Page 1

Leica DMI3000B,
DMI4000B, DMI6000B
Instructions · Bedienungsanleitung · Mode d’emploi
Page 2

Published August 2010 by:
Herausgegeben August 2010 von:
Edité en août 2010 par :
Leica Microsystems CMS GmbH
Ernst-Leitz-Straße 17-37
D-35578 Wetzlar (Germany)
Responsible for contents:
Verantwortlich für den Inhalt:
Responsable du contenu rédactionnel :
Bernard Kleine
(Marketing CMS, Life Science Research Microscopy, Product
Management)
(Marketing CMS, Life Science Research Microscopy, Produktmanagement)
(Marketing CMS, Life Science Research Microscopy,
chef de produit)
Dietmar Gnass
(R&D Manager)
In case of questions, please contact the hotline:
Bei Fragen wenden Sie sich bitte an die Hotline:
Pour toute question, contacter notre service d’assistance
téléphonique :
Phone: +49 (0) 64 41 - 29 42 53
Fax: +49 (0) 64 41 - 29 22 55
E-Mail: MQM-Hotline@leica-microsystems.com
Page 3

Leica DMI3000B,
DMI4000B, DMI6000B
Instructions
3
Page 4

Copyrights
Copyrights
All rights to this documentation are held by Leica Microsystems CMS GmbH. Reproduction of
text or illustrations (in whole or in part) by print,
photocopy, microfi lm or other method (including
electronic systems) is not allowed without express written permission from Leica Microsystems CMS GmbH.
The term „Windows“ may appear in the following
text without further identifi cation. It is, however,
a registered trademark of Microsoft Corporation. The names of companies and products used
herein may be trademarks of their respective
owners.
The instructions contained in the following documentation refl ect state-of-the-art technology
standards. We have compiled the texts and illustrations as accurately as possible. Nevertheless,
no liability of any kind may be assumed for the
accuracy of this manual’s contents. Still, we are
always grateful for comments and suggestions
regarding potential mistakes within this documentation.
The information in this manual is subject to modifi cation at any time and without notifi cation.
4
Page 5

Contents
Contents
1. Important Notes about this Manual ........ 7
2. Intended Purpose of the Microscope ..... 8
3. Safety Notes ................................................ 9
3.1 General Safety Notes ................................ 9
3.2 Electrical Safety.......................................... 10
3.3 Safety Instructions
for Handling the Light Sources ................ 12
3.4 Notes on handling laser devices ............. 12
3.5 Safety Instructions
for Handling Acids and Bases .................. 12
3.6 Disposal........................................................ 13
4. Overview of the Leica DMI Series .......... 14
5. Unpacking the Microscope ..................... 27
6. Assembling the Microscope .................... 30
6.1 Assembly Tools ........................................... 30
6.2 Installation of the Transmitted Light
Illumination Carrier (TL) ............................. 31
6.3 Installation of the DIC Module
and DIC Objective Prisms ......................... 32
6.4 Installation of Specimen Stages .............. 33
6.5 Installation of Condensers ........................ 38
6.6 Installation of Eyepieces ........................... 43
6.7 Installation of Objectives .......................... 43
6.8 Installation of Filters
in the Illumination Arm............................... 44
6.9 Installing the transmitted Light
Lamp Housing ............................................. 44
6.10 Installation and Replacement of the
transmitted Light Lamps:
107 or 107/2 Lamp Housing........................ 45
6.11 Installing the Lamp Housing Mount
and Mirror Housing) ................................... 46
6.12 Installation and Replacement of
Incident Light Lamps .................................. 48
6.13 Equipping the Incident Light
Turret Disk .................................................... 52
6.14 Inserting the Front Module Slider ............ 55
6.15 Installation of the Polarizer
and Analyzer................................................ 55
6.16 Optional Accessories................................. 57
6.17 Connection to the Electronics Box .......... 58
6.18 Connection to the Computer ..................... 59
6.19 Connection to the Power Supply ............. 59
7. Start-up ........................................................ 60
7.1 Functional Principle .................................. 60
7.2 Switching on the Microscope .................. 64
7.3 The LeicaDisplay ........................................ 65
7.4 The Function Buttons on the Stand ......... 66
7.5 The SmartMove
Remote Control Module ............................ 69
7.6 Illumination .................................................. 69
7.6.1 Transmitted light .............................. 69
7.6.2 Incident Light - Fluorescence ........ 73
7.7 Checking Phase Contrast Rings ............... 74
7.8 Checking modulation contrast
slit diaphragms............................................ 77
7.9 Setting the Motorized Polarizer ............... 77
7.10 Adjusting the Light Sources ..................... 78
5
Page 6

Contents
8. Operation ..................................................... 81
8.1 Switching on................................................ 81
8.2 Contrast Methods ....................................... 83
8.2.1 Bright Field (TL) ................................ 83
8.2.2 Phase Contrast (TL) ....................... 85
8.2.3 Dark Field (TL) .................................. 86
8.2.4 Polarization (TL) ............................... 87
8.2.5 Differential
Interference Contrast (TL) ............. 88
8.2.6 Integrated Phase Contrast (TL) ..... 89
8.2.7 Integrated
Modulation Contrast (TL) ................ 90
8.3 Fluorescence............................................... 91
8.4 Combination Methods ............................... 93
8.5 Focusing ....................................................... 94
8.6 Tubes ............................................................ 96
8.7 Port selection ............................................. 96
8.8 Eyepieces..................................................... 97
8.9 Objectives .................................................... 98
8.10 Stages and Object Displacement ............ 101
8.11 Magnifi cation Changer .............................. 102
8.12 Light sources ............................................... 103
8.13 Aperture and Field Diaphragm ................ 104
9. Troubleshooting .......................................... 105
10. Care of the Microscope ............................ 109
10.1 Dust Cover ................................................... 109
10.2 Cleaning ....................................................... 109
10.3 Handling Acids and Bases ........................ 110
11. Major Consumable
and Replacement Parts ............................. 111
12. Dimensions.................................................. 112
13. Abbreviations and Pictograms ................ 113
14. Index ............................................................ 115
15. EU Declaration of Conformity .................. 117
6
Page 7
1. Important Notes about this Manual
1. Important Notes about this Manual
Caution!
This operating manual is an essential component of the microscope, and must be read
carefully before the microscope is assembled, put into operation or used.
Text symbols, pictograms and their meanings:
This operating manual contains important instructions and information for the operational
safety and maintenance of the microscope and
accessories. It must therefore be kept safely for
future reference.
A separate manual is available on CD-ROM covering the operation of the Leica Application Suite
(LAS).
(1.2)
→ p. 20
!
*
Numbers in parentheses, such as „(1.2)“, correspond to illustrations (in the example, Figure 1,
Item 2).
Numbers with pointer arrows (for example
→ p. 20), point to a certain page of this manual.
Caution!
Special safety instructions within this manual
are indicated with the triangle symbol shown
here, and have a gray background.
Caution! The microscope and accessories can
be damaged when operated incorrectly.
Notes on the disposal of the microscope, accessories and consumable materials.
Explanatory note.
Item not contained in all confi gurations.
7
Page 8
2. Intended Purpose of the Microscope
2. Intended Purpose of the Microscope
The Leica DMI Series microscopes covered in
this manual are designed for biological, routine,
and research applications. This includes the examination of samples taken from the human body
in order to provide information on physiological
or pathological states or congenital abnormalities; to determine the safety and compatibility
with potential recipients; or to monitor therapeutic measures.
The Leica DMI Series is an additional development of Leica’s proven inverted research microscopes, designed for cellular and tissue
examination, micromanipulation and microinjection techniques, microdissection, and confocal
microscopy. The Leica DMI Series is suitable
for universal deployment. All contrast methods
such as dark fi eld, bright fi eld, phase contrast,
DIC, fl uorescence, and modulation contrast are
integral to the microscope and can be adapted
or changed quickly and easily. Variable illumination and imaging beam paths, as well as HCS
optics, modular accessories, and a comprehensive range of peripherals complement the Leica
Microsystems inverted research stand.
The above-named microscope series complies
with the Council Directive 98/79/EEC concerning in vitro diagnostics. They also conform to the
Council Directives 73/23/EEC concerning electrical apparatus and 89/336 /EEC concerning electromagnetic compatibility for use in an industrial
environment.
Caution!
The manufacturer assumes no liability for
damage caused by, or any risks arising from,
using the microscopes for purposes other
than those for which they are intended or not
using them within the specifi cations of Leica
Microsystems CMS GmbH.
In such cases the declaration of conformity
shall cease to be valid.
Caution!
These (IVD) devices are not intended for use
in the patient environment defi ned by DIN
VDE 0100-710. Neither are they intended for
combining with medical instruments according to EN 60601-1. If a microscope is electrically connected to a medical instrument
according to EN 60601-1, the requirements
defi ned in EN 60601-1-1 shall apply.
8
8
Page 9
3. Safety Notes
3.1 General Safety Notes
3. Safety Notes
This safety class 1 device is constructed and
tested in accordance with
EN 61010-2-101:2002,
EN 61010-1:2001,
IEC 61010-1:2001,
Safety regulations for electrical measuring, control, and laboratory devices.
Caution!
In order to maintain this condition and to ensure safe operation, the user must follow the
instructions and warnings contained in this
operating manual.
Caution!
The devices and accessories described in
this operating manual have been tested for
safety and potential hazards.
The responsible Leica affi liate or the main
plant in Wetzlar must be consulted whenever
the device is altered, modifi ed or used in conjunction with non-Leica components that are
outside of the scope of this manual.
Unauthorized alterations to the device or
noncompliant use shall void all rights to any
warranty claims!
9
9
Page 10

3. Safety Notes
3.2 Electrical Safety
General Specifi cations
Leica CTR4000, CTR5000, CTR5500, CTR6000,
CTR6500, CTR7000, CTR6500 HS, CTR7000 HS
Electronics Boxes
For indoor use only.
Supply voltage:
Frequency:
Power input:
Fuses:
Ambient temperature:
Relative humidity:
Over voltage category:
Pollution degree:
Microscope
For indoor use only.
Supply voltage:
Frequency:
Power input:
Fuses:
Ambient temperature:
Relative humidity:
Over voltage category:
Pollution degree:
90–250 V~
50–60 Hz
max. 290 VA
T6.3 A
(IEC 60127-2/3)
15–35°C
max. 80% to 30°C
II
2
90–250 V~
50–60 Hz
See CTR4000–7000 HS
See CTR4000–7000 HS
15–35°C
max. 80% to 30°C
II
2
ebq 100 supply unit*
For indoor use only.
Supply voltage:
Frequency:
Power input:
Fuses:
Ambient temperature:
Relative humidity:
Over voltage category:
Pollution degree:
(see enclosed manual)
Leica EL6000*
For indoor use only.
Supply voltage:
Frequency:
Power input:
Fuses:
Ambient temperature:
Relative humidity:
Overvoltage category:
Pollution degree:
(see enclosed manual)
90–250 V~
50–60 Hz
see CTR4000–7000
see CTR4000–7000
15–35°C
max. 80% to 30°C
II
2
100–240 VAC
50–60 Hz
max. 200 VA
5x20, 2.5 A, slow,
breaking capacity H
0°–40°C
10–90%
non-condensing
II
2
10
Page 11
3. Safety Notes
Caution!
Power plugs may only be plugged into an outlet equipped with a grounding contact.
Do not interfere with the grounding function
by using an extension cord without a ground
wire. Any interruption of the ground wire inside or outside of the device, or release of
the ground wire connection, can cause the
device to become hazardous. Intentional
ground interruption is not permitted!
Caution!
Peripheral devices with their own or separate power supplies that are connected to
the microscope can have the same protective conductor potential by connecting them
to the ground screw on the back of the Leica
CTR4000, CTR6000, CTR6500 and CTR7000
electronics boxes. For connections without
a ground connector, Leica Service must be
consulted.
Caution!
The microscope’s electrical accessory components are not protected against water. Water can cause electric shock.
Caution!
Protect the microscope from excessive temperature fl uctuations. Such fl uctuations can
lead to the accumulation of condensation,
which can damage the electrical and optical
components.
Ambient temperature: 15–35°C.
Caution!
Before exchanging the fuses or lamps, be absolutely certain to switch off the main power
switch and remove the power cable.
Caution!
Never use any fuses as replacements other
than those of the types and the current ratings listed here. Using patched fuses or
bridging the fuse holder is not permitted. The
use of incorrect fuses may result in a fi re hazard.
11
Page 12
3. Safety Notes
3.3 Safety Instructions
for Handling the Light Sources
Caution!
Light sources pose a potential irradiation risk
(glare, UV-radiation, IR-radiation). Therefore,
lamps have to be operated in closed housings.
Never look directly into the beam path (blinding hazard).
Connect the light guide to the microscope
fi rst to prevent exposing the user to the highenergy light output of the Leica EL6000 compact light source.
Never look directly into the light emitted by
the light guide.
3.4 Notes on handling laser devices
The microscope is not suitable for coupling laser
devices into the camera ports (refer to Chapter
4), as this creates a danger to the user from laser
radiation.
For use of the microscope with lasers, Leica
Microsystems offers special microscopes with
additional safety devices.
For further information, please contact your authorized Leica Microsystems representative.
Caution!
Follow safety instructions for immersion oil!
3.5 Safety Instructions
for Handling Acids and Bases
For examinations using acids or other aggressive
chemicals, particular caution must be taken.
12
Caution!
Be absolutely certain to prevent coming into
contact with these chemicals.
Page 13
3.6 Disposal
To dispose of the product at the end of its service
life, please contact Leica Service or Sales.
Please observe national laws and regulations,
such as those implementing and enforcing the
WEEE EU Directive.
Note!
Like other electronic devices, the microscope, its accessories and consumable materials must not be disposed of as regular
household waste.
3. Safety Notes
13
Page 14

4. Overview of the Instruments
4. Overview of the Leica DMI Series
4.1 Specifi cations
Contrast Methods
Transmitted Light Axis
Leica DMI Series
• transmitted light (TL): BF, DF, PH, DIC, Pol
• intermediate pupil:
IPH (Integrated phase contrast)
• incident light (IL): Fluo
Leica DMI4000 B and DMI6000 B
• combination (TL/IL): Fluo/DIC, Fluo/PH
Leica DMI Series
• Manual and coded transmitted light illumination arm with integrated mechanical tilt mechanism to provide adequate space for
specimens and micromanipulators, integrated fi eld dia phragm,
fi lter magazine for 2 replaceable fi lters, condenser quick-changer
Illumination Manager (aperture diaphragm, fi eld diaphragm, light in-
•
tensity)
• manual shutter
• lamp housing mount for interchangeable lamp housings.
• with integrated cable channel
Leica DMI4000 B and Leica DMI6000 B
• Motorized or manual/coded transmitted light illumination arm
with integrated mechanical tilt mechanism to provide adequate
space for specimens and micromanipulators, integrated motorized fi eld diaphragm, motorized fi lter magazine for 2 replace able
fi lters, condenser quick-changer
• with integrated cable channel
• automatic Illumination Manager
(aperture, fi eld diaphragm, intensity, process switching)
• manual or motorized shutter
• lamp housing mount for interchangeable lamp housings.
• automatic, electronic condenser identifi cation
IMC (integrated modulation contrast)
14
Page 15

4. Overview of the Instruments
Incident Light Axis
Tube
Leica DMI3000 B
• manual shutter
• lamp housing mount for up to 3 interchangeable light sources
• manual 5-place fi lter turret
• Fluorescence Intensity Manager (FIM)
(reduction of incident illumination intensity)
Leica DMI4000 B and Leica DMI6000 B
• automatic Illumination Manager
(aperture, fi eld diaphragm*, intensity, process switching)
• motorized shutter (switching speed < 50 ms)
• lamp housing mount for up to 3 interchangeable light sources
• motorized 6-place fi lter turret
• Fluorescence Intensity Manager (FIM)
(reduction of incident illumination intensity)
• Optional: Interface for structured illumination
• Leica DMI6000 B:
mechanical booster lens for central boosting of
fl uorescence or uniform distribution
• motorized Excitation Manager* to monitor fl uorescence emission
when using double and triple fi lter cubes
• ultra fast fi lter wheel for 3 excitation wavelengths
(switching speed < 50 ms)
Leica DMI Series
• ergonomic with or without camera port at left
• 2 switching positions: 100%VIS and 50%VIS / 50%CAM or
• 2 switching positions: 100%VIS and 0%VIS / 100%CAM
• optional Bertrand lens
• eye spacing adjustment
• height and angle adjustment (30° - 45°)
Magnifi cation Changer
Leica DMI4000 B and Leica DMI6000 B
• motorized
• 3 switching positions
(choice of magnifi cations: 1x; 1.5x; 1.6x or 2.0x)
• effective on all camera ports and eyepieces
or Leica DMI Series
• manual
• 2 switching positions
(choice of magnifi cations: 1x; 1.5x; 1.6x or 2.0x)
• effective on tube port and eyepieces
* not in combination with structured Illumination
15
Page 16
4. Overview of the Instruments
Objective Turret
Stages
Leica DMI6000 B
• motorized and coded
• 6x for objectives with M25 thread and 45 mm parfocal distance
• for DIC: motorized or manual/coded Wollaston prism carousel
• anti-vibration locking
Leica DMI4000 B
• manual and coded
• 6x for objectives with M25 thread and 45 mm parfocal distance
• for DIC: motorized or manual/coded Wollaston prism carousel
Leica DMI3000 B
• manual
• 6x for objectives with M25 thread and 45 mm parfocal distance
• for DIC: manual Wollaston prism carousel
Leica DMI Series
Fixed regular stages
• Ceramic-coated stage plate (248 mm x 204 mm)
• heating stage plate (3°C above room temperature to 60°C)
(248 x 212 mm)
• temperature-controlled stage plate (0°C to 60°C)
(248 mm x 212 mm)
• fi xed micromanipulation stages
• ceramic-coated stage plate (248 mm x 204/122 mm)
• heated stage plate (from 3°C above room temperature
to 60°C) (248 mm x 204/122 mm)
• temperature-controlled stage plate (0°C to 60°C)
(248 mm x 204/122 mm)
• regular manual and motorized 3-plate cross-stage
• positioning range: 83 mm x 127 mm
• 20 optional inserts (standard, heating, cooling) for a variety
of applications, size of inserts:160 mm x 110 mm
(compatible with scanning stages)
• narrow manual and motorized micromanipulation
3-plate cross-stage
• positioning range: 40 mm x 40 mm
• 3 optional inserts for a variety of applications
• Scanning stage 120 x 100 (motors on bottom)
• 1 mm, 2 mm, 4 mm spindle pitch
(higher resolution vs. higher speed)
• 20 optional inserts (standard, heating, cooling) for a variety
of applications, size of inserts:160 mm x 110 mm
16
Page 17

4. Overview of the Instruments
Condensers
Z Focus
Leica DMI4000 B and Leica DMI6000 B
(identical for Leica DMI3000 B, but manual)
• motorized and coded or manual and coded
• motorized or manual aperture diaphragm
• contrast methods: BF, DF, PH, DIC, Pol, IMC, IPH
• automatic method switching
• condenser turret with 7 positions for contrast methods
• 2 condenser housings (S1-S28 and S40,S70)
• condenser heads: S1/1.4 oil, S1/0.9 dry, S23/0.53, S28/0.55
• condenser heads can be swung out
• condenser S40/S70 with additional lens for low magnifi cations
• all condensers suitable for magnifi cations from 1.25x to 100x
• with or without motorized or manual polarizer
• with or without motorized or coded Wollaston prism disk
Leica DMI6000 B
• motorized and coded
• 9 mm travel (1 mm below, 8 mm above the stage)
• maximum travel speed: 5 mm/s
• 5 focus steps: 0.05 µm; 0.1 µm; 0.7 µm; 1.5 µm; 5.0 µm
• electronic focus repositioning
• automatic lowering prior to objective change
• electronic parfocality
• Optional: Adaptive Focus Control (AFC)
Leica DMI3000 B and Leica DMI4000 B
• manual
• 9 mm travel (1 mm below, 8 mm above the stage)
Observation Ports
Leica DMI6000 B
• motorized and coded
• left side ports (100%, 80% or 50% transmission)
• left side port dichroic splitting at 680 nm
• right side ports (100%, 80% or 50% transmission)
• bottom port
optional
• top port with 2 switching positions
• 100% to eyepieces
• 50% to eyepieces/ 50% to port
Leica DMI4000 B
left side port, manual (100% or 80% transmission)
17
Page 18

4. Overview of the Instruments
Observation Ports
Controls
Electronics Box
Leica DMI3000 B
(a manual side port is a standard feature of the Leica DMI3000 B stand)
• manual
• left side port (80% or 100% transmission)
Leica DMI4000 B and Leica DMI6000 B
• 7 fi xed control buttons for illumination and apertures
• 7 variable function buttons behind the focus controls
• 3 fi xed control buttons for focus stops (Leica DMI6000 B only)
• 2 focus hand wheels
• 7 buttons for fl uorescence cubes and shutters
• 4 buttons for magnifi cation changer and ports
• SmartMove: ergonomic remote control module for x,y,z control
and four additional variable function buttons
• STP6000
Leica DMI3000 B
• 2 focus hand wheels
• 1 illumination hand wheel
• 2 turning knobs for fi eld diaphragm and FIM adjustment
• 1 On/Off switch
• separate control unit for all motorized and electronic elements of
the microscope such as:
For CTR6500 (HS)/CTR7000 (HS) only
• scanning stages
18
For CTR6000 only
• motorized 3-plate cross-stages
For CTR6000/7000
• objective turret
• focus
• ports
• magnifi cation changer
• fl uorescence
• condenser
• power supply for SmartMove
For all CTR boxes
with
• power supply for 100W halogen lamps
Page 19
4. Overview of the Instruments
Interfaces
Software Tools
Leica DMI4000 B and Leica DMI6000 B
• 2 x RS232C
• 2 x USB
• 4 x external/internal peripherals
• CTR boxes
• SmartMove
• STP6000
Leica DMI4000 B and Leica DMI6000 B
• Leica Application Suite (LAS) for Windows
with plug-ins for:
• microscope and camera confi guration
• microscope and camera control
• image acquisition
TM
19
Page 20
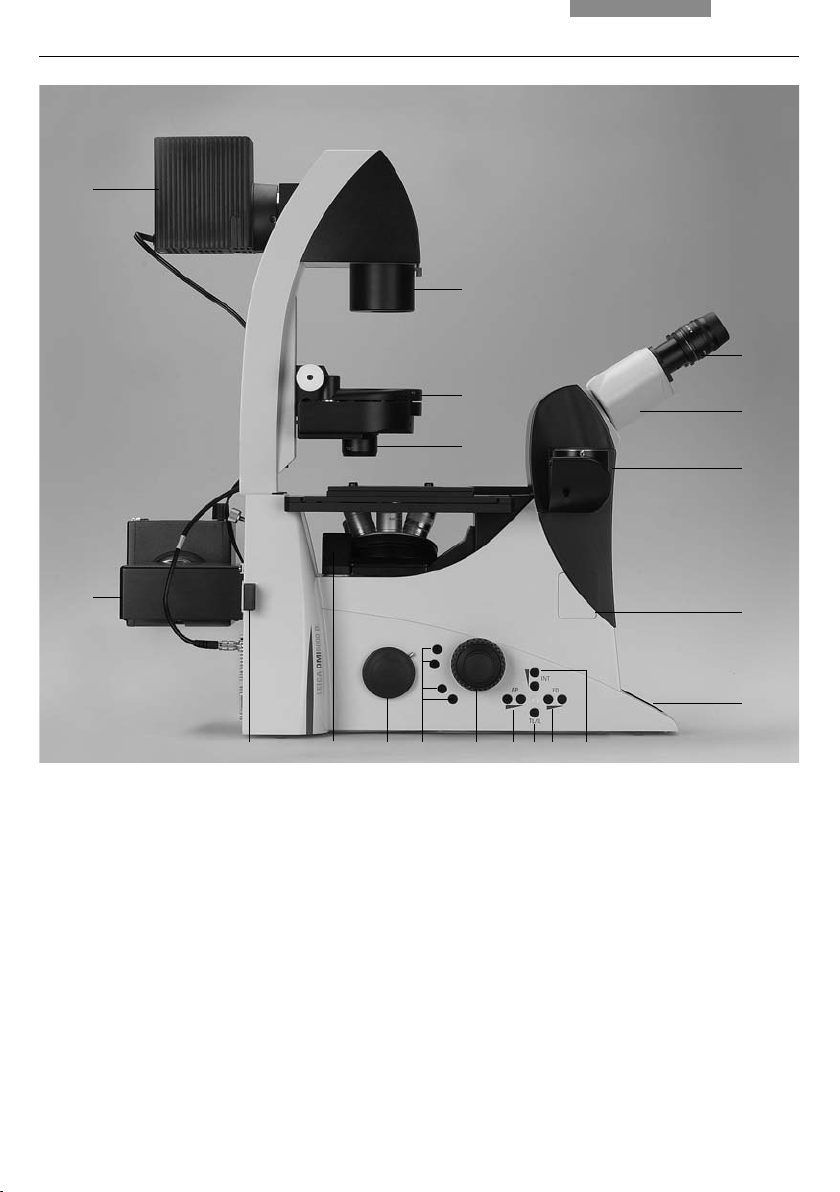
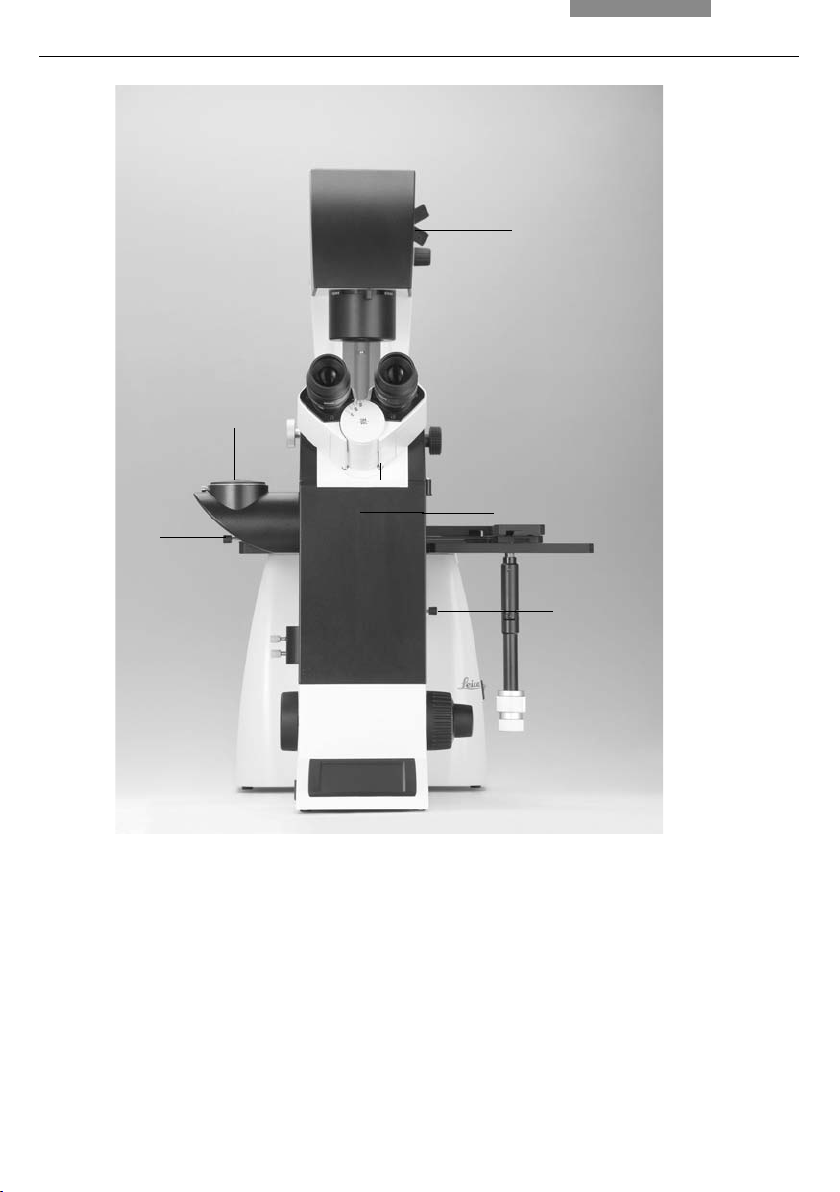
4. Overview of the Instruments
18
17
1
14
19
Fig. 1 Left side, Leica DMI4000 B and DMI6000 B
1 Eyepiece
2 Eyepiece tube
3 Top port
4 Intermediate pupil interface
5 LeicaScreen
6 Light intensity
7 Field diaphragm
8 TL/IL switching
9 Aperture diaphragm
10 Focus wheel (motorized Leica DMI6000 B,
manual (fi ne and coarse) Leica DMI4000 B)
16
2
15
3
4
5
678910111213
11 Variable function buttons
12 Left side port
13 Booster lens
(Leica DMI6000 B fl uorescence microscopes only)
14 Lamp mount (fl uorescence microscopes only)
15 Condenser head
16 Condenser base
17 Field diaphragm
18 Transmitted light lamp housing
19 DIC objective prism disk
20
Page 21
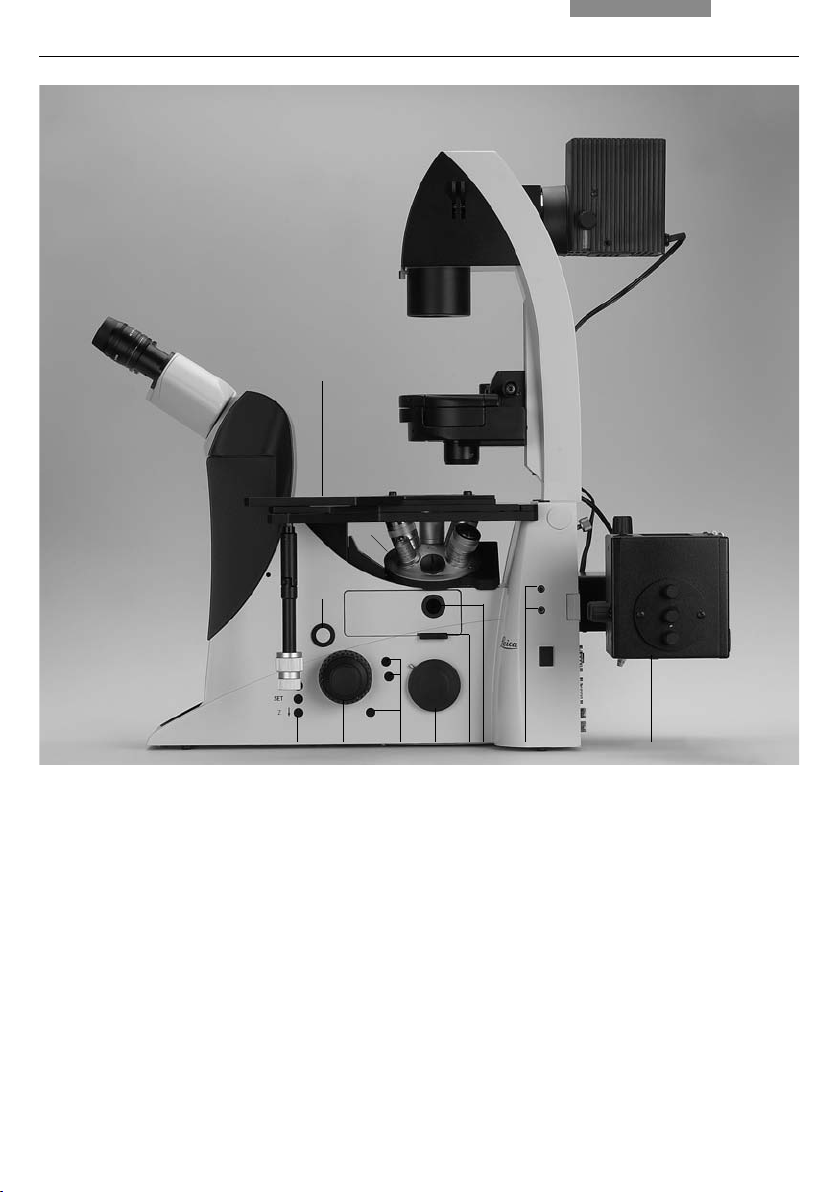
4. Overview of the Instruments
11
4
5
12 3
Fig. 2 R
1 E-Focus buttons (Leica DMI6000 B only)
2 Focus wheel (motorized Leica DMI6000 B,
manual (fi ne) Leica DMI4000 B)
3 Variable function buttons
4 Opener for drawer (fl uorescence microscopes only)
5 Drawer (fl uorescence microscopes only)
6 Right side port
7 Analyzer slot
ight side Leica DMI4000 B and DMI6000 B
6 78129 10
8 Centering window (fl uorescence microscopes only)
9 Field diaphragm centering
(fl uorescence microscopes only)
10
Incident light lamp housing (fl uorescence microscopes
only)
11 Objective turret
12 Stage with attachable mechanical stage
21
Page 22

4. Overview of the Instruments
4
3
5
6
2
1
Fig. 3 Front view Leica DMI4000 B and Leica DMI6000 B
1 LeicaScreen
2 Front control panel
3 Port switching
4 Top port
5 Manual transmitted light fi lters
6 Bertrand lens centering
22
Page 23

4. Overview of the Instruments
Fig. 3a Front control panel
1 Fluorescence cube
2 Shutter
3 100% light to all eyepieces
4 Port selection
5 Magnifi cation selection
6 1x tube lens
2
543
Fig. 3b SmartMove remote control module
1 Travel in x
2 Travel in y
3 Focus
4 Variable function buttons
(pre assigned at factory)
3
11
Fig. 4 General view Leica DMI4000 B and Leica DMI6000 B with SmartMove remote control module
1
2
4
23
Page 24
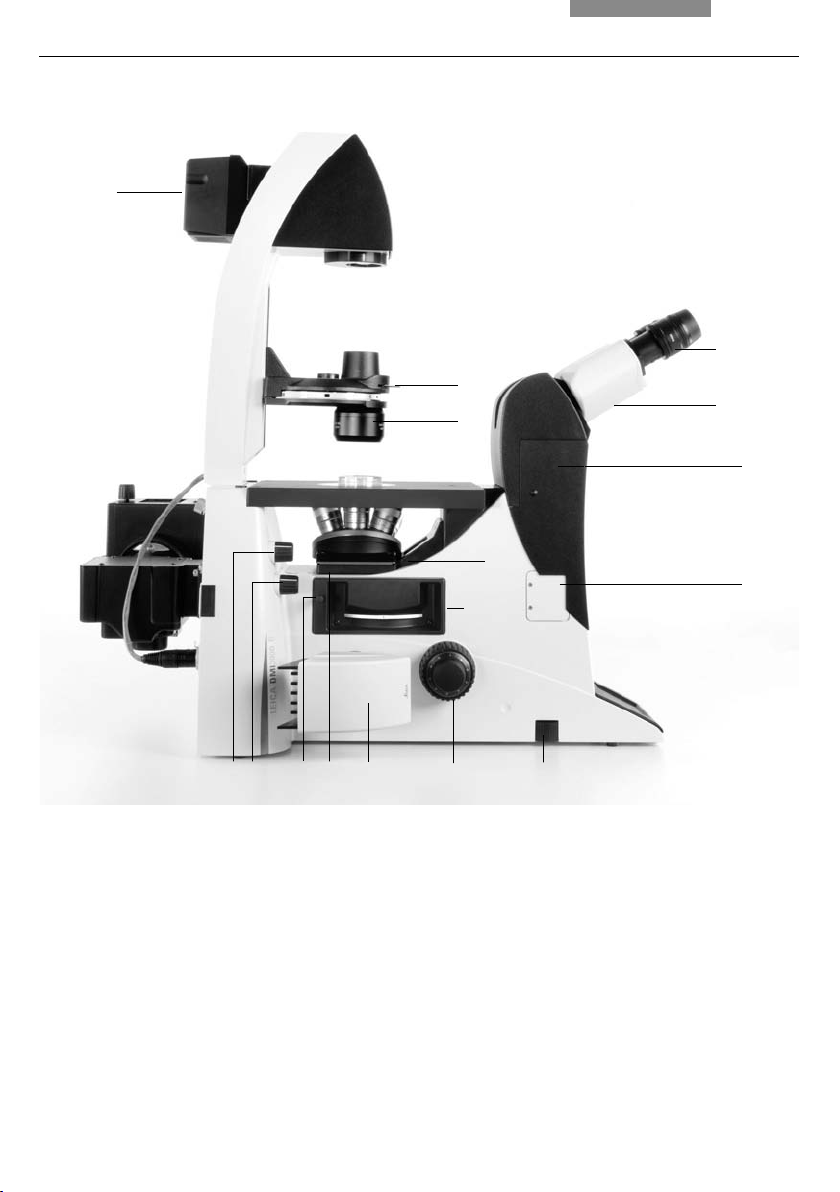
4. Overview of the Instruments
16
1
15
14
13
12
2
3
4
Fig. 5a Leica DMI3000 B left view
1 Eyepiece
2 Eyepiece tube
3 Top port
4 Intermediate pupil interface
5 Light intensity
6 Focus wheel
7 Left side port with camera
8 Objective turret
24
8
91011
9 Filter slider
10 Adjustment FIM
11 Adjustment fi eld diaphragm
12 Drawer (fl uorescence microscopes only)
13 DIC objective prism disk
14 Condenser head
15 Condenser base
16 Integrated 30W transmitted light lamp housing
567
Page 25
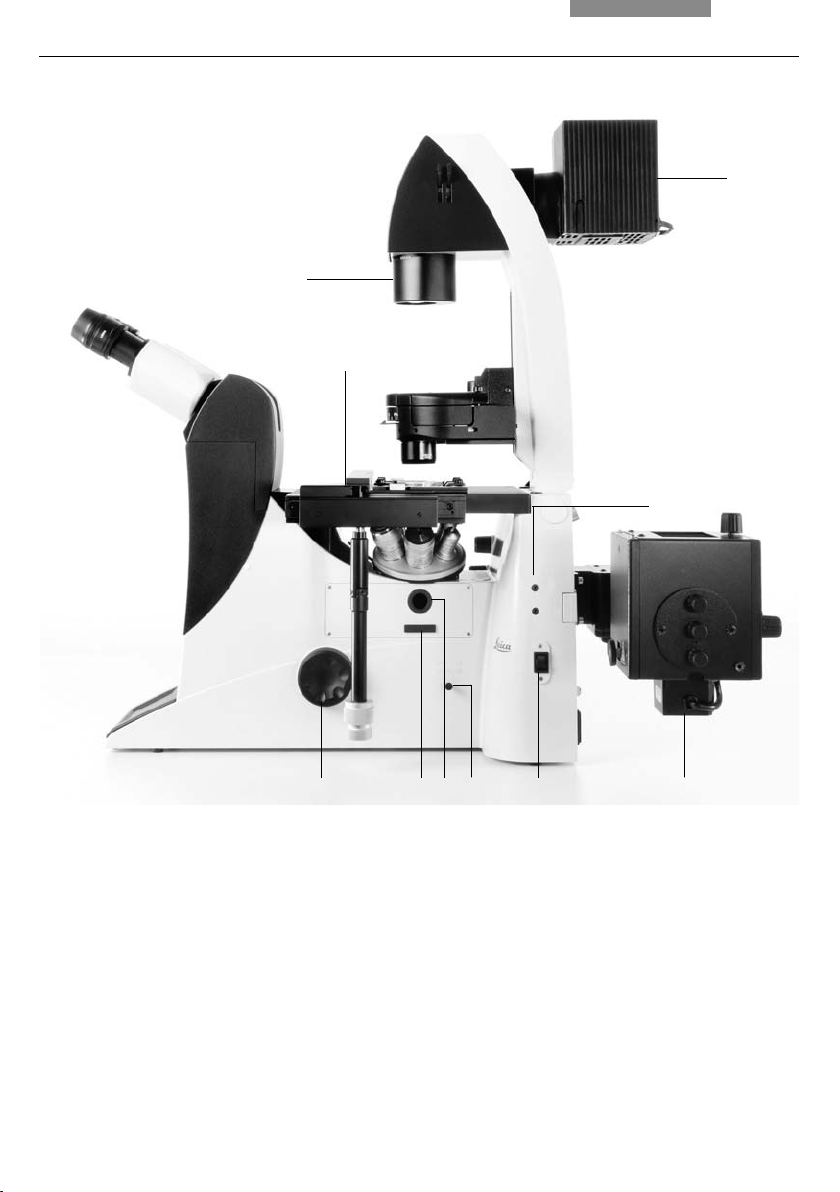
4. Overview of the Instruments
8
9
10
7
1
Fig. 5b
1 Focus wheel
2 Analyzer slot
3 Centering window (fl uorescence microscopes only)
4 Port switching
5 On/Off switch
6 Incident light lamp housing (fl uorescence microscopes only)
7 Field diaphragm centering
8 Transmitted light lamp housing
9 Field diaphragm
10 Stage with attachable mechanical stage
Leica DMI3000 B right view
2
35
4
6
25
Page 26
4. Overview of the Instruments
2
1
3
4
5
Fig. 6 Leica DMI3000 B front view
1 Port switching and Bertrand lens
2 Top port
3 Manual transmitted light fi lters
4 Bertrand lens centering
5 Manual magnifi cation changer
26
Page 27

5. Unpacking the Microscope
5. Unpacking the Microscope
The microscope is delivered in several packages.
The stand package contains the following components:
• Stand with integrated incident light axis,
objective turret, and tube
• Illumination arm
• Specimen stage
• CD with Leica Application Suite (LAS) software
package
• Instructions and list of microscope presets
(identifi cation sheet)
The system package contains the microscope‘s
accessories:
• Eyepieces
• Objectives
• Condenser
• Lamp housings with accessories
• Assembly tools
• Additional accessories such as fi lter cubes,
etc. depending on feature set
The Leica CTR4000, CTR5000, CTR5500, CTR6000,
CTR6500, CTR7000, CTR6500 HS, CTR7000 HS
electronics box, the SmartMove, STP6000 remote
control module,
sories,
the external ebq 100 supply unit and the
compact light source Leica EL6000 are provided
in separate packages.
movable stages, stage acces-
27
Page 28
5. Unpacking the Microscope
Please carefully compare the contents of the delivery to the packing slip, delivery note or invoice.
We strongly recommend storing a copy of these
documents with the manual to ensure that you
have information on the date and scope of delivery handy for subsequent orders or service work.
Please ensure that no small parts remain in the
packing material. Parts of the packing material
are marked by symbols to simplify recycling.
Caution!
Do not connect the microscope or peripherals to an AC power source at this time under
any circumstances!
Installation Location
First, carefully remove all components from the
transportation and packaging materials.
Caution!
Do not put the instrument into operation in the
event of visible damage to the components or
packing material.
Note:
If at all possible, avoid touching the lens surfaces
of the objectives. If fi ngerprints do appear on the
glass surfaces, remove them with a soft leather
or linen cloth. Even small traces of fi nger perspiration can damage the surfaces in a short time.
See the chapter „Care of the Microscope“ → p.
109, for additional instructions.
Work with the microscope should be performed
in a dust-free room, which is free of oil vapor and
other chemical vapor, as well as extreme humidity. At the workplace, large temperature fl uctuations, direct sunlight, and vibration should be
avoided. These may adversely affect measurements and long-term observations.
Allowable ambient conditions
Temperature 15–35°C
Relative humidity maximum 80% up to 30°C
Microscopes in warm and warm-damp climatic
zones require special care in order to prevent the
build up of fungus.
See the chapter „Care of the Microscope“
→ p. 109, for additional instructions.
Caution!
Electrical components must be placed at
least 10 cm from the wall and away from
fl ammable substances.
28
Page 29

Transport
For shipping or transporting the microscope and
its accessory components, the original packaging should be used.
As a precaution to prevent damage from vibrations, the following components should be disassembled and packaged separately:
• Unscrew the objectives.
• Remove the eyepieces.
• Remove the condenser.
• Remove the specimen stage.
• Remove the transmitted-light arm.
• Remove the lamp housings.
• Remove the lamp housing mount.
5. Unpacking the Microscope
• Disassemble the burner of 106 z lamp housing.
• Remove the fi lter cube.
• Remove all moving or loose parts.
29
Page 30

6. Assembly
6. Assembling the Microscope
The microscope components* are logically assembled in this order:
• Transmitted light illumination carrier
• DIC module and DIC objective prisms
• Condenser with condenser head
• Eyepieces
• Objectives
• Transmitted light lamps
• Lamp housing mount (mirror housings)
• Incident light lamps
• Assembly of incident light turret disk
• Specimen stage
• Polarizer and analyzer
The order may be vary when using
climate cham-
bers or other systems and optical accessories.
In this case, read Chapter
„6.16 Optional Accessories“ → p. 57.
6.1 Assembly Tools
If possible, the microscope should be assembled
and set up with the assistance of Leica sales or
service personnel.
A small number of universal screwdrivers which
are included in the scope of delivery are required
for assembly (Fig. 7).
Fig. 7 Assembly tools
1 Phillips screwdriver*
2
3 mm Allen key
3 1.5 mm centering key*
4 2 mm centering key*
5 3 mm hex key*
6 2.5 mm hex key* (short type)
7 2.5 mm hex key*
30
* depending on scope of delivery
2
5
1
3
4
6
2
7
Page 31

6. Assembly
6.2 Installation of the
Transmitted Light Illumination Carrier (TL)
Wipe the installation surface on the microscope
(8.3) with a dry cloth. Tip the illumination carrier
(8.1) back slightly and install it so that the pin (8.2)
engages the groove in the support surface (8.4).
Set the TL illumination carrier upright and fasten
it with the 4 screws.
When fastening the transmitted light illumination
carrier, do not hold it. This will ensure its optimal
alignment with the optical axis.
The tilt angle of the illumination carrier can be
varied with the knurled screw (9.1) or fi xed vertically.
Fig. 8 Installing the transmitted light illumination carrier
1 Transmitted light illumination carrier
2 Transmitted light illumination carrier pin
3 Support surface
4 Support surface groove
5 Support surface groove
6 EXT1-EXT4 sockets
7 Connector cable
Leica DMI4000 B and Leica DMI6000 B
Connect the electronics cable to one of the sockets, EXT1 – EXT4.
The transmitted light lamp housing for 12 V
100 W halogen lamps is a separate component.
For instructions on replacing the halogen lamp
→ Ch. 6.10, p. 45.
Fig. 9 Transmitted light illumination carrier, rear side
1 Knurled locking knob
of the transmitted light illumination carrier
2 Connector cable for the microscope rear side
1
2
1
3
7
4
2
6
1
5
1
5
31
Page 32

6. Assembly
6.3 Installation of the DIC Module
and DIC Objective Prisms
If your microscope is not equipped with DIC,
please continue with Chapter 6.4.
In the Leica DMI series microscopes, the DIC
prisms are already installed in the DIC disk below
the objective turret (Fig. 10b).
Motorized, manual
coded and manual DIC disks are available. The
installation is identical for all types.
Proceed as follows when making changes to the
IC prism disk:
• Remove the front cover (Fig. 11) below the
objective revolver after releasing the socket
screws (Fig. 10a).
Fig. 10a Removing the front cover
Fig. 11 Front cover, DIC prism disk
• Insert the DIC prism disk (Fig. 10b) squarely in
its receptacle. First, lightly tighten one screw
with the included 3 mm hex screwdriver, then
tighten both Allen screws.
Note: insert the prism disk with the electronics
board facing down. Do not touch the electronics (especially the contacts) with your bare fi ngers!
Replacing Individual IC Prisms:
• Release the two socket screws and remove
the prism disk.
• Place the prism against the stop pin (10b.3),
place the washer between the screw and the
prism, and tighten gently to prevent undue tension. Insert the prism so that its identifying letter, e.g. ID, is facing upward and is legible.
• After installing the prisms, replace the prism
disk in its receptacle.
Fig. 10b DIC objective prism turret (coded and motorized)
1 IC objective prism in frame
2 Identifi cation letter (ID)
3 Orientation pin
Fig. 12 IC objective prism
1 Objective prism in frame
2 Screw and washer
32
1
2
32 1
Page 33

6.4 Installation of Specimen Stages
A wide range of specimen stages are available.
The most important are the following:
• Fixed stage (248 mm x 204 mm) (Fig. 13):
normal, heating and temperature-controlled,
with and without attachable mechanical stage
• Fixed micromanipulation stage (248 mm x
204/112 mm) (Fig. 15): normal, heating, and
temperature-controlled, with and without attachable mechanical stage
• Standard manual (Fig. 14) and motorized
3-plate cross-stage, positioning range: 83 mm
x 127 mm
• Manual (Fig. 15) and motorized micromanipulation 3-plate cross-stage
positioning range: 40 mm x 40 mm
• manual rotating stage
• scanning stage 120 x 100
(motors on bottom)
6. Assembly
Fig. 14 Mechanical 3-plate stage
Fig. 15 Micromanipulation stage with attachable
mechanical stage
Fig. 13 Fixed stage (normal)
Fig. 16 3-plate micromanipulation stage
33
Page 34

6. Assembly
The assembly of these stages is identical. The
stages are solidly attached to the microscope by
three screws. In the case of fi xed stages, an attachable mechanical stage may be installed (Fig.
18). These are supplied in a separate package.
Multiple-plate stages are supplied separately.
Like the fi xed stages, these stages are mounted
as follows:
• If the screws for the stage are already in the
stand, remove them fi rst. In most cases, the
screws will be found in the packing material of
the stand.
Caution!
!
The screw lengths may vary. When using screws
of different lengths, use the shorter of the three
screws in the front hole and the equally long
ones in the rear holes.
• Use a clean cloth to remove dust and packing
material residue from the stand’s contact surface for the stage.
• Align the stage so that the pair of holes faces
back toward the illumination axis and the single hole faces forward toward the tube.
• Align the mounting holes in the stage with the
holes in the support surface. If the holes are
covered in the case of 3-plate cross-stages or
scanning stages, please shift the upper stage
plate until the opening becomes visible.
• First, tighten the single front screw with the included 3 mm hex screwdriver. Be sure to use
the shortest of the three screws in the front
hole, as an excessively long screw can interfere with the focus travel.
• Next, fi rmly tighten the two rear screws.
• Finally, give the front screw a fi nal fi rm tightening.
Fig. 17 Fixed micromanipulation stage
34
Fig. 18 Attachable mechanical stage for fi xed
micromanipulation stage
Page 35

6. Assembly
Fixed Stage
Attachable mechanical stages designed to accept a variety of culture dishes are also available
for fi xed stages (Fig. 18).
Two screws are included with the attachable
mechanical stage. Tighten these screws in the
threaded holes on the underside of the fi xed
stage with the 3 mm hex screwdriver. Retighten
these screws from time to time after frequent
use.
The attachable mechanical stage has been preadjusted in the factory. In the event that the attachable mechanical stage runs out of focus
when moving from right to left, this can be corrected by Leica’s technical service.
Next, remove one or more of the ordered insert
frames (Fig. 20) from their packaging and place
the insert frame into the precise retention system. The stage, the attachable mechanical stage,
and the insert frame are now ready for use.
Some (not all) inserts are provided with selfadhesive scales to permit the coordinates to be
read.
Apply these scales to the recesses of the attachable mechanical stage.
Fig. 20 a, b, c
Inserts for attachable mechanical stage (micromanipulation
stage)
a
Fig. 19 a, b Inserts for attachable mechanical stage
(fi xed stage)
a
b
b
c
35
Page 36

6. Assembly
Manual Fixed Micromanipulation Stage
To install the attachable mechanical stage for the
manual fi xed micromanipulation stage (Fig. 24),
proceed as you would for the attach able mechanical stage of the standard stage.
The insert frames (Fig. 20a to c) differ at this point.
These are held by two screws on the attachable
mechanical stage and changed by re leasing the
screws.
Fig. 21
Inserts for fi xed stages
Fig. 24 Installation of attachable mechanical stage
Fig. 25 Installation of attachable mechanical stage
Fig. 22
Glass insert for
3-plate cross-stage
and scanning stage
Fig. 23
Heater insert
36
Page 37

6. Assembly
Motorized 3-plate or Scanning Stages
3-plate stages and scanning stages: after installing the stage, connect the included stage cable
(for motorized stages) fi rst to the socket on the
stage, then to the CRT6000, CTR6500 or CTR7000
box. The correct place on the box is called „XY
Stage“.
A variety of inserts (including heating ones) are
available for the normal 3-plate and scanning
stages. Install these inserts diagonally from
above into the corner with the spring clips. The
insert will click into place when seated properly.
!
Caution:
Press the spring clip into place only from the
side.
Do not press the insert onto the spring clips diagonally from above, as the insert will not be
aligned parallel to the stage and may be bent in
the process.
Fig. 29 a, b Mounting screws for 3-plate cross-stage
ab
37
Page 38

6. Assembly
6.5 Installation of Condensers
All condensers of the Leica DMI Series are
equipped with a 7-position turret disk that can be
equipped with light rings phase contrast (PH) or
dark fi eld (DF), IC prisms for transmitted light interference contrast (DIC),
integrated modulation contrast (IMC).
or slit illuminators for
Light rings, slit diaphragms, and condenser
prisms are generally already factory-installed in
the turret, making the following assembly steps
unnecessary. Please continue on → page 41, Installation of Condensers.
Installing the Light Rings and Slit Diaphragms
• Switch the microscope off.
• Remove the condenser cover (38.1). Insert the
light ring in one of the condenser disk’s large
receptacles with guide grooves.
• Turn the right-hand centering screw back fully
with the adjusting key (39.2).To prevent the
condenser disk from turning further, insert the
adjusting key (39.2) into the left-hand centering
screw of the disk. It may protrude a maximum
of 1 mm into the opening.
Insert light rings for Phaco (marked with the ID
numbers 0, 1, 2, 3 and the focal intercept S of the
corresponding condenser head), DF diaphragms
(marked with a D for dark fi eld and the focal intercept S of the corresponding condenser head),
and slit diaphragms (marked M05, M10, M20,
M40 and M63)
in the location holes of the turret
disk as follows:
• Select a position and ensure that the two
mounting screws have been released to the
point that they no longer extend into the position. To adjust the screws, turn the desired light
ring position into the beam path. You can now
turn the screws using the two adjusting keys.
• Next, take the special condenser tool
(Fig. 39.1).
• If possible, install the light rings 0 to 3 in ascending order. The numbering of the openings is located at the edge of the crown gear
(4 large openings: 1-4; 3 small openings: 5-7).
Fig. 34
Condenser head S1
Fig. 33 Condenser base S1-S28
38
Fig. 35
Condenser head S28
Page 39

6. Assembly
• Grasp the light ring to be installed with the
condenser tool (the lettering must face upward
and be legible) so that the tab of the light ring
is positioned to the center of the tool’s cam and
the upper edge of the light ring is lying fl at in
the holder of the tool. The numbers should be
positioned toward the end of the tool. Press
the cheeks of the tool to grasp the light ring
(Fig. 39a).
• Two guide hooks are located on the underside
of the light rings. These must fi t into the two
grooves of the opening.
Insert the light ring (holding the condenser tool
angled slightly upward and at a 90° angle to
the housing) so that the mount fi ts under the
spring clip of the retainer (Fig. 3).
!
Caution:
Do not press the spring clip down under any
circumstances. This can destroy the clip or result in an unstable position of the light ring.
Turn the light ring to ensure that it snaps into
position and release the tool.
Remove fi ngerprints or dust from the prism
with care.
• Use the left centering screw to roughly center
the light ring. The right centering screw must
not restrict the range of adjustment under any
circumstances.
• Note the number of the opening and the light
ring designation for entry into the Leica Application Suite (LAS).
• Remove the adjusting key and close the condenser.
• Fine adjust with the Bertrand lens or telescope
after switching the unit on (Fig. 32).
Please continue reading if you also have to install
IC prisms. Otherwise, skip to the next section.
Fig. 36 Phase rings Fig. 37 Condenser prisms
39
Page 40

6. Assembly
Installation of IC Prisms
• Switch the microscope off.
• Remove the condenser cover (38.1). Insert the
prism in one of the condenser disk’s large receptacles with guide grooves.
• Turn the right-hand centering screw back fully
with the adjusting key (39.2). To prevent the
condenser disk from turning further, insert the
adjusting key (39.2) into the left-hand centering
screw of the disk. It may protrude a maximum
of 1 mm into the opening.
• Grasp the prism to be installed with the condenser tool (the lettering must face upward
and be legible) so that the tab of the prism ring
is positioned to the center of the tool’s cam,
and the upper edge of the prism is lying fl at in
the holder of the tool. The numbers K2 to K16
should be positioned toward the end of the
tool. Press the cheeks of the tool to grasp the
prism (Fig. 39a).
• Two guide hooks are located on the underside of the prisms. These must fi t into the two
grooves of the opening.
Insert the prism (holding the condenser tool
angled slightly upward and at a 90° angle to
the housing) so that the mount fi ts under the
spring clip of the retainer (Fig. 39a).
Fig. 38 Condenser
1 condenser cover, 2 centering opening
1
2
40
Fig. 39 Open condenser
1 condenser tool, 2 adjusting key
1
2
Fig. 39a Inserting the prism
The designation must be visible when
installed and oriented toward the center
of the condenser.
DIC images are not possible otherwise.
1
Page 41

6. Assembly
!
Caution:
Do not press the spring clip down under any
circumstances. This can destroy the clip or result in an unstable position of the prism.
Turn the prism to ensure that it snaps into po-
sition and release the tool.
Remove fi ngerprints or dust from the prism
with care.
• Use the left centering screw to roughly center
the prism. The right centering screw must not
restrict the range of adjustment under any circumstances.
• Note the number of the opening and the prism
designation for entry into the Leica Applica tion
Suite (LAS).
• Remove the adjusting key and close the condenser.
• Fine adjust with the Bertrand lens or telescope
after switching the unit on (Fig. 32).
Installation of Condensers
The installation procedure is identical for all condensers S1 to S70 (motorized or manual/coded not coded for S40).
Release the socket head screw at the right side
of the condenser holder. Place the condenser
on the retaining pins of the illumination arm and
move the condenser to the correct height. Use
the markings on the column and condenser to
determine the correct position.
Once you have reached the correct position,
tighten the socket head screw.
Fig. 40 Installation of condenser on
transmitted light illumination arm
41
Page 42

6. Assembly
Condenser Heads
Four different condenser heads are available:
1) S1/1.40 oil
2) S1/0.90 dry
3) S23/0.53
4) S28/0.55
Condenser heads 3 and 4 are screwed directly
into the condenser body. A spacer ring (42.2)
must be screwed into the thread at the bottom of
the condenser body prior to installing condenser
heads 1 and 2. The S1 condenser heads fi t into
this ring.
The S40 and S70 condensers are delivered complete with a condenser head, making additional
assembly unnecessary.
Fig. 41 Condenser on transmitted light illumination arm
Fig. 42 Installation of condenser heads S1
1 Condenser base
2 Spacer ring
3 Condenser head
42
Fig. 43 Installation of condenser head S28
1
2
3
Page 43

6. Assembly
6.6 Installation of Eyepieces
The eyepieces are inserted into the eyepiece
tubes.
Note:
We recommend running a teach-in via the Leica
Application Suite (LAS) software when using
eyepieces not included in the scope of delivery.
This will ensure that the total magnifi cation
shown on the LeicaScreen is correct.
Fig. 44 Eyepieces
6.7 Installation of Objectives
The positions in the objective turret disk are numbered (Fig. 45). Depending on your equipment,
the individual objectives have already been assigned to specifi c positions at the factory.
For details on the exact positions of the objectives, please refer to the enclosed identifi cation
sheet.
Caution:
!
Close vacant threads in the nosepiece with dust
protection caps!
Please note that the front lenses of the objectives
point upward and are therefore more vulnerable
to contamination than those of upright microscopes.
Check the front lenses for cleanliness frequently.
Note:
Leica DMI6000 B:
We recommend running a parfocality compensation via the Leica Application Suite (LAS) software.
Fig. 45a Objective turret Fig. 45b Objective turret, loaded
43
Page 44

6. Assembly
6.8 Installation of Filters in the Illumination Arm
The Leica DMI Series is equipped with a fi lter
magazine to accommodate two 40 mm dia. fi lters
as a standard feature. The fi lters are factoryinstalled. To change fi lters yourself, proceed as
follows:
• Release the screw (46.1) and remove the cover.
• Place the fi lter in the holder.
• Place the cover on transmitted light illumination
carrier and fasten with the locking screw.
Leica DMI6000 B:
• Activate the fi lters via the Leica Application Suite
(LAS/LAS AF).
Leica DMI3000 B and Leica DMI4000 B:
• Mark the 2 levers with the provided adhesive labels.
Fig. 46 Unscrewing the fi lter holder cover and inserting
fi lters in the transmitted light illumination arm
1 Screw
1
6.9 Installing the transmitted Light Lamp
Housing
• Place the lamp housing in the transmitted light
lamp housing mount (Fig. 47), and fasten it with
the clamping screw on the side.
• Thread the cable through the transmitted light
illumination arm (Fig. 48).
• Connect the lamp housing cable to the power supply for transmitted light on the Leica
CTR4000–7000 electronics box (Fig. 49.1).
Leica DMI3000 B:
• For the DMI3000 B, connect the cable directly
to the back of the microscope.
For instructions on changing the lamp, please
see Chapter 6.10.
These instructions also apply to installing an Hg
lamp on the transmitted light axis. For descriptions of the lamp housings and replacement of
the burner, please see Chapter 6.12, → p. 48ff.
Fig. 47 Mounting the lamp housing on the
transmitted light illumination arm
Fig. 48 Lamp housing cabling (cable duct)
44
Fig. 49 Connecting the lamp housing to the
Leica electronics box, example: Leica CTR6000
1
Page 45

6. Assembly
6.10 Installation and Replacement of the
transmitted Light Lamps: 107 or 107/2 Lamp
Housing
This lamp housing is used with a 12V 100W Halogen Lamp, which is already mounted.
In case the lamp has to be removed:
Changing the 12 V 100 W halogen lamp
Caution!
Ensure that the lamp housing has been disconnected from the power supply. Unplug the
power plug and the power supply during assembly.
Caution!
Light sources pose a potential irradiation risk
(glare, UV-radiation, IR-radiation). Therefore,
lamps have to be operated in closed housings.
• Lift the housing off (Fig. 50b).
• Remove the lamp.
Caution!
Do not remove the new lamp’s dust cover until you have installed the lamp. Avoid fi ngerprints on the lamp.
• Insert the new 12 V 100 W lamp (Fig. 51) with
the dust cover straight into the socket until it stops. Be sure that the lamp is inserted
straight.
• Remove the lamp’s dust cover.
• Replace the housing and fasten it in place using the fastening screw.
Fig. 50b
Removing housing
• Remove the fastener screw on the housing
(Fig. 50a).
Fig. 50a
Lamp housing 107/2
Releasing the
fastening screw
Fig. 50c
Lamp housing 107/2
opened
1 Mount with
halogen lamp
2 Collector
1
2
45
Page 46

6. Assembly
Fig. 51
Inserting
lamp with
cover
a right
b wrong
6.11 Installing the Lamp Housing Mount and
Mirror Housing (Leica DMI4000 B and
DMI6000 B)
Place lamp housing mount (Fig. 53) or mirror
housing on rear wall. Mount from front with socket head screws.
a
b
Fig. 53 Lamp housing mount
Next, attach the appropriate connector(s) (right,
left, straight) to the lamp housing mount. The
lamp housing or coupling is then mounted on the
connector, which is also held by four screws.
Fig. 52 Rear view, Leica DMI4000 B and DMI6000 B
1 Installation point for lamp housing mount
or mirror housing
2 Holes for lamp housing mount or mirror housing screws
2
1
2
46
Fig. 54 Lamp housing 106z
1 Collector adjustment
2 Vertical lamp adjustment
3 Horizontal lamp adjustment
4 Adapter ring
4
2
3
1
Page 47

If a booster lens is included in the scope of delivery, insert it into the rear stand opening at the left
or right, depending on the stand model.
The booster slide has several positions:
1. Slide pulled out:
no effect
2. Depending on orientation of slide:
a) symbol visible:
•
center orientation
The intensity of the fl uorescence is
increased by 50% in the center of the
fi eld of view (approx. 30% of the fi eld).
b) symbol
visible:
The overall intensity is reduced by
25%. The entire fi eld of view is evenly
illuminated, however.
6. Assembly
Fig. 56 Booster lens in stand
1 Booster lens
1
Fig. 55 Booster lens
Fig. 57 Hg-mercury burner
47
Page 48

6. Assembly
3
1
4
6.12 Installation and Replacement
of Incident Light Lamps
Caution!
Light sources pose a potential irradiation risk
(glare, UV-radiation, IR-radiation). Therefore,
lamps have to be operated in closed housings.
Ensure that the lamp housing has been disconnected from the power supply. Unplug the
power plug and the power supply during assembly.
During assembly work on xenon burners, always wear the supplied protective gloves and
face protection (Fig. 58) (risk of explosion).
Never touch the glass parts of the burner
with bare hands.
Never look directly into the beam path (blinding hazard).
Lamp Housing 106 z
This lamp housing is suitable for use with a 12 V
100 W halogen lamp or a variety of gas discharge
lamps.
Caution!
Make sure to follow the instructions and
safety notes of the lamp supplier.
Before changing lamps allow at least 30 minutes for cooling down!
Fig. 58
Protective gloves and mask
48
Fig. 59 Lamp housing 106 z L with Hg 100 W lamp
1 Collector focusing
2 Vertical lamp adjustment
3 Horizontal lamp adjustment
4 Hg lamp mount
5 Refl ector adjustment (not visible)
2
5
Page 49

6. Assembly
Inserting Gas Discharge Lamps (Hg and Xe) in
the 106z Lamp Housing
Hg and Xe lamps are powered by separate supply units.
Please also read the separate instruction manual
provided with these supply units.
The following gas discharge lamps may be used
and require different supply units and lamp
mounts (Fig. 60, 61):
Type Typical Bulb Life*
100W high-pressure mercury burner (direct current) 200 hrs.
100W high-pressure mercury burner (direct current, type 103 W/2) 300 hrs.
75W high-pressure xenon burner (direct current) 400 hrs.
* Please observe the data sheets of the lamp manufacturer.
Fig. 60 Lamp mounts for Hg 100 gas discharge lamp
1 Upper clamping system
2 Lower clamping system
3 Cooling element
Hg 100
3
1
2
Fig. 61 Lamp mounts for gas discharge lamp Xe 75
1 Upper clamping system
2 Lower clamping system
3 Cooling element
4 Protective cover of Xe 75 burner
a
Xe 75
1
2
b
3
4
49
Page 50

6. Assembly
Caution!
Caution!
Make sure to follow the safety notes on
page 48.
• To open the 106 z lamp housing, unscrew the
fastening screws on the cover l. Loosen the
contact plug somewhat and pull it out of the
socket (63.9). Flip the cover up (63.1).
• Loosen the mounting screws (63.8) on the lamp
socket and pull the socket out.
• Remove the transport anchorage (red plastic
rod in place of the burner) in the lamp mount.
To do so, remove the lower clamp (60.1, 61.1).
Pull up the cooling element (61.3, 60.3) and turn
it to the side. Detach the lower clamp system
(61.2, 60.2) and remove the tr
nsport an-
chorage.
Do not remove the burner’s dust cover until
you have installed the lamp. Avoid fi ngerprints on the lamp. Sweat from your fi ngers
on the glass will shorten the life of the lamp
signifi cantly.
• Install the burner in reverse order.
Caution!
Xe 75 burner:
Remove the burner’s dust cover (61.4) after
you have installed the burner.
Fig. 63 106 z lamp housing (on the side, open)
1 Cover raised
2 Collector
3 12V 100W lamp or
gas discharge lamp in mount
4 Refl ector (mirror)
5, 6, 7 Adjusting screw for x-y refl ector
8 Locking screws for lamp mount
9 Socket for contact plug
Fig. 62 Rear panel of ebq 100 supply unit
1 Lamp connection
1
50
1
2
4
5
3
7
898
Page 51

6. Assembly
• Insert the lamp mount, with the burner in-
stalled, into the lamp housing and tighten it
with the screws (63.8).
• Test the adjustment of the collector (63.2):
Do not touch the power supply while perform-
ing these actions. When closing the lamp
housing, ensure that the pins of the contact
plug engage in their sockets (63.9).
Tighten the screws of the cover and press the
contact plug home.
• Place the lamp housing in the incident light
lamp housing mount (Fig. 53) and fasten it with
the clamping screw on the side.
• Connect the lamp housing to the external pow-
er supply (62.1).
Caution!
The burner must be adjusted immediately after lighting.
Leica EL6000
Caution!
When using the compact light source Leica
EL6000, it is essential to observe the safety
information in the separate instructions.
51
Page 52

6. Assembly
6.13 Equipping the Incident Light Turret Disk
Caution:
!
Please read this section completely before beginning with the assembly of the turret disk.
Leica DMI4000 B and Leica DMI6000 B:
The fl uorescence drawer is located on the right
side of the stand. Before opening this drawer,
remove the cap below the drawer covering the
analyzer slot (65.1). Remove the analyzer if it is
already in the slot.
The replacement of individual cubes is more convenient with the microscope switched on. The
position to be changed then automatically turns
to the outside and you can be sure that the cube
is positioned in the correct holder. You can therefore postpone installing the fi lter cubes until after
the microscope has been switched on.
You can also insert the fi lter cubes while the instrument is switched off.
Press the white button next to the drawer. The
drawer will glide out into its initial position.
Fig. 65 Opening the fl uorescence drawer
1 Analyzer slot
1
Fig. 66 Open fl uorescence drawer
1 Lever for fi xing the loading position
Fig. 64a Filter cube,
front side
52
1
Fig. 67 Inserting or removing a fi lter cube
Fig. 64b Filter cube,
back side
Page 53

6. Assembly
The positions in the turret disk are numbered. Depending on your equipment, the individual fi lter
and refl ector cubes have already been assigned
to specifi c positions at the factory. For details,
check the identifi cation sheet included with your
order.
Now open the drawer several mm further until
it clicks into its end position. Actuate the lever
(66.1) to engage the turret disk in the loading position.
You can now insert a fi lter block. Proceed as follows:
• With the holder facing you squarely, insert
the fi lter or refl ector cubes into the holder in
accordance with the included identifi cation
sheet.
• The fl uorescence cubes are suitable for both
upright and inverted microscopes. When using
them with inverted microscopes, insert them
so that the writing is upside down along the
lower edge.
• Release the lever (66.1) again to turn the disk
on to the next loading position. Continue in this
way for all of the cubes.
• Once all fi lter and refl ector cubes have been
inserted, close the drawer and replace the
analyzer or cap.
Replacing Cubes with the Instrument Switched
On:
• Remove the analyzer or the cap of the analyzer
slot.
• Press and hold the Shutter button on the front
panel and press the button of the cube you
would like to insert or replace at the same
time.
• The fi lter changer will then rotate to the correct position to insert or replace the cube
when you open the drawer by pressing the
white button on the right side of the stand.
The following message will appear in the top
line of the LeicaScreen.
To do so, place the fi lter or refl ector cube on
the left side and press it to the right into the
mounting (Fig. 67).
• Ensure that the cube is correctly seated. A
loose cube can block the disk or be destroyed
by the turning disk.
Load!
To insert the cubes, proceed exactly as described
above.
53
Page 54

6. Assembly
Leica DMI3000 B:
To equip the turret disk with fi lter cubes, the turret disk must be removed from the stand (left side
of stand, Fig. 68).
The supports of the disk are labeled Pos1 to Pos5
(Fig. 69).
• Pull the fi lter slider out of the stand.
• Insert the fi lter cubes in the supports so that
the labeling is upside down.
To do this, position the fi lter cube at the left
side and engage it to the right in the mount.
One position of the turret disk must remain free
for transmitted light bright fi eld.
• When all fi lter cubes are inserted, push the
fi lter slider to the stop again in the left stand
side.
Fig. 68 Removing the fi lter slider Fig. 69 Filter slider
54
Page 55

6. Assembly
6.14 Inserting the Front Module Slider
If your microscope is prepared for integrated
modulation contrast or integrated phase contrast, a front module (possibly in conjunction with
a manual magnifi cation changer) will be integrated in the stand. This is recognizable by a 2 x 3 cm
opening at the left front side of the microscope.
If this opening is not present or closed, then your
microscope is not prepared for the integrated
processes.
A slider for integrated modulation contrast or integrated phase contrast fi ts in this opening. The
phase contrast slider may still require the installation of phase rings.
Insert the slider with the markings facing forward. It features a bright fi eld position and two
positions for contrast methods (position A and
position C).
(A and C designate the eyepoint of the used objective. Please refer to the included objective list
for the eyepoint of your objective. It can also be
found engraved on the objective.)
6.15 Installation of the Polarizer and Analyzer
Installed at the factory.
To change the components, proceed as follows:
Motorized condenser:
See included installation instructions.
Manual condenser:
Attach the single or triple position holder to the
top of the manual condenser. The holder has a
guide that must be inserted in the opening next
to the screw threads. The holder must be positioned so that the polarizer or fi lter to be used
covers the opening of the condenser.
Insert the polarizer or fi lter with the correct side
facing up into the holder (λ: lambda and polarizer; POL: polarizer only). A click mechanism will
indicate proper seating. The polarizer must turn
easily between the two stops (approx. 30°).
Fig. 70 Mechanical polarizer holder
1 Manual polarizer
2 Manual analyzer
1
Fig. 71 Condenser with motorized polarizer
2
55
Page 56

6. Assembly
Analyzer for Incident Light and Transmitted Light.
• Remove the cap (Fig. 72) on the right side of the
stand (under the fl uorescence drawer).
• Insert the analyzer into the receptacle until it
latches in place (Fig. 73.1).
Fig. 72 Analyzer slot cap
Fig. 73 Inserting the analyzer
1 Slot
2 Analyzer
56
Fig. 74 Inserting the analyzer
1
2
Page 57

Fig. 75 C-mount 0.63x
6. Assembly
6.16 Optional Accessories
Camera
Connecting a camera
A camera can be installed using a C-mount or
Vario mount.
• Place the C-mount or Vario mount onto one of
the camera ports and secure it with the locking
screw at the side.
• Screw on the camera.
Note:
When using a C-mount or Vario mount, run a
teach-in via the Leica Application Suite (LAS)
software.
Connecting multiple cameras
Two or more cameras – for example a digital and
an analog camera – can be adapted as required.
Fig. 76 C-mount 0.5x
• When using a DC type camera, connect the
camera to the PCI card of your PC.
• When using a DFC type camera, connect the
camera to the FireWire card of your PC.
Note:
Please read the separate operating manual of
your digital camera.
57
Page 58

6. Assembly
6.17 Connection to the Electronics Box
CTR4000, CTR5000, CTR5500, CTR6000,
CTR6500, CTR7000, CTR6500 HS, CTR7000 HS
The Leica DMI 3000 B is supplied without an
electronics box. The power supply is integrated
in the stand and a socket has been provided on
the back of the microscope to connect the transmitted light illumination. The illuminated ON/OFF
switch is located on the stand.
Fig. 77 Rear view of electronics box, example: CTR6000
1 AC power socket
2 XY Stage socket for motorized stage
3 Direct interface socket optional
4 Z Control for separate focus control
5 XYZ Control for SmartMove
6 Microscope socket for microscope
7
12 V, max 100 W for the lamp power cable of stand
8 DL: reset button
CTR 4000 Electronics Box
The Leica DMI 4000 B is supplied with the
CTR4000 electronics box. The power supply
for the microscope is located in this box. Two
sockets are located on the back of the CTR4000
electronics box for 12V/100W transmitted light
and 12V/100W incident light illuminators. The illuminated ON/OFF switch for the microscope is
located on the CTR4000 electronics box.
Fig. 78 Rear view of stand
1 RS232 ports
2 2 x USB
3 4 x EXT.
4 XYZ control for SmartMove
5 Electronic box connection
6 Condenser cable
7 Lamp power cable
58
8
5
4
3
6
2
7
6
1
1
7
2
5
4
3
Page 59

6. Assembly
CTR4000, CTR5000, CTR5500, CTR6000, CTR6500,
CTR7000, CTR6500 HS, CTR7000 HS Electronics Box:
Note:
These electronics boxes must not be used with
other stands. The serial number of the associated stand has been recorded on the back of the
electronics box.
A 3-axis control unit for focus and motor stages
is integrated in the CTR6000.
A 3-axis control unit for focus and a scanning
stage is integrated in the CTR6500/7000.
• Connect the Microscope (77.6) socket to the
back of the stand (78.5) using the 25-pin microscope cable.
• Connect the SmartMove remote control module to the XYZ-Control socket (77.5).
• Connect the motorized stage, if present, to the
XY-Stage socket (77.2).
Caution!
Ensure that the plugs are correctly inserted
and secured to prevent overheating of the
sockets.
6.18 Connection to the Computer
Note:
To start the Leica Application Suite (LAS/LAS AF),
ensure that the COM1 serial port is not in use by
another program or driver. This is frequently the
case when using Palms or other PDAs or when
using external modems or other devices. The
devices in question must therefore always be
disabled before using the Leica Application Suite
(LAS/LAS AF) software.
• Please use the included serial cable. Connect
the COM1 port of your PC with the RS232C port
(78.1) on the back of the stand.
Alternatively the PC can be connected via
USB.
• Connect the lamp power cable (78.7) to the 12
V, max 100 W socket (77.7).
Fig. 79 Rear panel of ebq 100 supply unit
1 AC power supply socket
1
6.19 Connection to the Power Supply
• Once all installation work is complete, connect
the electronics box to an AC power outlet with
the included power cable (socket 77.1).
• If you are using the external ebq 100 supply
unit or the compact light source Leica EL6000,
connect it to an AC power outlet at this time
(socket 79.1).
59
Page 60

7. Start-up
7. Start-up
7.1 Functional Principle (Leica DMI4000 B and Leica DMI6000 B)
Thanks to its intelligent automation, the Leica DMI4000 B and DMI6000 B can be controlled using a
variety of control elements.
1 . Intelligent Automation
• Switching between contrast methods at the touch of a button. Light rings, DIC prisms, etc.
are automatically positioned in the beam path.
• The microscope recognizes the selected objective and associated contrast method.
The intensity (INT), aperture diaphragm (AP) and fi eld diaphragm (FD) are always set to suitable values.
• The INT, AP and FD values are always based on the currently activated illumination axis
(transmitted light or incident light).
• The INT, AP, and FD values can be adjusted individually. Manual adjustments overwrite the
previous settings. The current setting is stored and is retained from one session to the next
when power is switched off.
2. Controls
• SmartMove knobs
for stage and focus control
• Fixed function buttons on stand
for INT, AP, and FD, as well as for switching between transmitted light and incident light axis
• Variable function buttons on stand, SmartMove, STP6000
These function buttons have functions suitable to the confi guration of your microscope as-
signed to them at the factory. The functions can be reprogrammed and/or adapted to your
specifi c requirements, however.
• Complete control of microscope and camera via software
(Leica Application Suite (LAS/LAS AF))
60
Page 61

Note: ( reset function)
The microscope can be reset to its factory default programming:
• With the stand switched off, press the top three
variable function buttons on the left side of the
stand.
• Switch on the power for the stand.
• Hold the buttons until the initialization is complete.
• The standard information display will now appear on the LeicaDisplay.
• Switch the instrument off and back on. The settings are now saved.
7. Start-up
The table on the following page provides an overview of the microscope functions and their controls.
61
Page 62

7. Start-up
Function Fixed Variable SmartMove Software
(DMI4000 B and DMI6000 B) Function Function Function Rotary and
buttons buttons buttons knobs STP6000
Stand Stand
4000 6000 4000 6000 4000 6000 4000 6000 4000/6000
Select contrast method - - + + + + - - +
Change transmitted light/incident light axis + + - - - - - - +
Change to objective - - - + - + - - +
Teach-in parfocality - - - - - - - - +
Change operating mode (dry/imm) - - + + + + - - +
Illumination Manager + + + + + + - - +
Magnifi cation changer (motorized) + + - - - - - - +
1)
Focusing - + - - - - - +
Set stops - + - - - - - - +
Go to stop - + - - - - - - +
Change step increment (coarse/fi ne) - - - + - + - - +
XY stage positioning - - - + +
Change speed - - - - - - - - +
Stage positions (store/go to) - - - - - - - - +
Change to fi lter/refl ector cube + + (+) + + - - +
Side and bottom port (DMI6000 B only) + (+) + - +
DIC fi ne adjustment + + - - - - - - +
+
Adapted Focus Control (AFC) - + - + - + - - +
+ always possible
(+) optional
- not possible
1)
Focusing alternatively via wheels
62
Page 63

7. Start-up
Possible Assignments for Variable Function Buttons on Stand and SmartMove
For Leica DMI4000 B and Leica DMI6000 B:
Function button Function
BF Bright fi eld transmitted light
PH Phase contrast transmitted light
ICT Interference contrast, transmitted light
DF Dark fi eld transmitted light
IMC Integrated modulation contrast
POL Polarization transmitted light
CHANGE TL Cycle through all contrast methods
INT ↑ Increase intensity (transmitted light)
INT ↓ Reduce intensity (transmitted light)
AP ↑ Open aperture diaphragm (transmitted light)
AP ↓ Close aperture diaphragm (transmitted light)
FD ↑ Open fi eld diaphragm (transmitted light)
FD ↓ Close fi eld diaphragm (transmitted light)
SHUTTER TL Open/close TL shutter
TL FLT 1 Enable/disable transmitted light fi lter at position 1
TL FLT 2 Enable/disable transmitted light fi lter at position 2
FLUO Fluorescence (last fi lter cube)
CUBE 1-6 Select fi lter cube in position 1-6
CHANGE CUBE CW Change cube clockwise (1 → 4)
CHANGE CUBE CCW Change cube counterclockwise (4 → 1)
INT FLUO ↑ Increase intensity (fl uorescence)
INT FLUO ↓ Reduce intensity (fl uorescence)
FD FLUO ↑ Open fi eld diaphragm (fl uorescence)
FD FLUO ↓ Close fi eld diaphragm (fl uorescence)
CHG FW Toggle fi lter functions
IFW Activate external fi lter wheel
ExMan Activate Excitation Manager
SHUTTER FL Open/close fl uoshutter
COMBI Combination method (PH fl uorescence or ICT fl uorescence)
CHANGE COMBI Cycle through all combination methods
CHANGE OBJ CW Cycle through objectives clockwise
CHANGE OBJ CCW Cycle through objectives counterclockwise
Z FINE Activate fi ne focusing (Leica DMI6000 B only)
Z COARSE Activate coarse focusing (Leica DMI6000 B only)
XY PRECISE Activate precise stage
XY FAST Activate fast stage
BTP ON/OFF Bottom port on/off (Leica DMI6000 B only)
DRY/IMM Switch dry/immersion
CHANGE FLT Switch TL fi lter
CHANGE CS Switch to confocal application
OBJ 1-6 Select objective at position 1-6
MEM 1-6 Memory activated stored functions
AFC ON/OFF Turns AFC on or off
AFC HOLD Holds current position
63
Page 64

7. Start-up
7.2 Switching on the Microscope
Leica DMI3000 B:
• Switch on the microscope’s power at the On/
Off switch. The signal lamp is lit when the instrument is ready. (For the Leica DMI3000 B
please continue at 7.4. Function Buttons on the
Stand)
Leica DMI4000 B and Leica DMI6000 B:
• Switch on the power of the electronics box at
the On/Off switch (80.1). The signal lamp (80.2)
is lit green when the unit is ready. All motor ized
microscope components will then run through
an initialization phase.
Note:
If a PC is connected, switch on the electronics
box fi rst, and then the computer.
After the initialization (Fig. 81) is complete, the
LeicaScreen will display the microscope’s current settings (Fig. 82).
If a component has not been installed correctly,
the LeicaScreen will display an error message.
See Troubleshooting chapter, → p. 105.
Components such as diaphragms, condensers,
light, and phase rings have been pre-centered
at the factory. It may be necessary to correct the
centering after the microscope has been transported and assembled.
Before performing the required steps, please familiarize yourself with the LeicaScreen and the
controls.
Caution!
After turning on the gas discharge lamp, the
burner must be immediately adjusted. Therefore, do not turn on the power supply unit
yet. First, work in transmitted light in order
to familiarize yourself with the microscope’s
controls.
Fig. 80
front side
Leica CTR6000
1 On/Off switch
2 Signal lamp
64
Fig. 81
LeicaScreen
Initialization
Fig. 82
LeicaScreen
after
Initialization
2
1
Page 65

7. Start-up
7.3 The LeicaDisplay
(Leica DMI 4000 B and DMI 6000 B)
The screen displays the microscope’s current
settings. The content of the display depends on
the features of the individual microscope.
For information on the abbreviations used, please
turn to the table of abbreviations → p. 113.
The screen has a number of areas and lines.
Line 1: contrast method
Line 2: objective/ magnifi cation
Line 3: illumination/ diaphragms
Line 4: active ports
Line 5: focus/ stops
(DMI 6000 B only)
The content of the display changes according to
the active function.
Fig. 83 Arrangement of the function buttons – overview
1 Four variable function buttons
2 Illumination Manager
3 Front control panel
4 Focus buttons (DMI6000 B only)
5 Three variable function buttons
6 SmartMove knobs
7 SmartMove function buttons
Pictograms
Contrast method
Objective/
Magnifi cation
Illumination
Diaphragm
Ports/Eyepiece
Focus/stops
(DMI6000 B only)
6
6
7
543321
7
65
Page 66

7. Start-up
The motorized DMI stands can be controlled
using the function buttons on the stand, the remote control SmartMove or the STP6000.
7.4 The Function Buttons on the Stand
Leica DMI3000 B:
• Focus wheels: the left-hand focus wheels can
be used for both coarse and fi ne focusing; the
right-hand focus wheel for fi ne focusing only
(a version of the Leica DMI3000 B with mirrored focus controls is also available)
• Light intensity: the transmitted light intensity
can be adjusted continuously from 0 to 12 V
using the potentiometer at the lower left of the
front of the microscope stand.
For the Leica DMI3000 B please continue at 7.6.
Illumination.
Leica DMI4000 B and Leica DMI6000 B:
A number of function buttons are located on both
sides of the stand. These can be broken down
into fi xed and variable buttons. The variable function buttons have different functions depending
on the features of the individual microscope.
Fixed function Buttons on the Left Side
The TL/IL button (84.1) toggles between the incident-light and transmitted light axis. The contrast
method last used with a given axis is restored
when switching.
The INT buttons (84.3) adjust the light intensity.
The adjustment can be made in coarse or fi ne
steps. Pressing both INT buttons at the same time
toggles between coarse and fi ne adjustment.
The AP buttons (84.2) for the aperture diaphragm
and FD (84.4) for the fi eld diaphragm open and
close their respective diaphragms.
Note:
Changes to the light intensity as well as aperture
and fi eld diaphragm settings are stored for the individual objectives and contrast methods.
Fig. 84 Fixed function buttons (left side of stand)
1 Toggle transmitted light/incident light
2 Aperture diaphragm
3 light intensity
4 fi eld diaphragm
3
2
4
66
1
Page 67

7. Start-up
Variable Function Buttons on the Stand
The variable function buttons are assigned functions at the factory that are appropriate to the
features of your microscope. They are labeled
accordingly. For details on button assignments,
please refer to the included identifi cation sheet.
For information on the abbreviations used, please
refer to the list → p. 63.
Note:
The Leica Application Suite (LAS) software is required for changing the button assignments.
Possible functions*:
BF
PH
ICT
DF
IMC
POL
CHANGE TL
INT ↑
INT ↓
AP ↑
AP ↓
FD ↑
FD ↓
SHUTTER TL
TL FLT 1
TL FLT 2
FLUO
CUBE 1
CUBE 2
CUBE 3
CUBE 4
CUBE 5
CUBE 6
AFC ON/OFF
AFC HOLD
CHANGE CUBE CW
CHANGE CUBE CCW
INT FLUO ↑
INT FLUO ↓
FD FLUO ↑
FD FLUO ↓
CHG FW
IFW
ExMan
COMBI
CHANGE COMBI
CHANGE OBJ CW
(only DMI6000 B)
CHANGE OBJ CCW (only DMI6000 B)
Z FINE
(only DMI6000 B)
Z COARSE
(only DMI6000 B)
XY PRECISE
XY FAST
BTP ON/OFF
(only DMI6000 B)
DRY/IMM
CHANGE FLT
CHANGE CS
OBJ 1-6
MEM 1-6
Turns AFC on or off
Holds current position
Fig. 85 Function buttons (left side of stand)
1 Variable function buttons
2 Open/close aperture diaphragm
3 TL/IL switching
4 Open/close fi eld diaphragm
5 Increase/decrease light intensity
54321
Fig. 86 Function buttons (right side of stand)
1 Variable function buttons
* See page 63 for abbreviations
1
67
Page 68

7. Start-up
Function Buttons on the Front Panel (Fig. 87)
100% of the light goes to the eye piece
(87.1).
→
Toggle function for the side ports
→
(87.2). This function depends on the
individual microscope confi guration.
Note:
Switching to the bottom port:
via the variable function buttons (Lei-
ca DMI6000 B only), switching to top
port: manually.
SHUTTER Opens and closes the shutter (87.3).
Switches between the possible
→
→
mag nifi cations of the magnifi cation
changer (87.4).
The magnifi cation changer is set to
1x
the magnifi cation 1x (87.5).
CUBE The CUBE 1 to CUBE 6 (87.6) buttons
permit the direct selection of individual fi lter cubes, provided the selected cube is valid for the selected
method.
Press the CUBE 3 and CUBE 4 but-
tons at the same time to display the
assignments of the variable function
buttons. To reset the display, press
the buttons again or wait 3 seconds.
Focus buttons (Fig. 88) (DMI6000 B only)
Z↑
Moves the Z drive in the indicated di-
Z↓
SET + Z
SET + Z
rection.
↑ Sets the upper focus stop.
↓ Sets the lower stop.
Fig. 87 Front control panel
1 100% light to eyepiece
2 Toggle ports
3 Shutter
4 Switch between subsequent magnifi cations
5 Subsequent magnifi cation 1x
6 Selecting fi lter cubes
4
2 5
13
66
68
Fig. 88
1 Focus control buttons
2 Open fi lter drawer
2
1
Page 69

7. Start-up
Shutter button + Cube buttons 1-6 (Leica
DMI4000 B and Leica DMI6000 B only)
The selected cube is moved to the
loading position for replacement.
„Load“ appears on the screen. After
pressing the button (88.2) the draw-
er is opened and the cube can be
changed. The next fi lter cube will be
moved to the loading position after
the drawer is closed.
7.5 The SmartMove Remote Control Module
SmartMove knobs
(Leica DMI4000 B and Leica DMI6000 B)
Use the knobs 89.1 and 89.2 to move the stage in
X and Y directions.
The image is focused using the knob 89.3 (Leica
DMI6000 B only).
The height of the knobs can be adjusted to a
comfortable working position by turning 89.4.
Variable function buttons on SmartMove
The variable function buttons are assigned functions at the factory that are appropriate to the
features of your microscope. They are labeled
accordingly. For details on button assignments,
please refer to the included identifi cation sheet.
For information on the abbreviations used, please
refer to the list → p. 63.
7.6 Illumination
7.6.1 Transmitted light
If your microscope has not yet been set up for
Koehler illumination, please continue with the
„Koehler Illumination“ section.
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select an objective with moderate magnifi cation
(10x–20x)
.
• Set the condenser to the bright fi eld position.
• Place a specimen on the stage.
• Focus on the specimen using the focus wheels.
• Adjust the light intensity.
• Close the fi eld diaphragm manually until the edge
of the diaphragm appears in the fi eld of view.
Fig. 89 SmartMove remote control module
1 travel in x
2 Travel in y
3 Focus
4 Individual adjustment of button height
5 Variable function buttons (factory preset)
Note:
The Leica Application Suite (LAS) software is required for changing the button assignments.
1
3
4
2
5
69
Page 70

7. Start-up
• Using the condenser height adjuster (90.2)
just the condenser until the edge of the fi eld
diaphragm appears in sharp relief (not S40 and
S70 condenser).
• Open the fi eld diaphragm until
it only just disappears from the fi eld of view
(91d).
Leica DMI4000 B and Leica DMI6000 B:
• Select an objective with moderate magnifi cation (10x–20x).
• Activate the transmitted light axis with the
TL/IL button (84.1).
• Press the BF button to activate the bright fi eld
contrast method (one of the variable function
buttons on the stand).
• Place a specimen on the stage.
• Focus the specimen using the SmartMove or
the focus wheels.
, ad-
Note:
The condenser height setting is dependent on
the thickness of the specimen and may require
adjustment for each new specimen.
Koehler illumination
(not for S40 and S70 condenser)
Suitable values for the motorized aperture diaphragm and motorized fi eld diaphragm have
been preset for each objective (Leica DMI4000 B
and Leica DMI6000 B).
been centered at the factory.
However, it may be necessary to readjust the
condenser in some cases. Therefore, check the
condenser centering.
The following procedure is provided for the transmitted light-bright fi eld illumination.
All required functions can be executed at the
touch of a button with the Leica DMI6000 B electronic microscope. (See Chapter 8, Operation).
The condenser has also
• Adjust the light intensity with the INT buttons
(84.3).
• Close the fi eld diaphragm with the FD button
(84.4) or manually until the edge of the diaphragm appears in the fi eld of view.
• Using the condenser height adjuster (90.2), adjust the condenser until the edge of the fi eld
diaphragm appears in sharp relief (not S70
condenser).
• Open the fi eld diaphragm just enough for it to
disappear from the fi eld of view (91d).
70
Preparation:
• Confi gure the microscope as follows:
Set up the illumination, condenser, objectives
and eyepieces correctly. (Please ensure that
the objectives are properly screwed in and
check the eyepiece settings.)
• Switch the microscope on and wait for the initialization phase to complete (automatic functions only).
• You will need either an empty Petri dish (preferably with a glass bottom) with a marking in
the middle or a stained specimen on a slide
with a coverslip.
Page 71

7. Start-up
• Switch to the 10x objective (if not present, the
20x objective).
• Ensure that the condenser is at the correct
height. The condenser height adjustment lets
you set the condenser head to the height of
the nominal free working distance. (For an S23
condenser, for example, the distance between
the surface of the stage and the front lens of
the condenser is approx. 23 mm).
• Hold a piece of white paper (approx. 3-10
cm) under the light source (fi eld diaphragm).
A light ring should appear on the paper – if
not, check the power cable, the light source
and the fuse of the supply unit (CTR box) and
ensure that all of the parts are correctly connected to one another.
• Open the fi eld diaphragm as far as possible until the light ring reaches its maximum diameter.
• Next, hold the paper under the condenser,
directly on the stage. Open the aperture diaphragm as far as possible, until the light ring
has reached its maximum brightness. In order
to achieve maximum brightness, ensure that
no port is activated. The full light should be directed to the VIS port.
• Check the magnifi cation changer to ensure
that the 1x tube lens is selected.
• Adjust the lenses of the eyepieces so that one
circle is visible in the eyepieces (not two!). If
you wear spectacles, remove the antiglare
hoods from the eyepiece tubes (or fold them
back).
• Ensure that the focus on the eyepieces is set
to ±0 (turn the upper part of the eyepiece tubes
until the silver ring is just covered).
• You should see light when looking through the
eyepieces at this point.
If the light is too bright, reduce it as required.
Remove all unneeded components from the light
path.
• Swing all fi lters (in the fi lter magazine of the
lamp housing or the fi lter holder of the condenser) out of the beam path.
• Set the condenser disk to the bright fi eld position.
• If your microscope is equipped for DIC:
• Remove the polarizer.
• Remove the analyzer.
• Remove the objective prism (move the mag-
azine to the „empty“ or „bright fi eld“ position).
• If your microscope is equipped for fl uorescence:
• Select an empty fi lter position (or a fi lter with
low transmission in the visible range, e.g. fi lter A).
Now to begin with the actual Koehler illumination:
• Place your specimen on the stage and focus
so that you can see its details as clearly as
possible. You probably will not get a perfect image at this point, as the illumination will not be
optimal (90a).
• Next, attempt to get a sharp image (or at least
a part of the image at the edge) by carefully
moving the condenser up and down (90.2). Try
this with a variety of fi eld diaphragm settings
until you get a clear, sharp image (91.b). This
may take a while!
• To center the sharp image, insert the centering
keys in the openings provided at either side of
the top part of the condenser (90.1). Move the
image into the center of the fi eld of view (91.c).
Next, open the fi eld dia phragm until the image
fi lls nearly the entire fi eld of view. The black
71
Page 72

7. Start-up
edges of the image should have the same distance to the outer edge of the fi eld of view on
all sides. If not, recenter the image with the
centering screws. Adjust the height of the condenser until the edges are sharp. Now open
the fi eld diaphragm until the image fi lls the
entire fi eld of view and the black edges have
disappeared completely (91.d).
• The last step is the adaptation of the contrast
settings. To improve the contrast, close the aperture diaphragm – if you close it too far, however, the resolution of the image details will
deteriorate.
To see the aperture diaphragm, remove an
eyepiece tube and look directly into the tube.
Your eye should be around 10 to 20 cm from
the tube. Change the size of the aperture diaphragm until its image is clearly visible in the
pupil of the objective.
• Set the aperture diaphragm to cover 2/3 to 4/5
of the pupil diameter. You will now have the
optimal balance between resolution and contrast.
7.6.2 Incident Light - Fluorescence
Leica DMI3000 B:
• Select an objective with moderate magnifi cation (10x–20x) and adjust the image.
• Close the fi eld diaphragm with the turning knob
until the edge of the diaphragm (round or angled) appears on the specimen level.
• If the limits of the fi eld diaphragm are not in the
center of the fi eld of view, move the position
of the fi eld diaphragm to the center with the
two centering screws on the right side of the
stand using a 3 mm Allen key. When centering, observe the position of the fi eld diaphragm
through the eyepieces or on the monitor.
• Open the light fi eld diaphragm until it just disappears from the fi eld of view.
Fig. 90 Condenser centering
1 Centering openings
2 Height adjuster
3 Prism and phase ring centering
2 1
3
72
Fig. 91 Koehler Illumination
a Field diaphragm not focused, not centered
b Field diaphragm focused, but not centered
c Field diaphragm focused and centered
Diameter is too small, however
d Illumination fi eld diameter = visible fi eld diameter
(Koehler illumination)
21
3
a
b
cd
Page 73

7. Start-up
Leica DMI4000 B and Leica DMI6000 B:
Suitable aperture and fi eld diaphragm values have
been preset for each objective. The inci dent light
module has also been centered at the factory.
However, it may be necessary to readjust the
in cident light module in some cases after transporting and setting up the stand. Therefore,
check the fi eld diaphragm centering.
The following procedure is provided for the incident light-bright fi eld illumination.
• Select an objective with moderate magnifi cation (10x–20x).
• Activate the incident light axis with the TL/IL
button (84.1).
• Press the IL-BF / Fluo button to activate the
bright fi eld contrast method (one of the variable function buttons on the stand).
• Place a specimen on the stage.
• Focus the specimen using the SmartMove or
the focus wheels.
• Adjust the light intensity with the INT buttons
(84.3).
Adjusting the fi eld diaphragm*
• Close the fi eld diaphragm with the FD button
(84.4) or manually until the edge of the diaphragm (round or rectangular) appears in the
fi eld of view.
•
If the limits of the fi eld diaphragm are not in the
center of the fi eld of view, move the posi tion of the
fi eld diaphragm to the center with the two centering screws (92.1) on the right side of the stand.
• Use the function buttons FD (84.4) to open the
fi eld diaphragm to the point that they just dis-
appear from the fi eld of view.
• We recommend the use of a rectangular fi eld
diaphragm when using a digital camera. Match
the size of the diaphragm to the chip size of the
camera.
7.7 Checking Phase Contrast Rings
If your microscope is equipped for phase contrast, light rings to match your objectives will be
installed in the condenser.
The light rings are already centered in the factory. As a result of transport and setup of the
stand, however, in some cases centering maybe
be required again. Therefore check the centering.
Note:
Each objective has its own light ring assigned to
it in the condenser. The test must therefore be
performed for each objective.
Regular phase contrast with phase objectives
When choosing an objective suitable for phase
contrast, the appropriate light ring is selected
automatically when using a motorized condenser. Otherwise, select the light ring manually.
Fig. 92
Adjusting the fi eld
diaphragm (incident lightfl uorescence)
1 Adjusting screws for
moving the fi eld
diaphragm
1
* not in combination with structured illumination
73
Page 74

7. Start-up
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Set the condenser to the bright fi eld position.
Leica DMI4000 B and Leica DMI6000 B:
• Press the BF (bright fi eld) button (one of the
variable function buttons on the stand).
• Instead of an eyepiece, place a focusing telescope (Fig. 93) in the observation tube or
ac tivate the Bertrand lens (pull rod (94.1) on
tube).
• Select the phase contrast objective with the
lowest magnifi cation.
• Focus on the specimen.
• Focus the ring structure (95a) by loosening
the clamping ring (93.2) somewhat and moving
the eyelens (93.1), or focus the Bertrand lens
(94.2).
• Retighten the clamping ring.
Leica DMI3000 B:
• Select the light ring for the active objective on
the condenser.
Leica DMI4000 B and Leica DMI6000 B:
• Press the PH (phase contrast) button (one of
the variable function buttons behind the focus
wheels). The light ring will be selected in the
condenser.
Fig. 94
1 Activating the Bertrand lens
2 Focusing the Bertrand lens
2
1
Fig. 93 Focusing telescope
1 Adjustable eyelens
2 Clamping ring for fi xing the focus position
1
2
74
Fig. 95 Phase contrast centering procedure
PH=phase contrast ring, LR=light ring
a Condenser in bright fi eld (BF) position
b Condenser in phase contrast (PH) position
Light ring (LR) not centered
c Light ring and phase ring centered
ab c
PH LR
Page 75

7. Start-up
• If the light ring and the phase ring are not
shown as arranged in Fig. 95c, the light ring
must be centered.
• Insert the centering keys into the openings
provided on both sides of the condenser (90.3).
• Turn the centering keys until the dark ring
(phase ring in the objective) is congruent with
the slightly narrower bright ring (light ring in
condenser) (95 c).
Caution!
The centering keys must be removed from
the centering openings before changing objectives. They may block the condenser.
• Repeat the process for all additional phase
contrast objectives.
• Remove the centering keys after centering.
Integrated phase contrast with bright fi eld
objectives via front slider
(For the eyepoint of your objective, please refer
to the included objective list or the engraving on
the objective itself.)
• Move the front slider with the phase rings into
the beam path.
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Set the condenser to the bright fi eld position.
Fig. 96 Condenser with motorized polarizer
1 Centering key for polarizer
1
When choosing an objective suitable for phase
contrast, the appropriate light ring is selected
automatically when using a motorized condenser. Otherwise, select the light ring manually.
Centering the phase rings is not required for objectives with eyepoint A. Checking the position
of the phase rings is essential only when using
objectives with eyepoint C.
Fig. 97 Condenser centering
1 Centering openings
2 Height adjuster
3 Prism and phase ring centering
2 1
3
21
3
75
Page 76

7. Start-up
Leica DMI4000 B and Leica DMI6000 B:
• Press the BF (bright fi eld) button (one of the
variable function buttons on the stand).
• Select the objective with the lowest magnifi cation.
• Focus on the specimen.
• Select the objective with the lowest magnifi cation and eyepoint C.
Leica DMI3000 B:
• Select the light ring for your current objective
on the condenser.
Leica DMI4000 B and Leica DMI6000 B:
• Press the PH (phase contrast) button (one of
the variable function buttons behind the focus
wheels). The light ring will be selected in the
condenser.
• Slide the front slider with the phase rings to
position C (A and C refer to the eyepoint of the
objective. For the eyepoint of your objective,
please refer to the included objective list or the
engraving on the objective itself.)
• If the light ring and the phase ring are not
shown as arranged in Fig. 95c, the light ring
must be centered.
• Insert the centering key in the opening provided on the front slider
• Turn the centering keys until the dark ring
(phase ring in the objective) is congruent with
the slightly narrower bright ring (light ring in
condenser) (95 c).
• Remove the centering keys after centering.
7.8 Checking modulation contrast slit dia-
phragms
If your microscope is prepared for integrated
modulation contrast, its condenser will be
equipped with slit diaphragms suitable for the
objectives.
The slit diaphragms have been centered at the
factory.
Their proper location should be checked, however.
• Instead of an eyepiece, place a focusing telescope (Fig. 93) in the observation tube or activate the Bertrand lens (pull rod (94.1) on tube).
• Focus the ring structure (95a) by loosening
the clamping ring (93.2) somewhat and moving
the eyelens (93.1), or focus the Bertrand lens
(94.2).
• Retighten the clamping ring.
76
Note:
Each objective has its own slit diaphragm assigned to it in the condenser disk. The test must
therefore be performed for each objective.
Open the cover at the top right side of the condenser. The various
numbered openings for the inserts are now visible. Ensure that all of the slit diaphragms are
fi rmly seated and that none of the retaining
screws are loose. If a part has loosened, please
see Chapter 6.5 Installation of Condensers.
Page 77

7. Start-up
7.9 Setting the Motorized Polarizer
Remove your specimen from the stage.
Leica DMI3000 B:
• Set the condenser to the bright fi eld position.
• Insert the analyzer into the analyzer slot on
right side of the stand.
• Activate the polarizer.
• Turn the polarizer until you have the optimal
dark position.
Leica DMI4000 B and Leica DMI6000 B:
For manual condensers, proceed as described
above for the DMI3000 B.
• Select the POL method (one of the variable
function buttons on the stand). If the analyzer
is present on the Fluo turret as an analyzer
block, it will move into position automatically. A
manual analyzer must be positioned by hand.
In the case of motorized condensers with mo-
torized polarizers, the polarizer will move into
position automatically.
• Insert the centering key in the opening provided on the condenser (Fig. 96).
7.10 Adjusting the Light Sources
Transmitted light axis (TL) with lamp housing
107/2
The lamp housing 107/2 with a 12V 100W halo gen
lamp is fi xed. Centering the lamp is not required.
Lamp housing 107 L
for 12V 100W halogen lamp
The lamp can be adjusted using the screws (98.2)
and the button (98.3).
• Place a sheet of white paper under the fi eld
diaphragm.
• Adjust the lamp to create an evenly bright spot
on the paper.
Incident light axis (IL) with lamp housing 106 z
• When a supply unit is used, it is turned on fi rst.
• Activate the incident light axis (for Leica
DMI4000/6000 B with the TL/IL function button.
FLUO will appear on the LeicaScreen).
• Insert the lamp adjustment refl ector (Fig. 99) in
the fi lter turret in place of a fi lter cube.
Make a note of the designation of the re placed
fi lter cube.
• Set up optimal darkening. (The analyzer must
be in place.)
• Remove the centering keys.
Replace your specimen on the stage.
77
Page 78

7. Start-up
Note:
To avoid adjustment errors, neighboring fi lter
cubes must also be removed.
• Turn the refl ector into the beam path.
For Leica DMI4000/6000 B: The refl ector is cor-
rectly positioned when the LeicaScreen shows
the designation of the replaced fi lter cube.
Caution!
Never look directly into the beam path!
Beware of the glare hazard when switching to
refl ector BF or Smith!
Fig. 98 Lamp housing 107 L
1 Mounting for housing
2 Screw for vertical adjustment
3 Button for horizontal adjustment
4 Collector focusing
4
12
78
Fig. 99 Refl ector cube for lamp adjustment
3
Page 79

Caution!
Light sources pose a potential irradiation risk
(glare, UV-radiation, IR-radiation).
7. Start-up
Centering the Hg 100 W and Xe 75 W
mercury lamps
• The adjustment window shows the direct image of the arc and its mirror image. These are
generally not in alignment with one another.
In the lamp housing 106 z, the direct image of the
fi lament (in halogen lamps) or the arc (in gas discharge lamps) and its refl ection are focused separately and adjusted in relation to one another.
An adjustment window (2.8, p. 21; 5b.3, S.25) in
which the light source is visible is located on the
right side of the microscope.
Adjust the lamp as follows while observing the
light source in the adjustment window.
• Focus the direct image with the collector
(100.6).
• Use the adjusting buttons to pivot the arc’s mirror image on the rear side of the lamp housing
(100.2, 100.4) to the side or completely out of
the beam path. The arc’s focused image remains visible (Fig. 101).
• Use the adjusting buttons (100.1 and 100.5) to
place the direct arc image in the middle of the
centering plane, whereby the bright tip of the
arc, the focal spot, should lie slightly outside
the center (Fig. 102).
Fig. 100 Lamp housing 106z L
1 Lamp adjustment, vertical
2 Vertical refl ector adjustment
3 Focusing the refl ector image
4 Horizontal refl ector adjustment
5 Lamp adjustment, horizontal
6 Collector focusing
7 Screw
7
1
6
5
2
3
4
79
Page 80

7. Start-up
• Then pivot the arc’s mirror image with the adjusting knobs (100.2) and (100.4) and focus it
using the refl ector (100.3).
• Use the adjusting knobs (100.2) and (100.4) to
orient the mirror image symmetrically to the direct image (Fig. 103).
The V-shaped irradiation of the direct image
and mirror image arcs can be superimposed.
Caution!
The bright tips of the arcs, the focal spots,
must never be projected onto each other, as
this results in a danger of explosion by overheating.
Caution!
The structure of the arc can no longer be
made out clearly in lamps that have been in
service for a long time. The image and mirror
image can no longer be superimposed exactly. In this case, align both images.
Fig. 101 Direct arc image focused but not centered
(in reality, the image is less focused)
Fig. 102 Direct arc image in target position
(in reality, the image is less focused)
• Using the collector, defocus the image with the
knob (100.6) until the arc image and mirror image are no longer recognizable and the image
is homogeneously illuminated.
• Replace the lamp adjustment refl ector with the
original fi lter cube.
80
Fig. 103 Direct arc image and mirror image in target
position (in reality, the image is less focused)
Page 81

8. Operation
8.1 Switching on
8. Operation
When using a gas discharge lamp, the ebq 100
external supply unit must be turned on separately
(104.1).
Leica DMI3000 B:
• Switch on the microscope’s power at the
On/Off switch. The signal lamp is lit when the
instrument is ready. (Continue with Chapter 8.2
Contrast Methods)
Leica DMI4000 B and Leica DMI6000 B:
• Switch on the power of the electronics box
at the On/Off switch (105.1). The signal lamp
(105.2) is lit green when the unit is ready.
All motorized microscope components will
then run through an initialization phase.
Fig. 104 Front panel of ebq 100 supply unit
1 Power switch
2 Lamp status
Note:
If a PC is connected, switch on the electronics
box fi rst, and then the computer.
All motorized microscope components will then
run through an initialization phase.
Note:
In the case of faulty initialization („Init Error“
message on LeicaScreen), see Troubleshooting
chapter, → p. 105.
Fig. 105
front side
Leica CTR6000
1 On/Off switch
2 Signal lamp
1 2
2
1
81
Page 82

8. Operation
All of the user’s previous settings are restored
during the initialization.
. Note: ( reset function)
Caution:
!
The focal position and the lower stop are also
retained from one session to the next when
power is switched off.
After the initialization is complete, the LeicaScreen will display the status screen with microscope’s current settings. Fig. 107 is an example.
The microscope can be reset to its factory default programming:
• With the stand switched off, press the top three
variable function buttons on the left side of the
stand.
• Switch on the power for the stand.
• Hold the buttons until the initialization is complete.
• The standard information display will now appear in the LeicaScreen (Fig. 106 and 107).
• Switch the instrument off and back on. The settings are now saved.
Fig. 106 LeicaScreen initialization Fig. 107 LeicaScreen following initialization
82
Page 83

8. Operation
8.2 Contrast Methods
All of the contrast methods
B and Leica DMI6000 B
trolled via the variable function buttons and the
Leica Application Suite (LAS). The only exceptions are methods that involve com ponents requiring manual control (e.g. systems with manual
analyzers). The following section describes the
use of the function buttons on the stand. For instructions on the use of the software, please refer to the separate manual.
of the Leica DMI4000
can be selected and con-
Contrast methods for the Leica DMI3000 B are
controlled via the manual condenser, the manual
objective turret, as well as turning knobs and
sliders at the microscope.
8.2.1 Bright Field (TL)
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Set the condenser to the bright fi eld position.
• Remove all other optical components such
as analyzers, polarizers or IC prisms from the
beam path.
• Insert a transmitted light specimen.
• Select your objective
• Set the brightness at the light potentiometer
• Focus the image with the focus wheels.
Leica DMI4000 B and Leica DMI6000B:
Note:
If all positions of the fi lter turret are occupied, fi lter cube „A“ can be swapped for fi lter cube „ATL“ using the Leica Application Suite (LAS/LAS
AF). TL contrast methods are possible with that
fi lter cube.
83
Page 84

8. Operation
• Use the TL/IL function button to switch to
transmitted light (TL).
• Select the BF (bright fi eld) contrast method
by pressing the variable button BF.
Alternatively: press the variable button
CHANGE TL .
(For details on button assignments, please see
the identifi cation sheet.)
BF will appear on the LeicaScreen.
Motorized condensers will now move to the
bright fi eld position. Coded condensers must
be switched manually.
The fl uorescence fi lter turret will automati cally
go to an empty position or to the „A-TL“ fi lter
cube.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
Fig. 108 Function buttons (left side of stand)
1 variable function buttons
2 Open/close aperture diaphragm
3 TL/IL switching
4 Open/close fi eld diaphragm
5 Increase/decrease light intensity
54321
84
Fig. 109 Function buttons (right side of stand)
1 variable function buttons
1
Page 85

8. Operation
8.2.2 Phase Contrast (TL)
(integrated phase contrast, see 8.2.6)
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select a phase contrast objective.
• Select the suitable light ring on the condenser.
• Open the aperture of the condenser completely.
• Remove all other optical components such
as analyzers, polarizers or IC prisms from the
beam path.
• Insert a phase contrast specimen.
• Set the brightness at the light potentiometer
Focus the image with the focus wheels.
•
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to
transmitted light (TL).
• Select the PH (phase contrast) contrast method
by pressing the variable button PH.
Alternatively: press the variable button
CHANGE TL .
(For details on button assignments, please see
the identifi cation sheet.)
PH will appear on the LeicaScreen.
Motorized condensers will now switch to the
correct light ring. Coded condensers must be
switched manually.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
Objectives that are suitable for phase contrast
are engraved with PH.
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
Note:
When selecting the phase contrast method, the
aperture diaphragm is opened fully and can not
be adjusted.
85
Page 86

8. Operation
8.2.3 Dark Field (TL)
Note:
The maximum usable objective aperture for dark
fi eld is for the condenser S1 0.70 and for the condenser S23/S28 0.40.
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select a dark fi eld objective.
• Select the suitable dark fi eld stop on the condenser.
• Open the aperture of the condenser completely.
• Remove all other optical components such
as analyzers, polarizers or IC prisms from the
beam path.
• Insert a dark fi eld specimen.
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to
transmitted light (TL).
• Select the DF (dark fi eld) contrast method
by pressing the variable button BF.
Alternatively: press the variable button
CHANGE TL .
(For details on button assignments, please see
the identifi cation sheet.)
DF will appear on the LeicaScreen.
Motorized condensers will now switch to the
dark fi eld ring. Coded condensers must be
switched manually.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
When selecting the dark fi eld method, the aperture diaphragm is opened fully and can not be
adjusted.
• Set the brightness at the light potentiometer
• Focus the image with the focus wheels.
86
Page 87

8. Operation
8.2.4 Polarization (TL)
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select an objective.
• Set the condenser to the bright fi eld position.
Remove all IC prisms from the light path.
• Move the polarizer on the condenser into the
beam path.
• Insert the analyzer into the right side of the
stand until it clicks into position.
• Bring the polarizer and analyzer into cross position until they reach maximum darkness.
• Insert a specimen.
• Set the brightness at the light potentiometer
• Focus the image with the focus wheels.
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to
transmitted light (TL).
Manual method:
• Move the polarizer on the condenser into the
beam path.
• Insert the analyzer into the right side of the
stand until it clicks into position (Fig. 110).
• Bring the polarizer and analyzer into cross position until they reach maximum darkness.
• Place a specimen on the stage and select a
suitable objective.
Motorized method:
• If the microscope is equipped with the relevant
components, the polarizer will be activated
automatically in the condenser when the POL
contrast method is selected. The analyzer
cube is also automatically positioned in the
beam path.
Combined methods:
• The Leica DMI4000 B and Leica DMI6000 B microscope permit purely mechanical and motorized components – such as a mechanical analyzer and motorized polarizer – to be combined.
• Select the POL (polarization) contrast method
by pressing the variable button POL.
Alternatively: press the variable button
CHANGE TL .
(For details on button assignments, please see
the identifi cation sheet.)
POL will appear on the LeicaScreen.
Fig. 110 Inserting the analyzer
87
Page 88

8. Operation
8.2.5 Differential Interference Contrast (TL)
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select an objective.
• At the condenser, select the appropriate Wollaston prism condenser.
• At the objective turret, select the appropriate
Wollaston prism objective.
• Move the polarizer on the condenser into the
beam path.
• Insert the analyzer into the right side of the
stand until it clicks into position.
• Insert a specimen.
• Set the brightness at the light potentiometer
• Focus the image with the focus wheels.
• Use the knurled wheel below the objective turret for fi ne adjustment (Fig. 111).
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to
transmitted light (TL).
• The polarizer in the condenser and the suitable
condenser prism are automatically positioned
in the beam path. The corresponding objective prism and the analyzer cube are also positioned automatically.
• Place a DIC specimen on the stage.
• Rotate an appropriate objective into place.
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
• Use the knurled wheel below the objective turret for fi ne adjustment (Fig. 111).
Manual alternative:
• Move the polarizer on the condenser into the
beam path manually.
• Insert the analyzer manually into the right side
of the stand until it clicks into position (Fig. 110).
Adjust the objective and condenser prisms
manually until a valid combination appears on
the display.
• Use the knurled wheel below the objective turret for fi ne adjustment (Fig. 111).
Fig. 111 DIC disk with knurled wheel for fi ne adjustment
• Select the DIC contrast method
by pressing the variable button DIC.
Alternatively: press the variable button
CHANGE TL .
(For details on button assignments, please see
the identifi cation sheet.)
DIC will appear on the LeicaScreen.
88
Page 89

8. Operation
8.2.6 Integrated Phase Contrast (TL)
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select a bright fi eld objective with eyepoint B
or C.
• Select the appropriate light ring at the condenser (see table).
• Open the aperture of the condenser completely.
• Remove all other optical components such
as analyzers, polarizers or IC prisms from the
beam path.
• Slide the phase contrast front module to the
correct eyepoint, B or C.
• Insert a phase contrast specimen.
• Set the brightness at the light potentiometer
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to
transmitted light (TL).
• Select the IPC contrast method (integrated
phase contrast). by pressing the variable button IPH. Alternatively: press the variable button
CHANGE TL . (For details on button assignments, please see the identifi cation sheet.)
PH will appear on the LeicaScreen. Motor-
ized condensers will now switch to the correct
light ring. Coded condensers must be switched
manually.
• Insert a transmitted light specimen.
• Select a suitable objective (eyepoint B or C).
• Slide the phase contrast front module to the
correct eyepoint, B or C.
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
• Focus the image with the focus wheels.
Note:
When selecting the phase contrast method, the
aperture diaphragm is opened fully and can not
be adjusted.
IP0 for 5x, e.g. NPlan 5x objective with eyepoint B
IP1 for 10x, e.g. NPlan 10 x objective with eyepoint B and
for 20x, e.g. NPlan L 20 x objective with eyepoint C
IP2 for 40x, e.g. HCX PL FL L 40 x objective with eyepoint C
IP3 for 63x, e.g. PL FL 63x/0.70 objective with eyepoint C
89
Page 90

8. Operation
8.2.7 Integrated Modulation Contrast (TL)
Leica DMI3000 B:
• If necessary, adjust the TL bright fi eld position
at the fi lter slider.
• Select a bright fi eld objective with eyepoint B
or C.
• Select the slit illumination suitable for the magnifi cation at the condenser.
• Move the polarizer on the condenser into the
beam path.
• Remove all other optical components such as
analyzers or IC prisms from the beam path.
• Slide the IMC front module to the correct eyepoint, B or C.
• Insert a specimen.
• Set the brightness at the light potentiometer
• Focus the image with the focus wheels.
• Use the knurled wheels on the slider and the
polarizer for fi ne adjustment.
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to
transmitted light (TL).
• Select the IMC contrast method (integrated
modulation contrast). by pressing the variable
button IMC.
Alternatively: press the variable button
CHANGE TL .
(For details on button assignments, please see
the identifi cation sheet.)
IMC will appear on the LeicaScreen. If you
have a motorized condenser, the correct slit
diaphragm and polarizer will be activated automatically. Coded condensers must be switched
manually.
• Insert a specimen.
• Select a suitable objective (eyepoint B or C).
• Slide the IMC front module to the correct eyepoint, B or C.
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
• Use the knurled wheels on the slider and the
polarizer for fi ne adjustment.
90
Page 91

8. Operation
8.3 Fluorescence
Leica DMI3000 B:
The fi lter slider (5a.9, S.24) is used to operate the
fl uorescence module.
• Pull the fi lter slider out completely to open the
beam path.
• Push the fi lter slider into the middle position
(1st detent) to bring the blue fi lter into the
beam path.
• Insert the fi lter slider fully in order to block the
beam path (shutter position).
• The fl uorescence illumination is controlled by
the rotary knob (5a.10, S.24).
• The fi lter cubes are swiveled manually into the
beam path by turning the incident light turret
disk.
Leica DMI4000 B and Leica DMI6000 B:
• Use the TL/IL function button to switch to fl uorescence FLUO.
• Place a specimen on the stage and select a
suitable objective.
• The current fl uorescence fi lter cube will be
displayed on the LeicaScreen.
• You may protect your specimen from fading by
closing the incident light shutter.
To do so, press the SHUTTER button (87.3) on
the front panel.
The following pictogram will appear on th Lei-
caScreen:
Changing the fl uorescence fi lter cube
Fixed function buttons on the front panel:
CUBE 1 to CUBE 6 or Cube CCW
Variable function buttons on the front panel
and SmartMove: CUBE CW or CUBE CCW
Leica Application Suite (LAS) Software
• Focus the image with the knob on the SmartMove or the focusing wheel and adjust the intensity with the INT function buttons.
91
Page 92

8. Operation
Options
• The intensity of the fl uorescence can be increased by using the booster lens (Fig. 112) on
the left rear side of the stand (Fig. 113).
If bright fl uorescence is required in the center
of the fi eld of view, slide the booster lens into
the receptacle with the marking
•
1.4x
facing the user. If a homogeneous distribution
over the entire fi eld of view is required, turn
the booster lens 180° so that the marking
0.7x
is facing forward.
• For multiple fl uorescence, we recommend using the Excitation Manager and/or the ultrafast
internal fi lter wheel. Excitation wavelengths
can thus be changed in milliseconds. They are
controlled by the function buttons.
Fig. 112 Booster lens
92
Fig. 113 Booster lens in stand
1
Page 93

8.4 Combination Methods
8. Operation
(Leica DMI4000 B and DMI6000 B)
Up to two combination methods are possible depending on the features of the individual microscope:
FLUO/PH and FLUO/DIC
• Select the combination method
by pressing the variable button COMBI .
Alternatively: press the variable button
CHANGE COMBI .
(For details on button assignments, please see
the identifi cation sheet.)
The content of the display changes accord-
ingly.
• Place a specimen on the stage and select a
suitable objective.
• Select the desired fi lter cube using the fi xed
function buttons on the front panel.
• The illumination settings for the fl uorescence
and transmitted light axes can be adjusted
separately.
Note:
The manual analyzer (Fig. 110) must be used for
the FLUO/DIC method as described in Chapter
8.2.5, p. 88.
• Toggle the illumination axes with the TL/IL
function button. The content of the LeicaScreen changes accordingly.
FLUO > DIC
The transmitted illumination is activated.
FLUO < DIC
The fl uorescence illumination is activated.
93
Page 94

8. Operation
8.5 Focusing
Leica DMI3000 B and Leica DMI4000 B:
The left-hand focus wheels can be used for both
coarse and fi ne focusing; the right-hand focus
wheel for fi ne focusing only (a version of the
Leica DMI3000 B with mirrored focus controls is
also available)
Leica DMI6000 B:
Note:
The parfocality teach-in has already been performed at the factory. However, it may be necessary to perform another teach-in after installing
the objectives when setting the microscope up.
We recommend checking parfocality before setting the stops and performing a teach-in with the
Leica Application Suite (LAS) if necessary.
Focusing the image
The focusing is controlled by the knobs (116.3,
p. 101) on the SmartMove remote control module.
Abb. 114
1 Focus operating keys
2 Open fi lter drawer
2
1
Alternatively, use the focus wheels on either side
of the stand.
The current Z position is shown on the LeicaScreen. In the case of motorized stages, the Z
drive will travel to its lowest position prior to the
stage initialization when switching the microscope on.
The focus buttons Z
the stand (Fig. 114) permit fast focusing or lowering of the objectives.
Setting stops
Set the lower focus stop by pressing and holding the SET button and pressing the Z
well.
The display will show .
Pressing the button combination again will delete
the stop.
The display will show .
The lower focus stop can also be set using the
Leica Application Suite (LAS).
The lower stop is the same for all objectives and
can not be traversed.
In addition, a focus position that can not be traversed can also be set.
To do so, press and hold the SET button and press
button as well.
the Z
The display will show .
Pressing the button combination again will delete
the stop.
The display will show .
The focus position can also be set using the Leica
Application Suite (LAS).
Set the focus position for the dry objective at the
highest magnifi cation. The focus positions will
then be set automatically for all other objectives,
taking parfocality and working distances into account.
↑ and Z↓ on the right side of
↓ button as
94
Page 95

8. Operation
Set the stops via
fi xed function buttons on stand
Leica Application Suite (LAS) Software
Summary of pictograms
lower focus stop not set
lower focus stop set
focus position not set
focus position set
Going to the stops
Go to the lower stop by pressing and holding the
↓ button.
Z
Go to the focus position by pressing and holding
↑ button.
the Z
These functions can be assigned to variable
function buttons on the stand or SmartMove, or
they can be controlled via software.
Go to stops via
Setting the step increments
It is possible to toggle between Fine and Coarse
step increments.
The Fine value varies to suit the current objective. Suitable values have been predefi ned. The assignments
can be changed with the Leica Application Suite (LAS).
When selecting Coarse, the positioning speed is
the same for all objectives. Coarse corresponds
to the maximum speed.
Note:
The assignment of a specifi c step increment to
an objective not only applies to the Z drive, but
also to the step increments assigned to the stage
when Precise (→ p. 101) is selected.
Switch between Fine and Coarse via
variable function buttons on stand and
SmartMove
Leica Application Suite (LAS) Software
Only for DMI6000 B with AFC (Adaptive Focus
Control)
fi xed function buttons on stand
variable function buttons on stand and
SmartMove
Leica Application Suite (LAS) Software
Note:
When going to the stops with the Z
buttons, hold the button until the stop has been
reached.
↑ and Z↓
AFC actively holds a pre-defi ned focus position
over time. This feature is especially useful, if
e.g. during a time-lapse experiment at 37°C the
climate chamber has to be opened and a drop of
the temperature may occur.
Activate the AFC function, focus on your specimen either with the hand wheel on the microscope stand or at the SmartMove and store the
current focus position as hold position.
AFC can be controlled either by.
•
Variable function keys at the stand or SmartMove
• STP6000
• Software (Leica LASAF)
95
Page 96

8. Operation
8.6 Tubes
Note:
Close any unused tube openings, as otherwise
stray light can interfere with observation.
Adjusting the viewing distance
• Adjust the viewing distance of the eyepieces so that a congruent total image is seen
(Fig. 115).
Adjusting the viewing angle
• Ergotubes feature a tilting binocular section for
a 30–45° viewing angle adjustment range.
Beam splitting in photo tubes
The beam splitting is set manually by pulling out
a control bar.
8.7 Port selection
Leica DMI 3000 B and Leica DMI4000 B:
Manual shifter rod activates and deactivates the
left-hand photo port.
VIS LEFT
100% 0%
20% 80%
alternatively: 0% 100%
Leica DMI 6000 B:
The
button on the front control panel switches 100%
of the light to the eyepieces.
Use the
→
→
button, also on the front control panel, to select
the side ports.
Observation photo
100% 0%
0% 100%
alternatively 50% 50%
BL activation of Bertrand lens*
Light distribution via
manual control bar
96
♦→
Fig. 115 Tube
setting
Page 97

8. Operation
Depending on the confi guration, the screen will
now display
- the active port (right or left) and
- the percentage of light going to the port (100%,
80%, 50%).
Optional Leica DMI 6000 B:
The bottom port selection function can be assigned to one of the variable function buttons on
the stand or the SmartMove.
The top port can only be selected manually.
Select ports via
fi xed function buttons on stand (side ports)
variable function buttons on stand and
SmartMove (bottom port)
manual action (top port)
8.8 Eyepieces
Note:
The eyepiece’s aperture protector must be removed or folded back, during microscopy while
wearing eyeglasses. We recommend removing
bifocals and spectacles with progressive-addition lenses when using the microscope.
• For the adjustable tubes with documentation
output, choose the 100% VIS position.
Eyepieces with inlaid reticle
• Focus the reticle by adjusting the eyelens.
• Focus on the object through this eyepiece.
• Then, close that eye and focus on the object
by adjusting only the second ocular.
Correction for Vision Problems
• With your right eye, look through the right eyepiece and bring the specimen into sharp focus.
• Then, with your left eye, view the same specimen and rotate the left eyepiece tube until
the object is brought into sharp focus. Do not
change the Z position in the process!
97
Page 98

8. Operation
8.9 Objectives
Note:
We recommend running a teach-in via the Leica
Application Suite (LAS) software when using
eyepieces not included in the scope of delivery. This will ensure that the total magnifi cation
shown in the LeicaScreen is correct.
Changing objectives
Leica DMI3000 B and Leica DMI4000 B:
Select objectives manually with the objective turret.
The objective turret of the DMI4000 B is coded
so that the selected objective is shown on the
display.
Leica DMI6000 B:
The objectives can be selected with the function buttons on the stand or the SmartMove, or
by manually turning the objective turret. When
changing objectives manually, please ensure
that the turret clicks into position.
The positions of the objectives in the objective
turret have been specifi ed at the factory and
must be observed when installing the objectives.
(→ also see Objectives, p. 43).
When selecting an objective, the microscope automatically selects:
• the optimal setting for the fi eld diaphragm
• the optimal setting for the aperture diaphragm
98
• the light intensity for the current contrast
method
The objective magnifi cation and total magnifi cation are displayed on the LeicaScreen.
Page 99

8. Operation
• For immersion objectives use the appropriate
immersion medium.
OIL: only use optical immersion oil
according to DIN/ISO standards.
Cleaning → p. 109.
W: Water immersion.
IMM: Universal objective for water, glycerol,
oil immersion.
Color coding of objectives
The magnifi cation of each objective is indicated by a color ring in accordance with DIN/ISO standards:
100x 63x 40x 25x 16x 10x 6.3x 4x 2.5x 1.6x
125x 50x 32x 20x 5x
150x
160x
Caution!
Follow safety instructions for immersion oil!
white dark- light- dark- light- yellow orange red brown gray
blue blue green green
Immersion objectives are marked by an additional, lower color ring.
black oil or Imm (universal objective for
oil, water or glycerin)
white water
orange glycerin
The various engraved markings of the objectives
provide information on their applications:
black or bright fi eld objectives,
dark blue strain-free
green phase contrast objectives,
strain-free
99
Page 100

8. Operation
Select objectives via
variable function buttons on stand and
SmartMove
Leica Application Suite (LAS) Software
Manual selection
Changing the operating modes
„dry“ ( DRY) and „immersion“ ( IMM)
Each objective is assigned to a specifi c objective
category:
1) Dry objectives (DRY)
2) Immersion objectives (IMM)
Note:
It is possible to use objectives for both operating
modes.
The mode can be assigned in the Leica Application Suite (LAS).
• Next, press the but on for the objective you intend to use.
Note:
If the Imm or Dry operating mode buttons are
pressed accidentally, the original mode can be
restored by pressing the appropriate button.
Change operating mode via
variable function buttons on stand and
SmartMove
Leica Application Suite (LAS) Software
Note:
When replacing objectives, you must perform
a teach-in for the new objectives in the Leica
Application Suite (LAS). A parfocality teach-in
should also be performed.
Changing the operating mode
• First, select the operating mode (Imm or Dry)
using the function buttons.
The operating mode may also be selected in
the Leica Application Suite (LAS).
• The objective turret is lowered to its bottom
stop. This is to permit the application of the
immersion liquid when changing from a dry to
an immersion objective. It also permits the removal of the liquid when changing to dry mode.
The current objective remains in the beam
path.
100
Note:
For lockable immersion objectives lock these by
pushing the front part upwards until it stops (approx. 2 mm). Then, after a gentle turning motion
to the right, the objective is locked.
For objectives with corrective mounts turn the
knurl to adjust the objective to the thickness of
the cover glass.
 Loading...
Loading...