Page 1
Model 422
Electro-Eluter
Instruction
Manual
Catalog Numbers
165-2976 and 165-2977
For Technical Service
Call Your Local Bio-Rad Office or
in the U.S. Call 1-800-4BIORAD
(1-800-424-6723)
Page 2

Note
To insure best performance from the Model 422 Electro-Eluter, become fully acquainted with these operating instructions before using the cell to separate samples. Bio-Rad recommends that you first read these instructions carefully. Then assemble and disassemble the
cell completely without casting a gel. After these preliminary steps, you should be ready to cast
and run a gel.
Bio-Rad also recommends that all Model 422 Electro-Eluter components and accessories
be cleaned with a suitable laboratory cleaner (such as Bio-Rad Cleaning Concentrate, catalog
number 161-0722) and rinsed thoroughly with distilled water, before use.
Warranty
Bio-Rad Laboratories warrants the Model 422 Electro-Eluter against defects in materials
and workmanship for 1 year. If any defects occur in the instrument during this warranty period, Bio-Rad Laboratories will repair or replace the defective parts free. The following defects,
however, are specifically excluded:
1. Defects caused by improper operation.
2. Repair or modification done by anyone other than Bio-Rad Laboratories or an authorized
agent.
3. Use of fittings or other spare parts supplied by anyone other than Bio-Rad Laboratories.
4. Damage caused by accident or misuse.
5. Damage caused by disaster.
6. Corrosion due to use of improper solvent or sample.
For any inquiry or request for repair service, contact Bio-Rad Laboratories after confirming the model and serial number of your instrument.
Model
Catalog No.
Date of Delivery
Warranty Period
Serial No.
Invoice No.
Purchase Order No.
Page 3

Table of Contents
Section 1 Introduction..................................................................................................1
1.1 Specifications.............................................................................................................1
Section 2 Equipment and Reagents............................................................................2
2.1 Equipment..................................................................................................................2
2.2 Modular Mini Electrophoresis System .....................................................................2
Section 3 Safety Instructions.......................................................................................3
Section 4 Assembly.......................................................................................................4
Section 5 Visualizing Proteins in SDS-Polyacrylamide Gels...................................5
5.1 Visualizing Proteins Stained with Coomassie Blue R-250 ......................................5
5.2 Visualizing Proteins Using Copper Chloride ...........................................................6
5.3 Visualizing Proteins Using Autoradiography ..........................................................6
Section 6 Protein Elution ............................................................................................6
6.1 Elution from SDS-PAGE Gels .................................................................................6
6.2 Elution of Fixed and Stained Proteins ......................................................................7
6.3 Concentration of Eluted Proteins Using Volatile Buffers.........................................7
6.4 Elution of Proteins from Native Gels .......................................................................7
6.5 Removal of SDS from Samples Subsequent to Elution............................................8
Section 7 Visualizing DNA.......................................................................................... 9
7.1 Visualizing DNA in Agarose Gels............................................................................9
7.2 Visualizing DNA in Acrylamide Gels.......................................................................9
Section 8 DNA Elution.................................................................................................9
8.1 Elution from Gel........................................................................................................9
8.2 DNA Purification.....................................................................................................10
Section 9 Recipies.......................................................................................................11
Section 10 Troubleshooting Guide..............................................................................11
i
Page 4

Section 1
Introduction
Elution of proteins and nucleic acids from gel slices is often a difficult and unreliable
procedure. The Model 422 Electro-Eluter solves many of the problems of conventional eluters
by providing easy assembly and eliminating sample loss due to leakage. The Model 422
Electro-Eluter consistently yields between 70% and 100% of the starting material, depending
on the type of elution. The samples are collected in 400-600 µl volumes. The small volume
of eluted material makes subsequent manipulations easy. The Model 422 Electro-Eluter can
run up to six samples at once, with no increase in running time or decrease in yield.
1.1 Specifications
Construction
Electrode module Precision machined acrylic
Buffer chamber and lid Molded polycarbonate
Glass tubes Borosilicate glass
Frits Porous polypropylene
Silicone adaptors Molded silicone
Grommets and stoppers Molded silicone
Membrane caps Molded polyethylene with dialysis membrane
Overall size 16 cm (L) x 12 cm (W) x 18 cm (H)
Elution capacity 1 to 6 samples
Glass tube dimensions 1 cm ID x 6 cm long
Collection volume 400-600 µl
Buffer volume 700 ml
Shipping weight 1.1 kg
Chemical compatibility The Model 422 Electro-Eluter is not
compatible with chlorinated hydrocarbons
(e.g. chloroform), aromatic hydrocarbons
(e.g. toluene, benzene), or acetone. Use of
organic solvents voids all warranties.
1
Page 5
Section 2
Equipment and Reagents
2.1 Equipment
Catalog
Number Product Description
165-2976 Model 422 Electro-Eluter, includes electro-eluter module, 12 Membrane
Caps with a molecular weight cut off (MWCO) of 12,000-15,000 daltons,
6 Glass Tubes with Frits, 6 Silicone Adaptors, 6 Grommets and Stoppers,
buffer tank with lid and cables, and instruction manual
165-2977 Model 422 Electro-Eluter Module, same as 165-2976 without buffer
tank and lid
Accessories
165-2985 Clear Membrane Caps (12), MWCO of 12,000-15,000 daltons
165-2986 Green Membrane Caps (12), MWCO of 3,500 daltons
165-2978 Glass Tubes (6)
165-2981 Silicone Adaptors (6)
165-1988 Grommets and Stoppers (8)
165-2987 Frits (12)
Related Accessories
161-0719 Tris, 1 kg
161-0718 Glycine, 1 kg
161-0400 Coomassie Blue R-250, 10 g
165-4710 Model 1000/500 Power Supply, 120 V, 50/60 Hz
165-4711 Model 1000/500 Power Supply, 220 V, 50/60 Hz
2.2 Modular Mini Electrophoresis System
165-2940 Mini-PROTEAN®II Cell, includes inner cooling core with gaskets,
lower buffer chamber, lid with power cables, Glass Plates (3 sets), clamp
assemblies (2), 10-well, 0.75 mm thick combs (2), casting stand with gaskets, leveling bubble, and instruction manual
170-3930 Mini Trans-Blot®Electrophoretic Transfer Cell, includes 2 gel hold-
ers, electrode assembly, fiber pads, Bio-Ice®cooling unit, and instruction
manual
170-3935 Mini Trans-Blot Module, for use with the Mini-PROTEAN II cell,
same as 170-6530 without buffer tank and lid
165-2960 Mini-PROTEAN II 2-D Cell, includes tube adaptor, sample reservoirs
and stoppers, sample reservoir/capillary tube connectors, Capillary Tubes
with Casting Tube, Tube Gel Ejector, Mini-PROTEAN II slab cell with
inner cooling core and gaskets, lower buffer chamber, lid with power
cables, 2-D combs with 1 standard well (2), 1.0 mm thick spacers (4),
Casting Stand with Gaskets, leveling bubble, and 2-D instruction manual.
165-2965 Mini Tube Cell Module, includes tube adaptor, sample reservoirs and
stoppers, sample reservoir/capillary tube connectors, Capillary Tubes
with Casting Tube, Tube Gel Ejector, and instructions.
2
Page 6

Section 3
Safety Instructions
Power to the Model 422 Electro-Eluter is to be supplied by an external DC voltage power
supply. This power supply must be ground isolated in such a way that the DC voltage out-
put floats with respect to ground. All of Bio-Rad's power supplies meet this important safety requirement. Regardless of which power supply is used, the maximum specified operating
parameters for the cell are:
600 VDC maximum voltage limit
24 Watts maximum power limit
50°C maximum ambient temperature limit
Current to the cell, provided from the external power supply, enters the unit through the
lid assembly, providing a safety interlock to the user. Current to the cell is broken when the
lid is removed. Do not attempt to circumvent this safety interlock, and always turn the
power supply off before removing the lid, or when working with the cell in any way.
Since hazardous voltages may be present in the electro-eluter, it is important that certain pre-
cautions are observed for the safety of the operator:
1. Always turn off the power supply before removing the safety cover. Do not use the safe-
ty cover as an on-off switch.
2. Never attempt to operate the cell without the safety cover.
3. Do not attempt to move the cell with the power on.
4. Do not leave an operating cell unattended for long periods of time.
5. Do not overfill the upper or lower buffer reservoirs. Refer to Section 4 for proper buffer
levels.
6. If the cell appears to be drawing excessive current, turn off the power supply and check
the entire system (for example, buffer chamber leaks, overfilled buffer chamber,
buffer too conductive, etc.).
Note: Please become familiar with these precautions, both for safe operation of this equip-
ment and to prevent loss of valuable experimental results.
Important:
This BIO-RAD instrument is designed and certified to meet IEC1010-1* safety stan-
dards. Certified products are safe to use when operated in accordance with the instruc-
tion manual. This instrument should not be modified or altered in any way. Alteration
of this instrument will:
• Void the manufacturer's warranty
• Void the IEC1010-1 safety certification
• Create a potential safety hazard
BIO-RAD is not responsible for any injury or damage caused by the use of this instrument
for purposes other than for which it is intended or by modifications of the instrument not
performed by BIO-RAD or an authorized agent.
*IEC1010-1 is an internationally accepted electrical safety standard for laboratory instruments.
3
!
Page 7

Section 4
Model 422 Electro-Eluter Assembly
1. Soak the Membrane Caps at 60 °C in elution buffer (see Section 9 for buffer recipes) for
at least 1 hour prior to use. Membrane caps may be soaked for longer than 1 hour. Wear
gloves when handling the membrane caps to prevent the dialysis membrane from becom-
ing contaminated. Membrane caps may be reused. If they are to be reused they must be
stored in elution buffer containing 0.05% sodium azide (NaN3) in the refrigerator. They
do not need to be reheated or resoaked when reused.
2. Take the appropriate number of Glass Tubes and place one Frit in the bottom of each
tube. Set the tube on a clean paper towel and, if necessary, push on the Frit from inside
the Glass Tube to insure that the Frit is flush with the bottom of the glass tube. Next, push
the Glass Tube and Frit into the electro-eluter module. It is easiest to do this by wetting
the grommet with elution buffer and sliding the Glass Tube in place. Make sure the Glass
Tube is even with the top of the grommet and the Frit is flush with the bottom of the
Glass Tube. Fill any empty grommet holes with stoppers.
3. Wear gloves when handling the Membrane Caps to prevent contamination of the dialy-
sis membrane. Place one pre-wetted membrane cap in the bottom of each Silicone
Adaptor. Fill the adaptor with elution buffer. Pipet the buffer up and down to remove any
air bubbles around the dialysis membrane.
4. Slide the Silicone Adaptor with the membrane cap onto the bottom of the Glass Tube
with Frit. Some tiny air bubbles may come out of the Frit. Pull the Silicone Adaptor par-
tially on and off a few times to make sure all the air bubbles are expelled. All air bubbles
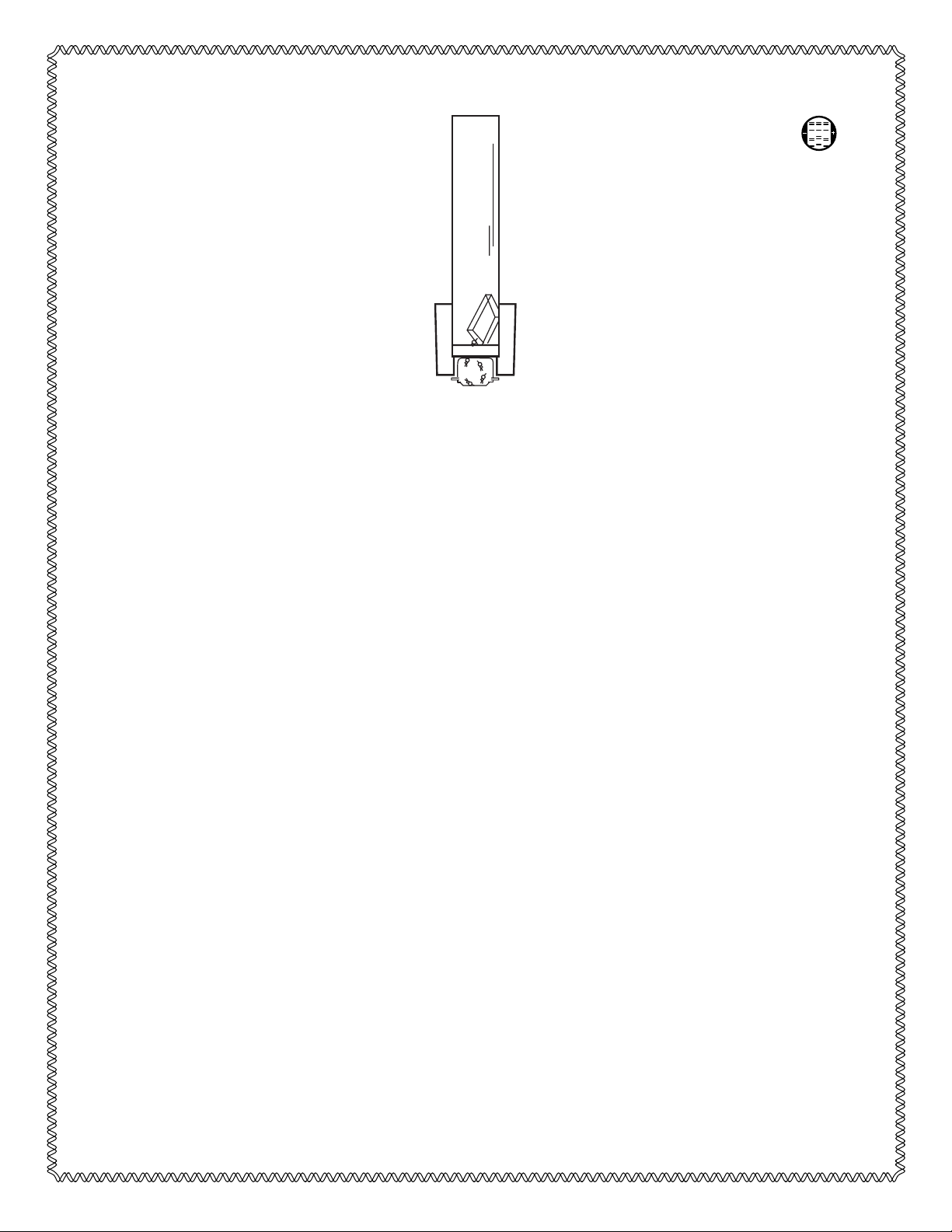
must be removed from the Frit for best elution yields. See Figure 1.
Note: The glass tubes for the Model 422 Electro-Eluter have one frosted end. The white
silicone adaptor must be placed on the frosted end, or it may slip off during electro-elution.
Fig. 1. Side view of Model 422 Electro-Eluter assembly. The vertical glass tube is filled with elution
buffer. The negative electrode is at the top. The positive electrode is below the membrane cap.
Macromolecules are carried by the electrical current out of the gel slice, through the Frit and into the
membrane cap. The molecules are retained by a dialysis membrane which is molded into the cap.
4
Glass tube
Silicone adaptor
Gel slice
Frit
Membrane cap
Page 8

5. Fill each tube with elution buffer and place the gel slice in the tube. To increase sample
recovery, several bands may be excised from the gel and minced, so long as the height of
the gel within the glass tube is approximately one centimeter or less. If the glass tube is
filled higher than 1 cm, elution times may increase.
6. Place the entire module into the buffer chamber. Fill the lower buffer chamber with ~600
ml of elution buffer. The level of the lower buffer must be above the top of the silicone
adaptors or bubbles may form on the bottom of the dialysis membrane. Fill the upper
buffer chamber with ~100 ml of elution buffer. To prevent electrical shorts the banana
plugs must be dry.
7. Place a stir bar in the bottom buffer tank. Vigorous stirring during the run will prevent bub-
bles from sticking to the bottom of the dialysis membrane.
8. Attach the lid with cables, orienting the red to red (anode) and black to black (cathode).
9. Elution is done at 8-10 mA/glass tube. See Sections 6 and 8 for specific conditions for elut-
ing protein and DNA. See Section 9 for elution buffer recipes.
Section 5
Protein Visualization in SDS-Polyacrylamide Gels
After the protein has been separated by gel electrophoresis, it is necessary to visualize
the band of interest. If the protein can be fixed, stained, and eluted see Section 6.1. Unstained
proteins can be isolated using one of the methods in Section 5.
5.1 Visualizing the Protein Using Coomassie Blue R-250
Using this method, a reference lane containing the same sample as the one being eluted
is run on the gel and stained to identify the band of interest. Prior to electrophoresis, the reference lane and the lane that will be eluted are treated exactly the same. The sample is prepared
as one large batch and equal amounts are loaded on the elution lane and the reference lane.
1. The reference lane should be run next to the elution lane. It should not be run on the end
lane because edge effects may distort the banding pattern. The gel is run at the optimal con-
ditions for the band of interest.
2. At the end of the run, cut a corner off the gel to maintain the correct orientation. Keep the
gel moist in a small amount of running buffer.
3. The reference lane is cut off, fixed, and stained with 10% acetic acid, 40% methanol,
0.1% Coomassie R-250 for 1 hour. It is destained using 10% acetic acid, 40% methanol
for 2 hours, or until the band of interest can be identified. It is not necessary to have a
completely clear background. The proteins will not diffuse in the 2-3 hours needed to do
the staining. Line up the stained gel lane with the unstained gel, making sure the corner
that was cut off is in the correct orientation. Cut out the appropriate area of the unstained
gel along with some extra gel above and below the band. The staining process may cause
the gel to shrink, so a larger area must be cut to insure that all of the protein is obtained.
Bands to be cut out and eluted may be spaced very close to other potentially “contami-
nating” bands. Shrinking of the reference lane may make it difficult to accurately deter-
mine the corresponding area to cut out from an unstained, unshrunk lane. In such a case,
the unstained lane(s) can be shrunk in 10% acetic acid (without methanol and Coomassie
R-250). In this way, the reduced size of the lane containing the band(s) to be eluted will
correspond to the stained gel.
5
Page 9

5.2 Visualizing Proteins Using Copper Chloride
An alternative method to Coomassie R-250 is the use of the new copper chloride non fixative-negative stain (Bio-Rad Copper Stain, catalog number 161-0471). This rapid, non-precipitating staining procedure surpasses Coomassie Blue in sensitivity. Following a single 5
minute incubation in CuCl2, proteins can be quantitatively eluted from gel slices at any time.
When the gel slice is obtained, proceed with the elution. See Section 6. [Copper Chloride: A
Five Minute Protein Stain for Sodium Dodecyl Sulfate-Polyacrylamide Gels, Anal. Biochem.,
166, 308-312 (1987).]
5.3 Visualizing Proteins Using Autoradiography
In this method, radioactively labeled proteins are used as the reference. An aliquot of the
sample is labeled and equal amounts of labeled and unlabeled protein are loaded on separate
lanes of an SDS-polyacrylamide gel. Using the labeled protein lane as a reference, the unlabeled protein of interest can be identified.
1. Load the labeled and unlabeled protein next to each other on the gel. Run the gel at the
optimal conditions for the band of interest. Both lanes should be run in the middle of the
gel because the end lanes may have edge effects that distort the banding pattern. Do not
allow the dye front to run off the gel.
2. After the run, cut a corner off the gel to maintain correct orientation.
3. Cut the reference lane off and place it on filter paper. Using radioactive ink (ink with
some extra isotope in it), label the top and bottom of the filter paper with the reference lane.
Dry the reference lane. Keep the rest of the gel moist by wrapping it in plastic wrap.
4. Place the dried gel in a cassette with X-ray film and expose overnight at -70 °C. After
the film has been developed, align the film and the dried lane from the gel using the marks
made with the radioactive ink. Using a lab marker, draw a line on the dried reference lane
indicating where the band is on the autoradiogram.
5. Put the rest of the gel on a glass plate. Align the gel using the cut-off corner. Put the glass
plate with the gel on top of the dried reference lane. Line up the top and bottom of the dried
lane and the gel. Using the mark made on the dried lane, cut out the appropriate area of
the gel. The dye front can be used to identify the lanes. Cut liberally to insure that all the
protein is excised. Protein diffusion should not be a problem if the band is excised the
next day.
6. After the gel slice has been cut out, proceed with the elution as described in Section 6.
Section 6
Protein Elution
6.1 Elution from SDS-PAGE Gels
1. Soak the membrane caps in protein elution buffer for at least 1 hour at 60 °C. See Section
9 for buffer recipes. Membrane caps may be soaked for longer than 1 hour. Wear gloves
when handling the Membrane Caps to prevent the dialysis membrane from becoming
contaminated.
2. Load the gel slice into the Model 422 Electro-Eluter as described in Section 4.
3. Elute at 8-10 mA/glass tube constant current for 3 to 5 hours. Elution times may vary
depending on gel percentage and the molecular weight of the protein.
6
Page 10

4. After the elution is completed, remove the electro-eluter module from the buffer tank.
Wear gloves to prevent contamination of the dialysis membrane.
5. Take the module, with the Glass Tubes attached, to a sink. If a stopper has been used,
remove the stopper from the upper buffer chamber and allow the buffer to drain out. If
there are no stoppers, carefully pull one glass tube out of the upper buffer chamber and
allow the upper buffer to drain.
6. Using a plastic pipet, remove the buffer left in the tube to the level of the Frit and discard
the liquid. Work quickly and carefully. Do not dislodge the Silicone Adaptor or the mem-
brane cap. Make sure that the liquid below the Frit is not disturbed or shaken up during
this process.
7. Remove the Silicone Adaptor together with the membrane cap from the bottom of the
glass tube. The liquid level should be slightly above the membrane cap. Using a new
plastic pipet, pipet the remaining liquid in the membrane cap into a microfuge tube. The
volume should be approximately 400 µl. With another 200 µl of fresh elution buffer, rinse
the membrane cap. Add the rinse solution to the microfuge tube. This solution will con-
tain the eluted protein. Do this for each Glass Tube. The yield for eluted proteins is
between 80-100%.
8. The membrane cap may be reused for at least five complete runs without decreasing the
yield. Refrigerate the membrane cap in elution buffer with 0.05% sodium azide (NaN3).
It is not necessary to reheat or resoak the membrane caps after the first use.
6.2 Elution of Fixed and Stained Proteins
With the Model 422 Electro-Eluter, it is possible to elute proteins that have been fixed and
stained. If the fixing and staining process will not affect subsequent experiments, this is the
preferred method. However, yields may be decreased slightly (10-20%) due to precipitation
of proteins by methanol and/or cross-linking of the molecules by the dyes or stains. If bands
are too closely spaced to allow estimation of their location in the gel by extrapolating from a
stained reference lane (see Section 5.3), the following method should be followed to elute
fixed and stained proteins.
1. Visualize the protein by staining the gel with Coomassie R-250 as described in Section
5.1.
2. Cut out the band of interest. Elute the proteins following the protocols in Section 6.
3. The yield of fixed and stained proteins is from 70% to 90%. Some protein may remain in
the gel slice.
6.3 Concentration of Eluted Proteins Using Volatile Buffers
1. The Model 422 Electro-Eluter is assembled as described in Section 4. The proteins are
visualized using one of the methods described in Section 5.1 or 5.2.
2. The protein is eluted from the gel as described in Section 6, except a volatile elution
buffer is used. See Section 9 for recipes for the volatile buffer.
3. After the elution, the volatile buffer is lyophilized in a spin-vacuum, leaving concentrat-
ed protein. Elution yields using the volatile buffer are from 80% to 100%.
6.4 Elution of Proteins from Native Gels
Native proteins separated on non-denaturing slab gels can be eluted using the same native
gel buffer system employed during electrophoresis in the slab (e.g. Ornstein-Davis buffer).
1
7
Page 11

Due to the absence of SDS in these buffer systems, expect the time required for elution to be
approximately 3-6 hours.
6.5 Removal of SDS from Samples Subsequent to Elution
SDS can be removed from the buffer and/or the sample in several ways:
1. Dialysis: Both electrodialysis,2and equilibrium dialysis,
2,3
have been used for SDS
removal. To electrodialyse, replace the lower buffer with fresh buffer made up without
SDS approximately half way through the run.
2. Triton X-100: This may be added to the elution buffer at approximately 0.5% concentration. Triton can then be removed after the elution as per conventional methods.
4
3. AG®11 A8 ion retardation resin: Using this ion-retardation resin, an average of 83%
recovery of proteins may be expected, while 0.1 to 1.4 moles of SDS remain on each
mole of protein.
5
4. Anion exchange separation: AG1-X2 anion exchange resin6 has been employed for SDS
removal from proteins. Use of AG 1-X2 resin has resulted in 95% protein recovery with
at most only 1% of all protein molecules still binding 1 molecule of SDS.
5. Bio-Beads®SM 2 beads. These macroporous support beads are mixed with the sample for
SDS removal.
1. Ornstein, L. and Davis, B. J., Ann N.Y., Acad. Sci., 121, 321 (1964).
2. Tuszynski, G. P., and Warren, L., Anal. Biochem., 67, 55 (1975).
3. Visser, L., and Blout, E. R., Biochem., 10, 743 (1971).
4. Holloway, P. W., Anal. Biochem., 53, 304 (1973).
5. Kapp, O. H., and Vinogradov, S. N., Anal. Biochem., 91, 230 (1978).
6. Weber, K., and Kuter, D. J., J. Biol.Chem., 246, 4504 (1971).
8
Page 12

Section 7
Visualization of DNA
7.1 Visualizing DNA in Agarose Gels
1. Electrophorese the DNA in an agarose gel. Use high quality agarose to prevent agarose
contaminants from affecting subsequent manipulations.
2. Stain the gel with an ethidium bromide solution. The concentration of the stain should be
0.5 µg/ml.
Caution: Ethidium bromide is a mutagen. Use gloves when handling ethidium bromide.
3. Place the stained gel on a UV light box. Cut out the band of interest with a scalpel.
7.2 Visualizing DNA in Acrylamide Gels
Often small pieces of DNA need to be eluted. For DNA of less than 1,000 base pairs,
acrylamide gels are recommended.
1. Electrophorese the DNA in a polyacrylamide gel.
2. After electrophoresis, stain the DNA using ethidium bromide at 0.5 µg/ml in 1x TBE.
Wear gloves when using ethidium bromide; it is a mutagen.
Note: Polyacrylamide quenches the fluorescence of ethidium bromide. Therefore, at least
10 ng of DNA/band must be loaded.
3. Excise the band of interest. Follow the Model 422 Electro-Eluter assembly procedures
given in Section 4.
Section 8
DNA Elution
8.1 Elution from Gel
1. Soak the membrane caps for at least 1 hour at 60 °C in DNA elution buffer. See Section
9 for recipes. Membrane caps may be soaked for longer than 1 hour. Wear gloves when
handling the membrane caps to prevent the dialysis membrane from becoming contaminated. Use Green Membrane Caps MWCO 3500 daltons (catalog number 165-2986).
2. Assemble the Model 422 Electro-Eluter as described in Section 4.
3. The elution is done at ~10 mA/Glass Tube constant current. Elute the DNA for 15 minutes to 1 hour. The optimal elution time is dependent on the size of the DNA fragment
being eluted. Elution times greater than the optimal time tend to drive the DNA into the
dialysis membrane, making it difficult or impossible to retrieve the DNA.
4. After elution, reverse the polarity for approximately 1 minute to remove the DNA from
the dialysis membrane. Take the electro-eluter module out of the buffer tank. Take the
module, with the Glass Tubes attached, to a sink. If a stopper has been used, remove the
stopper from the upper buffer chamber and allow the buffer to drain out. If there are no
stoppers, carefully pull one Glass Tube out of the upper buffer chamber and allow the
upper buffer to drain.
5. Using a plastic pipet, remove the buffer left in the tube down to the level of the frit and
discard the liquid. Work quickly and carefully. Do not dislodge the Silicone Adaptor or
9
Page 13

the membrane cap. Make sure that the liquid below the frit is not disturbed or shaken up
during this process.
6. Remove the Silicone Adaptor together with the membrane cap from the bottom of the
Glass Tube. The buffer level should be at the top of the membrane cap. Using a clean
plastic pipet, pipet the liquid remaining in the membrane cap into a microfuge tube. The
volume should be approximately 400 µl. Rinse the membrane cap with 200 µl of fresh elution buffer. Add this liquid to the centrifuge tube which contains the eluted DNA. The volume collected will be approximately 600 µl. Do this for each Glass Tube.
7. Typical elution yields for DNA are between 70% and 90%. Some DNA will be irreversibly bound to the dialysis membrane, even if the polarity is reversed at the end of
electro-elution.
8. Membrane caps should not be reused for DNA unless the same fragment is being eluted.
Contamination of different DNAs will occur if a membrane cap is used for more than
one fragment, because the DNA from the first elution will stick to the membrane.
9. If the same fragment of DNA will be eluted later, the membrane caps can be reused. Store
the caps in DNA elution buffer with 0.05% sodium azide (NaN3) in the refrigerator. It is
not necessary to reheat or resoak them after the first use.
8.2 DNA Purification
After the DNA has been eluted and collected, it will have to be purified for further use.
1. Add 1/10 volume 3M sodium acetate (NaOAc) to the DNA sample. Add DNase free
tRNA to the eluted DNA. The concentration of nucleic acids should be approximately
10 µg/ml. The tRNA is added as a carrier to insure that all of the DNA will be ethanol precipitated.
2. Extract the DNA with an equal volume of equilibrated phenol. Vortex for at least 30 seconds. Spin in a microfuge for 2 minutes. Pull off the aqueous phase and put it in a new
microfuge tube.
3. Extract a second time with an equal volume of phenol/chloroform. The chloroform is a
24:1 mixture of chloroform and isoamyl alcohol. Vortex for at least 30 seconds. Spin in
a microfuge for 2 minutes. Pull off the aqueous phase and put it in a new microfuge tube.
4. Add 2 to 2.5 volumes of ice-cold ethanol. Place at -70 °C for one half hour, or overnight
at 20°C.
5. Spin down for 15 minutes in a cold microfuge. Decant the supernatant.
6. Resuspend in 100 µl of 10 mM Tris pH 7.6, 1 mM EDTA (TE). Repeat the ethanol precipitation. Decant the supernatant and rinse the pellet with 70% ethanol. Allow the sample to dry. Resuspend in TE.
10
Page 14

Section 9
Recipes
1. Protein Elution Buffer
Tris base 3.0 g 25 mM
Glycine 14.4 g 192 mM
SDS 1.0 g 0.1%
to 1 liter with dH20
Store at 4 °C. Warm to 37 °C before use if precipitation occurs.
2. Volatile Buffer (for protein elution and concentration)
Ammonium bicarbonate (NH4HCO3) 3.95 g 50 mM
SDS 1.0 g 0.1%
to 1 liter with dH20
Make up only 1 liter at a time because the buffer will volatilize. Store at 4° C.
3. DNA Elution Buffer (50x) Working Concentration
Tris 242.g40.mM
Glacial acetic acid 57.1 ml 20.mM
0.5 M EDTA (pH 8.0) 100.ml 1.mM
SDS 5
.g 0.1%
to 1 liter with dH20
Section 10
Troubleshooting Guide
SDS is included in elution buffer recipes to insure that the net negative charge of eluted
molecules is maintained. As a result, the eluted molecules migrate toward the anode (positive
electrode) and end up in the elution cup, SDS concentrates in the lower buffer chamber. If high
SDS concentration is undesirable, at the end of the run the lower buffer can be exchanged
for fresh elution buffer made without SDS, and the run continued for approximately 1/2 hour.
This will effectively electrodialyze SDS from the collected sample.
Proteins originally separated in native gels can be eluted without inclusion of SDS in the
elution buffer. Because elution without SDS requires an approximately 25% increase in run
times, and the volume of buffer contained in the Model 422 Electro-Eluter is small relative to
a standard vertical slab electrophoresis apparatus, the buffer may require replenishment during these extended runs (very high MW proteins especially) to avoid “buffer depletion.”
Buffer depletion is also problematic when running the eluter with more than the recom-
mended 1 cm of gel per elution tube, which may require runs exceeding 6-7 hours. Even in
the presence of SDS, extended runs (6 hours to overnight) will require at least one change of
upper and lower elution buffer. The buffering capacity of the elution buffer maintains the pH
at which molecules maintain electrophoretic mobility. Alkaline pH (8-9) is standard for most
Tris-glycine or phosphate based native electrophoresis/elution buffers.
11
Page 15

12
Page 16

Eastern Regional Office,
85A Marcus Dr., Melville, New York 11747 • Phone (516) 756-2575 • Fax (516) 756-2594
European Headquarters,
Bio-Rad Laboratories, Dreve du Sénéchal, 19, B-1180 Brussels • Phone 02 375 59 70 • Fax 02 374 61 62
Australia,
Bio-Rad Laboratories Pty Limited, Unit 11, 112-118 Talavera Rd P.O. Box 371, North Ryde, N.S.W. 2113 • Phone 02-805-5000 • Fax 02-805-1920
Austria,
Bio-Rad Laboratories Ges.m.b.H., Auhofstrasse 78D, A-1130 Wien • Phone 0222-877 89 01 • Fax 0222-876 56 29
Belgium,
Bio-Rad Laboratories S.A./N.V., Begoniastraat 5, B-9810 Nazareth Eke • Phone 091-85 55 11 • Fax 091-85 65 54
Canada,
Bio-Rad Laboratories (Canada) Ltd., 5149 Bradco Boulevard, Mississauga, Ontario L4W 2A6 • Phone (416) 624-0713 • Fax (416) 624-3019
China,
Bio-Rad Laboratories, Yanshan Hotel Office Tower, #1307, 138A Haidian Road, Beijing • Phone 2563146 • Fax 2564308
France,
Bio-Rad S.A., 94/96 rue Victor Hugo, B.P. 220, 94203 Ivry Sur Seine Cedex • Phone 01-49 60 68 34 • Fax 01-46 71 24 67
Germany,
Bio-Rad Laboratories GmbH, Heidemannstraße 164, Postfach 45 01 33, D-8000 München 45 • Phone 089-318 84-0 • Fax 089-318 84 100
Italy,
Bio-Rad Laboratories S.r.l.,Via Cellini, 18A, 20090 Segrate Milano • Phone 02-21609.1 • Fax 02-21609-399
Japan,
Nippon Bio-Rad Laboratories, K. K., Sumitomo Seimei Kachidoki Bldg 5-3-6 Kachidoki, Chuo-Ku, Tokyo 104 • Phone 03-3534-7515 • Fax 03-3534-8027
The Netherlands,
Bio-Rad Laboratories B. V., Fokkerstraat 10, 3905 KV Veenendaal • Phone 08385-40666 • Fax 08385-42216
New Zealand,
Bio-Rad Laboratories, Pty Ltd., P. O. Box 100-051, North Shore Mail Centre, Auckland 10 • Phone 09-443 3099 • Fax 09-443 3097
Pacific,
Bio-Rad Laboratories, Unit 1111, 11/F., New Kowloon Plaza, 38, Tai Kok Tsui Road, Tai Kok Tsui, Kowloon, Hong Kong • Phone 7893300 • Fax 7891257
Scandinavia,
Bio-Rad Laboratories, Kanalvägen 10C, 19461 Upplands Väsby, Sweden • Phone 46 (0) 8 590-73489 • Fax 46 (0) 8 590-71781
Spain,
Bio-Rad Laboratories, S. A. Avda Valdelaparra 3, Pol. Ind. Alcobendas, E-28100 Alcobendas, Madrid • Phone (91) 661 70 85 • Fax (91) 661 96 98
Switzerland,
Bio-Rad Laboratories AG, Kanalstrasse, 17, 8152 Glattbrugg • Phone 01-810 16 77 • Fax 01-810 19 33
United Kingdom,
Bio-Rad Laboratories Ltd., Bio-Rad House, Maylands Avenue, Hemel Hempstead, Herts HP2 7TD • Phone 0800 181134 • Fax 0442 259118
Life Science
Group
2000 Alfred Nobel Drive
Hercules, California 94547
Telephone (510) 741-1000
Fax: (510) 741-1060
Printed in USA
M1652976 Rev B
Bio-Rad
Laboratories
 Loading...
Loading...