Page 1

Single use soft brush (MAJ-1888)
MH-553 MH-438MH-443MB-358 MB-142MAJ-1119
MH-856
MH-944 MH-946
MAJ-1339
BW-20T
BW-201T
MH-948 BW-412T
MAJ-1888
INSTRUCTIONS
EVIS EXERA II DUODENOVIDEOSCOPE
OLYMPUS TJF TYPE Q180V
Accessories:
• Water resistant cap (MH-553) • Chain for water-resistant cap (MAJ-1119)
• Biopsy valve (MB-358) • Suction valve (MH-443)
• Air/water valve (MH-438) • Mouthpiece (MB-142)
• Suction cleaning adapter (MH-856) • Channel plug (MH-944)
• Channel cleaning brush (BW-20T) • Injection tube (MH-946)
• Single use channel cleaning brush (BW-201T) • Single use channel-opening cleaning brush (MAJ-1339)
• AW channel cleaning adapter (MH-948) • Single use combination cleaning brush (BW-412T)
• Single use soft brush (MAJ-1888)
Refer to the endoscope’s companion manual, the “OPERATION MANUAL” with your endoscope model
listed on the cover, for operation information.
USA: CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Page 2
Revision History
Note: The Revision History shows the latest changes.
Version Date Description of Changes
RC2409 01 March, 2015 Cover
Section 1.4
Sections 2.10, 3.1, and 4.1
Section 4.2
Sections 4.3 and 5.1
Section 5.2
Section 5.3
Section 5.4
Section 5.5
Section 5.6
Chapter 7
GE8415 09 February,
2015
Single use soft brush (MAJ-1888) information.
Updated WARNING statements for MAJ-1888 and
using an AER.
Updated MAJ-1888 information.
Updated MAJ-1888 and AER information.
Updated MAJ-1888 information.
Updated “Aspirate water” section.
Updated “Detach the endoscope from the light
source” section.
Updated “Equipment needed” chart and CAUTION for
MAJ-1888, Step 5 on page 69, and added “Brush and
flush the forceps elevator recess” section.
Updated “Flush all channels and around the forceps
elevator with disinfectant solution” section.
Updated “Rinse the endoscope and accessories”
section; the “Alcohol flush” section.
Updated WARNING statements for AER.
Page 3

Contents
Contents
Chapter 1 General Policy ......................................................... 1
1.1 Instructions...................................................................................... 1
1.2 Importance of cleaning, disinfection, and sterilization..................... 2
1.3 Signal words ................................................................................... 2
1.4 Precautions ..................................................................................... 3
1.5 Reprocessing before the first use ................................................... 8
1.6 Reprocessing and storage after use ............................................... 9
1.7 Reprocessing before patient procedure.......................................... 9
Chapter 2 Function and Inspection of the Accessories for
Reprocessing........................................................... 10
2.1 Water resistant cap (MH-553)......................................................... 10
2.2 Channel plug (MH-944)................................................................... 12
2.3 Injection tube (MH-946) .................................................................. 14
2.4 Channel cleaning brush (BW-20T).................................................. 16
2.5 Suction cleaning adapter (MH-856) ................................................ 18
2.6 AW channel cleaning adapter (MH-948)......................................... 19
2.7 Single use channel cleaning brush (BW-201T)............................... 20
2.8 Single use channel-opening cleaning brush (MAJ-1339) ............... 22
2.9 Single use combination cleaning brush (BW-412T)........................ 24
2.10 Single use soft brush (MAJ-1888)................................................... 25
2.11 Chain for water-resistant cap (MAJ-1119) ...................................... 27
Chapter 3 Compatible Reprocessing Methods and
Chemical Agents .................................................... 28
3.1 Compatibility summary.................................................................... 28
3.2 Water (for reprocessing) ................................................................. 30
3.3 Detergent solution........................................................................... 31
3.4 Disinfectant solution........................................................................ 31
3.5 Rinse water ..................................................................................... 31
3.6 Alcohol ............................................................................................ 31
3.7 Ethylene oxide gas sterilization....................................................... 32
3.8 Steam sterilization (autoclaving) ..................................................... 33
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
i
Page 4

Contents
Chapter 4 Reprocessing Workflow for the Endoscope
and Accessories ...................................................... 35
4.1 Workflow for manually cleaning and disinfecting the endoscope
and accessories .............................................................................. 36
4.2 Workflow for cleaning and disinfecting the endoscope and
accessories using an AER .............................................................. 38
4.3 Workflow for manually cleaning and sterilizing the endoscope
and accessories .............................................................................. 40
Chapter 5 Reprocessing the Endoscope
(and related reprocessing accessories) ................ 42
5.1 Preparing the equipment for reprocessing ...................................... 44
5.2 Precleaning the endoscope and accessories.................................. 45
5.3 Leakage testing of the endoscope .................................................. 51
5.4 Manually cleaning the endoscope and accessories........................ 56
5.5 Manually disinfecting the endoscope and accessories ................... 80
5.6 Rinsing the endoscope and accessories following disinfection....... 86
5.7 Sterilizing the endoscope and accessories ..................................... 94
Chapter 6 Reprocessing the Accessories .............................. 96
6.1 Manually cleaning the accessories ................................................. 98
6.2 Manually disinfecting the accessories............................................. 101
6.3 Rinsing the accessories following disinfection ................................ 102
6.4 Sterilizing the accessories............................................................... 105
Chapter 7 Reprocessing Endoscopes and Accessories
using an Automated Endoscope Reprocessor..... 106
Chapter 8 Storage and Disposal .............................................. 108
8.1 Storing the disinfected endoscope and accessories ....................... 109
8.2 Storing the sterilized endoscope and accessories .......................... 111
8.3 Disposal .......................................................................................... 111
ii
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 5

Chapter 1 General Policy
1.1 Instructions
• This manual contains the cleaning, disinfection, and sterilization
methods recommended by Olympus for the endoscopes and
accessories listed on the front cover.
• This instruction manual contains essential information on reprocessing
endoscopes and accessories safely and effectively.
• Before reprocessing, thoroughly review this manual and the manuals for
the reprocessing equipment and chemicals that will be used for
reprocessing. Reprocess all the devices as instructed.
• Note that the complete instruction manual set for the endoscope and
accessories consists of this manual and the “OPERATION MANUAL”
with your endoscope model listed on the cover. Both manuals
accompanied the endoscope at shipment.
Chapter 1 General Policy
• Keep this manual and all related manuals in a safe and accessible
location (e.g., in the reprocessing area).
• If you have any questions or comments about any information in this
manual, or if a problem occurs while reprocessing that cannot be solved,
contact Olympus.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
1
Page 6

Chapter 1 General Policy
1.2 Importance of cleaning, disinfection, and
sterilization
The medical literature reports incidents of cross-contamination resulting from
improper cleaning, disinfection, or sterilization. It is strongly recommended that
all individuals engaged in reprocessing closely observe all instructions given in
this manual and the manuals for all ancillary equipment, and have a thorough
understanding of the following items:
• Professional health and safety policies of your hospital
• Instruction manuals for the endoscope, accessories, and all the other
reprocessing equipment
• Structure and handling of endoscope and accessories
• Handling of pertinent chemicals
When selecting appropriate methods and conditions for cleaning and disinfection
and sterilization, follow the policies at your institution, applicable national laws
and standards, and professional society guidelines and recommended practices,
in addition to the instructions given in this manual.
1.3 Signal words
The following signal words are used throughout this manual:
Indicates a potentially hazardous situation which, if not
avoided, could result in death or serious injury.
Indicates a potentially hazardous situation which, if not
avoided, may result in minor or moderate injury. It may also
be used to alert against unsafe practices or potential
equipment damage.
Indicates additional helpful information.
2
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 7

1.4 Precautions
Chapter 1 General Policy
• An insufficiently cleaned, disinfected, or sterilized endoscope
and/or accessories may pose an infection control risk to the
patients and/or operators who contact them.
• All disinfection methods (whether performed manually or by
an automated endoscope reprocessor), and all sterilization
methods (whether performed by ethylene oxide gas or
steam) require thorough prior cleaning of the instrument
being reprocessed. If the equipment is not adequately
cleaned prior to disinfection/sterilization, these processes will
be ineffective. Immediately after each patient procedure and
before disinfection/sterilization, thoroughly clean the
endoscope and the accessories used with the endoscope.
• All channels of the endoscope, including the instrument
channel and all accessories used with the endoscope during
the patient procedure, such as all valves, must be cleaned
and high-level disinfected or sterilized after each patient
procedure, even if the channels or accessories were not
used during the patient procedure. Insufficient cleaning and
disinfection or sterilization of these components may pose an
infection control risk to patients and/or operators.
• Disinfectant solutions are hazardous. After disinfection, rinse
all external surfaces and channels of the endoscope and
accessories thoroughly with water to remove residual
disinfectant solution.
• The results of sterilization depend on various factors. These
factors include how the equipment was packaged, and the
placing and loading of the package in the sterilization device.
Verify the sterilization process using biological and/or
chemical indicators. Follow the guidelines for sterilization
issued by national authorities, professional organizations and
infection control professionals, as well as the instruction
manual for the sterilization device.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
3
Page 8
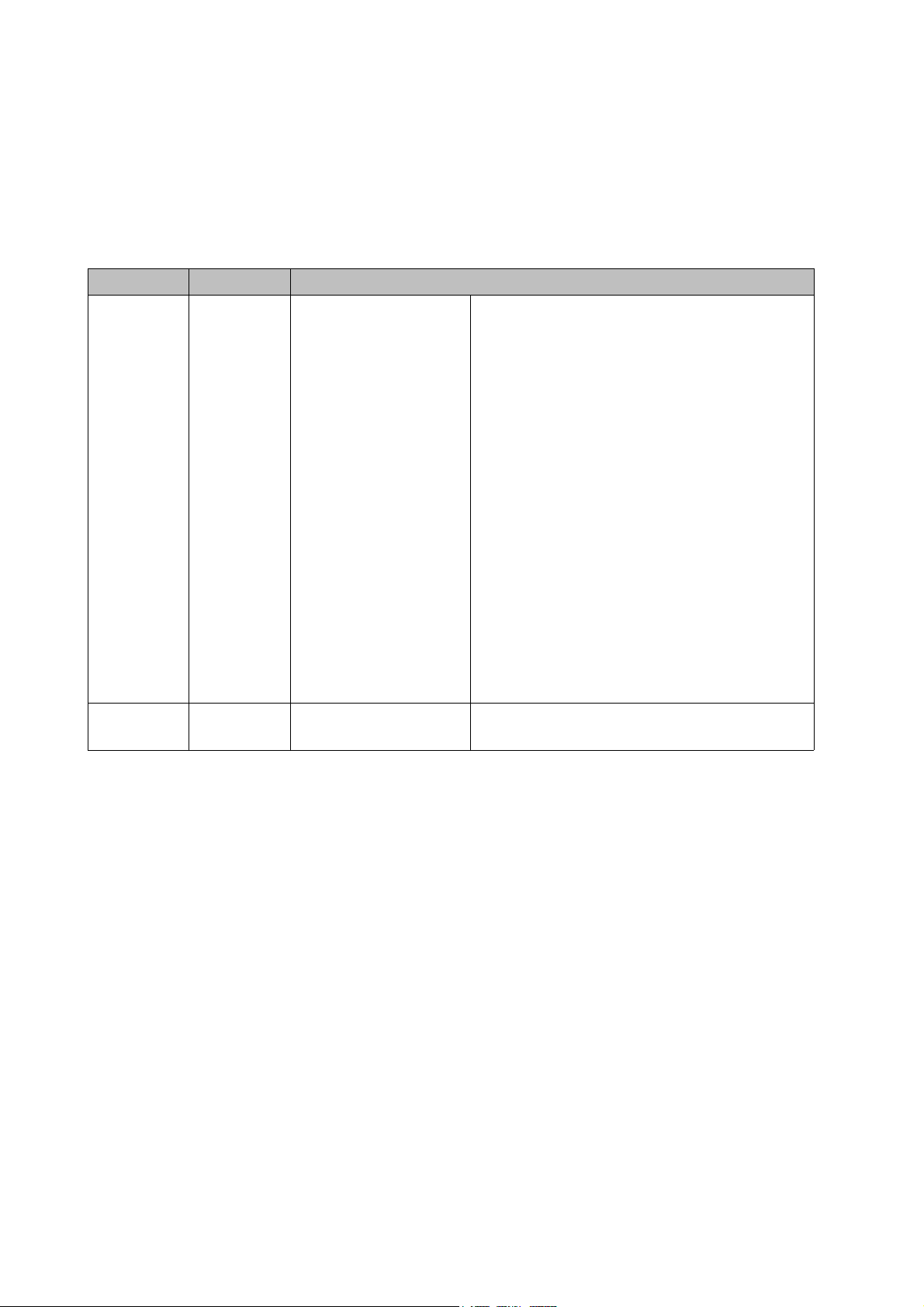
Chapter 1 General Policy
Leakage tester
• Establish an internal system of identifying contaminated
versus reprocessed endoscopes and accessories to prevent
both mix-ups and cross-contamination. Touching a
reprocessed endoscope and/or accessories with
contaminated gloves or placing them on a contaminated
hanger or surface, including letting them touch the floor, will
recontaminate them.
• Prior to each patient procedure, confirm that the endoscope
and accessories have been properly reprocessed and stored.
If there are any doubts or questions, reprocess them again
before the patient procedure, following the instructions given
in this manual.
• Perform a leakage test on the endoscope after each
precleaning procedure. Do not use the endoscope if a leak is
detected. Use of an endoscope with a leak may cause a
sudden loss of the endoscopic image, damage to the
bending mechanism, or other malfunctions. Use of a leaking
endoscope may also pose an infection control risk.
4
Figure 1.1
• Store alcohol in an airtight container. Alcohol stored in an
open container may cause a fire hazard and may result in a
loss of efficacy due to evaporation.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 9
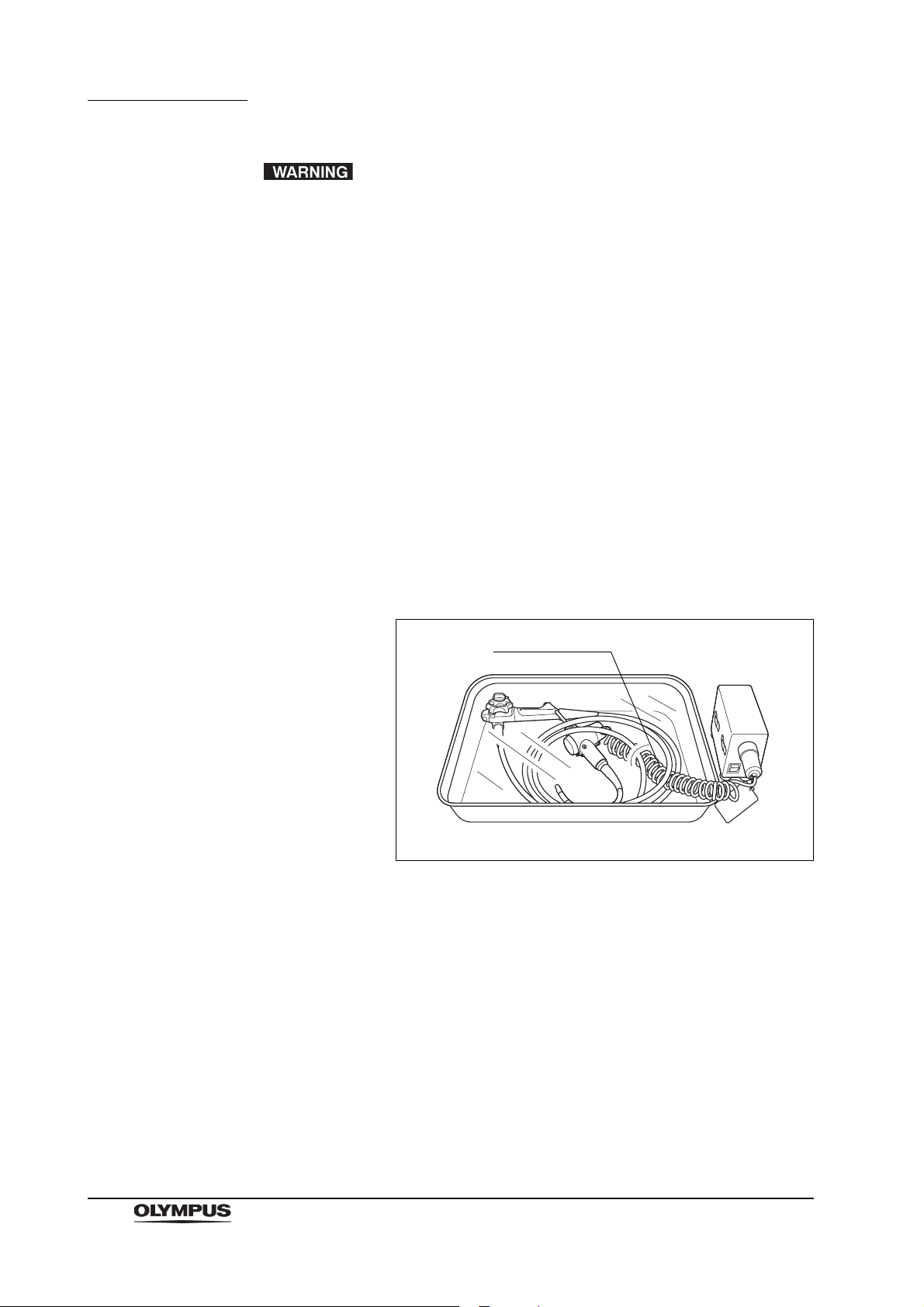
Chapter 1 General Policy
AW channel cleaning
adapter (MH-948)
• Do not use the AW channel cleaning adapter (MH-948) for
patient procedures. It will cause continuous insufflation and
could result in patient injury.
Figure 1.2
• The accessories listed on the front cover of this manual are
consumables, meaning that these accessories cannot be
refurbished or repaired and are intended to be replaced once
they show any signs of wear. Should any irregularity be
observed, use a replacement accessory instead. Using
defective accessories may cause equipment malfunction,
reduce the efficacy of reprocessing, present a risk to patients
and/or operators, or damage the endoscope and/or
accessories.
• Single-use brushes, such as the single use channel cleaning
brush (BW-201T), the single use combination cleaning brush
(BW-412T), the single use channel-opening cleaning brush
(MAJ-1339), and the single use soft brush (MAJ-1888), are
designed for cleaning only one endoscope and its related
accessories. Dispose of the single-use brush immediately
after use. Using a single-use brush to clean multiple
endoscopes and/or accessories may reduce its cleaning
efficacy and may damage the brush leading to brush
breakage or endoscope and/or accessory damage.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
5
Page 10

Chapter 1 General Policy
• Patient debris and reprocessing chemicals are hazardous.
To guard against contact with dangerous chemicals and
potentially infectious material, wear appropriate personal
protective equipment during cleaning, disinfection, and
sterilization. Such protective equipment should include
appropriate eyewear, face mask, cap, moisture-resistant
clothing, shoe covers, and chemical-resistant gloves that fit
properly and are long enough to prevent skin exposure.
• The reprocessing room must be adequately ventilated.
Adequate ventilation protects against the buildup of toxic
chemical fumes.
• Always remove contaminated personal protective equipment
before leaving the reprocessing area to prevent
contamination from spreading.
• Only Olympus-recommended or Olympus-endorsed
automated endoscope reprocessors (AERs) have been
validated by Olympus. When using an AER that is not
recommended by Olympus, the manufacturer of the AER is
responsible for validating compatibility of the AER with each
Olympus endoscope and accessory.
• Before using an AER, confirm that it is capable of
reprocessing the endoscope including all channels, the
forceps elevator recess, and accessories. Be sure to attach
all required connectors. Otherwise, insufficient reprocessing
may pose an infection control risk. If you are uncertain as to
the ability of your AER to reprocess the endoscope including
all channels, the forceps elevator recess, and accessories,
contact the manufacturer of the AER for specific instructions
and information on compatibility and required connectors.
When you use an AER which allows you skip some steps in
precleaning and manual cleaning of endoscopes, confirm
with the AER manufacturer that such skip is applicable to this
endoscope and establish detailed precleaning and manual
cleaning procedures of this endoscope according to both
instructions of this manual and the AER manufacturer.
• Put the forceps elevator in intermediate position of the range
of movement by turning the elevator control lever and set it in
your AER.
6
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 11

Chapter 1 General Policy
• Instructions provided in this manual are not valid for Olympus
devices repaired by a non-Olympus facility. The Olympus
recommended reprocessing procedures have not been
validated for reprocessing devices repaired by a
non-Olympus facility. In the event that your device has been
repaired by a non-Olympus facility, contact that repair facility
for instructions regarding reprocessing.
• Prions, which are the pathogenic agent of the
Creutzfeldt-Jakob disease (CJD), cannot be destroyed or
inactivated by the cleaning, disinfection, and sterilization
methods stated in this instruction manual. When using the
endoscope and accessories on patients with CJD or variant
Creutzfeldt-Jakob disease (vCJD), be sure to use them for
such patients only, or immediately dispose of them after use
in an appropriate manner to prevent the usage of exposed
devices on other patients. For methods to handle CJD, follow
the respective guidelines in your country.
• The endoscope and accessories may be damaged by
published methods for destroying or inactivating prions. For
information on the durability of Olympus equipment against a
particular reprocessing method, contact Olympus. In general,
Olympus cannot guarantee the effectiveness, safety, and
durability of cleaning, disinfection, or sterilization methods
not described in this reprocessing manual. If you chose to
use a reprocessing method not recommended in this manual,
the local institution and/or physicians must assume
responsibility for its safety and efficacy. Make sure to
carefully inspect each piece of endoscopic equipment for
irregularities (damage) prior to each patient procedure. Do
not use the equipment if any irregularity is found.
• Good quality control practices typically require appropriate
documentation. Items such as local SOPs (standard
operating procedures), confirmation of operator training,
routine testing of the disinfectant’s MEC (minimal effective
concentration), confirmation of the disinfectant’s use-life,
etc., should be documented as performed.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
7
Page 12
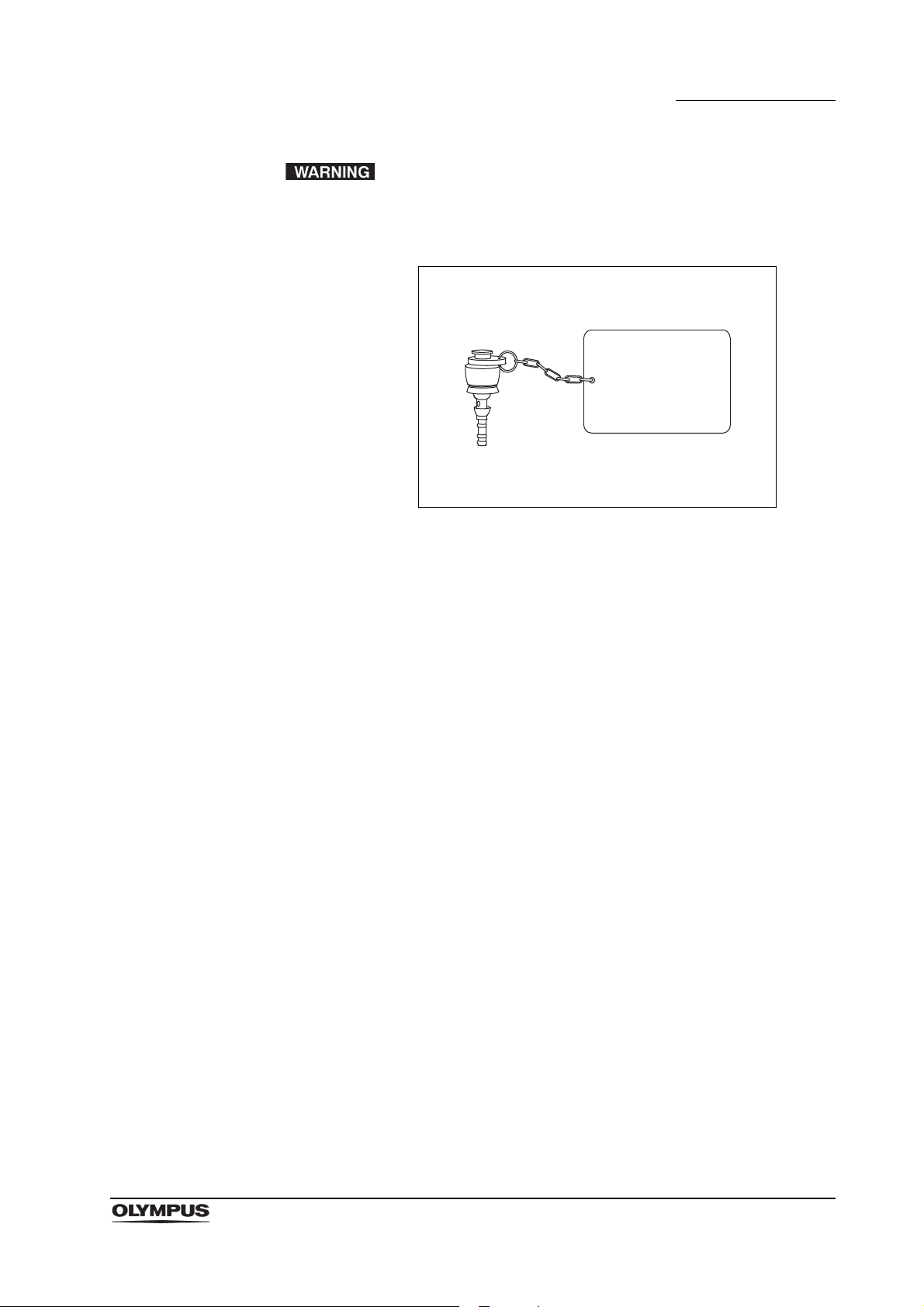
Chapter 1 General Policy
Water resistant
cap (MH-553)
Electrical connector
• When reprocessing the endoscope, confirm that the water
resistant cap (MH-553) is securely attached to the electrical
connector before immersing the endoscope in reprocessing
fluids. If the water resistant cap is not securely attached, the
reprocessing fluids could enter the endoscope and damage
the endoscope.
1.5 Reprocessing before the first use
Figure 1.3
• When aerating or irrigating the endoscope channels, the air
or water pressure must not exceed 0.5 MPa (5 kgf/cm
71 psig). Higher pressures may cause damage to the
endoscope.
• Store spare accessories in their original packaging to prevent
damage.
• To prevent damage, do not apply excessive force to the
endoscope and accessories during reprocessing.
• Vapors from disinfectant solutions and alcohol may damage
electronic devices such as computers.
New endoscopes, repaired endoscopes, accessories, and the carrying case for
endoscopes are not cleaned, disinfected, or sterilized prior to shipping from
Olympus, regardless of whether those instruments are for new purchase, demo
or loaner purposes. Reprocess all such endoscopes and accessories received
from Olympus according to the instructions given in this manual before storage
and before using them in a patient procedure.
2
,
8
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 13

1.6 Reprocessing and storage after use
• Do not reuse rinse water.
• High-level disinfectant solutions are only effective when used
according to the disinfectant manufacturer’s instructions.
Follow the manufacturer’s instructions regarding activation (if
required), concentration, temperature, contact time and use
life required to achieve high-level disinfection.
• If the disinfectant solution is reused, check its efficacy with a
test strip according to the disinfectant manufacturer’s
recommendations prior to use.
• Do not reuse alcohol.
• Alcohol is not a sterilant or high-level disinfectant.
• To maintain sterility of equipment following sterilization, use
sterile packaging and wraps according to national guidelines.
Chapter 1 General Policy
1.7 Reprocessing before patient procedure
• Improper storage practices, such as not thoroughly drying
external and internal surfaces (lumens) including the forceps
elevator recess prior to storage, will lead to an infection
control risk.
• Improper handling, such as touching a reprocessed
endoscope and/or accessories with contaminated gloves,
placing a reprocessed device on a contaminated hanger or
surface, allowing devices to touch the floor, etc., will
recontaminate the device.
Some national or professional guidelines recommend
reprocessing endoscopes prior to their first use of the day.
Confirm that the endoscope and accessories have undergone proper
reprocessing following their last use and that they have been stored properly.
Check the storage period of reprocessed endoscopes, and check for surface
contamination (e.g., dust). Check the sterilization expiration date(s) of all items
so marked and for tears or breaches in sterile packaging. If there are any doubts
or questions concerning whether a device is contaminated, reprocess it again
following the instructions given in this manual.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
9
Page 14
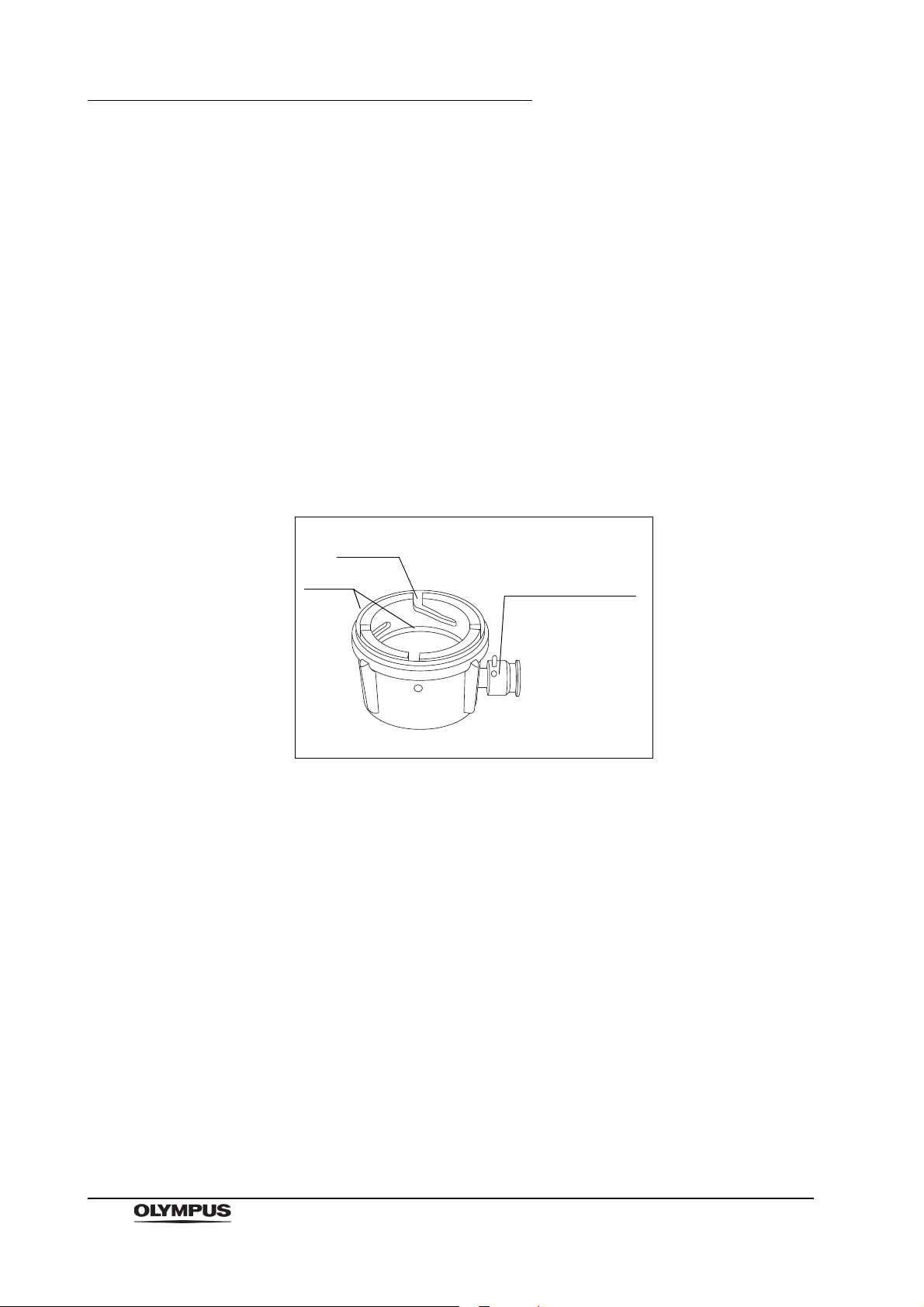
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Groove
Venting connector
Seals
Chapter 2 Function and Inspection of
the Accessories for
Reprocessing
Certain accessories are required for reprocessing the endoscope. This chapter
describes the function of these accessories. It also describes how to inspect
these accessories before using them to reprocess the endoscope.
2.1 Water resistant cap (MH-553)
10
Figure 2.1
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 15
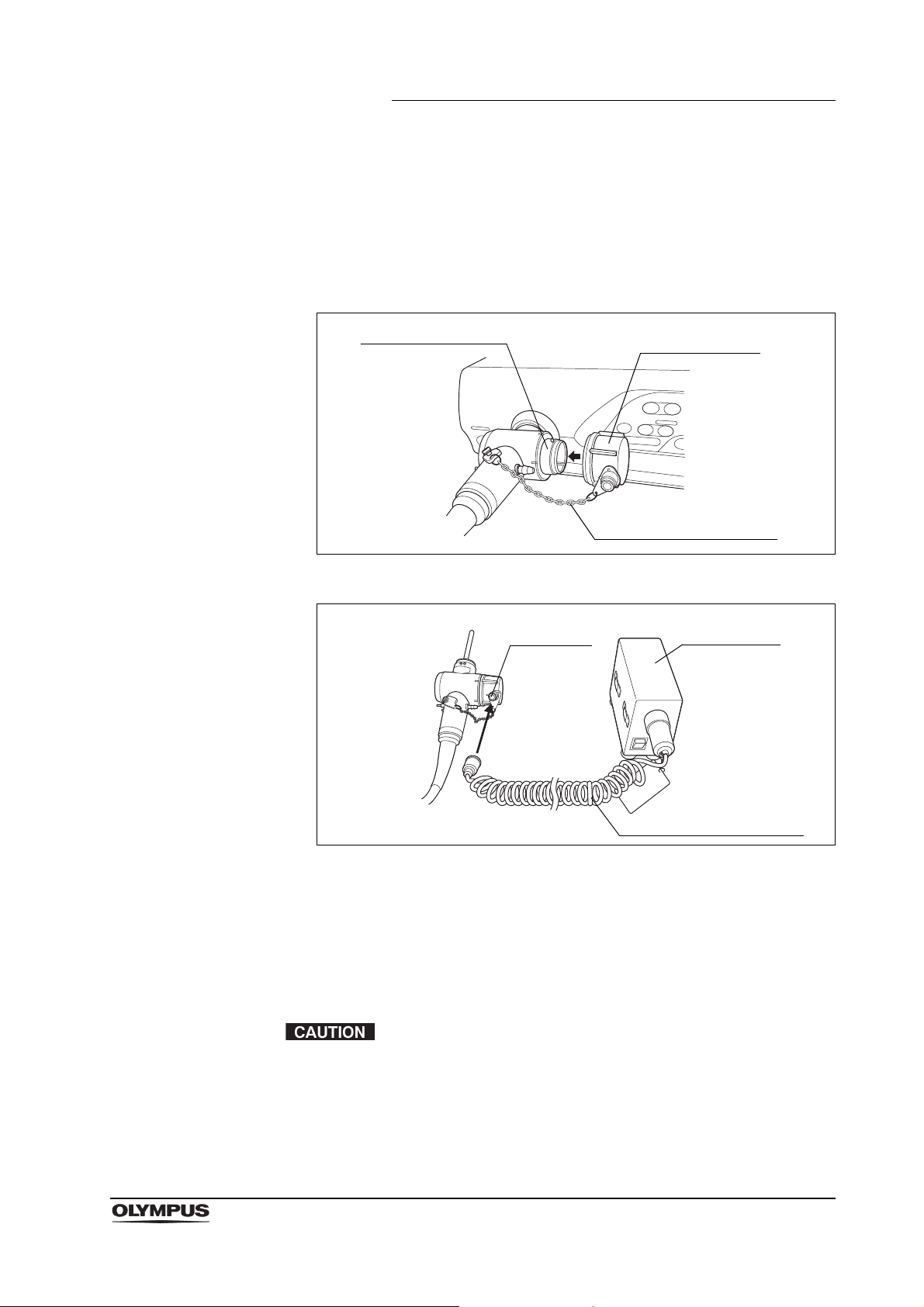
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Water resistant
cap (MH-553)
Chain for water-resistant
cap (MAJ-1119)
Electrical connector
Maintenance
unit (MU-1)
Venting
connector
Leakage tester (MB-155)
Function
The water resistant cap is attached to the electrical connector on the endoscope
to protect the connector and the endoscope from water penetration during
reprocessing. During leakage testing, the leakage tester (MB-155) is attached to
the venting connector of the water resistant cap.
Figure 2.2
Figure 2.3
The water resistant cap must be attached to the electrical connector of the
endoscope whenever the endoscope is immersed in reprocessing fluids. It is
detached from the connector whenever the endoscope is used for patient
procedures, being sterilized by ethylene oxide gas, or stored in an endoscope
storage cabinet.
Always use a dry water resistant cap. Any water remaining
inside the cap may cause damage to the endoscope.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
11
Page 16
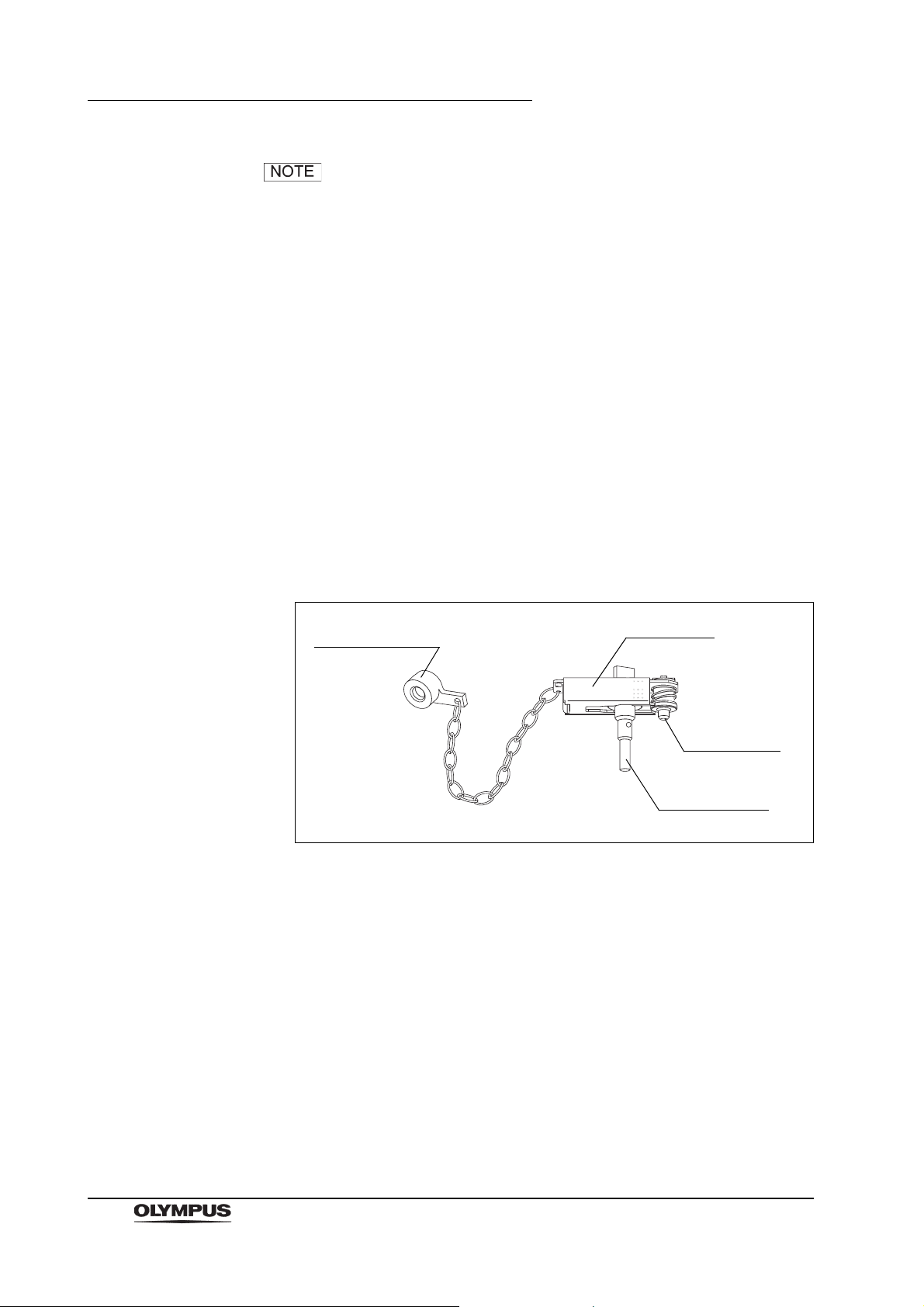
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Biopsy valve cap
Plug frame
Suction plug
Air/water plug
Use the chain for water-resistant cap (MAJ-1119) to connect
the water resistant cap to the endoscope. The water resistant
cap can remain connected to the endoscope by the chain at
all times (including during patient procedures, reprocessing,
and storage of the endoscope).
Inspection
1. Confirm that the inside of the cap is dry and free from debris. Wipe with a
dry, lint-free cloth if the inside of the cap is wet or if debris is detected.
2. Confirm that the seals inside the cap are free from scratches, cuts, and
debris.
3. Check to ensure that the venting connector on the cap is not loose.
2.2 Channel plug (MH-944)
Figure 2.4
12
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 17
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Instrument
channel port
Channel plug
Function
The channel plug is used to plug the openings of the instrument channel port
and the suction and air/water cylinders of the endoscope whenever the injection
tube (MH-946) is used to flush the suction and air/water channels of the
endoscope with reprocessing fluids.
Figure 2.5
When attached to the endoscope, the channel plug is
designed to allow a small amount of fluid to exit from the
openings of the endoscope. This enables reprocessing fluids
to contact the endoscope openings.
Inspection
Confirm that the suction plug, air/water plug, and the biopsy valve cap of the
channel plug are free from cracks, scratches, and debris.
The channel plug does not need to be cleaned, disinfected,
or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
13
Page 18
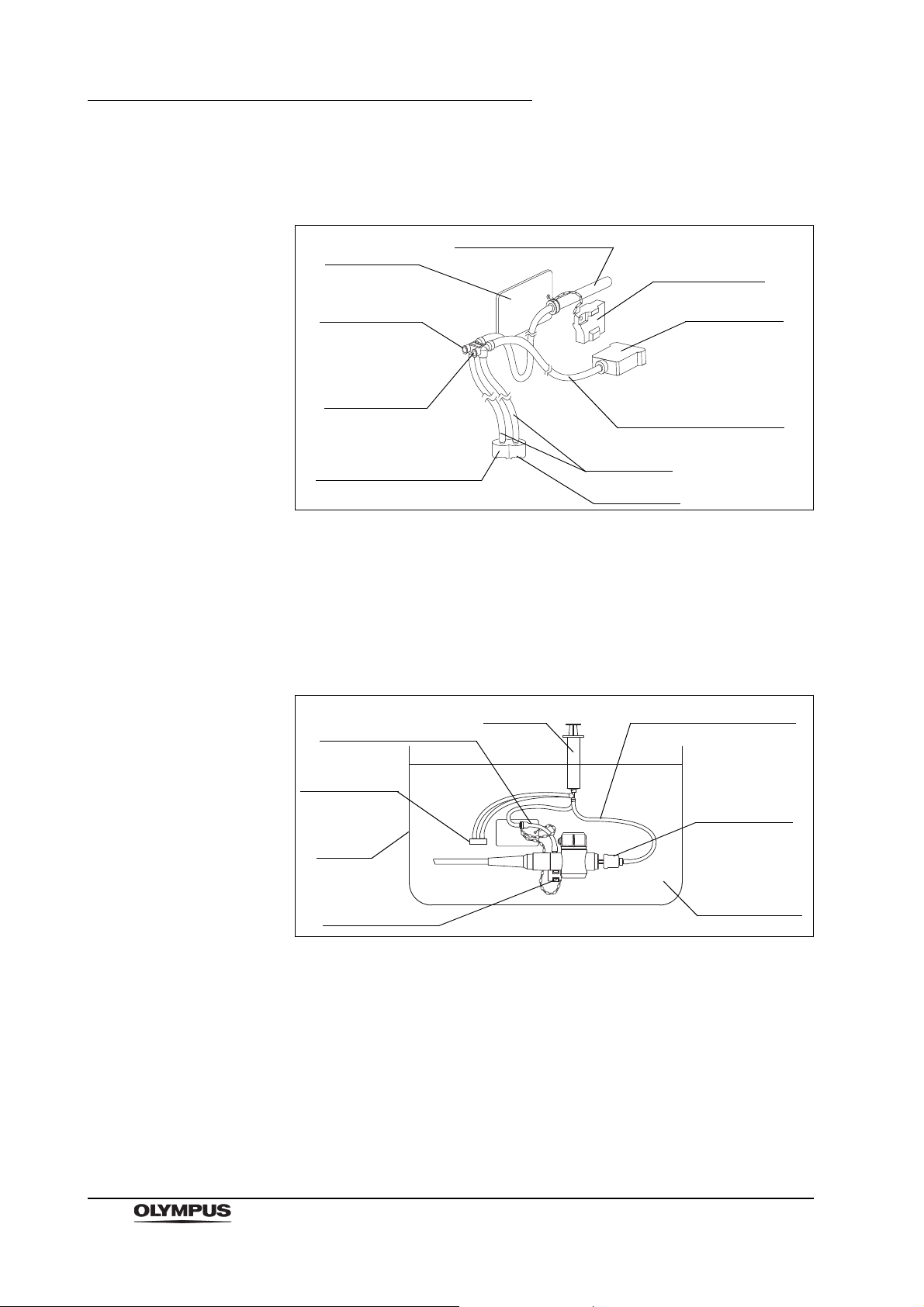
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Suction channel tube
Information
card
Connector plug
Suction
channel port
Air pipe port
Air/water
channel port
Suction port
Air/water channel tube
(including the filter mesh)
Filter tube
Filter mesh
Syringe
Air/water channel tube
Suction channel tube
Air pipe port
Suction port
Basin
Reprocessing
fluids
Connector plug
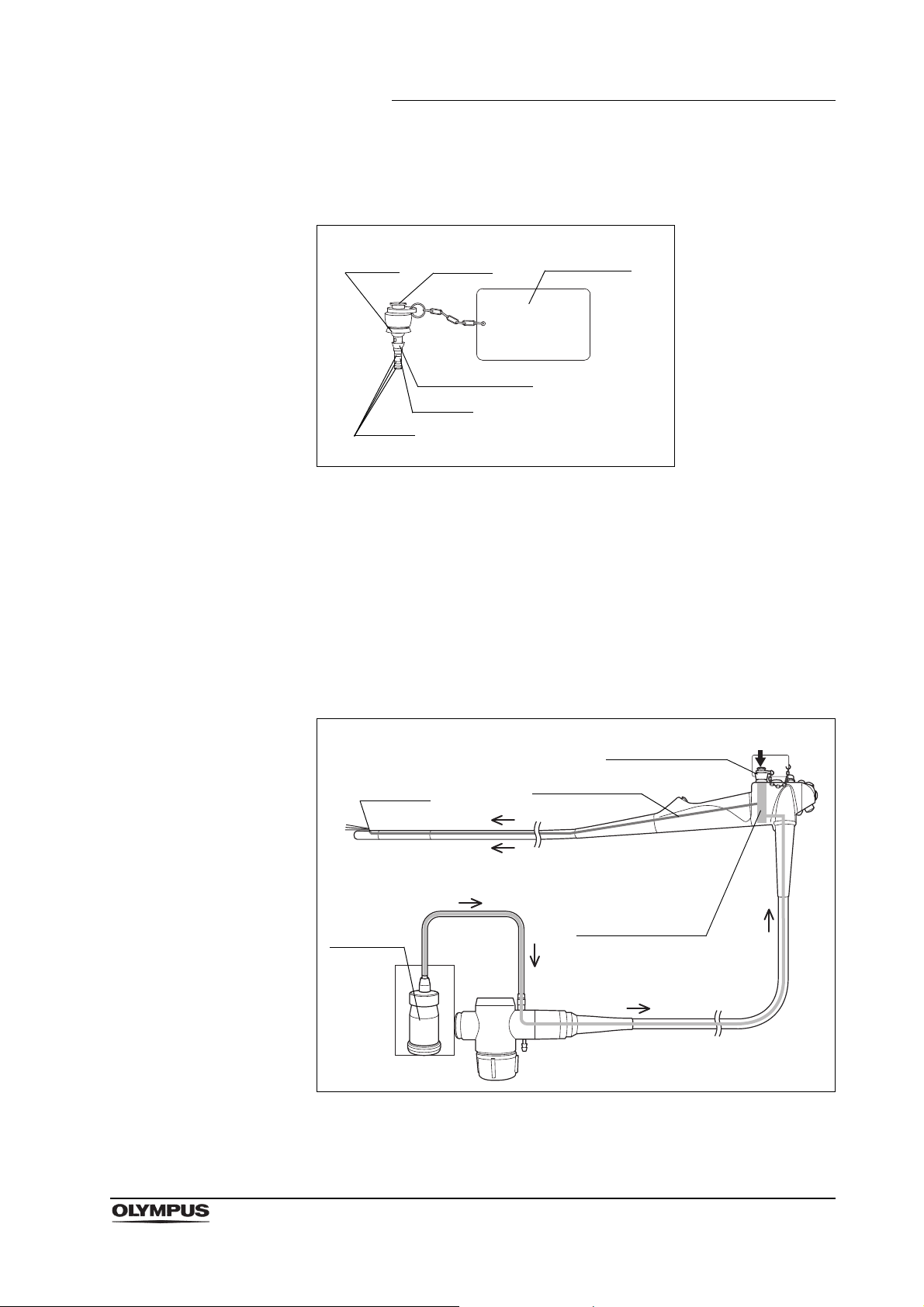
2.3 Injection tube (MH-946)
Figure 2.6
Function
The injection tube is used to inject reprocessing fluids into the instrument
channel, suction channel, and air/water channels of the endoscope. It is also
used to flush air through these channels to expel fluids.
Figure 2.7
Inspection
14
1. Confirm that all components of the injection tube are free from cracks,
scratches, flaws, and debris (see Figure 2.6).
2. Confirm that the filter mesh is in the suction port of the injection tube.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 19
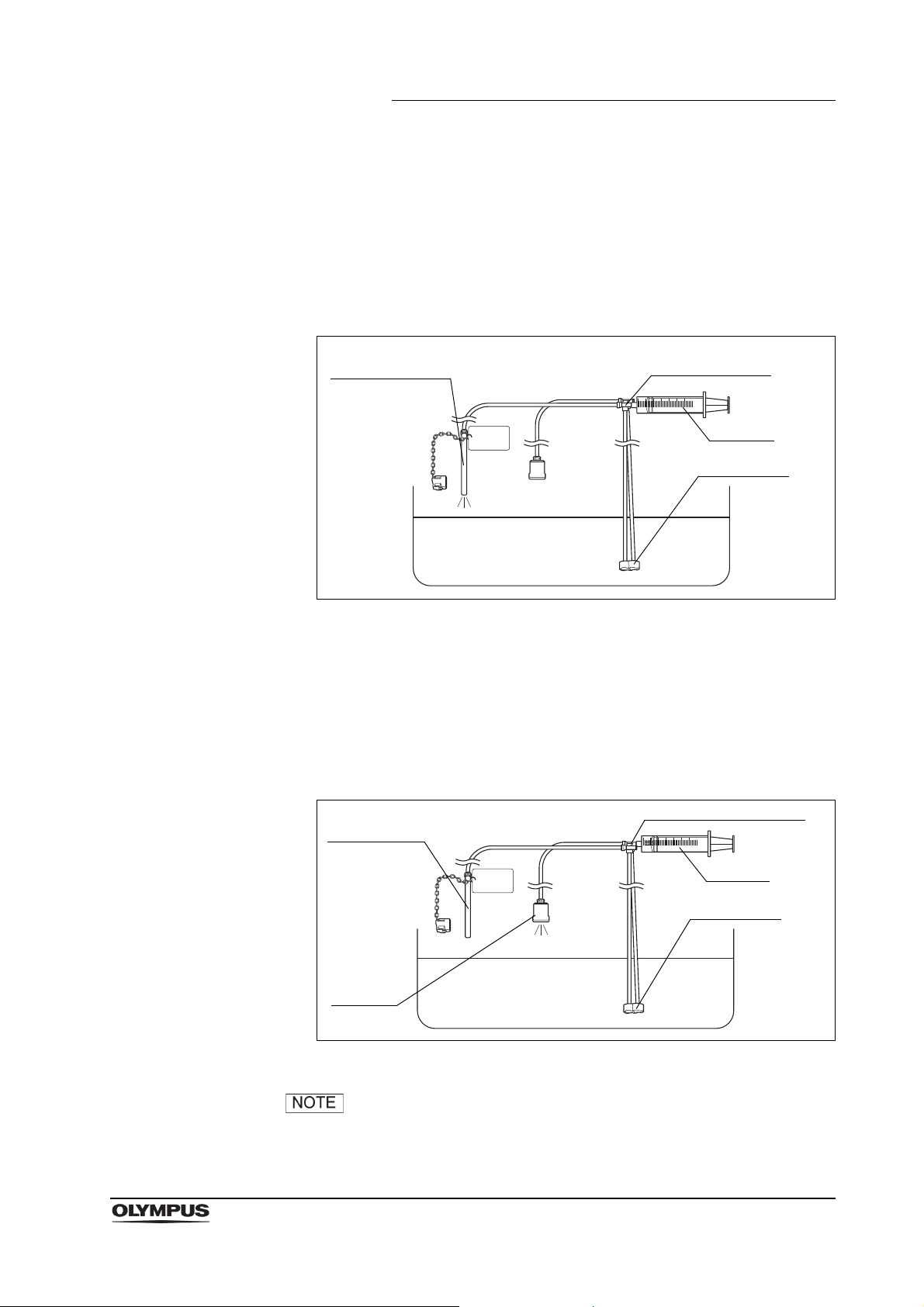
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Suction port
Suction channel
port
Suction channel
tube
Syringe
Suction port
Air/water channel port
Air pipe
port
Suction
channel tube
Syringe
3. Attach a clean 30 ml syringe to the suction channel port of the injection tube.
With the suction port of the injection tube immersed in the water referred to
in Section 3.2, withdraw the syringe plunger and confirm that the water is
drawn into the syringe. Depress the plunger and confirm that the water is
emitted from the suction channel tube of the injection tube. Confirm that the
water is not emitted from the suction port when removing the suction port
from the water.
Figure 2.8
4. Move the syringe to the air/water channel port of the injection tube. With the
suction port of the injection tube immersed in the water, withdraw the
syringe plunger and confirm that the water is drawn into the syringe.
Depress the plunger and confirm that the water is emitted from the air pipe
port of the injection tube. Confirm that the water is not emitted from the
suction port when removing the suction port from the water.
Figure 2.9
The injection tube does not need to be cleaned, disinfected,
or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
15
Page 20
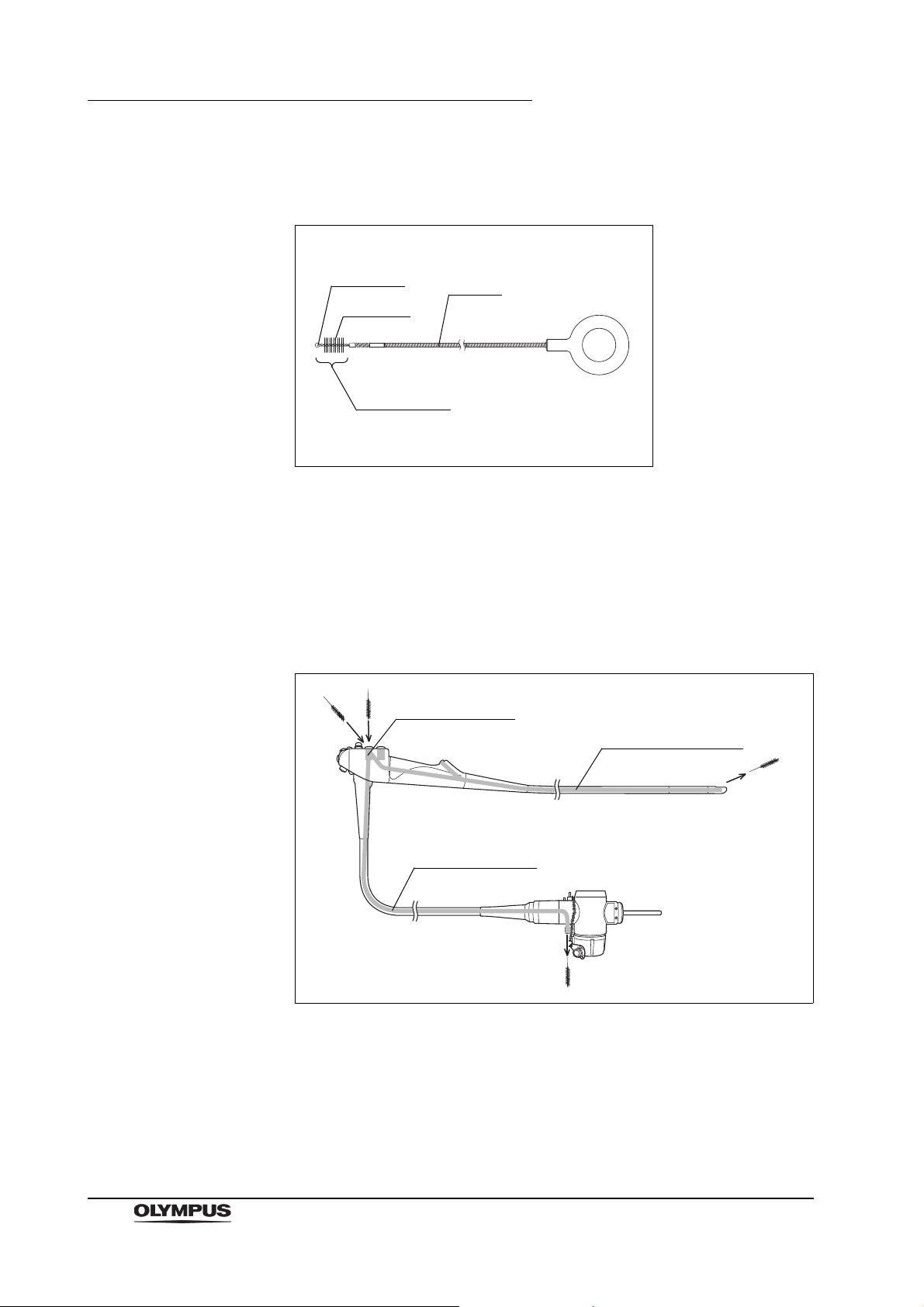
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Metal tip
Shaft
Brush head
Bristles
Suction cylinder
Instrument channel
Suction channel
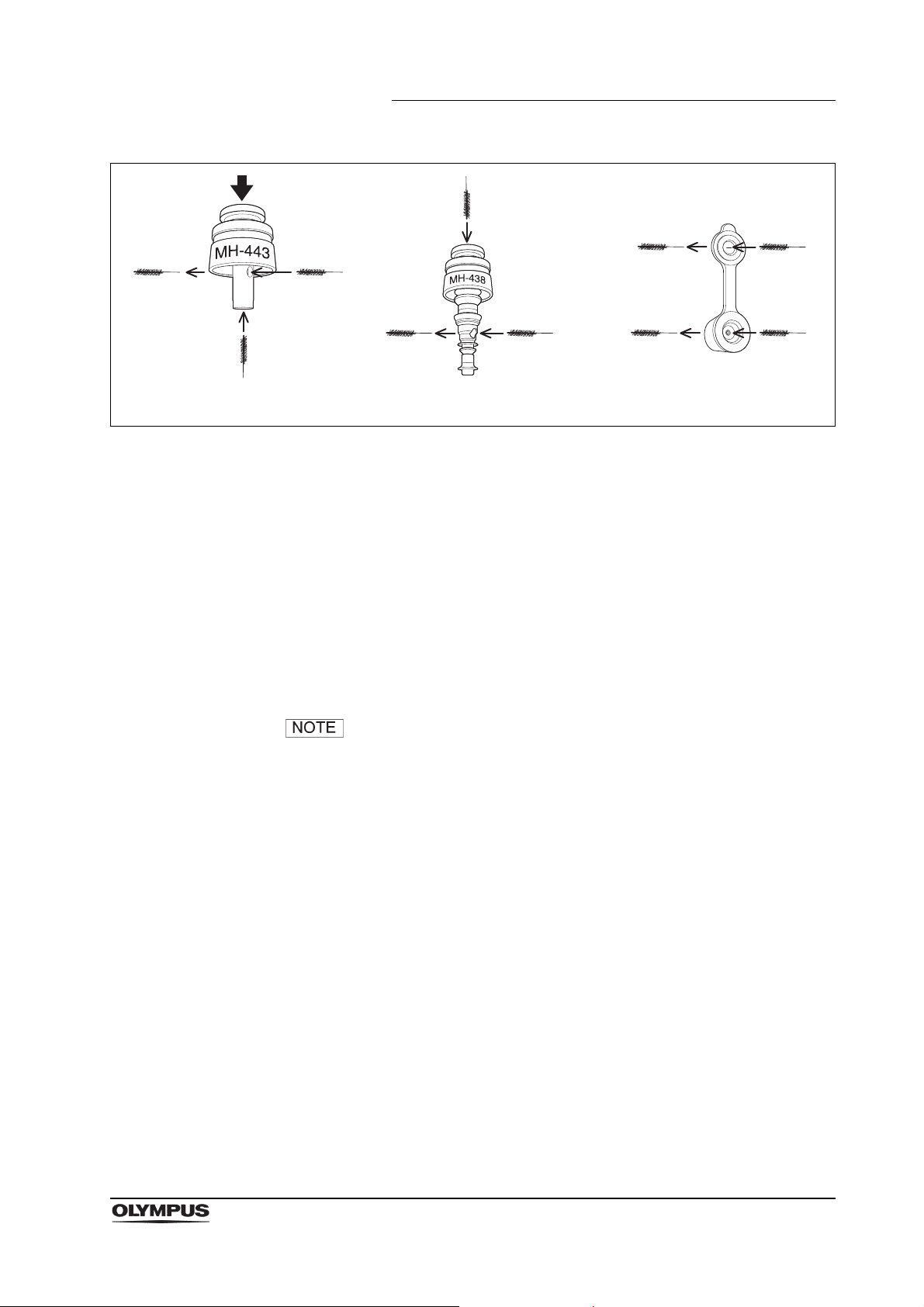
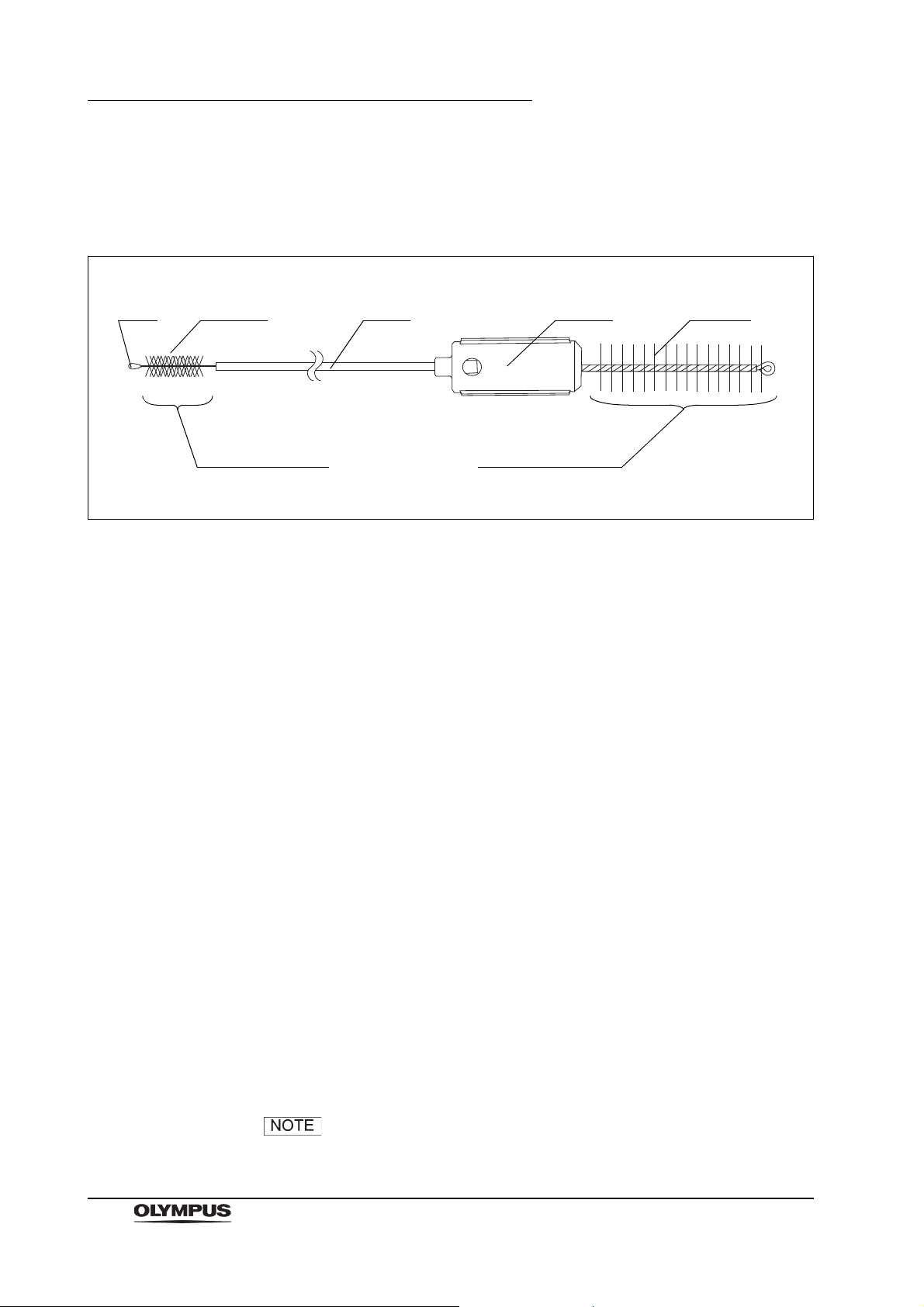
2.4 Channel cleaning brush (BW-20T)
Figure 2.10
Function
The channel cleaning brush is used to brush the inside of the instrument channel
and suction channel of the endoscope, and the interior and openings of the
suction valve (MH-443), the air/water valve (MH-438) and the biopsy valve
(MB-358).
Figure 2.11
16
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 21
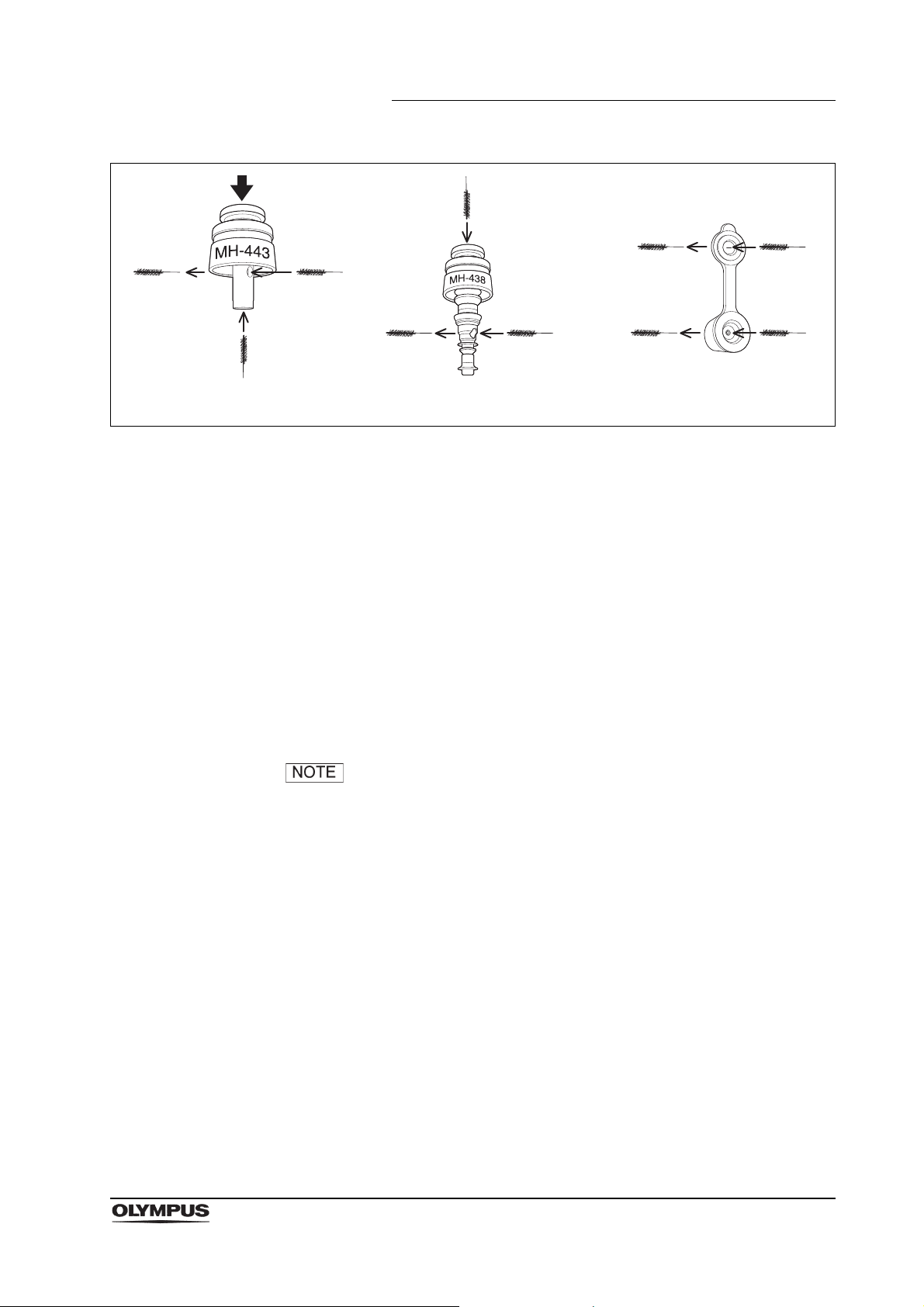
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Depress
Suction valve
(MH-443)
Air/water valve
(MH-438)
Biopsy valve (MB-358)
Figure 2.12
Inspection
1. Confirm that the brush head and the metal tip of the distal end are securely
attached. Check the brush head for loose or missing bristles.
2. Check the bristles for damage. If the bristles are crushed, gently straighten
them with your gloved fingertips.
3. Check for bends, scratches, and other damage to the shaft.
4. Visually check for debris on the shaft and/or the bristles of the brush head. If
there is debris on the brush, immerse the brush in the water referred to in
Section 3.2 and clean the brush until no debris is observed on the brush.
The channel cleaning brush does not need to be cleaned,
disinfected, or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
17
Page 22
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Weighted end
Connecting end
Suction cleaning
adapter
Connecting end
Weighted end
Suction pump
Instrument
channel port
Suction cylinder
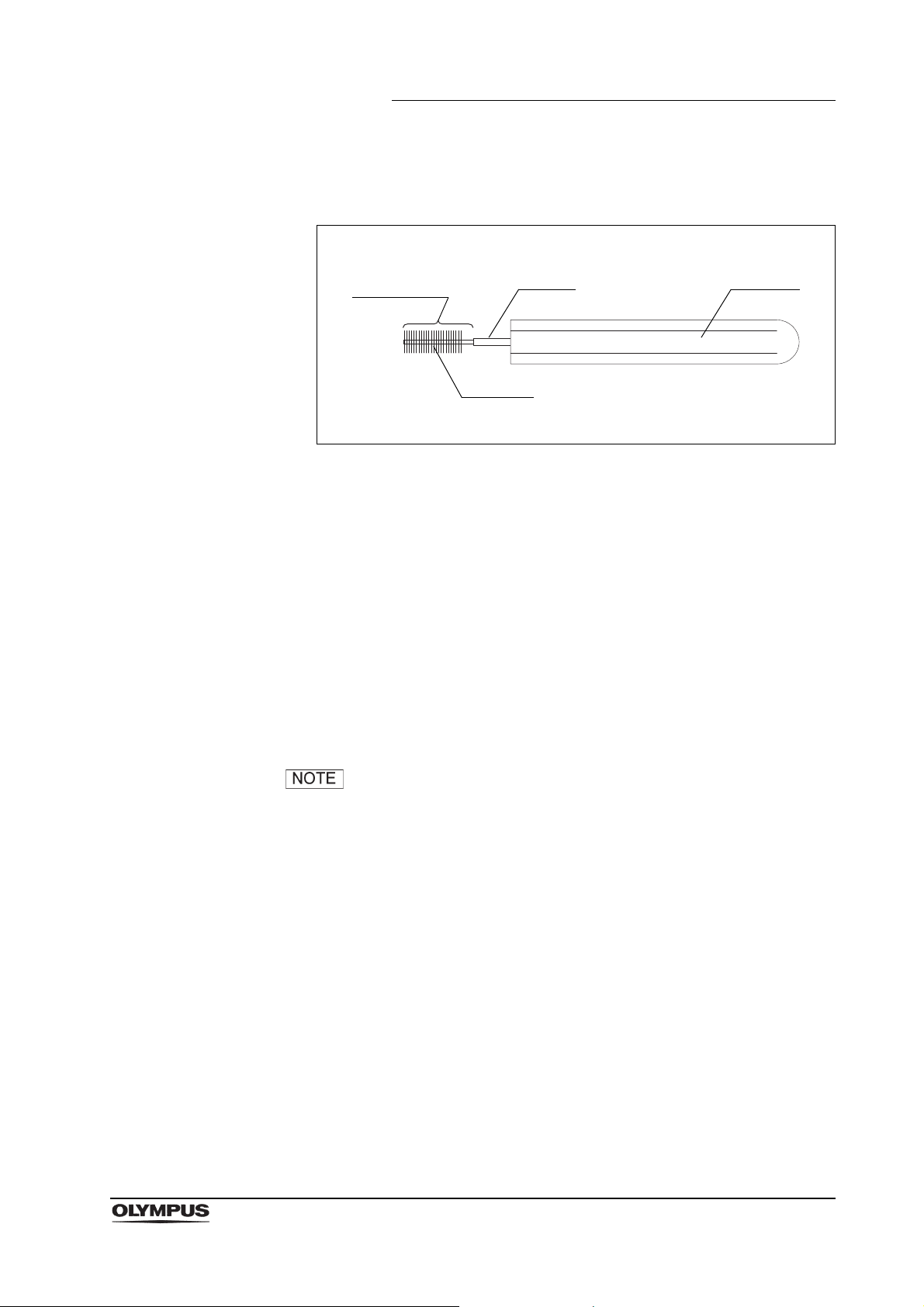
2.5 Suction cleaning adapter (MH-856)
Figure 2.13
Function
The suction cleaning adapter is used to aspirate reprocessing fluids through the
instrument channel port of the endoscope.
Figure 2.14
18
Inspection
Check for debris, cracks, scratches, and other damage.
The suction cleaning adapter does not need to be cleaned,
disinfected, or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 23
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Information
card
Slider
One-way valve
Piston
Seals
Button
AW channel
cleaning adapter
Air/water
nozzle
Depress
Air channel
Air/water cylinder
Water
container
2.6 AW channel cleaning adapter (MH-948)
Figure 2.15
Function
During precleaning of the endoscope, the AW channel cleaning adapter is
attached to the air/water cylinder of the endoscope. When the button of the
adapter is depressed, the water in the water container is fed through the air/
water nozzle of the endoscope to clean the nozzle and air/water channels of the
endoscope. Air is continuously fed through the air/water channels when the
button is not depressed.
Figure 2.16
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
19
Page 24
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Tip
Shaft
Brush head
Caution sticker
Bristles
Suction channel
Instrument channel
Suction cylinder
Inspection
Check for debris, cracks, scratches, and other damage.
The AW channel cleaning adapter does not need to be
cleaned, disinfected, or sterilized prior to its first use.
2.7 Single use channel cleaning brush (BW-201T)
Figure 2.17
Function
The single use channel cleaning brush is used to brush the inside of the
instrument channel and suction channel of the endoscope and the interior and/or
openings of the suction valve (MH-443), the air/water valve (MH-438), and the
biopsy valve (MB-358).
20
Figure 2.18
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 25
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Depress
Suction valve
(MH-443)
Air/water valve
(MH-438)
Biopsy valve (MB-358)
Figure 2.19
Inspection
1. Remove the brush from its packaging just prior to use.
2. Confirm that the tip and brush head at the distal end are securely attached.
Check the brush head for loose or missing bristles.
3. Check the bristles for any damage. If the bristles are crushed, gently
straighten them with your fingertips.
4. Check for bends, scratches, and other damage to the shaft.
The single use channel cleaning brush does not need to be
cleaned, disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
21
Page 26
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Brush head
Handle
Instrument
channel port
Suction
cylinder
2.8 Single use channel-opening cleaning brush
(MAJ-1339)
Figure 2.20
Function
The single use channel-opening cleaning brush is used to brush the suction
cylinder, the instrument channel port, the distal end, the forceps elevator, and the
forceps elevator recess.
Figure 2.21
22
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 27
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Forceps elevator
recess
Forceps elevator
Forceps elevator
recess
(a) Brushing when forceps elevator is lowered. (b) Brushing when forceps elevator is raised.
Figure 2.22
Inspection
1. Remove the brush from its packaging just prior to use.
2. Check the brush head for loose or missing bristles.
3. Check the bristles for any damage. If the bristles are crushed, gently
straighten them with your fingertips.
4. Check for bends, scratches, and other damage to the shaft.
The single use channel-opening cleaning brush does not
need to be cleaned, disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
23
Page 28
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Tip
Shaft
Channel cleaning
brush part
Handle
Channel-opening
cleaning brush part
Bristles Bristles
2.9 Single use combination cleaning brush
(BW-412T)
Figure 2.23
Function
The channel cleaning brush part of the single use combination cleaning brush is
used to brush the inside of the instrument channel and suction channel of the
endoscope, and the interior and/or openings of the suction valve (MH-443), the
air/water valve (MH-438), and the biopsy valve (MB-358). The channel-opening
cleaning brush part of the single use combination cleaning brush is used to
brush the suction cylinder, the instrument channel port, the distal end, the
forceps elevator, and the forceps elevator recess of the endoscope.
Inspection
1. Remove the brush from its packaging just prior to use.
2. Confirm that the channel cleaning brush part and tip at the distal end are
securely attached.
3. Check the channel cleaning brush and the channel-opening cleaning brush
parts for loose or missing bristles.
4. Check the bristles of the channel cleaning brush and the channel-opening
cleaning brush parts for any damage. If the bristles are crushed, gently
straighten them with your fingertips.
5. Check for bends, scratches, and other damage to the shaft.
24
The single use combination cleaning brush does not need to
be cleaned, disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 29
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Bristles
Shaft
Brush head
Handle
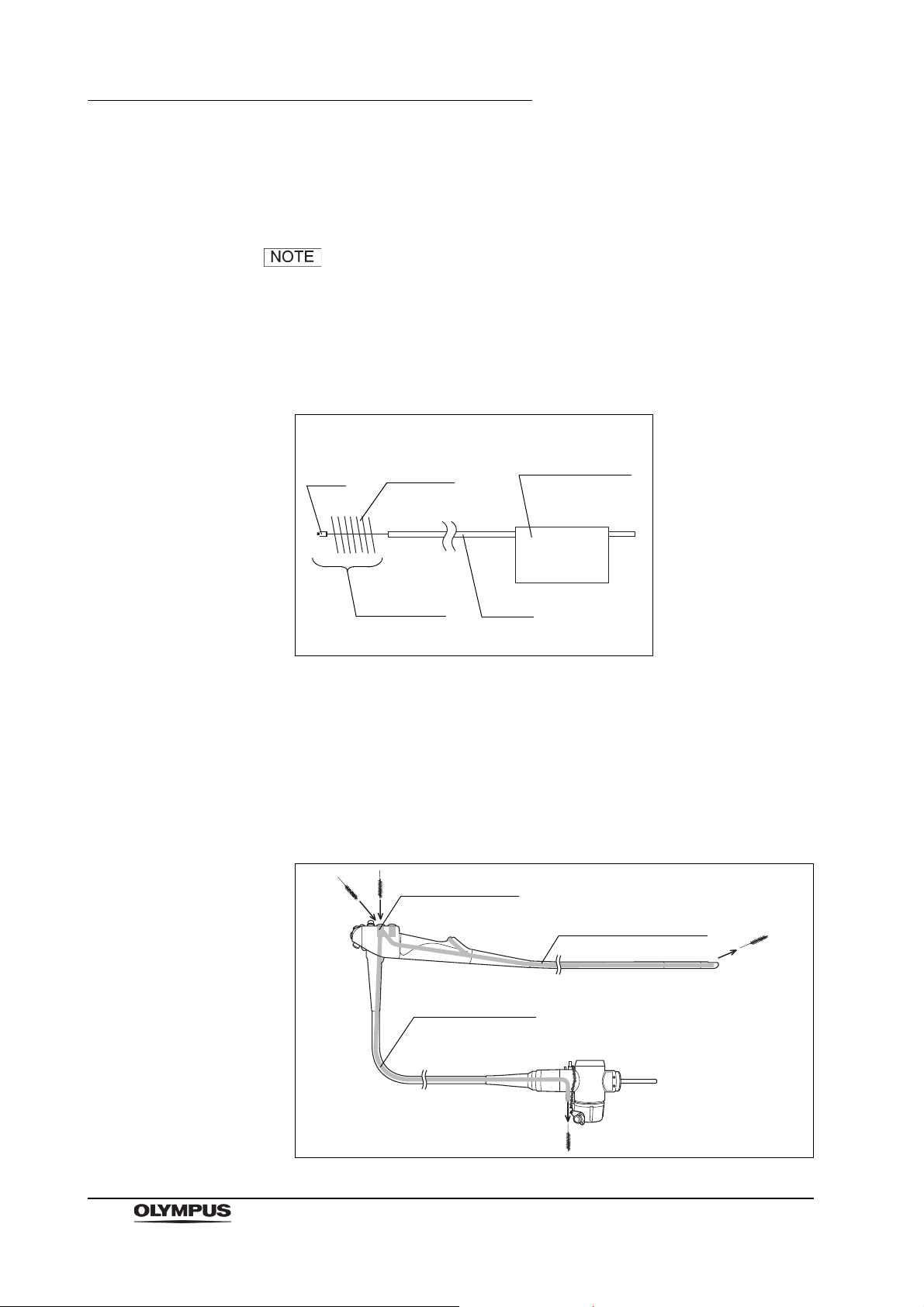
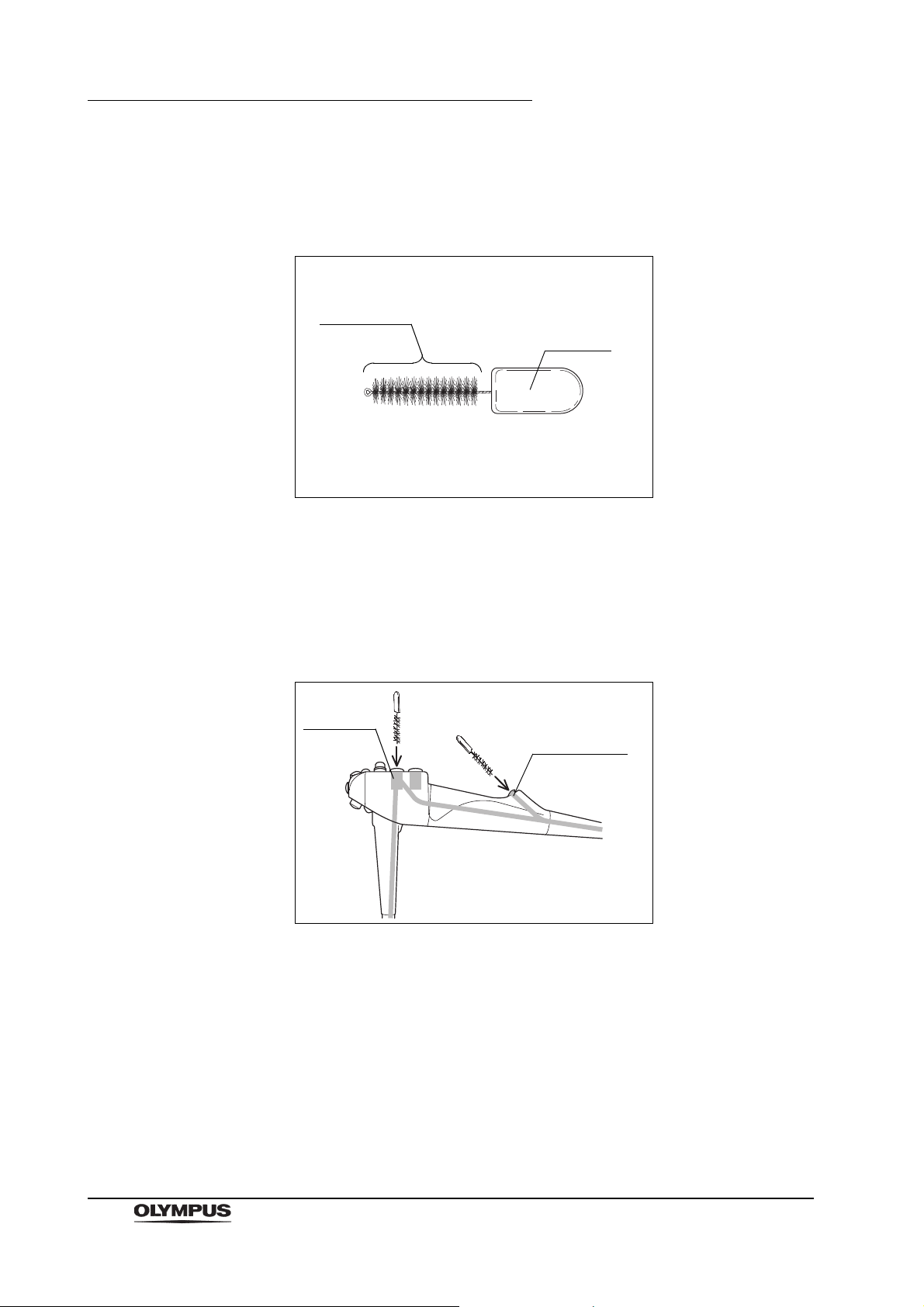
2.10 Single use soft brush (MAJ-1888)
Figure 2.24
Function
The single use soft brush is used to brush around the forceps elevator.
Inspection
1. Check the brush head for loose, missing bristles, and other damage. If the
bristles are crushed and/or bent, gently straighten them with your fingers.
2. Check for bends, scratches, and other damage to the shaft.
3. Check for debris on the shaft and or in the bristles of the brush head.
The single use soft brush does not need to be cleaned,
disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
25
Page 30
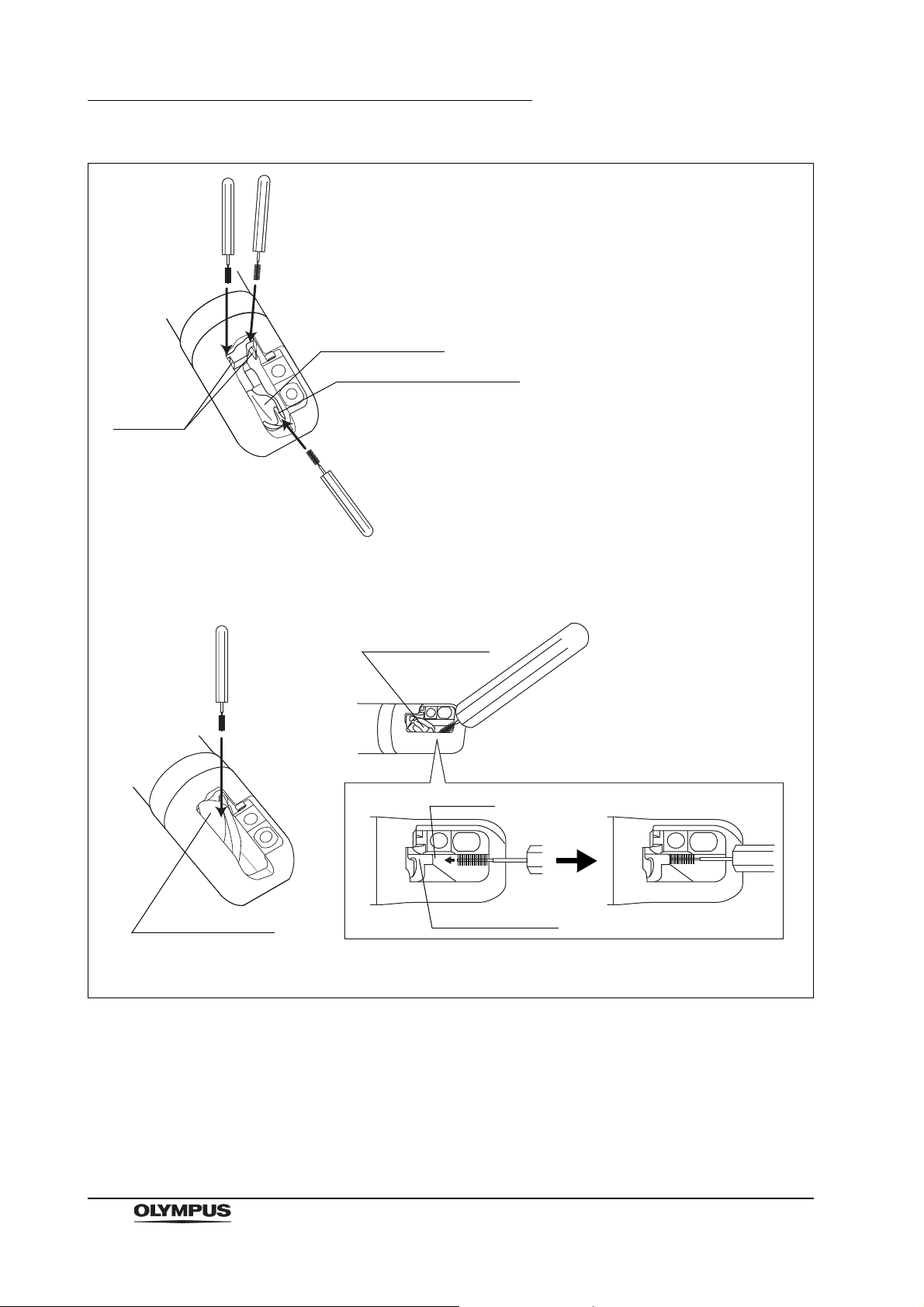
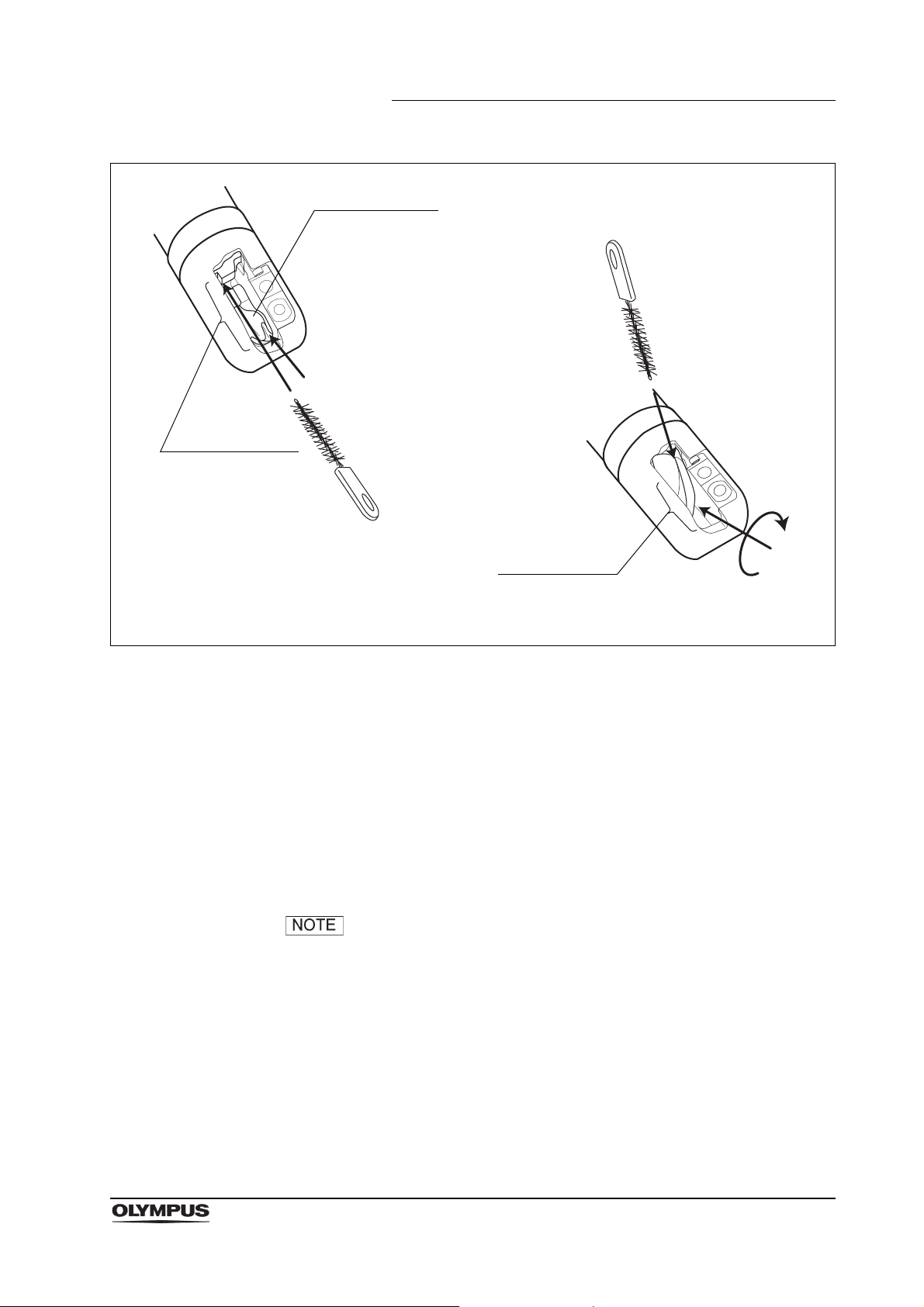
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Groove
Forceps elevator
Guidewire-locking groove
Forceps elevator
Forceps elevator
Forceps elevator
Groove
(a) Brushing when forceps elevator is lowered.
(b) Brushing when forceps elevator is raised.
Figure 2.25
26
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 31

Chapter 2 Function and Inspection of the Accessories for Reprocessing
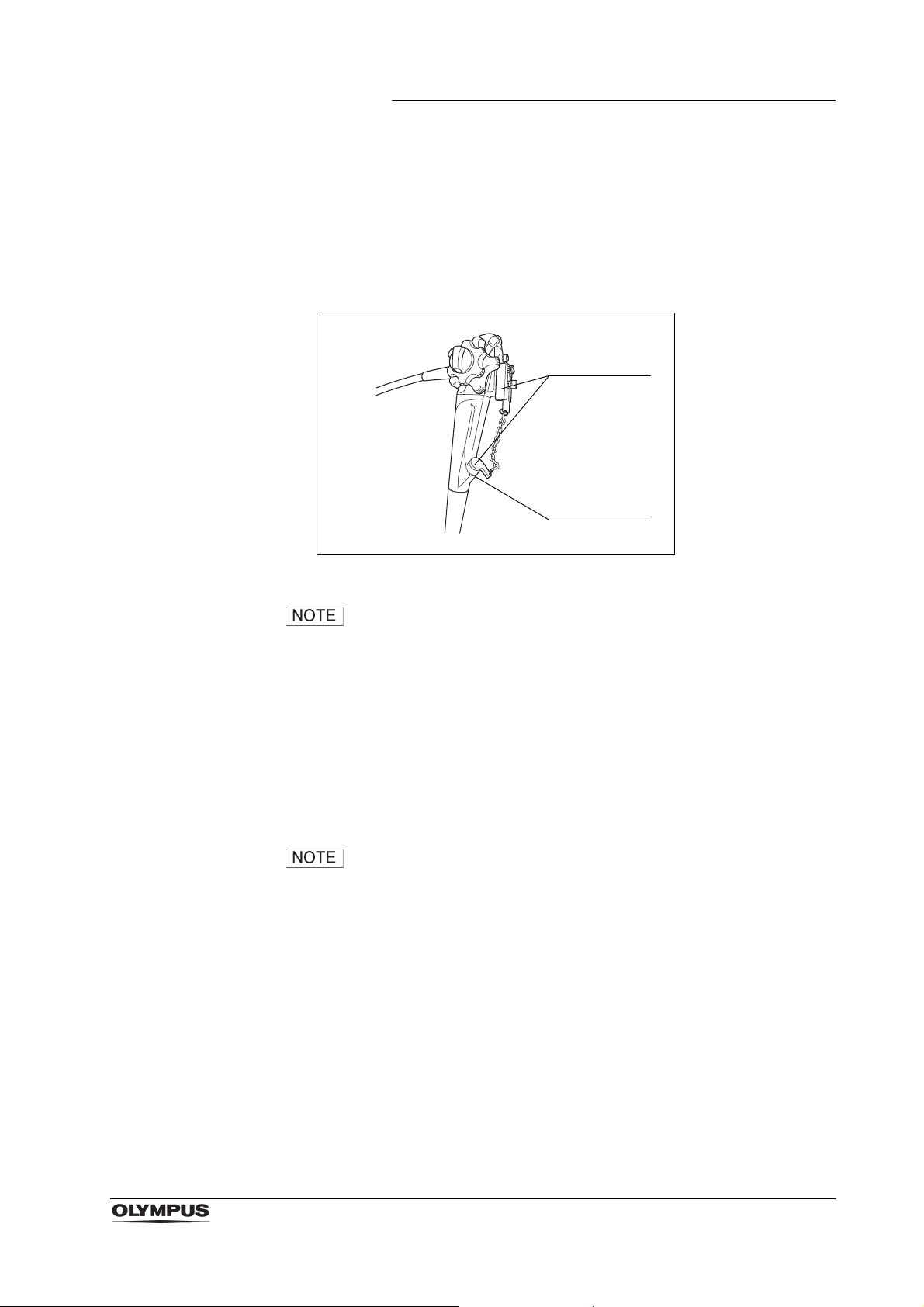
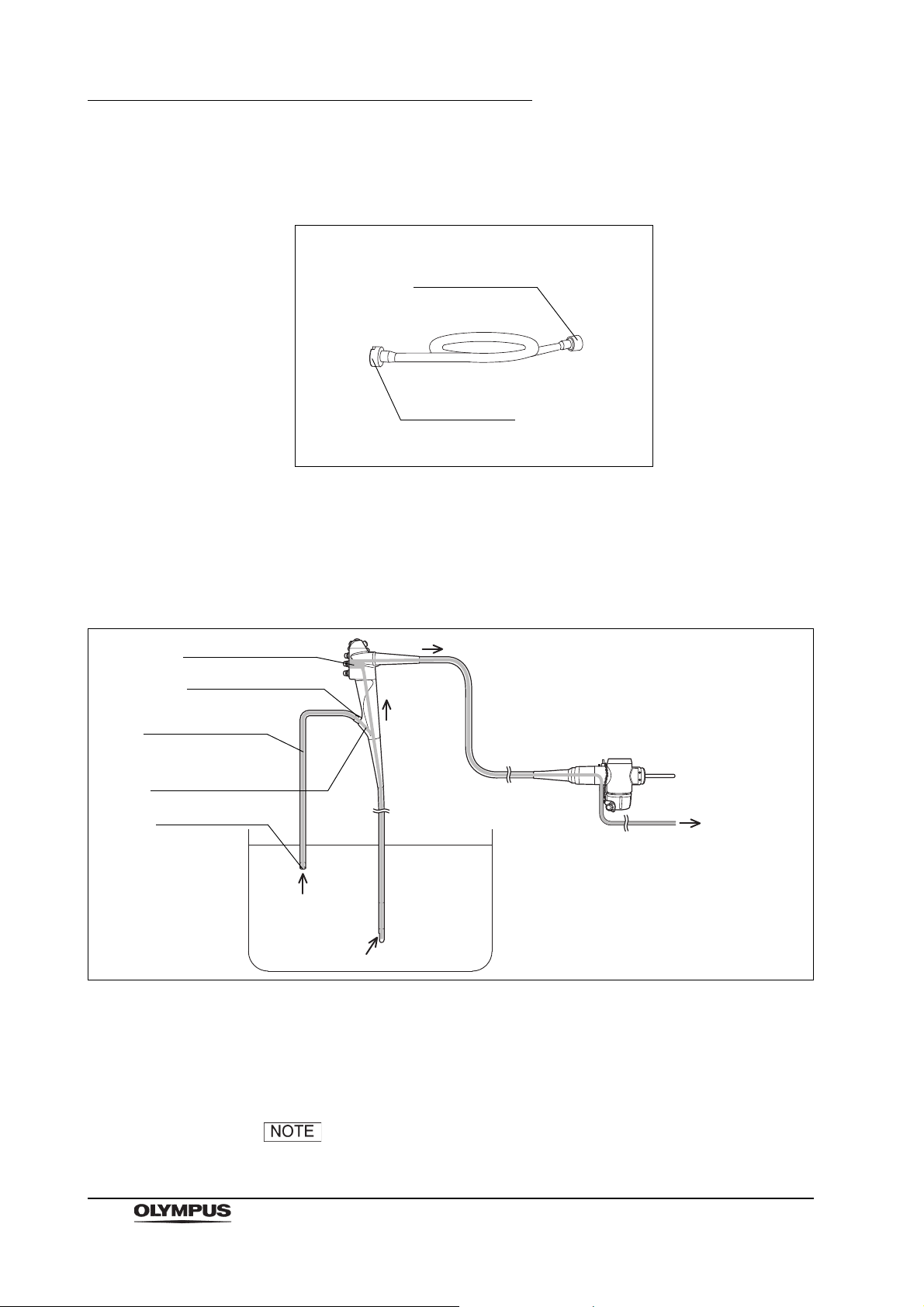
2.11 Chain for water-resistant cap (MAJ-1119)
Figure 2.26
Function
The chain for water-resistant cap is used to keep the water resistant cap
(MH-553) with the endoscope at all times.
Inspection
It is not necessary to inspect the chain before reprocessing.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
27
Page 32

Chapter 3 Compatible Reprocessing Methods and Chemical Agents
Chapter 3 Compatible Reprocessing
Methods and Chemical
Agents
3.1 Compatibility summary
The endoscope and accessories are compatible with several methods of
reprocessing. However, not all reprocessing methods are compatible with all
endoscopes and all accessories. Reprocessing with incompatible methods can
cause equipment damage even if the number of reprocessing cycles is small.
For appropriate reprocessing methods, see Table 3.1.
Follow the policies at your local institution when choosing which methods listed
in Table 3.1 to employ.
• Methods listed as “compatible” in Table 3.1 are compatible
for routine use only when used according to manufacturer’s
instructions. Repeated use and reprocessing of endoscopes
and accessories leads to gradual wear and tear. But
reprocessing methods that employ higher temperatures and
more caustic/corrosive materials may lead to faster
deterioration. In general, sterilization processes are harsher
on equipment than disinfection processes. Before each
patient procedure, inspect the endoscope and accessories
for damage, according to the instructions described in this
manual and its companion “OPERATION MANUAL”.
• Instructions provided in this manual regarding material
compatibility are not valid for Olympus devices repaired by a
non-Olympus facility. Olympus repairs devices to
manufacturer’s specifications using original equipment
manufacturer’s (OEM) materials. The use of non-OEM
materials to repair an Olympus device may affect the material
compatibility of the device with certain reprocessing
chemicals or methods. In the event that your device has
been repaired by a non-Olympus facility, contact that repair
facility for instructions regarding material compatibility.
28
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 33

.
Endoscope
Chapter 3 Compatible Reprocessing Methods and Chemical Agents
For sterilization Steam sterilization (autoclaving)
Ethylene oxide gas sterilization
(gas mixture 20% ethylene oxide gas/80% CO
for countries other than the USA)
Ethylene oxide gas sterilization
(100% ethylene oxide gas)
For disinfection
ACECIDE disinfectant solution
2 – 3.5% glutaraldehyde
For alcohol flush 70% ethyl or 70% isopropyl
alcohol
For cleaning Detergent solution
Ultrasonic
cleaning
1
,
2
3
Water resistant cap
(MH-553)
Chain for water-resistant
cap (MAJ-1119)
Channel cleaning brush
(BW-20T)
Air/water valve (MH-438)
Suction valve (MH-443)
Biopsy valve (MB-358)
Channel plug (MH-944)
Injection tube (MH-946)
AW channel cleaning
adapter (MH-948)
Suction cleaning adapter
(MH-856)
Mouthpiece (MB-142)
Single use channel
cleaning brush (BW-201T)
Single use channel-opening
cleaning brush (MAJ-1339)
Single use combination
cleaning brush (BW-412T)
Single use soft brush
(MAJ-1888)
2
compatible not compatible
Table 3.1
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
29
Page 34

Chapter 3 Compatible Reprocessing Methods and Chemical Agents
Green
marking
AUTOCLAVABLE
Air/water valve (MH-438)
1 The endoscope is only compatible with ultrasonic cleaning as performed
in an Olympus-recommended endoscope reprocessor such as OER-AW,
OER-Pro (OER-AW and OER-Pro are not available in some areas). When
using an AER that is recommended by Olympus other than listed above,
contact Olympus.
2 The water resistant caps (MH-553) and the chain for water-resistant cap
(MAJ-1119) can only be ultrasonically cleaned if attached to an endoscope
that is being cleaned in an automated endoscope reprocessor with an
ultrasonic cleaning phase.
3 ACECIDE are not available in some areas.
Accessories that are marked by the words “AUTOCLAVE” or
“AUTOCLAVABLE”, or with green markings (such as a green
component or label) are compatible with steam sterilization
(autoclaving).
3.2 Water (for reprocessing)
30
Figure 3.1
Water is used for leakage testing and manual cleaning of the endoscope and
accessories. For these purposes, use either fresh, potable tap water or water
that has been processed (e.g., filtered, deionized or purified) to improve its
chemical and/or microbiological quality. Consult with your hospital’s infection
control committee.
When rinsing the endoscope and accessories following high-level disinfection,
use the water referred to in Section 3.5.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 35

Chapter 3 Compatible Reprocessing Methods and Chemical Agents
3.3 Detergent solution
• Excessive foaming prevents detergent from properly
contacting the surfaces and channel walls of the endoscope
and accessories, and may impair effective cleaning.
• Do not reuse detergent solutions.
Use a medical-grade, low-foaming, neutral pH detergent. Follow the instructions
provided by the detergent manufacturer regarding concentration, temperature,
contact time, and expiration date. Contact Olympus for the names of specific
brands of detergent solution that have been tested for compatibility with
endoscopes and accessories.
3.4 Disinfectant solution
Use a high-level disinfectant cleared by your national regulatory agency for use
in reprocessing flexible endoscopes. Follow the disinfectant manufacturer’s
instructions regarding activation (if required), concentration, temperature,
contact time, and expiration date.
For further information regarding the compatibility of glutaraldehyde-based or
non glutaraldehyde-based disinfectant solutions, contact Olympus.
3.5 Rinse water
Use sterile water for rinsing the endoscope and accessories following high-level
disinfection.
If sterile water is not available, use either fresh, potable tap water or water that
has been processed (e.g., filtered, deionized or purified) to improve its chemical
and/or microbiological quality, and flush the endoscope and accessories with the
alcohol referred to in Section 3.6 after rinsing. Consult with your hospital’s
infection control committee regarding local policies on water quality.
3.6 Alcohol
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Use medical-grade 70% ethyl or 70% isopropyl alcohol.
31
Page 36

Chapter 3 Compatible Reprocessing Methods and Chemical Agents
3.7 Ethylene oxide gas sterilization
The endoscope and accessories listed as compatible with ethylene oxide gas
sterilization in Table 3.1 can be sterilized by ethylene oxide gas and aerated
within the parameters given in Table 3.2 and 3.3. When performing ethylene
oxide gas sterilization, follow all national, professional, and institutional
reprocessing protocols as well as the instructions provided by the manufacturer
of your sterilization equipment.
Exceeding the recommended parameters may cause
equipment damage (see Table 3.2 and 3.3).
Parameters for 100% ethylene oxide gas sterilization
cycles
Process phase Parameter Value
Sterilization Temperature
Vac uum
(Absolute pressure)
Relative humidity 50 – 80%
Ethylene oxide gas
concentration
Exposure time 60 minutes
Aeration Minimum aeration
parameters
55
C (130F)
0.05 – 0.07 MPa
(7.25 – 10.15 psia)
0.735 – 0.740 mg/cm
(735 – 740 mg/L)
12 hours in an aeration chamber
at 50 – 57C(122 – 135F) or
7 days at room temperature
3
32
Table 3.2
Parameters for 20% ethylene oxide gas/80% CO2 gas
sterilization cycles, for countries other than the USA
Process phase Parameter Value
Sterilization Temperature
Relative pressure 0.1 – 0.17 MPa
Relative humidity 55%
Ethylene oxide gas
concentration
Exposure time 105 minutes
Aeration Minimum aeration
parameters
Table 3.3
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
57
C (135F)
0.6 – 0.7 mg/cm
(600 – 700 mg/L)
12 hours in an aeration chamber
at 50 – 57C (122 – 135F) or
7 days at room temperature
3
Page 37

Chapter 3 Compatible Reprocessing Methods and Chemical Agents
3.8 Steam sterilization (autoclaving)
The accessories listed as compatible with steam sterilization in Table 3.1 can be
sterilized by steam within the parameters given in Table 3.4. When steam
sterilizing, follow all national, professional, and institutional reprocessing
protocols as well as the instructions provided by the manufacturer of your
sterilization equipment.
• Do not steam sterilize the endoscope. Steam sterilization will
cause severe damage.
• Exceeding the recommended parameters may damage the
accessories (see Table 3.4).
Process Parameters
Prevacuum Temperature 132 – 134C
Exposure time 5 minutes
(270 – 274F)
Table 3.4
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
33
Page 38

Chapter 3 Compatible Reprocessing Methods and Chemical Agents
34
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 39

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Chapter 4 Reprocessing Workflow for
the Endoscope and
Accessories
This chapter describes the workflow for reprocessing the endoscope and
accessories.
Deviation from the recommended workflow may pose an
infection control risk.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
35
Page 40

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Endoscope
Patient procedure
Section 5.2, “Precleaning
the endoscope and
accessories”
Section 5.3, “Leakage testing
of the endoscope”
Section 5.4, “Manually
cleaning the endoscope and
accessories”
Section 8.1, “Storing the disinfected endoscope and accessories”
Section 5.5, “Manually
disinfecting the endoscope
and accessories”
Section 5.6, “Rinsing the
endoscope and accessories
following disinfection”
Mouthpiece
(MB-142)
Air/water
valve
(MH-438)
Biopsy
valve
(MB-358)
Suction
valve
(MH-443)
Water
resistant cap
(MH-553)
Patient
procedure
Section 5.2
Section 6.1, “Manually cleaning the accessories”
Section 6.2, “Manually disinfecting the accessories”
Section 6.3, “Rinsing the accessories following disinfection”
Chain for
water-resistant
cap
(MAJ-1119)
1
AW channel
cleaning
adapter
(MH-948)
Section 5.2
4.1 Workflow for manually cleaning and disinfecting
the endoscope and accessories
The water resistant cap (MH-553) should remain connected to the endoscope at all times using the chain for water-resistant
1
cap (MAJ-1119).
36
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 41

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Injection
tube
(MH-946)
Suction
cleaning
adapter
(MH-856)
Single use
channelopening
cleaning
brush
(MAJ-1339)
Channel
cleaning
brush
(BW-20T)
Section 5.4
Section 6.1
Section 6.2
Section 6.3
Section 8.1
Section 5.5
Section 5.6
Single use
channel
cleaning
brush
(BW-201T)
Channel
plug
(MH-944)
Single use
combination
cleaning
brush
(BW-412T)
Single use
soft brush
(MAJ-1888)
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
37
Page 42

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Air/water
valve
(MH-438)
Endoscope
Patient procedure
Section 5.2, “Precleaning
the endoscope and
accessories”
2
Section 5.3, “Leakage
testing of the endoscope”
3
Section 5.4, “Manually cleaning the
endoscope and accessories”
4
Section 8.1, “Storing the disinfected endoscope and accessories”
Chapter 7, “Reprocessing Endoscopes and Accessories using an Automated Endoscope Reprocessor”
5
Section 5.5, “Manually disinfecting
the endoscope and accessories”
Mouthpiece
(MB-142)
7
Biopsy
valve
(MB-358)
Suction
valve
(MH-443)
Patient
procedure
Section 5.2
2
Section 6.1, “Manually cleaning the accessories”
Section 6.2, “Manually disinfecting the accessories”
Section 6.3, “Rinsing the accessories following disinfection”
Chain for
water-resistant
cap (MAJ-1119)
1
Some endoscopes can be cleaned and disinfected with an AER while others cannot. The endoscopes that can be
cleaned and disinfected vary, depending upon which model AER is used. Check the AER’s instruction manual to
confirm which endoscopes can be cleaned and disinfected in the AER.
AW channel
cleaning
adapter
(MH-948)
7
Section
5.2
2
Section 5.6, “Rinsing the
endoscope and accessories
following disinfection”
Water resistant
cap (MH-553)
When you use an AER which allows you skip some steps in precleaning and manual cleaning of
endoscopes, confirm with the AER manufacturer that such skip is applicable to this endoscope and
establish detailed precleaning and manual cleaning procedures of this endoscope according to both
instructions of this manual and the AER manufacturer.
4.2 Workflow for cleaning and disinfecting the
endoscope and accessories using an AER
The water resistant cap (MH-553) should remain connected to the endoscope at all times using the chain for water-resistant
1
cap (MAJ-1119).
When using Olympus AER OER-Pro or OER-AW for reprocessing this endoscope, you are able to simplify the standard
2
manual precleaning procedure. When simplifying the procedure, follow the instruction manual “Modified precleaning and
cleaning of TJF-Q180V when using OER-Pro/OER-AW”.
Check the instruction manual for your AER to determine how to leakage test the endoscope using the AER. When leakage
3
testing an endoscope within an AER basin it may be difficult to fully angulate the bending section. Perform leakage testing in
the AER and/or manually, depending upon the policy of your institution.
38
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 43

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Section 5.4
4
Section 6.1
Section 6.2
Section 6.3
Section 8.1
Chapter 7
5
Section 5.5 & 5.6
or
Section 6.2 & 6.3
6
Some accessories can be cleaned and disinfected with an AER while others cannot. The accessories that can be
cleaned and disinfected vary, depending upon which model AER is used. Check the AER’s instruction manual to
confirm which accessories can be cleaned and disinfected in the AER.
Manually disinfect all endoscopes and accessories that cannot be reprocessed in the AER.
Injection
tube
(MH-946)
7
Suction
cleaning
adapter
(MH-856)
7
Single use
channelopening
cleaning
brush
(MAJ-1339)
Channel
cleaning
brush
(BW-20T)
7
Single use
channel
cleaning
brush
(BW-201T)
Channel
plug
(MH-944)
7
Single use
combination
cleaning
brush
(BW-412T)
Single use
soft brush
(MAJ-1888)
4
5
6
7 The accessory(s) are not compatible with the OER-Pro.
When using Olympus AER OER-Pro or OER-AW for reprocessing this endoscope, you are able to simplify the standard
manual cleaning procedure. When simplifying the procedure, follow the instruction manual “Modified precleaning and
cleaning of TJF-Q180V when using OER-Pro/OER-AW”.
If the endoscope and/or the accessories are compatible with the AER, clean and disinfect them in the AER, following the
AER’s instruction manual. If the endoscope and/or the accessories are not compatible with the AER, manually clean,
disinfect and rinse them following the instructions of this manual, as shown in the dotted boxes.
If the endoscope and the accessory(s) are not compatible with the AER, manually disinfect and rinse them, according to
Sections 5.5 and 5.6. If the endoscope is compatible and the accessory(s) are not compatible, manually disinfect and rinse
the accessory(s) according to Section 6.2 and 6.3.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
39
Page 44

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Patient procedure
Section 5.2, “Precleaning
the endoscope and
accessories”
Section 5.3, “Leakage
testing of the endoscope”
Section 5.4, “Manually
cleaning the endoscope and
accessories”
Section 8.2, “Storing the sterilized endoscope and accessories”
Section 5.7, “Sterilizing the
endoscope and
accessories”
Patient
procedure
Section 5.2
Section 6.1, “Manually cleaning the accessories”
Section 6.4, “Sterilizing the accessories”
If required by the local policy of your institution, disinfect and rinse the endoscope and accessories
manually, or clean and disinfect them with an AER between manual cleaning and sterilization.
Section 5.2
Endoscope Mouthpiece
(MB-142)
Air/water
valve
(MH-438)
Biopsy
valve
(MB-358)
Suction
valve
(MH-443)
Water
resistant cap
(MH-553)
Chain for
water-resistant
cap
(MAJ-1119)
1
AW channel
cleaning
adapter
(MH-948)
4.3 Workflow for manually cleaning and sterilizing
the endoscope and accessories
The water resistant cap (MH-553) should remain connected to the endoscope at all times using the chain for water-resistant
1
cap (MAJ-1119).
40
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 45

Chapter 4 Reprocessing Workflow for the Endoscope and Accessories
Section 5.4
Section 8.2
Section 5.7
Injection
tube
(MH-946)
Suction
cleaning
adapter
(MH-856)
Single use
channelopening
cleaning
brush
(MAJ-1339)
Channel
cleaning
brush
(BW-20T)
Single use
channel
cleaning
brush
(BW-201T)
Channel
plug
(MH-944)
Single use
combination
cleaning
brush
(BW-412T)
Single use
soft brush
(MAJ-1888)
Section 6.1
Section 6.4
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
41
Page 46

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Guidewire-locking
groove
Groove
Forceps elevator recess
Chapter 5 Reprocessing the
Endoscope (and related
reprocessing accessories)
Certain accessories are required to manually reprocess the endoscope. Some of
these accessories are cleaned and disinfected along with the endoscope. The
steps for reprocessing the endoscope and these accessories are explained in
this chapter. Chapter 6, “Reprocessing the Accessories” describes the steps for
reprocessing accessories that are not reprocessed together with the endoscope.
The reprocessing workflow of all accessories is outlined in Chapter 4,
“Reprocessing Workflow for the Endoscope and Accessories”.
Figure 5.1
The TJF-Q180V endoscope has a forceps elevator. The
surface and surrounding area of the forceps elevator, such as
the groove, guidewire locking groove, and forceps elevator
recess, have a complex shape. Reprocess these parts
carefully following the procedure described in Chapter 5.
Insufficient reprocessing may pose an infection control risk to
patient and/or operators.
42
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 47

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Bending section
Insertion section
Insertion tube
Distal end
• The insertion section of the endoscope is composed of the
insertion tube, the bending section, and the distal end. The
bending section is covered by a thin, easily damaged elastic
covering. Do not allow reprocessing equipment to forcefully
contact the bending section. Do not allow any sharp edges,
such as the distal ends of EndoTherapy accessories
(needles, forceps, snares, etc. used in the instrument
channel of the endoscope) to contact the bending section.
Such improper handling may damage the covering and
cause the endoscope to leak.
Figure 5.2
• Handle the insertion section carefully. Tightly gripping or
sharply bending the insertion tube or the bending section can
stretch or severely damage the insertion tube and/or the
covering of the bending section.
• To prevent damage to the endoscope, do not immerse the
endoscope with objects other than the equipment used for
reprocessing the endoscope.
• To prevent damage, do not coil the insertion tube or the
universal cord of the endoscope with a diameter of less than
12 cm.
Use sterile equipment, such as sterile syringes and cloths, for all reprocessing
steps occurring after immersion of the endoscope and accessories in
disinfectant solution.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
43
Page 48

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Leakage tester (MB-155)
(Sold separately. Refer to
its instruction manual.)
Water resistant cap
(MH-553)
Channel cleaning
brush (BW-20T)
AW channel
cleaning adapter
(MH-948)
Suction cleaning
adapter (MH-856)
Channel plug
(MH-944)
Injection tube
(MH-946)
Maintenance unit (MU-1)
(Sold separately. Refer
to its instruction
manual.)
Suction pump
(KV-4, KV-5, SSU-2) and Tube
(Sold separately. Refer to its
instruction manual.)
Single use channel
cleaning brush
(BW-201T)
1
Single use channel-opening
cleaning brush
(MAJ-1339)
1
Single use combination
cleaning brush
(BW-412T)
1
Single use soft brush
(MAJ-1888)
5.1 Preparing the equipment for reprocessing
Equipment needed
The following equipment is necessary to perform the reprocessing steps
described in this chapter.
• Personal protective equipment • Water for cleaning (Refer to Section 3.2)
• Detergent solution (Refer to Section 3.3) • Disinfectant solution (Refer to Section 3.4)
• Rinse water (Refer to Section 3.5) • 70% ethyl or 70% isopropyl alcohol (Refer to Section
• Clean, soft brush(s)
• Sterile lint-free cloths
• Sterile cotton swabs • Clean 30 ml (30 cc) syringe(s)
• Sterile 30 ml (30 cc) syringe(s) • Clean, 500 ml containers
• Clean, large basins with tight-fitting lids
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
• Sterile, large basins
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
44
2
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
3.6)
• Clean lint-free cloths
• Clean sponge(s)
• Clean, large basins
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
• Sterile, small basins with tight-fitting lids
(size: 25 (W) × 10 (H) × 25 (D) cm or more)
2
Page 49

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
AW channel
cleaning adapter
(MH-948)
Suction pump (KV-4, KV-5, SSU-2)
and Tube (Sold separately. Refer
to its instruction manual.)
Water resistant cap
(MH-553)
1 These products may not be available in some areas.
2 All cloths used in reprocessing are recommended to be lint-free. Lint or cloth fibers shed into
reprocessing fluids may be injected into the endoscope channels. There is the potential for lint or
cloth fibers to lodge in channels or become trapped in the air/water nozzle. If gauze is used to
reprocess the endoscope, ensure that fibers do not get caught on or remain trapped by
protruding components like the air/water nozzle.
5.2 Precleaning the endoscope and accessories
If the endoscope and accessories used in the patient
procedure are not immediately cleaned after each patient
procedure, residual organic debris will begin to dry and
solidify, hindering effective removal and reprocessing
efficacy. Preclean the endoscope and the accessories at the
bedside in the patient procedure room immediately after
each patient procedure.
Equipment needed
Prepare the following equipment.
• Water for cleaning (Refer to Section 3.2) • Detergent solution (Refer to Section 3.3)
• Clean lint-free cloths • Clean sponge(s)
• Clean 30 ml (30 cc) syringe(s) • Clean, 1,000 ml containers
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
45
Page 50

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Cap
Suction valve
(MH-443)
Preparation
Immediately following the patient procedure, with the endoscope still connected
to the equipment used in the patient procedure (i.e., the light source, video
system center, suction pump), perform the following precleaning steps at the
patient bed side.
1. Turn the video system center and light source OFF.
2. Prepare a clean 1,000 ml container of the water referred to in Section 3.2.
Wipe the insertion section
Dip a clean, lint-free cloth or sponge in the water and wipe the entire insertion
section of the endoscope. Wipe from the boot at the control section toward the
distal end.
Aspirate water
1. Turn the suction pump ON.
2. Close the cap on the biopsy valve.
Monitor the suction bottle on the suction pump carefully to
ensure that it does not overflow.
46
Figure 5.3
3. Lower the forceps elevator by turning the elevator control lever and immerse
the distal end of the insertion section in the water. Depress the suction valve
(MH-443) on the endoscope and aspirate the water through the endoscope
for 30 seconds.
4. While continuing the immersion and the aspiration, raise and lower the
forceps elevator three times by turning the elevator control lever.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 51

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
AW channel
cleaning adapter
Air/water valve
5. Remove the distal end from the water. Depress the suction valve and
aspirate air for 10 seconds.
6. Turn the suction pump OFF.
Flush the air/water channel with water and air
• To prevent clogging of the air/water nozzle of the endoscope,
flush water into the air/water channel of the endoscope, using
the AW channel cleaning adapter (MH-948) after each
patient procedure.
• Do not apply lubricants to the AW channel cleaning adapter.
Lubricants may cause malfunction of the AW channel
cleaning adapter.
1. Turn the light source ON.
2. Switch “OFF” the airflow regulator on the light source.
3. Detach the air/water valve (MH-438) from the endoscope and place it in the
detergent solution. Attach the AW channel cleaning adapter (MH-948) to the
air/water cylinder of the endoscope.
Figure 5.4
• The air/water valve (MH-438) is to be reprocessed, according
to Chapter 6, “Reprocessing the Accessories”.
• Water may drip from the air/water cylinder when the air/water
valve is detached. The water dripping from the air/water
cylinder is clean (i.e., sterile water in the water container). If
water is dripping from the air/water cylinder, hold the control
section higher than the water container.
4. Immerse the distal end of the insertion section in the water.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
47
Page 52

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
5. Switch the airflow regulator on the light source to maximum output (“HIGH”
or “3”).
6. Depress the button of the AW channel cleaning adapter to flush the air
channel with water from the water container for 30 seconds.
7. Release the button to flush air for 10 seconds.
8. Turn the light source OFF.
Detach accessories from the endoscope
1.
Detach the videoscope cable (MAJ-1430, MAJ-843, or MH-976) from the
electrical connector of the endoscope.
2. Detach the suction tube from the suction connector on the endoscope
connector.
3. Detach the metal tip of the water container (MAJ-901 or MH-884) from the
air/water supply connector on the endoscope connector. Put the metal tip of
the water container tube into the receptacle on the lid of the water container,
as described in the instruction manual for the water container.
Attach the water resistant cap (MH-553)
If the exterior surface of the electrical connector of the
endoscope is scratched, the connection with the water
resistant cap (MH-553) may no longer be waterproof and the
cap’s seal may be damaged. If the electrical connector is
damaged, send the endoscope to Olympus for repair.
The water resistant cap can be attached in two different
positions. Attach the water resistant cap to the endoscope as
shown in Figure 5.5 to allow the endoscope and cap to lay
properly in the reprocessing basin.
48
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 53

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
2.
Rotate
Rotate
4.
4.
Attachment
completed
(b) When using KC-10/TD-20(a) When using an Olympus automated endoscope
reprocessor or an Olympus reprocessing
equipment other than KC-10/TD-20.
2.
Mark 2 (yellow)
Water resistant
cap
Mark 2 (yellow)
Attachment
completed
Figure 5.5
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
49
Page 54

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Pin
Groove
1. Confirm that the exterior surface of the electrical connector is free from
scratches.
2. When using an Olympus automated endoscope reprocessor, or Olympus
reprocessing equipment other than the KC-10/TD-20, align the OER/ETD/
EW characters or the EW characters on the water resistant cap with mark 2
on the electrical connector housing. When using the KC-10/TD-20, align the
KC/TD characters on the cap with mark 2 on the housing.
3. Align the pins on the electrical connector with the grooves on the cap.
Figure 5.6
4. Attach the cap to the electrical connector by pushing in and rotating the cap
clockwise until it stops (approximately 45 degrees).
50
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 55

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Leakage tester (MB-155)
Maintenance unit (MU-1)
(Sold separately. Refer to
its instruction manual.)
Leakage tester connector
Connector cap
5.3 Leakage testing of the endoscope
Equipment needed
Prepare the following equipment.
• Water for cleaning (Refer to Section 3.2) • Detergent solution (Refer to Section 3.3)
• Clean, large basins
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
Detach the endoscope from the light source
1. Detach the endoscope from the light source.
2. Transport the endoscope to the reprocessing area. Use a covered container
if required by local policy.
3. Detach the AW channel cleaning adapter (MH-948), the suction valve
(MH-443), and the biopsy valve (MB-358) from the endoscope and place
them in the detergent solution.
Do not touch the light guide of the endoscope connector
immediately after detaching it from the light source because it
is extremely hot. Injury may result.
The AW channel cleaning adapter, the suction valve, and the
biopsy valve are to be reprocessed according to Chapter 6,
“Reprocessing the Accessories”.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
4. When using the single use biopsy valve (MAJ-1555), detach the single use
biopsy valve from the endoscope and discard it.
51
Page 56

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Figure 5.7
• Remove the single use biopsy valve from the instrument
channel port after breaking off its lever. Otherwise, it may
spray patient debris or fluids, and it may pose an infection
control risk.
• Do not reuse the single use biopsy valve. Reusing the single
use biopsy valve could pose an infection control risk and
cause malfunction. After use, dispose of it in an appropriate
manner.
52
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 57

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Perform the leakage test
• If you identify a leak during leakage testing, remove the
endoscope from the water with both the water resistant cap
(MH-553) and the leakage tester (MB-155) still attached.
Contact Olympus regarding instructions for reprocessing a
leaking endoscope in preparation for returning the
endoscope to Olympus for repair.
• Do not attach/detach the water resistant cap or the leakage
tester while immersed. Attaching/detaching under water
could allow the water to enter the endoscope, resulting in
endoscope damage.
• When attaching the connector cap of the leakage tester to
the venting connector of the water resistant cap, make sure
that both the connector cap and the venting connector are
thoroughly dry. Water on the surface of either component
may enter the endoscope and could cause endoscope
damage.
• When attaching the connector cap of the leakage tester to
the venting connector of the water resistant cap, push on and
rotate the connector cap clockwise fully until it stops. If it is
not fully and properly attached, the interior of the endoscope
will not be properly pressurized and accurate leakage testing
will be impossible.
• Detach the leakage tester from the maintenance unit (MU-1)
or the light source before detaching the leakage tester from
the water resistant cap. If the leakage tester is detached from
the water resistant cap before detaching the leakage tester
from the maintenance unit or the light source, the air
pressure inside the endoscope will not vent properly. This
may damage the endoscope.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
53
Page 58

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Pin
Connector cap
1. Fill a clean, large basin with the water referred to in Section 3.2.
2. Attach the leakage tester connector of the leakage tester (MB-155) to the
output socket of the maintenance unit (MU-1) or the light source. Turn the
maintenance unit or the light source ON. Set the light source’s airflow
regulator switch to its maximum level.
3. Depress the pin located inside the connector cap of the leakage tester and
listen to confirm that air is emitted from the connector cap.
Figure 5.8
4. Confirm that both the connector cap of the leakage tester and the venting
connector of the water resistant cap are dry. If not, dry with a clean, lint free
cloth. Attach the connector cap to the venting connector by pushing on and
rotating clockwise until it stops.
5. With the leakage tester attached, immerse the endoscope in the water and
observe for approximately 30 seconds while deflecting the bending section
of the endoscope by turning the endoscope’s UP/DOWN and RIGHT/LEFT
angulation control knobs, and while raising and lowering the forceps
elevator by moving the endoscope’s elevator control lever. Confirm that
there is no location on the endoscope from which a continuous series of air
bubbles emerge.
54
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 59

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Air bubbles
Channel opening
Bending section
• A continuous series of air bubbles emerging from any
location on the endoscope indicates a leak at that location. If
there is a leak in the instrument channel or suction channel of
the endoscope, a continuous series of air bubbles will
emerge from one or more channel openings (e.g., distal end,
suction connector, suction cylinder, instrument channel port)
on the submerged endoscope.
Figure 5.9
• During the leakage test, the covering of the bending section
will expand as the air pressure inside the endoscope
increases. This is normal.
6. Remove the endoscope from the water with the leakage tester still attached.
7. Turn the maintenance unit or the light source OFF.
8. Detach the leakage tester from the maintenance unit or the light source.
9. Wait 30 seconds, or until the covering of the bending section contracts to its
pre-expansion size. Detach the leakage tester from the water resistant cap.
10. Thoroughly dry the leakage tester using a clean, lint-free cloth.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
55
Page 60

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Channel cleaning
brush (BW-20T)
1
Suction cleaning
adapter (MH-856)
Channel plug
(MH-944)
Injection tube
(MH-946)
Single use channel
cleaning brush
(BW-201T)
1
Single use soft brush
(MAJ-1888)
Suction pump
(KV-4, KV-5, SSU-2)
and Tube
Single use
combination cleaning
brush (BW-412T)
1
Single use channel-opening
cleaning brush
(MAJ-1339)
1
5.4 Manually cleaning the endoscope and
accessories
Prepare the following equipment.
Equipment needed
• Water for cleaning (Refer to Section 3.2) • Detergent solution (Refer to Section 3.3)
• Clean, soft brush(s) • Clean lint-free cloths
• Clean sponge(s) • Clean 30 ml (30 cc) syringe(s)
• Clean, large basins
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
1 Prepare either a single use combination cleaning brush (BW-412T) or a set of channel cleaning
brush (BW-20T) and single use channel-opening cleaning brush (MAJ-1339) or single use
channel cleaning brush (BW-201T) and single use channel-opening cleaning brush (MAJ-1339).
56
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 61

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Objective lens
Air/water nozzle
opening
Clean the external surface
1.
Fill a clean, large basin with the detergent solution at the concentration
recommended by the detergent manufacturer.
2. Immerse the endoscope in the detergent solution.
3. Thoroughly brush or wipe all external surfaces of the endoscope, using
clean lint-free cloths, brushes, or sponges. Pay particular attention to the air/
water nozzle opening and the objective lens on the distal end of the
insertion section, and ensure that all surfaces of the distal end are
thoroughly cleaned.
Figure 5.10
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
57
Page 62

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Elevator
control lever
Brush the forceps elevator
To avoid splashing the detergent solution when the brush is
pulled out from the endoscope, keep the endoscope
immersed in the detergent solution while brushing.
When brushing the forceps elevator and the forceps elevator
recess, gently use a single use channel-opening cleaning
brush (MAJ-1339) or a single use combination cleaning
brush (BW-412T) and single use soft brush (MAJ-1888).
Do not use a stiff brush or brush with excessive force even
with the brush MAJ-1339 or BW-412T and MAJ-1888.
Using a stiff brush or brushing with excessive force may
damage the distal end of the endoscope and cause the
endoscope to leak.
1. Lower the forceps elevator by turning the elevator control lever in the
opposite direction of the “ U” direction until the forceps elevator stops.
Perform the following brushing in the detergent solution.
Figure 5.11
58
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 63

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Guide-wire locking groove
Forceps elevator recess
a
b
Elevator
control lever
2. Straighten the bending section of the endoscope. Brush the forceps elevator
including the guidewire-locking groove and the forceps elevator recess with
the single use channel-opening cleaning brush (MAJ-1339) or the single use
combination cleaning brush (BW-412T) as follows:
a) Insert the brush into the forceps elevator recess along the forceps
elevator (insert the brush into the instrument channel) until the brush
handle touches the distal end of the endoscope and pull the brush out of
the forceps elevator recess. Repeat the insertion and pulling out three
times;
b) Brush the guidewire-locking groove;
Figure 5.12
3. Raise the forceps elevator by turning the elevator control lever in the “ U”
direction until the forceps elevator stops.
Figure 5.13
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
59
Page 64

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
a
b
Forceps elevator
Forceps
elevator recess
4. While holding the distal end, brush the forceps elevator and the forceps
elevator recess with the single use channel-opening cleaning brush
(MAJ-1339) or the single use combination cleaning brush (BW-412T) as
follows:
a) Insert the brush into the forceps elevator recess along the back of the
forceps elevator until the distal end of the brush touches the bottom of
the forceps elevator recess and pull the brush out of the forceps elevator
recess;
b) Insert the brush into the forceps elevator recess, rotate the brush one
full revolution, and pull the brush out of the forceps elevator recess.
60
Figure 5.14
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 65

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction channel
Instrument channel
Instrument channel port
Suction cylinder
5. Operate the elevator control lever to lower and raise the forceps elevator in
the detergent solution three times.
6. Clean the bristles of the brush in the detergent solution using your gloved
fingertips to remove any debris.
7. Lower the forceps elevator by turning the elevator control lever in the
opposite direction of the “ U” direction until the forceps elevator stops.
8. Brush the distal end of the endoscope except the forceps elevator and the
forceps elevator recess, using the single use channel-opening cleaning
brush (MAJ-1339) or the single use combination cleaning brush (BW-412T),
until no debris is observed upon inspection of the distal end of the
endoscope.
Brush the channels
• Be sure to thoroughly brush the inside of the instrument
channel, the instrument channel port, the suction channel,
and the suction cylinder of the endoscope. Insufficient
brushing may pose an infection control risk.
Figure 5.15
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
• To avoid splashing the detergent solution when the brush is
pulled out from the endoscope, keep the endoscope
immersed in the detergent solution while brushing.
61
Page 66

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction connector
Open end
• The channel cleaning brush (BW-20T) is consumable. The
single use channel cleaning brush (BW-201T) and the single
use combination cleaning brush (BW-412T) are for single
use. Repeated usage of these brushes may cause the brush
head to become bent or kinked, which could cause it to come
off during use. Confirm that the brush is free from any
damage or other irregularities before and after use. If a piece
of the brush comes off inside the endoscope channel,
immediately retrieve it. Confirm that no parts remain inside
either the instrument channel or the suction channel of the
endoscope by carefully passing a new brush through both
channels. Any part left in the channels can drop into the
patient during a subsequent patient procedure. Depending
on the location of the missing part, the part may not be
retrievable by passing a new brush. In this case, contact
Olympus.
• Do not attempt to pass the channel cleaning brush or the
single use channel cleaning brush backwards – i.e., by
inserting the brush directly into the open end of the
instrument channel at the distal end of the endoscope’s
insertion section or directly into the suction connector on the
endoscope connector. It may get caught, making retrieval
impossible.
62
Figure 5.16
• Do not coil the insertion section or the universal cord of the
endoscope with a diameter of less than 40 cm. If the
diameter is less than 40 cm, it may be difficult to pass the
brush completely through the channels.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 67

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
45
Suction cylinder
Distal
end
Instrument channel
Suction channel
Suction cylinder
Opening
Brush from the suction cylinder to the distal end of the
insertion section (This brushes the instrument channel in the
insertion section and the suction channel in the control section.)
When withdrawing the channel cleaning brush (BW-20T) or
the single use combination cleaning brush (BW-412T) from
the suction cylinder of the endoscope, make sure that the
shaft of the brush does not rub against the cylinder opening.
Excessive rubbing of the brush against the cylinder edge
may damage the cylinder.
1. Straighten the bending section of the endoscope. Grip the channel cleaning
brush (BW-20T), the channel cleaning brush part of the single use
combination cleaning brush (BW-412T), or the single use channel cleaning
brush (BW-201T) at a point 3 cm from the bristles.
2. Insert the brush at a 45 angle into the opening located in the side wall of
the suction cylinder. Using short strokes, feed the brush through the
instrument channel until it emerges from the distal end of the endoscope’s
insertion section.
Figure 5.17
Figure 5.18
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
63
Page 68

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction cylinder
Suction channel
3. Inspect whether there is debris on the bristles when the brush emerges from
the distal end. Clean the bristles in the detergent solution using your gloved
fingertips to remove any debris.
4. Carefully pull the brush back through the channel and out of the suction
cylinder.
5. Inspect whether there is debris on the bristles when the brush emerges from
the suction cylinder. Clean the bristles in the detergent solution using your
gloved fingertips to remove any debris.
6. Repeat Step 2 through 5 until no debris is observed upon inspection of the
brush.
Brush from the suction cylinder to the endoscope
connector (This brushes the suction channel in the universal
cord and the endoscope connector.)
1. Grip the channel cleaning brush (BW-20T), the channel cleaning brush part
of the single use combination cleaning brush (BW-412T), or the single use
channel cleaning brush (BW-201T) at a point 3 cm from the bristles.
2. Insert the brush straight into the suction cylinder. Using short strokes, feed
the brush through the suction channel until it emerges from the suction
connector on the endoscope connector.
Figure 5.19
64
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 69

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction cylinder
Figure 5.20
3. Inspect whether there is debris on the bristles when the brush emerges from
the suction connector. Clean the bristles in the detergent solution using your
gloved fingertips to remove any debris.
4. Carefully pull the brush back through the channel and out of the suction
cylinder.
5. Inspect whether there is debris on the bristles when the brush emerges from
the suction cylinder. Clean the bristles in the detergent solution using your
gloved fingertips to remove any debris.
6. Repeat Step 2 through 5 until no debris is observed upon inspection of the
brush.
The channel cleaning brush and the single use channel
cleaning brush will be used later to brush the accessories
described in Section 6.1.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
65
Page 70

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction cylinder
Brush the suction cylinder
When inserting the single use channel-opening cleaning
brush (MAJ-1339) or the channel-opening cleaning brush
part of the single use combination cleaning brush (BW-412T)
into the suction cylinder, do not forcibly insert the brush
beyond the mid portion of the brush, to prevent the brush
from getting stuck.
1. Insert the single use channel-opening cleaning brush (MAJ-1339) or the
channel-opening cleaning brush part of the single use combination cleaning
brush (BW-412T) into the suction cylinder, until half of the brush section is
inserted.
Figure 5.21
2. Rotate the inserted brush one full revolution.
3. Pull the brush out of the cylinder.
4. Inspect whether there is debris on the bristles. Clean the bristles in the
detergent solution using your gloved fingertips to remove any debris.
5. Repeat Step 1 through 4 until no debris is observed upon inspection of the
brush.
66
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 71

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Instrument
channel port
Brush the instrument channel port
1. Insert the single use channel-opening cleaning brush (MAJ-1339) or the
channel-opening cleaning brush part of the single use combination cleaning
brush (BW-412T) into the instrument channel port, until the brush handle
touches the channel opening.
Figure 5.22
2. Rotate the inserted brush one full revolution.
3. Pull the brush out of the instrument channel port.
4. Inspect whether there is debris on the bristles. Clean the bristles in the
detergent solution using your gloved fingertips to remove any debris.
5. Repeat Step 1 through 4 until no debris is observed upon inspection of the
brush.
6. Dispose of the single use channel-opening cleaning brush, according to
Section 8.3.
7. Remove the endoscope from the detergent solution.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
67
Page 72

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction connector
Suction tube
Suction pump
Aspirate detergent solution through the instrument channel
and the suction channel
Monitor the suction bottle on the suction pump carefully to
ensure that it does not overflow.
1. Attach the suction cleaning adapter (MH-856) to the instrument channel
port.
2. Attach the suction tube from the suction pump to the suction connector on
the endoscope connector. Turn the suction pump ON.
Figure 5.23
68
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 73

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Suction cleaning
adapter
Connecting end
Weighted end
Suction pump
Instrument
channel port
Suction cylinder
Suction cylinder
3. Confirm that the forceps elevator is lowered, and immerse both the distal
end of the insertion section and the weighted end of the suction cleaning
adapter in the detergent solution.
Figure 5.24
4. Cover the suction cylinder of the endoscope with your gloved finger and
aspirate the detergent solution through the instrument channel and the
suction channel of the endoscope for approximately 30 seconds.
Figure 5.25
5. While continuing the immersion and the aspiration, raise and lower the
forceps elevator three times by turning the elevator control lever.
6. Turn the suction pump OFF.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
69
Page 74

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
7. Detach the suction tube and the suction cleaning adapter from the
endoscope.
The suction cleaning adapter is to be reprocessed according
to Chapter 6, “Reprocessing the Accessories”.
Brush and flush the forceps elevator recess
• To avoid splashing the detergent solution when the brush is
pulled out from the endoscope, keep the endoscope
immersed in the detergent solution while brushing.
• After use, carefully check that the single use soft brush
(MAJ-1888) head and/or shaft has not fallen off inside of the
endoscope. If this inspection determines that the brush head
and/or shaft has come off during cleaning, check around the
forceps elevator and inside of the instrument channel, etc.,
then retrieve it. The brush head remaining in the groove
around the forceps elevator or inside of the endoscope can
drop into the patient’s body during a subsequent procedure
and/or may damage the equipment. Also, an EndoTherapy
accessory may not be withdrawn because the forceps
elevator may become immobile during the procedure.
Olympus endorsed brushes can be used instead of the single
use soft brush (MAJ-1888).
1. Confirm that the forceps elevator is lowered, and straighten the bending
section of the endoscope.
70
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 75

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Groove
Forceps elevator
Guidewire-locking groove
b
b
a
2. Brush the forceps elevator and the forceps elevator recess with the single
use soft brush (MAJ-1888) as follows, keeping the distal end of the
endoscope immersed in the detergent solution:
a) Brush the guidewire-locking groove with three strokes;
b) Brush both grooves with three strokes each.
Figure 5.26
3. Clean the bristles of the brush gently with your fingertips in the detergent
solution.
4. Raise the forceps elevator by turning the elevator control lever in the “ U”
direction until the forceps elevator stops.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
71
Page 76

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Forceps elevator
Forceps elevator
Groove
5. Brush the whole back side of the forceps elevator with the brush
(MAJ-1888) three times, keeping the distal end of the endoscope immersed
in the detergent solution.
Figure 5.27
6. Clean the bristles of the brush gently with your fingertips in the detergent
solution.
7. Insert the brush head slowly into the groove under the forceps elevator until
the end of the brush touches the wall behind the elevator. (See Figure 5.28)
To avoid damage to the brush, make sure that the brush is
gently inserted into the groove under the forceps elevator
shown as “Groove” in Figure 5.28.
72
Figure 5.28
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 77

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Detergent solution
Syringe
Insert
Forceps elevator recess
8. Rotate the inserted brush three revolutions while ensuring the end of the
brush contacting the wall behind the forceps elevator (see Figure 5.29)
while keeping the distal end of the endoscope immersed in the detergent
solution.
Figure 5.29
9. Pull the brush out slowly and gently clean the bristles in the detergent
solution with your fingertips.
10. Operate the elevator control lever to lower and raise the forceps elevator
three times, keeping the distal end of the endoscope immersed in the
detergent solution.
11. With the forceps elevator raised, insert the tip of the 30 ml syringe into the
interior of the forceps elevator recess in the detergent solution, and flush the
interior of the recess with 30 ml of the detergent solution.
Figure 5.30
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
73
Page 78

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Syringe
Forceps elevator
Syringe
Detergent solution Detergent solution
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
Detergent solution
Syringe
Insert
Forceps elevator recess
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
Figure 5.31
12. Lower the forceps elevator by turning the elevator control lever. Insert the tip
of the 30 ml syringe into the interior the forceps elevator recess in the
detergent solution, and flush the interior of the recess with 30 ml of the
detergent solution.
Figure 5.32
74
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 79

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Syringe
Forceps elevator
Syringe
Detergent solution Detergent solution
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
Figure 5.33
13. Repeat Step 7 through 12.
14. Inspect whether there is debris on the forceps elevator and in the forceps
elevator recess while raising and lowering the forceps elevator, and repeat
brushing and/or flushing the forceps elevator and the forceps elevator
recess until no debris is observed upon the inspection.
15. Dispose of the brush in an appropriate manner.
If the single use soft brush (MAJ-1888) is not properly
disposed of, it could pose a contamination and infection
control risk.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
75
Page 80

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Instrument
channel port
Channel
plug
Biopsy
valve cap
Plug frame
Air/water plug
Air/water cylinder
Switch 1
Suction cylinder
Suction plug
c
d
b
a
Control section
Flush the air/water channel with detergent solution
1.
Attach the biopsy valve cap of the channel plug (MH-944) to the instrument
channel port of the endoscope.
Figure 5.34
2. Attach the channel plug to the air/water and suction cylinders of the
endoscope as follows:
a) Insert the air/water plug of the channel plug into the air/water cylinder;
b) Insert the suction plug of the channel plug into the suction cylinder;
c) Push the plug frame towards the control section of the endoscope, until
the plug frame contacts the control section;
d) While pushing the plug frame towards the control section, slide the plug
frame towards switch 1, until the plug frame stops. The channel plug
should now be firmly locked in place.
76
Figure 5.35
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 81

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Air pipe
Injection tube
Suction
channel port
Suction
connector
Suction
channel tube
Air and water
supply connector
Connector plug
Air/water
channel port
Suction port
Air pipe port
b
a
c
Air/water channel port
Suction channel port
Syringe
Air/water
channel tube
3. Attach the injection tube (MH-946) to the endoscope connector, as follows:
a) Attach the connector plug of the injection tube to the air and water
supply connectors on the endoscope connector;
b) Attach the air pipe port of the injection tube to the air pipe on the
endoscope connector;
c) Attach the suction channel tube of the injection tube to the suction
connector on the endoscope connector.
Figure 5.36
4. Immerse the suction port of the injection tube in the detergent solution.
5. Attach a clean 30 ml syringe to the air/water channel port of the injection
tube.
Figure 5.37
6. Flush the air/water channel with 90 ml of the detergent solution by pumping
the syringe at least three times.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
77
Page 82

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Air/water channel port
Suction channel port
Syringe
Air/water
channel tube
Immerse the endoscope and accessories in detergent
solution
1.
Wipe all external surfaces of the endoscope, the channel plug (MH-944),
and the injection tube (MH-946) to remove debris while they are immersed
in the detergent solution, using a clean, lint-free cloth, brush or sponge.
2. Leave the endoscope with attached accessories immersed in the detergent
solution, according to the instructions of the detergent manufacturer.
3. Remove the endoscope with attached accessories from the detergent
solution.
Remove detergent solution from all channels
1.
Fill a clean, large basin with the water referred to in Section 3.2.
2. Immerse the endoscope with attached accessories in the water and gently
agitate them to thoroughly rinse.
3. Immerse the suction port of the injection tube (MH-946) in the water. (See
Figure 5.36)
4. Attach a clean 30 ml syringe to the suction channel port of the injection tube
and flush the suction channel with 90 ml of water (i.e., pump the syringe at
least three times).
Figure 5.38
78
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 83

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
5. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 90 ml of water (i.e., pump the syringe at least
three times). (See Figure 5.37)
6. Remove the endoscope with attached accessories from the water.
7. Place them in a clean basin and cover the distal end and the control section
of the endoscope with a clean, lint-free cloth(s) to prevent splashing from
the channel openings.
8. Attach the syringe to the suction channel port of the injection tube and flush
the suction channel with 90 ml of air. (See Figure 5.38)
9. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 90 ml of air. (See Figure 5.37)
10. Remove the cloth(s) from the endoscope.
11. Detach the channel plug and the injection tube from the endoscope.
Dry external surfaces
1.
Dry the external surfaces of the endoscope, the channel plug, and the
injection tube by wiping with a clean, lint-free cloth(s).
2. Inspect all items for residual debris. Should any debris remain, repeat the
entire cleaning procedure until all debris is removed.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
79
Page 84

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
5.5 Manually disinfecting the endoscope and
accessories
Equipment needed
Prepare the following equipment.
• Disinfectant solution (Refer to Section 3.4) • Clean 30 ml (30 cc) syringe(s)
• Sterile 30 ml (30 cc) syringe(s) • Clean, large basins with tight-fitting lids
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
Preparation
1.
Fill a clean, large basin with the disinfectant solution. Check the
concentration of the disinfectant solution according to the manufacturer’s
instructions to verify that the concentration is above the recommended
minimum.
2. Immerse the endoscope in the disinfectant solution.
3. Attach the channel plug (MH-944) and the injection tube (MH-946) to the
endoscope and immerse in the disinfectant solution. (See Figure 5.34, 5.35,
and 5.36)
Flush all channels and around the forceps elevator with
disinfectant solution
• Make sure that the disinfectant solution contacts all internal
channel surfaces of the endoscope and accessories by
completely removing all air bubbles from all channels. Air
bubbles may inhibit disinfection of the channel’s surfaces.
When filling the channels with the disinfectant solution, flush
until no more air bubbles are seen exiting the channel
openings.
• Make sure that no air bubbles in the disinfectant solution
when flushing using the syringe. Air bubbles may inhibit
disinfection of the parts that are flushed by the syringe.
80
Removal of air bubbles can be facilitated by forcefully
flushing the disinfectant solution through the channels.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 85

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Disinfectant solution
Syringe
Insert
Forceps elevator recess
1. Confirm that the suction port of the injection tube (MH-946) is immersed in
the disinfectant solution.
2. Attach a clean 30 ml syringe to the suction channel port of the injection tube
and forcefully flush the suction channel with 180 ml of the disinfectant
solution – i.e., by pumping the syringe at least six times. Confirm that no air
bubbles exit the distal end of the endoscope’s insertion section during the
sixth flush. If air bubbles still exit, flush the channel with the disinfectant
solution until no air bubbles exit. (See Figure 5.38)
3. Move the syringe to the air/water channel port of the injection tube and
forcefully flush the air/water channel with 180 ml of the disinfectant solution.
Confirm that no air bubbles exit the distal end during the sixth flush. If air
bubbles still exit, flush the channel with the disinfectant solution until no air
bubbles exit. (See Figure 5.37)
4. Remove the biopsy valve cap of the channel plug (MH-944) from the
instrument channel port of the endoscope, remaining the channel plug to be
attached to the air/water and suction cylinders of the endoscope. Forcefully
flush the instrument channel with 180 ml of the disinfectant solution, using
the 30 ml syringe - i.e., fill the syringe with the disinfectant solution without
air, put the distal end of the syringe in the instrument channel port in the
disinfectant solution, and forcefully flush at least six times, minimizing
disinfectant solution leakage from the port. Confirm that no air bubbles exit
the distal end of the endoscope’s insertion section during the sixth flush. If
air bubbles still exit, flush the channel with the disinfectant solution until no
air bubbles exit.
5. With the forceps elevator raised, insert the tip of the 30 ml syringe into the
interior of the forceps elevator recess in the disinfectant solution, and flush
the interior of the recess with 60 ml of the disinfectant solution.
Figure 5.39
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
81
Page 86

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Syringe
Forceps elevator
Syringe
Disinfectant solution Disinfectant solution
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
Disinfectant solution
Syringe
Insert
Forceps elevator recess
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
Figure 5.40
6. Lower the forceps elevator by turning the elevator control lever. Insert the tip
of the 30 ml syringe into the interior the forceps elevator recess in the
disinfectant solution, and flush the interior of the recess with 60 ml of the
disinfectant solution.
Figure 5.41
82
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 87

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Syringe
Forceps elevator
Syringe
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
Disinfectant solution
Disinfectant solution
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
Figure 5.42
7. Turn the elevator control lever to raise and lower the forceps elevator three
times, keeping the distal end of the endoscope immersed in the disinfectant
solution.
8. Forcefully flush the instrument channel with 90ml of the disinfectant solution,
using the 30ml syringe - i.e., fill the syringe with the disinfectant solution
without air, put the distal end of the syringe in the instrument channel port in
the disinfectant solution, and forcefully flush at least three times, minimizing
disinfectant solution leakage from the port. Confirm that no air bubbles exit
the distal end of the endoscope’s insertion section during the third flush. If
air bubbles still exit, flush the channel with the disinfectant solution until no
air bubbles exit.
9. Repeat Step 5 through 8 above.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
83
Page 88

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Immerse the endoscope and accessories in disinfectant
solution
Make sure that the disinfectant solution contacts all external
surfaces of the endoscope and accessories. If accessories,
such as the injection tube, remain attached to the endoscope
during disinfection, the disinfectant solution cannot
adequately contact the mated surfaces between the
endoscope and the accessory. Detach the channel plug and
the injection tube from the endoscope while immersed. If the
endoscope and accessories are not completely immersed,
any protruding section(s) of the device(s) will not be
adequately disinfected. Always check to confirm that the
endoscope and accessories are completely below the
surface of the disinfectant solution.
Do not immerse the endoscope and accessories in the
disinfectant solution for a longer contact time, at a higher
temperature, or at a greater concentration than
recommended by the disinfectant manufacturer. Such
immersion may cause damage to the endoscope and
accessories.
1. While immersed, detach the channel plug (MH-944) and the injection tube
(MH-946) from the endoscope. Confirm that the endoscope and all
accessories are completely submerged in the disinfectant solution.
2. Confirm that there are no air bubbles on the surfaces of the endoscope and
accessories. If air bubbles adhere to the surfaces, wipe them away using
your gloved finger or a clean, lint-free cloth.
3. Cover the basin of the disinfectant solution with a tight-fitting lid to minimize
the diffusion of disinfectant vapors.
4. Leave the endoscope, the channel plug, and the injection tube immersed in
the disinfectant solution according to the instructions of the disinfectant
manufacturer. Confirm the recommended contact time, temperature, and
concentration. Use a clock or timer to accurately measure the disinfection
contact time.
84
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 89

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Air pipe
Injection tube
Suction
channel port
Suction
connector
Suction
channel tube
Air and water
supply connector
Connector plug
Air/water
channel port
Suction port
Air pipe port
b
a
c
Remove the endoscope and accessories from disinfectant
solution
1.
Attach the channel plug (MH-944) and the injection tube (MH-946) to the
endoscope. (See Figure 5.34 and 5.35)
Figure 5.43
2. Remove the suction port of the injection tube from the disinfectant solution.
3. Attach a sterile 30 ml syringe to the suction channel port of the injection tube
and flush the suction channel with 90 ml of air – i.e., pump the syringe at
least three times. (See Figure 5.38)
4. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 90 ml of air. (See Figure 5.37)
5. Remove the endoscope with attached accessories from the disinfectant
solution.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
85
Page 90

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
5.6 Rinsing the endoscope and accessories
following disinfection
After rinsing, thoroughly dry the channels of the endoscope
and accessories. Otherwise, bacteria may proliferate in the
channels and pose an infection control risk.
Equipment needed
Prepare the following equipment.
• Rinse water (Refer to Section 3.5)
• Sterile cotton swabs
• Sterile, large basins
(size: 40 (W) × 40 (H) × 25 (D) cm or more)
• 70% ethyl or 70% isopropyl alcohol (Refer to Section
3.6)
1
1
• Sterile lint-free cloths
• Sterile 30 ml (30 cc) syringe(s)
• Sterile, small basins with tight-fitting lids
(size: 25 (W) × 10 (H) × 25 (D) cm or more)
1
1
1 Following high-level disinfection, it is very important not to recontaminate the endoscope and
accessories with potentially infectious microorganisms. When rinsing and drying the endoscope
and accessories after high-level disinfection, the use of sterile equipment (e.g., basin, cloths,
syringes, etc.) is recommended. If sterile equipment is not available, use clean equipment that
does not recontaminate the endoscope and accessories with potentially infectious
microorganisms. Consult with your hospital’s infection control committee regarding local policies
or requirements regarding reprocessing equipment.
1
86
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 91

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Rinse the endoscope and accessories
Use appropriate rinse water as instructed in Section 3.5. If nonsterile water is
used for rinsing the endoscope and accessories, flush the endoscope and
accessories with 70% ethyl or 70% isopropyl alcohol after rinsing, according to
the procedures described below.
• Some national or professional guidelines recommend
flushing all channels of the endoscope with 70% ethyl or 70%
isopropyl alcohol regardless of whether sterile or nonsterile
water is used to rinse the endoscope. Check with your local
infection control committee for advice.
• Flushing the interior and recessed parts of the endoscope
and accessories with alcohol facilitates drying. Olympus
recommends the use of alcohol.
1. Fill a sterile, large basin with the rinse water referred to in Section 3.5.
2. Immerse the endoscope with attached accessories in the rinse water.
Detach the channel plug (MH-944) and injection tube (MH-946) from the
endoscope.
3. Wipe all external surfaces of the endoscope and accessories, using a
sterile, lint-free cloth.
4. Attach the channel plug and the injection tube to the endoscope. Immerse
the suction port of the injection tube in the rinse water. (See Figure 5.34,
5.35, and 5.43)
5. Attach a sterile 30 ml syringe to the suction channel port of the injection tube
and flush the suction channel with 90 ml of the rinse water – i.e., pump the
syringe at least three times. (See Figure 5.38)
6. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 90 ml of the rinse water. (See Figure 5.37)
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
87
Page 92

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Rinse water
Syringe
Insert
Forceps elevator recess
Forceps elevator
Syringe
Forceps elevator
Syringe
Rinse water Rinse water
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
7. Raise the forceps elevator by turning the elevator control lever. Insert the tip
of the 30 ml syringe into the interior of the forceps elevator recess in the
rinse water, and flush the interior of the recess with 30 ml of the rinse water.
Figure 5.44
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
Figure 5.45
88
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 93

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Syringe
Insert
Forceps elevator recess
Rinse water
Forceps elevator
Syringe
Forceps elevator
Syringe
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
Rinse water
Rinse water
8. Lower the forceps elevator by turning the elevator control lever. Insert the tip
of the 30 ml syringe into the interior the forceps elevator recess in the rinse
water, and flush the interior of the recess with 30 ml of the rinse water.
Figure 5.46
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
Figure 5.47
9. Turn the elevator control lever to raise and lower the forceps elevator three
times, keeping the distal end of the endoscope immersed in the rinse water.
10. Repeat Step 1 through 9 above for the necessary number of times, following
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
the rinsing method described in the disinfectant solution manual.
89
Page 94

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Channel plug
Suction pump
Suction
connector
11. By turning the elevator control lever, put the forceps elevator in intermediate
position of the range of movement.
12. Remove the endoscope with attached accessories from the rinse water and
place them in a sterile basin.
13. Cover the distal end and the control section of the endoscope with a sterile,
lint-free cloth(s) to prevent splashing from the channel openings.
14. Attach the syringe to the suction channel port of the injection tube and flush
the suction channel with 90 ml of air. (See Figure 5.38)
15. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 90 ml of air. (See Figure 5.37)
16. Remove the cloth(s) from the endoscope.
17. Detach only the injection tube from the endoscope.
18. Attach a sterile suction tube from the suction pump to the suction connector
on the endoscope connector. Turn the suction pump ON and aspirate air for
at least 15 seconds. Air will flow through the instrument channel and the
suction channel of the endoscope.
90
Figure 5.48
19. While continuing the aspiration, raise and lower the forceps elevator three
times by turning the elevator control lever.
20. Turn the suction pump OFF.
21. Detach the suction tube and the channel plug from the endoscope.
22. Thoroughly dry the external surfaces of the endoscope, the channel plug,
and the injection tube, by wiping with a sterile, lint-free cloth(s).
23. Thoroughly dry the inside of the suction cylinder, the air/water cylinder, the
instrument channel port of the endoscope, and forceps elevator recess,
using a sterile cotton swab(s).
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 95

Alcohol flush
Syringe
Insert
Forceps elevator recess
1.
Fill a sterile small basin with the alcohol referred to in Section 3.6.
2. Attach the channel plug (MH-944) and the injection tube (MH-946) to the
endoscope. Immerse the suction port of the injection tube in the alcohol.
(See Figure 5.34, 5.35 and 5.43)
3. Cover the distal end and the control section of the endoscope with a sterile,
lint-free cloth(s) to prevent splashing alcohol from the channel openings.
4. Attach a sterile 30 ml syringe to the suction channel port of the injection tube
and flush the suction channel with 90 ml of the alcohol – i.e., pump the
syringe at least three times. (See Figure 5.38)
5. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 30 ml of the alcohol. (See Figure 5.37)
6. Raise the forceps elevator by turning the elevator control lever. Insert the tip
of the 30 ml syringe into the interior of the forceps elevator recess under
covering the cloth(s), and flush the interior of the recess with 30 ml of
alcohol.
Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Figure 5.49
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syinge over the interior of the
recess while the syringe contacts the surface of the
endoscope.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
91
Page 96

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Syringe
Forceps elevator
Syringe
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
Syringe
Insert
Forceps elevator recess
Figure 5.50
7. Lower the forceps elevator by turning the elevator control lever. Insert the tip
of the 30 ml syringe into the interior the forceps elevator recess under
covering the cloth(s), and flush the interior of the recess with 30 ml of the
alcohol.
Figure 5.51
When using a luer-lock type syringe, the tip of the syringe
may not be fit into the interior of the forceps elevator recess.
In this case, hold the tip of the syringe over the interior of the
recess while the syringe contacts the surface of the
endoscope.
92
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 97

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Forceps elevator
Syringe
Forceps elevator
Syringe
(a) Flushing using a syringe without luer-lock tip. (b) Flushing using a syringe with luer-lock tip.
Figure 5.52
8. Turn the elevator control lever to raise and lower the forceps elevator three
times.
9. Remove the suction port of the injection tube from the alcohol.
10. Attach the syringe to the suction channel port of the injection tube and flush
the suction channel with 90 ml of air. (See Figure 5.38)
11. Move the syringe to the air/water channel port of the injection tube and flush
the air/water channel with 90 ml of air. (See Figure 5.37)
12. Remove the cloth(s) from the endoscope.
13. Detach the channel plug and the injection tube from the endoscope.
14. Thoroughly dry the external surfaces of the endoscope, the channel plug,
and the injection tube, by wiping with a sterile, lint-free cloth(s).
15. Thoroughly dry the inside of the suction cylinder, the air/water cylinder, the
instrument channel port of the endoscope, and the forceps elevator recess,
using a sterile cotton swab(s).
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
93
Page 98

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
Chain for water-resistant
cap (MAJ-1119)
Water resistant cap
(MH-553)
5.7 Sterilizing the endoscope and accessories
Ethylene oxide gas sterilization of the endoscope and
accessories
• Thoroughly dry the endoscope and accessories before
sterilization.
• All instruments must be properly aerated following ethylene
oxide gas sterilization to remove toxic ethylene oxide
residuals.
• Exceeding the recommended sterilization parameters may
cause damage to the endoscope and/or accessories.
• Detach the water resistant cap (MH-553) from the electrical
connector on the endoscope connector prior to ethylene
oxide gas sterilization. The detached water resistant cap may
remain connected to the endoscope via the chain for
water-resistant cap (MAJ-1119). If the water resistant cap
(MH-553) is attached to the electrical connector during the
ethylene oxide gas sterilization cycle, the air inside the
endoscope will expand and rupture the covering of the
bending section.
94
Figure 5.53
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
Page 99

Chapter 5 Reprocessing the Endoscope (and related reprocessing accessories)
1. Flush and dry all channels of the endoscope, the channel plug (MH-944),
and the injection tube (MH-946), according to the “Alcohol flush” instructions
in Section 5.6.
2. Wipe and dry all external surfaces of the endoscope and accessories, using
a sterile, lint-free cloth(s) moistened with alcohol.
3. Thoroughly dry the inside of the suction cylinder, the air/water cylinder and
the instrument channel port of the endoscope and the forceps elevator
recess, using a sterile cotton swab(s).
4. Detach the water resistant cap (MH-553) from the electrical connector.
5. Seal the endoscope and accessories in individual packaging appropriate for
ethylene oxide gas sterilization, according to your institution’s protocol.
6. Sterilize and aerate the packaged endoscope and accessories, according to
the parameters described in Section 3.7. In addition, always comply with the
instructions of the sterilizer manufacturer.
Steam sterilization (autoclaving) of the accessories
Allow the accessories in the sterile packaging to dry within
the sterilization device, using the device’s prevacuum cycle.
If any water remains in the packaging after the sterilization
cycle, the cycle may have been ineffective. Remove the
accessory from the packaging, thoroughly dry, seal in new
sterile packaging, and sterilize again.
1. Seal the accessories in individual packaging appropriate for steam
sterilization, according to your institution’s protocol.
2. Sterilize the packaged accessories, according to the parameters described
in Section 3.8. In addition, always comply with the instructions of the
sterilizer manufacturer.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
95
Page 100

Chapter 6 Reprocessing the Accessories
Biopsy valve
(MB-358)
Mouthpiece
(MB-142)
Air/water valve
(MH-438)
Suction valve
(MH-443)
AW channel
cleaning adapter
(MH-948)
Suction cleaning
adapter (MH-856)
Channel cleaning
brush (BW-20T)
Injection tube
(MH-946)
1
Channel plug
(MH-944)
1
Single use combination
cleaning brush
(BW-412T)
2
Single use channel
cleaning brush
(BW-201T)
2
Chapter 6 Reprocessing the
Accessories
All accessories (except single-use accessories) must be
cleaned and high-level disinfected or sterilized after each
use, to prevent an infection control risk.
The following accessories are not cleaned or disinfected with the endoscope
during manual cleaning and disinfection of the endoscope. These accessories
must be reprocessed separately, as described in this Chapter.
1 The channel plug (MH-944) and the injection tube (MH-946) are manually cleaned and
2 It is needed when the single use combination cleaning brush (BW-412T) or the single use
96
disinfected with the endoscope during manual cleaning and disinfection of the endoscope, as
described in Chapter 5, “Reprocessing the Endoscope (and related reprocessing accessories)”.
However, in case the endoscope is compatible with an automated endoscope reprocessor (AER)
and these accessories are not compatible with the AER, these accessories must be cleaned and
disinfected manually apart from the endoscope. This chapter also describes how to reprocess
these accessories separate from the endoscope.
channel cleaning brush (BW-201T) has been used to reprocess the endoscope.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL
 Loading...
Loading...