Page 1

Service Manual
Nellcor
TM
Portable SpO2 Patient Monitoring System
Page 2

COVIDIEN, COVIDIEN with logo, the Covidien logo and positive results for life are U.S. and
internationally registered trademarks of Covidien AG. Other brands are trademarks of a Covidien company.
©2014 Covidien. All rights reserved.
Microsoft and Windows CE are registered trademarks of Microsoft Corporation in the United
States and other countries.
The information contained in this manual is the sole property of Covidien and may not be
duplicated without permission. This manual may be revised or replaced by Covidien at any time
and without notice. It is the responsibility of the reader to have the most current applicable
version of this manual. If in doubt, contact Covidien Technical Services.
While the information set forth herein is believed to be accurate, it is not a substitute for the
exercise of professional judgment.
The equipment and software should only be operated and serviced by trained professionals.
Covidien’s sole responsibility with respect to the equipment and software, and its use, is as
stated in the limited warranty provided.
Nothing in this manual shall limit or restrict in any way Covidien’s right to revise or otherwise
change or modify the equipment and software described herein, without notice. In the
absence of an express, written agreement to the contrary, Covidien has no obligation to
furnish any such revisions, changes, or modifications to the owner or user of the equipment
and software described herein.
Page 3

Table of Contents
1 Introduction
1.1 Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
1.2 Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
1.2.1 Safety Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
1.2.2 Explosion, Shock, and Toxicity Hazards . . . . . . . . . . . . . . . . . . . . 1-2
1.2.3 Service Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
1.2.4 Monitoring System Operation and Service . . . . . . . . . . . . . . . . . . 1-4
1.2.5 Patient Monitoring and Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
1.2.6 Monitoring System Readings . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
1.2.7 Sensors, Cables, and Other Accessories . . . . . . . . . . . . . . . . . . . . 1-6
1.2.8 Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
1.2.9 Connections with Other Equipment . . . . . . . . . . . . . . . . . . . . . . . 1-8
1.2.10 Monitoring System Storage, Transport, and Disposal . . . . . . . . . . 1-9
1.3 Obtaining Technical Assistance . . . . . . . . . . . . . . . . . . . . . . . . 1-10
1.3.1 Technical Services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
1.3.2 Related Documents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
1.4 Revision History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11
1.5 Warranty Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11
2 Data Management
2.1 Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
2.2 External Data Communication . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
2.2.1 Real-Time Data Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
2.2.2 Monitoring History Download . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
2.3 Firmware Upgrade . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-18
3 Modification and Testing
3.1 Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
3.2 Required Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
3.3 System Performance Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
3.3.1 Power-On Self-Test (POST) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
3.3.2 Battery Status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
3.3.3 Patient Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
3.3.4 Homecare Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5
3.3.5 Sleep Study Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
3.3.6 Dynamic Passwords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
3.3.7 Date and Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
3.3.8 Wireless Network Connectivity . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
3.4 Operational and Functional Tests . . . . . . . . . . . . . . . . . . . . . . 3-15
3.4.1 General Operation Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-15
iii
Page 4

3.4.2 Functional Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-33
3.5 Verification Check Sheets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-53
3.5.1 Performance, Operation, and Functional Test Results . . . . . . . . . 3-53
4 Troubleshooting
4.1 Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
4.2 Troubleshooting Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
4.2.1 Error Conditions by Category . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
4.2.2 System Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
4.3 Return . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
5 Repair
5.1 Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
5.2 Spare Parts and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
5.3 Required Tools . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
5.4 Battery Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
5.4.1 Remove the Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
5.4.2 Replace the Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
5.5 Disassembly and Reassembly . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
5.5.1 Front and Rear Assembly Replacement . . . . . . . . . . . . . . . . . . . . 5-7
5.5.2 NELL1SR Board Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
5.5.3 Main Board Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
5.5.4 Coin Cell Battery Replacement . . . . . . . . . . . . . . . . . . . . . . . . . 5-12
5.5.5 Wireless Board Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-13
5.5.6 LCD Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-15
5.5.7 PI Cable and Cable Housing Replacement . . . . . . . . . . . . . . . . . 5-16
iv
Page 5

List of Figures
Figure2-1. Communication Settings—Oxinet ............................................. 2-3
Figure2-2. Remote Connectivity Selections ................................................. 2-4
Figure2-3. New Connection Settings .......................................................... 2-5
Figure2-4. Wi-Fi On/Off Setting ................................................................. 2-6
Figure2-5. Wi-Fi On/Off Setting ................................................................. 2-7
Figure2-6. Mini-USB Port ........................................................................... 2-9
Figure2-7. Transfer Data Type .................................................................. 2-10
Figure2-8. Transfer Data by USB .............................................................. 2-10
Figure2-9. Sample Monitoring History Printout ........................................ 2-11
Figure2-10. Bridge Driver Installer Window ................................................ 2-13
Figure2-11. New Hardware Wizard Screen ................................................. 2-14
Figure2-12. Device Manager Button, Hardware Tab ................................... 2-15
Figure2-13. Hardware List in Device Manager Window .............................. 2-16
Figure2-14. Sample Initial USB to UART Bridge Properties Window ............ 2-17
Figure2-15. Baud Rate List, Port Settings Tab ............................................. 2-18
Figure2-16. Firmware Upgrade Mode ........................................................ 2-19
Figure2-17. Firmware Upgrade, PCSync Utility ........................................... 2-20
Figure2-18. Firmware Upgrade Process ...................................................... 2-21
Figure2-19. POST Screen and Firmware Version ......................................... 2-21
Figure3-1. Power-On Self-Test Sequence ................................................... 3-3
Figure3-2. Change Patient Mode Menu ..................................................... 3-4
Figure3-3. Homecare Mode Menu Item ..................................................... 3-6
Figure3-4. Homecare Mode Monitoring Screen .......................................... 3-7
Figure3-5. Sleep Study Mode Menu Item ................................................... 3-8
Figure3-6. Service Menu ............................................................................ 3-9
Figure3-7. Dynamic Password Menu (Homecare to Standard Example) ....... 3-9
Figure3-8. Password Set or Reset (Homecare to Standard Example) .......... 3-10
Figure3-9. Date/Time Settings .................................................................. 3-11
Figure3-10. Communications Settings ....................................................... 3-12
Figure3-11. Add New Connection Menu ................................................... 3-13
Figure3-12. Device Settings Menu ............................................................. 3-14
Figure3-13. Sensor Port ............................................................................. 3-15
Figure3-14. Sensor Port ............................................................................. 3-17
Figure3-15. Low SpO
Figure3-16. Low Pulse Alarm Limit of 160BPM ........................................... 3-18
Figure3-17. Alarm Audio Paused Setting of 30 Seconds ............................. 3-19
Figure3-18. Permission to Mute Alarm ....................................................... 3-20
Figure3-19. Confirmation for Muted Alarm ............................................... 3-21
Figure3-20. Alarm Volume Default Setting of 2 ......................................... 3-22
Figure3-21. Key Beep Volume Default Setting of 0 .................................... 3-23
Figure3-22. Pulse Volume Default Setting of 0 ........................................... 3-24
Figure3-23. Brightness Setting ................................................................... 3-25
Figure3-24. “Spot Reading Saved” Message .............................................. 3-26
Figure3-25. Monitoring History Spot Data .................................................. 3-27
2 Alarm Limit of 99% ................................................ 3-17
v
Page 6

Figure3-26. Sensor Disconnect Alarm Priority Setting ................................. 3-28
Figure3-27. Sensor Off Alarm .................................................................... 3-29
Figure3-28. Screen Saver Time Setting ....................................................... 3-30
Figure3-29. Auto Power Off Time Setting .................................................. 3-32
Figure3-30. SRC-MAX OxiMax Oximetry Tester .......................................... 3-35
Figure3-31. SRC-MAX Tester-Generated Waveform ................................... 3-36
Figure3-32. SRC-MAX Increase to 200 BPM ............................................... 3-37
Figure3-33. SRC-MAX Decrease to 60 BPM ............................................... 3-38
Figure3-34. SRC-MAX %SpO
Figure3-35. SRC-MAX %SpO
2 Increase to 90 ............................................ 3-39
2 Decrease to 75 ........................................... 3-40
Figure3-36. SRC-MAX High Modulation .................................................... 3-41
Figure3-37. BPM of 200 with High Modulation .......................................... 3-42
Figure3-38. BPM of 60 with High Modulation ............................................ 3-43
Figure3-39. %SpO
Figure3-40. %SpO
Figure3-41. %SpO
2 of 90 with High Modulation ....................................... 3-44
2 of 75 with High Modulation ....................................... 3-45
2 of 75 with Low Modulation ........................................ 3-46
Figure3-42. High Light Condition ............................................................... 3-47
Figure3-43. BPM of 200 with High Light Condition .................................... 3-48
Figure3-44. BPM of 60 with High Light Condition ...................................... 3-49
Figure3-45. %SpO
Figure3-46. %SpO
2 of 90 with High Light Condition ................................. 3-50
2 of 75 with High Light Condition ................................. 3-51
Figure3-47. High Modulation and High Light Condition ............................. 3-52
Figure4-1. Return Packaging .................................................................... 4-10
Figure5-1. Exploded View .......................................................................... 5-2
Figure5-2. Standard Cover (3 Shown) and Ambulatory Cover .................... 5-4
Figure5-3. Battery Replacement ................................................................. 5-5
Figure5-4. Front and Rear Assembly Replacement ...................................... 5-8
Figure5-5. NELL1SR Board Replacement ................................................... 5-10
Figure5-6. Main Board Replacement ........................................................ 5-11
Figure5-7. Coin Cell Battery Replacement ................................................ 5-13
Figure5-8. Wireless Board Replacement ................................................... 5-14
Figure5-9. LCD Replacement ................................................................... 5-15
Figure5-10. PI Cable and Cable Housing Replacement ............................... 5-17
vi
Page 7

List of Tables
Table1-1. Safety Symbol Definitions......................................................... 1-2
Table2-1. Monitoring Status Codes ........................................................ 2-12
Table3-1. Required Test Equipment ......................................................... 3-2
Table3-2. Patient Modes ........................................................................... 3-5
Table3-3. Wireless Status Icon................................................................. 3-14
Table3-4. Functional Tests with SRC-MAX ............................................. 3-33
Table4-1. Error Conditions and Resolutions............................................. 4-2
Table4-2. System Error Codes.................................................................... 4-6
Table5-1. Spare Parts List by Callout Number .......................................... 5-3
Table5-2. Monitoring System Accessories ................................................ 5-4
vii
Page 8

Page Left Intentionally Blank
viii
Page 9

1 Introduction
1.1 Overview
This manual contains information for servicing the Nellcor™ Portable SpO2
Patient Monitoring System.
This manual applies to the following products:
Note:
Before use, carefully read this manual, the Operator’s Manual, accessory Instructions
for Use, and all precautionary information and specifications.
PM10N
PM10N-W
Reference the Operator’s Manual for the following information:
• Intended Use statement
• Operations-related warnings and cautions
• Overviews of the display and operating buttons
• Descriptions of product and packaging symbols
• Installation instructions
• Alarms management
• Preventive maintenance
• Performance considerations
• Accessories
• Theory of operations
• Clinical studies
1-1
Page 10
Introduction
1.2.1 Safety Symbols
1.2 Safety Information
This section contains important safety information related to general use of the
Nellcor™ Portable SpO2 Patient Monitoring System. Other important safety
information appears throughout the manual. The Nellcor™ Portable SpO2
Patient Monitoring System is referred to as the “monitoring system” throughout this manual.
Table1-1.Safety Symbol Definitions
Symbol Definition
WARNING
Alerts users to potential serious outcomes (death, injury, or adverse events) to
the patient, user, or environment.
Caution
Identifies conditions or practices that could result in damage to the equipment
or other property.
Note
Provides additional guidelines or information.
1.2.2 Explosion, Shock, and Toxicity Hazards
WARNING:
Explosion hazard — Do not use the monitoring system in the presence of
flammable anesthetics.
WARNING:
Shock hazard—Do not pour or spill liquids onto the monitoring system.
WARNING:
Shock hazard—Firmly close the battery cover to prevent moisture from
entering the monitoring system.
1-2 Service Manual
Page 11

WARNING:
The LCD panel (display) contains toxic chemicals. Do not touch broken LCD
panels. Physical contact with a broken LCD panel can result in transmission or
ingestion of toxic substances.
1.2.3 Service Procedures
WARNING:
To avoid possible injury, do not attempt to service the monitoring system if
there are any signs of burning or smoking coming from the monitoring
system.
WARNING:
Before attempting to service the monitoring system, disconnect it from the
patient to avoid possible injury to the patient.
Safety Information
WARNING:
Before attempting to disassemble the monitoring system, remove the
batteries to prevent possible injury.
WARNING:
Ensure that conductive portions of the electrodes, leads, and cable do not
come into contact with any other conductive parts.
WARNING:
High voltage is generated by the LCD backlight driver. Exercise caution when
operating the monitoring system with covers open.
WARNING:
Extreme care must be taken in modifying default or other settings to ensure
they are appropriate to the intended use.
WARNING:
Make sure to complete all performance and safety tests outlined in Chapter
3, Modification and Testing before placing the monitoring system into
operation after repair or maintenance. Failure to perform all tests could result
in erroneous monitoring system readings.
Service Manual 1-3
Page 12

Introduction
WARNING:
WARNING:
1.2.4 Monitoring System Operation and Service
Any connections between this monitoring system and other devices must
comply with applicable medical systems safety standards such as IEC 60601-1.
Failure to do so could result in unsafe leakage current and grounding
conditions.
To ensure accurate performance and prevent device failure, do not expose
the monitoring system to extreme moisture, such as direct exposure to rain.
Such exposure may cause inaccurate performance or device failure. Reference
the Operator’s Manual for fluid ingress specifications.
WARNING:
Inspect the monitoring system and all accessories before use to ensure there
are no signs of physical damage or improper function. Do not use if damaged.
WARNING:
To ensure accurate performance and prevent device failure, do not expose
the monitoring system to extreme moisture, such as direct exposure to rain.
Such exposure may cause inaccurate performance or device failure. Do not
immerse in water, solvents, or cleaning solutions, since the monitoring
system and pulse oximetry sensors and connectors are not waterproof.
WARNING:
Do not sterilize the monitoring system
oxide.
WARNING:
The monitoring system should not be used adjacent to or stacked with other
equipment. If adjacent or stacked use is necessary, observe the monitoring
system to verify normal operation in the desired configuration.
by irradiation, steam, or ethylene
WARNING:
The only user-serviceable parts inside the monitoring system are the four AA
batteries. While users can open the battery cover to change the batteries,
1-4 Service Manual
Page 13

only qualified service personnel should remove the cover or access internal
components for any other reason. Users should not modify any components
of the monitoring system.
WARNING:
Do not spray, pour, or spill any liquid on the monitoring system, its
accessories, connectors, switches, or openings in the casing, since this may
cause damage to the monitoring system. Never place fluids on the monitoring
system. If fluid spills on the monitoring system, remove batteries, wipe all
components dry immediately, and have the monitoring system serviced to
ensure no hazard exists.
WARNING:
Do not damage the batteries by applying pressure. Do not throw, hit, or drop
or impact the batteries.
Safety Information
WARNING:
Keep the monitoring system and batteries out of reach of children to avoid
any accidents.
Caution:
The monitoring system may not operate properly if it is operated or stored at
conditions outside the ranges stated in this manual, or if it is subjected to
excessive shock or dropping.
1.2.5 Patient Monitoring and Safety
WARNING:
Always disconnect and remove the monitoring system and sensors during
magnetic resonance imaging (MRI) scanning. Attempting to use the
monitoring system during an MRI procedure could cause burns or adversely
affect the MRI image or the monitoring system's accuracy.
WARNING:
Keep patients under close surveillance when monitoring. It is possible,
although unlikely, that radiated electromagnetic signals from sources
external to the patient and the monitoring system can cause inaccurate
measurement readings.
Service Manual 1-5
Page 14

Introduction
WARNING:
WARNING:
1.2.6 Monitoring System Readings
WARNING:
As with all medical equipment, carefully route patient cabling to reduce the
possibility of patient entanglement or strangulation.
Do not lift or carry the monitoring system by the pulse oximetry sensor or
pulse oximetry interface cable. The cable may disconnect and cause the
monitoring system to drop on a patient or cause damage to monitoring
system surfaces.
The monitoring system may remain attached to the patient during
defibrillation or during use of an electrosurgical unit; however, the
monitoring system is not defibrillator-proof, and readings may be inaccurate
during defibrillation and shortly thereafter.
WARNING:
Check the patient's vital signs by alternate means should there be any doubt
about the accuracy of any measurement. Request a qualified service
technician confirm the monitoring system is functioning correctly.
WARNING:
For best product performance and measurement accuracy, use only
accessories supplied or recommended by Covidien. Use accessories according
to their respective Instructions for Use.
1.2.7 Sensors, Cables, and Other Accessories
WARNING:
Before use, carefully read the pulse oximetry sensor Instructions for Use,
including all warnings, cautions, and instructions.
1-6 Service Manual
Page 15

WARNING:
Use only the Covidien-approved pulse oximetry sensors, interface cables, and
accessories. Use of other sensors, cables, and accessories can result in
inaccurate readings and increased monitoring system
WARNING:
Do not use any other cables to extend the length of the Covidien-approved
interface cable. Increasing the length will degrade signal quality and may lead
to inaccurate measurements.
WARNING:
To prevent damage, avoid undue bending of the sensor cable.
WARNING:
The sensor disconnect error message and associated alarm indicate the pulse
oximetry sensor is either disconnected or has faulty wiring. Check the
connection and, if necessary, replace the sensor, the pulse oximetry cable, or
both.
Safety Information
emissions.
1.2.8 Electromagnetic Interference
WARNING:
Any radio frequency transmitting equipment or other nearby sources of
electrical noise may result in disruption of the monitoring system.
WARNING:
The monitoring system is designed for use in environments in which the
signal can be obscured by electromagnetic interference. During such
interference, measurements may seem inappropriate or the monitoring
system may not seem to operate correctly.
WARNING:
Large equipment using a switching relay for its power on/off may affect
monitoring system operation. Do not operate the monitoring system in such
environments.
Service Manual 1-7
Page 16

Introduction
Caution:
Caution:
Caution:
This device has been tested and found to comply with the limits for medical
devices related to IEC 60601-1-2: 2007. These limits are designed to provide
reasonable protection against harmful interference in a typical medical
installation.
This monitoring system generates, uses, and can radiate radio frequency
energy and, if not installed and used in accordance with the instructions, may
cause harmful interference to other devices in the vicinity. If interference is
suspected, move pulse oximetry cables away from the susceptible device.
Be aware of possible interference from sources of electromagnetic
interference, such as cellular phones, radio transmitters, motors, telephones,
lamps, electrosurgical units, defibrillators, and other medical devices. If pulse
oximetry readings are not as expected for the patient’s condition, remove the
sources of possible interference.
1.2.9 Connections with Other Equipment
Caution:
Accessory equipment connected to the monitoring system's data interface
must be certified according to IEC 60950-1 for data-processing equipment. All
combinations of equipment must be in compliance with IEC 60601-1:2005
Requirements for Medical Electrical Systems. Anyone who connects
additional equipment to the signal input or signal output port configures a
medical system and is therefore responsible for ensuring the system complies
with the requirements of IEC 60601-1:2005 and IEC 60601-1-2:2007.
Caution:
When connecting the monitoring system to any instrument, verify proper
operation before clinical use.
Caution:
Anyone who connects a PC to the data output port configures a medical
system and is therefore responsible for ensuring that the system complies
1-8 Service Manual
Page 17

with the requirements of IEC 60601-1-1 and the electromagnetic compatibility
IEC 60601-1-2.
1.2.10 Monitoring System Storage, Transport, and Disposal
Caution:
Remove the batteries from the monitoring system
or when not using it for a long period.
before placing it in storage
Caution:
Do not short-circuit the batteries, as they may generate heat. To avoid shortcircuiting, do not let the batteries come in contact with metal objects at any
time, especially during transport.
Safety Information
Caution:
Follow local government ordinances and recycling instructions regarding
disposal or recycling of the monitoring system
batteries and accessories.
and its components, including
Service Manual 1-9
Page 18

Introduction
1.3.1 Technical Services
1.3 Obtaining Technical Assistance
For technical information and assistance, contact Covidien or a local Covidien
representative.
Covidien Technical Services: Patient Monitoring
15 Hampshire Street
Mansfield, MA 02048 USA
1.800.635.5267, 1.925.463.4635,
or contact a local Covidien representative
www.covidien.com
When calling Covidien or a local Covidien representative, have the monitoring
system serial number available. The serial number label is located on the
bottom of the monitoring system. Provide the firmware version number displayed during the power-on self-test (POST).
1.3.2 Related Documents
Nellcor™ Portable SpO2 Patient Monitoring System
Operator’s Manual —
system and troubleshooting errors or malfunctions.
Nellcor™ Pulse Oximetry Sensor Instructions for Use — Guides sensor selection
and usage. Before attaching any of the various Covidien-approved pulse oximetry
sensors to the monitoring system, refer to the individual Directions for Use.
Saturation Accuracy Grid — Provides sensor-specific guidance related to desired
2 saturation accuracy measurements. Available online at www.covidien.com.
SpO
Provides basic information for operating the monitoring
1-10 Service Manual
Page 19

1.4 Revision History
The documentation part number and revision number indicate its current edition. The revision number changes when Covidien prints a new edition. Minor
corrections and updates incorporated at reprint do not cause a change in the
revision number. Extensive changes may require a new document part number.
1.5 Warranty Information
The information contained in this document is subject to change without
notice. Covidien makes no warranty of any kind with regard to this material,
including, but not limited to, the implied warranties of merchantability and
fitness for a particular purpose. Covidien shall not be liable for errors contained
herein or for incidental or consequential damages in connection with the furnishing, performance, or use of this material.
Revision History
Service Manual 1-11
Page 20

Introduction
Page Left Intentionally Blank
1-12 Service Manual
Page 21

2 Data Management
2.1 Overview
This chapter contains information for accessing, transmitting, and downloading patient monitoring data and history. This chapter also contains instructions
for upgrading firmware for the Nellcor™ Portable SpO2 Patient Monitoring
System. The monitoring system supports the following types of data viewing
and transmission:
• Transmit real-time data — Real-time patient data transmission by Wi-Fi to the
Oxinet III Remote Patient Monitoring System or the Vital Sync Virtual Patient Monitoring System. Reference the Operator’s Manual.
• Access stored monitoring history — Monitoring history (trend data) can be
viewed anytime it is stored in the monitoring system. Reference the Operator’s
Manual.
• Download stored monitoring history — Monitoring history can be down-
loaded to a PC using HyperTerminal or other data transmission and analysis tools.
• Upgrade the monitoring system’s firmware — Occasionally, Covidien will
provide upgrades to the firmware for the monitoring system, which must be
loaded via the mini-USB port.
Note:
The Wi-Fi feature is an option and does not exist on all Portable SpO
Monitoring Systems.
2.2 External Data Communication
WARNING:
Any connections between this monitoring system and other devices must
comply with applicable medical systems safety standards such as IEC 60601-1
and applicable collaterals. Failure to do so may result in unsafe leakage
current and grounding conditions.
2 Patient
2-1
Page 22

Data Management
Caution:
Do not attach any cable intended for computer use to the sensor port
connector.
Caution:
Connect the monitoring system to a medical grade PC that is on an isolated
AC circuit.
Note:
Reference the manuals for the Covidien Nellcor™ Oxinet III Remote Monitoring
System and Vital Sync™ System 2.0 or above for operation information and
recommendations for the placement of the monitoring system relative to the
distributed alarm system.
The monitoring system supports the following types of data communication:
• Real-time data transmission — Real-time data transmission by Wi-Fi to the
Covidien Nellcor™ Oxinet III Remote Monitoring System or the Vital Sync™
System 2.0 (or above) Virtual Patient Monitoring System.
• Monitoring history (trend data) download — Monitoring history downloads
by mini-USB connection to a PC using data transmission and analysis tools.
Note:
To use the Wi-Fi feature (if available), a qualified service technician must first configure
the monitoring system to connect to a wireless network, as described in the following
sections.
2.2.1 Real-Time Data Transmission
Transfer real-time monitoring data to Covidien Nellcor™ Oxinet III or Vital
Sync™ System
the monitoring system to one of these applications.
To set up a new connection for Oxinet or VitalSync
1. Start Oxinet III or VitalSync 2.0 or above.
2.0 or above. First, set up a new connection, and then connect
2. Turn on the monitoring system and go to the Service Menu (pass code required).
3. In the Service Menu, select Communication Settings.
2-2 Service Manual
Page 23
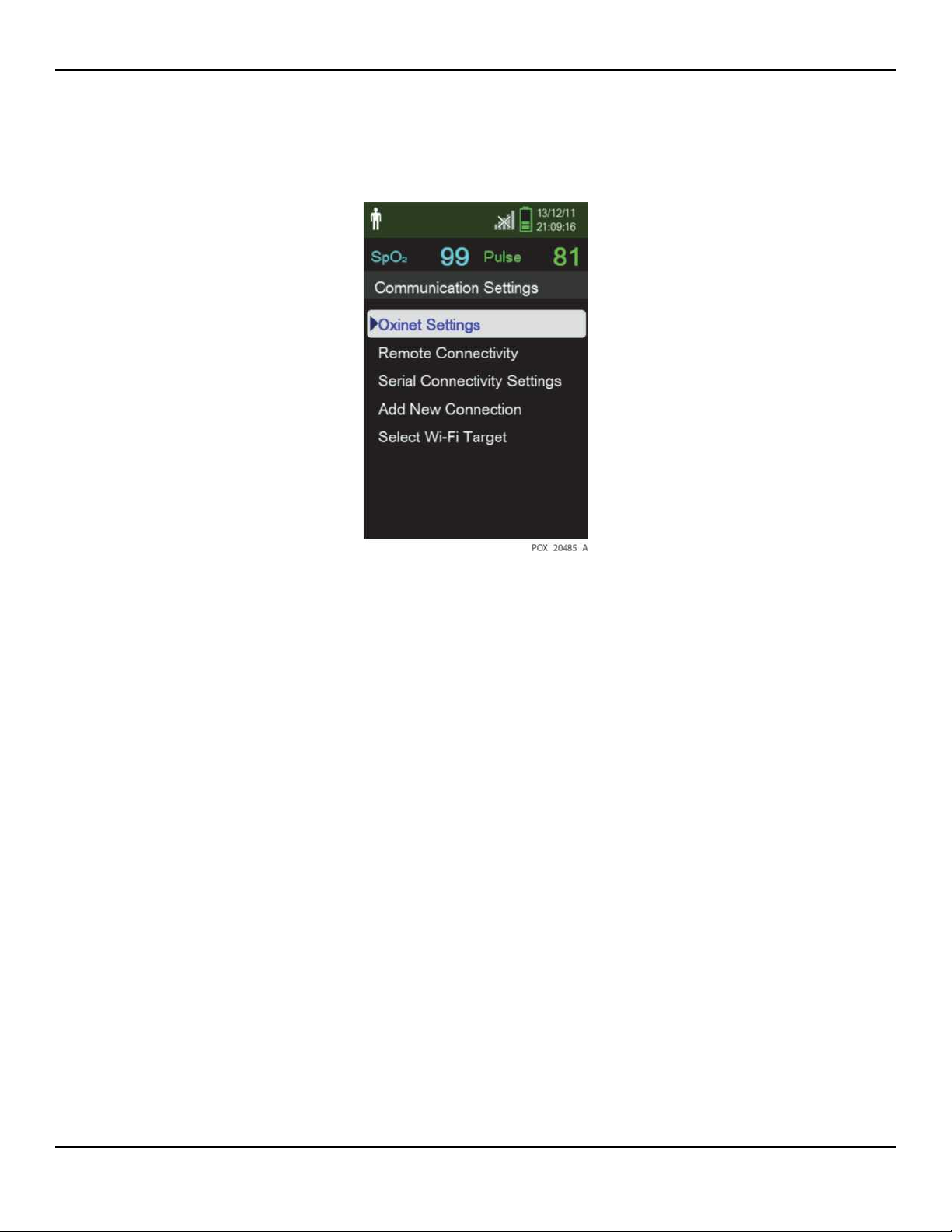
Figure2-1.Communication Settings—Oxinet
External Data Communication
4. Select Oxinet Settings and make the following settings:
• Destination IP Address — Set the IP address of the computer with the Oxinet or
VitalSync program installed
• Port — 18000 for Oxinet; 10001 for VitalSync
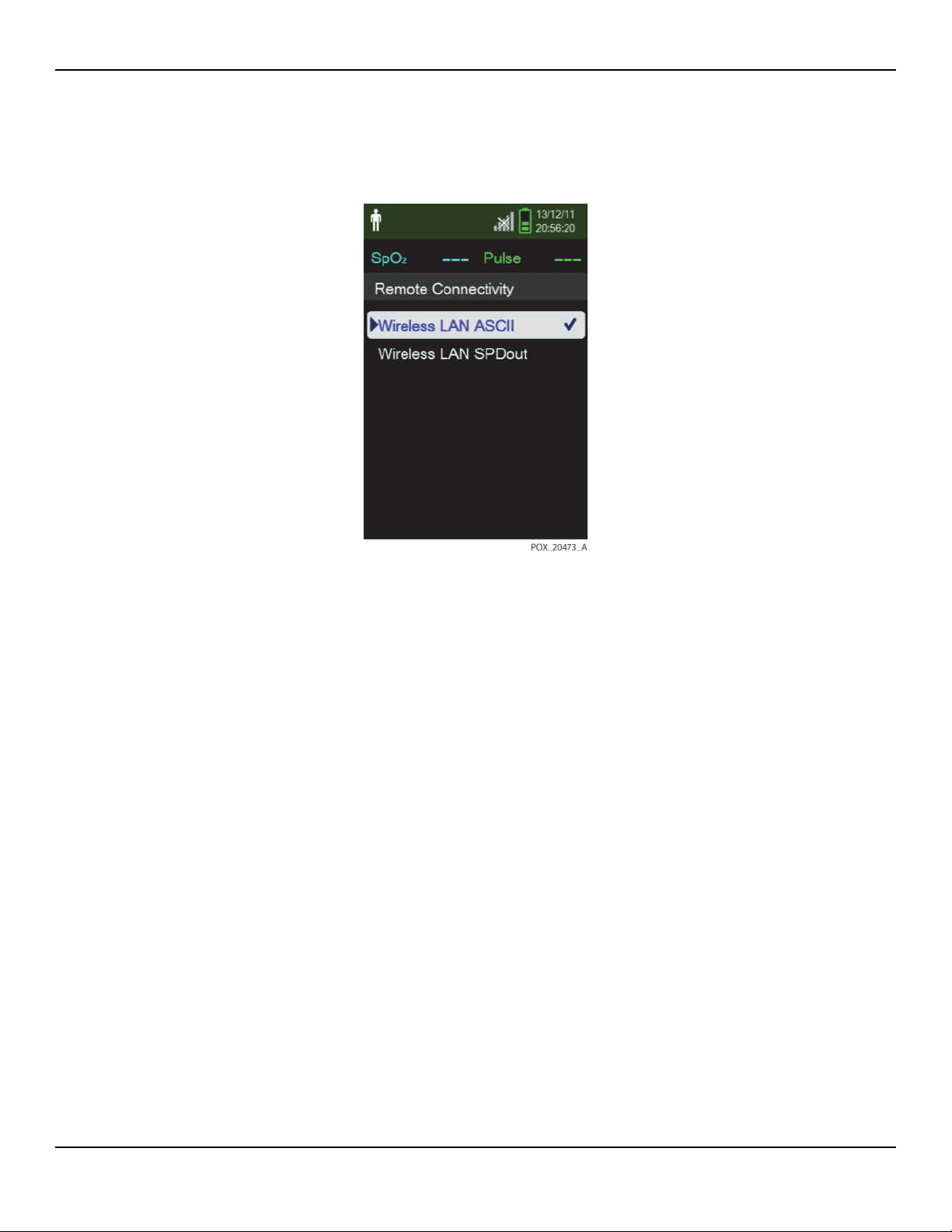
5. Select the data packet type (ASCII, SPDout) by selecting Remote Connectivity.
Service Manual 2-3
Page 24
Data Management
Figure2-2.Remote Connectivity Selections
6. Select Add New Connection and set as follows:
a. SSID: Enter the SSID name of the AP (access point).
b. Encryption: Select the encryption type of the current AP.
c. Encryption Type: Set the password for each encryption type.
d. DHCP: Turn DHCP On or Off. (When On, the IP Address, Subnet Mask, and
Gateway setting are automatically assigned using DHCP.)
e. IP Address: If DHCP is Off, set the IP address of the monitoring system.
f. Subnet Mask: If DHCP is Off, set the subnet mask of the monitoring system.
g. Gateway: If DHCP is Off, set the gateway of the monitoring system.
h. After setting the new connection, select Save Connection to add the settings
to the Wi-Fi Target.
2-4 Service Manual
Page 25

Figure2-3.New Connection Settings
External Data Communication
7. From the Select Wi-Fi Target menu, select the AP saved in the previous step.
8. Exit the Service Menu and go to the Device Settings Menu.
To transfer real-time data to Oxinet
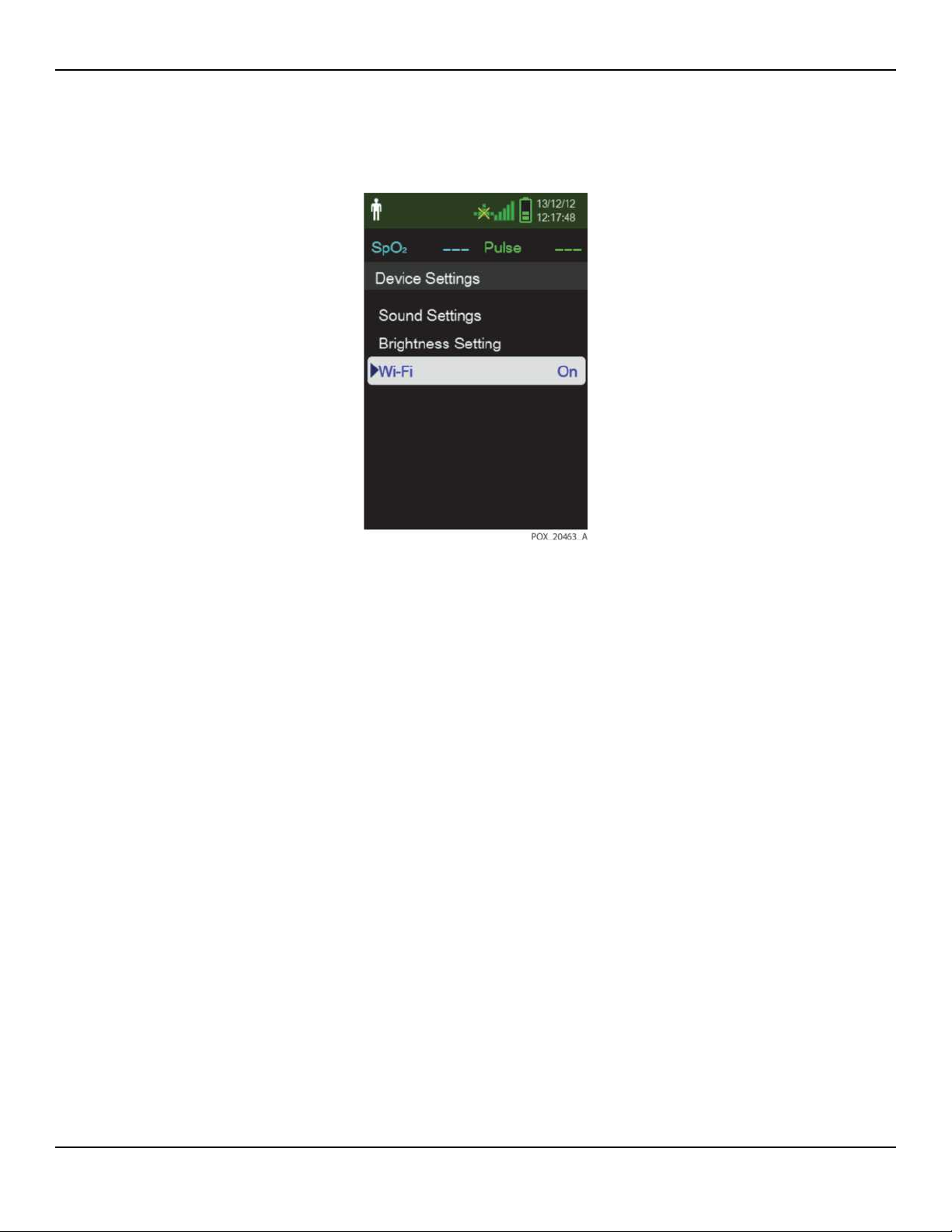
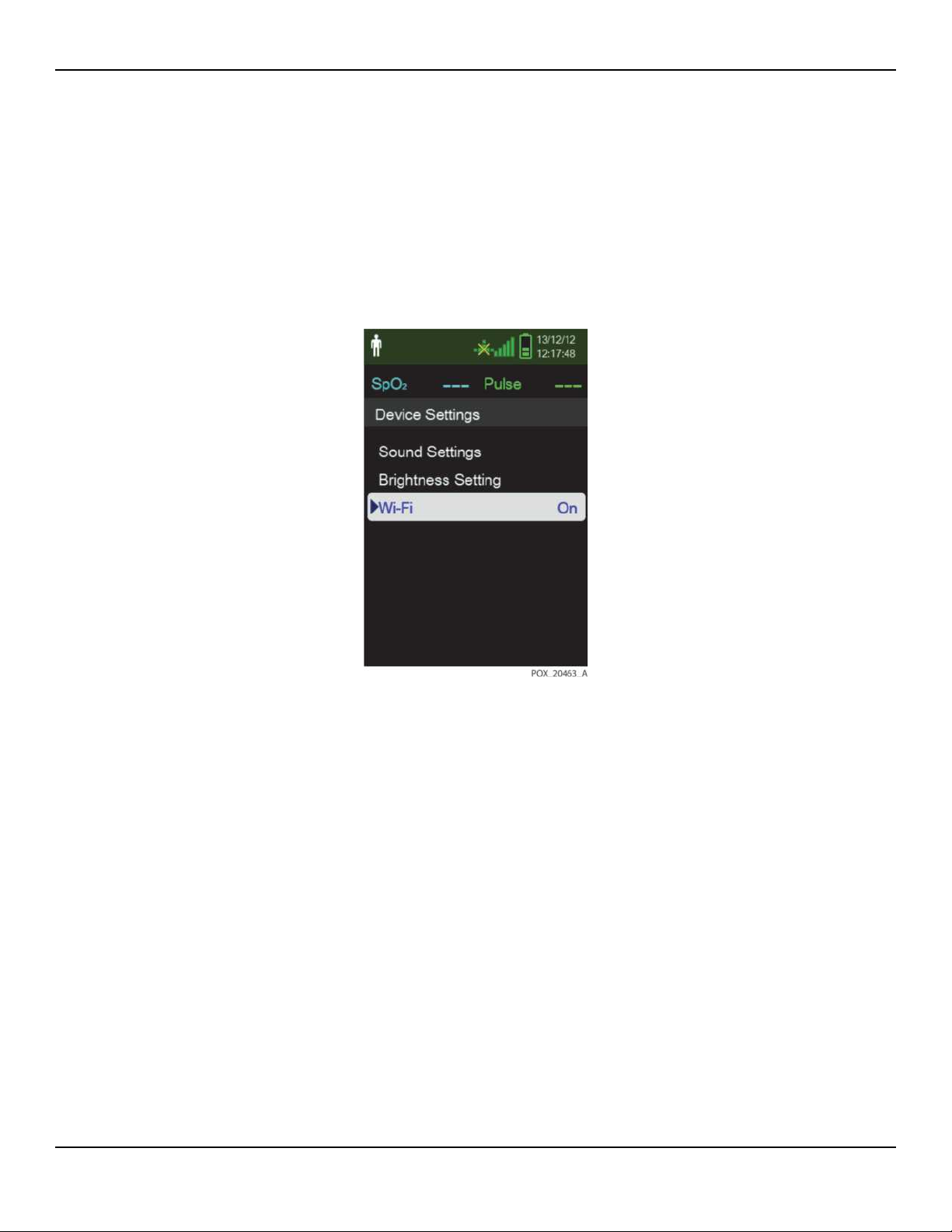
1. On the Device Settings menu, select Wi-Fi to activate the Wi-Fi connection.
Service Manual 2-5
Page 26
Data Management
Figure2-4.Wi-Fi On/Off Setting
2. On the Oxinet interface, click the Administration button at the bottom of the
screen and verify that the BEDs are connected. (When a connection is first established, a BED is displayed in an orange color.)
3. Select the connected BED and select the room number.
4. After verifying that the room is selected, register the patient information.
5. Click the Monitor button on the middle of the Oxinet screen and verify that the
selected room is displayed on the screen.
6. To confirm the settings of the monitoring system, select the measurement value
on the Oxinet screen. Then verify the following items:
a. The high and low limit values of the %SpO2 and pulse rate.
b. Patient type (Adult or Neonate).
c. Protocol: ASCII.
d. SatSeconds settings.
e. Model: NPSPMS (Nellcor™ Portable SpO2 Patient Monitoring System). To
check the model name, select the Addition Information window at the bottom
right of the Oxinet screen.
2-6 Service Manual
Page 27
External Data Communication
To transfer real-time data to Vital Sync 2.0 or above
1. Reference To set up a new connection for Oxinet or VitalSync, p. 2-2 to connect
to a wireless network.
2. On the Device Settings menu, select Wi-Fi to activate the Wi-Fi connection.
Figure2-5.Wi-Fi On/Off Setting
3. The real-time data displays on the Vital Sync screen.
2.2.2 Monitoring History Download
The monitoring system presents monitoring history (trend data) in tabular
format. The newest data values appear at the top.
WARNING:
Replacing the coin cell battery for the main board resets the monitoring
system’s date and time settings. Integrity of existing patient data will be
questionable. Reset the date and time after replacing this battery with a
known good battery.
Caution:
Anyone who connects a PC to the data output port configures a medical
system and is therefore responsible for ensuring that the system complies
Service Manual 2-7
Page 28

Data Management
with the requirements of IEC 60601-1-1 and the electromagnetic compatibility
IEC 60601-1-2.
Caution:
Signal artifacts, secondary to a variety of external factors, may compromise
the presence or accuracy of the displayed values.
Caution:
If the monitoring system does not contain its own isolation barrier, connect it
to a medical grade PC that is on an isolated AC circuit.
To download monitoring history (trend data), connect by mini-USB port to a
PC using HyperTerminal or other data transmission and analysis tools. Any PC
connected to the data port must be certified according to IEC 60950. All combinations of equipment must be in compliance with IEC 60601-1-1 system
requirements.
Note:
Users may choose to import patient monitoring history to a spreadsheet program. To
do so, export monitoring history using the ASCII format option. Have a qualified
service technician set this option prior to attempting a data download.
System Compatibility Prerequisites
• Windows-based PC
• HyperTerminal or equivalent software installed on PC
Hardware
• Mini-USB data download cable
• CD or thumb drive, if USB driver required
Data transfer by USB port relies on existing communication software drivers for
USB-based devices already on the computer, so should not require any modification of the drivers used by the USB interface. If, for some reason, the computer does not have the correct USB driver, use the device driver provided on
the product CD or from Technical Services. Reference COM port USB Driver
Alternatives, p. 2-13.
2-8 Service Manual
Page 29

Note:
Any monitoring history download relies on either factory default settings or
institutional default settings established by a qualified service technician prior to
usage. This includes baud rate and communication protocol selection.
To download monitoring history using HyperTerminal
1. Configure the monitoring system’s Serial Connectivity Settings appropriately.
2. Connect the monitoring system’s mini-USB port to the computer.
External Data Communication
Figure2-6.Mini-USB Port
3. Execute HyperTerminal.
Note:
If this is the first time the HyperTerminal program launches, it will prompt the user
to set it as the default Telnet program. Depending on institutional requirements,
choose Yes or No.
4. Set the appropriate values for HyperTerminal’s port settings:
a. Set the baud rate (bits per second) to match the monitoring system’s baud
rate.
b. Ensure the data bit is set to 8.
c. Ensure the parity bit is set to none.
d. Ensure the stop bit is set to 1.
e. Ensure the flow control is set to off.
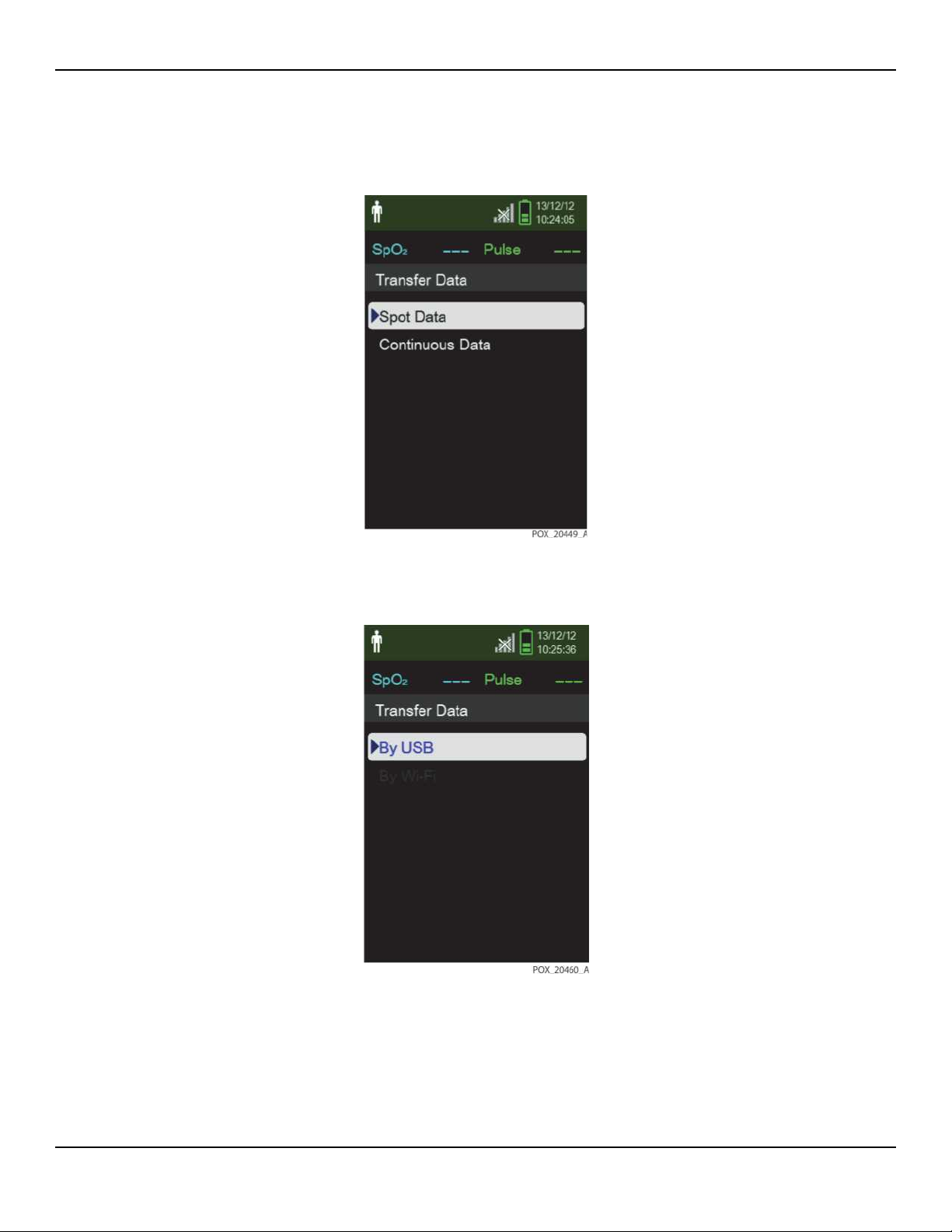
5. From the monitoring system’s Transfer Data menu, select Spot Data or Continu-
ous Data.
Service Manual 2-9
Page 30
Data Management
Figure2-7.Transfer Data Type
6. Select By USB.
Figure2-8.Transfer Data by USB
The data is transferred, and a progress bar is displayed. If desired, select Cancel to
abort the transmission.
2-10 Service Manual
Page 31

External Data Communication
The Output Complete message is displayed when the transmission is complete.
To interpret downloaded monitoring history
1. Examine monitoring history on the HyperTerminal screen, in a spreadsheet, or on
a printout from the personal computer.
Figure2-9.Sample Monitoring History Printout
1 Product column headings Data source, firmware version, and system settings
2 Patient data column headings Lists appropriate time and data headings
3 Time column Real-time clock date and time stamp
4 Output Complete Message indicating completion of monitoring history down-
load
5 %SpO
6 PR Current pulse rate
7 PA Current pulse ampltitude
8 Status Operating status of the monitoring system
2. Ensure patient data settings coincide with expected settings. This would include
2 Current saturation value
the version of firmware and its CRC code, which should be all zeros; alarm limit
settings; patient mode; and SatSeconds setting.
3. Scan the time, SpO2, or PR column until reaching the events of interest.
Service Manual 2-11
Page 32

Data Management
4. Reference Table 2-1 on page 2-12 for descriptions of the operating status codes.
Table2-1.Monitoring Status Codes
Status
Description
Code
LM Loss of pulse, patient motion
LP Loss of pulse
CB Critically low battery
LB Low battery
SO Sensor off
SD Sensor disconnect
AO Alarm off
AS Alarm paused
MO Signal interference, patient motion
PS Pulse search
2-12 Service Manual
Page 33

External Data Communication
COM port USB Driver Alternatives
• Load the appropriate driver from the product CD into the connected computer.
• Contact Technical Services or a local Covidien representative.
To install a USB driver from the compact disc
1. Insert the Nellcor™ Portable SpO2 Patient Monitoring System compact disc (CD)
into the designated personal computer (PC).
2. Copy the Covidien USB to UART Bridge Driver zip file to the PC, installing it in the
desired program folder.
3. Right-click on the zipped folder.
4. Select Extract All.
5. Open the extracted folder.
6. Launch the Driver Installer executable file.
Note:
To change the location of the driver, select the desired mapping by clicking
Change Install Location.
7. Click Install.
8. Reboot the PC for changes to take effect.
Figure2-10.Bridge Driver Installer Window
9. Connect the monitoring system to the PC, firmly engaging the USB end to the PC
and the mini-USB to the monitoring system.
Service Manual 2-13
Page 34

Data Management
10. Allow the PC to sense the new hardware and load the InstallShield Wizard, which
guides users through the entire setup process. Do not click the Cancel button.
Figure2-11.New Hardware Wizard Screen
11. At the prompt from the InstallShield Wizard, click the Next button to copy the
driver to the PC.
12. When the InstallShield Wizard provides the end-user license agreement, read it
carefully, then click the button for accepting the terms of the license.
13. Click Next to formally accept the agreement.
14. Review the Destination Folder mapping. To change the destination, click Browse
and select the desired mapping.
15. Click Next to formally accept the Destination Folder mapping.
16. Click Install in the resulting driver installer window. Do not click the Cancel
button.
Note:
If Windows Security pops up, select the option to install the driver anyway.
2-14 Service Manual
Page 35

External Data Communication
17. Click OK to complete the installation in the resulting Success window.
18. Reboot the PC for changes to take effect.
19. From the Start menu, click the Settings menu option and select the Control Panel
option.
20. Select the System option to open the System Properties window.
21. Click the Hardware tab, then the Device Manager button.
Figure2-12.Device Manager Button, Hardware Tab
22. Select the Ports option from the resulting list.
Service Manual 2-15
Page 36

Data Management
Figure2-13.Hardware List in Device Manager Window
23. Double click the Silicon Labs CP210x USB to UART Bridge option.
Note:
The listed COM port should match the HyperTerminal COM port designation.
Reference To download monitoring history using HyperTerminal, p. 2-9.
2-16 Service Manual
Page 37

Figure2-14.Sample Initial USB to UART Bridge Properties Window
External Data Communication
24. Click the Port Settings tab.
25. Set the bits per second to one of the possible baud rates: 19200 or 115200. The
factory default is 19200 bps.
Service Manual 2-17
Page 38

Data Management
Figure2-15.Baud Rate List, Port Settings Tab
26. Click the OK button to complete the process.
27. Reference To download monitoring history using HyperTerminal, p. 2-9 to down-
load monitoring history. Or, use a different data transmission and analysis tool.
2.3 Firmware Upgrade
This section describes how to upgrade the firmware for the monitoring system.
Firmware updates occur periodically, and the monitoring system should be
kept up to date to ensure proper operation. Reference System Error Codes, p.
4-6 if errors occur during firmware upgrade.
2-18 Service Manual
Page 39

Caution:
To help prevent the loss of power during the upgrade, make sure to use new
batteries.
Caution:
Do not press buttons other than those specified in the following instructions.
The firmware upgrade can be interrupted or canceled by pressing any button.
Caution:
When the firmware version (.bin) is not matched with the correct version of
the resource file (.res), the monitoring system may not operate properly.
To upgrade firmware
1. Install and run the PCSync utility on the PC to be used for the upgrade.
Firmware Upgrade
2. Turn off the monitoring system.
3. Connect a mini-USB cable from the monitoring system to the PC.
4. On the monitoring system, press the Power On/Off button and Down button
together.
5. The monitoring system powers on in firmware update mode as shown in Figure
2-16.
Figure2-16.Firmware Upgrade Mode
6. Start the PC Sync utility.
Service Manual 2-19
Page 40

Data Management
7. Complete the connection between the monitoring system and PCSync by using
Figure2-17.Firmware Upgrade, PCSync Utility
the PC’s Device Manager to determine the serial port number for the USB to UART
Bridge.
Enter the port number in the Serial Port box at the top left of the dialog box, then
click Connect.
To verify the connection, click the Get Software Version on the PC Sync dialog
box. A pop-up window containing the current version of the monitoring system
firmware is displayed.
8. If the Resource file needs to be updated, click the Resource Update button and
select the appropriate .res file.
When the file is selected, the resource upgrade procedure begins automatically.
Upon completion of the resource update, the Complete message is displayed in
the PC Sync utility.
If the Resource file does not need to be updated, skip this step.
9. Click the Firmware Update button and select the appropriate .bin file.
When the file is selected, the firmware update begins automatically.
Upon completion of the firmware update, the Complete message is displayed in
the PC Sync utility.
The screen shown in Figure 2-18 on page 2-21 is displayed as the monitoring
system is updated. Then the monitoring system is automatically reset.
2-20 Service Manual
Page 41

Figure2-18.Firmware Upgrade Process
Firmware Upgrade
10. Verify the upgrade is complete by checking the POST screen and firmware version.
Figure2-19.POST Screen and Firmware Version
Service Manual 2-21
Page 42

Data Management
Note:
The monitoring system’s previous settings are not modified during the upgrade.
Reference the Operator’s Manual for power-on settings.
2-22 Service Manual
Page 43

3 Modification and Testing
3.1 Overview
This chapter provides information to trained service technicians on verifying
Nellcor™ Portable SpO2 Patient Monitoring System performance following
repairs or during preventive maintenance. When performing tests, follow
these guidelines:
• All tests can be performed without removing the covers from the monitoring
system.
• For many of the tests, the PCSync utility shown in Figure 2-17, p. 2-20 provides
an efficient way to change the monitoring system’s settings.
• All tests must be performed as the last operation before the monitoring system is
returned to the user.
• If the monitoring system fails to perform as specified in any test, repairs must be
made to correct the problem before the monitoring system is returned to the user.
WARNING:
Only qualified service personnel should open the monitoring system, remove
and replace parts, or make adjustments. If your medical facility does not have
a qualified service technician, please contact Covidien Technical Services or
your local Covidien representative.
Note:
Many of the tests outlined in this chapter require access to the monitoring system’s
Service Menu. Contact Covidien Technical Services for the pass code to this menu.
3-1
Page 44

Modification and Testing
3.2 Required Equipment
SpO
SpO
SpO
Stop watch Manual or electronic/For alarm audio paused and alarm
Note:
Contact Covidien Technical Services for pricing and ordering information of the
required equipment. Reference Technical Services, p. 1-10.
Table3-1.Required Test Equipment
Equipment Description/Use
2 sensor (durable) DS-100A Durasensor™ Adult Finger Clip Sensor
2 extension cable DEC-4
2 simulator Nellcor model SRC-MAX
reminder intervals
3.3 System Performance Tests
Caution:
If using the PCSync utility to change settings for the monitoring system, make
sure all the settings on the PCSync screen are correct before clicking Send.
Otherwise, unexpected settings will result.
3.3.1 Power-On Self-Test (POST)
To check the power-on self-test (POST)
1. With the monitoring system off, press and hold the Power On/Off button for
approximately 1 second.
While the monitoring system performs power-on self-test (POST), a progress bar
appears at the bottom of the display.Verify that the following steps occur:
a. The product name and software version number are displayed on the POST
screen.
b. When POST completes successfully, one pass tone sounds, and the monitoring
system displays the main monitoring screen.
3-2 Service Manual
Page 45

Figure3-1.Power-On Self-Test Sequence
System Performance Tests
Note:
POST takes approximately 10 seconds to complete.
Note:
If an error occurs during POST, the monitoring system displays an error message.
Reference Chapter 4, Troubleshooting.
3.3.2 Battery Status
To verify battery status:
1. Insert the batteries into the monitoring system.
2. Press and hold the Power On/Off button for approximately 1 second.
3. Verify the monitoring system is turned on and operating normally.
• Verify the Battery status icon on the display. The battery status icon displays
the remaining battery capacity.
• If the monitoring system is in a critically low battery condition, the high priority
alarm occurs for about 5 minutes before the monitoring system shuts down
automatically. Replace the batteries.
Service Manual 3-3
Page 46

Modification and Testing
• When the monitoring system is in a low battery condition, the remaining
battery power is only enough for 15 minutes of operation. Replace the batteries.
4. Press and hold the Power On/Off button for approximately 1 second and verify
that the monitoring system turns off.
3.3.3 Patient Modes
To verify that patient modes can be selected
1. Select the Change Patient Mode Menu.
A check mark appears next to the selected patient mode.
Figure3-2.Change Patient Mode Menu
2. Press the Down button to highlight each patient mode and press OK to verify that
the correct patient mode icon appears.
Pass codes are required to change to Homecare Mode, Sleep Study Mode, and
back to Standard (Adult or Neonatal) Mode.
Table 3-2 on page 3-5 lists the patient modes and their icons. The icon for the
current patient mode setting appears in the upper left of the monitoring system’s
display.
3-4 Service Manual
Page 47

Note:
To change to Homecare Mode or Sleep Study Mode requires a unique pass code
for each mode. Contact Covidien Technical Services for these pass codes.
System Performance Tests
Table3-2.Patient Modes
Icon Patient Mode
Adult
Neonatal
Homecare
(requires Homecare pass code)
Sleep Study
(requires Sleep Study pass code)
3.3.4 Homecare Mode
To verify Homecare Mode:
1. Connect a sensor to the monitoring system and to a live subject.
2. Access the Change Patient Mode menu.
3. Press the Up or Down button to highlight Homecare Mode, then press OK to
select Homecare Mode.
Service Manual 3-5
Page 48

Modification and Testing
Figure3-3.Homecare Mode Menu Item
4.
Enter the four-digit pass code for Homecare Mode.
Use the Up and Down buttons to change the value for each digit, then press OK to
select the value.
5.
After entering the four digits, select Confirm to enter Homecare Mode.
6.
Verify that the Homecare Mode screen appears. The Homecare Mode icon appears at
top left of the screen, and the monitoring screen’s appearance is different from the
Standard Mode and Sleep Study Mode screens.
3-6 Service Manual
Page 49

Figure3-4.Homecare Mode Monitoring Screen
System Performance Tests
Sleep Study Mode
3.3.5
To verify Sleep Study Mode
1. Access the Change Patient Mode menu.
2. Press the Up or Down button to highlight Sleep Study Mode, then press OK to
select Sleep Study Mode.
Service Manual 3-7
Page 50

Modification and Testing
Figure3-5.Sleep Study Mode Menu Item
3.
Enter the four-digit pass code for Sleep Study Mode.
Use the Up and Down buttons to change the value for each digit, then press OK to
select the value.
4.
After entering the four digits, select Confirm to enter Sleep Study Mode.
5.
Verify that the Sleep Study Mode icon appears at top of screen.
3.3.6 Dynamic Passwords
The Dynamic Password menu allows a user to set new pass codes for switching
between care modes. The menu is accessible in Standard Mode, and requires
access to the Service Menu. Contact Technical Services to obtain the default
Service Menu pass code.
To change a dynamic password:
1. Select the Service Menu.
2. Enter the default pass code obtained from Technical Services.
3-8 Service Manual
Page 51

System Performance Tests
3. From the Service Menu, use the down button to highlight Dynamic Password,
then press OK.
Figure3-6.Service Menu
4. Highlight the mode transition for which the password is being changed, then
press OK.
Figure3-7.Dynamic Password Menu (Homecare to Standard Example)
Service Manual 3-9
Page 52

Modification and Testing
5. Perform one of the following to set a new dynamic password or to reset the exist-
ing one to the factory default:
• Enter a new password by pressing the Up and Down buttons to change the
value for each digit, then select Confirm.
• Select Reset to revert to the factory default password.
Figure3-8.Password Set or Reset (Homecare to Standard Example)
3.3.7
Date and Time
To verify the date and time are accurate:
1. Select the Service Menu.
2. From the Service Menu, use the Down button to select the Date/Time Settings
menu.
3-10 Service Manual
Page 53

Figure3-9.Date/Time Settings
System Performance Tests
3.
Set the correct year, month, day, hour, and minute.
4.
Use the Up button and Down button to scroll through the Date Format options. The
options allow for changes in the date format (yy/mm/dd, mm/dd/yy, dd/mm/yy).
5. Verify that the selected year, month, day, hour, and minute appear at top of
screen
.
Note:
The time format is 24 hours only.
3.3.8 Wireless Network Connectivity
The wireless network feature is an option and does not exist on all monitoring
systems.
To verify the wireless network connectivity:
1. Select the Service Menu.
2. From the Service Menu, use the Down button to select the Communication Set-
tings menu.
Service Manual 3-11
Page 54

Modification and Testing
Figure3-10.Communications Settings
3.
Select the Oxinet Settings menu.
4.
Set the IP address and Port for access to the server.
5.
Select Add New Connection to set the access point (AP) and IP address for accessing
the monitoring system.
•
Select the SSID menu and enter the SSID for the AP
• Select the Encryption Type menu and enter the security type of the current AP.
• Unless the security type is Disable, set the Password in the Encryption Type
.
menu.
• Set the desired setting in the IP Address menu, Subnet Mask menu, or
Gateway menu.
• Select the Save Connection menu.
3-12 Service Manual
Page 55

Figure3-11.Add New Connection Menu
System Performance Tests
6.
Select the Select Wi-Fi Target menu and choose the desired settings.
7.
To exit the Communication Settings menu, select Return.
8.
From the Service Menu, select the Device Settings menu.
9.
Use the Down button to activate the Wi-Fi.
Service Manual 3-13
Page 56

Modification and Testing
Figure3-12.Device Settings Menu
10.
Verify that the wireless status icon appears at the top of the screen and that the icon
matches the current state of the wireless connection.
Note:
If the monitoring system does not contain the wireless option, the wireless status
icons will not appear on the screen.
Table3-3.Wireless Status Icon
Icon Description
Wi-Fi On, access point (AP) disabled
or
Wi-Fi Off
Unable to connect to the host
Active wireless connection
3-14 Service Manual
Page 57

Operational and Functional Tests
Table3-3.Wireless Status Icon
Icon Description
Wireless signal strength
3.4 Operational and Functional Tests
3.4.1 General Operation Tests
To perform general operation tests:
1. Set the monitoring system to Factory Defaults, which removes institutional default
settings.
For more information about factory defaults and options for saving monitoring
system settings, reference the Operator’s Manual.
2. Use a durable, adult finger sensor as specified in Table 3-1 on page 3-2.
LED Excitation Test
The LED excitation test uses normal system components to test circuit operation. The test uses the red sensor LED to verify intensity modulation controlled
by the LED intensity control circuit. Use an adult finger sensor to examine LED
intensity control.
Figure3-13.Sensor Port
To test the circuit operation:
1. Open the sensor port cover.
2. Connect a DEC-4 pulse oximetry cable to the sensor port.
3. Connect the adult finger sensor to the DEC-4 cable.
Service Manual 3-15
Page 58

Modification and Testing
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. Pinch the ends of the finger sensor to open the sensor to its widest point.
6. After the monitoring system completes POST, verify the finger sensor LED is
brightly lit.
7. Allow the sensor clip to close slowly.
8. Verify the LED intensity decreases as the LED approaches the optical sensor.
9. Open the sensor again and verify that the LED intensity increases.
10. Repeat step 7 and verify the intensity continues to decrease. This variation is an
indication the microprocessor is in proper control of LED intensity.
11. Leave the monitoring system on for the next test.
Operation with a Live Subject
Use the same finger sensor used in the LED Excitation Test, p. 3-15 to perform
the following test with a live subject.
To test using a live subject:
1. Attach the finger sensor to a live subject as recommended in the OxiMax pulse
oximetry sensor's directions for use.
2. If not already done, press and hold the Power On/Off button to turn the moni-
toring system on and verify the monitoring system is operating.
3. The monitoring system should stabilize on the subject's physiological signal in
about 15 to 30 seconds. Verify the oxygen saturation and pulse rate values are
reasonable for the subject.
Alarms and Alarm Paused
Access these settings from the Service Menu by using the pass code provided
by Technical Services. Reference Technical Services, p. 1-10.
To adjust the audio (alarms and alarm paused) options:
1. Connect the DEC-4 pulse oximetry cable to the monitoring system’s sensor port.
3-16 Service Manual
Page 59

Operational and Functional Tests
Figure3-14.Sensor Port
2. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
3. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
Alarm Audio Paused
1. Verify the SpO2 and pulse rate appear.
2. Select the Alarm Limits Menu.
3. Select Low SpO2.
4. Change Low SpO2 to 99%.
Figure3-15.Low SpO2 Alarm Limit of 99%
Service Manual 3-17
Page 60

Modification and Testing
5. Select Low Pulse.
6. Change Low Pulse to 160 BPM.
Figure3-16.Low Pulse Alarm Limit of 160BPM
7. Confirm the following results:
• The waveform tracks the pulse rate.
• The pulse tone is audible.
• SpO2 and pulse rate values have flashing yellow highlights behind them.
Note:
Depending on the live subject, an alarm might not be triggered using the Low
SpO
• The audible alarm sounds.
• The following messages alternately appear in the display: Pulse Rate Low and
SpO
8. Access the Service Menu and set Alarm Audio Paused to 30 seconds.
2 alarm limit of 99%.
2 Low.
3-18 Service Manual
Page 61

Figure3-17.Alarm Audio Paused Setting of 30 Seconds
Operational and Functional Tests
9. Exit the Service Menu.
10. As soon as the SpO2 Low alarm and Pulse Rate Low alarms sound, press the
Audio Paused button to immediately pause the alarm tone.
11. With the alarm paused, verify the following:
• Alarm remains silent for 30 seconds before the alarm tone is audible.
• Audio Paused indicators light, and the Alarm Audio Paused messages appears
in the display.
• SpO2 and pulse rate values have flashing yellow highlights behind them.
• Pulse tone is audible.
Permission to Mute Alarm
1. Use the same values for Low SpO2 and PR Alarm Limit as described in “Alarm
Audio Paused” on page 17.
2. Confirm the following results:
• The Pulse Tone is audible.
• The SpO2 and pulse rate values are highlighted by flashing yellow boxes.
Service Manual 3-19
Page 62

Modification and Testing
• The Audible Alarm sounds.
• The following messages alternately appear in the display: Pulse Rate Low and
SpO
3. In the Service Menu, select the Permission to Mute Alarm.
2 Low.
Figure3-18.Permission to Mute Alarm
A confirmation message for the muted alarm setting appears.
4. Select Yes.
3-20 Service Manual
Page 63

Figure3-19.Confirmation for Muted Alarm
Operational and Functional Tests
5. Exit the Service Menu.
6. Access the Device Settings menu.
7. In the Device Settings Menu, access the Sound Settings menu.
8. In the Sound Settings menu, change the alarm volume to 0.
9. Verify the following:
• Alarm audio is silent.
• Audio Paused indicators appear.
• Pulse tone is audible.
Alarm Volume Control
After testing the Alarm Audio Paused and Alarm Audio Reminder settings,
perform the following alarm volume test procedure.
Note:
Turn the alarm audio back on for both SpO
procedure.
2 and pulse rate before performing this
Service Manual 3-21
Page 64

Modification and Testing
To test the alarm volume
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system off.
2. Connect the DEC-4 pulse oximetry cable to the sensor port.
3. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. Access the Device Settings menu.
6. In the Device Settings menu, access the Sound Settings menu.
7. Press the Down button to highlight Alarm Volume, then press OK.
8. Press the Up or Down button to adjust the alarm volume.
The Alarm Volume setting controls the volume (1-4) of alarms. The default setting
is 2.
9. To mute the alarm, change the Permission to Mute Alarm to Yes in the Service
Menu. Reference Permission to Mute Alarm, p. 3-19.
Figure3-20.Alarm Volume Default Setting of 2
3-22 Service Manual
Page 65

Operational and Functional Tests
Key Beep Volume Control
A beep sounds each time a button is pressed, unless the Key Beep Volume
setting is 0.
To test the Key Beep volume
1. Access the Device Settings menu.
2. In the Device Settings menu, access the Sound Settings menu.
3. Press Down button to highlight Key Beep Volume, then press OK.
4. Press Up or Down button to adjust the key beep volume.
The Key Beep Volume setting controls the volume (0-4) of key beeps. The default
setting is 0.
Figure3-21.Key Beep Volume Default Setting of 0
Pulse Volume Control
A pulse tone sounds with each detected heart beat.
To set the pulse volume
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system off.
Service Manual 3-23
Page 66

Modification and Testing
2. Connect the DEC-4 pulse oximetry cable to the monitoring system Sensor Port.
3. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. Access the Device Settings menu.
6. In the Device Settings menu, access the Sound Settings menu.
7. Press Down button to highlight Pulse Volume, then press OK.
8. Press Up or Down button to adjust the pulse volume.
The Pulse Volume setting controls the volume (0-4) of the pulse rate indicator. The
default setting is 0.
Brightness Control
Figure3-22.Pulse Volume Default Setting of 0
Adjust the brightness of the display.
To adjust the Brightness:
1. Access the Device Settings menu.
3-24 Service Manual
Page 67

Operational and Functional Tests
2. In the Device Settings menu, press the Up or Down button to highlight the Bright-
ness Setting menu and then press OK to select the Brightness Setting menu.
Figure3-23.Brightness Setting
Press Down button to decrease the brightness.
•
• Press Up button to increase the brightness.
3. Press OK to save the desired brightness.
Save Spot Reading
The Save Spot Reading function saves a point in time of the patient’s data.
To save a spot reading:
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system off.
2. Connect the DEC-4 pulse oximetry cable to the monitoring system Sensor Port.
3. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. Press Menu.
Service Manual 3-25
Page 68

Modification and Testing
6. Highlight Save Spot Reading and press OK.
The message “Spot Reading Saved” appears.
Figure3-24.“Spot Reading Saved” Message
7. Access the Monitoring History menu.
8. In the Monitoring History menu, select View Spot Data.
9. Verify the saved spot readings.
3-26 Service Manual
Page 69

Figure3-25.Monitoring History Spot Data
Operational and Functional Tests
Sensor Alarm Priority Settings
Access the Sensor Alarm Priority Settings menu to change the alarm priority to
Low, Medium, or High for:
• Sensor Disconnect alarm
• Sensor Off alarm
• Sensor Failure alarm
To change the Alarm Priority Setting for Sensor Disconnect:
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system off.
2. Connect the DEC-4 pulse oximetry cable to the monitoring system’s sensor port.
3. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. When the SpO2 saturation and pulse rate are displayed, disconnect the sensor
from the monitoring system’s sensor port.
Service Manual 3-27
Page 70

Modification and Testing
6. Verify that the Sensor Disconnect alarm occurs.
7. Access the Service Menu.
8. In the Service Menu, select the Sensor Alarm Priority Settings menu.
9. Set the Sensor Disconnect priority to High.
Figure3-26.Sensor Disconnect Alarm Priority Setting
10. Observe the monitoring screen.
11. Verify the following:
• The SpO2 and pulse rate values are highlighted by flashing red boxes.
• The High priority alarm sounds.
• The message “Sensor Disconnect” appears.
To change the Alarm Priority Setting for Sensor Off:
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system off.
2. Connect the DEC-4 pulse oximetry cable to the monitoring system’s sensor port.
3. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
3-28 Service Manual
Page 71

Operational and Functional Tests
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. When the monitoring system is powered on, disconnect the sensor from the
finger.
6. Verify that the Sensor Off alarm occurs.
Figure3-27.Sensor Off Alarm
7. Access the Service Menu.
8. In the Service Menu, Select the Sensor Alarm Priority Settings menu.
9. Set the High in the Sensor Off menu.
10. Go to the monitoring screen.
11. Verify the following:
• The SpO2 and pulse rate values are highlighted by flashing box boxes.
• The High priority alarm sounds.
• The message “Sensor Off” appears.
Service Manual 3-29
Page 72

Modification and Testing
Screen Saver
Screen Saver saves power by darkening the screen.
The Screen Saver functions during periods when no alarm condition exists and
the buttons are not pressed.
To set the Screen Saver:
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
2. Access the Service Menu.
3. In the Service Menu, Select the Power Saving Settings menu.
4. Select Screen Saver Time. The factory default is 3 minutes.
5. Set the desired number of minutes.
Screen Saver Time selections include Never and from 1 minute to 10 minutes.
Figure3-28.Screen Saver Time Setting
6. In the Power Saving Settings menu, select the Screen Saver Brightness menu.
7. Change the brightness to 0. The factory default is 20%.
3-30 Service Manual
Page 73

Operational and Functional Tests
8. After 1 minute, verify the following:
• The screen of the monitoring system is darkened.
• The LED is flashing green.
9. After pressing any button or when an alarm occurs, verify the following:
• The screen of the monitoring system is brightened.
• The LED lights green.
Power Saving
Power Saving turns the screen off after 10 minutes of inactivity.
Power Saving functions when no alarm condition exists and the buttons are
not pressed.
To test the Power Saving:
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system off.
2. Connect the DEC-4 pulse oximetry cable to the monitoring system’s sensor port.
3. Connect the OxiMax DS-100A sensor to the DEC-4 cable, then to a finger.
4. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
5. After 10 minutes, verify the following:
• The screen of the monitoring system is turned off.
• The LED is flashing at 2.5-second intervals.
6. After pressing any button except the Power On/Off or Alarm Paused button,
verify the following:
• The brightness of the screen is set to the desired brightness.
• The LED lights green.
Service Manual 3-31
Page 74

Modification and Testing
Auto Power Off
Auto Power Off turns the monitoring system off after the specified period of
inactivity.
Auto Power Off functions when no alarm condition exists, the buttons are not
pressed for the specified period, the sensor is disconnected, and the sensor
signal is lost.
To test Auto Power Off:
1. Press and hold the Power On/Off button for approximately 1 second to turn the
monitoring system on.
2. Access the Service Menu.
3. In the Service Menu, select the Power Saving Settings menu.
4. Select the Auto Power Off Time menu. The factory default is 3 minutes.
5. Set 1 minute in the Auto Power Off Time menu.
Auto Power Off selections include Never and from 1 minute to 10 minutes.
Figure3-29.Auto Power Off Time Setting
6. After 1 minute, verify the following:
3-32 Service Manual
Page 75

• The LED is off.
• The monitoring system turns off automatically.
3.4.2 Functional Tests
To perform functional tests:
1. Read SRC-MAX Overview, p. 3-33 to become familiar with the pulse oximetry
functional tester (Nellcor model SRC-MAX).
Note:
For the waveform tests, the display will show a pulse waveform of approximately
1/2-inch peak to peak (P-T-P) amplitude. Actual amplitude may vary but will be a
reference for low pulse amplitude/low light patients.
Operational and Functional Tests
2. Once the SRC-MAX is attached to the DEC-4 cable and the SRC-MAX and moni-
toring system are turned on, complete all of the tests in sequence, beginning with
BPM (PR), then %SpO
2, then Modulation, and finally Light Level.
SRC-MAX Overview
WARNING:
The SRC-MAX is a functional tester that verifies operation of the monitoring
system. It cannot be used to assess the accuracy of the monitoring system's
%SpO
The SRC-MAX functional tester enables qualified technicians to functionally
test Nellcor OxiMax technology-based monitoring systems and OEM OxiMax
technology-based monitoring systems. Figure 3-4 provides a brief description
of each test.
BPM Test The test procedure simulates an OxiMax pulse oximetry sensor attached to a
2 and pulse rate readings.
Table3-4.Functional Tests with SRC-MAX
Tests Descriptions
patient indicating a pulse rate of 60 BPM and 200 BPM.
%SpO
2 Test
Service Manual 3-33
The test procedure simulates an OxiMax pulse oximetry sensor attached to a
patient indicating a 75% blood oxygen saturation and 90% blood oxygen
saturation.
Page 76

Modification and Testing
Tests Descriptions
Modulation Level Test The test procedure simulates an OxiMax pulse oximetry sensor attached to a
Light Level Test The test procedure simulates an OxiMax pulse oximetry sensor attached to a
Note:
The SRC-MAX selectable indicator LEDs may extinguish if there is a delay in
proceeding through the above tests. This is normal operation to conserve battery
power.
Note:
Pressing a button on the SRC-MAX during the test procedures may be requested to
change a certain parameter. If the SRC-MAX LEDs are not lit, press the button twice.
Pressing the button once causes the indicators to relight and pressing twice initiates
the change.
Table3-4.Functional Tests with SRC-MAX (Continued)
patient indicating low and high pulse strength.
patient indicating low and high light level passing through the patient at the
sensor site.
Note:
As the SRC-MAX tests run, if the SatSeconds setting is any value other than 0, the
visual and audible alarms will not activate until the SatSeconds circle is full.
3-34 Service Manual
Page 77

Figure3-30.SRC-MAX OxiMax Oximetry Tester
Operational and Functional Tests
1 DEC-4 Cable Connector 5 %SpO2 Select Button
2 Infrared LED Drive Indicator 6 % Modulation Select Button
3 Pulse Rate Selection Button 7 Battery Low Indicator
4 Light Level Selection Button 8 Red LED Drive Indicator
BPM (PR) Test
1. With the monitoring system turned off, connect the DEC-4 pulse oximetry cable
to the sensor port.
2. Connect the SRC-MAX tester to the other end of the DEC-4 cable.
3. Turn on the monitoring system by pressing and holding the Power On/Off
button. Wait for the monitoring system to complete POST.
Service Manual 3-35
Page 78

Modification and Testing
Figure3-31.SRC-MAX Tester-Generated Waveform
4. Verify the following:
a. Audio alarm is active.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch peak to peak (P-T-P) amplitude.
5. Press the SRC-MAX PULSE RATE selection button. The SRC-MAX PULSE RATE
200 LED lights. The monitoring system registers that the BPM increases and stabilizes to a value in the range of 197 to 203 BPM.
3-36 Service Manual
Page 79

Figure3-32.SRC-MAX Increase to 200 BPM
Operational and Functional Tests
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. Flashing BPM indication in the range of 197 to 203.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
6. Press the SRC-MAX PULSE RATE selection button. The SRC-MAX PULSE RATE
60 LED lights. The pulse oximeter registers that the BPM decreases and stabilizes
to a value in the range of 57 to 63 BPM.
Service Manual 3-37
Page 80

Modification and Testing
Figure3-33.SRC-MAX Decrease to 60 BPM
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
3-38 Service Manual
Page 81

Operational and Functional Tests
SpO2 Test
1. Press the SRC-MAX %SpO2 selection button. The SRC-MAX %SpO2 90 LED
lights. The monitoring system displays three dashes [ - - - ] until the %SpO
2 stabi-
lizes at a value in the range of 88 to 92.
Figure3-34.SRC-MAX %SpO2 Increase to 90
Verify the following:
a. No audio alarm.
b. %SpO2 indication in the range of 88 to 92.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
2. Press the SRC-MAX %SpO2 selection button. The SRC-MAX %SpO2 75 LED
lights. The monitoring system displays three dashes [ - - - ] until the %SpO
2 stabi-
lizes at a value in the range of 73 to 77.
Service Manual 3-39
Page 82

Modification and Testing
Figure3-35.SRC-MAX %SpO2 Decrease to 75
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
3-40 Service Manual
Page 83

Operational and Functional Tests
Modulation Level Test
1. Press the SRC-MAX MODULATION selection button.
The SRC-MAX MODULATION LED lights. The pulse amplitude waveform increases
in amplitude and then stabilizes at a P-T-P amplitude of approximately 1 inch.
Figure3-36.SRC-MAX High Modulation
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1-inch P-T-P amplitude.
2. Press the SRC-MAX PULSE RATE selection button. The SRC-MAX PULSE RATE
200 LED lights. The pulse oximeter registers that the BPM increases and stabilizes
to a value in the range of 197 to 203.
Service Manual 3-41
Page 84

Modification and Testing
Figure3-37.BPM of 200 with High Modulation
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. Flashing BPM indication in the range of 197 to 203.
d. Pulse waveform of approximately 1-inch P-T-P amplitude.
3. Press the SRC-MAX PULSE RATE selection button. The SRC-MAX PULSE RATE
60 LED lights. The monitoring system registers that the pulse rate decreases and
stabilizes at a value in the range of 57 to 63.
3-42 Service Manual
Page 85

Figure3-38.BPM of 60 with High Modulation
Operational and Functional Tests
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1-inch P-T-P amplitude.
4. Press the SRC-MAX %SpO2 selection button. The SRC-MAX %SpO2 90 LED
lights. The monitoring system displays three dashes [ - - - ] until the %SpO
2 stabi-
lizes to a value in the range of 88 to 92.
Service Manual 3-43
Page 86

Modification and Testing
Figure3-39.%SpO2 of 90 with High Modulation
Verify the following:
a. No active audio alarm.
b. %SpO2 indication in the range of 88 to 92.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1-inch P-T-P amplitude.
5. Press the SRC-MAX %SpO2 selection button. The SRC-MAX %SpO2 75 LED
lights. The pulse oximeter displays three dashes [ - - - ] until the %SpO
2 stabilizes
to a value in the range of 73 to 77.
3-44 Service Manual
Page 87

Figure3-40.%SpO2 of 75 with High Modulation
Operational and Functional Tests
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1-inch P-T-P amplitude.
6. Press the SRC-MAX MODULATION selection button. The SRC-MAX MODULATION
LED lights. The pulse amplitude waveform decreases in amplitude and the stabilizes at a P-T-P amplitude of approximately 1/2 inch.
Note:
The readings may drop out temporarily.
Service Manual 3-45
Page 88

Modification and Testing
Figure3-41.%SpO2 of 75 with Low Modulation
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
3-46 Service Manual
Page 89

Light Level Test
1. Press the SRC-MAX LIGHT LEVEL selection button. The SRC-MAX LIGHT LEVEL LED
lights. The waveform amplitude initially flatlines and then stabilizes at the previous
amplitude.
Note:
Flat-lining is the only indication of a light change at the measurement site. For the
monitoring system to recover and display normally is an indication of proper
operation with light changes.
Operational and Functional Tests
Figure3-42.High Light Condition
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
2. Press the SRC-MAX PULSE RATE selection button. The SRC-MAX PULSE RATE
200 LED lights. The monitoring system registers that the BPM increases and then
stabilizes at a value in the range of 197 to 203.
Service Manual 3-47
Page 90

Modification and Testing
Figure3-43.BPM of 200 with High Light Condition
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. Flashing BPM indication in the range of 197 to 203.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
3. Press the SRC-MAX PULSE RATE selection button. The SRC-MAX PULSE RATE
60 LED lights. The monitoring system registers that the pulse rate decreases and
then stabilizes at a value in the range of 57 to 63.
3-48 Service Manual
Page 91

Figure3-44.BPM of 60 with High Light Condition
Operational and Functional Tests
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
4. Press the SRC-MAX %SpO2 selection button. The SRC-MAX %SpO2 90 LED
lights. The monitoring system displays three dashes [ - - - ] until the %SpO
2 stabi-
lizes to a value in the range of 88 to 92.
Service Manual 3-49
Page 92

Modification and Testing
Figure3-45.%SpO2 of 90 with High Light Condition
Verify the following:
a. No audio alarm.
b. %SpO2 indication in the range of 88 to 92.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
5. Press the SRC-MAX %SpO2 selection button. The SRC-MAX %SpO2 75 LED
lights. The monitoring system displays three dashes [ - - - ] until the %SpO
2 stabi-
lizes to a value in the range of 73 to 77.
3-50 Service Manual
Page 93

Figure3-46.%SpO2 of 75 with High Light Condition
Operational and Functional Tests
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1/2-inch P-T-P amplitude.
6. Press the SRC-MAX MODULATION selection button. The SRC-MAX MODULATION
LED lights. The monitoring system’s pulse waveform increases in amplitude and
then stabilizes at a P-T-P amplitude of approximately 1 inch.
Service Manual 3-51
Page 94

Modification and Testing
Figure3-47.High Modulation and High Light Condition
Verify the following:
a. Active audio alarm.
b. Flashing %SpO2 indication in the range of 73 to 77.
c. BPM indication in the range of 57 to 63.
d. Pulse waveform of approximately 1-inch P-T-P amplitude.
7. Reset the Power On Settings to Factory Defaults. Doing so removes any saved set-
tings and clears the Last Setting option.
8. Turn off the monitoring system.
3-52 Service Manual
Page 95

3.5 Verification Check Sheets
Verification Check Sheets
Model
Name
3.5.1 Performance, Operation, and Functional Test Results
Item Results Remarks
Performance Tests
Power-on self-test (POST) Pass / Fail
Battery Status Pass / Fail
Patient modes Pass / Fail
Date and time Pass / Fail
Wireless Network Connectivity Pass / Fail
Homecare Mode Pass / Fail
Sleep Study Mode Pass / Fail
General operation tests
LED excitation Pass / Fail
Serial
Number
Software
Version
Operation with a live subject Pass / Fail
Alarms and alarm paused Pass / Fail
Alarm volume control Pass / Fail
Key beep volume control Pass / Fail
Pulse volume control Pass / Fail
Brightness control Pass / Fail
Save Spot Reading Pass / Fail
Sensor Disconnect Alarm Priority Pass / Fail
Sensor Off Alarm Priority Pass / Fail
Service Manual 3-53
Page 96

Modification and Testing
Screen Saver Pass / Fail
Power Saving Pass / Fail
Auto Power Off Pass / Fail
Functional tests
2 90 +/- 2% Pass / Fail Value: %
SpO
SpO2 75 +/- 2% Pass / Fail Value: %
Pulse rate 200 +/- 3 bpm (high priority alarm condition) Pass / Fail Value: bpm
Pulse rate 60 +/- 3 bpm Pass / Fail Value: bpm
Modulation level Pass / Fail
Light level Pass / Fail
TESTS PERFORMED BY: SIGNATURE and DATE:
3-54 Service Manual
Page 97

4 Troubleshooting
4.1 Overview
This chapter describes how to troubleshoot common problems that may occur
while using the Nellcor™ Portable SpO2 Patient Monitoring System.
WARNING:
Only a qualified service technician should remove the cover or access or
replace any internal parts.
4.2 Troubleshooting Guide
Potential problems with the monitoring system are separated into categories
and listed in Table 4-1 on page 4-2.
General guidelines for troubleshooting:
• Taking the recommended actions discussed in this section will correct the majority
of problems that might be encountered. When the recommended action is to
replace a part, reference Chapter 5, Repair.
• Problems not covered in this section can be resolved by calling Covidien Technical
Services. Reference Technical Services, p. 1-10.
• If Technical Service recommends returning the monitoring system for repair, ref-
erence Return, p. 4-9, for packing instructions.
4-1
Page 98

Troubleshooting
4.2.1 Error Conditions by Category
Error Condition by Category Cause or Checkpoint Corrective Action
Power
Table4-1.Error Conditions and Resolutions
Monitoring system does not
turn on when Power On/Off
button is pressed.
Low battery/critically low
battery condition
Monitoring System Malfunction
Monitoring system is frozen Reset needed. Press and hold the Power On/Off button
Monitoring system message:
System Failure (920)
Monitoring system’s Power On/
Off button must be held for 1
second.
Battery is not properly seated. Check that the battery polarity is correct
Depleted battery or unsuitable
battery.
Monitoring system has been run
on battery power for the life of
the battery.
Unsuitable battery. Ensure all batteries are 1.5V.
Main board is malfunctioning. If problem persists, replace the main board.
Monitoring system opened and
harnesses disconnected.
Batteries depleted.
Battery disconnected while monitoring system was powered on.
Press and hold the Power On/Off button
for 1 second.
and that all batteries are seated.
Ensure all batteries are 1.5V.
If problem persists, replace the batteries.
Replace the batteries.
for 10 seconds to force the system to turn
off. Turn the monitoring system on again.
Press and hold the Power On/Off button
for 1 second to turn the monitoring system
off. Turn the monitoring system on again.
Verify normal POST.
Display
Blank display after normal
POST
Display does not function properly or looks distorted
4-2 Service Manual
LCD cable is disconnected. Reconnect the LCD cable.
LCD is damaged. Replace the LCD.
Electromagnetic interference. Isolate sources of electromagnetic interfer-
ence (electrosurgical device, cell phone).
LCD cable disconnected or
improperly connected.
Main board is malfunctioning. Replace the main board.
Reconnect or reseat LCD cable.
Page 99

Table4-1.Error Conditions and Resolutions (Continued)
Error Condition by Category Cause or Checkpoint Corrective Action
LCD is visibly cracked or broken Replace LCD.
Sound
Troubleshooting Guide
No sound during POST Speaker cable is loose or discon-
Reseat speaker cable in main board.
nected.
Speaker is malfunctioning. Ensure connector on main board is firmly
seated. If problem persists, replace speaker.
Main board is malfunctioning. Replace main board.
Buzzer sounds during POST,
monitoring system message:
Speaker cable is disconnected. Ensure the speaker cable is connected to
main board.
System Failure (910)
Speaker is malfunctioning or main
board is malfunctioning.
Test the short circuit in a speaker by using
the digital multi meter. If there is a short in
the speaker, replace the speaker. If not,
replace the main board.
Audio alarm cannot be paused Main board is malfunctioning. Replace the main board.
Keypad is damaged. Replace the front case assembly.
Audio alarm does not pause for
the time specified in the Institu-
Institutional defaults not set correctly.
Reset institutional defaults.
tional Default settings
Audio Paused button activator on
Replace the main board.
main board is malfunctioning.
Buttons
No response when control
buttons are pressed
Keypad ribbon cable not making
contact.
Check that the keypad ribbon cable is connected to main board.
System is frozen. Press and hold the Power On/Off button
for 10 seconds to restart.
Buttons are defective. Replace the front case assembly.
Button activators on main board
Replace the main board.
are malfunctioning.
SpO
2 / Sensor
Sensor Messages:
SpO
2 Pulse Search
2 Sensor Off
SpO
SpO
2 Cable/Sensor Disconnect
Sensor disconnected from cable
Check all connections.
or monitoring system.
Detached from patient. Reposition sensor and secure cable.
Signal Artifact Detected
Service Manual 4-3
Page 100

Troubleshooting
Error Condition by Category Cause or Checkpoint Corrective Action
Table4-1.Error Conditions and Resolutions (Continued)
Patient Data / USB Data Port
Questionable readouts of
patient physiological measurements; wrongly tagged or
missing patient data
Unable to get reliable readings
because of substances on
patient’s skin or nails, or because
of excessive patient motion.
Sensor not attached correctly. Check all connections and reposition if nec-
Sensor is malfunctioning. Replace sensor or cable.
Electromagnetic interference. Remove sources of electromagnetic inter-
Ambient light interfering with
proper sensing.
Coin cell battery was removed
from main board
WARNING: Replacing the coin
cell battery for the main board
resets the monitoring system’s
date and time settings. Integrity of
existing patient data will be questionable.
Remove items of interference (electrosurgical device, cell phone, nail polish, cream).
If problems persist, replace the cable and/or
sensor.
essary
ference (electrosurgical device, cell phone).
Remove excessive ambient light.
Replace coin cell battery with known good
battery.
Delete monitoring history.
Set date and time for locale and time zone.
USB data port does not function properly
USB cable not firmly connected. Ensure USB cable is firmly connected.
Disconnect USB cable, reset system power,
then reconnect.
Covidien bridge driver corrupted. Re-install the bridge driver provided by
Covidien.
Mismatched baud rates between
monitoring system and PC.
Mismatched COM port between
PC and HyperTerminal.
USB port damaged. Ensure no physical damage to USB port. If
Main board failure. Replace the main board.
Ensure baud rate settings for both monitoring system and PC are the same.
Ensure same COM port is selected on PC
and HyperTerminal or other data transmission tool.
damaged, replace main board.
4-4 Service Manual
 Loading...
Loading...