Page 1
BX-URA2
BX-RFA
U-LH100HGAPO
U-LH100HG
Power Supply Unit
U-25ND6-2
U-25ND25-2
U-25ND50-2
U-RSL6
U-RSL6EM
BX-RFSS
U-EXBABG
U-EXBAUB
U-EXBAUG
INSTRUCTIONS
REFLECTED
FLUORESCENCE
SYSTEM
This instruction manual is for the Olympus Reflected Fluorescence System. To ensure the safety,
obtain optimum performance and to familiarize yourself fully with the use of this system, we recommend that you study this manual thoroughly before operating the microscope. Retain this instruction manual in an easily accessible place near the work desk for future reference.
This publication is printed on 100% recycled paper
A X 7 6 2 5
Page 2

Page 3

CONTENTS
Correct assembly and adjustments are critical for the reflected fluorescence system to exhibit its full performance. If you are
going to assemble the reflected fluorescence system yourself, please carefully read section 9, “ASSEMBLY” (pages 30 to 35).
IMPORTANT — Be sure to read this section for safe use of the equipment. —
I. REFLECTED FLUORESCENCE OBSERVATION
1 NOMENCLATURE
REFLECTED FLUORESCENCE OBSERVATION PROCEDURE
2
3 USING THE CONTROLS
1 General Precautions for Observation....................................................................................................................... 8
2 Selecting the Fluorescence Mirror Unit....................................................................................................... 8-10
3 Objectives for Various Observation Modes........................................................................................ 10-11
4 Turning the Power Supply Unit On............................................................................................................................. 11
5 Centering the Field Iris Diaphragm.......................................................................................................................... 12
6 Centering the Aperture Iris Diaphragm.............................................................................................................. 13
1-3
4-5
6-7
8-15
7 Centering the Mercury Burner................................................................................................................................ 14-15
8 Mounting the ND Filters ............................................................................................................................................................ 15
4 SIMULTANEOUS FLUORESCENCE OBSERVATIONS
1
Simultaneous Reflected Fluorescence and Phase Contrast Observations
Simultaneous Reflected Fluorescence and Transmitted Light Nomarski
2
Differential Interference Contrast (DIC) Observations .................................................................. 16
5 TROUBLESHOOTING GUIDE
6 SPECTRAL CHARACTERISTICS OF FILTERS
7 SPECIFICATIONS
16
.............. 16
17
18-22
23
Page 4
8 OPTIONAL MODULES
1 6-Position Filter Slider U-RSL6........................................................................................................................... 24-25
2 6-Position Barrier Filter Slider U-RSL6EM .................................................................................................. 26
3 Rectangle Field Stop BX-RFSS (for exclusive use with the BX-RFA).................... 27
4
Exciter Balancers U-EXBABG/EXBAUB/EXBAUG (for exclusive use with the BX-RFA) ......
24-29
28-29
9 ASSEMBLY
9-1 Assembly Diagram ............................................................................................................................................................................ 30
9-2 Detailed Assembly Procedures ............................................................................................................................. 31-35
— See this section for the replacement of the light bulb. —
II. REFLECTED OBSERVATIONS (BX-URA2 Only)
1 CONFIGURATION OF REFLECTED OBSERVATION SYSTEM
2 ASSEMBLY
FIELD IRIS AND APERTURE IRIS DIAPHRAGM ADJUSTMENTS
3
4 OBSERVATIONS
4-1 Reflected Light Brightfield/Darkfield Observations ........................................................................... 39
30-35
36
37
37-38
39-42
Reflected Light Nomarski Differential Interference Contrast (DIC) Observation
4-2
4-3 Reflected Light Simple Polarized Light Observation....................................................................... 42
5
OPTICAL CHARACTERISTICS
«UIS2 (UIS) Series for Reflected Light Observation»
6 TROUBLESHOOTING GUIDE
■
PROPER SELECTION OF THE POWER SUPPLY CORD .........................................................................
...... 40-42
43-44
45
46-47
Page 5

IMPORTANT
This system employs a UIS2/UIS (Universal Infinity System) optical design, and should be used only with
UIS2/UIS microscopes, eyepieces, objectives and condensers for the BX2 series. (Some of the modules
designed for the BX series and objectives/eyepieces for the UIS series are also usable. For details,
please consult Olympus or the catalogues.) Less than optimum performance may result if inappropriate
accessories are used.
The use of a universal reflected fluorescence illuminator has enabled the installation of necessary fluorescence mirror units.
By combining the microscopy techniques as shown below, this system can efficiently be used to find fluorescence emission in any area of cells:
1. Reflected fluorescence observation + Transmitted light phase contrast observation
2. Reflected fluorescence observation + Transmitted Nomarski Differential Interference Contrast (DIC) observation
3. Reflected fluorescence observation + Transmitted Light Observation
In addition, the following observations are also by installing a general reflected light observation unit (BX-URA2 only):
1. Reflected brightfield/darkfield observations
2. Reflected Nomarski DIC observation
3. Reflected simplified polarized light observation
This manual describes the instructions for I. Reflected Fluorescence Observations in the first half and those for II. Reflected
Light Observations in the second half.
Please find the pages giving you the appropriate instructions for your observation.
SAFETY PRECAUTIONS
1. This system is composed of precision instruments. Handle it with care and avoid subjecting it to sudden or severe
impact.
2. The ultrahigh-pressure mercury burner used should be the USH-103OL DC burner (mfd. by USHIO, Inc.) or the HBO103W/
2 burner (mfd. by OSRAM) that Olympus supplies.
3. Make sure that a mercury burner is attached and that cables are plugged in firmly.
4. The inside of the lamp housing is very hot and hazardous during lighting and for about 10 minutes after turning off. Do not
open the lamp housing in this period. (Page 11)
5. Do not apply excessive force to the stoppers which are provided for some functions. Otherwise, the stopper or equipment
may be damaged.
6. Do not attempt to open or disassemble the power supply unit because it includes high voltage parts inside.
7. Always use the power cord provided by Olympus. If no power cord is provided, please select the proper power cord by
referring to the section “PROPER SELECTION OF THE POWER SUPPLY CORD” at the end of this instruction manual. If the
proper power cord is not used, product safety and performance cannot be guaranteed.
Before plugging the power cord to the power outlet, make sure that the main switch of the power supply unit is set to
“
” (OFF).
8. To ensure safety, be sure to ground the power supply unit. Otherwise, Olympus can no longer warrant the electrical safety
performance of the system.
9. Before opening the lamp housing for replacement of the burner or any other internal part, set the main switch to “
(OFF), then unplug the lamp housing connection cable from the power supply unit, and wait for more than 10 minutes
until the lamp housing cools down.
10
.
The top panel of the lamp housing becomes very hot during operation. To prevent fire hazard, do not block the ventilation
through the top panel.
”
1
Page 6

Safety Symbols
The following symbols are found on the microscope. Study the meaning of the symbols and always use the equipment
in the safest possible manner.
Symbol Explanation
Indicates the presence of high voltage (1 kV or more). Take caution to guard against electric
shock.
Indicates that the surface becomes hot, and should not be touched with bare hands.
Before use, carefully read the instruction manual. Improper use could result in personal injury to
the user and/or damage to the equipment.
l
Warning indications
Warning indications are placed at parts where special precaution is required when handling and using the System.
Always heed the warnings.
Warning indication
position:
Indicates that the main switch is ON.
Indicates that the main switch is OFF.
· Mercury burner lamp housing
(U-LH100HG, U-LH100HGAPO
· Power supply unit for 100 W
mercury burner
· ND filters
(U-25ND6, U-25ND25, U-25ND50)
[Warning against
high temperature]
[Warning against
high voltage]
1 Getting Ready
1. This manual pertains only to the reflected fluorescence system. Before using this system together with the BX2 microscope and associated options, make sure that you have carefully read and understood their manuals, and understand
how the system should be operated together.
2. The reflected fluorescence system is composed of precision instruments. Handle it with care and avoid subjecting it to
sudden or severe impact.
3. Do not use the system where it is subjected to direct sunlight, high temperature and humidity, dust or vibrations.
4. To allow heat from the unit to dissipate well, reserve a distance of at least 10 cm between the lamp housing and power
supply unit.
5. The power cord can also be used to cut the power supply in case of emergency. To make this possible, the power supply
unit should be installed so that the power cord connector (on the rear of the power supply unit) or the power outlet is
easily accessible for unplugging in case of emergency.
2
Page 7
2 Maintenance and Storage
1. To clean the lenses and other glass components, simply blow dirty away using a commercially available blower and wipe
gently using a piece of cleaning paper (or clean gauze).
If a lens is stained with fingerprints or oil smudges, wipe it gauze slightly moistened with commercially available absolute
alcohol.
!Since the absolute alcohol is highly flammable, it must be handled carefully.
Be sure to keep it away from open flames or potential sources of electrical sparks --- for example, electrical
equipment that is being switched on or off.
Also remember to always use it only in a well-ventilated room.
2. With any part of the system other than glass components gets dirty, do not use organic solvents but wipe it with a clean
cloth. If the part is extremely dirty, use a lint-free, soft cloth slightly moistened with a diluted neutral detergent.
3. Do not disassemble any part of the system. This could result in malfunctions or reduced performance.
4. The mercury burner has a service life period of 300 hours (USH-103OL, HBO103W/2). When the hour counter on the
power supply unit indicates this value, set the main switch to “
replacing the mercury burner (Page 33). Unlike electric bulbs, the mercury burner seals high-pressure gas inside. If it
continues to be used after the service life has expired, the glass tube may eventually explode due to accumulated
distortion.
5. When not using the microscope, be sure set the main switch to “
cooled down sufficiently, cover the microscope with the dust cover for storage.
6. When disposing of the microscope, check the regulations and rules of your local government and be sure to observe
them.
” (OFF) and wait for more than 10 minutes before
” (OFF). After confirming that the lamp housing has
3 Caution
If the system is used in a manner not specified by this manual, the safety of the user may be imperiled. In addition, the
system equipment may also be damaged. Always use the system as outlined in this instruction manual.
The following symbols are used to set off text in this instruction manual.
! : Indicates that failure to follow the instructions in the warning could result in bodily harm to the
user and/or damage to equipment (including objects in the vicinity of the equipment).
# : Indicates that failure to follow the instructions could result in damage to equipment.
} : Indicates commentary (for ease of operation and maintenance).
3
Page 8
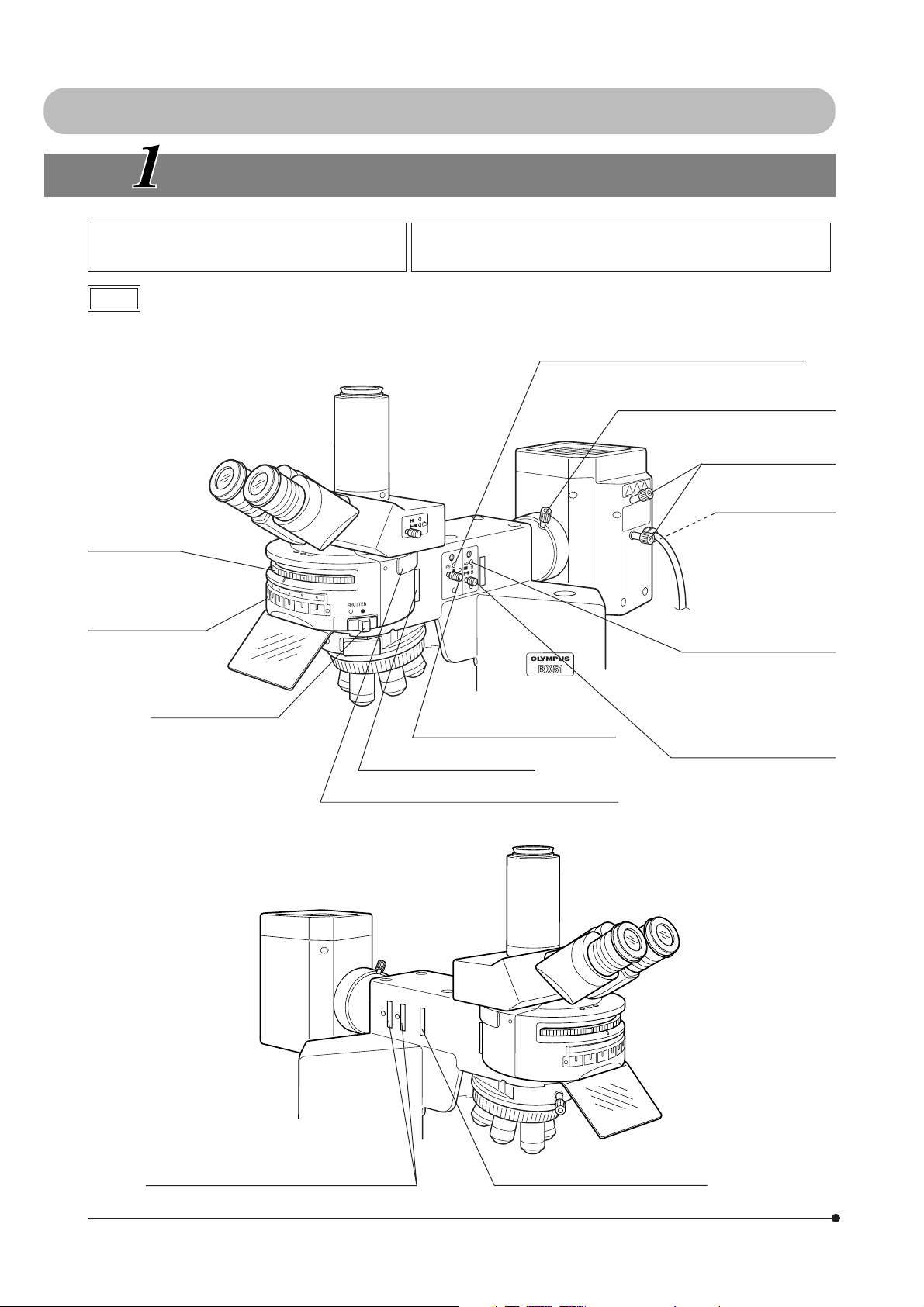
I. REFLECTED FLUORESCENCE OBSERVATION
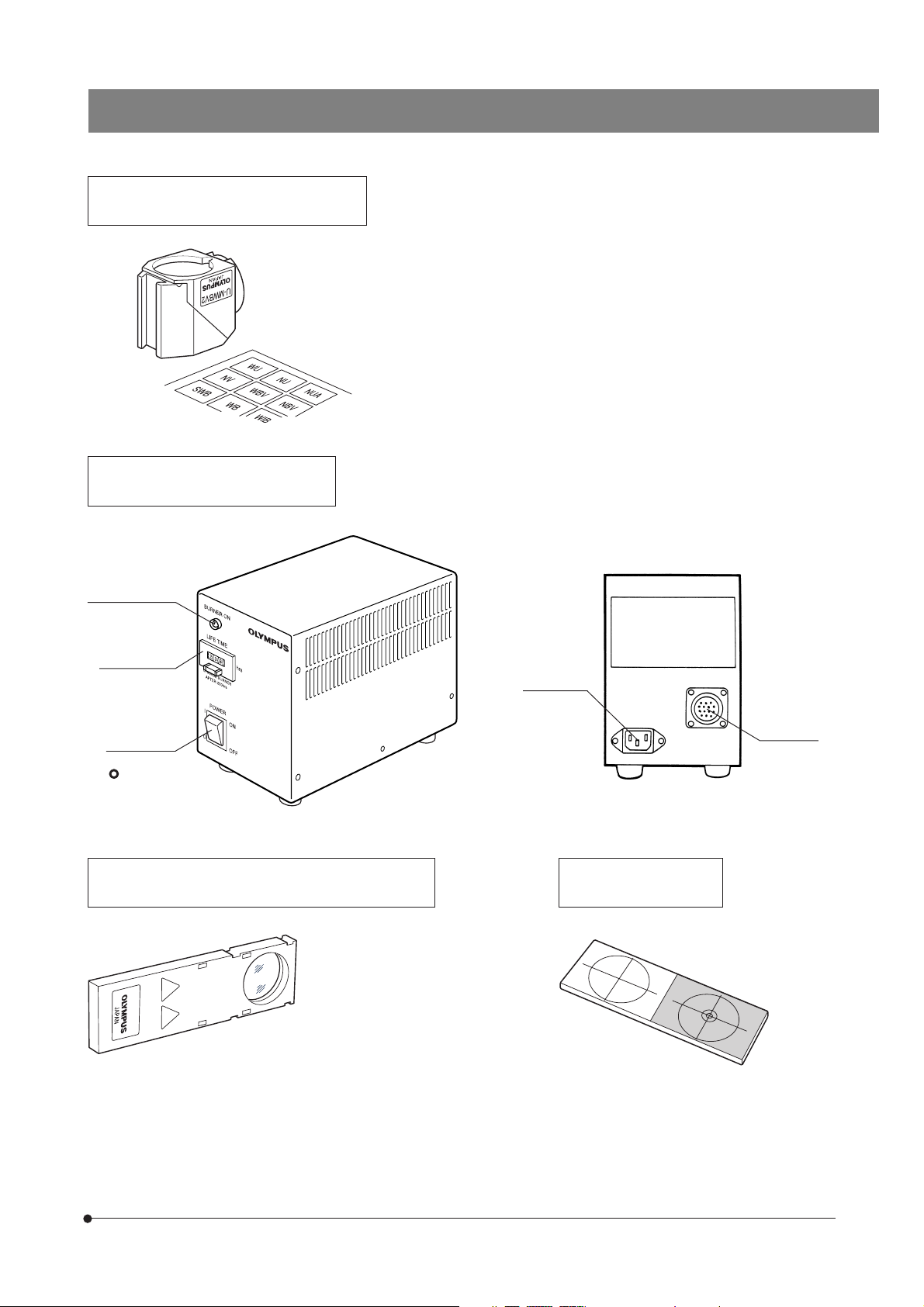
NOMENCLATURE
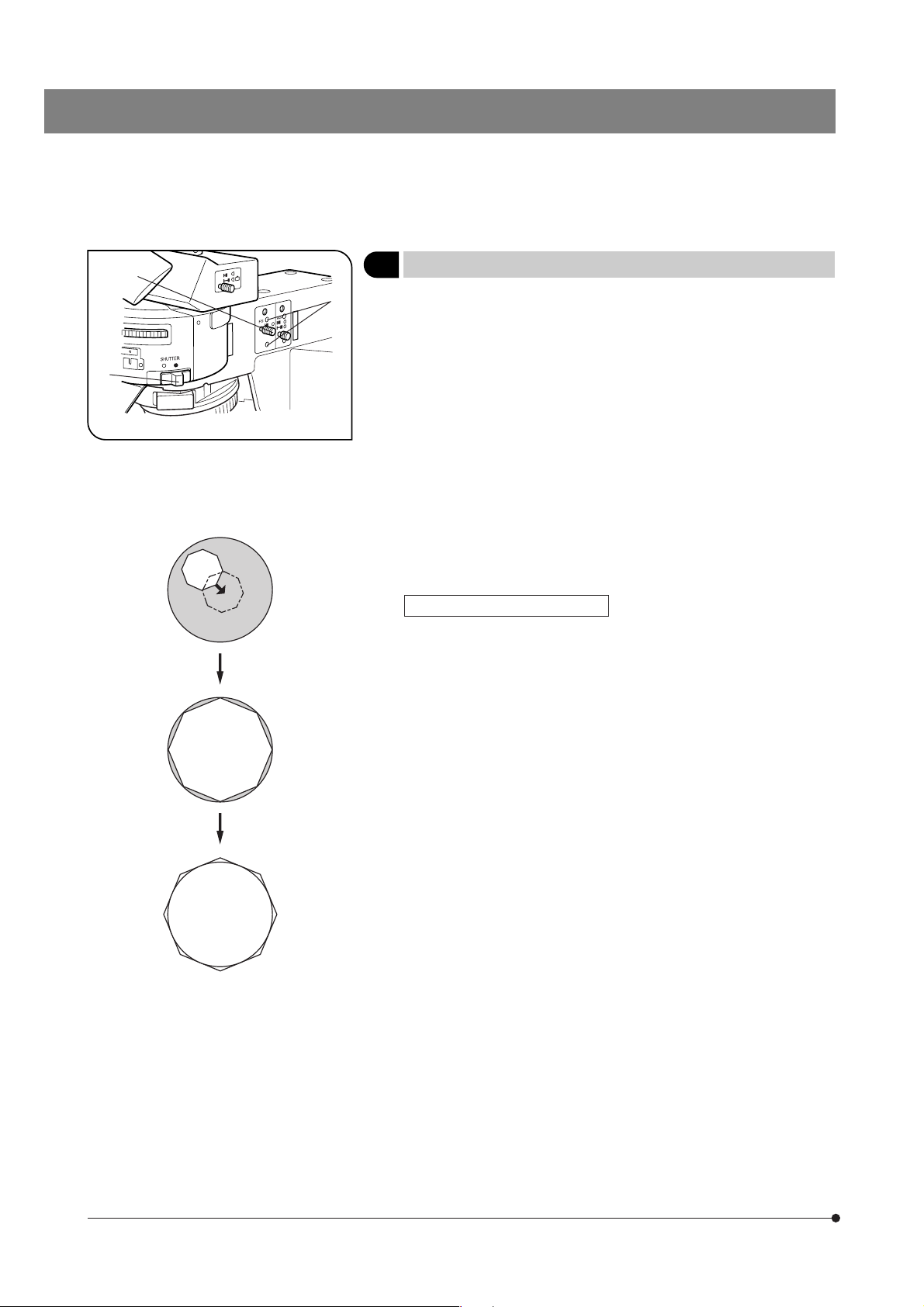
Reflected Illuminator BX-URA2
Fluorescence Illuminator BX-RFA
Note
Mirror unit turret
Mirror unit inscription
pocket (Page 31)
The diagram shows the BX-RFA. Parts
marked * are not provided on the BX-URA2.
100 W Mercury Apo Lamp Housing U-LH100HGAPO
100 W Mercury Lamp Housing U-LH100HG
Field iris diaphragm centering screws (Page 12)
x2 screws.
Collector lens focusing knob (Page 14)
Burner centering knobs
(Page 14)
Mirror focusing screw
(Page 15)
On the rear of the
lamp housing.
Aperture iris diaphragm
centering screws (Page 13)
x2 screws.
Shutter knob (Page 12)
\: Shutter OUT
{: Shutter IN
Field iris diaphragm knob (Page 12)
6-position filter inlet (Page 24)
Analyzer/6-position barrier filter slider inlet (Page 26)
3
3
2
1
Aperture iris diaphragm knob
6
5
4
(Page 13)
4
ND filter/* exciter balancer inlet (Pages 15 & 28)
* 6-Position filter slider inlet (Page 24)
Page 9
Fluorescence Mirror Units
U-MWU2, etc., total 24 models
Indicator sheets
Power Supply Unit
(for 100 W mercury burner)
Burner ON LED
}Up to six fluorescence mirror units can be mounted on the BX-RFA or BX-
URA2.
#Each filter unit includes a dichroic mirror, barrier filter and excitation
filter that have been combined according to the excitation method. It is
basically not recommended to open a fluorescence mirror unit.
}It is recommended that you use the U-MF2 dummy filter unit (which does
not contain a filter) when making your original fluorescence unit. (Page 32)
Blank indicator sheets provided with the illuminator can be used to write
the names of original fluorescence mirror units.
Hour counter
Main switch
I : ON
: OFF
ND Filters
U-25ND6-2, U-25ND25-2, U-25ND50-2
Input
receptacle
Output
connector
Centering Target
U-CST
5
Page 10
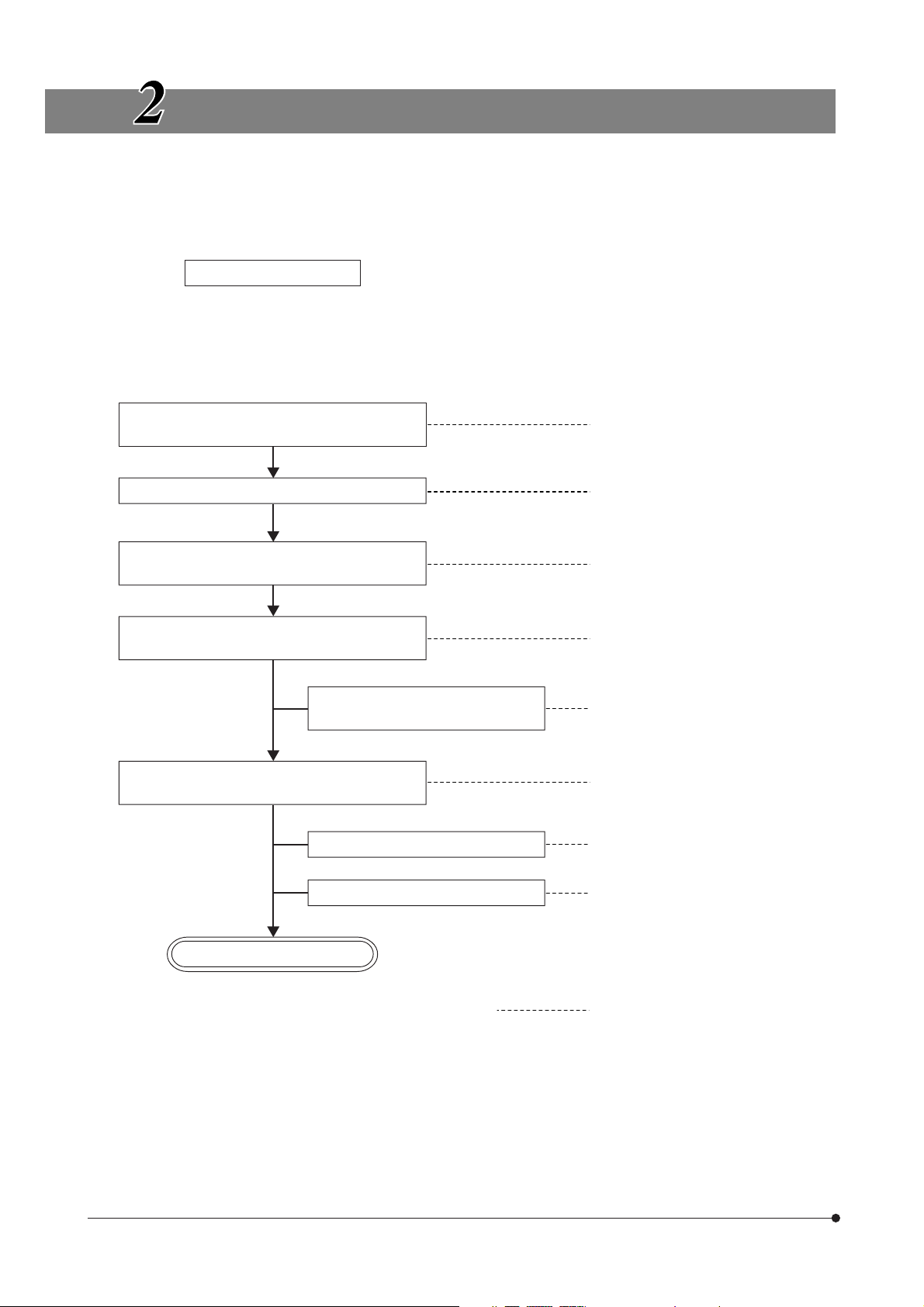
REFLECTED FLUORESCENCE OBSERVATION PROCEDURE
}If you need simultaneous observation of reflected fluorescence observation with the phase contrast observation or trans-
mitted light Nomarski Differential Interference Contrast (DIC) observation, please read Chapter 4, “SIMULTANEOUS FLUORESCENCE OBSERVATION”. (Page 16)
(Controls Used)
Preparation
· Attach the fluorescence mirror unit and objective matching the observation method. (Pages 8 to 11)
· Center the mercury burner. (Page 14 or 15)
Set the main switch to “ I ” (ON) and wait for
the arc to stabilize.
Place the specimen on the stage.
Engage the fluorescence mirror unit
matching the specimen in the light path.
Engage the objective in the light path and
focus on the specimen.
Engage an ND filter in the light
path as required.
@ Main switch
² Specimen holder
³ X-/Y-axis knobs
| Mirror unit turret
ƒ Revolving nosepiece
… Coarse/fine adjustment knobs
† ND filters
(Page)
(P. 11)
(P. 15)
Adjust so that the entire field is
uniform and brightest.
Adjust the field iris diaphragm.
Adjust the aperture iris diaphragm.
Start observation.
}Engage the shutter if you interrupt observation for a short time.
‡ Collector lens focusing knob
Š Field iris diaphragm knob
‰ Aperture iris diaphragm knob
‹ Shutter knob
(P. 14)
(P. 12)
(P. 13)
(P. 12)
6
Page 11
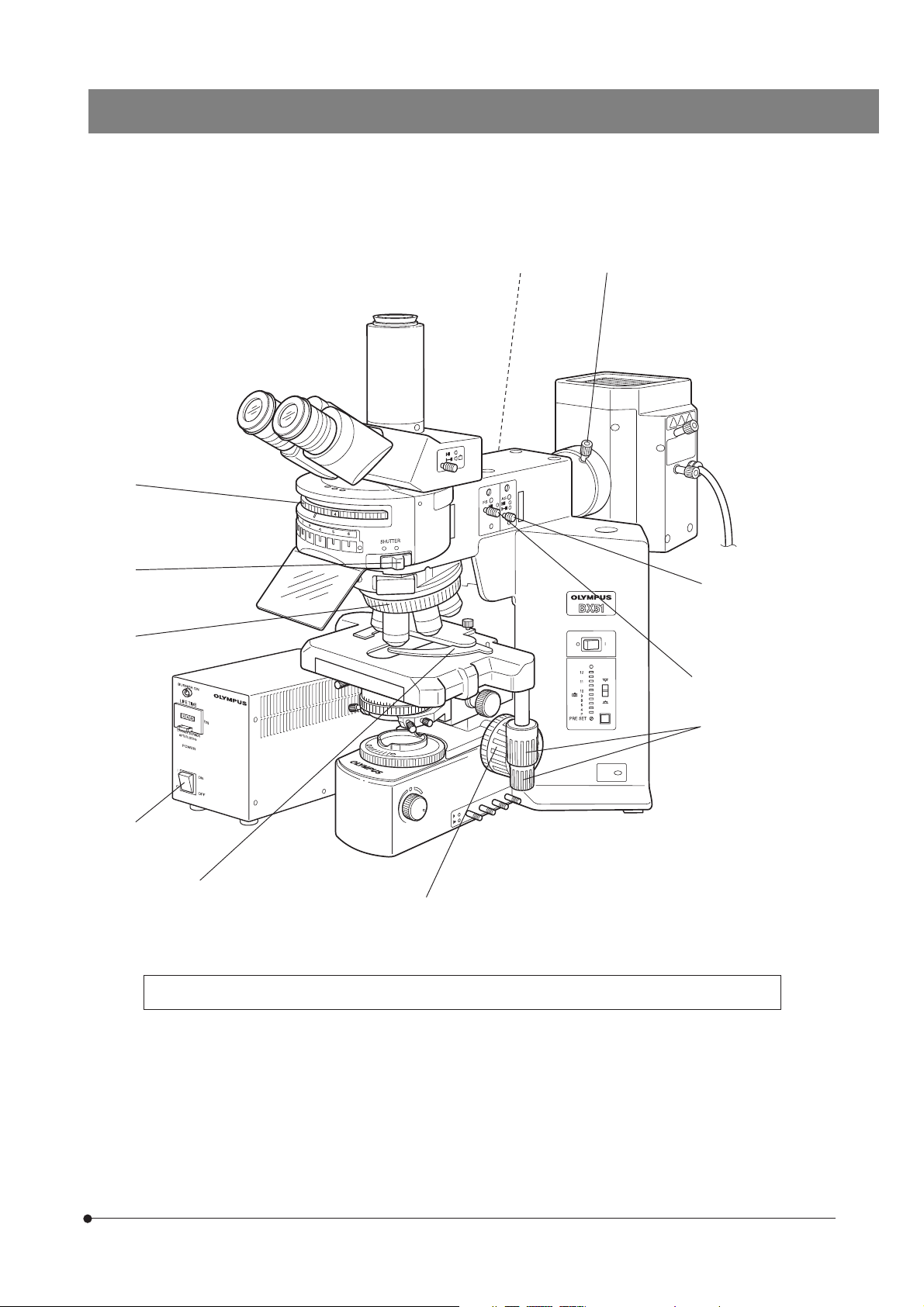
|
†
‡
‹
ƒ
@
²
…
} Make a photocopy of the observation procedure pages and post it near your microscope.
‰
Š
³
7
Page 12

USING THE CONTROLS
1 General Precautions for Observation
1. Verify that the power supply voltage and frequency match the requirements inscribed on the Rating plate.
2. Make sure that the power cord and connecting cables are plugged in securely.
3. If you perform only transmitted light phase contrast or transmitted light DIC observations, leave one cube position on the
turret empty. This allows for transmission of white light.
The turret must always be set to one of the click position. If it is deviated from a click position, the cover may be deformed
by heat.
4. Enlarge the field iris diaphragm so it just circumscribes the field of view. If decentered, center it using the Allen screwdriver.
5. Always use immersion for oil immersion objectives.
6. If you use an objective with correction collar such as the UPlanSApo40X, UPlanFLN60X, UPlanApo40X or PlanApo40X,
you can correct variations in cover glass thickness by adjusting the correction collar.
Correction procedure
If the cover glass thickness is known, match the correction collar to the cover glass thickness using the collar scale
provided. If the thickness is not known, turn the collection collar and adjust the fine adjustment knob to where the
image is as sharp as possible.
7. Engage the shutter if you interrupt observation for a short time.
(Turning the mercury burner ON and OFF repeatedly will significantly shorten the life span of the burner.)
8. Color fading of specimens
This system features high excitation light intensity to ensure bright observation of dark fluorescence specimens.
In consequence, after long period of observations using high-power objectives, the colors of specimens will fade quicker
than usual, causing the view (contrast) of fluorescent images to deteriorate.
In such a case, slightly reduce the excitation light intensity to slow color fading down and improve the fluorescence
images.
To reduce the excitation light intensity, use ND filters or aperture iris diaphragm as far as the observation is not affected or
use the shutter to limit the exposure of specimen to more than necessary light.
Commercially-marketed color fading protection agent (DABCO, etc.) can also delay fading of specimen colors. The use of
fading protection agent is recommended especially when you perform high-magnification observations frequently.
#Remember that the fading protection agents cannot be used with certain kinds of specimens.
2 Selecting the Fluorescence Mirror Unit
Select the fluorescence mirror unit which matches the fluorochrome in use.
#Never mount or use the U-MBF3 brightfield mirror unit together with a with a mirror unit for fluorescence. The U-
MBF3 brightness is excessive and injury to the eyes could occur. If this type of mirror unit is to be used together
with a mirror unit for fluorescence, use the U-MBFL3 mirror unit equipped with a built-in ND filter or add a 3% ND
filter to the U-MBF3.
}Use according to the excitation wevelength:
Olympus has prepared some sets of fluorescence mirror unit combined with appropriate filters which are variable depending on wavelengths.
The wide-band (W) set is normally used. There may be cases, however, where superwide-band (SW) or Narrow-band (N)
sets are recommendable.
@Extremely weak fluorescence brightness
(B- and G-excitation only):
² Specimens emitting strong autofluorescence: Use the narrow band (N).
Use the super-wide band (SW).
}With the SWB, strong autofluorescence may reduce
image contrast.
}The fluorescence bright is somewhat reduced.
8
Page 13
Dichroic Mirror and Filter Configurations of Fluorescence Mirror Units
Excitation
Method
U
V
BV
B
IB
G
IG
IY
Mirror Unit Dichroic Mirror Excitation Filter Barrier Filter Fluorochromes
U-MWU2
U-MNU2
U-MNV2
U-MWBV2
U-MNBV2 BP420-440
U-MWB2
U-MNB2
U-MSWB2
U-MWIB3
U-MNIB3
U-MWG2
U-MNG2
U-MSWG2
U-MWIG3 DM570 BP530-550 BA575IF
U-MWIY2 DM600
DM400
DM455 BP400-410 BA455
DM455
DM500
DM505
DM570
BP330-385
BP360-370
BP400-440
BP460-490
BP470-490
BP420-480
BP460-495
BP470-495
BP510-550
BP530-550
BP480-550
BP545-580 BA610IF
BA420
BA475
BA520IF
BA510IF
BA590
· Autofluorescence observation
· DAPI: DNA staining
· Hoechest 33258, 33342: Chromosome
· Catecholamine
· Serotonin
· Tetracyline: Bones, teeth
· Quinacrine, quinacrine mustard:
Chromosome
· Thioflavine S: Lymphocyte
· Acriflavine: Nucleic acid
· ECFP
· FITC: Fluorescent antibody
· Acridine orange: DNA, RNA
· Auramine: Tubercle bacillus
· EGFP, S65T, RSGFP
· Rhodamine, TRITC: Florescent antibody
· Propidium iodide: DNA
· RFP
Texas Red: Fluorescent antibody
Color Separation Filter Combinations
U U-MNUA2
U-MWIBA3
IB
U-MNIBA3
U-MWIGA3
G
U-MNIGA3
Mirror Unit Name Meaning
DM400 BP360-370 BA420-460
DM505
DM570
U- MN I B A 2
Mirror unit
Universal
For observing only the U-excitation stain,
when using U-excitation stain together
with FITC.
BP460-495
BA510-550
BP470-495
BP530-550
BA575-625
BP540-550
Model number (2 or 3)
For color separation
Excitation (U, V, BV, B, IB, G, IG or IY)
Bandwidth (SW: Superwide band. W: Wide band. N: Narrow band.)
For observing only the B-excitation stain,
when using B-excitation stain with TRITC
or Texas Red.
For observing only the G-excitation stain,
when using G-excitation stain together
with Cy5.
9
Page 14
Exclusively for Fluorescent Proteins
Excitation
Method
CFP U-MCFPHQ DM450HQ BP425-445HQ BA460-510HQ For ECFP
GFP U-MGFPHQ DM485HQ BP460-480HQ BA495-540HQ For EGFP
YFP U-MYFPHQ DM505HQ BP490-500HQ BA515-560HQ For EYFP
RFP U-MRFPHQ DM565HQ BP535-555HQ BA570-625HQ For RFP
Mirror Unit Name Meaning
Mirror Unit Dichroic Mirror Excitation Filter Barrier Filter Fluorochromes
U- MC F P HQ
High Quality
CFP/GFP/YFP/RFP
Mirror unit
Universal
3 Objectives for Various Observation Modes
UIS2 Series
Objective
UPlanSApo 4X
10X
20X
20X O
40X
60X W
60X O
100X O
PlanApoN 60X O
UPlanFLN 4X
10X
20X
40X
40X O
60X
60X OΙ
100X O2
100X OΙ2
Reflected light
fluorescence
¦
¦
¦
¦
¦
¦
¦
¦
¦*
¦
¦
¦
¦
¦
¦
¦
¦
¦
Phase contrast
difference
–
–
–
–
–
–
–
–
–¦
–
¦**
¦**
¦**
–
–
¦**
¦**
–
Transmitted light DIC
¦
¦
¦
¦
¦
¦
¦
¦
–
¦
¦
¦
¦
–
¦
¦
¦
10
¦ : Recommended combination.
¦* : Slightly inferior in U-excitation.
–– : Not usable, or applicable objective is not available.
¦** : A phase contrast (Ph) objective is necessary for phase contrast observation.
Page 15
UIS Series
Objective
UPlanApo 4X
10X
10X O
10X W
20X
20X O3
40X
40X O Ι 3
60X
60X W3
100X OΙ 3
PlanApo 40X
60X O3
100X O3
UPlanFI 4X
10X
20X
40X
60X O Ι 3
100X O, OΙ3
UApo
20X 3/340
20X W3/340
40X 3/340
40X
40X W3/340
OΙ 3/340
Reflected light fluorescence
U, V, BV B, IB, G, IY
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
–
¦
–
¦*
¦*
¦*
¦*
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦*
¦*
¦*
¦*
¦
¦
¦
¦
¦
¦
¦
Phase contrast
difference
–
¦**
–
–
¦**
–
–
¦**
–
–
¦**
–
¦**
–
–
¦**
¦**
¦**
¦**
¦**
–
–
–
–
–
Transmitted
light DIC
–
¦
¦
–
¦
¦
¦
¦
–
¦
¦
–
¦
–
–
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦
¦ : Recommended combination.
¦* : Usable, but image be dark depending on NA.
–– : Not usable, or applicable objective is not available.
¦** : A phase contrast (Ph) objective is necessary for phase contrast observation. The Ph objective is not
available for the UPlanFI100XOI3.
4 Turning the Power Supply Unit On
Set the main switch to “ I ” (ON). The arc will stabilize in 5 to 10 minutes after ignition.
}The discharge type mercury burner may not be ignited from the beginning on rare occasions due to its characteristics.
In this case, set the main switch to “ ” (OFF), wait for 5 to 10 seconds, then set it again to “ I ” (ON).
#To extend the mercury burner life, do not turn the mercury burner off for 15 minutes after ignition.
#The mercury burner cannot be reignited until the mercury vapor has cooled down and liquefied. Before re-igniting
a mercury burner, wait for about 10 minutes after the last time it was turned off.
}For the shake of safety, the power supply to the lamp housing is shut down if the lamp housing is opened while the burner
is on. If this happens, set the main switch to “ ” (OFF), wait for more than 10 minutes, then set it again to “ I ” (ON). Do not
open the lamp housing until it has cooled down enough.
#To reset the hour counter, hold its reset button till “000.0” is displayed.
11
Page 16
@
²
Fig. 1
³
5 Centering the Field Iris Diaphragm
1. Close the light path by sliding the shutter knob @ to position marked {.
2. Engage the B or IB mirror unit in the light path by rotating the turret.
(If these mirror units are not available, engage another fluorescence mirror unit in the light path.)
3. Open the light path by sliding the shutter knob to position marked \.
4 Engage the 10X objective in the light path, place the specimen on the
stage and bring the image into approximate focus.
5. Pull out the field iris diaphragm knob ² to minimize the field iris diameter.
6. Fit the Allen wrench provided with the microscope frame in the two field
iris centering screws ³ and adjust so that the iris image comes at the
center of the field of view.
7. While pushing in the field iris diaphragm knob ², enlarge the field iris
diaphragm until the field iris image inscribes the field of view. If eccentricity is found after this, try centering again.
8. Enlarge the iris diaphragm until the iris image becomes almost the same
size as (i.e. circumscribes) the field of view.
Effects of Field Iris Diaphragm
The field iris diaphragm restricts the diameter of the beam of light entering the objective and thus excludes extraneous light, improving image
contrast. The field iris diaphragm also functions to prevent color fading of
fluorescent light in other part than the observed region.
To exclude extra light, set the field iris diaphragm knob ² on the fluorescence illuminator according to the objective power, so that the image of
the field iris diaphragm just circumscribes the field of view.
(Fig. 1)
12
Page 17

@
Fig. 2
|
³
²
6 Centering the Aperture Iris Diaphragm
1. Close the light path by sliding the shutter knob @ to position marked {.
2. Engage the B or IB mirror unit in the light path by rotating the turret.
(If these mirror units are not available, engage another fluorescence mirror unit in the light path.)
3. Engage the 10X objective in the light path and lace the U-CST centering
target on the stage.
4. Open the light path by sliding the shutter knob to position marked \.
5. Move the white surface with crosslines of the U-CST until the crosslines
are overlaid on the center of field.
6. Turn the revolving nosepiece to engage the empty place (the objective
cap should be removed) in the light path.
7. Pull out the aperture iris diaphragm knob ² to minimize the aperture iris
diameter.
8. Pull out the field iris diaphragm knob ³ to minimize the field iris diaphragm. Now the aperture iris image should be visible on the U-CST.
9. Fit the Allen wrench in the two aperture iris centering screws | and
adjust so that the aperture iris image coincides with the crosslines.
Effects of Aperture Iris Diaphragm
The aperture iris diagram helps adjust the brightness of the observed
image and improve the contrast.
To execute normal fluorescence observation, enlarge the aperture iris
diaphragm by pushing in the aperture iris diaphragm knob ².
}If specimen colors tend to fade due to too high excitation light, first use
ND filters to reduce the brightness, and decrease the aperture iris diaphragm if the ND filters are not enough.
Do not decrease the aperture iris diaphragm too much. Do not use it as
a substitute to the shutter.
(Fig. 2)
13
Page 18

7 Centering the Mercury Burner
}Set the main switch to “ I ” (ON) and wait for 5 to 10 minutes until the arc
stabilizes before proceeding to the mercury burner centering.
1. Close the light path by sliding the shutter knob @ to position marked {.
2. Engage the B or IB mirror unit in the light path by rotating the turret.
(If these mirror units are not available, engage another fluorescence mirror unit in the light path. Also note that, when using a U-excitation fluorescence mirror unit, be sure to observe the specimen through a UV cut
plate.)
3. Engage the 10X objective in the light path, place the U-CST centering
target on the stage, and adjust the centering of the center of crosslines
on white surface of the U-CST with respect to the center of field of view.
4. Turn the revolving nosepiece to engage the empty position (the objective
cap should be removed) in the light path.
5. Pull out the field iris diaphragm knob ² (to minimize it) and push in the
aperture iris diaphragm knob ³ (to enlarge it).
6. Open the shutter by setting shutter knob @ to position marked \.
7. Project the arc image on the U-CST by turning the collector lens focusing
knob |. (A)
If the arc image is not protected, adjust the burner centering knobs ƒ.
8. ring the arc image on the center of the left (or right) half of the field by
turning the burner centering knobs ƒ. (B)
9. Focus on the mirror arc image by adjusting the mirror focus screw … (Fig
4) on the rear of the lamp housing using the Allen screwdriver. (C)
Overlay the arc image with the mirror arc image by turning the burner
10
.
centering knobs ƒ. (D)
}During observation, adjust the collector lens focusing knob | so that the
observed field is uniform.
}Hereafter, the mercury burner centering need not be adjusted until the
next time the mercury burner is replaced.
@
²
³
|
ƒ
Fig. 3
A
B
14
C
D
Page 19

@
‡
²
Fig. 4
Fig. 5
…
†
Precise Centering of the Mirror Arc Image
}The mirror arc image position has been adjusted and fixed at the factory.
Perform the centering of the mirror arc image after completing the centering of the mercury burner and only when you want to make your adjustments very strict and precise.
Note that, once this adjustment has been executed, the mirror can never
be returned to the same status as the factory shipment status.
1. Using a pair of tweezers, etc., peel off the two blind seals † from the rear
of the lamp housing.
2. Loosen the screws below the seals using the Allen screwdriver. The mirror is unclamped when these two screws are loosened.
3. Then peel off another couple of blind seals ‡. This exposes the mirror
arc image centering holes.
4. Adjust the centering of the mirror arc image using the Allen screwdriver
in these holes.
8 Mounting the ND Filters
}Specimen color fading can be delayed by reducing the excitation light
intensity with ND filters. Use the ND filters as far as they do not hinder
observations.
· As necessary, up to two ND filters (with ND of 6 and 25) may be
individually inserted into filter insertion positions @ and/or ². Insert
the ND filters (U-25ND6-2 and/or U-25ND25-2, U-25ND50-2) with the
marked side facing toward the observer.
The ND filters must be inserted in the correct orientation. Otherwise, the
ND filters may be damaged.
· As you insert a filter, you will hear two clicks. At the first, the filter is at the
at an empty position, and at the second click the filter enters the light
path.
Note that the metallic filter frame will be very hot if you leave the filter
inserted for a long time while the mercury burner is on.
Do not leave the filter insertion positions in other positions than the
click positions for a long period of time.
15
Page 20

SIMULTANEOUS FLUORESCENCE OBSERVATIONS
}By properly combining equipment, this system can be used in transmitted light brightfield observation, transmitted phase
contrast observation and transmitted light DIC observation in addition to the reflected fluorescence observation. With
specimens that fade rapidly, fading can be minimized by initially using transmitted light phase contrast or transmitted light
DIC observation for positioning. Reflected fluorescence observation can also be executed simultaneously with phase
contrast or DIC observation, making it easy to tell which portion of the specimen is fluorescing.
1 Simultaneous Reflected Fluorescence and Phase Contrast Observations
The phase contrast observation requires a phase contrast condenser (U-PCD2) or a universal condenser (U-UCD8) and
a Ph objective.
1. Engage a dummy mirror unit (or an empty position on the turret) in the light path.
2. Rotate the phase contrast turret to show the same number as the Ph number shown on the objective.
3. Adjust the optical axis between the ring sit and phase plate by centering them.
4. Engage the mirror unit corresponding to the desired excitation into the light path and open the shutter.
5. Adjust the transmitted light for the best balance of fluorescence and phase contrast brightness, and you are ready for
observation.
}Use ND filters or the light intensity control lever on the microscope base to adjust the transmitted light intensity.
}For details on using phase contrast observation, refer to the instruction manual provided with the phase contrast con-
denser or universal condenser.
Simultaneous Reflected Fluorescence and Transmitted Light Nomarski Differential
2
Interference Contrast (DIC) Observations
The transmitted light Nomarski DIC observation requires the following accessories; 1) universal condenser (U-UCD8); 2)
transmitted light DIC slider (U-DICT, U-DICTS, U-DICTHR or U-DICTHC); 2) analyzer (U-AN or U-AN360-3); 6- or 7-position
revolving nosepiece for DIC (U-D6RE or U-D7RE).
}In order for reflected fluorescence to be effective in the simultaneous observation, insert the analyzer (U-AN or U-AN360-
3) into the analyzer inlet slot above the dichroic mirror on the illuminator.
Do not insert the U-ANT analyzer in the transmitted light DIC slider, for this will dim the fluorescence observation image
and cause the analyzer to be burnt.
1. Engage the dummy mirror unit (or an empty position on the turret) in the light path.
2. Adjust the polarizer on the universal condenser to the “crossed Nicol” (complete extinction) status.
3. Insert the transmitted light DIC slider into the position provided on the nosepiece.
4. Rotate the turret on the universal condenser to select the Nomarski prism matching the objective to be used for observation.
5. Engage the objective to be used in the light path.
6. Place the specimen on the stage and focus on the specimen.
7. Adjust the field iris diaphragm of the transmitted light illumination unit (built into the microscope base) and the aperture iris
diaphragm of the universal condenser.
8. Turn the prism movement knob on the transmitted light DIC slider to adjust contrast of the DIC image.
9. Engage the mirror unit corresponding to the desired excitation in the light path and opent the shutter.
Adjust the transmitted light for optimum fluorescence and DIC image brightness.
10.
}For details on the transmitted light DIC observation, refer to the instruction manual provided with the U-UCD8 transmitted
light universal condenser.
Notes
}We recommend the use of the highly wear-resistant U-ANH analyzer-slider instead of the U-AN analyzer when you are
frequently switching between reflected fluorescence observation and transmitted light Nomarski DIC observation and
need to use both observations simultaneously.
}However, if you are frequency switching between reflected fluorescence observation and transmitted light Nomarski DIC
observation but you do not need to use both simultaneously, then it will be more convenient for you to use the M-DICT3
DIC mirror unit instead of an analyzer (U-AN or U-ANH). This facilitates the switching operation because the analyzer
simultaneously enters the light path when the fluorescence mirror unit is switched to the DIC mirror unit.
16
Page 21

TROUBLESHOOTING GUIDE
Under certain conditions, performance of the unit may be adversely affected by factors other than defects. If problems occur,
please review the following list and take remedial action as needed. If you cannot solve the problem after checking the
entire list, please contact your local Olympus representative for assistance.
Problem
1. Optical System
a) Burner is ON but light cannot be
seen from eyepiece of is dark.
b) Image is low quality, not sharp or
poor in contrast.
c) Field of view is obscured or not
evenly illuminated
d) Field contains dark, spot-like areas. Dirt or dust on burner or on burner side
2. Electrical System
Shutter is closed. Open the shutter.
ND filter is engaged in light path.
Fluorescence mirror unit is not correctly
engaged in light path.
Aperture and field iris diaphragms are not
fully enlarged.
Fluorescence mirror unit does not match
specimen.
Dirt/dust on objective or filter. Clean thoroughly.
Aperture and field iris diaphragms are not
properly enlarged.
Fluorescence mirror unit does not match
specimen.
Objective is not correctly engaged in light
path.
Fluorescence mirror unit is not correctly
engaged in light path.
Field iris diaphragm is set too small. Fully enlarge field iris diaphragm.
ND slider is not stopped at click position.
Mercury burner is not centered or focusing is defective.
of collector lens.
Cause Remedy Page
Remove ND filter as required.
Engage it correctly.
Fully enlarge aperture iris diaphragm
and enlarge field iris diaphragm until
it circumscribes field of view.
Use fluorescence mirror unit matching specimen.
Fully enlarge aperture iris diaphragm
and enlarge field iris diaphragm until
it circumscribes field of view.
Use fluorescence mirror unit matching specimen.
Make sure that revolving nosepiece
clicks properly into place.
Engage fluorescence mirror unit correctly in light path.
Make sure that ND slider clicks properly into place.
Center mercury burner or perform focusing adjustment.
Clean them.
4
15
4
13
9/10
3
12/13
9/10
––
––
12
15
14
3
a) Main switch cannot turn system
ON.
b) Main switch can turn system ON
but mercury burner will not light.
c) Mercury burner flickers or is dark. It is soon after ignition. Leave for 10 minutes or more after ig-
Power cord is not connected properly.
Connectors are not connected properly. Connect firmly.
Mercury burner is not attached. Attach mercury burner.
Safety device in lamp housing is active.
Auto ignition is malfunctioning. Set main switch of power supply unit
Burner life has expired.
Burner is deviated from optical axis. Center mercury burner.
Connect firmly.
Set up the lamp socket correctly.
to “
” (OFF) then “ I ” (ON) again. (OFF/
ON can be repeated.)
nition.
If hour counter indicates 300 hours
(USH-103OL,
mercury burner.
HBO103W/2), replace
35
35
33
33
11
11
34
14
17
Page 22

SPECTRAL CHARACTERISTICS OF FILTERS
1. U-excitation (Wide band)
U-MWU2
Transmittance %
Wavelength (nm)
2. U-excitation (Narrow band)
U-MNU2
4. BV-excitation (Wide band)
U-MWBV2
Transmittance %
Wavelength (nm)
5. BV-excitation (Narrow band)
U-MNBV2
Transmittance %
Wavelength (nm)
3. V-excitation (Narrow band)
U-MNV2
Transmittance %
Transmittance %
Wavelength (nm)
6. B-excitation (Wide band)
U-MWB2
Transmittance %
18
Wavelength (nm)
Wavelength (nm)
Page 23

7. B-excitation (Narrow band)
U-MNB2
10. IB-excitation (Narrow band)
U-MNIB3
100
BP470-495
DM505
Transmittance %
Wavelength (nm)
8. B-excitation (Superwide band)
U-MSWB2
Transmittance %Transmittance %
Transmittance %
BA510IF
0
400 600 800
Wavelength (nm)
11. G-excitation (Wide band)
U-MWG2
Transmittance %
Wavelength (nm)
9. IB-excitation (Wide band)
U-MWIB3
100
BP460-495
BA510IF
0
400 600 800
Wavelength (nm)
Wavelength (nm)
12. G-excitation (Narrow band)
U-MNG2
DM505
Transmittance %
Wavelength (nm)
19
Page 24

13. G-excitation (Superwide band)
U-MSWG2
16. U-excitation, color separation (Narrow band)
U-MNUA2
Transmittance %
14. IG-excitation (Wide band)
U-MWIG3
100
Transmittance %
0
BP530-550
400 600 800
Wavelength (nm)
BA575IF
DM570
Transmittance %
Wavelength (nm)Wavelength (nm)
17. IB-excitation, color separation (Wide band)
U-MWIBA3
100
BP460-495
DM505
Transmittance %Transmittance %
BA510-550
0
400 600 800
Wavelength (nm)
20
15. IY-excitation (Wide band)
U-MWIY2
Transmittance %
Wavelength (nm)
18. IB-excitation, color separation (Narrow band)
U-MNIBA3
100
BP470-495
DM505
BA510-550
0
400 600 800
Wavelength (nm)
Page 25

BA515-560HQ
19. G-excitation for color separation (Wide band)
U-MWIGA3
100
BP530-550
DM570
22. For green fluorescent protein (GFP)
U-MGFPHQ
100
BP460-480HQ
DM485HQ
BA495-540HQ
Transmittance %
0
400 600 800
Wavelength (nm)
BA575-625
20. G-excitation for color separation (Narrow band)
U-MNIGA3
100
BP540-550
DM570
Transmittance %
BA575-625
0
400 600 800
Wavelength (nm)
Transmittance %
0
400
450 500 550 600
Wavelength (nm)
23. For yellow fluorescent protein (YFP)
U-MYFPHQ
100
BP490-500HQ
BA515-560HQ
Transmittance %
0
400
450 500 550
Wavelength (nm)
DM505HQ
600
21. For cyan fluorescent protein (CFP)
U-MCFPHQ
100
BP425-445HQ
BA460-510HQ
Transmittance %
0
400
450 500 550 600
Wavelength (nm)
DM450HQ
24. For red fluorescent protein (RFP)
U-MRFPHQ
100
BP535-555HQ
Transmittance %
BA570-625HQ
0
400 600 800
Wavelength (nm)
DM565HQ
21
Page 26

Typical Example of Emission Spectrum of Ultra-High-Vacuum
Mercury Burner Spectrum
For fluorochrome emission, a light beam having a specific wavelength is selected from a
wide spectrum of wavelengths. The five major peaks of luminance are at wavelengths of
365/366, 404.7, 435, 546.1 and 577.0/579.1 nm. In addition, light beams having wavelengths of 334.2 and 490 nm (at rather low luminance) are also applicable to fluorochrome emission.
22
Page 27

SPECIFICATIONS
Item
Vertical illuminators
· UIS2/UIS (Universal Infinity System) optical system (featuring infinity correction)
· Magnification: 1X (Superwide field: NA 26.5)
· Observation switching: Mirror unit turret carrying max. 6 mirror units.
· Aperture iris diaphragm and field iris diaphragm (Both centerable) Detachable with the BXRFA.
· Shutter provided.
· Slider inlet
@ Analyzer/6-position barrier filter slider
² Polarizer/6-position filter slider
³ ND filters
· Available observation modes
@ Reflected fluorescence
² Reflected fluorescence + Transmitted DIC
³ Reflected fluorescence + Phase contrast
| Reflected light brightfield
ƒ Reflected light darkfield
… Reflected light DIC
† Reflected light simplified polarization
‡ Transmitted light
· Optional accessories
Mercury lamp housing · 100 W mercury lamp housing U-LH100HG
· 100 W mercury apo lamp housing U-LH100HGAPO
· Mercury burner: USH-103OL (USHIO) or HBO103W/2 (OSRAM)
Operating environment
· Indoor use.
· Altitude: Max. 2000 meters
· Ambient temperature: 5° to 40°C (41° to 104° F)
· Maximum relative humidity: 80% for temperatures up to 31°C (88°F), decreasing linearly
through 70% at 34°C (93°F), 60% at 37°C (99°F), to 50% relative humidity at 40°C (104°F).
· Supply voltage fluctuations; Not to exceed ±10% of the normal voltage.
· Pollution degree: 2 (in accordance with IEC60664)
· Installation/Overvoltage category: II (in accordance with IEC60664)
Reflected Illuminator
BX-URA2
Specification
@ Analyzer/6-position barrier filter slider
² 6-position filter slider
³ ND filters
| 6-position filter slider
@ Reflected fluorescence
² Reflected fluorescence + Transmitted DIC
³ Reflected fluorescence + Phase contrast
| Transmitted light
@ Exciter/balancer
² Rectangle field stop
Fluorescence Illuminator
BX-RFA
23
Page 28

OPTIONAL MODULES
#The sliding performance of the U-RSL6 or U-RSL6RM filter slider may drop when it has been used for 2000 or
more times of reciprocation. In this case, remove the dirt and contamination on the sliding surface. If it is expected
to use the slider for more 2000 times of reciprocation or more, apply a thin layer of lubricant, such as grease on
the sliding surface.
1 6-Position Filter Slider U-RSL6
}This filter slider is for use with the BX-URA2 or BX-RFA illuminator and accommodates a total of six excitation and ND
filters. It is designed to prevent centering deviation between the optical axes of the excitation filters when multiple
excitation mirror units are used and switched.
Slider knob
Inscription sticker position
Diameter 8 mm, depth 1 mm
Light shielding position
Diameter 25 mm, Thickness 2 to 6 mm
Light shield plate
Filter Mounting Procedure
1. Remove the slider knob on the opposite end to the extremity where the slider inscription is engraved, and place the filter
slider so that the surface with the slider inscription faces down.
2. Remove the filter holder rings from the filter insertion positions by turning it counterclockwise using the provided holder
ring driver.
#The insertion orientation of the holder rings should be changed according to the thickness of the mounted filters.
3. If the mounted filter includes an exciter filter, insert it so that the arrow inscription on the side faces down.
a)
b)
4. If it is required to attach a filter type inscription, attach a seal as described in the next section on the U-RSL6EM filter slider.
5. Insert the filter slider from the right of the 6-position filter slider inlet slot on the illuminator so that inscription “U-RSL6”
comes at the deep, then attach the slider knob which has been removed in the above.
a) Filter with thickness of 4 mm or more:
Place each filter so that it fits inside the holder ring.
b) Filter with thickness of 4 mm or less:
Place each filter so that it does not fit inside the holder ring.
If you perform transmitted light observation or you do not want to use a filter,
mount the provided light shield plates (having the same size as the filter) in
place.
If nothing is mounted, the scattered light of reflected lighting may enter your
eyes or the view in transmitted light observations will be deteriorated.
Holder ring driver
Joint plate
Filter insertion positions (x 6)
24
Page 29

Using the Joint Plates
@
Fig. 6
The joint plates @ can be attached and locked between the slider knob
and slider as shown in the figure. The joint plates should be attached on
both ends of the filter slider.
By locking with the joint plates, you can switch the barrier and excitation
filters together as a set.
NOTES
· When inserting the 6-position filter slider in the 6-position filter slider near
the rear panel, insert from the left so that the “U-RSL6” inscription comes
at the deep. Otherwise, the filters will not be set in the correct positioning.
· When the 6-position filter slider near the rear panel is used, avoid using
the interference type or color glass type filters. This is because the 6position filter slider near the rear panel is one of the positions where the
energy from the light source is concentrated. When an interference type
or color glass type filter is mounted in it, the filter interference film may
peel off or the color glass may be damaged.
· Make sure that the 6-position filter slider is set to a click position.
· For safety, insert the provided light shield plates in the unused filter positions.
Fig. 7
25
Page 30

2 6-Position Barrier Filter Slider U-RSL6EM
}This filter slider is for use with the BX-URA2 or BX-RFA illuminator and accommodates a total of six barrier filters.
Slider knob
Light shield plate (x 4)
Inscription sticker position
Diameter 8 mm, depth 1 mm
Light shield position
Barrier filter insertion positions (x 6)
Diameter 25 mm, thickness max. 5 mm
Filter Mounting Procedure
1 Remove the slider knob on the opposite end to the extremity where the slider inscription is engraved.
2 Gently place the barrier filters in filter insertion positions.
#Insert the filters so that their arrow inscriptions on the side face downward.
3. If it is required to inscribe the type of the inserted filter, write it on a commercially available round sticker with a diameter of
less than 8 mm, and attach it to the specified inscription sticker position.
#Make sure that the sticker does not deviate from the specified circular area. Otherwise, the slide will be caught in
motion.
4. Gently insert the filter slider from the right of the analyzer inlet slot on the illuminator, and attach the slider knob which has
been removed in the above.
5. Use the joint plate if you want to interlock this filter slider with the U-RSL6 fitter slider. (For the attaching method, see the
description on the U-RSL6.)
Joint plate
26
NOTES
· Be sure to insert each filter in the specified orientation. Otherwise, the filter cannot be set in the correct positioning.
· For safety, insert the provided light shield plates in the unused filter positions.
Page 31

3 Rectangle Field Stop BX-RFSS (for exclusive use with the BX-RFA)
}When fluorescence images are recorded with the TV camera for observation or image processing, this unit projects a
rectangular iris diaphragm image with size variable according to the captured image size. This helps prevent color fading
of specimen due to other reasons than image capturing.
Clamping screw hole
Adjustment screws
}The adjustment knobs can be stored in the
upper slots of the adjustment screws.
Installation Procedure (Fig. 8)
Adjustment knobs (x 2)
@
Fig. 8
Operation
1. Insert the provided adjustment knobs into the two adjustment screw holes near the front panel, and move the two sides
of the rectangle to the desired position by turning the knobs.
2. Insert the adjustment knobs into the two adjustment screw holes near the rear panel. and move the other two sides of the
rectangle by turning the knobs.
3. After the desired shape has been obtained by moving the sides, remove the adjustment knobs.
}Rectangle area: A rectangle which circumscribes the field with a number of 22 (the center of the rectangle should be
located at the center of field). The rectangle iris diaphragm cannot be rotated.
NOTE
The BX-RFA fluorescence illuminator cannot be attached or removed while the BX-RFSS is installed. IF you want to install
the BX-RFA, remove the BX-RFSS temporarily.
1. Using the Allen screwdriver, loosen and take out the field iris diaphragm
clamping screw @. of the BX-RFA.
2. Remove the field iris diaphragm by puling it out toward you.
3. Insert the BX-RFSS rectangle field stop into the position of the field iris
diaphragm, then tighten the clamping screw @.
27
Page 32

4
Exciter Balancers U-EXBABG/EXBAUB/EXBAUG (for exclusive use with the BX-RFA)
}When an image of fluorescence by multiple excitation of U/B/G is observed with dual- or triple-band fluorescence mirror
units, use the exciter balancer to select the balance between the excitation light intensities of the fluorochromes.
Clamping screw
Adjustment lever
Installation Procedure (Fig. 9)
1. Stand the adjustment lever @ of the exciter balancer vertically and insert
it in one of the ND filter inlets with the same number as the slider on the
@
U-EXBAUG
U-EXBABG/EXBAUB
left side of the illuminator, or into the one which is located near the illuminator rear panel.
· The insertion position is variable depending on the type of the exciter
balancer.
· With any type of exciter balancer, always insert so that the clamping
screw faces toward you.
2. Tighten the clamping screws using the Allen screwdriver.
28
Fig. 9
Operation
Observing a Double Stained Specimen
1. Set up normal reflected fluorescence observation.
2. Mount the fluorescence mirror units for double staining and engage them in the light path.
}Olympus standard products
#Due to its own characteristics, the G-exci-
tation has a narrower intensity control range
than the U- and B-excitation. The intensity
control range is also variable depending on
the status of specimen and mirror units.
#Lighting irregularities may be observed on
the upper and lower edges of the field due
to the rotation angles of filters and the variance in mirror units’ characteristics. However, these lighting irregularities does not
affect the photographed area.
U-EXBABG
U-EXBAUB
U-EXBAUG
Fluorescence Mirror UnitExciter Balancer
Fluorescence mirror units
for double staining
·U-DM-FI/TR2
·U-DM-FI/PI2
·U-DM-FI/TX2
·U-DM-DA/FI2
·U-DM-DA/TR2
·U-DM-DA/PI2
·U-DM-DA/TX2
Fluorescence mirror units
for triple staining
·U-DM-DA/FI/TR2
·U-DM-DA/FI/PI2
·U-DM-DA/FI/TX2
Page 33

3. Push in the adjustment lever of the balancer slider to be used to engage the filter in the light path.
}The angle of each adjustment lever can be adjusted in the range shown below, only when the lever is pushed in.
0˚
Front (Observer)
U-EXBAUG U-EXBABG or U-EXBAUB
4. While conducting fluorescence observation, adjust by tilting the adjustment lever of the exciter balancer which is currently
in the light path.
· With the U-EXBABG, setting the lever to 0° enhances the fluorescence of longer wavelengths (near red) and to 45°
enhances the fluorescence of shorter wavelengths (near green).
· With the U-EXBAUB, setting the lever to 0° enhances the fluorescence of shorter wavelengths (near blue) and to 45°
enhances the fluorescence of longer wavelengths (near green).
· With the U-EXBAUG, setting the lever to 0° enhances the fluorescence of longer wavelengths (near red) and to 45°
enhances the fluorescence of shorter wavelengths (near blue).
Observation of Triple Stained Specimen
}The operation is basically similar to the double stained specimens, but fluorescence mirror units for triple staining should
be used. The exciter balancers to be used are the U-EXBAUB (front inlet) and U-EXBAUG (rear inlet).
· While conducting fluorescence observation, adjust the intensities of the three fluorescence lights by tilting the two adjustment levers.
NOTES
1. When the adjustment lever of an exciter balancer is stood vertically, flare tends to occur easily due to the repeated
reflections on the filter surface. Be sure to disengage the exciter balancer from the light path when it is not used.
2. Be sure to stand the adjustment lever vertically when disengaging the filter from the light path or removing the exciter
balancer. (Otherwise, damage may result.)
3. To use the ND filters while the balancer is already used, insert the ND filters in the 6-position filter inlet slot which is near the
front panel (i.e. on the left).
29
Page 34

ASSEMBLY
9-1 Assembly Diagram
The diagram below shows the sequence of assembly of the various modules. The numbers indicate the order of assembly.
The module numbers shown in the following diagram are merely the typical examples. For the modules with which the
module numbers are not given, please consult your Olympus representative or the catalogues.
#When assembling the microscope, make sure that all parts are free of dust and dirt, and avoid scratching any parts
or touching glass surfaces.
Assembly steps enclosed in will be detailed on the subsequent pages.
}All assembly operations are possible by using the Allen screwdriver (
The Allen wrench (
) provided with the illuminator is used only for clamping the screws inside the illuminator. (To retain
the performance, have your Olympus representative conduct this work.)
) provided with the microscope.
NOTES
· Parts marked with # can be attached only to the BX-URA2 universal illuminator.
· Be sure to insert the sliders in the orientations shown in the diagram. Otherwise, they cannot be fitted in click
positions and engaged correctly in the light path.
Eyepiece
ND filters
U-25ND6-2
U-25ND25-2
U-25ND50-2
6-Position filter slider
U-RSL6
Turret
UV cut plate
Exciter balancer
U-EXBABG
U-EXBAUB
U-EXBAUG
Fluorescence
mirror unit
Observation
tube
Vertical
illuminator
BX-URA2
BX-RFA
Illuminator
clamping
screws
Mercury burner
USH-103OL
HBO103W/2
Lamp housing
clamping screw
Power cord
Mercury lamp
housing
U-LH100HG
U-LH100HGAPO
Power supply unit
(for 100W mercury burner)
Rectangle field stop
BX-RFSS
30
Objectives/Revolving
nosepiece
Light shield sheets
Microscope frame
BX51TRF
Analyzer
U-AN
U-AN360-3
6-Position filter slider
U-RSL6
Polarizer
U-PO3
6-Position barrier filter slider
U-RSL6EM
Page 35

9-2 Detailed Assembly Procedures
…
ƒ
Fig. 10
Fig. 11
@
|
2
Attaching the Fluorescence Mirror Units
1. Using the Allen screwdriver, loosen the clamping screw @ at the right
side of the vertical illuminator.
2. Pull out the turret and place it upside down.
}Dummy mirror units ² are mounted in the mirror unit positions. Remove
the dummy mirror units from the positions you want to mount mirror units
by loosening the clamping screw ³ of each mirror unit using the Allen
screwdriver.
(Figs. 10 & 11)
³
²
3. Hold the fluorescence mirror unit | to be mounted so that the model
name inscription on the side is upside down, align it with the mount
dovetail and insert all the way into the insertion position. Tighten the
clamping screw ƒ firmly.
#If the clamping screw ³ is loose, The turret will be unable to be
rotated due to interference with the cover.
4. Check the mount dovetail number ƒ and place the inscription sheet of
the mounted fluorescence mirror unit into the inscription pocket … with
the same number on the front of the turret.
5. Mount other the required fluorescence mirror units by repeating the above
steps for each of them.
6. Place the turret in the original position and tighten the clamping screw @.
while pushing the turret in.
31
Page 36

Making an Optional Fluorescence Mirror Unit
}You can also fabricate optional fluorescence mirror units by fitting a commercially available barrier filter, excitation filter or
dichroic mirror in the U-MF2 mirror unit frame.
Dimensions of Optical Parts
· Barrier filter
· Excitation filter
· Dichroic mirror 26 -0.1/-0.3 x 38 -0.1/-0.3 mm, thickness 1 ±0.05 mm
Dichroic mirror
(commercially available)
#When replacing the dichroic mirror, take special care not to stain it with fingerprints, etc.
Diameter
25 -0.1/-0.2 mm, max. thickness 6 mm.
Barrier filter (commercially available)
Interference film surface
Excitation filter
(commercially available)
32
Page 37

³
Fig. 12
Fig. 13
@
²
|
3 Attaching the Mercury Burner
1. Loosen the socket clamping screw @ using the Allen screwdriver.
2. Hold the upper section of lamp housing and pull it upward to remove the
socket section.
#To prevent malfunctions, do not hold the lamp housing by the cen-
tering knobs ².
3. Place the socket section upside down as shown in Fig. 13.
}The lamp housing is equipped with the holder for transportation in the
factory shipment condition, or with an old burner when the burner is
replaced. Remove the holder or old burner by loosening the two burner
holding screws ³.
4. Attach the + (positive) pole of a specified mercury burner | to the fixed
mount on the upper side, and the - (negative) pole to the mount on the
lower side.
#Be sure to use the USH-103OL (mfd, by USHIO Inc.) or HBO103W/2
(mfd. by OSRAM) burner.
!Be careful and avoid leaving fingerprints or contaminants on the
mercury burner. Otherwise, there is a danger of explosion due to
distortion of glass caused by the stains. If the burner is contaminated, clean it by wiping gently with gauze slightly moistened with
absolute alcohol.
5. Attach the socket section with burner to the original position and tighten
the clamping screw @.
#Align the external edges of the lamp housing with those on the
socket section, and push the lamp housing straight downward.
(Figs. 12 - 15)
33
Page 38

@
²
Fig. 14
Fig. 15
Resetting the Burner Hour Counter
1. Press the center section @ of the reset button ² on the power supply
unit’s front panel to reset the hour counter to 000.0.
}The hour counter shows elapsed time in hours. The service life of a
burner is 300 hours. For safety’s sake, replace the burner when the hour
counter indicates 300.0 hours.
Mercury Burner Replacement
1. In order not to impair the safety of the equipment, replace the burner
when it has been used for 300 hours. The burner may crack if used
beyond the specified life time.
When the end of the burner’s service life is near, flickering is likely
to increase. It is therefore recommended to replace the burner
according to the purpose of observation.
* This value assumes light cycles composed of 2 hours of lighting
and 30 minutes of extinction (with the USH-103OL). Do not turn it
on and off at a shorter cycle than the above, for this will shorten the
service life of the burner.
2. Before replacing the burner, wait at least 10 minutes or until the
lamp and lamp housing have cooled down after turning the burner
off. Before removing the burner, confirm that the main switch on the
power supply unit is “
plug from the output connector on the power supply unit. Refer to
page 33 for details on replacement procedure.
3. After replacing the burner, reset the hour counter to 000.0 as outlined above.
” (OFF) and unplug the connecting cord
34
Page 39

³
Fig. 16
|ƒ
Fig. 17
@
²
7 Setting the Power Supply Unit
!Cables and cords cam easily be damaged when bent or twisted. Do
not subject them to excessive force.
!Make sure that the main switch is set to “
ing the power cord.
!Always use the power cord provided by Olympus. If no power cord
is provided, please select the proper power cord by referring to
“PROPER SELECTION OF THE POWER SUPPLY CORD” at the end of
this instruction manual.
1. Verify that the voltage and frequency of the input power supply match the
requirements inscribed on the rating plate @.
(100 V systems can be used with voltages in the 100 to 120 V range and
200 V systems can be used with voltage in the 220 to 240 V range, both
with frequencies of 50 to 60 Hz.
2. Securely plug the burner socket connection cord into the power supply
unit’s connector ².
3. Plug the power supply unit’s power cord into its power input connector
³, then plug the power plug | into the wall power outlet ƒ.
!Be sure to supply power from a grounded 3-conductor power outlet
using the proper power cord. If the power outlet is not grounded
properly, Olympus can no longer warrant the electrical safety performance of the equipment.
(Figs. 16 & 17)
” (OFF) before connect-
35
Page 40

II. REFLECTED OBSERVATIONS (BX-URA2 Only)
CONFIGURATION OF REFLECTED OBSERVATION SYSTEM
The BX-URA2 universal illuminator can be used in a variety of brightfield observations, darkfield observation, DIC observation
and simplified polarized observation under reflected lighting when it is used in combination with a UIS2/UIS objective for
metallic specimens, the U-MBF3 brightfield mirror unit, U-MDF3 darkfield mirror unit, etc.
}Replace the standard stage with the stage for metallurgical specimens or the specimen holder with the stage plate for easier
observation.
Analyzer
· U-AN
· U-AN360-3
Mirror unit
· For brightfield: U-MBF3
· For darkfield: U-MDF3*
· For DIC: U-MDIC3**
· For brightfield,
with built-in ND filters: U-MBFL3
· For fluorescense: U-MWUS3
U-MWBS3
U-MWGS3
Filter
· U-25ND6-2
· U-25ND25-2
· U-25ND50-2
· U-25LBD
· U-25IF550
· U-25Y48
· U-25FR
· U-25L42
Objective
· UIS2/UIS series objectives
for metallic specimens
Conversion lens
· U-RCV*
Lamp housing
· Mercury lamp housing
U-LH100HG
U-LH100HGAPO
· Halogen lamp housing
U-LH100-3
U-LH100L-3
U-ULH (Halogen model)
· Xenon lamp housing
U-LH75XEAPO
Polarizer**
U-PO3
U-POTP3
Light shield tube
DIC prism
· U-DICR
· U-DICRH
· U-DICRHC
36
* The U-RCV conversion lens is required when the U-MDF3 mirror unit is used.
** When the U-MDIC3 mirror unit or the U-PO3 or U-POTP3 polarizer is used, combine the U-25L42 filter to prevent polarizing
optics from being deteriorated by UV rays from a high-intensity light source other than a halogen light source.
Page 41

ASSEMBLY
}This chapter pertains only to the assembly of items which cannot be assembled in the same way as the fluorescence
modules.
Fig. 18
@
Fig. 19
@
1 Attaching the U-RCV Conversion Lens
}Be sure to use this conversion lens when the U-MDF3 mirror unit for
darkfield observation is used.
· Insert the conversion lens @ between the reflected illuminator and lamp
housing.
#With ultrawide-field observation, the ambient lighting may be insuffi-
cient with certain types of specimens.
2 Attaching the Light Shield Tube
}The light shield tube must be used with darkfield observation (using DF
mirror unit).
1. Remove the turret.
2. Place the light shied tube in the reflected illuminator so that the positioning collar @ on the tube comes on the right.
(Fig. 18)
(Fig. 19)
@
FIELD IRIS AND APERTURE IRIS DIAPHRAGM ADJUSTMENTS
(Fig. 20)
²
Fig. 20
³
1 Centering the Field Iris Diaphragm
1. Rotate the turret to engage the mirror unit (BF) in the light path, then open
the shutter @.
2. Rotate the revolving nosepiece to engage the 10X objectlve, then place
the specimen on the stage and bring the image into approximate focus.
3. Pull out the field iris diaphragm knob ² on the reflected illuminator to
where the diameter of the diaphragm is at its smallest.
37
Page 42

Field iris image
Eyepiece’s field of view
4. Fit the Allen screwdrivers provided with the microscope frame into the
two field iris diaphragm centering screws ³ and adjust them so that the
field iris image of the diaphragm is centered on the field of view.
5. To check centering, enlarge the diaphragm by pushing in the field iris
diaphragm knob ² until the diaphragm image touches the perimeter of
the field of view. If the image is not centered precisely, center it again.
6. Further enlarge the iris diaphragm until its image just circumscribes the
field of view.
Effects of Field Iris Diaphragm
{Reflected light brightfield, DIC and simplified polarized light obser-
vations:
To obtain good image contrast, adjust the diameter of the illuminating
beam in accordance with the objective in use.
Using the field iris diaphragm knob ² on the reflected illuminator, adjust
the diaphragm so that the field of view is circumscribed by the field iris
diaphragm in order to exclude stray light.
{Reflected light darkfield observation:
Always keep the field iris diaphragm knob ² pushed in to leave the
diaphragm open.
@
²
Fig. 21
70-80%
30-20%
³
2 Centering the Aperture Iris Diaphragm
1. Engage the mirror unit (BF) in the light path by turning the turret, then
open the shutter @.
2. Rotate the revolving nosepiece to engage the 10X objective, then place
a highly flat specimen such as a mirror on the stage, and bring the
image into approximate focus.
3 Remove the eyepiece. While looking into the eyepiece sleeves, pull out
the aperture iris diaphragm knob ² so that the aperture iris image can
be seen in the field.
4. Fit the Allen screwdrivers provided with the microscope frame into the
two aperture iris diaphragm centering screws ³ and adjust them so that
the aperture iris image of the diaphragm is centered on the field of view.
Effects of Aperture Iris Diaphragm
{Reflected light brightfield observation:
In general, favorable observation is possible by setting the aperture iris of
the illumination system to 70% to 80% of the N.A. of the objective.
#The effects of aperture iris diaphragm cannot be obtained with 150X
and 250X objectives.
{Reflected light darkfield observation:
Always keep the aperture iris diaphragm knob ² pushed in to leave the
diaphragm open.
}With certain specimens, smaller aperture may sometimes offer images
with better contrast and smaller flare. Please also try such a setting.
(Fig. 21)
38
Page 43

OBSERVATIONS
4-1 Reflected
@
Fig. 22
@
²
Fig. 23
Light
Brightfield/Darkfield Observations
1 Selecting the Light Path for Observation
Rotate the turret @ to set the mirror unit matching the required observation method in the light path.
Inscription
Reflected light brightfield BF U-MBF3 Adjust as required.
Reflected light darkfield DF U-MDF3 Must be open.
2 Applications of Filters
As necessary up to two filters may be individually inserted into the filter
insertion positions @ and ². Insert each filter with the marked side facing
toward the observer.
As you insert the filter, you will hear two clicks. At the first, the filter is in the
empty position, and at the second the filter is engaged in the light path.
Usable Filters
@
U-25FR (Frost filter) To eliminate uneven illumination.
U-25LBD
(Color temperature conversion
filter)
U-25IF550 (Green filter)
U-25Y48 (Yellow filter)
²
U-25ND50-2
(Neutral Density filter)
U-25ND25-2
(Neutral Density filter)
U-25ND6-2
(Neutral Density filter)
Mirror Unit Field Iris
Applications
To convert the color temperature of
the source to the color temperature
of daylight. Used for comfortable
observation and when taking color
photographs.
To increase contrast during monochrome observation. Used when tak-
ing monochrome photographs.
To achieve good contrast for semi-
conductor wafers.
To adjust illumination brightness.
(Transmittance 50%)
To adjust illumination brightness.
(Transmittance 25%)
To adjust illumination brightness
(Transmittance 6%)
(Fig. 22)
Aperture Iris
(Fig. 23)
U-25L42
To prevent the polarizer burning
when a light source with high intensity is used.
39
Page 44

Reflected Light Nomarski Differential Interference Contrast (DIC) Observation
4-2
#The performance of polarizer may deteriorate when it has been exposed to light for a long period (about
continuous 2000 hours). If this happens, replace the polarizer.
#When using the high-intensity light source, be sure to use the U-25L42 filter for prevention of the polarizer burn.
}When performing sensitive color observation using the U-DICRH DIC slider, combine the U-POTP3 polarizer.
@
@
Fig. 24
²
|
³
1 Selecting the Light Path for Observation
1. Rotate the turret to engage the BF mirror unit @ in the light path.
Inscription
Reflected light Nomarski
DIC
}When the U-MDIC3 DIC mirror unit is mounted in the turret, engage the
DIC mirror unit in the light path. The analyzer and polarizer are set to the
“Crossed Nicol” position so adjustment is not required.
2. Engage the U-AN360-3 analyzer and U-PO3 polarizer in the light path.
3 Rotate the analyzer dial until complete extinction (crossed Nicol position)
is obtained.
DIC U-MDIC3 Analyzer/polarizer built in
Mirror Unit Note
BF
U-MBF3
(Fig. 24)
2 Installing the Nomarski Prism
1. Loosen the DIC clamping knob @ at the front of the DIC revolving nosepiece, and insert the DIC prism ² with the inscription facing upward.
2. With the U-DICR interference slider, set the slide lever ³ according to the
objective in use.
Lever ³ position Applicable Objectives
Pushed in
UIS2
MPLFLN/MPLFLN-BD series
(Fig. 25)
40
Fig. 25
Pulled out
UIS
UIS2
UIS
UMPlanFl/UMPlanFl-BD series
MPlanApo20X, 100X
MPlanApo100XBD
LMPLFLN/LMPLFLN-BD series
LMPlanFl/LMPlanFl-BD series
LMPlanApo/LMPlanApo-BD series
Page 45

3. With the U-DICRH or U-DICRHC slider that does not have the slide lever,
the applicable objectives are as follows.
DIC Slider Applicable Objectives
U-DICRH MPLFLN/MPLFLN-BD series
UIS2
UIS
U-DICRHC LMPLFLN/LMPLFLN-BD series
UIS2
UIS
UMPlanFl/UMPlanFl-BD series
MPlanFl-BD series
MPlanApo20X, 100X
LMPlanFl/LMPlanFl-BD series
LMPlanApo/LMPlanApo-BD series
3 Observation Procedure
1. Place the specimen on the stage and move the stage to bring the specimen into focus.
2. Adjust the field iris diaphragm until it circumscribes the field of view.
3. Stopping down the aperture iris diaphragm may increase the contrast
somewhat.
U-DICR
1. Rotate the prism control knob | for the DIC prism to adjust the background contrast as outlined below.
2. Rotating the prism control knob of the DIC prism will continuously change
the interference color of the background from the gray sensitive color to
magenta sensitive color (-100 to 600 nm). Select the interference color
offering optimum contrast for each specimen.
· If the background color is gray, a 3D-looking observation with good contrast is possible in the most sensitive gray colors.
· If the background color is sensitive magenta, even a minor optical retardation can be observed as a color change.
U-DICRHC
U-DICRH
1. Rotate the prism control knob | for the DIC prism to adjust the background contrast as outlined below.
2. Rotating the prism control knob of the U-DICRH DIC prism will continuously change the interference color of the background from -100 to 100
nm. Select the retardation offering optimum contrast.
· If the background color is gray, a 3D-looking observation with good contrast is possible in the most sensitive gray colors.
· If the background color is sensitive magenta, even a minor optical retardation can be observed as a color change.
To use the background color sensitive magenta, use the U-.POTP3 polarizer. Position the polarizer so that the
when the polarizer is inserted into the inlet slot.
#Care should be taken to keep the specimen surface clan, as even a
small amount of contamination on the surface may show up due to
the exceptionally high sensitivity of the DIC method.
}Since the detection sensitivity is variable depending on orientation, it is
recommended to use a rotary stage.
symbol can be seen from the front
41
Page 46

Switching Between Brightfield and
4
Darkfield Observation
1. Loosen the DIC clamping screw @ at the front of the revolving nosepiece, and gently pull the DIC prism ² outward until a click is heard.
Tighten the claming screw again.
2. Rotate the turret to disengage the U-MDIC3 DIC mirror unit from the light
path.
Or slide the analyzer/polarizer to disengage it from the light path.
4-3 Reflected Light Simple Polarized Light Observation
}To prepare for simple polarized light observation using the reflected illuminator, perform the operations in paragraph
“Selecting the Light Path” in section 4-2, “Reflected Light Nomarski DIC Observation” on page 40.
1 Observation Procedure
1. Place the specimen on the stage and move the stage to bring the specimen into focus. Simple polarized light observation
is now possible.
2. Adjust the field iris diaphragm until the diaphragm opening circumscribes the field of view.
3. Stopping down the aperture iris diaphragm may increase the contrast somewhat.
1
42
Page 47

OPTICAL CHARACTERISTICS
«UIS2 (UIS) Series for Reflected Light Observation»
-- The UIS series objectives that are not mentioned below can also be mounted on this microscope. --
The table below shows the optical characteristics of different eyepiece
Magnification
Objective series
and objective combinations. Objective specifications are marked on
the objective (as shown in the diagram on the right).
NOTE
Refer to the latest catalogue or consult Olympus for the updated information on the eyepieces and objectives that can be combined with
UIS marking
NA (Numerical Aperture)
Brightfield/darkfield
application
this unit.
Series
MPLN
UIS2
Plan Achromat
series
(FN22)
MPLN-BD
Brightfield/
darkfield
Plan Achromat
(FN22)
MPLFLN
Plan SemiApochromat
(FN26.5)
*1.25X:FN22
MPLFLN-BD
Brightfield/
darkfield
Plan SemiApochromat
(FN26.5)
MPLFLN-BDP
Reflected Polarized
Light Plan SemiApochromat
(FN26.5)
LMPLFLN
Long-WD Plan
Semi-Apochromat
(FN26.5)
LMPLFLN-BD
Brightfield/darkfield
long-WD Plan SemiApochromat
(FN26.5)
MPlanApo
UIS
Plan
series
Apochromat
MPlanApo-BD
Brightfield/darkfield
Plan Apochromat
SLMPlan
Superlong-WD
Plan Achromat
(FN26.5)
Optical
characteristics
Marking
MPlanN
MPlanN-BD
MPlanFLN
MPlanFLN-BD
MPlanFLN-BDP
LMPlanFLN
LMPlanFLN-BD
MPlanApo
MPlanApo-BD
SLMPlan
Cover glass thickness
—: May be used with our with-
out a cover glass.
0: Used without a cover glass.
Cover
Magnifi-
cation
100X 0.90 0.21 0 0.37 1000X 0.73 0.22
100X 0.90 0.21 0 0.37 1000X 0.73 0.22
1.25X 0.04 3.5 — 8.39 12.5X 870 17.6 — — —
100X 0.90 1.0 0 0.37 1000X 0.73 0.22 1000X 0.73 0.27
100X 0.90 1.0 0 0.37 1000X 0.73 0.22 1000X 0.73 0.27
150X 0.90 1.0 0 0.37 1500X 0.6 0.15 1500X 0.6 0.18
100X 0.90 1.0 0 0.37 1000X 0.73 0.22 1000X 0.73 0.27
100X 0.80 3.4 0 0.42 1000X 0.87 0.22 1000X 0.87 0.27
100X 0.80 3.3 0 0.42 1000X 0.87 0.22 1000X 0.87 0.27
100X 0.95 0.35 0 0.35 1000X 0.67 0.22 1000X 0.67 0.27
100X 0.90 0.31 0 0.37 1000X 0.73 0.22 1000X 0.73 0.27
N.A.
(mm)
5X 0.10 20.0 — 3.36 50X 98 4.4
10X 0.25 10.6 — 1.34 100X 18 2.2
20X 0.40 1.3 0 0.84 200X 6.1 1.1 — — —
50X 0.75 0.38 0 0.45 500X 1.4 0.44
5X 0.10 12.0 — 3.36 50X 98 4.4
10X 0.25 6.5 — 1.34 100X 18 2.2
20X 0.40 1.3 0 0.84 200X 6.1 1.1 — — —
50X 0.75 0.38 0 0.45 500X 1.4 0.44
2.5X 0.08 10.7 — 4.19 25X 220 8.8 25X 220 10.6
5X 0.15 20.0 — 2.24 50X 59 4.4 50X 59 5.3
10X 0.30 11.0 — 1.12 100X 15 2.2 100X 15 2.65
20X 0.45 3.1 0 0.75 200X 5.2 1.1 200X 5.2 1.33
50X 0.80 1.0 0 0.42 500X 1.3 0.44 500X 1.3 0.53
5X 0.15 12.0 — 2.24 50X 59 4.4 50X 59 5.3
10X 0.30 6.5 — 1.12 100X 15 2.2 100X 15 2.65
20X 0.45 3.0 0 0.75 200X 5.2 1.1 200X 5.2 1.33
50X 0.80 1.0 0 0.42 500X 1.3 0.44 500X 1.3 0.53
5X 0.15 12.0 — 2.24 50X 59 4.4 50X 59 5.3
10X 0.25 6.5 — 1.34 100X 18 2.2 100X 18 2.65
20X 0.40 3.0 0 0.84 200X 6.1 1.1 200X 6.1 1.33
50X 0.75 1.0 0 0.45 500X 1.4 0.44 500X 1.4 0.53
5X 0.13 22.5 — 2.58 50X 70 4.4 50X 70 5.3
10X 0.25 21.0 — 1.34 100X 18 2.2 100X 18 2.65
20X 0.40 12.0 0 0.84 200X 6.1 1.1 200X 6.1 1.33
50X 0.50 10.6 0 0.67 500X 2.5 0.44 500X 2.5 0.53
5X 0.13 15.0 — 2.58 50X 70 4.4 50X 70 5.3
10X 0.25 10.0 — 1.34 100X 18 2.2 100X 18 2.65
20X 0.40 12.0 0 0.84 200X 6.1 1.1 200X 6.1 1.33
50X 0.50 10.6 0 0.67 500X 2.5 0.44 500X 2.5 0.53
20X 0.60 0.9 0 0.56 200X 3.68 1.1 200X 3.68 1.33
50X 0.95 0.3 0 0.35 500X 1.04 0.44 500X 1.04 0.53
20X 0.35 21.0 0 0.96 200X 7.2 1.1 200X 7.2 1.33
50X 0.45 15.0 0 0.75 500X 2.9 0.44 500X 2.9 0.53
W.D.
glass
thick
ness
(mm)
tion
(µm)
Resolu-
WHN10X (FN22)
Depth
Total
of focus
mag.
(µm)
Eyepieces
Field
of view
(mm)
FN (Field Number)
SWH10X (FN26.5)
Depth
Total
of focus
mag.
(µm)
Note) When an MPLN-BD series objective is used in darkfield observation with a xenon light source, the peripheral area
may be obscured with certain specimens.
(PL = Plan)
Field
of view
(mm)
43
Page 48

Significance of Objective Name
(Examples) M PL FL N 100 BD
(Plan)
Figure : Magnification
None : UIS
N : UIS 2
None : Achromat, or aberration correction with 2 wavelengths (red and bleu).
FL : Semi-Apochromat, or color aberration correction with visual wave-
lengths (bluish purple to red).
APO : Apochromat, or color aberration correction with all visual-domain
wavelength (purple to red).
PL : Plan, or correction of image curving on peripheral area.
M : Metal observation (no cover)
LM : Long-WD metal observation
SLM : Superlong-WD metal observation
LC : Observation over glass plate
None : Brightfield
BD : Darkfield
BDP :
Brightfield or polarized
IR : IR light
Glossary of Terms Used in the Optical Characteristics Table
Working distance (WD) : The distance from the top of specimen and the front lens of objective.
Number of aperture (NA) : Important figure determining the objective characteristics (resolution, focal depth and bright-
ness).
Resolution ............. Increases in proportion with the NA.
Focal depth ......... Decreases in proportion with the NA.
Brightness ............. Proportional with the square of NA (comparison under the same magnification).
Resolution : The limit that an objective can identify the images of two points that are close to each other,
expressed as the distance between the two points on the specimen.
Depth of focus : The maximum depth of the specimen at which the entire specimen can be brought into focus
simultaneously. This value increases when the aperture iris diaphragm is narrowed and de-
creases when the objective NA is increased.
Field number : The diameter of the image area that can be observed through the eyepieces, expressed in mm.
Field of view : The diameter of the area observable on the specimen, expressed in mm.
44
Page 49

TROUBLESHOOTING GUIDE
Reflected Light Observation Modes
Problem
a) Bulb operates, but field of view re-
mains dark.
b) Field of view is obscured or not
evenly illuminated.
c) Image glares. Aperture iris diaphragm is stopped down
d) Visibility is poor.
· Image is not sharp.
· Contrast is poor.
· Details are indistinct.
e) One side of image is blurred.
Reflected light lamp is not on. Turn lamp on.
Aperture and field iris diaphragms are not
opened wide enough.
Mirror unit is not mounted. Mount mirror unit.
Mirror unit is not correctly engaged in
light path.
Optimum mirror unit for observation is
not engaged in light path.
Field iris diaphragm has not been centered.
Field iris diaphragm is stopped down too
far.
Mercury burner is not centered correctly. Center mercury burner.
Frost filter is not engaged in light path. Engage frost filter in light path.
Filter is not in click position. Push filter until it clicks properly.
too far.
A non-UIS2/UIS objective is used. Use only UIS2/UIS series objectives
Front lens of objective is dirty. Clean objective.
Immersion oil is not being used with an
oil immersion objective.
Recommended immersion oil is not
used.
Light shield tube is not attached. Attach light shield tube.
Specimen is tilted. Place specimen properly on stage and
Revolving nosepiece is not correctly
mounted.
Objective is not correctly engaged in light
path.
Cause Remedy Page
Enlarge them to proper sizes.
Engage mirror unit correctly in light
path.
Set turret so that optimum mirror unit
for observation is engaged in light path.
Center field iris diaphragm/
Enlarge field iris diaphragm until it circumscribes field of view.
Open aperture iris diaphragm.
with this microscope.
Use immersion oil.
Use provided immersion oil.
fix with specimen holders.
Attach revolving nosepiece correctly.
Engage objective correctly in light
path.
11
38
31
39/40
39/40
37
38
14
39
39
38
43
3
––
––
37
––
––
––
45
Page 50

PROPER SELECTION OF THE POWER SUPPLY CORD
If no power supply cord is provided, please select the proper power supply cord for the equipment by referring to “ Specifications ” and
“ Certified Cord ” below:
CAUTION:
In case you use a non-approved power supply cord for Olympus products, Olympus can no longer warrant the
electrical safety of the equipment.
Specifications
Voltage Rating
Current Rating
Temperature Rating
Length
Fittings Configuration
125V AC (for 100-120V AC area) or, 250V AC (for 220-240V AC area)
6A minimum
60°C minimum
3.05 m maximum
Grounding type attachment plug cap. Opposite terminates in molded-on IEC configuration appliance coupling.
Table 1 Certified Cord
A power supply cord should be certified by one of the agencies listed in Table 1 , or comprised of cordage marked with an
agency marking per Table 1 or marked per Table 2. The fittings are to be marked with at least one of agencies listed in
Table 1. In case you are unable to buy locally in your country the power supply cord which is approved by one of the
agencies mentioned in Table 1, please use replacements approved by any other equivalent and authorized agencies in
your country.
Country Agency
Argentina
Australia
IRAM
SAA
Certification
Mark
Country Agency
Italy
Japan
IMQ
JET, JQA, TÜV,
UL-APEX / MITI
Certification
Mark
46
Austria
Belgium
Canada
Denmark
Finland
France
Germany
Ireland
ÖVE
CEBEC
CSA
DEMKO
FEI
UTE
VDE
NSAI
Netherlands
Norway
Spain
Sweden
Switzerland
United
Kingdom
U.S.A.
KEMA
NEMKO
AEE
SEMKO
SEV
ASTA
BSI
UL
Page 51

Table 2 HAR Flexible Cord
APPROVAL ORGANIZATIONS AND CORDAGE HARMONIZATION MARKING METHODS
Approval Organization
Comite Electrotechnique Belge
(CEBEC)
Verband Deutscher Elektrotechniker
(VDE) e.V. Prüfstelle
Union Technique de l´Electricite´
(UTE)
Instituto Italiano del Marchio di
Qualita´ (IMQ)
British Approvals Service for Electric
Cables (BASEC)
N.V. KEMA
SEMKO AB Svenska Elektriska
Materielkontrollanstalter
Österreichischer Verband für
Elektrotechnik (ÖVE)
Danmarks Elektriske Materialkontroll
(DEMKO)
Printed or Embossed Harmonization Marking (May be located on
jacket or insulation of internal wiring)
CEBEC <HAR>
<VDE> <HAR>
USE <HAR>
IEMMEQU <HAR>
BASEC <HAR>
KEMA-KEUR <HAR>
SEMKO <HAR>
<ÖVE> <HAR>
<DEMKO> <HAR>
Alternative Marking Utilizing
Black-Red-Yellow Thread (Length
of color section in mm)
Black Red Yellow
10 30 10
30 10 10
30 10 30
10 30 50
10 10 30
10 30 30
10 10 50
30 10 50
30 10 30
National Standards Authority of Ireland
(NSAI)
Norges Elektriske Materiellkontroll
(NEMKO)
Asociacion Electrotecnica Y
Electronica Espanola (AEE)
Hellenic Organization for
Standardization (ELOT)
Instituto Portages da Qualidade
(IPQ)
Schweizerischer Elektro
Technischer Verein (SEV)
Elektriska Inspektoratet
Underwriters Laboratories Inc. (UL) SV, SVT, SJ or SJT, 3 X 18AWG
Canadian Standards Association (CSA) SV, SVT, SJ or SJT, 3 X 18AWG
This device complies with the requirements of both directive 89/336/EEC concerning electromagnetic compatibility
and directive 73/23/EEC concerning low voltage. The CE marking indicates compliance with the above directives.
<NSAI> <HAR>
NEMKO <HAR>
<UNED> <HAR>
ELOT <HAR>
np <HAR>
SEV <HAR>
SETI <HAR>
30 30 50
10 10 70
30 10 70
30 30 70
10 10 90
10 30 90
10 30 90
47
Page 52

Shinjuku Monolith, 3-1, Nishi Shinjuku 2-chome, Shinjuku-ku, Tokyo, Japan
Postfach 10 49 08, 20034, Hamburg, Germany
2 Corporate Center Drive, Melville, NY 11747-3157, U.S.A.
One Corporate Drive, Orangeburg, NY 10962, U.S.A.
491B River Valley Road, #12-01/04 Valley Point Office Tower, Singapore 248373
2-8 Honduras Street, London EC1Y OTX, United Kingdom.
31 Gilby Road, Mt. Waverley, VIC 3149, Melbourne, Australia.
Blue Lagoon Drive, Suite 390 Miami, FL 33126-2087, U.S.A.
6100
This publication is printed on recycled paper.
Printed in Japan 2006 10 M 020–@
 Loading...
Loading...