Methods
GST methods can be run using only native
purification protocols. Under native conditions,
proteins are purified using buffers that help retain
the natural folded structure of the target protein.
The recommended buffer compositions are provided
in Table 4.
12
Page 16

Table 4. Recommended Buffers and Storage
Solutions
Solution Composition
Lysis/wash
buffer 150 mM NaCl, 10 mM Na
2
HP04, 5 mM EDTA, pH 7.4
Elution buffer 20 mM glutathione, 100 mM Tris, 5 mM EDTA, pH 8.0
Desalting buffer 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na
2
HP04, 8.1 mM KH2P04, pH 7.4
Cleaning
solution 1 500 mM NaCl, 50 mM Tris, pH 8.0
Cleaning
solution 2 500 mM NaCl, 100 mM NaOAc, pH 4.5
Storage solution 2% Benzyl alcohol or 20% Ethanol
Section 6
Quick Solubility Screening
Protocols
Before choosing a purification protocol, it is useful
to determine the approximate expression level of a
protein, and to determine if the overexpressed target
protein partitions into the soluble or insoluble fraction.
Soluble proteins are purified with the native
purification procedure. The following procedure
provides a quick screen for solubility and expression
level.
13
Page 17

1. Pellet ~2 ml of E. coli culture by centrifugation
at 4,000 x g for 10 min at 4°C.
2. Resuspend the pellet in 500 µl of PBS and
sonicate for 60 sec, on ice, in 10 sec pulses.
Remove 50 µl of the sonicate and label as the
“Total” sample. Centrifuge the lysate at 12,000 x g
for 10 min at 4°C. Transfer the supernatant to a
clean tube. Remove 50 µl of the supernatant,
and label tube “Soluble”.
3. Resuspend the insoluble pellet in 500 µl of 6 M
urea in 1x PBS and sonicate for 60 sec, on ice,
in 10 sec pulses. Centrifuge the lysate at
12,000 x g for 10 min at 4°C. Remove 50 µl of
the supernatant, and label "Insoluble".
4. To each of the 50 µl samples, add 150 µl of
Laemmli buffer, and boil for 5 min at 95°C.
5. Load 10 µl of each sample on an SDS-PAGE
gel.
6. Examine the soluble and insoluble fractions for
the target protein. Approximate the expression
14
Page 18

level, and determine partitioning of the target
protein.
A partitioning profile of soluble and insoluble target
proteins, with approximate expression levels, can be
seen in Figure 5.
15
Page 19
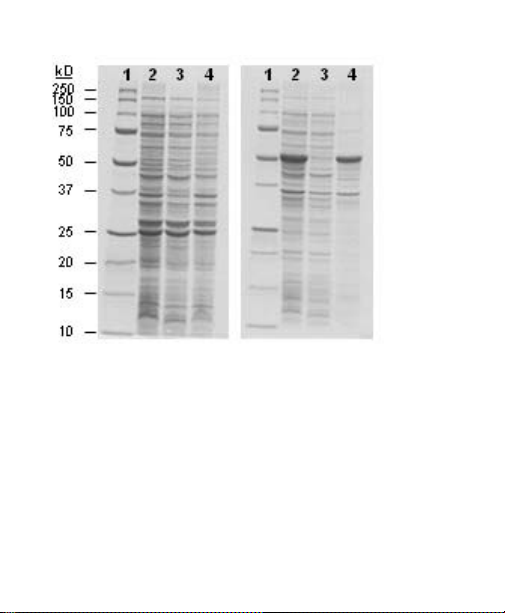
Fig. 5. Partioning profiles. Representative gels showing partitioning of the
target protein into the soluble fraction (left panel) or insoluble fraction (right
panel). For both gels, Precision Plus Protein
™
standards were loaded in lane 1,
followed by the total, soluble, and insoluble fractions in lanes 2–4 respectively.
The first panel depicts GST, a 26 kD protein, which partitions into the soluble
fraction. The second panel shows GST-tagged GFP, grown under conditions
that drive the fusion protein into inclusion bodies.
16
Page 20

Section 7
Preparation of E. coli
Lysates
Lysates from E. coli cultures can be prepared using
conventional sonication procedures with the lysis buffers
supplied in each kit, or can be prepared using chemical
lysis methods and the Profinia
™
bacterial lysis/extraction
reagent. For E. coli cultures expressing medium to high
levels of fusion proteins, (≥10% of total protein), 200 ml
of culture will normally yield sufficient material for a 1 ml
cartridge purification, and 1,000 ml of culture will yield
sufficient material for a 5 ml cartridge purification run.
For cultures expressing protein at low levels (≤10% of
total protein), the culture volumes will need to be
determined empirically for each protein. Bacterial cultures
can be grown in advance and centrifuged. The pellets
can be stored at –70°C for several months and lysed at
a convenient date for sample preparation.
17
Page 21

Basic Protocol
1. Harvest cell pellet by centrifugation at 8,000 x g for
10 min at 4°C.
2. Determine weight of pellet and resuspend in 10
volumes of lysis/wash buffer (200 ml of culture
typically yields 0.8–1.0 g of paste, or 8–10 ml of
lysate).
3. Thoroughly resuspend the pellet by pipetting or
vortexing.
4. As an optional step and to decrease the viscosity,
add a nuclease solution (DNase at 100 units/ml or
Benzonase at 25 units/ml) and incubate for
10 min at room temperature).
5. Sonicate the lysate (on ice, using 25% output) 4
times at 1 min intervals.
6. Centrifuge the lysate at 16,000 x g for 20 min at
4°C.
7. Remove the supernatant and filter through a
0.45 µm filter to remove particulates. The lysate is
now ready to be loaded into the Bio-Scale Mini
Profinity GST cartridge.
18
Page 22

If the lysate is not going to be used immediately, it
can be frozen at –20°C and thawed once to be
purified at a later date. However, proteolysis can
occur upon freezing and thawing, and the quality of
the purified product may be compromised. This will
have to be determined empirically for individual
proteins. Upon thawing, refilter through a 0.45 µm
filter, as precipitates often form after freezing.
Section 8
Preparing a Cartridge, and
Subsequent Purification
Prepare buffer sets for the purification protocols
using a single buffer set throughout the procedure.
To prepare the cartridge for the procedure, remove
the top closure and connect the cartridge to the
chromatography system. Open the bottom closure
and connect the cartridge outlet to the system.
Flush the packing solution (20% EtOH) from the
cartridge by running 2 column volumes (CV) of water
19
Page 23
or buffer of choice, at a flow rate of 2 ml/min (1 ml
cartridge) or 10 ml/min (5 ml cartridge). The cartridge
is now ready for the purification steps. Flow rates
are given in ml/min and are specific to the 1 ml
cartridge. If a 5 ml cartridge is used for a procedure,
substitute the higher flow rate in the method (refer to
the table below).
Table 5. Purification Method Suggestions
1 ml Cartridge 5 ml Cartridge
Step CV Flow Rate Flow Rate
Equilibrate 5 2 ml/min 10 ml/min
Lysate load 5 to 10 0.5–1 ml/min 2.5–5 ml/min
Wash 1 12 2 ml/min 10 ml/min
Elute 3 0.5 ml/min 2.5 ml/min
Standard methods that are compatible with any
type of chromatography system are listed below. To
maximize binding capacity, the lysate load flow rate
can be decreased to the minimum recommended
flow rate for 1 ml and 5 ml columns (Table 1). This
will have to be determined empirically for individual
proteins.
20
Page 24

1. Equilibrate the cartridge with 5 CV of
equilibration/wash buffer 1 at 2 ml/min.
2. Load the sample lysate at 0.5–1 ml/min.
3. Wash the cartridge with 12 CV of wash buffer
at 2 ml/min.
4. Elute the purified protein with 3 CV of elution
buffer at 0.5 ml/min.
5. Collect the fractions of eluted target protein for
analysis by SDS-PAGE and pool the fractions
that are satisfactory.
Cleaning the Cartridge
6. Wash the column with 5 CV of cleaning buffer
1 at 2 ml/min.
7. Wash the column with 5 CV of cleaning buffer
2 at 2 ml/min. Rinse the column with 5 CV of
high-purity deionized water at 2 ml/min.
8. Rinse the column with 5 CV of storage solution
at 1 ml/min.
9. Store the cleaned column well-sealed at 4ºC.
21
Page 25
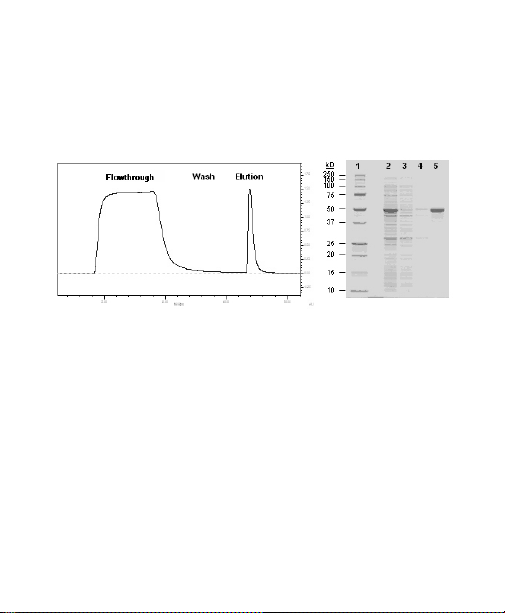
The chromatogram and gel in Figure 6 illustrate a
representative purification of a high-expressing
soluble protein purified using the GST buffer set and
method described in Tables 4 and 5.
Fig. 6. GST purification: A 51 kD GST-tagged protein was purified from
the soluble fraction of an E. coli lysate using a standard Profinity GST
purification protocol. 10 ml of lysate (10 CV) from a 100 ml E. coli culture was
loaded onto a 1 ml Profinity GST cartridge. The cartridge was washed with
12 CV of wash buffer and purified protein was eluted with 3 CV of elution
buffer (0.5 ml/min). The purified product was >80% pure by densitometric
scanning and Quantiy One
®
software analysis. Lane 1, Precision Plus Protein
unstained standards; lane 2, soluble lysate; lane 3, flowthrough; lane 4, wash
1; lane 5, purified product.
22
Page 26

Cleavage of GST fusion proteins
Design of an enzyme-cleavable fusion construct
requires splicing a recognition site for thrombin,
Factor Xa, or other sequence-specific proteolytic
enzyme into the linkage between the GST and the
target protein. The target protein can be obtained in
purified form post-elution, or while still on the column
(Dian C et al. 2002).
Section 9
Scaling Up
Bio-Scale Mini cartridges are available in 1 ml and
5 ml cartridge formats. The Profinity GST resin is
also available in larger amounts, from 25 ml bottles
to bulk quantities, for scaling up methods developed
using the cartridges.
For quick scale-up, two or three cartridges of the
same type can be connected in series; backpressure
will increase with cartridges in series, so care
should be taken to maintain an overall system
pressure ≤45 psi.
23
Page 27

In addition, Bio-Rad carries an extensive line of
empty chromatography columns from laboratory
scale to process scale. Inquire with your local
Bio-Rad representative, or go online to
wwwwww..bbiioo--rraadd..ccoomm
Section 10
Regenerating, Cleaning,
Sanitizing, and Storage
Protein cross-contamination, frit clogging, and
increased backpressure can result from repeating
the number of uses beyond the recommended
number. After repeated use, a cartridge may run
slower or produce higher backpressure, an expected
result due to the nature of the sample mixture. The
following cleaning and regeneration procedures may
be used; however, it is recommended to
dispose of the cartridge after several uses. To avoid
cross-contamination, single cartridges should be
designated for single proteins.
24
Page 28

To maintain good flow properties, the cartridges
should be cleaned between each use. For the 1 ml
cartridges, run the cleaning protocol at 3 ml/min. It
is recommended that the 5 ml cartridge cleaning
protocol be run at 15 ml/min.
High Salt/Acid Cleaning
1. Rinse the cartridge with 2 CV water at 2 ml/min.
2. Wash the cartridge with 5 CV 500 mM NaCl,
50 mM Tris, pH 8.0 at 2 ml/min.
3. Wash the cartridge with 5 CV 500 mM NaCl,
100 mM NaOAc, pH 4.5 at 2 ml/min.
4. Rinse the cartridge with 2 CV water at 2 ml/min.
5. Store the cartridge in 20% ETOH or 2% Benzyl
alcohol at 4–8°C.
Chaotropic Agent Cleaning
1. Rinse the cartridge with 2 CV water at 2 ml/min.
2. Wash the cartridge with 5 CV 6 M guanidine HCl
at 2 ml/min.
3. Rinse the cartridge with 2 CV water at 2 ml/min.
4. Store the cartridge in 20% ETOH or 2% Benzyl
alchol at 4–8°C.
25
Page 29

Section 11
Troubleshooting Guide
Problem Possible Cause Solution
Cartridge clogging Particulates in samples Filter all samples and buffers
or slow flow rate or buffers through 0.2 µm filter prior to
application
Sample too viscous Add nuclease to lysate to
degrade DNA. Centrifuge
and filter lysate again
No target protein Low level of target Check expression level by
in eluate protein in starting SDS-PAGE
material
Target protein not Check levels of target protein
binding, or eluting in in lysate, flowthrough, wash,
wash fractions and eluted fractions. Check
for presence of tag with
anti-GST antibody
Target protein in Tag not accessible Reclone GST-tagged protein
flowthrough onto opposite terminus (N- or
C-terminus)
Tag not accessible Purify protein under denaturing
conditions to expose
glutathione S-tranferase tag
Proteolysis and removal Include protease inhibitors in
of tag lysis buffer (or reaction), or
purify in the cold
26
Page 30

Problem Possible Cause Solution
Precipitation during Binding capacity of Load less sample
purification cartridge exceeded
Protein aggregating Include low levels of
detergent (0.1% Triton
X-100, Tween 20) in
purification. Include glycerol
up to 10%
Protein too concen- Eluate with elution buffer
trated during elution gradient
Eluted protein is Contaminants coeluting Decrease sonication time
impure
Target protein is Proteolysis of target Add protease inhibitors to
degraded protein lysate. Purify at 4°C or under
denaturing conditions
Low yield Low expression level Optimize expression system
or use different system
Insufficient extraction Use frozen bacterial pellet
instead of fresh pellet. Adding
lysozyme to the lysis buffer
may increase the efficiency of
extraction
Construct does not Fusion partner affects GST
bind to column conformation. Adding in 5
mM DTT to lysis buffer may
help
Low product purity Construct binds Adding DTT can reduce
other bacterial nonspecific interactions
27
Page 31

Problem Possible Cause Solution
A small amount of a
nondenaturing detergent can
be added to the wash buffer
Column not washed Increase number of column
sufficiently volumes in wash buffer step
Column runs slowly Overloading column Reduce lysate load volume
Sample is too viscous Dilute the lysate before
application to column
28
Page 32

Section 12
Ordering Information
Bio-Scale Mini Cartridges
Description 5 x 1 ml 1 x 5 ml 5 x 5 ml
UNOsphere Q Support 732-4100 731-4102 731-4104
UNOsphere S Support 732-4110 731-4112 731-4114
Macro-Prep
®
High Q Support 732-4120 732-4122 732-4124
Macro-Prep High S Support 732-4130 732-4132 732-4134
Macro-Prep DEAE Support 732-4140 732-4142 732-4144
Bio-Gel P-6 Support — 732-4502 732-4504
Affi-Prep
®
Protein A Support 732-4600 732-4602 —
Profinity IMAC Support 732-4610 732-4612 732-4614
Profinity GST Support 732-4620 732-4622 732-4624
Affi-Gel
®
DEAE Blue Support – 732-4642 732-4644
Affi-Gel Blue Support – 732-4632 732-4634
1x 5x
Bio-Gel P-6 Desalting, 10 ml — — 732-5304
Bio-Gel P-6 Desalting, 50 ml — 732-5312 732-5314
• Visit www.bio-rad.com/cartridges/ for current information on
prepacked cartridges.
• Larger package sizes of media are available for process-scale
chromatography. Inquire with your local Bio-Rad representative.
29
Page 33

Fittings Kits
Catalog # Description
732-0111 Luer to M6 Adaptor Fittings Kit, includes luer to
M6 fittings to connect 1 cartridge to an FPLC
system
732-0112 Luer to 10-32 Adaptor Fittings Kit, includes luer
to 10-32 fittings to connect 1 cartridge to an
HPLC system
732-0113 Luer to BioLogic System Fittings Kit, includes
1/4-28 female to male luer and 1/4-28 female to
female luer to connect 1 cartridge to a BioLogic
DuoFlow system
30
Page 34

Section 13
References
Dian C et al., Strategies for the purification and
on-column cleavage of glutathione S-transferase
fusion target proteins, J Chromatogr B Analyt
Technol Biomed Life Sci 769, 133–144 (2002)
Smith DB and Johnson KS, Single-step purification
of polypeptides expressed Escherichia coli as
fusions with glutathione S-transferase, Gene 67,
31–40 (1988)
31
Page 35

Section 14
32
Legal Notices
Benzonase is a trademark of Novage. FPLC is a
trademark of GE Healthcare. Luer-Lok is a
trademark of Becton, Dickenson & Co. Triton is a
trademark of Union Carbide. Tween is a trademark
of ICI Americas, Inc.
Purification and preparation of fusion proteins and
affinity peptides comprising at least two adjacent
Histidine residues may require a license under US
patent 5,284,933 and US patent 5,310,663, including
corresponding foreign patents (assignee HoffmanLa Roche, Inc).
Bio-Rad Laboratories, Inc. 925 Alfred Nobel Dr.
Hercules, CA 94547 USA 510-741-1000 or
1-800-424-6723
Page 36

Bio-Rad Laboratories, Inc.
2000 Alfred Nobel Dr.
Hercules, CA 94547 USA
510-741-1000
1-800-424-6723
10007406 Rev C
 Loading...
Loading...