Roche Cobas B221 User manual

cobas b 221 system
Instructions for Use

COBAS, COBAS B and LIFE NEEDS ANSWERS are trademarks of Roche.
©2009 Roche Diagnostics
Roche Diagnostics GmbH
D-68298 Mannheim
Germany
www.roche-diagnostics.com

cobas b 221 system
Revision History
Manual Version |
Software Version |
Revision date |
Changes |
2.0 |
1.0 |
May 2003 |
Launch |
3.0 |
1.0 |
June 2003 |
not delivered |
3.1 |
1.02 |
July 2003 |
|
4.0 |
2.0 |
March 2004 |
|
5.0 |
4.0 |
December 2004 |
|
6.0 |
5.0 |
November 2005 |
|
7.0 |
5.0 |
March 2006 |
cobas Branding |
8.0 |
6.0 |
December 2006 |
|
9.0 |
7.0 |
February 2008 |
|
10.0 |
>7.0 |
April 2009 |
|
|
|
|
|
Edition notice
cobas b 221 system
In the course of 2006 the Roche OMNI S system was relaunched under the
Roche Diagnostics professional IVD user brand cobas®.
Systems with a serial number of 5001 or above are cobas b 221 systems.
Systems with a serial number up to 5000 are Roche OMNI S systems.
Every effort has been made to ensure that all the information contained in this manual is correct at the time of printing. However, Roche Diagnostics GmbH reserves the right to make any changes necessary without notice as part of ongoing product development.
Any customer modification to the instrument will render the warranty or service agreement null and void.
Software updates are done by Roche Service representatives.
Copyright
© 2009, Roche Diagnostics GmbH, all rights reserved
The contents of this document may not be reproduced in any form or communicated to any third party without the prior written consent of Roche Diagnostics.
While every effort is made to ensure its correctness, Roche Diagnostics assumes no responsibility for errors or omissions which may appear in this document. Subject to change without notice.
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
1 |

cobas b 221 system
Brands
COBAS, COBAS B, LIFE NEEDS ANSWERS, ROCHE OMNI, AUTOQC,
ROCHE MICROSAMPLER, COMBITROL and AUTO-TROL are trademarks of
Roche.
Contact addresses
Manufacturer |
Roche Diagnostics GmbH |
|
|
|
D-68298 Mannheim / Germany |
|
www.roche.com |
Edition
Revision 10.0, April 2009
First edition: May 2003
REF/No. 03261395001
Roche Diagnostics |
April 2009 |
2 |
Instructions for Use · Revision 10.0 |

cobas b 221 system
Table of contents
|
Revision History |
|
1 |
|
|
Edition notice |
|
1 |
|
|
Copyright |
|
1 |
|
|
Brands |
|
2 |
|
|
Contact addresses |
|
2 |
|
|
Edition |
|
2 |
|
|
Table of contents |
|
3 |
|
|
Preface |
|
5 |
|
|
How to use this manual |
|
5 |
|
|
Where to find information |
|
5 |
|
|
Conventions used in this manual |
|
5 |
|
|
|
|
||
Introduction and specifications |
Part |
A |
||
|
|
|
|
|
1 |
Safety information |
|
|
|
|
Important information |
|
A-5 |
|
|
Operating safety information |
|
A-6 |
|
2 |
General descriptions |
|
|
|
|
Introduction |
|
A-9 |
|
|
General notes |
|
A-11 |
|
|
Measurement and calibration procedure |
|
A-13 |
|
|
Measurement evaluation |
|
A-14 |
|
|
Safety instructions for specific dangers |
|
A-14 |
|
|
Handling solutions |
|
A-15 |
|
|
Handling electrodes |
|
A-15 |
|
|
General notes on the use of the MSS cassette |
A-16 |
||
|
System description |
|
A-18 |
|
3 |
Installation and shutdown |
|
|
|
|
Installation |
|
A-27 |
|
|
Shutdown |
|
A-48 |
|
4 |
Specifications |
|
|
|
|
Performance data |
|
A-59 |
|
|
Sample throughput |
|
A-86 |
|
|
Measurement times of the samples |
|
A-86 |
|
|
Sample volumes |
|
A-87 |
|
|
Sample types |
|
A-87 |
|
|
Calibrations |
|
A-88 |
|
|
Environmental parameters |
|
A-89 |
|
|
Product data |
|
A-91 |
|
|
AutoQC |
|
A-92 |
|
|
Printer |
|
A-92 |
|
|
Touch screen-PC unit |
|
A-93 |
|
|
Barcode scanner |
|
A-94 |
|
5 |
Theoretical foundations |
|
|
|
|
Parameters and calculations |
|
A-97 |
|
|
Clinical significance |
|
A-109 |
|
Operation |
Part |
B |
|
|
|
|
|
6 |
Measurement |
|
|
|
Preanalytics |
|
B-5 |
|
Interferences |
B-10 |
|
|
Limitations of clinical analysis |
B-17 |
|
|
Measuring procedure |
B-19 |
|
7 |
Quality control |
|
|
|
Quality control - general |
B-33 |
|
|
General QC concept |
B-33 |
|
|
Important information concerning the analysis of QC |
||
|
measurement results |
B-35 |
|
|
Material setup |
B-36 |
|
|
QC setup wizard |
B-44 |
|
|
QC measurement |
B-51 |
|
|
Multirules |
B-53 |
|
|
QC consequences |
B-55 |
|
|
Remove the QC lock |
B-56 |
|
|
QC for Ready (with AutoQC module) |
B-57 |
|
|
QC for Ready (without AutoQC module) |
B-59 |
|
|
QC troubleshooting |
B-61 |
|
8 |
Calibration |
|
|
|
Calibration - general |
B-65 |
|
|
Automatic calibrations |
B-65 |
|
|
User-activated calibrations |
B-66 |
|
|
Display of parameters during calibration |
B-68 |
|
9 |
Software modes |
|
|
|
Software modes - general |
B-71 |
|
|
User interface |
B-71 |
|
|
Analyzer mode |
B-78 |
|
|
Setup |
B-80 |
|
|
Data manager |
B-81 |
|
|
Info |
B-87 |
|
Maintenance |
Part |
C |
|
|
|
|
|
10 Maintenance |
|
|
|
Maintenance - general |
|
C-5 |
|
Decontamination |
|
C-5 |
|
Daily |
|
C-7 |
|
Weekly |
|
C-8 |
|
Quarterly |
|
C-9 |
|
Sample-dependent maintenance procedures |
C-13 |
||
Unscheduled |
|
C-22 |
|
Additional maintenance procedures |
|
C-38 |
|
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
3 |

cobas b 221 system
Troubleshooting |
Part |
D |
|
|
|
11 Troubleshooting |
|
|
Troubleshooting - general |
D-5 |
|
System stops |
D-5 |
|
Module stops |
D-12 |
|
System warnings |
D-16 |
|
Status messages of measuring and calibration values |
||
D-20 |
|
|
Status messages on the measurement report |
D-39 |
|
Barcode |
D-40 |
|
Appendix |
Part |
E |
|
|
|
12 List of consumables |
|
|
Order information |
|
E-5 |
Glossary |
|
E-9 |
Index |
Part |
F |
|
|
|
Index |
|
F-3 |
Roche Diagnostics |
April 2009 |
4 |
Instructions for Use · Revision 10.0 |

cobas b 221 system
Preface
The cobas b 221 system is an analyzer with integrated AutoQC drawer option.
This manual has detailed descriptions of cobas b 221 system features and general operational concepts, specification functions and use of controls, operating techniques, emergency procedures, product labeling and maintenance procedures.
How to use this manual
o Keep this manual in a safe place to ensure that it is not damaged and remains available for use. o This Instructions for Use should be easily accessible at all times.
To help you find information quickly, there is a table of contents at the beginning of the book and each chapter. In addition, a complete index can be found at the end.
Where to find information
In addition to the Instructions for Use, the following documents are also provided to assist in finding desired information quickly:
o cobas b 221 system Reference Manual o cobas b 221 system Short Instruction
Conventions used in this manual
Visual cues are used to help locate and interpret information in this manual quickly.
This section explains formatting conventions used in this manual.
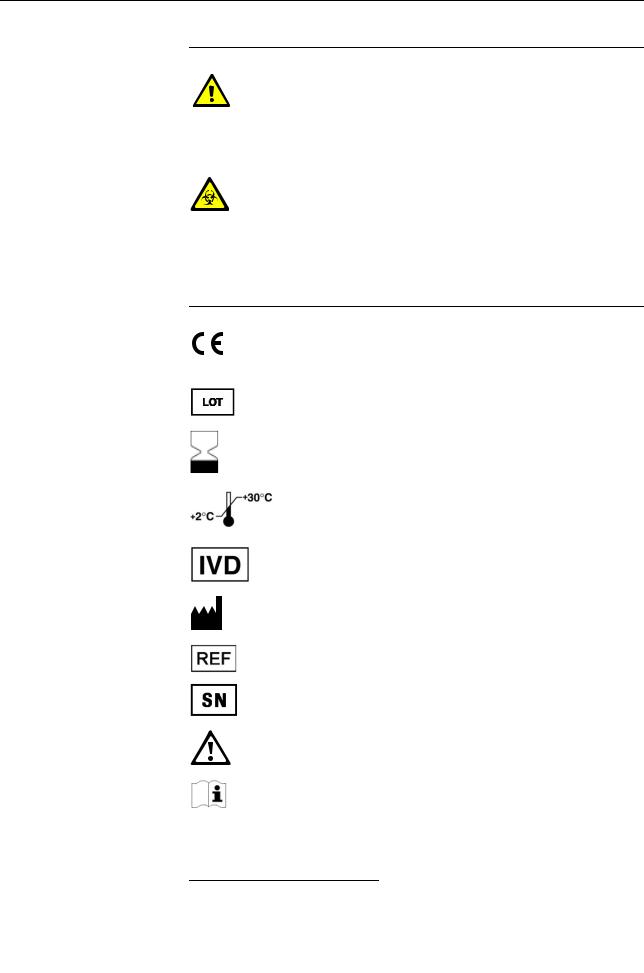
Symbols Helping to locate and interpret information in this manual the following symbols are used:
Symbol |
Used for |
|||||
a |
Procedural step |
|||||
o |
List item |
|||||
e |
Cross-reference |
|||||
h |
Call up of screen |
|||||
|
|
|
|
|
|
Note |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
5 |
cobas b 221 system
Symbol |
Used for |
|
Caution |
|
All sections / passages that are marked with this symbol describe procedures |
|
and/or indicate conditions or dangers that could damage or lead to a |
|
malfunction in the cobas b 221 system, and which therefore should never be |
|
attempted and contain information that must be observed to avoid potential |
|
injuries (to patients, users and third parties). |
|
Risk of infection |
|
All sections and parts of texts that are marked with this symbol describe |
|
procedures that may involve risk of infection. |
|
|
IVD symbols The symbols are used in accordance with DIN EN 980(a) and DIN EN ISO 780(b).
Symbol |
Description |
|
Conformité Européenne: |
|
This product complies with the requirements in the guideline for |
|
In Vitro Diagnostic 98/79/EC. |
|
Lot designation |
|
Use by... |
|
The product should not be used after expiry of the specified date. |
|
If a day is not indicated, apply the last day of the respective month. |
|
Temperature limitation |
|
The conditions necessary to preserve the product's shelf life before |
|
opening. |
|
In Vitro Diagnostic Medical Device |
|
Manufacturer |
|
(according to In Vitro Diagnostic guidelines 98/79/EG) |
|
Catalogue number |
|
Serial number (model plate) |
|
Caution, consult accompanying documents |
|
Please consult instructions for use |
|
|
(a)DIN EN 980: Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied (Part 1: General requirements)
(b)DIN EN ISO 780: Packaging - Pictorial marking for the handling of goods
Roche Diagnostics |
April 2009 |
6 |
Instructions for Use · Revision 10.0 |
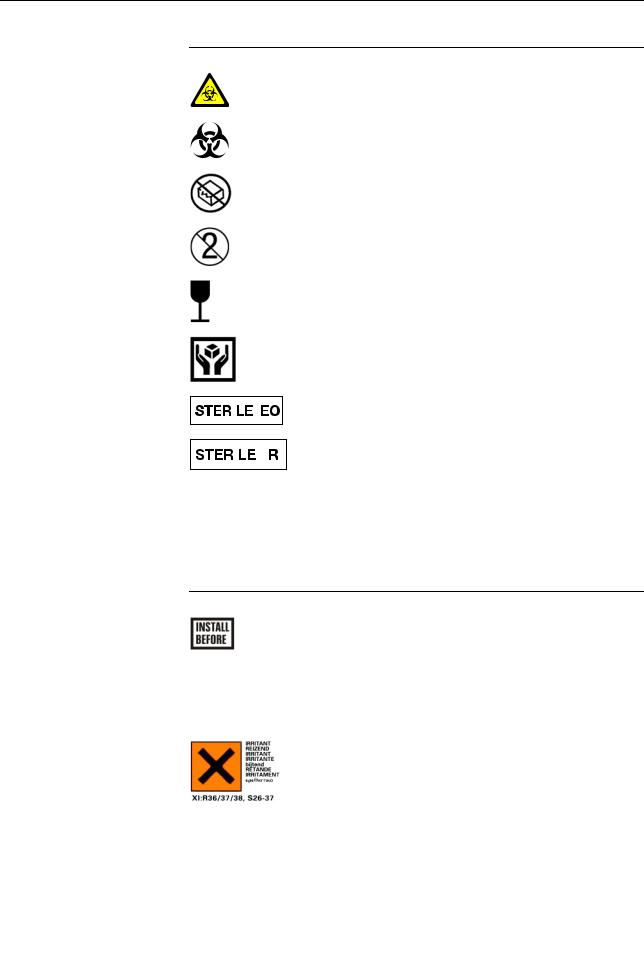
cobas b 221 system
Symbol |
|
|
Description |
|
|
|
Biological risk! |
|
|
|
(according to the standard IEC/EN 61010-2-101)(a) (Instrument) |
|
|
|
Biological risk! |
|
|
|
(according to the standard DIN EN ISO 980)(b) (Consumables) |
|
|
|
Do not use if package damaged |
|
|
|
Do not reuse |
|
|
|
Fragile. Handle with care |
|
|
|
Handle with care |
|
|
|
Valid only for Roche MICROSAMPLER: |
|
|
||
|
|
|
Method of sterilization using ethylene oxide |
|
|||
|
|
|
Valid only for BS2 Blood Sampler: |
|
|
|
|
|
|
|
Method of sterilization using irradiation |
|
|
||
|
|
|
|
(a)IEC/EN 61010-2-101: Safety requirements for electrical equipment for measurement, control, and laboratory use - (Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment).
(b)DIN EN ISO 980: Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied (Part 1: General requirements).
Other symbols The following symbols are listed as additional information:
Symbol |
Description |
|
Electrodes: |
|
This date indicates the limit of the maximum storage time of |
|
an electrode. The electrode must be installed in the instrument |
|
no later than the imprinted date. |
|
If the installation takes place on the imprinted date, it still falls |
|
within the specifications. The calculation of the “Install |
|
before” date is based on the production date of the elctrode. |
|
Danger symbol: "Irritant" (on the label and the packaging of |
|
S2 Fluid Pack) |
|
Rating: Although not corrosive, momentary, longer-lasting, or |
|
repeated contact with skin or mucous membrane may result in |
|
inflammation. Danger of sensitization during contact with |
|
skin (when classified with R 43). |
|
Caution: Avoid contact with eyes and skin, do not inhale |
|
vapors. |
|
|
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
7 |

cobas b 221 system
Symbol |
Description |
||||
|
|
|
|
|
Invisible Laser Radiation |
|
|
|
|
|
Avoid direct radiation to eyes! |
|
|
|
|
|
Laser Class 3R according to EN 60825-1 |
|
|
|
|
|
P0 ≤ 5 mW |
|
|
|
|
|
λ = 635 - 850 nm |
|
|
|
|
|
Store upright |
|
|
|
|
|
|
|
|
|
|
|
"Grüner Punkt" (in Germany) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Protective gloves, protective goggles and suitable protective |
|
|
|
|
|
clothing must be worn. |
|
|
|
|
|
|
Abbreviations The following abbreviations are used:
Abbreviation |
Definition |
A |
|
|
ANSI |
American National Standards Institute |
|
AQC |
Automatic Quality Control |
|
B |
|
|
BG |
Blood gas |
|
BUN |
Abbr. for blood urea nitrogen |
|
C |
|
|
CLIA |
Clinical Laboratory Improvement Amendments |
|
CLSI |
Clinical and Laboratory Standards Institute |
|
cond |
Conductivity |
|
CSA |
Canadian Standards Association |
|
D |
|
|
dBA |
Decibel weighted against the A-frequency response curve. This curve |
|
|
approximates the audible range of the human ear. |
|
DIL |
Diluent |
|
DNS |
Domain Name Server |
|
E |
|
|
EC |
European community |
|
e.g. |
exempli gratia – for example |
|
EN |
European standard |
|
F |
|
|
FMS |
Fluid mixing system |
|
H |
|
|
Hct |
Hematrocrit |
|
HIV |
Human immunodeficiency virus |
|
HW |
Hardware |
|
|
|
Roche Diagnostics |
April 2009 |
|
|
|
|
8 |
|
Instructions for Use · Revision 10.0 |

cobas b 221 system
Abbreviation |
Definition |
I |
|
i.e. |
id est – that is to say |
ISE |
Ion selective electrode |
IVD |
In vitro Diagnostic Directive |
L |
|
LCD |
Liquid cristal display |
LIS |
Laboratory Information System |
LJ |
Levey Jennings |
M |
|
MAC |
Media Access Control |
MC |
Measuring chamber |
MSDS |
Material safety data sheet |
MSS |
Metabolite sensitive sensor |
MV |
Mean value |
P |
|
PP |
Peristaltic pump |
Q |
|
QC |
Quality control |
R |
|
RCon |
Reference contact |
REF |
Reference solution |
S |
|
SIP |
Sample inlet path |
SDC |
Sample distributor cartridge |
S1 |
S1 Rinse Solution |
S2 |
S2 Fluid Pack |
S3 |
S3 Fluid Pack |
SCon |
Sensor contact |
SD |
Standard deviation |
SO2 |
Oxygen saturation |
T |
|
T&D |
Turn & dock |
tHb |
Total hemoglobin |
U |
|
UL |
Underwriters Laboratories Inc. |
V |
|
VDE |
Association of German Electrical Engineers (Verband Deutscher |
|
Elektrotechniker) |
|
|
eFor writing the measuring, calculated and input values see Chapter 9 Softwaremodi > Parameter on page B-75!
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
9 |

cobas b 221 system
Roche Diagnostics |
April 2009 |
10 |
Instructions for Use · Revision 10.0 |

Introduction and specifications A
1 |
Safety information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-3 |
2 |
General descriptions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-7 |
3 |
Installation and shutdown . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-25 |
4 |
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-57 |
5 |
Theoretical foundations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-95 |
cobas b 221 system |
1 Safety information |
|
Contents |
Safety information
The information provided in this chapter is essential for the safe, trouble-free operation of the instrument and must be read and understood by the user.
In this chapter
Chapter 1
Important information ................................................................................................... |
5 |
Operating safety information ......................................................................................... |
6 |
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-3 |

1 Safety information |
cobas b 221 system |
Contents
Roche Diagnostics |
April 2009 |
A-4 |
Instructions for Use · Revision 10.0 |
cobas b 221 system |
1 Safety information |
|
Important information |
Important information
These Instructions for Use contain vital warnings and safety information.
This instrument is intended to be used only for the specialized purpose described in the instructions. The most important prerequisites for use, operation, and safety are explained to ensure smooth operation. No warranty or liability claims will be covered if the machine is used in ways other than those described or if the necessary prerequisites and safety measures are not observed.
The instrument may be operated only by persons whose qualifications enable them to comply with the safety measures that are necessary during operation of the instrument.
Suitable protective equipment, like laboratory clothing, protective gloves, protective goggles and if necessary mouth protectors, must be worn to prevent direct contact with biological working materials. In addition, a face mask is required if there is a risk.
Adjustments and maintenance performed with covers removed and power connected may be attempted only by a qualified technician who is aware of the associated dangers.
Instrument repairs are to be performed only by the manufacturer or qualified service personnel.
Only accessories and supplies either delivered by or approved by Roche are to be used with the instrument. These items are manufactured especially for use with this instrument and meet the highest quality requirements.
Operation of the instrument with solutions whose composition is not consistent with that of the original solutions can negatively affect the long-term measurement accuracy. Deviations in the composition of the solutions can also decrease the service life of the electrodes.
In order to ensure the quality of the measurement results, complete a quality control test on 3 levels (low, normal, high) after each electrode exchange, after each exchange of solutions and packs and after startup of the instrument.
Additionally complete a quality control test on one level between two automatic 2P calibrations. The level have to be alternated (low, normal, high).
Since the measurements of the instrument depend not only on the correct characteristic function, but also on a series of marginal conditions (e.g. pre-analysis), results obtained from the instrument should be submitted for an expert opinion before taking additional measures based on the supplied measurements.
Caution (refer to accompanying documents)!
Please refer to safety-related notes in the manual accompanying this instrument.
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-5 |
1 Safety information |
cobas b 221 system |
Operating safety information
Operating safety information
The instrument has been constructed and tested according to the following European
Standards:
o IEC/EN 61010-1
o IEC/EN 61010-2-101
o IEC/EN 61010-2-081 + A1
It was delivered from the factory in flawless condition with regards to safety features. In order to preserve this condition and ensure safe operation, the user must respect the notices and warnings that are contained in these Instructions for Use.
oThis equipment is a Class I laser product, and it complies with FDA Radiation Performance Standards, 21 CFR Subchapter J (only valid for
cobas b 221<1> system, cobas b 221<3> system and cobas b 221<5> system with tHb/SO2 module).
oThis instrument is classified under the protection class I according to IEC /EN 61010-1.
o The instrument meets the conditions for overvoltage category II. o The instrument meets the conditions for contamination level 2.
oDo not operate the instrument in an explosive environment or in the vicinity of explosive anesthetic mixtures containing oxygen or nitrous oxide.
oIf objects or liquids enter the internal areas of the instrument, remove the instrument from its power supply and allow an expert to check it thoroughly before using it again.
o The instrument is suitable for long-term operation indoors.
o The power cord must be plugged into a grounded power receptacle. When using an extension cord, make sure it is properly grounded.
oAny rupture of the ground lead inside or outside the instrument or a loose ground connection may result in hazardous operating conditions for the operating personnel. Intentional disconnection of the grounding is not permitted.
oThe instrument is not suitable for operation with a direct current power supply. Use only the original power plug delivered with the cobas b 221 system.
oThe use of controls or adjustments or performance of procedures other than those specified herein may result in hazardous radiation exposure.
Roche Diagnostics |
April 2009 |
A-6 |
Instructions for Use · Revision 10.0 |
cobas b 221 system |
2 General descriptions |
|
Contents |
General descriptions
This chapter contains a general description of the instrument, as well as precautionary measures against special dangers and the proper handling of sensors, solutions and the MSS cassette.
In this chapter
Chapter 2
Introduction .................................................................................................................... |
9 |
General notes ................................................................................................................. |
11 |
Application area ....................................................................................................... |
11 |
Operating instructions ............................................................................................ |
11 |
Important buttons on the screen ............................................................................ |
12 |
Measurement and calibration procedure ..................................................................... |
13 |
Measurement procedure ......................................................................................... |
13 |
Calibration procedure ............................................................................................. |
13 |
Measurement evaluation ............................................................................................... |
14 |
Safety instructions for specific dangers ........................................................................ |
14 |
Handling samples .................................................................................................... |
14 |
Disposal of waste water, bottles, packs, electrodes and the instrument ............... |
14 |
Decontamination .................................................................................................... |
14 |
Handling solutions ........................................................................................................ |
15 |
Handling electrodes ...................................................................................................... |
15 |
General notes on the use of the MSS cassette .............................................................. |
16 |
MSS cassette removed from the measuring chamber ............................................ |
16 |
Incompatible substances ......................................................................................... |
16 |
Inserting the MSS cassette ...................................................................................... |
17 |
System description ........................................................................................................ |
18 |
Visual identification ................................................................................................ |
18 |
Screen/PC unit ......................................................................................................... |
19 |
Printer ...................................................................................................................... |
19 |
Measuring chamber ................................................................................................. |
19 |
tHb/SO2 module ..................................................................................................... |
19 |
COOX module ......................................................................................................... |
20 |
Pumps ...................................................................................................................... |
20 |
Input unit ................................................................................................................. |
20 |
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-7 |

2 General descriptions |
cobas b 221 system |
Contents |
|
Bottle compartment ................................................................................................ |
20 |
Reverse side .............................................................................................................. |
21 |
Power supply ...................................................................................................... |
21 |
Interfaces ............................................................................................................ |
22 |
Barcode scanner ................................................................................................. |
23 |
Warning and identification labels (incl. nameplate) ....................................... |
24 |
Roche Diagnostics |
April 2009 |
A-8 |
Instructions for Use · Revision 10.0 |

cobas b 221 system |
2 General descriptions |
|
Introduction |
Introduction
Figure A-1 |
cobas b 221 system |
The cobas b 221 system is an analyzer with integrated AutoQC drawer option. Depending on combination and configuration, the following parameters can be measured in whole blood, serum, plasma, acetate and bicarbonate containing dialysis solutions and QC materials:
|
o |
pH |
|
o |
Blood gas BG (PO2, PCO2) |
|
o |
Electrolyte ISE (Na+, K+, Cl–, Ca2+) |
|
o |
Hematocrit (Hct) |
|
o |
Metabolite MSS |
|
|
|
|
|
|
Urea/BUN - only cobas b 221<6> system
o Total hemoglobin (tHb) o Oxygen saturation (SO2)
o Hemoglobin derivative COOX (O2Hb, HHb, COHb, MetHb) o Bilirubin (neonatal)
The following configurations are available:
o cobas b 221<1> system(a) |
BG, pH, tHb/SO2 |
o cobas b 221<2> system |
BG, pH, COOX, Bili |
o cobas b 221<3> system (a) |
BG, pH, ISE, Hct, tHb/SO2 |
o cobas b 221<4> system |
BG, pH, ISE, Hct, COOX, Bili |
o cobas b 221<5> system(a) |
BG, pH, ISE, Hct, MSS, tHb/SO2 |
o cobas b 221<6> system |
BG, pH, ISE, Hct, MSS, COOX, Bili |
|
|
(a) are no longer manufactured or offered.
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-9 |

2 General descriptions |
cobas b 221 system |
Introduction
During the measurement or calibration or other processes, it is possible to conduct database operations, perform certain settings or call up general information at the same time.
e For details see Chapter 9 Software modes
The individual, mutually independent software modes are defined as follows:
o |
Analyzer |
Measuring, QC measurement, system, calibration, |
|
|
commonly used functions (quick access) |
o |
Setup |
Instrument settings |
o |
Database |
Data about patients, measurements, calibrations, QC, and |
|
|
the instrument |
o |
Info |
|
Roche Diagnostics |
April 2009 |
A-10 |
Instructions for Use · Revision 10.0 |

cobas b 221 system |
2 General descriptions |
|
General notes |
General notes
Application area
The instrument has been tested for measuring parameters in whole blood, serum, plasma and dialysis solutions (electrolytes only) and the validity of measurements was tested accordingly.
In order to achieve accurate measurements of recommended aqueous control solutions (with regards to deviations from biological samples), choose the proper components and make the corresponding corrections in the QC measurement mode.
The accuracy of measurement values of undefined aqueous solutions cannot be guaranteed (e.g. due to the possibility of interfering components and/or missing or insufficient buffer systems, and/or differences in ionic strength and diffusion potential when compared to biological samples).
Operating instructions
The cobas b 221 system should be switched on at all times!
If the instrument is switched off for an extended period of time (more than 24 hours), a shutdown must be performed.
eFor additional information, see Chapter 3 Installation and shutdown, section Installation on page A-27 and Shutdown on page A-48.
Prevent any other liquids from entering the instrument except samples and
QC material at the fill port.
In order to ensure the quality of the measurement results, complete a quality control test on 3 levels (low, normal, high) after each electrode exchange, after each exchange of solutions and packs and after startup of the instrument.
Additionally complete a quality control test on one level between two automatic 2P calibrations. The level have to be alternated (low, normal, high).
e For additional information, see Chapter 7 Quality control.
With Software V 6.0 onwards, using cobas bge link, the instrument can be monitored from one location, any disturbances can be remedied and the analytical quality monitored.
cobas bge link is a remote monitoring and remote maintenance software for Roche Point-of-Care analyzers.
e see Figure A-2 on page A-12!
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-11 |
2 General descriptions |
cobas b 221 system |
General notes
B
A
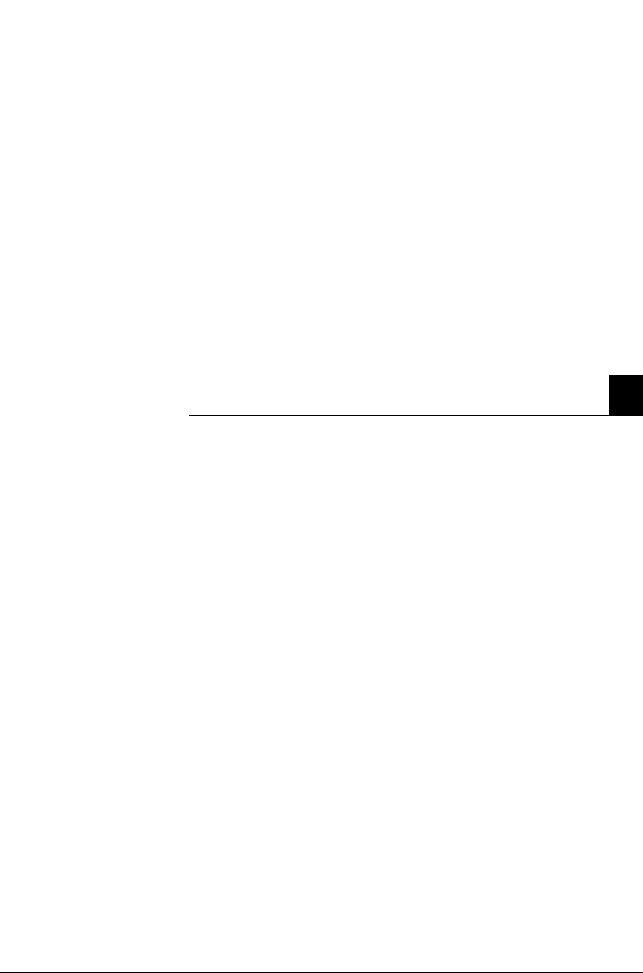
A "Screen sharing" Symbol |
B "Screen sharing" active |
|
|
Figure A-2
Confirm the message with [OK] either on the instrument or on the PC. The "screen sharing" symbol is added in the status line.
To avoid multiple operation of the instrument, the message "Screen sharing active" is displayed with a yellow background in the error and message window of the instrument.
As long as the "screen sharing" symbol is displayed in the status line, the service connection is active. In order to prevent multiple operation of the instrument, no buttons on the screen should be pressed!
Important buttons on the screen
Buttons |
Description |
|
"Analyzer" active / inactive |
|
"Database" active / inactive |
|
"Setup" active / inactive |
|
"Info" active / inactive |
e For additional information, see Chapter 9 Software modes, section Buttons on page B-76.
Roche Diagnostics |
April 2009 |
A-12 |
Instructions for Use · Revision 10.0 |

cobas b 221 system |
2 General descriptions |
|
Measurement and calibration procedure |
Measurement and calibration procedure
Measurement procedure
PO2: Use of the Clark measurement principle: measurement of current generated by the reduction of oxygen.
PCO2: Use of the Severinghouse principle: potentiometric measurement of the pH change in the electrode caused by CO2.
pH- , Na+-,K+-, Ca2+- und Cl- electrodes are potentiometric electrodes. Special glasses are used as the sensitive element for pH and Na+. The potassium and calcium membranes contain special neutral carriers. A special ion exchanger is used for chloride membranes. Calculation of these variables also requires the use of a reference electrode—a permanently contacted chloride electrode in the cobas b 221 system.
Glucose, lactate: Glucose oxidizes to form gluconolacton using atmospheric oxygen and the glucose-oxidase (GOD) enzyme, lactate oxidizes to form pyruvate using the lactate oxidase enzyme.
The generated H2O2 is determined amperometrically by using manganese dioxide/ carbon electrode at 350 mV.
Urea: Urea is broken into ammonia and carbon dioxide through urease. Ammonia and carbon dioxide react through hydrolysis with physiological pH to form ammonia or bicarbonate ions. The ammonia ions can be determined using a potentiometrical ammonia ion-selective electrode. This measurement requires a reference electrode such as those used in ion-selective electrodes.
tHb/SO2: Light absorption in whole blood is measured at four different wavelengths, the sample is subjected to light radiation and the dispersed light is also evaluated.
COOX: The hemoglobin derivatives and the total bilirubin (= neonatal) are determined spectrophotometrically based on the Lambert-Beer law.
Hematocrit: Measurement of the sample's conductivity in the ISE measuring chamber.
Calibration procedure
tHb and SO2 was calibrated when the instrument was manufactured.
Oxygen (O2): Ambient air and a zero point solution are used to calibrate oxygen.
PCO2, pH, ISE: are calibrated using two solutions mixed under different conditions, thereby avoiding the gas supply which is required by other instruments.
MSS: The calibration is carried out with four (Glu, Lac) or five solutions (Urea/BUN) whose weighing concentrations form the basis for measured value determination.
COOX: Determining the hemoglobin derivatives and the total bilirubin (= neonatal) are carried out spectral-photometrically using a cuvette.
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-13 |
2 General descriptions |
cobas b 221 system |
Measurement evaluation
Measurement evaluation
The validity of the test results from the cobas b 221 system must be carefully examined by a clinical-medical specialist who will take the patient's clinical condition into consideration before any clinical decisions are reached based on the test results.
In order to ensure the quality of the measurement results, complete a quality control test on 3 levels (low, normal, high) after each electrode exchange, after each exchange of solutions and packs and after startup of the instrument.
Additionally complete a quality control test on one level between two automatic 2P calibrations. The level have to be alternated (low, normal, high).
e For detailed information, see Chapter 7 Quality control.
Safety instructions for specific dangers
Handling samples
While handling samples, all necessary regulations concerning hygiene must be observed. Dangerous pathogenic agents could be present.
e For more detailed information, see Chapter 6 Measurement
Disposal of waste water, bottles, packs, electrodes and the instrument
Dispose of waste water, bottles, packs, electrodes and the instrument according to local and/or labor regulations (biologically contaminated—hazardous waste!).
Decontamination
The purpose of this decontamination is to minimize risk when handling items that were in contact with biological samples.
Roche recommends following a decontamination procedure in addition to regulations specific to the laboratory.
These decontamination procedures should be performed periodically to minimize the risk of infections.
Always wear gloves!
e For more detailed information about decontamination, see Chapter 10 Maintenance
Roche Diagnostics |
April 2009 |
A-14 |
Instructions for Use · Revision 10.0 |

cobas b 221 system |
2 General descriptions |
|
Handling solutions |
Handling solutions
Store the cobas b 221 system wash/calibrating solutions according to the specified packaging requirements. The temperature of the solutions should be adapted to the ambient temperature before use.
The shelf life of the solutions is limited.
Please read the bottle label and the packaging for the correct storage temperature and the maximum shelf life.
DO NOT FREEZE!
If frozen, the solution's concentration may change and cause calibration errors!
Do not use damaged fluid packs (S2 and S3)! Do not mix the individual components!
e For "Storage specifications", see Chapter 4 Specifications.
Handling electrodes
Store the electrodes according to the packaging specifications.
The shelf life of the electrodes is limited.
Please read the label and the packaging for the correct storage temperature and the maximum shelf life.
CAUTION! Installation note for the PCO2 electrode
Insert the electrode into the measuring chamber within 5 minutes of opening the ALU-PE packaging.
A special protective gas atmosphere designed to condition the PCO2 electrode during storage is found inside the ALU-PE packaging.
This gas atmosphere ensures immediate potential stability during insertion of the electrode into the measuring chamber and immediate readiness for measuring the first 2 point calibration.
If more than 5 minutes elapse after opening the ALU-PE packaging, the level of gas conditioning could be lost and the time required for the first-time calibration could be increased.
e For "Storage specifications", see Chapter 4 Specifications.
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-15 |

2 General descriptions |
cobas b 221 system |
General notes on the use of the MSS cassette
General notes on the use of the MSS cassette
For instrument versions with MSS module only!
Attention:
MSS cassette may only be brought into contact with liquids in the cobas b 221 system while electrodes are changed!
Replace the MSS cassette within 28 days of installation!
After initial contact with liquids, the MSS cassette may no longer be removed from the instrument.
It may lead to the destruction of the enzyme sensors.
Storage:
At 2 – 8 °C, maximum of 2 weeks at room temperature.
MSS cassette removed from the measuring chamber
Once an MSS cassette is exposed to liquid, it must not be allowed to dry out under any circumstances since this would destroy the enzymes. The enzymes are equipped with a special protectant prior to shipping for transportation purposes. This protectant is washed out inside the instrument during the warm-up phase and MSS polarization.
Incompatible substances
The following substances may not be introduced into the MSS measuring chamber under any circumstances since they would immediately destroy the MSS sensors or severely impact their functionality.
o Deproteinizer (NaOCl) o O2 zero point solution o Cleaning solution
o Na electrode conditioning solution o Rinse additive
o Solutions containing heavy metals (Ag, Hg, Au, etc., e.g. Thiomersal)
oCleaning solutions containing detergent (e.g. washing material or liquid detergents)
oAll solutions for disinfections (e.g. high-percentage alcohol, glutaric dialdehyde, cresol, etc.)
oSolutions with pH values that deviate greatly from neutral (e.g. pH value of < 6.0 and > 9.0)
The use of anticoagulants other than those approved by Roche Diagnostics (approved: heparin salts), such as EDTA, citrate, NH4 heparin and glycolysis inhibitor such as NaF and oxalate can lead to erroneous results.
Roche Diagnostics |
April 2009 |
A-16 |
Instructions for Use · Revision 10.0 |

cobas b 221 system |
2 General descriptions |
|
General notes on the use of the MSS cassette |
Inserting the MSS cassette
Hold the MSS cassette only at the designated handle and avoid touching the contacts.
eFor a detailed description see Chapter 10 Maintenance, section Changing the MSS cassette (cobas b 221<5> system and cobas b 221<6> system only) on page C-32.
Roche Diagnostics |
April 2009 |
Instructions for Use · Revision 10.0 |
A-17 |

2 General descriptions |
cobas b 221 system |
System description
System description
Visual identification
For example: cobas b 221<6> system
P
A
B
C
D
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F |
G |
H |
I |
|
|
||||
A |
Screen/PC unit |
|
G |
S1 |
Rinse Solution |
|
|
|
|
M |
Input unit |
|||||
B |
Reverse side |
|
H |
S2 |
Fluid Pack |
|
|
|
|
N |
Measuring chambers |
|||||
C |
Docking mechanism |
I |
S3 |
Fluid Pack |
|
|
|
|
O |
Printer |
||||||
D |
AutoQC drawer |
|
J |
Bottle compartement cover |
|
|
P |
Pumps |
||||||||
E |
Barcode scanner |
|
K |
Bottle compartement |
|
|
|
|
|
|
|
|
||||
F |
W Waste container |
L |
COOX module (tHb/SO2 module) |
|
|
|
|
|
|
|||||||
Figure A-3 |
cobas b 221<6> system |
|
|
|
|
|
|
|
|
|
|
|
|
|||
O
N
M
L
K
J
Roche Diagnostics |
April 2009 |
A-18 |
Instructions for Use · Revision 10.0 |
 Loading...
Loading...