ReliOn Ketone Test Strips Owner's Manual
SSUUMMMMAARRYY AANNDD EEXXPPLLAANNAATTIIOONN::
The strips provide a fast, convenient way of testing urine
for the presence and concentration of acetoacetic acid
(ketone). This substance when found in the urine provides
information on carbohydrate and fat metabolism.
1,2
Acetoacetic acid can be found in the urine from
diabetics and is more commonly referred to as a
“ketone body.” Use of KETONE Reagent Strips can
alert you and your doctor or diabetes educator to
changes in your condition for which adjustments
in your diet and/or medication may be needed.
Carefully follow the testing schedule your doctor or
educator establishes.
CCHHEEMMIICCAALL PPRRIINNCCIIPPLLEESS OOFF TTHHEE PPRROOCCEEDDUURREE::
The ketone test is specific for acetoacetic acid and
is based on the development of colors ranging from
buff-pink to maroon when acetoacetic acid reacts
with nitroprusside.
RREEAAGGEENNTTSS::
7.1% w/w sodium nitroprusside; 92.9% w/w buffer.
WWAARRNNIINNGGSS AANNDD PPRREECCAAUUTTIIOONNSS::
KETONE Reagent Strips are for
in vitro
diagnostic
use. They have been determined to be nonhazardous
under the guidelines issued by OSHA in 29CFR
1910.1200(d).
SSTTOORRAAGGEE AANNDD HHAANNDDLLIINNGG::
Never use KETONE Reagent Strips past the
expiration date printed on the bottle label.
To keep KETONE Reagent Strips fresh, follow these
suggestions carefully. Keep your finger or other
objects from touching the reagent area before testing.
Store at room temperatures between 59°– 86°F
(15°–30°C). Do not store the bottle in direct sunlight.
Keep unused reagent strips in the original bottle
with the cap tightly closed. Always replace the cap
immediately and tightly after removing a strip. A new
bottle of strips can be used for six months after being
first opened. Always write the date you first opened
the bottle on the bottle label.
DDoo nnoott uussee pprroodduucctt
((ooppeenneedd oorr uunnooppeenneedd)) aafftteerr eexxppiirraattiioonn ddaattee.. UUssee
ooff
ssttrriippss bbeeyyoonndd tthhee eexxppiirraattiioonn ddaattee mmaayy yyiieelldd
llooww rreessuullttss..
Never transfer reagent strips to another
bottle. Do not remove desiccant from bottle. The
desiccant absorbs moisture and keeps the strips dry.
Never put cotton or other materials in the bottle. If
the reagent area is ever discolored or darkened, throw
the strip away and use a strip from a new bottle.
SSPPEECCIIMMEENN CCOOLLLLEECCTTIIOONN AANNDD PPRREEPPAARRAATTIIOONN::
Collect fresh urine in a clean, dry container and test
it as soon as possible, or alternatively, pass the
reagent pad through the urine stream. If testing
cannot be done within an hour after collecting a urine
specimen, refrigerate the specimen immediately and
let it return to room temperature before testing.
RRE
ESSUULLTTSS::
Results with KETONE Reagent Strips are obtained
directly from comparison to the Color Chart. The
color blocks represent nominal values; actual values
will vary around the nominal values.
Ketone results are read from the Color Chart as
negative or varying degrees of positive which indicate
the relative amounts of ketone (acetoacetic acid)
present. The color blocks represent Negative, Trace
(5 mg/dL), Small (15 mg/dL), Moderate (40 mg/dL)
and Large (80–160 mg/dL). Proper read time is
critical for optimal results.
The ketone reagent area is most accurate when testing
urines of specific gravity between 1.010 and 1.020.
IIFF TTEESSTT RREESSUULLTTSS SSEEEEMM QQUUEESSTTIIOONNAABBLLEE::
1. Check the EXPIRATION DATE printed on the
bottle label. If the date has passed, discard and
retest with strips from a new bottle. Check the
date you first opened the bottle and if 6 months
have passed, throw the strips away and use a
new bottle of KETONE Reagent Strips.
2. Test the urine again with a strip from a new bottle
and compare results.
IIff aa pprroobblleemm ccaannnnoott bbee
iiddeennttiiffiieedd oorr ccoorrrreecctteedd,, ccaalll
l oouurr CCuussttoommeerr
SSeerrvviiccee DDeeppaarrttmmeenntt,, ttoollll ffrreeee 11--880000--334488--88110000
oorr ccoonnssuulltt yyoouurr ddooccttoorr,, ddiiaabbeetteess eedduuccaattoor
r,,
pphhaarrmmaacciisstt oorr SSeellff TTeessttiinngg CCeenntteerr ffoorr aaddvviiccee
oonn tteessttiinngg tteecchhnniiqquuee aanndd rreessuullttss..
EEXXPPEECCTTEEDD VVAALLUUEESS::
Normal urine specimens ordinarily yield negative
results with the KETONE reagent area. Detectable
levels of ketone may occur in urine during physiological
stress conditions such as fasting, pregnancy and
frequent strenuous exercise.
3,5
In ketoacidosis,
starvation or other abnormalities of carbohydrate or
fat metabolism, ketones may appear in urine in large
amounts before serum ketone is elevated.
6
LLIIMMIITTAATTIIOONNSS OOFF PPRROOCCEEDDUURREESS::
As with all laboratory tests, definitive diagnostic or
therapeutic decisions should not be based on any
single result or method. KETONE results should
never be used as the sole basis for adjusting
insulin dosage.
Substances that cause abnormal urine color, such
as drugs containing azo dyes (e.g., Pyridium
®
*,
Azo Gantrisin
®
*, Azo Gantanol®*), nitrofurantoin
(Macrodantin
®
*, Furadantin®*), and riboflavin, may
affect the readability of the ketone reagent area on
the KETONE Reagent Strips. The color development
on the reagent pad may be masked, or a color
reaction may be produced on the pad that could be
interpreted as a false positive.
False positive results (Trace or less) may occur
with highly pigmented urine specimens or those
containing large amounts of levodopa metabolites.
Compounds such as mesna (2-mercaptoethane
sulfonic acid) that contain sulfhydryl groups may
cause false positive results or an atypical color
reaction.
SSPPEECCIIFFIICC PPEERRFFOORRMMAANNCCEE CCHHAARRAACCTTEERRIISSTTIICCSS::
Specific performance characteristics are based on
clinical and analytical studies. In clinical specimens,
the sensitivity depends upon several factors: the
variability of color perception; the presence or absence
of inhibitory factors typically found in urine, the
specific gravity, and the pH (see LIMITATIONS OF
PROCEDURES section); and the lighting conditions
under which the product is read. Because the
color of each reagent area changes as the
analyte concentration increases, the percentage of
specimens detected as positive will increase with the
analyte concentration.
Each color block represents a range of values.
Because of specimen and reading variability, specimens with analyte concentrations that fall between
nominal levels may give results at either level.
Results at levels greater than the second positive
level will usually be within one level of the true
concentration.
The test reacts with acetoacetic acid in urine. It
does not react with acetone or beta-hydroxybutyric
acid. In a majority of cases, the reagent area detects
5 to 10 mg/dL acetoacetic acid. Some high specific
gravity-low pH urines may give reactions up to and
including Trace (5 mg/dL). Clinical judgment is needed
to determine the significance of reactions up to and
including Trace.
AAVVAAIILLAABBIILLIITTYY::
KETONE Reagent Strips in bottles of 50 strips
(#2523) are available only at Wal-Mart and SAM’s
Club pharmacies.
RREEFFEERREENNCCEESS::
1. Cecil, R. L. and Loeb, R. F.: A Testbook of Medicine, W. B. Saunders,
Co., Philadelphia, 1959, 10th ed., pp 618-619.
2. Engel, F. L. and Amatruda, T. T.: Ann. N. Y. Acad. Sci. 104, Art 2, 753-771
(1963).
3. McGarry, J. D., Lilly, Lecture 1978: New Perspectives in the Regulation of
Ketogenesis.
DIABETES
28, May, 1978, 517-523.
4. Williamson, D. H.: Physiological ketoses, or why ketone bodies?
Postgraduate Medical Journal
(June Suppl. 1971), 371-375.
5. Paterson, P., Sheath, J., Pincus, T. and Wood, C.: Maternal and Fetal
Ketone Concentrations in Plasmas and Urine,
The Lancet:
April 22, 1967,
862-865.
6. Fraser, J., Fetter, M.C., Mast, R. L. and Free, A. H.: Studies with a Simplified
Nitroprusside Test for Ketone Bodies in Urine, Serum, Plasma and Milk,
Clin. Chem. Acta II
(1965) 376-378.
* Trademarks
Pyridium®is a registered trademark of Parke-Davis.
Azo Gantrisin®and Azo Gantanol®are registered trademarks of Roche
Laboratories.
Macrodantin
®
and Furadantin®are registered trademarks of Procter &
Gamble Pharmaceuticals, Inc.
KKeettoonnee RReeaaggeenntt SSttrriippss//
TTeesstt IInnffoorrmmaattiioonn aanndd PPrroocceedduurree
RReeaaggeenntt SSttrriippss ffoorr UUrriinnaallyyssiiss
AN13216A USA © 2004 Bayer HealthCare LLC Rev. 7/04
Manufactured by:
Bayer HealthCare LLC
Mishawaka, IN 46544
WWHHYY DDOO II NNEEEEDD TTOO TTEESSTT FFOORR
KKEETTOONNEE??
Ketone is a substance which is usually not
harmful when found in small amounts in
the urine. This could happen in dieting and,
when identified, gives you information on
carbohydrate and fat metabolism.
However, when you have too much of
this substance in your body it can be
dangerous and especially dangerous for a
person with diabetes.
If you are a person with diabetes, you may
produce ketones in urine whether you are
insulin dependent (Type I) or non-insulin
dependent (Type II). A person with Type I
diabetes is more likely to spill ketones in
the urine because they are more likely to
burn too much fat.
If you burn too much fat and produce a lot
of ketones, your diabetes may be out of
control. Then you may develop a complication called diabetic KETOACIDOSIS. This
can lead to a coma. You need to take fast
action when you see the ketone warning
signal. You should contact your doctor or
diabetes educator immediately. Your doctor
or educator can tell you how to change your
medication/insulin dose or your food intake
to bring your diabetes back into control and
to prevent ketoacidosis.
WWHHEENN SSHHOOUULLDD II TTEESSTT FFOORR KKEETTOONNEE??
1. When you have a cold, the flu or any
other kind of illness.
2. When your urine sugar test results show
you are spilling large amounts of sugar
(2% or more for two or more tests in a
row or several days in a row).
3. When you “feel” the signs of high blood
sugar (over 240 mg/dL) or when your
blood sugar is well over the range your
doctor or educator has set for you (if
you do blood glucose monitoring).
4. When you are under stress e.g., at
home, at school, at work, or on a
vacation.
5. Regularly during pregnancy.
HHOOWW DDOO II DDOO TTHHEE TTE
ESSTT??
Materials needed:
• KETONE Reagent Strips
• Timer or watch with second hand
1. Remove the strip from bottle.
RReeppllaaccee
tthhee ccaapp iimmmmeeddiiaatteellyy aanndd ttiigghhttllyy..))
Do
not
touch the test area of the strip.
Check the EXP. DATE printed on the
bottle label. If the date has passed,
discard and retest with test strips
from a new bottle. If the bottle has
been opened, check the date you
recorded when you first opened the
bottle and if 6 months have passed,
throw the test strips away and use a
new bottle of KETONE Reagent Strips.
DDoo nnoott uussee pprroodduucctt ((ooppeenneedd oorr
uunnooppeenneedd)) aafftteerr eexxppiirraattiioonn ddaattee.. UUssee
ooff ssttrriippss bbeeyyoonndd tthhe
e eexxppiirraattiioonn ddaattee
mmaayy yyiieelldd llooww rreessuullttss..
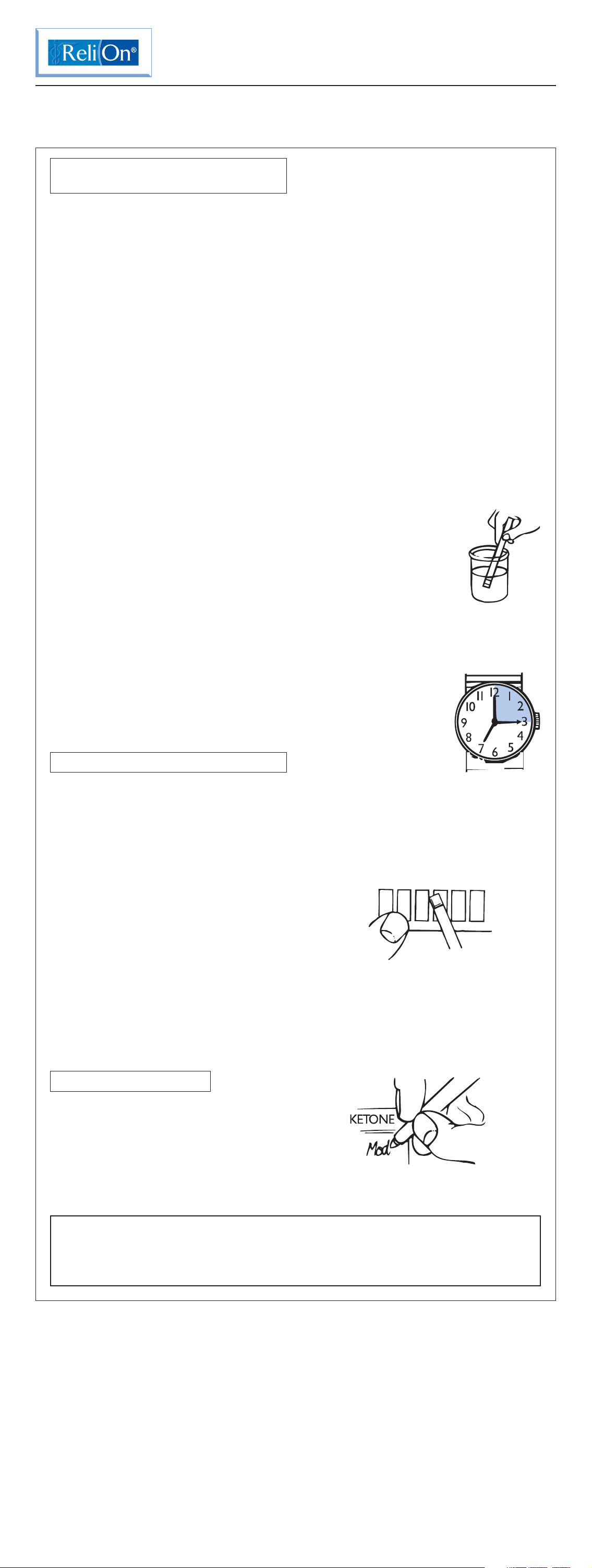
2. Dip the test end of the strip into a
fresh urine sample and
remove it immediately
(drawing edge of strip
against the rim of the
urine container to remove
excess urine), or pass
the test end of the strip
through a stream of urine.
3. Begin timing.
4. AT EXACTLY 15 SECONDS, match the
reagent area to the ketone color chart.
Ignore color changes that occur after
15 seconds.
5. Record the result.
NNOOTTEE::
If the test shows a moderate or
large amount of ketone, call your doctor
or diabetes educator.
If a problem cannot be identified or corrected, call our Customer Service Department, toll free
1-800-348-8100 or consult your doctor, diabetes educator, pharmacist or Self-Care Center for
advice on testing technique and results.
KKeettoonnee RReeaaggeenntt SSttrriippss//
TTeesstt IInnffoorrmmaattiioonn aanndd PPrroocceedduurree
UUrriinnee TTeesstt ffoorr KKeettoonnee ((AAcceettooaacceettiicc AAcciidd))
 Loading...
Loading...