Page 1

CLARUS® 500
Instructions for Use
Page 2

Copyright
©2019 , Carl Zeiss Meditec, Dublin, CA
Trademarks
All Zeiss products mentioned herein are either registered trademarks or trademarks of Carl Zeiss Meditec, Inc. in the United States
and/or other countries.
Windows, Windows Media, Windows Server, and Microsoft are
either registered trademarks or trademarks of Microsoft Corporation in the United States and/or other countries.
Mac OS, iMac, iPad, and QuickTime are either registered trademarks or trademarks of Apple Inc. in the United States and/or other
countries.
All other trademarks used in this document are the property of
their respective owners.
Patents
http://www.zeiss.com/meditec/en_us/imprint/patents
Page 3

Instructions for Use Table of Contents
CLARUS® 500
2660021171806 Rev. A 2019-01 3 / 192
Table of Contents
1 Safety and Certifications .............................................................................. 9
1.1 Compliance...........................................................................................................................9
1.1.1 Laser Safety and Compliance.............................................................................................................9
1.1.2 Optical Safety....................................................................................................................................9
1.1.3 RoHS Compliance..............................................................................................................................9
1.1.4 Electrical Safety .................................................................................................................................9
1.2 Symbols and Labels ............................................................................................................10
1.3 Warnings, Cautions, and Notes ..........................................................................................12
1.3.1 Definitions.......................................................................................................................................12
1.3.2 Warnings.........................................................................................................................................12
1.3.3 Cautions..........................................................................................................................................15
1.3.4 Notes ..............................................................................................................................................17
1.4 Report Serious Accidents....................................................................................................18
1.5 Essential Performance ........................................................................................................18
1.6 Electromagnetic Compatibility (EMC)..................................................................................18
1.6.1 Electromagnetic Emissions...............................................................................................................19
1.6.2 Electromagnetic Immunity...............................................................................................................19
1.7 Wireless Communications...................................................................................................20
2 Introduction ............................................................................................... 21
2.1 Scope..................................................................................................................................21
2.1.1 Intended Use / Indications for Use ...................................................................................................21
2.2 Usage .................................................................................................................................21
2.3 Intended Audience .............................................................................................................21
2.3.1 Patient Profile..................................................................................................................................21
2.3.2 Operator Profile...............................................................................................................................21
2.3.3 Doctor Profile..................................................................................................................................22
2.3.4 Administrator Profile .......................................................................................................................22
2.4 User Documentation...........................................................................................................23
2.4.1 Purpose...........................................................................................................................................23
2.4.2 Access.............................................................................................................................................23
2.4.3 Conventions Used in This Document................................................................................................23
2.5 Questions and Comments ...................................................................................................23
2.6 System Overview ................................................................................................................23
2.6.1 System Description ..........................................................................................................................23
2.6.2 Hardware Overview.........................................................................................................................25
2.6.3 Software Overview ..........................................................................................................................29
2.6.4 Networking Overview......................................................................................................................35
3 Installation ................................................................................................. 37
3.1 Hardware Installation .........................................................................................................37
3.1.1 Preparing to Install ..........................................................................................................................37
Page 4

Table of Contents Instructions for Use
CLARUS® 500
4 / 192 2660021171806 Rev. A 2019-01
3.2 Software Installation ..........................................................................................................38
3.2.1 Installing Review Software...............................................................................................................38
3.2.2 Upgrading Instrument and Review Software....................................................................................42
3.3 Installation Troubleshooting...............................................................................................43
4 Daily Startup .............................................................................................. 45
4.1 Turn on the Instrument ......................................................................................................45
4.2 Logging In ..........................................................................................................................46
5 Configuration ............................................................................................. 47
5.1 About User Roles................................................................................................................47
5.2 Opening Settings ................................................................................................................47
5.3 Setting All Options .............................................................................................................48
5.4 Managing Data...................................................................................................................49
5.4.1 Manage Patient Records..................................................................................................................49
5.4.2 Manage Users .................................................................................................................................61
5.4.3 Manage Backups.............................................................................................................................66
5.4.4 Log Files..........................................................................................................................................68
5.4.5 Data Storage ...................................................................................................................................69
5.5 Managing Connections .......................................................................................................69
5.5.1 Connecting to a Wireless Printer......................................................................................................69
5.5.2 Mapping Networked Drives.............................................................................................................70
5.5.3 Connecting to a LAN .......................................................................................................................71
5.6 Managing Licenses .............................................................................................................72
5.6.1 Activating Licenses Online ...............................................................................................................73
5.6.2 Activating Licenses Offline...............................................................................................................73
5.6.3 Returning Product Licenses..............................................................................................................75
5.6.4 Repairing Product Licenses ..............................................................................................................77
5.7 Configuring Local Settings..................................................................................................78
5.8 Configuring Report Settings ...............................................................................................79
5.9 Configuring Acquisition Settings ........................................................................................80
5.10 Clearing Alert History .........................................................................................................80
5.11 System Administration .......................................................................................................81
5.11.1 Windows Patches and Updates .......................................................................................................81
5.11.2 Data Safety......................................................................................................................................81
5.11.3 Networking .....................................................................................................................................82
5.11.4 Configuring Enhanced Security........................................................................................................83
6 About User Roles ....................................................................................... 97
7 Operation................................................................................................... 99
7.1 Safety During Operation.....................................................................................................99
7.2 Preparing the Device ..........................................................................................................99
7.2.1 Finding a Patient .............................................................................................................................99
Page 5

Instructions for Use Table of Contents
CLARUS® 500
2660021171806 Rev. A 2019-01 5 / 192
7.2.2 Selecting the Patient......................................................................................................................100
7.3 Preparing the Patient .......................................................................................................100
7.3.1 Dilating the Patient’s Eyes (Optional) .............................................................................................101
7.3.2 Lifting the Patient's Eyelid (Optional) .............................................................................................101
7.3.3 Positioning the Patient ..................................................................................................................102
7.3.4 Aligning and Focusing on the Patient's Eye....................................................................................104
7.4 Acquire Images.................................................................................................................107
7.4.1 About Acquisition Options.............................................................................................................107
7.4.2 Capturing a Widefield Image .........................................................................................................113
7.4.3 Capturing an Ultra-widefield Image (2-Image Montage) ................................................................114
7.4.4 Capturing an AutoMontage Image (4-Image Montage) .................................................................115
7.4.5 Capturing a Montage Image (2-6 Image Custom Montage) ...........................................................117
7.4.6 Capturing a Stereo Image..............................................................................................................118
7.4.7 Capturing an External Eye Image ...................................................................................................120
7.4.8 Blinking the Fixation Target ...........................................................................................................121
7.4.9 Manually Focusing the Image ........................................................................................................122
7.4.10 Manually Positioning the Internal Fixation Target ..........................................................................122
7.4.11 Using the External Fixation Target .................................................................................................123
7.4.12 Checking Scan Quality...................................................................................................................124
7.4.13 Deleting or Replacing an Image.....................................................................................................124
7.5 Analyze Images ................................................................................................................125
7.5.1 Proof.............................................................................................................................................125
7.5.2 Review ..........................................................................................................................................126
7.5.3 Opening the Analyze Window.......................................................................................................126
7.5.4 Sorting and Selecting Images.........................................................................................................126
7.5.5 Editing Scanned Images.................................................................................................................128
7.5.6 Manually Creating a Montage .......................................................................................................144
7.5.7 Viewing Stereo Images..................................................................................................................145
7.5.8 Saving Edited Images.....................................................................................................................145
7.5.9 Creating a Report ..........................................................................................................................146
7.6 Shutdown .........................................................................................................................146
7.6.1 Logging Off...................................................................................................................................147
7.6.2 Shutting Down..............................................................................................................................147
8 Cleaning and Disinfection......................................................................... 149
8.1 Safety During Cleaning .....................................................................................................149
8.2 Cleaning Agents ...............................................................................................................149
8.3 Cleaning the Front Lens....................................................................................................149
8.3.1 Removing Fluid Splashes................................................................................................................149
8.3.2 Removing Minor Dust Accumulation .............................................................................................149
8.3.3 Removing Severe Contamination ...................................................................................................150
8.4 Cleaning the Front Window Lens......................................................................................151
8.4.1 Cleaning Heavy Contamination......................................................................................................151
8.4.2 Brush Cleaning Method.................................................................................................................151
8.4.3 Wipe Cleaning Method..................................................................................................................152
8.4.4 Dust Cleaning................................................................................................................................152
8.5 Cleaning the Chinrest and Forehead Support ...................................................................153
8.6 Peripherals and Table .......................................................................................................153
Page 6

Table of Contents Instructions for Use
CLARUS® 500
6 / 192 2660021171806 Rev. A 2019-01
8.7 Cleaning the PC Screen.....................................................................................................153
9 Troubleshooting....................................................................................... 155
9.1 Safety During Troubleshooting.........................................................................................155
9.2 System Startup Errors.......................................................................................................156
9.3 Error Messages.................................................................................................................156
9.4 Information Messages ......................................................................................................158
9.5 Image Quality Troubleshooting ........................................................................................160
10 Specifications ........................................................................................... 163
10.1 Electrical Specifications ....................................................................................................163
10.2 Instrument Specifications .................................................................................................163
10.2.1 At-Instrument Computer Specifications .........................................................................................163
10.3 Dimensions and Weight....................................................................................................164
10.4 Ambient Requirements .....................................................................................................164
10.5 Fundus Camera Specifications ..........................................................................................164
10.6 Laser Classification ...........................................................................................................165
11 Legal Notices ........................................................................................... 167
12 Accessories and User Replaceable Spare Parts ......................................... 169
12.1 Parts Orders .....................................................................................................................169
12.1.1 U.S. Domestic Parts Ordering.........................................................................................................169
12.1.2 International Service Operations ....................................................................................................169
12.2 Accessory Kit Parts List (Replaceable Parts)......................................................................170
12.3 Additional Replaceable Parts............................................................................................171
12.4 Returning Defective Parts.................................................................................................172
12.4.1 Equipment Return Authorization ...................................................................................................172
12.4.2 Packing for Shipment ....................................................................................................................172
13 Decommissioning ..................................................................................... 173
13.1 Safety During Decommissioning .......................................................................................173
14 Packaging and Transport ......................................................................... 175
14.1 Safety During Transport ...................................................................................................175
14.2 Instrument Disposal..........................................................................................................175
15 Disposal ................................................................................................... 177
15.1 Packaging Disposal...........................................................................................................177
15.2 Device Disposal.................................................................................................................177
Page 7

Instructions for Use Table of Contents
CLARUS® 500
2660021171806 Rev. A 2019-01 7 / 192
Glossary ...................................................................................................179
Index ........................................................................................................ 181
Page 8

Empty page, for your notes
Page 9

Instructions for Use 1 Safety and Certifications
CLARUS® 500 1.1 Compliance
2660021171806 Rev. A 2019-01 9 / 192
1 Safety and Certifications
1.1 Compliance
CLARUS® 500 from ZEISS is compliant with ISO 10940:2009 and
ISO 15004-1:2006.
1.1.1 Laser Safety and Compliance
1.1.2 Optical Safety
CLARUS® 500 is compliant with the following optical safety
standards:
• IEC 60825-1
• ANSI Z80.36
• ISO 15004-2
• Classification: Group 1 Instrument - Per ANSI Z80.36 and ISO
15004-2
Group 1 instruments are ophthalmic instruments for which no
potential light hazard exists.
• Class 1 Laser Product – Per IEC 60825-1.
1.1.3 RoHS Compliance
The product is RoHS-compliant according to Directive 2011/65/EU.
1.1.4 Electrical Safety
Electrical Safety Class I Equipment - Protection against electrical
shock (per IEC 60601-1).
Page 10

1 Safety and Certifications Instructions for Use
CLARUS® 5001.2 Symbols and Labels
10 / 192 2660021171806 Rev. A 2019-01
1.2 Symbols and Labels
Symbol Meaning
Warning
Caution
Note
Electricity
Must follow Instructions for Use
Power On (computer)
Power Off (computer)
Protective Earth
Fuse
Direct Current
Type B Applied Parts
Manufacturer
Page 11

Instructions for Use 1 Safety and Certifications
CLARUS® 500 1.2 Symbols and Labels
2660021171806 Rev. A 2019-01 11 / 192
Symbol Meaning
Authorized European Community Representative
Serial Number
Catalog Number/Part Number
Model Number
Certification mark of CSA – Nationally Recognized
Testing Laboratory for US and Canada
Disposal of the Product within the E.U. Do not dispose
via domestic waste disposal system or communal waste
disposal facility.
Fragile
Keep Dry
This End Up
Transport conditions: humidity (10% to 95%)
Page 12

1 Safety and Certifications Instructions for Use
CLARUS® 5001.3 Warnings, Cautions, and Notes
12 / 192 2660021171806 Rev. A 2019-01
Symbol Meaning
Transport conditions: temperature (-40°C to 70°C)
Transport conditions: atmospheric pressure (500 hPa to
1060 hPa)
Caution: Federal law (or United States) restricts this
device to sale by or on the order of a licensed healthcare
practitioner
1.3 Warnings, Cautions, and Notes
1.3.1 Definitions
Warnings and cautions are defined as follows:
WARNING!
Indicates hazards that, if not avoided,
could cause severe injury or death.
u These are actions that can be taken to prevent the hazard.
CAUTION!
Indicates hazards that, if not avoided,
could cause minor or moderate injury.
u These are actions that can be taken to prevent the hazard.
1.3.2 Warnings
WARNING!
Opening the device covers
may result in injury.
u Only ZEISS authorized service technicians may remove device
covers.
WARNING!
Tipping the device
may result in injury.
u Do not allow the patient to lean on the table or use it as a
support to stand up.
Page 13

Instructions for Use 1 Safety and Certifications
CLARUS® 500 1.3 Warnings, Cautions, and Notes
2660021171806 Rev. A 2019-01 13 / 192
WARNING!
Modifying this device
may increase risks and decrease the service life of the device.
u Do not use in the presence of flammable gasses.
u Do not use in oxygen rich environments.
WARNING!
Using this device in the presence of flammable gases
may cause ignition and result in a fire hazard.
u Do not use this device in the presence of flammable anesthetics
or oxidizers such as pure oxygen or nitrous oxide.
WARNING!
Use of this equipment adjacent to or stacked with other
equipment
should be avoided because it could result in improper operation.
u If such use is necessary, this equipment and the other
equipment should be observed to verify that they are operating
normally.
WARNING!
Use of accessories, transducers, and cables other than those
specified or provided by the manufacturer of this equipment
could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper
operation.
u Use only the accessories, transducers, and cables specified or
provided by ZEISS.
WARNING!
Adding peripheral equipment or replacing parts
may result in noncompliance with the safety requirements of IEC
60601-1.
u You are responsible for ensuring that the system meets the
safety requirements of IEC 60601-1.
u Only approved equipment to their respective IEC/ISO standards
such as IEC 60601-1 or IEC 60950 are allowed to be connected
to the Signal Input/Output parts (SIP/SOPs).
u Place any AC-powered, non-medical device peripherals at least
1.5 m away from the instrument and connect them to a
separation device, unless an isolation transformer is used.
Page 14

1 Safety and Certifications Instructions for Use
CLARUS® 5001.3 Warnings, Cautions, and Notes
14 / 192 2660021171806 Rev. A 2019-01
WARNING!
Unplugging the power cord is the means of disconnecting
from the power supply.
Blocking access to the plug may result in an electrical hazard.
u Set up the instrument so that it can be unplugged easily.
WARNING!
Using parts that are not authorized by ZEISS
may compromise device safety during operation.
u Use only accessories authorized by ZEISS.
u In the U.S., call 800–341–6968. Outside the U.S., contact your
local Zeiss distributor. You can find the ZEISS contact partner
for your country on our website: www.zeiss.com.
WARNING!
Class 3B laser radiation
when open.
u Avoid exposure to beam.
WARNING!
Contact with the patient and a peripheral device
may cause an electric shock.
u Do not touch the patient and a peripheral device at the same
time.
WARNING!
Device produces visual stimuli, including flickering light and
flashing patterns, between 5 and 65 Hz
may adversely affect certain patients, although this effect is yet
unproven.
u Medical professionals need to determine whether this device
should be used for patients who may be photosensitive,
including those with epilepsy.
WARNING!
Unqualified personnel
may injure themselves or cause damage to the instrument.
u Only trained, qualified personnel may use this device.
u This device may be used only for its intended purpose.
u Only ZEISS authorized personnel may perform maintenance or
repair procedures not described in this manual.
Page 15

Instructions for Use 1 Safety and Certifications
CLARUS® 500 1.3 Warnings, Cautions, and Notes
2660021171806 Rev. A 2019-01 15 / 192
WARNING!
Use of the acquisition device, a printer, or the power table
with an extension cord or a power strip (multiple portable
socket outlet)
could cause electrical shock to the patient or operator.
u Do not use extension cords with the instrument.
u Do not use power strips with the instrument.
u Do not plug in any other equipment into the same wall outlet
as the instrument.
u To avoid the risk of electric shock, this equipment must only be
connected to a supply mains with protective earth.
1.3.3 Cautions
CAUTION!
Use of controls or adjustments or performance of procedures
other than those specified herein
may result in hazardous optical radiation exposure.
CAUTION!
Attempting to carry out activities not specifically endorsed
by ZEISS
may void your warranty and could result in damage to the
instrument.
u Read the user documentation.
u Follow directions carefully.
u Do not make upgrades, or carry out repairs or modifications,
without specific guidance and instruction from ZEISS or an
authorized ZEISS represenative.
CAUTION!
Improper cleaning
may result in inadequately disinfected equipment.
u Refer to the cleaning instructions in this Instructions for Use
and local and national disinfecting regulations.
Page 16

1 Safety and Certifications Instructions for Use
CLARUS® 5001.3 Warnings, Cautions, and Notes
16 / 192 2660021171806 Rev. A 2019-01
CAUTION!
Internet connection of the CLARUS® 500
increases its vulnerability to serious security risks, including viruses
and worms that could disable your system or adversely affect its
performance and may void the instrument warranty.
u Transfer data through internal networks.
u Ensure that all firewalls and internet security applications are
up-to-date and running.
u Connecting the device to the internet or transferring data via
USB devices may result in compromised patient privacy and
expose the network to malware.
CAUTION!
Installing or putting the device into service without regard to
EMC information provided
may void your Device warranty, result in damage to the instrument
and/or compromise safety for patients and operators.
u The CLARUS® 500 has special EMC precaution requirements
and needs to be installed and put into service according to the
EMC information provided herein.
CAUTION!
Using this instrument with patients who are light-sensitive,
including epileptics
may cause nausea or harm to the patient.
CAUTION!
Attempting to decommission your system
may result in damaged equipment and danger to personnel.
u Never attempt to decommission a ZEISS system or instrument.
Only ZEISS approved representatives are qualified to safely
decommission your system.
u Contact your ZEISS approved representatives to set up an
appointment for system or instrument decommissioning.
CAUTION!
Packaging and transport by non-ZEISS personnel
could result in damage, loss, or non-compliance within the country
of transit.
u Allow only change to Zeiss approved representative to prepare
the instrument and associated components for transport.
u Allow only ZEISS-approved personnel to transport the
instrument and associated components.
Page 17

Instructions for Use 1 Safety and Certifications
CLARUS® 500 1.3 Warnings, Cautions, and Notes
2660021171806 Rev. A 2019-01 17 / 192
CAUTION!
Do not lift or carry the instrument using the patient support.
The patient support could break if used as a carrying handle.
u Make sure you support the instrument from the base whenever
you carry the instrument.
CAUTION!
Portable RF communications equipment (including peripherals such as antenna cables and external antennas)
should be used no closer than 30 cm (12 inches) to any part of the
device, including cables specified by the manufacturer.
u Otherwise, degradation of the performance of this equipment
could result.
CAUTION!
The Zeiss Instrument is intended for use in a professional
healthcare facility environment.
Using the instrument in any other environment may void the
warranty and compromises the safety of the patient and/or
operator.
CAUTION!
Image Degradation
If image quality is degraded or image is absent from display, please
contact Zeiss customer service.
u The physician must decide whether the image is appropriate for
diagnosis
1.3.4 Notes
NOTE
Transferring data via USB devices
may result in compromised patient privacy and expose the network
to malware.
u Use access controls to ensure that only authorized personnel
use the device.
NOTE
Connecting the device to a network that includes other
equipment
may result in previously unidentified risks.
u You are responsible for analyzing and controlling these risks.
u You must reassess these risks when you make any changes to
the network, including connecting, disconnecting, or upgrading
equipment.
Page 18

1 Safety and Certifications Instructions for Use
CLARUS® 5001.4 Report Serious Accidents
18 / 192 2660021171806 Rev. A 2019-01
NOTE
Unprotected devices
may be at risk from unauthorized individuals.
u Select and set the Password strength setting in the User
management screen.
1.4 Report Serious Accidents
Report any serious incident related to this instrument, the operator,
patient, or anyone else:
• To the instrument manufacturer or distributor.
• (European Union only) To the competent authority in the state
where the instrument operator is established.
1.5 Essential Performance
The main clinical performance of the CLARUS® 500 is to capture,
display and store images to aid in the diagnosis and monitoring of
diseases and disorders occurring in the retina, ocular surface and
visible adnexa. Since there is are no surgical or treatment decisions
made solely on data obtained by the instrument, it was determined
that the CLARUS® 500 has no “essential performance” as defined
in IEC 60601-1 standard.
1.6 Electromagnetic Compatibility (EMC)
WARNING!
Installing or putting the device into service without regard to
EMC information provided
may void your ZEISS instrument warranty, result in damage to the
instrument and/or compromise safety for patients and operators.
u This instrument has special EMC precaution requirements and
needs to be installed and put into service according to the EMC
information provided herein.
NOTE
The emissions characteristics of this equipment
make it suitable for use in industrial areas and hospitals (CISPR 11
Class A).
u If it is used in a residential environment, this equipment might
not offer adequate protection to radio-frequency communication services.
u The user might need to take mitigation measures, such as
relocating or re-orienting the equipment.
Electromagnetic Emissions [}19] and Electromagnetic Immunity
[}19] are required per IEC 60601-1-2:2014.
Page 19

Instructions for Use 1 Safety and Certifications
CLARUS® 500 1.6 Electromagnetic Compatibility (EMC)
2660021171806 Rev. A 2019-01 19 / 192
1.6.1 Electromagnetic Emissions
The Zeiss Instrument complies with the following emission requirements:
Phenomenon Standard
Conducted and radiated RF
emissions
Group 1
CISPR 11 Class A
Harmonic distortion IEC 61000-3-2
Voltage fluctuations and flicker IEC 61000-3-3 Class A
Table1: Electromagnetic Emissions
1.6.2 Electromagnetic Immunity
The CLARUS® 500 complies with the following immunity requirements:
Phenomenon Basic EMC standard or test
method
Immunity test levels
Electrostatic Discharge IEC 61000-4-2 ± 8 kV contact
± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air
Radiated RF EM fields IEC 61000-4-3 3 V/m
80 MHz – 2,7 GHz
80 % AM at 1 kHz
Proximity fields from RF wireless
communications equipment
IEC 61000-4-3 See Wireless Communications
[}20]
Rated power frequency magnetic
fields
IEC 61000-4-8 30 A/m
50 Hz or 60 Hz
Electrical fast transients / bursts IEC 61000-4-4 ± 2 kV 100 kHz repetition
frequency
Surges line-to-line IEC 61000-4-5 ± 0,5 kV, ± 1 kV
Surges line-to-ground IEC 61000-4-5 ± 0,5 kV,± 1 kV, ± 2 kV
Conducted disturbances induced
by RF fields
IEC 61000-4-6 3 V
0,15 MHz – 80 MHz
6 V in ISM bands
between 0,15 MHz and 80 MHz
80 % AM at 1 kHz
Voltage dips, short interruptions,
and voltage variations on power
supply input lines
IEC 61000-4-11 0 % UT1; 0,5 cycle
At 0°, 45°, 90°, 135°, 180°, 225°,
270° and 315°
0 % UT1; 1 cycle
and
70 % UT1; 25/30 cycles
Single phase: at 0°
Voltage Interruptions IEC 61000-4-11 0 % UT1; 250/300 cycle
Table2: Electomagnetic Immunity
Page 20

1 Safety and Certifications Instructions for Use
CLARUS® 5001.7 Wireless Communications
20 / 192 2660021171806 Rev. A 2019-01
1
UT is the a.c. mains voltage prior to application of the test level.
1.7 Wireless Communications
Test
Frequency
(MHz)
Band (MHz) Service Modulation Maximum
Power (W)
Distance (m) Immunity
Test Level (V/
m)
385 380 - 390 TERTRA 400 Pulse 18 Hz 1.8 0.3 27
450 430 - 470 GMRS 460,
FRS 460
FM ± 5 kHz
deviation
1 kHz sine
2 0.3 28
710
745
780
704 - 787 LTE Band 13,17Pulse 217 Hz 0.2 0.3 9
810
870
930
800 - 960 GSM
800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse 18 Hz 2 0.3 28
1720
1845
1970
1700 - 1990 GSM 1800;
CDMA 1900;
GSM 1900;
DECT; LTE
Band
1,3,4,25;
UMTS
Pulse 217 Hz 2 0.3 28
2450 2400 - 2570 Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse 217 Hz 2 0.3 28
5240
5500
5785
5100 - 5800 WLAN
802.11 a/n
Pulse 217 Hz 0.2 0.3 9
Table3: Test specifications for enclosure port immunity to RF wireless communications equipment.
Page 21

Instructions for Use 2 Introduction
CLARUS® 500 2.1 Scope
2660021171806 Rev. A 2019-01 21 / 192
2 Introduction
2.1 Scope
2.1.1 Intended Use / Indications for Use
The CLARUS® 500 ophthalmic camera is indicated to capture,
display, annotate and store images to aid in the diagnosis and
monitoring of diseases and disorders occurring in the retina, ocular
surface and visible adnexa. It provides true color and autofluorescence imaging modes for stereo, widefield, ultra-widefield, and
montage fields of view.
2.2 Usage
The CLARUS® 500 is designed for continuous use in a professional
healthcare facility environment.
The mode of operation for the table is 2 minutes on and 20
minutes off.
2.3 Intended Audience
CLARUS® 500 users have a role, which is assigned when an
Administrator creates their username and password. These roles
are:
• Operator
• Doctor
• Administrator
For more information, refer to: About User Roles [}62].
2.3.1 Patient Profile
This device may be used on any patient who is able to sit upright
with his or her face in the instrument's chin and forehead rest,
independently or with assistance.
2.3.2 Operator Profile
2.3.2.1 Intended Demographic
This device is intended for use by the following trained professionals:
• Opticians
• Ophthalmic Photographers
• Optometrists
• Ophthalmologists
Page 22

2 Introduction Instructions for Use
CLARUS® 5002.3 Intended Audience
22 / 192 2660021171806 Rev. A 2019-01
• Medical Assistants
• Clinical Researchers
2.3.2.2 Required Skills
Operators are expected to have the following skills:
• Knowledge of how to capture fundus images
• Experience managing patients
• Experience with the Microsoft Windows operating system and
applications based on it
2.3.3 Doctor Profile
2.3.3.1 Intended Demographic
Doctors are ophthalmologisst, optometrists, or other heath care
professionals with training in interpretation of fundus images.
2.3.3.2 Required Skills
Doctors are expected to know how to evaluate fundus images.
2.3.4 Administrator Profile
2.3.4.1 Intended Demographic
Administrators maintain the facility's computers, instruments, and
networks.
2.3.4.2 Required Skills
Administrators are expected to know:
• Windows administration principles
• Networking principles
• Data management principles
• Permissions and user assignment principles
• The site or facility systems, including:
– Network(s)
– Computers and connections
– Medical record system connections
– Instrument connections
Page 23

Instructions for Use 2 Introduction
CLARUS® 500 2.4 User Documentation
2660021171806 Rev. A 2019-01 23 / 192
2.4 User Documentation
2.4.1 Purpose
The user documentation that comes with your device is provided to
ensure that all users operate and maintain it safely and successfully.
• Read all user documentation before starting and using the
device
• Keep all user documentation where it is accessible at all times
for all users
• Pass the user documentation on to the next owner of the
device
2.4.2 Access
User documentation for your device is provided on the USB drive
that came with the device as part of the Instrument Accessory Kit.
2.4.3 Conventions Used in This Document
Certain types of information are specially marked in this document
for better recognition.
• This is a list.
– This is a second level list.
This is a cross-reference: Questions and comments [}23].
This is software code or program text.
The name of software windows are Capitalized. For example:
"Patient Screen"
Names of menus, and buttons or other selectable items, are shown
in Bold.
• View menu.
• File > Save as
• My documents > Documents
2.5 Questions and Comments
If you have questions or comments about this user documentation
or the device, contact your ZEISS representative.
2.6 System Overview
2.6.1 System Description
CLARUS® 500 is a non-contact, high-resolution imaging device for
in vivo imaging of the human eye. Imaging modes include:
Page 24

2 Introduction Instructions for Use
CLARUS® 5002.6 System Overview
24 / 192 2660021171806 Rev. A 2019-01
• True color reflectance imaging
• Fundus autofluorescence with green or blue excitation (FAF-G
and FAF-B)
• Stereo imaging
• External eye imaging
2.6.1.1 Principles of Operation
Using a monochromatic camera, a broad line of illumination scans
across the retina.
Red, green and blue Light Emitting Diodes (LEDs) sequentially
illuminate to generate true color images. The broad bandwidth of
LEDs gives a true color rendering. Blue and green LED illumination
enables Fundus AutoFlourescence (FAF) imaging.
By illuminating only a narrow strip of the retina at a time, the
illumination stays out of the viewing path, keeping haze and
fluorescence of the anterior segment out of the retinal image and
allowing a clear view of much more of the retina than the annularring illumination used in traditional fundus cameras. The design
allows a single exposure to image an area of the retina previously
covered by the 7-fields in the Early Rreatment of Diabetic
Retinopathy Study (ETDRS).
2.6.1.2 Key Features
Scan Type Options:
• True color
• Fundus autofluorescence with green excitation
• Fundus autofluorescence with blue excitation
• Stereo imaging
• External eye imaging
Field of View Options:
• Widefield: A single image with a 133° FOV when measured
from the center of the eye.
(90° when measured as described in ISO 10940).
• Ultra-widefield: Two images stitched together into a montage
with a 200° FOV when measured from the center of the eye.
(135° when measured as described in ISO 10940).
• AutoMontage: a 4-scan montage using preset fixation targets.
• Montage: 2-6 widefield images stitched into a single,
combined retina image.
Page 25

Instructions for Use 2 Introduction
CLARUS® 500 2.6 System Overview
2660021171806 Rev. A 2019-01 25 / 192
2.6.2 Hardware Overview
The CLARUS® 500 is connected to the All-in-One PC using cables
mounted under the table. The operator controls the instrument and
software using the instrument joystick, the All-in-One PC touchscreen, wireless keyboard and touchpad.
2.6.2.1 System Hardware
1
2
3
4
5
6
Figure1: System Hardware
1 CLARUS® 500 Acquisition
Head
2 All-in-One PC
3 Keyboard Tray 4 Touchpad
5 Keyboard 6 Joystick
Page 26

2 Introduction Instructions for Use
CLARUS® 5002.6 System Overview
26 / 192 2660021171806 Rev. A 2019-01
2.6.2.1.1 USB Locations
1
2
Figure2: USB Port Locations (6 total)
Number Type Quantity Location
1 USB 2.0 2 Side
2 USB 3.0 4 Bottom
Page 27

Instructions for Use 2 Introduction
CLARUS® 500 2.6 System Overview
2660021171806 Rev. A 2019-01 27 / 192
2.6.2.2 Patient View
1
2
3
4
Figure3: Patient View
1 Forehead rest Helps keep the patient's head steady and in
place
2 Front lens Aperture for image acquisition
3 Chin rest Positions the patient's head at the correct
height
4 Chin rest
adjuster
Manually raises and lowers the chin rest
Page 28

2 Introduction Instructions for Use
CLARUS® 5002.6 System Overview
28 / 192 2660021171806 Rev. A 2019-01
2.6.2.3 Operator Controls
3
4
1
2
Figure4: Operator Controls
1 Focus knob Manually adjusts the focus
2 Friction knob Tightens or loosens the swivel movement of the
acquisition head
3 Joystick Moves the acquisition head side-to-side and
forward and back
(The joystick button triggers image capture)
4 Cross-table lock Locks the lateral movement of the acquisition
head
Page 29

Instructions for Use 2 Introduction
CLARUS® 500 2.6 System Overview
2660021171806 Rev. A 2019-01 29 / 192
2.6.2.4 External Fixation Target
2
1
3
Figure5: Installing the External Fixation Target
1 External Fixation
Target
Used when patient cannot see the internal
fixation target
2 External Fixation
Target Mount
Mount for the external fixation target
3 Patient Support Support for the headrest and chinrest
2.6.3 Software Overview
The instrument software includes three distinct areas: Patient,
Acquire, and Analyze windows. All windows use the same top
toolbar.
2.6.3.1 Main Toolbar
1
2
3
4
5 6
Figure6: Main Toolbar
Pos. Symbol Explanation
1 Indicates your current screen:
• Patient
• Patient > Acquire
• Patient > Analyze
2 Quickly hides the screen for patient privacy
Page 30

2 Introduction Instructions for Use
CLARUS® 5002.6 System Overview
30 / 192 2660021171806 Rev. A 2019-01
Pos. Symbol Explanation
3 Opens notification volume control
4 Opens the Settings to configure the instrument and set your
preferences
5 Minimizes the application on the computer's desktop
6 Allows you to log off or shut down
2.6.3.2 Patient Window
Use the Patient window to find, edit, and add patient records.
1
2
3
4
56789
Figure7: Patient Window
Pos. Name Explanation
1 Search field Searches for a patient record by patient name, ID, or date of birth
2 Advanced Opens the Advanced search, which allows a more detailed search
3 Add Allows you to create a new patient record
4 Patient data Displays data about the selected patient
5 Acquire Opens the Acquire window allowing you to acquire new scans for
the selected patient
Page 31

Instructions for Use 2 Introduction
CLARUS® 500 2.6 System Overview
2660021171806 Rev. A 2019-01 31 / 192
Pos. Name Explanation
6 Analyze Opens the Analyze window allowing you to analyze scans and
prepare reports
7 History Lists the selected patient's scans from prior visits
8 All Lists patient records available on the local database
9 Today Lists patient records for the patients scheduled today
2.6.3.3 Acquire Window
Use the Acquire window to set image capture options and capture
images.
1
2
3
4
5 6
78910
11
121314
Figure8: Acquire Window
Pos. Name Explanation
1 Patient data Information about the currently selected patient
2 Laterality Indicates which eye is being captured
3 Alignment preview A visual aid for aligning the instrument to the patient's pupil
4 Live preview Displays a real-time preview image prior to capture and a quality
check image following capture
5 Thumbnail filters Filters the capture bin by laterality or scan type
Page 32

2 Introduction Instructions for Use
CLARUS® 5002.6 System Overview
32 / 192 2660021171806 Rev. A 2019-01
Pos. Name Explanation
6 Capture bin Displays thumbnails of images that have already been captured, along
with the capture settings
7 Analyze Opens the Analyze window
8 Focus Displays the current focus and allows for manual focus adjustments.
Auto determines the best focus setting automatically.
9 Blink internal fixation
target
Blinks the internal fixation target for a configurable amount of time
10 Capture Captures the image
11 Scan Type Sets the type of scan
12 Pupil Size Toggles between mydriatic and non-mydriatic acquisition modes
13 Fixation Type Toggles between internal fixation and external fixation targets
14 Patients Returns to the Patients window
2.6.3.4 Analyze Window - Proof
The Analyze window has two screens:
• Proof to view and select images
• Review to compare and edit images
Page 33

Instructions for Use 2 Introduction
CLARUS® 500 2.6 System Overview
2660021171806 Rev. A 2019-01 33 / 192
1
2
3
4
5 6 7 8
9101112
Figure9: Analyze Window - Proof
Pos. Name Explanation
1 Patient data Displays information about the currently selected patient
2 Favorite Flags selected thumbnails with a yellow start for easy identifiication
3 Laterality filter Allows you to filter the thumbnails to show one or both eyes
4 Date filters Allows you to filter the thumbnails to show only those taken between
the start date and end date you set
5 Scan type filter Allows you to select which type(s) of scans to show
6 View Allows you to select what size to display the thumbnails in preview
7 Sort Allows you to group the thumbnails by scan type or laterality or show
thumbnails in the order they were taken (Date/Time)
8 Clear Remove all thumbnails from the Selection bin
9 Review Opens the Review screen for viewing and editing the images in the
Selection bin
10 Selection bin Displays thumbnails of selected images
11 Preview Displays available thumbnails as filtered
12 Patients Returns to the Patient window
Page 34
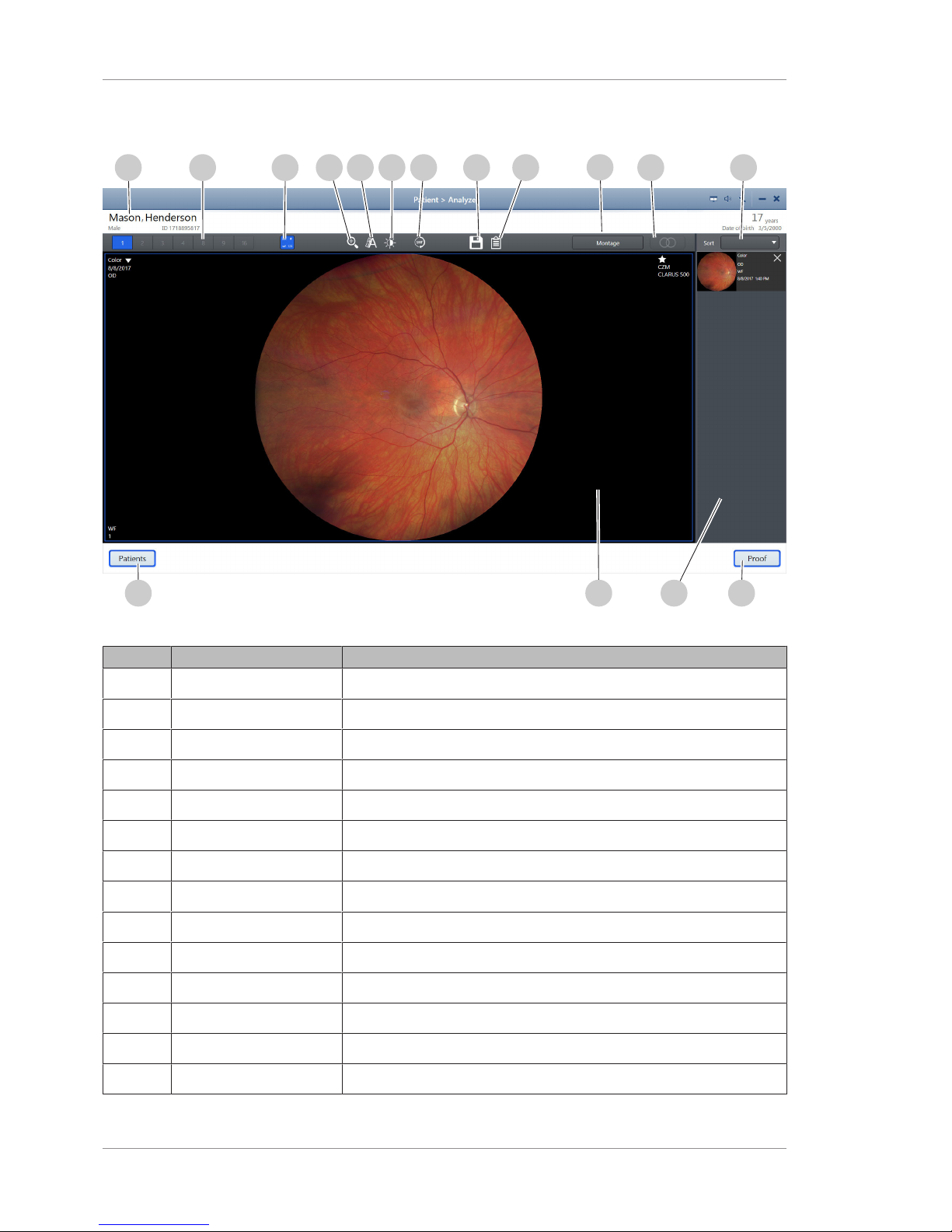
2 Introduction Instructions for Use
CLARUS® 5002.6 System Overview
34 / 192 2660021171806 Rev. A 2019-01
2.6.3.5 Analyze Window - Review
1
2
4
5
6 7 8 9
10
11 12
13
141516
3
Figure10: Analyze Window - Review
Pos. Name Explanation
1 Patient data Provides information about the selected patient
2 Number of viewports Controls how many viewports are in the main display area
3 Show / Hide metadata Shows or hides the image information in the corners
4 Zoom Opens the zoom slider
5 Annotate Opens the annotation tool panel
6 Adjust image Opens the brightness, contrast, saturation, and sharpness sliders
7 Rotate Rotates the image 180°
8 Save Saves the edited image
9 Print Report Opens a preview of the report to print
10 Montage Allows you to select and create a montage
11 Stereo Displays linked, side-by-side view of stereo image pairs
12 Sort Selection Allows you to select the way to sort the selected scans
13 Proof Returns to the Proofsheet
14 Selection bin Displays thumbnails of the selected images
Page 35

Instructions for Use 2 Introduction
CLARUS® 500 2.6 System Overview
2660021171806 Rev. A 2019-01 35 / 192
Pos. Name Explanation
15 Viewport Allows you to see the selected images.
16 Patients Returns to the Patient window
2.6.4 Networking Overview
CLARUS® 500 can run as a standalone instrument storing patient
and exam data locally, or it can be connected to a network server
or EMR system to share data and worklists in a central location.
WARNING!
Adding peripheral equipment
may result in noncompliance with the safety requirements of IEC
60601-1.
u You are responsible for ensuring that the system meets the
safety requirements of IEC60601-1.
u Place any AC-powered, non-medical device peripherals at least
1.5 m away from the device and connect them to a separation
device, or else use an isolation transformer.
WARNING!
Connection to It-Networks including other equipment
could result in previously unidentified risks related to patients,
operators or third parties.
Changes to the IT networks could introduce new risks that require
additional analysis.
Changes to the IT networks include:
u Changes to network configurations
u Connections of additional items
u Disconnection of items
u Update of equipment
u Upgrade of equipment
ð The Responsible Organization should identify, analyze, evaluate
and control these risks.
Page 36

Empty page, for your notes
Page 37

Instructions for Use 3 Installation
CLARUS® 500 3.1 Hardware Installation
2660021171806 Rev. A 2019-01 37 / 192
3 Installation
3.1 Hardware Installation
Your instrument will be installed by qualified ZEISS representatives.
Do not attempt to install the instrument yourself.
WARNING!
Unauthorized Installation
Unauthorized installation could lead to the injury of patients and
operators, as well as to property damage.
u Only ZEISS authorized personnel may install Zeiss products.
WARNING!
Powering peripherals directly through a wall socket
could result in electrical shock to the patient and/or examiner.
u When using a printer in the USB configuration, always power
the printer through an isolation transformer. Some ZEISS
equipment comes with an isolation transformer that may be
used by plugging into a special power strip provided with the
equipment. Talk to your ZEISS Service Representative to
determine if this is true for your equipment.
u If you are not sure, plug all peripherals (such as a printer), into
an isolation transformer. This requires a special power cable. In
North America, the required cable has an IEC–320–14
connector on one end and a NEMA S–15R connector on the
other end. This cable is included in the accessory kit shipped
with the instrument.
CAUTION!
Do not lift or carry the instrument using the patient support.
The patient support could break if used as a carrying handle.
u Make sure you support the instrument from the base whenever
you carry the instrument.
3.1.1 Preparing to Install
Install the CLARUS® 500 instrument in an environment that meets
the following requirements:
• no direct sunlight
• properly grounded, dedicated 15 A power source that meets all
local electrical codes
• not connected to a power strip
• the device's ventilation openings are not blocked
• the device is not exposed to water or other liquids
Do not modify the instrument or use cables not provided by ZEISS.
Page 38

3 Installation Instructions for Use
CLARUS® 5003.2 Software Installation
38 / 192 2660021171806 Rev. A 2019-01
The CLARUS® 500 instrument arrives on a pallet with three boxes
that contain all parts and accessories needed to assemble the
instrument and table.
3.2 Software Installation
The CLARUS® 500 system ships with software installed on your
instrument. From time to time you will be notified to upgrade
system software (Upgrading Instrument and Review Software
[}42]).
You can install review software on any compatible laptop or
computer that clinicians use to review scanned images (Installing
Review Software [}38]).
3.2.1 Installing Review Software
You can install review software on computers that clinicians use to
compare, analyze, annotate and save scans acquired on CLARUS®
500.
NOTE
If you need to uninstall review software, use the Windows
control panel for adding and removing programs.
The computer must meet the following minimum requirements:
• Runs on:
– Windows 7
– Windows 8
– Windows 10
1
– Windows 2008 Server R2
– Windows 2012 Server R2
• Has an Intel processor with a Passmark benchmark score of at
least 5675
• Has at least 8 GB of memory
• Has a monitor with resolution set to at least 1024 x 768
NOTE
Review software does not support: adding, editing, or
deleting a patient record.
Adding, editing, or deleting a patient record
Deleting a scan
Adding users or user accounts
Adding, editing, or deleting the Institution data
Page 39

Instructions for Use 3 Installation
CLARUS® 500 3.2 Software Installation
2660021171806 Rev. A 2019-01 39 / 192
NOTE
When installing review software on a review station running
Windows 10, make sure you set the resolution to 1280 x
1024 before opening the review software.
If you do not set the screen resolution properly, the screens can
appear distorted.
To install review software:
þ The computer and monitor meet the minimum requirements
listed above.
1. Insert the software media into the review station computer's
USB drive.
2. In the CLARUS® 500 software folder, double-click on
setup.exe.
ð A confirmation opens.
3. Click Next.
4. Accept the license agreement, then click Next.
5. Type the user name and organization name and click Next.
6. Select Review Software, then click Next.
Page 40

3 Installation Instructions for Use
CLARUS® 5003.2 Software Installation
40 / 192 2660021171806 Rev. A 2019-01
7. If you want to choose a different destination folder for the
review software, click Change... and browse to the desired
folder.
8. Click Next.
9. If this review software will use Remote Destop Services, check
Remote Desktop services will be used.
Page 41

Instructions for Use 3 Installation
CLARUS® 500 3.2 Software Installation
2660021171806 Rev. A 2019-01 41 / 192
10. Click Next.
11. Click Install.
12. If your system prompts you to confirm changes on the
computer, click Yes.
ð Review software installation begins. Installation takes
several mintues to complete.
13. When installation is complete, click Finish.
Page 42
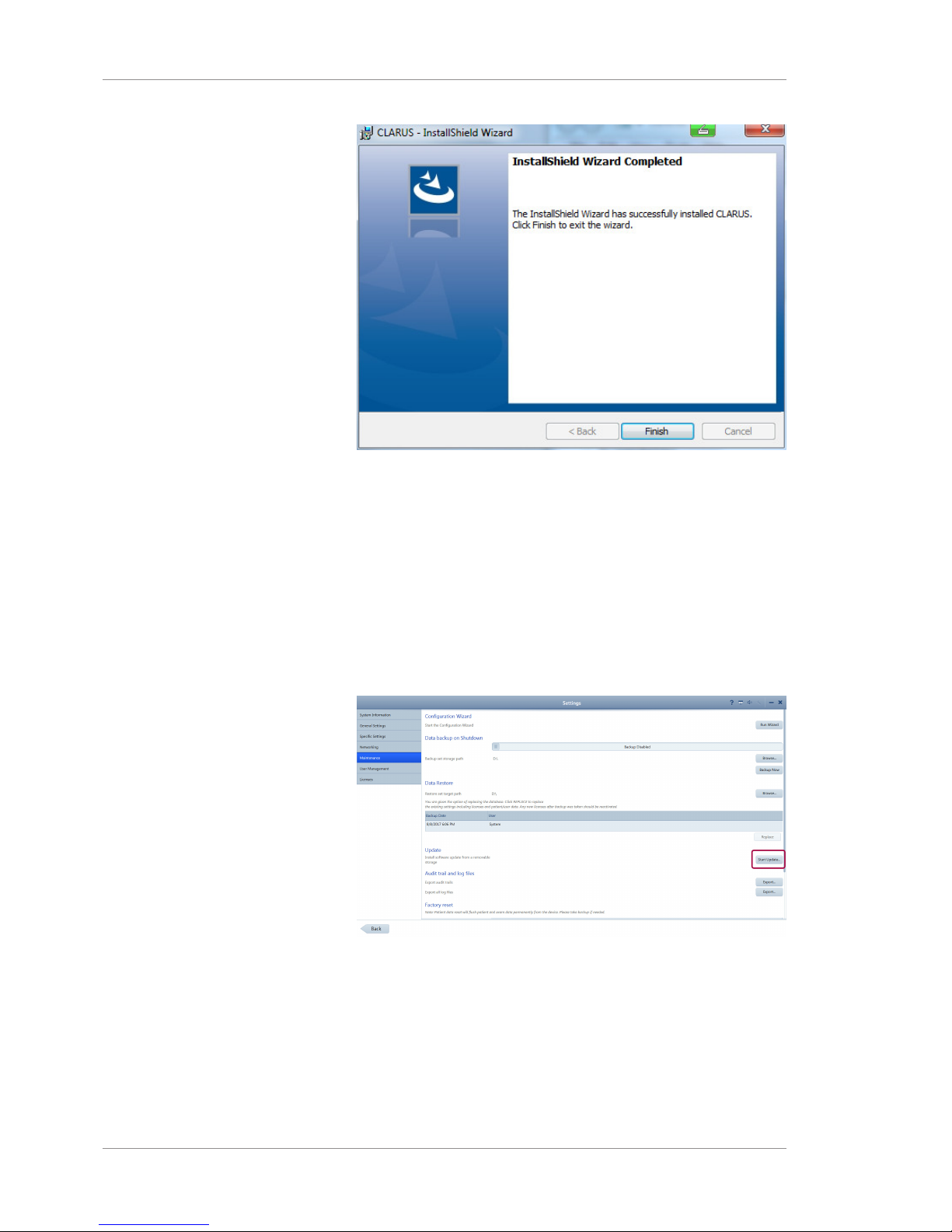
3 Installation Instructions for Use
CLARUS® 5003.2 Software Installation
42 / 192 2660021171806 Rev. A 2019-01
ð Installation completes and the configuration wizard opens.
14. If the review station is running Windows 10, set the screen
resolution to 1280 x 1024.
For more information about the configuration wizard, refer to:
Setting All Options [}48].
3.2.2 Upgrading Instrument and Review Software
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select the Maintenance tab.
2. Under Update, click Start Update....
ð A confirmation opens.
3. Click Yes.
Result ü The software installs and the system reboots.
When you upgrade instrument or review software,CLARUS® 500
retains stored information including:
Page 43

Instructions for Use 3 Installation
CLARUS® 500 3.3 Installation Troubleshooting
2660021171806 Rev. A 2019-01 43 / 192
• Logins and Passwords
• Patient Data
• Exam Data
3.3 Installation Troubleshooting
Fault Cause Rectification Qualification
Unexpected software
shutdown when
clicking on the
patient's name.
Antivirus software
may falsely
identify CLARUS®
500 installation
files as a potential
threat and cause
the CLARUS® 500
Review Software
installation to fail.
u Tempo-
rarily
suspend
onaccess
virus
scannin
g during
the
installation of
CLARUS
® 500
Review
Softwar
e.
Service
Technician
Review software does
not launch properly.
Antivirus software
may falsely
identify and
quarantine
binaries required
for the CLARUS
Review Software.
If required files
have been
quarantined, the
CLARUS® 500
Review application will fail to
launch properly.
Configure
Antivirus
software to
exclude "C:
\Program
Files\CZM
\CLARUS"
and "C:
\ProgramDat
a\Carl Zeiss
Meditec
\Review"
folders from
on-access
and ondemand
scanning.
Service
Technician
Table4: Review Software Installation Troubleshooting
Page 44

Empty page, for your notes
Page 45
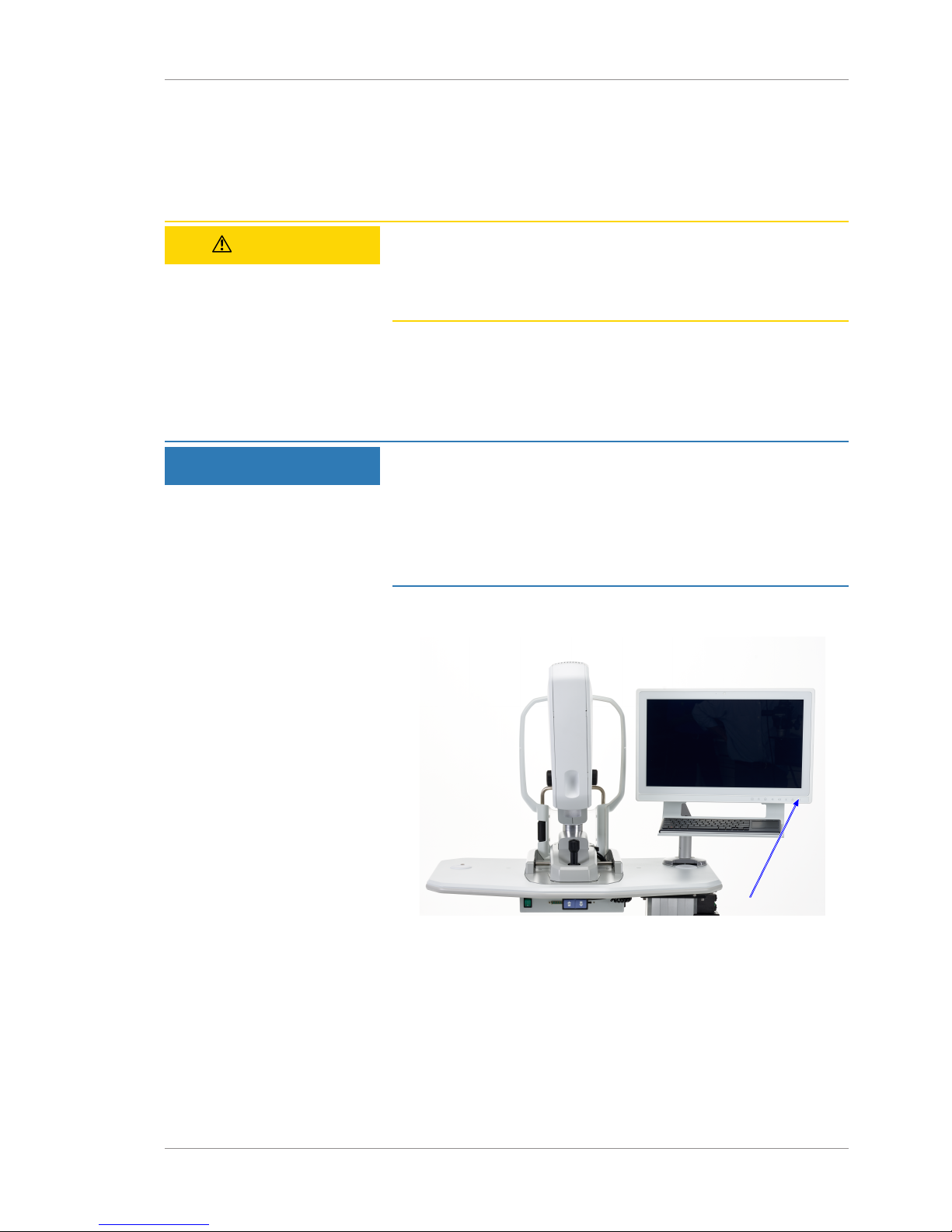
Instructions for Use 4 Daily Startup
CLARUS® 500 4.1 Turn on the Instrument
2660021171806 Rev. A 2019-01 45 / 192
4 Daily Startup
4.1 Turn on the Instrument
CAUTION!
Do not unplug the instrument during startup
or instrument may not start up successfully.
u Make sure the instrument has completed its system checks
before you unplug or remove power.
Each time you turn on the CLARUS® 500, system diagnostics
checks that the system initialized correctly and is running properly.
If the diagnostic check detects any problems, an error or alert
message displays. For more information about startup errors and
alerts, refer to: Troubleshooting [}155].
NOTE
If a critical system test fails
take the device out of service.
u Contact your ZEISS representative.
u If a non-critical test fails or a warning appears, the device can
still be used. Contact your ZEISS representative. (See Maintenance.)
To turn on the instrument:
Action 1. Turn on the instrument.
2. When the system test sequence completes, click Continue.
3. Log in (Logging In [}46]).
ð The Patient window displays.
Page 46
4 Daily Startup Instructions for Use
CLARUS® 5004.2 Logging In
46 / 192 2660021171806 Rev. A 2019-01
4.2 Logging In
After the system boots up and runs a series of system checks, the
login screen opens. The login screen also appears when a user logs
out.
NOTE
Passwords are case-sensitive.
An Administrator must add a user's name and password
before that user can log in. (Adding a New User [}62])
To log in:
Action
1. Double-click on the CLARUS® 500 application icon.
ð The login screen opens.
2. Select your user name.
3. Type your password.
Page 47
Instructions for Use 5 Configuration
CLARUS® 500 5.1 About User Roles
2660021171806 Rev. A 2019-01 47 / 192
5 Configuration
5.1 About User Roles
Not all users have access to all features. The table below list some
of the key differences for different types of users.
Operator Doctor Adminis-
trator
Acquire scans X X
Review scans X X
Select, edit and annotate scans X X
Use review software installed on a separate computer X X
Reset your own password X X X
Delete a local patient record X X X
Save and print reports X X X
Import and export data X X X
Configure import and export settings X X X
Configure general settings X X
Configure network settings X
Configure data backup X
Restore data from a backup X
Perform system maintenance X
Manage licenses X
Reset other user's passwords X
Add or delete users X
Export log files X
Table5: Permissions Levels
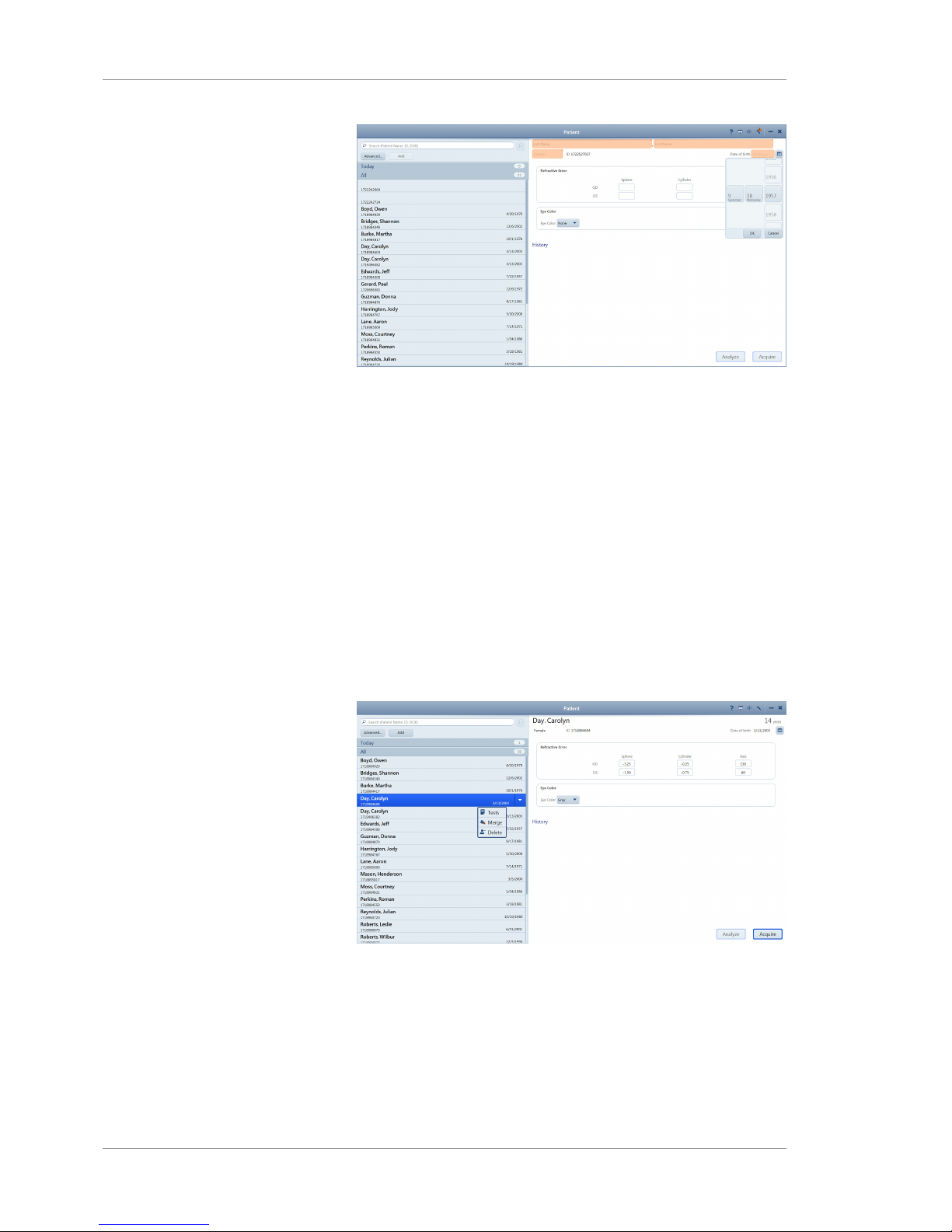
5.2 Opening Settings
The Settings icon is on the top toolbar of all application windows.
You can open settings from any screen in the application.
Setting options allows you to configure the application the way you
want to use it. Different types of users can set different options. For
more information about settings permissions, refer to: About User
Roles [}62].
Page 48
5 Configuration Instructions for Use
CLARUS® 5005.3 Setting All Options
48 / 192 2660021171806 Rev. A 2019-01
Setting Description
System Information Instrument and software information
General Settings • Your institution information
• Duration for displaying alerts
• Local date, time, and language
• Teleservice
• On-screen keyboard
• Patient ID creation
• Printer and reports
Specific Settings Acquisition settings
• Capture preview duration
• Fixation target blink duration
• Scan type presets
Import and Export Settings (DICOM and FORUM)
Networking Network connection settings
Maintenance • Record backup and restore
• Application update
• Log files
Factory reset
User Management • Create, edit, and delete users
• Change password
Licenses • View, activate, repair, and return licensed
features
Table6: Settings
To open settings:
Action 1. Click on the Settings icon on the toolbar.
Result ü The Settings window opens.
5.3 Setting All Options
CLARUS® 500 has an initial settings wizrds that you can also use
for checking or resetting options.
NOTE
Only Administrators can complete this task.
To set all options using the wizard:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select Maintenance and click Run Wizard.
Page 49

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 49 / 192
ð The initial settings wizard opens and steps you through
settings.
5.4 Managing Data
5.4.1 Manage Patient Records
When patient records are imported from an EMR, they are reconciled with local records as follows:
• Same ID and Issuer of ID, different name, date of birth, or
gender: The local record is updated to match the EMR record.
• Different ID or Issuer of ID: A new patient record is generated.
Some EMR systems allow records that are missing key identifying
information. The CLARUS® 500 automatically adds the default
"Unknown" if the name is missing, but rejects records that do not
have a Patient ID or date of birth.
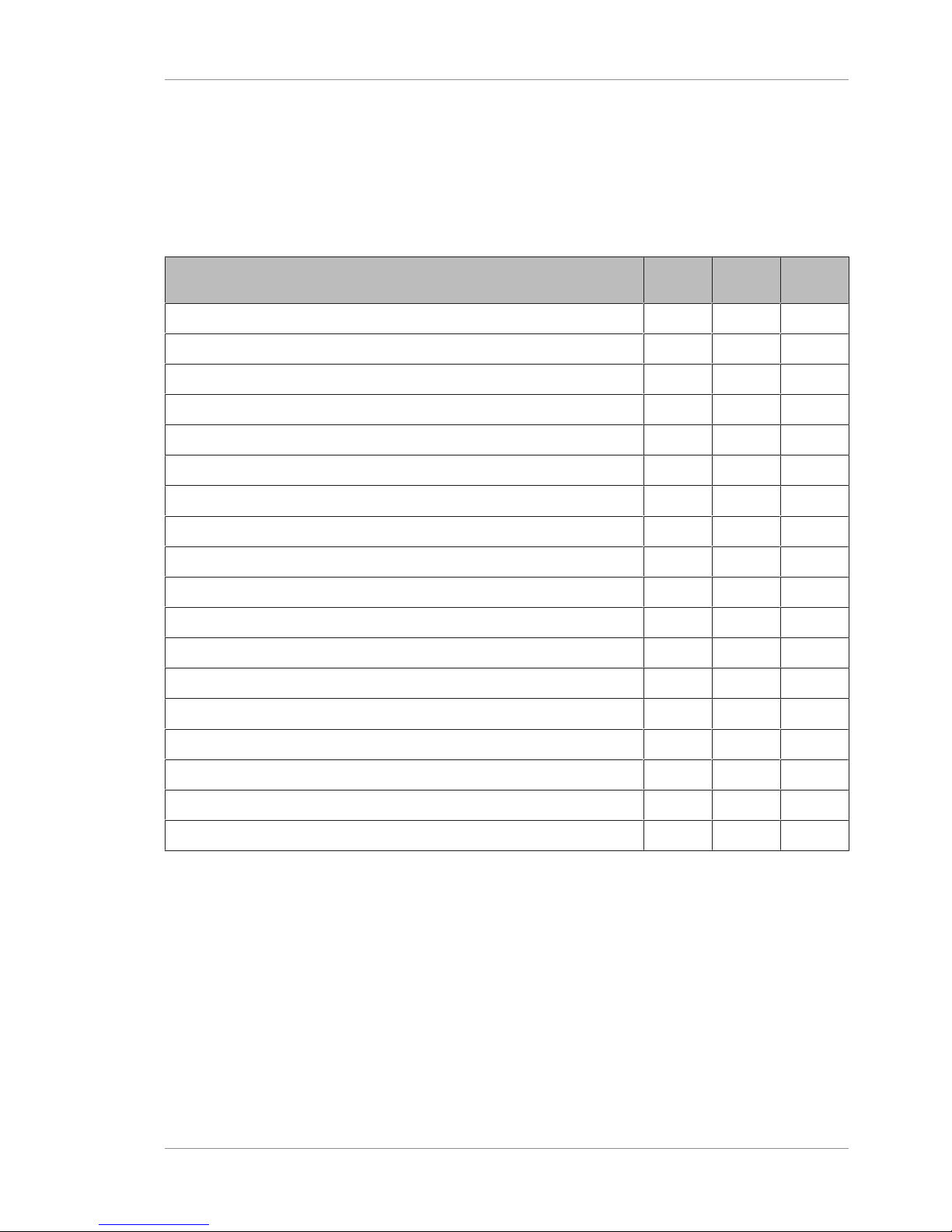
5.4.1.1 Adding a Patient
Adding the patient's name and date of birth are both required.
To add a patient:
Prerequisite þ The Patient window is open.
Action 1. Click Add.
Page 50
5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
50 / 192 2660021171806 Rev. A 2019-01
2. Type the patient's first and last name.
3. Select the patient's gender.
4. Select the patient's date of birth.
5. (Optional) Type the patient's Refractive Error information.
6. (Optional) Select the patient's Eye Color (optional).
7. To acquire a scan, click Acquire.
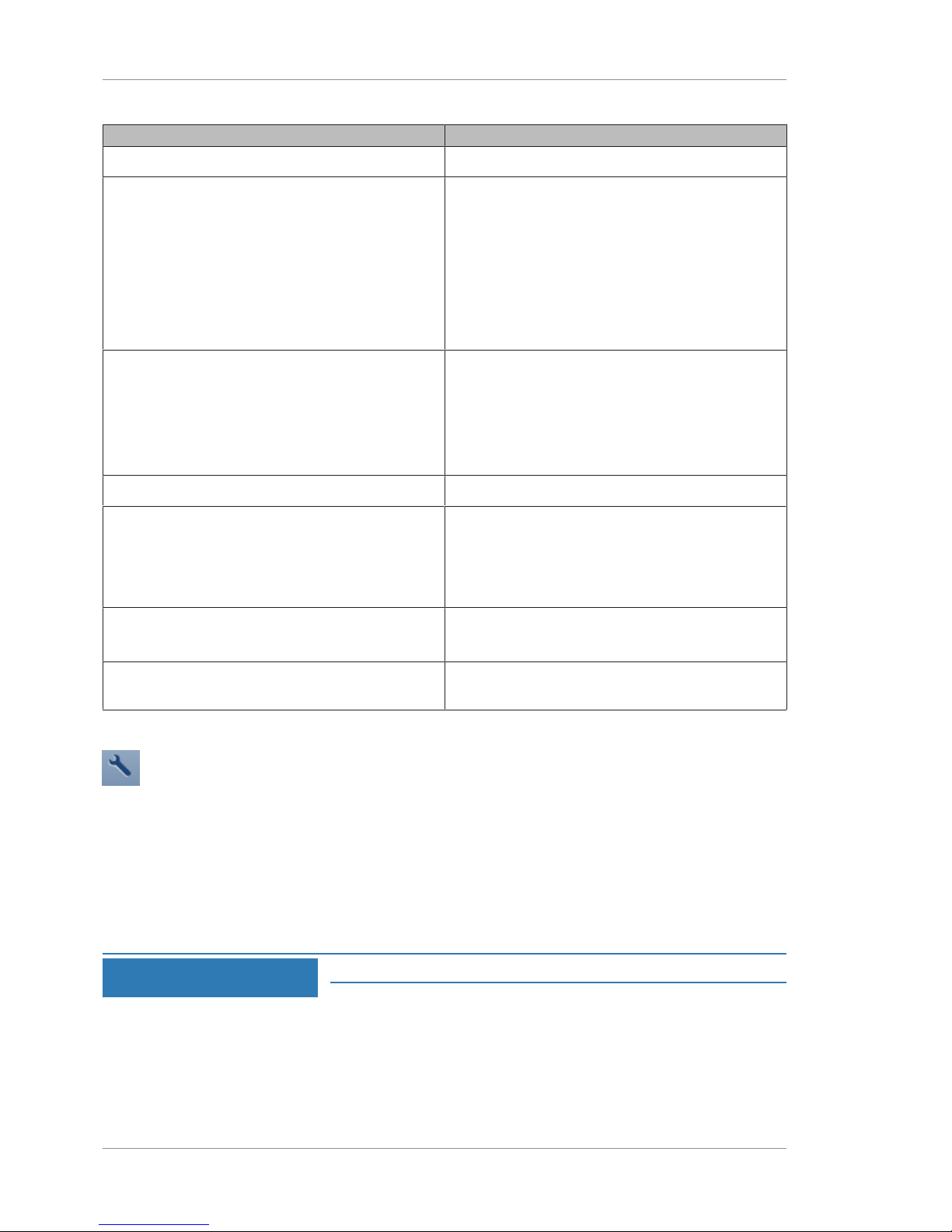
5.4.1.2 Merging Patient Records
If two records are inadvertently created for the same patient, they
can be merged to store all the patient data in one record.
To merge patient records:
Action 1. In the Patient window, select the patient name and click on
the down arrow.
2. Select Merge.
3. Type the name or ID for the duplicate patient record in the
search field and click Search.
Page 51

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 51 / 192
4. Select the duplicate patient record(s). You can merge up to 20
patient records.
5. To see the details of selected patient records side by side with
the original record, select Compare.
6. Select Merge.
7. Enter a reason for the merge, then select Merge again.
ð The exam data from the duplicate patient record is added
to the original patient record.
5.4.1.3 Reassigning Image Scan
If you inadvertently select the wrong patient record at the
beginning of an exam, you can reassign the data to the correct
patient record.
To reassign test data:
Prerequisite
þ Settings are open (Opening Settings [}47]).
þ The patient with incorrect test data is selected Finding a Patient
[}99].
Action 1. Click the down arrow for the patient with test data that you
want to reassign.
Page 52

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
52 / 192 2660021171806 Rev. A 2019-01
2. Select Tests.
ð A list of the tests assigned to the patient opens.
3. Select the incorrectly saved test and click Reassign.
ð The Reassign page opens.
4. In the search field, type the name, ID number, or DOB of the
patient the test data belongs to and click the search icon.
Page 53

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 53 / 192
5. Select the correct patient and click 2660021167882 Rev. A.pdf.
ð A confirmation opens.
6. Type a reason for resassigning the test.
7. Click Reassign.
5.4.1.4 Deleting a Patient Record
NOTE
Only Administrators can complete this task.
Patients deleted on the instrument are not deleted from the EMR
system.
To delete a patient record:
Prerequisite þ The instrument is not connected to FORUM or any other EMR
system.
Action 1. In the Patient window, select the patient name and click on
the down arrow and select Delete.
Page 54

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
54 / 192 2660021171806 Rev. A 2019-01
ð A confirmation opens.
2. Click Delete.
5.4.1.5 Manually Correcting Laterality
You can change the laterality of an image after it is saved using the
Review or Proof screen.
Laterality change feature does not work under the following conditions:
• After the data is exported
• In connected mode (FORUM)
• On imported data
To correct laterality manually in Proof:
Prerequisite þ Proof Sheet is open.
Action 1. Right-click on the image you want to change.
1
2. Select Change Laterality.
ð A confirmation opens.
3. Click OK.
Page 55

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 55 / 192
Result ü The thumbnail changes laterality.
To correct laterality manually in Review:
Prerequisite þ Review is open.
4. Select the image you want to change.
5. Click on the current laterality indicator in the preview panel.
ð The other laterality appears for selection.
1
6. Click the desired laterality.
ð The laterality indicator changes.
7. Click Save.
5.4.1.6 Importing Data
You can import data in the following individual exams or multiple
exams (bulk import).
You can import the following file formats:
• JPG
• TIFF
• PNG
• JPEG2000
• DICOM
• ZIP (compressed)
To import data:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Insert the removable media into the USB port.
2. Click Specific Settings.
ð The Specific Settings window opens.
Page 56

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
56 / 192 2660021171806 Rev. A 2019-01
3. Click Import.
4. Click Import... and navigate to the file stored on the external
removable media device.
5. Click Select.
ð A confirmation informs you when the import completes.
6. Click OK.
5.4.1.6.1 Data Integrity of Imported Records
For all imported patient records, it is possible to import new scan
data and update patient data, including obscured patient records. If
during import the device encounters information associated with a
patient that was already imported, the device does the following:
• imports all scan data (exams) not previously imported, but never
deletes nor overwrites any scan data already imported
• updates patient data only if it was created on a later date than
the data already imported. This prevents overwriting of newer
patient data with older patient data
5.4.1.7 Exporting Data
You can export data in multiple ways:
• In the Proof or Review screen, select an image and right-click.
• In the Proof screen, select multiple images and right-click.
Page 57

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 57 / 192
• Export all of a patient's data; select Settings > Specific
Settings > Export.
• Set automatic export (Configuring Export Settings [}59])
To export data:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. If you are exporting to removable media, insert the media into
the USB port.
2. Click Specific Settings.
ð The Specific Settings window opens.
3. Click Export.
ð A list of all patients opens.
4. To select all patients, click All.
5. To select one or more patients individually, check the patients
you want to export.
6. To deselect the selected patients, click None.
7. Click Export.
Page 58

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
58 / 192 2660021171806 Rev. A 2019-01
8. Navigate to the folder you want to use for export and click
Select.
ð A progress bar appears until export is complete.
9. Click OK.
ð The data exports and a confirmation opens.
10. Click OK.
5.4.1.8 Configuring Import Settings
You can choose automatic import of images and data from a
folder.
NOTE
If you use an EMR system, make sure you complete the
networking settings
to ensure that automatic export can connect to your EMR server.
To configure import settings:
Prerequisite
þ Settings are open (Opening Settings [}47]).
þ A folder for automatic imports is created and you know the
path to it.
Action 1. Select Specific Settings.
2. Click Import Export Settings.
Page 59

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 59 / 192
3. Under File import, set Auto import files to On.
4. Click Browse... and navigate to the folder to use for automatic
importing.
5. Click Select.
Result ü The folder path appears next to the Browse.... button and
files stored in that location will be automatically imported.
5.4.1.9 Configuring Export Settings
You can choose automatic exporting of images and data settings,
including:
• How often to automatically export images and patient data
(logout or shutdown)
• What type of EMR server to send the exported images and data
and the server path
• What image format to use for export
• What report format to use for export
• Whether to compress files on export
• Whether to remove patient demographic information from
exported exam data
NOTE
If you use an EMR system, make sure you complete the
networking settings
to ensure that automatic export can connect to your EMR server.
To configure export settings:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select Specific Settings.
Page 60
5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
60 / 192 2660021171806 Rev. A 2019-01
2. Click Import Export Settings.
3. Set the frequency of automatic exports:
To set automatic export when a user logs off, click Session.
To set automatic export when a user shuts down the ZEISS
application, click Shutdown.
4. If you use and EMR system, select the type (FORUM/PACS or
DICOM).
5. Under File Export, select the file format for images (DICOM,
JPEG, PNG, TIFF, JPEG2000).
6. To automatically removes patient demographic information
from exported exam data, set De-identify patient data to On.
7. To automatically compress data on export, set Compress Data
to On.
8. For Report Export, select whether to use PDF, ePDF or both
formats.
5.4.1.10 Configuring Scan Default Settings
You can change the default settings for images. You can apply
these changes to all existing color images after you set new
defaults.The ZEISS Default settings are:
Page 61
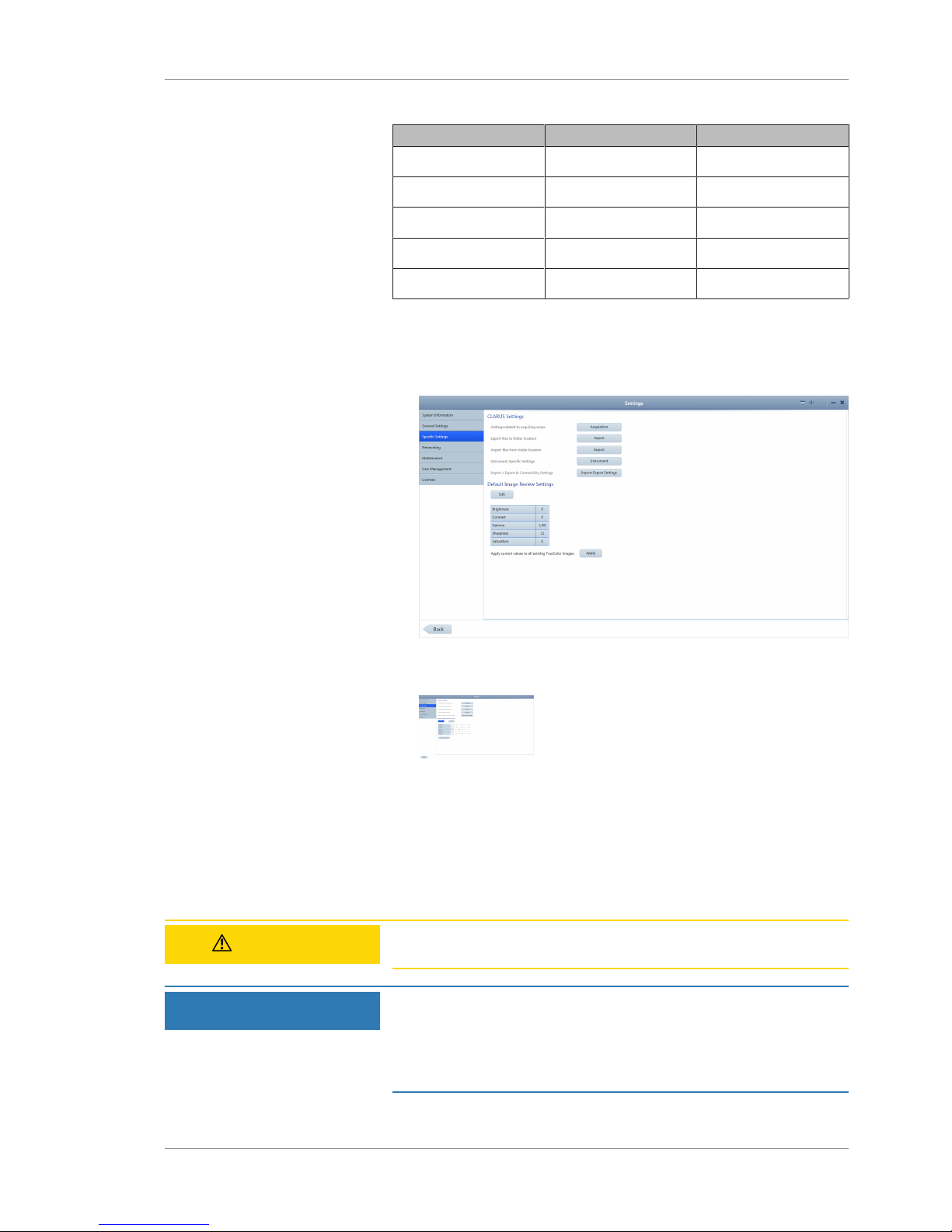
Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 61 / 192
Setting ZEISS Default Adjustment Levels
Brightness 0 -100 to 100
Contrast 0 -100 - 100
Gamma 1.00 0 to 5
Sharpness 15 0 to 100
Saturation 0 -100 to 100
To configure scan default settings:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select Specific Settings.
2. Click Edit.
ð A set of sliders for each setting opens.
3. Click in the box and type a new setting or slide to adjust the
default setting(s) you want to change.
4. Click Done.
5. To apply the newly set defaults to all color images, click Apply.
5.4.2 Manage Users
CAUTION!
Do not use Bitlocker Passkeys with this instrument or review
software.
NOTE
Unsecured Logins
may result in unauthorized access or inaccurate record-keeping.
u Create individual user accounts for each staff member.
u Staff members should log out after every use.
Page 62

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
62 / 192 2660021171806 Rev. A 2019-01
5.4.2.1 About User Roles
Not all users have access to all features. The table below list some
of the key differences for different types of users.
Operator Doctor Adminis-
trator
Acquire scans X X
Review scans X X
Select, edit and annotate scans X X
Use review software installed on a separate computer X X
Reset your own password X X X
Delete a local patient record X X X
Save and print reports X X X
Import and export data X X X
Configure import and export settings X X X
Configure general settings X X
Configure network settings X
Configure data backup X
Restore data from a backup X
Perform system maintenance X
Manage licenses X
Reset other user's passwords X
Add or delete users X
Export log files X
Table7: Permissions Levels
5.4.2.2 Adding a New User
NOTE
Only Administrators can complete this task.
For valid password format, see: Password Requirements [}65].
To add a new user:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Click Settings > User Management.
Page 63

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 63 / 192
2. Click Add User.
3. Type the information for the new user and select the user's
role.
4. Click Add User.
Result ü The new user can now log in.
5.4.2.3 Deleting a User
NOTE
Only Administrators can complete this task.
To delete a user:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Click Settings > User Management.
Page 64

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
64 / 192 2660021171806 Rev. A 2019-01
2. Select the Login name for the user you want to delete.
3. Click Delete User.
Result ü The user's Login is now disabled.
5.4.2.4 Changing a User's Password
NOTE
Only Administrators can complete this task.
For valid password format, see: (Password Requirements [}65])
To change a user's password:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select User Management.
2. Select the Login name for the user who needs a new
password and click Change Password ....
ð The password reset panel opens.
Page 65

Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 65 / 192
3. For New password, type a new password for the user.
4. For Repeat new password, retype the new password.
Result ü The new password for the user is now active.
5.4.2.5 Changing Your Own Password
For valid password format, see: (Password Requirements [}65])
To change your password:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Click Settings > User Management.
2. Click Change Password ....
3. For Old password, type your existing password.
4. For New password, type a new password.
5. For Repeat new password, retype the new password.
Result ü Your password is now changed to the new password.
5.4.2.6 Password Requirements
CLARUS® 500 users’ passwords expire every 60 days. All
passwords must follow these rules:
Page 66

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
66 / 192 2660021171806 Rev. A 2019-01
• Must be at least seven characters long.
• Must contain at least three of the following:
– English uppercase characters (A through Z)
– English lowercase characters (a through z)
– Numbers (0 through 9)
– Non-alphabetic characters (for example: !, $, #, %)
• Must not contain the user's account name.
• Must not contain two consecutive letters of the user's name.
5.4.3 Manage Backups
On the CLARUS® 500, you can enable data backup each time the
system shuts down (Configuring Automatic Backups [}66]). You
can also back up data manually at any time (Backing Up Data
Manually [}67]).
5.4.3.1 Configuring Automatic Backups
You can save a backup copy of selected data at any time. You can
save this data on removable media for import into other applications.
To configure automatic backups:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Insert the removable media into the USB port.
2. Click Settings > Maintenance.
ð The Maintenance window opens.
3. Slide Data backup on Shutdown to Backup Enabled.
4. Click Browse.
Page 67
Instructions for Use 5 Configuration
CLARUS® 500 5.4 Managing Data
2660021171806 Rev. A 2019-01 67 / 192
5. Navigate to the folder where you want to store the backups
and click Select.
Result ü Each time the system shuts down a backup will be saved in
the selected folder.
5.4.3.2 Backing Up Data Manually
To back up data manually:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Insert the removable media into the USB port.
2. Click Settings > Maintenance.
ð The Maintenance window opens.
3. Click Backup Now.
ð A progress bar opens.
ð When the backup completes, a confirmation opens.
4. Click OK.
Result ü A new data backup is stored in the folder you configured
to store automatic backups. (See Configuring Automatic
Backups [}66].)
Page 68

5 Configuration Instructions for Use
CLARUS® 5005.4 Managing Data
68 / 192 2660021171806 Rev. A 2019-01
5.4.3.3 Restoring Data from a Backup
NOTE
Only Administrators can complete this task.
If the CLARUS® 500 has automatic backups enabled or if there is a
saved manual backup, you can restore the system with the data
from the backup file.
After you restore data, you must restart the instrument.
To restore data from a backup:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select Maintenance.
2. Under Data Restore, select the backup you want to install.
3. Click Replace.
ð A confirmation opens.
4. Click Replace.
ð A progress bar opens. When restoration is complete, a
restart confirmation opens.
5. Click Restart.
Result ü The system restarts with the data that existed in the
backup.
5.4.4 Log Files
CLARUS® 500 records the following events and identifies them by
date, time, and User ID:
• Log on/log off
• Create, modify, delete data
• Import/export data from removable media
• Receipt/transmit data from/to the network
• Remote service activity
A new file is created when the application starts. Events are
automatically recorded in 1-5 audit files of 10 Mb each.
Page 69

Instructions for Use 5 Configuration
CLARUS® 500 5.5 Managing Connections
2660021171806 Rev. A 2019-01 69 / 192
The instrument retains up to 100 of the latest log files. When file
size limit is reached, the device overwrites existing files (starting
with the oldest file).
The default folder for the audit log files is C:\ProgramData\Carl
Zeiss Meditec\...\Logs.
NOTE
Save (export) audit log files regularly
to ensure events of consequence can be tracked should you
encounter a data error.
u Log on as Administrator.
u Click Tools > Export Audit Log File.
u In the Browse folder, browse to the export location.
u Click Save.
ð The log is exported as a .zip file with the format:
AuditLog_dd_mm_yyyy_hh_mm.
5.4.5 Data Storage
The CLARUS® 500 includes a some storage space on its PC. If you
need additional data storage capacity, add external networked
storage devices as requried.
5.5 Managing Connections
5.5.1 Connecting to a Wireless Printer
NOTE
Connect only to wireless printers recommended).
NOTE
Only Administrators and Doctors can complete this task.
To connect to a wireless printer:
Prerequisite þ The CLARUS® 500 and printer are on.
þ The printer is configured correctly.
þ Settings are open (Opening Settings [}47]).
Action 1. Select General Settings and scroll to the bottom.
2. Under Printer and Report, click Manage Printers.
Page 70

5 Configuration Instructions for Use
CLARUS® 5005.5 Managing Connections
70 / 192 2660021171806 Rev. A 2019-01
3. Select the printer you want to connect and click Add Printer.
4. To print a test page, select the printer and click Print test
page.
5. To view the properties of the printer, click Properties.
5.5.2 Mapping Networked Drives
To map a networked drive:
Prerequisite
þ Settings are open (Opening Settings [}47]).
þ You know the path of the drive you want to map in the format:
\\<servername>\<foldername>\<subfoldername>. For example:
\\Server_x543\ZEISS\Exported_Files
Action 1. Select Networking.
Page 71

Instructions for Use 5 Configuration
CLARUS® 500 5.5 Managing Connections
2660021171806 Rev. A 2019-01 71 / 192
2. Under Network Drive Configuration, click Map....
3. Type the path and select the Drive letter.
4. To save the login credentials, type a User name and
Password for the server.
5. Click Map.
Result ü The drive is now mapped.
5.5.3 Connecting to a LAN
To connect to a LAN:
Prerequisite þ The CLARUS® 500 must be connected to the local network
using a network cable. Be gentle with the connector when
plugging it into the CLARUS® 500 ethernet port.
þ Settings are open (Opening Settings [}47]).
Action 1. Select Networking.
Page 72

5 Configuration Instructions for Use
CLARUS® 5005.6 Managing Licenses
72 / 192 2660021171806 Rev. A 2019-01
2. To automatically assign an IP address to the instrument, set
DHCP to Enabled. For a network with static IP addresses, set
DHCP to Disabled and enter the relevant network infor-
mation.
3. Under Network Drive Configuration, select Map....
4. Enter the network path for the shared folder you'd like to
connect to. The shared folder must be a root directory or a
subdirectory of a root directory.
5. Select an unused drive letter.
6. If a password is required for access to the shared folder, enter
the username and password. Otherwise, leave these fields
blank.
7. Select Map.
ð Successfully mapped drives are indicated with a check mark
in a green circle. To disconnect a network drive, select the
drive, then click Unmap.
5.6 Managing Licenses
Your instrument is shipped with the software license installed. The
instrument's software license and ten Review Station licenses are
provided with the instrument. You can install CLARUS® 500 review
software on (up to) ten computers to use as Review Stations.
After you install software on each Review Station, you must
activate a license for it.
If you return a license from a Review Station computer, you can
install and activate the software on a different Review Station
computer using the same license.
NOTE
Only Administrators can complete this task.
Page 73

Instructions for Use 5 Configuration
CLARUS® 500 5.6 Managing Licenses
2660021171806 Rev. A 2019-01 73 / 192
5.6.1 Activating Licenses Online
If the Review Station is connected to the intranet, activating a
licenses is a simple, one-step process.
To activate a license online:
Prerequisite þ The Review Station is connected to the internet.
þ You located the Activation ID of the license you want to use
for this Review Station.
Action 1. Login to the CLARUS® 500 software on the Review Station
as an Administrator.
2. Select Settings > Licenses.
3. For Online License Activation, type the activation ID.
4. Click Create request file.
5. Click Activate.
Result ü The newly activated license appears as Active at the top of
the license settings page.
5.6.2 Activating Licenses Offline
If the Review Station is not connected to the internet, you can
activate the license offline. Using this method you exchange a
Request (XML file) for a Response (XML file) with your ZEISS
representative.
5.6.2.1 Requesting Licenses Offline
When you request a license, you create an XML license request file
and send it to your ZEISS representative.
To request a license offline:
Prerequisite þ You located the Activation ID of the license you want to use
for this Review Station.
Action 1. Login to the CLARUS® 500 software on the Review Station
as an Administrator.
Page 74

5 Configuration Instructions for Use
CLARUS® 5005.6 Managing Licenses
74 / 192 2660021171806 Rev. A 2019-01
2. Select Settings > Licenses.
3. For Offline License Activation, type the Activation ID.
4. Click Create request file.
5. Navigate to the network folder or flash drive location where
you want to save the request file.
6. Click Save.
7. From a computer connected to the internet, send the XML file
to your ZEISS representative.
5.6.2.2 Activating Licenses Offline
To activate a license offline:
Prerequisite þ You sent the request file for this license to your ZEISS represen-
tative.
þ Your ZEISS representative returned a response file for this
license.
þ You saved the response file on the network or a flash drive to
install on the Review Station.
Action 1. Login to the CLARUS® 500 software on the Review Station as
an Administrator.
2. Select Settings > Licenses.
Page 75

Instructions for Use 5 Configuration
CLARUS® 500 5.6 Managing Licenses
2660021171806 Rev. A 2019-01 75 / 192
3. For Offline License Activation, click Import Response file.
4. Navigate to the flash drive or network folder where the
response file is located.
5. Select the response file and click Activate.
Result ü The newly activated license appears as Active at the top of
the license settings page.
5.6.3 Returning Product Licenses
With each CLARUS® 500 instrument there are ten licenses for
Review Stations. If you want to move a license from one Review
Station computer to another, you must:
1. Deactivate (return) the license on the original Review Station
computer.
2. Activate the license on the new Review Station computer.
5.6.3.1 Returning Licenses Online
If the Review Station is connected to the intranet, deactivating a
licenses is a simple, one-step process.
To deactivate a license online:
Prerequisite þ The Review Station is connected to the internet.
Action 1. Login to the CLARUS® 500 software on the Review Station
as an Administrator.
2. Select Settings > Licenses.
3. For Online License Return, type the active Activation ID
listed at the top of the page.
4. Click Return.
Result ü The license no longer appears as Active at the top of the
license settings page.
Page 76
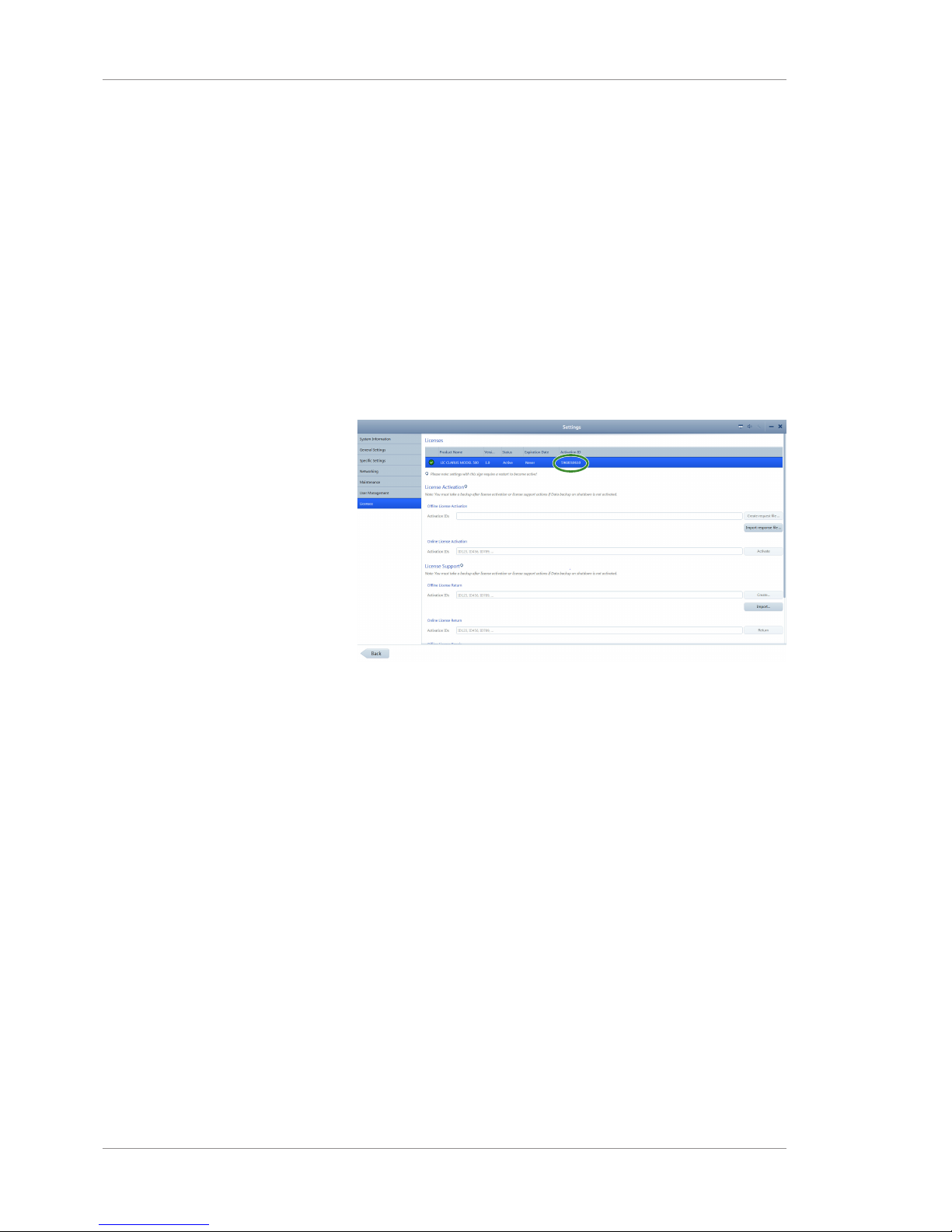
5 Configuration Instructions for Use
CLARUS® 5005.6 Managing Licenses
76 / 192 2660021171806 Rev. A 2019-01
5.6.3.2 Returning Licenses Offline
If the Review Station is not connected to the internet, you can
activate the license offline. Using this method you exchange a
Request (XML file) for a Response (XML file) with your ZEISS
representative.
5.6.3.2.1 Requesting License Return
When you deactivate a license, you create an XML file and send it
to your ZEISS representative.
To deactivate a license offline:
Action 1. Login to the CLARUS® 500 software on the Review Station
as an Administrator.
2. Select Settings > Licenses.
3. For Offline License Return, type the active Activation ID
listed at the top of the page.
4. Click Create.
5. Navigate to the network folder or flash drive location where
you want to save the request file.
6. Click Save.
7. From a computer connected to the internet, send the XML file
to your ZEISS representative.
5.6.3.2.2 Returning the License
To deactivate a license offline:
Prerequisite þ You sent the deactivation request file for this license to your
ZEISS representative.
þ Your ZEISS representative returned a deactivation response file
for this license.
þ You saved the response file on the network or a flash drive to
install on the Review Station.
Page 77

Instructions for Use 5 Configuration
CLARUS® 500 5.6 Managing Licenses
2660021171806 Rev. A 2019-01 77 / 192
Action 1. Login to the CLARUS® 500 software on the Review Station as
an Administrator.
2. Select Settings > Licenses.
3. For Offline License Return, click Import.
4. Navigate to the flash drive or network folder where the
response file is located.
5. Select the response file and click Select.
Result ü The license no longer appears as Active at the top of the
license settings page.
5.6.4 Repairing Product Licenses
If a license becomes corrupted, you may need to repair it. Connect
the Review Station to the intranet to repair the license.
To repair a license online:
Prerequisite þ The Review Station is connected to the internet.
þ You located the Activation ID of the license you want to use
for this Review Station.
Action 1. Login to the CLARUS® 500 software on the Review Station
as an Administrator.
2. Select Settings > Licenses.
3. Write down the Activation ID of the active license.
Page 78

5 Configuration Instructions for Use
CLARUS® 5005.7 Configuring Local Settings
78 / 192 2660021171806 Rev. A 2019-01
4. Scroll down to the bottom of the page.
5. For Online License Repair, type the activation ID.
6. Click Repair.
Result ü The active license is properly restored.
5.7 Configuring Local Settings
Local settings include information about your clinic such as address,
local language and local time zone.
To configure local settings:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select General Settings.
2. For Institution Information, complete the form for your clinic.
3. For Alert display time, select the number of seconds you
want system or instrument alerts to display.
4. For Locale Settings, select the language and date formats.
5. Scroll to set additional general settings.
6. For System Date and Time, select the appropriate settings.
Page 79

Instructions for Use 5 Configuration
CLARUS® 500 5.8 Configuring Report Settings
2660021171806 Rev. A 2019-01 79 / 192
7. To display the onscreen keyboard, slide On-Screen Keyboard
to Enabled.
8. For Patient Management, select Name or ID as patient data
identifier.
9. Select the method for patient ID assignment. If you select
CLARUS® 500, the instrument will assign unique IDs for
patients.
10. If your language requires Multi-Component Names, Enable
Multi component names and select the appropriate type.
5.8 Configuring Report Settings
You can add your logo and a signature field to reports. You can
also set the path for storing reports and manage printers.
To configure the report settings for automatic export, refer to:
Configuring Export Settings [}59].
To configure report settings:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select General Settings.
2. Scroll to the bottom of the General Settings panel.
Page 80

5 Configuration Instructions for Use
CLARUS® 5005.9 Configuring Acquisition Settings
80 / 192 2660021171806 Rev. A 2019-01
3. To enable a signature field on reports, slide Signature field on
reports to Show.
4. To add a logo to reports, click Browse... next to Report logo
and navigate to the logo file and select it.
5. To set a path to store reports, click Browse... next to Report
storage path.
5.9 Configuring Acquisition Settings
You can add your logo and a signature field to reports. You can
also set the path for storing reports and manage printers.
To configure acquisition settings:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select Specific Settings.
2. Click Acquisition.
5.10 Clearing Alert History
To clear alert history:
Prerequisite
þ Settings are open (Opening Settings [}47]).
Action 1. Select System Information.
Page 81

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 81 / 192
2. Under Alert History, click Clear.
ð The alerts are removed.
5.11 System Administration
NOTE
Only Administrators can complete this task.
This section contains detailed configuration information for system
administrators. Only system administrators should make changes to
these settings.
5.11.1 Windows Patches and Updates
Automatic Windows updates are disabled. Windows patches and
updates are distributed with CLARUS® 500 software after they are
tested and approved for use.
5.11.2 Data Safety
5.11.2.1 Auto-lock
After 15 minutes of inactivity, the CLARUS® 500 screen locks and
the user must log in again.
5.11.2.2 Anti-Malware
When configuring anti-malware applications, ensure that updates
and full system scans do not occur when data acquisition could be
in progress. Refer to the software release notes for a list of
approved anti-malware software.
5.11.2.3 User Names and Passwords
NOTE
Passwords for Tech Support users are unique for each
system.
Page 82

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
82 / 192 2660021171806 Rev. A 2019-01
Zeiss instruments initially have three user names and passwords.
Initial passwords are shown in the table below. Change these
passwords before using the instrument.
User Name Type Password Purpose
Zeiss Administrator November171846 Instrument User
ZeissAdmin Administrator November171846 Instrument Adminis-
tration
Technical Support Administrator <unique> Zeiss Technical Support
Table8: Initial User Names and Passwords
5.11.3 Networking
5.11.3.1 Network Controllers and IP Addressing
NOTE
The CLARUS® 500 is not compatible with networks using
IPv4 addressing in the range of 192.168.111.0 to
192.168.111.254.
The CLARUS 500 contains two network controllers; Internal
Network and External Network.
5.11.3.1.1 Internal Network Ports
NOTE
Do not rename the Internal Network.
Internal Network is for instrument use only with the following
assignments:
• Static IPv4 address: 192.168.111.2
• Subnet mask: 255.255.255.0
5.11.3.1.2 External Network Ports
The External Network is for CLARUS® 500 connectivity and
automatically picks up an IP address.
5.11.3.2 Required Network Ports
The network ports listed in this section are required for proper
operation of the CLARUS® 500.
5.11.3.2.1 Required Internal Network Ports
• Internal Communication Port: TCP 80
• Internal Communication Port: TCP 22
5.11.3.2.2 Required External Network Ports
Service TCP UDP
Database inbound 18081
Page 83

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 83 / 192
Service TCP UDP
Database outbound 18082
Database inbound/outbound (open port visible externally) 3346
Peer to Peer broadcast inbound/outbound 54183
Distributed Data Service inbound/outbound 54187
Table9: External Network Ports
5.11.3.2.3 Required External Network Ports for EMR and FORUM
Service TCP UDP
DICOM outbound 11119
FORUM outbound 8080
DICOM inbound 11112
FORUM inbound
The first available port in this range will be used.
8081 ~
8101
DNS
(AutoConnect for instruments outbound)
5353
Table10: External Network Ports for EMR / FORUM
5.11.3.3 Additional Network Ports
The following network ports are not required to operate the
instrument. These ports facilitate instrument configuration and
maintenance.
Service TCP UDP
Remote service (outbound) 80
DHCP 67 - 68
TCP/IP MS Networking 445
NTP 123
NETBIOS Name Service
(UDP open port visible externally)
137 137
NETBIOS Datagram Service 138 138
NETBIOS Session Service 139 139
Table11: Additional Network Ports
5.11.4 Configuring Enhanced Security
Enhanced security settings are not required to operate the
instrument properly. You can change these settings individually to
match your Windows installation.
Page 84

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
84 / 192 2660021171806 Rev. A 2019-01
To remove Enhanced Security, log in as ZeissAdmin and run the
“Remove Enhanced Security.CMD” script located on the desktop.
5.11.4.1 Disabling Enhanced Security Settings
When you received the CLARUS 500, enhanced security settings are
turned on. These settings are not required for the instrument to
operate properly.
You can change individual settings per your institution's requirements or you can disable all of these settings using the Remove
Enhanced Security command on the desktop.
Enhanced security settings are listed in the following sections:
• Enhanced Security Windows Firewall Rules [}85]
• Enhanced Security Services [}86]
• Enhanced Security Group Policy [}86]
NOTE
Only Administrators can complete this task.
To disable enhanced security settings:
Prerequisite
þ The CLARUS 500 application is minimized (Main Toolbar [}29])
Action 1. Location the following command on the Windows desktop:
Remove Enhanced Security.CMD.
2. Double-click Remove Enhanced Security.CMD.
ð All enhanced security settings are reset to the Windows
default settings.
5.11.4.2 Enabling Enhanced Security Settings
When you received the CLARUS 500, enhanced security settings are
turned on. These settings are not required for the instrument to
operate properly.
If there were changes to individual settings or if all enhanced
security settings were disabled, you can re-enable all of these
settings using the Install Enhanced Security command on the
desktop.
Enhanced security settings are listed in the following sections:
• Enhanced Security Windows Firewall Rules [}85]
• Enhanced Security Services [}86]
• Enhanced Security Group Policy [}86]
NOTE
Only Administrators can complete this task.
To enable enhanced security settings:
Prerequisite
þ The ZEISS application is minimized (Main Toolbar [}29])
Page 85
Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 85 / 192
Action 1. Location the following command on the Windows desktop:
Apply Enhanced Security.CMD.
2. Double-click Apply Enhanced Security.CMD.
ð All enhanced security settings are enabled.
5.11.4.3 Enhanced Security Windows Firewall Rules
Enhanced security disables the following Windows firewall rules:
Disabled Firewall Rules
AllJoyn Router (TCP-In)
AllJoyn Router (TCP-Out)
AllJoyn Router (UDP-In)
AllJoyn Router (UDP-Out)
Cast to Device functionality (qWave-TCP-In)
Cast to Device functionality (qWave-TCP-Out)
Cast to Device functionality (qWave-UDP-In)
Cast to Device functionality (qWave-UDP-Out)
Cast to Device SSDP Discovery (UDP-In)
Cast to Device streaming server (HTTP-Streaming-In)
Cast to Device streaming server (RTCP-Streaming-In)
Cast to Device streaming server (RTP-Streaming-Out)
Cast to Device streaming server (RTSP-Streaming-In)
Cast to Device UPnP Events (TCP-In)
Cortana
Delivery Optimization (TCP-In)
Delivery Optimization (UDP-In)
DIAL protocol server (HTTP-In)
Microsoft.AccountsControl
Microsoft.LockApp
Microsoft.Windows.ContentDeliveryManager
Microsoft.Windows.ParentalControls
Microsoft.Windows.Apprep
Network Discovery (WSD Events-In)
Proximity sharing over TCP (TCP sharing-In)
Proximity sharing over TCP (TCP sharing-Out)
Remote Assistance (DCOM-In)
Remote Assistance (PNRP-In)
Remote Assistance (RA Server TCP-In)
Remote Assistance (SSDP TCP-In)
Remote Assistance (SSDP UDP-In)
Remote Assistance (TCP-In)
SmartScreen
Windows.ShellExperience
Windows Spotlight
Wireless Display (TCP-In)
Wireless Display (TCP-Out)
Wireless Display (UDP-Out)
Wireless Display Infrastructure Back Channel (TCP-In)
Page 86

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
86 / 192 2660021171806 Rev. A 2019-01
5.11.4.4 Enhanced Security Services
Enhanced security disables the following services:
Disabled Security Services
AllJoyn Router Service
Application Layer Gateway Service
Bluetooth Handsfree Service
Bluetooth Support Service
BranchCache
Connected Devices Platform Service
Connected User Experiences and Telenetry
Downloaded Maps Manager
Fax
Function Discovery Resource Publication
Geolocation Service
HomeGroup Listener
HomeGroup Provider
Infrared Monitor Service
Internet Connection Sharing (ICS)
Microsoft iSCSI Initiator Service
Microsoft Storage Spaces SMP
Microsoft Windows SMS Router Service
Network Connection Broker
Phone Service
Program Compatibility Assistant Service
Quality Windows Audio Video Experience
Retail Demo Service
Shell Hardware Detection
Telephony
Touch Keyboard and Handwriting Panel Service
Windows Camera Frame Server
Windows Event Collector
Windows Image Acquisition (WIA)
Windows Insider Service
Windows Media Player Network Sharing Service
Windows Mobile Hotspot Service
Work Folders Xbox Live Auth Manager Xbox Live Game Save Xbox Live
Networking Service
5.11.4.5 Enhanced Security Group Policy
Policy Setting
Access Credential Manager as a trusted caller
No One
Access this computer from the network
Administrators
Account lockout duration )
60 minute(s
Account lockout threshold
30 invalid logon
attempt(s)
Accounts: Administrator account status
Enabled
Page 87

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 87 / 192
Policy Setting
Accounts: Guest account status
Disabled
Accounts: Limit local account use of blank passwords to console logon only
Enabled
Accounts: Rename administrator account
ZeissAdmin
Accounts: Rename guest account
Not Used
Act as part of the operating system
No One
Adjust memory quotas for a process
Administrators,
Local Service,
Network Service
Allow all trusted apps to install
Disabled
Allow Basic authentication
Disabled
Allow Cortana
Disabled
Allow indexing of encrypted files
Disabled
Allow log on locally
Administrators,
Users
Allow Microsoft accounts to be optional
Enabled
Allow Telemetry 0 - Off (Enterprise Only)
Enabled
Allow unencrypted traffic
Disabled
Allow user control over installs
Disabled
Allow users to connect remotely by using Remote Desktop Services
Disabled
Allow Windows to automatically connect to suggested open hotspots, to
networks shared by contacts, and to hotspots offering paid services
Disabled
Always install with elevated privileges
Disabled
Always prompt for password upon connection
Enabled
Apply UAC restrictions to local accounts on network logons
Enabled
Audit Policy: Account Logon: Credential Validation
Success and
Failure
Audit Policy: Account Management: Other Account Management Events
Success and
Failure
Audit Policy: Account Management: Security Group Management
Success and
Failure
Audit Policy: Account Management: User Account Management
Success and
Failure
Audit Policy: Detailed Tracking: Process Creation
Success
Audit Policy: Logon-Logoff: Account Lockout
Success
Page 88

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
88 / 192 2660021171806 Rev. A 2019-01
Policy Setting
Audit Policy: Logon-Logoff: Logoff
Success
Audit Policy: Logon-Logoff: Logon
Success and
Failure
Audit Policy: Logon-Logoff: Special Logon
Success
Audit Policy: Object Access: Removable Storage
Success and
Failure
Audit Policy: Policy Change: Audit Policy Change
Success and
Failure
Audit Policy: Policy Change: Authentication Policy Change
Success
Audit Policy: Privilege Use: Sensitive Privilege Use
Success and
Failure
Audit Policy: System: IPsec Driver
Success and
Failure
Audit Policy: System: Other System Events
Success and
Failure
Audit Policy: System: Security State Change
Success
Audit Policy: System: Security System Extension
Success and
Failure
Audit Policy: System: System Integrity
Success and
Failure
Audit: Audit the access of global system objects
Disabled
Audit: Audit the use of Backup and Restore privilege
Enabled
Audit: Force audit policy subcategory settings (Windows Vista or later) to override
audit policy category settings
Enabled
Audit: Shut down system immediately if unable to log security audits
Disabled
Back up files and directories
Administrators
Boot-Start Driver Initialization Policy
Choose the boot-start drivers that can be initialized
Enabled
Good, unknown and
bad but critical
Change the system time
LOCAL SERVICE,
Administrators
Configure Automatic Updates
Disabled
Configure Offer Remote Assistance
Disabled
Configure registry policy processing
Do not apply during periodic background processing
Process even if the Group Policy objects have not changed
Enabled
TRUE
TRUE
Page 89

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 89 / 192
Policy Setting
Configure Solicited Remote Assistance
Maximum ticket time (units)
Maximum ticket time (value)
Method for sending email invitations
Permit remote control of this computer
Enabled
Hours
1
Simple MAPI
Allow helpers to
remotely control
the computer
Enabled
Configure Windows SmartScreen
Disabled
Create a pagefile
Administrators
Create a token object
No One
Create global objects
Administrators,
SERVICE, LOCAL
SERVICE, NETWORK
SERVICE
Create permanent shared objects
No One
Create symbolic links
Administrators
Debug programs
Administrators
Deny access to this computer from the network
NT AUTHORITY
\Local
Account,GUESTS
Deny log on locally
Guests
Deny log on through Remote Desktop Services
NT AUTHORITY
\Local
Account,GUESTS
Detect compatibility issues for applications and drivers
Enabled
Devices: Allow undock without having to log on
Enabled
Disallow Autoplay for non-volume devices
Enabled
Disallow Digest authentication
Enabled
Disallow WinRM from storing RunAs credentials
Enabled
Do not allow drive redirection
Enabled
Do not allow passwords to be saved
Enabled
Do not display network selection UI
Enabled
Do not enumerate connected users on domain-joined computers
Enabled
Do not preserve zone information in file attachments
Disabled
Domain member: Digitally encrypt or sign secure channel data (always)
Enabled
Page 90

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
90 / 192 2660021171806 Rev. A 2019-01
Policy Setting
Domain member: Digitally encrypt secure channel data (when possible)
Enabled
Domain member: Digitally sign secure channel data (when possible)
Enabled
Domain member: Disable machine account password changes
Disabled
Domain member: Maximum machine account password age
30 day(s)
Domain member: Require strong (Windows 2000 or later) session key
Enabled
Don't allow SmartScreen Filter warning overrides
Disabled
Enable computer and user accounts to be trusted for delegation
No One
Enable insecure guest logons
Disabled
Enable local admin password management
Enabled
Enable RPC Endpoint Mapper Client Authentication
Enabled
Enable screen saver
Enabled
Enforce password history
24 password(s)
Enumerate administrator accounts on elevation
Disabled
Enumerate local users on domain-joined computers
Disabled
Force shutdown from a remote system
Administrators
Generate security audits
Local Service,
Network Service
Hardened UNC Paths
Hardened UNC Paths:
Hardened UNC Paths: = \\*\NETLOGON"
RequireMutualAuthentication = 1
RequireIntegrity = 1
Enabled
Impersonate a client after authentication
Administrators,
SERVICE, Local
Service, Network
Service
Include command line in process creation events
Enabled
Increase scheduling priority
Administrators
Interactive logon: Machine account lockout threshold
10 invalid logon
attempts
Interactive logon: Machine inactivity limit
900 seconds
Interactive logon: Number of previous logons to cache (in case domain controller
is not available)
10 logon(s)
Interactive logon: Prompt user to change password before expiration
5 day(s)
Page 91

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 91 / 192
Policy Setting
Interactive logon: Require Domain Controller authentication to unlock
workstation
Disabled
Interactive logon: Require smart card
Disabled
Interactive logon: Smart card removal behavior
No Action
Load and unload device drivers
Administrators
Lock pages in memory
No One
Manage auditing and security log Administrators Maximum password age
60 days
Microsoft network client: Digitally sign communications (always)
Disabled
Microsoft network client: Digitally sign communications (if server agrees)
Enabled
Microsoft network client: Send unencrypted password to third-party SMB servers
Disabled
Microsoft network server: Amount of idle time required before suspending
session
15 minute(s)
Microsoft network server: Digitally sign communications (if client agrees)
Enabled
Microsoft network server: Server SPN target name validation level
Accept if
provided by
client
Minimize the number of simultaneous connections to the Internet or a Windows
Domain
Enabled
Minimum password age
1 day(s)
Minimum password length
7 character(s)
Minimum PIN length Minimum
PIN length
Enabled
6
Modify an object label
No one
Modify firmware environment values
Administrators
MSS: (AutoAdminLogon) Enable Automatic Logon (not recommended)
Disabled
MSS: (DisableIPSourceRouting IPv6) IP source routing protection level (protects
against packet spoofing)
DisableIPSourceRoutingIPv6
Enabled
Highest
protection, source
routing is
completely
disabled
MSS: (DisableIPSourceRouting) IP source routing protection level (protects against
packet spoofing)
DisableIPSourceRouting
Enabled
Highest
protection, source
routing is
completely
disabled
Page 92
5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
92 / 192 2660021171806 Rev. A 2019-01
Policy Setting
MSS: (EnableICMPRedirect) Allow ICMP redirects to override OSPF generated
routes
Disabled
MSS: (NoNameReleaseOnDemand) Allow the computer to ignore NetBIOS name
release requests except from WINS servers
Enabled
Network access: Allow anonymous SID/Name translation
Disabled
Network access: Do not allow anonymous enumeration of SAM accounts
Enabled
Network access: Do not allow anonymous enumeration of SAM accounts and
shares
Enabled
Network access: Do not allow storage of passwords and credentials for network
authentication
Enabled
Network access: Let Everyone permissions apply to anonymous users
Disabled
Network access: Restrict anonymous access to Named Pipes and Shares
Enabled
Network access: Sharing and security model for local accounts
Classic - local
users authenticate as
themselves
Network security: Allow Local System to use computer identity for NTLM
Enabled
Network security: Allow LocalSystem NULL session fallback
Disabled
Network Security: Allow PKU2U authentication requests to this computer to use
online identities
Disabled
Network Security: Configure encryption types allowed for Kerberos
RC4\AES128\AES25
6\Future types
Network security: LAN Manager authentication level
Send NTLMv2
response only.
Refuse LM & NTLM
Network security: Do not store LAN Manager hash value on next password
change
Enabled
Network security: Force logoff when logon hours expire
Disabled
Network security: LDAP client signing requirements
Negotiate
signing
Network security: Minimum session security for NTLM SSP based (including
secure RPC) clients
Require NTLMv2
session
security,
Require 128-bit
encryption
Page 93

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 93 / 192
Policy Setting
Network security: Minimum session security for NTLM SSP based (including
secure RPC) servers
Require NTLMv2
session
security,
Require 128-bit
encryption
No auto-restart with logged on users for scheduled automatic updates installations
Enabled
Notify antivirus programs when opening attachments
Enabled
Password must meet complexity requirements
Enabled
Password protect the screen saver
Enabled
Perform volume maintenance tasks
Administrators
Prevent enabling lock screen camera
Enabled
Prevent enabling lock screen slide show
Disabled
Prevent the usage of OneDrive for file storage
Enabled
Profile single process
Administrators
Prohibit connection to non-domain networks when connected to domain authenticated network
Enabled
Recovery console: Allow automatic administrative logon
Disabled
Recovery console: Allow floppy copy and access to all drives and all folders
Disabled
Replace a process level token Local Service, Network Service Require a Password
When a Computer Wakes (On Battery)
Enabled
Require a Password When a Computer Wakes (Plugged In)
Enabled
Require secure RPC communication
Enabled
Reset account lockout counter after
60 minute(s)
Restrict Unauthenticated RPC clients
RPC Runtime Unauthenticated Client Restriction to Apply:
Enabled
Authenticated
Restore files and directories
Administrators
Screen saver timeout
Seconds
Enabled
900
Set client connection encryption level
Encryption Level
Enabled
High Level
Set the default behavior for AutoRun
Default AutoRun Behavior
Enabled
Do not execute any
autorun commands
Shutdown: Allow system to be shut down without having to log on
Enabled
Page 94

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
94 / 192 2660021171806 Rev. A 2019-01
Policy Setting
Shutdown: Clear virtual memory pagefile
Disabled
Sign-in last interactive user automatically after a system-initiated restart
Disabled
Specify the maximum log file size (KB) (Application Log)
Maximum Log Size (KB)
Enabled
32768
Specify the maximum log file size (KB) (Security Log)
Maximum Log Size (KB)
Enabled
196608
Specify the maximum log file size (KB) (System Log)
Maximum Log Size (KB)
Enabled
32768
Store passwords using reversible encryption
Disabled
System cryptography: Use FIPS compliant algorithms for encryption, hashing, and
signing
Disabled
System objects: Require case insensitivity for non-Windows subsystems
Enabled
System objects: Strengthen default permissions of internal system objects (e.g.
Symbolic Links)
Enabled
System settings: Use Certificate Rules on Windows Executables for Software
Restriction Policies
Disabled
Take ownership of files or other objects
Administrators
Turn off access to the Store
Enabled
Turn off app notifications on the lock screen
Enabled
Turn off Automatic Download and Install of updates
Enabled
Turn off Autoplay Turn off
Autoplay on
Enabled
All drives
Turn off Data Execution Prevention for Explorer
Disabled
Turn off downloading of print drivers over HTTP
Disabled
Turn off heap termination on corruption
Disabled
Turn off Internet download for Web publishing and online ordering wizards
Enabled
Turn off Microsoft consumer experiences
Enabled
Turn off Password Manager
Disabled
Turn off printing over HTTP
Disabled
Turn off the offer to update to the latest version of Windows
Enabled
Turn off the SmartScreen Filter
Enabled
Turn off toast notifications on the lock screen
Enabled
Turn off Windows Update device driver searching
Enabled
Page 95

Instructions for Use 5 Configuration
CLARUS® 500 5.11 System Administration
2660021171806 Rev. A 2019-01 95 / 192
Policy Setting
Turn on convenience PIN sign-in
Disabled
Turn on PowerShell Script Block Logging
Log script block invocation start / stop events:
Enabled
TRUE
Untrusted Font Blocking
Mitigation Options
Enabled
Block untrusted
fonts and log
events
Use a hardware security device
Enabled
Use enhanced anti-spoofing when available
Enabled
User Account Control: Admin Approval Mode for the Built-in Administrator
account
Enabled
User Account Control: Allow UIAccess applications to prompt for elevation
without using the secure desktop
Disabled
User Account Control: Behavior of the elevation prompt for administrators in
Admin Approval Mode
Elevate without
prompting
User Account Control: Behavior of the elevation prompt for standard users
Prompt for
credentials
User Account Control: Detect application installations and prompt for elevation
Enabled
User Account Control: Only elevate executables that are signed and validated
Disabled
User Account Control: Only elevate UIAccess applications that are installed in
secure locations
Enabled
User Account Control: Run all administrators in Admin Approval Mode
Enabled
User Account Control: Switch to the secure desktop when prompting for
elevation
Enabled
User Account Control: Virtualize file and registry write failures to per-user
locations
Enabled
WDigest Authentication (disabling may require KB2871997)
Disabled
Windows Firewall: Domain: Display a notification
No
Windows Firewall: Domain: Inbound connections
Inbound Connections
Enabled
Block
Windows Firewall: Domain: Logging: Log dropped packets
Yes
Windows Firewall: Domain: Logging: Log successful connections
Yes
Windows Firewall: Domain: Logging: Name
%SYSTEMROOT%
\System32\
logfiles
\firewall\doma
infw.log
Page 96

5 Configuration Instructions for Use
CLARUS® 5005.11 System Administration
96 / 192 2660021171806 Rev. A 2019-01
Policy Setting
Windows Firewall: Domain: Logging: Size limit (KB)
16384 KB
Windows Firewall: Domain: Outbound connections
Allow
Windows Firewall: Private: Display a notification
No
Windows Firewall: Private: Inbound connections
Inbound Connections
Enabled
Block
Windows Firewall: Private: Logging: Log dropped packets
Yes
Windows Firewall: Private: Logging: Log successful connections
Yes
Windows Firewall: Private: Logging: Name
%SYSTEMROOT%
\System32\
logfiles
\firewall\priv
atefw.log
Windows Firewall: Private: Logging: Size limit (KB)
16384 KB
Windows Firewall: Private: Outbound connections
Allow
Windows Firewall: Public: Apply local connection security rules
No
Windows Firewall: Public: Apply local firewall rules
No
Windows Firewall: Public: Display a notification
No
Windows Firewall: Public: Inbound connections
Inbound Connections
Enabled
Block
Windows Firewall: Public: Logging: Log dropped packets
Yes
Windows Firewall: Public: Logging: Log successful connections
Yes
Windows Firewall: Public: Logging: Name
%SYSTEMROOT%
\System32\
logfiles
\firewall\publ
icfw.log
Windows Firewall: Public: Logging: Size limit (KB)
16384 KB
Windows Firewall: Public: Outbound connections
Allow
Table12: Table Title
Page 97

Instructions for Use 6 About User Roles
CLARUS® 500
2660021171806 Rev. A 2019-01 97 / 192
6 About User Roles
Not all users have access to all features. The table below list some
of the key differences for different types of users.
Operator Doctor Adminis-
trator
Acquire scans X X
Review scans X X
Select, edit and annotate scans X X
Use review software installed on a separate computer X X
Reset your own password X X X
Delete a local patient record X X X
Save and print reports X X X
Import and export data X X X
Configure import and export settings X X X
Configure general settings X X
Configure network settings X
Configure data backup X
Restore data from a backup X
Perform system maintenance X
Manage licenses X
Reset other user's passwords X
Add or delete users X
Export log files X
Table13: Permissions Levels
Page 98

Empty page, for your notes
Page 99

Instructions for Use 7 Operation
CLARUS® 500 7.1 Safety During Operation
2660021171806 Rev. A 2019-01 99 / 192
7 Operation
NOTE
Third-party software updates and installations can slow the
system and impact software performance.
Anti-virus software an other applications perform periodic update
installations automatically, which use system resources that impact
the performance of other software.
u If the CLARUS® 500 software seems to be running slowly,
check whether other software (for example, anti-virus software)
is installing updates.
u Wait until the installations are complete or allow more time for
software response while the installation is in progress.
7.1 Safety During Operation
CAUTION!
Improper operator training
could lead to poor scan quality, damage to system components, or
inadvertent patient safety compromise.
u Train all operators fully.
u Ensure all personnel are familiar with the information contained
in the Safety and Certifications chapter.
u Ensure that routine maintenance has been properly carried out
in conformance with the Maintenance Schedules described in
the Maintenance chapter.
For lens cleaning instructions, refer to: Cleaning the Front Lens
[}149].
7.2 Preparing the Device
To prepare the device:
Action 1. Wipe the chin rest and forehead rest with an alcohol pad, and
allow the assembly to dry. (See Cleaning the Chinrest and
Forehead Support [}153].)
2. Carefully read and understand any instructions provided by the
officiating physician or researcher.
7.2.1 Finding a Patient
To find a patient:
Action 1. In the Search bar, type the first name, last name, patient ID, or
a scan date of the person you want to find.
2. Click the Search icon.
3. To refine your search criteria, use Advanced Search.
Page 100
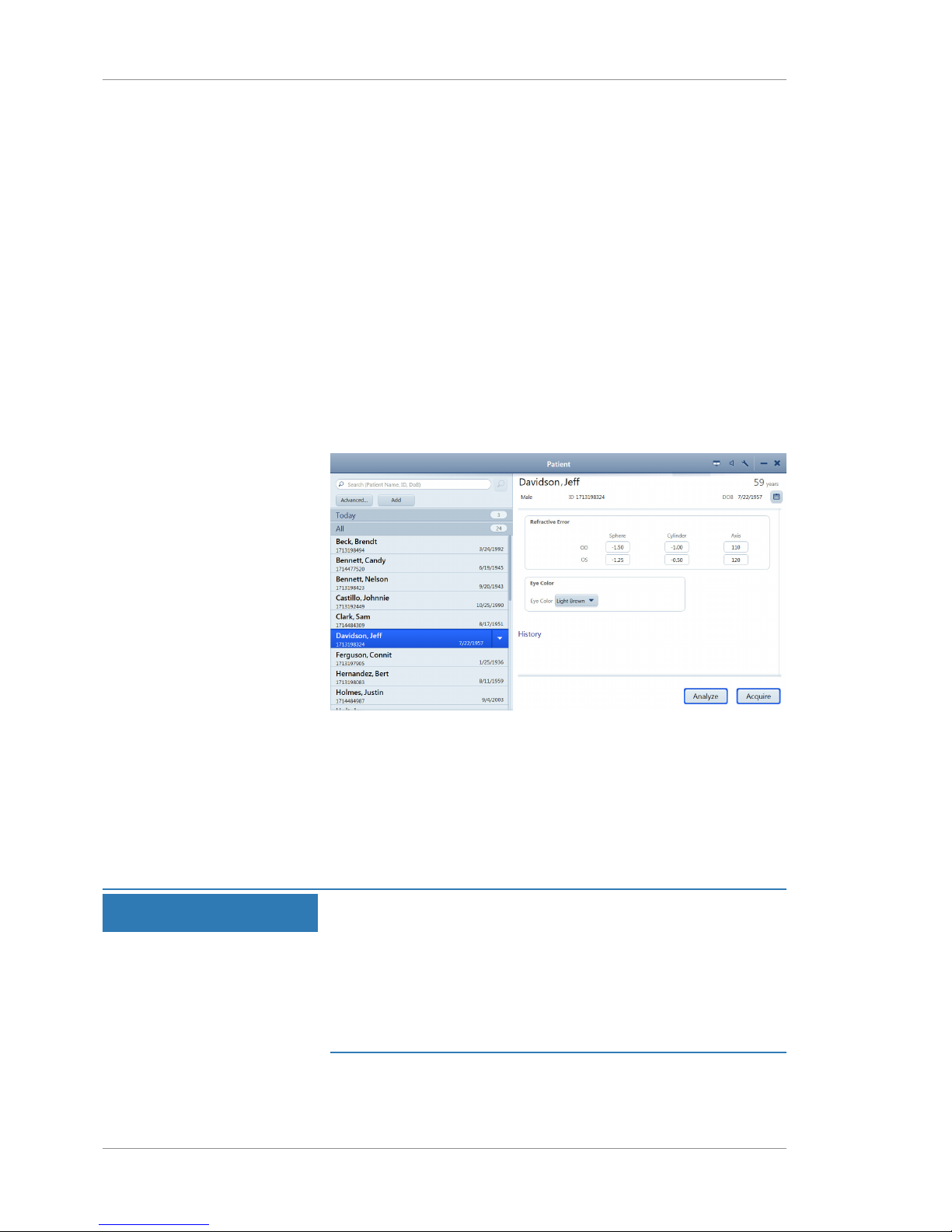
7 Operation Instructions for Use
CLARUS® 5007.3 Preparing the Patient
100 / 192 2660021171806 Rev. A 2019-01
7.2.2 Selecting the Patient
Finding a patient's name can vary depending on the systems you
use.
• If you use an EMR system:
– The patient's name might be listed under Today
– If the patient is not listed under Today, search for their
name or ID (See Finding a Patient [}99]).
– If the patient is not in the database, add the patient's infor-
mation. (See Adding a Patient [}49]).
• If you do not use an EMR system:
– Search for the patient's name or ID Search (See Finding a
Patient [}99]).
– If the patient is not in the database, add the patient's infor-
mation. (See Adding a Patient [}49]).
To select a patient:
Action 1. Find the patient's name using the list or search and select the
patient's name.
2. Click Acquire.
7.3 Preparing the Patient
NOTE
Keep the front of the lens clean.
The quality of image capture is affected by the cleanliness of the
front lens.
u Avoid touching the lens with anything but approved cleaning
equipment.
u Use the protective cover to keep dust off the lens when the
device is not in use.
For complete instructions on cleaning the lens, refer to: Cleaning
and Disinfection [}149]
 Loading...
Loading...