Stryker ProForm 281505550007, ProForm 281505550003, ProForm 281505550010, ProForm 281505550009, ProForm 281505550020 Operation Manual
...Page 1

PPrrooFFoorrmm™™ nnoonn--ppoowweerreedd ssuuppppoorrtt ssuurrffaaccee
OOppeerraattiioonnss MMaannuuaall
2815
2815-009-001 Rev D.1
EN
ES
FR
KO
PT
2019/04
Page 2

Page 3
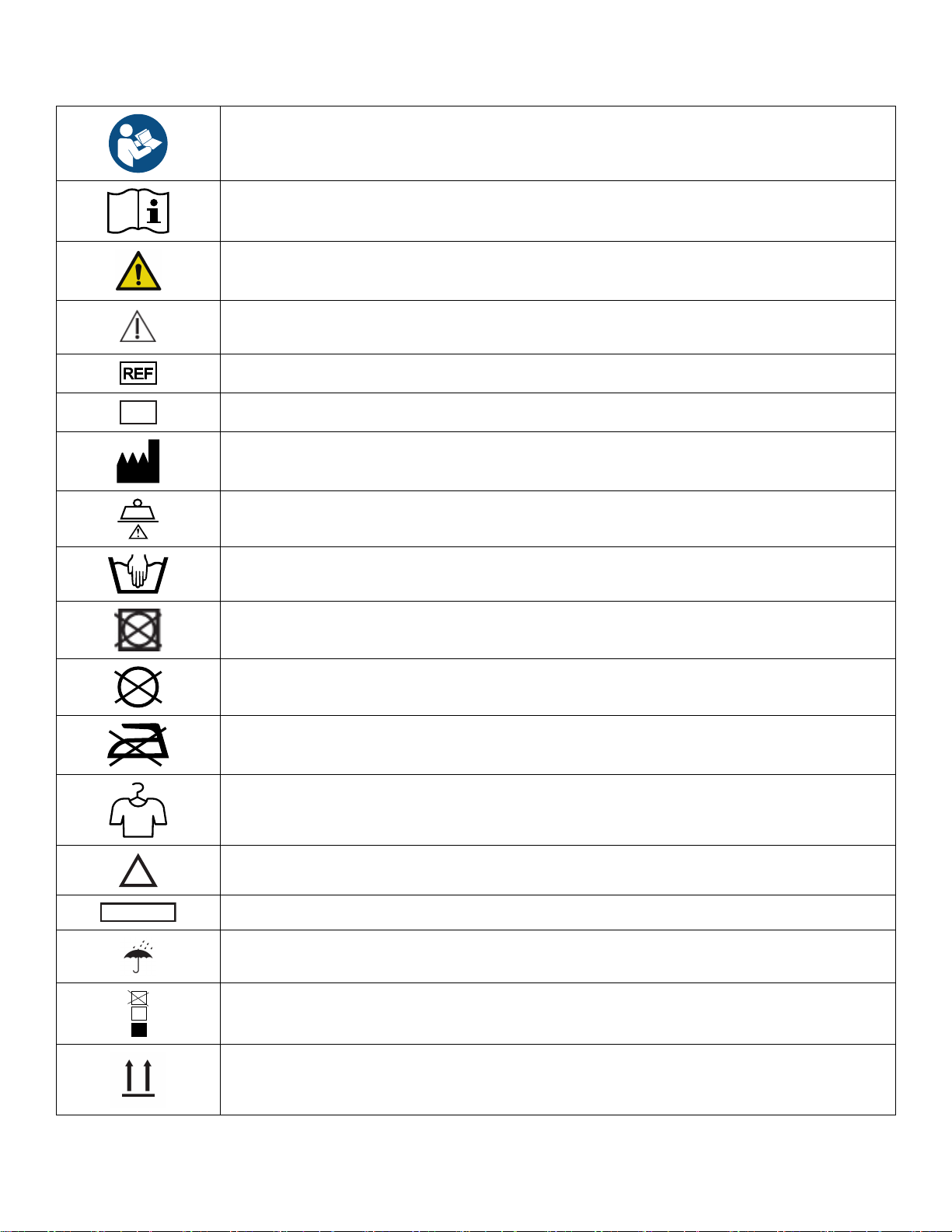
SSyymmbboollss
10
Refer to instruction manual/booklet
Operating instructions/Consult instructions for use
General warning
Caution
Catalogue number
Serial number
Manufacturer
Safe working load
Wash by hand
Do not tumble dry
Do not dry-clean
Do not iron
Allow to air dry
Chlorinated bleach
For US Patents see www.stryker.com/patents
Keep dry
Do not stack more than 10 high
2815-009-001 Rev D.1 EN
This side up
Page 4

Fragile
Do not use sharp objects to open the package
Mass of equipment
EN 2815-009-001 Rev D.1
Page 5

TTaabbllee ooff CCoonntteennttss
Symbols .............................................................................................................................................................1
Warning/Caution/Note Definition ....................................................................................................................2
Summary of safety precautions ...............................................................................................................2
Introduction ..................................................................................................................................................4
Product description ................................................................................................................................4
Intended use..........................................................................................................................................4
Expected service life - standard...............................................................................................................5
Expected life - behavioral health..............................................................................................................5
Contraindications ...................................................................................................................................5
Specifications ........................................................................................................................................5
Standard.........................................................................................................................................5
Behavioral health.............................................................................................................................6
Environmental conditions .................................................................................................................6
Contact information ................................................................................................................................6
Serial number location............................................................................................................................7
Date of manufacture...............................................................................................................................7
Setup...........................................................................................................................................................8
Operation.....................................................................................................................................................9
Transferring a patient from one patient support platform to another ............................................................9
Preventive maintenance ..............................................................................................................................10
Cleaning and disinfecting with wipes......................................................................................................10
Care and maintenance .........................................................................................................................11
Quick reference replacement parts...............................................................................................................12
Cover replacement - Standard model..................................................................................................... 12
2815-009-001 Rev D.1 1 EN
Page 6

WWaarrnniinngg//CCaauuttiioonn//NNoottee DDeeffiinniittiioonn
The words WWAARRNNIINNGG, CCAAUUTTIIOONN, and NNOOTTEE carry special meanings and should be carefully reviewed.
WWAARRNNIINNGG -- Alerts the reader about a situation which, if not avoided, could result in death or serious injury. It may also
describe potential serious adverse reactions and safety hazards.
CCAAUUTTIIOONN -- Alerts the reader of a potentially hazardous situation which, if not avoided, may result in minor or moderate
injury to the user or patient or damage to the product or other property. This includes special care necessary for the safe
and effective use of the device and the care necessary to avoid damage to a device that may occur as a result of use or
misuse.
NNoottee -- Provides special information to make maintenance easier or important instructions clearer.
SSuummmmaarryy ooff ssaaffeettyy pprreeccaauuttiioonnss
Always read and strictly follow the warnings and cautions listed on this page. Service only by qualified personnel.
WWAARRNNIINNGG
• Never use the standard model support surface in the behavioral health use environment. The zipper allows access to
the internal components which could be used for harm.
• Always check patient’s skin regularly. Consult a physician if erythema or skin breakdown occurs. Serious injury could
result if the patient’s skin condition is left untreated.
• Always use extra caution and supervision to help reduce the risk of a patient fall. Patient stability and siderail coverage
may be compromised with the use of an overlay.
• Always inspect for foreign objects between the support surface and the bed frame. Foreign objects may cause the
support surface to slide on the support platform.
• Always consider the use of siderails. The safe use of the support surface is maximized when used in conjunction with
siderails. There may be an increased risk of falls when siderails are not present. Serious injury or death can result from
the use (potential entrapment) or non-use (potential patient falls) of siderails or other restraints. Consider local policies
regarding the use of siderails. The physician, operator, or responsible parties should determine whether and how to use
siderails based on each patient’s individual needs.
• Always use extra caution with a patient at risk of a fall (such as agitated or confused) to help reduce the likelihood of a
fall.
• Do not use the support surface on a larger or smaller bed frame that does not fit the width, length, or thickness. This is to
avoid the risk of the support surface sliding, patient injury, or interference with moving parts of the bed.
• Do not use the support surface when gaps are present. The risk of entrapment can develop when the support surface is
placed on non-compatible bed frames.
• Do not stick needles into a support surface through the support surface cover. Holes may allow body fluids to enter the
inside (inner core) of the support surface and could cause cross-contamination, product damage, or product
malfunction.
• Do not use the support surface as a transfer device.
• Do not use the support surface handles to lift or move the support surface with a patient on board.
• Do not exceed the safe working load of the hospital bed frame when supporting both the patient and the support surface.
Excess weight could cause unpredictable safety and performance of this product.
• Always make sure that the patient support platforms and their respective transfer gaps are adequate to support the
patient. If the space between the two patient support platforms is greater than 3 in. (7.6 cm), use the transfer bridge to fill
the gap. The transfer bridge is meant to ease transfer of a patient from one patient support platform to another.
• Always make sure that the opposite siderail is raised when placing a patient on the support surface to reduce the risk of
patient fall.
• Do not wash the internal components of this support surface. Discard the support surface if a contamination is found
inside.
• Do not immerse the product in cleaning or disinfectant solutions.
• Do not allow liquid to pool on the product.
EN 2 2815-009-001 Rev D.1
Page 7

• Always inspect support surface covers (top and bottom) for tears, punctures, excessive wear, and misaligned zippers
before each use. If compromised immediately remove the support surface from service.
• Always make sure that you wipe each product with clean water and dry each product after you clean. Some cleaning
agents are corrosive in nature and may cause damage to the product. Failure to follow these cleaning instructions may
void your warranty.
CCAAUUTTIIOONN
• Improper usage of the product can cause injury to the patient or operator. Operate the product only as described in this
manual.
• Do not modify the product or any components of the product. Modifying the product can cause unpredictable operation
resulting in injury to patient or operator. Modifying the product also voids its warranty.
• Always be aware of devices or equipment that are placed on the top of the support surface. Damage to the surface may
occur due to the weight of the equipment, heat generated by the equipment, or sharp edges on the equipment.
• Do not put overlays or accessories inside the cover to avoid the risk of reducing pressure redistribution performance.
• Do not allow liquid to seep into the zipper area or watershed cover barrier when you clean the underside of the support
surface. Fluids allowed to come in contact with the zipper may leak into the support surface core.
• Do not iron, dry-clean, or tumble dry the support surface covers.
• Do not power wash the support surface as this may damage the product.
• Always dry the support surface covers before you store, add linens, or place a patient on the surface. A dry product
helps to prevent impaired performance.
• Do not over expose the covers to higher concentration chemical solutions as these may degrade the covers.
• Do not use accelerated hydrogen peroxides or quaternaries that contain glcyol ether >3% as these chemicals may
damage the support surface cover.
• Failure to follow manufacturing instructions may also affect useful life of the support surface cover.
• Always take caution when you replace the support surface cover. The internal fire barrier contains fiberglass fibers. Dust
particles from the fibers may cause skin irritation.
2815-009-001 Rev D.1 3 EN
Page 8
IInnttrroodduuccttiioonn
This manual assists you with the operation or maintenance of your Stryker product. Read this manual before operating or
maintaining this product. Set methods and procedures to educate and train your staff on the safe operation or maintenance
of this product.
CCAAUUTTIIOONN
• Improper usage of the product can cause injury to the patient or operator. Operate the product only as described in this
manual.
• Do not modify the product or any components of the product. Modifying the product can cause unpredictable operation
resulting in injury to patient or operator. Modifying the product also voids its warranty.
NNoottee
• This manual is a permanent part of the product and should remain with the product even if the product is sold.
• Stryker continually seeks advancements in product design and quality. This manual contains the most current product
information available at the time of printing. There may be minor discrepancies between your product and this manual. If
you have any questions, contact Stryker Customer Service or Technical Support at 1-800-327-0770.
PPrroodduucctt ddeessccrriippttiioonn
PPrrooFFoorrmm™™ is a 6” thick, non-powered foam support surface for the general patient population. There are articulation cuts
on the mattress core. The mattress cover material options include nylon material or END406 by DDaarrtteexx® fabric. All
configurations of the product include heel slope at the foot end of the support surface. The dimensions of this product are
compatible with SSttrryykkeerr bed frames. See the compatible bed frame list in the specifications section of this manual.
IInntteennddeedd uussee
PPrrooFFoorrmm uses foam to help with pressure redistribution and immersion.
This product is a non-powered support surface that assists in the prevention and treatment of pressure injuries or pressure
ulcers (all stages, Unstageable injury, and Deep Tissue injury). We recommend you implement this product in combination
with clinical evaluation of risk factors and skin assessments made by a healthcare professional.
The standard Model (281505550001, 281505550003, 281505550005, 281505550007, 281505550020) is intended for the
general hospital environment, specifically the acute care healthcare facilities.
The behavioral health Model (281505550009, 281505550010, 281505550011) are intended for use in behavioral care
facilities.
PPrrooFFoorrmm shall be used with a support surface cover at all times. The support surface cover can interact with all external
skin. A top sheet should be used with this product.
The standard general hospital environment models and the behavioral health models are not intended for:
• Patients that exceed the safe working load
• Bariatric patients
• Home health environment setting
• Not a sterile product
• Does not contain a measuring function
SSttrryykkeerr promotes the clinical assessment of each patient and appropriate usage by the operator.
EN 4 2815-009-001 Rev D.1
Page 9

EExxppeecctteedd sseerrvviiccee lliiffee -- ssttaannddaarrdd
The PPrrooFFoorrmm support surface core and covers have a 3 year expected service life under normal use, conditions, and with
appropriate periodic maintenance.
EExxppeecctteedd lliiffee -- bbeehhaavviioorraall hheeaalltthh
The PPrrooFFoorrmm support surface has a 3 year expected life under normal use, conditions, and with appropriate periodic
maintenance. There are no serviceable parts for this product.
CCoonnttrraaiinnddiiccaattiioonnss
None known.
SSppeecciiffiiccaattiioonnss
500 lb 226.8 kg
Safe working load
Minimum patient weight 50 lb 26.8 kg
NNoottee -- The patient must not exceed safe working load specified by the support surface.
SSttaannddaarrdd
MMooddeell ggeenneerraall
ppaattiieenntt
Length 84 in. 213.4 cm 80 in. 203.2 cm
Width 35 in. 88.9 cm 35 in. 88.9 cm
Thickness 6 in. 15.2 cm 6 in. 15.2 cm
Product weight 30 lb 13.6 kg 29 lb 13.2 kg
Top cover material END406 by DDaarrtteexx®
MMooddeell ggeenneerraall
ppaattiieenntt
Length 84 in. 213.4 cm 80 in. 203.2 cm
Width 35 in. 88.9 cm 35 in. 88.9 cm
Thickness 6 in. 15.2 cm 6 in. 15.2 cm
Product weight 30 lb 13.6 kg 29 lb 13.2 kg
228811550055555500000011 oorr 228811550055555500002200 bbaaggggeedd 228811550055555500000033
228811550055555500000055 228811550055555500000077
Top cover material Nylon
Mattress material Polyurethane foam
Product compliance with fire barrier 16CFR1632, 16CFR1633, CGSB CAN 2-4.2 Method 27.7-
M77, CAL TB129, BFD IX-11
Compatible frames 3002 SS33™™, 3005 SS33™™, GGooBBeedd® II, SSppiirriitt SSeelleecctt™™with
high quad 4 zone siderails
2815-009-001 Rev D.1 5 EN
Page 10
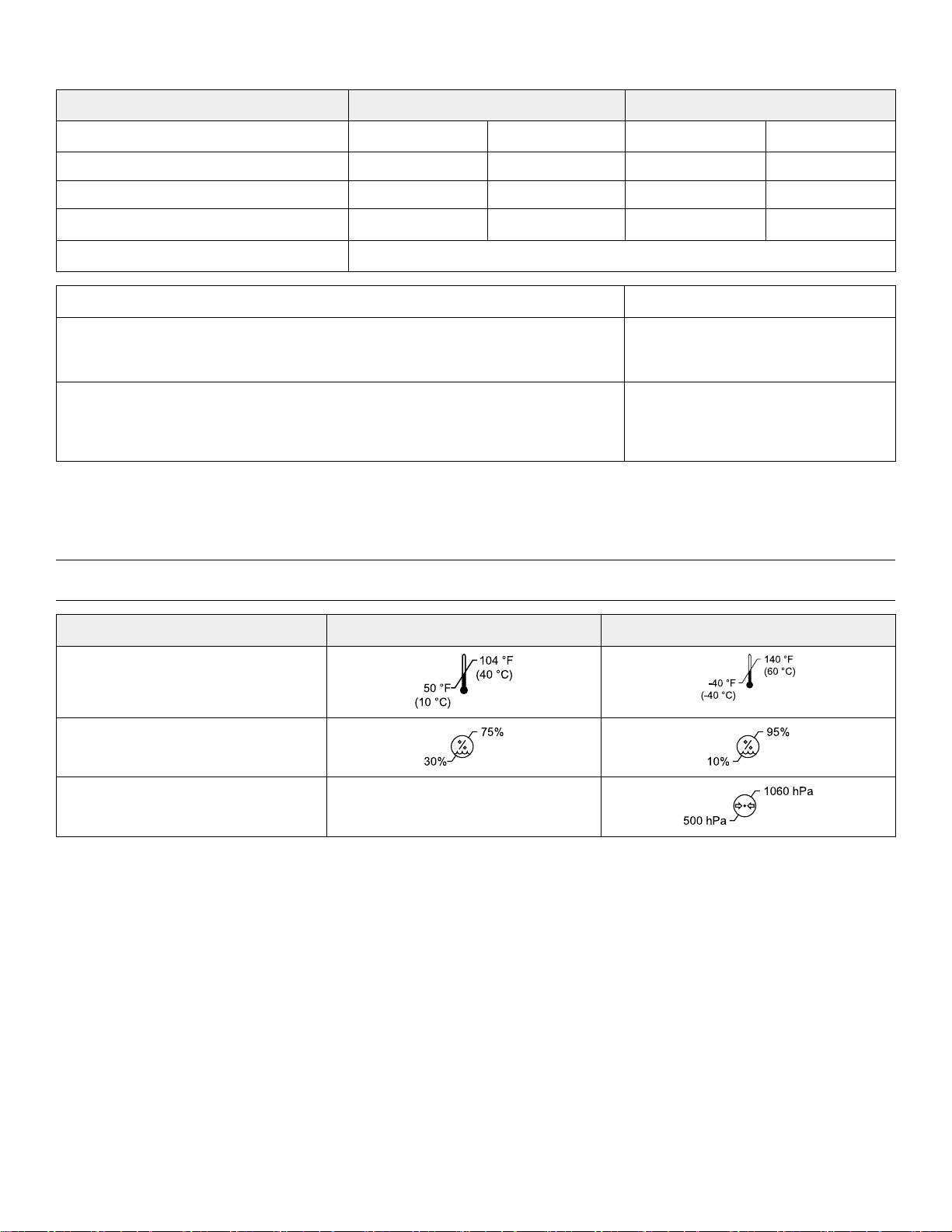
BBeehhaavviioorraall hheeaalltthh
MMooddeell 228811550055555500001100,, 228811550055555500001111 228811550055555500000099
Length 80 in. 203.2 cm 84 in. 213.4 cm
Width 35 in. 88.9 cm 35 in. 76 cm
Thickness 6 in. 15.2 cm 6 in. 7.6 cm
Product weight 29 lb 13.2 kg 30 lb 13.6 cm
Top cover material END406 by DDaarrtteexx®®
Mattress material Polyurethane foam
Product compliance with fire barrier 16CFR1632, 16CFR1633, CGSB
CAN 2-4.2 Method 27.7-M77, CAL
TB129, BFD IX-11
Compatible frames 3002 SS33™™, 3005 SS33™™, GGooBBeedd®® II,
SSppiirriitt SSeelleecctt™™ with high quad 4
zone siderails, SSppiirriitt®® Behavioral
Health Platform Bed
NNoottee -- The Behavioral health covers do not have zippers. The product is not serviceable.
EEnnvviirroonnmmeennttaall ccoonnddiittiioonnss
WWAARRNNIINNGG -- Never use the standard model support surface in the behavioral health use environment. The zipper allows
access to the internal components which could be used for harm.
EEnnvviirroonnmmeennttaall ccoonnddiittiioonnss
Ambient temperature
Relative humidity (non-condensing)
Atmospheric pressure
Stryker reserves the right to change specifications without notice.
OOppeerraattiioonn SSttoorraaggee aanndd ttrraannssppoorrttaattiioonn
CCoonnttaacctt iinnffoorrmmaattiioonn
Contact Stryker Customer Service or Technical Support at: 1-800-327-0770.
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
USA
To view your operations or maintenance manual online, see https://techweb.stryker.com/.
EN 6 2815-009-001 Rev D.1
Page 11
Have the serial number (A) of your Stryker product available when calling Stryker Customer Service or Technical Support.
A
Include the serial number in all written communication.
SSeerriiaall nnuummbbeerr llooccaattiioonn
DDaattee ooff mmaannuuffaaccttuurree
The year of manufacture is the first four digits of the serial number.
2815-009-001 Rev D.1 7 EN
Page 12
SSeettuupp
WWAARRNNIINNGG
• Always check patient’s skin regularly. Consult a physician if erythema or skin breakdown occurs. Serious injury could
result if the patient’s skin condition is left untreated.
• Always use extra caution and supervision to help reduce the risk of a patient fall. Patient stability and siderail coverage
may be compromised with the use of an overlay.
• Always inspect for foreign objects between the support surface and the bed frame. Foreign objects may cause the
support surface to slide on the support platform.
• Always consider the use of siderails. The safe use of the support surface is maximized when used in conjunction with
siderails. There may be an increased risk of falls when siderails are not present. Serious injury or death can result from
the use (potential entrapment) or non-use (potential patient falls) of siderails or other restraints. Consider local policies
regarding the use of siderails. The physician, operator, or responsible parties should determine whether and how to use
siderails based on each patient’s individual needs.
• Always use extra caution with a patient at risk of a fall (such as agitated or confused) to help reduce the likelihood of a
fall.
• Do not use the support surface on a larger or smaller bed frame that does not fit the width, length, or thickness. This is to
avoid the risk of the support surface sliding, patient injury, or interference with moving parts of the bed.
• Do not use the support surface when gaps are present. The risk of entrapment can develop when the support surface is
placed on non-compatible bed frames.
• Do not stick needles into a support surface through the support surface cover. Holes may allow body fluids to enter the
inside (inner core) of the support surface and could cause cross-contamination, product damage, or product
malfunction.
CCAAUUTTIIOONN
• Always be aware of devices or equipment that are placed on the top of the support surface. Damage to the surface may
occur due to the weight of the equipment, heat generated by the equipment, or sharp edges on the equipment.
• Do not put overlays or accessories inside the cover to avoid the risk of reducing pressure redistribution performance.
To setup the support surface:
1. Make sure that the support surface fits the bed frame.
2. Place the support surface with the artwork face up at the head end of the bed frame.
3. Make sure that the mattress is placed between the mattress retainers on the bed frame.
4. Place linens on the support surface per hospital protocols.
EN 8 2815-009-001 Rev D.1
Page 13

OOppeerraattiioonn
TTrraannssffeerrrriinngg aa ppaattiieenntt ffrroomm oonnee ppaattiieenntt ssuuppppoorrtt ppllaattffoorrmm ttoo aannootthheerr
WWAARRNNIINNGG
• Do not use the support surface as a transfer device.
• Do not use the support surface handles to lift or move the support surface with a patient on board.
• Do not stick needles into a support surface through the support surface cover. Holes may allow body fluids to enter the
inside (inner core) of the support surface and could cause cross-contamination, product damage, or product
malfunction.
• Do not exceed the safe working load of the hospital bed frame when supporting both the patient and the support surface.
Excess weight could cause unpredictable safety and performance of this product.
• Always make sure that the patient support platforms and their respective transfer gaps are adequate to support the
patient. If the space between the two patient support platforms is greater than 3 in. (7.6 cm), use the transfer bridge to fill
the gap. The transfer bridge is meant to ease transfer of a patient from one patient support platform to another.
• Always make sure that the opposite siderail is raised when placing a patient on the support surface to reduce the risk of
patient fall.
To transfer the patient from one patient support surface to another:
PPrreerreeqquuiissiittee:: Follow hospital protocols required to transfer a patient from one surface to another.
1. Position one patient support platform alongside the other patient support platform while minimizing the gap between the
two platforms.
2. Set the brakes to on for both patient support platforms.
3. Adjust the patient support platform heights so that they are level with one another.
4. Transfer the patient following all applicable safety rules and institution protocols for patient and operator safety.
2815-009-001 Rev D.1 9 EN
Page 14

PPrreevveennttiivvee mmaaiinntteennaannccee
At a minimum, check all items listed during annual preventive maintenance for all Stryker Medical products. You may need
to perform preventive maintenance checks more often based on your level of product usage.
Remove product from service before performing preventive maintenance.
NNoottee -- Clean and disinfect the exterior of the support surface before inspection, if applicable.
Inspect the following items for the standard models:
Zipper and covers (top and bottom) are free of tears, cuts, holes, or other openings
Internal components for signs of staining from fluid ingress or contamination by fully unzipping the covers
Labels for legibility, proper adherence, and integrity
Handles are free of rips or cracks and stitching is intact
Foam has not degraded or come apart
Fire barrier for rips, cracks, or other visible signs of damage
Inspect the following items for the behavioral health models:
Covers (top and bottom) are free of tears, cuts, holes, or other openings
Labels for legibility, proper adherence, and integrity
Product serial number:
Completed by:
Date:
CClleeaanniinngg aanndd ddiissiinnffeeccttiinngg wwiitthh wwiippeess
For United States only. Confirm availability for your configuration or region. Call Stryker Customer Service: 1-800-327-
0770.
Stryker’s preferred wipes (2060-000-001 6'' x 10'' or 2060-000-002 9'' x 12'') include the following active ingredients:
• n-Alkyl (60% C14, 30% C16, 5% C12, 5% C18) dimethyl benzyl ammonium chloride - 0.154%
• n-Alkyl (68% C12, 32% C14) dimethyl ethylbenzyl ammonium chloride - 0.154%
• Isopropanol - 21.000%
Non-active ingredient: Ethylene Glycol Monobutyl Ether – < 3%
NNoottee -- For safety information, read the product label.
To clean or disinfect the external product surface:
1. To clean, wipe external surfaces with a fresh, clean wipe to remove all visible soils. Repeat as necessary until the
product is clean.
NNoottee
• Use as many wipes as necessary.
• Complete step 1 before you disinfect.
2. To disinfect, wipe external surfaces with a fresh, clean wipe until wet. Allow the external surface to remain wet for two
minutes at room temperature.
3. Allow the product to dry before you return it to service.
EN 10 2815-009-001 Rev D.1
Page 15

CCaarree aanndd mmaaiinntteennaannccee
WWAARRNNIINNGG
• Do not wash the internal components of this support surface. Discard the support surface if a contamination is found
inside.
• Do not immerse the product in cleaning or disinfectant solutions.
• Do not allow liquid to pool on the product.
• Always inspect support surface covers (top and bottom) for tears, punctures, excessive wear, and misaligned zippers
before each use. If compromised immediately remove the support surface from service.
• Always make sure that you wipe each product with clean water and dry each product after you clean. Some cleaning
agents are corrosive in nature and may cause damage to the product. Failure to follow these cleaning instructions may
void your warranty.
CCAAUUTTIIOONN
• Do not allow liquid to seep into the zipper area or watershed cover barrier when you clean the underside of the support
surface. Fluids allowed to come in contact with the zipper may leak into the support surface core.
• Do not iron, dry-clean, or tumble dry the support surface covers.
• Do not power wash the support surface as this may damage the product.
• Always dry the support surface covers before you store, add linens, or place a patient on the surface. A dry product
helps to prevent impaired performance.
• Do not over expose the covers to higher concentration chemical solutions as these may degrade the covers.
• Do not use accelerated hydrogen peroxides or quaternaries that contain glcyol ether >3% as these chemicals may
damage the support surface cover.
• Failure to follow manufacturing instructions may also affect useful life of the support surface cover.
The support surface cover is resistant to the following chemical solutions:
• Quaternaries (active ingredient - ammonium chloride) that contain less than 3% glycol ether
• Phenolic solution (Matar)
• Chlorinated bleach solution (10000 ppm) for use with END406 mattress covers
• Chlorinated bleach solution (1000 ppm) for use with Nylon mattress covers
• 70% isopropyl alcohol
Follow hospital protocol for support surface care between patients, to avoid the risk of cross-contamination and infection.
NNoottee -- Chlorinated bleach solution may cause discoloration of the Nylon mattress covers.
2815-009-001 Rev D.1 11 EN
Page 16
QQuuiicckk rreeffeerreennccee rreeppllaacceemmeenntt ppaarrttss
These parts are available for purchase for the standard models, but subject to change. Call Stryker Customer Service: 1800-327-0770 for available parts and prices.
NNaammee NNuummbbeerr
Cover assembly, heel slope, END406 80 in. 281505550012
Cover assembly, heel slope, END406, 84 in. 281505550014
Cover assembly, heel slope, Nylon 80 in. 281505550018
Cover assembly, heel slope, Nylon 84 in. 281505550016
CCoovveerr rreeppllaacceemmeenntt -- SSttaannddaarrdd mmooddeell
CCAAUUTTIIOONN -- Always take caution when you replace the support surface cover. The internal fire barrier contains fiberglass
fibers. Dust particles from the fibers may cause skin irritation.
TToooollss rreeqquuiirreedd::
• None
PPrroocceedduurree::
1. Raise the bed height to the full up position.
2. Lower the fowler and gatch sections to the full down positions.
3. Unzip the cover. Start at the foot end patient right corner of the support surface and stop at the head end patient right
corner.
4. Fold the top of the cover to the patient’s right side and then remove the foam crib assembly from the bed and set aside.
5. Remove and discard the cover.
6. Place the replacement cover, unzipped and open, on the bed with the black bottom cover on the litter and the top cover
folded over the patient’s right side of the bed.
7. Place the foam crib assembly on top of the bottom part of the cover to make sure that the foam crib is aligned with the
cover.
8. Fold the top cover over the top of the foam crib assembly to make sure that the top cover is aligned with the foam crib
assembly.
9. Zip the cover to close. Start at the head end patient right corner and stop at the foot end patient right corner.
10.Verify proper operation before you return the product to service.
EN 12 2815-009-001 Rev D.1
Page 17

SSuuppeerrffiicciiee ddee ssooppoorrttee nnoo mmoottoorriizzaaddaa PPrrooFFoorrmm™™
MMaannuuaall ddee uussoo
2815
2815-009-001 Rev D.1
ES
2019/04
Page 18

Page 19
SSíímmbboollooss
10
Consultar el manual/folleto de instrucciones
Instrucciones de utilización/Consultar las instrucciones de uso
Advertencia general
Precaución
Número de catálogo
Número de serie
Fabricante
Carga de trabajo segura
Lavar a mano
No secar en secadora
No limpiar a seco
No planchar
Dejar secar al aire
Lejía clorada
Para ver las patentes estadounidenses, visite www.stryker.com/patents
Mantener seco
No apilar más de 10 unidades
2815-009-001 Rev D.1 ES
Este lado hacia arriba
Page 20

Frágil
No utilizar objetos afilados para abrir el paquete
Peso del equipo
ES 2815-009-001 Rev D.1
Page 21

ÍÍnnddiiccee
Símbolos ..........................................................................................................................................................15
Definición de advertencia, precaución y nota ..................................................................................................2
Resumen de las precauciones de seguridad ............................................................................................2
Introducción .................................................................................................................................................4
Descripción del producto ........................................................................................................................4
Uso previsto ..........................................................................................................................................4
Vida útil prevista, estándar ......................................................................................................................5
Vida útil prevista, salud conductual..........................................................................................................5
Contraindicaciones.................................................................................................................................5
Especificaciones ....................................................................................................................................5
Estándar.........................................................................................................................................5
Salud conductual .............................................................................................................................6
Condiciones ambientales .................................................................................................................6
Información de contacto..........................................................................................................................7
Ubicación del número de serie ................................................................................................................7
Fecha de fabricación ..............................................................................................................................7
Instalación....................................................................................................................................................8
Funcionamiento............................................................................................................................................9
Transferencia de un paciente de una plataforma de soporte del paciente a otra ..........................................9
Mantenimiento preventivo............................................................................................................................10
Limpieza y desinfección con paños........................................................................................................10
Cuidado y mantenimiento .....................................................................................................................11
Referencia rápida a las piezas de repuesto................................................................................................... 12
Sustitución de la funda, modelo estándar ...............................................................................................12
2815-009-001 Rev D.1 1 ES
Page 22

DDeeffiinniicciióónn ddee aaddvveerrtteenncciiaa,, pprreeccaauucciióónn yy nnoottaa
Las palabras AADDVVEERRTTEENNCCIIAA, PPRREECCAAUUCCIIÓÓNN y NNOOTTAA tienen un significado especial y deberán considerarse
detenidamente.
AADDVVEERRTTEENNCCIIAA -- Advierten al lector sobre situaciones que, si no se evitan, podrían producir la muerte o lesiones graves.
También pueden describir posibles reacciones adversas graves y peligros para la seguridad.
PPRREECCAAUUCCIIÓÓNN -- Advierten al lector sobre situaciones potencialmente peligrosas que, si no se evitan, pueden producir
lesiones leves o moderadas al usuario o al paciente, o daños al equipo u otras propiedades. Incluyen cuidados especiales
necesarios para el uso seguro y eficaz del dispositivo, y para evitar dañarlo con el uso o el mal uso.
NNoottaa -- Ofrecen información especial que facilita el mantenimiento o aclara instrucciones importantes.
RReessuummeenn ddee llaass pprreeccaauucciioonneess ddee sseegguurriiddaadd
Lea siempre las advertencias y precauciones indicadas en esta página, y sígalas escrupulosamente. Las reparaciones
solo puede realizarlas personal cualificado.
AADDVVEERRTTEENNCCIIAA
• Nunca utilice la superficie de soporte del modelo estándar en entornos de salud conductual. La cremallera permite
acceder a componentes internos que podrían utilizarse para producir daños.
• Compruebe siempre con regularidad la piel del paciente. Consulte a un médico si se produce un eritema o una lesión
cutánea. Podrían producirse lesiones graves si no se trata la afección de la piel del paciente.
• Extreme las precauciones y la vigilancia para ayudar a reducir el riesgo de caída del paciente. La estabilidad del
paciente y la protección que brindan las barras laterales pueden reducirse por el uso de un protector.
• Compruebe siempre si hay objetos extraños entre la superficie de soporte y el bastidor de la cama. Los objetos extraños
pueden hacer que la superficie de soporte se deslice sobre la plataforma de soporte.
• Considere siempre el uso de las barras laterales. El uso seguro de la superficie de soporte se maximiza cuando se
utiliza junto con las barras laterales. Cuando las barras laterales no están presentes, el riesgo de caída es mayor.
Pueden producirse lesiones graves o la muerte debido al uso (posible atrapamiento) o al no uso (posibles caídas de
pacientes) de las barras laterales o de otros elementos restrictivos. Tenga en cuenta las políticas locales sobre el uso
de las barras laterales. El médico, el operador o las partes responsables deberán determinar si utilizar o no las barras
laterales, y cómo hacerlo en función de las necesidades individuales de cada paciente.
• Tenga siempre más cuidado con los pacientes con riesgo de caer (como cuando están agitados o confusos) para
reducir la probabilidad de una caída.
• No utilice la superficie de soporte sobre bastidores de cama más grandes o más pequeños que no coincidan en
anchura, longitud o grosor. Esto es para evitar el riesgo de deslizamiento de la superficie de soporte, de lesiones en el
paciente o de interferencia con las piezas móviles de la cama.
• No utilice la superficie de soporte cuando haya huecos. Cuando la superficie de soporte se coloque en bastidores de
cama no compatibles, es posible que aumente el riesgo de atrapamiento.
• No pase agujas a través de la funda de la superficie de soporte. Los orificios pueden permitir el paso de líquidos
corporales al interior (parte central interna) de la superficie de soporte, lo que podría causar contaminación cruzada y
daños o mal funcionamiento del producto.
• No utilice la superficie de soporte como un dispositivo de transferencia.
• No utilice las agarraderas de la superficie de soporte para levantar o desplazar la superficie de soporte con un paciente
encima.
• No supere la carga de trabajo segura del bastidor de la cama del hospital cuando sostenga al paciente y la superficie de
soporte. El peso excesivo puede provocar resultados impredecibles en el funcionamiento y la seguridad de este
producto.
• Asegúrese siempre de que las plataformas de soporte del paciente y sus correspondientes huecos de transferencia
sean los adecuados para dar soporte al paciente. Si el hueco entre las dos plataformas de soporte del paciente es de
más de 3 pulgadas (7,6 cm), utilice el puente de transferencia para rellenar el hueco. El puente de transferencia está
diseñado para facilitar la transferencia de un paciente de una plataforma de soporte del paciente a otra.
• Para reducir el riesgo de caída del paciente, cuando coloque al paciente sobre la superficie de soporte, asegúrese
siempre de que la barra lateral opuesta esté levantada.
ES 2 2815-009-001 Rev D.1
Page 23

• No lave los componentes internos de esta superficie de soporte. Si encuentra contaminación en su interior, deseche la
superficie de soporte.
• No sumerja el producto en soluciones de limpieza o desinfectantes.
• No deje que se acumule líquido sobre el producto.
• Antes de cada uso, inspeccione siempre las fundas (superior e inferior) de la superficie de soporte para detectar
desgarros, perforaciones, desgaste excesivo y cremalleras mal alineadas. Si observa algún deterioro, retire del servicio
de inmediato la superficie de soporte.
• Asegúrese siempre de limpiar todos los productos con agua limpia y de secarlos después de la limpieza. Algunos
productos de limpieza son corrosivos por naturaleza y pueden provocar daños al producto. Si no se siguen estas
instrucciones de limpieza, se podría anular la garantía del producto.
PPRREECCAAUUCCIIÓÓNN
• El uso inadecuado del producto puede provocar lesiones al paciente o al operador. Utilice el producto únicamente como
se describe en este manual.
• No modifique el producto ni ninguno de sus componentes. La modificación del producto puede provocar un
funcionamiento impredecible que, a su vez, cause lesiones al paciente o al operador. La modificación del producto
también anula su garantía.
• Tenga siempre cuidado con los dispositivos o el equipo colocados sobre la superficie de soporte. La superficie podría
dañarse debido al peso del equipo, el calor generado por este o sus bordes afilados.
• No coloque protectores ni accesorios dentro de la funda para evitar el riesgo de reducir el rendimiento de la
redistribución de la presión.
• No permita que se filtre líquido por la zona de la cremallera ni por la barrera de la funda que protege a la cremallera de
la entrada de líquidos cuando limpie la parte inferior de la superficie de soporte. Los líquidos que entren en contacto con
la cremallera podrían pasar a la la parte central interna de la superficie de soporte.
• Las fundas de la superficie de soporte no pueden plancharse, limpiarse en seco ni secarse en una secadora de ropa.
• No lave a presión la superficie de soporte porque podría dañar el producto.
• Deje siempre que las fundas de la superficie de soporte se sequen antes de guardar la superficie de soporte, ponerle
sábanas o colocar un paciente sobre ella. El secado del producto ayuda a evitar que su rendimiento disminuya.
• No exponga excesivamente las fundas a soluciones químicas muy concentradas, ya que estas pueden degradarlas.
• No utilice peróxidos de hidrógeno acelerados ni compuestos cuaternarias que contengan >3 % de éteres de glicol, ya
que estos productos químicos podrían dañar la funda de la superficie de soporte.
• El incumplimiento de las instrucciones de fabricación también puede afectar a la vida útil de la funda de la superficie de
soporte.
• Tenga cuidado siempre que sustituya la funda de la superficie de soporte. La barrera ignífuga interna contiene fibras de
fibra de vidrio. Las partículas de polvo de las fibras pueden provocar irritación cutánea.
2815-009-001 Rev D.1 3 ES
Page 24
IInnttrroodduucccciióónn
Este manual le ayudará a utilizar o mantener su producto de Stryker. Lea este manual antes de utilizar este producto o de
realizar su mantenimiento. Establezca métodos y procedimientos para formar a su personal en el uso o el mantenimiento
seguros de este producto.
PPRREECCAAUUCCIIÓÓNN
• El uso inadecuado del producto puede provocar lesiones al paciente o al operador. Utilice el producto únicamente como
se describe en este manual.
• No modifique el producto ni ninguno de sus componentes. La modificación del producto puede provocar un
funcionamiento impredecible que a su vez cause lesiones al paciente o al operador. La modificación del producto
también anula su garantía.
NNoottaa
• Este manual es un componente permanente del producto y debe permanecer con él si se vende.
• Stryker busca continuamente el avance en el diseño y la calidad de sus productos. Este manual contiene la información
sobre el producto más actualizada disponible en el momento de la impresión. Puede haber ligeras discrepancias entre
su producto y este manual. Si tiene preguntas, póngase en contacto con el Servicio de Atención al Cliente o con el
Servicio de Asistencia Técnica de Stryker, en el +1-800-327-0770 (llamada gratuita en EE. UU.).
DDeessccrriippcciióónn ddeell pprroodduuccttoo
PPrrooFFoorrmm™™ es una superficie de soporte de espuma no motorizada de 6 pulgadas (15,2 cm) de grosor para la población
general de pacientes. La parte central interna del colchón tiene cortes de articulación. Las opciones del material de la
funda del colchón incluyen material de nailon o tejido END406 de DDaarrtteexx®. Todas las configuraciones del producto
incluyen inclinación de talón en el extremo de los pies de la superficie de soporte. Las dimensiones de este producto son
compatibles con los bastidores de cama de SSttrryykkeerr. Consulte la lista de bastidores de cama compatibles en el apartado de
especificaciones de este manual.
UUssoo pprreevviissttoo
PPrrooFFoorrmm utiliza espuma para facilitar la redistribución de la presión y la inmersión.
Este producto es una superficie de soporte no motorizada que ayuda a prevenir y tratar lesiones por presión o úlceras por
presión (todas las fases, lesiones no clasificables y lesiones tisulares profundas). Le recomendamos que utilice este
producto junto con la evaluación clínica de factores de riesgo y la evaluación cutánea realizadas por un profesional
sanitario.
El modelo estándar (281505550001, 281505550003, 281505550005, 281505550007, 281505550020) está indicado para
entornos hospitalarios generales, concretamente para los centros sanitarios de agudos.
El modelo de salud conductual (281505550009, 281505550010, 281505550011) está indicado para utilizarse en centros
de salud conductual.
PPrrooFFoorrmm deberá utilizarse siempre con una funda para la superficie de soporte. La funda de la superficie de soporte puede
interactuar con toda la piel externa. Este producto deberá utilizarse con una sábana encimera.
Los modelos estándar para entornos hospitalarios generales y los modelos de salud conductual no están indicados para:
• Pacientes que superen la carga de trabajo segura
• Pacientes bariátricos
• Entornos sanitarios domésticos
• No es un producto estéril
• No incluye una función de medición
SSttrryykkeerr fomenta la evaluación clínica de cada paciente y el uso adecuado por parte del operador.
ES 4 2815-009-001 Rev D.1
Page 25
VViiddaa úúttiill pprreevviissttaa,, eessttáánnddaarr
La parte central interna y las fundas de la superficie de soporte del PPrrooFFoorrmm tienen una vida útil prevista de 3 años en
condiciones de uso normales y con el mantenimiento periódico adecuado.
VViiddaa úúttiill pprreevviissttaa,, ssaalluudd ccoonndduuccttuuaall
La superficie de soporte del PPrrooFFoorrmm tiene una vida útil prevista de 3 años en condiciones de uso normales y con el
mantenimiento periódico adecuado. Este producto no tiene piezas que requieran servicio técnico.
CCoonnttrraaiinnddiiccaacciioonneess
Ninguna conocida.
EEssppeecciiffiiccaacciioonneess
500 libras 226,8 kg
Carga de trabajo segura
NNoottaa -- El paciente no deberá superar la carga de trabajo segura especificada para la superficie de soporte.
EEssttáánnddaarr
MMooddeelloo ppaarraa
ppaacciieennttee ggeenneerraall
Longitud 84 pulgadas 213,4 cm 80 pulgadas 203,2 cm
Anchura 35 pulgadas 88,9 cm 35 pulgadas 88,9 cm
Grosor 6 pulgadas 15,2 cm 6 pulgadas 15,2 cm
Peso del producto 30 libras 13,6 kg 29 libras 13,2 kg
Material de la funda
superior
MMooddeelloo ppaarraa
ppaacciieennttee ggeenneerraall
Longitud 84 pulgadas 213,4 cm 80 pulgadas 203,2 cm
Anchura 35 pulgadas 88,9 cm 35 pulgadas 88,9 cm
Grosor 6 pulgadas 15,2 cm 6 pulgadas 15,2 cm
Peso del producto 30 libras 13,6 kg 29 libras 13,2 kg
Material de la funda
superior
228811550055555500000011 oo 228811550055555500002200 eenn bboollssaa 228811550055555500000033
END406 de DDaarrtteexx®
228811550055555500000055 228811550055555500000077
Nailon
2815-009-001 Rev D.1 5 ES
Page 26

Material del colchón Espuma de poliuretano
Conformidad del producto con la barrera ignífuga 16CFR1632, 16CFR1633, CGSB CAN 2-4.2 Método 27.7-
M77, CAL TB129, BFD IX-11
Bastidores compatibles 3002 SS33™™, 3005 SS33™™, GGooBBeedd® II, SSppiirriitt SSeelleecctt™™con
barras laterales cuádruples altas de 4 zonas
SSaalluudd ccoonndduuccttuuaall
MMooddeelloo 228811550055555500001100,, 228811550055555500001111 228811550055555500000099
Longitud 80 pulgadas 203,2 cm 84 pulgadas 213,4 cm
Anchura 35 pulgadas 88,9 cm 35 pulgadas 76 cm
Grosor 6 pulgadas 15,2 cm 6 pulgadas 7,6 cm
Peso del producto 29 libras 13,2 kg 30 libras 13,6 cm
Material de la funda superior END406 de DDaarrtteexx®
Material del colchón Espuma de poliuretano
Conformidad del producto con la barrera ignífuga 16CFR1632, 16CFR1633, CGSB
CAN 2-4.2 Método 27.7-M77, CAL
TB129, BFD IX-11
Bastidores compatibles 3002 SS33™™, 3005 SS33™™, GGooBBeedd®® II,
SSppiirriitt SSeelleecctt™™ con barras laterales
cuádruples altas de 4 zonas, cama
con plataforma de salud conductual
SSppiirriitt®®
NNoottaa -- Las fundas de salud conductual no tienen cremalleras. En el producto no se realizan tareas de servicio técnico.
CCoonnddiicciioonneess aammbbiieennttaalleess
AADDVVEERRTTEENNCCIIAA -- Nunca utilice la superficie de soporte del modelo estándar en entornos de salud conductual. La
cremallera permite acceder a componentes internos que podrían utilizarse para producir daños.
CCoonnddiicciioonneess aammbbiieennttaalleess FFuunncciioonnaammiieennttoo
Temperatura ambiente
Humedad relativa (sin condensación)
Presión atmosférica
AAllmmaacceennaammiieennttoo yy ttrraannssppoorrttee
Stryker se reserva el derecho a cambiar las especificaciones sin previo aviso.
ES 6 2815-009-001 Rev D.1
Page 27

IInnffoorrmmaacciióónn ddee ccoonnttaaccttoo
A
Póngase en contacto con el Servicio de Atención al Cliente o con el Servicio de Asistencia Técnica de Stryker llamando al:
. 1-800-327-0770.
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
EE. UU.
Para ver en línea el manual de uso o de mantenimiento de su producto, visite https://techweb.stryker.com/.
Tenga a mano el número de serie (A) del producto de Stryker cuando llame al Servicio de Atención al Cliente o al Servicio
de Asistencia Técnica de Stryker. Incluya el número de serie en todas las comunicaciones escritas.
UUbbiiccaacciióónn ddeell nnúúmmeerroo ddee sseerriiee
FFeecchhaa ddee ffaabbrriiccaacciióónn
El año de fabricación corresponde a los cuatro primeros dígitos del número de serie.
2815-009-001 Rev D.1 7 ES
Page 28

IInnssttaallaacciióónn
AADDVVEERRTTEENNCCIIAA
• Compruebe siempre con regularidad la piel del paciente. Consulte a un médico si se produce un eritema o una lesión
cutánea. Podrían producirse lesiones graves si no se trata la afección de la piel del paciente.
• Extreme las precauciones y la vigilancia para ayudar a reducir el riesgo de caída del paciente. La estabilidad del
paciente y la protección que brindan las barras laterales pueden reducirse por el uso de un protector.
• Compruebe siempre si hay objetos extraños entre la superficie de soporte y el bastidor de la cama. Los objetos extraños
pueden hacer que la superficie de soporte se deslice sobre la plataforma de soporte.
• Considere siempre el uso de las barras laterales. El uso seguro de la superficie de soporte se maximiza cuando se
utiliza junto con las barras laterales. Cuando las barras laterales no están presentes, el riesgo de caída es mayor.
Pueden producirse lesiones graves o la muerte debido al uso (posible atrapamiento) o al no uso (posibles caídas de
pacientes) de las barras laterales o de otros elementos restrictivos. Tenga en cuenta las políticas locales sobre el uso
de las barras laterales. El médico, el operador o las partes responsables deberán determinar si utilizar o no las barras
laterales, y cómo hacerlo en función de las necesidades individuales de cada paciente.
• Tenga siempre más cuidado con los pacientes con riesgo de caer (como cuando están agitados o confusos) para
reducir la probabilidad de una caída.
• No utilice la superficie de soporte sobre bastidores de cama más grandes o más pequeños que no coincidan en
anchura, longitud o grosor. Esto es para evitar el riesgo de deslizamiento de la superficie de soporte, de lesiones en el
paciente o de interferencia con las piezas móviles de la cama.
• No utilice la superficie de soporte cuando haya huecos. Cuando la superficie de soporte se coloque en bastidores de
cama no compatibles, es posible que aumente el riesgo de atrapamiento.
• No pase agujas a través de la funda de la superficie de soporte. Los orificios pueden permitir el paso de líquidos
corporales al interior (parte central interna) de la superficie de soporte, lo que podría causar contaminación cruzada y
daños o mal funcionamiento del producto.
PPRREECCAAUUCCIIÓÓNN
• Tenga siempre cuidado con los dispositivos o el equipo colocados sobre la superficie de soporte. La superficie podría
dañarse debido al peso del equipo, el calor generado por este o sus bordes afilados.
• No coloque protectores ni accesorios dentro de la funda para evitar el riesgo de reducir el rendimiento de la
redistribución de la presión.
Para instalar la superficie de soporte:
1. Asegúrese de que la superficie de soporte se ajuste al bastidor de la cama.
2. Coloque la superficie de soporte con el material gráfico mirando hacia arriba en el extremo de la cabeza del bastidor de
la cama.
3. Asegúrese de que colchón quede colocado entre los dispositivos de retención del colchón del bastidor de la cama.
4. Coloque las sábanas en la superficie de soporte de acuerdo con los protocolos hospitalarios.
ES 8 2815-009-001 Rev D.1
Page 29

FFuunncciioonnaammiieennttoo
TTrraannssffeerreenncciiaa ddee uunn ppaacciieennttee ddee uunnaa ppllaattaaffoorrmmaa ddee ssooppoorrttee ddeell ppaacciieennttee aa oottrraa
AADDVVEERRTTEENNCCIIAA
• No utilice la superficie de soporte como un dispositivo de transferencia.
• No utilice las agarraderas de la superficie de soporte para levantar o desplazar la superficie de soporte con un paciente
encima.
• No pase agujas a través de la funda de la superficie de soporte. Los orificios pueden permitir el paso de líquidos
corporales al interior (parte central interna) de la superficie de soporte, lo que podría causar contaminación cruzada y
daños o mal funcionamiento del producto.
• No supere la carga de trabajo segura del bastidor de la cama del hospital cuando sostenga al paciente y la superficie de
soporte. El peso excesivo puede provocar resultados impredecibles en el funcionamiento y la seguridad de este
producto.
• Asegúrese siempre de que las plataformas de soporte del paciente y sus correspondientes huecos de transferencia
sean los adecuados para dar soporte al paciente. Si el hueco entre las dos plataformas de soporte del paciente es de
más de 3 pulgadas (7,6 cm), utilice el puente de transferencia para rellenar el hueco. El puente de transferencia está
diseñado para facilitar la transferencia de un paciente de una plataforma de soporte del paciente a otra.
• Para reducir el riesgo de caída del paciente, cuando coloque al paciente sobre la superficie de soporte, asegúrese
siempre de que la barra lateral opuesta esté levantada.
Para transferir el paciente de una superficie de soporte del paciente a otra:
PPrreerrrreeqquuiissiittoo:: Siga los protocolos hospitalarios necesarios para transferir un paciente de una superficie a otra.
1. Coloque una plataforma de soporte del paciente junto a la otra plataforma de soporte del paciente, dejando un hueco
mínimo entre las dos plataformas.
2. Active los frenos de ambas plataformas de soporte del paciente.
3. Ajuste la altura de las plataformas de soporte del paciente, de modo que queden al mismo nivel.
4. Traslade al paciente siguiendo todas las reglas de seguridad y protocolos del hospital aplicables, para garantizar la
seguridad del paciente y del operador.
2815-009-001 Rev D.1 9 ES
Page 30

MMaanntteenniimmiieennttoo pprreevveennttiivvoo
Como mínimo, revise todos los componentes incluidos en la lista durante el mantenimiento preventivo anual de todos los
productos de Stryker Medical. Es posible que deba realizar revisiones de mantenimiento preventivo con más frecuencia en
función de su nivel de uso del producto.
Retire el producto del servicio antes de realizar el mantenimiento preventivo.
NNoottaa -- Si es necesario, limpie y desinfecte el exterior de la superficie de soporte antes de la inspección.
En el caso de los modelos estándar, revise lo siguiente:
La cremallera y las fundas (superior e inferior) no presentan rasgaduras, cortes, orificios ni otras aberturas.
Los componentes internos no presentan signos de manchas por entrada de líquidos o contaminación (para ello,
abra completamente la cremallera de las fundas).
Las etiquetas se leen sin dificultad, están bien pegadas y están íntegras.
Las agarraderas no presentan rasguños ni roturas, y las costuras están intactas.
La espuma no se ha degradado ni disgregado.
La barrera ignífuga no presenta desgarros, grietas ni otros signos visibles de daño.
En el caso de los modelos de salud conductual, revise lo siguiente:
Las fundas (superior e inferior) no presentan rasgaduras, cortes, orificios ni otras aberturas.
Las etiquetas se leen sin dificultad, están bien pegadas y están íntegras.
Número de serie del producto:
Cumplimentado por:
Fecha:
LLiimmppiieezzaa yy ddeessiinnffeecccciióónn ccoonn ppaaññooss
Para EE. UU. solamente. Confirme la disponibilidad para su configuración o región. Llame al Servicio de Atención al
Cliente de Stryker: 1-800-327-0770.
Los paños preferidos de Stryker (2060-000-001 6 x 10 pulgadas [15,2 x 25,4 cm] o 2060-000-002 9 x 12 pulgadas
[22,9 x 30,5 cm]) incluyen los principios activos siguientes:
• Cloruro de n-alquilo (60 % de C14, 30 % de C16, 5 % de C12, 5 % de C18) dimetilbencil amonio - 0,154 %
• Cloruro de n-alquilo (68 % de C12, 32 % de C14) dimetiletilbencil amonio - 0,154 %
• Isopropanol - 21,000 %
Principio no activo: Etilenglicol monobutil éter - <3 %
NNoottaa -- Para obtener información sobre la seguridad, consulte la etiqueta del producto.
Para limpiar o desinfectar la superficie externa del producto:
1. Para limpiar, frote las superficies externas con un paño nuevo limpio para retirar toda la suciedad visible. Repita el
proceso hasta que el producto esté limpio.
NNoottaa
• Utilice tantos paños como sea necesario.
• Finalice el paso 1 antes de desinfectar.
2. Para desinfectar, frote las superficies externas con un paño nuevo limpio hasta humedecerlas por completo. Deje que
la superficie externa permanezca húmeda dos minutos a temperatura ambiente.
ES 10 2815-009-001 Rev D.1
Page 31

3. Deje que el producto se seque antes de volverlo a utilizar.
CCuuiiddaaddoo yy mmaanntteenniimmiieennttoo
AADDVVEERRTTEENNCCIIAA
• No lave los componentes internos de esta superficie de soporte. Si encuentra contaminación en su interior, deseche la
superficie de soporte.
• No sumerja el producto en soluciones de limpieza o desinfectantes.
• No deje que se acumule líquido sobre el producto.
• Antes de cada uso, inspeccione siempre las fundas (superior e inferior) de la superficie de soporte para detectar
desgarros, perforaciones, desgaste excesivo y cremalleras mal alineadas. Si observa algún deterioro, retire del servicio
de inmediato la superficie de soporte.
• Asegúrese siempre de limpiar todos los productos con agua limpia y de secarlos después de la limpieza. Algunos
productos de limpieza son corrosivos por naturaleza y pueden provocar daños al producto. Si no se siguen estas
instrucciones de limpieza, se podría anular la garantía del producto.
PPRREECCAAUUCCIIÓÓNN
• No permita que se filtre líquido por la zona de la cremallera ni por la barrera de la funda que protege a la cremallera de
la entrada de líquidos cuando limpie la parte inferior de la superficie de soporte. Los líquidos que entren en contacto con
la cremallera podrían pasar a la parte central interna de la superficie de soporte.
• Las fundas de la superficie de soporte no pueden plancharse, limpiarse en seco ni secarse en una secadora de ropa.
• No lave a presión la superficie de soporte porque podría dañar el producto.
• Deje siempre que las fundas de la superficie de soporte se sequen antes de guardar la superficie de soporte, ponerle
sábanas o colocar un paciente sobre ella. El secado del producto ayuda a evitar que su rendimiento disminuya.
• No exponga excesivamente las fundas a soluciones químicas muy concentradas, ya que estas pueden degradarlas.
• No utilice peróxidos de hidrógeno acelerados ni compuestos cuaternarios que contengan >3 % de éter de glicol, ya que
estos productos químicos podrían dañar la funda de la superficie de soporte.
• El incumplimiento de las instrucciones de fabricación también puede afectar a la vida útil de la funda de la superficie de
soporte.
La funda de la superficie de soporte es resistente a las soluciones químicas siguientes:
• Compuestos cuaternarios (principio activo - cloruro de amonio) que contengan menos de un 3 % de éter de glicol
• Solución fenólica (Matar)
• Solución de lejía clorada (10 000 ppm) para utilizarse con las fundas END406 para colchón
• Solución de lejía clorada (1000 ppm) para utilizarse con las fundas de nailon para colchón
• Alcohol isopropílico al 70 %
Siga el protocolo del hospital para el cuidado de la superficie de soporte entre un paciente y el siguiente para evitar el
riesgo de contaminación cruzada y de infección.
NNoottaa -- La solución de lejía clorada puede causar decoloración de las fundas de nailon para colchón.
2815-009-001 Rev D.1 11 ES
Page 32

RReeffeerreenncciiaa rrááppiiddaa aa llaass ppiieezzaass ddee rreeppuueessttoo
Se comercializan las siguientes piezas de repuesto para los modelos estándar, pero esto puede cambiar. Llame al Servicio
de Atención al Cliente de Stryker: +1-800-327-0770 (línea gratuita en los Estados Unidos), para confirmar la disponibilidad
de las piezas y los precios.
NNoommbbrree NNúúmmeerroo
Conjunto de funda, inclinación de talón, END406, 80
pulgadas (203,2 cm)
Conjunto de funda, inclinación de talón, END406, 84
pulgadas (213,4 cm)
Conjunto de funda, inclinación de talón, nailon, 80
pulgadas (203,2 cm)
Conjunto de funda, inclinación de talón, nailon, 84
pulgadas (213,4 cm)
281505550012
281505550014
281505550018
281505550016
SSuussttiittuucciióónn ddee llaa ffuunnddaa,, mmooddeelloo eessttáánnddaarr
PPRREECCAAUUCCIIÓÓNN -- Tenga cuidado siempre que sustituya la funda de la superficie de soporte. La barrera ignífuga interna
contiene fibras de fibra de vidrio. Las partículas de polvo de las fibras pueden provocar irritación cutánea.
HHeerrrraammiieennttaass nneecceessaarriiaass::
• None
PPrroocceeddiimmiieennttoo::
1. Levante la cama hasta la posición completamente erguida.
2. Baje el respaldo Fowler y las secciones de elevación de las rodillas hasta las posiciones más inferiores.
3. Abra la cremallera de la cubierta. Empiece por la esquina derecha del extremo de los pies del paciente de la superficie
de soporte y deténgase en la esquina derecha del extremo de la cabeza del paciente.
4. Doble la parte superior de la cubierta hacia el lado derecho del paciente, a continuación, retire el conjunto del colchón
de espuma de la cama y déjelo a un lado.
5. Retire y deseche la funda.
6. Coloque la funda de sustitución, con la cremallera abierta y desplegada, sobre la cama con la funda inferior negra sobre
la superficie de soporte del colchón y la funda superior doblada sobre el lado derecho del paciente de la cama.
7. Coloque el conjunto del colchón de espuma sobre la parte inferior de la funda para asegurarse de que ambos queden
alineados.
8. Doble la funda superior sobre el conjunto del colchón de espuma para asegurarse de que ambos queden alineados.
9. Cierre la cubierta con la cremallera. Empiece por la esquina derecha del extremo de la cabeza del paciente y
deténgase en la esquina derecha del extremo de los pies del paciente.
10.Verifique que el producto funciona correctamente antes de ponerlo de nuevo en servicio.
ES 12 2815-009-001 Rev D.1
Page 33

SSuurrffaaccee ddee ssuuppppoorrtt nnoonn mmoottoorriissééee PPrrooFFoorrmm™™
MMaannuueell dd''uuttiilliissaattiioonn
2815
2815-009-001 Rev D.1
FR
2019/04
Page 34

Page 35

SSyymmbboolleess
10
Consulter le manuel d'utilisation/notice
Mode d’emploi/Consulter le mode d’emploi
Avertissement général
Mise en garde
Numéro de référence
Numéro de série
Fabricant
Charge maximale admissible
Nettoyer à la main
Ne pas sécher au sèche-linge
Ne pas nettoyer à sec
Ne pas repasser
Laisser sécher à l’air
Eau de Javel
Pour les brevets américains, consulter www.stryker.com/patents
Maintenir au sec
Ne pas empiler plus de 10 dispositifs
2815-009-001 Rev D.1 FR
Ce côté-ci vers le haut
Page 36

Fragile
Ne pas utiliser un objet acéré pour ouvrir l’emballage
Poids de l’équipement
FR 2815-009-001 Rev D.1
Page 37

TTaabbllee ddeess mmaattiièèrreess
Symboles.......................................................................................................................................................... 15
Définition de « Avertissement », « Mise en garde » et « Remarque » ................................................................2
Résumé des mesures de sécurité............................................................................................................2
Introduction ..................................................................................................................................................4
Description du produit.............................................................................................................................4
Utilisation ..............................................................................................................................................4
Durée de vie utile – standard ...................................................................................................................5
Durée de vie utile – santé comportementale .............................................................................................5
Contre-indications ..................................................................................................................................5
Caractéristiques techniques ....................................................................................................................5
Standard.........................................................................................................................................5
Santé comportementale ...................................................................................................................6
Conditions ambiantes ......................................................................................................................6
Informations de contact ..........................................................................................................................7
Emplacement du numéro de série ...........................................................................................................7
Date de fabrication .................................................................................................................................7
Installation....................................................................................................................................................8
Fonctionnement............................................................................................................................................9
Transfert d’un patient d’une plate-forme de support à une autre .................................................................9
Entretien préventif....................................................................................................................................... 10
Nettoyage et désinfection avec des lingettes ..........................................................................................10
Entretien et maintenance ......................................................................................................................11
Référence rapide des pièces de rechange ....................................................................................................12
Housse de remplacement – Modèle standard ......................................................................................... 12
2815-009-001 Rev D.1 1 FR
Page 38

DDééffiinniittiioonn ddee «« AAvveerrttiisssseemmeenntt »»,, «« MMiissee eenn ggaarrddee »» eett «« RReemmaarrqquuee »»
Les termes AAVVEERRTTIISSSSEEMMEENNTT, MMIISSEE EENN GGAARRDDEE et RREEMMAARRQQUUEE ont une signification particulière et doivent faire
l’objet d’une lecture attentive.
AAVVEERRTTIISSSSEEMMEENNTT -- Avertit le lecteur d’une situation qui, si elle n’est pas évitée, pourrait entraîner la mort ou des
blessures graves. Peut également attirer l’attention sur l’existence potentielle d’effets indésirables graves ou de risques
d’accident.
MMIISSEE EENN GGAARRDDEE -- Avertit le lecteur d’une situation potentiellement dangereuse qui, si elle n’est pas évitée, peut causer
des blessures mineures ou modérées à l’utilisateur ou au patient ou endommager le matériel en question ou d’autres biens.
Couvre notamment les précautions à prendre afin d’assurer l’utilisation sûre et efficace du dispositif et d’éviter les
dommages qui pourraient découler de l’usage ou du mésusage du matériel.
RReemmaarrqquuee -- Fournit des informations spécifiques destinées à faciliter l’entretien ou à clarifier des instructions importantes.
RRééssuumméé ddeess mmeessuurreess ddee ssééccuurriittéé
Toujours lire et respecter scrupuleusement les avertissements et les mises en garde indiqués sur cette page. Toute
réparation doit être effectuée exclusivement par du personnel qualifié.
AAVVEERRTTIISSSSEEMMEENNTT
• Ne jamais utiliser le modèle standard de la surface de support dans un contexte de santé comportementale. La
fermeture éclair permet l'accès aux composants internes qui pourraient être utilisés pour infliger des mutilations.
• Toujours vérifier régulièrement la peau du patient. Consulter un médecin si un érythème ou une plaie cutanée apparaît.
Une lésion grave peut se produire si l’affection cutanée du patient n’est pas traitée.
• Toujours faire preuve de prudence et de supervision particulières pour limiter le risque de chute du patient. La stabilité
du patient et la protection assurées par les barrières peuvent être compromises lors de l’utilisation d’un surmatelas.
• Toujours vérifier l’absence de corps étrangers entre la surface de support et le cadre de lit. Les corps étrangers peuvent
provoquer le glissement de la surface de support sur la plate-forme de support.
• Toujours penser à utiliser les barrières. Il est conseillé d’utiliser la surface de support en conjonction avec les barrières
latérales pour une utilisation sans danger. Un risque accru de chutes peut exister en l'absence de barrières latérales.
Une blessure grave ou le décès peut résulter de l’utilisation (possibilité de coincement) ou de la non-utilisation (chute
potentielle du patient) des barrières ou d’autres dispositifs de maintien. Tenir compte des politiques locales en ce qui
concerne l’utilisation des barrières. Le médecin, l’opérateur ou les parties responsables doivent déterminer si et
comment les barrières doivent être utilisées en fonction des besoins spécifiques de chaque patient.
• Toujours faire preuve d’une prudence particulière avec un patient présentant un risque de chute (patient agité ou confus,
par exemple) pour réduire le risque de chute.
• Ne pas utiliser la surface de support sur un cadre de lit dont la taille n’est pas adaptée à sa largeur, longueur ou
épaisseur. Cela permet d'éviter le risque de glissement de la surface de support, de blessure du patient ou
d'interférence avec les parties mobiles du lit.
• Ne pas utiliser la surface de support lorsque des espaces sont présents. Un risque de coincement peut survenir lorsque
la surface de support est placée sur des cadres de lit non compatibles.
• Ne pas planter d’aiguille dans une surface de support à travers la housse. La formation de trous risque de provoquer
l’infiltration de liquides corporels à l’intérieur de la surface de support (dans la partie interne), ce qui pourrait entraîner
une contamination croisée ou un dysfonctionnement du produit, ou endommager ce dernier.
• Ne pas utiliser la surface de support comme dispositif de transfert.
• Ne pas utiliser les poignées de la surface de support pour soulever ou déplacer la surface de support avec le patient
installé dessus.
• Ne pas dépasser la charge maximale admissible du cadre de lit d’hôpital lorsqu’il supporte le poids du patient et de la
surface de support. Une charge excessive pourrait rendre imprévisibles la sécurité et les performances de ce produit.
• Toujours veiller à ce que les plates-formes de support du patient et les espaces de transfert respectifs entre ces platesformes soient adéquats pour supporter le patient. Si l’espace entre les deux plates-formes de support du patient est
supérieur à 3” (7,6 cm), utiliser la planche de transfert pour combler l’interstice. La planche de transfert est destinée à
faciliter le transfert d’un patient d’une plate-forme de support à une autre.
FR 2 2815-009-001 Rev D.1
Page 39

• Toujours s’assurer que la barrière opposée est levée lorsque l’on place un patient sur la surface de support afin de
réduire le risque de chute du patient.
• Ne pas laver les composants internes de cette surface de support. Jeter la surface de support si une contamination est
observée à l’intérieur.
• Ne pas immerger le matelas dans des solutions de nettoyage ou désinfectantes.
• Éviter tout déversement de liquide sur le matelas.
• Toujours inspecter les housses de la surface de support (supérieure et inférieure) pour déceler toute déchirure,
perforation, usure excessive, ainsi qu’un mauvais alignement des dents de la fermeture éclair, avant chaque utilisation.
Si l'intégrité de la surface de support est compromise, la mettre immédiatement hors service.
• Toujours veiller à nettoyer chaque produit avec de l’eau claire et à sécher soigneusement chaque produit après le
nettoyage. Certains produits de nettoyage sont de nature corrosive et peuvent endommager le produit. Le non-respect
de ces instructions de nettoyage peut annuler la garantie.
MMIISSEE EENN GGAARRDDEE
• L’utilisation incorrecte du produit est susceptible de causer des blessures au patient ou à l’utilisateur. Utiliser le produit
uniquement de la manière décrite dans ce manuel.
• Ne pas modifier le produit ni aucun de ses composants. Toute modification du produit peut entraîner un fonctionnement
imprévisible, susceptible d'occasionner des blessures chez le patient ou l’opérateur. La garantie du produit serait en
outre annulée par toute modification du produit.
• Toujours faire attention aux dispositifs ou équipements qui sont posés sur la surface de support. Un endommagement
de la surface peut se produire à cause du poids ou des bords tranchants de l’équipement ou de la chaleur générée par
celui-ci.
• Ne pas placer de surmatelas ou d’accessoires à l’intérieur de la housse pour éviter le risque de réduire la performance
de redistribution de la pression.
• Ne pas laisser de liquide s’infiltrer dans la zone de la fermeture éclair ou dans le rabat de la fermeture éclair lors du
nettoyage du côté inférieur de la surface de support. Les liquides qui parviennent à entrer en contact avec la fermeture
éclair peuvent s’infiltrer dans le noyau de la surface de support.
• Ne pas repasser, ni nettoyer à sec, ni sécher au sèche-linge les housses de la surface de support.
• Ne pas laver la surface de support sous pression sous risque d'endommager le produit.
• Toujours sécher complètement les housses de la surface de support avant de les stocker, de poser des draps ou
d’installer un patient dessus. Le séchage du produit permet d'éviter une altération de sa performance.
• Ne pas surexposer les housses à des solutions chimiques de concentration plus élevée car ces solutions peuvent
dégrader les housses.
• Ne pas utiliser de peroxydes d’hydrogène accélérés ni de composés quaternaires contenant de l’éther glycolique > 3 %
car ces substances chimiques peuvent endommager la housse de la surface de support.
• Le non-respect des instructions de fabrication peut également avoir un impact sur la durée de vie utile de la housse de la
surface de support.
• Toujours être prudent lors du remplacement de la housse de la surface de support. La protection anti-feu interne
contient de la fibre de verre. Les particules de poussière provenant de ces fibres peuvent causer une irritation cutanée.
2815-009-001 Rev D.1 3 FR
Page 40

IInnttrroodduuccttiioonn
Ce manuel vous aide à utiliser ou entretenir votre produit Stryker. Lire ce manuel avant d’utiliser ce produit ou d’en effectuer
la maintenance. Il convient d'établir des procédures et techniques visant à éduquer et à former le personnel quant au
fonctionnement et à l’entretien sécuritaires de ce produit.
MMIISSEE EENN GGAARRDDEE
• L’utilisation incorrecte du produit est susceptible de causer des blessures au patient ou à l’utilisateur. Utiliser le produit
uniquement de la manière décrite dans ce manuel.
• Ne pas modifier le produit ni aucun de ses composants. Toute modification du produit peut entraîner un fonctionnement
imprévisible, susceptible de causer des blessures au patient ou à l’utilisateur. La garantie du produit serait en outre
invalidée par toute modification du produit.
RReemmaarrqquuee
• Ce manuel doit être considéré comme faisant partie du produit et doit l’accompagner à tout moment, même en cas de
vente ultérieure du produit.
• Stryker cherche continuellement à améliorer le design et la qualité de ses produits. Ce manuel contient les informations
produit les plus récentes disponibles au moment de l’impression. Il peut y avoir de légères divergences entre le produit
et ce manuel. Pour toute question, contacter le service clientèle ou le support technique de Stryker au +1-800-327-
0770.
DDeessccrriippttiioonn dduu pprroodduuiitt
PPrrooFFoorrmm™™ est une surface de support en mousse non motorisée de 6 po (15,2 cm) d'épaisseur destinée à une population
générale de patients. Il y a des découpes pour permettre au noyau du matelas de s'articuler. Les options de matériau pour
la housse de matelas comprennent le nylon ou le tissu END406 par DDaarrtteexx®. Toutes les configurations du produit
comprennent une inclinaison pour les talons côté pieds de la surface de support. Les dimensions de ce produit sont
compatibles avec les cadres de lit SSttrryykkeerr. Voir la liste des cadres de lit compatibles dans la partie de ce manuel consacrée
aux caractéristiques techniques.
UUttiilliissaattiioonn
PPrrooFFoorrmm utilise de la mousse pour faciliter la redistribution de la pression et l'immersion.
Ce produit est une surface de support non motorisée qui contribue à la prévention et au traitement des lésions de pression
ou des plaies de pression (tous les stades, lésions non stadifiables et lésions tissulaires profondes). Nous vous
recommandons d'utiliser ce produit en combinaison avec une évaluation clinique des facteurs de risque et des évaluations
cutanées effectuées par un professionnel de la santé.
Le modèle standard (281505550001, 281505550003, 281505550005, 281505550007, 281505550020) est destiné à un
environnement hospitalier général, en particulier les établissements de soins aigus.
Les modèles de santé comportementale (281505550009, 281505550010, 281505550011) sont destinés aux
environnements de soins de santé comportementale.
PPrrooFFoorrmm doit être utilisée avec une housse de surface de support à tout moment. La housse de la surface de support peut
interagir avec toute la peau externe. Un drap à mettre au-dessus devrait être utilisé avec ce produit.
Les modèles standard destinés à un environnement hospitalier général et les modèles de santé comportementale ne sont
pas prévus pour :
• Patients qui dépassent la charge maximale admissible
• Patients bariatriques
• Environnement de soins à domicile
• Produit non stérile
• N'est pas doté d'une fonction de mesure
FR 4 2815-009-001 Rev D.1
Page 41

SSttrryykkeerr recommande l’évaluation clinique de chaque patient et une utilisation adéquate de la part de l’opérateur.
DDuurrééee ddee vviiee uuttiillee –– ssttaannddaarrdd
Le noyau de la surface de support et les housses PPrrooFFoorrmm ont une durée de vie utile de trois ans dans des conditions
normales d’utilisation et d’entretien périodique.
DDuurrééee ddee vviiee uuttiillee –– ssaannttéé ccoommppoorrtteemmeennttaallee
La PPrrooFFoorrmm surface de support a une durée de vie utile de trois ans dans des conditions normales d'utilisation et
d'entretien périodique. Il n'y a pas de pièces réparables pour ce produit.
CCoonnttrree--iinnddiiccaattiioonnss
Aucune connue.
CCaarraaccttéérriissttiiqquueess tteecchhnniiqquueess
500 livres 226,8 kg
Charge maximale admissible
RReemmaarrqquuee -- Le patient ne doit pas dépasser la charge maximale admissible spécifiée pour la surface de support.
SSttaannddaarrdd
MMooddèèllee ddeessttiinnéé àà
uunnee ppooppuullaattiioonn
ggéénnéérraallee ddee ppaattiieennttss
Longueur 84 po 213,4 cm 80 po 203,2 cm
Largeur 35 po 88,9 cm 35 po 88,9 cm
Épaisseur
Poids du produit 30 livres 13,6 kg 29 livres 13,2 kg
Matériau de la housse
supérieure
MMooddèèllee ddeessttiinnéé àà
uunnee ppooppuullaattiioonn
ggéénnéérraallee ddee ppaattiieennttss
Longueur 84 po 213,4 cm 80 po 203,2 cm
Largeur 35 po 88,9 cm 35 po 88,9 cm
228811550055555500000011 oouu 228811550055555500002200 eenn ssaacc 228811550055555500000033
6 po 15,2 cm 6 po 15,2 cm
END406 par DDaarrtteexx®
228811550055555500000055 228811550055555500000077
Épaisseur
Poids du produit 30 livres 13,6 kg 29 livres 13,2 kg
Matériau de la housse
supérieure
2815-009-001 Rev D.1 5 FR
6 po 15,2 cm 6 po 15,2 cm
Nylon
Page 42

Matériau du matelas Mousse de polyuréthane
Conformité de protection anti-feu du produit 16CFR1632, 16CFR1633, CGSB CAN 2-4.2 Méthode
27.7-M77, CAL TB129, BFD IX-11
Cadres compatibles 3002 SS33™™, 3005 SS33™™, GGooBBeedd® II, SSppiirriitt SSeelleecctt™™avec
barrières latérales hautes à 4 zones
SSaannttéé ccoommppoorrtteemmeennttaallee
MMooddèèllee 228811550055555500001100,, 228811550055555500001111 228811550055555500000099
Longueur 80 po 203,2 cm 84 po 213,4 cm
Largeur 35 po 88,9 cm 35 po 76 cm
Épaisseur
Poids du produit 29 livres 13,2 kg 30 livres 13,6 cm
Matériau de la housse supérieure END406 par DDaarrtteexx®®
Matériau du matelas Mousse de polyuréthane
Conformité de protection anti-feu du produit 16CFR1632, 16CFR1633, CGSB
Cadres compatibles 3002 SS33™™, 3005 SS33™™, GGooBBeedd®® II,
RReemmaarrqquuee -- Les housses pour modèles de santé comportementale ne disposent pas de fermeture éclair. Le produit n’est
pas réparable.
6 po 15,2 cm 6 po 7,6 cm
CAN 2-4.2 Méthode 27.7-M77, CAL
TB129, BFD IX-11
SSppiirriitt SSeelleecctt™™ avec barrières
latérales hautes à 4 zones, Plateforme de lit SSppiirriitt®® destinée à la
santé comportementale
CCoonnddiittiioonnss aammbbiiaanntteess
AAVVEERRTTIISSSSEEMMEENNTT -- Ne jamais utiliser le modèle standard de la surface de support dans un contexte de santé
comportementale. La fermeture éclair permet l'accès aux composants internes qui pourraient être utilisés pour infliger des
mutilations.
CCoonnddiittiioonnss aammbbiiaanntteess FFoonnccttiioonnnneemmeenntt
Température ambiante
Humidité relative (sans
condensation)
Pression atmosphérique
Stryker se réserve le droit de modifier ces caractéristiques sans préavis.
FR 6 2815-009-001 Rev D.1
SSttoocckkaaggee eett ttrraannssppoorrtt
Page 43

IInnffoorrmmaattiioonnss ddee ccoonnttaacctt
A
Contacter le service clientèle ou le support technique de Stryker au : 1-800-327-0770.
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
États-Unis
Pour consulter votre mode d'emploi ou votre manuel d'entretien en ligne, rendez-vous sur https://techweb.stryker.com/.
Avoir le numéro de série (A) du produit Stryker à disposition avant d’appeler le service clientèle ou le support technique de
Stryker. Inclure le numéro de série dans toutes les communications écrites.
EEmmppllaacceemmeenntt dduu nnuumméérroo ddee sséérriiee
DDaattee ddee ffaabbrriiccaattiioonn
Les quatre premiers chiffres du numéro de série correspondent à l'année de fabrication.
2815-009-001 Rev D.1 7 FR
Page 44

IInnssttaallllaattiioonn
AAVVEERRTTIISSSSEEMMEENNTT
• Toujours vérifier régulièrement la peau du patient. Consulter un médecin si un érythème ou une plaie cutanée apparaît.
Une lésion grave peut se produire si l’affection cutanée du patient n’est pas traitée.
• Toujours faire preuve de prudence et de supervision particulières pour limiter le risque de chute du patient. La stabilité
du patient et la protection assurées par les barrières peuvent être compromises lors de l’utilisation d’un surmatelas.
• Toujours vérifier l’absence de corps étrangers entre la surface de support et le cadre de lit. Les corps étrangers peuvent
provoquer le glissement de la surface de support sur la plate-forme de support.
• Toujours penser à utiliser les barrières. Il est conseillé d’utiliser la surface de support en conjonction avec les barrières
latérales pour une utilisation sans danger. Un risque accru de chutes peut exister en l'absence de barrières latérales.
Une blessure grave ou le décès peut résulter de l’utilisation (possibilité de coincement) ou de la non-utilisation (chute
potentielle du patient) des barrières ou d’autres dispositifs de maintien. Tenir compte des politiques locales en ce qui
concerne l’utilisation des barrières. Le médecin, l’opérateur ou les parties responsables doivent déterminer si et
comment les barrières doivent être utilisées en fonction des besoins spécifiques de chaque patient.
• Toujours faire preuve d’une prudence particulière avec un patient présentant un risque de chute (patient agité ou confus,
par exemple) pour réduire le risque de chute.
• Ne pas utiliser la surface de support sur un cadre de lit dont la taille n’est pas adaptée à sa largeur, longueur ou
épaisseur. Cela permet d'éviter le risque de glissement de la surface de support, de blessure du patient ou
d'interférence avec les parties mobiles du lit.
• Ne pas utiliser la surface de support lorsque des espaces sont présents. Un risque de coincement peut survenir lorsque
la surface de support est placée sur des cadres de lit non compatibles.
• Ne pas planter d’aiguille dans une surface de support à travers la housse. La formation de trous risque de provoquer
l’infiltration de liquides corporels à l’intérieur de la surface de support (dans la partie interne), ce qui pourrait entraîner
une contamination croisée ou un dysfonctionnement du produit, ou endommager ce dernier.
MMIISSEE EENN GGAARRDDEE
• Toujours faire attention aux dispositifs ou équipements qui sont posés sur la surface de support. Un endommagement
de la surface peut se produire à cause du poids ou des bords tranchants de l’équipement ou de la chaleur générée par
celui-ci.
• Ne pas placer de surmatelas ou d’accessoires à l’intérieur de la housse pour éviter le risque de réduire la performance
de redistribution de la pression.
Pour mettre en place la surface de support :
1. Veiller à ce que la surface de support s'adapte au cadre de lit.
2. Placer la surface de support avec le logo face vers le haut au niveau de la tête du cadre de lit.
3. Veiller à ce que le matelas soit placé entre les supports de matelas sur le cadre de lit.
4. Placer les draps sur la surface de support selon les protocoles hospitaliers.
FR 8 2815-009-001 Rev D.1
Page 45

FFoonnccttiioonnnneemmeenntt
TTrraannssffeerrtt dd’’uunn ppaattiieenntt dd’’uunnee ppllaattee--ffoorrmmee ddee ssuuppppoorrtt àà uunnee aauuttrree
AAVVEERRTTIISSSSEEMMEENNTT
• Ne pas utiliser la surface de support comme dispositif de transfert.
• Ne pas utiliser les poignées de la surface de support pour soulever ou déplacer la surface de support avec le patient
installé dessus.
• Ne pas planter d’aiguille dans une surface de support à travers la housse. La formation de trous risque de provoquer
l’infiltration de liquides corporels à l’intérieur de la surface de support (dans la partie interne), ce qui pourrait entraîner
une contamination croisée ou un dysfonctionnement du produit, ou endommager ce dernier.
• Ne pas dépasser la charge maximale admissible du cadre de lit d’hôpital lorsqu’il supporte le poids du patient et de la
surface de support. Une charge excessive pourrait rendre imprévisibles la sécurité et les performances de ce produit.
• Toujours veiller à ce que les plates-formes de support du patient et les espaces de transfert respectifs entre ces platesformes soient adéquats pour supporter le patient. Si l’espace entre les deux plates-formes de support du patient est
supérieur à 3” (7,6 cm), utiliser la planche de transfert pour combler l’interstice. La planche de transfert est destinée à
faciliter le transfert d’un patient d’une plate-forme de support à une autre.
• Toujours s’assurer que la barrière opposée est levée lorsque l’on place un patient sur la surface de support afin de
réduire le risque de chute du patient.
Pour transférer un patient entre deux surfaces de support :
CCoonnddiittiioonn pprrééaallaabbllee :: Respecter le protocole hospitalier relatif au transfert de patients entre deux surfaces de support.
1. Placer une plate-forme de support de patient le long d’une autre plate-forme de support, en veillant à minimiser l'espace
entre elles.
2. Serrer les freins sur les deux plates-formes de support de patient.
3. Régler les deux plates-formes de support de patient à la même hauteur.
4. Pour assurer la sécurité du patient et de l’opérateur, observer toutes les règles de sécurité et protocoles hospitaliers en
vigueur lors du transfert du patient.
2815-009-001 Rev D.1 9 FR
Page 46

EEnnttrreettiieenn pprréévveennttiiff
Au minimum, vérifier tous les éléments mentionnés pendant l’entretien préventif annuel pour tous les produits Stryker
Medical. Il peut être nécessaire d’effectuer les vérifications de maintenance préventive plus fréquemment en fonction du
degré d’utilisation du produit.
Mettre le produit hors service avant de procéder à l’entretien préventif.
RReemmaarrqquuee -- Nettoyer et désinfecter l’extérieur de la surface de support avant inspection, le cas échéant.
Inspecter les éléments suivants pour les modèles standard :
La fermeture éclair et les housses (supérieure et inférieure) sont exemptes de déchirures, entailles, trous ou autres
ouvertures
Les composants internes ne présentent pas de signes de taches liées à la pénétration de liquide ou la contamination
(vérification avec les housses entièrement ouvertes)
Bonne lisibilité, adhésion et intégrité des étiquettes
Les poignées sont exemptes d’accrocs ou de fentes et les coutures sont intactes
La mousse ne s’est pas dégradée ou détachée
Protection anti-feu pour les déchirures, fissures ou autres signes visibles d’endommagement
Inspecter les éléments suivants pour les modèles de santé comportementale :
Les housses (supérieure et inférieure) sont exemptes de déchirures, entailles, trous ou autres ouvertures
Bonne lisibilité, adhésion et intégrité des étiquettes
Numéro de série du produit :
Effectué par :
Date :
NNeettttooyyaaggee eett ddééssiinnffeeccttiioonn aavveecc ddeess lliinnggeetttteess
Destiné aux États-Unis uniquement. Confirmer la disponibilité en fonction de la configuration du produit ou du pays.
Contacter le service clientèle de Stryker : +1-800-327-0770.
Les lingettes recommandées par Stryker (2060-000-001 6” x 10” (15,2 cm x 25,4 cm) ou 2060-000-002 9” x 12” (22,9 cm x
30,5 cm)) contiennent les principes actifs suivants :
• Chlorure d’alkyldiméthylbenzyl ammonium (60 % C14, 30 % C16, 5 % C12, 5 % C18) – 0,154 %
• Chlorure d’alkyldiméthyléthylbenzyl ammonium (68 % C12, 32 % C14) – 0,154 %
• Isopropanol – 21,000 %
Principe non actif : Éther monobutylique de l'éthylène glycol – <3 %
RReemmaarrqquuee -- Pour obtenir des renseignements relatifs à la sécurité, lire l’étiquette du produit.
Pour nettoyer ou désinfecter la surface externe du produit :
1. Pour nettoyer, essuyer les surfaces externes avec une lingette fraîche et propre pour enlever toutes les salissures
visibles. Répéter au besoin jusqu'à ce que le produit soit propre.
RReemmaarrqquuee
• Utiliser autant de lingettes que nécessaire.
• Terminer l'étape 1 avant de désinfecter.
2. Pour désinfecter, essuyer les surfaces externes avec une lingette fraîche et propre jusqu'à ce qu'elles soient humides.
Laisser la surface extérieure humide pendant deux minutes à température ambiante.
FR 10 2815-009-001 Rev D.1
Page 47

3. Laisser le produit sécher avant de le remettre en service.
EEnnttrreettiieenn eett mmaaiinntteennaannccee
AAVVEERRTTIISSSSEEMMEENNTT
• Ne pas laver les composants internes de cette surface de support. Jeter la surface de support si une contamination est
observée à l’intérieur.
• Ne pas immerger le produit dans des solutions de nettoyage ou désinfectantes.
• Éviter tout déversement de liquide sur le produit.
• Toujours inspecter les housses de la surface de support (supérieure et inférieure) pour déceler toute déchirure,
perforation, usure excessive, ainsi qu’un mauvais alignement des dents de la fermeture éclair, avant chaque utilisation.
Si l'intégrité de la surface de support est compromise, la mettre immédiatement hors service.
• Toujours veiller à nettoyer chaque produit avec de l’eau claire et à sécher soigneusement chaque produit après le
nettoyage. Certains agents nettoyants sont de nature corrosive et peuvent endommager le produit. Le non-respect de
ces instructions de nettoyage peut annuler la garantie.
MMIISSEE EENN GGAARRDDEE
• Ne pas laisser de liquide s’infiltrer dans la zone de la fermeture éclair ou dans le rabat de la fermeture éclair lors du
nettoyage du côté inférieur de la surface de support. Les liquides qui parviennent à entrer en contact avec la fermeture
éclair peuvent s’infiltrer dans le noyau de la surface de support.
• Ne pas repasser, ni nettoyer à sec, ni sécher au sèche-linge les housses de la surface de support.
• Ne pas laver la surface de support sous pression sous risque d'endommager le produit.
• Toujours sécher complètement les housses de la surface de support avant de les stocker, de poser des draps ou
d’installer un patient dessus. Le séchage du produit permet d'éviter une altération de sa performance.
• Ne pas surexposer les housses à des solutions chimiques de concentration plus élevée car ces solutions peuvent
dégrader les housses.
• Ne pas utiliser de peroxydes d’hydrogène accélérés ni de composés quaternaires contenant de l’éther glycolique >3 %
car ces substances chimiques peuvent endommager la housse de la surface de support.
• Le non-respect des instructions de fabrication peut également avoir un impact sur la durée de vie utile de la housse de la
surface de support.
La housse de la surface de support résiste aux solutions chimiques suivantes :
• Composés quaternaires (principe actif : chlorure d’ammonium) contenant moins de 3 % d’éther de glycol
• Solution phénolique (Matar)
• Solution chlorée à base d’eau de Javel (10 000 ppm) à utiliser avec les housses de matelas END406
• Solution chlorée à base d’eau de Javel (1 000 ppm) à utiliser avec les housses de matelas en nylon
• Alcool isopropylique à 70 %
Toujours respecter le protocole hospitalier pour l'entretien de la surface de support entre deux patients pour éviter le risque
de contamination croisée et d’infection.
RReemmaarrqquuee -- La solution chlorée à base d’eau de Javel peut entraîner une décoloration des housses de matelas en nylon.
2815-009-001 Rev D.1 11 FR
Page 48

RRééfféérreennccee rraappiiddee ddeess ppiièècceess ddee rreecchhaannggee
Ces pièces sont disponibles à l'achat pour les modèles standard, mais sous réserve de modifications. Contacter le service
clientèle de Stryker : +1-800-327-0770 pour connaître les pièces disponibles et leur prix.
NNoomm NNuumméérroo
Ensemble de housses, inclinaison pour les talons, END406
80 po (203,2 cm)
Ensemble de housses, inclinaison pour les talons, END406
84 po (213,4 cm)
Ensemble de housses, inclinaison pour les talons, Nylon
80 po (203,2 cm)
Ensemble de housses, inclinaison pour les talons, Nylon
84 po (213,4 cm)
281505550012
281505550014
281505550018
281505550016
HHoouussssee ddee rreemmppllaacceemmeenntt –– MMooddèèllee ssttaannddaarrdd
MMIISSEE EENN GGAARRDDEE -- Toujours être prudent lors du remplacement de la housse de la surface de support. La protection antifeu interne contient de la fibre de verre. Les particules de poussière provenant de ces fibres peuvent causer une irritation
cutanée.
OOuuttiillss rreeqquuiiss ::
• None
PPrrooccéédduurree ::
1. Élever la hauteur du lit jusqu’à la position haute maximale.
2. Abaisser les sections du relève-buste et du relève-jambes à la position basse maximale.
3. Ouvrir la fermeture éclair de la housse. Commencer au niveau des pieds dans l’angle droit par rapport au patient de la
surface de support et terminer au niveau de la tête dans l’angle droit par rapport au patient.
4. Plier le haut de la housse sur le côté droit par rapport au patient puis retirer le matelas en mousse du lit et laisser de
côté.
5. Retirer et éliminer la housse.
6. Placer la nouvelle housse, fermeture éclair ouverte, sur le lit avec la housse inférieure noire sur le plan de couchage et
la housse supérieure pliée sur le côté droit du lit par rapport au patient.
7. Placer le matelas en mousse sur la partie inférieure de la housse pour s’assurer que le matelas en mousse est aligné
avec la housse.
8. Plier la housse supérieure au-dessus du matelas en mousse pour s’assurer que la housse supérieure est alignée avec
le matelas en mousse.
9. Fermer la fermeture éclair de la housse. Commencer au niveau de la tête dans l’angle droit par rapport au patient et
terminer au niveau des pieds dans l’angle droit par rapport au patient.
10.Vérifier le fonctionnement correct du produit avant de le remettre en service.
FR 12 2815-009-001 Rev D.1
Page 49

PPrrooFFoorrmm™™ 비비전전동동식식 지지지지면면
작작동동 설설명명서서
2815
2815-009-001 Rev D.1
KO
2019/04
Page 50

Page 51

기기호호
10
지침 매뉴얼/소책자를 참조할 것
조작 지침/사용 설명서를 참고할 것
일반 경고
주의
카탈로그 번호
일련번호
제조업체
안전 작업 하중
손으로 세척할 것
회전식 건조 금지
드라이클리닝 금지
다림질 금지
자연 건조
염소 표백
미국 특허는 www.stryker.com/patents를 참조할 것
건조한 상태 유지
10개 높이 넘게 적재하지 말 것
2815-009-001 Rev D.1 KO
이 쪽이 위임
Page 52

파손 주의
포장 개봉 시 날카로운 물체를 사용하지 말 것
장비 질량
KO 2815-009-001 Rev D.1
Page 53

목목차차
기호..................................................................................................................................................................15
경고/주의/참고 정의 ......................................................................................................................................2
안전 예방 조치 요약................................................................................................................................2
소개.............................................................................................................................................................4
제품 설명...............................................................................................................................................4
용도 ......................................................................................................................................................4
예상 사용 수명 - 표준..............................................................................................................................4
예상 수명 - 행동 건강..............................................................................................................................5
금기 사항...............................................................................................................................................5
규격 ......................................................................................................................................................5
표준................................................................................................................................................5
행동 건강 ........................................................................................................................................5
환경 조건 ........................................................................................................................................6
연락처 정보 ...........................................................................................................................................6
일련번호 위치 ........................................................................................................................................7
제조일...................................................................................................................................................7
설치.............................................................................................................................................................8
작동.............................................................................................................................................................9
환자 지지 플랫폼 간 환자 이동.................................................................................................................9
예방정비 ....................................................................................................................................................10
물티슈를 사용한 세척 및 소독................................................................................................................ 10
관리 및 유지보수..................................................................................................................................11
교체 부품 빠른 참조 ....................................................................................................................................12
커버 교체 - 표준 모델............................................................................................................................12
2815-009-001 Rev D.1 1 KO
Page 54

경경고고//주주의의//참참고고 정정의의
경경고고, 주주의의 및 참참고고는 특별한 의미를 담고 있으므로 주의 깊게 검토해야 합니다.
경경고고 -- 준수하지 않을 경우 사망 또는 중상을 초래할 수 있는 상황을 알립니다. 또한 발생할 수 있는 심각한 부작용 및 안전상
의 위험에 대해서 설명할 수도 있습니다.
주주의의 -- 준수하지 않을 경우, 사용자 또는 환자에게 경증 또는 중등도의 부상을 초래하거나 또는 제품이나 다른 재산에 손상을
초래할 수 있는 잠재적으로 위험한 상황에 대해 알립니다. 여기에는 장비를 안전하고 효과적으로 사용하기 위한 특별 사항
그리고 사용 또는 오용의 결과로 장비에 발생할 수 있는 손상을 방지하기 위한 특별 사항이 포함됩니다.
참참고고 -- 유지 보수를 용이하게 하고 중요한 지침이 명백하게 전달되도록 하는 특정 정보를 제공합니다.
안안전전 예예방방 조조치치 요요약약
항상 이 페이지에 기재된 경고 및 주의를 읽고 엄격히 준수해야 합니다. 자격을 갖춘 요원만이 정비를 실행합니다.
경경고고
• 표준 모델 지지면을 행동 건강 사용 환경에서 사용하지 마십시오. 지퍼는 유해스러운 경우 사용할 수 있는 내부 구성품에
접근할 수 있도록 합니다.
• 항상 환자의 피부를 정기적으로 점검하십시오. 홍반 또는 피부 손상이 발생할 경우 의사에게 알리십시오. 환자의 피부 상
태를 치료하지 않고 방치할 경우 중상을 초래할 수 있습니다.
• 환자의 낙상 위험을 줄이기 위해 항상 각별한 주의를 기울이고 감독하십시오. 덮개를 사용하면 환자 안정성과 사이드레일
사용 범위에 부정적인 영향을 줄 수 있습니다.
• 지지면과 베드 프레임의 사이에 이물질이 있는지 항상 검사하십시오. 이물체가 있으면 지지 플랫폼에서 지지면이 미끄러
질 수 있습니다.
• 항상 사이드레일 사용을 고려하십시오. 지지면은 사이드레일과 함께 사용할 때 안전성이 극대화됩니다. 사이드레일이 없
을 때 낙상 위험이 증가할 수 있습니다. 사이드레일 또는 기타 구속 장치의 사용(끼일 가능성) 또는 비사용(환자 낙상 가능
성)으로 인해 중상 또는 사망이 발생할 수 있습니다. 사이드레일 사용에 관한 현지 정책을 고려하십시오. 의사, 작동자 또
는 책임있는 당사자는 사이드레일 사용 여부와 사용 방법을 각 환자의 개별적 필요에 기반하여 결정해야 합니다.
• 낙상의 가능성을 줄이기 위해, 낙상의 위험이 있는 환자(예: 격앙 또는 혼동 상태에 있는 환자)에 대해서는 항상 각별한 주
의를 기울이십시오.
• 너비, 길이 또는 두께가 맞지 않는 더 크거나 더 작은 베드 프레임에 지지면을 사용하지 마십시오. 지지면 미끄러짐, 환자
부상 또는 베드의 움직이는 부품과의 간섭 위험을 피하기 위함입니다.
• 간격이 있는 경우에는 지지면을 사용하지 마십시오. 함께 사용할 수 없는 베드 프레임에 지지면을 설치할 때 걸릴 위험이
발생할 수 있습니다.
• 지지면 커버를 통해 지지면에 바늘을 꽂지 마십시오. 구멍을 통해 체액이 지지면의 내부(내핵)로 유입될 수 있으며, 이 경
우 교차 오염, 제품 손상 또는 제품 오작동이 발생할 수 있습니다.
• 지지면을 운반 장치로 사용하지 마십시오.
• 환자가 탑승한 상태에서 지지면 핸들을 사용하여 지지면을 올리거나 이동하지 마십시오.
• 병원 베드 프레임이 환자와 지지면을 모두 지지할 경우, 프레임의 안전 사용 하중을 초과하지 마십시오. 과도한 중량은 이
제품의 예상치 못한 안전 및 성능 문제를 유발할 수 있습니다.
• 환자 지지 플랫폼과 플랫폼 간의 해당 운반 간격이 환자를 지지하기에 충분한지 항상 확인하십시오. 두 환자 지지 플랫폼
간의 간격이 3인치(7.6cm) 를 초과하는 경우, 이전 브리지를 사용하여 간격을 메꾸십시오. 운반 다리는 환자 지지 플랫폼
간 환자 이동을 용이하게 하기 위한 용도입니다.
• 환자를 지지면에 놓을 때에는 환자의 낙상 위험을 줄이기 위해 항상 반대쪽 사이드레일이 올려져 있는지 확인하십시오.
• 이 지지면의 내부 구성 요소를 세척하지 마십시오. 내부 오염이 확인될 경우 지지면을 폐기하십시오.
• 본 제품을 세정액이나 소독액에 담그지 마십시오.
• 이 제품에 액체가 고이지 않도록 하십시오.
• 각 사용 전에 지지면 커버(위 및 아래)에 찢긴 곳, 구멍, 과도한 마모, 지퍼 오정렬 등이 있는지 항상 검사하십시오. 손상된
경우, 지지면 사용을 즉시 중단하십시오.
• 항상 각 제품을 깨끗한 물로 닦고, 세척 후에는 각 제품을 건조시키십시오. 일부 세정제는 부식성이 있기 때문에 본 제품
에 손상을 초래할 수 있습니다. 이러한 세척 지침을 따르지 않으면 보증이 무효가 될 수 있습니다.
KO 2 2815-009-001 Rev D.1
Page 55

주주의의
• 제품을 부적절하게 사용하면 환자나 작동자가 부상을 입을 수 있습니다. 본 설명서에 기술된 대로만 제품을 사용하십시
오.
• 제품 또는 제품의 구성 요소 일체를 개조하지 마십시오. 제품을 개조하면 예상치 않게 작동하여 환자 또는 작동자가 부상
을 입을 수 있습니다. 제품을 개조하면 보증이 무효가 될 수도 있습니다.
• 지지면 상단에 배치된 장치 또는 장비에 항상 주의하십시오. 장비의 무게, 장비에 의해 발생한 열 또는 장비의 날카로운 가
장자리로 인해 표면 손상이 발생할 수 있습니다.
• 압력 재분산 성능이 저하될 위험이 있으니 커버 내부에 덮개나 부속장치를 놓지 마십시오.
• 지지면의 하부를 세척할 때 액체가 지퍼 영역 또는 분수령 커버에 스며들지 않도록 하십시오. 지퍼에 닿은 액체는 지지면
코어 안으로 유입될 수 있습니다.
• 다리미, 드라이크리닝 또는 회전식 건조를 이용하여 지지면 커버를 건조시키지 마십시오.
• 제품 손상을 유발할 수 있으므로 지지면을 고압 세척하지 마십시오.
• 지지면 커버를 보관하거나 리넨을 추가하거나 환자를 지지면에 두기 전에 항상 지지면 커버를 건조시키십시오. 제품을 건
조시키면 성능 저하를 예방하는 데 도움이 됩니다.
• 커버가 분해될 수 있으므로 커버를 고농도 화학물질 용액에 과도하게 노출시키지 마십시오.
• 지지면 커버가 손상될 수 있으므로 글리콜에테르 >3%가 함유된 가속 과산화수소 또는 4차 화합물을 사용하지 마십시오.
• 또한 제조업체 지침을 따르지 않으면 지지면 커버의 사용 수면 기간에 영향을 줄 수 있습니다.
• 지지면 커버를 교체할 때는 항상 주의하십시오. 내부 내화격막에는 유리섬유가 함유되어 있습니다. 유리섬유의 분진 입자
가 피부 자극을 야기할 수 있습니다.
2815-009-001 Rev D.1 3 KO
Page 56

소소개개
본 설명서는 Stryker 제품의 사용 또는 유지보수에 도움이 되도록 제작되었습니다. 본 제품을 작동 또는 유지보수하기 전에
본 설명서를 읽어보십시오. 이 제품의 안전한 작동 또는 유지보수에 관해 직원을 교육하고 훈련시키는 방법과 절차를 마련하
십시오.
주주의의
• 제품을 부적절하게 사용하면 환자나 작동자가 부상을 입을 수 있습니다. 본 설명서에 기술된 대로만 제품을 사용하십시
오.
• 제품 또는 제품의 구성 요소 일체를 개조하지 마십시오. 제품을 개조하면 예상치 않게 작동하여 환자 또는 작동자가 부상
을 입을 수 있습니다. 제품을 개조하면 보증이 무효가 될 수도 있습니다.
참참고고
• 본 설명서는 영구적인 제품의 일부로 간주되어야 하며 제품이 추후 판매되더라도 제품과 함께 동봉되어야 합니다.
• Stryker는 제품 설계와 품질의 발전을 지속적으로 추구합니다. 본 설명서에는 인쇄 시점에서 제공되어 있는 가장 최근의
제품 정보가 실려 있습니다. 해당 제품과 본 설명서 간에 사소한 차이가 있을 수 있습니다. 질문이 있는 경우, Stryker 고객
서비스 또는 기술 지원부(1-800-327-0770)에 문의하시기 바랍니다.
제제품품 설설명명
PPrrooFFoorrmm™™은 일반 환자용 6인치(15.2cm) 두께의 비전동식 폼 지지면입니다. 매트리스 코어에 굴절 커트가 있습니다. 매트
리스 커버 소재는 나일론 또는 DDaarrtteexx®의 END406 섬유 중에서 선택할 수 있습니다. 제품의 모든 구성에는 지지면의 발쪽에
힐 슬로프가 포함되어 있습니다. 본 제품의 크기들은 SSttrryykkeerr 베드 프레임에 적합합니다. 본 설명서의 사양 절에 나와 있는
함께 사용할 수 있는 베드 프레임의 목록을 참조하십시오.
용용도도
PPrrooFFoorrmm은 압력 재분배와 가라앉음에 도움이 되도록 폼을 사용합니다.
본 제품은 압박 손상 또는 욕창(모든 단계, 미분류 손상 및 심조직 손상)의 예방 및 치료를 돕는 비전동식 지지면입니다. 의료
전문가의 위험 요인에 대한 임상 평가 및 피부 평가와 함께 이 제품을 사용하는 것이 좋습니다.
표준 모델(281505550001, 281505550003, 281505550005, 281505550007, 281505550020)은 일반 병원 환경, 특히 급성 간
호 의료 시설을 위한 것입니다.
행동 건강 모델(281505550009, 281505550010, 281505550011)은 행동 의료 시설에서 사용하기 위한 것입니다.
PPrrooFFoorrmm은 항상 지지면 커버와 함께 사용해야 합니다. 지지면 커버는 모든 외부 피부와 닿아 상호작용할 수 있습니다. 이 제
품과 함께 윗시트를 사용해야 합니다.
표준 일반 병원 환경 모델과 행동 건강 모델은 다음을 위한 용도가 아닙니다.
• 안전 작업 하중을 초과하는 환자
• 비만 환자
• 가정 건강 환경 세팅
• 멸균 제품 아님
• 측정 기능 불포함
SSttrryykkeerr는 작동자가 각 환자를 임상 평가하여 적절하게 사용할 것을 권장합니다.
예예상상 사사용용 수수명명 -- 표표준준
PPrrooFFoorrmm지지면 코어 및 커버는 정상적인 사용, 조건하에서 적절한 주기적 정비를 할 경우 예상 사용 수명이 3년입니다.
KO 4 2815-009-001 Rev D.1
Page 57

예예상상 수수명명 -- 행행동동 건건강강
PPrrooFFoorrmm지지면은 정상적인 사용, 조건하에서 적절한 주기적 정비를 할 경우 예상 수명이 3년입니다. 이 제품에는 정비 가능
한 부품이 없습니다.
금금기기 사사항항
알려진 바 없음.
규규격격
500파운드
안전 사용 하중
참참고고 -- 환자의 체중이 지지면에 명시된 안전 사용 하중을 초과해서는 안 됩니다.
226.8kg
표표준준
일일반반 환환자자 모모델델 228811550055555500000011 또또는는 228811550055555500002200((포포장장된된
상상태태))
길이 84인치
폭
두께 6인치
제품 중량 30파운드
상단 커버 소재 DDaarrtteexx®의 END406
일일반반 환환자자 모모델델
길이 84인치
폭
두께 6인치
35인치
228811550055555500000055 228811550055555500000077
35인치
213.4cm
88.9cm
15.2cm
13.6kg
213.4cm
88.9cm
15.2cm
228811550055555500000033
80인치
35인치
6인치
29파운드
80인치
35인치
6인치
203.2cm
88.9cm
15.2cm
13.2kg
203.2cm
88.9cm
15.2cm
제품 중량 30파운드
상단 커버 소재 나일론
매트리스 소재 폴리우레탄 폼
제품의 방화재 준수 16CFR1632, 16CFR1633, CGSB CAN 2-4.2 방법 27.7-
함께 사용할 수 있는 프레임 3002 SS33™™, 3005 SS33™™, GGooBBeedd® II, SSppiirriitt SSeelleecctt™™ 및 높
13.6kg
29파운드
M77, CAL TB129, BFD IX-11
은 4존 사이드레일
13.2kg
행행동동 건건강강
모모델델
길이 80인치
폭
2815-009-001 Rev D.1 5 KO
228811550055555500001100,, 228811550055555500001111 228811550055555500000099
35인치
203.2cm
88.9cm
84인치
35인치
213.4cm
76cm
Page 58

모모델델
228811550055555500001100,, 228811550055555500001111 228811550055555500000099
두께 6인치
제품 중량 29파운드
상단 커버 소재 DDaarrtteexx®®의 END406
매트리스 소재 폴리우레탄 폼
제품의 내화 격막 준수
함께 사용할 수 있는 프레임
참참고고 -- 행동건강 커버에는 지퍼가 없습니다. 본 제품은 수리가 불가능합니다.
15.2cm
13.2kg
6인치
30파운드
16CFR1632, 16CFR1633, CGSB
CAN 2-4.2 방법 27.7-M77, CAL
TB129, BFD IX-11
3002 SS33™™, 3005 SS33™™, GGooBBeedd®® II,
SSppiirriitt SSeelleecctt™™ 및 높은 4존 사이드레
일, SSppiirriitt®® 행동건강 플랫폼 베드
7.6cm
13.6cm
환환경경 조조건건
경경고고 -- 표준 모델 지지면을 행동 건강 사용 환경에서 사용하지 마십시오. 지퍼는 유해스러운 경우 사용할 수 있는 내부 구성
품에 접근할 수 있도록 합니다.
환환경경 조조건건 작작동동 보보관관 및및 운운반반
주위 온도
상대 습도(비응축)
대기압
Stryker는 통지 없이 사양을 변경할 권리를 보유합니다.
연연락락처처 정정보보
Stryker 고객서비스부 또는 기술지원부 연락처: 1-800-327-0770.
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
USA
온라인에서 조작 또는 정비 매뉴얼을 보려면 https://techweb.stryker.com/을 방문하십시오.
Stryker 고객서비스 또는 기술지원 부서에 전화할 때는 Stryker 제품의 일련번호(A)를 준비해 두십시오. 모든 서면 통신문에
일련번호를 기재하십시오.
KO 6 2815-009-001 Rev D.1
Page 59

일일련련번번호호 위위치치
A
제제조조일일
일련번호의 첫 네 자리가 제조 연도입니다.
2815-009-001 Rev D.1 7 KO
Page 60

설설치치
경경고고
• 항상 환자의 피부를 정기적으로 점검하십시오. 홍반 또는 피부 손상이 발생할 경우 의사에게 알리십시오. 환자의 피부 상
태를 치료하지 않고 방치할 경우 중상을 초래할 수 있습니다.
• 환자의 낙상 위험을 줄이기 위해 항상 각별한 주의를 기울이고 감독하십시오. 덮개를 사용하면 환자 안정성과 사이드레일
사용 범위에 부정적인 영향을 줄 수 있습니다.
• 지지면과 베드 프레임의 사이에 이물질이 있는지 항상 검사하십시오. 이물체가 있으면 지지 플랫폼에서 지지면이 미끄러
질 수 있습니다.
• 항상 사이드레일 사용을 고려하십시오. 지지면은 사이드레일과 함께 사용할 때 안전성이 극대화됩니다. 사이드레일이 없
을 때 낙상 위험이 증가할 수 있습니다. 사이드레일 또는 기타 구속 장치의 사용(끼일 가능성) 또는 비사용(환자 낙상 가능
성)으로 인해 중상 또는 사망이 발생할 수 있습니다. 사이드레일 사용에 관한 현지 정책을 고려하십시오. 의사, 작동자 또
는 책임있는 당사자는 사이드레일 사용 여부와 사용 방법을 각 환자의 개별적 필요에 기반하여 결정해야 합니다.
• 낙상의 가능성을 줄이기 위해, 낙상의 위험이 있는 환자(예: 격앙 또는 혼동 상태에 있는 환자)에 대해서는 항상 각별한 주
의를 기울이십시오.
• 너비, 길이 또는 두께가 맞지 않는 더 크거나 더 작은 베드 프레임에 지지면을 사용하지 마십시오. 지지면 미끄러짐, 환자
부상 또는 베드의 움직이는 부품과의 간섭 위험을 피하기 위함입니다.
• 간격이 있는 경우에는 지지면을 사용하지 마십시오. 함께 사용할 수 없는 베드 프레임에 지지면을 설치할 때 걸릴 위험이
발생할 수 있습니다.
• 지지면 커버를 통해 지지면에 바늘을 꽂지 마십시오. 구멍을 통해 체액이 지지면의 내부(내핵)로 유입될 수 있으며, 이 경
우 교차 오염, 제품 손상 또는 제품 오작동이 발생할 수 있습니다.
주주의의
• 지지면 상단에 배치된 장치 또는 장비에 항상 주의하십시오. 장비의 무게, 장비에 의해 발생한 열 또는 장비의 날카로운 가
장자리로 인해 표면 손상이 발생할 수 있습니다.
• 압력 재분산 성능이 저하될 위험이 있으니 커버 내부에 덮개나 부속장치를 놓지 마십시오.
지지면을 설치하는 방법:
1. 지지면이 베드 프레임에 맞는지 확인합니다.
2. 지지면의 무늬쪽을 위로 하여 베드 프레임 머리쪽에 놓습니다.
3. 매트리스가 베드 프레임의 매트리스 리테이너들 사이에 놓였는지 확인합니다.
4. 병원 표준 규정에 따라 리넨을 지지면에 놓습니다.
KO 8 2815-009-001 Rev D.1
Page 61

작작동동
환환자자 지지지지 플플랫랫폼폼 간간 환환자자 이이동동
경경고고
• 지지면을 운반 장치로 사용하지 마십시오.
• 환자가 탑승한 상태에서 지지면 핸들을 사용하여 지지면을 올리거나 이동하지 마십시오.
• 지지면 커버를 통해 지지면에 바늘을 꽂지 마십시오. 구멍을 통해 체액이 지지면의 내부(내핵)로 유입될 수 있으며, 이 경
우 교차 오염, 제품 손상 또는 제품 오작동이 발생할 수 있습니다.
• 병원 베드 프레임이 환자와 지지면을 모두 지지할 경우, 프레임의 안전 사용 하중을 초과하지 마십시오. 과도한 중량은 이
제품의 예상치 못한 안전 및 성능 문제를 유발할 수 있습니다.
• 환자 지지 플랫폼과 플랫폼 간의 해당 운반 간격이 환자를 지지하기에 충분한지 항상 확인하십시오. 두 환자 지지 플랫폼
간의 간격이 3인치(7.6cm) 를 초과하는 경우, 이전 브리지를 사용하여 간격을 메꾸십시오. 운반 다리는 환자 지지 플랫폼
간 환자 이동을 용이하게 하기 위한 용도입니다.
• 환자를 지지면에 놓을 때에는 환자의 낙상 위험을 줄이기 위해 항상 반대쪽 사이드레일이 올려져 있는지 확인하십시오.
한 환자 지지면으로부터 다른 지지면으로 환자를 이동하는 방법:
선선행행 조조건건:: 지지면 간 환자 이동에 필요한 병원 표준 규정을 준수합니다.
1. 한 환자 지지 플랫폼을 다른 환자 지지 플랫폼과 나란히 놓아 두 플랫폼 사이의 간격을 최소화합니다.
2. 두 환자 지지 플랫폼의 브레이크를 체결합니다.
3. 환자 지지 플랫폼의 높이가 다른 플랫폼과 같아지도록 높이를 조절합니다.
4. 환자 및 작동자 안전을 위한 모든 해당 안전 규칙과 병원 표준 규정에 따라 환자를 이동합니다.
2815-009-001 Rev D.1 9 KO
Page 62

예예방방정정비비
모든 Stryker Medical 제품에 대한 연례 예방정비시에는 적어도 나열된 모든 항목을 점검하십시오. 제품 사용 수준에 따라 예
방정비 점검을 더 자주 수행해야 할 수도 있습니다.
예방정비를 실시하기 전에는 제품을 사용하지 마십시오.
참참고고 -- 해당되는 경우 점검 전에 지지면 외부를 세척하고 소독하십시오.
표준 모델의 경우 다음 항목을 검사하십시오.
지퍼와 커버(위 및 아래)에 찢어진 곳이나 갈라진 곳 또는 구멍이나 기타 틈새가 없는지 점검
커버의 지퍼를 완전히 열고 내부 구성 요소에 액체 유입 또는 오염으로 인한 얼룩의 징후가 있는지 점검
라벨이 읽기 쉬운지, 적절하게 부착되어 있는지, 온전한 상태인지 점검
핸들에 뜯어지거나 갈라진 곳이 없는지, 바느질이 온전한지 점검
폼이 부식되었거나 부서지지 않았는지 점검
내화격막의 파열, 균열 또는 기타 육안으로 보이는 손상 징후
행동 건강 모델의 경우 다음 항목을 검사하십시오.
커버(위 및 아래)에 찢어진 곳이나 갈라진 곳 또는 구멍이나 기타 틈새가 없는지 점검
라벨이 읽기 쉬운지, 적절하게 부착되어 있는지, 온전한 상태인지 점검
제품 일련번호:
시행자:
일자:
물물티티슈슈를를 사사용용한한 세세척척 및및 소소독독
미국에만 해당. 해당 구성 또는 지역에 대한 이용 가능 여부를 확인하십시오. 가용 부품 및 가격에 대해서는 Stryker 고객서비
스부에 전화하십시오: 1-800-327-0770.
Stryker의 선호 물티슈(2060-000-001 6'' x 10''(15cm x 25cm) 또는 2060-000-002 9'' x 12''(23cm x 30cm))에는 다음 활성 성
분이 들어있습니다:
• n-알킬(60% C14, 30% C16, 5% C12, 5% C18) 디메틸 벤질 암모늄 클로라이드 - 0.154%
• n-알킬(68% C12, 32% C14) 디메틸 에틸벤질 암모늄 클로라이드 - 0.154%
• 이소프로판올 - 21.000%
비활성 성분: 에틸렌 글리콜 모노부틸 에테르 – < 3%
참참고고 -- 안전 정보는 제품 라벨을 참조하십시오.
제품 외면을 세척 또는 소독하는 방법:
1. 세척하려면 깨끗한 새 물티슈로 외면을 닦아 눈에 보이는 모든 오물을 제거하십시오. 제품이 깨끗해질 때까지 필요한 만
큼 반복합니다.
참참고고
• 필요만 숫자 만큼의 물티슈를 사용하십시오.
• 단계 1을 완료한 후에 소독합니다.
2. 소독하려면 외면이 젖을 때까지 깨끗한 새 물티슈로 닦으십시오. 외면이 실온에서 2분 동안 젖은 상태로 두십시오.
3. 제품을 자연 건조시킨 후에 다시 사용합니다.
KO 10 2815-009-001 Rev D.1
Page 63

관관리리 및및 유유지지보보수수
경경고고
• 이 지지면의 내부 구성 요소를 세척하지 마십시오. 내부에 오염된 부분이 확인될 경우 지지면을 폐기하십시오.
• 본 제품을 세정액이나 소독액에 담그지 마십시오.
• 제품에 액체가 고이지 않도록 하십시오.
• 매 사용 전에 지지면 커버(상단 및 하단)에 찢김, 구멍, 과도한 마모 및 오정렬된 지퍼 등이 있는지 항상 점검하십시오. 손
상된 경우, 지지면 사용을 즉시 중단하십시오.
• 항상 각 제품을 깨끗한 물을 사용하여 닦고, 세척 후에는 각 제품을 건조시키십시오. 일부 세정제는 부식성이 있기 때문에
본 제품에 손상을 초래할 수 있습니다. 이러한 세척 지침을 따르지 않으면 보증이 무효가 될 수 있습니다.
주주의의
• 지지면의 밑면을 세척할 때 액체가 지퍼 영역 또는 지퍼 덮개 보호막에 스며들지 않도록 하십시오. 지퍼에 닿은 액체는 지
지면 코어 안으로 유입될 수 있습니다.
• 다리미, 드라이크리닝 또는 회전식 건조기를 이용하여 지지면 커버를 건조시키지 마십시오.
• 제품 손상을 유발할 수 있으므로 지지면을 고압 세척하지 마십시오.
• 지지면 커버를 보관하거나 린넨을 더 깔거나 또는 환자를 지지면 위에 배치시키기 전에 항상 지지면 커버를 건조시키십시
오. 제품을 건조시키면 성능 저하를 예방하는 데 도움이 됩니다.
• 커버의 질이 저하될 수 있으므로 커버를 고농도 화학물질 용액에 과도하게 노출시키지 마십시오.
• 지지면 커버가 손상될 수 있으므로 글리콜에테르 >3%가 함유된 가속 과산화수소 또는 4차 화합물을 사용하지 마십시오.
• 제조업체 지침을 따르지 않으면 지지면 커버의 사용 수명 기간에도 영향을 줄 수 있습니다.
지지면 커버는 다음 화학물질 용액에 대한 저항성이 있습니다.
• 4차 세정제(활성 성분 - 염화암모늄), 글리콜에테르 3% 미만 함유
• 페놀 용액(Matar)
• 염소계 표백 용액(10000 ppm) - END406 매트리스 커버에 사용
• 염소계 표백 용액(1000 ppm) - 나일론 매트리스 커버에 사용
• 70% 아이소프로필 알코올
교차 오염 및 감염의 위험을 예방하기 위해, 환자가 바뀔 때마다 지지면에 대한 병원 표준 규정을 따르십시오.
참참고고 -- 염소계 표백 용액은 나일론 매트리스 커버의 변색을 초래할 수 있습니다.
2815-009-001 Rev D.1 11 KO
Page 64

교교체체 부부품품 빠빠른른 참참조조
이러한 부품은 표준 모델로 구입할 수 있지만, 변경될 수 있습니다. 가용 부품 및 가격에 대해서는 Stryker 고객서비스부에 전
화하십시오: 1-800-327-0770.
명명칭칭 번번호호
커버 어셈블리, 힐 슬로프, END406 80인치(203.2cm)
커버 어셈블리, 힐 슬로프, END406 84인치(213.4cm)
커버 어셈블리, 힐 슬로프, 나일론 80인치(203.2cm)
커버 어셈블리, 힐 슬로프, 나일론 84인치(213.4cm)
281505550012
281505550014
281505550018
281505550016
커커버버 교교체체 -- 표표준준 모모델델
주주의의 -- 지지면 커버를 교체할 때는 항상 주의하십시오. 내부 내화격막에는 유리섬유가 함유되어 있습니다. 유리섬유의 분진
입자가 피부 자극을 야기할 수 있습니다.
필필수수 도도구구::
• None
절절차차::
1. 침대 높이를 최고 위치로 올립니다.
2. 등판부와 무릎부를 최저 위치로 내립니다.
3. 커버의 지퍼를 엽니다. 지지면의 발끝 환자 오른쪽 코너에서 시작하여 머리끝 환자 오른쪽 코너에서 중지합니다.
4. 커버의 상단을 환자의 오른쪽으로 접은 후 폼 침대 어셈블리를 침대에서 치워둡니다.
5. 커버를 벗겨 폐기하십시오.
6. 지퍼가 열린 교체용 커버를 검은색 하단 커버가 매트리스 위에 놓인 상태에서 베드 위에 놓고 상단 커버를 환자의 베드 오
른쪽에 접어 놓습니다.
7. 폼 크립이 커버와 정렬되도록 폼 크립 어셈블리를 커버 하단부 위에 놓습니다.
8. 상단 커버가 폼 크립 어셈블리와 아귀가 맞도록 상단 커버를 폼 크립 어셈블리 위에 포갭니다.
9. 커버의 지퍼를 닫습니다. 머리끝 환자 오른쪽 코너에서 시작하여 발끝 환자 오른쪽 코너에서 중지합니다.
10.제품을 다시 사용하기 전에 제대로 작동하는지 확인하십시오.
KO 12 2815-009-001 Rev D.1
Page 65

SSuuppeerrffíícciiee ddee aappooiioo nnããoo aalliimmeennttaaddaa PPrrooFFoorrmm™™
MMaannuuaall ddee uuttiilliizzaaççããoo
2815
2815-009-001 Rev D.1
PT
2019/04
Page 66

Page 67

SSíímmbboollooss
10
Consultar o manual/folheto de instruções
Instruções de funcionamento/Consultar as instruções de utilização
Advertência geral
Precaução
Número de catálogo
Número de série
Fabricante
Carga de trabalho segura
Lavar à mão
Não secar na máquina
Não limpar a seco
Não passar a ferro
Deixar secar ao ar
Solução clorada (lixívia)
Para patentes dos EUA, consulte www.stryker.com/patents
Manter seco
Não empilhar mais de 10 unidades
2815-009-001 Rev D.1 PT
Este lado para cima
Page 68

Frágil
Não usar objetos cortantes para abrir a embalagem
Peso do equipamento
PT 2815-009-001 Rev D.1
Page 69

ÍÍnnddiiccee
Símbolos ..........................................................................................................................................................15
Definição de Advertência/Precaução/Nota......................................................................................................2
Resumo das precauções de segurança ...................................................................................................2
Introdução....................................................................................................................................................4
Descrição do produto .............................................................................................................................4
Utilização prevista ..................................................................................................................................4
Vida útil prevista - padrão .......................................................................................................................5
Vida útil prevista - saúde comportamental ................................................................................................5
Contraindicações ...................................................................................................................................5
Especificações.......................................................................................................................................5
Padrão ...........................................................................................................................................5
Saúde comportamental ....................................................................................................................6
Condições ambientais......................................................................................................................6
Informações para contacto......................................................................................................................7
Localização do número de série ..............................................................................................................7
Data de fabrico ......................................................................................................................................7
Preparação ..................................................................................................................................................8
Funcionamento.............................................................................................................................................9
Transferência de um doente de uma plataforma de apoio para outra..........................................................9
Manutenção preventiva ...............................................................................................................................10
Cleaning and disinfecting with wipes......................................................................................................10
Cuidados e manutenção .......................................................................................................................11
Lista de consulta rápida de peças de substituição ......................................................................................... 12
Substituição da cobertura - Modelo padrão ............................................................................................12
2815-009-001 Rev D.1 1 PT
Page 70

DDeeffiinniiççããoo ddee AAddvveerrttêênncciiaa//PPrreeccaauuççããoo//NNoottaa
Os termos AADDVVEERRTTÊÊNNCCIIAA, PPRREECCAAUUÇÇÃÃOO e NNOOTTAA possuem significados especiais aos quais se deve prestar a devida
atenção.
AADDVVEERRTTÊÊNNCCIIAA -- Alerta o leitor para uma situação que, se não for evitada, pode resultar em morte ou lesões graves.
Pode igualmente descrever possíveis reacções adversas graves e perigos de segurança.
PPRREECCAAUUÇÇÃÃOO -- Alerta o leitor para uma situação potencialmente perigosa que, se não for evitada, poderá resultar em
lesões ligeiras ou moderadas no utilizador ou no doente, ou danos no equipamento ou noutros bens. Inclui os cuidados
especiais necessários para uma utilização segura e eficaz do dispositivo, e os cuidados necessários para evitar a
possibilidade de danos no dispositivo em resultado da utilização correcta ou incorrecta do mesmo.
NNoottaa -- Fornece informações especiais, que se destinam a facilitar a manutenção ou a clarificar instruções importantes.
RReessuummoo ddaass pprreeccaauuççõõeess ddee sseegguurraannççaa
Leia sempre e cumpra com rigor as advertências e precauções indicadas nesta página. A assistência deve ser feita
apenas por pessoal qualificado.
AADDVVEERRTTÊÊNNCCIIAA
• Nunca utilize o modelo padrão da superfície de apoio em ambientes de saúde comportamental. O fecho permite o
acesso aos componentes internos que podem ser utilizados para provocar dano.
• Examine sempre a pele do doente com regularidade. Consulte um médico caso ocorra eritema ou ulceração cutânea.
Poderão ocorrer lesões graves se o problema de pele do doente não for tratado.
• Tenha sempre muita precaução e supervisione para ajudar a reduzir o risco de queda do doente. A estabilidade do
doente e a cobertura protetora fornecida pelas grades laterais poderão ficar comprometidas com a utilização de uma
capa.
• Inspecione sempre a existência de objetos estranhos entre a superfície de apoio e a estrutura da cama. Os objetos
estranhos podem fazer com que a superfície de apoio deslize na plataforma de apoio.
• Considere sempre a utilização de grades laterais. A utilização segura da superfície de apoio é maximizada quando
utilizada em conjunto com as grades laterais. Pode existir um risco elevado de quedas quando as grades laterais não
estão presentes. Poderão ocorrer lesões graves ou mesmo a morte devido à utilização (possível aprisionamento) ou
não utilização (potenciais quedas do doente) de grades laterais ou outros dispositivos de contenção. Considere sempre
as políticas locais relativas à utilização de grades laterais. O médico, o operador ou outros responsáveis devem
determinar se e como se deverão utilizar grades laterais com base nas necessidades de cada doente.
• Tenha sempre muita precaução com um doente em risco de queda (tal como um doente agitado ou confuso) para
ajudar a reduzir a probabilidade de queda.
• Não utilize a superfície de apoio numa estrutura de cama maior ou mais pequena, que não se adeque à largura,
comprimento ou espessura. Isto serve para evitar o risco do deslizamento da superfície de apoio, de lesão no doente,
ou interferência com partes móveis da cama.
• Não utilize a superfície de apoio quando existirem espaços.
O risco de entalamento pode surgir quando a superfície de apoio está colocada em estruturas de cama não
compatíveis.
• Não espete agulhas na superfície de apoio através da respetiva cobertura. Os orifícios poderão permitir a entrada de
fluidos corporais no interior (núcleo interior) da superfície de apoio e poderão causar contaminação cruzada, danos ou
avaria do produto.
• Não utilize a superfície de apoio como um dispositivo de transferência.
• Não utilize as pegas da superfície de apoio para elevar ou mover a superfície de apoio com um doente colocado.
• Não exceda a carga de trabalho segura da estrutura da cama hospitalar quando suportar o doente e a superfície de
apoio. O excesso de peso poderá originar segurança e desempenho imprevisíveis deste produto.
• Certifique-se sempre de que as plataformas que suportam o doente e os respetivos espaços de transferência são
adequados para suportar o doente. Se o espaço entre as duas plataformas de apoio do doente for superior a 3 pol. (7,6
cm), utilize a ponte de transferência para preencher o espaço. A ponte de transferência destina-se a facilitar a
transferência de uma plataforma de apoio do doente para outra.
PT 2 2815-009-001 Rev D.1
Page 71

• Ao colocar o doente na superfície de apoio, certifique-se sempre de que a grade lateral oposta está elevada para
reduzir o risco de queda do doente.
• Não lave os componentes internos desta superfície de apoio. Elimine a superfície de apoio caso se descubra
contaminação no seu interior.
• Não mergulhe o produto em soluções de limpeza ou desinfetantes.
• Não permita a acumulação de líquido no produto.
• Inspecione sempre as coberturas da superfície de apoio (superior e inferior) quanto a rasgões, perfurações, desgaste
excessivo e fechos mal alinhados, antes de cada utilização. Caso esteja comprometida, deixe imediatamente de utilizar
a superfície de apoio.
• Certifique-se sempre de que limpa com água limpa e de que seca cada produto após a sua limpeza. Alguns produtos de
limpeza são de natureza corrosiva e podem danificar o produto. O incumprimento destas instruções de limpeza poderá
anular a sua garantia.
PPRREECCAAUUÇÇÃÃOO
• A utilização inadequada do produto pode provocar lesões no doente ou no operador. Utilize o produto apenas conforme
descrito neste manual.
• Não modifique o produto nem qualquer componente do mesmo. A modificação do produto poderá originar um
funcionamento imprevisível, o que poderá provocar lesões no doente ou no operador. A modificação do produto
também anula a garantia.
• Esteja sempre atento aos dispositivos ou equipamentos que são colocados no topo da superfície de apoio. Poderão
ocorrer danos na superfície devido ao peso do equipamento, ao calor gerado pelo equipamento ou a bordos
perfurocortantes do equipamento.
• Não coloque capas ou acessórios no interior da cobertura para evitar o risco de reduzir o desempenho da redistribuição
da pressão.
• Não permita que penetrem líquidos na zona do fecho ou na barreira de separação da cobertura quando limpar a parte
inferior da superfície de apoio. Os fluidos cujo contacto com o fecho for permitido, podem penetrar no núcleo da
superfície de apoio.
• Não passe a ferro, não limpe a seco nem seque na máquina as coberturas da superfície de apoio.
• Não lave a superfície de apoio com água sob pressão, pois poderá danificar o produto.
• Seque sempre as coberturas da superfície de apoio antes de as guardar, adicionar lençóis ou de colocar um doente na
superfície. Um produto seco ajuda ajuda a prevenir que o seu desempenho seja alterado.
• Não exponha excessivamente as coberturas a soluções desinfetantes de concentração mais elevada, pois isto poderá
degradar as coberturas.
• Não utilize peróxidos de hidrogénio acelerados ou quaternários que contenham éter de glicol >3% já que estes
químicos podem danificar a cobertura da superfície de apoio.
• O não cumprimento das instruções do fabricante também poderá afetar a vida útil da cobertura da superfície de apoio.
• Tenha sempre precaução ao substituir a cobertura da superfície de apoio. A barreira anti-fogo interna contém fibras de
fibra de vidro. As partículas de poeira das fibras podem provocar irritação cutânea.
2815-009-001 Rev D.1 3 PT
Page 72

IInnttrroodduuççããoo
Este manual ajuda no funcionamento e manutenção do seu produto da Stryker. Leia este manual antes de utilizar ou
proceder à manutenção deste produto. Defina métodos e procedimentos para educar e formar a sua equipa no que diz
respeito ao funcionamento ou manutenção seguros deste produto.
PPRREECCAAUUÇÇÃÃOO
• A utilização inadequada do produto pode provocar lesões no doente ou no operador. Utilize o produto apenas conforme
descrito neste manual.
• Não modifique o produto nem qualquer componente do mesmo. A modificação do produto poderá originar um
funcionamento imprevisível, o que poderá provocar lesões no doente ou no operador. A modificação do produto
também anula a garantia.
NNoottaa
• Este manual deve ser considerado uma parte permanente do produto e deve ser guardado juntamente com o produto,
mesmo que este seja vendido.
• A Stryker procura continuamente avanços no design e na qualidade do produto. Este manual contém as informações
mais atuais sobre o produto disponíveis no momento da impressão. Poderão existir pequenas discrepâncias entre o
seu produto e este manual. Se tiver alguma questão, contacte o Serviço de Apoio ao Cliente ou a Assistência Técnica
da Stryker através do número de telefone +1-800-327-0770.
DDeessccrriiççããoo ddoo pprroodduuttoo
A PPrrooFFoorrmm™™ é uma superfície de apoio de espuma com espessura de 6” (15,2 cm), não alimentada, para a população
geral de doentes. Existem cortes de articulação no núcleo do colchão. As opções para o material da cobertura do colchão
incluem nylon ou tecido END406 da DDaarrtteexx®. Todas as configurações do produto incluem inclinação para calcanhar na
extremidade do lado dos pés da superfície de apoio. As dimensões deste produto são compatíveis com as estrutura de
cama da SSttrryykkeerr. Consulte a lista completa de estruturas de cama na secção de especificações deste manual.
UUttiilliizzaaççããoo pprreevviissttaa
PPrrooFFoorrmm utiliza espuma para ajudar na redistribuição da pressão e imersão.
Este produto é uma superfície de apoio não alimentada que ajuda na prevenção e tratamento de lesões de pressão ou
úlceras de pressão (todas as fases, lesões inclassificáveis e lesões dos tecidos profundos). Recomendamos que
implemente este produto em combinação com a avaliação clínica dos fatores de risco e avaliações da pele realizadas por
um profissional de cuidados de saúde.
O Modelo padrão (281505550001, 281505550003, 281505550005, 281505550007, 281505550020) destina-se ao
ambiente hospitalar em geral, especificamente para instalações de cuidados de saúde agudos.
O Modelo de saúde comportamental (281505550009, 281505550010, 281505550011) destina-se a ser utilizado em
instalações de cuidados comportamentais.
A PPrrooFFoorrmm deve ser sempre utilizada com uma cobertura da superfície de apoio. A cobertura da superfície de apoio pode
interagir com toda a pele externa. Deve ser utilizado um lençol superior com este produto.
Os modelos padrão de ambiente hospitalar em geral e os modelos de saúde comportamental não são destinados a:
• Doentes que excedam a carga de trabalho segura
• Doentes bariátricos
• Ambiente de saúde domiciliária
• Produto não-esterilizado
• Não contém uma função de medição
A SSttrryykkeerr promove a avaliação clínica de cada doente e a utilização adequada pelo operador.
PT 4 2815-009-001 Rev D.1
Page 73

VViiddaa úúttiill pprreevviissttaa -- ppaaddrrããoo
A PPrrooFFoorrmm núcleo e coberturas da superfície de apoio têm uma vida útil esperada de 3 anos em condições e utilização
normais e com a manutenção periódica apropriada.
VViiddaa úúttiill pprreevviissttaa -- ssaaúúddee ccoommppoorrttaammeennttaall
A PPrrooFFoorrmm superfície de apoio tem uma vida útil esperada de 3 anos em condições e utilização normais e com a
manutenção periódica apropriada. Não existem peças passíveis de manutenção para este produto.
CCoonnttrraaiinnddiiccaaççõõeess
Não são conhecidos.
EEssppeecciiffiiccaaççõõeess
500 lb 226,8 kg
Carga de trabalho segura
NNoottaa -- O peso do doente não deve exceder a carga de trabalho segura especificada pela superfície de apoio.
PPaaddrrããoo
MMooddeelloo ppaarraa oo
ddooeennttee eemm ggeerraall
Comprimento 84 pol. 213,4 cm 80 pol. 203,2 cm
Largura 35 pol. 88,9 cm 35 pol. 88,9 cm
Espessura 6 pol. 15,2 cm 6 pol. 15,2 cm
Peso do produto 30 lb 13,6 kg 29 lb 13,2 kg
Material da cobertura
superior
MMooddeelloo ppaarraa oo
ddooeennttee eemm ggeerraall
Comprimento 84 pol. 213,4 cm 80 pol. 203,2 cm
Largura 35 pol. 88,9 cm 35 pol. 88,9 cm
Espessura 6 pol. 15,2 cm 6 pol. 15,2 cm
Peso do produto 30 lb 13,6 kg 29 lb 13,2 kg
Material da cobertura
superior
228811550055555500000011 oouu 228811550055555500002200
eennssaaccaaddoo
END406 da DDaarrtteexx®
228811550055555500000055 228811550055555500000077
Nylon
228811550055555500000033
2815-009-001 Rev D.1 5 PT
Page 74

Material do colchão Espuma de poliuretano
Conformidade do produto com barreira anti-fogo 16CFR1632, 16CFR1633, CGSB CAN 2-4.2 Método 27.7-
-M77, CAL TB129, BFD IX-11
Estruturas de cama compatíveis 3002 SS33™™, 3005 SS33™™, GGooBBeedd® II, SSppiirriitt SSeelleecctt™™com
grades laterais elevadas com 4 zonas
SSaaúúddee ccoommppoorrttaammeennttaall
MMooddeellooss 228811550055555500001100,, 228811550055555500001111 228811550055555500000099
Comprimento 80 pol. 203,2 cm 84 pol. 213,4 cm
Largura 35 pol. 88,9 cm 35 pol. 76 cm
Espessura 6 pol. 15,2 cm 6 pol. 7,6 cm
Peso do produto 29 lb 13,2 kg 30 lb 13,6 cm
Material da cobertura superior END406 da DDaarrtteexx®®
Material do colchão Espuma de poliuretano
Conformidade do produto com barreira anti-fogo 16CFR1632, 16CFR1633, CGSB
CAN 2-4.2 Método 27.7-M77, CAL
TB129, BFD IX-11
Estruturas de cama compatíveis 3002 SS33™™, 3005 SS33™™, GGooBBeedd®® II,
SSppiirriitt SSeelleecctt™™ com grades laterais
elevadas com 4 zonas, Plataforma
de cama de saúde comportamental
SSppiirriitt®®
NNoottaa -- As coberturas de saúde comportamental não têm fechos de correr. O produto não tem manutenção.
CCoonnddiiççõõeess aammbbiieennttaaiiss
AADDVVEERRTTÊÊNNCCIIAA -- Nunca utilize o modelo padrão da superfície de apoio em ambientes de saúde comportamental. O fecho
permite o acesso aos componentes internos que podem ser utilizados para provocar dano.
CCoonnddiiççõõeess aammbbiieennttaaiiss
Temperatura ambiente
Humidade relativa (sem
condensação)
Pressão atmosférica
FFuunncciioonnaammeennttoo
AArrmmaazzeennaammeennttoo ee ttrraannssppoorrttee
A Stryker reserva-se o direito de alterar as especificações sem aviso prévio.
PT 6 2815-009-001 Rev D.1
Page 75

IInnffoorrmmaaççõõeess ppaarraa ccoonnttaaccttoo
A
Contacte o Serviço de Apoio ao Cliente ou a Assistência Técnica da Stryker, através do número: 1-800-327-0770.
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
EUA
Para consultar o manual de operações ou manutenção online, vá a https://techweb.stryker.com/.
Tenha o número de série (A) do seu produto da Stryker à mão quando telefonar para o Serviço de Apoio ao Cliente ou
Assistência Técnica da Stryker. Inclua o número de série em toda a comunicação escrita.
LLooccaalliizzaaççããoo ddoo nnúúmmeerroo ddee sséérriiee
DDaattaa ddee ffaabbrriiccoo
O ano de fabrico é indicado pelos primeiros quatro dígitos do número de série.
2815-009-001 Rev D.1 7 PT
Page 76

PPrreeppaarraaççããoo
AADDVVEERRTTÊÊNNCCIIAA
• Examine sempre a pele do doente com regularidade. Consulte um médico caso ocorra eritema ou ulceração cutânea.
Poderão ocorrer lesões graves se o problema de pele do doente não for tratado.
• Tenha sempre muita precaução e supervisione para ajudar a reduzir o risco de queda do doente. A estabilidade do
doente e a cobertura protetora fornecida pelas grades laterais poderão ficar comprometidas com a utilização de uma
capa.
• Inspecione sempre a existência de objetos estranhos entre a superfície de apoio e a estrutura da cama. Os objetos
estranhos podem fazer com que a superfície de apoio deslize na plataforma de apoio.
• Considere sempre a utilização de grades laterais. A utilização segura da superfície de apoio é maximizada quando
utilizada em conjunto com as grades laterais. Pode existir um risco elevado de quedas quando as grades laterais não
estão presentes. Poderão ocorrer lesões graves ou mesmo a morte devido à utilização (possível aprisionamento) ou
não utilização (potenciais quedas do doente) de grades laterais ou outros dispositivos de contenção. Considere sempre
as políticas locais relativas à utilização de grades laterais. O médico, o operador ou outros responsáveis devem
determinar se e como se deverão utilizar grades laterais com base nas necessidades de cada doente.
• Tenha sempre muita precaução com um doente em risco de queda (tal como um doente agitado ou confuso) para
ajudar a reduzir a probabilidade de queda.
• Não utilize a superfície de apoio numa estrutura de cama maior ou mais pequena, que não se adeque à largura,
comprimento ou espessura. Isto serve para evitar o risco do deslizamento da superfície de apoio, de lesão no doente,
ou interferência com partes móveis da cama.
• Não utilize a superfície de apoio quando existirem espaços.
O risco de entalamento pode surgir quando a superfície de apoio está colocada em estruturas de cama não
compatíveis.
• Não espete agulhas na superfície de apoio através da respetiva cobertura. Os orifícios poderão permitir a entrada de
fluidos corporais no interior (núcleo interior) da superfície de apoio e poderão causar contaminação cruzada, danos ou
avaria do produto.
PPRREECCAAUUÇÇÃÃOO
• Esteja sempre atento aos dispositivos ou equipamentos que são colocados no topo da superfície de apoio. Poderão
ocorrer danos na superfície devido ao peso do equipamento, ao calor gerado pelo equipamento ou a bordos
perfurocortantes do equipamento.
• Não coloque capas ou acessórios no interior da cobertura para evitar o risco de reduzir o desempenho da redistribuição
da pressão.
Para preparar a superfície de apoio:
1. Certifique-se de que a superfície de apoio se adequa à estrutura da cama.
2. Coloque a superfície de apoio com a face decorada voltada para cima na extremidade do lado da cabeça da estrutura
da cama.
3. Certifique-se de que o colchão está colocado entre o dispositivo de retenção do colchão na estrutura da cama.
4. Coloque a roupa de cama na superfície de apoio de acordo com os protocolos hospitalares.
PT 8 2815-009-001 Rev D.1
Page 77

FFuunncciioonnaammeennttoo
TTrraannssffeerrêênncciiaa ddee uumm ddooeennttee ddee uummaa ppllaattaaffoorrmmaa ddee aappooiioo ppaarraa oouuttrraa
AADDVVEERRTTÊÊNNCCIIAA
• Não utilize a superfície de apoio como um dispositivo de transferência.
• Não utilize as pegas da superfície de apoio para elevar ou mover a superfície de apoio com um doente colocado.
• Não espete agulhas na superfície de apoio através da respetiva cobertura. Os orifícios poderão permitir a entrada de
fluidos corporais no interior (núcleo interior) da superfície de apoio e poderão causar contaminação cruzada, danos ou
avaria do produto.
• Não exceda a carga de trabalho segura da estrutura da cama hospitalar quando suportar o doente e a superfície de
apoio. O excesso de peso poderá originar segurança e desempenho imprevisíveis deste produto.
• Certifique-se sempre de que as plataformas que suportam o doente e os respetivos espaços de transferência são
adequados para suportar o doente. Se o espaço entre as duas plataformas de apoio do doente for superior a 3 pol. (7,6
cm), utilize a ponte de transferência para preencher o espaço. A ponte de transferência destina-se a facilitar a
transferência de uma plataforma de apoio do doente para outra.
• Ao colocar o doente na superfície de apoio, certifique-se sempre de que a grade lateral oposta está elevada para
reduzir o risco de queda do doente.
Para transferir o doente de uma superfície de apoio para outra:
PPrréé--rreeqquuiissiittoo:: Siga os protocolos hospitalares necessários para transferir um doente de uma superfície para outra.
1. Posicione uma plataforma de apoio do doente ao longo de outra plataforma enquanto minimiza o espaço entre as duas
plataformas.
2. Acione os travões de ambas as plataformas de apoio do doente.
3. Ajuste a altura das plataformas de apoio do doente para que fiquem niveladas uma com a outra.
4. Transfira o doente, seguindo todas as regras de segurança e protocolos da instituição aplicáveis para garantir a
segurança do doente e do operador.
2815-009-001 Rev D.1 9 PT
Page 78

MMaannuutteennççããoo pprreevveennttiivvaa
No mínimo, verifique todos os itens indicados durante a manutenção preventiva anual para todos os produtos da Stryker
Medical. Poderá ter de realizar verificações da manutenção preventiva mais frequentemente, com base no nível de
utilização do produto.
Deixe de utilizar o produto antes da realização da manutenção preventiva.
NNoottaa -- Limpe e desinfete o exterior da superfície de apoio antes da inspeção, caso se aplique.
Inspecione os seguintes itens nos modelos padrão:
Os fechos e as coberturas (superior e inferior) não estão rasgados, cortados, não têm orifícios nem outras aberturas
Os componentes internos apresentam sinais de manchas devido à entrada de líquidos ou contaminação, abrindo
totalmente o fecho da cobertura do colchão
As etiquetas, verificando se estão legíveis, bem aderentes e íntegras
As pegas não apresentam rasgões nem rachas e as costuras estão intactas
A espuma não se degradou ou separou
A barreira anti-fogo quanto a rasgões, rachas ou outros sinais visíveis de danos
Inspecione os seguintes itens para os modelos de saúde comportamental:
As coberturas (superior e inferior) não estão rasgadas, cortadas, não têm orifícios nem outras aberturas
As etiquetas, verificando se estão legíveis, bem aderentes e íntegras
Número de série do produto:
Preenchido por:
Data:
CClleeaanniinngg aanndd ddiissiinnffeeccttiinngg wwiitthh wwiippeess
For United States only. Confirm availability for your configuration or region. Call Stryker Customer Service: 1-800-327-
-0770.
Stryker’s preferred wipes (2060-000-001 6'' x 10'' or 2060-000-002 9'' x 12'') include the following active ingredients:
• n-Alkyl (60% C14, 30% C16, 5% C12, 5% C18) dimethyl benzyl ammonium chloride - 0.154%
• n-Alkyl (68% C12, 32% C14) dimethyl ethylbenzyl ammonium chloride - 0.154%
• Isopropanol - 21.000%
Non-active ingredient: Ethylene Glycol Monobutyl Ether – < 3%
NNoottaa -- For safety information, read the product label.
To clean or disinfect the external product surface:
1. To clean, wipe external surfaces with a fresh, clean wipe to remove all visible soils. Repeat as necessary until the
product is clean.
NNoottaa
• Use as many wipes as necessary.
• Complete step 1 before you disinfect.
2. To disinfect, wipe external surfaces with a fresh, clean wipe until wet. Allow the external surface to remain wet for two
minutes at room temperature.
3. Allow the product to dry before you return it to service.
PT 10 2815-009-001 Rev D.1
Page 79

CCuuiiddaaddooss ee mmaannuutteennççããoo
AADDVVEERRTTÊÊNNCCIIAA
• Não lave os componentes internos desta superfície de apoio. Elimine a superfície de apoio caso se descubra
contaminação no seu interior.
• Não mergulhe o produto em soluções de limpeza ou desinfetantes.
• Não permita a acumulação de líquido no produto.
• Inspecione sempre as coberturas da superfície de apoio (superior e inferior) quanto a rasgões, perfurações, desgaste
excessivo e fechos mal alinhados, antes de cada utilização. Caso esteja comprometida, deixe imediatamente de utilizar
a superfície de apoio.
• Certifique-se sempre de que limpa com água limpa e de que seca cada produto após a sua limpeza. Alguns produtos de
limpeza são de natureza corrosiva e podem danificar o produto. O incumprimento destas instruções de limpeza poderá
anular a sua garantia.
PPRREECCAAUUÇÇÃÃOO
• Não permita que penetrem líquidos na zona do fecho ou na barreira de separação da cobertura quando limpar a parte
inferior da superfície de apoio. Os fluidos cujo contacto com o fecho for permitido, podem penetrar no núcleo da
superfície de apoio.
• Não passe a ferro, não limpe a seco nem seque na máquina as coberturas da superfície de apoio.
• Não lave a superfície de apoio com água sob pressão, pois poderá danificar o produto.
• Seque sempre as coberturas da superfície de apoio antes de as guardar, adicionar lençóis ou de colocar um doente na
superfície. Um produto seco ajuda a prevenir que o seu desempenho seja alterado.
• Não exponha excessivamente as coberturas a soluções desinfetantes de concentração mais elevada, pois isto poderá
degradar as coberturas.
• Não utilize peróxidos de hidrogénio acelerados ou quaternários que contenham éter de glicol >3% já que estes
químicos podem danificar a cobertura da superfície de apoio.
• O não cumprimento das instruções do fabricante também poderá afetar a vida útil da cobertura da superfície de apoio.
A cobertura da superfície de apoio é resistente às seguintes soluções químicas:
• Quaternários (ingrediente ativo – cloreto de amónia) que contenham menos de 3% de éter de glicol
• Solução fenólica (Matar)
• Solução de lixívia clorada (10000 ppm) para utilização com coberturas de colchão END406
• Solução clorada de lixívia (1000 ppm) para utilização com coberturas de colchão de Nylon
• Álcool isopropílico a 70%
Siga o protocolo hospitalar para os cuidados com a superfície de apoio entre doentes, para evitar o risco de contaminação
cruzada e infeção.
NNoottaa -- A solução de lixívia clorada pode causar descoloração das coberturas de colchão de Nylon.
2815-009-001 Rev D.1 11 PT
Page 80

LLiissttaa ddee ccoonnssuullttaa rrááppiiddaa ddee ppeeççaass ddee ssuubbssttiittuuiiççããoo
Estas peças estão disponíveis para compra para os modelos padrão, mas sujeitas a alterações. Contacte a assistência ao
cliente da Stryker: 1-800-327-0770 para saber as peças disponíveis e preços.
NNoommee NNúúmmeerroo
Cobertura, inclinação para calcanhar, END406 80 pol.
(203,2 cm)
Cobertura, inclinação para calcanhar, END406, 84 pol.
(213,4 cm)
Cobertura, inclinação para calcanhar, Nylon 80 pol. (203,2
cm)
Cobertura, inclinação para calcanhar, Nylon 84 pol. (213,4
cm)
281505550012
281505550014
281505550018
281505550016
SSuubbssttiittuuiiççããoo ddaa ccoobbeerrttuurraa -- MMooddeelloo ppaaddrrããoo
PPRREECCAAUUÇÇÃÃOO -- Tenha sempre precaução ao substituir a cobertura da superfície de apoio. A barreira anti-fogo interna
contém fibras de fibra de vidro. As partículas de poeira das fibras podem provocar irritação cutânea.
FFeerrrraammeennttaass nneecceessssáárriiaass::
• None
PPrroocceeddiimmeennttoo::
1. Eleve a altura da cama até à máxima possível.
2. Baixe as secções do apoio de costas de Fowler e da plataforma articulada para os joelhos até às posições mais baixas
possíveis.
3. Abra o fecho da cobertura. Comece pelo canto direito (em relação ao doente) da superfície de apoio, do lado da
extremidade dos pés, e pare no canto direito (em relação ao doente) da extremidade do lado da cabeça.
4. Dobre o topo da cobertura para o lado direito do doente e, em seguida, remova o colchão de espuma da cama e ponha-
-o de lado.
5. Remova e descarte a cobertura.
6. Coloque a cobertura de substituição, com o fecho aberto e estendida sobre a cama, com a cobertura preta inferior
sobre a estrutura da cama e a cobertura superior dobrada sobre o lado da cama à direita do doente.
7. Coloque cuidadosamente a estrutura de grades da espuma sobre o topo da parte inferior da cobertura para se certificar
de que o colchão de espuma está alinhado com a cobertura.
8. Dobre a cobertura superior sobre o topo da estrutura de grades da espuma para se certificar de que a cobertura
superior está alinhada com a estrutura de grades da espuma.
9. Feche o fecho da cobertura. Comece pelo canto direito (em relação ao doente) do lado da extremidade da cabeça e
pare no canto direito (em relação ao doente) da extremidade do lado dos pés.
10.Confirme que o produto está a funcionar corretamente antes de o voltar a utilizar.
PT 12 2815-009-001 Rev D.1
Page 81

Page 82

Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
USA
2815-009-001 Rev D.1
WCR:
2019/04
 Loading...
Loading...