Page 1

Instruction Manual
M24/7 Ambulatory Blood Pressure Monitor
www.omron-healthcare.com
EN
1
Page 2

Introduction
Thank you for purchasing the OMRON M24/7 ambulatory blood pressure
monitor.
The OMRON M24/7 is a silent, compact and very reliable ambulatory blood
pressure monitor.
A large LCD screen insures easy readability and its lightweight design provides
for maximum patient comfort.
The OMRON M24/7 operates with two 1.5V AA batteries (included in the
package) or two 1.2V AA rechargeable batteries.
The device can store more than 400 automatic readings along with
approximately 200 manual measurements and events in its solid-state memory
for an unlimited length of time.
Extra measurements can be triggered, and events can be marked manually.
The device can be adjusted to the patient’s lifestyle with a push of the
DAY/NIGHT button.
It provides a voltage display function to show the status of the batteries ensures that fresh batteries or properly charged batteries are used for the
monitoring period.
The OMRON M24/7 is quiet and equipped with multiple patient safety functions.
It can be connected to practically any standard PC with its optical USB interface
cable.
The user-friendly software provides exible programming as well as
comprehensive analysis, presentation and reporting functions.
The OMRON M24/7 has been clinically validated. Quality, safety and reliability
of the product are demonstrated by its CE mark.
2
Page 3

Content
Introduction ........................................................................................................2
Important Safety Information ...........................................................................4
Recommended use of ambulatory blood pressure monitoring ....................6
1. Overview .....................................................................................................7
1.1. Main Unit......................................................................................................7
1.2. Arm Cuffs .....................................................................................................7
1.3. Display .........................................................................................................8
1.4. Package Contents ......................................................................................8
2. Operating Instructions ..............................................................................9
2.1. Working with the OMRON M24/7 Unit .........................................................9
2.2. Using the Buttons ........................................................................................9
2.3. Overview Display ....................................................................................... 11
2.4. Rules of Monitoring ....................................................................................12
2.5. Monitoring Step by Step ............................................................................12
2.6. Manual Programming ................................................................................14
2.7. Batteries.....................................................................................................15
2.8. Cuffs and their application ......................................................................... 16
3. Care and Maintenance.............................................................................17
3.1. Handling Errors and Problems...................................................................17
3.2. Protection, cleaning and washing ..............................................................18
3.3. Maintenance and Storage Conditions........................................................ 19
3.4. Correct Disposal of this Product ................................................................19
3.5. Accessories ...............................................................................................19
4. Technical Data ..........................................................................................20
4.1. Technical parameters.................................................................................20
4.2. Safety Concerns ........................................................................................21
4.3. EMC Information ........................................................................................22
Patient Diary.....................................................................................................26
4.4. Product Warranty Information ....................................................................27
3
Page 4

Important Safety Information
Important Safety Information
This symbol on the Omron M24/7 monitor is a warning that you
should read the accompanying documentation (this manual).
Warning:
Indicates a potentially hazardous situation which, if not avoided,
could result in death or serious injury.
The ambulatory blood pressure monitor should not be used if
any of the following cases apply:
• patients without an indication for ambulatory blood pressure
monitoring
• non-cooperative patients
• patients in any way unable to operate a monitor as intended
• patients requiring urgent / emergency cardiac care
• unconscious or otherwise incapable patients
• patients with serious mobility impairments without supervision
• patients with coagulation disturbances
• children without supervision
• children under the age of 8 years
Though the blood pressure measurement algorithm used in the
OMRON M24/7 has been tested and found to function properly
on patients with atrial brillation or other common arrhythmias,
the oscillometric blood pressure measurement method is
generally recommended for use only with special caution in
patients with arrhythmias, Parkinson’s disease, or other diseases
with tremor.
Always consult a physician for the interpretation of the blood
pressure measurements. Note that any blood pressure recording
may be affected by the body position, the physiological condition
of the patient, and other factors.
Take care to avoid blocking the air ow in the tube of the cuff
and twisting the tube. Make sure the cuff and its tubing do not
cause strangulation or a circulation problem. Should the patient
experience arm numbness or pain remaining after any blood
pressure reading is completed, the cuff should be removed to
avoid permanent vascular or neural injury.
4
Page 5

Caution:
Indicates a potentially hazardous situation which, if not avoided,
may result in minor or moderate injury to the user or patient or
damage to the equipment or other property.
(General Usage)
No user serviceable parts inside. The OMRON M24/7 monitor
contains high complexity electronic and ne mechanical
components. If you have any problems, please refer your
monitor to quali ed service personnel.
OMRON M24/7 described in this manual complies with the
requirements of the EU Medical Devices Directive (93/42 EEC).
0120 is the identi er of Noti ed Body (SGS UK)
MDD
IIa
YYYY/nnnnnn
MDD classi cation IIa. EMC class B. EMC group 1.
The rst four digits of the serial number of a monitor show the
year of production. The rest is the serial number.
This symbol shows that according to regulations OMRON M24/7
should be handled as electronic waste during disposal.
Classi cation of Applied Parts - Type CF
5
Page 6

Recommended use of ambulatory blood pressure
monitoring
The European Society of Hypertension has published recommendations
for ambulatory blood pressure measurement (O’Brien, E. et al. on Behalf of
the European Society of Hypertension Working Group on Blood Pressure
Monitoring, European Society of Hypertension recommendations for
conventional, ambulatory and home blood pressure measurement, Journal of
Hypertension 2003, 21: 821-848).
Ambulatory blood pressure monitoring can bene t patients with in many
situations.
Indications
• Suspected white-coat hypertension
• Suspected nocturnal hypertension
• To establish dipper status
• Resistant hypertension
• Elderly patient
• As a guide to antihypertensive drug treatment
• Type 1 diabetes
• Hypertension of pregnancy
• Evaluation of hypotension
• Autonomic failure
On the contrary, ambulatory blood pressure monitoring should be avoided in
several situations.
Contraindications
• Non-cooperative patients, unconscious or otherwise incapable patients
• Patients requiring urgent / emergency cardiac care
• Patients with coagulation disturbances
• Patients with serious mobility or other impairments without supervision
• Children without supervision; children younger than 8 years
• Though the blood pressure measurement algorithm used in the
OMRON M24/7 has been found to function properly on patients with
atrial brillation or other common arrhythmias, the oscillometric blood
pressure measurement method is generally recommended for use only
with special caution in patients with arrhythmias, Parkinson’s disease, or
other diseases with tremors.
6
Page 7
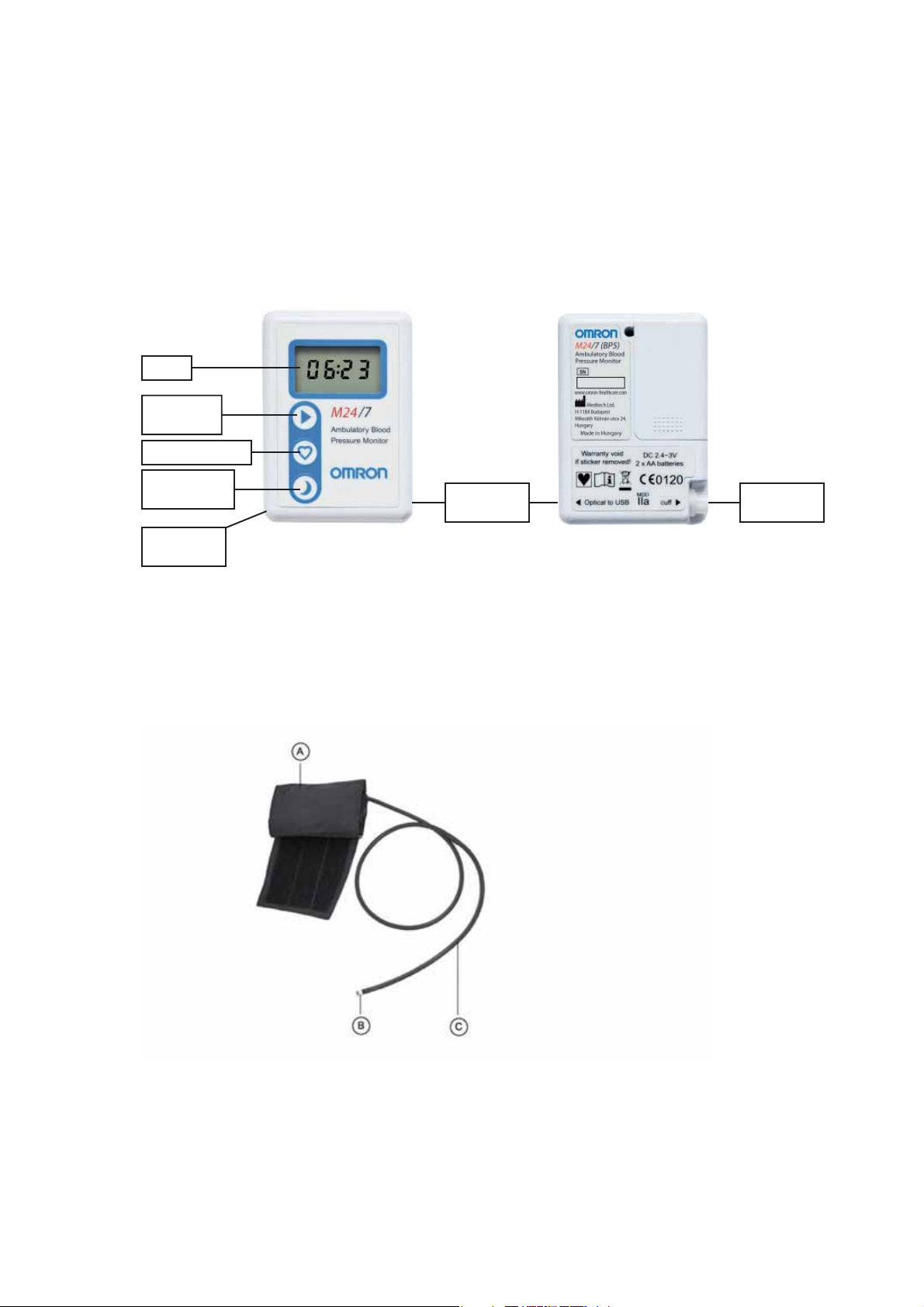
1. Overview
1.1. Main Unit
Front side Back side
LCD
START
button
EVENT button
DAY/NIGHT
button
CUFF
connection
Interface
connection
CUFF
connection
1.2. Arm Cuffs
The set contains 2 adult cuffs: normal size (24-32cm) and large size (32-42cm)
A. Arm Cuff
B. Air Plug
C. Air Tube
7
Page 8

1.3. Display
Heart symbol for
Heart symbol for
pulse indication
pulse indication
Communication
Communication
with PC
with PC
Time display
Time display
Battery voltage
Battery voltage
display
display
Night mode: moon
Night mode: moon
sign is lit
sign is lit
Crossed battery
Crossed battery
symbol warns
symbol warns
about low battery
about low battery
For details of different display options see p. 11
1.4. Package Contents
one (1) OMRON M24/7 monitor unit
one (1) optical USB cable
one (1) carry pouch for monitor, with shoulder and waist straps
one (1) normal size neoprene cuff (latex-free, hand washable sleeve)
one (1) large size neoprene cuff (latex-free, hand washable sleeve)
four (4) 1.5V AA alkaline batteries (LR6)
one (1) OMRON BP Tracker Software CD-Rom or DVD including all
manuals
one (1) M24/7 Instruction Manual
one (1) BP Tracker Software Instruction Manual
Important Note:
Doctor’s Quick Guide and Patient’s Quick Guide ONLY available on the CD-Rom
Declaration of Conformity – to obtain a copy please contact your local OMRON
Distributor.
incl. template for patient diary
8
Page 9

2. Operating Instructions
2.1. Working with the OMRON M24/7 Unit
The OMRON M24/7 device is a compact, lightweight monitoring unit typically
worn by the patient for 24 hours. The OMRON M24/7 operates with two AA
size batteries. The memory is capable of storing more than 600 measurements.
Stepwise pressure de ation ensures quality measurements, even if disturbing
environmental factors occur. You may nd the battery compartment and the rating
label on the backside housing. The serial number is placed on the rating label,
however it is also stored electronically in the solid state memory of the device. On
the front side of the housing, there is the LCD, the buttons of the device and the
name of the device. The device can be connected to the USB port of a PC with
an optoelectronic interface. The device has its socket positioned on the opposite
side from the cuff connector. Patients can start extra blood pressure readings or
mark symptomatic events.
2.2. Using the Buttons
On the front side of the housing, below the LCD, you may nd three buttons: the
START button is marked with a triangle, the EVENT button marked by a heart
and the DAY/NIGHT button indicated by a crescent moon. If the device is turned
on, then every button press is accompanied by a short beeping sound.
START
button
EVENT button
DAY/NIGHT
button
Cancel a blood pressure measurement
The patient can interrupt a blood pressure measurement by pressing a button at
any time while the cuff is in ated. This will result in immediate fast cuff de ation.
Such interruption is limited to the measurement in progress only and has no
effect on further operation. This function is available with all three buttons.
Use this function only if the cuff is hurting the patient’s arm.
Manual blood pressure measurement
If it seems necessary, the patient can start an additional, manual blood pressure
measurement by pressing the START button for less than 3 seconds. The result
with a manual measurement marker will be stored in the memory of the device.
Typical causes for this use: dizziness, palpitations (angina pectoris or headache).
9
Page 10

Switching the device off
Press and hold the START button for more than 10 seconds then release it when
two horizontal segments appear on the LCD, this way the OMRON M24/7 will be
switched off. If you do not release the button in 2 seconds after the two horizontal
segments appeared, the monitor will return to normal operation. This feature helps
to avoid unintended power-off. The device can only be switched on manually.
While the monitor is switched off normal functions are not available, even
prescheduled measurements will not be triggered. Therefore, only in case
of a valid reason should the device be switched off.
Switching the device on
The OMRON M24/7 is switched on to normal operation if the START button is
pressed and held for more than 3 seconds. If the device is switched off, no other
functions are available.
LCD check
Press and hold the START button 3 to 5 seconds to light up all segments of the
LCD to check if they all work correctly.
Battery voltage check
Press and hold the START button for more than 5 seconds to display battery
voltage on the LCD (e.g. 2_37, equal to 2.37 V, see example). After checking
the voltage, please release the button, because after additional 5 seconds, the
device may turn off. The unit will then return to displaying time. The voltage for
fresh alkaline batteries should be over 3 V and for fully charged accumulators
over 2.5 V.
Set a patient event marker
The patient can mark any event without starting a manual blood pressure
measurement by pressing the EVENT button brie y. A typical causes for this
use is taking medicine. The patient should be instructed to record the reason for
setting an event marker in a diary (see Patient’s Quick Guide and template for
patient diary at the end of this user manual).
Mark time of going to bed and rising from bed
If the DAY/NIGHT shift function is disabled during programming, the patient can
press the DAY/NIGHT button to mark the time of going to bed (in the evening)
and rising from bed (in the morning).
10
Manual DAY/NIGHT shift
If this function is enabled during the programming, then the patient can manually
shift the measurement frequency period (day or night) by pressing the DAY/NIGHT
button. The shift is available in the two hour period before the prescheduled shift.
Page 11

2.3. Overview Display
The OMRON M24/7 shows important status information, the processes and results
of individual readings on its LCD. The most important displays are listed here. In
addition to these, a lot of extraordinary situations and errors have their own code
displayed on the LCD. These codes are stored together with recorded data and
listed in the OMRON BP Tracker Software. This helps service personnel in case
of support issues.
Normal status: time is
displayed
. Automatically
set by PC
Blood pressure
measurement initiated.
[mmHg]
Pumping for measurement,
current pressure is
displayed [mmHg]
Heart symbol blinking:
measurement in progress.
[mmHg]
De ation during
measurement, current
pressure displayed[mmHg]
Systolic value of just
completed measurement
[mmHg]
Diastolic value of just
completed measurement
[mmHg]
Night mode: time is
displayed, moon sign is lit
Blood pressure
measurement initiated.
[kPa]
Pumping for measurement,
current pressure is
displayed [kPa]
Heart symbol blinking:
measurement in progress.
[kPa]
De ation during
measurement, current
pressure displayed [kPa]
Systolic value of just
completed measurement
[19,2 kPa]
Diastolic value of just
completed measurement
[12,3 kPa]
Pulse rate value of just
completed measurement
[beats per minute]
Event marker set due to
button push
Communication with
personal computer
Error code display LCD check: all segments
The monitor is switched off
Blood pressure
measurement cancelled by
pressing a button
The crossed battery symbol
warns about low battery
Battery voltage display
(2.37 V)
are displayed
11
Page 12

The monitor must be programmed with the OMRON BP Tracker Software
installed on the computer (for details see Software Manual). Once the preprogrammed time is reached, the monitor will start operating automatically and
perform blood pressure measurements based on the monitoring plan. To obtain
reliable BP readings, certain rules must be observed.
2.4. Rules of Monitoring
1. Inform the patient about the goal and expected results of the monitoring.
Provide an event/patient diary and rules to observe. The Patient’s Quick
Guide will help the patient to remember what has been discussed with you.
The Patient’s Quick Guide can be given to the patient. It also includes a
patient diary. This can be found at the end of this user manual, electronic
versions are available on the CD-ROM or DVD.
2. Patients can t the unit comfortably with the adjustable straps of the carry
pouch.
3. It is advisable to wear a thin shirt under the ABP cuff. This does not in uence
the accuracy of blood pressure measurement, but it prevents problems
caused by long-time wear of the cuff (sweat, itching, soreness, etc.).
4. The cuff should be properly placed and connected.
5. Patients should avoid excess movement during blood pressure measurements.
They should hold their arm loose, slightly away from their chest.
6. Should the blood pressure measurements cause blood shots, torpidity or
pain in the hand, the cuff should be removed from the arm immediately and
disconnected from the monitor. Such occurrence should be reported to the
physician as soon as possible or at the latest after the monitoring session.
7. Patients should not remove the monitor even at night. By loosening the straps,
they can avoid problems when turning in their sleep. The monitor does not
disturb most patients at night.
8. Patients may start extra blood pressure measurements with the START
button of the OMRON M24/7 monitor, marked with a triangle. They should
mark events such as taking medication with the EVENT button, marked with
a heart. They should also mark the time of going to bed and rising from bed,
with the DAY/NIGHT button marked with a crescent moon. They may interrupt
any single blood pressure measurement if necessary by pressing any button.
9. Should the batteries run down during a monitoring session, they can be simply
replaced. Monitoring will continue, and data will not be lost.
10. Patients should never measure anybody else’s blood pressure with an
OMRON M24/7 during an ambulatory blood pressure monitoring session.
12
2.5. Monitoring Step by Step
Before you begin, you must have the OMRON BP Tracker Software properly
installed and con gured on your computer, and the monitor correctly connected
(for details see Software Manual). To program your monitor, you will need the
optical interface cable which is included in the set properly connected to your
computer’s USB port and the communication port (USB) correctly selected in
the OMRON BP Tracker Software.
Page 13

A successful monitoring session consists of the following steps:
1. Connecting the OMRON M24/7 monitor to the PC (First connection only
– not required to repeat with each session)
1. Connect the optical interface, which was delivered with the device, to the
USB port of the PC.
2. The other side of the optical USB interface cable, which is a two-point
plastic connection, should be connected to the socket of the OMRON
M24/7, in a way that the red plastic ring is directed to the lower side of the
device.
3. Start the OMRON BP Tracker Software and open the Options menu, then
click on the Communication tab on the left side of the screen.
4. On the appearing window, click on the USB option. Please connect the
device to the PC (fresh/charged batteries, device switched on) as noted
above then click on the Test button. Upon successful communication the
software will display the serial number and the rmware version of the
device.
2. Preparation of Monitoring Session
1. Inform your patient about the monitoring rules well in advance.
2. Programming the measurement plan into the device:
• Using PC: start the OMRON BP Tracker Software program
• Optionally the device can be programmed without using a PC, for
details see Manual Programming section.
3. Enter new patient data or select patient from the database.
4. Create a monitoring plan with respect to the patient’s lifestyle.
5. Insert two fresh or fully charged, AA size batteries into the battery
compartment and check their voltage.
6. Connect the monitor to the computer.
7. Send the monitoring plan from the computer to the monitor unit.
8. Apply the cuff to the patient with the device placed in the carry pouch.
9. Give the Patient’s Quick Guide to the patient along with detailed
instructions about the rules and the usage of the device.
3. Evaluation
1. Remove the unit and cuff from the patient on his/her return.
2. Ask for the Patient’s Quick Guide /patient diary, and ask the patient
for any events, symptoms, observations or complaints.
3. Start the OMRON BP Tracker Software
4. Connect the device to the PC and then transfer collected data from
the monitor to your database.
5. Analyze the blood pressure pro le.
6. Create and print a report.
13
Page 14

2.6. Manual Programming
The OMRON M24/7 can be programmed with using its buttons, without using a
PC.
Programming Options
There are three different measurement plans which may be selected in case
of manual programming. These plans are stored in the device’s inside memory
and they cannot be changed. The rst plan which is also the default one in
the OMRON BP Tracker Software, measurements every 15 minutes during
daytime and 30 minutes during night. The second one provides less frequent
measurements with 20 and 40 minutes respectively. The third version is based on
30 minute measurement intervals, independent of the time of day.
Other settings are the same in case of all three plans: cuff size not set, pressure
limit 300 mmHg, enabled LCD display and disabled day/night shift. Daytime starts
at 6:00, night time starts at 22:00, special session is disabled. Measurement
period is exactly 24 hours.
Measurement Timing
The rst measurement has a controlling purpose and it starts in the second
minute after the programming, then in the next ve minutes there are no
measurements. The rest of the measurements are taken at speci c 15/20/30/40
minute intervals and there are measurements at the 6:00 and 22:00 hour shifts.
The last measurement is exactly 24 hours after the second measurement. Patient
information can be selected or added later in the OMRON BP Tracker Software.
Manual Programming Step by Step
1. Push the measurement (triangle) and the day/night shift (moon) buttons
simultaneously.
2. Keep them pushed, in 10 seconds the measurement options will be
displayed for 3 seconds each along with the measurement frequencies.
Then the clock will be displayed.
3. In order to choose the measurement plan, you must release the buttons
while that plan is displayed. You will hear two beeps and the LCD will display
four blinking “o” (oooo for four seconds). The process of programming
takes approximately four seconds, then you will hear ve beeps. For
ve seconds, the chosen plan will be displayed again for con rmation. If
programming fails for some reason, the E90 error code will be displayed
on the LCD.
In case of manual programming there is no time setting, as the PC would be
responsible for time synchronizing. If the time setting is imprecise then the time
of measurements may be false. If you would like to use the manual programming
function then do not leave the device without batteries for a longer period. If
you do so then program the device with the PC once and then leave the batteries
in it, so the time can be set by the PC.
14
Page 15

Software
The OMROM M24/7 programmed manually can be used with the OMRON BP
Tracker Software version 1.14.6 or newer.
Manual programming LCD displays:
Normal status: time is
displayed
1. measurement plan:
15/30 minute day/night
intervals
3. measurement plan:
30/30 minute day/night
intervals
10 second delay state: time
is displayed but with no
double-dot
2. measurement plan:
20/40 minute day/night
intervals
Programming in progress:
blinking signal
2.7. Batteries
The OMRON M24/7 ambulatory blood pressure monitor operates with two 1.5V
AA batteries or two 1.2V AA rechargeable batteries. Use only standard longlife (alkaline) batteries, or standard NiCd or NiMH rechargeable batteries of the
proper size. Do not use lithium batteries. Do not mix different battery types, do not
mix new and old batteries. Never use batteries of low or unknown quality or preused batteries, as they may not cover the power needs of the monitor, and they
may damage the monitor, as they may contain acidic electrolytes which may leak
and corrode electronic components. Never use batteries damaged in any way.
Should the batteries run down during a monitoring session, they can be replaced.
Monitoring will continue and data will not be lost. If you do not use the monitor, it is
advisable to remove batteries since they may run down due to the constant small
power consumption of the integrated circuits of the device. Data in the monitor
are not lost even if batteries run down or are removed. Used batteries may fall
under the category of hazardous waste and should be disposed of properly.
Important! It is strongly recommended to use freshly charged accumulators or new
batteries with every patient so that batteries do not run down during monitoring,
even in case of very high blood pressure values and/or a long monitoring session.
After inserting batteries in M24/7monitors, it is advised to check their voltage
before programming the monitors. Do not start a new monitoring session with
low batteries. The typical voltage for two fully charged rechargeable batteries
should be over 2,5 V, and for fresh alkaline batteries, over 3 V. It is possible
to check battery voltage with the START button. (Please check the Using the
buttons section for more details.)
Important! If a monitor is not used for a long period, the in-built backup cell
ensuring the operation of the internal clock may get discharged. In this case keep
freshly charged batteries in the monitor for at least one day; this will recharge the
backup cell. It is possible to use the monitor afterwards. If the backup cell is not
properly charged, the internal clock may work incorrectly, and the monitor may
not start measurements in due time.
A set of new, high capacity batteries will enable the OMRON M24/7 monitor to
perform 250 blood pressure measurements during a 24-48 hours long monitoring
session. If you opt to use alkaline batteries, choose high capacity, long-life
products to enable reliable operation. A small crossed battery sign on the LCD
shows low battery voltage.
15
Page 16

In order to change batteries, take the monitor out of the holder carry pouch and
remove the battery compartment cover on the back-side. Place two new, longlife AA alkaline batteries or two properly charged, high capacity AA rechargeable
batteries into the compartment as shown in the polarity drawing. Close the
compartment.
2.8. Cuffs and their application
The OMRON M24/7 comes with 2 cuffs, normal and large adult size (see below).
The bladders are made of neoprene and are latex-free. It is advisable to wear a
thin shirt or blouse under the cuff. This does not in uence the accuracy of blood
pressure measurements but it prevents possible problems caused by long-time
wear (sweating, itching, etc.). Place the cuff on the upper arm so that the
rubber tube points towards the patient’s shoulder and the bladder is placed
above the brachial artery, if possible. Contrary to the usual placement with
the tube pointing downwards, the advantage is that the patient can wear a loose
jacket over the cuff. Connect the rubber tube of the cuff into the air plug connector,
which you can nd on the long edge closer to the buttons of the OMRON M24/7
monitor. Connect the cuff turning it clockwise with slight pressure.
16
Page 17

Note:
Note: It is recommended that the cuff be applied as tightly as acceptable for the
patient. A too loose cuff will cause much longer blood pressure measurement
times and possibly aborted measurements. With an overly loose cuff, the
monitor must pump to tighten the cuff on the arm and then it must reach the
pressure necessary for measurement. This causes considerable inconvenience
for the patient and results in less data for evaluation. If the patient removes
the cuff for a period during the monitoring session, it should be re-applied
with appropriate tightness, with help from another person, if necessary. Should
blood pressure measurements cause blood shots, torpidity or pain in the hand
after an individual measurement, the cuff should be removed from the arm and
disconnected from the monitor. Such occurrence should be reported to the
physician at once after the monitoring session.
OMRON M24/7 recognizes and functions with three different cuff sizes. Please
set the appropriate cuff size to be used during the programming of the device.
Attention: inappropriate setting of the cuff size may lead to device malfunctioning,
which is inconvenient for the patient and may lead to an unsuccessful measurement.
Name Bladder
dimensions
Sleeve
dimensions
Arm circumference
range*
Small cuff (child) 9 x 18 cm 11 x 32 cm under 24 cm
Normal cuff 12 x 25 cm 15 x 56 cm 24-32 cm
Large cuff 15 x 33 cm 17 x 77 cm 32-42 cm
* When properly applied, the end of the sleeve (the one closer to the tube) should
fall in the indicated range.
The cuff is the component which, by de nition of the relevant standard, is
protected against a de brillator discharge.
Caution!
Substitution of a cuff different from that supplied might result in measurement
error and/or in certain cases cause damage to the main monitor unit.
3. Care and Maintenance
3.1. Handling Errors and Problems
Below you can nd a list of potential error code displays, their meaning and a
description of the error code.
Unsuccessful measurements
E 1 aborted measurement the measurement timeout is over, the
measurement had to be aborted (the
patient was moving)
E 2 (Off) manually interrupted the measurement was stopped by pressing
a button (the display differs from others:
“OFF” on the LCD)
17
Page 18

E 3 battery rundown the AA batteries exhausted during
measurement
E 4 batteries changed the AA batteries were replaced during the
measurement – not shown on LCD
E 8 pressure limit exceeded the pressure in the pneumatic system
exceeded the preset pressure limit
E 9 temporary disturbance external electric signals (e.g., static
discharge) disturbed the operation of the
device
Cuff related errors
E 31 cuff missing or loose; there was no cuff
connected to the device; maybe the cuff is
too loose on the patient’s arm
E 32 cuff tubbing clogged; the cuff is clogged or
the rubber tube is broken
E 33 device or cuff leakage or cuff is loose.
There is a hole in the cuff or it is very loose
on the patient’s arm
E 34 cuff not on patient’s arm or not connected
Faulty device
E 90 device error: the device could not measure
due to a hardware error
E 99 device error: the device could not measure
3.2. Protection, cleaning and washing
OMRON M24/7 ambulatory blood pressure monitors are not specially protected
against spills or ingression of water or other liquids. Do not immerse the monitor
in water or any cleaning uid, and protect it from spills and splashes. Do not
expose it to heavy rain or steam, and do not wear it in a wet environment
e.g: shower, bath, or swimming pool. In case of the minor effects of a wet
environment, wipe off water drops with a dry cloth. Keep the monitor in a normal
dry room for at least one hour before use if condensation is suspected. In case
of ingress of water in the monitor, remove batteries from the unit, and refer
the unit to authorized service. Never place a monitor unit in a disinfecting or
sterilizing machine! Recommended cleaning method is to wipe the monitor with
a disinfectant cleaning tissue, e.g., Henkel Ecolab Incides, or a similar product.
Alternatively, wipe with a slightly damp cloth then dry it with an antistatic tissue.
Do not expose monitors to extreme heat or radiation, including long exposure to
direct strong sunlight.
To wash the cuff please do the following:
1. Remove the bladder.
2. Wash by hand the sleeve with lukewarm water and regular washing liquid
suitable for black material. Rinse well.
3. If required, wipe the bladder with a disinfectant cleaning tissue.
4. Allow both bladder and sleeve to air dry.
5. Replace bladder in the sleeve.
18
Page 19

3.3. Maintenance and Storage Conditions
Temperature: -20 - 50 °C
Humidity: 10 - 95 %, non condensing
Regular checks, warranty, service
Veri cation of pressure measurement accuracy is recommended biannually.
OMRON M24/7 monitors are covered by a two-year warranty. This warranty
does not cover any malfunction or defects arising from improper use, the use of
inadequate accessories, accident, theft, or use of the device outside operating
environmental speci cations or intended measurement range. Removing the
closing label from the back side of the device voids this warranty. There are no
user serviceable parts inside the OMRON M24/7 monitors; they contain high
complexity electronic and ne mechanical components. If you have any problems,
please refer the monitor to quali ed service personnel. All consequences of
improper servicing are the sole responsibility of the user. Contact OMRON or
your distributor for service information.
3.4. Correct Disposal of this Product
OMRON M24/7 should be disposed of according to your local regulations for
disposal of hazardous waste.
3.5. Accessories
Art. Nr. Product Description
BP4-A012-NP M24/7 cuff, small, neoprene, white plug
BP4-A010-NP M24/7 cuff, normal, neoprene, white plug
BP4-A011-NP M24/7 cuff, large, neoprene, white plug
BP4-A011-LNP M24/7 cuff, Extra large, neoprene, white plug
GPC-A015 M24/7 sleeve, small
GPC-A013 M24/7 sleeve, normal
GPC-A014 M24/7 sleeve, large
GPC-A001 M24/7 carry pouch
19
Page 20

4. Technical Data
4.1. Technical parameters
Power supply:
2 AA rechargeable NiCd or NiMH
batteries or 2 AA alkaline batteries
Display:
liquid-crystal
Data storage:
internal solid state memory
Data transmission:
USB optical cable
PC interface:
USB interface
Operating environment:
Temperature: 10 - 45 °C
Humidity: 10 - 95 %, non condensing
Atmospheric pressure: 70 - 106 kPa
Storage conditions:
Temperature: -20 - 50 °C
Humidity: 10 - 95 %, non condensing
Size (H x W x D):
70 x 99 x 30 mm
Weight:
app. 240 g (batteries included)
Blood pressure measurement method:
oscillometric
Blood pressure maximum storage:
over 400 automatic measurements
Pressure measurement range:
0-300 mmHg
0-40 kPa*
Static accuracy:
± 3 mmHg or 0,4 kPa or ± 2% of
measured value (stability: 2 years)
Blood pressure measurement range
indication:
30-260 mmHg
4-35 kPa*
Pulse rate measurement range:
40-200 beat per minute
Blood pressure measurement accuracy:
The OMRON M24/7 has been clinically
validated
Pressure sensor:
piezo-resistive
In ation:
automatically controlled pump
Safety:
maximum in ation 300 mmHg
(40 kPa*);
independent safety release valve
De ation and rapid air release:
automatic pressure release valve
Please note that the OMRON M24/7 might not meet its performance speci cations
if stored or used outside the speci ed environmental conditions.
*Measuring and LCD displaying in kPa values is an option which can be selected
in the OMRON BP Tracker Software. The unit of measurement can be changed
later in the database.
For information on cuffs and their application, see 2.8.
This symbol shows that according to regulations OMRON M24/7
should be handled as electronic waste during disposal.
Blood pressure measurements determined with the algorithm
of an OMRON M24/7 monitor on adults are equivalent to those
obtained by a trained observer using the cuff/stethoscope
auscultation method Korotkoff phase V, within the limits prescribed
by the American National Standard for Electronic or Automated
;
Sphygmomanometers. The algorithm used in the OMRON M24/7
also ful ls the requirements of the British Hypertension Society
Validation Protocol for Automated Blood Pressure Measuring
Devices.
20
Page 21

4.2. Safety Concerns
Electric shock hazard protection
OMRON M24/7 monitors meet relevant shock hazard protection standards.
OMRON M24/7 monitors operate with two 1.5V AA batteries or two 1.2V AA
rechargeable batteries. This excludes all electric shock hazards, even in the
unlikely case of multiple device errors. Use only standard long-life (alkaline)
batteries, or standard NiCd or NiMH rechargeable batteries of the proper size.
Do not use lithium batteries. Do not mix different battery types, do not mix new
and old batteries, do not use damaged batteries.
Many personal computers do not meet certain shock hazard protection standards
or strict safety regulations applicable to medical devices. Therefore, during the
computer-based use of OMRON monitors, keep at least a 2 meter distance
between patient and computer. This is the required minimum safety distance.
OMRON M24/7 monitors communicate using a plastic optical cable, whose 4 m
standard length allows for the required safety distance. The plastic optical cable
ensures perfect electric separation and reduces the effects of external electric
noise. It does not conduct electricity.
Biocompatibility
To avoid infection risks, and for general hygienic reasons, the device, cuff and
tubing should never contact the patient’s skin directly.
Hazardous materials
Used batteries qualify as hazardous waste and should be disposed of properly.
OMRON monitors do not contain any materials quali ed as pharmaceutical
substance or tissue of animal origin. They emit no material hazardous to humans.
Risk of incorrect diagnosis
The basic intended use of OMRON M24/7 monitors is to record blood pressure
and pulse rate values. Patients should be informed about rules of cooperative
behaviour; proper handling of the monitor used, and expected results of
monitoring in advance. OMRON M24/7 monitors only provide data to support
diagnostic decisions of a quali ed physician; they do not automatically provide a
diagnosis of any kind. During the evaluation of recorded blood pressure values,
possible artefacts due to external disturbances, motion artefacts, and electrical
noise should be observed and handled with caution.
21
Page 22

4.3. EMC Information
Medical electrical equipment should be used with precautions according to EMC,
and must be installed according to the EMC notices disclosed in this manual as
mobile RF transceivers could adversely affect it.
Directive and declaration of manufacturer – Electromagnetic Emission
The OMRON M24/7 is suitable for use in the speci ed electromagnetic environment. The
purchaser or user of the OMRON M24/7 should assure that it is used in an electromagnetic
environment as described below
Emission test Compliance Electromagnetic Environment
Radiated and conducted
RF emission
CISPR 11
Radiated and conducted
RF emission
CISPR 11
Harmonic emission
IEC61000-3-2
Voltage uctuations /
Flickers
Group 1 OMRON M24/7 uses RF energy only for its
internal function. Therefore, the emission
is very low and not likely to cause any
interference in nearby electronic equipment.
Class B OMRON M24/7 is suitable for use in domestic
establishments and in establishments directly
connected to the low voltage power supply
network which supplies buildings used for
domestic purposes.
Not applicable ---
Not applicable ---
IEC61000-3-3
22
Page 23

Directive and declaration of manufacturer – Electromagnetic immunity
OMRON M24/7 is suitable for use in the speci ed electromagnetic environment. The purchaser
or user of OMRON M24/7 should assure that it is used in an electromagnetic environment as
described below.
Immunity test IEC60601-1-2
test level
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
Power frequency
(50/60Hz) magnetic
eld
IEC 61000-4-8
Note: U
is the nominal voltage of mains.
T
±6KV contact
±8KV air
±2KV for power
supply lines
±1KV for input/
output lines
±1KV differential
mode
±2KV common
mode
<5% U
(>95%
T
dip) for 0.5 cycle;
40% U
(60% dip)
T
for 5 cycles;
70% UT (30% dip)
for 25 cycles;
<5% U
(>95%
T
dip) for 5 sec.
3A/m 3A/m Power frequency magnetic elds
Compliance
level
Electromagnetic
environment
±8KV air Floors are wood, concrete or
ceramic tile, or oors are covered
with synthetic material and the
relative humidity is at least 30
percent.
Not applicable Mains power quality is that of a
typical commercial and/or hospital
environment.
Not applicable Mains power quality is that of a
typical commercial and/or hospital
environment.
Not applicable Mains power quality is that of
a typical commercial and/or
hospital environment. If the user of
OMRON M24/7 requires CLINICAL
UTILITY during power mains
interruptions, it is recommended
that parts of the OMRON M24/7
system where applicable be
powered from an uninterruptible
power supply.
are at levels characteristic of
a typical location in a typical
commercial and/or hospital
environment.
23
Page 24

Directive and declaration of manufacturer – Electromagnetic immunity
OMRON M24/7 is suitable for use in the speci ed electromagnetic environment. The
purchaser or user of OMRON M24/7 should assure that it is used in an electromagnetic
environment as described below.
Immunity test IEC60601-1-2 Compliance
level
Conducted RF
IEC 6100-4-6
3V
eff
150KHz - 80MHz
Not applicable Portable and mobile RF
Electromagnetic
environment
communications equipment
are used no closer to any part
of OMRON M24/7, including
cables, than the Recommended
Separation Distance calculated the
formula written below.
Recommended Separation
distance:
d=[3.5/V
Radiated RF
IEC 61000-4-3
1. note: in case of frequency 80MHz or 800 MHz, the formula for the higher range is
applicable.
2. note: These are guidelines. Actual conditions may vary.
3V/m
80MHz – 2.5GHz
3V/m d=[3.5/3V/m]P;
(80MHz – 800MHz)
d=[7/3V/m]P;
(800MHz – 2.5GHz)
where:
P is the highest radiated power
disclosed by the manufacturer of
transmitter [W];
d is the recommended separation
distance [m].
]P
1
24
Page 25

Recommended separation distance
OMRON M24/7 is intended to be used in electromagnetic environment with controlled RF
disturbances. The purchaser or user of M24/7may help to reduce electromagnetic disturbances
by de ning the separation distance between the transportable or mobile RF telecommunication
equipment (transmitters) and OMRON M24/7, depending on the highest output power of the
telecommunication equipment.
Separation distance in function of the frequency of the
transmitter [m]
The highest
output
power of the
transmitter [W]
0.01 Not applicable 0.12 0.23
0.1 Not applicable 0.38 0.73
1 Not applicable 1.2 2.3
10 Not applicable 3.8 7.3
100 Not applicable 12 23
If this table does not contain the highest output power of the transmitter, the d separation
distance [m] can be calculated by the formula, depending on the frequency of the transmitter,
where P is the rated highest output power of the transmitter [W].
1. note: in case of frequency 80MHz or 800 MHz, the formula for the higher range is
applicable.
150KHz –
80MHz
d=[3.5/V1]P
80MHz –
800MHz
d=[3.5/E
]P
1
800MHz – 2.5GHz
d=[7/E
]P
1
2. note: These are guidelines. Actual conditions may vary.
25
Page 26

AMBULATORY BLOOD PRESSURE MONITORING
PATIENT DIARY
Name:
Date of birth:
Monitoring date:
8:00-8:30 20:00-20:30
8:30-9:00 20:30-21:00
9:00-9:30 21:00-21:30
9:30-10:00 21:30-22:00
10:00-10:30 22:00-22:30
10:30-11:00 22:30-23:00
11:00-11:30 23:00-23:30
11:30-12:00 23:30-24:00
12:00-12:30 0:00-0:30
12:30-13:00 0:30-1:00
13:00-13:30 1:00-1:30
13:30-14:00 1:30-2:00
14:00-14:30 2:00-2:30
14:30-15:00 2:30-3:00
15:00-15:30 3:00-3:30
15:30-16:00 3:30-4:00
16:00-16:30 4:00-4:30
16:30-17:00 4:30-5:00
17:00-17:30 5:00-5:30
17:30-18:00 5:30-6:00
18:00-18:30 6:00-6:30
18:30-19:00 6:30-7:00
19:00-19:30 7:00-7:30
19:30-20:00 7:30-8:00
Complete the activity diary during the monitoring session using the numbered guide as
shown below to reduce your work. Careful completion will help the physician evaluate
the data recorded. Do not forget to record the time you awake and go to sleep.
1 = Working 2 = Housework (what kind) 3 = Walking
4 = Exercise (what kind) 5 = Driving 6 = Travelling
7 = Eating 8 = Watching TV 9 = Relaxing
10 = Sleeping
Medication taken during the measurement:push the button marked with heart.
1.
2.
3.
4.
5.
26
Page 27

4.4. Product Warranty Information
(a) MONITOR WARRANTY. The main monitor unit will be free from defects in materials
and workmanship under normal use and service for a period of two (2) years from
the date of receipt. This warranty covers the monitor unit only. This warranty does not
cover any accessories that might come with the monitor unit.
(b) ACCESSORIES WARRANTY. The non-disposable accessories delivered with the
monitor unit will be free from defects in materials and workmanship under normal use
and service for a period of one (1) year from the date of receipt. This warranty does
not cover disposable accessories, packaging materials, accumulators and batteries,
cuffs, or any of their components.
(c) CUFF WARRANTY. The cuff(s) will be free from defects in materials and workmanship
under normal use and service for a period of six (6) months from the date of receipt.
This warranty covers the cuff(s) delivered with a monitor unit exclusively.
(d) SOFTWARE WARRANTY. The OMRON BP Tracker Software under normal use
will perform substantially in accordance with the accompanying written/electronic
documents for a period of ninety (90) days from the date of receipt.
This warranty does not cover any malfunction or defects of the monitor unit or any of its
accessories arising from improper use, the use of inadequate accessories, accident, theft,
or use of the monitor unit outside its operating speci cations and intended measurement
range. Removing the closing label from the back side of the monitor unit, or opening the
unit any other way voids this warranty.
EXCLUSION OF BIOHAZARD. OMRON will not accept for repair potentially infectious
products or accessories, especially pouches and cuffs, that might have been in direct
contact with the patient, and could not be, or (potentially) were not, properly disinfected,
even within the warranty period. If a problem occurs within the warranty period, such
accessories will be replaced without any physical inspection, reserving the rights to hold
an inspection when found necessary.
NO OTHER WARRANTIES. OMRON disclaims all other warranties, either expressed or
implied, including, but not limited to, implied warranties of merchantability and tness for a
particular purpose, with regard to the monitor unit, any accessory or other accompanying
hardware, and the OMRON BP Tracker Software.
NO LIABILITY FOR CONSEQUENTIAL DAMAGES. In no event shall OMRON be liable
for any special, incidental, indirect, or consequential damages whatsoever (including,
without limitation, damages for loss of business pro ts, business interruption, loss of
business information, loss of data, or any other pecuniary loss) arising out of the use
of or inability to use the monitor unit, its accessories and/or the OMRON BP Tracker
Software, even if OMRON
Product Serial number _____________________________________
Details of fault _____________________________________
has been advised of the possibility of such damages.
Name & Address _____________________________________
_____________________________________
_____________________________________
_____________________________________
OMRON Customer Services becomes the owner of all exchanged units
27
Page 28

Remarks
2010. All rights reserved
Made in Hungary
Meditech Ltd.
H-1184 Budapest,
Mikszáth Kálmán utca 24, Hungary
Scorpius 33
2132 LR Hoofddorp
28
IM-ABPM-BP5-EN-04-06/2012
 Loading...
Loading...