Page 1

Service Guide
IntelliVue Patient Monitor
MX400/MX450/MX500/MX550
Release K.2x.xx
Patient Monitoring
Page 2

Page 3
1Table of Contents
1 Introduction 5
Who Should Use This Guide 5
How to Use This Guide 5
Abbreviations 5
Responsibility of the Manufacturer 6
Passwords 6
Safety Information 7
2 Theory of Operation 11
Integrated Monitor Theory of Operation 11
3 Testing and Maintenance 31
Introduction 31
Terminology and Definitions 31
Recommended Frequency 32
When to Perform Tests 33
Testing Sequence 36
Visual Inspection 36
Safety Tests 37
System Test 52
Preventive Maintenance Procedures 63
Performance Assurance Tests 63
Reporting of Test Results 90
Other Regular Tests 93
Touchscreen Calibration 93
Disabling/Enabling Touch Operation 94
Printer Test Report 94
Battery Handling, Maintenance and Good Practices 95
After Installation, Testing or Repair 102
4 Troubleshooting 103
Introduction 103
How To Use This Section 103
Who Should Perform Repairs 103
Replacement Level Supported 103
Software Revision Check 104
Software Compatibility Matrix 104
Obtaining Replacement Parts 104
Troubleshooting Guide 104
3
Page 4

5 Repair and Disassembly 139
Tools Required 139
Monitor Disassembly 139
Plug-in Modules 182
Multi-Measurement Module (MMS) Disassembly 186
MMS Extensions - Exchanging the Top Cover, MSL Flex Cable and the Dual Link Bar 203
6 Parts 217
MX400/450/500/550 Parts 218
Remote Control Parts 225
Multi-Measurement Module (MMS) Parts 225
MMS Extension Parts (M3012A, M3014A, M3015A/B) 233
IntelliVue X2 Part Numbers 235
Plug-in Modules Part Numbers 235
Smart Battery Charger Part Numbers 241
External Display Part Numbers 241
Test and Service Tools 242
7 Installation Instructions 245
Electromagnetic Emissions 245
Installation Checklist 246
Unpacking the Equipment 246
Initial Inspection 247
Installing the MX400/450/500/550 Monitor 247
Connecting the Monitor to AC Mains 259
8 Site Preparation 289
Introduction 289
Monitor MX400/450/500/550 Site Requirements 292
Electrical and Safety Requirements (Customer or Philips) 293
Remote Device Site Requirements 293
RS232/MIB/LAN Interface 300
Nurse Call Paging Cable 301
ECG Out Interface 302
9 Gas Analyzers 303
10 Specifications 305
Essential Performance Characteristics 305
MDD Classification 308
Safety and Regulatory Information 308
11 IntelliVue MX400-550 Product Structure 309
4
Page 5

1Introduction
This Service Guide contains technical details for the IntelliVue MX400/450/500/550 Patient Monitor,
the measurement modules, the Multi-Measurement Module (MMS), the IntelliVue X2, and the
Measurement Server Extensions.
This guide provides a technical foundation to support installation, effective troubleshooting and repair.
It is not a comprehensive, in-depth explanation of the product architecture or technical
implementation. It offers enough information on the functions and operations of the monitoring
systems so that engineers who install or repair them are better able to understand how they work.
It covers the physiological measurements that the products provide, the Measurement Server that
acquires those measurements, and the monitoring system that displays them.
Who Should Use This Guide
1
This guide is for biomedical engineers or technicians responsible for installing, troubleshooting,
repairing, and maintaining Philips’ patient monitoring systems.
How to Use This Guide
Navigate through the table of contents at the left of the screen to select the desired topic. Links to
other relevant sections are also provided within the individual topics. You can also scroll through the
topics using the page up and page down keys.
Abbreviations
Abbreviations used throughout this guide are:
Name Abbreviation
IntelliVue MX400/450/500/550 Patient Monitor the monitor
Multi-Measurement Module MMS
Measurement Link MSL
Medical Information Bus MIB
IntelliVue G1/G5 Gas Analyzers G1/G5, the gas analyzer
5
Page 6

1Introduction
Responsibility of the Manufacturer
Philips only considers itself responsible for any effects on safety, EMC, reliability and performance of
the equipment if:
• assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by Philips, and
• the electrical installation of the relevant room complies with national standards, and
• the instrument is used in accordance with the instructions for use.
To ensure safety and EMC, use only those Philips parts and accessories specified for use with the
monitor. If non-Philips parts are used, Philips is not liable for any damage that these parts may cause to
the equipment.
This document contains proprietary information which is protected by copyright. All Rights Reserved.
Reproduction, adaptation, or translation without prior written permission is prohibited, except as
allowed under the copyright laws.
Philips Medizin Systeme Böblingen GmbH
Hewlett-Packard Str. 2
71034 Böblingen, Germany
The information contained in this document is subject to change without notice.
Philips makes no warranty of any kind with regard to this material, including, but not limited to, the
implied warranties or merchantability and fitness for a particular purpose.
Philips shall not be liable for errors contained herein or for incidental or consequential damages in
connection with the furnishing, performance, or use of this material.
Passwords
In order to access different modes within the monitor a password may be required. The passwords are
listed below.
CAUTION
Your hospital/organization is responsible that the passwords listed below are revealed to authorized
personnel only.
Monitoring Mode: No password required
Configuration Mode: 71034
Demo Mode: 14432
Service Mode: 1345
Consult the configuration guide before making any changes to the monitor configuration.
6
Page 7

Safety Information
Warnings and Cautions
In this guide:
•A warning alerts you to a potential serious outcome, adverse event or safety hazard. Failure to
observe a warning may result in death or serious injury to the user or patient.
•A caution alerts you where special care is necessary for the safe and effective use of the product.
Failure to observe a caution may result in minor or moderate personal injury or damage to the
product or other property, and possibly in a remote risk of more serious injury.
Electrical Hazards and Interference
WARNING
Grounding: To avoid the risk of electric shock, the monitor must be grounded during operation. If a
three-wire receptacle is not available, consult the hospital electrician. Never use a three-wire to twowire adapter.
Electrical shock hazard: Do not open the monitor or measurement device. Contact with exposed
electrical components may cause electrical shock. Refer servicing to qualified service personnel.
1 Introduction
Leakage currents: If multiple instruments are connected to a patient, the sum of the leakage currents
may exceed the limits given in IEC/EN 60601-1, IEC 60601-1-1, UL 60601-1. Consult your service
personnel.
Radio frequency interference: The monitor generates, uses and radiates radio-frequency energy, and
if it is not installed and used in accordance with its accompanying documentation, may cause
interference to radio communications.
Use Environment
WARNING
Explosion Hazard: Do not use in the presence of flammable anesthetics or gases, such as a
flammable anesthetic mixture with air, oxygen or nitrous oxide. Use of the devices in such an
environment may present an explosion hazard.
Positioning Equipment: The monitor should not be used next to or stacked with other equipment.
If you must stack the monitor, check that normal operation is possible in the necessary configuration
before you start monitoring patients.
Environmental Specifications: The performance specifications for the monitors, measurements and
accessories apply only for use within the temperature, humidity and altitude ranges specified in .
Liquid Ingress: If you spill liquid on the equipment, battery, or accessories, or they are accidentally
immersed in liquid, contact your service personnel or Philips service engineer. Do not operate the
equipment before it has been tested and approved for further use.
7
Page 8

1Introduction
Alarms
Prohibited Environments: The monitors are not intended for use in an MRI environment or in an
oxygen-enriched environment (for example, hyperbaric chambers).
WARNING
• Do not rely exclusively on the audible alarm system for patient monitoring. Adjustment of alarm
volume to a low level or off during patient monitoring may result in patient danger. Remember
that the most reliable method of patient monitoring combines close personal surveillance with
correct operation of monitoring equipment.
• Be aware that the monitors in your care area may each have different alarm settings, to suit
different patients. Always check that the alarm settings are appropriate for your patient before you
start monitoring.
Accessories
WARNING
Philips' approval: Use only Philips-approved accessories. Using other accessories may compromise
device functionality and system performance and cause a potential hazard.
Reuse: Never reuse disposable transducers, sensors, accessories and so forth that are intended for
single use, or single patient use only. Reuse may compromise device functionality and system
performance and cause a potential hazard.
Electromagnetic compatibility: Using accessories other than those specified may result in increased
electromagnetic emission or decreased electromagnetic immunity of the monitoring equipment.
Damage: Do not use a damaged sensor or one with exposed electrical contacts.
Cables and tubing: Always position cables and tubing carefully to avoid entanglement or potential
strangulation.
MR Imaging: During MR imaging, remove all transducers, sensors and cables from the patient.
Induced currents could cause burns.
8
Page 9

Maintenance, Repair and Care
WARNING
Maintenance and Repair:
• Do not maintain or repair the device in patient vicinity.
• Failure on the part of the responsible individual hospital or institution using this equipment to
implement a satisfactory maintenance schedule may cause undue equipment failure and possible
health hazards.
• Performance verification: do not place the system into operation after repair or maintenance has
been performed, until all performance tests and safety tests listed in Testing and Maintenance of
this service manual have been performed. Failure to perform all tests could result in erroneous
parameter readings, or patient/operator injury.
Care and Disinfection:
• To avoid contaminating or infecting personnel, the environment or other equipment, make sure
you disinfect and decontaminate the monitor appropriately before disposing of it in accordance
with your country's laws for equipment containing electrical and electronic parts.
• For disposal of parts and accessories such as thermometers, where not otherwise specified, follow
local regulations regarding disposal of hospital waste.
1 Introduction
9
Page 10

1Introduction
10
Page 11

2Theory of Operation
Integrated Monitor Theory of Operation
The IntelliVue MX400/450/500/550 Patient Monitor:
• displays real-time data
• controls the attached multi-measurement modules
• alarms in the case of patient or equipment problems
• offers limited data storage and retrieval (trending)
• interfaces to the Philips Clinical Network and other equipment
A monitor with just a single integrated measurement module can be connected to additional building
blocks to form a monitoring system with a large number of measurements and additional interface
capabilities and multiple slave displays. These elements cooperate as one single integrated real-time
measurement system.
2
11
Page 12
2 Theory of Operation
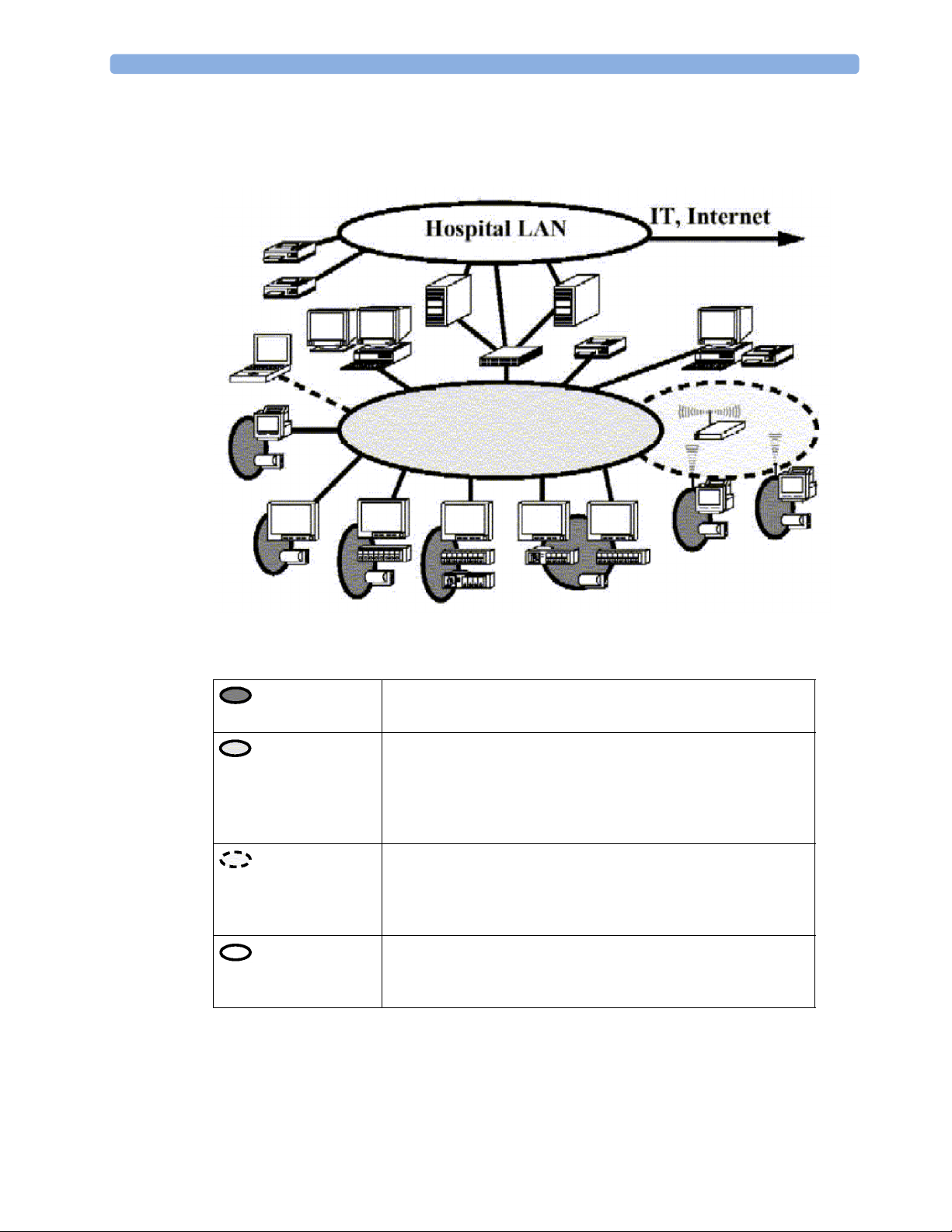
System Boundaries
The following diagram discusses specific boundaries within the overall system with respect to their
openness and real-time requirements:
System Boundaries
Measurement connections
Built-in measurement block
Philips Clinical Network (wired LAN)
connects multiple patient monitors, information centers,
application servers; closed system, only Philips qualified products
(tested and with regulatory approval) are connected, Philips is
responsible for guaranteed real-time functionality and performance
Philips Clinical Network (wireless)
like Philips Clinical Network (wired) LAN, however due to current
wireless technologies available it has reduced bandwidth, longer
latencies, reduced functionality
Hospital LAN, Internet
Standard Network, not under Philips control, no guaranteed
service, no real-time requirements
12
Page 13

Hardware Building Blocks
The following hardware building blocks make up the monitoring system:
IntelliVue MX400/450/500/550
The IntelliVue MX400/450/500/550 Monitor:
• integrates the display and processing unit into a single package
• uses a 9" TFT WVGA Color display (MX400)
• uses a 12" TFT WXGA Color display (MX450/500)
• uses a 15" TFT WXGA Color Display (MX550)
• uses the touch screen as primary input device; a remote control and computer devices such as
mice, trackball, and keyboard can be added optionally.
• has an optional built-in recorder (MX400/450 only)
• has an integrated 3-slot rack (MX500/550 only)
NOTE
The 802.11 Bedside Adapter (WLAN) and IIT are mutually exclusive.
2 Theory of Operation
13
Page 14
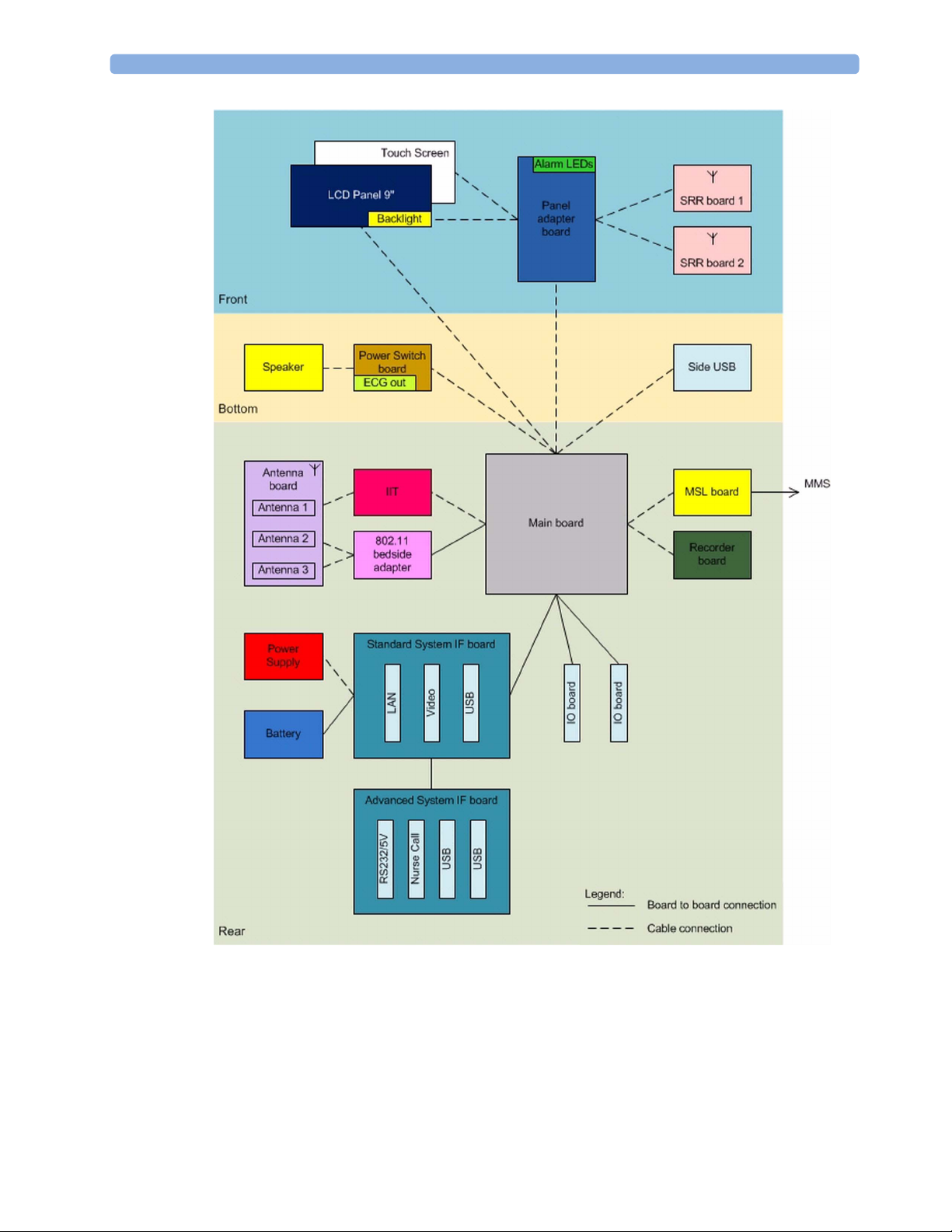
2 Theory of Operation
14
MX400 Hardware Building Blocks
Page 15
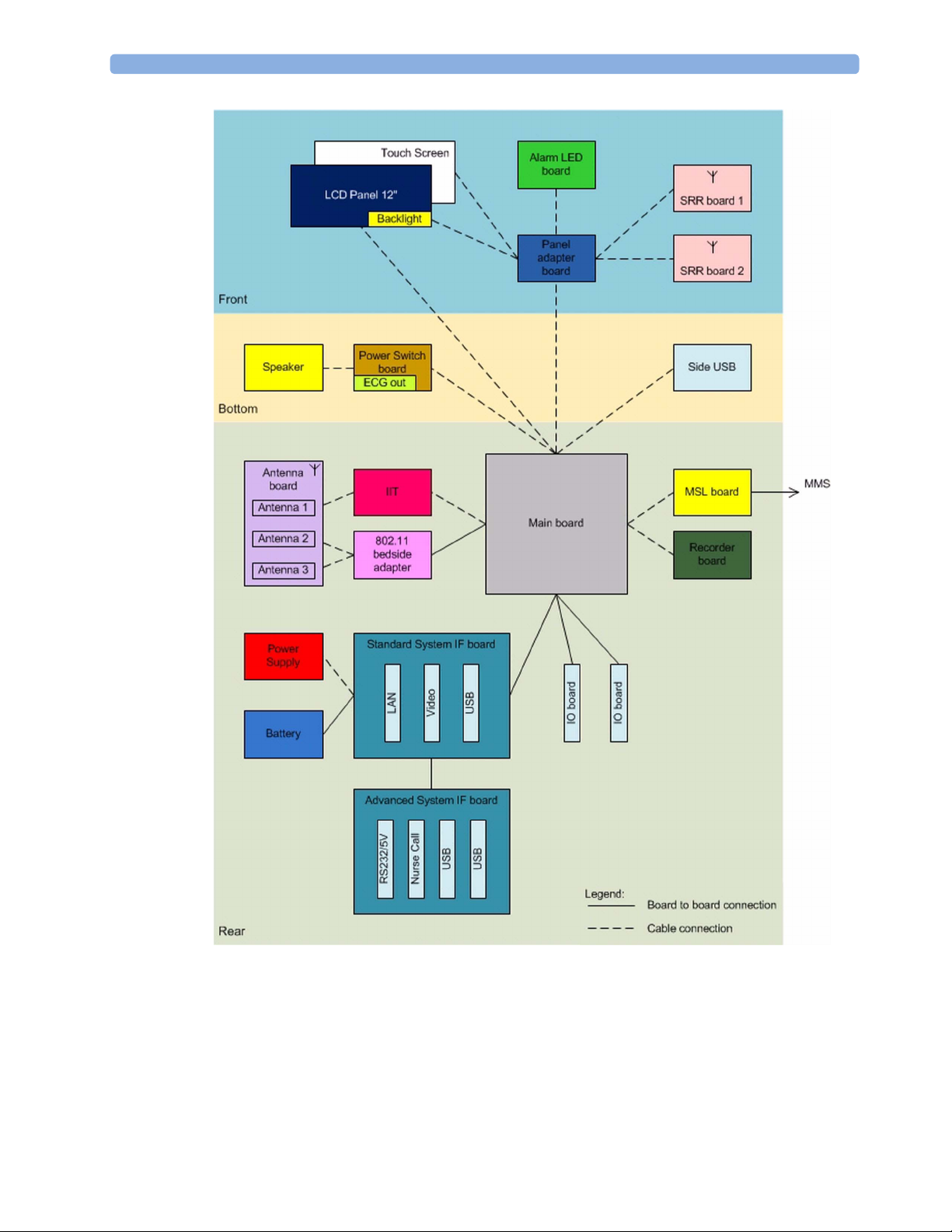
2 Theory of Operation
MX450 Hardware Building Blocks
15
Page 16
2 Theory of Operation
16
MX500/MX550 Hardware Building Blocks
Page 17
Compatible Devices
M3001A Multi-Measurement
Module (MMS)
M3002A IntelliVue X2
2 Theory of Operation
M3012A MMS Extension
M3014A MMS Extension
17
Page 18

2 Theory of Operation
M3015A/B MMS Extension
865244 Remote Control
18
Page 19
Power Supply
2 Theory of Operation
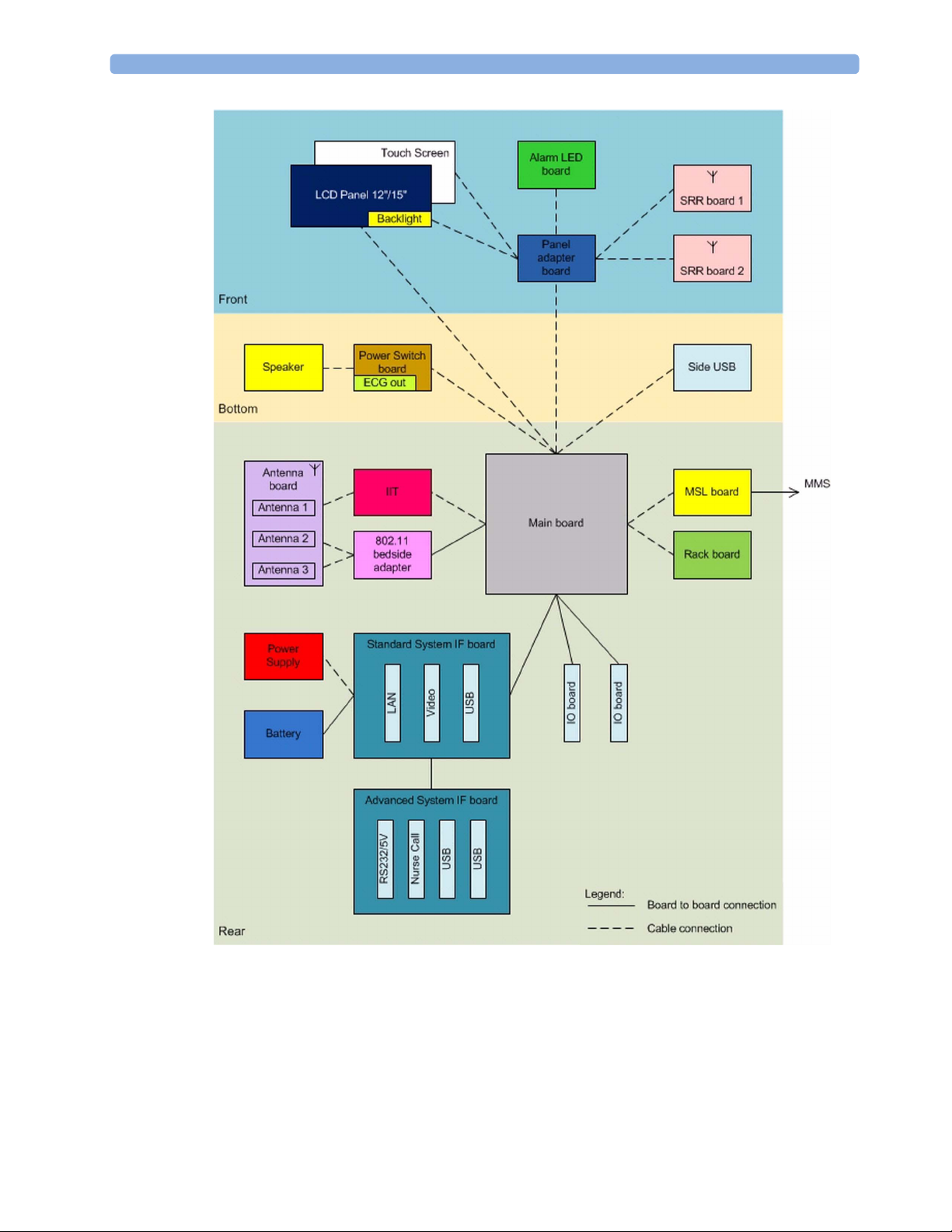
Main Board
I/O Boards
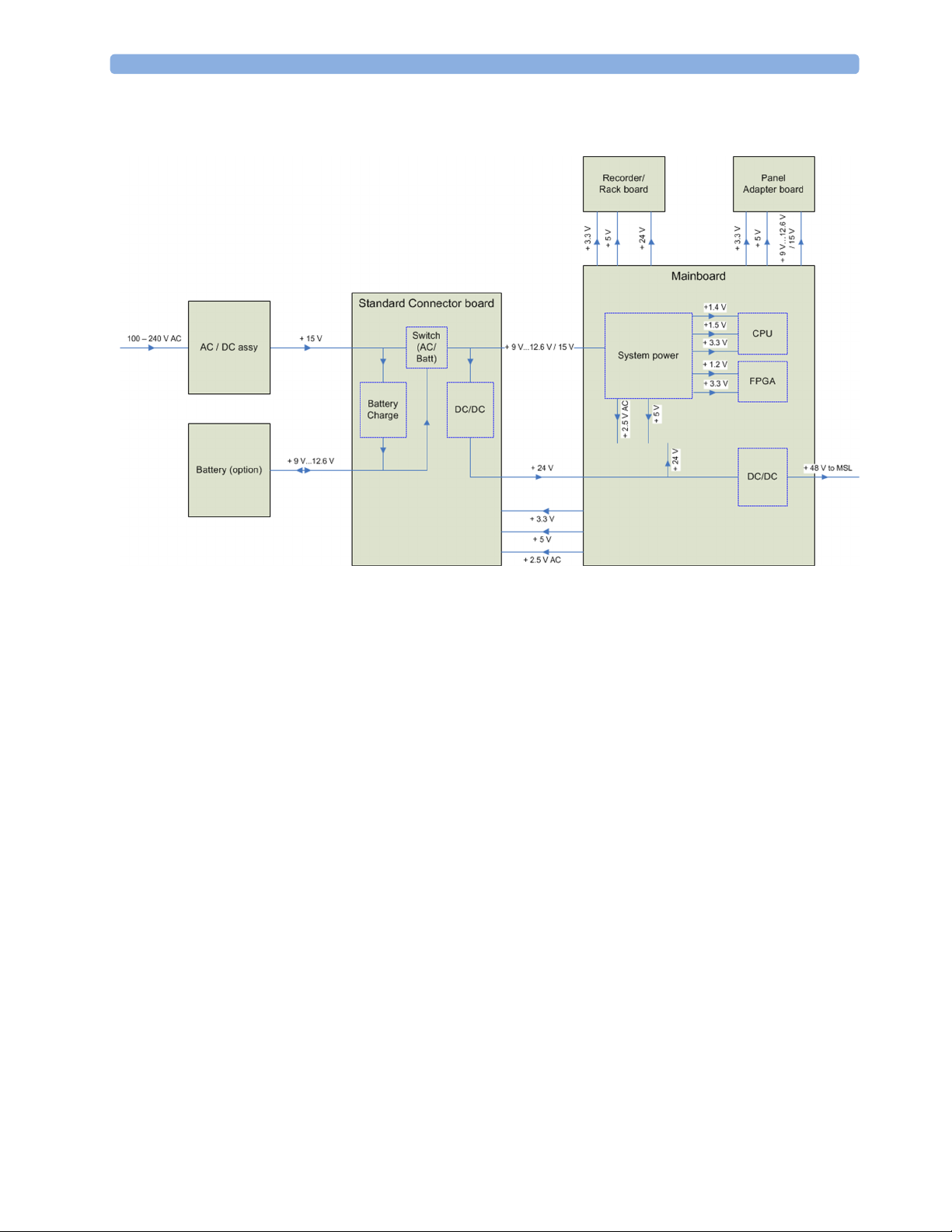
The AC/DC converter transforms the AC power coming from the plug into 15V/ 70W DC source
and isolates the monitoring system from the AC power mains. The 15V is distributed via power bus
either directly or over additional converters to all components of the system:
The battery charger is supplied with 15V and switches between AC/DC supply and battery depending
on whether AC power cord is plugged or unplugged.
The 48V DC power needed for the MSL is created by an isolating DC/DC converter.
The LED backlight converter located on the panel adapter board is supplied with 9V - 12.6V / 15V.
The isolated interfaces are supplied with 2.5V AC. The main board is supplied with 5V, 3.3V, 1.5V,
1.4V and 1.2V.
Additionally, for some infrastructural functions 3.6V is provided to the main board.
The main board contains the CPU which includes the graphic processing unit and USB controller. The
main memory, a system FPGA, a system controller including watchdogs and various power supplies
are located on this board. Additionally, this board contains the MSL interface, the recorder interface,
the ECG Out hardware and various other interfaces.
System information is stored in serial EEPROMs to support the automatic configuration of the
operating system at boot time.
A dual MIB/RS232 board, a Flexible Nurse Call Relay board or an IntelliBridge board can be added
optionally.
19
Page 20
2 Theory of Operation
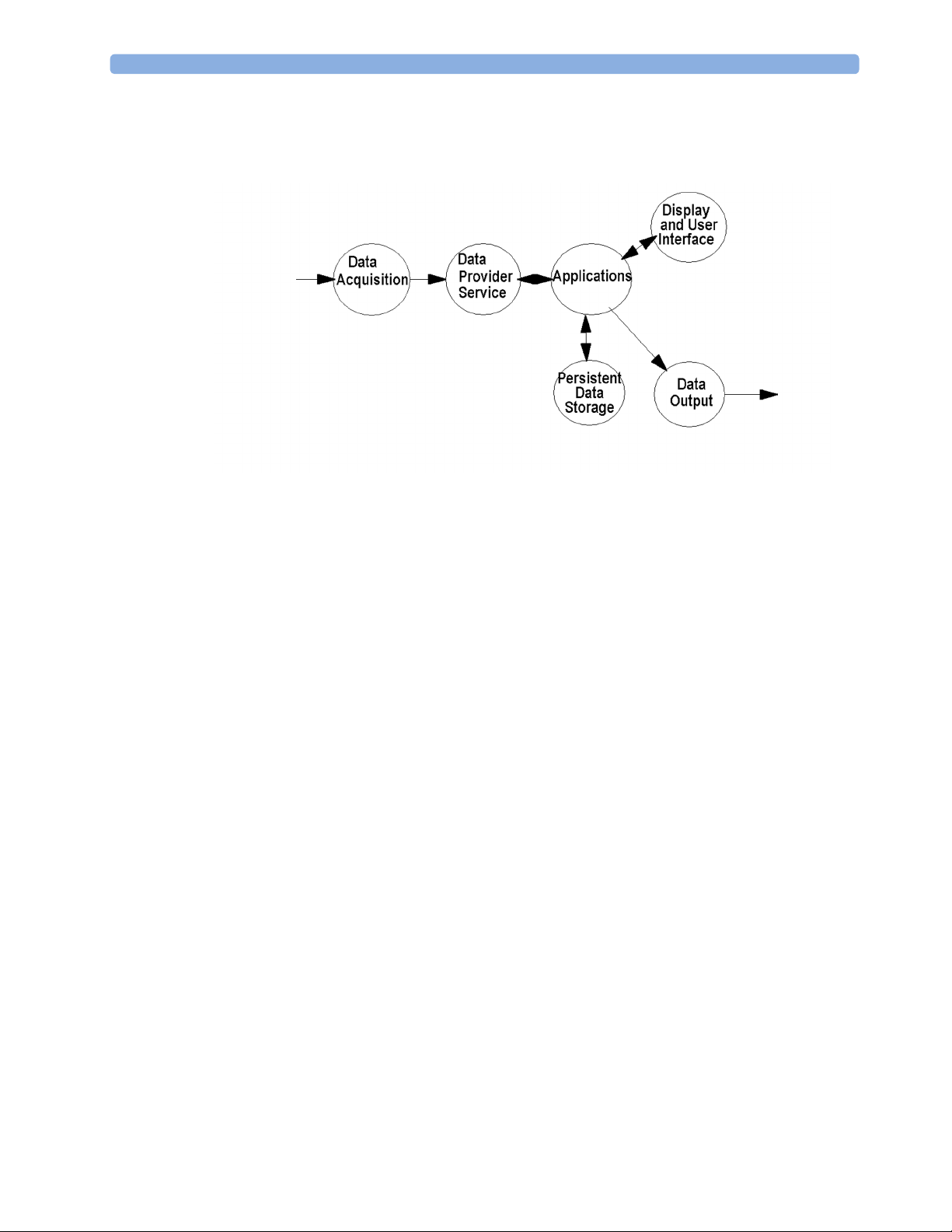
Data Flow
The following diagram shows how data is passed through the monitoring system. The individual stages
of data flow are explained below.
Data Flow
Data Acquisition
Monitoring data (for example patient measurement data in the form of waves, numerics and alerts) is
acquired from a variety of sources:
• Measurement Servers
The Measurement Servers connected to the internal LAN convert patient signals to digital data
and apply measurement algorithms to analyze the signals.
• External measurement devices
Data can be also acquired from devices connected to interface boards of the monitor. Software
modules dedicated to such specific devices convert the data received from an external device to
the format used internally. This applies to parameter modules and the Anesthetic Gas Module.
• Server systems on the Philips Clinical Network
To enable networked applications such as the other bed overview, data can be acquired from server
systems attached to the Philips Clinical Network, for example a Philips Information Center
Data Provider System Service
All data that is acquired from measurement servers or external measurement devices is temporarily
stored by a dedicated data provider system service. All monitor applications use this central service to
access the data in a consistent and synchronized way rather than talking to the interfaces directly.
This service makes the applications independent of the actual type of data acquisition device.
The amount of data stored in the data provider system service varies for the different data types. For
example several seconds of wave forms and the full set of current numerical values are temorarily
stored in RAM.
20
Page 21

Persistent Data Storage System Service
Some applications require storage of data over longer periods of time. They can use the persistent data
storage system service. Dependent on the application requirements, this service can store data either in
battery backed-up (buffered) memory or in flash memory. The buffered memory will lose its contents
if the monitor is without power (not connected to mains) for an extended period of time. The flash
memory does not lose its contents.
The trend application for example stores vital signs data in a combination of flash memory and
buffered memory, while the system configuration information (profiles) is kept purely in flash
memory.
Display and User Interface Service
Applications can use high level commands to display monitoring data or status and command windows
on the internal LCD panel. These commands are interpreted by the display manager application. This
application controls the dedicated video hardware.
User input is acquired from a variety of input devices, for example the touchscreen or other standard
input devices (keyboard, mouse). The system software makes sure that the user input is directed to the
application which has the operating focus.
2 Theory of Operation
Data Output
The monitoring system is very flexible and customizable regarding its data output devices. Built-in
devices (for example LAN, video) provide the basic output capabilities.
These capabilities can be enhanced by adding additional I/O boards, as required in the specific enduser setup. The additional I/O boards typically provide data to externally attached devices, for example
to RS232 based data collection devices.
The monitor can identify I/O boards by means of a serial EEPROM device that stores type and
version information. The operating system detects the I/O boards and automatically connects them
with the associated (interface driver) application. For some multi-purpose boards it is necessary to
configure the board for a particular purpose first (for example the MIB/RS232 board can support
external touch display , data import, data export).
Monitor Applications
The monitor applications provide additional system functionality over the basic measurement and
monitoring capabilities. This includes for example trending, report generating, event storage or derived
measurements.
In general, the monitor applications use the data provider system service to access the measurement
data. Application interfaces to the other system services allow the application to visualize data, to store
data over extended periods of time or to output data to other devices.
Internal LAN (Measurement Link)
The monitor and multi-measurement modules communicate using an IEEE802.3/ Ethernet LAN in
the Measurement Link (MSL). This network is used to distribute data between the components, for
example:
• Digitized patient signals including wave data, numerical data and status information (typically from
the measurement server to a display unit)
21
Page 22
2 Theory of Operation
• Control data representing user interactions (typically from the display unit to a measurement
server)
• Shared data structures, for example representing patient demographical data and global
configuration items
The internal LAN allows plug and play configuration of the monitoring system. The system
automatically detects plugging or unplugging of measurement servers and configures the system
accordingly.
The components on the internal LAN are time-synchronized to keep signal data consistent in the
system. Dedicated hardware support for synchronization eliminates any latency of the network driver
software.
The integrated LAN provides deterministic bandwidth allocation/reservation mechanisms so that the
real-time characteristic of signal data and control data exchange is guaranteed. This applies to the data
flow from the measurement server to the monitor (for example measurement signal data) and the data
flow from the monitor to a measurement server (for example to feed data to a recorder module).
Philips Clinical Network
The monitoring system may be connected to the Philips Clinical Network, for example to provide
central monitoring capabilities or other network services. This connection may be through a normal
wired connection or through a wireless connection.
The monitor supports the connection of an internal wireless adapter (#J35). Switching between wired
and wireless networks is automatically triggered by the plugging or unplugging of the network cable.
The Philips Clinical Network protocols function very similarly to the protocols used on the internal
LAN.
After configuration, the monitoring system sends the digitized patient signals including wave data,
numerical data and status information onto the network. Control data representing user interactions
can be exchanged between the monitoring system and a central station bi-directionally.
Additional protocols are supported for networked applications, for example for the other bed
overview function, which allows viewing of monitoring data from other patients on the network.
For plug and play operation, the monitoring system uses the standard BootP protocol to automatically
acquire a network address.
Ambient Light Sensor
22
The monitor adjusts its display brightness depending on the ambient light level. Therefore an Ambient
Light Sensor is integrated in the front bezel of the display.
Page 23
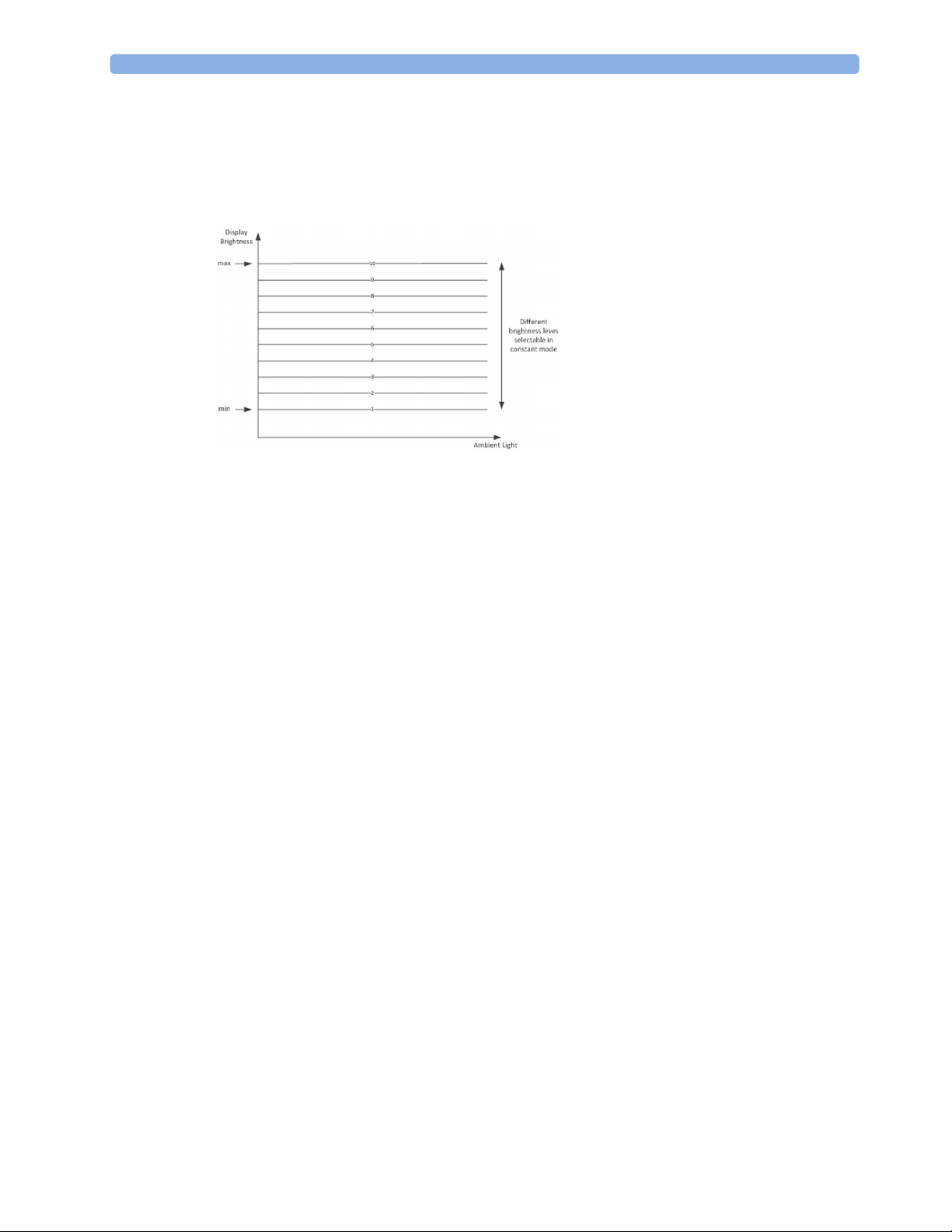
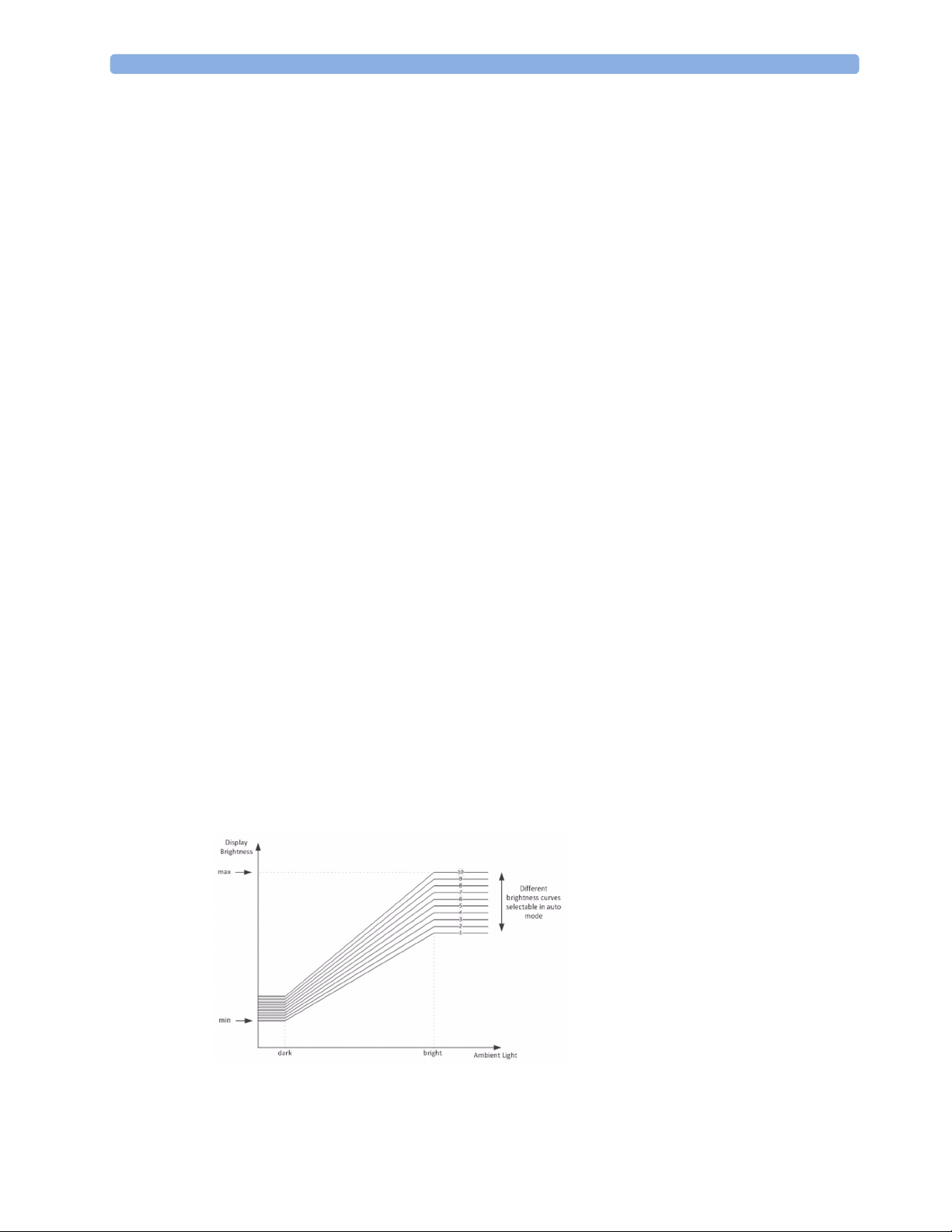
Although there is an automatic brightness adjustment, it is still possible for the user to change the
brightness. As shown in the figure above, the user can select between different brightness level curves.
If a constant brightness is desired, it is possible to deactivate the automatic brightness control via the
Config mode of the monitor. Without automatic brightness control, the user can select between
different constant brightness levels as shown below.
Microstream CO2
CO2 sample rate: 20 samples/second
2 Theory of Operation
Calculation of end tidial CO2 (etCO2)
The M3015A/B MMS Extensions use Microstream® non–dispersive infrared (NDIR) spectroscopy
to continuously measure the amount of CO2 during every breath, the amount of CO2 present at the
end of exhalation (etCO2), the amount of CO2 present during inhalation (imCO2), and the respiratory
rate. The displayed etCO2 is the maximum etCO2 over the previous peak-picking interval as defined
by the Max Hold setting (configuration mode). It can be set to no peak picking (off), 10 seconds and
20 seconds.
Test method for respiration rate range
A breath simulator system combined with CO2 and N2 gases was used to simulate respiration rates
covering the specified range. The resulting end tidal CO2 values were compared to the expected value.
Differences between actual and expected end tidal CO2 values were within the limits of the specified
accuracy for the respective respiration rate, i.e. there was no effect of the respiration rate on the end
tidal CO2 values beyond those limits.
How does the Support Tool Work with the Monitor
The Support Tool Mark2 is a Windows application typically installed on the laptop of a customer
engineer or a biomedical engineer working in the customer’s own service department.
The purpose of the Support Tool Mark2 is to upgrade, configure and diagnose all monitoring
components (modules, measurement servers, and monitors) in the system over the network.
The tool allows access to internal service information and to serial numbers. It can be remotecontrolled, for example via a dial-up connection from a response center, provided the proper
infrastructure is in place.
For details see the Instructions for Use for the Support Tool Mark2.
23
Page 24

2 Theory of Operation
Monitor Software Block Diagram
Block Diagram Legend
Functional Block Description
Services
Operating System The Operating System (OS) provides a layer of isolation between
the specific hardware implementation and the application
software. The OS performs system checks and allocates resources
to ensure safe operation when the system is first started. This
includes internal self-tests on several hardware modules and
configuration checks for validity of configuration with the
operating software. During normal operation, the OS continues to
run checks on system integrity. If error conditions are detected the
OS will halt monitoring operations and inform the operator about
the error condition.
24
Page 25
2 Theory of Operation
Functional Block Description
System Services The System Services provide generic common system services.
In particular:
They use a real-time clock component to track time. They
synchronize to network time sources and verify the accuracy of the
system time information. They are also responsible for managing
persistent user configuration data for all Measurement Servers and
IntelliVue Patient Monitoring System software modules. User
configuration data is stored in a non-volatile read/write storage
device
Applications
Reports The Reports Service retrieves current and stored physiological
data and status data to format reports for printing paper
documentation. The following reports are supported:
• Vital Signs Report
• Graphical Trend Report
• Event Review Report
• Event Episode Report
• ECG Report (12 Lead/Multi-Lead)
• Cardiac Output Report
• Calculations Report (Hemodynamic/Oxygenation/
Ventilation)
• Calculations Review Report
•Wedge Report
•Test Report
• Other reports (e.g. Loops, Review Applications, Drug report)
The Reports service generates report data which can be printed on
a local or a central printer.
Record The Record Service retrieves current and stored physiological data
and status data to format a continuous strip recording. A recording
can be triggered manually by the operator or automatically by an
alarm condition. The Record Service can also send data to a
recorder.
25
Page 26
2 Theory of Operation
Functional Block Description
Alarm The Alarm Service contains logic that prioritizes alarm conditions
Trend The Trend service stores the sample values of physiological data
OxyCRG The OxyCRG (Oxygen CardioRespiroGram) service derives a
ADT The ADT (Admit/Discharge/Transmit) service maintains the
Events The Events Application captures physiological data from episodes
that are generated either by the Measurement Servers or by
IntelliVue Patient Monitoring System software modules. Visual
alarm signals (messages) are displayed at the top of the IntelliVue
Patient Monitoring System display and alarm sounds are generated
by a loudspeaker. Alarm conditions may be generated when a
physiological parameter exceeds preselected alarm limits or when a
physiological parameter or any other software module reports an
inoperative status (technical alarm, for example, the ECG leads
may have fallen off the patient). The Alarm service manages the
alarm inactivation states, for example suspension of alarms,
silencing of alarms, and alarm reminder. Alarm signals may also be
configured as latching (alarm signals are issued until they are
acknowledged by the operator, even when the alarm condition is
no longer true). The Alarm service controls the visual alarm
signals (alarm lamps).
and status data with a resolution of 12 seconds, 1 minute or 5
minutes for a period of up to 48 hours. The data is kept in battery
buffered read/write storage and flash memory devices to be
preserved across power failures. The stored data is protected via
consistency checks and checksums. When a new patient is
admitted, the trend database erases all data of the previous patient.
high-resolution trend graph from the Beat-to-Beat Heart Rate,
SpO2 or tcpO2, and Respiration physiological data. The OxyCRG
is specialized for neonatal applications, allowing the operator to
identify sudden drops in Heart Rate (Bradycardia) and SpO2 or
tcpO2 (Desaturations), and supporting the operator in visualizing
Apnea situations.
patient demographics information. The operator may admit a new
patient, discharge the old patient and enter or modify the patient
demographics. The ADT service also supports the transport of a
patient (trend database) with the M3001A Multi-Measurement
Module. The ADT service controls the deletion of old patient
data, the upload of trend data from the M3001A and the switching
back of all settings to user defaults. It also synchronizes patient
information with a central station on the network.
for later review and documentation purposes. Events can be
triggered automatically by an alarm condition, by user-defined
conditions or manually by the operator.
26
Page 27
2 Theory of Operation
Functional Block Description
Protocol Watch ProtocolWatch allows the execution of pre-defined clinical
protocols in the IntelliVue patient monitor by combining events
such as automatically triggered events, time and manually triggered
events with textbook knowledge thus aiding the operator to follow
clinical guidelines. ProtocolWatch notifies the operator when
certain combinations of clinical conditions occur and it documents
the developments and clinician actions in a log which can be
reviewed on the monitor and documented on a printer.
Calc Param The Calc Param (Calculated Parameters) service accesses current,
stored and manually entered physiological data as input to
calculation formulas. With these formulas, derived hemodynamic,
oxygenation and ventilation variables are computed. The
calculation results, including the input parameters, are stored for
later review using the Trend service.
Heart Mgr. The Heart Manager Application allows the selection of the
alarming source to be either heart rate (from ECG) or the system
pulse rate. The system pulse rate can be chosen from any of the
possible pulse rate sources (e.g., SpO2 and invasive pres-sures).
The module implements automatic fall-backs when selected signal
sources are not available.
Drug Calc The Drug Calc application aids in calculating drug dosages for
patients.
EGM EGM (extensible Gas Module) interface aneasthesia gas
measurement devices. The EGM Module interfaces the M1013A
or M1019A Gas Analyzer devices. The EGM Module retrieves the
measurement data and controls the external device. It provides
numerical data, wave form data and alarm data for the gas
parameters measured by the attached analyzers.
PV Loops The PV Loops application compares graphic representations of
airway waves to help detect changes in the patient airway
condition.
Interface Managers
MDSE The MDSE (Medical Data Service Element) Interface Manager is
responsible for the exchange of real-time data between the
IntelliVue Patient Monitoring System display unit and the
Measurement Servers as well as between the IntelliVue Patient
Monitoring System display unit and other devices attached to the
network. MDSE establishes and maintains a data communication
link between the devices. It provides configuration information
about the remote device to applications in the local device and it
allows the exchange of measurement data and status information
between the devices.
27
Page 28
2 Theory of Operation
Functional Block Description
Printer The Printer Interface Manager provides a high level interface to a
Display & Operator Interface The Display and Operator Interface Manager performs the
printer. It provides means to:
• establish a connection to the printer
• transfer data to the printer
• get status of the printer
• close connection to the printer
The Printer Interface Manager also supervises the connection to
the printer and whether the printer accepts data (for example
paper out). The Printer Interface Manager notifies the operator in
such cases.
following tasks:
• Screen presentation of real-time and stored physiological
measurement data, alarm condition data and status
information received from the MDSE interface manager, the
Alarm service or other IntelliVue Patient Monitoring System
modules
• Screen presentation of operating controls (control windows)
• Processing of operating control commands received from
HIF Control interface. The module verifies and interprets the
received commands and forwards them to other software
modules of the IntelliVue Patient Monitoring System display
unit or Measurement Servers
• Sound generation (issues audible alarm signals and generates
audible information signals, for example QRS and SpO2
tones, operator audible feedback)
LabData/Manual Data The Laboratory Data/ Manual Data Entry Interface Manager
allows acquisition of laboratory data (e.g. acquired by the central
station from a laboratory information system). It also allows to
manually enter measurement data to make additional, manually
acquired measurements available to internal applications and to
the system.
Wireless Measurement
Manager (WMM)
The WMM Interface Manager provides connectivity to the SRR
interface. It establishes communication between SRR enabled
devices and the ASW module that manages the data provided by
the device
Interfaces
LAN The LAN interface implements the physical layer of IEEE 802.3,
electrical isolation, and ESD protection. Electronically separated
interfaces are used for communication to the Measurement
Servers and to the network.
WLAN The WLAN Interface is a network interface that provides access
to an IEEE 802.11 wireless Local Area Network. The
configuration of this interface is done by an OS Service.
28
Page 29
2 Theory of Operation
Functional Block Description
Display Controller The display controller is integrated into the CPU. The video RAM
is shared with the main memory. The display controller processes
the high level display commands (character and graphic
generation, wave drawing) and translates them into pixels, which
are written into the video RAM, where the display controller
generates the video synchronization signals and the pixel stream
for the internal and external display.
HIF Control The HIF (Human Interface Control) interface scans the Human
Interface devices for operator controls (Touch Screen, and USB
devices), formats the collected data and sends it to the display and
Operating Interface.
ECG-Out The ECG Out interface receives the ECG waveform directly from
the ECG/Resp Arrhythmia ST-Segment physiological algorithm
via an RS-422 serial interface and converts the digital ECG signal
to an analog ECG signal.
Sync Out (ECG) A pulse signal is provided on the Sync Out connector to allow
synchronization with other medical devices.
RS-232 The RS-232 component represents a generic serial communication
interface to connect external devices as shown in the diagram, also
providing power in MP5, MX400/450/500/550.
RS-422 The serial link RS-422 interface communicates the ECG signal to
the ECG Output of the IntelliVue Patient Monitoring System
display unit. The interface is a serial, differential, full-duplex link.
The interface is ESD protected.
Nurse Call The Nurse Call has a modular jack 6P6C connector. The
connector has an open and close contact on alarm.
MIB The MIB interface allows full-duplex, short-haul asynchronous
binary communication between the monitor and an arbitrary
(medical/non-medical) device using an eight-pin RJ45 modular
connector. Switching between MIB and RS232 protocol is
possible.
IIT Interface The IIT Interface allows operation of the monitors with IntelliVue
Instrument Telemetry
SRR The SRR interface allows operation of the monitor with an
IntelliVue Remote Control.
USB Interface The USB interface allows connection of USB devices (Mouse,
Keyboard, Barcode Scanner, Printer) to the monitor.
Remote Control The remote control allows remote operation of the monitor via a
USB cable or SRR connection.
29
Page 30

2 Theory of Operation
30
Page 31

3Testing and Maintenance
Introduction
This chapter provides a checklist of the testing and maintenance procedures to ensure the performance
and safety of the monitor, the Multi-Measurement Module (MMS), the MMS Extensions and the
parameter modules.
These tests must be performed only by qualified personnel certified by the responsible organization.
Qualifications required are: training on the subject, knowledge, experience and acquaintance with the
relevant technologies, standards and local regulations. The personnel assessing safety must be able to
recognize possible consequences and risks arising from non-conforming equipment.
All recurring safety and performance assurance tests must be performed under equal environmental
conditions to be comparable.
3
Preventive Maintenance refers specifically to the series of tests required to make sure the measurement
results are accurate. The accuracy and performance procedures are designed to be completed as
specified in the following sections or when readings are in question.
For detailed instructions on the maintenance and cleaning of the monitor and its accessories, see Care
and Cleaning, Using Batteries and Maintenance and Troubleshooting in the monitor's Instructions for Use.
Terminology and Definitions
The following terms and definitions are used throughout this chapter and taken from the international
standards IEC 60601-1, IEC 60601-1-1 and IEC 62353.
• Medical System: a medical electrical system is a combination of at least one medical electrical
device and other electrical equipment, interconnected by functional connection or use of a
multiple portable socket-outlet.
• Patient Environment: any area in which intentional or unintentional contact can occur between
the patient and parts of the medical system or between the patient and other persons who have
had contact with parts of the medical system. The patient environment is defined anywhere within
1.5m (5 feet) of the perimeter of the patient's bed and 2.5m (8.2 feet) from the floor.
• Separation Device/Transformer: a component or arrangement of components with input parts
and output parts that, for safety reasons, prevent a transfer of unwanted voltage or current
between parts of a medical system.
• Multiple Portable Socket-Outlet: a combination of two or more socket-outlets intended to be
connected to or integrated with flexible cables or cords, which can easily be moved from one place
to another while connected to the power mains.
31
Page 32

3 Testing and Maintenance
• Functional Connection: an electrical connection for transfer of signals and/or power.
• Tests: Safety or Performance Assurance test procedures which may consist of several steps.
Recommended Frequency
Perform the procedures as indicated in the suggested testing timetable. These timetable
recommendations do not supersede local requirements.
Table 1 Suggested Testing Timetable
Tests Frequency
Preventive
Maintenance*
Other Regular Tests
Performance Assurance
Tests
NBP Performance Once every two years, or more often
if specified by local laws.
Microstream CO2 Calibration Once a year or after 4000 hours of
continuous use and following any
instrument repairs or the replacement
of any instrument parts.
Visual Inspection Before each use.
Power On Test
ECG/Resp Performance Once every two years, or if you
ECG Out Performance
SpO2 Performance
NBP Performance
Invasive Pressure Performance
Temperature Accuracy
M3014A Capnography Extension
Performance Tests
Microstream CO2 Performance
Test
Spirometry Accuracy Test
C.O. Performance
NMT Performance
IntelliBridge Performance Test
Nurse Call Relay Performance
MSL Assurance Test
Power Loss Alarm Buzzer
Performance Test
Recorder M1116C Performance
Test
Mounting Integrity Test
suspect the measurement is incorrect,
except Mainstream CO
Check, Sidestream CO
Check and Flow Check - required
once a year.
Accuracy
2
Accuracy
2
32
Page 33

3 Testing and Maintenance
Tests Frequency
Safety Tests Visual
Electric
al
NOTE
The EEG parameter does not require performance testing. See “EEG, SvO2 (SO2) and tcGas
Performance Tests” on page 84 for details.
Visual Inspection After each service event
Protective Earth Once every two years and after
Equipment Leakage Current
Applied Part Leakage Current
System Test Once every two years
When to Perform Tests
This table tells you when to perform specific tests.The corresponding test procedures are described in
the following sections All tests listed below must be performed on the monitor itself and any
attached MMS/X2 and parameter modules.
Table 2 When to perform tests
Service Event
(When performing...
repairs where the power supply has
been removed or replaced or the
monitor has been damaged by impact.
Tests Required
...Complete these tests)
Installation
Installation of a monitor in combination with a
medical or non-medical device connected to the
same multiple socket outlet.
Installation of a monitor with no display
connected to the video output
Installation of a monitor with a medical display
specified by Philips
Installation of a monitor with an off-the-shelf
display (non-compliant with IEC 60601-1)
Installation of a monitor with IntelliVue G1/
G5, connected to separate mains sockets.
Installation of a monitor with an IntelliBridge
connection to another medical device (compliant
with IEC 60601-1), connected to separate mains
sockets.
Installation of a monitor with recorder module
M1116C
Installation of a monitor with IT equipment e.g.
printer, PC connected via a functional connection
USB.
Perform Visual Inspection, Power On and System
Tests
Perform Visual Inspection and Power On Test
Perform Visual Inspection and Power On Test
Perform Visual Inspection, Power On and System
Test (per each affected video port)
Perform Visual Inspection and Power On Tests
Perform Visual Inspection and Power On Tests
Perform Visual Inspection, Power On and
Recorder Performance Test
Perform Visual Inspection, Power On and System
Tests
33
Page 34

3 Testing and Maintenance
Service Event
(When performing...
Installation of monitor with IntelliVue 802.11
Bedside Adapter
Tests Required
...Complete these tests)
Perform Visual Inspection, Power On and
IntelliVue 802.11 Bedside Adapter
Communication Test
Installation of a monitor with IntelliVue
Instrument Telemetry
Installation of monitor with Short Range Radio
(SRR)
Perform Visual Inspection, Power On and IIT
Communication Test
Perform Visual Inspection, Power On and SRR
Communication Test.
Installation of networked monitor (LAN) Perform Visual Inspection and Power On Test
Preventive Maintenance
Preventive Maintenance* Perform preventive maintenance tests and
procedures:
NBP calibration
Microstream CO
calibration
2
Other Regular Tests and Tasks
Visual Inspection Perform Visual Inspection
Power On Test Perform Power On test
Repairs
Repairs where the monitor, parameter modules,
MMS or X2 have been damaged by impact, liquid
Perform Visual Inspection, Power On, all Safety
Tests and Full Performance Assurance Tests
ingression, fire, short circuit or electrical surge.
Repairs where the power supply, the mains
socket or an interface board of the monitor is
Perform Visual Inspection, Power On, all Safety
Tests and Basic Performance Assurance Test
removed or replaced or the protective earth
ground connection is disrupted.
Repairs of IntelliVue 802.11 Bedside Adapter Perform Visual Inspection, Power On and
IntelliVue 802.11 Bedside Adapter
Communication Test
Repairs of IntelliVue Instrument Telemetry (IIT)
Module
Perform Visual Inspection, Power On and IIT
Communication Test
Repairs of Short Range Radio (SRR) Interface Perform Visual Inspection, Power On and SRR
Communication Test
Repairs of the parameter modules, MMS or X2
(all service events where the parameter modules
MMS or X2 have been opened)
Perform Visual Inspection, Power On, all Safety
Tests and Basic Performance Assurance Test.
If a certain parameter seems suspicious, perform
Full Performance Assurance Test for this
parameter.
Repairs where the NBP pump of the MMS or
X2 has been replaced
Perform Visual Inspection, Power On, all Safety
Tests, Basic Performance Assurance Test and NBP
Performance Test and Calibration
Repairs where the parameter module, MMS or
X2 has been replaced.
Perform Visual Inspection, Power On and Basic
Performance Assurance
34
Page 35

3 Testing and Maintenance
Service Event
(When performing...
Repairs where the recorder module M1116C has
been replaced or repaired.
Tests Required
...Complete these tests)
Perform Visual Inspection, Power On and
Recorder Performance Test
Repairs of the IntelliVue G1/G5 Perform Basic Performance Assurance Test. For
further testing requirements, see IntelliVue G1/
G5 Service Guide
Repairs where the printer connected to the
monitor via connector board has been replaced.
All other IntelliVue Monitoring System repairs
(except when power supply is removed)
Perform Visual Inspection, Power On, System
Test and Printer Test.
Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test
Performance Assurance
Basic Performance Assurance Perform basic performance assurance tests for the
respective monitoring system component.
Full Performance Assurance Perform all accuracy and performance test
procedures listed in the following sections. If a
particular measurement is in question, perform the
measurement performance test only.
Upgrades
Software Upgrades Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test unless
otherwise specified in the Upgrade Installation
Notes shipped with the upgrade.
Hardware Upgrades Perform Visual Inspection, Power On Test and
Basic Performance Assurance Test unless
otherwise specified in the Upgrade Installation
Notes shipped with the upgrade.
Hardware Upgrades where IntelliVue 802.11
Bedside Adapter is installed
Perform Visual Inspection, Power On Test, Basic
Performance Assurance Test and IntelliVue 802.11
Bedside Adapter Communication Test
Hardware Upgrades where IntelliVue
Instrument Telemetry (IIT) is installed
Perform Visual Inspection, Power On Test, Basic
Performance Assurance Test and IIT
Communication Test
Hardware Upgrades where Short Range Radio
(SRR) is installed
Perform Visual Inspection, Power On Test, Basic
Performance Assurance Test and SRR
Communication Test
Installation of Interfaces or Hardware Upgrades
where the power supply of the monitor or
Perform Visual Inspection, Power On Test, Basic
Performance Tests and all Safety Tests
interface boards of the monitor need to be
removed.
Combining or Exchanging System
Components (non-medical equipment
Perform the System Test for the respective system
components
connected to an IntelliVue monitor or medical
system equipment operated on a multiple socket
outlet)
35
Page 36

3 Testing and Maintenance
NOTE
It is the responsibility of the facility operator or their designee to obtain reference values for recurring
safety and system tests. These reference values are the results of the first test cycles after an installation.
You may also purchase this service from Philips.
Testing Sequence
Summary of the recommended sequence of testing:
NOTE
If any single test fails, testing must be discontinued immediately and the device under test must be
repaired or labeled as defective.
Visual Inspection
Before Each Use
Check all exterior housings for cracks and damage. Check the condition of all external cables,
especially for splits or cracks and signs of twisting. If serious damage is evident, the cable should be
replaced immediately. Check that all mountings are correctly installed and secure. Refer to the
instructions that accompany the relevant mounting solution.
36
Page 37

After Each Service, Maintenance or Repair Event
Check:
• the integrity of mechanical parts, internally and externally.
• any damage or contamination, internally and externally
• that no loose parts or foreign bodies remain in the device after servicing or repair.
• the integrity of all relevant accessories.
Power On Test
1 Connect the monitoring system to mains and switch it on. This includes connected displays, MMS,
MMS Extensions, X2, and parameter modules, gas analyzers and IntelliBridge devices.
2 Make sure that all steps listed in the table Initial Instrument Boot Phase in the Troubleshooting section
are completed successfully and that an ECG wave appears on the screen.
The expected test result is pass: the monitor boots up and displays an ECG wave. The wave might be
a flat line if no simulator is attached.
Safety Tests
3 Testing and Maintenance
Safety tests are comprised of the following tests performed on the monitoring system:
• protective earth resistance
• equipment leakage current
• applied part leakage current
• system test (if applicable)
Safety test requirements are set according to international standards, their national deviations and
specific local requirements. The safety tests detailed in this Service Guide are derived from
international standards but may not be sufficient to meet local requirements. We recommend that you
file the results of safety tests. This may help to identify a problem early particularly if the test results
deteriorate over a period of time.
Each individual piece of equipment which has its own connection to mains or which can be connected
or disconnected from mains without the use of a tool must be tested individually. The monitoring
system as a whole must be tested according to the procedure described in “System Test” on page 52.
Accessories which can affect the safety of the equipment under test or the results of the safety test
must be included in the tests and documented.
Warnings, Cautions, and Safety Precautions
• These tests are well established procedures of detecting abnormalities that, if undetected, could
result in danger to either the patient or the operator.
• Disconnect the device under test from the patient before performing safety tests.
• Disconnect the device under test from mains before performing safety tests. If this is not possible,
ensure that the performance of these tests does not result in danger to the safety analyzer operator,
patients or other individuals.
• Test equipment (for example, a Safety Analyzer) is required to perform the safety tests. Please refer
to Annex C of IEC/EN 62353 for exact requirements for the measurement equipment and for
measurement circuits for protective earth resistance and leakage currents. Refer to the
37
Page 38

3 Testing and Maintenance
documentation that accompanies the test equipment. Only certified technicians should perform
safety testing.
• The consistent use of a Safety Analyzer as a routine step in closing a repair or upgrade is
emphasized as a mandatory step to maintain user and patient safety. You can also use the Safety
Analyzer as a troubleshooting tool to detect abnormalities of line voltage and grounding plus total
current loads.
• During safety testing, mains voltage and electrical currents are applied to the device under test.
Ensure that there are no open electrical conductive parts during the performance of these tests.
Avoid that users, patients or other individuals come into contact with touch voltage.
• For Europe and Asia/Pacific, the monitor complies with:
IEC 60601-1:1988 + A1:1991 + A2:1995(Ed.2); EN60601-1:1990 + A1:1993 + A2:1995(Ed.2);
IEC 60601-1-1:2001; EN 60601-1-1:2001; IEC 60601-1-2:2001+A1:2004; EN 60601-12:2001+A1:2006.
For USA, the monitor complies with:
UL60601-1:2003
For Canada, CAN/CSA C22.2#601.1-M90+S1+A2
• Local regulations supersede the testing requirements listed in this chapter.
• If a non-medical electrical device is connected to a medical electrical device, the resulting medical
electrical system must comply IEC 60601-1-1:2000/ EN 60601-1-1:2001 or IEC 60601-1:2005/
EN 60601-1:2006+A1:2012 (Ed.3) Section 16 "ME Systems"
• Perform safety tests as described on the following pages.
Safety Test Procedures
Use the test procedures outlined here only for verifying and recording the initial values prior to or at
installation, safe installation or service of the product, and for periodic recurrent testing. The setups
used for these tests and the acceptable ranges of values are derived from local and international
standards but may not be equivalent. These tests are not a substitute for local safety testing where it is
required for an installation or a service event. If using an approved safety tester, perform the tests in
accordance with the information provided by the manufacturer of the tester and in accordance with
your local regulations, for example IEC/EN 60601-1, UL60601-1 (US), IEC/EN 62353, and IEC/EN
60601-1-1. The safety tester should print results as detailed in this chapter, together with other data.
Please refer to Annex C of IEC/EN 62353 for requirements for the measurement equipment and for
measurement circuits for protective earth resistance and leakage currents.
The following symbols are used in the diagrams illustrating the safety tests:
Supply mains Protective earth
L, N Supply mains terminals PE Protective earth terminal
38
Page 39

Mains part Applied part
F-type applied part Measuring device
Resistance measuring device Connection to accessible
......... Optional connection
3 Testing and Maintenance
conductive parts
CAUTION
After each service, maintenance or repair event:
Ensure all fuses accessible from the outside comply with the manufacturer’s specification.
Check:
• the integrity of mechanical parts, internally and externally.
• any damage or contamination, internally and externally.
• that no loose parts or foreign bodies remain in the device after servicing or repair.
• the integrity of all relevant accessories.
Hints for Correct Performance of Safety Tests
• Perform a visual inspection on all detachable power cords used with the monitoring system and
include these in all safety test procedures.
• Connection lines such as data lines or functional earth conductors may appear to act like protective
earth connections. These may lead to incorrect measurements and need to be considered during
testing. If necessary, unplug these connections.
• During measurements, the device under test shall be isolated from earth (e.g. test on an insulated
work bench), except the protective earth conductor in the power supply cord.
• Position all cables and cords in such a manner that they do not influence the safety tests.
• Measurement of insulation resistance is not required.
• When testing a medical electrical system, where possible, test it such that potential ground voltage
variations are present as they may be during actual use.
39
Page 40

3 Testing and Maintenance
Guideline for Performance of Safety Tests
This section introduces the general principle of performing recurrent safety tests. Product specific test
descriptions are described in the following sections.
Connect the detachable power cord of the device under test to the safety analyzer's test mains port.
Connect the enclosure test lead of the safety analyzer to the enclosure of the device under test, e.g. to
the equipotential connector or unearthed conductive accessible parts where applicable during
Equipment Leakage Current Tests and Applied Part Leakage Current Tests. For testing the applied
part leakage current, connect all applied parts to the safety analyzer using the appropriate patient lead
or adapter cable. For the ECG parameter all ten ECG-leads need to be connected to the safety
analyzer. If necessary, use an adapter cable to connect all ten ECG-leads. If necessary, repeat the safety
test procedure until all available applied parts have been tested. Refer to the documentation that
accompanies the safety analyzer for further details on how to set up and perform the test.
Protective Earth Resistance Test - Setup Example
NOTE
The test lead needs to go to parts that require protective earthing. This may be a single connection or
several tested after each other
Equipment Leakage Current Test - Setup Example
NOTE
The test lead needs to go to the grounded enclosure parts, the ungrounded enclosure parts and all of
the applied parts connected together.
40
Page 41

Applied Part Current Test - Setup Example
NOTE
The above graphics resemble the Metron QA-90 setup and are protected by copyright. Copyright
owned by Fluke (Metron).
Safety Test Adapter Cable - Schematics
The following graphics provide schematics of safety test (patient lead) adapter cables which can be
used for electrical safety testing. These schematics can also be used as a guideline for making your own
safety test adapter cables. Alternatively, other methods to make safety test adapter cables can be used,
e.g. using a modified accessory cable.
3 Testing and Maintenance
NOTE
You may not need all of the cables displayed below for electrical safety testing of your respective
monitor.
41
Page 42

3 Testing and Maintenance
ECG
SpO2 (MP2/X2, MP5, M3001A & M1020B #A01, #A02, #A03, #A04)
42
Page 43

Invasive Pressure
Invasive Pressure (M1006B #C01)
3 Testing and Maintenance
Temperature
43
Page 44

3 Testing and Maintenance
CO2 (MP5, M3014A)
Cardiac Output
44
Page 45

NMT
3 Testing and Maintenance
IntelliBridge
45
Page 46

3 Testing and Maintenance
EEG
ScVO2 (M1011A)
46
Page 47

TcG10
3 Testing and Maintenance
Electrical Safety Testing
S(1): Protective Earth Resistance Test
Test to perform:
Measuring circuit for the measurement of Protective Earth Resistance in medical electrical
equipment that is disconnected from the supply mains.
47
Page 48

3 Testing and Maintenance
This measures the impedance of the Protective Earth (PE) terminal to all exposed metal parts of the
Device under Test (DUT), which are for safety reasons connected to the Protective Earth (PE).
You can find metal parts of the device at the equipotential connector.
Measurements shall be performed using a measuring device capable to deliver a current of at least
200 mA into 500 mOhms with maximum open circuit voltage of 24V
This safety test is based on IEC/EN 62353.
Report the highest value (X1).
Test Expected test results
Protective Earth Resistance Test (with
X1 <= 300mOhms
mains cable)
NOTE
• If the protective earth resistance test fails, testing must be discontinued immediately and the device
under test must be repaired or labeled as defective.
• All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
• Flex the power cord during the protective earth resistance test to evaluate its integrity. If it does
not pass the test, exchange the power cord. Then repeat the test. If it still does not pass, follow the
instructions in the first bullet point of this note above.
S(2): Equipment Leakage Current Test - Normal Condition
Test to perform:
48
Measuring circuit for the measurement of Equipment Leakage Current - Direct method
according to IEC/EN 62353.
This test measures leakage current of accessible conductive and non-conductive metal parts of the
monitor and the functional earth leakage current. It tests normal and reversed polarity. Perform the
test with S1 closed (Normal Condition).
There are no parts of the equipment that are not protectively earthed. Disconnect any data cables and
any connections that may provide an extraneous earth path. Test the device under test (DUT) on an
insulated surface. Do not touch the DUT during testing.
This safety test is based on IEC/EN 62353.
Page 49

Report the highest value (X1).
Test Expected test results
3 Testing and Maintenance
Equipment Leakage Current Test
X1 <= 100μA
(Normal Condition - with mains cable)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
S(3): Equipment Leakage Current Test - Single Fault Condition
Test to perform:
Measuring circuit for the measurement of Equipment Leakage Current - Direct method
according to IEC/EN 62353.
This test measures leakage current of accessible conductive and non-conductive metal parts of the
monitor and the functional earth leakage current. It tests normal and reversed polarity. Perform the
test with S1 open (Single Fault Condition).
There are no parts of the equipment that are not protectively earthed. Disconnect any data cables and
any connections that may provide an extraneous earth path. Test the device under test (DUT) on an
insulated surface. Do not touch the DUT during testing.
This safety test is based on IEC/EN 62353.
Report the highest value (X2).
Test Expected test results
Equipment Leakage Current Test (Single
X2 <= 300μA
Fault Condition - with mains cable)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
49
Page 50

3 Testing and Maintenance
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
S(4): Applied Part Leakage Current - Mains on Applied Part
NOTE
During measurement of the Applied Part Leakage Current it is possible that the measured current can
exceed the allowed limit (per IEC/EN 60601-1 or IEC/EN 62353).
This can occur when the safety tester is connected to the invasive blood pressure and temperature
connectors at the same time during the applied leakage current measurement.
The connectors for the invasive blood pressure and temperature are independently functioning
connectors.
Although there are individual connectors on the front end, internally those parameters use the same
electrical insulation interface and are hardwired to each other. This results in an electrical short of
those connectors during measurement if a test current is applied simultaneously. Therefore this should
be avoided.
Due to the combined insulation interface, it is sufficient to connect to only one parameter interface
(that is, Invasive Blood Pressure or Temperature) of the invasive blood pressure/temperature
measurement block. This avoids a short and the potential of exceeding the limit for the current.
Test to perform:
Measuring circuit for the measurement of Applied Part Leakage Current - Direct method
according to IEC/EN 62353.
50
Page 51

3 Testing and Maintenance
This test measures applied part leakage current from applied part to earth caused by external main
voltage on the applied part. Each polarity combination possible shall be tested. This test is applicable
to each Applied Part tested and results recorded in turn with all other Applied Parts left floating.
Applied Parts with multiple connections (e.g. ECG) are tested with the connections short-circuited.
There are no parts of the equipment that are not protectively earthed.
This safety test is based on IEC/EN 62353.
For measurement limits and test voltage, refer to Safety (4) test, Test and Inspection Matrix.
Report the highest value. (X1).
Test Expected test results
Applied Part Leakage Current Test
(Single Fault Condition - mains on
applied part)
NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
In case of an IT-power system, this safety test measurement requires a special measuring circuit, for
example with its own integrated TN-system or use of an external isolation transformer attached to the
safety test device.
X1 <= 50μA (CF)
Reference: Allowable Values for IEC 60601-1:1998 and UL 60601-1 Measurements
Protective Earth resistance (between the PROTECTIVE EARTH TERMINAL and any
ACCESSIBLE METAL PART which is PROTECTIVELY EARTHED, w/o power cord):
100mOhms
Protective Earth resistance of power cord: 100mOhms
Enclosure leakage current (IEC 60601-1 and UL60601-1): 100 μA (N.C.)
Enclosure leakage current:(IEC 60601-1): 500 μA (S.F.C)
Enclosure leakage current (UL 60601-1): 300 μA (S.F.C)
Patient leakage current: (IEC 60601-1 and UL60601-1): 100 μA (N.C.) for BF
Patient leakage current: (IEC 60601-1 and UL60601-1): 500 μA (S.F.C.) for BF
Patient leakage current: (IEC 60601-1 and UL60601-1): 10 μA (N.C.) for CF
Patient leakage current: (IEC 60601-1 and UL60601-1): 50 μA (S.F.C.) for CF
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated
Insulation Resistance
It is not recommended to perform measurements of the insulation resistance. Refer to IEC 62353 for
details about methods of the insulation resistance measurement.
51
Page 52

3 Testing and Maintenance
System Test
After mounting and setting up a system, perform system safety tests according to IEC/EN 60601-1-1.
What is a Medical Electrical System?
A medical electrical system is a combination of at least one medical electrical piece of equipment and
other electrical equipment, interconnected by functional connection or use of a multiple portable
socket-outlet.
• Devices forming a medical electrical system must comply either with IEC/EN 60601-1-1 or IEC/
EN 60601-1+A1 Ed.3 clause 16.
• Any electrical device such as IT equipment that is connected to the medical electrical equipment
must comply either with IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16 and be
tested accordingly.
• Non-medical electrical equipment may require connection through a separating device (e.g. an
isolation transformer).
General Requirements for a System
After installation or subsequent modification, a system must comply with the requirements of the
system standard IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16. Compliance is checked
by inspection, testing or analysis, as specified in the IEC/EN 60601-1-1 or in this book.
Medical electrical equipment must comply with the requirements of the general standard IEC/EN
60601-1, its relevant particular standards and specific national deviations. Non-medical electrical
equipment shall comply with IEC safety standards that are relevant to that equipment.
Relevant standards for some non-medical electrical equipment may have limits for equipment leakage
currents higher than required by the standard IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3
clause 16. These higher limits are acceptable only outside the patient environment. It is essential to
reduce equipment leakage currents to values specified in IEC/EN 60601-1 when non-medical
electrical equipment is to be used within the patient environment.
52
Page 53

System Example
This illustration shows a system where both the medical electrical equipment and the non-medical
electrical equipment are situated at the patient’s bedside.
3 Testing and Maintenance
WARNING
• Do not use additional AC mains extension cords or multiple portable socket-outlets. If a multiple
portable socket-outlet is used, the resulting system must be compliant with IEC/EN 60601-1-1 or
IEC/EN 60601-1+A1 Ed.3 clause 16. Do not place multiple socket-outlets on the floor. Do not
exceed the maximum permitted load for multiple socket-outlets used with the system. Do not plug
additional multiple socket outlets or extension cords into multiple socket outlets or extension
cords used within the medical electrical system.
• Do not connect any devices that are not supported as part of a system.
• Do not use a device in the patient vicinity if it does not comply with IEC/EN 60601-1 or IEC
60601-1 edition 3 clause 16. The whole installation, including devices outside of the patient
vicinity, must comply with IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16. Any nonmedical device placed and operated in the patient’s vicinity must be powered via a separating
transformer (compliant with IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16) that
ensures mechanical fixing of the power cords and covering of any unused power outlets.
System Installation Requirements
• Ensure that the medical electrical system is installed in a way that the user achieves optimal use.
• Make sure the user is informed about the required cleaning, adjustment, sterilization and
disinfection procedures listed in the Instructions for Use.
• The medical electrical system must be installed in such a way that the user is able to carry out the
necessary cleaning, adjustment, sterilization and disinfection procedures listed in the Instructions
for Use.
53
Page 54

3 Testing and Maintenance
• Ensure that the medical electrical system is installed in a way that an interruption and restoration
of power to any part of the medical electrical system does not result in a safety hazard.
• We recommend using fixed mains socket outlets to power the medical system or parts thereof.
Avoid using multiple portable socket-outlets.
• Any multiple portable socket outlets used must be compliant with IEC 60884-1 and IEC/EN
60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16.
• Ensure that any part of the system connected to multiple portable socket-outlets is only removable
with a tool, i.e. the multiple portable socket-outlet provides a locking mechanism to prevent power
cords from being plugged or unplugged unintentionally. Otherwise, the multiple portable socketoutlet must be connected to a separation device. Multiple Socket Outlets used within the medical
electrical system must only be used for powering medical electrical equipment which is part of the
system.
• Ensure that any functional connections between parts of the medical electrical system are isolated
by a separation device according to IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16
to limit increased equipment leakage currents caused by current flow through the signal
connections where necessary (e.g. leakage current coming from non-medical electrical equipment
into medical electrical equipment or building ground voltage differences providing leakage current
through grounded data cables). This only works if the equipment leakage current of the respective
medical electrical system parts is not exceeded under normal conditions. This isolation is especially
important where the non-medical electrical equipment leakage currents can pass to the medical
electrical equipment in the system or building ground voltage differences can pass to the medical
electrical equipment via ground in a data cable connection in the system
• Avoid increase of equipment leakage currents when non-medical electrical equipment within the
medical electrical system is used. This only applies when if the equipment leakage current of the
respective medical electrical system parts is not exceeded under normal conditions. Use of an
additional protective earth connection, separation device or additional non-conductive enclosures
are options that can prevent a problem.
• Within the patient environment it is important to limit electrical potential differences between
different parts of a system. If necessary, use potential equalization equipment (equipotential cable)
or additional protective earth connections.
• Medical electrical equipment used in medical rooms must be connected to potential equalization
equipment (equipotential cable) to avoid electrical potential differences. Check your local
requirements for details.
Required Protective Measures at System Installation
For any IT equipment (IEC60950-1) operated in the patient environment ensure that the equipment
leakage current does not exceed the limits described in IEC 60601-1. Use a separation device to ensure
compliance. After installation of IT equipment in the patient environment, an equipment leakage
current test is required.
54
Page 55

3 Testing and Maintenance
Case 1: Medical Device Combined with Medical Device
If you combine a medical device with another medical device (incl. Philips specified displays) to form a
medical electrical system according to IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16,
no additional protective measures are required. The medical electrical devices may be located in or
outside the patient vicinity in a medically used room. This is valid as long as the medical devices are
connected to separate mains outlets. No system test is required.
NOTE
The pictures below and in the following chapters show the MX800 monitor as an example. All cases
apply to the MX400/450/500/550/600/700 monitors as well.
55
Page 56

3 Testing and Maintenance
56
If the combined medical devices are connected to the same multiple portable socket outlet an
enclosure leakage current test of the entire device combination on the multiple portable socket outlet is
required to ensure that the resulting protective earth leakage current and equipment leakage current
does not exceed the limits of IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16. Avoid
using multiple portable socket outlets. The medical electrical devices may be located in or outside the
patient vicinity in a medically used room. If the limits are exceeded, additional protective measures are
required, e.g. a separation device or the connection of each device to separate mains.
Page 57

3 Testing and Maintenance
Case 2: Medical Device Combined with a Non-Medical Device
If you combine a medical device with a non-medical device to form a medical electrical system
according to IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16, additional protective
measures are required, e.g. usage of a separation device. The medical electrical devices or the IT
equipment may be located in or outside the patient vicinity in a medically used room. After system
installation incl. protective measures, a system test is required to ensure that the resulting equipment
leakage current and applied part leakage current does not exceed the limits of IEC/EN 60601-1-1 or
IEC/EN 60601-1+A1 Ed.3 clause 16.
57
Page 58

3 Testing and Maintenance
For any IT equipment (IEC60950-1) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3
clause 16. Use a separation device to ensure compliance. After installation of IT equipment in patient
vicinity, an equipment leakage current test is required.
58
If the combined devices forming the medical electrical system are connected to the same multiple
portable socket outlet, ensure that the resulting protective earth leakage current and equipment leakage
current do not exceed the limits of IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16. The
medical electrical devices or IT equipment may be located in or outside the patient vicinity in a
medically used room. Avoid using multiple portable socket outlets. If the limits of IEC/EN 60601-1-1
Page 59

3 Testing and Maintenance
or IEC/EN 60601-1+A1 Ed.3 clause 16 are exceeded, additional protective measures are required, e.g.
a separation device or the connection of each device to separate mains.
For any IT equipment (IEC60950-1) operated in patient vicinity ensure that the equipment leakage
current does not exceed the limits described in IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3
clause 16. Use a separation device to ensure compliance. After installation of IT equipment in patient
vicinity, an equipment leakage current test is required.
59
Page 60

3 Testing and Maintenance
Case 3: Medical Device Combined with a Medical or Non-Medical Device with one Device in a Non-Medically-Used Room
If you combine a medical device with a medical or non-medical device to form a medical electrical
system according to IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16 using a common
protective earth connection and one of the devices is located in a non-medically used room, additional
protective measures are required, e.g. usage of a separation device or additional protective earth
connection. The medical electrical devices or IT equipment may be located in or outside the patient
vicinity. After system installation incl. protective measures, a system test is required to ensure that the
resulting equipment leakage current does not exceed the limits of IEC/EN 60601-1-1 or IEC/EN
60601-1+A1 Ed.3 clause 16.
60
Page 61

3 Testing and Maintenance
If you combine a medical device with a medical or non-medical device to form a medical electrical
system according to IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16 using two separate
protective earth connections and one of the devices is located in a non-medically used room creating a
potential voltage difference, additional protective measures are required, e.g. usage of a separation
device or additional protective earth connection. The medical electrical devices or IT equipment may
be located in or outside the patient vicinity. After system installation incl. protective measures, a system
test is required to ensure that the resulting equipment leakage current does not exceed the limits of
IEC/EN 60601-1-1 or IEC/EN 60601-1+A1 Ed.3 clause 16.
61
Page 62

3 Testing and Maintenance
System Test Procedure
If the medical electrical device has already been tested as a standalone device e.g. during factory safety
testing, an equipment leakage current test must only be performed once the device is connected to
another electrical device/system. If the medical electrical system has not been tested as a standalone
device, the device has to be tested as a standalone device (without connection to the system) and as
part of the system (with connection to the system).
Connect the detachable power cord of the device under test to the safety analyzer's test mains port.
Connect the enclosure test lead of the safety analyzer to the enclosure of the device under test as
described in the "Equipment Leakage Test" section . Refer to the documentation that accompanies the
safety analyzer for further details on how to set up the test.
Test Expected test results
Equipment Leakage Current Test
(Normal Condition)
Equipment Leakage Current Test (Single
Fault Condition)
After the testing of the device as a standalone device and as part of the system, check that the resulting
values (without connection and with connection to the system) do not differ by more than +/- 10%
from each other.
If the devices in the medical electrical system are connected to a multiple portable socket outlet the
resulting protective earth leakage current needs to be determined. All system components must be
connected to the multiple portable socket outlet and be switched on during this measurement.
Sys1 <= 100μA
Sys2 <= 300μA
62
Test Expected test results
Protective Earth Leakage Current of
Multiple Socket Outlets
Sys3 <= 300μA
Page 63

3 Testing and Maintenance
Refer to the documentation that accompanies the safety analyzer for further details on how to set up
the test.
Preventive Maintenance Procedures
Noninvasive Blood Pressure Measurement Calibration
Carry out the noninvasive blood pressure measurement performance tests at least every two years , or
as specified by local laws (whichever comes first).
Microstream CO2 Calibration
Carry out the Microstream CO2 calibration once a year or after 4000 hours of continuous use and
following any instrument repairs or the replacement of any instrument parts.
Performance Assurance Tests
Some of the following test procedures must be performed in service mode. To enter service mode
Operating Modes in the main menu. Then select Service Mode and enter the password.
select
If required, open the screen menu in the monitor info line at the top of the screen and select
access the service screen. This is required particularly for Anesthetic Gas Module testing procedures.
Basic Performance Assurance Test
This section describes the basic performance test procedure. Please refer to the section for detailed
information on when which test procedure is required.
Procedure:
Power on the monitoring system and go into demo mode. Check that each connected parameter
(module, MMS, Gas Analyzer, IntelliBridge connected device) displays values.
Full Performance Assurance Test
The following sections describe the full performance testing procedures i.e. detailed testing of each
parameter with a patient simulator or specified tools. Please refer to the section for information on
when which testing procedure is required.
ECG/Resp Performance Test
This test checks the performance of the ECG and respiration measurements.
Tools required: Patient simulator.
ECG Performance
1 Connect the patient simulator to the ECG/Resp connector on the MMS/IntelliVue X2.
Service to
2 Configure the patient simulator as follows:
– ECG sinus rhythm.
– HR = 100 bpm or 120 bpm (depending on your patient simulator).
3 Check the displayed ECG wave and HR value against the simulator configuration.
4 The value should be 100bpm or 120 bpm+/- 2 bpm.
63
Page 64

3 Testing and Maintenance
Respiration Performance
1 Change the Patient Simulator configuration to:
– Base impedance line 1500 Ohm.
– Delta impedance 0.5 Ohm.
– Respiration rate 40 rpm or 45 rpm.
2 The value should be 40 rpm +/- 2 rpm or 45 rpm +/- 2 rpm.
Test Expected test results
ECG Performance Test 100 bpm +/- 2 bpm or
Respiration Performance Test 40 rpm +/- 2 rpm or
ECG Out Performance Test
This test checks the performance of ECG synchronization between the monitor and a defibrillator. It
only needs to be performed when this feature is in use as a protocol at the customer site.
Tools required:
• Defibrillator with ECG Input.
120 bpm +/- 2 bpm
45 rpm +/- 2 rpm
• Patient simulator.
1 Connect the patient simulator to the ECG connector of the MMS and the defibrillator to the ECG
Output on the monitor with the ECG Sync cable.
2 Set the patient simulator to the following configuration:
– HR = 100 bpm or 120 bpm (depending on your patient simulator).
– ECG sinus rhythm.
3 Switch the defibrillator to simulation mode.
4 Check that the ECG signal is displayed.
Test Expected test results
ECG Out Performance Test ECG signal is displayed (pass/fail)
SpO2 Performance Test
This test checks the performance of the SpO2 measurement.
Procedure for Philips FAST SpO
Tools required: none
1 Connect an adult SpO
2 Measure the SpO
3 The value should be between 95% and 100%.
value on your finger (this assumes that you are healthy).
2
Technology:
2
transducer to the SpO2 connector.
2
64
Test Expected test results
SpO
Performance Test 95% and 100%
2
Page 65

3 Testing and Maintenance
Procedure for Nellcor OxiMax SpO2 Technology:
Nellcor recommends that the functionality of this parameter be verified using the SRC-MAX.
A possible performance assurance check requiring no tools would be:
1 Connect an adult SpO
2 Measure the SpO
3 The value should be between 95% and 100%.
Test Expected test results
SpO2 Performance Test 95% and 100%
Procedure for Masimo SET SpO
The end user may verify SpO
designed to work with Masimo Pulse Oximeter technology. Optical simulators are recommended as
they use the patient cable and sensor as part of the test setup. Additionally, a test that includes placing
the sensor on a healthy subject and confirming the device reads a normal saturation and pulse rate and
displays a clean pleth waveform (while the subject is still) may further increase the confidence that the
device is functioning properly.
Measurement Validation
NOTE
A functional tester cannot be used to assess the accuracy of a pulse oximeter monitor or sensor.
However, it can be used to demonstrate that a particular pulse oximeter monitor reproduces a
calibration curve that has been independently demonstrated to fulfill a particular accuracy
specification.
transducer to the SpO2 connector.
2
value on your finger (this assumes that you are healthy).
2
Technology:
2
performance via commercially available SpO2 simulators specifically
2
Philips FAST SpO
The SpO
accuracy has been validated in human studies against arterial blood sample reference
2
Technology
2
measured with a CO-oximeter. In a controlled desaturation study, healthy adult volunteers with
saturation levels between 70% and 100% SaO
were studied. The population characteristics for those
2
studies were:
• about 50% female and 50% male subjects
• age range: 19 to 39
• skin tone: from light to dark brown
Pulse rate accuracy has been validated with an electronic pulse simulator.
Nellcor OxiMax Technology
Accuracy specifications are based on controlled hypoxia studies with healthy non-smoking adult
volunteers over the specified saturation SpO2 range(s). Pulse oximeter SpO2 readings were compared
to SaO2 values of drawn blood samples measured by hemoximetry. All accuracies are expressed as ±
"X" digits. Pulse oximeter equipment measurements are statistically distributed; about two-thirds of
pulse oximeter measurements can be expected to fall in this accuracy (ARMS) range. Because scatter
and bias of pulse oximeter SpO2 and blood SaO2 comparisons commonly increase as the saturation
decreases, and accuracy specifications are calculated from data spanning the stated range, different
accuracy values may result when describing partially overlapping ranges.
65
Page 66

3 Testing and Maintenance
Subjects used to validate SpO2 measurement accuracies were healthy and recruited from the local
population. Comprised of both men and women, subjects spanned a range of skin pigmentations and
ranged in age from 18-50 years old.
Oxygen saturation accuracy can be affected by certain environmental, equipment, and patient
physiologic conditions (as discussed in the Instructions for Use for the monitor) that influence
readings of SpO2, SaO2, or both. Accordingly, observations of clinical accuracy may not achieve the
same levels as those obtained under controlled laboratory conditions.
Pulse rate accuracy has been validated with an electronic pulse simulator.
Masimo SET SpO2 Technology
The SpO2 accuracy (except for LNOP Blue sensors) has been validated in human studies against
arterial blood sample reference measured with a CO-oximeter. In a controlled desaturation study,
healthy adult volunteers with saturation levels between 70% and 100% SpO2 were studied.
The population characteristics for those studies were:
• healthy female subjects: 22 to 39 years of age; light to dark skin pigmentation
• healthy male subjects: 19 to 37 years of age; light to dark skin pigmentation
The LNOP Blue SpO2 accuracy has been validated in human blood studies on neonatal, infant and
pediatric patients with congenital cyanotic cardiac lesions in the range of 60%-100% SpO2 against a
laboratory CO-oximeter.
The population characteristics for those studies were:
• female patients: 5 days to 20 months of age; light and dark skin pigmentation
• male patients: 1 day to 13 months of age; light and dark skin pigmentation
Pulse rate accuracy has been validated with an electronic pulse simulator or ECG as reference.
For further information please refer to the Instructions for Use of the Device and Accessories.
NBP PerformanceTest
This section describes NBP test procedures.The monitor must be in service mode and the screen
“Service A” must be selected to perform these tests. The NBP Performance Test consists of:
• NBP Accuracy Test
• NBP Leakage Test
•NBP Linearity Test
•Valve Test
NBP Accuracy Test and Calibration
This test checks the performance of the non-invasive blood pressure measurement. Connect the
equipment as shown:
66
Page 67

3 Testing and Maintenance
Tools required:
• Reference manometer (includes hand pump and valve), accuracy +/-0.8mmHg.
• Expansion chamber (volume 250 ml +/- 10%)
• Appropriate tubing.
In service mode, the systolic and diastolic readings indicate the noise of NBP channels 1 and 2
respectively. When static pressure is applied, the reading in NBP channel 1 should be below 50. The
value in parentheses indicates the actual pressure applied to the system.
1 Connect the manometer and the pump with tubing to the NBP connector on the MMS and to the
expansion chamber.
2 In service mode, select the Setup NBP menu.
3 Select Close Valves: On
4 Raise the pressure to 280 mmHg with the manometer pump.
5 Wait 10 seconds for the measurement to stabilize.
6 Compare the manometer values with the displayed values.
7 Document the value displayed by the monitor (x1).
8 If the difference between the manometer and displayed values is greater than 3 mmHg or if the
NBP pump assembly has been exchanged, calibrate the MMS. If not, proceed to the leakage test.
9 To calibrate the MMS, select Close Valves off then Calibrate NBP and wait for the instrument to
pump up the expansion chamber.Wait a few seconds after pumping stops until
highlighted and then move the cursor to the value shown on the manometer. If one of the
following prompt messages appears during this step, check whether there is leakage in the setup:
– NBP unable to calibrate–cannot adjust pressure
– NBP unable to calibrate–unstable signal
10 Press Confirm.
If the INOP NBP Equipment Malfunction message occurs in monitoring mode, go back to service
mode and repeat the calibration procedure.
NBP Leakage Test
The NBP leakage test checks the integrity of the system and of the valve. It is required once every two
years and when you repair the MMS or X2 or replace parts.
EnterPrVal is
1 If you have calibrated, repeat steps 2 to 6 from the accuracy test procedure so that you have 280
mmHg pressure on the expansion chamber.
2 Watch the pressure value for 60 seconds.
67
Page 68

3 Testing and Maintenance
3
Calculate and document the leakage test value (x2).
x2 = P1 - P2
where P1 is the pressure at the beginning of the leakage test and P2 is the pressure displayed after
60 seconds.
The leakage test value should be less than 6 mmHg.
NOTE
The leakage test value of 6 mmHg applies for an expansion chamber of 250ml. When using a different
size of expansion chamber, the expected test result needs to be adapted accordingly. E.g for an
expansion chamber of 500ml, the leakage test value should be less than 3 mmHg. All other NBP
performance tests are independent of the expansion chamber size.
NBP Linearity Test
1 Reduce the manometer pressure to 150 mmHg.
2 Wait 10 seconds for the measurement to stabilize.
3 After these 10 seconds, compare the manometer value with the displayed value.
4 Document the value displayed by the monitor (x3)
5 If the difference is greater than 3 mmHg, calibrate the MMS or X2 (see steps 9 to 10 in the
accuracy test procedure).
Valve Test
1 Raise the pressure again to 280 mmHg.
2 Select Close valves: Off.
3 Wait five seconds and then document the value displayed. The value should be less than 10 mmHg.
4 Document the value displayed by the monitor (x4).
Test Expected test results
Accuracy test x1 = 280 ± 3mmHg
Difference ≤ 3mmHg
Leakage test x2 = leakage test value
x2 < 6 mmHg (with 250ml
expansion chamber)
Linearity test x3 = 150 ± 3mmHg
Difference ≤ 3mmHg
Valve Test x4 = value < 10 mmHg
Invasive Pressure Performance Test
This test checks the performance of the invasive pressure measurement.
Tools required: Patient simulator.
68
1 Connect the patient simulator to the pressure connector.
2 Set the patient simulator to 0 pressure.
3 Make a zero calibration.
Page 69

4
Configure the patient simulator as P(static) = 200 mmHg.
5 Wait for the display.
6 The value should be 200 mmHg ± 5 mmHg. If the value is outside these tolerances, calibrate the
Invasive Pressure measurement. If the measurement was calibrated with a dedicated reusable
catheter, check the calibration together with this catheter.
Test Expected test results
Invasive Pressure Performance Test 200 mmHg ± 5 mmHg
Temperature Performance Test
This test checks the performance of the temperature measurement.
Tools required: Patient simulator (with 0.1°C or 0.2°F tolerance).
1 Connect the patient simulator to the temperature connector.
2 Configure the patient simulator to 40°C or 100°F.
3 The value should be 40°C ± 0.2°C or 100°F ± 0.4°F.
Test Expected test results
Temperature Performance Test 40°C ± 0.2°C or 100°F ± 0.4°F
3 Testing and Maintenance
M3014A Capnography Extension Performance Tests
The procedures below describe the mainstream and sidestream CO2 performance tests for the
M3014A Capnography Extension.
Mainstream CO2 Accuracy Check
Tools Required:
• three airway adapters
• Verification Gas M2506A
• Gas cylinder regulator M2505A
You also need a local barometric pressure rating received from a reliable local source (airport, regional
weather station or hospital weather station) which is located at the same altitude as the hospital.
Procedure:
1 Attach the M2501A CO
Make sure that the sensor is disconnected from the patient circuit.
2 Switch on the patient monitor.
3 Enter the monitor’s Service Mode.
4 Using the sensor status provided in the M2501A Serial protocol, wait for the M2501A sensor to
warm up to its operating temperature.
sensor to the patient monitor. Attach an airway adapter to the sensor.
2
5 The default setting for gas temperature is 22°C. If the gas temperature is significantly above or
below this value, correct the gas temperature setting.
6 Zero the sensor on the airway adapter being used in this test. Ensure Zero Gas is set to Room Air
7 Attach a regulated flowing gas mixture of 5% CO2, balance N2 to the airway adapter.
8 Set the gas correction to off.
69
Page 70
3 Testing and Maintenance
9
Allow a few seconds for the gas mixture to stabilize and observe the CO2 value. The expected
value is 5% of the ambient pressure ±2mmHg
NOTE
Make sure that you follow the above steps correctly. If the sensor fails this check it must be exchanged.
The sensor cannot be calibrated.
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x1) Acceptance Range
Mainstream CO2 Accuracy
Test
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient pressure).
Sidestream CO2 Accuracy Check
Tools Required:
• Cal gas flow regulator M2267A
• Cal tube 13907A
• Verification Gas M2506A
• Straight Sample Line M2776A
You also need a local barometric pressure rating received from a reliable local source (airport, regional
weather station or hospital weather station) which is located at the same altitude as the hospital.
Procedure:
1 Attach the M2741A CO2 sensor to the patient monitor. Attach the sample line and the cal tube to
the sensor. Make sure that the sensor is disconnected from the patient circuit.
2 Switch on the patient monitor.
3 Enter the monitor’s Service Mode.
5% of 760 mmHg pressure ±2mmHg 36 mmHg - 40 mmHg
70
4 Using the sensor status provided in the M2741A Serial protocol, wait for the M2741A sensor to
warm up to its operating temperature.
5 Zero the sensor. Ensure Zero Gas is set to Room Air
6 Attach a regulated flowing gas mixture of 5% CO2, balance N2 to the cal tube.
7 Set the gas correction to off.
8 Allow a few seconds for the gas mixture to stabilize and observe the CO2 value. The expected
value is 5% of the ambient pressure ±2mmHg
NOTE
Make sure that you follow the above steps correctly. If the sensor fails this check it must be exchanged.
The sensor cannot be calibrated
Page 71

Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x2) Acceptance Range
Sidestream CO2 Accuracy Test 5% of 760 mmHg pressure ±2mmHg 36 mmHg - 40 mmHg
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient pressure).
Sidestream CO2 Flow Check
Check the flow rate in the Sidestream CO2 extension as follows:
1 Connect the flowmeter to the sample line
3 Testing and Maintenance
2 Check on the flowmeter the flow that the Sidestream CO
ml/min ± 10 ml/min. If the value is not within tolerance check your setup again and perform
another flow check. If it fails again, the sensor must be replaced. The sensor cannot be calibrated.
Example for an expected test result:
The expected test result for an altitude of 0 m (sea level) at approximately 760 mmHg ambient
pressure is:
Test Expected test results (x3) Acceptance Range
Sidestream CO2 Flow Check 50 ml/min ±10 ml/min 40 ml/min - 60 ml/min
NOTE
The expected test results will differ depending on the conditions (i.e. altitude or ambient pressure).
Microstream CO2 Performance Test
Allow five seconds between individual service procedures to ensure stable equipment conditions.
When certain monitor procedures are running, service procedures are not possible and trying to start
them will result in a message
completes the current operation, then restart the service procedure.
This test checks the performance of the Microstream CO2 measurement. The Microstream CO2
measurement can either be integrated into the IntelliVue MP5 monitor or, for other IntelliVue
monitors, into the M3015A/B MMS Extensions. The Microstream CO2 performance test is required
once per year or after 4000 hours of continuous use and when the instrument is repaired or when parts
are replaced.
Service Operation Failed in the monitor’s status line. Wait until the monitor
extension pump draws. It should be 50
2
This test uses calibration equipment that you can order (see the Parts section for the part number). The
procedure is summarized in the following steps. Refer to the documentation accompanying the
equipment for detailed instructions.
Tools Required:
• Standard tools, such as screwdriver, tweezers
• Electronic flowmeter, M1026-60144 or Mass Flowmeter 453564178121
• Digital Barometer ±2mbar or better
• Gas calibration equipment:
71
Page 72

3 Testing and Maintenance
• Cal 1 gas 15210-64010 (5% CO2)
• Cal 2 gas 15210-64020 (10% CO
)
2
• Cal gas flow regulator M2267A
• Cal tube 13907A
• Calibration Line M3015-47301
• Leakage Test Kit M1013-64002 (451261014851) (only required for leakage test without M102660144 Flowmeter)
• Flexible Connecting Tube
You also need a local barometric pressure rating received from a reliable local source (airport, regional
weather station or hospital weather station) which is located at the same altitude as the hospital.
The CO2 calibration for the Microstream extension consists of the following steps:
• Leakage check, either with M1026-60144 Flowmeter or with 453564178121 Mass Flowmeter*
• Barometric pressure check and calibration, if required.*
• Flow check and calibration, if required
•Noise check
• CO2 Cal check and calibration, if required
•CO2 Cal verification
Perform all checks in the same session.
* Not applicable for all HW Revisions. See individual test sections for details.
NOTE
The M3015A/B HW Rev C is indicated as HW Rev. Q.xx.xx in the IntelliVue Revision Screen.
Leakage Check with M1026-60144 Flowmeter (only for M3015A with HW Rev. A and B and Firmware Revision < P.01.32)
The leakage check consists of checking the tubing between:
• the pump outlet and the mCO
• the pump inlet and calibration line inlet.
Check the user’s guide of the flowmeter for details on how to make a correct flow reading.
Part 1
1 Go into service mode and select Setup CO2 menu.
2 Connect a calibration line to the Microstream CO
3 Check the ambient pressure and the cell pressure shown in the monitor’s status line. The cell
pressure should be approximately 20 mmHg lower than ambient pressure. (This test is only to
check that the pump starts and is running, which is also indicated by the noise generated by the
running pump.)
4 Connect the flowmeter outlet to the calibration line inlet using a flexible connecting tube.
5 Block the mCO
outlet using your fingertip and observe the flowmeter display. The value on the
2
flowmeter (x1) should decrease to between 0 and 4 ml/min, accompanied by an audible increase in
pump noise. If the value is within the tolerance limits, continue with part 2 of the leakage check.
outlet and
2
input to start the pump running.
2
72
Page 73

3 Testing and Maintenance
6
If the value is outside the tolerance limits, there is a leakage between the pump outlet and the
outlet.
mCO
2
7 Open the MMS Extension or MP5 and check the tubing connections at the pump outlet and the
extension gas outlet. If the connections are good, then there is a leakage in the tubing and you
must exchange the MMS Extension or the mCO
Assembly of the MP5 respectively.
2
Part 2
1 Disconnect the flowmeter from the Part 1 setup and connect the flowmeter inlet to the M3015A
gas outlet or the MP5 mCO
2 Leave the calibration line connected to the M3015A inlet or the MP5 mCO
3 Block the inlet of the calibration line using your fingertip and observe the flowmeter display. The
gas outlet.
2
inlet..
2
value on the flowmeter (x2) should decrease to between 0 and 4 ml/min, accompanied by an
audible increase in pump noise. The cell pressure shown in the status line on the display should
decrease to between 300 and 500 mmHg. Do not block the inlet for longer than 25 seconds as this
will lead to an “Occlusion” INOP. If the value is within the tolerance limits, there are no leakages
and the leakage check is completed; proceed to the pump check.
4 If the value is not within the tolerance limits, there is a leakage between the calibration line inlet
and the pump inlet.
5 Check the calibration line connections and open the M3015A or MP5 to check the tubing
connections at the pump inlet and the M3015A or MP5 mCO
gas inlet. If the connections are
2
good, try replacing the calibration line and repeating the leakage check. If the situation remains,
there is a leakage in the tubing and the M3015A or the mCO
assembly of the MP5 must be
2
exchanged.
Test Expected test results
Leakage Check Parts 1 and 2 x1 = value of part 1 leakage check on flowmeter
(x1< 4.0 ml/min)
x2 = value of part 2 leakage check on flowmeter
(x2< 4.0 ml/min)
Leakage Check for M3015B and M3015A with HW Rev C or M3015A with HW Rev. A/B without M1026-60144 Flowmeter
Preparation of Leakage Test Kit:
Remove two Luer connectors from the Leakage Test Kit, as shown in the following picture.
NOTE
These Luer connectors are not required for the actual Leakage Check. However, you should keep
them, as they are required for other tests (e.g. for the kit leak test as documented later in this section).
73
Page 74

3 Testing and Maintenance
Test Setup:
1 Connect the Calibration Line (M3015-47301) to the inlet of the M8105A/M3015A/B (the
M8105A/M3015A/B must be switched off, either by disconnecting from the host monitor or by
switching off the monitor).
2 Connect the leakage test tubing to the outlet of the M8105A/M3015A/B, to the digital barometer,
to the calibration line, and the (empty) syringe as shown below. Make sure all connections have a
tight fit!
74
Test Procedure:
1 Open the 3-way stopcock for all three limbs.
2 Switch on the digital barometer (the digital barometer should now display the actual ambient
pressure).
Page 75

3 Testing and Maintenance
3
Now slowly draw at the syringe, as if filling the syringe, until the pressure (as displayed on the
digital barometer) drops to approximately 350 mbar below ambient pressure.Then close the line to
the syringe at the 3-way stopcock to syringe (circled in picture below).
4 Let the reading on the digital barometer stabilize for a moment and then perform the leakage
check: for 30 seconds the change of the pressure reading should be less than 20 mbar.
5 If the leakage test is NOT passed, check all connections once more and repeat the test.
Test Expected test results
Leakage Check Reading on the digital barometer
change is less than 20 mbar for 30
seconds (pass/fail)
NOTE
To ensure the integrity of the Leakage Test Kit (M1013-64002, 451261014851) the following Kit Leak
Test Procedure must be performed:
a. Form a loop with the leakage test kit as shown in the picture below.
b. Connect the syringe to the 3-way stopcock and the digital barometer to the open tubing.
c. Draw at the syringe until the digital barometer shows approximately 350 mbar below ambient
pressure.
d. Close the 3-way stopcock to the syringe and wait 5 - 10 seconds. In this time, the overall
pressure should stabilize.
e. After 1 minute, check the pressure. The pressure should not increase more than 8 mbar in 1
minute for the test to pass.
75
Page 76

3 Testing and Maintenance
f. If this test fails, exchange the leakage test kit.
Barometric Pressure Check and Calibration
NOTE
The M3015A with HW Rev C and the M3015B do not require calibration of the barometric pressure.
Therefore you will not be able to activate a barometric pressure calibration. If you are using a HW Rev
C M3015A or M3015B, perform the barometric pressure check as described below, making sure that
only a sample line is connected to the MMS Extension. If the pressure check fails, the M3015A/B
needs to be exchanged.
Check the barometric pressure value in the M3015A/B MMS Extension or the MP5 as follows:
1 Go into service mode and select Setup CO
2 Connect a calibration line to the Microstream CO
menu.
2
input. This activates the pump in the M3015A/
2
B MMS Extension or the MP5.
3 The status line at the bottom of the screen displays “CO
pressure reading (ambient/cell) xxx/
2
yyy” where xxx is the ambient pressure and yyy is the measured cell pressure. Check whether the
ambient pressure value (x3) matches (within the acceptable tolerance of ±12mm Hg) the reference
value you have received. If so, proceed to the leakage check.
If the value is not correct, calibrate as follows.
CO
a. Select
then select Barom.Press to activate a table of values.
2
b. Select the value in the table which matches the reference value received from a reliable local source
(airport, regional weather station or hospital weather station). (The values are displayed with a
resolution of 2 mmHg up to 500 mmHg and a resolution of 1 mmHg from 500 mmHg to 825
mmHg.) Note: the selected value must be within ±10% of the current measured ambient pressure,
otherwise an error message will occur at restarting the monitor.
c. Confirm the barometric pressure setting.
d. Check that the ambient pressure displayed in the status line at the bottom of the screen is the same
as the value which you selected from the list in step b.
Test Expected test results
Barometric Pressure Check x3 = difference between the reference
pressure and the measured ambient
pressure displayed on the monitor
Flow Rate Check and Calibration
Check the flow rate in the M3015A/B MMS Extension or the MP5 as follows:
1 Connect the calibration line to the mCO
2 Check on the flowmeter the flow that the M3015A/B MMS Extension or MP5 mCO2 pump
draws (x5). It should be 50 +15/-7.5 ml/min. If the value is within tolerance, proceed to the CO
Gas calibration check.
If the value is not within tolerance, calibrate as follows.
a. Adjust the flow in the instrument by selecting
possible to 50 ml per minute as indicated on the flowmeter gauge.
76
(x3<12 mmHg)
inlet and the flowmeter outlet to the calibration line.
2
Increase Flow or Decrease Flow until it is as close as
2
Page 77

Noise Check
3 Testing and Maintenance
b. When you are satisfied that the flow is set as close as possible to 50 ml per minute, select Store Flow
and confirm the setting. If you do not store the adjusted flow within 60 seconds of the adjustment,
the old flow setting is restored.
c. If you cannot adjust the flow to within tolerance, replace the pump . If you still cannot make the
flow adjustment, this indicates a fault in the measurement extension, which must be replaced.
Note that the pump can only be replaced on M3015A with the old hardware Rev. A (i.e. Serial No.
DE020xxxxx)
Test Expected test results
Flow Rate Check Flow rate is 50 +15/-7.5 ml/min
1 With the monitor in service mode, select Setup CO
2 Connect the calibration line, the cal tube, the flow regulator and the 5% calibration gas to the
inlet.
mCO
2
menu.
2
3 Open the valve to apply the 5% calibration gas and wait until the value is stable.
4 Check the noise index (x6) displayed next to the CO
of noise on the CO
wave). If the value exceeds 3 mmHg, replace the measurement extension.
2
Test Expected test results
Noise Check x6 = noise index displayed on monitor
CO2 Cal Check and Calibration
After switching the measurement extension on, wait at least 20 minutes before checking the
calibration. Check the calibration of the CO
value on the display (this indicates the level
2
(x6<3.0)
gas measurement as follows:
2
77
Page 78

3 Testing and Maintenance
1
Connect the calibration line, the cal tube, the flow regulator and the 5% calibration gas to the
inlet.
mCO
2
2 Calculate the expected measurement value in mmHg as follows:
0.05 x (ambient pressure) = value mmHg
for example 0.05 x 736 = 36.8 mmHg (with an ambient pressure of 736 mmHg)
3 Open the valve on the flow regulator to allow 5% CO
gas to flow into the extension. Allow the
2
value to stabilize.
4 Check that the value on the instrument (measurement value on the main screen, x7) matches the
calculated mmHg value ± 2.6 mmHg.
If the value is outside the tolerance, calibrate as described in step 8a to 8e below.
5 Disconnect the 5% calibration gas and connect the 10% calibration gas.
6 Calculate the expected measurement value and tolerance in mmHg as follows:
0.1 x (ambient pressure) = value mmHg
±0.07 x (value mmHg) = tolerance
for example 0.1 x 737 mmHg = 73.7 mmHg (with an ambient pressure of 737 mmHg)
±0.07 x 73.7 mmHg = ±5.16 mmHg tolerance
7 Open the valve on the flow regulator to allow 10% CO
gas to flow into the extension. Allow the
2
value to stabilize.
8 Check that the value on the instrument (x8) matches the calculated mmHg value within the
calculated tolerance. If so, the measurement extension is correctly calibrated.
If the value is outside the tolerance, calibrate as follows.
a. Keep the same setup and connect the 5% calibration gas.
b. Select
Cal. CO
.
2
c. Select the value for the calibration gas. (The default value is 5.0%.)
78
d. Open the valve on the calibration gas to allow CO2 gas to flow into the extension. Allow the value
to stabilize before the start of the calibration. Leave the valve open until the instrument gives a
prompt that gas can be removed.
Page 79

e. The extension calibrates and prompts when calibration is successful.
Test Expected test results
CO2 Cal Check x7 = calculated mmHg value ±2.6 mmHg
Calibration Verification
1 Keep the same setup as described in “CO2 Cal Check and Calibration” on page 77.
2 Reopen the 5% gas valve and allow the value to stabilize.
3 Check that the value displayed on the monitor is correct within the tolerance (see step above).
4 Disconnect the 5% calibration gas and connect the 10% calibration gas.
5 Open the valve on the flow regulator to allow 10% CO2 gas to flow into the extension. Allow the
value to stabilize.
6 Check that the value displayed on the monitor is correct within the tolerance (see step above).
If one or both values are not within tolerances, you must exchange the M3015A/B MMS Extension or
the MP5 mCO
Assembly.
2
3 Testing and Maintenance
x8 = calculated mmHg value within
calculated tolerance
Test Expected Test Results
Leakage Check parts
1 and 2*
x1 = value of part 1 leakage check on flowmeter
(x1< 4.0 ml/min)
x2 = value of part 2 leakage check on flowmeter
(x2< 4.0 ml/min)
Leakage Check
without Flowmeter
Barometric Pressure
Check
reading on the digital barometer change is less than 20
mbar for 30 seconds
x3 = difference between the reference pressure and the
measured ambient pressure displayed on the monitor
(x3<12 mmHg)
Flow Check x4 = difference between measured value and 50.0 ml/min
(x4 = 50+15/-7.5 ml/min)
Noise Check x5 = noise index displayed on monitor (x5<3.0)
Gas Calibration
CO
2
Check
x6 = difference between measured CO2 value and
calculated value, based on 5% CO
cal. gas. (x6 < 2.6
2
mmHg)
CO2 Cal Verification x7 = difference between measured CO2 value and
calculated value, based on 10% CO
cal. gas.
2
(x7 < ± {0.07 x value calculated})
* M3015A HW Rev. B and FW Revision < P.01.32 only
79
Page 80

3 Testing and Maintenance
Spirometry Performance Tests
These tests verify the performance accuracy of the M1014A Spirometry module.
Equipment Required
• Leak test kit (Part number: M1014-64100)
• calibrated barometer
• M2785A Pediatric/Adult Flow Sensor
• 500ml calibration syringe, Hans Rudolph model 5550 or equivalent
Flow Test
1 Connect the M1014A Spirometry Module to the host monitor and go into service mode.
2 Connect the flow sensor to the module.
3 Connect the 500ml calibration syringe to the flow sensor. Make sure the syringe is set to the
“empty” position.
4 Press the Setup key on the module and select Show all Values in the Setup Spirometry menu.
5 Pump the calibration syringe back and forth with a steady motion at a rate of 20 cycles and verify
that the readings for TVexp and TVin are 500 ± 25 ml.
If the readings are not within the specified range, try another flow sensor. Ensure that the syringe is
calibrated correctly and that the procedure is performed exactly as described above. If the test fails
again, replace the module.
Leakage Test
1 Connect the M1014A Spirometry Module to the host monitor and go into service mode.
2 Connect the leak test adapter to the module.
3 Press the Setup key on the module and then select Show all Values in the Setup Spirometry menu.
4 Press the Purge key on the module and start a purge cycle. At the end of the purge cycle, the values
5 Verify that the pressure difference between Ppeak and Paw remains less than 10 cmH2O after 30
If the readings are not within the specified range or if an INOP (e.g. SPIRO PURGE FAILED) is
issued, check the leak test adapter for any leaks. Disconnect the adapter from the module and start the
test procedure from the beginning. If the test fails again, replace the module.
Test Expected test results
Flow Test TVexp and TVin are 500 ± 25 ml
for Paw and Ppeak should both be above 100 cmH2O.
seconds.
Test Expected test results
Leakage Test Paw and Ppeak >100 cmH2O
Barometer Check
1 Connect the M1014A Spirometry Module to the host monitor and go into service mode.
2 Attach any airway adapter to the module.
80
Page 81

3
Press the Setup key on the module and then select Show all Values in the Setup Spirometry menu.
4 Check that the barometric reading (PB) is within ± 5 mmHg of a reference barometer.
5 If the readings are not within the specified range, check the accuracy of the barometric pressure
reference again. If the test fails again, replace the module.
Test Expected test results
Barometer Check PB is within ± 5 mmHg of a reference
barometer
NOTE
The built-in barometer cannot be recalibrated.
Cardiac Output (C.O.) Performance Test
These tests check the performance of the cardiac output measurement.
1 Connect the patient simulator to the C.O. module using the patient cable.
2 Configure the patient simulator as follows:
Injection temperature: 2 °C
Computation Const: 0.542
(Edward's Catheter)
Flow: 5 l/min
3 Testing and Maintenance
3 Check displayed value against the simulator configuration.
4 Expected test result: C.O. = 5 +/– 1 l/min.
Test Expected test results
Cardiac Output Performance Test C.O. = 5 +/– 1 l/min
Service Tool Procedure, Version 1
This procedure applies for Service Tool M1012-61601 in combination with C.O. modules without
option C10 and M3012A MMS extensions with option C05.
1 In monitoring mode, connect the C.O. interface cable to the module.
2 Connect one side of the service tool to the injectate receptacle of C.O. interface cable and the
other side to catheter cable receptacle.
3 Enter the C.O. Procedure window and check the results. The expected test result is:
Tblood = 37.0
o
C +/- 0.1oC
Test Expected test results
Cardiac Output Service Tool Procedure
Version 1
Service Tool Procedure, Version 2
This procedure applies only for Service Tool M1012-61601 in combination with C.O. modules with
option C10 and for the M3012A MMS Extension with option C10.
Tblood = 37.0
o
C +/- 0.1oC
1 In monitoring mode, connect the C.O. interface cable to the module.
81
Page 82
3 Testing and Maintenance
2
Connect one side of the service tool to the injectate receptacle of the C.O. interface cable and the
other side to the catheter cable receptacle.
3 Enter C.O. Procedure window and check results for:
– Method of measurement
– Arterial Catheter constant
– Tblood
The expected results are:
– Transpulmonary
– 341
– Tblood = 37.0
4 Make sure the main alarms are switched on.
5 Disconnect the Catheter cable receptacle from the service tool
6 Enter the Setup C.O Window and change the method of measurement to “Right Heart”
7 Enter the C.O. Procedure window and check the Tinj value. The expected result is:
Tinj = 0.0
o
Test Expected test results
o
C +/- 0.1oC
C +/- 0.1oC
Cardiac Output Service Tool Procedure
Version 2
NMT Performance Test
NMT Stimulation Output Test
1 Short circuit the stimulation cables by connecting the two cable clamps to each other as shown
below.
Tinj = 0.0oC +/- 0.1oC
82
2 In service mode, select the Setup NMT menu.
3 Select Start Test.
4 Select Confirm.
Test Expected test results
NMT Stimulation Output Test
NMT Stimulation Output Test passed is
displayed on the monitor.
Page 83

NMT Transducer Test
1 Go into Service Mode. In Service Mode the NMT Bar Graph only contains three bars instead of
four.
2 Place the NMT Transducer on a flat surface with the flat side facing downwards. Two of the three
bars in the NMT bar graph should be at the same level and the third one should be higher than the
other two.
3 Testing and Maintenance
3 Turn the NMT Transducer 180° and place it on a flat surface with the flat side facing upwards.
The bar that was higher than the other two before should now be lower than the other two by
approximately the same amount.
Test Expected test results
NMT Transducer Test First two bars in the NMT bar graph
are at the same level, third bar is
higher when the flat side of the
transducer is facing downwards and
lower by the same amount when the
transducer is facing upwards.
83
Page 84

3 Testing and Maintenance
IntelliBridge Performance Test
This test checks the performance of the IntelliBridge EC10 & EC5 modules.
Tools required: none / external device (i.e. ventilator) and the required IntelliBridge EC5 Module
1 Plug the IntelliBridge EC10 module into the Philips patient monitor or run the test with the built-
in EC10 I/O board.
2 Connect the Service PC to the IntelliBridge EC10 module or I/O board and make sure the correct
drivers for the external devices are installed. (See the chapter for details).
3 Depending on your external device, connect the appropriate EC5 ID module (indicated on the
EC5 label) to the external device.
4 Connect the EC5 to the EC10 module or I/O board using the supplied cable.
5 Switch the external device on. The connection status LED will flash green until it has correctly
identified the external device and started communication. Check that the connection status LED
then lights green continuously indicating that communication has been established. Information
from the external device should now be available on the Philips patient monitor.
6 Select Main Setup -> Measurements -> <External Device Name> to enter the setup menu for the
connected device.
7 Select Setup Waves or Setup Numerics and make any required changes.
8 Close the setup menu.
9 Select the wave segment on the screen, in which you want the waves to be displayed. In the pop-up
menu, select Change Wave, and then select WAVE.
10 We recommend that you confirm with the user that waves and numerics required from the
external device are being accurately received. If the external device has a demo mode, use this.
Test Expected test results
IntelliBridge Performance Test Numerics are visible on screen (pass/
fail)
Recorder Performance Test - M1116C
This test checks the performance of the recorder module M1116C.
1 Load paper into the recorder (for paper loading instructions, refer to your monitor's Instructions
for Use).
2 Start a recording, e.g. an Alarm Limits Recording.
3 If no print-out appears, the paper may be loaded backwards or the wrong paper may be inserted.
4 Try reloading the paper. Make sure you are using the correct paper.
Test Expected test results
Recorder Performance Test Recording is printed correctly
EEG, SvO2 (SO2) and tcGas Performance Tests
The EEG and SVO2 (SO2) parameters do not require performance tests because the modules perform
internal self-tests regularly. These tests suffice for performance testing of these three parameters.
Since the tcGas Module is calibrated regularly it also does not require a separate performance test.
84
Page 85

Nurse Call Relay Performance Test
The nurse call relay performance test can be performed at the Modular Jack 6P6C connector.
This test checks the operation of the Nurse Call Relay. The Nurse Call Relay test is recommended for
customer sites where the nurse call is in use. The Nurse Call relay functions as follows:
• Standard Operation—connector contact 1-2 open; 1-5 closed.
• Alarm Condition—connector contact 1-2 closed; 1-5 open.
Tools required: Ohmmeter.
1 Plug a 6P6C Modular Plug into the Nurse Call Relay connector.
2 Connect the ohmmeter.
3 When no alarm occurs, connector contacts 1-2 are open and connector contacts 1-5 are closed.
When an alarm occurs, connector contacts 1-2 are closed and connector contacts 1-5 are open.
3 Testing and Maintenance
4 The expected test result is: Alarm condition - Connector contacts 1-2 are closed and connector
contacts 1-5 are open.
Test Expected test results
Nurse Call Relay Performance Test Alarm Condition—
Connector contacts 1-2 are closed and
Connector contacts 1-5 are open
Multi-Port Nurse Call Connector Test (Flexible Nurse Call)
This test checks the operation of the Flexible Nurse Call Relay. The Nurse Call Relay test is
recommended for customer sites where the nurse call is in use. The following diagram and table show
the pins and relay identifiers of the connector:
85
Page 86

3 Testing and Maintenance
Pin Cable Color
Relay
Coding
1black R2-closure
2brown R2-middle
3red R2-opener
4orange R3-closure
5 yellow R3-middle
6 green R3-opener
7blue n/a
8purple n/a
9gray n/a
10 white n/a
11 pink R1-closure
12 light green R1-middle
13 black/white R1-opener
14 brown/white n/a
15 red/white n/a
16 orange/white n/a
17 blue/white R_failure_closure
18 purple/white R_failure_middle
19 green/white R_failure_opener
20 red/black n/a
The Nurse Call relay functions as follows:
• During standard operation R1,R2,R3_opener are closed; R1,R2,R3_closure are open.
• During alarm condition—R1,R2,R3_opener are open; R1,R2,R3_closure are closed.
Tools required: Ohmmeter.
1 Plug an M8087-61001 cable into the Nurse Call Relay connector.
2 Connect the ohmmeter and measure the pins as indicated in the diagram and table.
3 The relay contacts should behave as described above. The behavior may vary depending on
configuration choices. See the Configuration Guide for details on Alarm Relay settings.
4 The expected test results depend on the relay contact used. Please check that the correct relay
activity is initiated during alarm condition.
Test Expected test results
Multi-Port Nurse Call Connector Test Correct relay activity is initiated during
alarm condition (pass/fail)
86
Page 87

MSL Assurance Test
Visually inspect all MSL connector sockets (cable/monitor/MMS).
1 Make sure that the pins of the connectors are not jolted.
2 Make sure that no pin is bent inwards or outwards.
3 Exchange connectors that show any evidence of damage or breakage
3 Testing and Maintenance
Examples of damaged connectors
Test Expected test results
MSL Assurance Test Pins of connector not jolted/bent
(pass/fail)
Power Loss Alarm Buzzer Performance Test
1 Switch on the monitor.
2 Disconnect the monitor from AC power.
3 The Power Loss Alarm Buzzer should beep for about one minute.
4 To switch off the alarm sound, either press the power button or connect the monitor to AC power
Test Expected test results
Power Loss Alarm Buzzer Performance
Beep for one minute
Test
IntelliVue 802.11 Bedside Adapter Communication Test
1 Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2 Go into Service Mode and select Main Setup -> Network -> Setup WLAN. In the Setup WLAN menu:
–set
–set
– set the
Mode to either 802.11Ah, 802.11G, 802.11Bg (not recommended), Auto (not
recommended) or
None (this setting disables the wireless LAN functionality permanently), to
match your wireless infrastructure installation.
SSID to match your installation.
Country code to “1000”. Setting the country code to this value will automatically adjust
the regulatory domain to match the configuration of the infrastructure. Do not set the country
code to values other than “1000” unless otherwise instructed.
87
Page 88
3 Testing and Maintenance
– set the Security Mode to match your installation.
– Enter the required keys/passwords.
3 Select Main Setup -> Network -> WLAN Diagnostic to access the WLAN Diagnostic window.
4 Proper installation of the IntelliVue 802.11 Bedside Adapter is assured by connecting to an access
point over the wireless link. Place the monitor with the IntelliVue 802.11 Bedside Adapter installed
in close proximity to the access point (e.g. if the access point is mounted on the ceiling, place the
monitor directly below). Wait until the
(for Rel. C.0 monitors) or Connected (for Rel D.0 or higher). Take the monitor approximately 5 m
away from the access point. There should be no walls or other obstacles between the monitor and
the access point. The following should apply:
– Observe the
RSSI value will fluctuate but should stay above 30 in a 5 m distance from the access point used.
The wireless link should be active, i.e. the
monitors) or Connected (for Rel D.0 or higher), and the other fields should contain values. If the
RSSI value is significantly lower, check the distance to the access point and the antenna
orientation at the monitor. The antenna orientation should be vertical, but the physical
placement of the monitor or other equipment within its vicinity as well as walls or other
obstacles may influence the antenna orientation required to receive the best RSSI value.
5 If this test fails, retry in a different physical area with a different access point and/or check the
credential settings in the monitor.
Conn.Status field in the service window shows Authenticatd
RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds. The
Conn.Status field should be Authenticatd (for Rel. C.0
Test Expected test results
IntelliVue 802.11 Bedside Adapter
Performance Test
IIT Communication Test
1 Make sure the LAN cable is disconnected from the rear of the monitor, then switch on the
monitor.
2 Go into Service mode and, select Main Setup -> Network -> Setup IIT. In the Setup IIT menu, set
RF Access Code in each profile to match your installation.
the
3 Go into Service Mode. Select Main Setup -> Network -> IIT Diagnostic to access the Instrument
Telemetry Diagnostic window.
4 Proper installation of the IIT module is assured by connecting to an access point over the wireless
link. Place the monitor with the IIT module installed in close proximity to the access point (e.g. if
the access point is mounted on the ceiling, place the monitor directly below). Wait until the
Conn.Status field in the Instrument Telemetry Service window shows Active. Take the monitor
approximately 5 m away from the access point. There should be no walls or other obstacles between the monitor and
the access point. The following should apply:
– Observe the
RSSI value should be around -50 ±10 in a 5 m distance from the access point used and the IIT
link should be active, i.e. the
contain values. If the
and the antenna orientation at both the monitor and the access point (both should be vertical).
– Remove the antenna. The
the connection could be unreliable. The
Seeking. If the difference between the
significantly lower, check the antenna and the antenna connector for damage and verify that
the cable fom the IIT adapter to the antenna connector plate is connected properly.
RSSI (Received Signal Strength Indicator) value for at least 5 - 10 seconds. The
RSSI value above 30
Conn.Status field should be Active and the other fields should
RSSI value is significantly lower, check the distance to the access point
RSSI value should be around -90 ±10. The IIT link may be active but
Conn. Status field may toggle between Inactive and
RSSI values measured with and without antenna is
88
Page 89

5
If this test fails, retry in a different physical area with a different access point.
Error Conditions:
–The field MAC IIT should show a value unequal to 0000 0000 0000. If it does not, there is a
communication problem between the monitor and the IIT adapter.
– With an incorrect RF Access Code or an incorrect or defective antenna installation, the fields
IP Address,Server IP, Subnet Mask, and RSSI in the Instrument Telemetry Service window will
stay blank. The field
6 Perform the Access Point Controller (APC) test blocks as described in the Philips IntelliVue
Wireless Network Installation and Configuration Guide.
Test Expected test results
IIT Communication Test IIT Communication without
Conn. Status will slowly toggle between Inactive and Seeking.
interference
Short Range Radio (SRR) Performance Test
1 Make sure that the short range radio interface is configured as follows: SRR On and appropriate
channel selected.
2 Assign a wireless remote control to the monitor as described in .
3 Check that you can operate the monitor with the remote control.
3 Testing and Maintenance
Test Expected test results
SRR Performance Test Wireless Remote Control functions
Mounting Integrity Test
Perform the Mounting Integrity Test
– whenever you have removed and reassembled a quick mount
– if one or both of the quick mount screws are loose
– if there is a clearance between the quick mount and the monitor bottom housing
– if the monitor mounting is unstable
Remove the monitor from the mount and disassemble the quick mount. Ensure that the threading of
the MX400/450/500/550 is not damaged or separated from the chassis.
If the quick mount is damaged, exchange the quick mount.
Ensure that all quick mount screws are tight (1.3 Nm). Test the quick mount by pressing the quick
release button. If it comes back out gradually and regularly, the quick mount is inserted correctly. If it
gets stuck, the quick mount is not centered and must be reinserted correctly.
If you notice any damage to the threading of the MX400/450/500/550 chassis, send the MX400/450/
500/550 in for bench repair.
correctly. Monitor can be operated
with Remote Control
Test Expected test results
Mounting Integrity Test All quick mount screws are tight. No
damage to quick mount. No damage
to threading of MX400/450/500/550.
Quick release button comes back out
gradually and regularly.
89
Page 90

3 Testing and Maintenance
Reporting of Test Results
Philips recommends all test results are documented in accordance with local laws. Authorized Philips
personnel report the test result back to Philips. While hospital personnel (biomedical engineers or
technicians) do not need to report results to Philips, Philips recommends that they record and store the
test results in accordance with local laws.
The following table lists what to record after completing the tests in this chapter. Record the results in
the empty column in the Test and Inspection Matrix.
The following is a guide as to what your documentation should include:
• Identification of the testing body (for example, which company or department carried out the
tests).
• Name of the person(s) who performed the tests and the concluding evaluation.
• Identification of the device(s) and accessories being tested (serial number, etc.).
• The actual tests (incl. visual inspections, performance tests, safety and system tests) and
measurements required
• Date of testing and of the concluding evaluation.
• A record of the actual values of the test results, and whether these values passed or failed the tests.
• Date and confirmation of the person who performed the tests and evaluation.
The device under test should be marked according to the test result: passed or failed.
90
Page 91

Carrying Out and Reporting Tests
Test Report
3 Testing and Maintenance
Testing Organization:
Name of testing person:
Date:
Responsible Organization:
Device Under Test: ID-Number
Product Number: Serial No.:
Accessories:
Measurement Equipment (Manufacturer, Type, Serial
No., Calibration Date):
Safety Test Method used
Functional Test (parameters tested):
Mains voltage and frequency used during safety
testing:
(Check one of the following three options)
Test before putting into service (reference value)
Recurrent Test
Test after Repair
Test and Inspection Matrix
Test Test or Inspection
to be Performed
Expected Test Results Record the Results (mandatory for
Philips Personnel only)
What to record Actual Results
Visual
Inspection
Power On Power on the unit.
Noninvasive
Blood Pressure
Performance
Tests
Temperature
Performance
Test
Perform Visual
Inspection
Does the self-test
complete
successfully
Perform the
Accuracy Test
Performance
Leakage Test
Performance
Linearity Test
Performance Valve
Test
Perform the
Temperature
Performance Test
Pass or Fail V:P or V:F
If Yes, Power On test is passed PO:P or PO:F
X1 = value displayed by monitor
Difference <= 3mmHg
X2 = leakage test value
X2 < 6 mmHg
X3 = value displayed by monitor
Difference <= 3mmHg
X4 = value < 10 mmHg PN:P/X4 or
X1= 40°C ± 0.2°C or 100°F ±
0.4°F
PN:P/X1 or
PN:F/X1
PN:P/X2 or
PN:F/X2
PN:P/X3 or
PN:F/X3
PN:F/X4
PT: P/X1 or
PT: F/X1
91
Page 92

3 Testing and Maintenance
Test Test or Inspection
to be Performed
Expected Test Results Record the Results (mandatory for
Philips Personnel only)
What to record Actual Results
All other
performance
tests
Perform the
remaining
parameter
See expected results in test
procedures
P: P or
P: F
performance tests,
if applicable
Safety (1) Perform Safety Test
(1): Protective
Earth Resistance
Safety (2) Perform Safety Test
(2): Equipment
Leakage Current -
With mains cable:
Maximum impedance (X1):
<=300 mOhms
With mains cable:
Maximum leakage current
(X1):<= 100 μA
S(1):P/X1 or
S(1):F/X1
S(2): P/X1 or
S(2): F/X1
*
*
Normal Condition.
Safety (3) Perform Safety Test
(3): Equipment
Leakage Current -
With mains cable:
Maximum leakage current
(X2):<= 300 μA
S(3): P/X2 or
S(3): F/X2
*
Single Fault
Condition (Open
Earth)
Safety (4) Perform Safety Test
(4): Applied Part
Leakage Current -
Maximum leakage current (X1):
<=50 μA (CF)
S(4): P/X1 or
S(4): F/X1
*
Single Fault
Condition, mains
on applied part.
System
(Sys 1-2)
Perform the system
test according to
subclause 19.201 of
IEC/EN 60601-1-1
or IEC/EN 606011+A1 Ed.3 clause
Equipment Leakage Current:
Sys1 <= 100 μA (Normal
Condition)
Sys2 <= 300μA (Single Fault
Condition
Sys: PSys1/PSys2
or
Sys: FSys1/Fsys2
*
16, if applicable,
after forming a
system
System
(Sys 3)
Perform the system
test according to
subclause 19.201 of
IEC/EN 60601-1-1
or IEC/EN 606011+A1 Ed.3 clause
16, if applicable,
Protective Earth Leakage
Current if medical electrical
system components are
connected to the same Multiple
Portable Socket Outlet:
Sys3 <= 300 μA
Sys: PSys3
or
Sys: FSys3
*
after forming a
system
Key: P = Pass, F = Fail, X or Sys = test value to be recorded, * = Record the worst-case results and the associated
switch positions (e.g. normal/reverse polarity)
92
Page 93

NOTE
All values for current and voltage are the root mean square (r.m.s.) values, unless otherwise stated.
Evaluation
Safety and Functional Test passed
Repair required at a later date, safety and functional test passed
Device must be taken out of operation until repair and passed tests
Device failed and must be taken out of operation.
Notes:
Next Recurrent Test:
Name:____________________________________________________
Date/Signature:_____________________________________________
3 Testing and Maintenance
Yes No
Evaluation of Test Results
The evaluation of the test results must be performed by appropriately trained personnel with sufficient
product, safety testing and application knowledge.
If any test results are between 90% and 100% of the respective expected result, the previously
measured reference values must be taken into consideration for the assessment of the electrical safety
of the device under test. If no reference values are available, you should consider shorter intervals
between upcoming recurrent tests.
NOTE
If any single test fails, testing must be discontinued immediately and the device under test must be
repaired or labeled as defective. Be sure to inform the user about the test failure in writing.
Other Regular Tests
The care and cleaning requirements that apply to the monitor and its accessories are described in the
Instructions for Use. This section `details periodic maintenance procedures recommended for the
monitor and its accessories.
Touchscreen Calibration
To access the touchscreen calibration screen:
1 Enter service mode
2 Select Main Setup
3 Select Hardware
93
Page 94

3 Testing and Maintenance
4
Select Touch Calibration
Touchscreen Calibration Screen
Make sure you complete the calibration procedure without powering off the monitor mid-way. If the
monitor is powered off after the first point is touched, the touch panel will be deactivated until the
touch calibration is performed again.
If the touchscreen is accidentally mis-calibrated by selecting the wrong spot, you must use another
input device to re-enter calibration mode. If you have the Support Tool, you can initiate a touch
calibration from there.
Please refer to the documentation shipped with your selected display for further details on touchscreen
calibration procedures.
NOTE
If a touchscreen calibration is started on a multiple display system, the calibration is started for all
displays at the same time.
Disabling/Enabling Touch Operation
To temporarily disable touchscreen operation of the monitor, press and hold the Main Screen key. A
padlock symbol will appear on the key. Press and hold the
touchscreen operation.
Main Screen key again to re-enable
Printer Test Report
94
To verify your printer configuration you may want to print a test report.
To print a test report select
Your test report should look like this:
Main Setup -> Reports -> Setup Printers -> Print Test Rep.
Page 95

3 Testing and Maintenance
Battery Handling, Maintenance and Good Practices
This section provides some information on how to handle and maintain the battery in order to get the
best usage from it. Additionally, some good working practices are also given regarding the correct
disposal of the batteries.
Battery Care
Battery care begins when you receive a new battery and continues throughout the life of the battery.
The table below lists battery care activities and when they should be performed.
Activity When to Perform
Perform a visual inspection Before inserting a battery into the
Charge the battery Upon receipt, after use, or if a low
Condition the battery When the "battery requires
Store the battery in a state of
charge in the range of 40% to
50%
monitor
battery state is indicated. To optimize
performance, a fully (or almost fully)
discharged battery should be charged
as soon as possible.
maintenance" symbol appears
When not in use for an extended
period of time
Refer to your monitor's Instructions for Use for details on how to perform battery care activities,
including charging and conditioning. We recommend using the Philips Smart Battery Conditioner LG
1480 (865432)
95
Page 96

3 Testing and Maintenance
Handling Precautions
Lithium ion batteries store a large amount of energy in a small package.Use caution when handling the
batteries; misuse or abuse could cause bodily injury and/or property damage.
• Do not short circuit - take care that the terminals do not contact metal or other conductive
materials during transport and storage
• Do not crush, drop or puncture - mechanical abuse can lead to internal damage and internal short
circuits which may not be visible externally
• Do not apply reverse polarity
• Do not expose batteries to liquids
• Do not incinerate batteries or expose them to temperatures above 60°C (140°F)
• Do not attempt to disassemble a battery.
If a battery has been dropped or banged against a hard surface, whether damage is visible externally or
not:
• discontinue use
• dispose of the battery in accordance with the disposal instructions
If a battery shows damage or signs of leakage, replace it immediately. Do not use a faulty
battery in the monitor.
Storage
When storing batteries, make sure that the battery terminals do not come into contact with metallic
objects, or other conductive materials.
If batteries are stored for an extended period of time, they should be stored in a cool place, ideally at
15°C (60°F), with a state of charge of 20% to 40%. Storing batteries in a cool place slows the aging
process.
The batteries should not be stored at a temperature outside the range of -20°C (-4°F) to 60°C (140°F).
Do not store batteries in direct sunlight.
Stored batteries should be partially charged to 20% to 40% of their capacity every 6 months. They
should be charged to full capacity prior to use.
NOTE
Storing batteries at temperatures above 38°C (100°F) for extended periods of time could significantly
reduce the batteries’ life expectancy.
Battery Lifetime Management
The lifetime of a Lithium Ion battery depends on the frequency and duration of use. When properly
cared for, the useful life is approximately 3 years or 500 charge-discharge cycles, whichever comes first.
In addition, experience indicates that the incidence of failure may increase with battery service life due
to the accumulated stresses of daily use. We therefore strongly recommend that lithium ion batteries be
replaced after 3 years or 500 charge-discharge cycles.
96
The age of a lithium ion battery begins at the date of manufacture. To see the date of manufacture and
the number of charge-discharge cycles, select the battery symbol on the patient monitor screen.
The date of manufacture and the number of charge-discharge cycles are listed with other battery data
on the screen.
Page 97

WARNING
The risk of battery failure increases with age, when a battery remains in use longer than 3 years or 500
charge-discharge cycles. Such failures can result in overheating that in rare cases can cause the battery
to ignite or explode.
Disposal
Batteries should be disposed of in an environmentally-responsible manner. Consult the hospital
administrator or your local Philips representative for local arrangements.
Discharge the batteries and insulate the terminals with tape before disposal. Dispose of used batteries
promptly and in accordance with local recycling regulations.
About the Battery
The rechargeable Lithium-Ion batteries used in the monitor are regarded as Smart batteries because
they have built-in circuitry. (This circuitry communicates battery status information to the monitor.)
Actual current/voltage: depends on smart battery request and monitor configuration. The approximate
charging time is 3 hours with the monitor switched off and up to 5 hours during monitor operation,
depending on the monitor configuration.
3 Testing and Maintenance
NOTE
Batteries will discharge within about 20 days if they are stored inside the monitor without AC power
connection.
Checking the Battery Status
When the Monitor is connected to the AC power supply, the battery charges automatically. The battery
can be charged remotely from the Monitor by using the battery charger. Use only the 865432 Smart
battery conditioner.
Battery status (level of charge) is indicated in several ways:
• LED on the front panel of the Monitor.
• Battery gauge.
• Display of battery time below gauge.
• Battery status window.
• INOP messages.
The AC Power LED is only on when the power cord is connected and AC power is available to the
Monitor. In this case, the battery can be either charging or fully charged.
The battery LED can be green, yellow, or red depending on the following conditions:
Battery LED Colors If the monitor is connected to
AC power, this means
If the monitor is running on
battery power, this means
Green
battery full (≥90%)
97
Page 98

3 Testing and Maintenance
Battery LED Colors If the monitor is connected to
AC power, this means
Yellow
Red, flashing
Red, flashes intermittently
Red, flashing when on/
standby switch is pressed
1 indicated by malfunction symbol and INOP
2 for further details see Troubleshooting section
battery charging
(battery power < 90%)
battery or charger malfunction1,2 battery or charger malfunction1,2
Battery Status on the Main Screen
Battery status information can be configured to display permanently on all Screens. It shows the status
of the battery and the battery power and battery time remaining. The battery time is only displayed
when the monitor is not running on AC power. Note that the battery status information may take a
few minutes after the monitor is switched on to stabilize and show correct values.
If the monitor is running on
battery power, this means
≤ 10 minutes power remaining
not enough battery power left to
power monitor
Battery power gauge:
This shows the remaining battery power. It is divided into sections, each representing 20% of the total
power. If three and a half sections are shaded, as in this example, this indicates that 70% battery power
remains. If no battery is detected, the battery gauge is greyed out.
Battery malfunction symbols:
If a problem is detected with the battery, these symbols are displayed. They may be accompanied by an
INOP message or by a battery status message in the monitor information line (if battery window is
open) providing more details.
Battery Status Symbols
Battery requires maintenance
Battery is empty
Battery not charging as the temperature is above or below the specified range
Charging stopped to protect the battery
98
Battery Malfunction Symbols
Incompatible Battery
Page 99

3 Testing and Maintenance
Battery Malfunction
Battery temperature too high
Battery has no power left
Explanations of Battery Status and Malfunction Symbols:
Battery requires maintenance: The battery requires conditioning. Refer to “Conditioning Batteries” for
details.
Battery is empty: The capacity of the battery is ≤10 min. Recharge the battery as soon as possible.
Temperature outside specified range: The charging of the battery is stopped if the temperature is below 15°C
or above 50°C in order to protect the battery. Charging is resumed as soon as the temperature is within
this range.
Incompatible Battery: The inserted battery is checked for certain battery internal parameters. If these are
not correct, the incompatible battery symbol is displayed. Please use only M4605A batteries with the
MX400/450/500/550 monitor. Note that the incompatible battery symbol may also appear if there is
a communication problem between the battery and the Standard System Interface board.
Battery Malfunction:Communication between the battery and the Standard System Interface board could
not be established or battery internal data indicates malfunction. Please see the “Troubleshooting”
section for remedies.
Battery Temperature too high: This symbol is displayed if the battery temperature goes above 65°C. In
addition the INOP message CHECK BATT TEMP is displayed. If the battery temperature increases
further above 70°C the batteries will switch off for safety reasons. Allow the battery to cool down to
avoid the monitor switching off.
Battery has no power left: If the monitor is not running on AC power: battery will switch off power
delivery at any moment - in this case recharge the battery immediately - or, if the monitor is running on
AC power, the battery is in deep discharge and requires pre-charging to restore communication. To
avoid this condition charge batteries to 50% for storage. Note that the battery malfunction INOP will
eventually be issued if the pre-charging does not restore battery communication within about 4
minutes.
99
Page 100

3 Testing and Maintenance
Battery Status Window
♦ To access the Battery Status window and its associated pop-up keys, select the battery status
information on the Screen, or select
State of Charge tells you the state of charge of the battery.
Time To Empty tells you approximately how long you can continue to use the monitor with this battery.
Note that this time fluctuates depending on the system load (how many measurements and recordings
you carry out), the age of the battery, and the remaining capacity of the battery. The time indication
appears after AC has been unplugged for about 10 seconds (after finishing calculation of the Time to
Empty)
Main Setup -> Battery.
Time To Full is shown in place of Time To Empty if the monitor is connected to AC power, and tells you
how much time is left until the battery is charged to 90%. Please allow indication to stabilize for 3 to 5
minutes after beginning the charging cycle. If the battery is charged over 90%
displayed until they are charged to 100%. Then
Documenting Battery Status
To print all battery information in the Battery Status window,
1 Select the battery status information on the Screen or select Main Setup -> Battery to open the
Battery Status window
2 Select the Record Status pop-up key to print the information on a recorder
or
Select the
Print Status pop-up key to print the information on a connected printer.
Conditioning a Battery
What is Battery Conditioning?
Battery conditioning recalibrates the battery to ensure that it has accurate information on the actual
battery capacity.
Why is Battery Conditioning Necessary?
The capacity of a battery decreases gradually over the lifetime of a battery. Each time a battery is
charged its capacity decreases slightly. Therefore, the operating time of a monitor running on batteries
also decreases with each charge cycle.
Battery Full (>90%) is
Batt Fully Charged is displayed.
100
 Loading...
Loading...