ENGLISH
Document No. CSD-SMESAR3
Revision 01
CopyrightⓒSAMSUNG MEDISON Co., L TD.


SSaaffeettyy RReeqquuiirreemmeennttss
Classifications:
- T ype of protection against electrical shock: Class I
- Degree of protection against electrical shock (Patient connection):T ype BF equipme nt
- Degree of protection against harmful ingress of water: Ordinary equipment
- Degree of safety of application in the presence of a flammable anesthetic material with
air or with oxygen or nitrous oxide: Equipment not suitable for use in the presence of a
flammable anesthetic mixture with air or with oxygen or nitrous oxide.
- Mode of operation: Continuous operation
Electromechanical safety standards met:
- IEC/EN 60601-1 Medical Electrical Equipment, Part 1General Requirements for Safety.
- IEC/EN 60601-1-1 Safety requirements for medical electrical sy stems.
- IEC/EN 60601-1-2 Electromagnetic compatibility -Requi rements and te sts.
- IEC/EN 60601-2-37 Particular requirements for the safety of ultrasonic medical
diagnostic and monitoring equipment.
- IEC 61 157 Declaration of acoustic output p arameters.
- ISO 10993-1 Biological evaluation of medical devices.
- UL 60601-1 Medical Electrical Equipment , Part 1 General Requirements for Safety.
- CSA 22.2, 601.1 Medical Electri cal Equipment, Part 1 General Requirement s for
Safety .
Declarations:
0123
This is CSA symbol for Canada and United States of America
This is manufacturer’s declaration of product compliance
with applicable EEC directive(s) and the European notified
body.
This is manufacturer’s declaration of product compliance
with applicable EEC directive(s).
This is GMP symbol for Good Manufacturing Practice of
Korea quality system regulation.

RREEAADD TTHHIISS FFIIRRSSTT
Before asking for the product to be repaired, read this service manual thoroughly, learn
how to troubleshoot, and make sure you understand the precautions fully.
The repair of the system and the replacement of parts must be carried out by an authorized
dealer or the customer service department of SAMSUNG MEDISON Co., Ltd.
The company is shall not be held liable for any injury and damage caused by not
following this warning.
For safe use of this product, you should read ‘Chapter 2. Safety’ in this manual, prior to
starting to
useing this system.
˙˙˙˙˙˙˙˙˙˙˙
DDAANNGGEER
˙˙˙˙˙˙˙˙˙˙˙˙˙
WWAARRNNIINNG
˙˙˙˙˙˙˙˙˙˙˙˙˙
CCAAUUTTIIOON
˙˙˙˙˙˙˙
NNOOTTE
˙
R
Describes precautions necessary to prevent user hazards of great urgency.
Ignoring a DANGER warning will risk life-threatening injury.
˙
G
Used to indicate the presence of a hazard that can cause serious personal injury,
or substantial property damage.
˙
N
Indicates the presence of a hazard that can cause equipment damage.
˙
E
A piece of information useful for installing, operating and maintaining a system.
Not related to any hazard.

Contents
Chapter 1. General Information
1.1 Overview ....................................................................................................... 1-1
1.2 Features and Advantages of SonoAce R3 .................................................. 1-2
1.3 Product Configuration .................................................................................... 1-3
1.3.1 Console ......................................................................................... 1-3
1.3.2 LCD Monitor ................................................................................. 1-4
1.3.3 Control Panel ................................................................................ 1-5
1.3.4 Probe ............................................................................................ 1-5
1.4 Specifications .................................................................................................. 1-6
Chapter 2. Safety
2.1 Overview ....................................................................................................... 2-1
2.2 Safety-Related Information ............................................................................ 2-2
2.2.1 Safety Symbols ............................................................................ 2-2
2.2.2 LABEL ........................................................................................... 2-3
2.3 Electrical Safety .............................................................................................. 2-4
2.3.1 Prevention Electric Shock ........................................................... 2-4
2.3.2 ESD ............................................................................................... 2-4
2.3.3 EMI ................................................................................................ 2-5
2.3.4 EMC .............................................................................................. 2-5
2.4 Mechanical Safety .......................................................................................... 2-11
2.4.1 Moving Equipment ....................................................................... 2-11
2.4.2 Safety Note ................................................................................... 2-11
2.5 Biological Safety ............................................................................................. 2-12
2.5.1 ALARA Principle .......................................................................... 2-12
2.6 Environmental Protection .............................................................................. 2-24
목차

Contents
Chapter 3. Installing the Product
3.1 Overview ....................................................................................................... 3-1
3.2 Transportation ................................................................................................ 3-3
3.2.1 Precautions for Transportation .................................................... 3-3
3.2.2 Temperature and Humidity ......................................................... 3-3
3.3 Unpacking ....................................................................................................... 3-4
3.3.1 Unpacking the Box ....................................................................... 3-4
3.3.2 Checking Package contents ....................................................... 3-5
3.4 Precautions for Installation ............................................................................ 3-6
3.4.1 Precautions ................................................................................... 3-6
3.5 Installations Procedure .................................................................................. 3-7
3.5.1 Installation Safety ......................................................................... 3-7
3.5.2 Connecting the Power Cord ........................................................ 3-8
3.5.3 Connecting the Network Cable ................................................... 3-9
3.5.4 Connecting the Probe .................................................................. 3-9
3.6 Starting the Product ....................................................................................... 3-10
3.7 Shutting down the Product ............................................................................ 3-11
3.8 Connecting the Peripherals ........................................................................... 3-12
3.8.1 External Peripherals .................................................................... 3-12
3.9 System Setting ............................................................................................... 3-14
3.9.1 General System Setup ................................................................ 3-14
3.9.2 Display Setup ............................................................................... 3-16
3.9.3 Misc ............................................................................................... 3-18
3.10 Measure Setting ............................................................................................. 3-19
3.10.1 General ......................................................................................... 3-19
3.10.2 Doppler ......................................................................................... 3-20
3.10.3 Report ........................................................................................... 3-21
3.10.4 OB ................................................................................................. 3-22
3.10.5 Fetal Echo .................................................................................... 3-25
3.10.6 Cardiac.......................................................................................... 3-26
3.10.7 Urology.......................................................................................... 3-26
3.10.8 Vascular ........................................................................................ 3-27
3.11 Setting DICOM ( Optional ) ............................................................................ 3-28
3.11.1 Setting DICOM Information ......................................................... 3-28
3.11.2 Network Setup .............................................................................. 3-29
3.11.3 Adding or Changing the DICOM Server .................................... 3-29
3.11.4 Editing the DICOM Server ........................................................... 3-32
3.11.5 Deleting DICOM Server ............................................................... 3 - 3 2
목차

3.11.6 Testing DICOM Server ................................................................ 3-32
3.11.7 DICOM Log .................................................................................. 3-32
3.12 Setting Option ................................................................................................. 3-34
3.13 Setting Peripheral Devices ............................................................................ 3-35
3.13.1 Video Out Type ............................................................................ 3-35
3.13.2 Foot Switch ................................................................................... 3-35
3.13.3 Printer 1 ........................................................................................ 3-35
3.13.4 Printer 2 ........................................................................................ 3-35
3.14 Information ...................................................................................................... 3-36
목차

Contents
Chapter 4. Checking the Product
4.1 Overview ..................................................................................................... 4-1
4.2 Starting the Product ....................................................................................... 4-2
4.3 Monitor ....................................................................................................... 4-3
4.3.1 Monitor Display ............................................................................ 4-3
4.4 Control Panel .................................................................................................. 4-5
4.4.1 Detail Control Panel ..................................................................... 4-6
4.4.2 Soft Menu ..................................................................................... 4-8
4.4.3 Keyboards .................................................................................... 4-9
4.5 Checking the Performance .......................................................................... 4-11
4.5.1 Basic Check ................................................................................. 4-11
4.5.2 Detail Check ................................................................................. 4-12
목차

Contents
제5장 제품 구조
5.1 개요 5-1
5.2 System Block Diagram .................................................................................. 5-3
5.3 EKO7의 기본 구조 ........................................................................................ 5-4
5.3.1 개요 ............................................................................................... 5-4
5.3.2 Ultrasound System Part .............................................................. 5-4
5.3.3 PC Part ......................................................................................... 5-5
5.3.4 User Interface Part ....................................................................... 5-5
5.3.5 AC to DC Power Module ............................................................. 5-5
5.4 PSA 5-6
5.5 Beamformer Board ......................................................................................... 5-8
5.6 CW Board 5-11
5.7 Back End Board ............................................................................................. 5-14
5.8 PCI Board 5-18
5.9 DVI Board 5-19
5.10 VGA Card 5-21
5.11 VCRIN Board .................................................................................................. 5-22
5.12 PC Mother Board ........................................................................................... 5-23
5.13 Software DSC ................................................................................................. 5-24
5.14 LCD IF Board .................................................................................................. 5-26
5.15 Rear Board ..................................................................................................... 5-27
5.16 Control Panel .................................................................................................. 5-28
5.17 Power Supply ................................................................................................. 5-30
목차

Contents
Chapter 6. Basic Maintenance
6.1 Overview ....................................................................................................... 6-1
6.2 System Information ........................................................................................ 6-2
6.3 Admin Mode ................................................................................................... 6-3
6.3.1 Entering Admin Mode .................................................................. 6-3
6.3.2 Admin Mode Functions................................................................ 6-4
6.4 Upgrade . .................................................................................................... 6-6
6.4.1 Software Upgrade ........................................................................ 6-6
6.4.2 Hardware Upgrade ...................................................................... 6-9
6.5 Backup & Restore .......................................................................................... 6-10
6.5.1 Backup User Setting .................................................................... 6-10
6.5.2 Restore User Setting ................................................................... 6-12
6.6 Adding and Deleting Options......................................................................... 6-14
6.6.1 Option type ................................................................................... 6-14
6.6.2 Registering Option ....................................................................... 6-14
6.7 Control Panel .................................................................................................. 6-16
목차

Contents
Chapter 7. Troubleshooting
7.1 Overview ....................................................................................................... 7-1
7.2 Power ....................................................................................................... 7-2
7.2.1 Power Failure ............................................................................... 7-2
7.2.2 Power cannot turned off .............................................................. 7-2
7.2.3 Power is automatically turned off ................................................ 7-2
7.3 Monitro ....................................................................................................... 7-3
7.3.1 Blank Screen ................................................................................ 7-3
7.3.2 Screen Color Abnomal ................................................................ 7-3
7.4 Error Messages .............................................................................................. 7-4
7.4.1 System hangs after an error during booting............................... 7-4
7.4.2 System works even if error occurred .......................................... 7-4
7.5 Image ....................................................................................................... 7-5
7.5.1 No BW Image Echo & No BW Mode Image Format ......................... 7-5
7.5.2 Noise Link Rain over the BW Mode Image (Noise) ........................... 7-5
7.5.3 PW & Color Doppler Mode Trouble ................................................... 7-5
목차

Contents
Chapter 8. Disassembly and Reassembly
8.1 Overview ....................................................................................................... 8-1
8.2 Disassembly and Reassembly of the Body Cover ...................................... 8-3
8.2.1 Preparations ................................................................................. 8-3
8.2.2 Body Rear & Middle Cover ......................................................... 8-3
8.2.3 Disassembly of the Main Part and LCD/Control Panel ............. 8-4
8.3 Disassembly and Reassembly of the Main System Part ............................ 8-5
8.3.1 Preparations ................................................................................. 8-5
8.3.2 Power & HDD & Side Fan ........................................................... 8-5
8.3.3 PSA & Rear & Rear Fan & Handle ............................................. 8-6
8.3.4 LCD & Inverter & USB Board ...................................................... 8-7
8.4 Disassembly and Reassembly of the Control Panel ................................... 8-8
8.4.1 Preparations ................................................................................. 8-8
8.4.2 Disassembly Control Panel and LCD ......................................... 8-8
8.4.3 Control Panel ................................................................................ 8-9
목차

Contents
Chapter 9. Probe
9.1 Overview ....................................................................................................... 9-1
9.2 Probe List ....................................................................................................... 9-2
9.3 Thermal Index (TI Table) ............................................................................... 9-5
9.4 Ultrasound Transmission Gel ........................................................................ 9-6
9.5 Sheaths ....................................................................................................... 9-7
9.6 Probe Precautions .......................................................................................... 9-9
Chapter 10. User Maintenance
10.1 Overview ....................................................................................................... 10-1
10.2 System Maintenance ..................................................................................... 10-2
10.2.1 Installation Maintenance .............................................................. 10-2
10.2.2 Cleaning and Disinfections .......................................................... 10-2
10.2.3 Fuse Replacement ...................................................................... 10-3
10.2.4 Accuracy Check ........................................................................... 10-4
10.3 Administration of Information ......................................................................... 10-5
10.3.1 User Setting Back-up ................................................................... 10-5
10.3.2 Patient Information Back-up ........................................................ 10-5
10.3.3 Software ........................................................................................ 10-5
Chapter 11. Service Part List
11.1 Overview ....................................................................................................... 11-1
11.2 Body Cover ..................................................................................................... 11-2
11.3 Ultrasound System Part ............................................................................... 11-4
11.4 LCD Part ....................................................................................................... 11-5
11.5 PC Part ....................................................................................................... 11-6
11.6 Power Part ...................................................................................................... 11-8
11.7 User Interface Part ......................................................................................... 11-9
11.8 ETC Part ................................................................................................. 11-10
11.9 Options ................................................................................................... 11-11
11.10 Probes ................................................................................................... 11-12
목차

CChhaapptteerr 11.. GGeenneerraall IInnffoorrmmaattiioonn
1.1 Overview
Chapter 1 contains the informatio n nece ssary to pl an the Tr oubles hooting of
SonoAceR3.
The SonoAceR3 is a high-resolution color ultrasound scanner with high penetration
and a variety of measurement fu nctions.
Contents
1.1 Overview ....................................................................................................1-1
1.2 Features and Advantages of SonoAceR3 ...............................................1-2
1.3 Product Configuration ...............................................................................1-3
1.3.1 Console ....................................................................................1-3
1.3.2 LCD Monitor ............................................................................1-4
1.3.3 Control Panel ...........................................................................1-5
1.3.4 Probes......................................................................................1-5
1.4 Specifications .............................................................................................1-6
Chapter 1. General Information 1-1

1.2 Features and Advantages of SonoAceR3
z High-end Digital Beamforming : The SonoAce R3 utilize s the ne wly
developed Digital Beam fo rming te chnolo gy.
z A variety of applications : The SonoAceR3 is optimized for use in a variety
of ultrasound departments, cardiac, vascular, abdomen, Obsterics, Urology,
Gynecology.
z Various diagnostic Modes : 2D Mode, M Mode, Color Doppler Mode, Power
Doppler Mode, PW Spectral Doppler Mode, etc.
z Measurement and Report Functions : Besides the basic distance, area,
circumference and volume measurement functions, the SonoAceR3 also
provides application-specific measurement functions. The report function
collates measurement data.
z Review of Scanned Images : The SonoAceR3 displays Cine images of 512
frames and loop images of 4096 lines.
z SonoViewTM : This is a total ultrasound image management system, which
allows a user to archive, view and exchange documents.
z Digital Imaging and Communication in Medicine (DICOM) Function : This is
used to archive, transmit and print DICOM images through a network.
z Peripheral/Accessory Connection : A variety of peripheral devices including
VCRs and printers can be easily connected to the SonoAceR3.
Chapter 1. General Information 1-2

1.3 Product Configuration
This Product consists of the monitor, the control panel, the console and, the probes
and the cart(optional).
1.3.2 Console
The console consists of two parts – the in ner unit and the outer unit .
The interior of the console mainly contains devices that produce ultrasound images.
The outside of the console consists of various connection ports and handles.
Handle
[Figure 1-1] Console of SonoAce R3
LCD Monitor
Control Panel
Chapter 1. General Information 1-3

1.3.2 LCD Monitor
The monitor of this system is a colo r VGA monito r, which di splays ultr asound im ages
and additional information. This monitor is connected to the main body through a
central pivot, allowing it to be tilte d to the optimal vi ewing an gle.
Probe Connector
[Figure 1-2] Front and Back of SonoAce R3
[Figure 1-3] LCD Monitor
Chapter 1. General Information 1-4

1.3.3 Control Panel
The control panel can be used for cont rolling th e system.
Alpha-Numeric
Button
Dial Button
Track Ball
Slide Volume
1.3.4 Probe
Probes are devices that generate ultrasound waves and process reflected wave data
for the purpose of image formatio n.
[Figure 1-4] Control Panel
˙
˙˙˙˙˙˙˙
E
NNOOTTE
For more information, refer to ‘Chapter 9. Probes’.
Chapter 1. General Information 1-5

1.3.5 SonoAceR3 Cart (Optional)
The SonoAce R3 System can be placed on a cart during use or for transport. For
more information on installing and using the SonoAce R3, please refer to the
installation guide that come s with it.
[Figure 1-5] SonoAce R3 Cart
Chapter 1. General Information 1-6
1.4 Specifications
Height: 375mm (with handle)
Physical Dimensions
Imaging modes
Gray Scale 256 (8 bits)
Focusing
Width: 402mm (with probe holder)
Depth: 188mm(with control panel)
Weight: More than 8.7kg
2D real-time
Dual 2D real-time
2D/M-mode
Power Doppler
Color Doppler for Option
Pulsed-wave Doppler for Option
3D-mode (Freehand) for Option
Simultaneous
Dynamic transmit focusing, maximum of eight points (four points
simultaneously selectable)
Digital dynamic receive focusing (continuous)
Curved Linear Array : C2-4/20, CN2-8, CN4-9
Probes
Probe connections
Monitor 15 inch LCD monitor
Rear Panel
Input/Output
Connections
Image Storage
Application
Linear Array : L5-12/60, LN5-12/4 0
Endocavity Curved Linear Array : EC4-9
One probe connectors
Two probe connectors for option
USB 3ports
LAN(10/100 BASE-T)
DVI Output
BW Printer remote control
BW Output
S-VHS Output
Sound Output
Maximum 512 frames for CINE memory
Maximum 4096 Lines for LOOP memory
Image filing system
Gynecology, Abdomen, OB, Renal, Urology, Vascular, Small
Part, Fetal Heart, Breast, Musculoskeletal, Pediatric, Neonatal,
Cardiac
Electrical Parameters 100-120V/200-240V, 250VA, 50/60Hz
Chapter 1. General Information 1-7
Automatic Calculation
and Quantification
Signal processing
(Pre-processing)
Signal processing
(Post-processing)
Obssterics
Gynecology
Cardiology
Fetal Echo
Vascular
Urology
*Refer the Chapter 5 for additional information
TGC control
Mode-independent gain control
Acoustic power control (adjustable)
Dynamic aperture
Dynamic apodization
Dynamic range control (adjustable)
Image view area control
M-mode sweep speed control
HD zoom
Frame average
Gamma-scale windowing
Image orientation (left/right and up/down, rot ation)
White on black/black on white
Trackball operation of multiple cursors
2D mode: Linear measurements and area measurements
Measurement
using elliptical approximation or trace
M mode: Continuous readout of distance, time, and slope rate
Doppler mode: Velocity and trace
Black-and white printer
Color printer
Auxiliary
VCR
Monitor
Foot switch
User Interface English, German, French, S panish, Italian, Russian, Chinese
Pressure Limits
Humidity Limits
Temperature Limits
Operating: 700hPa to 1060hPa
Storage: 700hPa to 1060h Pa
Operating: 30% to 75%
Storage & Shipping: 20% to 90%
Operating: 10
Storage & Shipping: -25
O
C ~ 35OC
O
C ~ 60OC
Chapter 1. General Information 1-8

CChhaapptteerr 22.. SSaaffeettyy
2.1 Overview
Chapter 2 contains the information necessary to Safety
Please read this chapter before using the SAMSUNG MEDISON ultrasound
system. It is relevant to the ultrasound system, the probes, the recording devices,
and any of the optional equipment.
SonoAce R3 is intended for use by, or by the order of, and under the supervision
of, a licensed physician who is qualified for direct use of the medical device.
Contents
2.1 Overview ....................................................................................................2-1
2.2 Safety – Related Information ....................................................................2-2
2.2.1 Safety Symbols .......................................................................2-2
2.2.2 LABEL ......................................................................................2-3
2.3 Electrical Safety .........................................................................................2-4
2.3.1 Prevention Electric Shock ......................................................2-4
2.3.2 ESD ..........................................................................................2-4
2.3.3 EMI ...........................................................................................2-5
2.3.4 EMC .........................................................................................2-5
2.4 Mechanical Safety ................................................................................... 2-11
2.4.1 Moving Equipment ................................................................ 2-11
2.4.2 Safety Note ............................................................................ 2-11
2.5 Biological Safety ..................................................................................... 2-12
2.5.1 ALARA Principle ................................................................... 2-12
2.6 Environmental Protection....................................................................... 2-24
Chapter 2. Safety 2-1
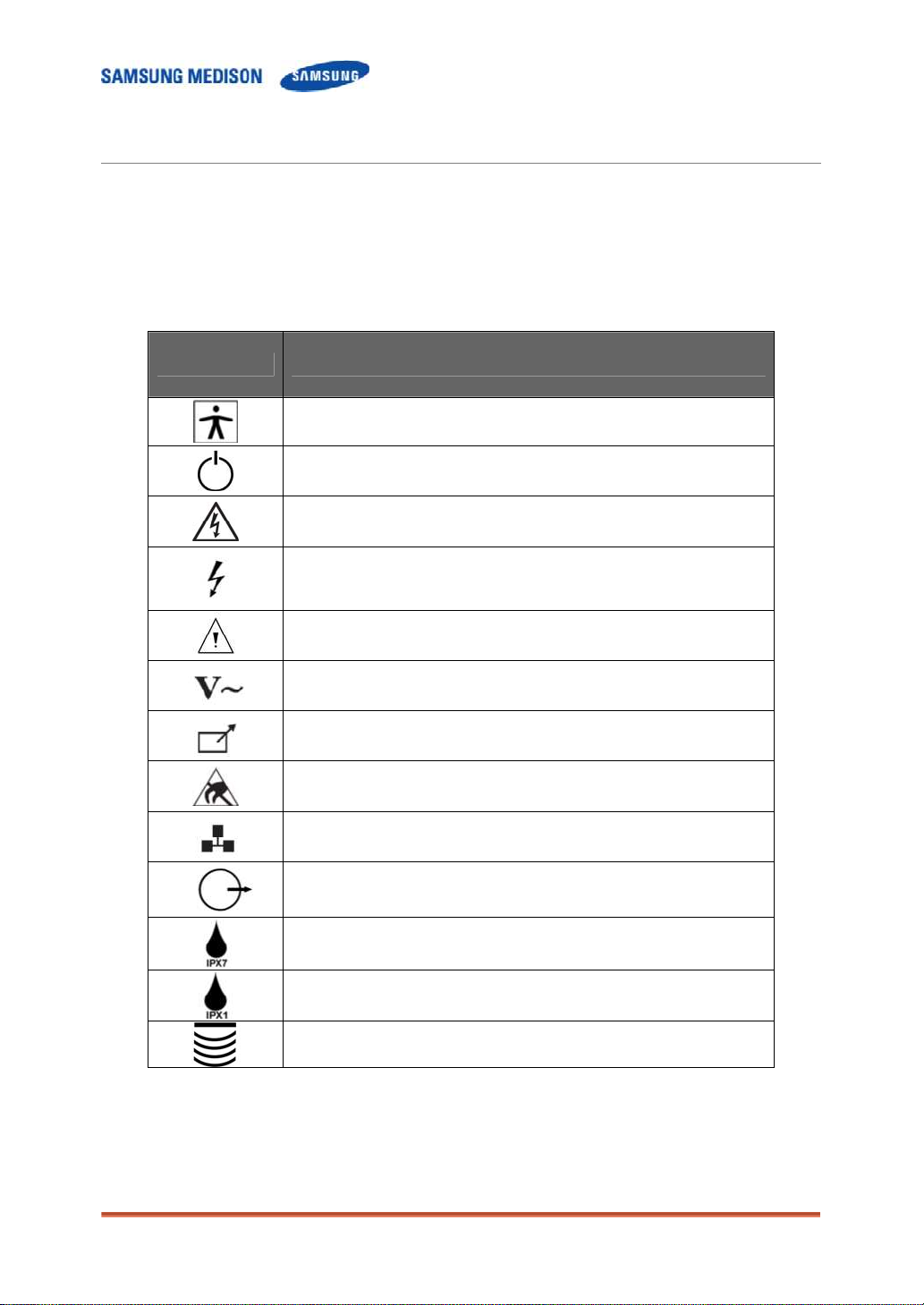
2.2 Safety – Related Information
2.2.1 Safety Symbols
The International Electro Technical Commission (IEC) has established a set of
symbols for medical electronic equipment, which classifies a connection or warn
of potential hazards. The classifications and symbols are shown below.
Symbols Description
Power switch (Supplies/cuts the power for product).
Indicates a caution for risk of electric shock.
Indicates dangerous voltages over 1000V AC or over
1500V DC.
Warning, Caution
AC (alternating current) voltage source
Print remote output
Electrostatic discharge
Protection against the effects of immersion.
Protection against dripping water.
Probe connector
Isolated patient connection (Type BF applied part).
Network port
Output port ( DVI, RGB, B/W, S-VHS, SOUND )
Chapter 2. Safety 2-2

2.2.2 LABEL
To protect the system, you may see ‘Warning’ or ‘Caution’ marked on the
surface of the product
[Figure 2-1]Marked on the product
Chapter 2. Safety 2-3
2.3 Electrical Safety
This equipment has been verified as a Class I device with Type BF applied parts.
2.3.1 Prevention of Electric Shock
Additional equipment connected to medical electrical equipment must comply with
the respective IEC or ISO standards (e.g. IEC 60950 for data processing
equipment). Furthermore all configurations shall comply with the requirements for
medical electrical systems (see IEC 60601-1-1 or clause 16 of the 3 Ed. of IEC
60601-1, respectively). Anybody connecting additional equipment to medical
electrical equipment configures a medical system and is therefore responsible
that the system complies with the requirements for medical electrical systems.
Attention is drawn to the fact that local laws take priority over the abovementioned
requirements. If in doubt, consult your local representative or the technical service
department.
˙˙˙˙˙˙˙˙˙˙˙˙˙
WWAARRNNIINNG
z Electric shock may exist result if this system, including and all of its externally mounted
recording and monitoring devices, is not properly grounded.
z Do not remove the covers on the system; hazardous voltages are present inside. Cabinet
panels
must be made by a qualified SAMSUNG MEDISON Customer Service Department
z Check the face, housing, and cable before use. Do not use, if the face is cracked, chipped, or
torn, the housing is damaged, or if the cable is abraded
z Always disconnect the system from the wall outlet prior to cleaning the system.
z All patient contact devices, such as probes and ECG leads, must be removed from the patient
prior to application of a high voltage defibrillation pulse
z The use of flammable anesthetic gas or oxidizing gases (N20) should be avoided.
˙˙˙˙˙˙˙˙˙˙˙˙˙
CCAAUUTTIIOON
z The system has been designed for 100-120VAC and 200-240VAC; you should select the
input
that the voltage indicated on the power cord matches the voltage rating of the OEM device
z An isolation transformer protects the system from power surges. The isolation transformer continues
to operate when the system is in standby
z Do not immerse the cable in liquids. Cables are not waterproof.
z The operator does not contact the parts (SIP/SOP) and the patient simultaneously.
˙
G
must be in place while the system is in use. All internal adjustments and replacements
.
.
.
˙
N
Outlet voltage of monitor, printer and VCR. Prior to connecting an OEM power cord, verify
.
.
Chapter 2. Safety 2-4
2.3.2 ESD
Electrostatic discharge (ESD), commonly referred to as a static shock, is a
naturally occurring phenomenon. ESD is most prevalent during conditions of low
humidity, which can be caused by heating or air conditioning. During low humidity
conditions, electrical charges naturally build up on individuals, creating static
electricity. An ESD occurs when an individual with an electrical energy build-up
comes in contact with conductive objects such as metal doorknobs, file cabinets,
computer equipment, and even other individuals. The static shock or ESD is a
discharge of the electrical energy build-up from a charged individual to a lesser or
non-charged individual or object.
˙˙˙˙˙˙˙˙˙˙˙˙˙
CCAAUUTTIIOONN
z The level of electrical energy discharged from a system user or patient to an ultrasound system
can be significant enough to cause damage to the system or probes
z Always perform the pre-ESD preventive procedures before using connectors marked with the ESD
warning label
˙
.
.
- Apply anti-static spray on carpets or linoleum.
- Use anti-static mats.
- Ground the product to the patient table or bed.
z It is highly recommended that the user be given training on ESD-related warning symbols and
preventive procedures
.
[Figure 2-7] ESD symbol
2.3.3 EMI
Although this system has been manufactured in compliance with existing EMI
(Electromagnetic Interference) requirements, use of this system in the presence
of an electromagnetic field can cause momentary degradation of the ultrasound
image.
If this occurs often, SAMSUNG MEDISON suggests a review of the environment
in which the
system is being used, to identify possible sources of radiated emissions. These
emissions could be from other electrical devices used within the same room or an
adjacent room. Communication devices such as cellular phones and pagers can
cause these emissions. The existence of radios, TVs, or microwave transmission
equipment nearby can also cause interference.
˙˙˙˙˙˙˙˙˙˙˙˙˙
CCAAUUTTIIOON
˙
N
In cases where EMI is causing disturbances, it may be necessary to relocate this
system.
Chapter 2. Safety 2-5
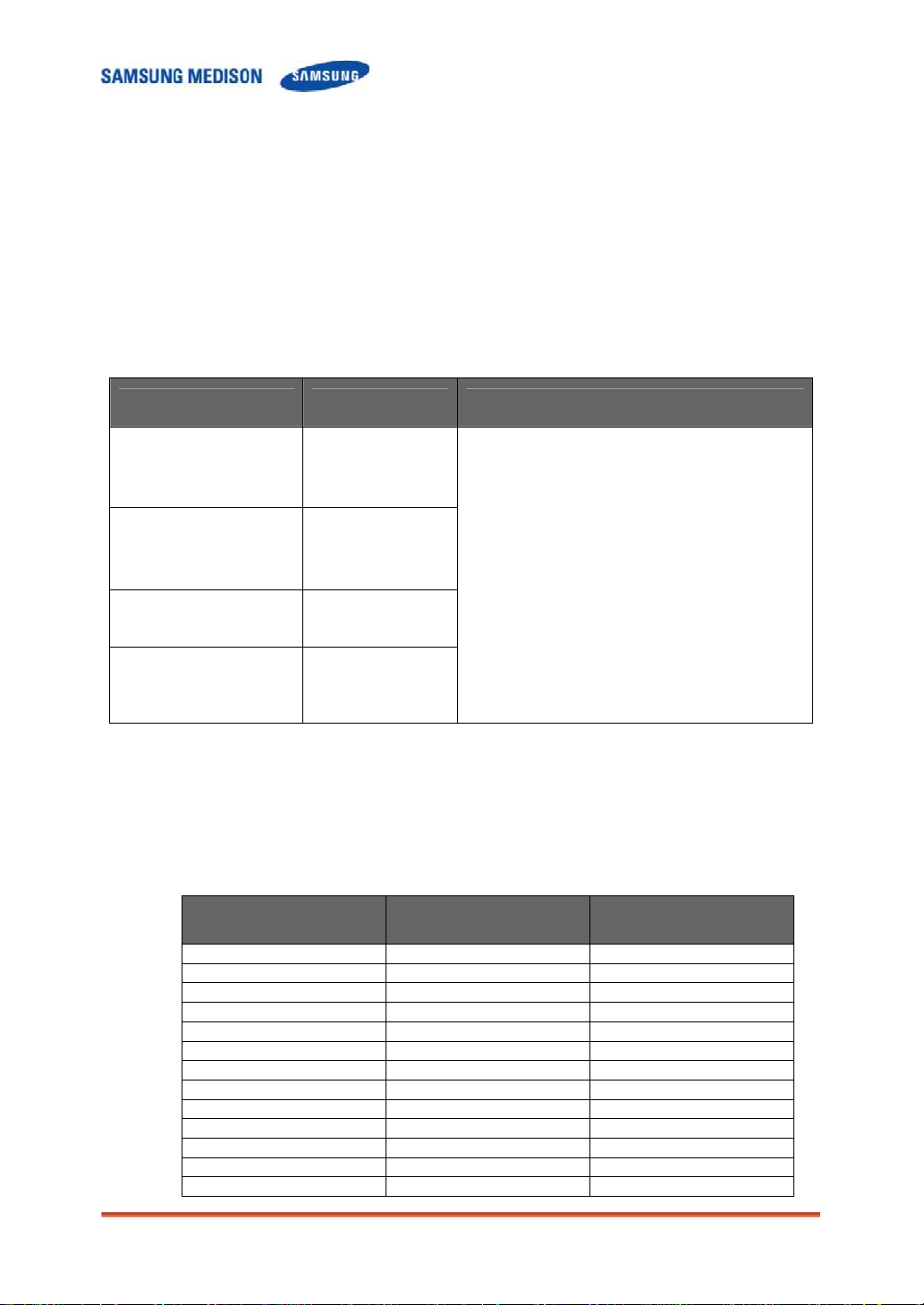
2.3.4 EMC
The testing for EMC(Electromagnetic Compatibility) of this system has been
performed according to the international standard for EMC with medical devices
(IEC60601-1-2). This IEC standard was adopted in Europe as the European norm
(EN60601-1-2).
2.3.4.1 Guidance and manufacturer’s declaration - electromagnetic emission
This product is intended for use in the electromagnetic environment specified
below. The customer or the user of this product should assure that it is used in
such an environment.
Emission test Compliance Electromagnetic environment -guidance
RF Emission
(Radiation)
CISPR 11
RF Emission
(Radiation)
CISPR 11
Harmonic Emission
IEC 61000-3-2
Flicker Emission
IEC 61000-3-3
Group 1
Class B
Group 1
Class B
Class A
Complies
The Ultrasound System uses RF energy only
for its internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
equipment.
The Ultrasound System is suitable for use in all
establishments, including domestic
establishments and those directly connected to
the public low-voltage power supply network
that supplies building used for domestic
purpose.
2.3.4.2 Approved Cables, Transducers and Accessories for EMC
1) Approved Cable for Electromagnetic Compliance
Cables connected to this product may affect its emissions;
Use only the cable types and lengths listed below table.
Cable Type Length
VGA Shielded Normal
Parallel Shielded Normal
RS232C Shielded Normal
USB Shielded Normal
LAN(RJ45) Twisted pair Any
S-Video Shielded Normal
Foot Switch Shielded 2.5m
B/W Printer Unshielded Coaxial Normal
MIC Unshielded Any
Printer Remote Unshielded Any
Audio R.L Shielded Normal
VHS Shielded Normal
ECG AUX input Shielded < 3m
Chapter 2. Safety 2-6
2) Approved Transducer for Electromagnetic Compliance
The probe listed in ‘Chapter 8. Probes’ when used with this product, have
been tested to comply with the group1 class B emission as required by
International Standard CISPR 11.
3) Approved Accessories for Electromagnetic Compliance
Accessories used with this product may effect its emissions
˙˙˙˙˙˙˙˙˙˙˙˙˙
CCAAUUTTIIOON
˙
N
When connecting other customer-supplied accessories to the system, such as a
remote printer or VCR, it is the user’s responsibility to ensure the electromagnetic
compatibility of the system. Use only CISPR 11 or CISPR 22, CLASS B compliant
devices
˙˙˙˙˙˙˙˙˙˙˙˙˙
WWAARRNNIINNG
˙
G
The use of cables, transducers, and accessories other than those specified may
result inincreased emission or decreased Immunity of the Ultrasound System.
Immunity test
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
IEC 60601
Test level
±6KV Contact
±8KV air
±2KV for power supply
lines
±1KV for input/output
lines
Compliance level
±6KV Contact
±8KV air
±2KV for power
supply lines
±1KV for input/
output lines
Electromagnetic environment -
guidance
Floors should be wood, concrete
or ceramic tile. If floors are
covered with synthetic material,
the relative humidity should be at
least 30%.
Mains power quality should be that
of a typical commercial or hospital
environment.
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
±1KV differential mode
±2KV common mode
<5% Uт
(>95% dip in Uт)
for 0.5cycle
40% Uт
(60% dip in Uт )
for 5 cycle
70% Uт
(30% dip in Uт)
for 25 cycle
<5% Uт
(<95% dip in Uт )
for 5 s
±1KV differential mode
±2KV common mode
<5% Uт
(>95% dip in Uт)
for 0.5cycle
40% Uт
(60% dip in Uт )
for 5 cycle
70% Uт
(30% dip in Uт)
for 25 cycle
<5% Uт
(<95% dip in Uт )
for 5 s
Mains power quality should be that
of a typical commercial or hospital
environment.
Mains power quality should be that
of a typical commercial or hospital
environment. If the user of this
product requires continued
operation during power mains
interruptions, it is recommended
that this product be powered from
an uninterruptible power supply or
a battery.
Chapter 2. Safety 2-7
Power frequency
(50/60Hz)
magnetic field
3 A/m 3 A/m
IEC 61000-4-8
NOTE Uт is the a.c. mains voltage prior to application of the test level.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80MHz
3 V/m
80 MHz to 2.5GHz
0.01V
3 V/m
Power frequency magnetic fields
should be at levels characteristic
of a typical location in a typical
commercial or hospital
environment.
Portable and mobile RF communications
equipment should be used no closer to any part
of the Ultrasound System, including cables,
than the recommended separation distance
calculated from the equation applicable to the
frequency of the transmitter.
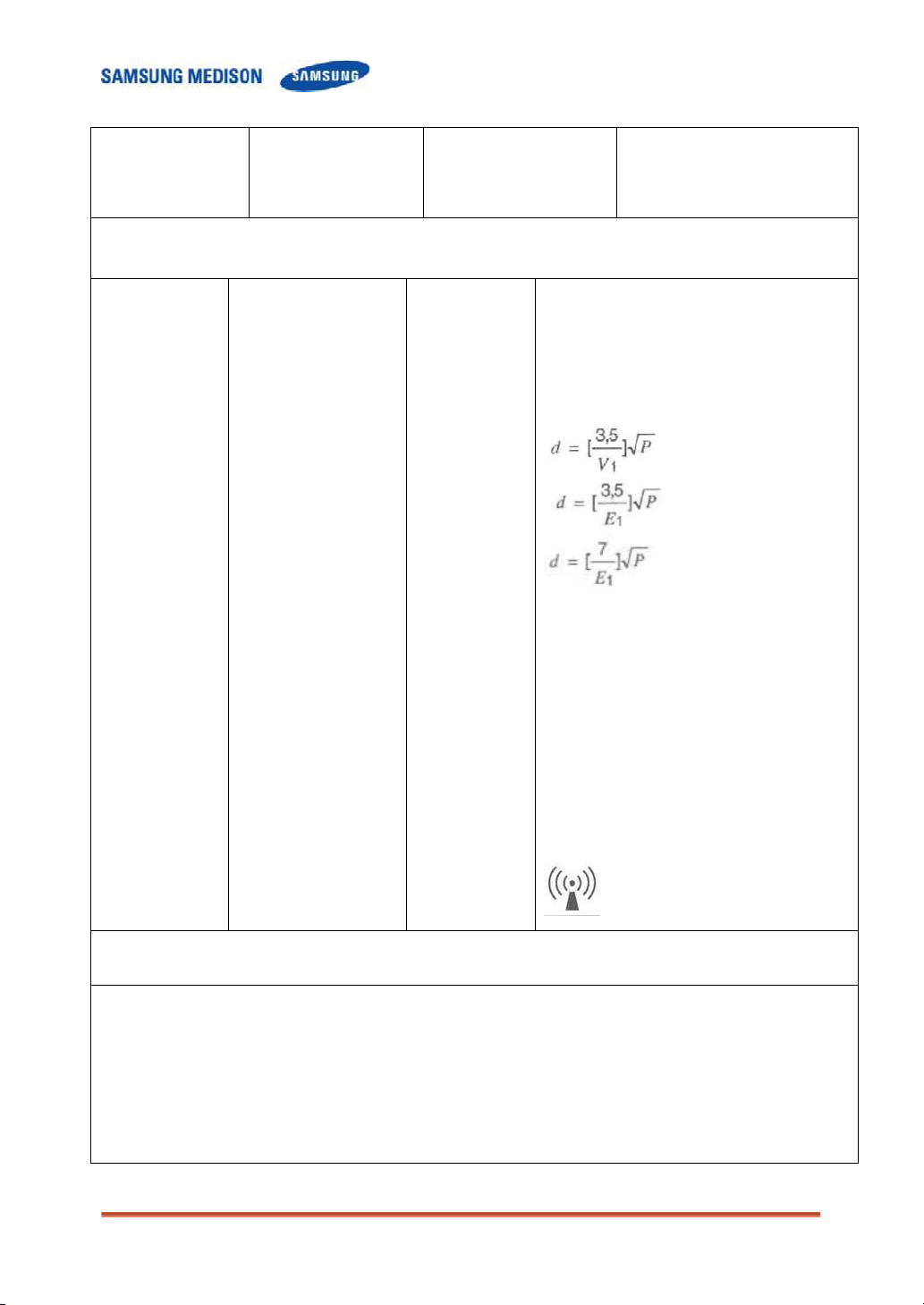
Recommended separation distance
80MHz to 800MHZ
800MHz to 2.5GHz
Where P is the maximum output power rating of
the transmitter in watts (W) according to the
transmitter manufacturer and d is the
recommended separation distance in meters
(m).
Field strengths from fixed RF transmitters, as
deter-mined by an electromagnetic site survey,
should be less than the compliance level in
each frequency range.
b
Interference may occur in the vicinity of
equipment marked with the following symbol :
a
NOTE 1) At 80MHz and 800MHz, the higher frequency range applies.
NOTE 2) These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which the Ultrasound System is used exceeds
the applicable RF compliance level above, the Ultrasound System should be observed to verify normal operation. If
abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the
Ultrasound System or using a shielded location with a higher RF shielding effectiveness and filter attenuation.
b Over the frequency range 150kHz to 80MHz, field strengths should be less than [V
] V/m.
1
Chapter 2. Safety 2-8
 Loading...
Loading...